Abstract
Hepatitis E virus (HEV) is a major cause of viral hepatitis globally. There is growing concern about transfusion-transmitted HEV (TT-HEV) as an emerging global health problem. HEV can potentially result in chronic infection in immunocompromised patients, leading to a higher risk of liver cirrhosis and even death. Between 0.0013% and 0.281% of asymptomatic blood donors around the world have HEV viremia, and 0.27% to 60.5% have anti-HEV immunoglobulin G. HEV is infectious even at very low blood concentrations of the virus. Immunosuppressed patients who develop persistent hepatitis E infection should have their immunosuppressant regimen reduced; ribavirin may be considered as treatment. Pegylated interferon can be considered in those who are refractory or intolerant to ribavirin. Sofosbuvir, a nucleotide analog, showed modest antiviral activity in some clinical studies but sustained viral response was not achieved. Therefore, rescue treatment remains an unmet need. The need for HEV screening of all blood donations remains controversial. Universal screening has been adopted in some countries after consideration of risk and resource availability. Various pathogen reduction methods have also been proposed to reduce the risk of TT-HEV. Future studies are needed to define the incidence of transmission through transfusion, their clinical features, outcomes and prognosis.
Keywords: Hepatitis E virus, Acute and chronic hepatitis, Immunosuppression, Blood transfusion, Transplantation
Core Tip: Transfusion-transmitted hepatitis E virus (HEV) is an emerging global health concern. In immunocompromised patients, chronic HEV infection increases the risk of liver cirrhosis. The prevalence of viremia and anti-HEV immunoglobulin G in asymptomatic blood donors varies widely between countries but even low concentrations of HEV in blood components are infectious, and in most countries blood donations are not routinely screened for HEV. Treatment of persistent infection includes modification of the immunosuppressant regimen followed by ribavirin. The need for screening of HEV in all blood donations remains controversial. Strategies to reduce de novo HEV infection should also be emphasized.
INTRODUCTION
Hepatitis E virus (HEV) was first discovered as an epidemic of non-A, non-B hepatitis in the 1980s[1], and has since become one of the major global causes of viral hepatitis. The World Health Organization estimated that HEV caused approximately 44000 deaths in 2015, and accounted for 3.3% of global deaths related to viral hepatitis[2]. A recent meta-analysis concluded that approximately 939 million of the global population have ever experienced HEV infection, and 15 to 110 million individuals have recent or ongoing infection[3]. The infection is generally self-limiting; however, it poses a threat to some vulnerable patients resulting in a significant burden of in-patient admissions, chronic infection, organ failure, and death[4]. The mortality rate can be greater than 20% in patients with chronic liver disease, cirrhosis, or pregnancy[4,5]. With a high HEV serological prevalence among the global population, the safety of blood products has become a public health concern. Herein, we review existing evidence on transfusion-transmitted HEV (TT-HEV), and the implications for screening of blood donations.
VIROLOGY
HEV is a positive-sense, single-stranded RNA icosahedral virus belonging to the genus Orthohepevirus within the Hepeviridae family[6]. Orthohepevirus A has eight distinct genotypes, of which HEV-1, -2, -3 and -4 infect humans[7]. HEV genotype C1, belonging to the species Orthohepevirus C, circulates in rats and can cause cross-species infection and sporadic zoonotic transmission to humans[8].
HEV exists in urine or feces as non-enveloped virions encased by a capsid. It circulates in blood in a membrane-associated, quasi-enveloped form (eHEV) which is considered to be less contagious[9]. The entry mechanisms for HEV are not well characterized, but once the genomic RNA is uncoated and delivered to the cytosol, the replication cycle is initiated[10]. The viral release that initiates subsequent infection requires multivesicular bodies through endosomal sorting complexes required for transport[11].
EPIDEMIOLOGY
The prevalence rates of HEV antibody are higher in developing countries than in developed countries[12]. The highest anti-HEV immunoglobulin G (IgG) seropositivity rate has been reported in Africa with a mean of 21.76%, followed by Asia (15.80%), Europe (9.31%), North America (8.05%), South America (7.28%), and Oceania (5.99%). In addition, the reported anti-HEV immunoglobulin M (IgM) seroprevalence rate was 3.09%, 1.86%, 0.79%, 0.22% and 2.43% in Africa, Asia, Europe, North America, and South America, respectively[3].
Among the four major genotypes that can infect humans, HEV-1 and -2 are mostly found in developing countries including Asia, Africa, Latin America, and Mexico. Infection is mainly transmitted via fecally contaminated water, but occasionally also by person-to-person and vertical transmission[13]. Hepatitis E occurs as outbreaks as well as sporadic cases of acute hepatitis, with the preponderance of cases among adolescents and young adults. When stratified by age, the estimated incidence of HEV-1 and -2 infection is roughly between 0.5% and 1.0% for ages 0 to 15 years, with rates increasing to between 1.0% and 1.4% for ages 15 years to 20 years, then falling rapidly to a lower rate of 0.2% and below in individuals older than 30 years[14].
HEV-3 accounts for most of the autochthonous infection in developed countries while HEV-4 is mainly found in Asia and sporadically in Europe[15,16]. The reported seroprevalence of HEV-3 ranged from 0.6% to 52.5% in Europe, 6% in United States, 3 to 16% in United Kingdom and up to 52% in some regions of France[17]. HEV-3 and HEV-4 are zoonotic viruses which are frequently transmitted via food, close contact with animals, or transfusion of viremic blood units[18].
CLINICAL FEATURES AND EXTRAHEPATIC MANIFESTATIONS
The incubation period following exposure to HEV ranges from 2 to 6 wks. HEV infection commonly takes a clinically silent, asymptomatic course with around 5% to 30% of infected individuals developing acute hepatitis[19]. Symptoms of acute hepatitis include fever, malaise, anorexia, vomiting, followed by jaundice, tea-colored urine, and hepatomegaly[20]. It is then followed by a convalescent phase with gradual recovery within a few weeks in immunocompetent patients[21]. Acute liver failure is rare and occurs more frequently in middle-aged/elderly patients[22]. Fulminant hepatitis with fatal outcome is uncommon, but has been observed in pregnant women or in patients with pre-existing liver disease. The development of fulminant hepatitis appears to be related to host-specific factors rather than virus genotype, variants, or specific substitutions[23]. HEV superinfection may trigger liver decompensation in patients with chronic liver disease or cirrhosis, resulting in acute-on-chronic liver failure, which is associated with significant short-term mortality[24,25]. Further research is needed to clarify the clinical features, course of illness, and prognosis of patients with decompensated cirrhosis who develop HEV infection.
HEV-3 and HEV-4 can persist in immunocompromised patients resulting in chronic infection, defined as viral replication lasting for more than 3 to 6 mo[26]. It has been well described in patients after solid organ or stem cell transplant, hematology patients receiving chemotherapy, or HIV-infected patients[27-32]. The prevalence of anti-HEV IgG was about 11.6% and viral RNA was 2% in solid organ transplant recipients[33]. In solid organ transplant recipients who were positive for HEV RNA, more than 60% developed chronic hepatitis[33].
The natural history of chronic hepatitis E infection is not well understood[34]. In liver transplant recipients infected by HEV, histological analyses of liver biopsy revealed atypical morphology that is distinct from those in immunocompetent patients during early phases of infection[35]. Proliferation of, and cytokine production by, CD4+ and CD8+ T-cells were impaired in patients with persistent HEV viremia[36]. Chronic hepatitis E leads to liver fibrosis and cirrhosis. Cases of HEV-related hepatocellular carcinoma have been reported[37].
Although HEV predominantly infects hepatocytes, it may also affect other organs and present as extrahepatic manifestations. The mechanisms by which HEV can induce extrahepatic manifestations are not fully understood, but hypotheses include direct cytopathic tissue damage by extrahepatic replication, or immunological processes induced by an overwhelming host immune response[38]. Details of extrahepatic manifestations are shown in Table 1[39-44].
Table 1.
Extrahepatic manifestations associated with hepatitis E virus infection
|
System
|
Extrahepatic manifestations
|
| Neurological | Guillain-Barré syndrome (GBS) |
| Neuralgic amyotrophy | |
| Neuropathy | |
| Bell’s palsy | |
| Encephalitis | |
| Transverse myelitis | |
| Myositis | |
| Myasthenia gravis | |
| Pseudotumor cerebri | |
| Seizure | |
| Renal | Decrease glomerular filtration rate |
| Glomerulonephritis | |
| Nephrotic syndrome | |
| Mixed cryoglobulinemia | |
| Hematological | Thrombocytopenia |
| Hemolytic anemia | |
| Aplastic anemia | |
| Hemophagocytic syndrome | |
| Monoclonal gammopathy of uncertain significance (MGUS) | |
| Others | Thyroiditis |
| Pancreatitis | |
| Myocarditis | |
| Polyarthritis |
PREVALENCE IN BLOOD DONORS
Viremia
The prevalence of HEV RNA in blood donors varies around the world. (Table 2)[45-78]. Most countries have a low prevalence of HEV viremia, ranging from 0.0013% to 0.086%. A relatively higher rate of viremia was reported in Germany (0.12%) and China (0.281%)[49,70]. A meta-analysis of 10 studies from China showed a pooled prevalence of HEV RNA of 0.1%[79]. The actual prevalence might have been underestimated as some studies included in the meta-analysis conducted RNA detection only in those donors who were positive for anti-HEV IgM or antigen[79].
Table 2.
Hepatitis E virus ribonucleic acid prevalence in donor, only studies include more than 1000 study subjects are included
|
Ref.
|
Country
|
Initial screening method
|
Number of donations screened
|
Number positive donations
|
Prevalence (95%CI)
|
HEV genotype: n/N
|
Median (range) viral load, IU/mL
|
Outcome of recipient
|
| Europe | ||||||||
| Fischer et al[45], 2015 | Austria | RT-PCR (plasma pool of 96 samples) with 95% LOD 11.6 IU/mL | 58915 | 7 | 0.012% | 3: 7/7 | (2200 to 290000) | N/A |
| Vercouter et al[46], 2019 | Belgium | RT-PCR (plasma pool of 6 samples) with 95% LOD 18.6 IU/mL | 38137 | 7 | 0.018% | N/A | (153 to 8710) | N/A |
| Harritshøj et al[47], 2016 | Denmark | TMA assay on individual plasma with 95% LOD 7.9 IU/mL | 25637 | 11 | 0.043% (0.02% - 0.07%) | 3 (in 2 samples) | 13 (unquantifiable to 920) | (1) Look-back testing was performed in 7 recipients; all were tested negative for HEV RNA and anti-HEV IgM; (2) No recipient developed transaminitis; and (3) One patient had strongly positive anti-HEV IgG assay which may indicate recent HEV infection or secondary immune response by HEV re-exposure. |
| Gallian et al[48], 2014 | France | RT-PCR (plasma pool of 96 samples) with 95% LOD 23 IU/mL | 53234 | 22 | 0.045% (0.043%-0.047%). | 3c: 5/14; 3f: 8/14; 3 1/14 | (468 to 5155800) | N/A |
| Westhölter et al[49], 2018 | Germany | RT-PCR (plasma pool of 24 samples) with 95% LOD 18.6 IU/mL | 18737 | 23 | 0.123% | 3: 6/7 | (120 to 11200000) | (1) Retrospective analysis of 4 viremic donors showed that they were HEV-positive in previous donations; (2) In 3 donors, testing of the previously donated blood in pools of 24 samples failed to identify viremic donations but were tpositive in unpooled samples; (3) Fourteen recipients had received HEV RNA positive blood products; (4) One immunosuppressed recipient tested positive for HEV RNA, developed acute on chronic liver failure, and died; and (5) One immunocompetent recipient developed acute self-limited episode of hepatitis E |
| Dreier et al[50], 2018 | Germany | RT-PCR with 95% LOD 4.7 IU/ml for FFP, platelet concentrates, and RBC supernatant; 95% LOD 8.9 IU/mL for RBCs. | 235524 | 182 | 0.077% | 3: 4/4 | (< 25 to 69.4) | (1) Nine viremic donations were transfused to 6 different recipients; (2) Two recipients were immunocompromised (heart transplantation and leukemia); (3) Two recipients died shortly after transfusion for reasons other than HEV infection; and (4) None of the other 4 recipients developed acute HEV infection or had detectable HEV RNA / anti-HEV IgG |
| Corman et al[51], 2013 | Germany | RT-PCR (plasma pool of 96 samples mixed in metapools of 20) | 93955 | 14 | 0.015% | 3: 14/14 | (3.1 to 4.8 Log10 IU/mL) | N/A |
| Vollmer et al[52], 2012 | Germany | RT-PCR (plasma pool of 48 samples) with 95% LOD 4.7 IU/ml | 16125 | 13 | 0.081% | 3: 13/13 | (13 to 68100) | N/A |
| Baylis et al[53], 2012 | Germany | RT-PCR (plasma pool of 96 samples) with 95% LOD 250 IU/mL | 18,100 | 4 | 0.022% | 3 | (3.26 to 5.35 log10 copies/mL) | Donations screened positive for HEV were excluded from pharmaceutical production |
| O'Riordan et al[54], 2016 | Ireland | TMA assay with 95% LOD 5.5 IU/mL | 24985 | 5 | 0.020% (0.0065%-0.0467%) | 3: 3/3 | (10 to 44550) | N/A |
| Spreafico et al[55], 2020 | Italy | TMA assay on individual plasma with 95% LOD 7.9 IU/mL | 9726 | 1 | 0.010% (0.00%-0.06%) | N/A | N/A | N/A |
| Spada et al[56], 2018 | Italy | RT-PCR, plasma pool and sensitivity varies according to anti-HEV IgG and IgM status | 10011 | 0 | N/A | N/A | N/A | N/A |
| Hogema et al[57], 2015 | Netherlands | RT-PCR (plasma pool of 96 samples) with 95% LOD 38.4 IU/mL with the EasyMag extraction method and 10.3 IU/mL using Qiagen extracts | 59474 | 41 | 0.069% | 3c: 15/17; 3f: 2/17 | N/A | N/A |
| Slot et al[58], 2013 | Netherlands | RT-PCR (plasma pool of 48 or 480 samples) with 95% LOD 25 IU/mL | 40176 | 13 | 0.032% | 3: 16/17 | (< 25 to 470000) | N/A |
| Grabarczyk et al[59], 2018 | Poland | TMA assay on individual plasma with 95% LOD 7.9 IU/mL | 12664 | 10 | 0.079% (0.043%-0.145%) | 3i: 2/3; 3c: 1/3 | (16 to 6586 in 4 patients with positive results in qPCR) | N/A |
| Rivero-Juarez et al[60], 2019 | Spain | RT-PCR (plasma pool of 8 samples) with sensitivity 670 IU/mL | 11313 | 4 | 0.035% (0.01%-0.09%) | 3: 4/4 | (10788 to 2000000) | (1) Five patients received transfusions from HEV-infected donors; and (2) None of them showed an increase in alanine aminotransferase levels after transfusion |
| Sauleda et al[61], 2015 | Spain | TMA assay on individual plasma with 95% LOD 7.9 IU/mL | 9998 | 3 | 0.030% (0.01%-0.09%) | 3f (in 1 sample) | (250 to 2755) | N/A |
| Baylis et al[53], 2012 | Sweden | RT-PCR (plasma pool of 96 samples) with 95% LOD 250 IU/mL | 95835 | 12 | 0.013% | 3 | (3.20 to 5.68 log10 copies/mL) | Donations screened positive for HEV were excluded from pharmaceutical production |
| Harvala et al[62], 2019 | United Kingdom | RT-PCR (plasma pool of 24 samples) with 95% LOD 18.6 IU/mL | 1838747 | 480 | 0.026% | 3c: 112/149; 3e: 21/1493f: 12/149; 3a: 1/149; 2 distantly related to 3h, and 1 clustered distantly with 3a | 883 (1 to 3230000) | N/A |
| Thom et al[63], 2018 | United Kingdom | RT-PCR (plasma pool of 24 samples) | 94302 | 38 | 0.040% | 3: 10/10 | N/A | N/A |
| Hewitt et al[64], 2014 | United Kingdom | RT-PCR (plasma pool of 24 samples) | 225000 | 79 | 0.035% | 3: 79/79 | 3900 (50 to 2.37 × 106) | (1) Forty-three patients who had received blood components from HEV-infected donors were followed up; (2) The overall transmission rate was 42% (18 of 43 exposed patients); (3) One recipient developed clinical hepatitis and 4 recipients developed asymptomatic transaminitis; and (4) Four heavily immunosuppressed patients had delayed (37-38 wk) seroconversion or no antibodies detected |
| Cleland et al[65], 2013 | United Kingdom | Nested PCR (plasma pool of 24 samples) with 95% LOD 201 IU/mL | 43560 | 3 | 0.0069% | 3: 3/3 | N/A | N/A |
| North America | ||||||||
| Delage et al[66], 2019 | United States | RT-PCR on individual samples with 95% LOD 18.6 IU/mL | 50724 | 3 | 0.0059% | 3: 2/3; genotyping was unsuccessful in 1 patient | (23 to 1420) | N/A |
| Canada | 50765 | 11 | 0.022% | 3 (in 1 sample) | (< 10 to 3080) | |||
| Roth et al[67], 2017 | United States | RT-PCR (plasma pool of 96 samples) with 95% LOD 18.6 IU/mL | 128021 | 4 | 0.003% | 3a: 3/3 | (3.0 to 3.8 log IU/mL) | N/A |
| Stramer et al[68], 2016 | United States | TMA assay on individual plasma with 95% LOD 7.9 IU/mL | 18829 | 2 | 0.011% (0.0018%-0.351%) | N/A | 14 IU/mL in one sample | N/A |
| Xu et al[69], 2013 | United States | RT-PCR (plasma pool of 7 to 8 samples) with 95% LOD 400 IU/mL and nested PCR with 95% LOD 200 IU/mL | 1939 | 0 | N/A | N/A | N/A | N/A |
| Baylis et al[53], 2012 | United States | RT-PCR (plasma pool of 96 samples) with 95% LOD 250 IU/mL | 51075 | 0 | N/A | N/A | N/A | N/A |
| Asia | ||||||||
| Wen et al[70], 2018 | China | RT-PCR on individual plasma | 5345 | 15 | 0.281% | One 4h, another one clustered between genotype 2 and 4i | N/A | N/A |
| Tsoi et al[71], 2019 | Hong Kong | RT-PCR with 95% LOD 7.89 IU/mL | 10000 | 2 | 0.02% | 4 (in 1 sample) | N/A | N/A |
| Katiyar H et al[72], 2018 | India | RT-PCR (plasma pool of 3 samples) with LOD 100 IU/mL | 1799 | 0 | N/A | N/A | N/A | N/A |
| Minagi T et al[73], 2016 | Japan | RT-PCR (plasma pool of 50 or 500 samples) with 95% LOD 152 IU/mL | 620140 | 36 | 0.0058% | 3: 36/36 | (< 1.69 to 7.22 log10 copies/mL) | N/A |
| Intharasongkroh et al[74], 2019 | Thailand | RT-PCR (plasma pool of 6 samples) with 95% LOD 53.5 IU/mL | 30115 | 26 | 0.086% | 3: 6/6 | N/A | N/A |
| Others | ||||||||
| Hoad et al[75], 2017 | Australia | TMA (plasma pool of 6 samples) | 74131 | 1 | 0.0013% | N/A | 180 | N/A |
| Shrestha et al[76], 2016 | Australia | TMA assay on individual plasma with 95% LOD 7.9 IU/mL | 14799 | 1 | 0.0068% (0.0002%-0.0376% | 3 | 15000 | N/A |
| Hewitt et al[77], 2018 | New Zealand | RT-PCR (plasma pool of 8 to 12 samples) | 5000 | 0 | N/A | N/A | N/A | N/A |
| Maponga et al[78], 2020 | South Africa | TMA assay on individual plasma with 95% LOD 7.9IU/mL | 10000 | 1 | 0.01% | 3 | 79000 | All donations from donors with active HEV infection were discarded |
CI: Confidence interval; FFP: Fresh frozen plasma; HEV: Hepatitis E virus; Ig: Immunoglobulin; LOD: Limit of detection; RBC: Red blood cells; RNA: Ribonucleic acid; RT-PCR: Real time polymerase chain reaction; TMA: Transcription mediated amplification.
The prevalence of HEV-3 and -4 is affected by dietary habits[80]. Consumption of raw pork tartare and undercooked pork liver may represent a relevant risk factor for HEV infection in Germany[49]. Regular consumption of pork meat and shellfish were also reported in the viremic donors in China[70].
Since 70% of infections with HEV-3 and -4 are asymptomatic[81], it can be difficult to identify infected blood donors, as viremia occurs primarily during the pre-icteric phase[82]. Katiyar et al[72] described anti-HEV IgG positivity in 60.5% of the tested donors in India and yet none of them were positive for HEV RNA. In India, human HEV is caused exclusively by the HEV-1 genotype, which causes brief hepatitis and seldom results in chronic infection[83,84]. The difference in endemicity between HEV genotypes may affect the propensity to cause symptomatic disease and viral persistence, which in turn influences the likelihood of viremia among blood donors.
Other factors influencing the reported prevalence of HEV viremia are the sensitivity and plasma pool size of the various nucleic acid test screening platforms used[85]. For example, 33 of 90 donations with a viral load of 20-750 IU/mL were positive when tested individually but missed in the pooled screening in a study by Hogema et al[57]. Delage et al[66] revealed a low prevalence (n = 11/50765) and viral loads of HEV-RNA in Canadian blood donors based on individual nucleic acid amplification techniques (NAT). They postulated that if pooled NAT was used, only two positive donations with viral loads > 1000 IU/mL would have been detected. The true frequency of viremia in blood donors in studies using pooled NAT could be underestimated due to a dilution effect. Vollmer et al[86] found that screening using individual NAT yielded an approximately 50% higher detection frequency compared with NAT of a mini-pool of 96 samples; nevertheless, samples exclusively positive for individual NAT had a corresponding viral load of < 25 IU/mL. High-sensitivity individual NAT can yield false-positive results[55]. Whether the identification of low-level HEV-positive donors translates into clinical significance and whether a single individual NAT is adequate remain undefined.
Antibodies
In addition to direct detection of HEV RNA, another important indirect assessment of HEV burden is the prevalence of anti-HEV IgM and IgG in blood donors (Table 3)[45,46,54-56,58,59,61,63,65,68,69,71,72,77,87-124]. HEV IgG prevalence increases with age which likely represents the cumulative effect of HEV exposure over a lifetime, especially as IgG antibodies can persist for decades[81]. The absence of detectable antibodies in donors was related to an increased risk of transfusion transmission of HEV[64]. However, the presence of anti-HEV IgG may not always be protective as multiple HEV reinfections could occur despite pre-existing antibodies[125]. Various HEV strains in serum are capable of replication in cell culture and generate infectious particles in the culture supernatant despite the coexistence of antibodies[126]. Anti-HEV IgM could be used to detect recent infection yet it failed to identify infected donors during the window period. For example, a meta-analysis of data from 28 countries found that only 26.6% of viremic blood units had positive anti-HEV antibodies[127]. In another study by Tedder et[128] al, a significant portion of viremic individuals (n = 57/79) were seronegative at the time of donation. Anti-HEV IgM sometimes exhibits unexpectedly long persistence for up to 3 years after a self-limiting acute hepatitis E episode[129]. Only a minority of anti-HEV IgM-positive donors have detectable RNA[58,93,103,109]. All these findings suggest that detection of anti-HEV IgG or IgM alone may not provide effective screening of HEV in blood donors.
Table 3.
Seroprevalence of hepatitis E in blood donors
|
Ref.
|
Country
|
Number of donations screened
|
Assay used
|
Number of samples positive for HEV IgG antibodies
|
Anti-HEV IgG prevalence (95%CI)
|
Number of samples positive for HEV IgM antibodies
|
Anti-HEV IgM prevalence (95%CI)
|
Number of samples positive for HEV RNA in anti-HEV IgM positive
|
Viral load, IU/mL
|
Genotype
|
| Europe | ||||||||||
| Fischer et al[45], 2015 | Austria | 1203 (from HEV RNA negative donors) | Wantai | 163 | 13.55% (11.6%-15.5%) | N/A | N/A | 0 | N/A | N/A |
| Vercouter et al[46], 2019 | Belgium | 356 (from HEV RNA negative donors) | Wantai | 31 | 8.71% (6.20%-12.10%) | 0 | N/A | 0 | N/A | N/A |
| Miletić et al[87], 2019 | Croatia | 1036 | 3 commercial ELISA assays were used, only findings with highest prevalence are shown | 209 | 20.17% | 46 | 4.44% | 0 | N/A | N/A |
| Holm et al[88], 2015 | Denmark | 504 | In-house NIH assay | 54 | 10.7% (8.2%-13.7%) | N/A | N/A | N/A | N/A | N/A |
| Wantai | 100 | 19.8% (16.4%-23.6%) | ||||||||
| Dimeglio et al[89], 2018 | France | 300 | Wantai | 23 | 7.7% (4.9%-11.3%) | 2 | 0.6% (0.1%-2.4%) | 0 | N/A | N/A |
| Juhl et al[90], 2013 | Germany | 1019 | RecomWell assay and Western blot | 69 | 6.8% (5.3%-8.3%) | N/A | N/A | N/A | N/A | N/A |
| Dalekos et al[91], 1998 | Greece | 3016 | Abbott assay and Western blot | 8 | 0.27% | 0 | N/A | N/A | N/A | N/A |
| O'Riordan et al[54], 2016 | Ireland | 1076 | Wantai | 57 | 5.3% (4.0%-6.8%) | 2 | 0.19% | 0 | N/A | N/A |
| Spreafico et al[55], 2020 | Italy | 767 | DiaPro | 52 | 6.8% (5.1%-8.8%) | 0 | N/A | 0 | N/A | N/A |
| Spada et al[56], 2018 | Italy | 10011 | Wantai | 869 | 8.7% (8.14%-9.25%) | 46 | 0.4% (0.34% - 0.61%) | 0 | N/A | N/A |
| De Sabato et al[92], 2017 | Italy | 170 | Bio-Chain Institute and Western blot | 15 | 8.82% | 3 | 1.76% | 0 | N/A | N/A |
| Lucarelli et al[93], 2016 | Italy | 313 | Wantai | 153 | 48.9% (43%-54%) | 2 | 0.6% (0.08%-2.3%) | 1 | 100 | 3 |
| Puttini et al[94], 2015 | Italy | 132 | EIAgen HEV IgG kit | 12 | 9.1% | N/A | N/A | N/A | N/A | N/A |
| Hogema et al[95], 2014 | Netherlands | 513 | Wantai | 58 | 11.31% | N/A | N/A | N/A | N/A | N/A |
| Slot et al[58], 2013 | Netherlands | 5239 | Wantai | 1401 | 26.7% (25.6%-28.0%) | 49 | 0.94% | 4 | Range: < 25 to 3700 | 3 |
| Grabarczyk et al[59], 2018 | Poland | 3079 | Wantai | 1340 | 43.52% (41.78%-45.28%) | 39 | 1.27% (0.93%-1.73%) | N/A | N/A | N/A |
| Sauleda et al[61], 2015 | Spain | 1082 | Wantai | 216 | 19.96% (17.60%-22.32%) | 13 | 1.20% | 0 | N/A | N/A |
| Mikrogen | 116 | 10.72% (8.90%-12.60%) | ||||||||
| Mateos et al[96], 1999 | Spain | 863 | Abbott assay and Western blot | 34 | 3.9% | 0 | N/A | N/A | N/A | N/A |
| Niederhauser et al[97], 2018 | Switzerland | 3609 | Wantai | 737 | 20.4% (19.1%-21.8%) | N/A | N/A | N/A | N/A | N/A |
| Kaufmann et al[98], 2011 | Switzerland | 550 | MP Biomedicals | 27 | 4.9% | N/A | N/A | N/A | N/A | N/A |
| Thom et al[63], 2018 | United Kingdom | 1714 | Wantai | 104 | 6.1% (5.0%-7.3%) | N/A | N/A | N/A | N/A | N/A |
| Cleland et al[65], 2013 | United Kingdom | 1559 | Wantai | 73 | 4.7% (3.6%-5.8%) | 0 | N/A | N/A | N/A | N/A |
| Beale et al[99], 2011 | United Kingdom | 262 | Wantai | 31 | 11.8% | 4 | 1.5% | 0 | N/A | N/A |
| North America | ||||||||||
| Zafrullah et al[100], 2018 | United States | 5040 (from HEV RNA negative donor) | DSI | 569 | 11.29% | 146 | 2.90% | 0 | N/A | N/A |
| MP Biomedicals | 537 | 10.65% | 93 | 1.85% | ||||||
| Wantai | 619 | 12.28% | 34 | 0.67% | ||||||
| Stramer et al[68], 2016 | United States | 4499 | MP Biomedicals | 329 | 7.3% (6.6%-8.1%) | 26 | 0.58% (0.39%-0.85%) | N/A | N/A | N/A |
| Xu et al[69], 2013 | United States | 1939 | Wantai | 364 | 18.8% (17.0%-20.5%) | 8 | 0.4% (0.1%-0.7%) | 0 | N/A | N/A |
| South America | ||||||||||
| Di Lello et al[101], 2020 | Argentina | 391 | DiaPro | 44 | 11.3% | 8 | 2.0% | 0 | N/A | N/A |
| Bangueses et al[102], 2020 | Uruguay | 400 | DiaPro | 40 | 10% | 19 | 4.75% | 3 | N/A | 3 |
| Asia | ||||||||||
| Nouhin et al[103], 2016 | Cambodia | 301 | Wantai | 85 | 28.2% (23.4%-33.5%) | 3 | 1.0% (0.01%-1.8%) | 1 | 956 | 3 |
| Chen et al[104], 2019 | China | 4044 | Wantai | 799 | 19.8% (18.6%-21.0%) | 43 | 1.1% (0.8%-1.4%) | 2 | N/A | 4 |
| Wen et al[70], 2018 | China | 5345 | Wantai | 1227 | 22.96% | 38 | 0.71% | 15 | N/A | N/A |
| Wang et al[105], 2017 | China | 9069 | Wantai | 2682 | 29.57% | 131 | 1.44% | 5 | N/A | N/A |
| Ma et al[106], 2015 | China | 816 | Wantai | 172 | 21.1% | 4 | 0.5% | 0 | N/A | N/A |
| Ren et al[107], 2014 | China | 10741 | Wantai | 2945 | 27.42% | 109 | 1.01% | 0 | N/A | N/A |
| Zhuang et al[108], 2014 | China | 486 | ELISA based on antigen protein pB166 and MPII | 113 | 23.3% | N/A | N/A | N/A | N/A | N/A |
| Tsoi et al[71], 2019 | Hong Kong | 2000 | Wantai | 315 | 15.8% (14.2%-17.4%) | 16 | 0.8% | 0 | N/A | N/A |
| Tripathy et al[109], 2019 | India | 2447 | Wantai | 433 | 17.70% (16.23%-19.26%) | 5 | 0.20% | 2 | Ranged from 3.5 × 104 to 4.6 × 105 copies/mL | 1 |
| Katiyar et al[72], 2018 | India | 633 | Wantai | 383 | 60.5% | N/A | N/A | 0 | N/A | N/A |
| Gajjar et al[110], 2014 | India | 460 | DiaPro | N/A | N/A | 22 | 4.78% | N/A | N/A | N/A |
| Parsa et al[111], 2016 | Iran | 700 | DiaPro | 42 | 6.0% | 5 | 0.71% | 5 (only 50 seropositive blood donors were tested) | N/A | 1 |
| Hesamizadeh et al[112], 2016 | Iran | 559 | DiaPro | 45 | 8.05% | N/A | N/A | N/A | N/A | N/A |
| Naeimi et al[113], 2015 | Iran | 628 | HEV IgG, Pasto, Iran | 105 | 16.72% | N/A | N/A | N/A | N/A | N/A |
| Ehteram et al[114], 2013 | Iran | 530 | DiaPro | 76 | 14.3% | N/A | N/A | N/A | N/A | N/A |
| Taremi et al[115], 2007 | Iran | 399 | DiaPro | 31 | 7.8% | N/A | N/A | N/A | N/A | N/A |
| Takeda et al[116], 2010 | Japan | 12600 | in-house ELISA | 431 | 3.42% | N/A | N/A | N/A | N/A | N/A |
| Shrestha et al[117], 2016 | Nepal | 1845 | Wantai | 773 | 41.9% (39.7%-44.2%) | 55 | 3.0% (2.2%-3.8%) | N/A | N/A | N/A |
| Nasrallah et al[118], 2017 | Qatar | 5854 | Wantai | 1198 | 20.46% | 34 | 0.58% | 4 | N/A | N/A |
| Jupattanasin et al[119], 2019 | Thailand | 630 | EUROIMMUN test kit | 187 | 29.7% (26.2%-33.4%) | N/A | N/A | N/A | N/A | N/A |
| Africa | ||||||||||
| Traoré et al[120], 2016 | Burkina Faso | 1497 | DiaPro and Wantai | 584 | 39% | 13 | 0.87% | N/A | N/A | N/A |
| Ibrahim et al[121], 2011 | Egypt | 760 | N/A | N/A | N/A | 3 | 0.39% | 2 | N/A | N/A |
| Meldal et al[122], 2013 | Ghana | 239 | 4 commercial assays were used, findings reactive in; at least two serological assays are shown | 11 | 4.6% | 14 | 5.9% | 0 | N/A | N/A |
| Lopes et al[123], 2017 | South Africa | 300 | Fortress Diagnostics | 76 | 25.3% | 0 | N/A | N/A | N/A | N/A |
| Ben-Ayed et al[124], 2015 | Tunisia | 426 | Globe; Diagnostics Srl ELISA | 19 | 4.46% | N/A | N/A | N/A | N/A | N/A |
| Others | ||||||||||
| Hewitt et al[77], 2018 | New Zealand | 1013 | Wantai | 98 | 9.7% (7.9%-11.7%) | N/A | N/A | N/A | N/A | N/A |
| MP Biomedicals | 82 | 8.1% (6.5%-10.0%) | ||||||||
ALT: Alanine aminotransferase; CI: Confidence interval; DSI: Diagnostic Systems Incorporated; ELISA: Enzyme-linked immunosorbent assay; HEV: Hepatitis E virus; NIH: National Institutes of Health.
Geographical variation, racial differences, and diverse study methodology and laboratory techniques all contribute to differences in HEV seroprevalence. More than one-third of donors had evidence of past HEV infection in Poland, India, Nepal and Burkina Faso[59,72,117,120]. Lucarelli et al[93] reported an unexpectedly high prevalence (48.9%) of anti-HEV IgG among 313 donors in central Italy. Eating raw dried pig liver sausage was the only independent risk factor for HEV IgG in their study, but the authors speculated that the uncontrolled expansion of the wild boar population had resulted in contamination of the soil and watercourses for people living in rural areas, and this may also have also contributed to the high prevalence of HEV[93].
Caution is needed when interpreting the HEV serology results because commercial kits for serological detection show marked variation in sensitivity and specificity. Despite the relatively high sensitivity of the IgM assay, the sensitivity of IgG detection kits is highly dependent on a patient’s immune status, being 80% to 90% in immunocompetent individuals, but falling dramatically to 15% to 45% in immunocompromised patients[130]. In a meta-analysis conducted in Europe, the pooled anti-HEV IgG seroprevalence rates determined by different commercial assays showed large variability with reported seroprevalence rates ranging from 2% to 17%[131]. Poor concordance of test results between the Wantai, Dia.Pro and MP Diagnostics HEV enzyme-linked immunosorbent assays (ELISA) were observed[132,133]. This may partly explain the broad ranges of anti-HEV IgG prevalence (5.3% to 48.9%) reported in Italy[55,56,92-94]. In contrast, most studies conducted in China used the Wantai assay and revealed a similar seroprevalence of around 20% to 30%. This assay is believed to be more sensitive than other commercial assays in detecting anti-HEV IgG[134,135].
TRANSFUSION-TRANSMITTED HEPATITIS E
HEV transmission via transfusion has been reported since 2004[136] and there has been increasing recognition of the risk of transmitting HEV by transfusion in recent years. Cases of TT-HEV are shown in Table 4[137-150]. Identical genomic sequences were identified in most infected patients and blood donors. Table 4 Likely only represents the tip of the iceberg as other probable or possible cases have been reported in the literature[151,152]. At the same time, patients with mild symptoms of hepatitis E may have gone undiagnosed. Physicians should stay vigilant for HEV infection in patients who have received a blood transfusion.
Table 4.
Reported cases of transfusion transmitted hepatitis E
|
Study
|
Number of patients
|
Comorbidity
|
Blood component received (n)
|
Viral load of transfused blood product IU/mL
|
Genotype
|
Treatment
|
Outcome
|
| Okano et al[137], 2020 | 1 | AML on chemotherapy | Plt | N/A | 3b | Nil | Spontaneous resolution and developed HEV antibodies after cessation of chemotherapy for AML |
| Gallian et al[138], 2019 | 23 | Solid organ transplant, n = 9; allogeneic hematopoietic stem cells transplant, n = 4; hematologic malignancies, n = 5; immunosuppressant, n = 2; immunocompetent, n = 3 | RBC n = 7; apheresis Plt n = 3; whole blood-derived pooled Plt n = 6; FFP n = 7 | Ranged from 1.14 × 103 to 31 × 62.106 | 3a, n = 1; 3c, n = 4; 3f, n = 16; 3, n = 1; 4d, n = 1 | Ribavirin, n = 15 | Acute HEV infection, n = 8; spontaneous resolution, n = 4; ribavirin treatment, n = 3; immunosuppressant reduction, n = 1; chronic HEV infection, n = 14, all immunosuppressed; resolution with ribavirin, n = 10; resolution with immunosuppressant reduction, n = 4; One solid organ transplant recipient did not clear HEV infection despite ribavirin and died of multiorgan failure |
| Ledesma et al[139], 2019 | 2 | Allogeneic BMT, n = 1; liver transplant, n = 1 | Plt | 3 × 104 | 3e | Ribavirin, n = 1 | The patient received BMT remained HEV-infected and IgM/IgG-negative until death; the patient with liver transplant was treated successfully with a course of ribavirin |
| Satake et al[140]a, 2017 | 19 | Hematologic malignancies, n = 6; organ transplant, n = 2; systemic disease, n = 8; no major comorbidity, n = 3 | RBC n = 10; Plt n = 6; FFP n = 3 | Ranged from 1.5 × 102 to 5.3 × 106 | 4, n = 2 | N/A | Two patients with malignant lymphoma and two who had received liver transplant developed chronic hepatitis E; the two liver transplant recipients were successfully cleared of HEV by ribavirin |
| Lhomme et al[141], 2017 | 3 | Solid organ transplant | One patient received RBC; one patient received RBC and Plt; one patient received Plt and FFP | Ranged from 3.6 to 8.2 log IU | 3, n = 1; 3f, n = 2 | N/A | N/A |
| Yamazaki et al[142], 2017 | 2 | Hematologic malignancies treated with chemotherapy | N/A | N/A | 3b | N/A | Did not become chronic hepatitis E |
| Belliere et al[143], 2017 | 1 | Heart transplant | RBC | 1430 copies/mL | 3 | Ribavirin | Died from multi-organ failure despite treatment |
| Riveiro-Barciela et al[144], 2017 | 1 | Immunocompetent, admitted for disseminated infection | RBC | 75000 | 3 | Nil | Spontaneous resolution |
| Hoad et al[145], 2017 | 1 | Liver transplant | FFP | 947 | 3 | Ribavirin | Resolved with treatment |
| Matsui et al[146], 2015 | 1 | AMI post CABG with hemorrhagic cardiac tamponade | Plt | 106.8 copies | 3 | Nil | Spontaneous resolution |
| Huzly et al[147], 2013 | 1 | Immunocompromised | Apheresis Plt | 30888-37273 | 3f | N/A | N/A |
| Coilly et al[148], 2013 | 1 | Liver transplant | RBC | 3.5 log10 | 3c | Ribavirin | Resolved with treatment |
| Boxall et al[149], 2006 | 1 | Lymphoma on chemotherapy | RBC | N/A | 3 | Nil | Spontaneous resolution |
| Mitsui et al[150], 2004 | 1 | Hemodialysis | RBC | N/A | 3 | Nil | Subclinical infection without elevated ALT |
Two cases were not confirmed by sequence identity and should only be considered as probable TT-HEV.
ALT: Alanine aminotransferase; AMI: Acute myocardial infarction; AML: Acute myeloid leukemia; BMT: Bone marrow transplant; CABG: Coronary artery bypass graft; FFP: Fresh-frozen plasma; HEV: Hepatitis E virus; Ig: Immunoglobulin; Plt: Platelet concentrates; RBC: Red blood cell.
Although blood components that contain larger plasma volumes, principally fresh frozen plasma and platelet components, are believed to transmit HEV more readily[64], a number of TT-HEV cases associated with red blood cell transfusion have also been described[138,140,141,143,144,148-150]. Red blood cell transfusion was a significant risk factor for HEV seropositivity in patients on hemodialysis in Croatia[153]. Twenty percent (n = 8/40) of multiply transfused thalassemia patients were anti-HEV IgG positive compared with 11.0% (n = 10/91) in blood donors[154]. In contrast, a study in Iran found anti-HEV antibodies in only 1.67% of patients with thalassemia, suggesting a low rate of TT-HEV in that country[155]. Results from these two studies in thalassemia patients were limited by the small sample size. Ankcorn et al[156] analyzed 1591 patients with hematologic malignancy and found that the more transfusions of non-HEV screened blood products the patients had received, the higher their likelihood of being IgG seroreactive was, suggesting HEV acquisition via transfusion in these patients.
A study by Hewitt et al[64] indicated that a viral concentration of between 407 and 257039 IU/mL in blood products was associated with TT-HEV, and that a high viral load in donors rendered infection more likely (P < 0.0001). However, this may not be true in immunocompromised patients. In a systematic review, Dreier et al[50] calculated the median transfused viral load in HEV-infected and non-infected immunocompromised patients. Although the transfused viral load was higher in the infected than the non-infected individuals (4.80 × 105 IU vs 1.55 × 104 IU), the between-group difference was not statistically significant (P = 0.1006)[50]. A potential reason for this finding is that a low viral concentration (150 IU/mL) of the blood component could already be infectious[140].
Most cases of TT-HEV occur in immunocompromised recipients, such as patients with hematologic malignancies, or recipients of solid organ or hematopoietic stem cell transplants. However, patients on simple immunosuppressants like corticosteroids and cyclosporine or even immunocompetent individuals are also at risk[157]. Massive transfusion increased the risk of HEV transmission in an immunocompetent trauma patient[158]. Spontaneous resolution, viral eradication by immunosuppressant reduction and/or ribavirin are possible[159] but occasionally there are cases which have progressed into chronic hepatitis, liver cirrhosis or multi-organ failure. Transfusion recipients are more vulnerable to chronic liver injury than the general population as a result of foodborne infection[140]. More than 60% (n = 56/85) of solid organ transplant recipients infected with HEV developed chronic hepatitis, with tacrolimus use as an independent predictive factor[160]. Pas et al[161] screened 1200 solid-organ transplant recipients in the Netherlands for HEV RNA and identified 12 patients with HEV infection. Nine of these 12 patients had been treated with a tacrolimus-based regimen postoperatively. In liver transplant recipients, graft hepatitis with rapid histological disease progression and requirement of re-transplantation due to liver cirrhosis has been reported[162,163]. The rapid progression of HEV infection to advanced fibrosis and cirrhosis has also been observed in individuals receiving kidney or heart transplants[33]. In 50 patients with hematologic malignancy and clinically overt hepatitis E, the mortality rate was 16% (n = 8), with liver-related death occurring in 4 patients[164]. HEV could actively suppress the cellular immune response and increase levels of immunosuppressive interleukin-10 that may perpetuate chronic infection and subsequent liver damage[165,166].
TREATMENT
The management strategy for HEV infection should be determined by the clinical presentation. Currently, there is limited information in the published literature that describes the clinical features of TT-HEV, or the optimal approach to management. Acute TT-HEV infections are usually subclinical or mild, with no severe or fulminant cases reported[140]. Therefore, most acute HEV infections should be treated conservatively, while waiting for spontaneous clearance, although a short course of ribavirin may also be considered. In 21 patients with acute HEV infection who were at high risk of liver failure, receiving immunosuppressive therapy for an autoimmune disease or undergoing chemotherapy, a short course of ribavirin for up to 3 mo was associated with rapid virological response and normalization of liver enzymes[167].
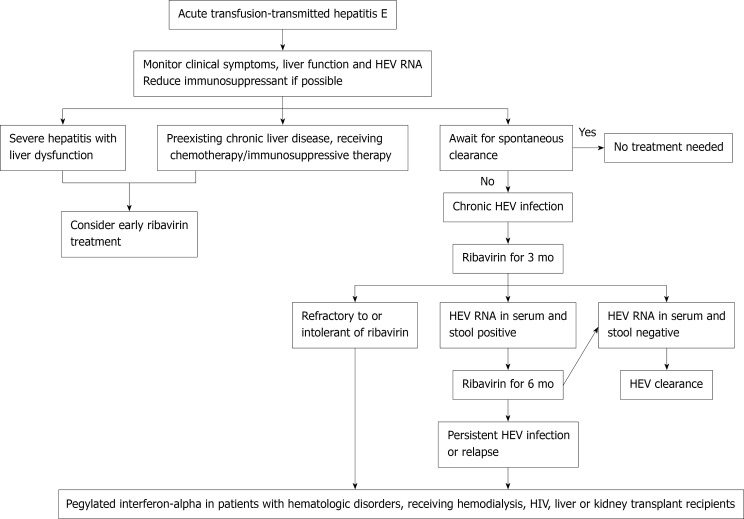
The current practice for management of chronic HEV infection is mainly based on observational data[18]; Figure 1 shows a proposed algorithm for management. In patients who are on immunosuppressants, the first-line intervention should be a dose reduction or discontinuation of the immunosuppressive drug[168,169]. In solid organ transplant recipients, reducing the dose of immunosuppressive therapies that principally target T-cells can achieve HEV clearance in nearly one third of patients[160]. Most immunosuppressive drugs such as cyclosporine and tacrolimus increase HEV replication in vitro; mycophenolate mofetil is the only immunosuppressant agent demonstrated to have an anti-viral effect[170].
Figure 1.
Recommended algorithm for management of transfusion-transmitted hepatitis E.
If modification of the immunosuppressant regimen is not possible or is unsuccessful, pharmacological agents such as ribavirin and/or pegylated interferon-alpha (peg-IFN) can be used[171]. In a meta-analysis that included 395 patients with chronic hepatitis E, ribavirin monotherapy for a median of 3 mo achieved sustained virological response (SVR) in 76% of patients[172]. The reported dose of ribavirin in the literature ranged from 29 to 1200 mg/d, and the duration from 1 to 18 mo. Data on the optimal treatment regimen are needed[173]. HEV RNA should be assessed in the serum and in the stool before treatment discontinuation[169]. A second course of ribavirin for 6 mo can be attempted in cases of treatment failure[172]. HEV RNA concentrations decrease within the first week of initiating ribavirin therapy, and a greater reduction in viral load on day 7 is an independent predictor of SVR[174]. Ribavirin failure has been linked to the presence of certain single nucleotide variants (SNVs) and in-frame insertions in the hypervariable region of open reading frame (ORF) 1 in the HEV genome[175].
For those who are refractory to, or intolerant of, ribavirin, peg-IFN can be considered. Its efficacy has been documented in patients with hematologic disorders, patients receiving hemodialysis, and in combination with ribavirin in patients with HIV[176-178]. Close monitoring is needed if it is used in transplant recipients because of an increased risk of acute humoral and cellular rejection[179,180]. Peg-IFN was thought to be safe only in liver transplant recipients until recent case reports described its successful use in a kidney transplant recipient[181-183].
Sofosbuvir is a nucleotide analog shown to decrease replication of HEV-3 in vitro[184]. However, in clinical studies, only modest antiviral activity was observed and SVR was not achieved[185-187]. Rescue treatment for patients who are not eligible for, or not responding to, ribavirin and/or peg-IFN remains an unmet need.
HOW TO REDUCE TRANSFUSION-TRANSMITTED HEPATITIS E
The background risk of foodborne HEV transmission to both donors and recipients of blood products is not negligible. The transfusion-related risk of infection only exceeds the annual dietary risk when more than 13 individual donor components are transfused[188]. Strategies to reduce de novo infection, such as modifying eating habits and eliminating HEV from pigs and other animals that are used for food production are essential[189]. The one available vaccine (HEV 239, Hecolin, Xiamen, China) is licensed only in China, and has yet to play a fundamental role in global outbreaks or pandemic control[190]. Nonetheless, the transmissibility and disease phenotype may not be the same for a person who acquires the virus orally and a person who gets infected intravenously, as there may be some protection provided by the acidic environment of the stomach and the mucosal barrier in the gut[191]. The infectivity of the non-enveloped form is different to that of enveloped HEV[9]. Data reporting outcomes of recipients of HEV-infected blood products are sparse[47,49,50,60,64].
Policies on screening HEV in blood products differ between countries. Universal screening was adopted in the United Kingdom, Ireland, and the Netherlands. Germany and France implemented targeted screening of donated plasma intended for use in high-risk patients[192]. In Japan, the use of nucleic acid-based screening is limited to Hokkaido[193]. Blood donors are not routinely tested for HEV infection in China including Hong Kong[70,71,194]. There has been much debate on mandatory HEV screening in blood donations[195]. Key questions, such as whether or not to screen, which laboratory assay to use, which donors to screen (universal or selective screening), and which types of blood components to screen should be assessed based on risk assessment, resource availability, health economics, and political or other influences. The answers may vary considerably by geographical location[169,196]. In areas where HEV is highly endemic, most donors and/or recipients have probably been exposed to HEV previously and would have positive IgG antibodies. Therefore, the decision on serological screening should also take into consideration the prevalence of HEV infection in that particular region.
All donors should answer a questionnaire about symptoms of clinical hepatitis and potential exposure to HEV prior to blood donation. Donation should be deferred in any donors with a history of clinical hepatitis[197]. Neither alanine aminotransferase (ALT) nor anti-HEV IgM testing correlate with the presence of HEV RNA, supporting the use of NAT for screening of blood donations[60,61,105]. A simulation study by Kamp et al[198] reported that testing for HEV RNA by NAT with a pool size of 96, and a 95% limit of detection of 20 IU/mL will result in an 80% reduction in expected HEV transmissions as well as of consequent chronic infections with severe complications. The risk of transmission could be reduced by 90% in NAT using a mini-pool of 24 samples[198].
If opting for selective screening instead of universal screening, a clear definition of at-risk patients is warranted[199]. Targeted screening should be contemplated for blood components that will be supplied to transplant recipients, or patients with hematologic malignancies or chronic liver disease, as these individuals are at high risk of developing fulminant hepatitis, acute on chronic liver failure, or chronic hepatitis. However, it is not yet clear whether patients with rheumatologic diseases, those on low-intensity immunosuppression, or elderly individuals should only receive HEV-negative blood products. A multicenter retrospective study in Europe including 21 rheumatology and internal medicine patients found that patients with rheumatoid arthritis who were receiving methotrexate or biologics were at risk of chronic hepatitis E infection[200]. However, another study in France did not find worse hepatitis E severity or increased risk of chronicity in 23 patients with inflammatory arthritis treated with immunosuppressants[201].
Patients co-infected with HIV with CD4+ count < 200/mm3 are at risk for persistent HEV infection[29]. In HIV patients with low CD4+ count, anti-HEV IgG seroconversion was delayed until immune reconstitution occurred[202]. A recent meta-analysis found that the HEV RNA positivity rate was significantly higher in transplant recipients than in HIV-positive patients [1.2% (95%CI: 0.9-1.6) vs 0.39% (95%CI: 0.2-0.7); P = 0.0011], possibly due to better immune status in the HIV-positive individuals using anti-retroviral therapy[203].
HEV-1 and -2 infections can take a fulminant course in pregnancy, resulting in liver failure, membrane rupture, spontaneous abortions, and stillbirths[204]. HEV-3 infection in pregnancy appears to be less virulent without significant maternal, fetal, or neonatal complications[205-207]. During pregnancy, a reduced cellular immunity and a high level of steroid hormones, in particular estrogen, progesterone, and human chorionic gonadotropin, influence viral replication/expression and possibly explain the disease severity[208]. The immune response could be influenced by HEV genotype, translating into different outcomes[209]. Ribavirin and peg-IFN are contraindicated in pregnancy due to concerns of teratogenicity[210]. Further studies are needed to clarify the risk of transmission of HEV to pregnant women via blood transfusion; however, in view of the potentially serious disease course and absence of a safe treatment, pregnant women are a priority group for HEV-negative blood products.
Roth et al[67] evaluated the safety of plasma-derived medicinal products (PDMP) and found a very low prevalence of HEV RNA (0.002%) in plasma donors. Since viral reduction methods are used in the manufacturing processes of PDMP, these data do not support routine screening of all plasma pools intended for producing PDMP. Currently there is a lack of evidence to suggest that human serum albumin or coagulation factor concentrates are a major source of HEV infection[211,212].
The cost effectiveness of HEV screening of blood donations was analyzed in the Netherlands. Screening of whole blood donations in pools of 24 would prevent 4.52 of the 4.94 TT-HEV infections annually at a cost of approximately €310000 (Euro) per prevented chronic case. The estimated cost per incurable case prevented was 10-fold higher. Costs could potentially be reduced by 85% if only the blood products intended for use by immunocompromised patients were screened. Additional costs for selective screening may arise for logistic reasons and a possible increase in the number of blood products that expire before use. They concluded that preventing HEV transmission by screening of blood donations appears not excessively expensive compared with other blood-screening measures but the impact on disease burden may be small as only a minority of all HEV cases are transmitted by blood transfusion[213]. Another economic analysis performed in North America found a very low estimated risk of TT-HEV infection risk leading to severe liver disease. When compared with no screening, the costs were $2.68 (USD) per component for a selective screening approach, and $6.68 per component for universal screening. The respective costs per quality-adjusted life-year gained were $225546 and $561810, respectively, which exceeded the threshold for what is considered as “cost-effective”[66].
In addition to screening, various pathogen reduction methods have been proposed to reduce risk of TT-HEV. Solvent/detergent treatment could not eliminate non-enveloped HEV in plasma[214]. Non-enveloped HEV is also resistant to the Intercept method, which combines a synthetic psoralen amotosalen HCl treatment with ultraviolet A light illumination to block the replication of DNA and RNA[215]. However, substantial viral reduction has been demonstrated during the manufacturing process of plasma products using immunoaffinity chromatography, nanofiltration, cold ethanol fractionation and heat treatment[216]. Anti-HEV antibodies enhanced HEV removal by nanofiltration[217]. Furthermore, ultraviolet C light provided effective inactivation of HEV in platelet concentrates[218].
CONCLUSION
To conclude, TT-HEV is gaining attention worldwide. Although the overall prevalence of viremic blood donations is low, HEV can cause sinister consequences in immunocompromised recipients. Future studies are needed to define the incidence of transmission through transfusion, clinical features, outcomes, and prognosis. The decision on a screening policy in asymptomatic blood donors should be based on local risk assessment and health economics.
Footnotes
Conflict-of-interest statement: The authors declare no conflict of interest.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: April 10, 2021
First decision: June 24, 2021
Article in press: December 22, 2021
Specialty type: Gastroenterology and Hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Anand AC, Kulkarni AV S-Editor: Chang KL L-Editor: Filipodia P-Editor: Chang KL
Contributor Information
Carmen Ka Man Cheung, Medicine and Therapeutics, Prince of Wales Hospital, Hong Kong 852, China.
Sunny Hei Wong, Institute of Digestive Disease and Department of Medicine and Therapeutics, the Chinese University of Hong Kong, Hong Kong 852, China; Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore 639798, Singapore.
Alvin Wing Hin Law, West Island School, Hong Kong 852, China.
Man Fai Law, Medicine and Therapeutics, Prince of Wales Hospital, Hong Kong 852, China. mflaw99@yahoo.com.hk.
References
- 1.Khuroo MS. Chronic liver disease after non-A, non-B hepatitis. Lancet. 1980;2:860–861. [PubMed] [Google Scholar]
- 2.World Health Organization. Hepatitis E Fact sheet. [Google Scholar]
- 3.Li P, Liu J, Li Y, Su J, Ma Z, Bramer WM, Cao W, de Man RA, Peppelenbosch MP, Pan Q. The global epidemiology of hepatitis E virus infection: A systematic review and meta-analysis. Liver Int. 2020;40:1516–1528. doi: 10.1111/liv.14468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wallace SJ, Swann R, Donnelly M, Kemp L, Guaci J, Murray A, Spoor J, Lin N, Miller M, Dalton HR, Hussaini SH, Gunson R, Simpson K, Stanley A, Fraser A. Mortality and morbidity of locally acquired hepatitis E in the national Scottish cohort: a multicentre retrospective study. Aliment Pharmacol Ther. 2020;51:974–986. doi: 10.1111/apt.15704. [DOI] [PubMed] [Google Scholar]
- 5.Bergløv A, Hallager S, Weis N. Hepatitis E during pregnancy: Maternal and foetal case-fatality rates and adverse outcomes-A systematic review. J Viral Hepat. 2019;26:1240–1248. doi: 10.1111/jvh.13129. [DOI] [PubMed] [Google Scholar]
- 6.Purdy MA, Harrison TJ, Jameel S, Meng XJ, Okamoto H, Van der Poel WHM, Smith DB Ictv Report Consortium. ICTV Virus Taxonomy Profile: Hepeviridae. J Gen Virol. 2017;98:2645–2646. doi: 10.1099/jgv.0.000940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Primadharsini PP, Nagashima S, Okamoto H. Genetic Variability and Evolution of Hepatitis E Virus. Viruses. 2019;11 doi: 10.3390/v11050456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sridhar S, Yip CC, Wu S, Chew NF, Leung KH, Chan JF, Zhao PS, Chan WM, Poon RW, Tsoi HW, Cai JP, Chan HS, Leung AW, Tse CW, Zee JS, Tsang OT, Cheng VC, Lau SK, Woo PC, Tsang DN, Yuen KY. Transmission of Rat Hepatitis E Virus Infection to Humans in Hong Kong: A Clinical and Epidemiological Analysis. Hepatology. 2021;73:10–22. doi: 10.1002/hep.31138. [DOI] [PubMed] [Google Scholar]
- 9.Yin X, Ambardekar C, Lu Y, Feng Z. Distinct Entry Mechanisms for Nonenveloped and Quasi-Enveloped Hepatitis E Viruses. J Virol. 2016;90:4232–4242. doi: 10.1128/JVI.02804-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Himmelsbach K, Bender D, Hildt E. Life cycle and morphogenesis of the hepatitis E virus. Emerg Microbes Infect. 2018;7:196. doi: 10.1038/s41426-018-0198-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kenney SP, Meng XJ. Hepatitis E Virus Genome Structure and Replication Strategy. Cold Spring Harb Perspect Med. 2019;9 doi: 10.1101/cshperspect.a031724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kmush B, Wierzba T, Krain L, Nelson K, Labrique AB. Epidemiology of hepatitis E in low- and middle-income countries of Asia and Africa. Semin Liver Dis. 2013;33:15–29. doi: 10.1055/s-0033-1338111. [DOI] [PubMed] [Google Scholar]
- 13.Khuroo MS, Khuroo MS, Khuroo NS. Hepatitis E: Discovery, global impact, control and cure. World J Gastroenterol. 2016;22:7030–7045. doi: 10.3748/wjg.v22.i31.7030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rein DB, Stevens GA, Theaker J, Wittenborn JS, Wiersma ST. The global burden of hepatitis E virus genotypes 1 and 2 in 2005. Hepatology. 2012;55:988–997. doi: 10.1002/hep.25505. [DOI] [PubMed] [Google Scholar]
- 15.Hakze-van der Honing RW, van Coillie E, Antonis AF, van der Poel WH. First isolation of hepatitis E virus genotype 4 in Europe through swine surveillance in the Netherlands and Belgium. PLoS One. 2011;6:e22673. doi: 10.1371/journal.pone.0022673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garbuglia AR, Scognamiglio P, Petrosillo N, Mastroianni CM, Sordillo P, Gentile D, La Scala P, Girardi E, Capobianchi MR. Hepatitis E virus genotype 4 outbreak, Italy, 2011. Emerg Infect Dis. 2013;19:110–114. doi: 10.3201/eid1901.120983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donnelly MC, Scobie L, Crossan CL, Dalton H, Hayes PC, Simpson KJ. Review article: hepatitis E-a concise review of virology, epidemiology, clinical presentation and therapy. Aliment Pharmacol Ther. 2017;46:126–141. doi: 10.1111/apt.14109. [DOI] [PubMed] [Google Scholar]
- 18.Goel A, Aggarwal R. Hepatitis E: Epidemiology, Clinical Course, Prevention, and Treatment. Gastroenterol Clin North Am. 2020;49:315–330. doi: 10.1016/j.gtc.2020.01.011. [DOI] [PubMed] [Google Scholar]
- 19.Kamar N, Izopet J, Pavio N, Aggarwal R, Labrique A, Wedemeyer H, Dalton HR. Hepatitis E virus infection. Nat Rev Dis Primers. 2017;3:17086. doi: 10.1038/nrdp.2017.86. [DOI] [PubMed] [Google Scholar]
- 20.Dalton HR, Stableforth W, Thurairajah P, Hazeldine S, Remnarace R, Usama W, Farrington L, Hamad N, Sieberhagen C, Ellis V, Mitchell J, Hussaini SH, Banks M, Ijaz S, Bendall RP. Autochthonous hepatitis E in Southwest England: natural history, complications and seasonal variation, and hepatitis E virus IgG seroprevalence in blood donors, the elderly and patients with chronic liver disease. Eur J Gastroenterol Hepatol. 2008;20:784–790. doi: 10.1097/MEG.0b013e3282f5195a. [DOI] [PubMed] [Google Scholar]
- 21.Lhomme S, Marion O, Abravanel F, Izopet J, Kamar N. Clinical Manifestations, Pathogenesis and Treatment of Hepatitis E Virus Infections. J Clin Med. 2020;9 doi: 10.3390/jcm9020331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haffar S, Shalimar , Kaur RJ, Wang Z, Prokop LJ, Murad MH, Bazerbachi F. Acute liver failure caused by hepatitis E virus genotype 3 and 4: A systematic review and pooled analysis. Liver Int. 2018;38:1965–1973. doi: 10.1111/liv.13861. [DOI] [PubMed] [Google Scholar]
- 23.Smith DB, Simmonds P. Hepatitis E virus and fulminant hepatitis--a virus or host-specific pathology? Liver Int. 2015;35:1334–1340. doi: 10.1111/liv.12629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frias M, López-López P, Rivero A, Rivero-Juarez A. Role of Hepatitis E Virus Infection in Acute-on-Chronic Liver Failure. Biomed Res Int. 2018;2018:9098535. doi: 10.1155/2018/9098535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Radha Krishna Y, Saraswat VA, Das K, Himanshu G, Yachha SK, Aggarwal R, Choudhuri G. Clinical features and predictors of outcome in acute hepatitis A and hepatitis E virus hepatitis on cirrhosis. Liver Int. 2009;29:392–398. doi: 10.1111/j.1478-3231.2008.01887.x. [DOI] [PubMed] [Google Scholar]
- 26.Kamar N, Rostaing L, Legrand-Abravanel F, Izopet J. How should hepatitis E virus infection be defined in organ-transplant recipients? Am J Transplant. 2013;13:1935–1936. doi: 10.1111/ajt.12253. [DOI] [PubMed] [Google Scholar]
- 27.Pischke S, Stiefel P, Franz B, Bremer B, Suneetha PV, Heim A, Ganzenmueller T, Schlue J, Horn-Wichmann R, Raupach R, Darnedde M, Scheibner Y, Taubert R, Haverich A, Manns MP, Wedemeyer H, Bara CL. Chronic hepatitis e in heart transplant recipients. Am J Transplant. 2012;12:3128–3133. doi: 10.1111/j.1600-6143.2012.04200.x. [DOI] [PubMed] [Google Scholar]
- 28.Ollier L, Tieulie N, Sanderson F, Heudier P, Giordanengo V, Fuzibet JG, Nicand E. Chronic hepatitis after hepatitis E virus infection in a patient with non-Hodgkin lymphoma taking rituximab. Ann Intern Med. 2009;150:430–431. doi: 10.7326/0003-4819-150-6-200903170-00026. [DOI] [PubMed] [Google Scholar]
- 29.Dalton HR, Bendall RP, Keane FE, Tedder RS, Ijaz S. Persistent carriage of hepatitis E virus in patients with HIV infection. N Engl J Med. 2009;361:1025–1027. doi: 10.1056/NEJMc0903778. [DOI] [PubMed] [Google Scholar]
- 30.Sridhar S, Chan JFW, Yap DYH, Teng JLL, Huang C, Yip CCY, Hung IFN, Tang SCW, Lau SKP, Woo PCY, Yuen KY. Genotype 4 hepatitis E virus is a cause of chronic hepatitis in renal transplant recipients in Hong Kong. J Viral Hepat. 2018;25:209–213. doi: 10.1111/jvh.12799. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Chen G, Pan Q, Zhao J. Chronic Hepatitis E in a Renal Transplant Recipient: The First Report of Genotype 4 Hepatitis E Virus Caused Chronic Infection in Organ Recipient. Gastroenterology. 2018;154:1199–1201. doi: 10.1053/j.gastro.2017.12.028. [DOI] [PubMed] [Google Scholar]
- 32.Owada Y, Oshiro Y, Inagaki Y, Harada H, Fujiyama N, Kawagishi N, Yagisawa T, Usui J, Akutsu N, Itabashi Y, Saito K, Watarai Y, Ichimaru N, Imamura R, Kyakuno M, Ide K, Shibuya Y, Okabe Y, Ono M, Sasaki K, Shiose A, Yamagishi K, Ohnishi H, Nagashima S, Takahashi M, Yuzawa K, Okamoto H, Ohkohchi N. A Nationwide Survey of Hepatitis E Virus Infection and Chronic Hepatitis in Heart and Kidney Transplant Recipients in Japan. Transplantation. 2020;104:437–444. doi: 10.1097/TP.0000000000002801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou X, de Man RA, de Knegt RJ, Metselaar HJ, Peppelenbosch MP, Pan Q. Epidemiology and management of chronic hepatitis E infection in solid organ transplantation: a comprehensive literature review. Rev Med Virol. 2013;23:295–304. doi: 10.1002/rmv.1751. [DOI] [PubMed] [Google Scholar]
- 34.Mirazo S, Arbiza J. Hepatitis E and chronic liver damage in apparently immunocompetent individuals: Now what? Ann Hepatol. 2019;18:539–540. doi: 10.1016/j.aohep.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 35.Protzer U, Böhm F, Longerich T, Seebach J, Heidary Navid M, Friemel J, Marques-Maggio E, Bawohl M, Heikenwalder M, Schirmacher P, Dutkowski P, Clavien PA, Schemmer P, Schnitzler P, Gotthardt D, Müllhaupt B, Weber A. Molecular detection of hepatitis E virus (HEV) in liver biopsies after liver transplantation. Mod Pathol. 2015;28:523–532. doi: 10.1038/modpathol.2014.147. [DOI] [PubMed] [Google Scholar]
- 36.Suneetha PV, Pischke S, Schlaphoff V, Grabowski J, Fytili P, Gronert A, Bremer B, Markova A, Jaroszewicz J, Bara C, Manns MP, Cornberg M, Wedemeyer H. Hepatitis E virus (HEV)-specific T-cell responses are associated with control of HEV infection. Hepatology. 2012;55:695–708. doi: 10.1002/hep.24738. [DOI] [PubMed] [Google Scholar]
- 37.Borentain P, Colson P, Bolon E, Gauchez P, Coso D, Gérolami R. Hepatocellular carcinoma complicating hepatitis E virus-related cirrhosis. Hepatology. 2018;67:446–448. doi: 10.1002/hep.29508. [DOI] [PubMed] [Google Scholar]
- 38.Pischke S, Hartl J, Pas SD, Lohse AW, Jacobs BC, Van der Eijk AA. Hepatitis E virus: Infection beyond the liver? J Hepatol. 2017;66:1082–1095. doi: 10.1016/j.jhep.2016.11.016. [DOI] [PubMed] [Google Scholar]
- 39.Fousekis FS, Mitselos IV, Christodoulou DK. Extrahepatic manifestations of hepatitis E virus: An overview. Clin Mol Hepatol. 2020;26:16–23. doi: 10.3350/cmh.2019.0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kamar N, Weclawiak H, Guilbeau-Frugier C, Legrand-Abravanel F, Cointault O, Ribes D, Esposito L, Cardeau-Desangles I, Guitard J, Sallusto F, Muscari F, Peron JM, Alric L, Izopet J, Rostaing L. Hepatitis E virus and the kidney in solid-organ transplant patients. Transplantation. 2012;93:617–623. doi: 10.1097/TP.0b013e318245f14c. [DOI] [PubMed] [Google Scholar]
- 41.Noble J, Jouve T, Malvezzi P, Rostaing L. Renal complications of liver diseases. Expert Rev Gastroenterol Hepatol. 2018;12:1135–1142. doi: 10.1080/17474124.2018.1530984. [DOI] [PubMed] [Google Scholar]
- 42.Kamar N, Marion O, Abravanel F, Izopet J, Dalton HR. Extrahepatic manifestations of hepatitis E virus. Liver Int. 2016;36:467–472. doi: 10.1111/liv.13037. [DOI] [PubMed] [Google Scholar]
- 43.Liu H, Ma Y. Hepatitis E virus-associated Guillain-Barre syndrome: Revision of the literature. Brain Behav. 2020;10:e01496. doi: 10.1002/brb3.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ripellino P, Pasi E, Melli G, Staedler C, Fraga M, Moradpour D, Sahli R, Aubert V, Martinetti G, Bihl F, Bernasconi E, Terziroli Beretta-Piccoli B, Cerny A, Dalton HR, Zehnder C, Mathis B, Zecca C, Disanto G, Kaelin-Lang A, Gobbi C. Neurologic complications of acute hepatitis E virus infection. Neurol Neuroimmunol Neuroinflamm. 2020;7 doi: 10.1212/NXI.0000000000000643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fischer C, Hofmann M, Danzer M, Hofer K, Kaar J, Gabriel C. Seroprevalence and Incidence of hepatitis E in blood donors in Upper Austria. PLoS One. 2015;10:e0119576. doi: 10.1371/journal.pone.0119576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vercouter AS, Van Houtte F, Verhoye L, González Fraile I, Blanco L, Compernolle V, Meuleman P. Hepatitis E virus prevalence in Flemish blood donors. J Viral Hepat. 2019;26:1218–1223. doi: 10.1111/jvh.13161. [DOI] [PubMed] [Google Scholar]
- 47.Harritshøj LH, Holm DK, Saekmose SG, Jensen BA, Hogema BM, Fischer TK, Midgley SE, Krog JS, Erikstrup C, Ullum H. Low transfusion transmission of hepatitis E among 25,637 single-donation, nucleic acid-tested blood donors. Transfusion. 2016;56:2225–2232. doi: 10.1111/trf.13700. [DOI] [PubMed] [Google Scholar]
- 48.Gallian P, Lhomme S, Piquet Y, Sauné K, Abravanel F, Assal A, Tiberghien P, Izopet J. Hepatitis E virus infections in blood donors, France. Emerg Infect Dis. 2014;20:1914–1917. doi: 10.3201/eid2011.140516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Westhölter D, Hiller J, Denzer U, Polywka S, Ayuk F, Rybczynski M, Horvatits T, Gundlach S, Blöcker J, Schulze Zur Wiesch J, Fischer N, Addo MM, Peine S, Göke B, Lohse AW, Lütgehetmann M, Pischke S. HEV-positive blood donations represent a relevant infection risk for immunosuppressed recipients. J Hepatol. 2018;69:36–42. doi: 10.1016/j.jhep.2018.02.031. [DOI] [PubMed] [Google Scholar]
- 50.Dreier J, Knabbe C, Vollmer T. Transfusion-Transmitted Hepatitis E: NAT Screening of Blood Donations and Infectious Dose. Front Med (Lausanne) 2018;5:5. doi: 10.3389/fmed.2018.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Corman VM, Drexler JF, Eckerle I, Roth WK, Drosten C, Eis-Hübinger AM. Zoonotic hepatitis E virus strains in German blood donors. Vox Sang. 2013;104:179–180. doi: 10.1111/j.1423-0410.2012.01638.x. [DOI] [PubMed] [Google Scholar]
- 52.Vollmer T, Diekmann J, Johne R, Eberhardt M, Knabbe C, Dreier J. Novel approach for detection of hepatitis E virus infection in German blood donors. J Clin Microbiol. 2012;50:2708–2713. doi: 10.1128/JCM.01119-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baylis SA, Gärtner T, Nick S, Ovemyr J, Blümel J. Occurrence of hepatitis E virus RNA in plasma donations from Sweden, Germany and the United States. Vox Sang. 2012;103:89–90. doi: 10.1111/j.1423-0410.2011.01583.x. [DOI] [PubMed] [Google Scholar]
- 54.O'Riordan J, Boland F, Williams P, Donnellan J, Hogema BM, Ijaz S, Murphy WG. Hepatitis E virus infection in the Irish blood donor population. Transfusion. 2016;56:2868–2876. doi: 10.1111/trf.13757. [DOI] [PubMed] [Google Scholar]
- 55.Spreafico M, Raffaele L, Guarnori I, Foglieni B, Berzuini A, Valenti L, Gerosa A, Colli A, Prati D. Prevalence and 9-year incidence of hepatitis E virus infection among North Italian blood donors: Estimated transfusion risk. J Viral Hepat. 2020;27:858–861. doi: 10.1111/jvh.13296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Spada E, Pupella S, Pisani G, Bruni R, Chionne P, Madonna E, Villano U, Simeoni M, Fabi S, Marano G, Marcantonio C, Pezzotti P, Ciccaglione AR, Liumbruno GM. A nationwide retrospective study on prevalence of hepatitis E virus infection in Italian blood donors. Blood Transfus. 2018;16:413–421. doi: 10.2450/2018.0033-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hogema BM, Molier M, Sjerps M, de Waal M, van Swieten P, van de Laar T, Molenaar-de Backer M, Zaaijer HL. Incidence and duration of hepatitis E virus infection in Dutch blood donors. Transfusion. 2016;56:722–728. doi: 10.1111/trf.13402. [DOI] [PubMed] [Google Scholar]
- 58.Slot E, Hogema BM, Riezebos-Brilman A, Kok TM, Molier M, Zaaijer HL. Silent hepatitis E virus infection in Dutch blood donors, 2011 to 2012. Euro Surveill. 2013;18 doi: 10.2807/1560-7917.es2013.18.31.20550. [DOI] [PubMed] [Google Scholar]
- 59.Grabarczyk P, Sulkowska E, Gdowska J, Kopacz A, Liszewski G, Kubicka-Russel D, Baylis SA, Corman VM, Noceń E, Piotrowski D, Antoniewicz-Papis J, Łętowska M. Molecular and serological infection marker screening in blood donors indicates high endemicity of hepatitis E virus in Poland. Transfusion. 2018;58:1245–1253. doi: 10.1111/trf.14531. [DOI] [PubMed] [Google Scholar]
- 60.Rivero-Juarez A, Jarilla-Fernandez M, Frias M, Madrigal-Sanchez E, López-López P, Andújar-Troncoso G, Machuca I, Camacho A, Muñoz-Valbuena P, Rivero A. Hepatitis E virus in Spanish donors and the necessity for screening. J Viral Hepat. 2019;26:603–608. doi: 10.1111/jvh.13064. [DOI] [PubMed] [Google Scholar]
- 61.Sauleda S, Ong E, Bes M, Janssen A, Cory R, Babizki M, Shin T, Lindquist A, Hoang A, Vang L, Piron M, Casamitjana N, Koppelman M, Danzig L, Linnen JM. Seroprevalence of hepatitis E virus (HEV) and detection of HEV RNA with a transcription-mediated amplification assay in blood donors from Catalonia (Spain) Transfusion. 2015;55:972–979. doi: 10.1111/trf.12929. [DOI] [PubMed] [Google Scholar]
- 62.Harvala H, Hewitt PE, Reynolds C, Pearson C, Haywood B, Tettmar KI, Ushiro-Lumb I, Brailsford SR, Tedder R, Ijaz S. Hepatitis E virus in blood donors in England, 2016 to 2017: from selective to universal screening. Euro Surveill. 2019;24 doi: 10.2807/1560-7917.ES.2019.24.10.1800386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thom K, Gilhooly P, McGowan K, Malloy K, Jarvis LM, Crossan C, Scobie L, Blatchford O, Smith-Palmer A, Donnelly MC, Davidson JS, Johannessen I, Simpson KJ, Dalton HR, Petrik J. Hepatitis E virus (HEV) in Scotland: evidence of recent increase in viral circulation in humans. Euro Surveill. 2018;23 doi: 10.2807/1560-7917.ES.2018.23.12.17-00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hewitt PE, Ijaz S, Brailsford SR, Brett R, Dicks S, Haywood B, Kennedy IT, Kitchen A, Patel P, Poh J, Russell K, Tettmar KI, Tossell J, Ushiro-Lumb I, Tedder RS. Hepatitis E virus in blood components: a prevalence and transmission study in southeast England. Lancet. 2014;384:1766–1773. doi: 10.1016/S0140-6736(14)61034-5. [DOI] [PubMed] [Google Scholar]
- 65.Cleland A, Smith L, Crossan C, Blatchford O, Dalton HR, Scobie L, Petrik J. Hepatitis E virus in Scottish blood donors. Vox Sang. 2013;105:283–289. doi: 10.1111/vox.12056. [DOI] [PubMed] [Google Scholar]
- 66.Delage G, Fearon M, Gregoire Y, Hogema BM, Custer B, Scalia V, Hawes G, Bernier F, Nguyen ML, Stramer SL. Hepatitis E Virus Infection in Blood Donors and Risk to Patients in the United States and Canada. Transfus Med Rev. 2019;33:139–145. doi: 10.1016/j.tmrv.2019.05.017. [DOI] [PubMed] [Google Scholar]
- 67.Roth NJ, Schäfer W, Alexander R, Elliott K, Elliott-Browne W, Knowles J, Wenzel JJ, Simon TL. Low hepatitis E virus RNA prevalence in a large-scale survey of United States source plasma donors. Transfusion. 2017;57:2958–2964. doi: 10.1111/trf.14285. [DOI] [PubMed] [Google Scholar]
- 68.Stramer SL, Moritz ED, Foster GA, Ong E, Linnen JM, Hogema BM, Mak M, Chia CP, Dodd RY. Hepatitis E virus: seroprevalence and frequency of viral RNA detection among US blood donors. Transfusion. 2016;56:481–488. doi: 10.1111/trf.13355. [DOI] [PubMed] [Google Scholar]
- 69.Xu C, Wang RY, Schechterly CA, Ge S, Shih JW, Xia NS, Luban NL, Alter HJ. An assessment of hepatitis E virus (HEV) in US blood donors and recipients: no detectable HEV RNA in 1939 donors tested and no evidence for HEV transmission to 362 prospectively followed recipients. Transfusion. 2013;53:2505–2511. doi: 10.1111/trf.12326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wen GP, Chen CR, Song XY, Tang ZM, Ji WF, Wang SL, Zhang K, Zhang J, Ou SH, Zheng ZZ, Xia NS. Long-term HEV carriers without antibody seroconversion among eligible immunocompetent blood donors. Emerg Microbes Infect. 2018;7:125. doi: 10.1038/s41426-018-0125-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tsoi WC, Zhu X, To AP, Holmberg J. Hepatitis E virus infection in Hong Kong blood donors. Vox Sang. 2020;115:11–17. doi: 10.1111/vox.12846. [DOI] [PubMed] [Google Scholar]
- 72.Katiyar H, Goel A, Sonker A, Yadav V, Sapun S, Chaudhary R, Aggarwal R. Prevalence of hepatitis E virus viremia and antibodies among healthy blood donors in India. Indian J Gastroenterol. 2018;37:342–346. doi: 10.1007/s12664-018-0880-7. [DOI] [PubMed] [Google Scholar]
- 73.Minagi T, Okamoto H, Ikegawa M, Ideno S, Takahashi K, Sakai K, Hagiwara K, Yunoki M, Wakisaka A. Hepatitis E virus in donor plasma collected in Japan. Vox Sang. 2016;111:242–246. doi: 10.1111/vox.12425. [DOI] [PubMed] [Google Scholar]
- 74.Intharasongkroh D, Thongmee T, Sa-Nguanmoo P, Klinfueng S, Duang-In A, Wasitthankasem R, Theamboonlers A, Charoonruangrit U, Oota S, Payungporn S, Vongpunsawad S, Chirathaworn C, Poovorawan Y. Hepatitis E virus infection in Thai blood donors. Transfusion. 2019;59:1035–1043. doi: 10.1111/trf.15041. [DOI] [PubMed] [Google Scholar]
- 75.Hoad VC, Seed CR, Fryk JJ, Harley R, Flower RLP, Hogema BM, Kiely P, Faddy HM. Hepatitis E virus RNA in Australian blood donors: prevalence and risk assessment. Vox Sang. 2017;112:614–621. doi: 10.1111/vox.12559. [DOI] [PubMed] [Google Scholar]
- 76.Shrestha AC, Flower RL, Seed CR, Keller AJ, Harley R, Chan HT, Hoad V, Warrilow D, Northill J, Holmberg JA, Faddy HM. Hepatitis E virus RNA in Australian blood donations. Transfusion. 2016;56:3086–3093. doi: 10.1111/trf.13799. [DOI] [PubMed] [Google Scholar]
- 77.Hewitt J, Harte D, Sutherland M, Croucher D, Fouche L, Flanagan P, Williamson D. Prevalence of hepatitis E virus antibodies and infection in New Zealand blood donors. N Z Med J. 2018;131:38–43. [PubMed] [Google Scholar]
- 78.Maponga TG, Lopes T, Cable R, Pistorius C, Preiser W, Andersson MI. Prevalence and risks of hepatitis E virus infection in blood donors from the Western Cape, South Africa. Vox Sang. 2020;115:695–702. doi: 10.1111/vox.12966. [DOI] [PubMed] [Google Scholar]
- 79.Wang M, Fu P, Yin Y, He M, Liu Y. Acute, Recent and Past HEV Infection among Voluntary Blood Donors in China: A Systematic Review and Meta-Analysis. PLoS One. 2016;11:e0161089. doi: 10.1371/journal.pone.0161089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Di Cola G, Fantilli AC, Pisano MB, Ré VE. Foodborne transmission of hepatitis A and hepatitis E viruses: A literature review. Int J Food Microbiol. 2021;338:108986. doi: 10.1016/j.ijfoodmicro.2020.108986. [DOI] [PubMed] [Google Scholar]
- 81.Kamar N, Bendall R, Legrand-Abravanel F, Xia NS, Ijaz S, Izopet J, Dalton HR. Hepatitis E. Lancet. 2012;379:2477–2488. doi: 10.1016/S0140-6736(11)61849-7. [DOI] [PubMed] [Google Scholar]
- 82.Pérez-Gracia MT, Suay B, Mateos-Lindemann ML. Hepatitis E: an emerging disease. Infect Genet Evol. 2014;22:40–59. doi: 10.1016/j.meegid.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 83.Gupta N, Sarangi AN, Dadhich S, Dixit VK, Chetri K, Goel A, Aggarwal R. Acute hepatitis E in India appears to be caused exclusively by genotype 1 hepatitis E virus. Indian J Gastroenterol. 2018;37:44–49. doi: 10.1007/s12664-018-0819-z. [DOI] [PubMed] [Google Scholar]
- 84.Mevis FM, Sabeena S, Sanjay R, Robin S, Devadiga S, Prasad V, Oliver D, Ameen A, Arunkumar G. Currently circulating genotypes of hepatitis E virus in India, 2014-2018. Indian J Med Microbiol. 2019;37:563–568. doi: 10.4103/ijmm.IJMM_19_449. [DOI] [PubMed] [Google Scholar]
- 85.Gallian P, Couchouron A, Dupont I, Fabra C, Piquet Y, Djoudi R, Assal A, Tiberghien P. Comparison of hepatitis E virus nucleic acid test screening platforms and RNA prevalence in French blood donors. Transfusion. 2017;57:223–224. doi: 10.1111/trf.13889. [DOI] [PubMed] [Google Scholar]
- 86.Vollmer T, Diekmann J, Knabbe C, Dreier J. Hepatitis E virus blood donor NAT screening: as much as possible or as much as needed? Transfusion. 2019;59:612–622. doi: 10.1111/trf.15058. [DOI] [PubMed] [Google Scholar]
- 87.Miletić M, Vuk T, Hećimović A, Stojić Vidović M, Jemeršić L, Jukić I. Estimation of the hepatitis E assay-dependent seroprevalence among Croatian blood donors. Transfus Clin Biol. 2019;26:229–233. doi: 10.1016/j.tracli.2019.06.234. [DOI] [PubMed] [Google Scholar]
- 88.Holm DK, Moessner BK, Engle RE, Zaaijer HL, Georgsen J, Purcell RH, Christensen PB. Declining prevalence of hepatitis E antibodies among Danish blood donors. Transfusion. 2015;55:1662–1667. doi: 10.1111/trf.13028. [DOI] [PubMed] [Google Scholar]
- 89.Dimeglio C, Beau F, Broult J, Gouy P, Izopet J, Lastère S, Abravanel F. Hepatitis E prevalence in French Polynesian blood donors. PLoS One. 2018;13:e0208934. doi: 10.1371/journal.pone.0208934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Juhl D, Baylis SA, Blümel J, Görg S, Hennig H. Seroprevalence and incidence of hepatitis E virus infection in German blood donors. Transfusion. 2014;54:49–56. doi: 10.1111/trf.12121. [DOI] [PubMed] [Google Scholar]
- 91.Dalekos GN, Zervou E, Elisaf M, Germanos N, Galanakis E, Bourantas K, Siamopoulos KC, Tsianos EV. Antibodies to hepatitis E virus among several populations in Greece: increased prevalence in an hemodialysis unit. Transfusion. 1998;38:589–595. doi: 10.1046/j.1537-2995.1998.38698326339.x. [DOI] [PubMed] [Google Scholar]
- 92.De Sabato L, Di Bartolo I, Montomoli E, Trombetta C, Ruggeri FM, Ostanello F. Retrospective Study Evaluating Seroprevalence of Hepatitis E Virus in Blood Donors and in Swine Veterinarians in Italy (2004) Zoonoses Public Health. 2017;64:308–312. doi: 10.1111/zph.12332. [DOI] [PubMed] [Google Scholar]
- 93.Lucarelli C, Spada E, Taliani G, Chionne P, Madonna E, Marcantonio C, Pezzotti P, Bruni R, La Rosa G, Pisani G, Dell'Orso L, Ragone K, Tomei C, Ciccaglione AR. High prevalence of anti-hepatitis E virus antibodies among blood donors in central Italy, February to March 2014. Euro Surveill. 2016;21 doi: 10.2807/1560-7917.ES.2016.21.30.30299. [DOI] [PubMed] [Google Scholar]
- 94.Puttini C, Riccio ML, Redi D, Tordini G, Cenerini M, Romanello F, De Luca A, Carmellini M, Fossombroni V, Cusi MG, Zanelli G. Seroprevalence of hepatitis E virus (HEV) infection in blood donors and renal transplant recipients: a retrospective study from central Italy. Infez Med. 2015;23:253–256. [PubMed] [Google Scholar]
- 95.Hogema BM, Molier M, Slot E, Zaaijer HL. Past and present of hepatitis E in the Netherlands. Transfusion. 2014;54:3092–3096. doi: 10.1111/trf.12733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mateos ML, Camarero C, Lasa E, Teruel JL, Mir N, Baquero F. Hepatitis E virus: relevance in blood donors and risk groups. Vox Sang. 1999;76:78–80. doi: 10.1159/000031024. [DOI] [PubMed] [Google Scholar]
- 97.Niederhauser C, Widmer N, Hotz M, Tinguely C, Fontana S, Allemann G, Borri M, Infanti L, Sarraj A, Sigle J, Stalder M, Thierbach J, Waldvogel S, Wiengand T, Züger M, Gowland P. Current hepatitis E virus seroprevalence in Swiss blood donors and apparent decline from 1997 to 2016. Euro Surveill. 2018;23 doi: 10.2807/1560-7917.ES.2018.23.35.1700616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kaufmann A, Kenfak-Foguena A, André C, Canellini G, Bürgisser P, Moradpour D, Darling KE, Cavassini M. Hepatitis E virus seroprevalence among blood donors in southwest Switzerland. PLoS One. 2011;6:e21150. doi: 10.1371/journal.pone.0021150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Beale MA, Tettmar K, Szypulska R, Tedder RS, Ijaz S. Is there evidence of recent hepatitis E virus infection in English and North Welsh blood donors? Vox Sang. 2011;100:340–342. doi: 10.1111/j.1423-0410.2010.01412.x. [DOI] [PubMed] [Google Scholar]
- 100.Zafrullah M, Zhang X, Tran C, Nguyen M, Kamili S, Purdy MA, Stramer SL. Disparities in detection of antibodies against hepatitis E virus in US blood donor samples using commercial assays. Transfusion. 2018;58:1254–1263. doi: 10.1111/trf.14553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Di Lello FA, Blejer J, Alter A, Bartoli S, Vargas F, Ruiz R, Galli C, Blanco S, Carrizo LH, Gallego S, Fernández R, Martínez AP, Flichman DM. Seroprevalence of hepatitis E virus in Argentinean blood donors. Eur J Gastroenterol Hepatol. 2020 doi: 10.1097/MEG.0000000000001853. [DOI] [PubMed] [Google Scholar]
- 102.Bangueses F, Abin-Carriquiry JA, Cancela F, Curbelo J, Mirazo S. Serological and molecular prevalence of hepatitis E virus among blood donors from Uruguay. J Med Virol. 2021;93:4010–4014. doi: 10.1002/jmv.26231. [DOI] [PubMed] [Google Scholar]
- 103.Nouhin J, Prak S, Madec Y, Barennes H, Weissel R, Hok K, Pavio N, Rouet F. Hepatitis E virus antibody prevalence, RNA frequency, and genotype among blood donors in Cambodia (Southeast Asia) Transfusion. 2016;56:2597–2601. doi: 10.1111/trf.13731. [DOI] [PubMed] [Google Scholar]
- 104.Chen X, Gong P, Wagner AL, Li Y, Wang G, Lu Y. Identification of hepatitis E virus subtype 4f in blood donors in Shanghai, China. Virus Res. 2019;265:30–33. doi: 10.1016/j.virusres.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 105.Wang M, He M, Wu B, Ke L, Han T, Wang J, Shan H, Ness P, Guo N, Liu Y, Nelson KE. The association of elevated alanine aminotransferase levels with hepatitis E virus infections among blood donors in China. Transfusion. 2017;57:273–279. doi: 10.1111/trf.13991. [DOI] [PubMed] [Google Scholar]
- 106.Ma L, Sun P, Lin F, Wang H, Rong X, Dai Y, Liu J, Qian L, Fang M, Su N, Xiao W, Ye S, Li C. Prevalence of hepatitis E virus in Chinese blood donors. J Int Med Res. 2015;43:257–262. doi: 10.1177/0300060514562054. [DOI] [PubMed] [Google Scholar]
- 107.Ren F, Zhao C, Wang L, Wang Z, Gong X, Song M, Zhuang H, Huang Y, Shan H, Wang J, Liu Q, Ness P, Nelson KE, Wang Y. Hepatitis E virus seroprevalence and molecular study among blood donors in China. Transfusion. 2014;54:910–917. doi: 10.1111/trf.12530. [DOI] [PubMed] [Google Scholar]
- 108.Zhuang W, Ding X, Lyu C, Xiang L, Teng H, Li J. Hepatitis E virus seroprevalence among blood donors in Jiangsu Province, East China. Int J Infect Dis. 2014;26:9–11. doi: 10.1016/j.ijid.2014.04.022. [DOI] [PubMed] [Google Scholar]
- 109.Tripathy AS, Puranik S, Sharma M, Chakraborty S, Devakate UR. Hepatitis E virus seroprevalence among blood donors in Pune, India. J Med Virol. 2019;91:813–819. doi: 10.1002/jmv.25370. [DOI] [PubMed] [Google Scholar]
- 110.Gajjar MD, Bhatnagar NM, Sonani RV, Gupta S, Patel T. Hepatitis E seroprevalence among blood donors: A pilot study from Western India. Asian J Transfus Sci. 2014;8:29–31. doi: 10.4103/0973-6247.126685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Parsa R, Adibzadeh S, Behzad Behbahani A, Farhadi A, Yaghobi R, Rafiei Dehbidi GR, Hajizamani S, Rahbar S, Nikouyan N, Okhovat MA, Naderi S, Salehi S, Alizadeh M, Ranjbaran R, Zarnegar G, Alavi P. Detection of Hepatitis E Virus Genotype 1 Among Blood Donors From Southwest of Iran. Hepat Mon. 2016;16:e34202. doi: 10.5812/hepatmon.34202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hesamizadeh K, Sharafi H, Keyvani H, Alavian SM, Najafi-Tireh Shabankareh A, Sharifi Olyaie R, Keshvari M. Hepatitis A Virus and Hepatitis E Virus Seroprevalence Among Blood Donors in Tehran, Iran. Hepat Mon. 2016;16:e32215. doi: 10.5812/hepatmon.32215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Naeimi B, Mazloom Kalimani F, Pourfatolah AA, Azimzadeh M, Mankhian A, Akbarzadeh S, Hajiani G, Kooshesh F, Khamisipour G. Hepatitis E Virus Seroprevalence Among Blood Donors in Bushehr, South of Iran. Hepat Mon. 2015;15:e29219. doi: 10.5812/hepatmon.29219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ehteram H, Ramezani A, Eslamifar A, Sofian M, Banifazl M, Ghassemi S, Aghakhani A, Mashayekhi P. Seroprevalence of Hepatitis E Virus infection among volunteer blood donors in central province of Iran in 2012. Iran J Microbiol. 2013;5:172–176. [PMC free article] [PubMed] [Google Scholar]
- 115.Taremi M, Gachkar L, MahmoudArabi S, Kheradpezhouh M, Khoshbaten M. Prevalence of antibodies to hepatitis E virus among male blood donors in Tabriz, Islamic Republic of Iran. East Mediterr Health J. 2007;13:98–102. [PubMed] [Google Scholar]
- 116.Takeda H, Matsubayashi K, Sakata H, Sato S, Kato T, Hino S, Tadokoro K, Ikeda H. A nationwide survey for prevalence of hepatitis E virus antibody in qualified blood donors in Japan. Vox Sang. 2010;99:307–313. doi: 10.1111/j.1423-0410.2010.01362.x. [DOI] [PubMed] [Google Scholar]
- 117.Shrestha AC, Flower RL, Seed CR, Rajkarnikar M, Shrestha SK, Thapa U, Hoad VC, Faddy HM. Hepatitis E virus seroepidemiology: a post-earthquake study among blood donors in Nepal. BMC Infect Dis. 2016;16:707. doi: 10.1186/s12879-016-2043-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nasrallah GK, Al Absi ES, Ghandour R, Ali NH, Taleb S, Hedaya L, Ali F, Huwaidy M, Husseini A. Seroprevalence of hepatitis E virus among blood donors in Qatar (2013-2016) Transfusion. 2017;57:1801–1807. doi: 10.1111/trf.14116. [DOI] [PubMed] [Google Scholar]
- 119.Jupattanasin S, Chainuvati S, Chotiyaputta W, Chanmanee T, Supapueng O, Charoonruangrit U, Oota S, Louisirirotchanakul S. A Nationwide Survey of the Seroprevalence of Hepatitis E Virus Infections Among Blood Donors in Thailand. Viral Immunol. 2019;32:302–307. doi: 10.1089/vim.2018.0146. [DOI] [PubMed] [Google Scholar]
- 120.Traoré KA, Ouoba JB, Rouamba H, Nébié YK, Dahourou H, Rossetto F, Traoré AS, Barro N, Roques P. Hepatitis E Virus Prevalence among Blood Donors, Ouagadougou, Burkina Faso. Emerg Infect Dis. 2016;22:755–757. doi: 10.3201/eid2204.151728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ibrahim EH, Abdelwahab SF, Nady S, Hashem M, Galal G, Sobhy M, Saleh AS, Shata MT. Prevalence of anti-HEV IgM among blood donors in Egypt. Egypt J Immunol. 2011;18:47–58. [PubMed] [Google Scholar]
- 122.Meldal BH, Sarkodie F, Owusu-Ofori S, Allain JP. Hepatitis E virus infection in Ghanaian blood donors - the importance of immunoassay selection and confirmation. Vox Sang. 2013;104:30–36. doi: 10.1111/j.1423-0410.2012.01637.x. [DOI] [PubMed] [Google Scholar]
- 123.Lopes T, Cable R, Pistorius C, Maponga T, Ijaz S, Preiser W, Tedder R, Andersson MI. Racial differences in seroprevalence of HAV and HEV in blood donors in the Western Cape, South Africa: a clue to the predominant HEV genotype? Epidemiol Infect. 2017;145:1910–1912. doi: 10.1017/S0950268817000565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ben-Ayed Y, Hannachi H, Ben-Alaya-Bouafif N, Gouider E, Triki H, Bahri O. Hepatitis E virus seroprevalence among hemodialysis and hemophiliac patients in Tunisia (North Africa) J Med Virol. 2015;87:441–445. doi: 10.1002/jmv.24082. [DOI] [PubMed] [Google Scholar]
- 125.Schemmerer M, Rauh C, Jilg W, Wenzel JJ. Time course of hepatitis E-specific antibodies in adults. J Viral Hepat. 2017;24:75–79. doi: 10.1111/jvh.12621. [DOI] [PubMed] [Google Scholar]
- 126.Takahashi M, Tanaka T, Takahashi H, Hoshino Y, Nagashima S, Jirintai , Mizuo H, Yazaki Y, Takagi T, Azuma M, Kusano E, Isoda N, Sugano K, Okamoto H. Hepatitis E Virus (HEV) strains in serum samples can replicate efficiently in cultured cells despite the coexistence of HEV antibodies: characterization of HEV virions in blood circulation. J Clin Microbiol. 2010;48:1112–1125. doi: 10.1128/JCM.02002-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Goel A, Vijay HJ, Katiyar H, Aggarwal R. Prevalence of hepatitis E viraemia among blood donors: a systematic review. Vox Sang. 2020;115:120–132. doi: 10.1111/vox.12887. [DOI] [PubMed] [Google Scholar]
- 128.Tedder RS, Tettmar KI, Brailsford SR, Said B, Ushiro-Lumb I, Kitchen A, Morgan D, Lattimore S, Tossell J, Ijaz S, Hewitt PE. Virology, serology, and demography of hepatitis E viremic blood donors in South East England. Transfusion. 2016;56:1529–1536. doi: 10.1111/trf.13498. [DOI] [PubMed] [Google Scholar]
- 129.Riveiro-Barciela M, Rando-Segura A, Barreira-Díaz A, Bes M, P Ruzo S, Piron M, Quer J, Sauleda S, Rodríguez-Frías F, Esteban R, Buti M. Unexpected long-lasting anti-HEV IgM positivity: Is HEV antigen a better serological marker for hepatitis E infection diagnosis? J Viral Hepat. 2020;27:747–753. doi: 10.1111/jvh.13285. [DOI] [PubMed] [Google Scholar]
- 130.Kar P, Karna R. A Review of the Diagnosis and Management of Hepatitis E. Infect Dis. 2020:1–11. doi: 10.1007/s40506-020-00235-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hartl J, Otto B, Madden RG, Webb G, Woolson KL, Kriston L, Vettorazzi E, Lohse AW, Dalton HR, Pischke S. Hepatitis E Seroprevalence in Europe: A Meta-Analysis. Viruses. 2016;8 doi: 10.3390/v8080211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shrestha AC, Flower RL, Seed CR, Stramer SL, Faddy HM. A Comparative Study of Assay Performance of Commercial Hepatitis E Virus Enzyme-Linked Immunosorbent Assay Kits in Australian Blood Donor Samples. J Blood Transfus. 2016;2016:9647675. doi: 10.1155/2016/9647675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Galli C, Fomiatti L, Tagliacarne C, Velati C, Zanetti AR, Castaldi S, Romanò L. Seroprevalence of hepatitis E virus among blood donors in northern Italy (Sondrio, Lombardy) determined by three different assays. Blood Transfus. 2017;15:502–505. doi: 10.2450/2017.0089-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Park HK, Jeong SH, Kim JW, Woo BH, Lee DH, Kim HY, Ahn S. Seroprevalence of anti-hepatitis E virus (HEV) in a Korean population: comparison of two commercial anti-HEV assays. BMC Infect Dis. 2012;12:142. doi: 10.1186/1471-2334-12-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bendall R, Ellis V, Ijaz S, Ali R, Dalton H. A comparison of two commercially available anti-HEV IgG kits and a re-evaluation of anti-HEV IgG seroprevalence data in developed countries. J Med Virol. 2010;82:799–805. doi: 10.1002/jmv.21656. [DOI] [PubMed] [Google Scholar]
- 136.Matsubayashi K, Nagaoka Y, Sakata H, Sato S, Fukai K, Kato T, Takahashi K, Mishiro S, Imai M, Takeda N, Ikeda H. Transfusion-transmitted hepatitis E caused by apparently indigenous hepatitis E virus strain in Hokkaido, Japan. Transfusion. 2004;44:934–940. doi: 10.1111/j.1537-2995.2004.03300.x. [DOI] [PubMed] [Google Scholar]
- 137.Okano H, Nakano T, Ito R, Tanaka A, Hoshi Y, Matsubayashi K, Asakawa H, Nose K, Tsuruga S, Tochio T, Kumazawa H, Isono Y, Tanaka H, Matsusaki S, Sase T, Saito T, Mukai K, Nishimura A, Kawakami K, Nagashima S, Takahashi M, Okamoto H. The spontaneous clearance of hepatitis E virus (HEV) and emergence of HEV antibodies in a transfusion-transmitted chronic hepatitis E case after completion of chemotherapy for acute myeloid leukemia. Clin J Gastroenterol. 2020;13:252–259. doi: 10.1007/s12328-019-01024-3. [DOI] [PubMed] [Google Scholar]
- 138.Gallian P, Pouchol E, Djoudi R, Lhomme S, Mouna L, Gross S, Bierling P, Assal A, Kamar N, Mallet V, Roque-Afonso AM, Izopet J, Tiberghien P. Transfusion-Transmitted Hepatitis E Virus Infection in France. Transfus Med Rev. 2019;33:146–153. doi: 10.1016/j.tmrv.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 139.Ledesma J, Williams D, Stanford FA, Hewitt PE, Zuckerman M, Bansal S, Dhawan A, Mbisa JL, Tedder R, Ijaz S. Resolution by deep sequencing of a dual hepatitis E virus infection transmitted via blood components. J Gen Virol. 2019;100:1491–1500. doi: 10.1099/jgv.0.001302. [DOI] [PubMed] [Google Scholar]
- 140.Satake M, Matsubayashi K, Hoshi Y, Taira R, Furui Y, Kokudo N, Akamatsu N, Yoshizumi T, Ohkohchi N, Okamoto H, Miyoshi M, Tamura A, Fuse K, Tadokoro K. Unique clinical courses of transfusion-transmitted hepatitis E in patients with immunosuppression. Transfusion. 2017;57:280–288. doi: 10.1111/trf.13994. [DOI] [PubMed] [Google Scholar]
- 141.Lhomme S, Bardiaux L, Abravanel F, Gallian P, Kamar N, Izopet J. Hepatitis E Virus Infection in Solid Organ Transplant Recipients, France. Emerg Infect Dis. 2017;23:353–356. doi: 10.3201/eid2302.161094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yamazaki Y, Naganuma A, Arai Y, Takeuchi S, Kobayashi T, Takakusagi S, Hatanaka T, Hoshino T, Namikawa M, Hashizume H, Takizawa D, Ohyama T, Suzuki H, Horiguchi N, Takagi H, Sato K, Kakizaki S, Kusano M, Nagashima S, Takahashi M, Okamoto H, Yamada M. Clinical and virological features of acute hepatitis E in Gunma prefecture, Japan between 2004 and 2015. Hepatol Res. 2017;47:435–445. doi: 10.1111/hepr.12765. [DOI] [PubMed] [Google Scholar]
- 143.Belliere J, Abravanel F, Nogier MB, Martinez S, Cintas P, Lhomme S, Lavayssière L, Cointault O, Faguer S, Izopet J, Kamar N. Transfusion-acquired hepatitis E infection misdiagnosed as severe critical illness polyneuromyopathy in a heart transplant patient. Transpl Infect Dis. 2017;19 doi: 10.1111/tid.12784. [DOI] [PubMed] [Google Scholar]
- 144.Riveiro-Barciela M, Sauleda S, Quer J, Salvador F, Gregori J, Pirón M, Rodríguez-Frías F, Buti M. Red blood cell transfusion-transmitted acute hepatitis E in an immunocompetent subject in Europe: a case report. Transfusion. 2017;57:244–247. doi: 10.1111/trf.13876. [DOI] [PubMed] [Google Scholar]
- 145.Hoad VC, Gibbs T, Ravikumara M, Nash M, Levy A, Tracy SL, Mews C, Perkowska-Guse Z, Faddy HM, Bowden S. First confirmed case of transfusion-transmitted hepatitis E in Australia. Med J Aust. 2017;206:289–290. doi: 10.5694/mja16.01090. [DOI] [PubMed] [Google Scholar]
- 146.Matsui T, Kang JH, Matsubayashi K, Yamazaki H, Nagai K, Sakata H, Tsuji K, Maguchi H. Rare case of transfusion-transmitted hepatitis E from the blood of a donor infected with the hepatitis E virus genotype 3 indigenous to Japan: Viral dynamics from onset to recovery. Hepatol Res. 2015;45:698–704. doi: 10.1111/hepr.12390. [DOI] [PubMed] [Google Scholar]
- 147.Huzly D, Umhau M, Bettinger D, Cathomen T, Emmerich F, Hasselblatt P, Hengel H, Herzog R, Kappert O, Maassen S, Schorb E, Schulz-Huotari C, Thimme R, Unmüssig R, Wenzel JJ, Panning M. Transfusion-transmitted hepatitis E in Germany, 2013. Euro Surveill. 2014;19 doi: 10.2807/1560-7917.es2014.19.21.20812. [DOI] [PubMed] [Google Scholar]
- 148.Coilly A, Haïm-Boukobza S, Roche B, Antonini TM, Pause A, Mokhtari C, Becq A, Farahmand H, Hauser L, Duclos-Vallée JC, Samuel D, Adam R, Roque-Afonso AM. Posttransplantation hepatitis E: transfusion-transmitted hepatitis rising from the ashes. Transplantation. 2013;96:e4–e6. doi: 10.1097/TP.0b013e318296c9f7. [DOI] [PubMed] [Google Scholar]
- 149.Boxall E, Herborn A, Kochethu G, Pratt G, Adams D, Ijaz S, Teo CG. Transfusion-transmitted hepatitis E in a 'nonhyperendemic' country. Transfus Med. 2006;16:79–83. doi: 10.1111/j.1365-3148.2006.00652.x. [DOI] [PubMed] [Google Scholar]
- 150.Mitsui T, Tsukamoto Y, Yamazaki C, Masuko K, Tsuda F, Takahashi M, Nishizawa T, Okamoto H. Prevalence of hepatitis E virus infection among hemodialysis patients in Japan: evidence for infection with a genotype 3 HEV by blood transfusion. J Med Virol. 2004;74:563–572. doi: 10.1002/jmv.20215. [DOI] [PubMed] [Google Scholar]
- 151.Ticehurst JR, Pisanic N, Forman MS, Ordak C, Heaney CD, Ong E, Linnen JM, Ness PM, Guo N, Shan H, Nelson KE. Probable transmission of hepatitis E virus (HEV) via transfusion in the United States. Transfusion. 2019;59:1024–1034. doi: 10.1111/trf.15140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Andonov A, Rock G, Lin L, Borlang J, Hooper J, Grudeski E, Wu J Members of the Canadian Apheresis Group (CAG) Serological and molecular evidence of a plausible transmission of hepatitis E virus through pooled plasma. Vox Sang. 2014;107:213–219. doi: 10.1111/vox.12156. [DOI] [PubMed] [Google Scholar]
- 153.Mrzljak A, Dinjar-Kujundzic P, Knotek M, Kudumija B, Ilic M, Gulin M, Zibar L, Hrstic I, Jurekovic Z, Kolaric B, Jemersic L, Prpic J, Tomljenovic M, Vilibic-Cavlek T. Seroepidemiology of hepatitis E in patients on haemodialysis in Croatia. Int Urol Nephrol. 2020;52:371–378. doi: 10.1007/s11255-019-02363-3. [DOI] [PubMed] [Google Scholar]
- 154.Slavov SN, Maçonetto JDM, Martinez EZ, Silva-Pinto AC, Covas DT, Eis-Hübinger AM, Kashima S. Prevalence of hepatitis E virus infection in multiple transfused Brazilian patients with thalassemia and sickle cell disease. J Med Virol. 2019;91:1693–1697. doi: 10.1002/jmv.25498. [DOI] [PubMed] [Google Scholar]
- 155.Dalvand N, Dalvand A, Sharifi Z, Hosseini SM. Prevalence of hepatitis E virus in thalassemia patients with hepatitis C in Tehran, Iran. Iran J Microbiol. 2019;11:535–540. [PMC free article] [PubMed] [Google Scholar]
- 156.Ankcorn MJ, Fox TA, Ijaz S, Nicholas C, Houston E, Longair I, Suri D, Mattes FM, Walker JL, Tedder RS, Sekhar M. Characterising the risk of Hepatitis E virus infection in haematological malignancies: a UK prospective prevalence study. Br J Haematol. 2019;186:191–195. doi: 10.1111/bjh.15796. [DOI] [PubMed] [Google Scholar]
- 157.Haïm-Boukobza S, Ferey MP, Vétillard AL, Jeblaoui A, Pélissier E, Pelletier G, Teillet L, Roque-Afonso AM. Transfusion-transmitted hepatitis E in a misleading context of autoimmunity and drug-induced toxicity. J Hepatol. 2012;57:1374–1378. doi: 10.1016/j.jhep.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 158.Loyrion E, Trouve-Buisson T, Pouzol P, Larrat S, Decaens T, Payen JF. Hepatitis E Virus Infection after Platelet Transfusion in an Immunocompetent Trauma Patient. Emerg Infect Dis. 2017;23:146–147. doi: 10.3201/eid2301.160923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Peters van Ton AM, Gevers TJ, Drenth JP. Antiviral therapy in chronic hepatitis E: a systematic review. J Viral Hepat. 2015;22:965–973. doi: 10.1111/jvh.12403. [DOI] [PubMed] [Google Scholar]
- 160.Kamar N, Garrouste C, Haagsma EB, Garrigue V, Pischke S, Chauvet C, Dumortier J, Cannesson A, Cassuto-Viguier E, Thervet E, Conti F, Lebray P, Dalton HR, Santella R, Kanaan N, Essig M, Mousson C, Radenne S, Roque-Afonso AM, Izopet J, Rostaing L. Factors associated with chronic hepatitis in patients with hepatitis E virus infection who have received solid organ transplants. Gastroenterology. 2011;140:1481–1489. doi: 10.1053/j.gastro.2011.02.050. [DOI] [PubMed] [Google Scholar]
- 161.Pas SD, de Man RA, Mulders C, Balk AH, van Hal PT, Weimar W, Koopmans MP, Osterhaus AD, van der Eijk AA. Hepatitis E virus infection among solid organ transplant recipients, the Netherlands. Emerg Infect Dis. 2012;18:869–872. doi: 10.3201/eid1805.111712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kamar N, Selves J, Mansuy JM, Ouezzani L, Péron JM, Guitard J, Cointault O, Esposito L, Abravanel F, Danjoux M, Durand D, Vinel JP, Izopet J, Rostaing L. Hepatitis E virus and chronic hepatitis in organ-transplant recipients. N Engl J Med. 2008;358:811–817. doi: 10.1056/NEJMoa0706992. [DOI] [PubMed] [Google Scholar]
- 163.Haagsma EB, van den Berg AP, Porte RJ, Benne CA, Vennema H, Reimerink JH, Koopmans MP. Chronic hepatitis E virus infection in liver transplant recipients. Liver Transpl. 2008;14:547–553. doi: 10.1002/lt.21480. [DOI] [PubMed] [Google Scholar]
- 164.von Felden J, Alric L, Pischke S, Aitken C, Schlabe S, Spengler U, Giordani MT, Schnitzler P, Bettinger D, Thimme R, Xhaard A, Binder M, Ayuk F, Lohse AW, Cornelissen JJ, de Man RA, Mallet V. The burden of hepatitis E among patients with haematological malignancies: A retrospective European cohort study. J Hepatol. 2019;71:465–472. doi: 10.1016/j.jhep.2019.04.022. [DOI] [PubMed] [Google Scholar]
- 165.Cao D, Cao QM, Subramaniam S, Yugo DM, Heffron CL, Rogers AJ, Kenney SP, Tian D, Matzinger SR, Overend C, Catanzaro N, LeRoith T, Wang H, Piñeyro P, Lindstrom N, Clark-Deener S, Yuan L, Meng XJ. Pig model mimicking chronic hepatitis E virus infection in immunocompromised patients to assess immune correlates during chronicity. Proc Natl Acad Sci U S A. 2017;114:6914–6923. doi: 10.1073/pnas.1705446114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Saravanabalaji S, Tripathy AS, Dhoot RR, Chadha MS, Kakrani AL, Arankalle VA. Viral load, antibody titers and recombinant open reading frame 2 protein-induced TH1/TH2 cytokines and cellular immune responses in self-limiting and fulminant hepatitis e. Intervirology. 2009;52:78–85. doi: 10.1159/000214862. [DOI] [PubMed] [Google Scholar]
- 167.Péron JM, Abravanel F, Guillaume M, Gérolami R, Nana J, Anty R, Pariente A, Renou C, Bureau C, Robic MA, Alric L, Vinel JP, Izopet J, Kamar N. Treatment of autochthonous acute hepatitis E with short-term ribavirin: a multicenter retrospective study. Liver Int. 2016;36:328–333. doi: 10.1111/liv.12911. [DOI] [PubMed] [Google Scholar]
- 168.McPherson S, Elsharkawy AM, Ankcorn M, Ijaz S, Powell J, Rowe I, Tedder R, Andrews PA. Summary of the British Transplantation Society UK Guidelines for Hepatitis E and Solid Organ Transplantation. Transplantation. 2018;102:15–20. doi: 10.1097/TP.0000000000001908. [DOI] [PubMed] [Google Scholar]
- 169.European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu.; European Association for the Study of the Liver. EASL Clinical Practice Guidelines on hepatitis E virus infection. J Hepatol. 2018;68:1256–1271. doi: 10.1016/j.jhep.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 170.Wang Y, Zhou X, Debing Y, Chen K, Van Der Laan LJ, Neyts J, Janssen HL, Metselaar HJ, Peppelenbosch MP, Pan Q. Calcineurin inhibitors stimulate and mycophenolic acid inhibits replication of hepatitis E virus. Gastroenterology. 2014;146:1775–1783. doi: 10.1053/j.gastro.2014.02.036. [DOI] [PubMed] [Google Scholar]
- 171.Kamar N, Lhomme S, Abravanel F, Marion O, Peron JM, Alric L, Izopet J. Treatment of HEV Infection in Patients with a Solid-Organ Transplant and Chronic Hepatitis. Viruses. 2016;8 doi: 10.3390/v8080222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Gorris M, van der Lecq BM, van Erpecum KJ, de Bruijne J. Treatment for chronic hepatitis E virus infection: A systematic review and meta-analysis. J Viral Hepat. 2021;28:454–463. doi: 10.1111/jvh.13456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.De Winter BCM, Hesselink DA, Kamar N. Dosing ribavirin in hepatitis E-infected solid organ transplant recipients. Pharmacol Res. 2018;130:308–315. doi: 10.1016/j.phrs.2018.02.030. [DOI] [PubMed] [Google Scholar]
- 174.Kamar N, Lhomme S, Abravanel F, Cointault O, Esposito L, Cardeau-Desangles I, Del Bello A, Dörr G, Lavayssière L, Nogier MB, Guitard J, Ribes D, Goin AL, Broué P, Metsu D, Sauné K, Rostaing L, Izopet J. An Early Viral Response Predicts the Virological Response to Ribavirin in Hepatitis E Virus Organ Transplant Patients. Transplantation. 2015;99:2124–2131. doi: 10.1097/TP.0000000000000850. [DOI] [PubMed] [Google Scholar]
- 175.Todt D, Meister TL, Steinmann E. Hepatitis E virus treatment and ribavirin therapy: viral mechanisms of nonresponse. Curr Opin Virol. 2018;32:80–87. doi: 10.1016/j.coviro.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 176.Alric L, Bonnet D, Laurent G, Kamar N, Izopet J. Chronic hepatitis E virus infection: successful virologic response to pegylated interferon-alpha therapy. Ann Intern Med. 2010;153:135–136. doi: 10.7326/0003-4819-153-2-201007200-00256. [DOI] [PubMed] [Google Scholar]
- 177.Kamar N, Abravanel F, Garrouste C, Cardeau-Desangles I, Mansuy JM, Weclawiak H, Izopet J, Rostaing L. Three-month pegylated interferon-alpha-2a therapy for chronic hepatitis E virus infection in a haemodialysis patient. Nephrol Dial Transplant. 2010;25:2792–2795. doi: 10.1093/ndt/gfq282. [DOI] [PubMed] [Google Scholar]
- 178.Rivero-Juarez A, Lopez-Lopez P, Frias M, Rivero A. Hepatitis E Infection in HIV-Infected Patients. Front Microbiol. 2019;10:1425. doi: 10.3389/fmicb.2019.01425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Nakano R, Ohira M, Ishiyama K, Ide K, Kobayashi T, Tahara H, Shimizu S, Arihiro K, Imamura M, Chayama K, Tanaka Y, Ohdan H. Acute Graft Rejection and Formation of De Novo Donor-Specific Antibodies Triggered by Low Cyclosporine Levels and Interferon Therapy for Recurrent Hepatitis C Infection After Liver Transplantation: A Case Report. Transplant Proc. 2017;49:1634–1638. doi: 10.1016/j.transproceed.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 180.Selzner N, Guindi M, Renner EL, Berenguer M. Immune-mediated complications of the graft in interferon-treated hepatitis C positive liver transplant recipients. J Hepatol. 2011;55:207–217. doi: 10.1016/j.jhep.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 181.Kamar N, Rostaing L, Abravanel F, Garrouste C, Esposito L, Cardeau-Desangles I, Mansuy JM, Selves J, Peron JM, Otal P, Muscari F, Izopet J. Pegylated interferon-alpha for treating chronic hepatitis E virus infection after liver transplantation. Clin Infect Dis. 2010;50:e30–e33. doi: 10.1086/650488. [DOI] [PubMed] [Google Scholar]
- 182.Haagsma EB, Riezebos-Brilman A, van den Berg AP, Porte RJ, Niesters HG. Treatment of chronic hepatitis E in liver transplant recipients with pegylated interferon alpha-2b. Liver Transpl. 2010;16:474–477. doi: 10.1002/lt.22014. [DOI] [PubMed] [Google Scholar]
- 183.Ollivier-Hourmand I, Lebedel L, Lecouf A, Allaire M, Nguyen TTN, Lier C, Dao T. Pegylated interferon may be considered in chronic viral hepatitis E resistant to ribavirin in kidney transplant recipients. BMC Infect Dis. 2020;20:522. doi: 10.1186/s12879-020-05212-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Dao Thi VL, Debing Y, Wu X, Rice CM, Neyts J, Moradpour D, Gouttenoire J. Sofosbuvir Inhibits Hepatitis E Virus Replication In Vitro and Results in an Additive Effect When Combined With Ribavirin. Gastroenterology. 2016;150:82–85.e4. doi: 10.1053/j.gastro.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 185.van der Valk M, Zaaijer HL, Kater AP, Schinkel J. Sofosbuvir shows antiviral activity in a patient with chronic hepatitis E virus infection. J Hepatol. 2017;66:242–243. doi: 10.1016/j.jhep.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 186.van Wezel EM, de Bruijne J, Damman K, Bijmolen M, van den Berg AP, Verschuuren EAM, Ruigrok GA, Riezebos-Brilman A, Knoester M. Sofosbuvir Add-on to Ribavirin Treatment for Chronic Hepatitis E Virus Infection in Solid Organ Transplant Recipients Does Not Result in Sustained Virological Response. Open Forum Infect Di. 2019;6 doi: 10.1093/ofid/ofz346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Cornberg M, Pischke S, Müller T, Behrendt P, Piecha F, Benckert J, Todt D, Steinmann E, Papkalla A, von Karpowitz M, Koch A, Lohse A, Hardtke S, Manns MP, Wedemeyer H. Sofosbuvir monotherapy fails to achieve HEV RNA elimination in patients with chronic hepatitis E - The HepNet SofE pilot study. J Hepatol. 2020;73:696–699. doi: 10.1016/j.jhep.2020.05.020. [DOI] [PubMed] [Google Scholar]
- 188.Tedder RS, Ijaz S, Kitchen A, Ushiro-Lumb I, Tettmar KI, Hewitt P, Andrews N. Hepatitis E risks: pigs or blood-that is the question. Transfusion. 2017;57:267–272. doi: 10.1111/trf.13976. [DOI] [PubMed] [Google Scholar]
- 189.Denner J. Hepatitis E virus (HEV)-The Future. Viruses. 2019;11 doi: 10.3390/v11030251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wu X, Chen P, Lin H, Hao X, Liang Z. Hepatitis E virus: Current epidemiology and vaccine. Hum Vaccin Immunother. 2016;12:2603–2610. doi: 10.1080/21645515.2016.1184806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Denner J, Pischke S, Steinmann E, Blümel J, Glebe D. Why all blood donations should be tested for hepatitis E virus (HEV) BMC Infect Dis. 2019;19:541. doi: 10.1186/s12879-019-4190-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Domanović D, Tedder R, Blümel J, Zaaijer H, Gallian P, Niederhauser C, Sauleda Oliveras S, O'Riordan J, Boland F, Harritshøj L, Nascimento MSJ, Ciccaglione AR, Politis C, Adlhoch C, Flan B, Oualikene-Gonin W, Rautmann G, Strengers P, Hewitt P. Hepatitis E and blood donation safety in selected European countries: a shift to screening? Euro Surveill. 2017;22 doi: 10.2807/1560-7917.ES.2017.22.16.30514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Matsubayashi K, Sakata H, Ikeda H. Hepatitis E virus infection and blood transfusion in Japan. ISBT Sci Ser. 2011;6:344–349. [Google Scholar]
- 194.Lee CK, Chau TN, Lim W, Tsoi WC, Lai ST, Lin CK. Prevention of transfusion-transmitted hepatitis E by donor-initiated self exclusion. Transfus Med. 2005;15:133–135. doi: 10.1111/j.0958-7578.2005.00563.x. [DOI] [PubMed] [Google Scholar]
- 195.Pawlotsky JM. Hepatitis E screening for blood donations: an urgent need? Lancet. 2014;384:1729–1730. doi: 10.1016/S0140-6736(14)61187-9. [DOI] [PubMed] [Google Scholar]
- 196.Boland F, Martinez A, Pomeroy L, O'Flaherty N. Blood Donor Screening for Hepatitis E Virus in the European Union. Transfus Med Hemother. 2019;46:95–103. doi: 10.1159/000499121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. American Association of Blood Banks. (2014) Hepatitis E virus [cited 20 March 2021]. Available from: https://www.aabb.org/docs/default-source/default-document-library/regulatory/eid/hepatitis-e-virus.pdf?sfvrsn=9f532d0e_2 .
- 198.Kamp C, Blümel J, Baylis SA, Bekeredjian-Ding I, Chudy M, Heiden M, Henseler O, Keller-Stanislawski B, de Vos AS, Funk MB. Impact of hepatitis E virus testing on the safety of blood components in Germany - results of a simulation study. Vox Sang. 2018;113:811–813. doi: 10.1111/vox.12719. [DOI] [PubMed] [Google Scholar]
- 199.Bi H, Yang R, Wu C, Xia J. Hepatitis E virus and blood transfusion safety. Epidemiol Infect. 2020;148:e158. doi: 10.1017/S0950268820001429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Pischke S, Peron JM, von Wulffen M, von Felden J, Höner Zu Siederdissen C, Fournier S, Lütgehetmann M, Iking-Konert C, Bettinger D, Par G, Thimme R, Cantagrel A, Lohse AW, Wedemeyer H, de Man R, Mallet V. Chronic Hepatitis E in Rheumatology and Internal Medicine Patients: A Retrospective Multicenter European Cohort Study. Viruses. 2019;11 doi: 10.3390/v11020186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Bauer H, Luxembourger C, Gottenberg JE, Fournier S, Abravanel F, Cantagrel A, Chatelus E, Claudepierre P, Hudry C, Izopet J, Fabre S, Lefevre G, Marguerie L, Martin A, Messer L, Molto A, Pallot-Prades B, Pers YM, Roque-Afonso AM, Roux C, Sordet C, Soubrier M, Veissier C, Wendling D, Péron JM, Sibilia J Club Rhumatismes et Inflammation, a section of the French Society of Rheumatology. Outcome of hepatitis E virus infection in patients with inflammatory arthritides treated with immunosuppressants: a French retrospective multicenter study. Medicine (Baltimore) 2015;94:e675. doi: 10.1097/MD.0000000000000675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Kenfak-Foguena A, Schöni-Affolter F, Bürgisser P, Witteck A, Darling KE, Kovari H, Kaiser L, Evison JM, Elzi L, Gurter-De La Fuente V, Jost J, Moradpour D, Abravanel F, Izpopet J, Cavassini M Data Center of the Swiss HIV Cohort Study, Lausanne, Switzerland. Hepatitis E Virus seroprevalence and chronic infections in patients with HIV, Switzerland. Emerg Infect Dis. 2011;17:1074–1078. doi: 10.3201/eid1706.101067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Buescher G, Ozga AK, Lorenz E, Pischke S, May J, Addo MM, Horvatits T. Hepatitis E seroprevalence and viremia rate in immunocompromised patients: a systematic review and meta-analysis. Liver Int. 2021;41:449–455. doi: 10.1111/liv.14695. [DOI] [PubMed] [Google Scholar]
- 204.Navaneethan U, Al Mohajer M, Shata MT. Hepatitis E and pregnancy: understanding the pathogenesis. Liver Int. 2008;28:1190–1199. doi: 10.1111/j.1478-3231.2008.01840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Anty R, Ollier L, Péron JM, Nicand E, Cannavo I, Bongain A, Giordanengo V, Tran A. First case report of an acute genotype 3 hepatitis E infected pregnant woman living in South-Eastern France. J Clin Virol. 2012;54:76–78. doi: 10.1016/j.jcv.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 206.Tabatabai J, Wenzel JJ, Soboletzki M, Flux C, Navid MH, Schnitzler P. First case report of an acute hepatitis E subgenotype 3c infection during pregnancy in Germany. J Clin Virol. 2014;61:170–172. doi: 10.1016/j.jcv.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 207.Bouthry E, Benachi A, Vivanti AJ, Letamendia E, Vauloup-Fellous C, Roque-Afonso AM. Autochthonous Hepatitis E during Pregnancy, France. Emerg Infect Dis. 2018;24:1586–1587. doi: 10.3201/eid2408.180105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Jilani N, Das BC, Husain SA, Baweja UK, Chattopadhya D, Gupta RK, Sardana S, Kar P. Hepatitis E virus infection and fulminant hepatic failure during pregnancy. J Gastroenterol Hepatol. 2007;22:676–682. doi: 10.1111/j.1440-1746.2007.04913.x. [DOI] [PubMed] [Google Scholar]
- 209.Kar P, Jilani N, Husain SA, Pasha ST, Anand R, Rai A, Das BC. Does hepatitis E viral load and genotypes influence the final outcome of acute liver failure during pregnancy? Am J Gastroenterol. 2008;103:2495–2501. doi: 10.1111/j.1572-0241.2008.02032.x. [DOI] [PubMed] [Google Scholar]
- 210.Kar P, Sengupta A. A guide to the management of hepatitis E infection during pregnancy. Expert Rev Gastroenterol Hepatol. 2019;13:205–211. doi: 10.1080/17474124.2019.1568869. [DOI] [PubMed] [Google Scholar]
- 211.Horvatits T, Westhölter D, Peine S, Schulze Zur Wiesch J, Lohse AW, Lütgehetmann M, Pischke S. Lack of evidence for human serum albumin as major source of HEV infections. Transfus Med. 2018;28:470–471. doi: 10.1111/tme.12536. [DOI] [PubMed] [Google Scholar]
- 212.Juhl D, Nowak-Göttl U, Blümel J, Görg S, Hennig H. Lack of evidence for the transmission of hepatitis E virus by coagulation factor concentrates based on seroprevalence data. Transfus Med. 2018;28:427–432. doi: 10.1111/tme.12498. [DOI] [PubMed] [Google Scholar]
- 213.de Vos AS, Janssen MP, Zaaijer HL, Hogema BM. Cost-effectiveness of the screening of blood donations for hepatitis E virus in the Netherlands. Transfusion. 2017;57:258–266. doi: 10.1111/trf.13978. [DOI] [PubMed] [Google Scholar]
- 214.Gallian P, Lhomme S, Morel P, Gross S, Mantovani C, Hauser L, Tinard X, Pouchol E, Djoudi R, Assal A, Abravanel F, Izopet J, Tiberghien P. Risk for Hepatitis E Virus Transmission by Solvent/Detergent-Treated Plasma. Emerg Infect Dis. 2020;26:2881–2886. doi: 10.3201/eid2612.191482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Hauser L, Roque-Afonso AM, Beylouné A, Simonet M, Deau Fischer B, Burin des Roziers N, Mallet V, Tiberghien P, Bierling P. Hepatitis E transmission by transfusion of Intercept blood system-treated plasma. Blood. 2014;123:796–797. doi: 10.1182/blood-2013-09-524348. [DOI] [PubMed] [Google Scholar]
- 216.Farcet MR, Lackner C, Antoine G, Rabel PO, Wieser A, Flicker A, Unger U, Modrof J, Kreil TR. Hepatitis E virus and the safety of plasma products: investigations into the reduction capacity of manufacturing processes. Transfusion. 2016;56:383–391. doi: 10.1111/trf.13343. [DOI] [PubMed] [Google Scholar]
- 217.Kapsch AM, Farcet MR, Wieser A, Ahmad MQ, Miyabayashi T, Baylis SA, Blümel J, Kreil TR. Antibody-enhanced hepatitis E virus nanofiltration during the manufacture of human immunoglobulin. Transfusion. 2020;60:2500–2507. doi: 10.1111/trf.16014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Praditya D, Friesland M, Gravemann U, Handke W, Todt D, Behrendt P, Müller TH, Steinmann E, Seltsam A. Hepatitis E virus is effectively inactivated in platelet concentrates by ultraviolet C light. Vox Sang. 2020;115:555–561. doi: 10.1111/vox.12936. [DOI] [PubMed] [Google Scholar]