Abstract
Endoscopic submucosal dissection (ESD) has been developed as a treatment for superficial gastrointestinal neoplasms, which can achieve en bloc resection regardless of the lesion size. However, ESD is technically difficult because endoscopists cannot bring their hand into the gastrointestinal tract, unlike surgeons in regular surgery. It is difficult to obtain sufficient tension in the dissection plane and a good field of vision. Therefore, ESD is associated with a long procedure time and a high risk of adverse events in comparison with endoscopic mucosal resection. Traction methods have been developed to provide sufficient tension for the dissection plane and a good field of vision during the ESD procedure. However, traction direction is limited in most traction methods, resulting in insufficient effect in some cases. Although traction direction is considered important, there have been few investigations of its effect. In the first half of this review, important traction methods are discussed, including traction direction. In second half, appropriate traction methods for each organ are considered. Other important considerations for traction method, such as ability to adjust traction strength, interference between traction device and endoscope, and the need for specialized devices are also discussed.
Keywords: Endoscopic submucosal dissection, Traction method, Countertraction, Traction direction, Vertical traction
Core Tip: Endoscopic submucosal dissection is associated with a long procedure time and adverse events (e.g., perforation) due to technical difficulty—the absence of tension for the dissection plane and poor field of vision. Traction methods allow efficient dissection and a good field of vision. Although many traction methods have been developed, traction direction is limited in most. Each traction method has advantages and disadvantages. It is important to select an appropriate traction method to obtain proper traction direction, depending on lesion location. We discuss the characteristics of different traction methods and their effects depending on traction direction.
INTRODUCTION
Endoscopic submucosal dissection (ESD) allows en bloc resection, regardless of lesion size, where endoscopic mucosal resection (EMR) is considered impossible, enabling accurate pathological assessment and a lower recurrence[1]. However, ESD is still a challenging therapy due to technical difficulty, which results in long procedure time and high perforation rate[2]. Surgeons may use the nondominant hand to provide traction for the lesion while they dissect using the dominant hand. By contrast, endoscopists cannot use their nondominant hand to provide traction for the lesion during ESD because they cannot put their hand into the gastrointestinal tract—it is like cutting a steak using only a knife, with no fork. It is important to obtain traction during ESD because this enables two important effects: creating a visual field by turning over the mucosal flap, and facilitating dissection by providing tension for the dissection plane. A basic hood attached to the endoscope can be used to obtain traction. However, this is occasionally insufficient.
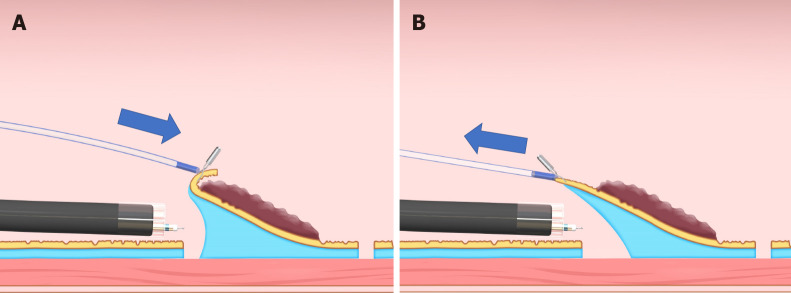
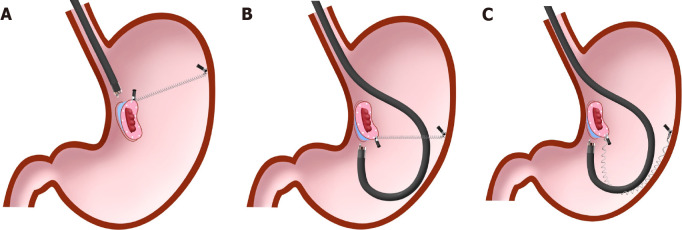
The clip-with-line method (Figure 1), which may be the first traction method ever used, was reported in 2002[3,4]. Many other methods have since been developed. Although the clip-with-line method is simple and low cost, its traction direction is limited to the direction in which the line is pulled. In a multicenter prospective randomized controlled trial comparing the conventional and the clip-with-line methods, the clip-with-line method did not demonstrate a reduction in procedure time for gastric ESD[5] but did for esophageal ESD[6]. These results suggest the efficacy of the traction method is different depending on traction direction, because traction direction in the clip-with-line method is limited to the direction toward the endoscope in esophageal ESD, while it changes depending on the lesion location in gastric ESD.
Figure 1.
Clip-with-line method. This method provides traction for the lesion by pulling the line. The traction direction is limited to the direction in which the line is pulled.
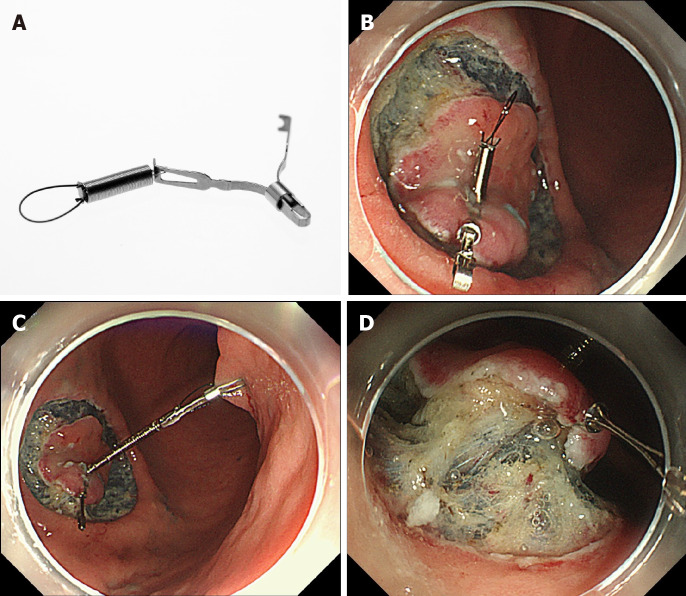
Unlike the clip-with-line method, several other traction methods can provide traction in any direction. These include the internal traction method, which uses an spring-and-loop with clip (S–O clip; Zeon Medical, Tokyo, Japan)[7-10] (Figure 2). We reported a single-center prospective randomized controlled trial comparing the conventional and the S–O clip-assisted methods in gastric ESD, which demonstrated that the S–O clip-assisted method reduced the median gastric ESD procedure time (29.1 min vs 52.6 min; P = 0.005)[11]. In this study, a direction vertical to the gastric wall was selected for the S–O clip-assisted method, using its multidirectional traction function.
Figure 2.
Internal traction method using the spring-and-loop with clip (Zeon Medical, Tokyo, Japan). A: The spring-and-loop with clip (S–O clip) has a 5-mm long spring and a 4-mm long loop at one side of the clip claws; B: The S–O clip is attached to the lesion; C: The regular clip anchors the loop of the S–O clip on the opposite side of the lesion; D: The extension of the spring provides traction on the lesion. Citation: Mitsuru Nagata. Internal traction method using a spring-and-loop with clip (S–O clip) allows countertraction in gastric endoscopic submucosal dissection. Surg Endosc 2020; 34(8): 3722–3733. Copyright © 2020 Mitsuru Nagata[10].
These outcomes suggest that traction direction is the important factor for traction-assisted ESD. However, little study has been done to explore the influence of traction direction during the procedure. Each traction method has characteristics other than traction direction, and it is necessary to understand the characteristics in order to use the methods effectively. The purpose of this article is to review the characteristic of traction methods. Then follows a discussion of appropriate traction methods for each organ, based on the results of clinical trials.
BASICS OF TRACTION
Definition of traction
In published literature of ESD, the terms traction and countertraction are used interchangeably; the unclear distinction results in a potential for confusion[12]. In this article, we do not use the term countertraction. We define traction as force acting on the target lesion.
Classification of traction direction
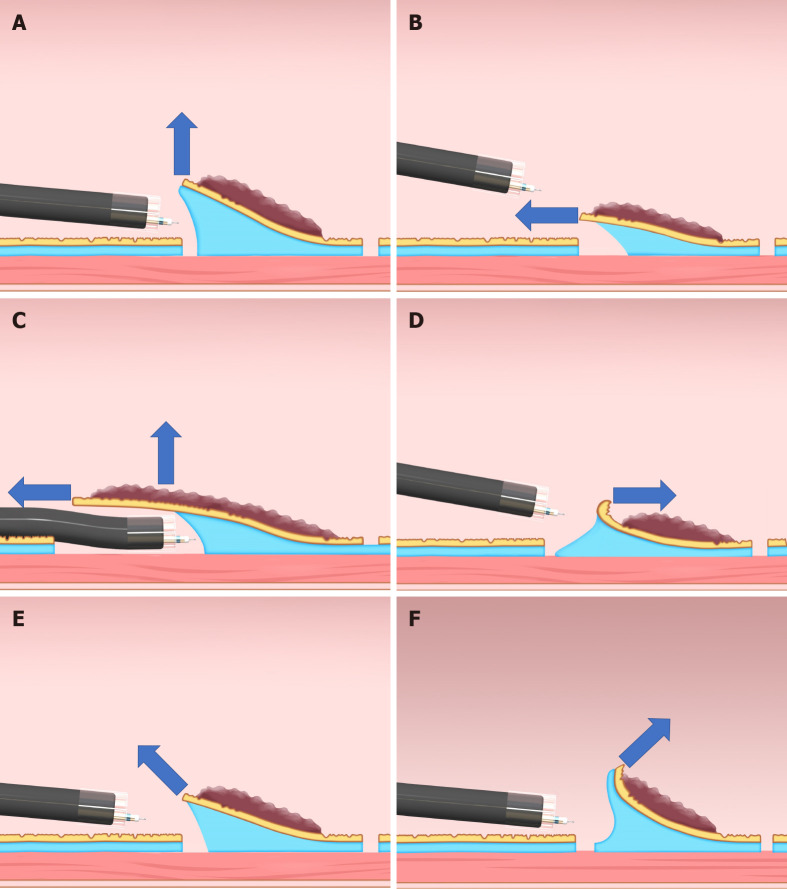
As a force, traction can be represented by a vector, characterized by size and direction. Traction direction can be divided into the following five categories, according to the relationship with the endoscope tip and the gastrointestinal wall: Proximal, diagonally proximal, vertical, diagonally distal, and distal (Figure 3). Of these five categories, vertical traction may be appropriate in any situation because it provides two important effects: Enabling visualization of the submucosa, by turning over the mucosa; and facilitating submucosal dissection by providing tension to submucosa (Figure 3A).
Figure 3.
Classification of the traction direction. A: Vertical traction; B: Proximal traction; C: Proximal traction combined with hood traction; D: Distal traction; E: Diagonally proximal traction; F: Diagonally distal traction.
Proximal traction can provide sufficient tension to the submucosa. However, the mucosal flap falls toward the endoscope (Figure 3B). If the endoscope tip is not parallel to the gastrointestinal wall, it can be difficult to approach the submucosa, even with proximal traction. If the endoscope tip is parallel to the gastrointestinal wall, it is easy to approach the submucosa, even if the mucosal flap falls down. Moreover, once the endoscope tip gets under the mucosal flap, proximal traction is combined with hood traction, resulting in diagonally proximal or vertical traction (Figure 3C). Proximal traction is suitable for situations where the endoscope tip can be placed parallel to the gastrointestinal wall, for example, in esophageal ESD.
Distal traction can cause the submucosal dissection plane to fall distally as submucosal dissection advances, resulting in submucosal thinning and subsequently, cutting the muscle layer or mucosa because of misrecognition of the layer (Figure 3D). Moreover, distal traction may decrease the effectiveness of the tension for the submucosal dissection plane, leading to inefficient dissection. Hence, distal traction may be the least useful approach for submucosal dissection in most cases.
Diagonally proximal traction (Figure 3E) and diagonally distal traction (Figure 3F) can be decomposed into horizontal and vertical vectors. The larger the horizontal component, the closer to the proximal or distal traction. The larger the vertical component, the closer to vertical traction.
MODALITY OF TRACTION
Traction can be roughly classified into hood traction, natural traction, and device-assisted traction. Natural traction and device-assisted traction can be further subdivided.
Hood traction
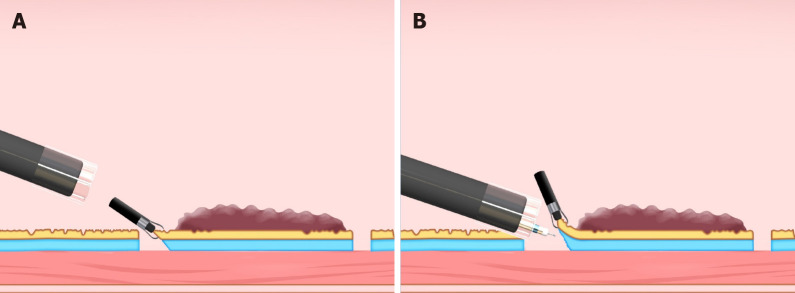
A hood attached to the endoscope tip is used in ESD procedures primarily to secure the visual field. The hood also can be used to obtain traction; it can turn the mucosal flap and provide tension for the submucosa after the endoscope tip is inserted under the mucosa. The straight hood has a wide field of view but is sometimes difficult to get under the mucosa. A hood with a tapered tip may be effective in such a situation (Figure 4)[13,14]. However, in a situation where it is difficult to keep the endoscope tip under the mucosa, such as in severe submucosal fibrosis, substantial lesion movement (due to patient respiration), and vertical confrontation with the lesion, the hood alone is not effective.
Figure 4.
Small-caliber tip transparent hood (Fujifilm, Tokyo, Japan). A: DH-33GR [small-caliber tip transparent (ST) hood] with 7-mm tip opening diameter and 7-mm tip protruding length; B: DH-28GR (short ST hood) with 8-mm tip opening diameter and 7-mm tip protruding length.
Natural traction
Natural traction is defined as traction using natural power, such as gravity, mucosal tension, buoyancy, and water pressure. The advantage of natural traction is that it is easy to switch to other methods and there is no need for any special device.
Gravity: When the lesion is gravitationally upward, gravity keeps the mucosal flap open and provides tension for the submucosa (Figure 5). Changing the patient’s posture to raise the lesion against gravity is the basic strategy for ESD. However, in esophageal, gastric, and duodenal ESD, the patient’s posture is primarily the left lateral decubitus position, which is difficult to change. By contrast, it is easy to change the patient’s posture in colorectal ESD (e.g., left lateral, supine, right lateral, and prone positions). However, changing the patient’s posture sometimes makes the ESD procedure complicated, for example, through poor maneuverability of the endoscope, a vertical approach to the lesion, and difficulty opening the lumen.
Figure 5.

Gravity provides favorable traction when the lesion is located at the upper side of the gravitational force.
Mucosal tension, pocket creation method, and endoscopic submucosal tunnel dissection: When the mucosa around the lesion is incised circumferentially, the lesion loses tension from the surrounding mucosa and submucosa, making it difficult to get the endoscope tip under the mucosal flap. By leaving a part of the mucosa around the lesion, the remaining mucosa gives tension to the lesion. In conventional ESD, traction at the lesion can be maintained by using mucosal tension, as follows. A C-shaped or inverted C-shaped mucosal incision is made. Next, the submucosa under the lesion is dissected, while the remaining mucosa gives tension at the dissection plane. Finally, a circumferential mucosal incision is made and the remaining submucosa dissected.
The pocket creation method (PCM) and endoscopic submucosal tunnel dissection (ESTD) use the same principle, using mucosal tension for traction[15-19]. In PCM (Figure 6), an initial mucosal incision on the proximal side of the lesion is first performed, to make entry to the submucosa. Then, the submucosa under the lesion is dissected, followed by creation of a submucosal space. Finally, the mucosa and submucosa around the submucosal space is dissected to achieve en bloc resection. In ESTD, a mucosal incision on the distal side of the lesion is performed before creation of a submucosal space is completed, unlike the PCM procedure.
Figure 6.
Procedure for the pocket creation method of endoscopic submucosal dissection. A: A minimal initial mucosal incision (approximately 20-mm in width) is made for entry into the submucosa; B: Submucosal dissection is performed, followed by creation of a submucosal pocket under the lesion; C: Additional mucosal incision of the gravitational lower side of the submucosal pocket; D: Opening around the submucosal pocket, then, en bloc resection is achieved.
PCM and ESTD procedures have similar advantages, as follows. The endoscope inside the submucosal space provides tension for the dissection plane. The endoscope tip can take a parallel approach to the muscle layer. The submucosal space holds the endoscope, which achieves stabilization of the endoscope. Thus, PCM and ESTD may be particularly suitable for lesions that are located where maneuverability of the endoscope is poor. Moreover, minimal mucosal incision until completion of submucosal dissection may prevent leakage of the injected solution.
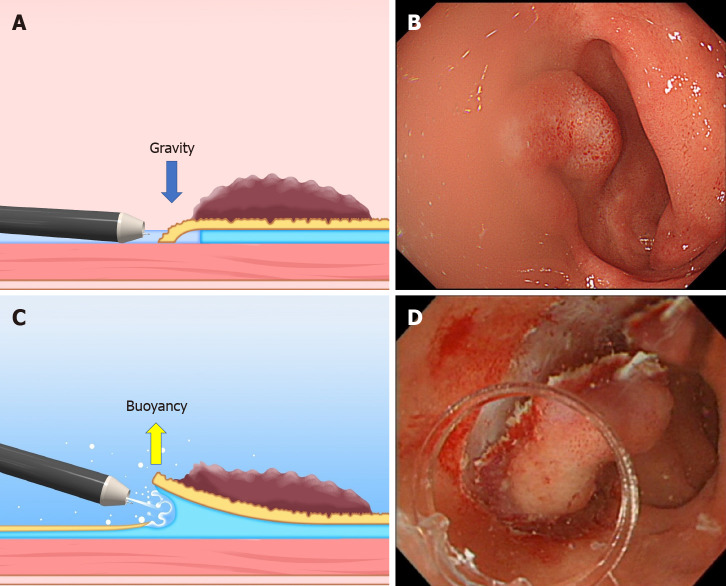
Buoyancy and water pressure: We have reported the usefulness of underwater ESD for buoyancy, easier use of water pressure from an endoscope that has a water supply function, clear visual field, and heat sink effect[20,21]. Buoyancy and water pressure can provide a traction function, where buoyancy acts opposite to gravitational pull.
In conventional ESD, when the lesion is located at the gravitationally lower side, the opening of the mucosal flap is obstructed by gravity. Moreover, the lesion is half-way submerged due to gravity, while the boundary between gas and liquid obstructs the visual field (Figure 7A and B). By switching from conventional ESD to underwater ESD, buoyancy aids opening of the mucosal flap and provides tension for the submucosa, while the visual field is unaffected by a gas-liquid boundary (Figure 7C and D). Water pressure from the endoscope (using its water supply function) also assists opening the mucosal flap. Whereas water pressure can temporarily deteriorate the visual field, due to splashing under gas insufflated conditions, it can be used without splashing in underwater conditions, allowing seamless submucosal dissection.
Figure 7.
The difference between the conventional endoscopic submucosal dissection and the underwater endoscopic submucosal dissection. A and B: The conventional endoscopic submucosal dissection for the lesion is located at the gravitational lower side. Gravity obstructs the opening of the mucosal flap. Incomplete submersion deteriorates the visual field; C and D: The underwater condition aids the opening of the mucosal flap by buoyancy. Water pressure from the endoscope (using its water supply function) also assists in opening the mucosal flap. Complete submersion improves the visual field. Citation: Mitsuru Nagata. Underwater endoscopic submucosal dissection in saline solution using a bent-type knife for duodenal tumor. VideoGIE 2018; 3(12): 375–377. Copyright © 2018 American Society for Gastrointestinal Endoscopy. Published by Elsevier Inc[21].
Saline solution is preferable for underwater ESD due to saline solution’s higher specific gravity, compared with water, which provides a greater flotation effect.
Device-assisted traction method
A traction method using a device other than a hood can be defined as device-assisted. Device-assisted traction method implies traction in the narrow sense. They are broadly classified into external, internal, and other methods; each of these is further sub-classified (Table 1).
Table 1.
Classification of device-assisted traction method
|
|
Traction direction
|
Control of traction force
|
Withdrawal of the endoscope
|
Recommended lesion location
|
| External traction methods | ||||
| Clip-with-line method | One | Strengthen | Required | Esophagus, Greater curvature of the upper and middle third of the stomach, Colorectum |
| Pulley method | Any | Strengthen | Required | unclear because of fewer reports |
| Sheath traction method | ||||
| Clip-and-snare method | Two | Strengthen and weaken | Required | Stomach, Rectum |
| Endo Trac | Two | Strengthen and weaken | Required | Stomach, Rectum |
| External forceps method | Two | Strengthen and weaken | Required | Esophagus; Stomach except for cardia, lesser curvature or posterior wall of the upper gastric body; Rectum |
| Double scope method | Any | Strengthen and weaken | None | Stomach |
| Magnetic anchor method | Any | Strengthen and weaken | Required | Stomach, Colorectum |
| Internal traction method | ||||
| S–O clip | Any | Strengthen and weaken | None | Stomach, Colorectum |
| Ring thread | Any | Strengthen and weaken | None | Colorectum |
| Multiloop | Any | Strengthen and weaken | None | Colorectum |
| Double clip and rubber band | Any | Strengthen and weaken | None | Colorectum |
| Others |
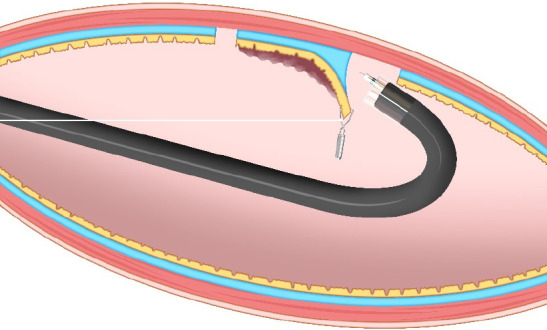
External traction methods: External traction can be defined as a method where the traction device acts from outside the body. Representative external traction method includes the clip-with-line method, pulley method, sheath traction method, external forceps method, double scope method, and magnetic anchor method.
Clip-with-line method: The clip-with-line method was reported by Oyama et al[3,4] in 2002 (Figure 1). It is performed as follows. After circumferential mucosal incision, the endoscope is withdrawn. The clip applicator is deployed into the accessory channel of the endoscope. The clip-with-line (a clip with a line tied to its arm) is attached to the clip applicator. The endoscope is inserted, then the clip-with-line is attached to the edge of the lesion. Using this procedure, the line comes out of the body without passing through the accessory channel of the endoscope, while pulling the line provides traction at the lesion.
The advantages of this method are its simplicity, low cost, and no requirement for a special device. The disadvantage is that traction direction is limited to the direction in which the line is pulled; therefore, submucosal dissection may be difficult, depending on traction direction. Although increasing traction force is possible, by pulling the line, it is difficult to weaken traction force. Moreover, friction between the endoscope and the line in the narrow space generates interference, which sometimes causes strong traction resulting in slip-off of the clip. In fact, the slip-off rate is reported to be 16.4% in esophageal ESD[6] and 13.2% in gastric ESD[5].
Pulley method: The pulley method is a modified clip-with-line method. By anchoring the line to the gastrointestinal wall, the direction of traction can be controlled in any direction (Figure 8). The pulley method can be classified into two types according to the pulley system used: Clip pulley[22] or suture pulley[23,24]. There are only a few reports on the pulley method, and its effectiveness needs to be verified.
Figure 8.
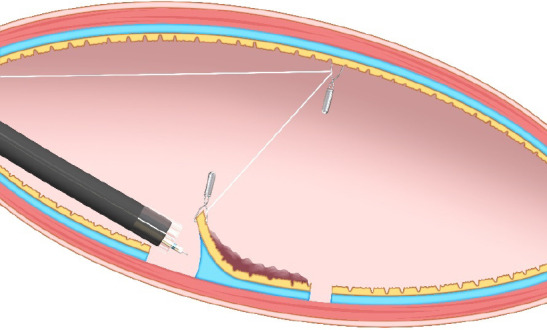
Pulley method. This method is the modified clip-with-line method, which can provide traction in any direction depending on the pulley site.
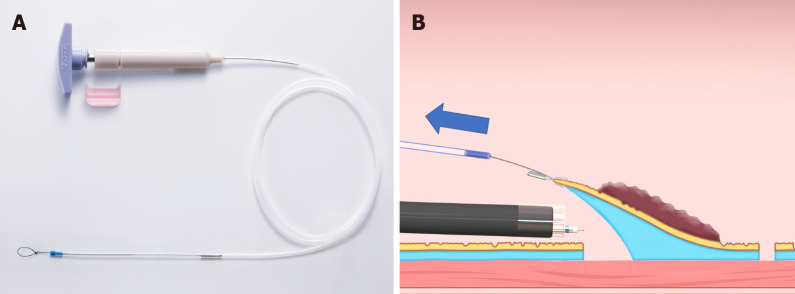
Sheath traction method: In the sheath traction method, the line part of the clip-with-line method is replaced with a sheath. Since the sheath is harder than the line, it can provide not only pulling force but also pushing force to the lesion, thus allowing two traction directions (Figure 9). Sheath traction method includes the clip-and-snare method[25-29] and the Endo Trac[30,31] (TOP, Tokyo, Japan) (Figure 10A).
Figure 9.
Sheath traction method. This method has two traction directions by pushing or pulling the sheath. A: Pushing the sheath; B: Pulling the sheath.
Figure 10.
Endo Trac (TOP, Tokyo, Japan). A: This device can be used for the sheath traction method; B: This device has a structure that can release the sheath from the lesion to avoid interference between the sheath and the endoscope tip.
The clip-and-snare method requires only a polypectomy snare and a clip; therefore, this method may be performed anywhere. In the conventional clip-and-snare method, the snare is grasped with forceps and delivered to the lesion[25]. However, this procedure is sometimes difficult. The prelooping technique was developed to improve the delivery of the snare[26-29]. The prelooping technique for the clip-and-snare method is performed as follows. After circumferential mucosal incision, the endoscope is withdrawn to preloop the snare on the tip of the endoscope. Then, the endoscope is inserted to the lesion along with the snare sheath. The clip is attached to the edge of the lesion. Then, the snare is loosened so that it can slide over the clip applicator toward the clip. Finally, the snare holds the clip, and the clip applicator is withdrawn.
Endo Trac is a product developed for the sheath traction method. Whereas interference between the sheath and the tip of the endoscope sometimes makes access to the submucosa difficult when the clip-and-snare method is used, the Endo Trac has a structure that can release the sheath from the lesion, to avoid interference between the sheath and the endoscope tip (Figure 10B).
The clip-and-snare method and the Endo Trac require withdrawal and reinsertion of the endoscope to set the traction system. Therefore, these methods are not suitable for colonic lesions, in which insertion of the endoscope is difficult and time-consuming. Moreover, interference between the sheath and the endoscope, due to friction, is possibly greater than with the clip-with-line method, due to the sheath being thicker than the line. In fact, it has been reported that even with a thin sheath (with a maximum diameter of 1.8 mm), interference with the endoscope can occur to some extent; the operator needs to move the endoscope carefully to avoid detachment of the snare from the clipped lesion[29].
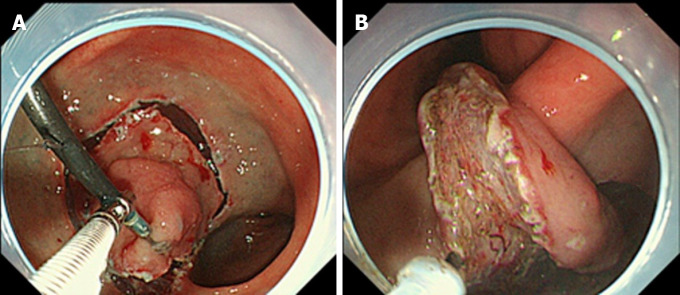
External forceps method: The external forceps method is performed as follows[32-36]. After circumferential mucosal incision, the endoscope is withdrawn. The endoscope is reinserted with external forceps that are grasped by second forceps inserted through the accessory channel of the endoscope. External forceps grasp the edge of the lesion (Figure 11A) while the second forceps are withdrawn. The external forceps provide traction to two directions by pulling or pushing the lesion (Figure 11B). This method allows changing the traction point, by releasing and re-grasping the lesion.
Figure 11.
Endoscopic submucosal dissection using external forceps. A: External grasping forceps was anchored at the distal margin of the lesion in the lesser curvature of the antrum under the control of the endoscope and a second grasping forceps; B: With gentle oral traction applied with the external grasping forceps, the submucosal layer was dissected in retroversion from the aboral side. Citation: Imaeda H, Hosoe N, Kashiwagi K, Ohmori T, Yahagi N, Kanai T, Ogata H. Advanced endoscopic submucosal dissection with traction. World J Gastrointest Endosc 2014; 6(7): 286-295. Copyright © 2014 Baishideng Publishing Group Inc. Published by Baishideng Publishing Group Inc[35].
However, this method has some disadvantages. It is difficult to deliver the external forceps, depending on lesion location, such as the cardia, lesser curvature or posterior wall of the upper gastric body, duodenum, and colon. Interference between the endoscope and the external forceps may be relatively strong compared with that of the clip-with-line and the sheath traction methods, because the forceps is thicker than the line and the sheath. Great care should be taken regarding potential damage to mucosa grasped by the external forceps, because of the strong traction.
Double scope method: The double scope method is performed by two experienced endoscopists with main and second endoscopes[37,38]. The second endoscope is inserted alongside the main endoscope. Then, the second endoscope deploys the forceps, through the accessory channel, and grasps the lesion to provide traction. A thin endoscope is recommended for the second endoscope, to avoid interference with the main endoscope.
This method has the great advantage that traction direction can be easily controlled by the second endoscope. Although the indication may be limited, as this method requires two experienced endoscopists and two endoscope systems, it may be a useful option for difficult cases, such as gastric cancers with ulcer scar[39]. This method has been reported to be useful in the treatment of superficial pharyngeal cancers[40] and gastric submucosal tumors[41].
Magnetic anchor method: The magnetic anchor method, as initially reported, used a large external electromagnet to provide traction, by moving an internal magnet attached to the lesion[42,43]. However, it was necessary to miniaturize the external electromagnet in clinical practice. Recently, use of neodymium rare earth magnets has allowed the external electromagnet to be minimized[44,45]; the feasibility of this method in clinical practice has been demonstrated in a prospective trial[46]. Although this method requires a special magnetic device and is not yet widespread, it is a promising method for the future due to the great advantage that it can provide traction in any direction and control traction force. It should be noted that patients with a cardiac pacemaker or implantable cardioverter-defibrillator are not indicated for this method.
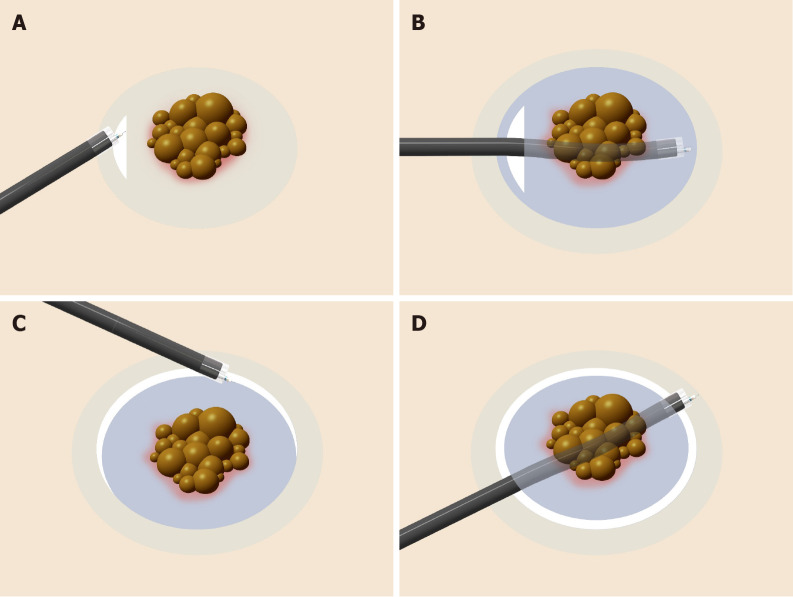
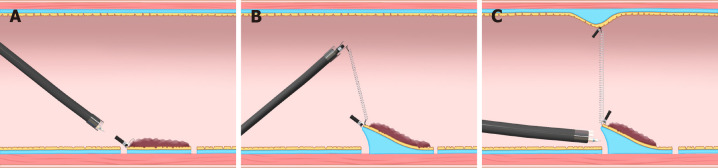
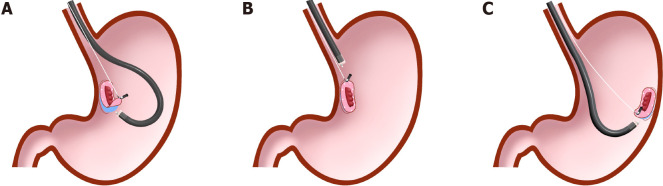
Internal traction methods: An internal traction method can be defined as a method in which the traction device acts only inside the gastrointestinal tract. Devices for internal traction include the S–O clip[7,8] (Zeon Medical, Tokyo, Japan) (Figure 2), ring thread[47], multiloop[48], double clip and rubber band[49], and clip band[50]. The principle for generating traction is the same in these devices, as follows. First, the clip with the specific mechanism (e.g., spring, thread, and band) for generating the traction is attached to the lesion (Figure 12A). Second, the regular clip anchors the tip of the mechanism to the gastrointestinal wall (Figure 12B). Finally, extension of the mechanism provides traction to the lesion (Figure 12C).
Figure 12.
Internal traction method using the spring-and-loop with clip (Zeon Medical, Tokyo, Japan). A: The spring-and-loop with clip (S–O clip) is attached to the lesion; B: The regular clip anchors the loop part of the S–O clip on the gastrointestinal wall; C: The extension of the spring provides traction on the lesion. The traction direction can be controlled by the anchor site. Citation: Nagata M, Fujikawa T, Munakata H. Comparing a conventional and a spring-and-loop with clip traction method of endoscopic submucosal dissection for superficial gastric neoplasms: a randomized controlled trial (with videos). Gastrointest Endosc 2021; 93(5): 1097-1109. Copyright © 2021 American Society for Gastrointestinal Endoscopy. Published by Elsevier Inc[11].
In these devices, the traction direction can be controlled in any direction by the anchor site. Traction force can also be controlled to some extent by inflating or collapsing the lumen. These devices are useful especially for colorectal ESD, as withdrawal of the endoscope is not required. The disadvantage is that a certain distance between the anchor clip and the clip attached to the lesion is required to generate the traction force. Therefore, these devices are usually utilized for gastric ESD[9-11] or colorectal ESD[47-50].
These devices are designed primarily for use with the forward endoscopic position, because there is a possibility that, in the retroflexed position, the endoscope may stretch the traction device, resulting in laceration of the mucosal flap or slip-off or breakage of the traction device. In gastric ESD, the retroflexed endoscopic position is as common as the forward position, due to the large lumen, unlike colorectal ESD. Therefore, we developed a modified method for attaching the S–O clip, to avoid stretching of the spring by the endoscope[9-11] (Figure 13). Although there are several devices for internal traction, the S–O clip may be the most appropriate in gastric ESD, as the S–O clip has a spring with higher elasticity than a thread or band. The elasticity of the spring can be easily adjusted for a large lumen, preventing laceration of the mucosal flap, slip-off, or breakage of the traction device. The S–O clip is sold only in Japan currently. However, it will be sold in future in Asian countries under the brand name “Countertraction CLIP”.
Figure 13.
Spring-and-loop with clip-assisted gastric endoscopic submucosal dissection. A: Forward endoscopic position; B: Retroflexed endoscopic position; C: The endoscope has the possibility to stretch the spring, resulting in a loss of spring elasticity in the retroflexed endoscopic position. In this situation, the modified attachment method that we described in detail in the previous papers[9-11] is required to avoid this problem. Citation: Nagata M, Fujikawa T, Munakata H. Comparing a conventional and a spring-and-loop with clip traction method of endoscopic submucosal dissection for superficial gastric neoplasms: a randomized controlled trial (with videos). Gastrointest Endosc 2021; 93(5): 1097-1109. Copyright © 2021 American Society for Gastrointestinal Endoscopy. Published by Elsevier Inc[11].
The management of the anchor clip after traction is not standardized. Conventionally, the traction mechanism of the device (e.g., thread, band) is cut to detach the resected specimen, while the anchor clip remains on the gastrointestinal wall. In colorectal ESD, the anchor clip may naturally drop by vermiculation. In contrast, vermiculation of the stomach is poor, except in the pars pylorica; there is a possibility of a permanent residual of the anchor clip after gastric ESD[51]. Therefore, we usually detach the anchor clip with forceps. So far, we have not experienced any adverse events from detaching the anchor clip (e.g., perforation, post-ESD bleeding from the anchor site), probably because of the thicker stomach wall, compared with other organ of the gastrointestinal tract[10,11]. The safety of this management method for the anchor clip should be assessed in many gastric cases.
Others
Pocket creation method with traction device: The combination of the PCM and traction device (TD) for internal traction has been reported to facilitate better mucosal flap formation and opening of the submucosal pocket, compared with conventional PCM[52]. A retrospective study demonstrated that the median dissection speed in PCM with TD was significantly greater than in conventional ESD with TD (16.6 mm2/min vs 12.2 mm2/min; P = 0.003)[53]. Additional studies are needed to confirm whether TD has an additional effect in PCM, by comparing PCM alone against PCM with TD.
Clip flap method: The clip flap method has been reported as using a clip attached to the edge of the lesion to substitute for the mucosal flap until it is made (Figure 14)[54-56]. By using the clip flap method together with clip-based traction (e.g., clip-with-line), the procedure of getting under the mucosal flap can be facilitated, especially when proximal traction makes the mucosal flap fall down (Figure 3B). Although a randomized controlled trial comparing the conventional method and clip flap method in gastric ESD demonstrated that the clip flap method had no advantage in efficacy and safety[57], this method may be effective when it is used along with other clip-based traction methods.
Figure 14.
Clip flap method. A: The clip is attached to the edge of the lesion; B: The clip can be used as a substitute for the mucosal flap.
TRACTION METHODS ASSOCIATED WITH LESION SITE
Esophageal ESD
Representative traction methods that are reported to be effective in esophageal ESD include the clip-with-line method and ESTD. A multicenter randomized controlled trial demonstrated that the median ESD procedure time was significantly shorter with the clip-with-line method (n = 116) than with the conventional method (n = 117) (44.5 min vs 60.5 min; P < 0.001)[6]. Although traction using the clip-with-line method in esophageal ESD is limited to proximal traction, because the forward endoscopic position is predominantly used, due to the narrow cylindrical esophageal lumen, proximal traction may be effective, because the endoscope tip can approach parallel to the esophageal wall and can easily access the submucosa without vertical traction. After getting under the mucosal flap, hood traction and proximal traction using the clip-with-line method are combined, providing diagonally proximal or vertical traction to the submucosa (Figure 3C). Remarkably, the conventional method was changed to the clip-with-line method in six patients (5.2%) because of technical difficulties. Moreover, five patients (4.3%) experienced perforation under the conventional method, whereas one patient (0.9%) could not complete the ESD procedure because of perforation. Conversely, no perforations were observed in the clip-with-line method.
In a multicenter randomized controlled trial, the clip slip-off rate was reported as 16.4%[6]. If clip slip-off occurs, there is a possibility that histopathological evaluation for the margin is made difficult due to damage to the specimen. Moreover, clip slip-off requires reattaching the clip-with-line, which is time-consuming. Interference between the endoscope and the line due to friction causes slip-off. Therefore, once the clip-with-line is attached to the lesion, unnecessary movement and withdrawal of the endoscope should be avoided.
A propensity score matching analysis[58] showed that ESTD had a shorter median ESD procedure time (38.0 min vs 48.0 min; P = 0.006) and lower muscle injury rate (28.9% vs 52.6%; P = 0.036) compared with conventional ESD. Furthermore, a meta-analysis including 17 studies[59] showed that ESTD had significantly higher en bloc resection rate, shorter ESD procedure time, and lower muscle injury rate. In ESTD, the endoscope tip is held by the submucosal tunnel, which allows stabilization of the endoscope and a parallel approach to the muscle layer. The endoscope tip inside the submucosal tunnel pushes up the lesion, providing sufficient tension at the dissection plane. These advantages of the ESTD may provide a shorter ESD procedure time and a lower muscle injury rate.
In conclusion, many promising results have been reported for the clip-with-line method and ESTD. At present, it may be better to select either of these two methods. Most studies on traction method for esophageal ESD have been reported from Asia, mainly targeting squamous cell carcinoma. There are not many reports of traction-assisted ESD for Barrett’s esophageal adenocarcinoma, located around the esophagogastric junction; future studies should focus on this issue.
Gastric ESD
As the stomach lumen is large, both the forward and retroflexed endoscopic positions are common, unlike in esophageal, duodenal, and colonic ESD. Therefore, it is desirable that a traction method for gastric ESD is easy to utilize in both forward and retroflexed endoscopic positions. The popular traction methods for gastric ESD include clip-with-line, internal traction, sheath traction, and ESTD.
The clip-with-line method may be the first traction method for gastric ESD, and was reported in 2002[3,4]. However, a multicenter randomized controlled trial[5] comparing the conventional ESD (n = 316) and the clip-with-line method (n = 319) failed to show a reduction in the mean procedure time for gastric ESD in the total population (conventional ESD, 60.7 min vs clip-with-line method, 58.1 min; P = 0.45). Since traction by the clip-with-line method in gastric ESD is limited to the cardia, the direction of traction varies depending on the lesion location. In the retroflexed endoscopic position, the traction is likely to be distal, especially for lesions located at the lesser couverture side of the gastric body (Figure 15A). Distal traction may cause the submucosal dissection plane to fall distally, making the procedure difficult and prolonging the procedure time in some cases. In the forward endoscopic position, the traction may be proximal or diagonally proximal (Figure 15B). If the endoscope tip cannot be parallel to the gastric wall, proximal traction may cause the mucosal flap to fall proximally, making it difficult to approach the submucosal layer. In contrast, a subgroup analysis based on lesion location demonstrated that the mean ESD procedure time for lesions located at the greater curvature of the upper and middle third of the stomach was significantly shorter in the clip-with-line method (104.1 min vs 57.2 min; P = 0.01). From an anatomical point of view, these results seem reasonable, because it is difficult for the clip-with-line method to provide vertical traction unless the lesion is located at the greater curvature (Figure 15C). In a subgroup analysis based on operator experience, the mean ESD procedure time was not significantly different between the conventional and clip-with-line methods in an expert group (58.0 min vs 58.0 min; P = 1.00). Conversely, the mean ESD procedure time in a trainee group tended to be better in the clip-with-line method (68.9 min vs 58.3 min; P = 0.13). However, the lesions managed by trainees were primarily easy cases; therefore, a simple comparison may be inaccurate. Nonetheless, the analysis result suggests that the benefit from clip-with-line method differs depending on the operator experience.
Figure 15.
Difference in traction direction depending on the lesion location in the clip-with-line method. A: Distal traction; B: Proximal traction; C: Vertical traction.
S–O clip-assisted ESD is classified as internal traction, and can provide traction in any direction. Since the use of the S–O clip in gastric ESD has a potential for the endoscope in the retroflexed position to stretch the spring, we have developed a modified attachment method for the S–O clip, to avoid interference between the endoscope and spring part of the clip[9,10]. We reported a single-center randomized controlled trial comparing conventional (n = 40) and S–O clip-assisted ESD (n = 40), which showed that the median ESD procedure time was significantly shorter in S–O clip-assisted ESD than in conventional ESD (29.1 min vs 52.6 min; P = 0.005)[11]. According to the subgroup analysis comparing the ESD procedure time by lesion location, the median ESD procedure time of S–O clip-assisted ESD was significantly shorter than that of conventional ESD in the upper and middle third of the stomach (39.4 min vs 58.3 min; P = 0.005). In the lower third of the stomach, the two methods were not significantly different (22.8 min vs 36.2 min; P = 0.146). Essentially, the ESD procedure performed in the lower third of the stomach is easier than that performed in the upper and middle third of the stomach[60,61]. The difference in the difficulty of the ESD procedure may explain the difference in the subgroup analysis outcomes. Therefore, the S–O clip-assisted ESD is especially recommended for lesions in the upper and middle third of the stomach. Meanwhile, en bloc resection, R0 resection, perforation, and post-ESD bleeding showed no significant difference between the two groups. The S–O clip slip-off rate was only 2.5%, probably because the modified attachment method prevented interference between the endoscope and spring part of the clip. In this trial, vertical traction was selected for the S–O clip-assisted ESD using its multidirectional traction function. Considering this result and the result of subgroup analysis of the above-mentioned multicenter randomized controlled trial of the clip-with-line method, vertical traction may be the optimal traction direction for gastric ESD. Although other internal traction methods, including the pulley, double scope, and magnetic anchor methods, may be able to provide vertical traction, their feasibility in gastric ESD is unclear and needed to be assessed.
The usefulness of the sheath traction method in gastric ESD has been reported. Unlike the clip-with-line method, the sheath traction method allows traction not only in the pulling direction but also in the pushing direction, so distal traction can be avoided in both forward and retroflexed endoscopic positions. A retrospective study comparing conventional ESD (n = 20) and the clip-and-snare method (n = 20) demonstrated that the clip-and-snare method significantly reduced the median ESD procedure time (38.5 min vs 59.5 min; P = 0.023)[29]. En bloc resection was achieved without perforation in all the patients in both groups. A case series of 21 challenging gastric ESD cases treated using the Endo Trac reported that the ability to change the traction direction in both proximal and distal sides was 100%[31]. Although these results are promising, the stress on the operator due to possible interference between the sheath and the endoscope is concerning. Moreover, these studies have the limitations of being retrospective studies with small numbers of cases. Therefore, evaluation in large-scale studies is warranted.
ESTD was reported to be useful not only for esophageal ESD but also for gastric ESD. A retrospective study evaluating 799 consecutive cases of gastric ESD in single institution showed that resection speed using ESTD was greater than with conventional ESD (19.3 mm2/min vs 17.7 mm2/min; P = 0.009)[62]. Perforation was significantly less frequent in ESTD (0.9% vs 6.0%; P = 0.035). However, the creation of a submucosal tunnel in the stomach may be more difficult compared to the esophagus because the stomach lumen is not straight. It has been reported difficult to form a submucosal tunnel for a lesion located at the pylorus ring or the greater curvature side of the fornix[62]. On the other hand, lesions located at the cardia, the lesser curvature of the gastric corpus, and the greater curvature of the antrum are reported to be suitable for ESTD[63].
In summary, the internal traction method using the S–O clip with modified attachment method has the potential to be the most appropriate traction method for gastric ESD. The clip-with-line method and ESTD may be effective methods in gastric ESD if the lesion location is appropriate for these methods.
Colon and rectal ESD
Colonic ESD is more challenging than esophageal and gastric ESD because the maneuverability of the endoscope is limited, the colorectal lumen is angulated, and the muscle layer is thin and easy to perforate. Withdrawal and reinsertion of the endoscope is time-consuming in colonic ESD, unlike ESD in the upper gastrointestinal tract and rectum. Therefore, for colonic ESD, a traction method that does not require withdrawal and reinsertion of the endoscope is suitable. By contrast, it is easy to utilize most traction methods in rectal ESD.
Internal traction methods are suitable not only for rectal ESD but also colonic ESD because they do not require withdrawal and reinsertion of the endoscope. Recently, several novel devices for internal traction in colorectal ESD have been reported, such as S–O clip[7,8], ring thread[47], multiloop[48], double clip and rubber band[49], and clip band[50]. These devices have the common advantage of controlling traction direction at anchor site. Among them, the S–O clip is made of a highly elastic spring that can be used flexibly, regardless of the lesion location. A prospective randomized controlled trial comparing conventional (n = 27) and S–O clip-assisted ESD (n = 23) demonstrated that the mean ESD procedure time for S–O clip-assisted ESD was significantly shorter than that for conventional ESD (37.4 min vs 67.1 min; P = 0.03)[64]. No significant differences were observed in en bloc resection, perforation, and post-ESD bleeding. Although the conventional ESD was converted into the S–O clip-assisted ESD in eight cases, these cases remained in the conventional ESD group. In most of these conversion cases, the lesions were located in flexural areas where endoscope maneuverability is poor; these areas were the sigmoid colon, hepatic flexure, and splenic flexure. In these areas, reaching under the mucosal flap by the endoscope tip is difficult when only used with hood traction. The S–O clip helps the endoscope tip reach under the mucosal flap, providing proper visualization of the submucosa, despite poor endoscope maneuverability. Traction-assisted ESD using ring thread[47], multiloop[65], or double clip and rubber band[49] also showed promising treatment results in clinical trial, compared with conventional ESD. Further studies should focus on which traction direction is appropriate for colorectal ESD by using multidirectional traction function of internal traction methods.
PCM is another traction method that does not require withdrawal and reinsertion of the endoscope. In this method, the submucosal pocket holds the endoscope, allowing stable endoscope maneuverability. Moreover, the endoscope inside the submucosal pocket pushes up the lesion and provides sufficient tension at the dissection plane. A prospective randomized controlled trial comparing PCM (n = 59) and the conventional method (n = 55), conducted at three Japanese institutions, reported that the rate of ESD completion (defined as completion of colorectal ESD in three hours with en bloc resection using the assigned ESD method without changing to other methods or other devices and without perforation during the procedure) was significantly higher in PCM compared with conventional ESD (93% vs 73%; P = 0.01)[66]. By contrast, the median dissection speed was not significantly different between the two methods (15.9 mm2/min vs 17.4 mm2/min; P = 0.81). This was unforeseen, as several retrospective studies had reported dissection speed significantly greater in PCM than in the conventional method[67,68]. A novel method that combines the PCM and internal traction has been developed[52,53], and it can possibly accelerate the dissection speed. A meta-analysis including five studies (two randomized controlled trials and three retrospective studies) evaluated the efficacy and safety of PCM in comparison with the conventional method for superficial colorectal neoplasms[69]. PCM achieved a higher R0 resection rate (93.5% vs 78.1%; OR, 3.4; 95%: 1.3–8.9; I2 = 58%), a higher en bloc resection rate (99.8% vs 92.8%; OR, 9.9; 95%CI: 2.7–36.2; I2 = 0), a shorter procedure time (min) [mean difference (MD), -11.5; 95%CI: -19.9 to -3.1; I2 = 72%], a faster dissection speed (mm2/min) (MD, 3.6; 95%CI: 2.8–4.5; I2 = 0), and a lower overall adverse event rate (4.4% vs 6.6%; OR, 0.6; 95%CI: 0.3–1.0; I2 = 0) than the conventional method. However, all the included studies were conducted in Japan, with only two randomized controlled trials. Hence, further study is needed, especially regarding dissection speed.
The conventional clip-with-line method requires withdrawal and reinsertion of the endoscope, which may be troublesome during colonic ESD for lesions located where it is difficult to insert the endoscope. Modified preparation techniques for the clip-with-line method have been developed that eliminate withdrawal and reinsertion of the endoscope[70]. A single-center prospective randomized controlled trial comparing the clip-with-line method with modified preparation technique (n = 42) against the conventional method (n = 42) demonstrated that the median colorectal ESD procedure time was significantly shorter in the modified clip-with-line method than in the conventional method (40 min vs 70 min; P < 0.0001)[71]. No significant differences were noted in en bloc resection, R0 resection, perforation, and post-ESD bleeding. In this study, two experts and two intermediates performed the colorectal ESD procedures. When the intermediates encountered difficult situations, the experts took over the procedure. The intermediates’ self-completion rate was significantly higher in the modified clip-with-line method than in the conventional method (100% vs 90%; P = 0.04). Although this modified preparation technique is a little tricky, and the clip-with-line method is not able to control the traction direction, it has the advantage that does not require any special device. Since colorectal ESD is generally performed in the forward endoscopic position, the clip-with-line method may provide diagonally proximal or proximal traction for colorectal ESD. The clip-with-line method may be effective, as long as the endoscope has a parallel approach to colorectal wall.
The sheath traction, clip-and-snare, and Endo Trac methods also requires withdrawal and reinsertion of the endoscope. However, the sheath traction method was reported to be utilized even for colonic lesions[27,31]. A retrospective study reported that the clip-and-snare method (n = 17) significantly reduced mean colorectal ESD procedure time compared with conventional method (n = 123) (45.6 min vs 70.1 min; P = 0.047)[28]. There were no significant differences in en bloc resection, curative resection and adverse events (perforation and post-ESD bleeding). The sheath traction method has the great advantage that it can control traction direction to some extent by pushing or pulling the sheath, which may facilitate the ESD procedure. Moreover, the clip-and-snare method may be useful in any country, because it does not require any special device. Although reinsertion of the endoscope during the sheath traction method is occasionally troublesome, a balloon overtube may help address the issue, simplifying insertion of the endoscope.
The usefulness of underwater techniques during colorectal ESD has been reported[20,72-75]. Underwater conditions provide buoyancy (classified as natural traction) which can help turn over the mucosal flap of a lesion located lower gravitationally (Figure 7C and D). Although colorectal ESD is generally performed with the patient’s posture such that the target lesion is on the upper side of gravity, to open the mucosal flap by gravity, it is difficult to select this posture in some cases due to poor endoscope maneuverability, a vertical approach to the lesion, and difficulty opening the lumen. Water pressure from the endoscope using its water supply function can be used as a traction method at any time. Water pressure can be used even in the conventional method. However, splashing can sometimes obstruct the visual field. In underwater conditions, splashing can be avoidable, which makes it easier to get under the mucosal flap. The underwater condition provides a good field of vision through a zoom effect and the disappearance of halation; this facilitates colorectal ESD in a poor field of vision due to severe submucosal fibrosis or fat tissue (Figure 16). We reported a case series study that demonstrated the feasibility and safety of underwater techniques for colorectal ESD[20]. However, additional studies are needed to evaluate whether underwater techniques improve colorectal ESD procedures compared with conventional methods.
Figure 16.
Comparison of aerial and underwater endoscopic view. A: Severe fibrosis and halation makes the boundary between the submucosal layer and muscle layer unclear; B: Submergence enables a detailed observation through a natural zoom effect and causes halation disappearance, thereby clarifying the boundary between the submucosal layer and muscle layer. Citation: Nagata M. Usefulness of underwater endoscopic submucosal dissection in saline solution with a monopolar knife for colorectal tumors (with videos). Gastrointest Endosc 2018; 87(5): 1345-1353. Copyright © 2018 American Society for Gastrointestinal Endoscopy. Published by Elsevier Inc[20].
In conclusion, PCM and internal traction methods (e.g., S–O clip, ring thread, multiloop, double clip and rubber band, clip band) are especially recommended for colorectal ESD, based on the results of recent studies.
Duodenal ESD
Duodenal ESD is extremely challenging due to the fragile muscle layer, thin submucosal layer, and poor maneuverability of the endoscope, resulting in a high perforation rate which has been reported as 8.8% to 27.0%[76-78]. However, there are few reports of effective methods for reducing the procedural difficulty of duodenal ESD, because superficial duodenal epithelial tumors indicated for ESD are rare.
PCM has been reported to be useful for the treatment of superficial duodenal epithelial tumors. A retrospective study showed that perforation was significantly less frequent in PCM [7% (2/28)] than in the conventional method [29% (5/17; P = 0.046)][79]. PCM demonstrated a faster dissection speed (9.4 mm2/min vs 6.5 mm2/min; P = 0.09) and a higher en bloc resection rate (100% vs 88%; P = 0.07) than the conventional method, although the differences were not statistically significant. PCM may prevent leakage of the injected solution from the submucosa due to minimal mucosal incision until completion of submucosal dissection. Moreover, the submucosal pocket holds the endoscope, providing stable endoscope maneuverability. In the conventional method for duodenal ESD, the mucosa around the lesion is incised widely before the completion of submucosal dissection, while the injected solution in the submucosa may easily flow out and the endoscope operability deteriorates.
Underwater techniques are another option for the treatment of superficial duodenal epithelial tumors[21,80]. Severe submucosal fibrosis in duodenal submucosa occasionally exists and causes insufficient submucosal elevation, even with a large quantity of injection. Although submucosal fibrosis generally makes it difficult to get under the mucosal flap during the first half of duodenal ESD, water pressure from the endoscope using its water supply function helps turn over the mucosal flap and enable the endoscope to get under the mucosal flap. Since the underwater condition eliminates splashing, unlike under gas supply conditions, the endoscope can get under the mucosal flap seamlessly after generating water pressure. This technique is classified as natural traction, and can be used repeatedly at any time.
The underwater condition has several useful effects based on nature, such as a zoom effect, the disappearance of halation, buoyancy, and a heat sink effect. The zoom effect and the disappearance of halation allow recognition of the proper dissection plane despite severe submucosal fibrosis. Buoyancy can be classified as natural traction and aids the opening of the mucosal flap when the lesion is gravitationally lower (Figure 7C and D). The heat sink effect minimizes thermal damage to the muscle layer from the ESD procedure. Thermal damage may increase the risk of delayed perforation after duodenal ESD, which leads to serious complications[81]; therefore, the underwater techniques may be suitable for duodenal ESD.
The underwater techniques may be able to be combined with other traction methods. In fact, a case report showed the usefulness of the underwater techniques with PCM and internal traction during duodenal ESD[82]. Although underwater techniques have major disadvantages, such as visual field loss due to active bleeding, gel immersion endoscopy, which secures the visual field during bleeding, may help address this issue[83]. Underwater techniques have the potential to reduce difficulty in ESD procedures through unique effects not found in conventional methods under gas supply condition. Further study should focus on the efficacy of ESD with underwater techniques, especially in the duodenum.
Several traction methods other than PCM and underwater techniques for duodenal ESD have been reported, such as internal traction using the S–O clip[84] and the sheath traction method[31]. However, there are few reports about these methods, and the efficacy of these methods is still unclear.
In summary, PCM and underwater techniques have the potential to facilitate the duodenal ESD procedure, decreasing the risk of perforation.
CONCLUSION
The purpose of traction during ESD is to create a visual field by turning over the mucosal flap and facilitate dissection by providing tension for the dissection plane. In order to achieve these effects, it is important to understand the advantages and disadvantages of each traction method and to use a traction method that is most appropriate as per the situation. The results of previous studies suggest that traction direction affects the effectiveness of the traction method. Therefore, traction direction should be considered when choosing a traction method. Although there are increasing reports of methods that can control the traction direction, further studies should focus on investigating the optimal traction direction and its influence on the effectiveness of the traction method.
Footnotes
Conflict-of-interest statement: No financial relationships with a commercial entity producing health-care related products and/or services relevant to this article.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: March 18, 2021
First decision: July 3, 2021
Article in press: December 28, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Li XB, Libânio D, Noh CK, Tsou YK S-Editor: Zhang H L-Editor: A P-Editor: Zhang H
References
- 1.Suzuki H, Oda I, Abe S, Sekiguchi M, Mori G, Nonaka S, Yoshinaga S, Saito Y. High rate of 5-year survival among patients with early gastric cancer undergoing curative endoscopic submucosal dissection. Gastric Cancer. 2016;19:198–205. doi: 10.1007/s10120-015-0469-0. [DOI] [PubMed] [Google Scholar]
- 2.Rösch T, Sarbia M, Schumacher B, Deinert K, Frimberger E, Toermer T, Stolte M, Neuhaus H. Attempted endoscopic en bloc resection of mucosal and submucosal tumors using insulated-tip knives: a pilot series. Endoscopy. 2004;36:788–801. doi: 10.1055/s-2004-825838. [DOI] [PubMed] [Google Scholar]
- 3.Oyama T, Kikuchi Y, Shimaya S, Tomori T, Hotta K, Miyata Y, Yamada S. Endoscopic mucosal resection using a hooking knife (hooking EMR) [in Japanese] Stomach Intest. 2002;37:1151–1161. [Google Scholar]
- 4.Oyama T. Counter traction makes endoscopic submucosal dissection easier. Clin Endosc. 2012;45:375–378. doi: 10.5946/ce.2012.45.4.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoshida M, Takizawa K, Suzuki S, Koike Y, Nonaka S, Yamasaki Y, Minagawa T, Sato C, Takeuchi C, Watanabe K, Kanzaki H, Morimoto H, Yano T, Sudo K, Mori K, Gotoda T, Ono H CONNECT-G Study Group. Conventional versus traction-assisted endoscopic submucosal dissection for gastric neoplasms: a multicenter, randomized controlled trial (with video) Gastrointest Endosc. 2018;87:1231–1240. doi: 10.1016/j.gie.2017.11.031. [DOI] [PubMed] [Google Scholar]
- 6.Yoshida M, Takizawa K, Nonaka S, Shichijo S, Suzuki S, Sato C, Komori H, Minagawa T, Oda I, Uedo N, Hirasawa K, Matsumoto K, Sumiyoshi T, Mori K, Gotoda T, Ono H CONNECT-E Study Group. Conventional versus traction-assisted endoscopic submucosal dissection for large esophageal cancers: a multicenter, randomized controlled trial (with video) Gastrointest Endosc. 2020;91:55–65.e2. doi: 10.1016/j.gie.2019.08.014. [DOI] [PubMed] [Google Scholar]
- 7.Sakamoto N, Osada T, Shibuya T, Beppu K, Matsumoto K, Shimada Y, Konno A, Kurosawa A, Nagahara A, Ohkusa T, Ogihara T, Watanabe S. The facilitation of a new traction device (S-O clip) assisting endoscopic submucosal dissection for superficial colorectal neoplasms. Endoscopy. 2008;40 Suppl 2:E94–E95. doi: 10.1055/s-2007-995603. [DOI] [PubMed] [Google Scholar]
- 8.Sakamoto N, Osada T, Shibuya T, Beppu K, Matsumoto K, Mori H, Kawabe M, Nagahara A, Otaka M, Ogihara T, Watanabe S. Endoscopic submucosal dissection of large colorectal tumors by using a novel spring-action S-O clip for traction (with video) Gastrointest Endosc. 2009;69:1370–1374. doi: 10.1016/j.gie.2008.12.245. [DOI] [PubMed] [Google Scholar]
- 9.Nagata M. Modified attachment method using an S-O clip for gastric endoscopic submucosal dissection. VideoGIE. 2019;4:151–153. doi: 10.1016/j.vgie.2018.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagata M. Internal traction method using a spring-and-loop with clip (S-O clip) allows countertraction in gastric endoscopic submucosal dissection. Surg Endosc. 2020;34:3722–3733. doi: 10.1007/s00464-020-07590-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagata M, Fujikawa T, Munakata H. Comparing a conventional and a spring-and-loop with clip traction method of endoscopic submucosal dissection for superficial gastric neoplasms: a randomized controlled trial (with videos) Gastrointest Endosc. 2021;93:1097–1109. doi: 10.1016/j.gie.2020.09.049. [DOI] [PubMed] [Google Scholar]
- 12.Uppal DS, Wang AY. Traction-assisted endoscopic submucosal dissection in the esophagus: Should we all be flossing? Gastrointest Endosc. 2020;91:66–69. doi: 10.1016/j.gie.2019.09.038. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto H, Kawata H, Sunada K, Sasaki A, Nakazawa K, Miyata T, Sekine Y, Yano T, Satoh K, Ido K, Sugano K. Successful en-bloc resection of large superficial tumors in the stomach and colon using sodium hyaluronate and small-caliber-tip transparent hood. Endoscopy. 2003;35:690–694. doi: 10.1055/s-2003-41516. [DOI] [PubMed] [Google Scholar]
- 14.Hayashi Y, Nomura T, Lee RF, Miura Y, Shinozaki S, Sunada K, Yamamoto H. Introducing the next evolution of the small-caliber-tip transparent hood: enhancing the pocket-creation method by building on previous successes. Endoscopy. 2020;52:E297–E299. doi: 10.1055/a-1093-0621. [DOI] [PubMed] [Google Scholar]
- 15.Arantes V, Albuquerque W, Freitas Dias CA, Demas Alvares Cabral MM, Yamamoto H. Standardized endoscopic submucosal tunnel dissection for management of early esophageal tumors (with video) Gastrointest Endosc. 2013;78:946–952. doi: 10.1016/j.gie.2013.05.031. [DOI] [PubMed] [Google Scholar]
- 16.Linghu E, Feng X, Wang X, Meng J, Du H, Wang H. Endoscopic submucosal tunnel dissection for large esophageal neoplastic lesions. Endoscopy. 2013;45:60–62. doi: 10.1055/s-0032-1325965. [DOI] [PubMed] [Google Scholar]
- 17.Choi HS, Chun HJ, Seo MH, Kim ES, Keum B, Seo YS, Jeen YT, Lee HS, Um SH, Kim CD, Ryu HS. Endoscopic submucosal tunnel dissection salvage technique for ulcerative early gastric cancer. World J Gastroenterol. 2014;20:9210–9214. doi: 10.3748/wjg.v20.i27.9210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayashi Y, Sunada K, Takahashi H, Shinhata H, Lefor AT, Tanaka A, Yamamoto H. Pocket-creation method of endoscopic submucosal dissection to achieve en bloc resection of giant colorectal subpedunculated neoplastic lesions. Endoscopy. 2014;46 Suppl 1:E421–E422. doi: 10.1055/s-0034-1377438. [DOI] [PubMed] [Google Scholar]
- 19.Miura Y, Hayashi Y, Lefor AK, Osawa H, Yamamoto H. The pocket-creation method of ESD for gastric neoplasms. Gastrointest Endosc. 2016;83:457–458. doi: 10.1016/j.gie.2015.08.068. [DOI] [PubMed] [Google Scholar]
- 20.Nagata M. Usefulness of underwater endoscopic submucosal dissection in saline solution with a monopolar knife for colorectal tumors (with videos) Gastrointest Endosc. 2018;87:1345–1353. doi: 10.1016/j.gie.2017.11.032. [DOI] [PubMed] [Google Scholar]
- 21.Nagata M. Underwater endoscopic submucosal dissection in saline solution using a bent-type knife for duodenal tumor. VideoGIE. 2018;3:375–377. doi: 10.1016/j.vgie.2018.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li CH, Chen PJ, Chu HC, Huang TY, Shih YL, Chang WK, Hsieh TY. Endoscopic submucosal dissection with the pulley method for early-stage gastric cancer (with video) Gastrointest Endosc. 2011;73:163–167. doi: 10.1016/j.gie.2010.08.041. [DOI] [PubMed] [Google Scholar]
- 23.Aihara H, Kumar N, Ryou M, Abidi W, Ryan MB, Thompson CC. Facilitating endoscopic submucosal dissection: the suture-pulley method significantly improves procedure time and minimizes technical difficulty compared with conventional technique: an ex vivo study (with video) Gastrointest Endosc. 2014;80:495–502. doi: 10.1016/j.gie.2014.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ge PS, Thompson CC, Jirapinyo P, Aihara H. Suture pulley countertraction method reduces procedure time and technical demand of endoscopic submucosal dissection among novice endoscopists learning endoscopic submucosal dissection: a prospective randomized ex vivo study. Gastrointest Endosc. 2019;89:177–184. doi: 10.1016/j.gie.2018.08.032. [DOI] [PubMed] [Google Scholar]
- 25.Yasuda M, Naito Y, Kokura S, Yoshida N, Yoshikawa T. Newly-developed ESD (CSL-ESD) for early gastric cancer using convenient and low-cost lifting method (lifting method using clips and snares) for lesions is clinically useful. Gastrointest Endosc. 2012;75:AB244. [Google Scholar]
- 26.Yoshida N, Doyama H, Ota R, Tsuji K. The clip-and-snare method with a pre-looping technique during gastric endoscopic submucosal dissection. Endoscopy. 2014;46 Suppl 1 UCTN:E611–E612. doi: 10.1055/s-0034-1390752. [DOI] [PubMed] [Google Scholar]
- 27.Ota R, Doyama H, Tsuji K, Yamada S. Deep colonic endoscopic submucosal dissection using a modified clip and snare method incorporating a pre-looping technique. BMJ Case Rep. 2015;2015 doi: 10.1136/bcr-2014-207918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamada S, Doyama H, Ota R, Takeda Y, Tsuji K, Tsuji S, Yoshida N. Impact of the clip and snare method using the prelooping technique for colorectal endoscopic submucosal dissection. Endoscopy. 2016;48:281–285. doi: 10.1055/s-0034-1393241. [DOI] [PubMed] [Google Scholar]
- 29.Yoshida N, Doyama H, Ota R, Takeda Y, Nakanishi H, Tominaga K, Tsuji S, Takemura K. Effectiveness of clip-and-snare method using pre-looping technique for gastric endoscopic submucosal dissection. World J Gastrointest Endosc. 2016;8:451–457. doi: 10.4253/wjge.v8.i12.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanaka S, Toyonaga T, Kaku H, Sakaguchi H, Baba S, Takao T, Kodama Y. A novel traction device (EndoTrac) for use during endoscopic submucosal dissection. Endoscopy. 2019;51:E90–E91. doi: 10.1055/a-0830-4556. [DOI] [PubMed] [Google Scholar]
- 31.Kaku H, Toyonaga T, Tanaka S, Takihara H, Baba S, Tsubouchi E, Ikeda Y, Orita H, Nakamoto M, Horikawa Y, Chiba H, Ban H, Furumoto Y, Morita R, Kodama Y. Endoscopic Submucosal Dissection Using EndoTrac, a Novel Traction Device. Digestion. 2021;102:714–721. doi: 10.1159/000511731. [DOI] [PubMed] [Google Scholar]
- 32.Imaeda H, Iwao Y, Ogata H, Ichikawa H, Mori M, Hosoe N, Masaoka T, Nakashita M, Suzuki H, Inoue N, Aiura K, Nagata H, Kumai K, Hibi T. A new technique for endoscopic submucosal dissection for early gastric cancer using an external grasping forceps. Endoscopy. 2006;38:1007–1010. doi: 10.1055/s-2006-925264. [DOI] [PubMed] [Google Scholar]
- 33.Imaeda H, Hosoe N, Ida Y, Kashiwagi K, Morohoshi Y, Suganuma K, Nagakubo S, Komatsu K, Suzuki H, Saito Y, Aiura K, Ogata H, Iwao Y, Kumai K, Kitagawa Y, Hibi T. Novel technique of endoscopic submucosal dissection using an external grasping forceps for superficial gastric neoplasia. Dig Endosc. 2009;21:122–127. doi: 10.1111/j.1443-1661.2009.00842.x. [DOI] [PubMed] [Google Scholar]
- 34.Imaeda H, Hosoe N, Ida Y, Nakamizo H, Kashiwagi K, Kanai T, Iwao Y, Hibi T, Ogata H. Novel technique of endoscopic submucosal dissection by using external forceps for early rectal cancer (with videos) Gastrointest Endosc. 2012;75:1253–1257. doi: 10.1016/j.gie.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 35.Imaeda H, Hosoe N, Kashiwagi K, Ohmori T, Yahagi N, Kanai T, Ogata H. Advanced endoscopic submucosal dissection with traction. World J Gastrointest Endosc. 2014;6:286–295. doi: 10.4253/wjge.v6.i7.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang F, Leng X, Gao Y, Zhao K, Sun Y, Bian H, Liu H, Liu P. Endoscopic submucosal dissection of distal intestinal tumors using grasping forceps for traction. Tech Coloproctol. 2019;23:1079–1083. doi: 10.1007/s10151-019-02102-x. [DOI] [PubMed] [Google Scholar]
- 37.Uraoka T, Kato J, Ishikawa S, Harada K, Kuriyama M, Takemoto K, Kawahara Y, Saito Y, Okada H. Thin endoscope-assisted endoscopic submucosal dissection for large colorectal tumors (with videos) Gastrointest Endosc. 2007;66:836–839. doi: 10.1016/j.gie.2007.04.028. [DOI] [PubMed] [Google Scholar]
- 38.Uraoka T, Ishikawa S, Kato J, Higashi R, Suzuki H, Kaji E, Kuriyama M, Saito S, Akita M, Hori K, Harada K, Ishiyama S, Shiode J, Kawahara Y, Yamamoto K. Advantages of using thin endoscope-assisted endoscopic submucosal dissection technique for large colorectal tumors. Dig Endosc. 2010;22:186–191. doi: 10.1111/j.1443-1661.2010.00992.x. [DOI] [PubMed] [Google Scholar]
- 39.Higuchi K, Tanabe S, Azuma M, Sasaki T, Katada C, Ishido K, Naruke A, Mikami T, Koizumi W. Double-endoscope endoscopic submucosal dissection for the treatment of early gastric cancer accompanied by an ulcer scar (with video) Gastrointest Endosc. 2013;78:266–273. doi: 10.1016/j.gie.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 40.Yoshio T, Tsuchida T, Ishiyama A, Omae M, Hirasawa T, Yamamoto Y, Fujisaki J, Sato Y, Sasaki T, Kawabata K, Igarashi M. Efficacy of double-scope endoscopic submucosal dissection and long-term outcomes of endoscopic resection for superficial pharyngeal cancer. Dig Endosc. 2017;29:152–159. doi: 10.1111/den.12712. [DOI] [PubMed] [Google Scholar]
- 41.Fujii L, Onkendi EO, Bingener-Casey J, Levy MJ, Gostout CJ. Dual-scope endoscopic deep dissection of proximal gastric tumors (with video) Gastrointest Endosc. 2013;78:365–369. doi: 10.1016/j.gie.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 42.Kobayashi T, Gotohda T, Tamakawa K, Ueda H, Kakizoe T. Magnetic anchor for more effective endoscopic mucosal resection. Jpn J Clin Oncol. 2004;34:118–123. doi: 10.1093/jjco/hyh025. [DOI] [PubMed] [Google Scholar]
- 43.Gotoda T, Oda I, Tamakawa K, Ueda H, Kobayashi T, Kakizoe T. Prospective clinical trial of magnetic-anchor-guided endoscopic submucosal dissection for large early gastric cancer (with videos) Gastrointest Endosc. 2009;69:10–15. doi: 10.1016/j.gie.2008.03.1127. [DOI] [PubMed] [Google Scholar]
- 44.Aihara H, Ryou M, Kumar N, Ryan MB, Thompson CC. A novel magnetic countertraction device for endoscopic submucosal dissection significantly reduces procedure time and minimizes technical difficulty. Endoscopy. 2014;46:422–425. doi: 10.1055/s-0034-1364940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matsuzaki I, Miyahara R, Hirooka Y, Funasaka K, Furukawa K, Ohno E, Nakamura M, Kawashima H, Maeda O, Watanabe O, Ando T, Kobayashi M, Goto H. Simplified magnetic anchor-guided endoscopic submucosal dissection in dogs (with videos) Gastrointest Endosc. 2014;80:712–716. doi: 10.1016/j.gie.2014.05.334. [DOI] [PubMed] [Google Scholar]
- 46.Matsuzaki I, Hattori M, Hirose K, Esaki M, Yoshikawa M, Yokoi T, Kobayashi M, Miyahara R, Hirooka Y, Goto H. Magnetic anchor-guided endoscopic submucosal dissection for gastric lesions (with video) Gastrointest Endosc. 2018;87:1576–1580. doi: 10.1016/j.gie.2018.01.015. [DOI] [PubMed] [Google Scholar]
- 47.Mori H, Kobara H, Nishiyama N, Fujihara S, Matsunaga T, Masaki T. Novel effective and repeatedly available ring-thread counter traction for safer colorectal endoscopic submucosal dissection. Surg Endosc. 2017;31:3040–3047. doi: 10.1007/s00464-016-5326-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sudo G, Tanuma T, Suzuki Y, Nakase H. Multiloop method for traction during colorectal endoscopic submucosal dissection. VideoGIE. 2019;4:11–13. doi: 10.1016/j.vgie.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jacques J, Charissoux A, Bordillon P, Legros R, Rivory J, Hervieu V, Albouys J, Guyot A, Ponchon T, Sautereau D, Kerever S, Pioche M. High proficiency of colonic endoscopic submucosal dissection in Europe thanks to countertraction strategy using a double clip and rubber band. Endosc Int Open. 2019;7:E1166–E1174. doi: 10.1055/a-0965-8531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ge PS, Aihara H. A novel clip-band traction device to facilitate colorectal endoscopic submucosal dissection and defect closure. VideoGIE. 2020;5:180–186. doi: 10.1016/j.vgie.2020.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim SH, Lee JK, Lim YJ, Kim JH. The risk factors for prolonged hemostatic clip retention after endoscopic submucosal dissection for gastric neoplasm. Surg Endosc. 2021 doi: 10.1007/s00464-021-08379-0. [DOI] [PubMed] [Google Scholar]
- 52.Ide D, Saito S, Chino A, Ohya TR. Submucosal pocket creation using a traction device in colorectal endoscopic submucosal dissection. Ann Gastroenterol. 2018;31:380. doi: 10.20524/aog.2018.0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ide D, Ohya TR, Saito S, Mitsuyoshi Y, Hatamori H, Ikenoyama Y, Suzuki K, Ishioka M, Yakabi S, Yasue C, Chino A, Igarashi M, Saruta M, Fujisaki J. Clinical utility of the pocket-creation method with a traction device for colorectal endoscopic submucosal dissection. Surg Endosc. 2021;35:2110–2118. doi: 10.1007/s00464-020-07614-4. [DOI] [PubMed] [Google Scholar]
- 54.Yamamoto K, Hayashi S, Nakabori T, Shibuya M, Ichiba M, Inada M. Endoscopic submucosal dissection using endoclips to assist in mucosal flap formation (novel technique: "clip flap method") Endoscopy. 2012;44 Suppl 2:E334–E335. doi: 10.1055/s-0032-1309860. [DOI] [PubMed] [Google Scholar]
- 55.Yamamoto K, Hayashi S, Nishida T, Saiki H, Naito M, Michida T, Ito T. Effective use of the "clip-flap" method for the endoscopic submucosal dissection of a difficult-to-approach superficial gastric tumor. Endoscopy. 2015;47 Suppl 1:E318–E319. doi: 10.1055/s-0034-1392319. [DOI] [PubMed] [Google Scholar]
- 56.Yamamoto K, Hayashi S, Saiki H, Indo N, Nakabori T, Yamamoto M, Shibuya M, Nishida T, Ichiba M, Inada M. Endoscopic submucosal dissection for large superficial colorectal tumors using the "clip-flap method". Endoscopy. 2015;47:262–265. doi: 10.1055/s-0034-1390739. [DOI] [PubMed] [Google Scholar]
- 57.Ban H, Sugimoto M, Otsuka T, Murata M, Nakata T, Hasegawa H, Inatomi O, Bamba S, Andoh A. Usefulness of the clip-flap method of endoscopic submucosal dissection: A randomized controlled trial. World J Gastroenterol. 2018;24:4077–4085. doi: 10.3748/wjg.v24.i35.4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huang R, Cai H, Zhao X, Lu X, Liu M, Lv W, Liu Z, Wu K, Han Y. Efficacy and safety of endoscopic submucosal tunnel dissection for superficial esophageal squamous cell carcinoma: a propensity score matching analysis. Gastrointest Endosc. 2017;86:831–838. doi: 10.1016/j.gie.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 59.Zhang T, Zhang H, Zhong F, Wang X. Efficacy of endoscopic submucosal tunnel dissection versus endoscopic submucosal dissection for superficial esophageal neoplastic lesions: a systematic review and meta-analysis. Surg Endosc. 2021;35:52–62. doi: 10.1007/s00464-020-07925-6. [DOI] [PubMed] [Google Scholar]
- 60.Imagawa A, Okada H, Kawahara Y, Takenaka R, Kato J, Kawamoto H, Fujiki S, Takata R, Yoshino T, Shiratori Y. Endoscopic submucosal dissection for early gastric cancer: results and degrees of technical difficulty as well as success. Endoscopy. 2006;38:987–990. doi: 10.1055/s-2006-944716. [DOI] [PubMed] [Google Scholar]
- 61.Kim SJ, Choi CW, Nam HS, Kang DH, Kim HW, Park SB, Ryu DG. Factors associated with conversion to snare resection during gastric endoscopic submucosal dissection. Surg Endosc. 2020;34:1585–1591. doi: 10.1007/s00464-019-06918-4. [DOI] [PubMed] [Google Scholar]
- 62.Ojima T, Takifuji K, Nakamura M, Nakamori M, Hayata K, Kitadani J, Yamaue H. Endoscopic submucosal tunnel dissection versus conventional endoscopic submucosal dissection for early gastric cancers: outcomes of 799 consecutive cases in a single institution. Surg Endosc. 2020;34:5625–5631. doi: 10.1007/s00464-020-07849-1. [DOI] [PubMed] [Google Scholar]
- 63.Feng X, Linghu E, Chai N, Lu Z, Wang X, Tang P, Meng J, Du H, Wang H. Endoscopic Submucosal Tunnel Dissection for Large Gastric Neoplastic Lesions: A Case-Matched Controlled Study. Gastroenterol Res Pract. 2018;2018:1419369. doi: 10.1155/2018/1419369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ritsuno H, Sakamoto N, Osada T, Goto SP, Murakami T, Ueyama H, Mori H, Matsumoto K, Beppu K, Shibuya T, Nagahara A, Ogihara T, Watanabe S. Prospective clinical trial of traction device-assisted endoscopic submucosal dissection of large superficial colorectal tumors using the S-O clip. Surg Endosc. 2014;28:3143–3149. doi: 10.1007/s00464-014-3572-0. [DOI] [PubMed] [Google Scholar]
- 65.Suzuki Y, Tanuma T, Nojima M, Sudo G, Murakami Y, Ishii T, Akahonai M, Kobayashi Y, Hamamoto H, Aoki H, Harada T, Katanuma A, Nakase H. Comparison of dissection speed during colorectal ESD between the novel Multiloop (M-loop) traction method and ESD methods without traction. Endosc Int Open. 2020;8:E840–E847. doi: 10.1055/a-1161-8596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yamashina T, Nemoto D, Hayashi Y, Fukuda H, Okada M, Takezawa T, Aizawa M, Sakamoto H, Miura Y, Sunada K, Lefor AK, Togashi K, Yamamoto H. Prospective randomized trial comparing the pocket-creation method and conventional method of colorectal endoscopic submucosal dissection. Gastrointest Endosc. 2020;92:368–379. doi: 10.1016/j.gie.2020.02.034. [DOI] [PubMed] [Google Scholar]
- 67.Sakamoto H, Hayashi Y, Miura Y, Shinozaki S, Takahashi H, Fukuda H, Okada M, Ino Y, Takezawa T, Sunada K, Lefor AK, Yamamoto H. Pocket-creation method facilitates endoscopic submucosal dissection of colorectal laterally spreading tumors, non-granular type. Endosc Int Open. 2017;5:E123–E129. doi: 10.1055/s-0042-122778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Takezawa T, Hayashi Y, Shinozaki S, Sagara Y, Okada M, Kobayashi Y, Sakamoto H, Miura Y, Sunada K, Lefor AK, Yamamoto H. The pocket-creation method facilitates colonic endoscopic submucosal dissection (with video) Gastrointest Endosc. 2019;89:1045–1053. doi: 10.1016/j.gie.2019.01.022. [DOI] [PubMed] [Google Scholar]
- 69.Pei Q, Qiao H, Zhang M, Wang G, Feng H, Pan J, Shi Y. Pocket-creation method versus conventional method of endoscopic submucosal dissection for superficial colorectal neoplasms: a meta-analysis. Gastrointest Endosc. 2021;93:1038–1046.e4. doi: 10.1016/j.gie.2021.01.007. [DOI] [PubMed] [Google Scholar]
- 70.Yamasaki Y, Takeuchi Y, Hanaoka N, Higashino K, Uedo N, Ishihara R, Iishi H. A novel traction method using an endoclip attached to a nylon string during colonic endoscopic submucosal dissection. Endoscopy. 2015;47 Suppl 1:E238–E239. doi: 10.1055/s-0034-1391868. [DOI] [PubMed] [Google Scholar]
- 71.Yamasaki Y, Takeuchi Y, Uedo N, Kanesaka T, Kato M, Hamada K, Tonai Y, Matsuura N, Akasaka T, Hanaoka N, Higashino K, Ishihara R, Okada H, Iishi H. Efficacy of traction-assisted colorectal endoscopic submucosal dissection using a clip-and-thread technique: A prospective randomized study. Dig Endosc. 2018;30:467–476. doi: 10.1111/den.13036. [DOI] [PubMed] [Google Scholar]
- 72.Despott EJ, Hirayama Y, Lazaridis N, Koukias N, Telese A, Hayashi Y, Miura Y, Yamamoto H, Murino A. Saline immersion therapeutic endoscopy facilitated pocket-creation method for endoscopic submucosal dissection (with video) Gastrointest Endosc. 2019;89:652–653. doi: 10.1016/j.gie.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 73.Mavrogenis G, Mavrogenis I, Anastasiadis S, Bazerbachi F. Underwater endoscopic submucosal dissection in saline solution with rubber-band countertraction for a cecal polyp extending into a diverticulum. Ann Gastroenterol. 2019;32:527. doi: 10.20524/aog.2019.0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ramos-Zabala F, García-Mayor M, Domínguez-Pino A, Alzina-Pérez A, Moreno-Almazán L. Combination of immersion in saline solution, pocket-creation method, water-jet hydrodissection, and hybrid knife "probe mode" simplifies endoscopic submucosal dissection in giant rectal polyp. VideoGIE. 2019;4:478–480. doi: 10.1016/j.vgie.2019.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Caruso A, Sgamato C, Bertani H, Pigò F, Mangiafico S, Grande G, Conigliaro RL. Underwater endoscopic submucosal dissection of an obstructing giant colonic lipoma. Endoscopy. 2020;52:E90–E91. doi: 10.1055/a-1011-3617. [DOI] [PubMed] [Google Scholar]
- 76.Hoteya S, Furuhata T, Takahito T, Fukuma Y, Suzuki Y, Kikuchi D, Mitani T, Matsui A, Yamashita S, Nomura K, Kuribayashi Y, Iizuka T, Kaise M. Endoscopic Submucosal Dissection and Endoscopic Mucosal Resection for Non-Ampullary Superficial Duodenal Tumor. Digestion. 2017;95:36–42. doi: 10.1159/000452363. [DOI] [PubMed] [Google Scholar]
- 77.Matsuda Y, Sakamoto K, Kataoka N, Yamaguchi T, Tomita M, Makimoto S. Perforation associated with endoscopic submucosal dissection for duodenal neoplasm without a papillary portion. World J Gastrointest Surg. 2017;9:161–166. doi: 10.4240/wjgs.v9.i7.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yahagi N, Kato M, Ochiai Y, Maehata T, Sasaki M, Kiguchi Y, Akimoto T, Nakayama A, Fujimoto A, Goto O, Uraoka T. Outcomes of endoscopic resection for superficial duodenal epithelial neoplasia. Gastrointest Endosc. 2018;88:676–682. doi: 10.1016/j.gie.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 79.Miura Y, Shinozaki S, Hayashi Y, Sakamoto H, Lefor AK, Yamamoto H. Duodenal endoscopic submucosal dissection is feasible using the pocket-creation method. Endoscopy. 2017;49:8–14. doi: 10.1055/s-0042-116315. [DOI] [PubMed] [Google Scholar]
- 80.Yahagi N, Nishizawa T, Sasaki M, Ochiai Y, Uraoka T. Water pressure method for duodenal endoscopic submucosal dissection. Endoscopy. 2017;49:E227–E228. doi: 10.1055/s-0043-113556. [DOI] [PubMed] [Google Scholar]
- 81.Inoue T, Uedo N, Yamashina T, Yamamoto S, Hanaoka N, Takeuchi Y, Higashino K, Ishihara R, Iishi H, Tatsuta M, Takahashi H, Eguchi H, Ohigashi H. Delayed perforation: a hazardous complication of endoscopic resection for non-ampullary duodenal neoplasm. Dig Endosc. 2014;26:220–227. doi: 10.1111/den.12104. [DOI] [PubMed] [Google Scholar]
- 82.Kono M, Nagami Y, Kitagawa D, Manabe T, Ominami M, Fukunaga S, Fujiwara Y. Underwater endoscopic submucosal dissection for a duodenal neuroendocrine tumor using pocket creation and ring-shaped thread countertraction methods. Endoscopy. 2021;53:E110–E111. doi: 10.1055/a-1198-4153. [DOI] [PubMed] [Google Scholar]
- 83.Yano T, Nemoto D, Ono K, Miyata Y, Numao N, Iwashita C, Nagayama M, Takahashi H, Lefor AK, Yamamoto H. Gel immersion endoscopy: a novel method to secure the visual field during endoscopy in bleeding patients (with videos) Gastrointest Endosc. 2016;83:809–811. doi: 10.1016/j.gie.2015.09.048. [DOI] [PubMed] [Google Scholar]
- 84.Hashimoto R, Hirasawa D. Duodenal endoscopic submucosal dissection with traction method using the S-O clip. Dig Endosc. 2017;29:635. doi: 10.1111/den.12860. [DOI] [PubMed] [Google Scholar]