Abstract
Hepatitis C virus (HCV) is a significant cause of hepatocellular carcinoma (HCC). The direct-acting antivirals marked a new era of HCV therapy and are associated with greater than 95% cure rate. Successful treatment of chronic hepatitis C greatly reduces the risk of HCC. A proportion of patients, especially those with pre-existing cirrhosis, remain at risk for HCC despite sustained virologic response (SVR). Diabetes mellitus, hepatic steatosis, alcohol consumption and lack of fibrosis regression are associated with risks of HCC after HCV cure. Noninvasive modalities such as aspartate aminotransferase to platelet ratio index and fibrosis-4 index and transient elastography have been used to monitor hepatic fibrosis. More recently, various fibrosis scores have been combined with clinical parameters and other novel biomarkers to predict risks of HCC for patients who achieved SVR. These models still need to be validated and standardized prior to applying to routine clinical care.
Keywords: Hepatitis C virus cure, Hepatocellular carcinoma, Hepatocellular carcinoma risk models, Fibrosis markers, Transient elastography
Core Tip: Direct-acting antivirals (DAA) therapy has revolutionized the treatment for chronic hepatitis C. However, the development of hepatocellular carcinoma (HCC) after achieving DAA-induced sustained virologic response remains a significant concern, especially those with advanced fibrosis. It is critically important to monitor hepatic fibrosis and continue HCC surveillance for patients with pre-existing cirrhosis. Lack of hepatic regression and several comorbid conditions are associated with HCC risks. Some promising models for predicting HCC risks after hepatitis C virus cure are in development.
INTRODUCTION
Hepatitis C virus (HCV) is a global health issue affecting 160-170 million people worldwide[1]. According to recent National Health and Nutrition Examination Survey data, there are approximately 2.4 million people with chronic hepatitis C (CHC) in the United States[2]. There are 6 major genotypes of HCV[3]. Globally, G1 is most common accounting for 49.1% of all infections among adults, followed by G3 (17.9%), G4 (16.8%), G2 (11.0%), G5 (2.0%) and G6 (1.4%)[3]. There are significant geographic variations in the 6 HCV genotypes (Table 1). G1 is the predominant HCV genotype, for example, in North America, Europe, Caribbean and Latin America. G4 is most common in North Africa especially Egypt and the Middle East. The high prevalence of G3 in Asia is largely contributed by South Asia in particular India and Pakistan[3].
Table 1.
Regional prevalence of hepatitis C virus genotypes
|
Regions
|
G1 (%)
|
G2 (%)
|
G3 (%)
|
G4 (%)
|
G5 (%)
|
G6 (%)
|
Mixed
|
| Africa | 26.3 | 23.7 | 6.3 | 28.1 | 12.2 | - | 3.4 |
| North Africa/Middle East | 27.3 | 0.8 | 6.3 | 65.3 | 0.3 | - | - |
| North America | 66.3 | 13.1 | 15.7 | 4.3 | - | 0.6 | - |
| Caribbean | 83 | 7.2 | 2.1 | 0.6 | - | 0.1 | 7.0 |
| Central Latin America | 74.6 | 21.6 | 3.3 | 0.1 | 0.1 | - | 0.3 |
| Central Asia | 70.4 | 8.6 | 19.6 | - | - | - | 1.4 |
| South Asia | 15.5 | 1.9 | 66.7 | 3.7 | 0.1 | 0.5 | 11.6 |
| Europe | 64.4 | 5.5 | 25.5 | 3.7 | 0.1 | 0.1 | 0.7 |
| Australasia | 55.0 | 6.5 | 36.0 | 1.2 | - | 1.3 | - |
HCV-related hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide, accounting for 85%-90% of primary liver cancers[4]. Advanced stage liver fibrosis (Metavir stage F3) carries an increased risk of HCC, and patients with cirrhosis (Metavir stage F4) have an annual HCC incidence of approximately 4%[4]. With the advent of direct-acting antivirals (DAA) therapy, over 95% of the treated patients were able to achieve sustained virologic response (SVR) or HCV cure[5]. HCV cure reduces the HCC risk but those with preexisting cirrhosis remain at risk[6,7]. This review focused on the pathogenesis and risk factors of HCC after HCV cure, and the applications of noninvasive modalities and models to predict HCC.
NATURAL HISTORY OF HCV INFECTION
The transmission of HCV occurs mainly via blood with the majority due to unsafe injection use (intravenous drug use, healthcare workers in underdeveloped countries) and blood transfusion recipients before 1992[8]. Moreover, sexual transmission of HCV has significantly increased in human immunodeficiency virus-infected MSM in recent years[9,10].
After the virus transmission, HCV RNA reaches a detectable level in the serum in 7 to 21 d[11,12]. HCV RNA levels rise rapidly during acute infection but it generally takes 4-12 wk for the elevation of alanine aminotransferase (ALT) (indicative of hepatic injury) with an associated increase of serum bilirubin[13]. HCV itself is not cytolytic, but it generates potent innate and adaptive immune responses with cytotoxic cytokines production and hepatic injury[14]. Acute liver failure due to HCV is rare, but its incidence increases especially in patients with pre-existing chronic liver diseases[12].
Spontaneous eradication of HCV with recovery occurs only in only 15%-25% of patients with acute hepatitis C. The presence of homozygous rs12979860-C alleles in the interferon lambda gene, however, is associated with about 80% of spontaneous recovery[15,16].
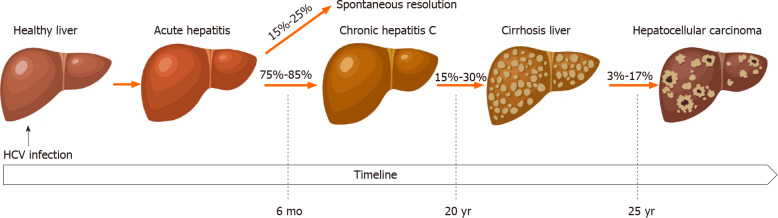
CHC is defined as the persistence of HCV RNA six months after the initial infection. CHC can lead to progressive fibrosis, cirrhosis, end-stage liver disease and complicated with HCC (Figure 1). It is estimated that 20%-30% of patients with CHC will develop cirrhosis after a period of 20 years[17]. Monitoring the development of fibrosis over time can provide a more accurate progression to cirrhosis. A study of paired liver biopsies scored by the same pathologists suggested the time to develop cirrhosis from diagnosis is about 30 to 40 years[17].
Figure 1.
Natural history of chronic hepatitis. HCV: Hepatitis C virus.
After the progression to cirrhosis, patients are at increased risk of decompensated liver disease with associated complications such as ascites, spontaneous bacterial peritonitis, variceal bleeding and hepatic encephalopathy. The development of any of these complications is an indicator of increased risk of death or need for liver transplantation. Among patients with compensated cirrhosis, the 5-year and 10-year survival was 85%-91% and 60%-79% respectively[18]. The rate of clinical decompensation was 2%-5% per year and incidence of HCC was 1%-4% in these patients[18]. Generally, the risk for HCC and death increases significantly once decompensation develops[18].
HCV CURE
Treatment for HCV has revolutionized in the last decade. Before 2011, interferon was the mainstay of the therapy for HCV. Pegylated interferon combined with ribavirin had a success rate of 70% and 80% for genotype 2 and 3, respectively. However, the efficacy of interferon in HCV genotype 1 was low at 10%-20% only[19]. The advent of DAA marked the new era of HCV cure (Table 2). Boceprevir (Victrelis®) and Telaprevir (Incivek®) were the first DAA agents approved for the treatment of genotype 1 HCV infection and multiple other regimens obtained approval in the ensuing years. Since 2016, there are three pangenotypic combination therapies against genotype 1 to 6 with potent efficacy.
Table 2.
Current therapies for treatment of chronic hepatitis C
|
Year approved
|
FDA approved therapy
|
Genotype
|
Trade name
|
| 2011 | PegIFN/RBV + Boceprevir | Genotype-1 | Victrelis® |
| 2011 | Telaprevir + PegIFNα/RBV | Genotype-1 | Incivek® |
| 2013 | Sofosbuvir + RBV or Sofosbuvir + PegIFNα/RBV | Genotype-1, 2, 3 and 4 | Sovaldi® |
| 2014 | Ledipasvir + Sofosbuvirwith or without RBV | Genotype-1, 4, 5 and 6 | Harvoni® |
| 2015 | Daclatasvir + Sofosbuvir with or without RBV | Genotype-1, and 3 | Daklinza™ + Sovaldi® |
| 2016 | Grazoprevir + Elbasvir + RBV | Genotype-1, and 4 | Zepatier™ |
| 2016 | Velpatasvir + Sofosbuvir | Genotype 1 to 6 | Epclusa® |
| 2017 | Glecaprevir + Pibrentasvir | Genotype 1 to 6 | Mavyret™ |
| 2017 | Sofosbuvir + Velpatasvir + Voxilaprevir | Genotype 1 to 6 | Vosevi® |
FDA: Food and Drug Administration; RBV: Ribavirin; IFN: Interferon.
HCV cure or SVR is characterized by the absence of detectable HCV RNA in the serum 12 wk after the completion of DAA therapy[20]. In a meta-analysis of 43 studies, the risk of relapse or reinfection in the low-risk patients was 0.95% [95% confidence interval (CI): 0.35%-1.69%] over a 5-year period. Among the high-risk populations, such as injecting drug users or prisoners, the reinfection rate increased to 10.67% (95%CI: 6.38%-15.66%) in 5 years[21].
REGRESSION OF FIBROSIS AFTER DAA THERAPY
Liver biopsy is the gold standard to estimate liver fibrosis regression after DAA therapy. In a study by Cheng et al[22], the Metavir fibrosis score decreased from F3-F4 to F0-2 in more than 50% of the patients from baseline to post-therapy. Since liver biopsy is an invasive procedure that can be associated with potential adverse events, non-invasive modalities have been developed to monitor hepatic fibrosis[23,24].
Fibrosis markers: Fibrosis-4 and aminotransferase to platelet ratio index
Aminotransferase to platelet ratio index (APRI) and fibrosis-4 (FIB-4) are non-invasive serum fibrosis markers. FIB-4 and APRI values have been shown to decrease significantly during the first four weeks of DAA therapy[22]. The initial reduction in fibrosis may be related to a decrease in hepatic inflammation. They reported that aspartate aminotransferase (AST) and ALT values significantly decreased by 50.8% and 64.1% respectively after 4 wk of DAA therapy and ultimately reaching normal values[22].
Fibroscan or vibration-controlled transient elastography
Vibration-controlled transient elastography is a non-invasive and accurate measuring tool of liver fibrosis. Liver stiffness scores significantly decreased in patients who responded to DAA. Several studies have shown long-term regression of fibrosis over a follow-up period of 2 years.
Rout et al[25] reported that high baseline liver stiffness measurements (LSM), low platelet count, and low body mass index (BMI) were independently associated with improvement of LSM values one year after successful therapy. Furthermore, the levels of serum transaminases were not significantly associated with a reduction of LSM on multivariate analysis.
Chan et al[26] monitored a cohort of patients for at least a year after completion of DAA therapy to exclude the confounding effect of liver inflammation on LSM. They observed the median intra-patient LSM reduction was 0.5 kPa between the end of therapy and 12 mo after treatment.
Stasi et al[27] observed the greatest reduction in stiffness values at end of DAA therapy. The reduction in fibrosis was more gradual thereafter. In this group of patients, the liver stiffness values reduced progressively at 1 year, 2 years after treatment, respectively. Their findings suggested a continued reduction of fibrosis beyond the initial resolution of inflammation.
Several studies reported that patients with advanced fibrosis had significant fibrosis regression after achieving SVR. The reduction was approximately 3.1 kPa in 6-12 mo after achieving HCV cure, and the median decline in liver stiffness was 28.2% (interquartile range of 21.8% to 34.8%)[28]. Despite a reduction from baseline LSM, more than half of the patients remained cirrhotic at week 24 after treatment completion[29]. This result is consistent with previous observations that advanced fibrosis often persists after SVR[30,31].
RISKS OF HCC AFTER HCV CURE
Lack of fibrosis regression
It is crucial to explore the relationship between the lack of fibrosis regression and HCC risk especially in patients with advanced fibrosis and cirrhosis[32,33]. In a study by Ravaioli et al[34], 139 patients with HCV-related cirrhosis who achieved SVR after DAA treatment were included to evaluate their HCC risk by comparing LSM at baseline to end of treatment. The majority of the patients were male (65.5%) and genotype 1b (58.3%). Those who developed HCC had in average an 18% reduction in LSM compared to 28.9% among those without HCC (P = 0.005). At multivariate analysis, a less than 30% reduction in LSM was an independent HCC risk factor.
In another study, Kawagishi et al[35] evaluated fibrosis regression by LSM in 110 HCV patients who achieved SVR. Regression of liver fibrosis was defined as: A decrease by > 1 stage after DAA therapy in patients with liver fibrosis stage F2 to F4; and no deterioration of fibrosis in patients with liver fibrosis F0/1. They found the rate of regression was lower at 96 wk after SVR among those with higher baseline fibrosis stages.
Hepatic steatosis and non-alcoholic fatty liver disease
Hepatic steatosis is one of the histopathologic features of CHC[36]. Both in vitro and in vivo studies have shown that HCV core protein expression either in cell cultures or in transgenic mice led to the development of hepatic steatosis, contributing to carcinogenesis[37-39]. Cholet et al[40] in their study demonstrated a significant relationship between steatosis and hepatic fibrosis in CHC highlighting the important role played by steatosis in liver disease progression in CHC. This relationship remained significant in multivariate analysis as well[40].
Hepatic steatosis is among the factors associated with increased risk of developing HCC in HCV patients after DAA therapy[41,42]. In a large retrospective study conducted by Peleg et al[43] on 515 CHC patients treated with interferon-free DAA regimens, baseline liver steatosis (LS) was significantly associated with all-cause mortality and the development of HCC after treatment. Patients with LS had higher incidence rates of HCC (5.23 cases per 100 person-years, 95%CI: 4.85-5.71) compared to patients with advanced fibrosis (3.51 cases per 100 persons-years, 95%CI: 3.33-3.67). Moreover, patients with LS without advanced fibrosis had higher rates of mortality and HCC compared to those with advanced fibrosis but without steatosis[42]. Kono et al[44] concluded in their study of 286 CHC patients that fatty liver along with advanced liver fibrosis is associated with sustained liver damage with abnormal alpha-feto protein (AFP) and ALT levels even after HCV cure. In a prospective study conducted by Noureddin et al[45], 47.5% of the HCV patients with SVR had evidence of LS. Long-term follow-up of these patients is critically important to monitor progressive liver disease.
Diabetes mellitus
Diabetes mellitus (DM) is identified as a significant risk factor for HCC in HCV patients after SVR but the mechanism remains unclear[46-48]. There is some evidence suggesting hyperinsulinemia and insulin-dependent signaling pathways are linked to the pathogenesis and progression of HCC. Insulin resistance increases the rate of fibrosis progression in HCV infected patients. Hyperinsulinemia and insulin resistance as a result of cirrhosis can further promote the development of HCC[49]. HCC risk after interferon-induced SVR in patients with DM and cirrhosis had been reported. Subsequently, this association was also noted after DAA therapy. A 3-year follow-up study including 565 CHC patients with cirrhosis treated with DAAs identified diabetes as an independent predictor of de novo HCC[50-54]. Degasperi et al[47] identified diabetes as a strong independent predictor for de novo HCC development and also HCC recurrence in a cohort of 546 HCV patients treated with DAA. On the multivariate analysis, diabetes [hazard ratio (HR): 2.52, 95%CI: 1.08-5.87, P = 0.03] predicted de novo HCC as well as HCC recurrence (HR: 4.12, 95%CI: 1.55-10.93, P = 0.004)[47]. Similarly, in another study, Lu et al[48] also found that DM had a significant effect on the risk of HCC [adjusted HR (aHR): 1.65, 95%CI: 1.09-2.49]. In contrast, DM was not associated with an increased risk of developing HCC after DAA-induced SVR in studies by Kanwal et al[41].
Alcohol
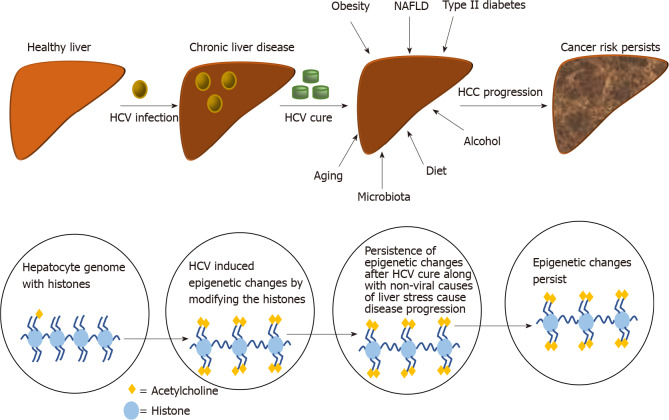
Alcohol is an important HCC risk factor regardless of the presence of HCV. The annual incidence of HCC is higher among patients with alcohol use compared to those without (aHR: 4.73, 95%CI: 3.34-6.68)[41]. Alcohol-induced oxidative stress and the hepatic metabolism of ethanol could increase the conversion of pro-carcinogens to active carcinogens that results in HCC[42]. Caldwell et al[55] found that history of heavy alcohol consumption, defined as consumption of more than 2 drinks per day or 14 drinks per week for female; 3 drinks per day or 21 drinks per week for male, had a direct impact on FIB-4 score. It was significantly higher in the group with heavy alcohol abuse compared to no alcohol abuse. A daily intake of ≥ 80 g of ethanol for > 10 years is thought to increase the risk of HCC by approximately five-fold and women are more susceptible to alcohol toxicity than men[56,57]. Alcohol acts synergistically with HCV in accelerating the progression to cirrhosis and liver-related complications[58]. The ethanol’s effects on hepatic fibrogenesis persist after HCV cure for those who continue to consume alcohol. A study by Kanwal et al[41] reported a higher annual incidence of HCC among patients with alcohol use (1.01%, 95%CI: 0.83-1.19) compared to those without (0.72%, 95%CI: 0.54%-0.91%; aHR: 1.56, 95%CI: 1.11-2.18) after achieving SVR post DAA therapy[41] (Figure 2).
Figure 2.
Pathogenesis of hepatocellular carcinoma. HCV: Hepatitis C virus; NAFLD: Non-alcoholic fatty liver disease; HCC: Hepatocellular carcinoma.
PATHOGENESIS OF HCC AFTER HCV CURE
A number of key pathways are involved in the development of HCV-related HCC: (1) Fibrosis due to continuous necrosis; (2) Immune-surveillance failures attributable to persistent viral replication with immune system escape mechanisms; and (3) Direct carcinogenic effect of HCV proteins which deregulate host cell cycle checkpoints leading to DNA mutations in liver cells[59]. The pathogenesis of HCC after HCV cure remains elusive. A 186-gene expression signature in liver tissue of CHC patients with HCC suggested virus-induced transcriptional reprogramming in the liver leading to carcinogenesis[60,61]. Epigenetic modifications of histones, for example, can lead to chromatin opening and compacting which, in turn, affect gene regulation[62]. Hamdane et al[63] investigated HCV-induced epigenetic alterations that might increase HCC risk after DAA treatment in patients and mice with humanized livers. They found that chronic HCV infection induced specific genome-wide changes in H3K27ac. The 5318 modified genes associated with CHC correlated with changes in the expression of mRNAs and proteins. A number of the altered pathways resulting from epigenetic changes persisted after HCV cure with DAAs. Namely, molecular pathways involving tumor necrosis factor α signaling, inflammatory response, G2M checkpoint, epithelial-mesenchymal transition, phosphoinositide 3-kinase, Akt, and mammalian target of rapamycin[63]. This analysis showed that H3K27ac changes observed in HCV-infected patients were partly reversed after cure for those with stage F2-3 fibrosis. This group shared only 42.5% of the HCV-modified genes. In contrast, in DAA-cured patients with cirrhosis (stage F4), 96.6% of the HCV-induced H3K27ac changes persisted[63]. By performing chromatin immunoprecipitation followed by next-generation sequencing of histone post-translational modifications that are epigenetic markers for active and repressed chromatin, Perez et al[64] also demonstrated that HCV infection induces genome-wide epigenetic changes. The "epigenetic signature" persisted after achieving DAA- related cure. Santangelo et al[65] examined the impact of DAAs on the ability of exosomal microRNAs (miRs) to modulate the innate immune response in patients with CHC. miR-122 was selectively studied as it is involved in HCV replication and its loss has been associated with HCC development. The study showed that miR-122-5p, miR-222-3p, miR146-5p, miR-150-5p, miR-30C-5p, miR-378a-3p, miR-20a5p were enriched in exosomes derived from the HCV-infected cells. The liver-specific miR-122 levels and the expression of the aforementioned miRs significantly decreased after DAAs therapy[65]. Human HCC cells express vascular endothelial growth factor (VEGF) that functions as a cytokine and affects cancer cell growth and survival[66]. The VEGF expression correlates with liver cancer angiogenesis and proliferative activity. Villani et al[66] studied the effect of DAA treatment-induced VEGF on HCC angiogenesis. In this study on 117 cirrhotic patients treated with DAA, a 4-fold increase in VEGF was observed compared to baseline. This significant increase in VEGF could potentially lead to an acceleration of cancer cell proliferation prior to HCV cure and the carcinogenesis remained after DAA even though the VEGF decreased to normal levels 12 wk after DAA treatment (Figure 2).
IDENTIFYING PATIENTS WITH HCC RISK AFTER HCV CURE
Although achieving SVR is the goal of HCV treatment, the risk of developing HCC remains high particularly in patients with advanced fibrosis and cirrhosis[67]. This risk ranges between 1.8% and 2.5% annually. The current guidelines suggest that these patients should undergo HCC surveillance every six months by ultrasound with or without alfa-fetoprotein indefinitely. On the contrary, patients with no or moderate fibrosis who achieved SVR and have no risk behavior could be discharged from specialty care[68]. Methods to identify patients with differential HCC risks can be challenging.
APRI and FIB-4 have been used to assess the HCC risks. These scores, however, were not developed specifically for HCC indication; thus, their accuracy is limited. Transient elastography, similarly, was not designed to detect HCC[68]. Specific score systems designed to predict HCC after HCV cure remain an unmet need.
A group in Japan developed a simple score to identify HCV patients at risk of HCC after achieving SVR[69]. The majority were HCV serotype 1 or 2 patients. They use multivariate analysis to identify predictive variables. They found that age (cutoff 75 years) and post-treatment AFP (cutoff 6 ng/mL) values were independent factors for HCC. Thus, they used a score with 0 and 1 point for each factor: < 75 and > 75 years were set as 0 and 1 point; < 6 and > 6 ng/mL were set as 0 and 1 points respectively. The sum of each factor was considered as the final score. HCC incidence increased significantly with higher scores. In the 0-point group, the incidence of HCC was 0% at 6 mo; 0.3% at 12, 18 and 24 mo; and only 1.26% at 36 mo. In contrast, the risk increased in the 2-point group: 2.88% at 6 mo; 4.92% at 12 mo; 11.61% at 18 mo; and up to 18.37% after 24 mo. This scoring system is simple to apply but needs to be validated prospectively in different patient populations.
In Egypt, Shiha et al[70] conducted a prospective study to develop an HCC risk model after SVR. Their model used clinical variables to create scores for low, intermediate and high HCC risk. Each variable was given a score according to its HR. This General Evaluation Score included age (< 54 = 0; > 54 = 1), gender (male = 3.5; female = 0), fibrosis stage (F3 = 1.5; F4 = 3), albumin (> 3.8 g/dL = 0; < 3.8 g/dL = 2) and alpha-fetoprotein levels (< 20 ng/mL = 0; > 20 ng/mL = 3). The score range was between 0 and 12.5. The low-risk group (score < 6) had a 1-year HCC incidence of 0.1%, 1.2% at 2 years and 1.9% at 3 years. The intermediate-risk group (score 6-7.5) had a 1-year incidence of 0.7%, 3.3% at 2 years and 5.8% at 3 years. Finally, the high-risk group (score > 7.5) had a 1-year HCC incidence of 1.2% which increased to 7.1% at 2 years and 9.5% at 3 years. The advantage of this tool is that it uses commonly available clinical variables that can be applied in different settings including low and medium-income populations. This study included only patients with HCV genotype 4. If it is validated in other HCV genotypes and populations, it can be a cost-effective tool for HCC surveillance.
Ioannou et al[71] developed different sets of models according to treatment modalities for CHC. For those with DAA-induced HCV cure, the regression model showed that age > 60, platelet count < 61 × 104, serum AST/ALT ratio > 8.8 in non-cirrhotic and > 11.01 in cirrhotic; and albumin < 2.9 were major predictive variables for the development of HCC. By applying these variables in the models, the cirrhotic/non-SVR group was predicted to have a 13.1% HCC risk at 2.6-year follow-up; the cirrhotic/SVR group had a 4.5% incidence at 2-year follow-up; the non-cirrhotic/non-SVR had a 4.2% incidence at 3.7-year follow-up; whereas the non-cirrhotic/SVR group had only a low 0.7% HCC risk at 2.3-year follow-up. Given the differential risks according to the clinical characteristics, the HCC screening guidelines could potentially be narrowed to specific risk groups. Although this model was internally validated and is easily available as a web-calculator tool, external validation would be necessary since it was performed using the Veterans Affairs healthcare data only and the majority of patients had HCV genotype 1.
Recently, a model using transient elastography was developed in Spain by Alonso López et al[72], they built two dynamic models for patients with advanced fibrosis and cirrhosis who achieved SVR. Their objective was to identify very low HCC risk patients who may not require continued HCC surveillance despite the presence of advanced fibrosis prior to therapy. The first model included baseline albumin, baseline and 1-year follow-up elastography. Given that elastography may not be available in every setting, the second model included serological markers only: Baseline albumin, baseline and 1-year follow-up FIB-4 and 1-year gamma-glutamyl transferase. They found that both models were useful as predictors of HCC. Moreover, after stratification of risk assigned by scoring each variable in both models, the ones who scored 0 had 0%-0.4% risk of developing HCC. The ability to accurately identify those at very low HCC risk could effectively stratify patients for HCC surveillance.
Alpha-fetoprotein is the most available HCC biomarker. Its sensitivity and specificity are very variable[73]. Recent studies have shown sphingolipids as potential biomarkers to detect hepatic decompensation in cirrhotic patients[74]. Two types of sphingolipids - C16-ceramide and sphingosine-1-phosphate - have been applied as HCC biomarkers in cirrhotic patients. Mücke et al[75] in Germany evaluated sphingolipids as early predictive HCC biomarkers in HCV patients with cirrhosis who had achieved SVR. They identified C16Cer as an independent biomarker for early detection of de novo HCC in both AFP-positive or AFP-negative patients. Although this finding seems novel and promising, prospective studies are needed to clarify the association between sphingolipids and carcinogenesis.
In the area of deep learning, Ioannou et al[76] utilized recurrent neural network (RNN) models to identify patients at high risk of developing HCC for at least a 3-year follow-up period after HCV cure. They used two types of variables: Baseline and longitudinal ones to evaluate the risk progression. They compared three models: Cross-sectional logistic regression (LR), longitudinal LR and RNN. The area under the receiver operating characteristic curve for these groups was 0.67, 0.70 and 0.80 respectively. The RNN model was superior to the conventional LR models and could be a promising tool after computational refinement.
CONCLUSION
In the DAA era, the development of HCC remains a significant concern especially among those with advanced hepatic fibrosis. A number of factors including diabetes mellitus, underlying non-alcoholic fatty liver disease and alcohol consumption have been associated with progression to HCC after HCV cure. Promising HCC predictive models are being developed but most require validation and standardization. The pathogenesis of HCC after HCV cure remains poorly understood. The understanding of the molecular mechanisms leading to HCC could facilitate the identification of novel biomarkers for early HCC detection.
Footnotes
Conflict-of-interest statement: Authors declare no conflict of interests for this article.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: May 29, 2021
First decision: June 22, 2021
Article in press: December 25, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Xu J, Yang L S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
Contributor Information
Maria Alejandra Luna-Cuadros, Liver Center, Division of Gastroenterology and Hepatology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA 02215, United States.
Hao-Wei Chen, Liver Center, Division of Gastroenterology and Hepatology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA 02215, United States.
Hira Hanif, Liver Center, Division of Gastroenterology and Hepatology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA 02215, United States.
Mukarram Jamat Ali, Liver Center, Division of Gastroenterology and Hepatology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA 02215, United States.
Muzammil Muhammad Khan, Liver Center, Division of Gastroenterology and Hepatology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA 02215, United States.
Daryl Tan-Yeung Lau, Liver Center, Division of Gastroenterology and Hepatology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA 02215, United States. dlau@bidmc.harvard.edu.
References
- 1.Lavanchy D. Evolving epidemiology of hepatitis C virus. Clin Microbiol Infect. 2011;17:107–115. doi: 10.1111/j.1469-0691.2010.03432.x. [DOI] [PubMed] [Google Scholar]
- 2.Hofmeister MG, Rosenthal EM, Barker LK, Rosenberg ES, Barranco MA, Hall EW, Edlin BR, Mermin J, Ward JW, Ryerson AB. Estimating Prevalence of Hepatitis C Virus Infection in the United States, 2013-2016. Hepatology. 2019;69:1020–1031. doi: 10.1002/hep.30297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petruzziello A, Marigliano S, Loquercio G, Cozzolino A, Cacciapuoti C. Global epidemiology of hepatitis C virus infection: An up-date of the distribution and circulation of hepatitis C virus genotypes. World J Gastroenterol. 2016;22:7824–7840. doi: 10.3748/wjg.v22.i34.7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoshida Y, Fuchs BC, Bardeesy N, Baumert TF, Chung RT. Pathogenesis and prevention of hepatitis C virus-induced hepatocellular carcinoma. J Hepatol. 2014;61:S79–S90. doi: 10.1016/j.jhep.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Falade-Nwulia O, Suarez-Cuervo C, Nelson DR, Fried MW, Segal JB, Sulkowski MS. Oral Direct-Acting Agent Therapy for Hepatitis C Virus Infection: A Systematic Review. Ann Intern Med. 2017;166:637–648. doi: 10.7326/M16-2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singal AK, Singh A, Jaganmohan S, Guturu P, Mummadi R, Kuo YF, Sood GK. Antiviral therapy reduces risk of hepatocellular carcinoma in patients with hepatitis C virus-related cirrhosis. Clin Gastroenterol Hepatol. 2010;8:192–199. doi: 10.1016/j.cgh.2009.10.026. [DOI] [PubMed] [Google Scholar]
- 7.van der Meer AJ, Veldt BJ, Feld JJ, Wedemeyer H, Dufour JF, Lammert F, Duarte-Rojo A, Heathcote EJ, Manns MP, Kuske L, Zeuzem S, Hofmann WP, de Knegt RJ, Hansen BE, Janssen HL. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308:2584–2593. doi: 10.1001/jama.2012.144878. [DOI] [PubMed] [Google Scholar]
- 8.Grebely J, Prins M, Hellard M, Cox AL, Osburn WO, Lauer G, Page K, Lloyd AR, Dore GJ International Collaboration of Incident HIV and Hepatitis C in Injecting Cohorts (InC3) Hepatitis C virus clearance, reinfection, and persistence, with insights from studies of injecting drug users: towards a vaccine. Lancet Infect Dis. 2012;12:408–414. doi: 10.1016/S1473-3099(12)70010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.López-Diéguez M, Montes ML, Pascual-Pareja JF, Quereda C, Von Wichmann MA, Berenguer J, Tural C, Hernando A, González-García J, Serrano L, Arribas JR GESIDA 37/03-FIPSE 36465/03-NEAT IG5 Study Group. The natural history of liver cirrhosis in HIV-hepatitis C virus-coinfected patients. AIDS. 2011;25:899–904. doi: 10.1097/QAD.0b013e3283454174. [DOI] [PubMed] [Google Scholar]
- 10.Hagan H, Jordan AE, Neurer J, Cleland CM. Incidence of sexually transmitted hepatitis C virus infection in HIV-positive men who have sex with men. AIDS. 2015;29:2335–2345. doi: 10.1097/QAD.0000000000000834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mosley JW, Operskalski EA, Tobler LH, Andrews WW, Phelps B, Dockter J, Giachetti C, Busch MP. Viral and host factors in early hepatitis C virus infection. Hepatology. 2005;42:86–92. doi: 10.1002/hep.20742. [DOI] [PubMed] [Google Scholar]
- 12.Hajarizadeh B, Grebely J, Dore GJ. Epidemiology and natural history of HCV infection. Nat Rev Gastroenterol Hepatol. 2013;10:553–562. doi: 10.1038/nrgastro.2013.107. [DOI] [PubMed] [Google Scholar]
- 13.Shin EC, Sung PS, Park SH. Immune responses and immunopathology in acute and chronic viral hepatitis. Nat Rev Immunol. 2016;16:509–523. doi: 10.1038/nri.2016.69. [DOI] [PubMed] [Google Scholar]
- 14.Negro F. Natural History of Hepatic and Extrahepatic Hepatitis C Virus Diseases and Impact of Interferon-Free HCV Therapy. Cold Spring Harb Perspect Med. 2020;10 doi: 10.1101/cshperspect.a036921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ge D, Fellay J, Thompson AJ, Simon JS, Shianna KV, Urban TJ, Heinzen EL, Qiu P, Bertelsen AH, Muir AJ, Sulkowski M, McHutchison JG, Goldstein DB. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399–401. doi: 10.1038/nature08309. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka Y, Nishida N, Sugiyama M, Kurosaki M, Matsuura K, Sakamoto N, Nakagawa M, Korenaga M, Hino K, Hige S, Ito Y, Mita E, Tanaka E, Mochida S, Murawaki Y, Honda M, Sakai A, Hiasa Y, Nishiguchi S, Koike A, Sakaida I, Imamura M, Ito K, Yano K, Masaki N, Sugauchi F, Izumi N, Tokunaga K, Mizokami M. Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat Genet. 2009;41:1105–1109. doi: 10.1038/ng.449. [DOI] [PubMed] [Google Scholar]
- 17.Ryder SD, Irving WL, Jones DA, Neal KR, Underwood JC Trent Hepatitis C Study Group. Progression of hepatic fibrosis in patients with hepatitis C: a prospective repeat liver biopsy study. Gut. 2004;53:451–455. doi: 10.1136/gut.2003.021691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lingala S, Ghany MG. Natural History of Hepatitis C. Gastroenterol Clin North Am. 2015;44:717–734. doi: 10.1016/j.gtc.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zając M, Muszalska I, Sobczak A, Dadej A, Tomczak S, Jelińska A. Hepatitis C - New drugs and treatment prospects. Eur J Med Chem. 2019;165:225–249. doi: 10.1016/j.ejmech.2019.01.025. [DOI] [PubMed] [Google Scholar]
- 20.Pearlman BL, Traub N. Sustained virologic response to antiviral therapy for chronic hepatitis C virus infection: a cure and so much more. Clin Infect Dis. 2011;52:889–900. doi: 10.1093/cid/cir076. [DOI] [PubMed] [Google Scholar]
- 21.Simmons B, Saleem J, Hill A, Riley RD, Cooke GS. Risk of Late Relapse or Reinfection With Hepatitis C Virus After Achieving a Sustained Virological Response: A Systematic Review and Meta-analysis. Clin Infect Dis. 2016;62:683–694. doi: 10.1093/cid/civ948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng CH, Chu CY, Chen HL, Lin IT, Wu CH, Lee YK, Hu PJ, Bair MJ. Direct-acting antiviral therapy of chronic hepatitis C improves liver fibrosis, assessed by histological examination and laboratory markers. J Formos Med Assoc. 2021;120:1259–1268. doi: 10.1016/j.jfma.2020.11.018. [DOI] [PubMed] [Google Scholar]
- 23.Sharma S, Khalili K, Nguyen GC. Non-invasive diagnosis of advanced fibrosis and cirrhosis. World J Gastroenterol. 2014;20:16820–16830. doi: 10.3748/wjg.v20.i45.16820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Papastergiou V, Tsochatzis E, Burroughs AK. Non-invasive assessment of liver fibrosis. Ann Gastroenterol. 2012;25:218–231. [PMC free article] [PubMed] [Google Scholar]
- 25.Rout G, Nayak B, Patel AH, Gunjan D, Singh V, Kedia S, Shalimar Therapy with Oral Directly Acting Agents in Hepatitis C Infection Is Associated with Reduction in Fibrosis and Increase in Hepatic Steatosis on Transient Elastography. J Clin Exp Hepatol. 2019;9:207–214. doi: 10.1016/j.jceh.2018.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan J, Gogela N, Zheng H, Lammert S, Ajayi T, Fricker Z, Kim AY, Robbins GK, Chung RT. Direct-Acting Antiviral Therapy for Chronic HCV Infection Results in Liver Stiffness Regression Over 12 Months Post-treatment. Dig Dis Sci. 2018;63:486–492. doi: 10.1007/s10620-017-4749-x. [DOI] [PubMed] [Google Scholar]
- 27.Stasi C, Sadalla S, Carradori E, Monti M, Petraccia L, Madia F, Gragnani L, Zignego AL. Longitudinal evaluation of liver stiffness and outcomes in patients with chronic hepatitis C before and after short- and long-term IFN-free antiviral treatment. Curr Med Res Opin. 2020;36:245–249. doi: 10.1080/03007995.2019.1691517. [DOI] [PubMed] [Google Scholar]
- 28.Singh S, Facciorusso A, Loomba R, Falck-Ytter YT. Magnitude and Kinetics of Decrease in Liver Stiffness After Antiviral Therapy in Patients With Chronic Hepatitis C: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2018;16:27–38.e4. doi: 10.1016/j.cgh.2017.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dolmazashvili E, Abutidze A, Chkhartishvili N, Karchava M, Sharvadze L, Tsertsvadze T. Regression of liver fibrosis over a 24-week period after completing direct-acting antiviral therapy in patients with chronic hepatitis C receiving care within the national hepatitis C elimination program in Georgia: results of hepatology clinic HEPA experience. Eur J Gastroenterol Hepatol. 2017;29:1223–1230. doi: 10.1097/MEG.0000000000000964. [DOI] [PubMed] [Google Scholar]
- 30.Balart LA, Lisker-Melman M, Hamzeh FM, Kwok A, Lentz E, Rodriguez-Torres M LATINO study investigators. Peginterferon α-2a plus ribavirin in Latino and Non-Latino Whites with HCV genotype 1: Histologic outcomes and tolerability from the LATINO Study. Am J Gastroenterol. 2010;105:2177–2185. doi: 10.1038/ajg.2010.157. [DOI] [PubMed] [Google Scholar]
- 31.Wei H, Song B. Elastography for Longitudinal Assessment of Liver Fibrosis after Antiviral Therapy: A Review. J Clin Transl Hepatol. 2020;8:445–453. doi: 10.14218/JCTH.2020.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Macek Jílková Z, Seigneurin A, Coppard C, Ouaguia L, Aspord C, Marche PN, Leroy V, Decaens T. Circulating IL-13 Is Associated with De Novo Development of HCC in HCV-Infected Patients Responding to Direct-Acting Antivirals. Cancers (Basel) 2020;12 doi: 10.3390/cancers12123820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hamoir C, Horsmans Y, Stärkel P, Dahlqvist G, Negrin Dastis S, Lanthier N. Risk of hepatocellular carcinoma and fibrosis evolution in hepatitis C patients with severe fibrosis or cirrhosis treated with direct acting antiviral agents. Acta Gastroenterol Belg. 2021;84:25–32. doi: 10.51821/84.1.420. [DOI] [PubMed] [Google Scholar]
- 34.Ravaioli F, Conti F, Brillanti S, Andreone P, Mazzella G, Buonfiglioli F, Serio I, Verrucchi G, Bacchi Reggiani ML, Colli A, Marasco G, Colecchia A, Festi D. Hepatocellular carcinoma risk assessment by the measurement of liver stiffness variations in HCV cirrhotics treated with direct acting antivirals. Dig Liver Dis. 2018;50:573–579. doi: 10.1016/j.dld.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 35.Kawagishi N, Suda G, Kimura M, Maehara O, Yamada R, Tokuchi Y, Kubo A, Kitagataya T, Shigesawa T, Suzuki K, Ohara M, Nakai M, Sho T, Natsuizaka M, Morikawa K, Ogawa K, Kudo Y, Nishida M, Sakamoto N. Baseline elevated serum angiopoietin-2 predicts long-term non-regression of liver fibrosis after direct-acting antiviral therapy for hepatitis C. Sci Rep. 2021;11:9207. doi: 10.1038/s41598-021-88632-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kralj D, Virović Jukić L, Stojsavljević S, Duvnjak M, Smolić M, Čurčić IB. Hepatitis C Virus, Insulin Resistance, and Steatosis. J Clin Transl Hepatol. 2016;4:66–75. doi: 10.14218/JCTH.2015.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moradpour D, Englert C, Wakita T, Wands JR. Characterization of cell lines allowing tightly regulated expression of hepatitis C virus core protein. Virology. 1996;222:51–63. doi: 10.1006/viro.1996.0397. [DOI] [PubMed] [Google Scholar]
- 38.Barba G, Harper F, Harada T, Kohara M, Goulinet S, Matsuura Y, Eder G, Schaff Z, Chapman MJ, Miyamura T, Bréchot C. Hepatitis C virus core protein shows a cytoplasmic localization and associates to cellular lipid storage droplets. Proc Natl Acad Sci U S A. 1997;94:1200–1205. doi: 10.1073/pnas.94.4.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moriya K, Fujie H, Shintani Y, Yotsuyanagi H, Tsutsumi T, Ishibashi K, Matsuura Y, Kimura S, Miyamura T, Koike K. The core protein of hepatitis C virus induces hepatocellular carcinoma in transgenic mice. Nat Med. 1998;4:1065–1067. doi: 10.1038/2053. [DOI] [PubMed] [Google Scholar]
- 40.Cholet F, Nousbaum JB, Richecoeur M, Oger E, Cauvin JM, Lagarde N, Robaszkiewicz M, Gouérou H. Factors associated with liver steatosis and fibrosis in chronic hepatitis C patients. Gastroenterol Clin Biol. 2004;28:272–278. doi: 10.1016/s0399-8320(04)94918-4. [DOI] [PubMed] [Google Scholar]
- 41.Kanwal F, Kramer J, Asch SM, Chayanupatkul M, Cao Y, El-Serag HB. Risk of Hepatocellular Cancer in HCV Patients Treated With Direct-Acting Antiviral Agents. Gastroenterology. 2017;153:996–1005.e1. doi: 10.1053/j.gastro.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 42.Lieber CS, Seitz HK, Garro AJ, Worner TM. Alcohol-related diseases and carcinogenesis. Cancer Res. 1979;39:2863–2886. [PubMed] [Google Scholar]
- 43.Peleg N, Issachar A, Sneh Arbib O, Cohen-Naftaly M, Harif Y, Oxtrud E, Braun M, Leshno M, Barsheshet A, Shlomai A. Liver steatosis is a major predictor of poor outcomes in chronic hepatitis C patients with sustained virological response. J Viral Hepat. 2019;26:1257–1265. doi: 10.1111/jvh.13167. [DOI] [PubMed] [Google Scholar]
- 44.Kono M, Nishida N, Hagiwara S, Minami T, Chishina H, Arizumi T, Minaga K, Kamata K, Komeda Y, Sakurai T, Takenaka M, Takita M, Yada N, Ida H, Minami Y, Ueshima K, Watanabe T, Kudo M. Unique Characteristics Associated with Sustained Liver Damage in Chronic Hepatitis C Patients Treated with Direct Acting Antivirals. Dig Dis. 2017;35:556–564. doi: 10.1159/000480148. [DOI] [PubMed] [Google Scholar]
- 45.Noureddin M, Wong MM, Todo T, Lu SC, Sanyal AJ, Mena EA. Fatty liver in hepatitis C patients post-sustained virological response with direct-acting antivirals. World J Gastroenterol. 2018;24:1269–1277. doi: 10.3748/wjg.v24.i11.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hedenstierna M, Nangarhari A, Weiland O, Aleman S. Diabetes and Cirrhosis Are Risk Factors for Hepatocellular Carcinoma After Successful Treatment of Chronic Hepatitis C. Clin Infect Dis. 2016;63:723–729. doi: 10.1093/cid/ciw362. [DOI] [PubMed] [Google Scholar]
- 47.Degasperi E, D'Ambrosio R, Iavarone M, Sangiovanni A, Aghemo A, Soffredini R, Borghi M, Lunghi G, Colombo M, Lampertico P. Factors Associated With Increased Risk of De Novo or Recurrent Hepatocellular Carcinoma in Patients With Cirrhosis Treated With Direct-Acting Antivirals for HCV Infection. Clin Gastroenterol Hepatol. 2019;17:1183–1191.e7. doi: 10.1016/j.cgh.2018.10.038. [DOI] [PubMed] [Google Scholar]
- 48.Lu M, Li J, Rupp LB, Holmberg SD, Moorman AC, Spradling PR, Teshale EH, Zhou Y, Boscarino JA, Schmidt MA, Lamerato LE, Trinacty C, Trudeau S, Gordon SC CHeCS Investigators. Hepatitis C treatment failure is associated with increased risk of hepatocellular carcinoma. J Viral Hepat. 2016;23:718–729. doi: 10.1111/jvh.12538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kukla M, Piotrowski D, Waluga M, Hartleb M. Insulin resistance and its consequences in chronic hepatitis C. Clin Exp Hepatol. 2015;1:17–29. doi: 10.5114/ceh.2015.51375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Polesel J, Zucchetto A, Montella M, Dal Maso L, Crispo A, La Vecchia C, Serraino D, Franceschi S, Talamini R. The impact of obesity and diabetes mellitus on the risk of hepatocellular carcinoma. Ann Oncol. 2009;20:353–357. doi: 10.1093/annonc/mdn565. [DOI] [PubMed] [Google Scholar]
- 51.Regimbeau JM, Colombat M, Mognol P, Durand F, Abdalla E, Degott C, Degos F, Farges O, Belghiti J. Obesity and diabetes as a risk factor for hepatocellular carcinoma. Liver Transpl. 2004;10:S69–S73. doi: 10.1002/lt.20033. [DOI] [PubMed] [Google Scholar]
- 52.Karagozian R, Derdák Z, Baffy G. Obesity-associated mechanisms of hepatocarcinogenesis. Metabolism. 2014;63:607–617. doi: 10.1016/j.metabol.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 53.Khan MM, Saito S, Takagi S, Ohnishi H, Izumi H, Sakauchi F, Washio M, Sonoda T, Nagata Y, Asakura S, Kobayashi K, Mori M, Shimamoto K. Relationship between hepatocellular carcinoma and impaired glucose tolerance among Japanese. Hepatogastroenterology. 2006;53:742–746. [PubMed] [Google Scholar]
- 54.Baffy G. Hepatocellular Carcinoma in Non-alcoholic Fatty Liver Disease: Epidemiology, Pathogenesis, and Prevention. J Clin Transl Hepatol. 2013;1:131–137. doi: 10.14218/JCTH.2013.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Caldwell SH, Li X, Rourk RM, Millar A, Sosnowski KM, Sue M, Barritt AS, McCallum RW, Schiff ER. Hepatitis C infection by polymerase chain reaction in alcoholics: false-positive ELISA results and the influence of infection on a clinical prognostic score. Am J Gastroenterol. 1993;88:1016–1021. [PubMed] [Google Scholar]
- 56.El-Serag HB, Mason AC. Risk factors for the rising rates of primary liver cancer in the United States. Arch Intern Med. 2000;160:3227–3230. doi: 10.1001/archinte.160.21.3227. [DOI] [PubMed] [Google Scholar]
- 57.Allen NE, Beral V, Casabonne D, Kan SW, Reeves GK, Brown A, Green J Million Women Study Collaborators. Moderate alcohol intake and cancer incidence in women. J Natl Cancer Inst. 2009;101:296–305. doi: 10.1093/jnci/djn514. [DOI] [PubMed] [Google Scholar]
- 58.Lieber CS. Mechanism of ethanol induced hepatic injury. Pharmacol Ther. 1990;46:1–41. doi: 10.1016/0163-7258(90)90032-w. [DOI] [PubMed] [Google Scholar]
- 59.Wirth TC, Manns MP. The impact of the revolution in hepatitis C treatment on hepatocellular carcinoma. Ann Oncol. 2016;27:1467–1474. doi: 10.1093/annonc/mdw219. [DOI] [PubMed] [Google Scholar]
- 60.Nakagawa S, Wei L, Song WM, Higashi T, Ghoshal S, Kim RS, Bian CB, Yamada S, Sun X, Venkatesh A, Goossens N, Bain G, Lauwers GY, Koh AP, El-Abtah M, Ahmad NB, Hoshida H, Erstad DJ, Gunasekaran G, Lee Y, Yu ML, Chuang WL, Dai CY, Kobayashi M, Kumada H, Beppu T, Baba H, Mahajan M, Nair VD, Lanuti M, Villanueva A, Sangiovanni A, Iavarone M, Colombo M, Llovet JM, Subramanian A, Tager AM, Friedman SL, Baumert TF, Schwarz ME, Chung RT, Tanabe KK, Zhang B, Fuchs BC, Hoshida Y Precision Liver Cancer Prevention Consortium. Molecular Liver Cancer Prevention in Cirrhosis by Organ Transcriptome Analysis and Lysophosphatidic Acid Pathway Inhibition. Cancer Cell. 2016;30:879–890. doi: 10.1016/j.ccell.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hoshida Y, Villanueva A, Sangiovanni A, Sole M, Hur C, Andersson KL, Chung RT, Gould J, Kojima K, Gupta S, Taylor B, Crenshaw A, Gabriel S, Minguez B, Iavarone M, Friedman SL, Colombo M, Llovet JM, Golub TR. Prognostic gene expression signature for patients with hepatitis C-related early-stage cirrhosis. Gastroenterology. 2013;144:1024–1030. doi: 10.1053/j.gastro.2013.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jones PA, Issa JP, Baylin S. Targeting the cancer epigenome for therapy. Nat Rev Genet. 2016;17:630–641. doi: 10.1038/nrg.2016.93. [DOI] [PubMed] [Google Scholar]
- 63.Hamdane N, Jühling F, Crouchet E, El Saghire H, Thumann C, Oudot MA, Bandiera S, Saviano A, Ponsolles C, Roca Suarez AA, Li S, Fujiwara N, Ono A, Davidson I, Bardeesy N, Schmidl C, Bock C, Schuster C, Lupberger J, Habersetzer F, Doffoël M, Piardi T, Sommacale D, Imamura M, Uchida T, Ohdan H, Aikata H, Chayama K, Boldanova T, Pessaux P, Fuchs BC, Hoshida Y, Zeisel MB, Duong FHT, Baumert TF. HCV-Induced Epigenetic Changes Associated With Liver Cancer Risk Persist After Sustained Virologic Response. Gastroenterology. 2019;156:2313–2329.e7. doi: 10.1053/j.gastro.2019.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Perez S, Kaspi A, Domovitz T, Davidovich A, Lavi-Itzkovitz A, Meirson T, Alison Holmes J, Dai CY, Huang CF, Chung RT, Nimer A, El-Osta A, Yaari G, Stemmer SM, Yu ML, Haviv I, Gal-Tanamy M. Hepatitis C virus leaves an epigenetic signature post cure of infection by direct-acting antivirals. PLoS Genet. 2019;15:e1008181. doi: 10.1371/journal.pgen.1008181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Santangelo L, Bordoni V, Montaldo C, Cimini E, Zingoni A, Battistelli C, D'Offizi G, Capobianchi MR, Santoni A, Tripodi M, Agrati C. Hepatitis C virus direct-acting antivirals therapy impacts on extracellular vesicles microRNAs content and on their immunomodulating properties. Liver Int. 2018;38:1741–1750. doi: 10.1111/liv.13700. [DOI] [PubMed] [Google Scholar]
- 66.Villani R, Facciorusso A, Bellanti F, Tamborra R, Piscazzi A, Landriscina M, Vendemiale G, Serviddio G. DAAs Rapidly Reduce Inflammation but Increase Serum VEGF Level: A Rationale for Tumor Risk during Anti-HCV Treatment. PLoS One. 2016;11:e0167934. doi: 10.1371/journal.pone.0167934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Setiawan VW, Rosen HR. Stratification of Residual Risk of HCC Following HCV Clearance With Direct-Acting Antivirals in Patients With Advanced Fibrosis and Cirrhosis. Hepatology. 2020;72:1897–1899. doi: 10.1002/hep.31639. [DOI] [PubMed] [Google Scholar]
- 68.European Association for the Study of the Liver. EASL Recommendations on Treatment of Hepatitis C 2018. J Hepatol. 2018;69:461–511. doi: 10.1016/j.jhep.2018.03.026. [DOI] [PubMed] [Google Scholar]
- 69.Tani J, Morishita A, Sakamoto T, Takuma K, Nakahara M, Fujita K, Oura K, Tadokoro T, Mimura S, Nomura T, Yoneyama H, Kobara H, Himoto T, Tsutsui A, Senoh T, Nagano T, Ogawa C, Moriya A, Deguchi A, Takaguchi K, Masaki T. Simple scoring system for prediction of hepatocellular carcinoma occurrence after hepatitis C virus eradication by direct-acting antiviral treatment: All Kagawa Liver Disease Group Study. Oncol Lett. 2020;19:2205–2212. doi: 10.3892/ol.2020.11341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shiha G, Waked I, Soliman R, Elbasiony M, Gomaa A, Mikhail NNH, Eslam M. GES: A validated simple score to predict the risk of HCC in patients with HCV-GT4-associated advanced liver fibrosis after oral antivirals. Liver Int. 2020;40:2828–2833. doi: 10.1111/liv.14666. [DOI] [PubMed] [Google Scholar]
- 71.Ioannou GN, Green PK, Beste LA, Mun EJ, Kerr KF, Berry K. Development of models estimating the risk of hepatocellular carcinoma after antiviral treatment for hepatitis C. J Hepatol. 2018;69:1088–1098. doi: 10.1016/j.jhep.2018.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alonso López S, Manzano ML, Gea F, Gutiérrez ML, Ahumada AM, Devesa MJ, Olveira A, Polo BA, Márquez L, Fernández I, Cobo JCR, Rayón L, Riado D, Izquierdo S, Usón C, Real Y, Rincón D, Fernández-Rodríguez CM, Bañares R. A Model Based on Noninvasive Markers Predicts Very Low Hepatocellular Carcinoma Risk After Viral Response in Hepatitis C Virus-Advanced Fibrosis. Hepatology. 2020;72:1924–1934. doi: 10.1002/hep.31588. [DOI] [PubMed] [Google Scholar]
- 73.Farinati F, Marino D, De Giorgio M, Baldan A, Cantarini M, Cursaro C, Rapaccini G, Del Poggio P, Di Nolfo MA, Benvegnù L, Zoli M, Borzio F, Bernardi M, Trevisani F. Diagnostic and prognostic role of alpha-fetoprotein in hepatocellular carcinoma: both or neither? Am J Gastroenterol. 2006;101:524–532. doi: 10.1111/j.1572-0241.2006.00443.x. [DOI] [PubMed] [Google Scholar]
- 74.Grammatikos G, Ferreiròs N, Waidmann O, Bon D, Schroeter S, Koch A, Herrmann E, Zeuzem S, Kronenberger B, Pfeilschifter J. Serum Sphingolipid Variations Associate with Hepatic Decompensation and Survival in Patients with Cirrhosis. PLoS One. 2015;10:e0138130. doi: 10.1371/journal.pone.0138130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mücke VT, Thomas D, Mücke MM, Waidmann O, Zeuzem S, Sarrazin C, Pfeilschifter J, Vermehren J, Finkelmeier F, Grammatikos G. Serum sphingolipids predict de novo hepatocellular carcinoma in hepatitis C cirrhotic patients with sustained virologic response. Liver Int. 2019;39:2174–2183. doi: 10.1111/liv.14178. [DOI] [PubMed] [Google Scholar]
- 76.Ioannou GN, Tang W, Beste LA, Tincopa MA, Su GL, Van T, Tapper EB, Singal AG, Zhu J, Waljee AK. Assessment of a Deep Learning Model to Predict Hepatocellular Carcinoma in Patients With Hepatitis C Cirrhosis. JAMA Netw Open. 2020;3:e2015626. doi: 10.1001/jamanetworkopen.2020.15626. [DOI] [PMC free article] [PubMed] [Google Scholar]