Abstract
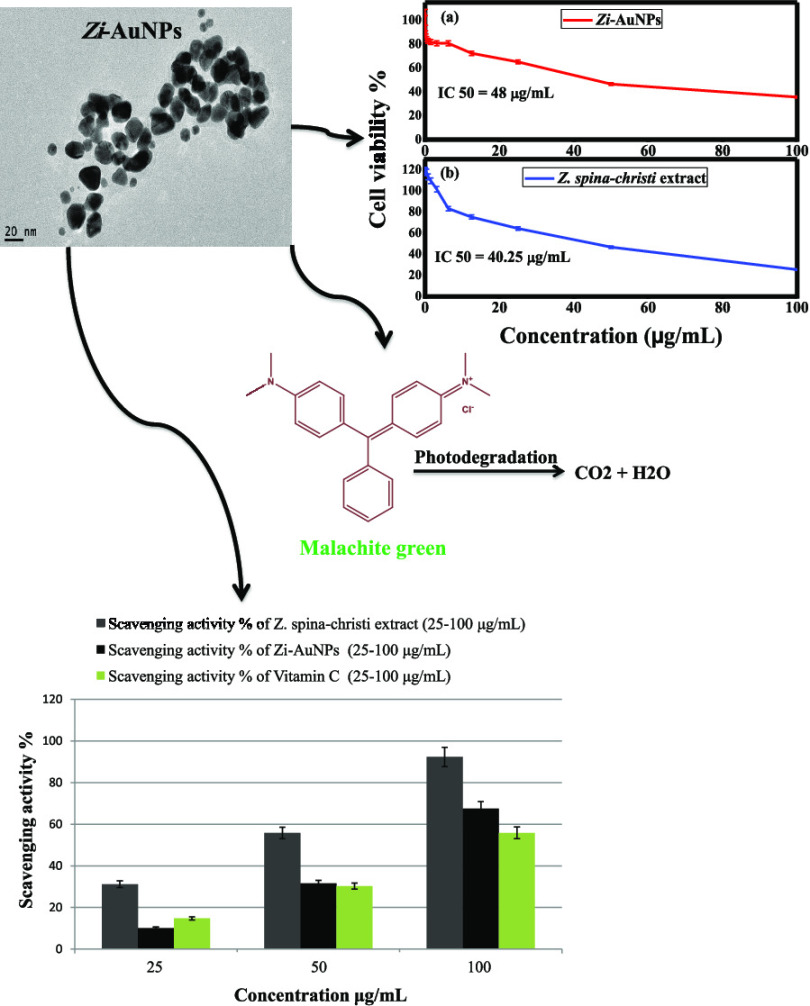
In the current study, a facile, rapid, and ecologically safe photosynthesis of gold nanoparticles (AuNPs) that remained stable for 3 months is reported to advocate the main aspects of green chemistry, such as safer solvents and auxiliaries, and the use of renewable feedstock. Zi-AuNPs were phytosynthesized by the aqueous extract of Ziziphus spina-christi leaves, and numerous techniques were employed for their characterization. The results demonstrated the successful phytofabrication of crystalline AuNPs with brownish-black color, spherical nanoparticles with a size between 0 and 10 nm, a plasmon peak at 540 nm, and a surface charge of −25.7 mV. Zi-AuNPs showed an effective photodegradation efficiency (81.14%) against malachite green and a good recycling capacity of 69.2% after five cycles of regeneration. The cytotoxicity test by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay signified a high anticancer efficiency for both Zi-AuNPs and Z. spina-christi extract against human breast cancer cells (MCF7 cell line) with IC50’s of 48 and 40.25 μg/mL, respectively. Highly efficient antioxidant capabilities were proven with 2,2-diphenyl-1-picrylhydrazyl (DPPH) removal percentages of 67.5% for Zi-AuNPs and 92.34% for Z. spina-christi extract.
1. Introduction
Nanotechnology has been postulated as a research area that combines the principles of biology, physics, and chemistry to synthesize new materials with unique properties at a very small scale, which is the nanoscale (0–100 nm).1,2 It is an interdisciplinary research area that involves developing, controlling, and utilizing these nanomaterials at the molecular level and in a lot of different applications.3,4 Optoelectronic devices, catalysis, cancer treatment, drug delivery, energy, space industry, and wastewater treatment are just some examples of the applications that nanotechnology is transforming through harnessing nanomaterials owing to their extremely small particle size and high surface area that render them unique characteristics.5,6
Various techniques could be employed in the fabrication of nanomaterials such as chemical reduction, electrochemical synthesis, physical synthesis, and lithography. These techniques are usually expensive and hazardous because they frequently involve the use of noxious reducing agents and harsh reaction conditions.7 On the other hand, biosynthesis of nanostructures via plants and microorganisms aroused as a safe, inexpensive, and more sustainable alternative.8 Relying on microorganisms requires cautious handling and maintenance in culturing and growing of these organisms, which to some extent adds to the expenses.9 Therefore, relying on plant extracts in the synthesis of nanomaterials has garnered most of the workers’ interests owing to the existence of various phytoactive constituents in plant extracts that are working as reducing and stabilizing agents at the same time, resulting in lower cost, facile, rapid, and efficient synthesis.10 Extracts of numerous plant species were utilized for nanomaterials production including Potamogeton pectinatus, Scutellaria barbata, and Phragmites australis to obtain different sized and shaped nanomaterials that could be harnessed in many applications.
Owing to their special properties such as quick synthesis, catalytic and size-dependent properties, and low toxicity, gold nanoparticles (AuNPs) are attracting more attention today in numerous applications.11,12 AuNPs could be employed in imaging, drug delivery, and photocatalysis.13 Production of AuNPs with different morphologies and characteristics could be accomplished through variation in the preparation conditions including pH and other factors.5
AuNPs and other nanomaterials have been widely employed in wastewater treatment to remove different types of pollutants as a result of their outstanding characteristics such as low cost and recyclability.14,15 These pollutants have seriously threatened human health and water quality.16 One of these toxic pollutants is malachite green (MG) dye, which is used in food coloring as well as in cloths, cotton, and wool industries.17
Currently, there is a high death rate from cancer in different countries and the treatment protocols are not very efficient.18 Despite imposing deleterious impacts on human health, conventional cancer treatments are also highly expensive.19 On this basis, there is an urgent requirement for less costly and safer methods of improving anticancer drugs and therapies.20 Consequently, the synthesis of sustainable and low-cost anticancer agents like AuNPs was targeted in the current study.
The role of free radicals’ scavengers, which are commonly known as antioxidants, was confirmed to be quintessential because of the detrimental impacts of these radicals.21 Free radicals such as 2,2-diphenyl-1-picrylhydrazyl (DPPH) are usually removed by antioxidants.22
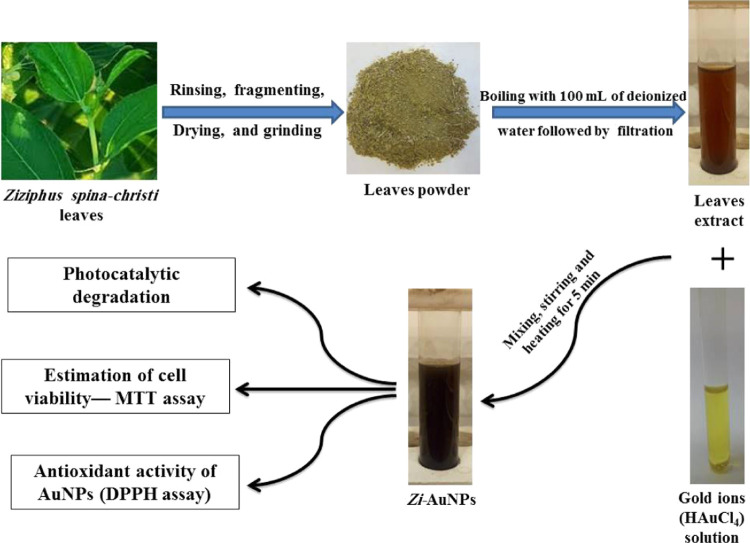
Ziziphus spina-christi is a deciduous tree that is commonly found in warm temperate and subtropical regions including the Middle East.23 Its antimicrobial, antifungal, antioxidant, and antidiabetic potentials were meticulously investigated, and they were accredited to its phytoconstituents including flavonoids, saponins, alkaloids, glycosides, and terpenoids.24 Therefore, the aqueous extract of Z. spina-christi was used in this work to phytofabricate AuNPs (Zi-AuNPs). Thus, the novel part of the current work is the rapid phytosynthesis of AuNPs using Z. spina-christi extract that were stable for about 3 months and further investigate the photocatalytic, anticancer, and antioxidant potentialities of these nanoparticles.
2. Results and Discussion
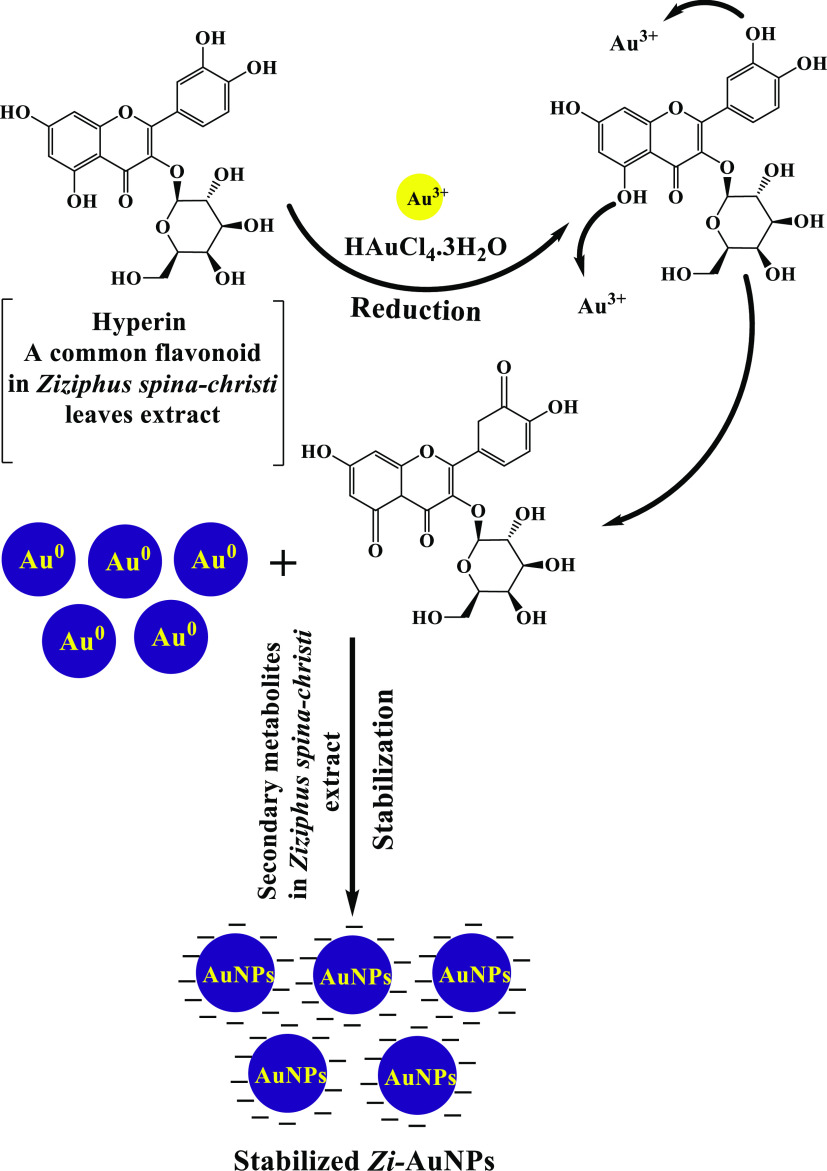
Z. spina-christi was proved to possess antinociceptive, antidiabetic, antioxidant, and antibacterial properties.24 The examination of the chemical composition of Z. spina-christi unraveled the existence of numerous phytoactive constituents such as alkaloids, flavonoids, glycosides, terpenoids, and tannins.25,26 The biosynthesis procedures are provided in Scheme 1. In addition, Figure 1 displays a facile probable mechanism for the phytofabrication of Zi-AuNPs via hyperin, which is a common flavonoid in Z. spina-christi leaves. Hyperin and other phytoconstituents such as rutin, quercetin, isovitexin, lucoside-7-O-rhamnoside, and apigenin in Z. spina-christi aqueous extract24 are reducing gold ions (Au3+) into nano gold (Au0). Furthermore, they contribute to the stabilization of Zi-AuNPs by capping them, with other phytoactive components, leading to the formation of stable Zi-AuNPs.
Scheme 1. Biosynthesis Procedures of Zi-AuNPs Colloidal Solution.
Figure 1.
Proposed mechanism of Zi-AuNPs synthesis.
2.1. UV–Visible Analysis
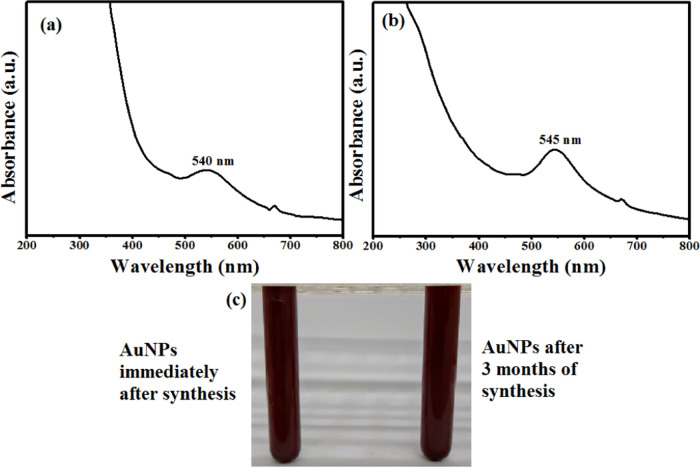
In the current study, a transformation in the color from light brown to brownish-black after adding gold ions to the extract of Z. spina-christi and a surface plasmon resonance (SPR) peak at 540 nm denoted the successful formation of Zi-AuNPs (Figure 2a). The obtained results were concomitant with others stated in previous works that aimed at the phytofabrication of AuNPs using other extracts such as Sunderam et al.27 who synthesized AuNPs using Anacardium occidentale leaf extract and reported a wavelength of 540 nm as well as Chen et al.28 who observed an SPR peak at 539 nm when synthesizing AuNPs by the extract of Curcumae kwangsiensis leaves due to the disappearance of d–d transitions of gold ions, indicating the successful green synthesis process. Additionally, it should be stated that the synthesized Zi-AuNPs were stabilized for almost 3 months as presented in Figure 2b,c, demonstrating the high efficacy of Z. spina-christi extract in the biosynthesis of AuNPs and also its promising application in the preparation of other nanomaterials.
Figure 2.
(a) UV–visible spectrum of immediately synthesized Zi-AuNPs. (b) UV–visible spectrum of Zi-AuNPs after 3 months. (c) Image of Zi-AuNPs promptly after synthesis and after 3 months.
2.2. Fourier Transform Infrared (FT-IR) Analysis
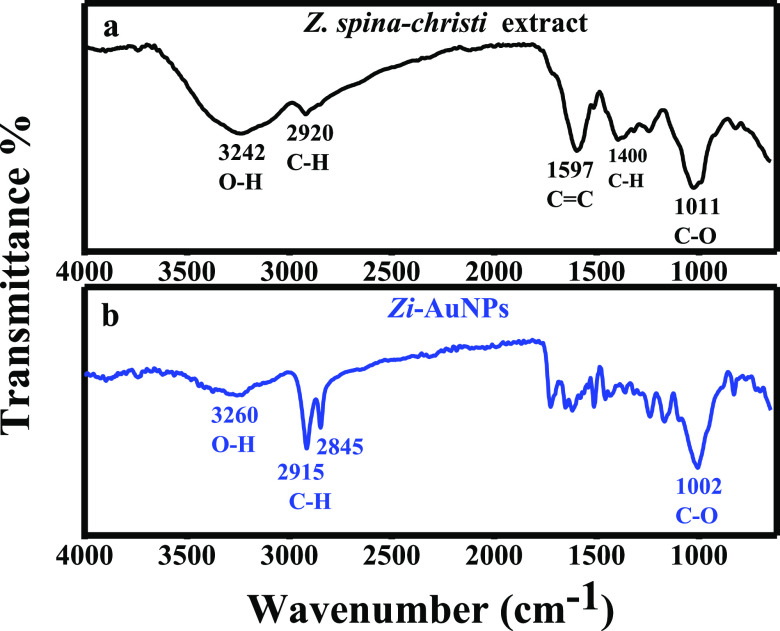
FT-IR spectroscopy is often employed in the detection of functional groups, which are likely participating in the reduction and stabilization of phytosynthesized nanomaterials. Several bands appeared in the IR spectrum of Z. spina-christi extract (Figure 3a) that are characteristic for different functional groups including the H-bonded O–H band at 3242 cm–1 that shifted to 3260 cm–1 in Zi-AuNPs accompanied with a dramatically lower intensity as shown in Figure 3b. A C–H stretch was observed at 2920 cm–1, which shifted to doublet C–H at 2915 and 2845 cm–1. Moreover, C=C band and C–H bending were identified at 1597 and 1400 cm–1, respectively. Subsequently, they completely disappeared in the spectrum of Zi-AuNPs. C–O stretching appeared at 1011 cm–1, which then slightly shifted to a lower wavenumber of 1002 cm–1 in Zi-AuNPs. Similar results to the current one were reported in other recent works.29,30 Therefore, it was concluded that various phytoconstituents, especially those containing O–H functional groups, such as alkaloids, flavonoids, and tannins, were responsible for the reduction and stabilization of Zi-AuNPs.
Figure 3.
FT-IR spectra of (a) Z. spina-christi extract and (b) Zi-AuNPs.
2.3. High-Resolution Transmission Electron Microscopy (HRTEM) Analysis
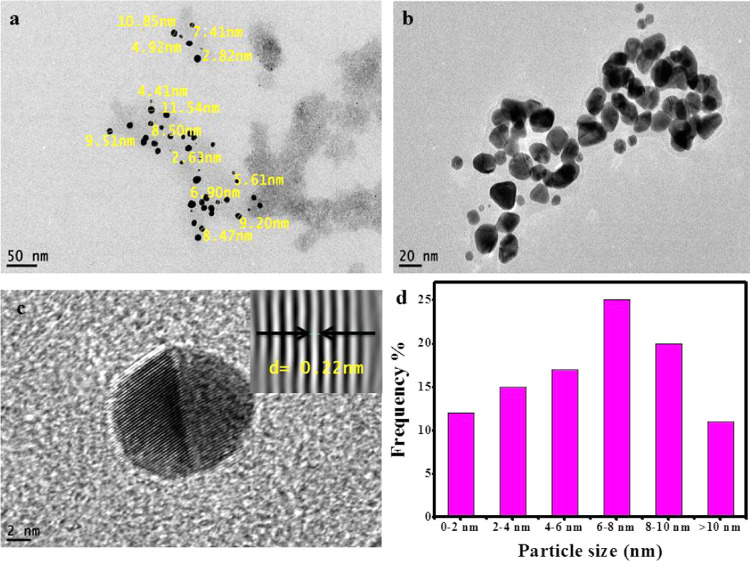
Size, shape, and other morphological characteristics of nanoparticles could be provided by microscopy techniques. Data collected from HRTEM analysis indicated that the size of Zi-AuNPs ranged from less than 2 nm to more than 10 nm and the principal shape formed was spherical as shown in Figure 4a–c. Furthermore, a histogram demonstrating the various sizes of Zi-AuNPs is displayed in Figure 4d, indicating that the average size is 6–8 nm with around 25%. The detected particle size in this study was concluded to be smaller than that observed in many studies such as AuNPs phytosynthesized by the Convolvulus fruticosus extract that was around 30 nm.31 Moreover, other shapes were observed, including rod, triangle, and others, and it was concluded that diverse shapes of phytosynthesized AuNPs are mainly formed through the various phytocomponents present in the plant extracts that have different reducing capacities.32 Crystalline Zi-AuNPs had an interplanar spacing of d = 0.22 nm, which is presented in Figure 4c (inset).
Figure 4.
(a–c) HRTEM images of Zi-AuNPs and (d) particle size distribution histogram of Zi-AuNPs.
2.4. X-ray Diffraction (XRD) Analysis
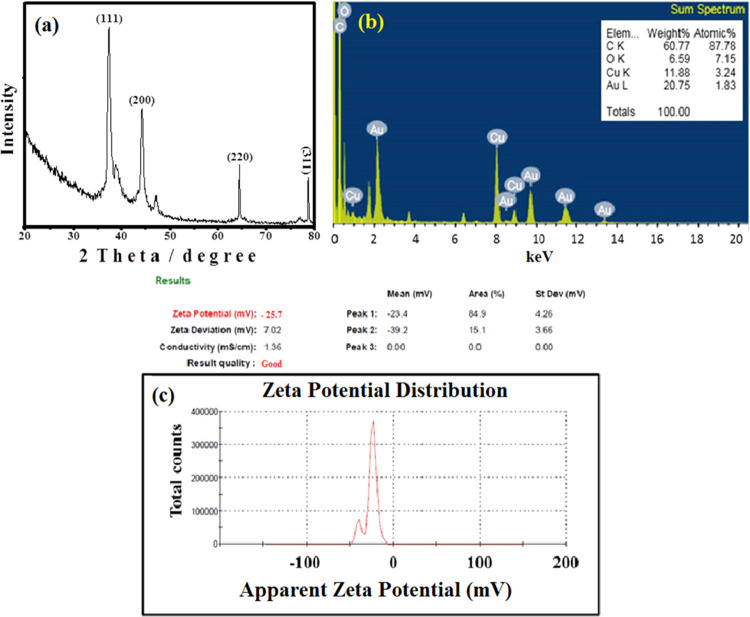
The face-centered cubic (FCC) crystalline structure of Zi-AuNPs was confirmed via XRD analysis that is displayed in Figure 5a as this analysis is reckoned as an essential step in determining the crystal structure of nanomaterials.33,34 Four distinguishing peaks of Au were observed at 37.31, 44.31, 64.51, and 78.73° matching the planes of (111), (200), (220), and (311), respectively. Moreover, the main growth orientation was the (111) plane as previously reported in different works.35 These values were matching the reported standards JCPDS file no. 04-0784 for crystalline Au. The crystallite size of Zi-AuNPs was measured by the Scherrer equation and found to be nearly 4.18 nm that was within the size range (0–10 nm) measured by HRTEM. This crystallite size was similar to that reported in another study that targeted the formation of AuNPs via the extract of Atriplex halimus,(36) which was 6.31 nm.
Figure 5.
(a) XRD spectrum of Zi-AuNPs. (b) Energy-dispersive X-ray (EDX) spectrum of Zi-AuNPs: inset: elemental analysis. (c) ζ-Potential of Zi-AuNPs.
2.5. EDX Analysis
In the current research, the bioreduction of Au3+ into Zi-AuNPs was ensured by EDX analysis that was designated by the strong peaks of Au0 at 2.3, 1.8, 8.5, 9.7, 11.5, and 13.4 keV as displayed in Figure 5b. These signals were concomitant with other previously reported results.37 Signals for carbon and oxygen were observed that are deemed to be resulting from the plant extract. The production yield of Zi-AuNPs was found to be 20.75%, as demonstrated in Figure 5b (inset), indicating the high efficiency of the Z. spina-christi extract in reducing Au3+ into Zi-AuNPs.
2.6. ζ Measurement
The existence of negative charge on the Zi-AuNPs surface is commonly attributed to the presence of several biological constituents in Z. spina-christi extract that preserves the colloidal stability of Zi-AuNPs. The ζ-potential of Zi-AuNPs was −25.7 mV, as displayed in Figure 5c, at a pH value around 6 denoting the prominent role of the phytoconstituents found in Z. spina-christi leaf aqueous extract in preserving the stability of the phytofabricated Zi-AuNPs. When the obtained results were compared to previously published ones such as ref (29), it was concluded that the current ζ-potential value is better as they detected a ζ value of −7.47 mV. Thus, the high degree of stability of Zi-AuNPs was confirmed.
2.7. Photocatalytic Degradation
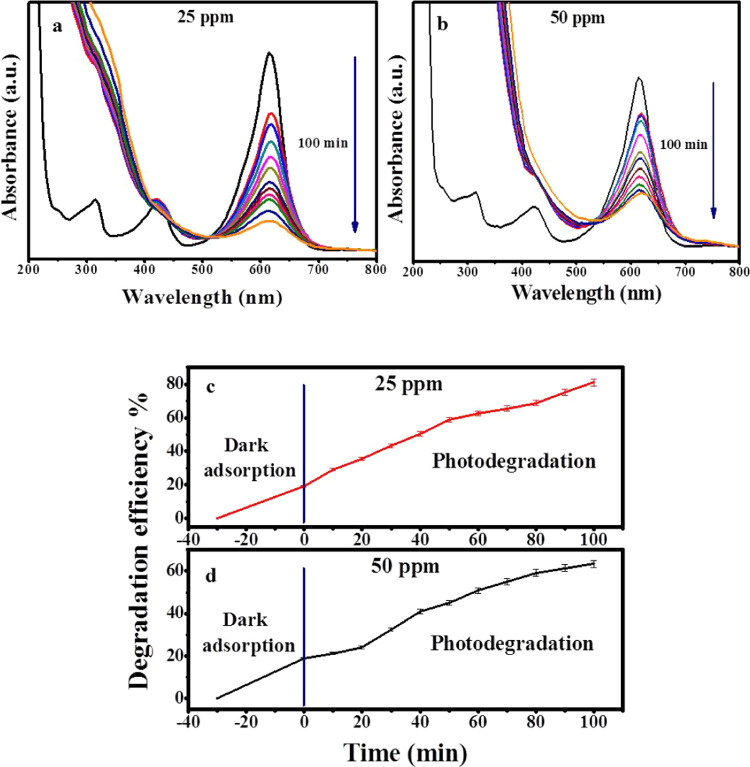
In the current study, the photodegradation of MG, which is a toxic organic pollutant usually resulting from various manmade sources via Zi-AuNPs, was examined using UV light irradiation. Two concentrations of MG were tested in the current work: 25 and 50 ppm. Regarding the lower concentration, its degradation percentage was about 81.14% after 100 min under UV irradiation, as displayed in Figure 6a. However, the photodegradation efficiency in the case of the higher concentration (Figure 6b) was 63.29% during the same time under pH 6 and 25 °C temperature and without the addition of free radicals.
Figure 6.
UV–visible absorption spectra of photodegradation of MG by Zi-AuNPs using different MG concentrations: (a) 25 ppm and (b) 50 ppm. Effect of time on the photodegradation of MG at concentrations of (c) 25 ppm and (d) 50 ppm.
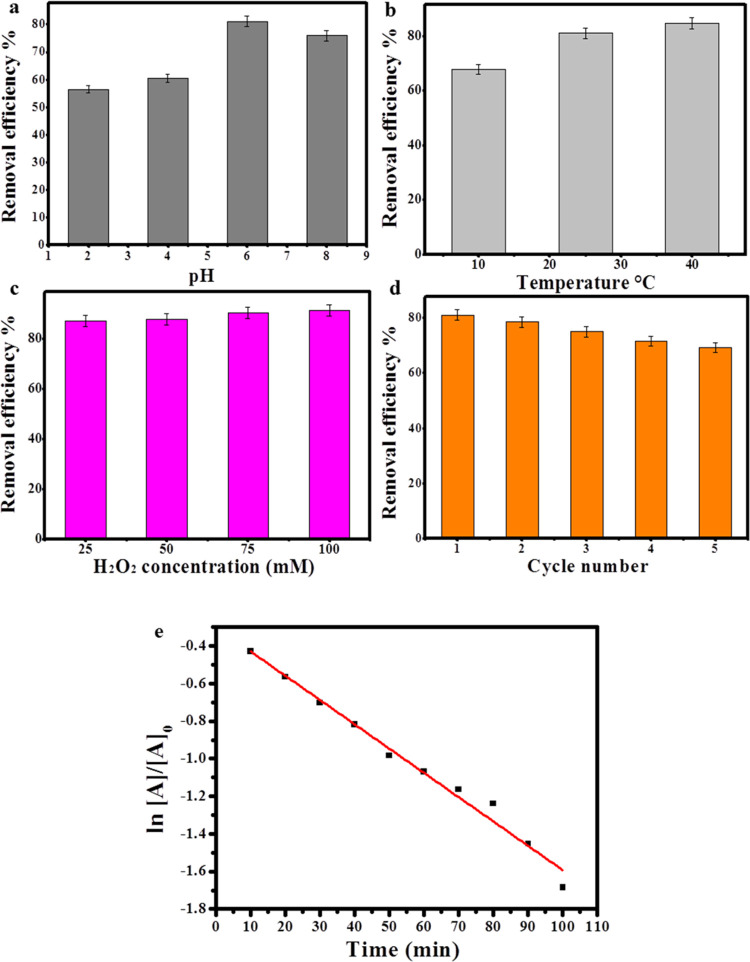
When the pH of the solution was first examined within 100 min of photodegradation of MG with a concentration of 25 ppm since the pH is postulated to be the most influential factor on the photodegradation process because the variation in pH usually influences the surface charge, adsorption ability, and electron transfer capability of the photocatalyst, consequently affecting the photodegradation rate.38 When the photodegradation process was carried out under highly acidic conditions (pH 2), the removal efficiency was about 56.5%. Afterward, the removal efficiency increased to 60.5 and 81.14% with increasing the pH value to 4 and 6. However, the degradation efficiency diminished to approximately 76% when the pH value was 8, denoting that the optimum pH level is 6. Thus, it was concluded that Zi-AuNPs could be applied as a photocatalyst for the photodegradation of MG over various pH levels as shown in Figure 7a.
Figure 7.
Effect of (a) pH, (b) temperature, and (c) H2O2 concentration on the removal efficiency of MG. (d) Recycling of Zi-AuNPs in MG removal. (e) Kinetics of MG removal by Zi-AuNPs.
Concerning the influence of temperature on MG removal with the concentration of 25 ppm in 100 min and under the optimum pH that is 6 (Figure 7b), the removal efficiency was recorded to be 67.85% when the temperature was 10 °C. Yet, the efficiency increased to approximately 81 and 84.7% when the temperature was set at 25 and 40 °C, respectively. Therefore, it was determined that by increasing temperature, the photodegradation process becomes enhanced due to the excessive release of hydroxyl radicals, which is in agreement with other previously published works.39 However, the average temperature (25 °C) was used for the rest of the experimental work to simulate the natural conditions, which are usually found in wastewater.
To determine the effect of free radicals such as H2O2 on the photodegradation of MG, four different concentrations of H2O2 (25, 50, 75, and 100 mM) were applied to the MG solutions (25 ppm) with a pH of 6 for 100 min as presented in Figure 7c. The obtained photodegradation percentage was approximately 87.22% when the radical concentration was 25 mM. Then, on increasing the concentration of H2O2 to 50, 75, and 100 mM, the removal efficiency was enhanced to 87.88, 90.38, and 91.42%, respectively. Therefore, the positive effect of H2O2 addition was proven through the improvement of MG removal by increasing the concentration of H2O2 that could be accredited to the release of hydroxyl groups that led to the provision of more hydroxyl radicals to the photodegradation system.40
When the stability and recycling of Zi-AuNPs as a photocatalyst was investigated (Figure 7d) within 100 min of photocatalytic degradation of 25 ppm MG under pH 6, it was observed that the efficiency diminished from 81 to 69.2% after five recycling times, signifying the high applicability of Zi-AuNPs regeneration. The kinetics study of MG removal with the concentration of 25 ppm by Zi-AuNPs is presented in Figure 7e, in which the rate constant (K) was found to be 0.0129 min–1. The obtained K in this study was suggested to be better than other previously reported studies on the photodegradation of MG, such as Tsvetkov et al.,41 who reported rate constants of 0.0083 and 0.0073 min–1 for MgFO/AgNPs and ZnFe2O4 nanocomposites, respectively, as well as better than Sharma et al.42 who reported a rate constant of 0.0102 min–1 using a nanocomposite hydrogel.
According to the above-mentioned results, it was determined that Zi-AuNPs is a promising photocatalyst that could proficiently be employed in wastewater treatment via the degradation of toxic organic pollutants. Furthermore, the effect of time on the photodegradation process of the two concentrations of MG is presented in Figure 6c,d. A comparison of the photodegradation efficacies between Zi-AuNPs and other photocatalysts utilized in MG photodegradation is presented in Table 1.
Table 1. Comparison of Photodegradation Efficacies between Zi-AuNPs and Other Photocatalysts against MG.
| catalyst | dye concentration (ppm) | degradation efficiency (%) | time (min) | ref |
|---|---|---|---|---|
| CuFe2O4 | 18 | 62.37 | 210 | (47) |
| ZnO | 5 | 49 | 60 | (48) |
| ZnO modified with EDTA | 94.14 | |||
| CdSe/TiO2 | 10 | 99 | 360 | (49) |
| chitosan-supported ZnO | 5 | ∼100 | 90 | (50) |
| Pd/WO3 photocatalyst | 5 | 66.66 | 360 | (51) |
| Au–ZnO nanofilm | 10 | 76.92 | 240 | (52) |
| Zi-AuNPs | 25 | 81.14 | 100 | current study |
| 50 | 63.29 |
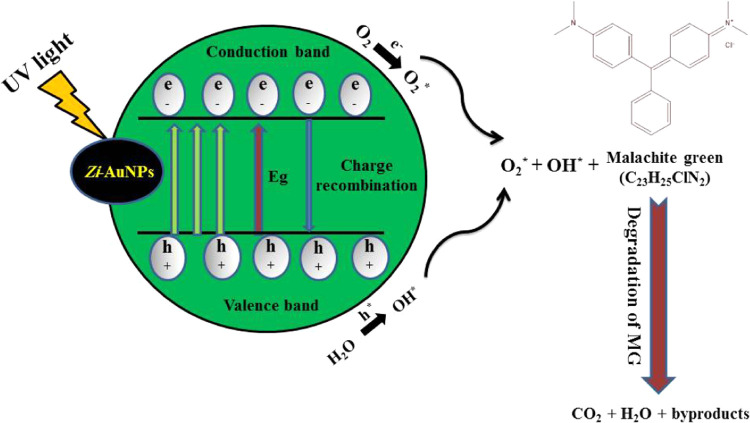
A mechanism for the photodegradation of MG is demonstrated in Figure 8, in which Zi-AuNPs resulted in MG degradation into simple, nontoxic, and inorganic products such as CO2 and H2O. The photodegradation process of MG is simply demonstrated through charge transfer procedures, which were previously elaborated in refs (43) and (44) through the following equations
| 1 |
| 2 |
| 3 |
| 4 |
Figure 8.
Probable mechanism of the photodegradation of MG by Zi-AuNPs.
By absorbing UV light, MG adsorbed on the surface of Zi-AuNPs became excited and commenced to donate its electrons to the conduction band of Zi-AuNPs. Afterward, these electrons were scavenged by dissolved oxygen and resulted in the formation of a highly reactive superoxide oxygen radical (O2*). Moreover, the O2* can react with H2O adsorbed on the MG surface to form H2O2 that is promptly transformed into OH*. Eventually, both O2* and OH* led to the degradation of MG into CO2, H2O, and byproducts. The obtained results are similar to Huang et al.45 and Li et al.46 who clarified the important role of reactive oxygen species (ROS) in the photocatalytic degradation of different organic pollutants.
2.8. Estimation of Cell Viability—3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium Bromide (MTT) Assay
A variety of unique properties of AuNPs enabled them to be utilized as drug delivery carriers. These properties include small particle size, nontoxicity, and low levels of immunogenicity.53 Therefore, they were extensively utilized in cancer treatment. As tumor-targeting delivery materials get smaller like AuNPs, the possibility of them bypassing the body’s natural barriers becomes better.54
Apoptosis is inhibited by physiological ATP levels, so ATP is thought to control apoptotic signals.55 Caspase is supposed to play a crucial role in programmed cell death in a group of protease enzymes, and its activation is believed to depend on intracellular ATP concentrations,56 so a drop in the ATP level after the addition of Zi-AuNPs might be presumed accountable for causing apoptosis via caspase pathways and resulting in cell death. Green synthesized AuNPs were also proved to be responsible for the release of ROS in a dose-dependent manner.57 ROS are generally toxic to cancer cells, and their role in the cellular death process is deemed to be indispensable.58 Additionally, affecting cell membrane integrity, disrupting ATP synthesis, and obstructing electron transfer are deemed to be the most commonly induced disorders when Zi-AuNPs interacted with MCF7 cancer cells that led to cell shrinkage and apoptosis.59
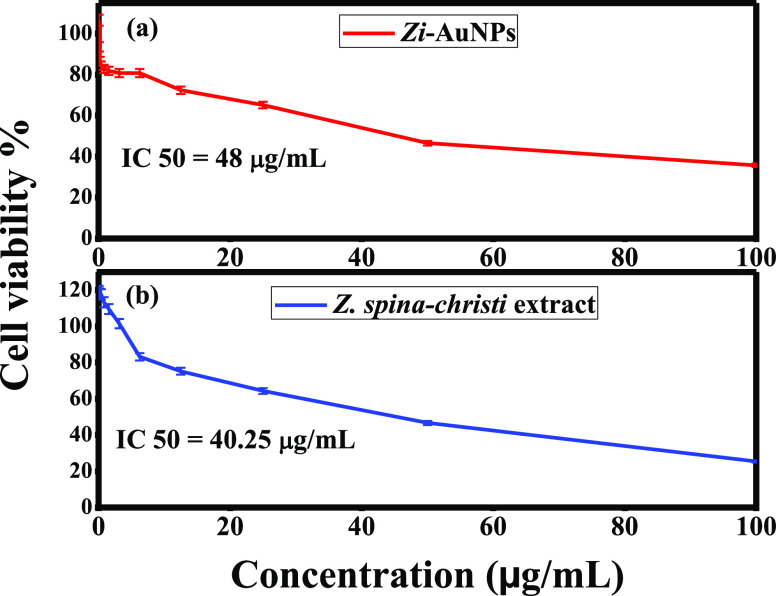
In this study, the anticancer efficacies of Zi-AuNPs and Z. spina-christi extract were examined against MCF7 utilizing MTT assay. The acquired results indicated that both samples exhibited good anticancer performance as the recorded cell viabilities were 35.73 and 25.44% at the highest concentration (100 μg/mL) of Zi-AuNPs (Figure 9a) and Z. spina-christi extract (Figure 9b), respectively. This may possibly be accredited to their capability of penetrating cell membrane, interacting with and disrupting proteins and other biomolecules.
Figure 9.
Anticancer efficiency % of (a) Zi-AuNPs and (b) Z. spina-christi extract against MCF7 cancer cells.
Zi-AuNPs and Z. spina-christi extract acquired IC50 concentrations of 48 and 40.25 μg/mL, respectively, signifying good efficacy in cancer treatment. A comparison between Zi-AuNPs and other synthesized AuNPs to indicate the higher cytotoxic efficiency of Zi-AuNPs is presented in Table 2.
Table 2. Comparison of Cytotoxic Efficiencies between Zi-AuNPs and Other Gold Nanoparticles.
2.9. Antioxidant Test
Antioxidants, such as metallic nanoparticles, play a significant role in the fight against free radicals like DPPH which is a commonly harmful free radical.65 Regarding the mechanism of the DPPH assay, it was concluded that via receiving a hydrogen atom or an electron from an antioxidant such as Zi-AuNPs, the OH-DPPH solution was altered into the nonradical form of DPPH-H, thus leading to DPPH removal. The assay was intended to detect the difference in DPPH concentration resulted from the reaction of DPPH with Zi-AuNPs using a spectrophotometer. Accordingly, the reception of an electron given by Zi-AuNPs led to the degradation of DPPH, which was determined quantitatively via the absorbance changes at 517 nm.
Using Zi-AuNPs to prevent the oxidative chain reaction from starting results in the generation of nonreactive radicals, which is what the antioxidant activity concept refers to. It was also found that AuNPs, particularly those made using biological or green methods, have significant antioxidant potential to control oxidative damage.28 Another important point to keep in mind is that Zi-AuNPs’ antioxidant activity is reliant on the phytoconstituents that are capping the nanoparticles.
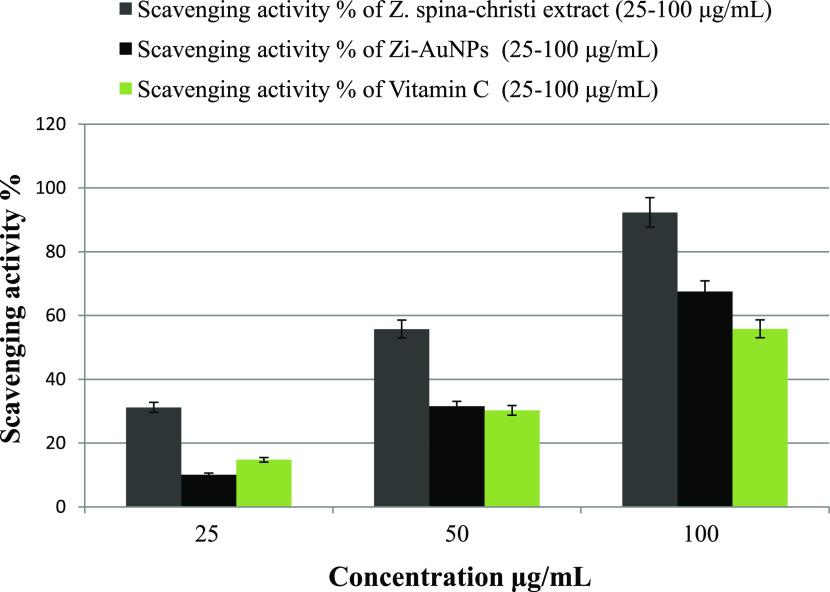
In the current work, Figure 10 indicates that all of the samples had an inhibitory effect against DPPH in a concentration-dependent manner. Both Z. spina-christi extract and Zi-AuNPs demonstrated high efficacies of DPPH removal of about 92.34 and 67.5%, respectively, at a concentration of 100 μg/mL. The scavenging activity of vitamin C that was used as a reference was found to be less efficient since it removed about 55.84% of DPPH at the same concentration. It should be noted that the presence of numerous phytoconstituents such as flavonoids on the surface of Zi-AuNPs could be liable for their boosted antioxidant efficacy as previously deduced.59
Figure 10.
DPPH scavenging activity % of Z. spina-christi extract, Zi-AuNPs, and vitamin C (reference).
Other workers have concluded that plants with high reducing ability also have a high antioxidant efficiency.66,67 The antioxidant potency of Z. spina-christi is well reported.24 As a result, the aqueous extract of Z. spina-christi was found to be a better antioxidant, as evidenced by the rapid transformation of Au3+ into Zi-AuNPs after the addition of the extract.
Similarly to the obtained results, other workers reported higher antioxidant efficacies of aqueous plant extracts than AuNPs fabricated via these extracts, such as Zayed et al.,68 who mentioned that Pimpinella anisum extract was a more efficient antioxidant compared to AuNPs, and Sathishkumar et al.,69 who indicated a higher inhibitory efficacy for Couroupita guianensis extract compared to phytosynthesized AuNPs. Additionally, Nakkala et al.70 reported a higher antioxidant efficiency for Costus pictus extract than AuNPs phytoproduced by the same extract. A comparison among Zi-AuNPs and other metallic nanoparticles in the scavenging efficiency of DPPH is presented in Table 3.
Table 3. Comparison of Antioxidant Efficiencies between Zi-AuNPs and Other Metal Nanoparticles.
3. Conclusions
According to the aforementioned results, the following conclusions could be drawn. Z. spina-christi extract played a dual role of a reducing agent and a stabilizing agent in a single-step process for the synthesis of Zi-AuNPs that remained stable for almost 3 months. FT-IR results revealed that the phytoconstituents of Z. spina-christi extract such as flavonoids, glycosides, terpenoids, and tannins were accountable for reducing and preserving the stability of the phytosynthesized AuNPs, which were majorly spherical with a surface plasmon peak at 540 nm and a surface charge of −25.7 mV. Moreover, rod, triangle, rhomboid, pentagonal, and other irregular shapes were also detected. Crystalline nature with an FCC structure and a small particle size range of 0–10 nm were also confirmed. Furthermore, the crystallite size was measured and found to be 4.18 nm. Photodegradation results indicated the good applicability of Zi-AuNPs as a photocatalyst against MG with a photodegradation efficiency of 81.14% and good recycling capacity that was 69.2% after five cycles of regeneration. The high efficiencies of both Zi-AuNPs and Z. spina-christi extract as efficient anticancer agents against MCF7 cancer cells with cell viabilities of 35.73 and 25.44% for Zi-AuNPs and Z. spina-christi, respectively, were confirmed. Prominent antioxidant efficiencies were proven with DPPH scavenging percentages of 67.5% for Zi-AuNPs and 92.34% for Z. spina-christi extract.
4. Materials and Methods
4.1. Chemicals and Reagents
All of the reagents employed in the current work were of high purity, including gold tetrachloroaurate solution (HAuCl4·3H2O), 2,2-diphenyl-1-picrylhydrazyl (DPPH) (99%), ascorbic acid, and methylene blue (MB), that were purchased from Merck. Dimethyl sulfoxide (DMSO) was purchased from HiMedia (India), and the MCF7 cancer cell line was purchased from Vacsera center, Giza, Egypt.
4.2. Preparation of Plant Extract
To remove impurities and undesired elements, Z. spina-christi leaves were repeatedly rinsed with deionized water (DW). After that, it was fractured and let to dry in the open air before being dried in the oven overnight at 60 °C. After that, dry leaves were pulverized into a fine powder in a stainless steel mixer. The resulting powder (5 g) was mixed in 100 mL of DW, and then the mixture was agitated and heated for about 20 min until it was boiling.22 Finally, the heated solution was filtered, and the filtrate extract was kept at a temperature of 4.0 °C for further use.59
4.3. Biosynthesis of Zi-AuNPs
In brief, 10 mL of the leaves extract was mixed with 1 mL of HAuCl4·3H2O (0.011 M). As a result, after 5 min of heating and stirring, the color changed from light brown to brownish-black. The purification phase involved centrifuging the Zi-AuNPs colloidal solution, collecting the precipitated pellets, and washing them with DW. To purify AuNPs once they were generated, this step was done three times. The colloidal solution was then kept at 4.0 °C for later use.59
4.4. Characterization Techniques
The size and shape of AuNPs were determined by HRTEM measurements performed on a JOEL, JEM-2100F, Japan, with an accelerating voltage of 200 kV. The formation of AuNPs was monitored by the UV–vis spectroscopy measurements on a double-beam T70/T80 series UV/vis spectrophotometer (PG Instruments Ltd., U.K.).36 Furthermore, the FT-IR spectrum measurements were conducted for the ground sample with KBr on a JASCO spectrometer over the range 4000–600 cm–1. The ζ-potential of AuNPs was determined by a ζ-potential analyzer (Zetasizer Nano ZS Malvern). XRD measurements of powdered AuNPs were conducted on an X-ray diffractometer (X’Pert PRO, the Netherlands) operated at a voltage of 45 kV and current of 40 mA with Cu Kα1 radiation (λ = 1.54056 Å) in the 2θ range of 20–80°. The crystallite size was calculated from the width of the XRD peaks using the Scherrer formula59 given by
where D is the average crystallite size, β indicates the line broadening the value of the full width at half-maximum (FWHM) of a peak, λ is the wavelength of irradiated X-rays, and θ is the maximum peak position value. Elements were determined by energy-dispersive X-ray (EDX) spectroscopy, JEOL model JSM-IT100.
4.5. Photocatalytic Experiments
The photocatalytic activity of the green synthesized Zi-AuNPs against MG dye was evaluated. Zi-AuNPs (10 mg) was added to 10 mL of two different concentrations of MG solution (25 and 50 ppm). Various levels of pH were examined, including 2, 4, 6, and 8. Three different temperatures were tested comprising 10, 25, and 40 °C. Furthermore, the effect of free radicals such as H2O2 was inspected using four different concentrations (25, 50, 75, and 100 mM). All of these factors were optimized to achieve the best photocatalytic removal of MG. Test solutions were mixed for 30 min in the dark for adsorption/desorption equilibration.75 Subsequently, the solutions were stirred under a xenon lamp as a UV light source (λ > 400 nm) and monitored.76 Next, 2 mL aliquots were removed and centrifuged at 17 000 rpm for 1 min to separate the solid nanocatalyst.77 The absorbance of the resultant supernatant of MG solutions was measured at a 610 nm wavelength using UV–vis spectroscopy (T70/T80 series UV/vis spectrophotometer, PG Instruments Ltd., U.K.).36 The percentage of MG degradation was calculated by the following formula
| 5 |
where A0 represents the initial absorbance and A refers to the final absorbance.
Regarding the recycling process, Zi-AuNPs were first removed from the solution by centrifugation at 17 000 rpm for 1 min, then thoroughly washed with DW, and eventually dried overnight in the oven.
4.6. Estimation of Cell Viability—MTT Assay
The effect of AuNPs on MCF7 cells was assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay.32 Briefly, the cells (2 × 105 cells) were treated with various concentrations of Zi-AuNPs and Z. spina-christi extract (0.097–100 μg/mL) separately and incubated at 37 °C for 48 h using a CO2 incubator. Over the incubation period, 10 μL of MTT solution (5 mg/mL) was added and the cells were incubated again for 4 h. The formed purple color formazone crystals were dissolved in 100 μL of dimethyl sulfoxide (DMSO), and the intensity was measured at 570 nm. The experiment was conducted in triplicate, and the percentage of cell viability was calculated by eq 2
| 6 |
where OD stands for the optical density that was measured at average room temperature 25 °C and atmospheric pressure 1 atm.
4.7. Antioxidant Activity of AuNPs (DPPH Assay)
The free radical scavenging activity was examined via DPPH assay to determine the antioxidant efficiency of Zi-AuNPs and Z. spina-christi extract. The assay was conducted in triplicate. In the process, 1 mL of Zi-AuNPs and Z. spina-christi extract was mixed separately with 1 mL of DPPH (0.2 mM) along with control DPPH that does not contain any nanoparticles. The two mixtures were blended for 3 min in the dark at ambient temperature. Then, after 20 min, the concentration of radical is examined by the reduction in absorbance percentage of the mixture at 517 nm wavelength. Vitamin C (ascorbic acid) was used as a reference. The radical scavenging activity was determined by the following equation
| 7 |
where control absorbance is the absorbance in the absence of antioxidants and sample absorbance is the absorbance in the presence of antioxidants (Zi-AuNPs, Z. spina-christi extract, and vitamin C) at 517 nm.
4.8. Statistical Analysis
All experiments were conducted in triplicate (n = 3), while the gained data were presented as a mean value corrected by the standard deviation (±SD).
Acknowledgments
The authors would like to acknowledge the financial support provided from Taif University Researchers Supporting Project Number (TURSP-2020/106) Taif University, Taif, Saudi Arabia.
Author Contributions
M.H. carried out the experimental work, data analysis, and writing the original draft. A.S.E. was responsible for the investigation, methodology, visualization, and supervision. M.M. was responsible for conducting the experimental work and data curation. Y.A.E.-B. was responsible for visualization and supervision. E.E.H. was responsible for data analysis and data curation. A.M.O. was responsible for supervision. M.F. was responsible for conceptualization, supervision, validation, and reviewing the final manuscript.
The authors declare no competing financial interest.
References
- Jadoun S.; Arif R.; Jangid N. K.; Meena R. K. Green synthesis of nanoparticles using plant extracts: A review. Environ. Chem. Lett. 2021, 19, 355–374. 10.1007/s10311-020-01074-x. [DOI] [Google Scholar]
- Liu S.; Tian J.; Wang L.; Luo Y.; Sun X. One-pot synthesis of CuO nanoflower-decorated reduced graphene oxide and its application to photocatalytic degradation of dyes. Catal. Sci. Technol. 2012, 2, 339–344. 10.1039/C1CY00374G. [DOI] [Google Scholar]
- Hosny M.; Fawzy M.; El-Fakharany E. M.; Omer A. M.; El-Monaem E. M. A.; Khalifa R. E.; Eltaweil A. S. Biogenic synthesis, characterization, antimicrobial, antioxidant, antidiabetic, and catalytic applications of platinum nanoparticles synthesized from Polygonum salicifolium leaves. J. Environ. Chem. Eng. 2022, 10, 106806 10.1016/j.jece.2021.106806. [DOI] [Google Scholar]
- Attia N. F.; Park J.; Oh H. Facile tool for green synthesis of graphene sheets and their smart free-standing UV protective film. Appl. Surf. Sci. 2018, 458, 425–430. 10.1016/j.apsusc.2018.07.066. [DOI] [Google Scholar]
- Asiya S.; Pal K.; Kralj S.; El-Sayyad G.; de Souza F.; Narayanan T. Sustainable preparation of gold nanoparticles via green chemistry approach for biogenic applications. Mater. Today Chem. 2020, 17, 100327 10.1016/j.mtchem.2020.100327. [DOI] [Google Scholar]
- Attia N. F.; Eid A. M.; Soliman M. A.; Nagy M. Exfoliation and decoration of graphene sheets with silver nanoparticles and their antibacterial properties. J. Polym. Environ. 2018, 26, 1072–1077. 10.1007/s10924-017-1014-5. [DOI] [Google Scholar]
- Hameed S.; Ali Shah S.; Iqbal J.; Numan M.; Muhammad W.; Junaid M.; Shah S.; Khurshid R.; Umer F. Cannabis sativa-mediated synthesis of gold nanoparticles and their biomedical properties. Bioinspired, Biomimetic Nanobiomater. 2020, 9, 95–102. 10.1680/jbibn.19.00023. [DOI] [Google Scholar]
- Guo Y.; Jiang N.; Zhang L.; Yin M. Green synthesis of gold nanoparticles from Fritillaria cirrhosa and its anti-diabetic activity on Streptozotocin induced rats. Arab. J. Chem. 2020, 13, 5096–5106. 10.1016/j.arabjc.2020.02.009. [DOI] [Google Scholar]
- El-Borady O. M.; Fawzy M.; Hosny M. Antioxidant, anticancer and enhanced photocatalytic potentials of gold nanoparticles biosynthesized by common reed leaf extract. Appl. Nanosci. 2021, 1–12. 10.1007/s13204-021-01776-w. [DOI] [Google Scholar]
- Abdelfatah A. M.; Fawzy M.; Eltaweil A. S.; El-Khouly M. E. Green Synthesis of Nano-Zero-Valent Iron Using Ricinus communis Seeds Extract: Characterization and Application in the Treatment of Methylene Blue-Polluted Water. ACS Omega 2021, 25397. 10.1021/acsomega.1c03355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elahi N.; Kamali M.; Baghersad M. H. Recent biomedical applications of gold nanoparticles: A review. Talanta 2018, 184, 537–556. 10.1016/j.talanta.2018.02.088. [DOI] [PubMed] [Google Scholar]
- El-Maghrabi N.; El-Borady O. M.; Hosny M.; Fawzy M. Catalytic and Medical Potential of a Phyto-Functionalized Reduced Graphene Oxide–Gold Nanocomposite Using Willow-Leaved Knotgrass. ACS Omega 2021, 34954. 10.1021/acsomega.1c05596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajeshkumar S.; Malarkodi C.; Al Farraj D. A.; Elshikh M. S.; Roopan S. M. Employing sulphated polysaccharide (fucoidan) as medium for gold nanoparticles preparation and its anticancer study against HepG2 cell lines. Mater. Today Commun. 2021, 26, 101975 10.1016/j.mtcomm.2020.101975. [DOI] [Google Scholar]
- Sadegh H.; Ali G. A.; Gupta V. K.; Makhlouf A. S. H.; Shahryari-Ghoshekandi R.; Nadagouda M. N.; Sillanpää M.; Megiel E. The role of nanomaterials as effective adsorbents and their applications in wastewater treatment. J. Nanostruct. Chem. 2017, 7, 1–14. 10.1007/s40097-017-0219-4. [DOI] [Google Scholar]
- Cheng N.; Tian J.; Liu Q.; Ge C.; Qusti A. H.; Asiri A. M.; Al-Youbi A. O.; Sun X. Au-nanoparticle-loaded graphitic carbon nitride nanosheets: green photocatalytic synthesis and application toward the degradation of organic pollutants. ACS Appl. Mater. Interfaces 2013, 5, 6815–6819. 10.1021/am401802r. [DOI] [PubMed] [Google Scholar]
- Jiang Y.; Zhao H.; Liang J.; Yue L.; Li T.; Luo Y.; Liu Q.; Lu S.; Asiri A. M.; Gong Z.; Sun X. Anodic oxidation for the degradation of organic pollutants: anode materials, operating conditions and mechanisms. A mini review. Electrochem. Commun. 2021, 106912 10.1016/j.elecom.2020.106912. [DOI] [Google Scholar]
- Srivastava S.; Sinha R.; Roy D. Toxicological effects of malachite green. Aquat. Toxicol. 2004, 66, 319–329. 10.1016/j.aquatox.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Shunmugam R.; Balusamy S. R.; Kumar V.; Menon S.; Lakshmi T.; Perumalsamy H. Biosynthesis of gold nanoparticles using marine microbe (Vibrio alginolyticus) and its anticancer and antioxidant analysis. J. King Saud Univ., Sci. 2021, 33, 101260 10.1016/j.jksus.2020.101260. [DOI] [Google Scholar]
- Rajeshkumar S. Anticancer activity of eco-friendly gold nanoparticles against lung and liver cancer cells. J. Genet. Eng. Biotechnol. 2016, 14, 195–202. 10.1016/j.jgeb.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y.; You P.; Xu F.; Liu J.; Xing F. Novel functionalized selenium nanoparticles for enhanced anti-hepatocarcinoma activity in vitro. Nanoscale Res. Lett. 2015, 10, 349 10.1186/s11671-015-1051-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu S.; Li J.; Sun L.; Zhong L.; Ma Q. An in vitro comparison of the antioxidant activities of chitosan and green synthesized gold nanoparticles. Carbohydr. Polym. 2019, 211, 161–172. 10.1016/j.carbpol.2019.02.007. [DOI] [PubMed] [Google Scholar]
- Eltaweil A. S.; Fawzy M.; Hosny M.; Abd El-Monaem E. M.; Tamer T. M.; Omer A. M. Green synthesis of platinum nanoparticles using Atriplex halimus leaves for potential antimicrobial, antioxidant, and catalytic applications. Arab. J. Chem. 2021, 103517 10.1016/j.arabjc.2021.103517. [DOI] [Google Scholar]
- Yossef H.; Khedr A. A.; Mahran M. Z. Hepatoprotective activity and antioxidant effects of El Nabka (Zizyphus spina-christi) fruits on rats hepatotoxicity induced by carbon tetrachloride. Nat. Sci. 2011, 9, 1–7. [Google Scholar]
- Asgarpanah J.; Haghighat E. Phytochemistry and pharmacologic properties of Ziziphus spina christi (L.) Willd. Afr. J. Pharm. Pharmacol. 2012, 6, 2332–2339. 10.5897/AJPP12.509. [DOI] [Google Scholar]
- Aly S. A.; El-Rigal N. S.; Rizk M. Z. Nutritional Supplementation with Ailanthus altissima and Ziziphus spina christi to Compensate for Some Metabolic. Pak. J. Biol. Sci. 2006, 9, 1700–1706. 10.3923/pjbs.2006.1700.1706. [DOI] [Google Scholar]
- Glombitza K.-W.; Mahran G.; Mirhom Y.; Michel K.; Motawi T. Hypoglycemic and antihyperglycemic effects of Zizyphus spina-christi in rats. Planta Med. 1994, 60, 244–247. 10.1055/s-2006-959468. [DOI] [PubMed] [Google Scholar]
- Sunderam V.; Thiyagarajan D.; Lawrence A. V.; Mohammed S. S. S.; Selvaraj A. In-vitro antimicrobial and anticancer properties of green synthesized gold nanoparticles using Anacardium occidentale leaves extract. Saudi J. Biol. Sci. 2019, 26, 455–459. 10.1016/j.sjbs.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.; Li Y.; Fang G.; Cao Z.; Shang Y.; Alfarraj S.; Alharbi S. A.; Li J.; Yang S.; Duan X. Green synthesis, characterization, cytotoxicity, antioxidant, and anti-human ovarian cancer activities of Curcumae kwangsiensis leaf aqueous extract green-synthesized gold nanoparticles. Arab. J. Chem. 2021, 14, 103000 10.1016/j.arabjc.2021.103000. [DOI] [Google Scholar]
- Rabeea M. A.; Owaid M. N.; Aziz A. A.; Jameel M. S.; Dheyab M. A. Mycosynthesis of gold nanoparticles using the extract of Flammulina velutipes, Physalacriaceae, and their efficacy for decolorization of methylene blue. J. Environ. Chem. Eng. 2020, 8, 103841 10.1016/j.jece.2020.103841. [DOI] [Google Scholar]
- Akintelu S. A.; Yao B.; Folorunso A. S. Green synthesis, characterization, and antibacterial investigation of synthesized gold nanoparticles (AuNPs) from Garcinia kola pulp extract. Plasmonics 2021, 16, 157–165. 10.1007/s11468-020-01274-9. [DOI] [Google Scholar]
- Ebrahimzadeh M. A.; Naghizadeh A.; Mohammadi-Aghdam S.; Khojasteh H.; Ghoreishi S. M.; Mortazavi-Derazkola S. Enhanced catalytic and antibacterial efficiency of biosynthesized Convolvulus fruticosus extract capped gold nanoparticles (CFE@ AuNPs). J. Photochem. Photobiol., B 2020, 209, 111949 10.1016/j.jphotobiol.2020.111949. [DOI] [PubMed] [Google Scholar]
- Manikandakrishnan M.; Palanisamy S.; Vinosha M.; Kalanjiaraja B.; Mohandoss S.; Manikandan R.; Tabarsa M.; You S.; Prabhu N. M. Facile green route synthesis of gold nanoparticles using Caulerpa racemosa for biomedical applications. J. Drug Delivery Sci. Technol. 2019, 54, 101345 10.1016/j.jddst.2019.101345. [DOI] [Google Scholar]
- Omer A. M.; El-Monaem E. M. A.; El-Subruiti G. M.; El-Latif M. M.; Eltaweil A. S. Fabrication of easy separable and reusable MIL-125 (Ti)/MIL-53 (Fe) binary MOF/CNT/Alginate composite microbeads for tetracycline removal from water bodies. Sci. Rep. 2021, 11, 23818 10.1038/s41598-021-03428-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltaweil A. S.; Mamdouh I. M.; Abd El-Monaem E. M.; El-Subruiti G. M. Highly efficient removal for methylene blue and Cu2+ onto UiO-66 metal–organic framework/carboxylated graphene oxide-incorporated sodium alginate beads. ACS Omega 2021, 6, 23528–23541. 10.1021/acsomega.1c03479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P.; El-kott A.; Ahmed A. E.; Khames A.; Zein M. A. Green synthesis of gold nanoparticles (Au NPs) using Tribulus terrestris extract: Investigation of its catalytic activity in the oxidation of sulfides to sulfoxides and study of its anti-acute leukemia activity. Inorg. Chem. Commun. 2021, 131, 108781 10.1016/j.inoche.2021.108781. [DOI] [Google Scholar]
- Hosny M.; Fawzy M.; Abdelfatah A. M.; Fawzy E. E.; Eltaweil A. S. Comparative study on the potentialities of two halophytic species in the green synthesis of gold nanoparticles and their anticancer, antioxidant and catalytic efficiencies. Adv. Powder Technol. 2021, 10.1016/j.apt.2021.07.008. [DOI] [Google Scholar]
- Sathiyaraj S.; Suriyakala G.; Gandhi A. D.; Babujanarthanam R.; Almaary K. S.; Chen T.-W.; Kaviyarasu K. Biosynthesis, characterization, and antibacterial activity of gold nanoparticles. J. Infect. Public Health 2021, 1842. 10.1016/j.jiph.2021.10.007. [DOI] [PubMed] [Google Scholar]
- Kim H.-J.; Phenrat T.; Tilton R. D.; Lowry G. V. Effect of kaolinite, silica fines and pH on transport of polymer-modified zero valent iron nano-particles in heterogeneous porous media. J. Colloid Interface Sci. 2012, 370, 1–10. 10.1016/j.jcis.2011.12.059. [DOI] [PubMed] [Google Scholar]
- Huang Y.; Lu Y.; Lin Y.; Mao Y.; Ouyang G.; Liu H.; Zhang S.; Tong Y. Cerium-based hybrid nanorods for synergetic photo-thermocatalytic degradation of organic pollutants. J. Mater. Chem. A 2018, 6, 24740–24747. 10.1039/C8TA06565A. [DOI] [Google Scholar]
- Liu W.; Li Z.; Kang Q.; Wen L. Efficient photocatalytic degradation of doxycycline by coupling α-Bi2O3/g-C3N4 composite and H2O2 under visible light. Environ. Res. 2021, 197, 110925 10.1016/j.envres.2021.110925. [DOI] [PubMed] [Google Scholar]
- Tsvetkov M.; Zaharieva J.; Milanova M. Ferrites, modified with silver nanoparticles, for photocatalytic degradation of malachite green in aqueous solutions. Catal. Today 2020, 357, 453–459. 10.1016/j.cattod.2019.07.052. [DOI] [Google Scholar]
- Sharma G.; ALOthman Z. A.; Kumar A.; Sharma S.; Ponnusamy S. K.; Naushad M. Fabrication and characterization of a nanocomposite hydrogel for combined photocatalytic degradation of a mixture of malachite green and fast green dye. Nanotechnol. Environ. Eng. 2017, 2, 4 10.1007/s41204-017-0014-y. [DOI] [Google Scholar]
- Li F.; Tao R.; Cao B.; Yang L.; Wang Z. Manipulating the Light-Matter Interaction of PtS/MoS2 p–n Junctions for High Performance Broadband Photodetection. Adv. Funct. Mater. 2021, 31, 2104367 10.1002/adfm.202104367. [DOI] [Google Scholar]
- Qin T.; Wang Z.; Wang Y.; Besenbacher F.; Otyepka M.; Dong M. Recent progress in emerging two-dimensional transition metal carbides. Nanomicro Lett. 2021, 13, 183 10.1007/s40820-021-00710-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.; Xu H.; Yang H.; Lin Y.; Liu H.; Tong Y. Efficient charges separation using advanced BiOI-based hollow spheres decorated with palladium and manganese dioxide nanoparticles. ACS Sustainable Chem. Eng. 2018, 6, 2751–2757. 10.1021/acssuschemeng.7b04435. [DOI] [Google Scholar]
- Li Y.; Xia Y.; Liu K.; Ye K.; Wang Q.; Zhang S.; Huang Y.; Liu H. Constructing Fe-MOF-derived Z-scheme photocatalysts with enhanced charge transport: nanointerface and carbon sheath synergistic effect. ACS Appl. Mater. Interfaces 2020, 12, 25494–25502. 10.1021/acsami.0c06601. [DOI] [PubMed] [Google Scholar]
- Yadav P.; Surolia P. K.; Vaya D. Synthesis and application of copper ferrite-graphene oxide nanocomposite photocatalyst for the degradation of malachite green. Mater. Today: Proc. 2021, 43, 2949–2953. 10.1016/j.matpr.2021.01.301. [DOI] [Google Scholar]
- Meena S.; Dipti V.; Das B. Photocatalytic degradation of Malachite Green dye by modified ZnO nanomaterial. Bull. Mater. Sci. 2016, 39, 1735–1743. 10.1007/s12034-016-1318-4. [DOI] [Google Scholar]
- Wang P.; Li D.; Chen J.; Zhang X.; Xian J.; Yang X.; Zheng X.; Li X.; Shao Y. A novel and green method to synthesize CdSe quantum dots-modified TiO2 and its enhanced visible light photocatalytic activity. Appl. Catal., B 2014, 160–161, 217–226. 10.1016/j.apcatb.2014.05.032. [DOI] [Google Scholar]
- Saad A. M.; Abukhadra M. R.; Ahmed S. A.-K.; Elzanaty A. M.; Mady A. H.; Betiha M. A.; Shim J.-J.; Rabie A. M. Photocatalytic degradation of malachite green dye using chitosan supported ZnO and Ce–ZnO nano-flowers under visible light. J. Environ. Manage. 2020, 258, 110043 10.1016/j.jenvman.2019.110043. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Ohko Y.; Zhang R.; Yang Y.; Zhang Z. Degradation of malachite green on Pd/WO3 photocatalysts under simulated solar light. J. Hazard. Mater. 2010, 184, 386–391. 10.1016/j.jhazmat.2010.08.047. [DOI] [PubMed] [Google Scholar]
- Georgiev P.; Kaneva N.; Bojinova A.; Papazova K.; Mircheva K.; Balashev K. Effect of gold nanoparticles on the photocatalytic efficiency of ZnO films. Colloids Surf., A 2014, 460, 240–247. 10.1016/j.colsurfa.2014.02.004. [DOI] [Google Scholar]
- Parveen A.; Rao S. Cytotoxicity and genotoxicity of biosynthesized gold and silver nanoparticles on human cancer cell lines. J. Clust. Sci. 2015, 26, 775–788. 10.1007/s10876-014-0744-y. [DOI] [Google Scholar]
- Hosny M.; Fawzy M.; El-Borady O. M.; Mahmoud A. E. D. Comparative study between Phragmites australis root and rhizome extracts for mediating gold nanoparticles synthesis and their medical and environmental applications. Adv. Powder Technol. 2021, 32, 2268–2279. 10.1016/j.apt.2021.05.004. [DOI] [Google Scholar]
- Garedew A.; Henderson S. O.; Moncada S. Activated macrophages utilize glycolytic ATP to maintain mitochondrial membrane potential and prevent apoptotic cell death. Cell Death Differ. 2010, 17, 1540–1550. 10.1038/cdd.2010.27. [DOI] [PubMed] [Google Scholar]
- Song Y.; Dong Y.; Liao Y.; Liang Z.; Yao J.; Zhou X. Apoptotic caspases suppress Mycobacterium bovis-induced IFN-β production in murine macrophage. J. Infect. 2021, 61. 10.1016/j.jinf.2021.04.014. [DOI] [PubMed] [Google Scholar]
- Pan Y.; Neuss S.; Leifert A.; Fischler M.; Wen F.; Simon U.; Schmid G.; Brandau W.; Jahnen-Dechent W. Size-dependent cytotoxicity of gold nanoparticles. Small 2007, 3, 1941–1949. 10.1002/smll.200700378. [DOI] [PubMed] [Google Scholar]
- Li T.; Li F.; Xiang W.; Yi Y.; Chen Y.; Cheng L.; Liu Z.; Xu H. Selenium-containing amphiphiles reduced and stabilized gold nanoparticles: kill cancer cells via reactive oxygen species. ACS Appl. Mater. Interfaces. 2016, 8, 22106–22112. 10.1021/acsami.6b08282. [DOI] [PubMed] [Google Scholar]
- Hosny M.; Fawzy M. Instantaneous phytosynthesis of gold nanoparticles via Persicaria salicifolia leaf extract, and their medical applications. Adv. Powder Technol. 2021, 32, 2891–2904. 10.1016/j.apt.2021.06.004. [DOI] [Google Scholar]
- Muthukumar T.; Sudhakumari; Sambandam B.; Aravinthan A.; Sastry T. P.; Kim J.-H. Green synthesis of gold nanoparticles and their enhanced synergistic antitumor activity using HepG2 and MCF7 cells and its antibacterial effects. Process Biochem. 2016, 51, 384–391. 10.1016/j.procbio.2015.12.017. [DOI] [Google Scholar]
- Uzma M.; Sunayana N.; Raghavendra V. B.; Madhu C. S.; Shanmuganathan R.; Brindhadevi K. Biogenic synthesis of gold nanoparticles using Commiphora wightii and their cytotoxic effects on breast cancer cell line (MCF-7). Process Biochem. 2020, 92, 269–276. 10.1016/j.procbio.2020.01.019. [DOI] [Google Scholar]
- Uma Suganya K. S.; Govindaraju K.; Ganesh Kumar V.; Prabhu D.; Arulvasu C.; Stalin Dhas T.; Karthick V.; Changmai N. Anti-proliferative effect of biogenic gold nanoparticles against breast cancer cell lines (MDA-MB-231 & MCF-7). Appl. Surf. Sci. 2016, 371, 415–424. 10.1016/j.apsusc.2016.03.004. [DOI] [Google Scholar]
- Vijayakumar S. Eco-friendly synthesis of gold nanoparticles using fruit extracts and in vitro anticancer studies. J. Saudi Chem. Soc. 2019, 23, 753–761. 10.1016/j.jscs.2018.12.002. [DOI] [Google Scholar]
- Barai A. C.; Paul K.; Dey A.; Manna S.; Roy S.; Bag B. G.; Mukhopadhyay C. Green synthesis of Nerium oleander-conjugated gold nanoparticles and study of its in vitro anticancer activity on MCF-7 cell lines and catalytic activity. Nano Convergence 2018, 5, 10 10.1186/s40580-018-0142-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakya S.; Muthukrishnan S.; Sukumaran M.; Muthukumar M. Biogenic synthesis of silver nanoparticles and their antioxidant and antibacterial activity. Appl. Nanosci. 2016, 6, 755–766. 10.1007/s13204-015-0473-z. [DOI] [Google Scholar]
- Karuppanapandian T.; Moon J.-C.; Kim C.; Manoharan K.; Kim W. Reactive oxygen species in plants: their generation, signal transduction, and scavenging mechanisms. Aust. J. Crop Sci. 2011, 5, 709–725. [Google Scholar]
- Apel K.; Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- Zayed M. F.; Mahfoze R. A.; El-kousy S. M.; Al-Ashkar E. A. In-vitro antioxidant and antimicrobial activities of metal nanoparticles biosynthesized using optimized Pimpinella anisum extract. Colloids Surf., A 2020, 585, 124167 10.1016/j.colsurfa.2019.124167. [DOI] [Google Scholar]
- Sathishkumar G.; Jha P. K.; Vignesh V.; Rajkuberan C.; Jeyaraj M.; Selvakumar M.; Jha R.; Sivaramakrishnan S. Cannonball fruit (Couroupita guianensis, Aubl.) extract mediated synthesis of gold nanoparticles and evaluation of its antioxidant activity. J. Mol. Liq. 2016, 215, 229–236. 10.1016/j.molliq.2015.12.043. [DOI] [Google Scholar]
- Nakkala J. R.; Bhagat E.; Suchiang K.; Sadras S. R. Comparative study of antioxidant and catalytic activity of silver and gold nanoparticles synthesized from Costus pictus leaf extract. J. Mater. Sci. Technol. 2015, 31, 986–994. 10.1016/j.jmst.2015.07.002. [DOI] [Google Scholar]
- Ramachandiran D.; Elangovan M.; Rajesh K. Structural, optical, biological and photocatalytic activities of platinum nanoparticles using salixtetraspeama leaf extract via hydrothermal and ultrasonic methods. Optik 2021, 167494 10.1016/j.ijleo.2021.167494. [DOI] [Google Scholar]
- Ramkumar V. S.; Pugazhendhi A.; Prakash S.; Ahila N.; Vinoj G.; Selvam S.; Kumar G.; Kannapiran E.; Rajendran R. B. Synthesis of platinum nanoparticles using seaweed Padina gymnospora and their catalytic activity as PVP/PtNPs nanocomposite towards biological applications. Biomed. Pharmacother. 2017, 92, 479–490. 10.1016/j.biopha.2017.05.076. [DOI] [PubMed] [Google Scholar]
- Sreelekha E.; George B.; Shyam A.; Sajina N.; Mathew B. A comparative study on the synthesis, characterization, and antioxidant activity of green and chemically synthesized silver nanoparticles. BioNanoScience 2021, 11, 489–496. 10.1007/s12668-021-00824-7. [DOI] [Google Scholar]
- Kharat S. N.; Mendhulkar V. D. Synthesis, characterization and studies on antioxidant activity of silver nanoparticles using Elephantopus scaber leaf extract. Mater. Sci. Eng., C 2016, 62, 719–724. 10.1016/j.msec.2016.02.024. [DOI] [PubMed] [Google Scholar]
- Bhati A.; Anand S. R.; Gunture; Garg A. K.; Khare P.; Sonkar S. K. Sunlight-induced photocatalytic degradation of pollutant dye by highly fluorescent red-emitting Mg-N-embedded carbon dots. ACS Sustainable Chem. Eng. 2018, 6, 9246–9256. 10.1021/acssuschemeng.8b01559. [DOI] [Google Scholar]
- Paramanik L.; Reddy K. H.; Parida K. Stupendous photocatalytic activity of p-BiOI/n-PbTiO3 heterojunction: the significant role of oxygen vacancies and interface coupling. J. Phys. Chem. C 2019, 123, 21593–21606. 10.1021/acs.jpcc.9b05747. [DOI] [Google Scholar]
- Zhang J.; Wang Y.; Jin J.; Zhang J.; Lin Z.; Huang F.; Yu J. Efficient visible-light photocatalytic hydrogen evolution and enhanced photostability of core/shell CdS/g-C3N4 nanowires. ACS Appl. Mater. Interfaces 2013, 5, 10317–10324. 10.1021/am403327g. [DOI] [PubMed] [Google Scholar]