Abstract
Background and Objective
The objectives of this study were to compare adherence to antiseizure medications (ASMs) vs non-ASMs among individuals with epilepsy, to assess the degree to which variation in adherence is due to differences between individuals vs between medication classes among individuals with epilepsy, and to compare adherence in individuals with vs without epilepsy.
Methods
This was a retrospective cohort study using Medicare. We included beneficiaries with epilepsy (≥1 ASM, plus ICD-9-CM diagnostic codes) and a 20% random sample without epilepsy. Adherence for each medication class was measured by the proportion of days covered (PDC) in 2013 to 2015. We used Spearman correlation coefficients, Cohen κ statistics, and multilevel logistic regressions.
Results
There were 83,819 beneficiaries with epilepsy. Spearman correlation coefficients between ASM PDCs and each of the 5 non-ASM PDCs ranged from 0.44 to 0.50; Cohen κ ranged from 0.33 to 0.38; and within-person differences between the PDC of each ASM minus the PDC of each non-ASM were all statistically significant (p < 0.01), although median differences were all very close to 0. Fifty-four percent of variation in adherence across medications was due to differences between individuals. Adjusted predicted probabilities of adherence were as follows: ASMs 74% (95% confidence interval [CI] 73%–74%), proton pump inhibitors 74% (95% CI 74%–74%), antihypertensives 77% (95% CI 77%–78%), selective serotonin reuptake inhibitors 77% (95% CI 77%–78%), statins 78% (95% CI 78%–79%), and levothyroxine 82% (95% CI 81%–82%). Adjusted predicted probabilities of adherence to non-ASMs were 80% (95% CI 80%–81%) for beneficiaries with epilepsy vs 77% (95% CI 77%–77%) for beneficiaries without epilepsy.
Discussion
Among individuals with epilepsy, ASM adherence and non-ASM adherence were moderately correlated, half of the variation in adherence was due to between-person rather than between-medication differences, adjusted adherence was slightly lower for ASMs than several non-ASMs, and epilepsy was associated with a quite small increase in adherence to non-ASMs. Nonadherence to ASMs may provide an important cue to the clinician to inquire about adherence to other potentially life-prolonging medications as well. Although efforts should focus on improving ASM adherence, patient-level rather than purely medication-specific behaviors are also critical to consider when developing interventions to optimize adherence.
Between 20% and 50% of individuals with epilepsy are classified as nonadherent to their antiseizure medications (ASMs).1 Nonadherence to ASMs is associated with adverse consequences, including increased seizures,2 mortality,3 health care costs,4-6 and acute care visits.4 However, because adults with epilepsy often have a wide variety of treatable chronic conditions7 and most medications taken by individuals with epilepsy are taken for indications other than epilepsy,8 optimizing adherence to non-ASMs in people with epilepsy would also reduce preventable adverse outcomes.
Although prior work has explored risk factors and prevalence of ASM nonadherence,1,6,9-14 little is known about how adherence to ASMs compares to adherence to non-ASMs among individuals with epilepsy. Understanding if differences exist would inform whether interventions to improve adherence in adults with epilepsy should target ASMs specifically or more global patient-level behaviors across medication classes. ASM nonadherence may correlate with general attitudes toward medications,15 although it is plausible that the unique side effect profiles, monitoring regimens, and psychosocial constructs16 surrounding ASMs and the unique consequences of seizures may lead to different drivers and prevalence of nonadherence to ASMs vs non-ASMs.
Furthermore, it remains unknown whether individuals with epilepsy demonstrate different rates of adherence across medication classes compared to individuals without epilepsy. Individuals with epilepsy have heightened risk for cognitive, psychiatric, and physical comorbid conditions,7 as well as disparities in health care access,17-19 all of which could increase risk for nonadherence compared to individuals without epilepsy. Still, such barriers are common across individuals with chronic conditions. Determining whether adherence differs between people with and those without epilepsy could inform whether epilepsy-specific interventions are needed.
Using Medicare data, we compared adherence to ASMs vs non-ASMs among individuals with epilepsy, assessed the degree to which variation in adherence is due to differences between individuals vs between medication classes among individuals with epilepsy, and compared adherence in individuals with and those without epilepsy. We hypothesized that ASM adherence would be partially correlated with non-ASM adherence, that within-person correlation rather than between-medication differences may explain a substantive amount of variation in adherence, and that individuals with epilepsy may have worse adherence compared with the general population.
Methods
Study Design and Dataset
We performed a retrospective cohort study of beneficiaries in fee-for-service Medicare across the entire United States, incorporating data from 2011 to 2015.
Standard Protocol Approvals, Registrations, and Patient Consents
This study was deemed exempt by the University of Michigan Institutional Review Board.
Patient Selection
Similar to prior work,20 we included patients with epilepsy defined as filling ≥1 ASM plus ICD-9-CM criteria for inpatient, outpatient, or emergency evaluation and management or consultation codes—≥1 for epilepsy (ICD-9-CM 345. xx) or (2) ≥2 for convulsions (ICD-9-CM 780.3x)—at least 30 days apart in 2013. Recent work in Medicare21 demonstrated good performance of combining ICD codes plus ASM to identify patients with epilepsy (area under the curve [AUC] 0.93, sensitivity 88%, and specificity 98%). Because we required ICD codes to determine the diagnosis of epilepsy (2013) and refractory (2011–2013) or prevalent (2011–2012) epilepsy, we excluded beneficiaries without continuous enrollment in Medicare Part A and B, or with managed care plans (whose claims do not appear in Medicare carrier files) in 2011 to 2013. Because we required medication fill data to determine proportion of days covered (PDC; 2013–2015), we also excluded beneficiaries without continuous enrollment in Medicare Part D in 2013 to 2015. We included all individuals qualifying for Medicare. Medicare criteria include age >65 years, disability, or end-stage renal disease.
In addition to the cohort with epilepsy, we included a 20% random Medicare sample of beneficiaries without epilepsy.
Variables
Adherence was measured with PDC. PDC represents the proportion of days (0%–100%) in an observation period during which an individual has medication supply. It is a widely accepted measure for claims-based analysis of medication adherence22,23 and is a standard measure in ASM adherence studies.4,6,9,10,24 We also dichotomized <80% (nonadherent) vs ≥80% (adherent) as is typically performed in adherence literature for analysis.4,6,9,10,22-24 We calculated 1 PDC for each medication class for each beneficiary. If a beneficiary took >1 unique medication in a given class, we summed the numerators and denominators for all medications within a class. Numerators were the number of days with medication supply (determined from the days’ supply field in the prescription claim) during the total observation period of July 2013 to June 2015. We did not double-count days if a fill occurred before the last day of the prior fill. Denominators were the total number of days summed across quarters unless 1 of the following was true. If there was no supply of a medication 180 days before a given fill, we considered that a newly started medication, and we started counting the denominator at the time of the first fill rather than July 1, 2013. If a prescription did not have enough days to last through the end of the observation period and there was no fill 180 days after the end of a given prescription, we stopped counting the denominator at the end of the last fill rather than stopping at the end of the period. Other investigators4 have similarly used this methodology to acknowledge that a medication could lapse for valid medical reasons (i.e., intolerance, remission) rather than nonadherence. We counted medications toward PDC calculations only if there was >1 fill for each medication during the observation period, given that it is not possible to calculate a valid PDC if a medication is filled just once; hence, sample sizes to calculate PDCs may be slightly smaller than the total population on at least 1 medication in a given class. An alternative to the PDC in administrative claims research is the medication possession ratio, which represents the summed days' supply of medication divided by the number of days in the observation window. However, we chose the PDC because the medication possession ratio can overestimate adherence (e.g., if refilling a medication before the end of the previous fill or if changing doses or switching agents), theoretically even producing values >100%, and the PDC is the standard approach used by the Centers for Medicare & Medicaid Services.25
We recorded the PDC for ASMs plus 5 non-ASM medication classes: antihypertensives, levothyroxine, proton pump inhibitors (PPIs), selective serotonin reuptake inhibitors (SSRIs), and statins. Non-ASM medication classes were chosen to represent a broad range of the most common medications for chronic conditions taken by individuals with epilepsy.8 eTable 1, links.lww.com/WNL/B673, lists all ASMs and the most common considered non-ASMs.
We captured baseline variables, including age, sex, race, Medicaid dual eligibility, rural zip code,26 and reason for entitlement. We calculated the Charlson Comorbidity Index27-29 in 2013 (a weighted sum of 22 comorbid conditions for which higher numbers indicate greater comorbidity), refractory epilepsy (≥1 claims for refractory epilepsy30: ICD-9-CM 345.01, 345.11, 345.41, 345.51, 345.61, 345.71, 345.81, 345.91 in 2011–2013), prevalent epilepsy (≥1 claims for seizures or epilepsy in 2011–2012), and number of unique medications or unique ASMs and total out-of-pocket drug expenses in 2013.
Statistical Analysis
We described baseline variables using medians (interquartile ranges) and frequencies (percentage).
In the first part, we assessed the PDC for each medication class among individuals with epilepsy. The distribution of the PDC of each medication class was compared first by use of violin plots. Violin plots31 are a modification of boxplots (which display quartiles) by superimposing plots of the estimated kernel density. We also repeated violin plots except we stratified all classes further in terms of brand name vs generic medications and stratified ASMs in terms of older (carbamazepine, ethosuximide, phenytoin, phenobarbital, primidone, valproate) vs newer generation (all others). We then displayed a separate scatterplot comparing each individual's ASM PDC and non-ASM PDC among each beneficiary who filled any of the 4 non-ASMs. We assessed correlations using Spearman correlation coefficients because PDCs were monotonically but not linearly related. One thousand bootstrapped samples were used to calculate empirical confidence intervals (CIs) around correlation coefficients. We subtracted the ASM PDC minus the PDC of each non-ASM to further depict within-person differences and assessed the significance of each difference using Wilcoxon signed-rank tests. We then performed χ2 tests to assess differences between adherence to ASMs and adherence to non-ASM classes and the Cohen κ statistics to assess agreement beyond chance.
In the second part, we performed multilevel models to calculate intraclass correlation coefficients (ICCs) among beneficiaries with epilepsy. An ICC represents the percentage (0%–100%) of variation in an outcome explained by between-person differences independently of other covariates.32 Stated another way, an ICC represents the within-person correlation (range 0–1, equivalent to 0%–100%) for the adherence outcome of each medication. If PDCs for each medication were identical within each individual but differed between individuals, the ICC would be 100%; that would imply that adherence was determined totally by individual factors rather than differences between medications. In these models, each person could have between 1 and 6 rows (depending on whether they filled only ASMs or filled any of the 5 other medication classes as well), and there was a person-level random intercept. The main outcome was binary adherence, PDC ≥80%. We calculated an unadjusted ICC and adjusted for medication class; in the fully adjusted model, we then adjusted for medication class in addition to age, sex, race, dual eligibility, rural zip code, reason for Medicare entitlement, neurologist visit, refractory epilepsy, prevalent epilepsy, number of unique medications, number of unique ASMs, total Part D out-of-pocket drug costs in 2013, maximal doses per day of long-term medications with ≥2 fills 30 days apart with ≥90-day supply in 2013, and Charlson Comorbidity Index. We displayed the predicted percent adherent to each medication class from this fully adjusted mixed-effects logistic regression. We conducted sensitivity analyses in which we evaluated robustness of model discrimination when varying the PDC cutoff to ≥80%, ≥70%, or ≥60% and then considered brand name and generic medications within each class as a separate row of data.
In the third part, we compared non-ASM adherence in beneficiaries with epilepsy vs without epilepsy. We repeated a mixed-effects logistic regression with a fixed effect for epilepsy and a random effect accounting for variability between individual beneficiaries. We performed an unadjusted model and then adjusted for the same covariates as in the previous fully adjusted model, except we omitted variables for prevalent epilepsy, refractory epilepsy, and number of ASMs because these variables were perfectly collinear with epilepsy.
Data were analyzed with SAS 9.4 (SAS Institute Inc, Cary, NC) and Stata 16.0 (StatCorp, College Station, TX).
Data Accessibility
All datasets are available to purchase at resdac.org/. Aggregated deidentified data may be shared on request.
Results
Cohort Description
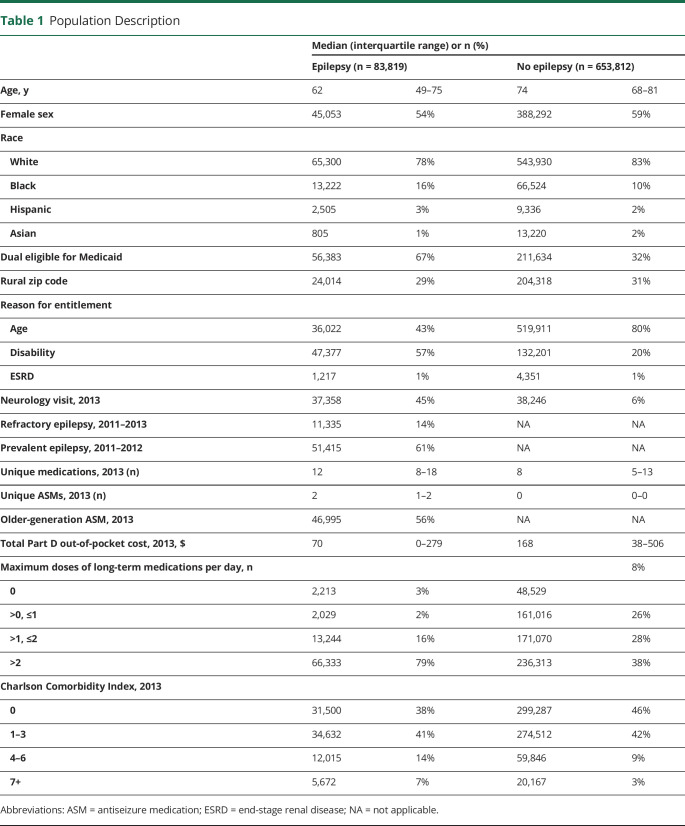
The cohort included 83,819 eligible beneficiaries with epilepsy and 653,812 from our 20% sample without epilepsy (eFigure 1, links.lww.com/WNL/B673). There were 77,261 eligible beneficiaries with epilepsy who filled an ASM at least twice for whom we could calculate an ASM PDC. Among beneficiaries with epilepsy, median age was 62 years (interquartile range 49–75 years), 54% were female, 78% were White, 67% were dual eligible for Medicaid, and 43% qualified for Medicare due to age and 57% qualified due to disability (Table 1).
Table 1.
Population Description
Comparing Adherence to ASMs and Non-ASMs Among Beneficiaries With Epilepsy
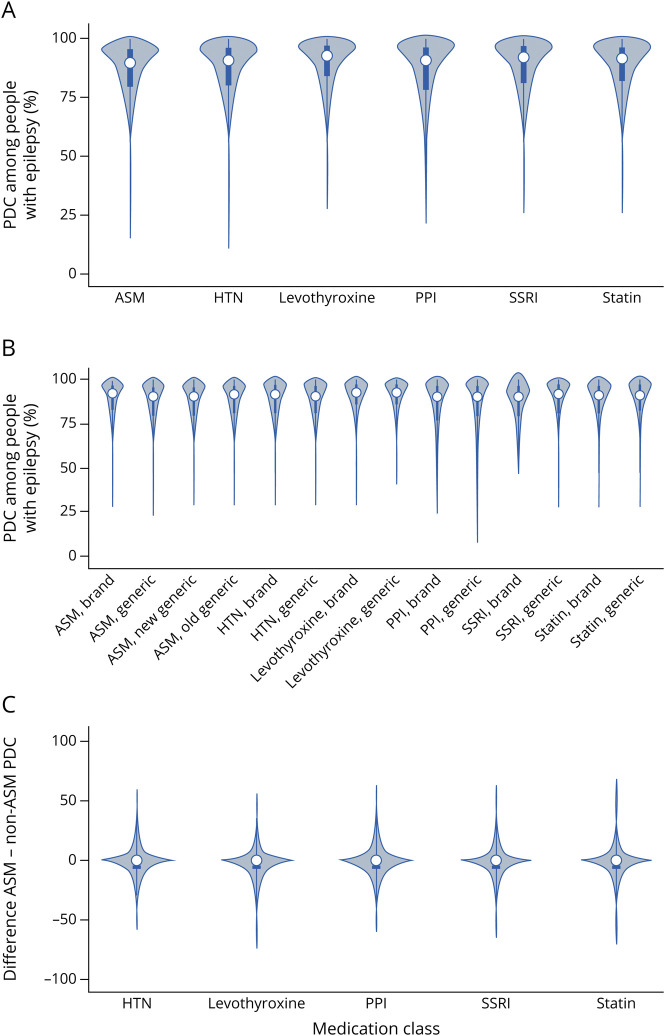
Median PDCs for each of the 6 classes ranged from 0.90 to 0.93 (Figure 1A). Distributions appeared similar when stratified according to older- vs newer-generation ASM and brand name vs generic (Figure 1B).
Figure 1. Distribution of PDCs by Medication Class.
Among beneficiaries with epilepsy, violin plots of proportion of days covered (PDC) for (A) each medication class (antiseizure medications [ASMs] and 4 non-ASMs) summed across all quarters, (B) each medication class stratified by older vs newer generation for ASMs and brand name vs generic for all classes, and (C) within-individual ASM minus non-ASM PDCs. HTN = antihypertensive; PPI = proton pump inhibitor.
Wilcoxon signed-rank tests for the within-person differences between the PDC of each ASM minus the PDC of each non-ASM were all statistically significant (p < 0.01). However, the median values for differences were all very close to 0 (−0.01 for each; Figure 1C, sample sizes are the same as in Figure 2).
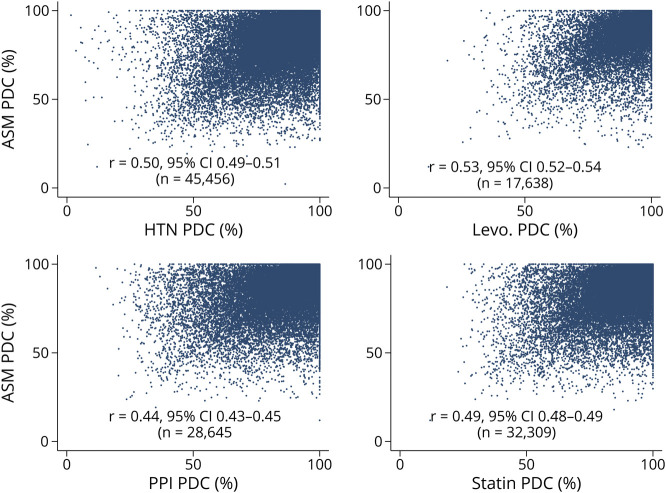
Figure 2. Antiseizure Medication (ASM) vs Non-ASM PDC.
Among beneficiaries with epilepsy, separate scatterplots of antiseizure medication (ASM) proportion of days covered (PDC) (x-axis) vs each non-ASM PDC (y-axis). Each plot contains a Spearman correlation coefficient (r), sample size (N), and a superimposed regression line with 95% confidence interval and regression equation. Note that selective serotonin reuptake inhibitors are not included due to space constraints, but results are similar to displayed panels (r = 0.50, 95% CI 0.49–0.51, N = 24,738). HTN = antihypertensive; Levo. = levothyroxine; PPI = proton pump inhibitor.
Scatterplots demonstrated a positive relationship between the PDC for ASM and for each non-ASM (Figure 2). Spearman correlation coefficients quantified this relationship from a minimum of 0.44 (PPIs) to a maximum of 0.53 (levothyroxine), which all represented moderate positive correlations between ASM and non-ASM PDCs.
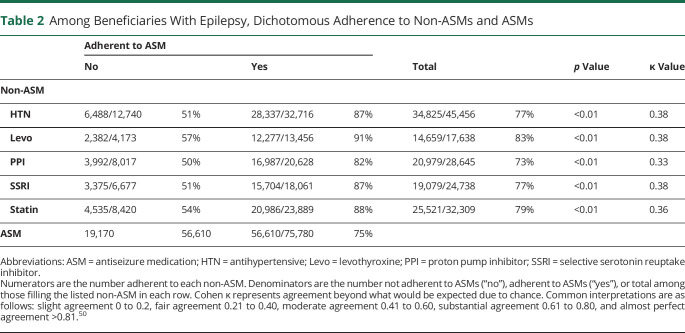
Associations between ASM and non-ASM dichotomized adherence are presented in Table 2 (populations are the same as in Figures 1 and 2). Seventy-five percent of beneficiaries were adherent to ASMs. Beneficiaries who were adherent to ASMs were more likely to be adherent to non-ASMs (all p < 0.01). For example, among beneficiaries filling antihypertensives and ASMs, 6,488 of 12,740 (51%) of those who were not adherent to their ASM were adherent to their antihypertensives, whereas 28,337 of 32,716 (87%) of those who were adherent to their ASM were adherent to their antihypertensive. The Cohen κ ranged from 0.33 to 0.38, which represented fair agreement beyond chance between ASM and non-ASM adherence.
Table 2.
Among Beneficiaries With Epilepsy, Dichotomous Adherence to Non-ASMs and ASMs
Assessing the Degree to Which Variation in Adherence Is Due to Differences Between Beneficiaries vs Between Medication Classes Among Beneficiaries With Epilepsy
In mixed-effects logistic models predicting adherence, ICCs were 57% (95% CI 56%–57%; nobservations = 230,939; nbeneficiaries = 79,585) in an unadjusted model, 57% (95% CI 56%–58%; nobservations = 230,939; nbeneficiaries = 79,585) in a model adjusted for medication class, and 54% (95% CI 53%–55%; nobservations = 230,374; nbeneficiaries = 79,379; AUC 0.95, 95% CI 0.95–0.95) in the fully adjusted model.
Marginal predicted proportions for adherence rates from the fully adjusted mixed-effects logistic model in ascending order were as follows: ASMs 74% (95% CI 73%–74%), PPIs 74% (95% CI 74%–74%), antihypertensives 77% (95% CI 77%–78%), SSRIs 77% (95% CI 77%–78%), statins 78% (95% CI 78%–79%), and levothyroxine 82% (95% CI 81%–82%). Each non-ASM proportion was significantly different from the ASM proportion (p < 0.05).
In sensitivity analyses, ICCs and AUCs were similar when the adherence cutoff was changed to ≥70% (ICC 55%; AUC 0.96) or ≥60% (ICC 54%; AUC 0.98) or whether distinguishing between brand name and generic medications (ICC 52%).
Comparing Adherence to Non-ASMs in Beneficiaries With Epilepsy vs Without Epilepsy
We repeated a mixed-effects logistic model except we included beneficiaries both with and without epilepsy and included only non-ASMs. Epilepsy had an unadjusted odds ratio (OR) for adherence of 1.00 (95% CI 0.96–1.02; nobservations = 1,342,456; nbeneficiaries = 605,492). This OR was 1.03 (95% CI 01.01–1.06; nobservations = 1,342,456; nbeneficiaries = 605,492) after adjustment for medication class and 1.35 (95% CI 1.32–1.39; nobservations = 1,331,642, nbeneficiaries = 598,967) in the fully adjusted model. The adjusted marginal predicted probability of adherence was 0.80 (95% CI 0.80–0.81) for beneficiaries with epilepsy vs 0.77 (0.77–0.77) for beneficiaries without epilepsy (p < 0.01).
In sensitivity analyses, ORs for epilepsy were similar when the adherence cutoff was changed to ≥70% (OR 1.39, 95% CI 1.34–1.43) or ≥60% (OR 1.38, 95% CI 1.32–1.43) or whether distinguishing between brand name and generic medications (OR 1.34, 95% CI 1.31–1.38). eTable 2, links.lww.com/WNL/B673, displays all ORs for the model including brand name as a variable (OR 0.85, 95% CI 0.84–0.87).
Discussion
In this large retrospective Medicare database study, ASM adherence and non-ASM adherence were moderately positively correlated with fair agreement, and individual patient-level factors accounted for slightly more than half of variation in adherence. While unadjusted median adherence was similar across medical classes and within-individual differences between ASM and non-ASM adherence were very close to 0, adjusted ASM adherence nonetheless was significantly lower than adherence for all non-ASMs, but absolute differences were quite small. Last, while individuals with epilepsy had unadjusted adherence across non-ASMs similar to that of individuals without epilepsy, after adjustment for demographics and comorbid conditions, individuals with epilepsy demonstrated 40% increased odds of adherence, although the absolute difference was small (4%).
Prior work has placed adherence within the context of the Necessity-Concerns Framework,33,34 whereby adherence is a complex interplay between general or medication-specific beliefs regarding need for treatment and concern about potential adverse consequences of medications. For example, in 1 study,15 expressing concern about long-term ASM harms predicted ASM nonadherence (OR 1.4). However, when respondents were asked about their attitudes toward medications in general, general concern about medications similarly predicted ASM nonadherence (OR 1.6). In our study, 54% of variation in adherence was due to person-to-person differences rather than medication-to-medication differences or other patient factors related to demographics or comorbid conditions. While Medicare lacks individual data about medication attitudes and beliefs, our findings are concordant with the concept that mechanisms underlying ASM adherence may not be totally unique to ASMs, seizures, or epilepsy. Rather, this result could reflect that adherence barriers unique to each individual (i.e., forgetting doses and cognitive function, difficulty swallowing, difficulty affording medications or getting to the pharmacy, health literacy, patient-provider relationship35-37) apply to all medication classes, and the perceived importance of medications in general varies between individuals. Evidence-based interventions36,38 targeting common features (i.e., calendar or text reminders) may prove useful for both ASM and non-ASM classes alike, and nonadherence to ASMs may provide an important cue to the clinician to inquire about adherence to other potentially life-prolonging medications. Nonadherence is a problem across chronic conditions,39-41 generally lower for brand name drugs similar to our findings rather than unique to any single medication class,42 and individual, family, health care system, and community factors all may play a role in adherence behaviors compared to the single chronic condition alone.
Still, in our study, ASM adherence was not perfectly correlated with non-ASM adherence; ≈50% to 60% of beneficiaries who were not adherent to ASMs were still adherent to non-ASMs, and agreement beyond chance between ASM and non-ASM adherence was only fair. Even if common belief structures or individual-level barriers influenced adherence to all of a patient's medications, one would still not expect perfect correlation between ASM and non-ASM adherence, given vastly different consequences of nonadherence to each studied medication class. For example, we studied both symptomatic medications (i.e., PPIs, SSRIs) and prevention medications (i.e., antihypertensives, statins). Increased side effects, monitoring, and psychosocial implications all could explain lower adjusted ASM adherence compared with other medication classes despite similar unadjusted PDCs, although these are not captured in Medicare data. Thus, these data do not inform the mechanisms underlying differences.
We also found that while adherence to non-ASMs was higher in individuals with epilepsy compared to those without epilepsy, the absolute magnitude of such differences was small. We initially hypothesized that patients with epilepsy may exhibit suboptimal adherence due to increased underlying memory dysfunction or more complex polypharmacy making adherence to any single medication more challenging. However, our data suggested the opposite. Prior work has shown that individuals with epilepsy are more likely to have a regular place of care and have more frequent health visits than patients without epilepsy,43 which could lead to more rapid detection of nonadherence across medications.
Our adherence rates were somewhat higher than those in previous literature. One comparable study in Medicare13 found that 68% were adherent to ASMs compared to our 74%. Differences could have emerged due to slightly different methodologies used to calculate PDCs in absence of a single gold-standard methodology. (1) Their study did not restrict to medications with >1 fill. While we acknowledge that this exclusion would not detect early nonpersistence after a single fill, we applied this exclusion because counting medications filled only once could misclassify a poorly tolerated, discontinued medication as nonadherence and because at least 2 pharmacy fills are required to understand adherence over time. (2) That study counted all days toward the denominator between the first and end of their follow-up period. While Medicare data do not explicitly inform reasons for extended lapses in medications, we did not count days at the end of each quarter toward the denominator if there was no subsequent 180-day fill, similar to other literature,4 to allow for the possibility that medications could be intentionally discontinued for valid medical reasons such as seizure remission44 rather than nonadherence. (3) Their study counted the proportion of days with at least 1 ASM prescription. However, that method would not detect nonadherence for patients on polytherapy who were fully adherent to 1 medication but not their other ASMs, whereas our method summing the numerators and denominators across all medications within a class would do so. Other studies have found adherence rates ranging from 50% to 79%, although it is difficult to directly compare across populations, study designs, and adherence measures (e.g., privately insured adults using retrospective claims and PDCs [61%]6,39; children at a single academic hospital using longitudinal follow-up of electronic pill caps [79%]45).
Our study has several limitations. Measuring adherence using PDCs from claims data could overestimate adherence; filling a medication does not guarantee ingestion. It also could underestimate adherence; a beneficiary could obtain medications over the counter (PPIs) that would not appear as a Part D claim. Regardless, we calculated PDCs using the exact same methodology across all medication classes; thus, it is unlikely that measurement error affected between-medication or between-person comparisons. In addition, PDC represents an integral component of how the Centers for Medicare & Medicaid evaluates Medicare Advantage and Part D plan performance25 and thus is a clinically relevant accepted metric driving policy. In addition, while Medicare is a large, diverse national database optimally suited to study older Americans in addition to those with disabilities (individuals with epilepsy demonstrate 3-fold increased rates of physical disability compared with the general population46), future studies may seek to reproduce our findings in younger, nondisabled, and less well-insured populations. While many studies using Medicare reduce heterogeneity by restricting to those eligible only due to age ≥65 years, that strategy sacrifices generalizability. We included all ages, which is a strength to make inferences about a wider population range, and we entered both age and reason for Medicare eligibility as covariates to account for this variation. It is also well known that identifying epilepsy cases in administrative datasets using ICD codes risks some degree of misclassification.47 Patients could receive a diagnosis but not fill an ASM prescription48 and thus not enter into our case definition, and prior work determining the accuracy of identifying epilepsy based on different numbers of ASM fills is limited. Still, recent work has suggested good sensitivity (up to 88%) and specificity (98%) of Medicare data compared with chart review epilepsy diagnoses,21 and it is also known that the positive predictive value of identifying epilepsy cases improves when ≥1 ASM fills are required.49 Furthermore, while 2013 to 2015 prescription data may not reflect contemporary advances, the medications studied here remain in widespread use.
These results suggest that while unique features of seizures and ASMs may drive a small portion of ASM nonadherence, a substantive portion of adherence is not ASM or epilepsy specific but rather person specific. While adjusted ASM adherence was slightly lower than non-ASM adherence and people with epilepsy demonstrated significantly higher adherence to non-ASMs compared to those without epilepsy, these absolute differences were quite small. Nonadherence to ASMs may provide an important cue to the clinician to inquire about adherence to other potentially life-prolonging medications as well. Acknowledging that many medications and chronic conditions likely share common adherence barriers, future interventions aimed at improving adherence in patients with epilepsy may more broadly target underlying patient-level barriers beyond ASM-specific concerns. This work could be addressed in the context of Epilepsy Learning Health Systems focusing on ensuring that providers assess medication barriers that may or may not be unique to ASMs to improve outcomes.
Glossary
- ASM
antiseizure medication
- AUC
area under the curve
- CI
confidence interval
- ICC
intraclass correlation coefficient
- ICD-9-CM
International Classification of Disease, 9th Revision, Clinical Modification
- OR
odds ratio
- PCD
proportion of days covered
- PPI
proton pump inhibitor
- SSRI
selective serotonin reuptake inhibitor
Appendix. Authors
Study Funding
S.W. Terman is supported by the Susan S Spencer Clinical Research Training Scholarship and the Michigan Institute for Clinical and Health Research J Award UL1TR002240. He was recently supported by the University of Michigan Department of Neurology Training Grant 5T32NS007222-38. W.T. Kerr is supported by NIH R25NS065723. C.E. Aubert is supported by an Early Postdoc Mobility grant from the Swiss National Science Foundation (grant P2LAP3_184042). C.E. Hill is supported by NIH KL2TR002241. Z.A. Marcum is supported by the National Institute of Aging K76AG059929. J.F. Burke is supported by the National Institute of Minority Health and Health Disparities R01 MD008879.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Faught E. Adherence to antiepilepsy drug therapy. Epilepsy Behav. 2012;25(3):297-302. [DOI] [PubMed] [Google Scholar]
- 2.Modi AC, Wu YP, Rausch JR, Peugh JL, Glauser TA. Antiepileptic drug nonadherence predicts pediatric epilepsy seizure outcomes. Neurology. 2014;83(22):2085-2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Faught E, Duh MS, Weiner JR, Guérin A, Cunnington MC. Nonadherence to antiepileptic drugs and increased mortality: findings from the RANSOM study. Neurology. 2008;71(20):1572-1578. [DOI] [PubMed] [Google Scholar]
- 4.Faught RE, Weiner JR, Guérin A, Cunnington MC, Duh MS. Impact of nonadherence to antiepileptic drugs on health care utilization and costs: findings from the RANSOM study. Epilepsia. 2009;50(3):501-509. [DOI] [PubMed] [Google Scholar]
- 5.Ettinger AB, Manjunath R, Candrilli SD, Davis KL. Prevalence and cost of nonadherence to antiepileptic drugs in elderly patients with epilepsy. Epilepsy Behav. 2009;14(2):324-329. [DOI] [PubMed] [Google Scholar]
- 6.Davis KL, Candrilli SD, Edin HM. Prevalence and cost of nonadherence with antiepileptic drugs in an adult managed care population. Epilepsia. 2008;49(3):446-454. [DOI] [PubMed] [Google Scholar]
- 7.Weatherburn CJ, Heath CA, Mercer SW, Guthrie B. Physical and mental health comorbidities of epilepsy: population-based cross-sectional analysis of 1.5 million people in Scotland. Seizure. 2017;45:125-131. [DOI] [PubMed] [Google Scholar]
- 8.Terman SW, Aubert CE, Hill CE, et al. Polypharmacy in patients with epilepsy: a nationally representative cross-sectional study. Epilepsy Behav. 2020;111:107261-107267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gollwitzer S, Kostev K, Hagge M, Lang J, Graf W, Hamer HM. Nonadherence to antiepileptic drugs in Germany: a retrospective, population-based study. Neurology. 2016;87(5):466-472. [DOI] [PubMed] [Google Scholar]
- 10.Alsous M, Hamdan I, Saleh M, McElnay J, Horne R, Masri A. Predictors of nonadherence in children and adolescents with epilepsy: a multimethod assessment approach. Epilepsy Behav. 2018;85:205-211. [DOI] [PubMed] [Google Scholar]
- 11.Zeber JE, Copeland LA, Pugh MJV. Variation in antiepileptic drug adherence among older patients with new-onset epilepsy. Ann Pharmacother. 2010;44(12):1896-1904. [DOI] [PubMed] [Google Scholar]
- 12.O'Rourke G, O'Brien JJ. Identifying the barriers to antiepileptic drug adherence among adults with epilepsy. Seizure. 2017;45:160-168. [DOI] [PubMed] [Google Scholar]
- 13.Piper K, Richman J, Faught E, et al. Adherence to antiepileptic drugs among diverse older Americans on Part D Medicare. Epilepsy Behav. 2017;66:68-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Terman SW, Burke JF, Kerr WT, Marcum ZA, Wang L. Antiseizure medication adherence trajectories in Medicare beneficiaries with newly treated epilepsy. Epilepsy. 2017;62(11):2778-2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chapman SC, Horne R, Chater A, Hukins D, Smithson WH. Patients' perspectives on antiepileptic medication: relationships between beliefs about medicines and adherence among patients with epilepsy in UK primary care. Epilepsy Behav. 2014;31:312-320. [DOI] [PubMed] [Google Scholar]
- 16.Montouris G, Hohler AD. Cultural barriers to medication adherence in epilepsy. Continuum. 2016;22(1 Epilepsy):266-269. [DOI] [PubMed] [Google Scholar]
- 17.Schiltz NK, Koroukian SM, Singer ME, Love TE, Kaiboriboon K. Disparities in access to specialized epilepsy care. Epilepsy Res. 2013;107(1-2):172-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamer HM, Kostev K. Sociodemographic disparities in administration of antiepileptic drugs to adults with epilepsy in Germany: a retrospective, database study of drug prescriptions. CNS Drugs. 2014;28(8):753-759. [DOI] [PubMed] [Google Scholar]
- 19.Bautista RE, Graham C, Mukardamwala S. Health disparities in medication adherence between African-Americans and Caucasians with epilepsy. Epilepsy Behav. 2011;22(3):495-498. [DOI] [PubMed] [Google Scholar]
- 20.Faught E, Richman J, Martin R, et al. Incidence and prevalence of epilepsy among older U.S. Medicare beneficiaries. Neurology. 2012;78(7):448-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moura LMVR, Smith JR, Blacker D, et al. Epilepsy among elderly Medicare beneficiaries. Med Care. 2019;57(4):318-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sikka R, Xia F, Aubert RE. Estimating medication persistency using administrative claims data. Am J Manag Care. 2005;11(7):449-457. [PubMed] [Google Scholar]
- 23.Andrade SE, Kahler KH, Frech F, Chan KA. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol Drug Saf. 2006;15(8):565-567. [DOI] [PubMed] [Google Scholar]
- 24.Manjunath R, Davis KL, Candrilli SD, Ettinger AB. Association of antiepileptic drug nonadherence with risk of seizures in adults with epilepsy. Epilepsy Behav. 2009;14(2):372-378. [DOI] [PubMed] [Google Scholar]
- 25.Centers for Medicare & Medicaid Services. Medicare 2021 Part C & D star rating technical notes. 2020. Accessed August 31, 2021. cms.gov/files/document/2021technotes20201001.pdf-0
- 26.Centers for Medicare & Medicaid Services. ZIP code to carrier locality file. Accessed August 31, 2021. cms.gov/Medicare/Medicare-Fee-For-Service-Payment/FeeScheduleGenInfo
- 27.Charlson M, Pompei P, Ales K, MacKenzie R. A new method of classifying prognostic in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. [DOI] [PubMed] [Google Scholar]
- 28.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245-1251. [DOI] [PubMed] [Google Scholar]
- 29.National Cancer Institute. Comorbidity SAS macro. 2014. Accessed August 31, 2021. healthcaredelivery.cancer.gov/seermedicare/considerations/macro-2014.html
- 30.Hill CE, Lin CC, Terman SW, et al. Definitions of drug-resistant epilepsy for administrative claims data research. Neurology. 2021;97(13):e1343-e1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hintze JL, Nelson RD. Violin plots: a box plot-density trace synergism. Am Stat. 1998;52(2):181-184. [Google Scholar]
- 32.Merlo J, Chaix B, Yang M, Lynch J, Råstam L. A brief conceptual tutorial of multilevel analysis in social epidemiology: linking the statistical concept of clustering to the idea of contextual phenomenon. J Epidemiol Community Health. 2005;59(6):443-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horne R, Weinman J. Patients' beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res. 1999;47:555-567. [DOI] [PubMed] [Google Scholar]
- 34.Horne R, Chapman SCE, Parham R, Freemantle N, Forbes A, Cooper V. Understanding patients' adherence-related beliefs about medicines prescribed for long-term conditions: a meta-analytic review of the Necessity-Concerns Framework. PLoS One. 2013;8(12):e80633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gellad WF, Grenard JL, Marcum ZA. A systematic review of barriers to medication adherence in the elderly: looking beyond cost and regimen complexity. Am J Geriatr Pharmacother. 2011;9(1):11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cross A, Elliott R, Petrie K, Kuruvilla L, George J. Adherence in older adults prescribed multiple medications. Cochrane Database Syst Rev. 2020;5(5):CD012419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gutierrez-Colina AM, Smith AW, Mara CA, Modi AC. Adherence barriers in pediatric epilepsy: from toddlers to young adults. Epilepsy Behav. 2018;80:229-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McGrady ME, Ryan JL, Gutiérrez-Colina AM, Fredericks EM, Towner EK, Pai AL. The impact of effective paediatric adherence promotion interventions: systematic review and meta-analysis. Child Care Health Dev. 2015;41(6):789-802. [DOI] [PubMed] [Google Scholar]
- 39.Briesacher BA, Andrade SE, Fouayzi H, Chan KA. Comparison of drug adherence rates among patients with seven different medical conditions. Pharmacotherapy. 2008;28(4):437-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Naderi SH, Bestwick JP, Wald DS. Adherence to drugs that prevent cardiovascular disease: meta-analysis on 376,162 patients. Am J Med. 2012;125(9):882–e1. [DOI] [PubMed] [Google Scholar]
- 41.Ho PM, Bryson CL, Rumsfeld JS. Medication adherence: its importance in cardiovascular outcomes. Circulation. 2009;119(23):3028-3035. [DOI] [PubMed] [Google Scholar]
- 42.Choudhry NK, Denberg TD, Qaseem A. Improving adherence to therapy and clinical outcomes while containing costs: opportunities from the greater use of generic medications: best practice advice from the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med. 2016;164(1):41-49. [DOI] [PubMed] [Google Scholar]
- 43.Terman SW, Aubert CE, Hill CE, Skvarce J, Burke JF, Mintzer S. Cardiovascular disease risk, awareness, and treatment in people with epilepsy. Epilepsy Behav. 2021;117:107878. [DOI] [PubMed] [Google Scholar]
- 44.Beghi E, Giussani G, Grosso S, et al. Withdrawal of antiepileptic drugs: guidelines of the Italian League Against Epilepsy. Epilepsia. 2013;54(suppl 7):2-12. [DOI] [PubMed] [Google Scholar]
- 45.Modi AC, Rausch JR, Glauser TA. Patterns of nonadherence to antiepileptic drug therapy in children with newly diagnosed epilepsy. JAMA. 2011;305(16):1669-1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Terman SW, Hill CE, Burke JF. Disability in people with epilepsy: a nationally representative cross-sectional study. Epilepsy Behav. 2020;112:107429. [DOI] [PubMed] [Google Scholar]
- 47.Jette N, Beghi E, Hesdorffer D, et al. ICD coding for epilepsy: past, present, and future: a report by the International League Against Epilepsy Task Force on ICD codes in epilepsy. Epilepsia. 2015;56(3):348-355. [DOI] [PubMed] [Google Scholar]
- 48.Kalilani L, Faught E, Kim H, et al. Assessment and effect of a gap between new-onset epilepsy diagnosis and treatment in the US. Neurology. 2019;92(19):E2197–E2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Holden EW, Grossman E, Nguyen HT, et al. Developing a computer algorithm to identify epilepsy cases in managed care organizations. Dis Manag. 2005;8(1):1-14. [DOI] [PubMed] [Google Scholar]
- 50.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159-174. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All datasets are available to purchase at resdac.org/. Aggregated deidentified data may be shared on request.