Abstract
Background and Objectives
A significant overlap between amyotrophic lateral sclerosis (ALS) and behavioral variant of frontotemporal dementia (bvFTD) has been observed at clinical, genetic, and pathologic levels. Within this continuum of presentations, the presence of mild cognitive or behavioral symptoms in patients with ALS has been consistently reported, although it is unclear whether this is to be considered a distinct phenotype or rather a natural evolution of ALS. Here, we used mathematical modeling of MRI connectomic data to decipher common and divergent neural correlates across the ALS–frontotemporal dementia (FTD) spectrum.
Methods
We included 83 patients with ALS, 35 patients with bvFTD, and 61 healthy controls, who underwent clinical, cognitive, and MRI assessments. Patients with ALS were classified according to the revised Strong criteria into 54 ALS with only motor deficits (ALS-cn), 21 ALS with cognitive or behavioral involvement (ALS-ci/bi), and 8 ALS with bvFTD (ALS-FTD). First, we assessed the functional and structural connectivity patterns across the ALS-FTD spectrum. Second, we investigated whether and where MRI connectivity alterations of patients with ALS with any degree of cognitive impairment (i.e., ALS-ci/bi and ALS-FTD) resembled more the pattern of damage of one (ALS-cn) or the other end (bvFTD) of the spectrum, moving from group-level to single-subject analysis.
Results
As compared with controls, extensive structural and functional disruption of the frontotemporal and parietal networks characterized bvFTD (bvFTD-like pattern), while a more focal structural damage within the sensorimotor-basal ganglia areas characterized ALS-cn (ALS-cn-like pattern). ALS-ci/bi patients demonstrated an ALS-cn-like pattern of structural damage, diverging from ALS-cn with similar motor impairment for the presence of enhanced functional connectivity within sensorimotor areas and decreased functional connectivity within the bvFTD-like pattern. On the other hand, patients with ALS-FTD resembled both structurally and functionally the bvFTD-like pattern of damage with, in addition, the structural ALS-cn-like damage in the motor areas.
Discussion
Our findings suggest a maladaptive role of functional rearrangements in ALS-ci/bi concomitantly with similar structural alterations compared to ALS-cn, supporting the hypothesis that ALS-ci/bi might be considered as a phenotypic variant of ALS, rather than a consequence of disease worsening.
Amyotrophic lateral sclerosis (ALS) is the most common clinical presentation of motor neuron disease, characterized by progressive neurodegeneration of upper and lower motor neurons. A growing body of evidence supports the notion of clinical, pathologic, and genetic overlap between ALS and the wide spectrum of frontotemporal dementia (FTD).1 Indeed, at least 50% of patients with ALS develop cognitive symptoms—mostly affecting executive functions—and behavioral alterations along the course of the disease, leading to a full-blown diagnosis of FTD in 5%–25% of cases.2,3 Considering that comorbid cognitive impairment is a known negative prognostic factor associated with more rapid progression to death or tracheostomy in patients with ALS,4,5 a better definition and understanding of this condition has clear clinical relevance.
The revised Strong criteria2 established a recognized nomenclature for the ALS-FTD clinical continuum ranging from ALS cognitively normal (ALS-cn) to ALS with FTD (ALS-FTD), including ALS with cognitive impairment (ALS-ci), ALS with behavioral impairment (ALS-bi), and ALS with combined cognitive and behavioral impairment (ALS-cbi). Nevertheless, there is debate regarding the pathologic underpinnings distinguishing ALS-cn from ALS-ci/bi and ALS-FTD cases, and whether this is to be considered a distinct phenotype or a natural evolution of ALS. Cross-sectional studies reported an increasing percentage of ALS-ci/bi in disease stages with more severe motor impairment,6 and a sequential cognitive staging system has been proposed for ALS,7 mirroring regions involved in pathologic stages of TDP-43 deposition.8 However, findings of the few available longitudinal neuropsychological studies in ALS diverge, as some support a stability of cognitive and behavioral changes over time, when present,9,10 whereas others suggest a subtle progression of cognitive deficits.11,12 The largest study in this context5 showed that patients who were cognitively impaired at baseline had a faster decline, in contrast with a tendency to remain cognitively intact in those who were cognitively unimpaired at study entry.
In this context, advanced MRI has provided a useful tool to investigate brain architecture in ALS and FTD. Several MRI studies evaluated patients with behavioral variant of FTD (bvFTD), using both conventional MRI13-16 and connectomic approaches,17,18 reporting specific patterns of structural and functional damage within frontoinsular and temporal networks. In ALS, widespread gray matter (GM)19-21 and white matter (WM) damage19,20,22 has been shown in cognitively impaired patients relative to patients with ALS-cn, involving not only motor but also extramotor areas, including frontotemporal, parietal, insular, and cingulate regions. A recent study using a connectomic approach revealed widespread cerebral WM changes affecting frontotemporal regions in patients with ALS-ci/bi relative to patients with ALS-cn.23 Available functional MRI studies have reported conflicting results, as executive dysfunction and behavioral disturbances in ALS have been associated with either enhanced functional connectivity in frontoparietal and temporal networks24-26 or suppressed connectivity within frontoparietal, salience, and executive networks.15,27 However, in the current literature, there is a lack of MRI studies specifically assessing functional brain alterations in ALS with mild cognitive/behavioral decline, as only one study suggested an enhanced functional connectivity in patients with cognitive decline relative to ALS-cn.28
A direct evaluation of brain network reorganization in ALS-ci/bi compared with the opposite ends of the ALS-FTD spectrum (i.e., ALS-cn and full-blown FTD) is needed. No studies have combined structural and functional information using graph analysis and connectomics to investigate neural correlates of cognitive and behavioral decline within patients of the spectrum. The aim of the present study was to bridge this gap, investigating structural and functional network correlates of cognitive/behavioral impairment in patients within the ALS-FTD continuum, who were fully characterized according to the revised Strong criteria.2 Using up-to-date MRI approaches, we assessed distinctive patterns of network disruption (i.e., “ALS-cn-like pattern” and “bvFTD-like pattern”) that may prove useful for accurate classification at a single-patient level.
Methods
An overview of the Methods is provided in Figure 1.
Figure 1. Study Framework.
(A) Patient classification. Revised Strong criteria were applied to identify patients with amyotrophic lateral sclerosis (ALS) with and without cognitive/behavioral impairments or dementia deficits. (B) Connectome reconstruction. Connectomics was applied on diffusion tensor MRI (DTI) and resting-state fMRI after parcellating the brain into 220 regions. Structural and functional connectomes of all participants were reconstructed. (C) Regional connectivity analysis. Network-based statistics was performed, performing all possible comparisons between groups. (D) Distribution analysis. After reconstructing the structural and functional connectome of each patient and control, all connections per each patient were normalized relative to controls and grouped into 6 macro-areas. Intra-area and interarea connectivity distribution were plotted and statistically compared between groups. (E) Classification analysis. Receiver operator characteristic (ROC) curve analysis was performed to discriminate ALS with motor impairment only (ALS-cn) from behavioral variant of frontotemporal dementia (bvFTD) and vice versa, considering intra-area and interarea connectivity that resulted significantly different between these 2 groups in the distribution analysis. (F) Frequency analysis. After ROC curve analysis, the optimal cutoff was identified using the Youden index. ALS with cognitive or behavioral impairment (ALS-ci/bi) and ALS with frontotemporal dementia (ALS-FTD) cases were then subdivided into those under and above the optimal cutoff. χ2 test was performed in order to identify the behavior of these 2 groups. ANOVA = analysis of variance; HC = healthy control.
Participants
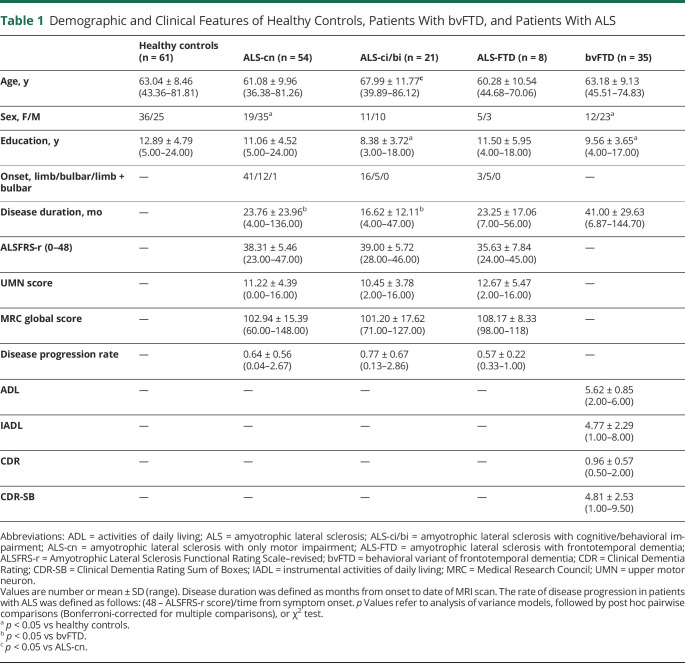
Eighty-three patients with ALS and 35 patients with bvFTD were recruited at the IRCCS Ospedale San Raffaele, Milan, Italy, in the framework of an observational study (Figure 1A). Only sporadic cases (i.e., no family history of dementia or motor neuron disease) in patients who proved negative for mutations in the major genes associated with ALS/FTD (i.e., C9ORF72, GRN, MAPT, TARDBP, SOD1, FUS, TBK1, TREM2, OPTN, and VCP) were included. The diagnosis of ALS was based on the revised El Escorial criteria,29 whereas bvFTD was diagnosed according to Rascovsky criteria.30 Patients underwent a comprehensive evaluation including neurologic history, clinical assessment (Table 1), neuropsychological testing (eTable 1, links.lww.com/WNL/B674), and MRI scan. For patients with ALS, the site of disease onset was recorded; disease severity was assessed using the Amyotrophic Lateral Sclerosis Functional Rating Scale–revised (ALSFRS-r)31; rate of disease progression was defined as (48 – ALSFRS-r score)/time from symptom onset; and muscular strength was assessed by manual muscle testing based on the Medical Research Council (MRC) scale. Patients with ALS were receiving riluzole at study entry. For patients with bvFTD, disease severity was assessed using the Clinical Dementia Rating scale.32
Table 1.
Demographic and Clinical Features of Healthy Controls, Patients With bvFTD, and Patients With ALS
Sixty-one healthy controls were recruited by word of mouth, based on the following criteria: no history of neurologic and psychiatric diseases, no family history of neurodegenerative diseases, and a normal neurologic assessment (Table 1).
Exclusion criteria for all participants were other significant medical illnesses or substance abuse that could interfere with cognitive functioning; any other major systemic, psychiatric, or neurologic illnesses; and other causes of focal or diffuse brain damage, including lacunae and extensive cerebrovascular disorders at routine MRI.
Cognitive and Behavioral Assessment
Patient Classification
Comprehensive multidomain cognitive testing was performed by trained neuropsychologists unaware of MRI results (Figure 1A). Tested cognitive domains were global cognitive functioning, memory, executive function, visuospatial abilities, fluency, language, mood, and behaviors, as previously described18,25 (eTable 1, links.lww.com/WNL/B674). According to the revised Strong criteria,2 patients with ALS were classified into 54 cases with motor impairment only (ALS-cn), 21 cases with cognitive or behavioral deficits (ALS-ci/bi), and 8 patients with ALS with bvFTD (ALS-FTD).
MRI Acquisition and Preprocessing
MRI scans were obtained using a 3T Philips Medical Systems Intera machine. T1-weighted, T2-weighted, fluid-attenuated inversion recovery, diffusion tensor MRI (DTI), and resting-state functional MRI (RS-fMRI) sequences were acquired. Full details of the MRI acquisition protocol are reported in eTable 2 (links.lww.com/WNL/B674). MRI analyses were performed by experienced observers blinded to participants' identity.
Connectome Reconstruction
Brain parcellation, DTI, and RS fMRI preprocessing and construction of brain structural and functional connectome have been described previously (Figure 1B).18,25 Briefly, brain was parcellated into 220 similarly sized GM cortical and subcortical regions (eTable 3, links.lww.com/WNL/B674). Applying a graph theoretical approach, the 220 brain regions are represented as nodes and structural or functional connections linking each pair of nodes as edges. Edges for structural connectivity are represented by fractional anisotropy (FA), whereas functional edges are represented by Pearson correlation coefficients between each pair of nodes. Once the structural macroscale connectome was reconstructed per each participant, we applied the structural connectome of an independent healthy control group as a comprehensive brain connection mask.18 Then, the masked structural connectome of each participant was used as mask for the respective functional connectome, in order to investigate the functional alterations only where structural connections exist, enhancing the biological interpretation of the results.33
Statistical Analysis
Characterization of Functional and Structural Connectivity Across the ALS-FTD Spectrum
Regional Connectivity Analysis
We investigated structural and functional network features in the different participant groups at regional level (Figure 1C). Network-based statistic (NBS)34 was performed to assess regional structural and functional connectivity strength at the level of significance p < 0.05. All possible combinations of comparisons between groups were performed. The largest (or principal) connected component and the smaller clusters of altered connections were identified.25,34 A corrected p value was calculated for each contrast using an age-, sex-, and education-adjusted permutation analysis (10,000 permutations).
Investigation of ALS-cn-like or bvFTD-like Patterns of Alterations in ALS-Ci/bi and ALS-FTD
The following analyses were focused firstly on identifying the specific structural and functional connectivity patterns that characterize the ends of the ALS-FTD spectrum (ALS-cn and bvFTD). We also investigated whether and where patients with ALS-ci/bi and patients with ALS-FTD showed an ALS-cn-like or a bvFTD-like connectivity pattern.
Distribution Analysis
Distribution analysis was performed to assess structural and functional connectivity alterations in patient groups (Figure 1D). Connectivity values of each connection for each patient were normalized relative to controls as follows:
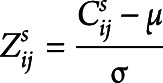 |
where 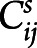 is the structural/functional connectivity value of the connection between node i and j for subject s;
is the structural/functional connectivity value of the connection between node i and j for subject s;  is the mean structural/functional connectivity value of the considered connection in the control group; and
is the mean structural/functional connectivity value of the considered connection in the control group; and  is the SD of the structural/functional connectivity value of such connection in the control group. Subsequently, the 220 regions from both hemispheres were grouped into 6 anatomical macro-areas (hereafter referred to as brain areas): temporal, parietal, occipital, fronto-insular, basal ganglia, and sensorimotor. Per each patient group (ALS-cn, ALS-ci/bi, ALS-FTD, and bvFTD), the mean values of intra-area and interarea connectivity were calculated averaging the normalized structural or functional connections belonging to an area (intra) or linking 2 distinct areas (inter), respectively. The percentage of patients with connectivity value below the reference value (i.e., control mean value) was calculated per each intra-area and interarea network. Finally, the intra-area and interarea connectivity values were compared between patient groups using age-, sex-, and education-adjusted analysis of variance models, followed by post hoc pairwise comparisons, Bonferroni-corrected for multiple comparisons (p < 0.05, SPSS Statistics 26.0 [SPSS Inc.]).
is the SD of the structural/functional connectivity value of such connection in the control group. Subsequently, the 220 regions from both hemispheres were grouped into 6 anatomical macro-areas (hereafter referred to as brain areas): temporal, parietal, occipital, fronto-insular, basal ganglia, and sensorimotor. Per each patient group (ALS-cn, ALS-ci/bi, ALS-FTD, and bvFTD), the mean values of intra-area and interarea connectivity were calculated averaging the normalized structural or functional connections belonging to an area (intra) or linking 2 distinct areas (inter), respectively. The percentage of patients with connectivity value below the reference value (i.e., control mean value) was calculated per each intra-area and interarea network. Finally, the intra-area and interarea connectivity values were compared between patient groups using age-, sex-, and education-adjusted analysis of variance models, followed by post hoc pairwise comparisons, Bonferroni-corrected for multiple comparisons (p < 0.05, SPSS Statistics 26.0 [SPSS Inc.]).
Classification Analysis
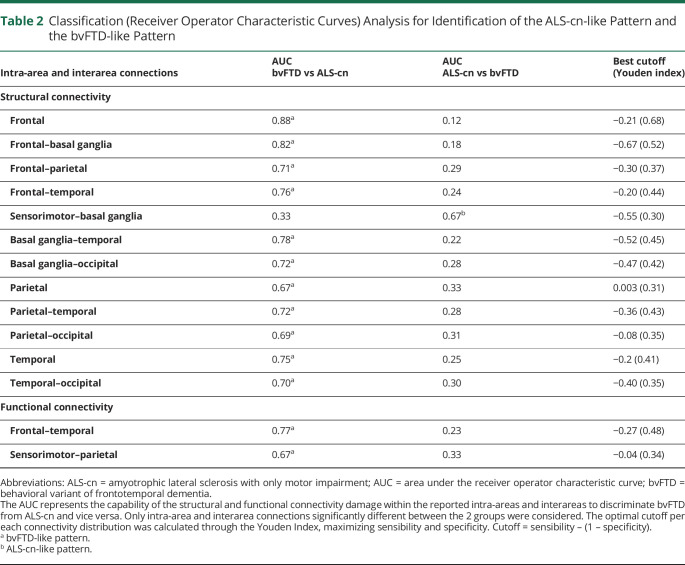
Classification analysis was performed to define the characteristic structural/functional patterns of damage of the 2 ends of the spectrum (ALS-cn and bvFTD) (Figure 1E). For this purpose, we selected structural and functional connectivity values only in those intra-area and interarea networks where ALS-cn and bvFTD showed significantly different patterns in the distribution analysis. Receiver operator characteristic (ROC) curve analysis was performed in these selected networks. The area under the curve (AUC), as derived measure of accuracy, was considered to assign a specific set of structural/functional alterations to ALS-cn (ALS-cn-like pattern) or to bvFTD (bvFTD-like pattern). Per each intra-area or interarea connectivity value involved in one of the 2 patterns, Youden Index was calculated, providing the best tradeoff between sensitivity and specificity. Finally, patients of each group were classified in those with connectivity values above or below the identified optimal cutoffs.
Frequency Analysis
Aiming to assess, at the single-subject level, whether and where patients with ALS-ci/bi and patients with ALS-FTD showed commonalities and differences with ALS-cn-like or bvFTD-like patterns, we performed a frequency analysis using the χ2 test (p < 0.05) (Figure 1F). Specifically, we identified and compared between groups the frequency of participants with connectivity values above and below the optimal cutoffs belonging to the ALS-cn-like and the bvFTD-like pattern. The ALS-cn group was excluded in the frequency analysis of the ALS-cn-like pattern; the bvFTD group was not considered in the bvFTD-like pattern analysis.
Data Availability
The dataset used during the study will be made available by the corresponding author upon request to qualified researchers (i.e., affiliated with a university or research institution/hospital).
Standard Protocol Approvals, Registrations, and Patient Consents
Local ethical standards committee on human experimentation approved the study protocol and all participants (or their caregivers) provided written informed consent.
Results
Clinical and Neuropsychological Features
Demographic and clinical characteristics of study groups are reported in Table 1 and neuropsychological features in eTable 1 (links.lww.com/WNL/B674). Relative to controls, patients with ALS-cn and patients with bvFTD showed a larger proportion of male individuals. Patients with ALS-ci/bi and patients with bvFTD showed lower education relative to controls. ALS groups and patients with bvFTD were different for disease duration at MRI, which was shorter in patients with ALS. ALS groups were comparable in terms of disease severity, as assessed by ALSFRS-r and MRC global score, disease progression rate, and site of clinical onset, although patients with ALS-ci/bi were older than patients with ALS-cn. The neuropsychological assessment did not reveal differences between controls and patients with ALS-cn. Patients with bvFTD and patients with ALS-FTD performed worse than controls and patients with ALS-cn in all investigated cognitive domains. The ALS-ci/bi group performed worse than controls in naming (actions) and better than patients with bvFTD and patients with ALS-FTD in fluency tests, with additional higher performance in global cognition, verbal memory, and abstract reasoning compared to the bvFTD group (eTable 1).
Characterization of Functional and Structural Connectivity Across the ALS-FTD Spectrum
Structural Connectivity
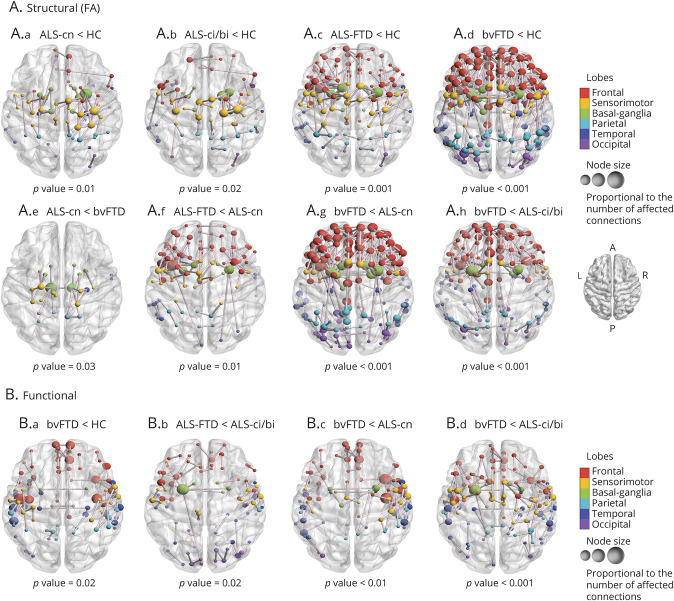
Regional connectivity analysis showed alterations involving the connections within and among the sensorimotor network, basal ganglia, frontal, temporal, and parietal areas, in addition to minimal involvement of occipital connections, in patients with ALS-cn relative to controls (p = 0.01; Figure 2A.a). This structural pattern of damage was also found in patients with ALS-ci/bi and patients with ALS-FTD relative to controls (p = 0.02 and p = 0.001, Figure 2A.b,c, respectively), with a more widespread disruption of the same networks in ALS-FTD reflecting increasing severity of impaired behavior and cognition (Figure 2A.c). Patients with ALS-FTD also showed more severe structural damage, mainly within frontal areas, relative to patients with ALS-cn (p = 0.01; Figure 2A.f). In addition, patients with ALS-cn showed greater structural alterations relative to bvFTD (p = 0.03; Figure 2A.e) in few connections within and among sensorimotor regions, parietal areas, and basal ganglia, especially involving thalamus and those connections from pallidum and putamen towards precentral, postcentral, and precuneus bilaterally. Patients with bvFTD showed widespread structural damage relative to controls, patients with ALS-cn, and patients with ALS-ci/bi across the whole brain (p < 0.001; Figure 2A.d,g,h, respectively). No further differences were observed in the remaining comparisons.
Figure 2. Alterations in Structural and Functional Connectivity in Patients With ALS and Patients With bvFTD Relative to Healthy Controls and Each Other.
Altered structural (A) and functional (B) connections are represented per each significant contrast, respectively (p < 0.05). Comparisons were adjusted for age, sex, and education. The node color represents its belonging to specific macro-areas (frontal, sensorimotor, basal ganglia, parietal, temporal, and occipital). The node size is proportional to the number of affected connections (the higher the number of disrupted connections, the larger the node). A = anterior; ALS = amyotrophic lateral sclerosis; ALS-ci/bi = amyotrophic lateral sclerosis with cognitive or behavioral impairment; ALS-cn = amyotrophic lateral sclerosis with motor impairment only; ALS-FTD = amyotrophic lateral sclerosis with frontotemporal dementia; bvFTD = behavioral variant of frontotemporal dementia; FA = fractional anisotropy; HC = healthy controls; P = posterior.
Functional Connectivity
NBS analysis did not show differences in functional connectivity in ALS groups relative to controls, although patients with ALS-ci/bi showed a trend toward an enhanced functional connectivity relative to controls within frontal and basal ganglia areas (p = 0.06). On the other hand, patients with bvFTD were characterized by reduced functional connectivity relative to controls (p = 0.02; Figure 2B.a), patients with ALS-cn (p = 0.01; Figure 2B.c), and patients with ALS-ci/bi (p < 0.001; Figure 2B.d), mainly involving the connections within the frontotemporal regions and between frontal and sensorimotor areas. Patients with ALS-FTD relative to patients with ALS-ci/bi showed reduced functional connectivity within and between the frontal, temporal, and motor areas similarly to bvFTD cases (p = 0.02; Figure 2B.b). No further differences were observed in the remaining comparisons.
Investigation of ALS-cn-like and bvFTD-like Patterns of Alterations in ALS-ci/bi and ALS-FTD: Structural Connectivity
Distribution Analysis
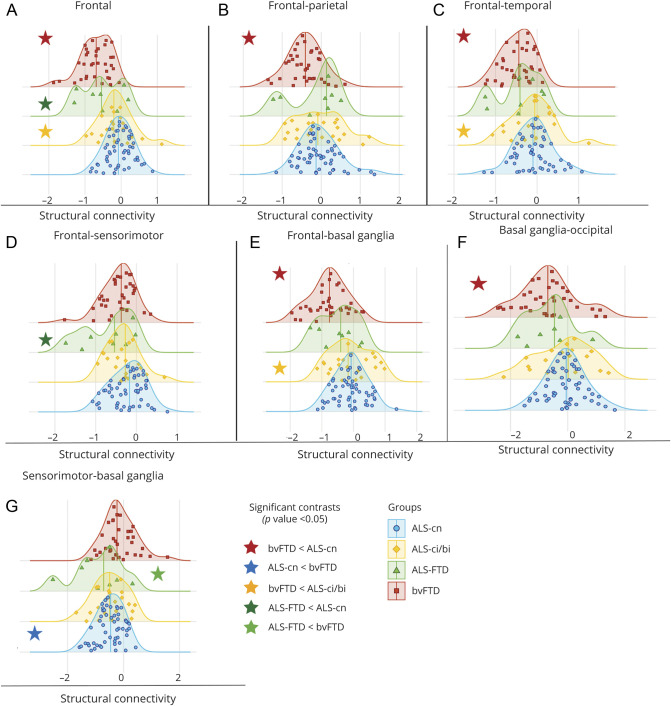
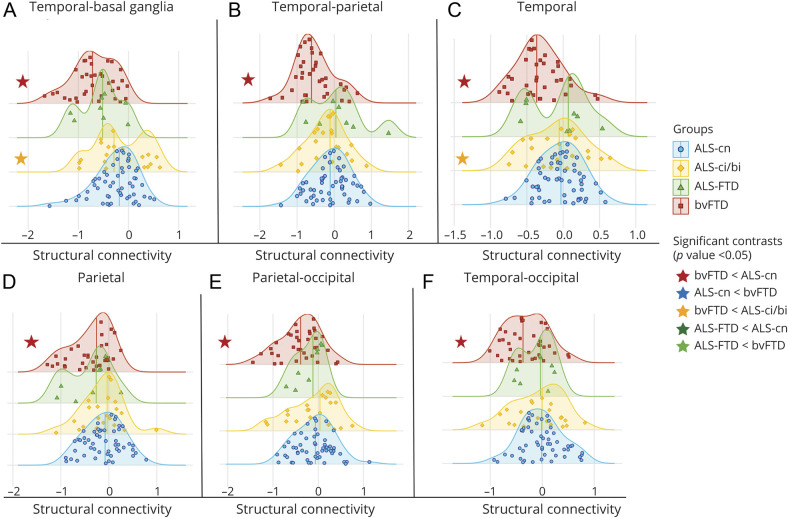
Compared with patients with ALS-cn, patients with bvFTD showed greater structural intra-area disruption within frontal (Figures 3 and 4), temporal, and parietal areas (Figure 3A, Figure 4, C and D, and eTable 4, links.lww.com/WNL/B674; p < 0.05) and interarea disruption in the frontal, temporal, and occipital connections toward parietal lobe (p = 0.01, Figure 3B and Figure 4, B and E), in the frontal, basal ganglia, and occipital connections toward temporal areas (p = 0.002, p < 0.001, and p = 0.03,Figure 3C and Figure 4, A and F, respectively), and in the connections between frontal and basal ganglia (p < 0.001) (Figure 3E and eTable 4). Most patients with bvFTD (from 83% to 100%) were severely disrupted in these networks (eTable 4). On the other hand, most patients with ALS-cn (81%) were characterized by greater damage within the motor network, specifically among the sensorimotor–basal ganglia connections, relative to bvFTD cases (p = 0.01, Figure 3G). In addition, patients with ALS-FTD showed structural connectivity alterations within the motor areas, resembling the ALS-cn damage. In particular, 88% of ALS-FTD cases revealed a significant structural disruption in the sensorimotor–basal ganglia connections compared with patients with bvFTD (p = 0.01; Figure 3G and eTable 4). Among the other brain regions, patients with ALS-ci/bi and patients with ALS-FTD behaved differently. Patients with ALS-ci/bi showed significant structural connectivity differences within frontal and temporal lobe (Figure 3A and Figure 4C) and between frontal, temporal, and basal ganglia areas compared to patients with bvFTD (p < 0.05; Figure 3, C and E, and Figure 4A), embracing a pattern of damage more like ALS-cn. On the other hand, ALS-FTD revealed a behavior more like bvFTD, showing a greater structural disruption within frontal (p = 0.03) and in frontal–sensorimotor connections (p = 0.02) compared to ALS-cn (Figure 3, A and D, and eTable 4).
Figure 3. Distribution Analysis of the Structural Connectivity Damage in Patient Groups.
A–G) The distribution of structural connectivity alterations within frontal and motor areas and in the connections towards these areas is displayed. Distribution curves are normalized relative to control values. The more the curve is shifted towards negative values, the greater the structural damage. All significant contrasts (p < 0.05)—displayed with colored stars—are reported according to age-, sex-, and education-adjusted analysis of variance models, Bonferroni-corrected for multiple comparisons. ALS = amyotrophic lateral sclerosis; ALS-ci/bi = amyotrophic lateral sclerosis with cognitive or behavioral impairment; ALS-cn = amyotrophic lateral sclerosis with motor impairment only; ALS-FTD = amyotrophic lateral sclerosis with frontotemporal dementia; bvFTD = behavioral variant of frontotemporal dementia.
Figure 4. Distribution Analysis of the Structural Connectivity Damage in Patient Groups.
A–F) The distribution of structural connectivity alterations within parietal and temporal areas and in the connections towards these areas is displayed. Distribution curves are normalized relative to control values. The more the curve is shifted towards negative values, the greater the structural damage. All significant contrasts (p < 0.05)—displayed with colored stars—are reported according to age-, sex-, and education-adjusted analysis of variance models, Bonferroni-corrected for multiple comparisons. ALS = amyotrophic lateral sclerosis; ALS-ci/bi = amyotrophic lateral sclerosis with cognitive or behavioral impairment; ALS-cn = amyotrophic lateral sclerosis with motor impairment only; ALS-FTD = amyotrophic lateral sclerosis with frontotemporal dementia; bvFTD = behavioral variant of frontotemporal dementia.
Classification Analysis
From ROC curve analysis, 2 characteristic patterns of damage were identified: the ALS-cn-like pattern defined by focal structural damage within sensorimotor–basal ganglia areas that distinguished ALS-cn from bvFTD (accuracy [AUC] = 0.67, eFigure 1A, blue line, links.lww.com/WNL/B674), and the bvFTD-like pattern characterized by structural alterations of the frontotemporal and parietal networks that discriminated bvFTD from ALS-cn with AUC ranging from 0.67 to 0.88 (eFigure 1A, red lines). The best cutoffs of structural connectivity per each significant network are reported in Table 2.
Table 2.
Classification (Receiver Operator Characteristic Curves) Analysis for Identification of the ALS-cn-like Pattern and the bvFTD-like Pattern
Frequency Analysis
The ALS-cn-like pattern was identified more frequently in ALS-ci/bi and ALS-FTD compared with bvFTD (ALS-ci/bi vs bvFTD, p = 0.04; ALS-FTD vs bvFTD, nonsignificant trend, p = 0.07) (eTable 5, links.lww.com/WNL/B674). On the other hand, the bvFTD-like pattern was found to be more frequent neither in ALS-ci/bi nor ALS-FTD compared to ALS-cn, except for a nonsignificant trend (p = 0.08) within frontal and among frontal-basal ganglia, temporal-occipital areas in ALS-FTD relative to ALS-cn (eTable 5).
Investigation of ALS-cn-like and bvFTD-like Patterns of Alterations in ALS-ci/bi and ALS-FTD: Functional Connectivity
Distribution Analysis
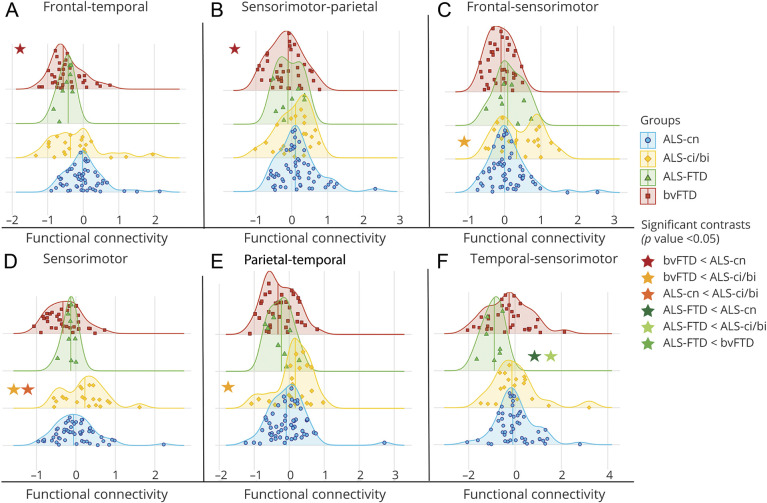
Regarding functional connectivity distribution analysis (Figure 5), decreased functional connectivity within frontotemporal (p = 0.001) and between sensorimotor and parietal connections (p < 0.02) was found in bvFTD compared with ALS-cn (Figure 5, A and B, and eTable 4, links.lww.com/WNL/B674). Patients with ALS-ci/bi showed significant enhanced functional connectivity relative to bvFTD in frontal–sensorimotor connections (p = 0.001), parietotemporal connections (p = 0.03), and within sensorimotor areas (p < 0.001, Figure 5, C–E, and eTable 4). In addition, patients with ALS-ci/bi showed increased functional connectivity within sensorimotor areas relative to ALS-cn (p < 0.04, Figure 5D and eTable 4). Of note, most patients with ALS-ci/bi (a percentage ranging from 67% to 76%) revealed normalized values of functional connectivity greater than zero in these abovementioned networks (i.e., frontal-sensorimotor, parietotemporal, and sensorimotor). Moreover, patients with ALS-FTD showed a significant greater reduced functional connectivity in temporal–sensorimotor connections compared to patients with ALS-cn (p = 0.03) and patients with ALS-ci/bi (p < 0.01, Figure 5F and eTable 4).
Figure 5. Distribution Analysis of the Functional Connectivity Damage in Patient Groups.
A–F) Functional connectivity damage distribution within area and among areas is reported. Distribution curves are normalized relative to control values. The more the curve is shifted towards negative values, the more reduced the functional connectivity. All significant contrasts (p < 0.05)—displayed with colored stars—are reported according to age-, sex-, and education-adjusted analysis of variance models, Bonferroni-corrected for multiple comparisons. ALS = amyotrophic lateral sclerosis; ALS-ci/bi = amyotrophic lateral sclerosis with cognitive or behavioral impairment; ALS-cn = amyotrophic lateral sclerosis with motor impairment only; ALS-FTD = amyotrophic lateral sclerosis with frontotemporal dementia; bvFTD = behavioral variant of frontotemporal dementia.
Classification Analysis
The ROC curve analysis on functional connectivity data identified only a bvFTD-like pattern of functional damage, involving frontotemporal and sensorimotor–parietal connections, with an AUC of 0.77 and 0.67 in discriminating bvFTD from ALS-cn, respectively (eFigure 1B, red lines, links.lww.com/WNL/B674). The best cutoff values of functional connectivity for each significant network are reported in Table 2.
Frequency Analysis
Within frontotemporal connections, patients with ALS-ci/bi were characterized by a greater proportion of cases showing bvFTD-like decreased functional connectivity compared with ALS-cn (p = 0.03; eTable 5, links.lww.com/WNL/B674), but a lower proportion compared with patients with ALS-FTD (p = 0.02), who mostly showed a typical bvFTD-like pattern with a decreased functional connectivity relative to patients with ALS-cn (p < 0.001; eTable 5).
Discussion
The present multiparametric MRI study provides a comprehensive characterization of neural correlates across the spectrum of ALS-FTD clinical presentations. A connectome-based approach was adopted, first to identify the connectivity signatures of ALS-cn and bvFTD (i.e., the 2 ends of this spectrum) and subsequently to characterize the alterations underlying mild cognitive/behavioral deficits and full-blown dementia in patients with ALS, with the aid of mathematical models and single-subject analysis. An ALS-cn-like pattern was defined by a focused structural damage within the motor areas. By contrast, a bvFTD-like pattern was delineated by widespread structural damage and decreased functional connectivity, specifically in frontal, temporal, and parietal areas. Patients with ALS-ci/bi showed a pattern of structural damage mostly overlapping with the ALS-cn-like pattern, whereas functional data diverged from ALS-cn for the presence of enhanced functional connectivity within sensorimotor regions and decreased functional connectivity in frontotemporal areas (i.e., mirroring a bvFTD-like pattern). ALS-FTD resembled the bvFTD-like pattern of damage both structurally and functionally, with, in addition, the structural ALS-cn-like damage in the motor areas. Although connectivity data alone cannot fully address the homogeneity or heterogeneity of this spectrum, our findings suggest a maladaptive role of functional rearrangements in ALS-ci/bi concomitantly with similar structural alterations compared to ALS-cn, supporting the hypothesis that ALS-ci/bi might be considered as a phenotypic variant of ALS, rather than a consequence of disease worsening.
When considering the results of the present study, some limitations should be noted. Despite the robust size of the overall ALS cohort, some subgroups were small (i.e., ALS-FTD), although this is indicative of the relative incidence of cognitive alterations. This aspect has also influenced our choice to bring together patients with mild cognitive dysfunction (i.e., ALS-ci) and patients with mild behavioral disturbances (i.e., ALS-bi), to avoid dispersion of data and the reduced statistical power that would result. Furthermore, the lack of information of a definite pathologic diagnosis for patients with bvFTD is an important limitation of the present study, even though the aim of the work was to explore the neural correlates of the clinical rather than the pathologic heterogeneity of the ALS-FTD spectrum. Another issue lies in the cross-sectional nature of the study. In this context, longitudinal studies are warranted to verify whether cognitive/behavioral dysfunction is a stable or progressive feature of the ALS trajectory, and to assess the evolution of associated network alterations over time.
The inherent limitations of MRI connectomics should be acknowledged,35,36 including, among others, the lack of an optimal framework, that is, a reference standard for the regional parcellation of brain MRI. The accuracy of any attempt to model the connectome is biased by the intrinsic limitations of the imaging techniques used. For example, fiber tracking based on DTI is known to be poor at points where only limited information about the WM fiber direction is available, such as where multiple tracts cross. This results in incomplete reconstruction of tracts and a general underrepresentation of long-distance connections in the brain. Despite these shortcomings, our study highlights the potential of multiparametric connectome-based approaches for providing novel pathophysiologic insights and biomarkers of cognitive dysfunction in the context of ALS-FTD. A key point of our study was the demonstration of characteristic brain structural damage and functional rearrangements across ALS cognitive phenotypes, as defined based on the application of revised Strong criteria to a sizeable monocentric cohort. Our conclusions were made possible by the extensive clinical and neuropsychological characterization of the sample, as well as by the multiparametric nature of this study. Current MRI literature has generally provided results based on the assessment of structural and functional alterations separately, at voxel or regional level, without a straightforward investigation of their relationship. Conversely, a connectomic approach gave us the potential to bridge the gap of the anatomo-functional link owing to the application of the same parcellation system, connectome reconstruction framework, and statistical approach. Whereas the capability of the connectome-based approach to provide information on the brain network architecture was achieved by a group-level analysis, smoothing out the interindividual variability, a further innovative aspect of our study was the transition to single-level analysis by the help of mathematical models. Indeed, the study framework was able to identify the ALS-cn-like or bvFTD-like patterns of damage, and to characterize the type of damage that each ALS-ci/bi and ALS-FTD case shared with such signatures of network alterations.
The selective involvement of motor WM regions in the ALS-cn sample is largely consistent with previous literature,21,25,37 confirming a signature pattern of frank decline in FA of the subnetworks connecting primary motor, supplementary motor, and premotor areas, as well as basal ganglia—specifically, the thalamus.38 The structural disruption of the sensorimotor network supports the current view of this network as the epicenter of degenerative process of the disease, in line with proposed neuropathologic and MRI-based disease staging systems.8,39 As for functional MRI findings, the current literature counts on a number of studies reporting reduced15,27 or increased functional connectivity in patients with ALS,24-26 or a mixed picture.40 Nevertheless, there is a shortage of MRI studies focusing on functional brain rearrangements in ALS related to cognitive status, and our findings contribute to fill this gap. Of note, both regional (i.e., NBS) and distribution analyses suggest that ALS-cn is characterized by preserved functional connectivity comparable to the functional healthy brain organization.
The bvFTD-like pattern included widespread brain structural disruption, with predominant damage in the frontotemporoparietal network and the involvement of the striatum, and functional connectivity breakdown within the same networks. Our findings confirm previous evidence that notes the disconnection of frontoinsular and temporal regions as hallmark of the behavioral clinical syndrome of FTD both at structural and functional levels.14,16-18 Herein, we extend these results by highlighting the relative preservation of motor areas in bvFTD, in contrast with a widespread structural and functional involvement of the anterior frontal lobes, as well as a differential involvement of basal ganglia circuits when compared with ALS-cn (i.e., greater involvement of striatal connections in bvFTD, in contrast with thalamic involvement in ALS-cn). These findings are in line with previous reports38,41 and support the notion of a diverging network vulnerability to disease pathology in the 2 opposite ends of the ALS-FTD spectrum.
The focus of the current study was on elucidating MRI connectomic underpinnings of mild or full-blown cognitive deficits in ALS, possibly addressing the long-standing debate on the nature of cognitive deficits in the course of the disease, as an early or rather a late-stage feature. Regarding the structural brain network, the presence of mild cognitive or behavioral impairment in patients with ALS did not contribute significantly to additional microstructural damage relative to ALS-cn with otherwise comparable clinical characteristics, including measures of motor impairment and disease duration. Although previous literature has suggested greater structural damage related to cognitive impairment in ALS,19-23 such damage was generally subtle and possibly driven by the inclusion of participants with ALS-FTD. By contrast, our study highlighted shared structural damage between patients with ALS-ci/bi and patients with ALS-cn, involving mainly the motor networks. On the other hand, the analysis of functional connectivity alterations played an important role for the differentiation of ALS-ci/bi from ALS-cn. Indeed, patients with ALS-ci/bi showed a rearrangement of functional networks, which was divergent from ALS-cn, with enhanced functional connectivity within motor areas and decreased connectivity in the frontotemporal networks. The concomitant absence of significant structural alterations, compared with the ALS-cn group, apparently supports a maladaptive role of such functional rearrangements in ALS-ci/bi, as previously hypothesized.25,42 The biological underpinnings of such functional disequilibrium have been suggested to lie in the known excitatory/inhibitory imbalance due to interneuron pathology in ALS, causing a reduction in recurring inhibition that has been associated with disease severity.43,44 We argue that functional imbalance between motor and extramotor frontal networks might be particularly severe in ALS-ci/bi, causing mild cognitive disturbances even in early phases of the disease, consistent with the relatively short disease duration of the current cohort. Therefore, our data suggest that ALS-ci/bi might be considered as a phenotypic variant of ALS, rather than a consequence of disease worsening.6,7 These findings may find support in one of the few longitudinal neuropsychological studies in this context,5 in which cognition decline was faster in patients who were already cognitively impaired at baseline, while normal cognition tended to remain intact with slower motor and cognitive progression. Of note, education levels of patients with ALS-ci/bi were lower than in ALS-cn in our sample, consistent with the recently highlighted influence of environmental factors that collectively constitute the cognitive reserve (i.e., education, occupation, and physical activity) over an early development of cognitive symptoms in ALS.45
In contrast with ALS-ci/bi cases, when patients with ALS had co-occurrent dementia (ALS-FTD), our study has outlined not only a pattern of microstructural damage involving motor networks (i.e., the characteristic ALS-cn-like pattern), but also a disruption of frontal, temporal, parietal, and striatal circuits, both from a structural and a functional point of view—therefore, resembling the bvFTD-like pattern.46 These findings agree with the pattern of widespread hypometabolism recently demonstrated in ALS cases with severe cognitive impairment,47 possibly mirroring the most advanced stages of TDP-43 neuropathologic models, which have been proposed both in ALS8 and bvFTD,48 here sharing the same pathologic signature.49,50 Similar to patients with ALS-ci/bi, patients with ALS-FTD showed similar severity of motor symptoms and disease duration when compared with patients with ALS-cn, supporting a view of this clinical presentation as a specific phenotype within the frontotemporal lobar degeneration spectrum, characterized by a combined, severe involvement of both motor and extramotor brain networks, rather than an evolution of either ALS or bvFTD.
Acknowledgment
The authors thank the patients and their families for their participation.
Glossary
- ALS
amyotrophic lateral sclerosis
- ALS-bi
amyotrophic lateral sclerosis with behavioral impairment
- ALS-cbi
amyotrophic lateral sclerosis with combined cognitive and behavioral impairment
- ALS-ci
amyotrophic lateral sclerosis with cognitive impairment
- ALS-cn
amyotrophic lateral sclerosis with only motor impairment (cognitively normal)
- ALS-FTD
amyotrophic lateral sclerosis with frontotemporal dementia
- ALSFRS-r
Amyotrophic Lateral Sclerosis Functional Rating Scale–revised
- AUC
area under the curve
- bvFTD
behavioral variant of frontotemporal dementia
- DTI
diffusion tensor MRI
- FA
fractional anisotropy
- FTD
frontotemporal dementia
- GM
gray matter
- MRC
Medical Research Council
- NBS
network-based statistic
- ROC
receiver operator characteristic
- RS-fMRI
resting-state functional MRI
- UMN
upper motor neuron
- WM
white matter
Appendix. Authors
Footnotes
CME Course: NPub.org/cmelist
Study Funding
Supported by the Italian Ministry of Health (GR-2011-02351217; GR-2013-02357415; RF-2011-02351193), AriSLA (ConnectALS), and European Research Council (StG-2016_714388_NeuroTRACK).
Disclosure
C. Cividini, S. Basaia, E.G. Spinelli, E. Canu, V. Castelnovo, N. Riva, G. Cecchetti, F. Caso, G. Magnani, and A. Falini report no disclosures relevant to the manuscript. M. Filippi is Editor-in-Chief of the Journal of Neurology, Associate Editor of Human Brain Mapping, Associate Editor of Radiology, and Associate Editor of Neurologic Sciences; received compensation for consulting services and/or speaking activities from Alexion, Almirall, Bayer, Biogen, Celgene, Eli Lilly, Genzyme, Merck-Serono, Novartis, Roche, Sanofi, Takeda, and Teva Pharmaceutical Industries; and receives research support from Biogen Idec, Merck-Serono, Novartis, Roche, Teva Pharmaceutical Industries, Italian Ministry of Health, Fondazione Italiana Sclerosi Multipla, and ARiSLA (Fondazione Italiana di Ricerca per la SLA). F. Agosta is Associate Editor of NeuroImage: Clinical; received compensation for consulting services and/or speaking activities from Novartis, Biogen Idec, Philips, and Roche; and receives or has received research support from the Italian Ministry of Health, AriSLA (Fondazione Italiana di Ricerca per la SLA), and the European Research Council. Go to Neurology.org/N for full disclosures.
References
- 1.Burrell JR, Halliday GM, Kril JJ, et al. . The frontotemporal dementia–motor neuron disease continuum. Lancet. 2016;388(10047):919-931. [DOI] [PubMed] [Google Scholar]
- 2.Strong MJ, Abrahams S, Goldstein LH, et al. . Amyotrophic lateral sclerosis–frontotemporal spectrum disorder (ALS-FTSD): revised diagnostic criteria. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18(3-4):153-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saxon JA, Thompson JC, Jones M, et al. . Examining the language and behavioural profile in FTD and ALS-FTD. J Neurol Neurosurg Psychiatry. 2017;88(8):675-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calvo A, Moglia C, Lunetta C, et al. . Factors predicting survival in ALS: a multicenter Italian study. J Neurol. 2017;264(1):54-63. [DOI] [PubMed] [Google Scholar]
- 5.Elamin M, Bede P, Byrne S, et al. . Cognitive changes predict functional decline in ALS: a population-based longitudinal study. Neurology. 2013;80(17):1590-1597. [DOI] [PubMed] [Google Scholar]
- 6.Chio A, Moglia C, Canosa A, et al. . Cognitive impairment across ALS clinical stages in a population-based cohort. Neurology. 2019;93(10):e984-e994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lule D, Bohm S, Muller HP, et al. . Cognitive phenotypes of sequential staging in amyotrophic lateral sclerosis. Cortex. 2018;101:163-171. [DOI] [PubMed] [Google Scholar]
- 8.Brettschneider J, Del Tredici K, Toledo JB, et al. . Stages of pTDP-43 pathology in amyotrophic lateral sclerosis. Ann Neurol. 2013;74(1):20-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kasper E, Schuster C, Machts J, et al. . Dysexecutive functioning in ALS patients and its clinical implications. Amyotroph Lateral Scler Frontotemporal Degener. 2015;16(3-4):160-171. [DOI] [PubMed] [Google Scholar]
- 10.Kilani M, Micallef J, Soubrouillard C, et al. . A longitudinal study of the evolution of cognitive function and affective state in patients with amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Mot Neuron Disord. 2004;5(1):46-54. [DOI] [PubMed] [Google Scholar]
- 11.Beeldman E, Govaarts R, de Visser M, et al. . Progression of cognitive and behavioural impairment in early amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2020;91(7):779-780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castelnovo V, Canu E, Riva N, et al. . Progression of cognitive and behavioral disturbances in motor neuron diseases assessed using standard and computer-based batteries. Amyotroph Lateral Scler Frontotemporal Degener. 2021;22(3-4):223-236. [DOI] [PubMed] [Google Scholar]
- 13.Seeley WW, Crawford R, Rascovsky K, et al. . Frontal paralimbic network atrophy in very mild behavioral variant frontotemporal dementia. Arch Neurol. 2008;65(2):249-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whitwell JL, Jack CR Jr, Parisi JE, et al. . Imaging signatures of molecular pathology in behavioral variant frontotemporal dementia. J Mol Neurosci. 2011;45(3):372-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trojsi F, Esposito F, de Stefano M, et al. . Functional overlap and divergence between ALS and bvFTD. Neurobiol Aging. 2015;36(1):413-423. [DOI] [PubMed] [Google Scholar]
- 16.Gordon E, Rohrer JD, Fox NC. Advances in neuroimaging in frontotemporal dementia. J Neurochem. 2016;138(suppl 1):193-210. [DOI] [PubMed] [Google Scholar]
- 17.Agosta F, Sala S, Valsasina P, et al. . Brain network connectivity assessed using graph theory in frontotemporal dementia. Neurology. 2013;81(2):134-143. [DOI] [PubMed] [Google Scholar]
- 18.Filippi M, Basaia S, Canu E, et al. . Brain network connectivity differs in early-onset neurodegenerative dementia. Neurology. 2017;89(17):1764-1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agosta F, Ferraro PM, Riva N, et al. . Structural brain correlates of cognitive and behavioral impairment in MND. Hum Brain Mapp. 2016;37(4):1614-1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alruwaili AR, Pannek K, Coulthard A, Henderson R, Kurniawan ND, McCombe P. A combined tract-based spatial statistics and voxel-based morphometry study of the first MRI scan after diagnosis of amyotrophic lateral sclerosis with subgroup analysis. J Neuroradiol. 2018;45(1):41-48. [DOI] [PubMed] [Google Scholar]
- 21.Illan-Gala I, Montal V, Pegueroles J, et al. . Cortical microstructure in the amyotrophic lateral sclerosis-frontotemporal dementia continuum. Neurology. 2020;95(18):e2565-e2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kasper E, Schuster C, Machts J, et al. . Microstructural white matter changes underlying cognitive and behavioural impairment in ALS: an in vivo study using DTI. PLoS One. 2014;9(12):e114543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van der Burgh HK, Westeneng HJ, Walhout R, et al. . Multimodal longitudinal study of structural brain involvement in amyotrophic lateral sclerosis. Neurology. 2020;94(24):e2592-e2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schulthess I, Gorges M, Muller HP, et al. . Functional connectivity changes resemble patterns of pTDP-43 pathology in amyotrophic lateral sclerosis. Sci Rep. 2016;6(1):38391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Basaia S, Agosta F, Cividini C, et al. . Structural and functional brain connectome in motor neuron diseases: a multicenter MRI study. Neurology. 2020;95(18):e2552-e2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Castelnovo V, Canu E, Calderaro D, et al. . Progression of brain functional connectivity and frontal cognitive dysfunction in ALS. Neuroimage Clin. 2020;28:102509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohammadi B, Kollewe K, Samii A, Krampfl K, Dengler R, Munte TF. Changes of resting state brain networks in amyotrophic lateral sclerosis. Exp Neurol. 2009;217(1):147-153. [DOI] [PubMed] [Google Scholar]
- 28.Hu T, Hou Y, Wei Q, et al. . Patterns of brain regional functional coherence in cognitive impaired ALS. Int J Neurosci. 2020;130(8):751-758. [DOI] [PubMed] [Google Scholar]
- 29.Brooks BR, Miller RG, Swash M, Munsat TL; World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Mot Neuron Disord. 2000;1(5):293-299. [DOI] [PubMed] [Google Scholar]
- 30.Rascovsky K, Hodges JR, Knopman D, et al. . Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134(pt 9):2456-2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cedarbaum JM, Stambler N, Malta E, et al. . The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function: BDNF ALS Study Group (phase III). J Neurol Sci. 1999;169(1-2):13-21. [DOI] [PubMed] [Google Scholar]
- 32.Knopman DS, Kramer JH, Boeve BF, et al. . Development of methodology for conducting clinical trials in frontotemporal lobar degeneration. Brain. 2008;131(pt 11):2957-2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmidt R, Verstraete E, de Reus MA, Veldink JH, van den Berg LH, van den Heuvel MP. Correlation between structural and functional connectivity impairment in amyotrophic lateral sclerosis. Hum Brain Mapp. 2014;35(9):4386-4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zalesky A, Fornito A, Bullmore ET. Network-based statistic: identifying differences in brain networks. Neuroimage. 2010;53(4):1197-1207. [DOI] [PubMed] [Google Scholar]
- 35.Pandya S, Kuceyeski A, Raj A; Alzheimer's Disease Neuroimaging Initiative. The brain's structural connectome mediates the relationship between regional neuroimaging biomarkers in Alzheimer’s disease. J Alzheimers Dis. 2017;55(4):1639-1657. [DOI] [PubMed] [Google Scholar]
- 36.Reyes P, Ortega-Merchan MP, Rueda A, et al. . Functional connectivity changes in behavioral, semantic, and nonfluent variants of frontotemporal dementia. Behav Neurol. 2018;2018:9684129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muller HP, Lule D, Roselli F, Behler A, Ludolph AC, Kassubek J. Segmental involvement of the corpus callosum in C9orf72-associated ALS: a tract of interest-based DTI study. Ther Adv Chronic Dis. 2021;12:20406223211002969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tu S, Menke RAL, Talbot K, Kiernan MC, Turner MR. Regional thalamic MRI as a marker of widespread cortical pathology and progressive frontotemporal involvement in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2018;89(12):1250-1258. [DOI] [PubMed] [Google Scholar]
- 39.Meier JM, van der Burgh HK, Nitert AD, et al. . Connectome-based propagation model in amyotrophic lateral sclerosis. Ann Neurol. 2020;87(5):725-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Agosta F, Canu E, Valsasina P, et al. . Divergent brain network connectivity in amyotrophic lateral sclerosis. Neurobiol Aging. 2013;34(2):419-427. [DOI] [PubMed] [Google Scholar]
- 41.Bede P, Omer T, Finegan E, et al. . Connectivity-based characterisation of subcortical grey matter pathology in frontotemporal dementia and ALS: a multimodal neuroimaging study. Brain Imaging Behav. 2018;12(6):1696-1707. [DOI] [PubMed] [Google Scholar]
- 42.Menke RAL, Proudfoot M, Talbot K, Turner MR. The two-year progression of structural and functional cerebral MRI in amyotrophic lateral sclerosis. Neuroimage Clin. 2018;17:953-961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van den Bos MAJ, Higashihara M, Geevasinga N, Menon P, Kiernan MC, Vucic S. Imbalance of cortical facilitatory and inhibitory circuits underlies hyperexcitability in ALS. Neurology. 2018;91(18):e1669-e1676. [DOI] [PubMed] [Google Scholar]
- 44.Crabe R, Aimond F, Gosset P, Scamps F, Raoul C. How degeneration of cells surrounding motoneurons contributes to amyotrophic lateral sclerosis. Cells. 2020;9(12):2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Costello E, Rooney J, Pinto-Grau M, et al. . Cognitive reserve in amyotrophic lateral sclerosis (ALS): a population-based longitudinal study. J Neurol Neurosurg Psychiatry. 2021;92(5):460-465. [DOI] [PubMed] [Google Scholar]
- 46.Saxon JA, Thompson JC, Harris JM, et al. . Cognition and behaviour in frontotemporal dementia with and without amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2020;91(12):1304-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Canosa A, Moglia C, Manera U, et al. . Metabolic brain changes across different levels of cognitive impairment in ALS: a (18)F-FDG-PET study. J Neurol Neurosurg Psychiatry. 2020. [DOI] [PubMed] [Google Scholar]
- 48.Braak H, Brettschneider J, Ludolph AC, Lee VM, Trojanowski JQ, Del Tredici K. Amyotrophic lateral sclerosis: a model of corticofugal axonal spread. Nat Rev Neurol. 2013;9(12):708-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rohrer JD, Geser F, Zhou J, et al. . TDP-43 subtypes are associated with distinct atrophy patterns in frontotemporal dementia. Neurology. 2010;75(24):2204-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Omer T, Finegan E, Hutchinson S, et al. . Neuroimaging patterns along the ALS-FTD spectrum: a multiparametric imaging study. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18(7-8):611-623. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset used during the study will be made available by the corresponding author upon request to qualified researchers (i.e., affiliated with a university or research institution/hospital).