Abstract
Background:
Although there is a presence of governmental and non-governmental organizations running to provide quality HIV care services to reduce HIV-related mortality, there is rapid disease progression and death among children in developing countries including Ethiopia. Thus, this study was aimed to assess the mortality predictors of children living with HIV at Bahir Dar town public health facilities.
Method:
A facility-based retrospective follow-up study was conducted among 588 children who were enrolled in the HIV care clinic from 1 September 2010 to 30 August 2019. Data were entered into the Epi-Data entry 3.1 and then exported to STATA version 14 for analysis. Multiple imputation models were employed to handle missing data using the multivariate imputation Chained Equations technique. The Kaplan–Meier survival curve and log-rank test were used to estimate and compare the survival time of categorical variables.
Result:
About 27 (4.6%) (95% confidence interval: 2.9–6.5) deaths were observed from the 30,062.3 person-months follow-up period, and the overall incidence density rate of 0.9 per 1000 child-months (95% confidence interval: 0.6–1.3). Advanced WHO clinical stage (adjusted hazard ratio = 3.18; 95% confidence interval: 1.07–9.43), hemoglobin level less than 8 g/dL (adjusted hazard ratio = 3.54; 95% confidence interval: 1.27–8.85), children having a weight for age of <−2z (adjusted hazard ratio = 2.81; 95% confidence interval: 1.19–6.6), children with poor adherence (adjusted hazard ratio = 3.91; 95% confidence interval: 1.41–10.8), and starting the treatment beyond 1 week of being eligible (adjusted hazard ratio = 3.22; 95% confidence interval: 1.21–8.53) were predictors of HIV-related mortality among children initiated antiretroviral therapy.
Conclusion:
The hazard of mortality was higher among HIV-infected children in the early period of initiation. Enhancing antiretroviral therapy drug adherence, monitoring Hgb level, and timely initiation of antiretroviral therapy reduce HIV-related mortality.
Keywords: Survival status, mortality, time to death
Introduction
According to the 2018 UNAIDS report, globally there was a total of 1.8 million children living with HIV with an estimated 110,000 AIDS-related deaths. In Ethiopia, the epidemic estimate of children living with HIV and HIV-related death among children was 62,000 and 3600. 1 There were evidence from different countries regarding the survival status of children living with HIV. From the tertiary hospital of Benin, 76.4% of survival probability after 60 months of antiretroviral therapy (ART) initiation was evidenced from a 5-year survival trend and outcome among children living with HIV. 2 The median survival time for children living with HIV in Swaziland was 78 months. 3 The mortality rate from the observational study in Zambia was 1.6/100 person-years of observation. 4 In Ethiopia, mortality rates of children living with HIV after initiating ART ranged from 4.8% to 22.9%.5,6
The pre-treatment mortality rate was high among children eligible for ART.7,8 Prompt initiation of ART within the beginning week of eligibility was associated with better treatment outcome in terms of virologic outcome, 9 but its effect in mortality aspect is not addressed; this research undergoes ascertaining its effect on mortality.
The Ethiopia government has adopted UNAIDS treatment 90-90-90 targets which aimed at ending the HIV epidemics by 2030 with specific targets, 90% of individuals living with HIV know their HIV status, 90% of those eligible are initiated ART, and 90% of individuals on ART will have viral load suppression by 2020. 10
Guidelines that Ethiopia have been implementing since 2014 consider that all children living with HIV should start ART regardless of CD4 count and World Health Organization (WHO) clinical stage. 11 But the guideline that was implemented before had different eligibility criteria like WHO clinical stage 3 and 4 regardless of CD4, WHO clinical stage 1 and 2 with CD4 below the threshold of %CD4+ ⩽25, or CD4 absolute count of ⩽750 cells/mm3 all children with the age of 2–5 years. 12
While researches are conducted to look at survival status in the different areas of Ethiopia, most of them considered the previous guideline. In resource-limited settings like Ethiopia, there is a pressing need for research to identify factors and further refine HIV treatment strategies among children to have better survival after initiation of ART. The survival outcomes might be affected by different potential factors; however, those factors are poorly understood in the study area, especially after the implementation of the new guideline that was launched in 2014. The finding of this study will help the researchers to uncover the different areas of factors by going further based on the factors and outcomes identified in this study and to achieve a good treatment outcome by understanding the individual variability in the treatment outcome and acting on the modifiable factors. This study assessed the survival status and predictors of mortality among children living with HIV and who initiated ART in Bahir Dar town public health facilities.
Methods and materials
Study design and setting
A retrospective cohort study was conducted to assess survival status and predictors among children living with HIV and initiated ART in Bahirdar town public health facility. Follow-up time for each patient was calculated from the date of ART initiation to the date of file closure which was due to death, transfer out, and loss to follow-up and the last clinic visit before 30 August 2019.
The study was conducted in Bahirdar city from 1 September 2010 to 30 August 2019. Bahirdar is the administrative capital of Amhara regional state and located 565 km northeast of Addis Ababa, Ethiopia’s capital.
Study population
All medical records of children living with HIV aged less than 15 years old at initiation and who were enrolled from 1 September 2010 to 30 August 2019 at Bahir Dar city public health facilities were study participants in this follow-up study.
Inclusion criteria
All children living with HIV enrolled on ART from 1 September 2010 to 30 August 2019, and if ART was initiated in the selected health facilities.
Exclusion criteria
Patients with incomplete baseline information (at least the child’s socio-demographics, initiation date, and exit date from the cohort).
Sample size and sampling procedure
The sample size was determined using double population proportion from factors in different studies using Epi info version 7 assuming: 95% confidence interval, power 80%.
The sample size for this study was 588, CD4 percentage less than 10% as a main explanatory variable for HIV-related death gave a higher sample size from the information available in the previous literature. 5
Sampling procedure
From all public health facilities providing ART services in Bahir Dar city, a random sampling method was employed. The sampling frame (808) was constructed at selected health facilities. Then from the sampling frame constructed, participants who fulfilled the inclusion criteria were selected randomly using excel (computer-generated random sample).
The allocation of the study subjects to each health facility was computed based on the proportion of the number of medical records of HIV-positive children.
Data collection tools and procedures
In the checklist, socio-demographic characteristics, baseline clinical, laboratory, and ART information were included. Data were extracted through chart review using a structured checklist, adapted from ART intake, and follow-up form. Before the actual data collection, data collectors and the supervisor were oriented about the objectives of the study and contents of the tools. Then, all medical records of children living with HIV that fulfilled the inclusion criteria were reviewed by four trained nurses as data collectors and 1 BSc nurse as a supervisor.
Data quality assurance
Both supervisors and data collectors were closely following the data collection process. Consistency cross-checking was conducted in Bahir Dar health center for 5% of the study population 2 weeks before the actual data collection to evaluate the validity of the checklist. Every day all checklists were reviewed and checked during the data collection and any errors were corrected accordingly.
Statistical analysis
All filled checklists were entered into EpiData entry 3.1 and checked for completeness and consistency and exported to STATA version 14. The data were processed by STATA version 14 to estimate the survival time of children living with HIV.
Cox proportional hazard model assumption was checked graphically using log-log and Kaplan–Meier versus predicted survival plot and by running a global test using the Schoenfeld residual test. Bivariable Cox proportional hazard model was employed. Variables found to have a p-value less than 0.2 in the bivariate analysis were a candidate to the multi-variable Cox proportional hazards regression model. Variables having a p-value ⩽0.05 were considered statistically significant.
Missing values were identified for some of the variables. The percentage of missing values across all variables varied between 0% and 6.1%. The missing data were expressed in terms of proportion, mechanisms under which the missing data occurs, and missing data patterns, to apply appropriate procedures to handle the existing missing data. The missing mechanism of the data was found to be missing at random by using little’s test and the need to do imputation was ascertained. Multiple imputations were used to create and analyze multiply imputed data sets. Complete variables were imputed under fully conditional specification, by using multivariate imputation Chained Equations (MICE) technique. For comparison, a complete case analysis was also performed.
Operational definition
Survival time: defined as the length of time between ART initiation and death/censure.
Censure: Patients still alive at the end of the study or lost to follow-up or transfer out to other health facilities and deaths with other comorbidities.
Event: death of children after the initiation of ART.
Functional status: Was described as working, ambulatory, bedridden. 13
Working: Go to school, do normal activities, or play.
Ambulatory: Able to perform activities of daily living.
Bedridden: Not able to perform activities of daily living.
GOOD adherence: equal or greater than 95% adherence—missing only 1 out of 30 doses or missing 2 from the 60 doses.
Fair adherence: 85%–94% adherence—missing 2–4 doses out of 30 doses or 4–9 doses from 60 doses.
Poor adherence: <85% adherence—missing >5 doses out of 30 doses or >10 doses from 60 doses. 13
Results
Baseline socio-demographic characteristics
Among 588 patients, 308 (52.4%) of study participants were females and the majority (79.4%, n = 467) of the participants were urban residents. The mean age at the commencement of ART was 7.2 (± 3.9) years with extremes of 0.5 and 14 years (Table 1).
Table 1.
Baseline socio-demographic characteristics of children on ART at Bahirdar city public health facility, Amhara regional state, Northwest Ethiopia (2010–2019).
| Variables | Category | Frequency | Percent |
|---|---|---|---|
| Sex (n = 588) | Male | 280 | 47.6 |
| Female | 308 | 52.4 | |
| Age (n = 588) | <2 years | 70 | 11.9 |
| 2–5 years | 101 | 17.2 | |
| 5–10 years | 234 | 39.8 | |
| ⩾10 years | 183 | 31.1 | |
| Residence (n = 588) | Urban | 467 | 79.4 |
| Rural | 121 | 20.6 | |
| Follow-up place (n = 588) | Health Center | 172 | 29.3 |
| Hospital | 416 | 70.7 | |
| HIV disclosure status (n = 566) | Disclosed | 340 | 60.1 |
| Not disclosed | 226 | 39.9 |
ART: antiretroviral therapy.
Baseline clinical, laboratory, and ART information
Regarding opportunistic infection, 188 (32%) of the children experience an opportunistic infection. 26.29% had recurrent upper respiratory tract infection (URTI) followed by bacterial pneumonia (19.5%) and herpes zoster (15.5%). About 60.4% of children start the treatment within 1 week of eligibility. The majority of the children have had a regimen change and treatment failure and drug side effects account for 14.4%, and 10% of the reason for regimen change (Table 2).
Table 2.
Baseline clinical, laboratory, and ART information of children on ART at Bahir Dar city public health facility, Amhara regional state, Northwest Ethiopia (2010–2019).
| Variables | Category | Frequency | Percent |
|---|---|---|---|
| Baseline WHO stage (n = 588) | Stage 1 | 171 | 29.1 |
| Stage 2 | 263 | 44.7 | |
| Stage 3 | 132 | 22.5 | |
| Stage 4 | 22 | 3.7 | |
| Hemoglobin (n = 588) | <8 g/dL | 25 | 4.3 |
| ⩾8 g/dL | 554 | 94.2 | |
| Missing | 9 | 1.5 | |
| W/A (n = 588) | ⩾–2z | 497 | 84.5 |
| <–2z | 91 | 15.5 | |
| H/A (n = 588) | ⩾–2z | 475 | 80.8 |
| <–2z | 111 | 18.9 | |
| Missing | 2 | 0.3 | |
| W/H/L (n = 588) | ⩾–2z | 488 | 83 |
| <–2z | 98 | 16.7 | |
| Missing | 2 | 0.3 | |
| Developmental millstones (n = 588) | Appropriate | 172 | 29.3 |
| Delayed | 17 | 2.9 | |
| Missing | 399 | 67.9 | |
| Functional status (n = 588) | Working | 213 | 36.2 |
| Ambulatory | 178 | 30.3 | |
| Missing | 197 | 33.5 | |
| Opportunistic infection (n = 588) | Yes | 188 | 32 |
| No | 400 | 68 | |
| INH prophylaxis (n = 588) | Yes | 293 | 49.8 |
| No | 290 | 49.3 | |
| Missing | 5 | 0.9 | |
| Cotrimoxazole prophylaxis (n = 588) | Yes | 422 | 71.8 |
| No | 166 | 28.2 | |
| Drug side effect (n = 588) | Yes | 24 | 4.1 |
| No | 564 | 95.9 | |
| Regimen change (n = 588) | Yes | 364 | 61.9 |
| No | 197 | 33.5 | |
| Missing | 27 | 4.6 | |
| Change status (n = 364) | Within the first line | 324 | 55.1 |
| To the second line | 40 | 6.8 | |
| Missing | 224 | 38.1 | |
| CD4 (n = 588) | <200 cells/mm3 | 95 | 16.1 |
| ⩾200 cells/mm3 | 486 | 82.7 | |
| Missing | 7 | 1.2 | |
| Adherence level (n = 588) | Good | 542 | 9.2 |
| Fair | 20 | 3.4 | |
| Poor | 26 | 4.4 | |
| Viral load (n = 588) | <1000 | 472 | 80.3 |
| ⩾1000 | 80 | 13.6 | |
| Missing | 36 | 6.1 |
ART: antiretroviral therapy; WHO: World Health Organization; CD4: cluster of differentiation 4; n: number; INH: isonicotinic acid hydrazide/isoniazide; W/A: weight for age; W/H/L: weight for height/length; H/A: height for age.
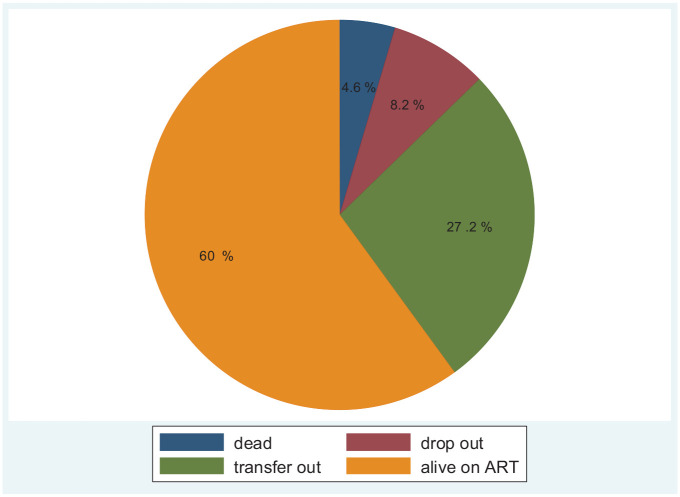
Among 588 patients, 362 (61.6%) of study participants have initiated their treatment after 2014. From the whole cohort, more than half (60%) of the children living with HIV are live on ART (Figure 1).
Figure 1.
Outcomes of HIV-positive children initiated ART at Bahir Dar city public health facility, Amhara regional state, Northwest Ethiopia (2010–2019).
Survival status after initiation of ART
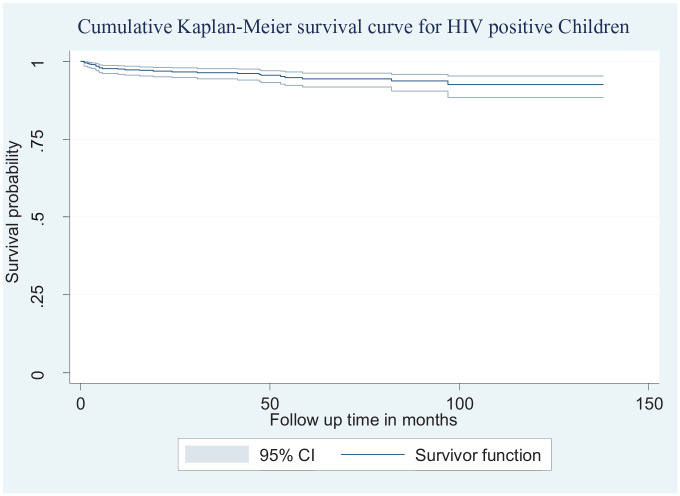
A total of 588 children living with HIV enrolled in care were followed and given 30,062.3 person-months observation. When the survival was stratified by the turning point for guideline change happened in 2014 (before and after 2014), there was no significant difference, but the proportion of deaths before and after 2014 was 5.7% and 3.9%, respectively. At the end of the follow-up, more than half (60%) of the patients were retained in care in those public health facilities, and they initiate their treatment. The median follow-up time was 51 months. In this study, 4.6% (95% confidence interval, CI: 2.9–6.5) of the study participant died during the follow-up period. The death incidence rate was calculated using person-months of follow-up. The overall incidence density rate (IDR) of the cohort was 0.9 per 1000 child-month observations (95% CI: 0.6–1.3). About half (48.2%) of the deaths occurred within the first 6 months of ART initiation which gives a mortality incidence of 3.9 per 1000 child months. The probability of death was estimated to be 2.3%, 2.7%, and 3.1% at 6, 12, and 24 months after initiation, respectively. The cumulative probabilities of survival at 6, 12, and 24 months of ART initiation were found to be 97.7%, 97.3%, and 97.1%, respectively (Figure 2).
Figure 2.
Cumulative Kaplan–Meier survival curve with 95% confidence intervals of children on ART at Bahir Dar city public health facility (2010–2019).
Predictors of mortality after ART initiation
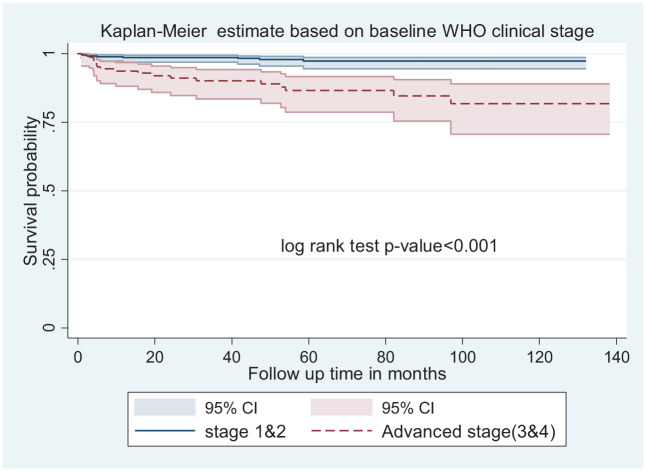
In the multivariable Cox proportional hazard adjusted model, five factors were identified (Table 3). The hazard of child mortality in children with advanced WHO clinical stage was 3.2 times higher when compared with those who were on WHO clinical stage 1 and 2 at initiation (adjusted hazard ratio (AHR): 3.2; CI: 1.1–9.4) (Figure 3).
Table 3.
Bivariable and multivariable Cox regression among HIV-positive children on ART at Bahir Dar public health facility Amhara regional state, North West Ethiopia (N = 588) (2010–2019).
| Variables | Category | Status | CHR (95% CI) | AHR (95% CI) | |
|---|---|---|---|---|---|
| Censured N (%) n = 561 |
Death N (%) n = 27 |
||||
| Age of the child | <2 years | 64 (91.4) | 6 (8.6) | 3.5 (1.1, 10.8) | 3.0 (0.8, 11.6) |
| 2–5 years | 96 (95.1) | 5 (4.9) | 1.5 (0.5,4.9) | 0.8 (0.2, 3.2) | |
| 5–10 years | 224 (95.7) | 10 (4.3) | 1.3 (0.5,3.7) | 1.2 (0.4, 3.5) | |
| ⩾10 years | 177 (96.7) | 6 (3.3) | 1 | 1 | |
| Duration between start and eligible date | <7 days | 349 (98.3) | 6 (1.7) | 1 | 1 |
| ⩾7 days | 212 (91) | 21 (9) | 5.0 (2.0, 12.5) | 3.2 (1.2, 8.5)* | |
| Baseline hemoglobin | <8 g/dL | 18 (72) | 7 (28) | 11 (6.7, 18.5) | 3.5 (1.3, 8.9)* |
| ⩾8 g/dL | 534 (96.4) | 20 (3.6) | 1 | 1 | |
| Missing | 7 (1.2%) | 2 (0.3%) | |||
| Initial regimen | NVP based | 352 (93.6) | 24 (6.4) | 1 | 1 |
| EFV based | 168 (99.4) | 1 (0.6) | 0.1 (0.1, 0.7) | 0.2 (0.0, 1.2) | |
| Others | 41 (95.3) | 2 (4.7) | 0.9 (0.2, 3.8) | 0.7 (0.1, 3.8) | |
| Baseline WHO | Stage 1 and 2 | 425 (97.9) | 9 (2.1) | 1 | 1 |
| Advanced | 136 (88.3) | 18 (11.7) | 5.7 (2.6, 12.7) | 3.2 (1.1, 9.4)* | |
| Baseline W/A | ⩾–2z | 482 (97) | 15(3) | 1 | 1 |
| <–2z | 79 (86.8) | 12 (13.2) | 4.3 (2.0, 9.2) | 2.8 (1.2, 6.6)* | |
| Opportunistic infection | Yes | 170 (90.4) | 18 (9.6) | 4.2 (1.9, 9.3) | 1.4 (0.5, 4.3) |
| No | 391 (97.7) | 9 (2.3) | 1 | 1 | |
| Recent adherence | Good | 523 (96.49) | 19 (3.51) | 1 | 1 |
| Fair | 19 (95) | 1 (5) | 1.3 (0.2, 9.8) | 0.8 (0.1, 6.8) | |
| Poor | 19 (73.1) | 7 (26.9) | 7.3 (3.1, 17.5) | 3.9 (1.4, 10.8)* | |
| Recent viral load | <1000 | 459 (97.3) | 13 (2.7) | 1 | 1 |
| ⩾1000 | 71 (88.7) | 9 (11.3) | 4.5 (1.9, 10.5) | 2.0 (0.7, 6.1) | |
| Missing | 31 (86.1) | 5 (13.9) | |||
ART: antiretroviral therapy; CHR: crude hazard ratio; AHR: adjusted hazard ratio; CI: confidence interval; Others in the initial regimen: (ABC-3TC-LPV, TDF-3TC-ATV, and ABC-3TC-LPV); W/A: weight for age; n: number; WHO: World Health Organization; NVP: Nevirapine; EFV: Efavirenz.
Significant (p-value < 0.05).
Figure 3.
Kaplan–Meier survival estimate of mortality-free survival proportion based on baseline WHO clinical stage at enrollment at Bahir Dar public health facility (2010–2019).
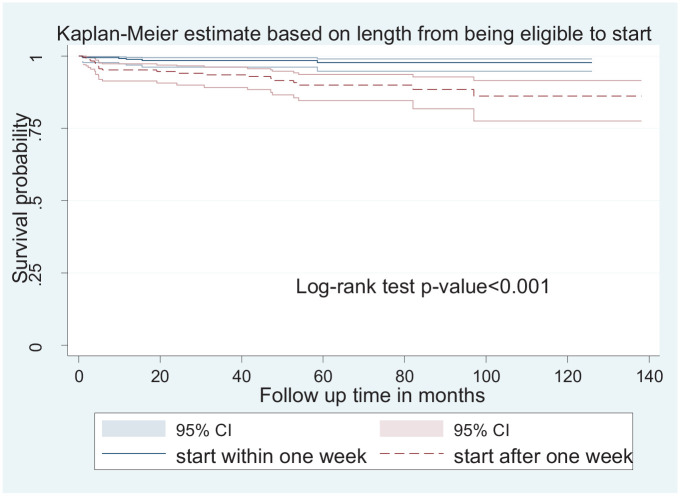
The hazard of child mortality in children with poor adherence was 3.9 times higher among children when compared with those with good adherence ((AHR: 3.9; CI: 1.4–10.8). The hazard of child mortality was 2.8 times higher among children who had a low W/A (<−2z) when compared with those who had ⩾−2z (AHR: 2.8; 95% CI: 1.2–6.6). The hazard of mortality in children who had a hemoglobin level less than 8 g/dL was 3.5 times higher when compared with those who had hemoglobin levels greater than 8 g/dL (AHR: 3.5; 95% CI: 1.3–8.9). Furthermore, the hazard of mortality in children who started the treatment after 1 week of being eligible was 3.2 times higher when compared with those who started within 1 week (AHR: 3.2; 95% CI: 1.2–8.5) (Figure 4).
Figure 4.
Kaplan–Meier survival estimate of mortality-free survival proportion based on the length of time from eligibility to initiation at Bahirdar public health facility (2010–2019).
Discussion
The findings of this study indicated that the overall incidence of death was 0.9 per 1000 child-months observation and cumulative incidence of 4.6% (95% CI: 2.9–6.5). The cumulative incidence finding was consistent with the comparative analysis of outcomes of ART in children in East Africa (4.3%), Asia (5.4%), and South Africa (5.7%). 14 But this finding is lower than study findings from West Africa (7.4%), 14 Dessie referral hospital, and Debre tabor general hospital (22.9%), 6 and studies conducted in Addis Ababa (10.4%). 15 This difference may be due to the study period variability since there was a change in treatment modality and care of children on ART starting from eligibility criteria to medication changes.
The overall incidence rate of death was found to be 0.9 per 1000 child-months. This finding is lower than studies conducted in China, 16 Asia, 17 South Africa, 18 Zambia, 19 Wolaita zone health facilities, Ethiopia. 20 The possible reason may be the variety in study populations; some studies consider only younger age groups, which tend to have a high incidence rate of mortality.
About half of the deaths occurred in the first 6 months of ART. This is consistent with surveillance data for children less than 5 years of age receiving ART in 48 HIV/AIDS treatment programs in Africa and Asia. 21 It is also similar to the finding from Addis Ababa, in which 70% of deaths occurred in the first 6 months of ART. 15
Related to the probability of death, the probability of death was estimated to be 2.3%, 2.7%, and 3.1% at 6, 12, and 24 months after initiation, respectively. This finding was consistent with a systematic review report from 29 articles in African countries (5%) at 6 months and(6%) at 12 months of initiation 22 and from 70 health facilities, Ethiopia, which was 3.4% at 6 months, 4.1% at 12 months, and 4.8% at 24 months. 23
In this study, the overall cumulative survival probability was found to be 92.5% at 138 months after ART initiation. The survival probability was also found to be 97.7% and 97.3%, at the first, 6th, and 12th months of initiation, this finding was higher than studies done in Adama referral hospital (93.9%) at 6 months and (92.8%) at 12 months 24 and study conducted in Debre Tabor referral hospital and Dessie referral hospital (85%) at 12 months. 6 This difference might be due to the characteristics of the population included in our follow-up. In this study, most of the study participants initiated ART regardless of any criteria; they started the treatment as soon as their status is known. It is well known that those children who have initiated ART at an early phase had good clinical and immunological improvement and the risk of severe morbidity is lower in those children.25,26
Baseline advanced WHO clinical stages at the initiation of ART was found to be a significant predictor of mortality among HIV-positive children on ART. The hazard of child mortality was higher among children with advanced WHO clinical stage compared with WHO clinical stages 1 and 2 at the initiation. This finding is supported with other studies conducted in China (HR = 2.4), 16 sub-Saharan Africa, 22 Adama, 24 Mekelle, 5 and Zambia (WHO stage 4 (AHR = 4.8), compared to WHO stage 1 and 2). 4 The mortality is high when the disease is advanced more because the viral load is high and it destroyed body defense mechanisms and exposes to several opportunistic infections. It finally results in death.
Mortality was closely linked to the children’s level of adherence to ART. The hazard of mortality among HIV-positive children who had end-line poor treatment adherence was higher than those children who had good treatment adherence.
This finding is supported by several studies. A study in Benin revealed an increased hazard of death among patients with poor adherence to therapy. 2 Also, a study in Addis Ababa, Ethiopia, provides evidence that suboptimal ART adherence increases the risk of mortality. 15 It is due to the development of drug resistance and an increase in the risk of virologic failure and subsequent disease progression.27,28
In this study, the hazard of mortality was higher among HIV-positive children who had a baseline (hemoglobin level of less than 8 g/dL) than those children who had greater than 8 g/dL during initiation of ART. This finding is supported by other studies that were conducted in Mekelle, Ethiopia, 5 and Addis Ababa, Ethiopia. 29 This could be due to the impact of anemia in HIV-infected patients on their physical functioning and quality of life. 30
The hazard of mortality in children who did not start the treatment within 1 week of being eligible was higher when compared with those who started within 1 week. This finding is supported by different studies done in resource-limited settings as mortality was associated with delays between clinic entry and ART initiation there was evidence of an increase in mortality from 11.0% to 14.7% with a 10-week delay in ART for patients entering care. 31 It could be because of the benefits of immediate initiation in terms of good treatment response as there is evidence of low risk of treatment failure in infants. 32 Prompt initiation might help in decreasing the burden of anemia and its subsequent complications. 33 Only the discussed factors were identified in our study, but there was evidence that children with young age (age < 1.5 years), 34 age group of <1 year, 3 age less than 18 months and children with chronic diarrhea 5 had a high chance of mortality and children who took isoniazide prophylaxis and Cotrimoxazole prophylactic therapy 35 and participants who had psychosocial support during follow-up 6 had higher survival probability. The survival status of HIV-positive children needs to be studied with a prospective study design to include different areas of factors, including the reasons for delay after being eligible for ART.
However, the follow-up period was long, which increases the patient’s period of observation and enabled us to know the long-term impact of ART on mortality and appropriate measures taken to handle the missing value; the present study does have some limitations. As the data were collected from medical records, socioeconomic factors were not possible to collect for the whole cohort, as they might be associated with mortality; therefore, this variable was not included in analyses.
Conclusion and recommendation
The survival rate of children living with HIV was higher as compared to other previous studies. The hazard of mortality was relatively higher among HIV-positive children in the early period of initiation. The risk of mortality is increased if the child was at a young age at the initiation of ART, lower baseline CD4 count, advanced WHO Clinical stage, lower hemoglobin values, and who have had poor adherence. However, prompt initiation of ART within 1 week of being eligible may minimize mortality. There was a considerable delay that can affect the long-term survival outcome, so health facilities that have been giving ART service better initiate ART as soon as the case is confirmed followed by strengthening careful and regular monitoring.
Acknowledgments
Our gratitude goes to supervisors, the data collectors, and the staff at the ART clinic of Felege hiwot comprehensive and specialized hospital, Bahir Dar, Abay, and Han health centers. We would also like to thank Mr Getnet Dessie (MSc, Adult health nursing) who supported us a lot in model development and the technical issues related to the software.
Footnotes
Author contributions: B.C. prepared the proposal, provided training for data collectors, supervised data collection, analyzed and interpreted the data, and wrote the main manuscript. A.B. supervised and evaluated the overall research process and reviewed the whole manuscript. E.A. prepared the proposal and reviewed the manuscript. A.G. reviewed the manuscript. A.T. prepared graphs and tables.
Availability of data materials: The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Ethical approval for this study was obtained from Research, Ethical Clearance Committee of Bahirdar University with a protocol number of 0046/2020. Following the approval, the official letter was obtained from the College of Medicine and Health Sciences of Bahir Dar University and given to the hospital managers, medical directors, and ART focal persons. Permission was obtained from the hospital’s administration and ART focal persons before data collection.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research has been financially supported by Wolkite University for data collection.
Informed consent: Written informed consent was not sought from the legally authorized representatives of the patients directly since we used a medical record.
ORCID iDs: Bogale Chekole  https://orcid.org/0000-0003-4366-2875
https://orcid.org/0000-0003-4366-2875
Agmasie Tigabu  https://orcid.org/0000-0001-9085-9625
https://orcid.org/0000-0001-9085-9625
References
- 1. UNAIDS. 2018, https://www.unaids.org/sites/default/files/media_asset/unaids-data-2018_en.pdf
- 2. Adedemy J, Agbeille M, Agossou J, et al. Five years survival trend and outcome among HIV infected children followed up in the paediatric department in a tertiary hospital. Int J Pediat Res 2019; 5(2): 52. [Google Scholar]
- 3. Shabangu P, Beke A, Manda S, et al. Predictors of survival among HIV-positive children on ART in Swaziland. Afr J AIDS Res 2017; 16(4): 335–343. [DOI] [PubMed] [Google Scholar]
- 4. Mutanga JN, Mutembo S, Ezeamama AE, et al. Long-term survival outcomes of HIV infected children receiving antiretroviral therapy: an observational study from Zambia (2003–2015). BMC Publ Health 2019; 19(1): 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gebremedhin A, Gebremariam S, Haile F, et al. Predictors of mortality among HIV infected children on anti-retroviral therapy in Mekelle Hospital, Northern Ethiopia: a retrospective cohort study. BMC Publ Health 2013; 13(1): 1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arage G, Assefa M, Worku T, et al. Survival rate of HIV-infected children after initiation of the antiretroviral therapy and its predictors in Ethiopia: a facility-based retrospective cohort. SAGE Open Med 2019; 7: 838957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sutcliffe CG, van Dijk JH, Munsanje B, et al. Risk factors for pre-treatment, mortality among HIV-infected children in rural Zambia: a cohort study. PLoS ONE 2011; 6(12): e29294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Okomo U, Togun T, Oko F, et al. Mortality and loss to programme before antiretroviral therapy among HIV-infected children eligible for treatment in The Gambia, West Africa. AIDS Res Ther 2012; 9(1): 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ssebunya R, Wanyenze RK, Lukolyo H, et al. Antiretroviral therapy initiation within seven days of enrolment: outcomes and time to undetectable viral load among children at an urban HIV clinic in Uganda. BMC Infect Dis 2017; 17(1): 439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. The 90 90 90 strategy to end the HIV and pandemic by 2030, 2019, https://www.researchgate.net/publication/304670232_The_90_90_90_strategy_to_end_the_HIV_Pandemic_by_2030_Can_the_supply_chain_handle_it (accessed 26 November 2019). [DOI] [PMC free article] [PubMed]
- 11. Ethiopia national guidelines for comprehensive HIV, 2014, https://aidsfree.usaid.gov/sites/default/files/ethiopia_natl_gl_2014.pdf
- 12. World Health Organization. Antiretroviral therapy of HIV infection in infants and children: towards universal access: recommendations for a public health approach-2010 revision. Geneva: World Health Organization, 2010. [PubMed] [Google Scholar]
- 13. National consolidated guidelines for comprehensive HIV, 2018, https://www.afro.who.int/sites/default/files/201904/National%20Comprehensive%20HIV%20Care%20%20Guideline%202018.pdf
- 14. Leroy V, Malateste K, Rabie H, et al. Outcomes of antiretroviral therapy in children in Asia and Africa: a comparative analysis of the IeDEA pediatric multiregional collaboration. J Acq Immune Def Synd 2013; 62(2): 208–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ebissa G, Deyessa N, Biadgilign S. Predictors of early mortality in a cohort of HIV-infected children receiving high active antiretroviral treatment in public hospitals in Ethiopia. AIDS Care 2015; 27(6): 723–730. [DOI] [PubMed] [Google Scholar]
- 16. Zhao Y, Li C, Sun X, et al. Mortality and treatment outcomes of China’s national pediatric antiretroviral therapy program. Clin Infect Dis 2013; 56(5): 735–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hansudewechakul R, Sirisanthana V, Kurniati N, et al. Antiretroviral therapy outcomes of HIV-infected children in the TREAT Asia pediatric HIV observational database. J Acq Immune Def Synd 2010; 55(4): 503–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zanoni BC, Phungula T, Zanoni HM, et al. Risk factors associated with increased mortality among HIV infected children initiating antiretroviral therapy (ART) in South Africa. PLoS ONE 2011; 6(7): e22706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Van Dijk JH, Sutcliffe CG, Munsanje B, et al. HIV-infected children in rural Zambia achieve good immunologic and virologic outcomes two years after initiating antiretroviral therapy. PLoS ONE 2011; 6(4): e19006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bitew S, Mekonen A, Assegid M. Predictors on mortality of human immunodeficiency virus infected children after initiation of antiretroviral treatment in Wolaita zone health facilities, Ethiopia: retrospective cohort study. J AIDS HIV Res 2017; 9(4): 89–97. [Google Scholar]
- 21. Sauvageot D, Schaefer M, Olson D, et al. Antiretroviral therapy outcomes in resource-limited settings for HIV-infected children <5 years of age. Pediatrics 2010; 125(5): e1039. [DOI] [PubMed] [Google Scholar]
- 22. Ahmed I, Lemma S. Mortality among pediatric patients on HIV treatment in sub-Saharan African countries: a systematic review and meta-analysis. BMC Publ Health 2019; 19(1): 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zenebe Melaku SL, Wang C, Lamb MR, et al. Outcomes among HIV-infected children initiating HIV care and antiretroviral treatment in Ethiopia. Trop Med Int Health 2013; 22: 474–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Aman Kedir A. Factors affecting survival of HIV positive children taking antiretroviral therapy at Adama Referral Hospital and Medical College, Ethiopia. J AIDS Clin Res 2014; 5(3): 1000289. [Google Scholar]
- 25. Zheng J, Zhao D. Clinical, immunological, and virological outcomes of pediatric antiretroviral therapy in central China. BMC Res Note 2014; 7(1): 419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Eholié SP, Badje A, Kouame GM, et al. Antiretroviral treatment regardless of CD4 count: the universal answer to a contextual question. AIDS Res Ther 2016; 13: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lodha R, Manglani M. Antiretroviral therapy in children: recent advances. Ind J Pediat 2012; 79(12): 1625–1633. [DOI] [PubMed] [Google Scholar]
- 28. Simoni JM, Montgomery A, Martin E, et al. Adherence to antiretroviral therapy for pediatric HIV infection: a qualitative systematic review with recommendations for research and clinical management. Pediatrics 2007; 119(6): e1371–e1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Asfawesen G, Solomie J, Bisirat T, et al. Outcome in a paediatric cohort receiving ART in Addis Abeba, Ethiopia. Acta Paediatr 2011; 100(8): 1164–1167. [DOI] [PubMed] [Google Scholar]
- 30. Breitbart W, McDonald MV, Rosenfeld B, et al. Fatigue in ambulatory AIDS patients. J Pain Symptom Manage 1998; 15(3): 159–167. [DOI] [PubMed] [Google Scholar]
- 31. Hoffmann CJ, Lewis JJ, Dowdy DW, et al. Mortality associated with delays between clinic entry and ART initiation in resource-limited-settings: Results of a transition-state model. J Acq Immune Def Synd 2013; 63(1): 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Collins IJ, Judd A, Gibb DM. Immediate antiretroviral therapy in young HIV-infected children: benefits and risks. Curr Opin HIV AIDS 2014; 9(1): 87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wagnew F, Eshetie S, Alebel A, et al. Burden of anemia and its association with HAART in HIV infected children in Ethiopia: a systematic review and meta-analysis. BMC Infect Dis 2019; 19(1): 1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Andargie AA, Asmleash Y. Survival time of human immunodeficiency virus (HIV) infected children under 15 years of age after initiation of antiretroviral therapy in the University of Gondar Comprehensive Specialized Hospital, Ethiopia. J AIDS HIV Res 2018; 10: 49–55. [Google Scholar]
- 35. Sidamo NB, Hebo SH. Survival time and its predictors among HIV-infected children after antiretroviral therapy in public health facilities of Arba Minch town, Gamo Gofa Zone, Southern Ethiopia. Ethiop J Health Dev 2018; 32(2), https://www.ajol.info/index.php/ejhd/article/view/174889 [Google Scholar]