Abstract
Adult marrow-derived mesenchymal stem cells (MSCs) are able to differentiate into bone, cartilage, muscle, marrow stroma, tendon–ligament, fat and other connective tissues. The questions can be asked, what do MSCs do naturally and where is the MSC niche? New insight and clinical experience suggest that MSCs are naturally found as perivascular cells, summarily referred to as pericytes, which are released at sites of injury, where they secrete large quantities of bioactive factors that are both immunomodulatory and trophic. The trophic activity inhibits ischaemia-caused apoptosis and scarring while stimulating angiogenesis and the mitosis of tissue intrinsic progenitor cells. The immunomodulation inhibits lymphocyte surveillance of the injured tissue, thus preventing autoimmunity, and allows allogeneic MSCs to be used in a variety of clinical situations. Thus, a new, enlightened era of experimentation and clinical trials has been initiated with xenogenic and allogeneic MSCs.
Keywords: mesenchymal stem cell, CFU-F, bone marrow, therapeutics
Introduction
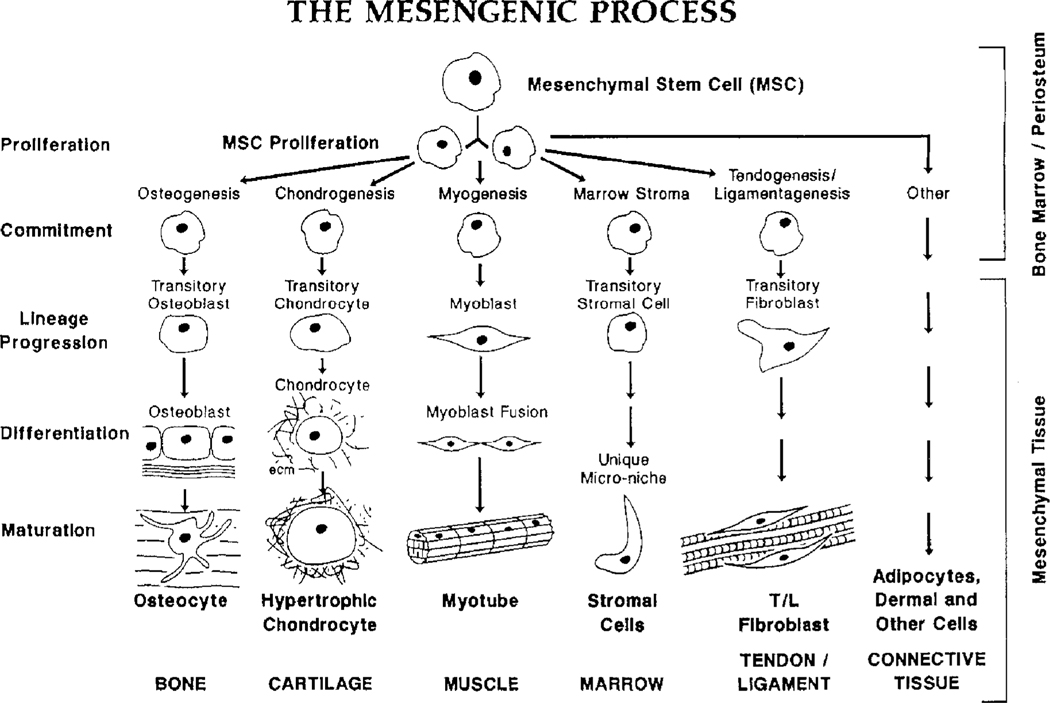
Adult mesenchymal stem cells (MSCs) isolated from bone marrow and mitotically expanded in culture are able to differentiate into a number of mesenchymal phenotypes, including those that form bone, cartilage, muscle, fat and other connective tissues (Figure 1) [1–3]. These observed events led me to suggest that MSCs were responsible for the normal turnover and maintenance of adult mesenchymal tissues [3]. This suggestion was based on the fact that all cells have half-lives and their natural expiration must be matched by their replacement, with the MSCs as the suggested source of these new replacement cells. This replacement logic was an extension from the known sequence of events involved in the turnover and maintenance of blood cells that are formed from haematopoietic stem cells (HSCs) [4,5]. The original mesengenic process pathway (Figure 1) was fashioned after the haematopoiesis lineage diagrams of the 1980s. Indeed, we knew that osteoblasts and chondrocytes were derived in lineage progression pathways, as shown in studies from a variety of laboratories, including our own [6,7].
Figure 1.
The mesengenic process. Adult mesenchymal stem cells (MSCs) are able to differentiate into bone, cartilage, muscle, marrow stroma, tendon/ligament, fat and other connective tissues in a sequence of lineage transitions. This figure was first drawn to mirror the sequence of events observed in haematopoietic differentiation. The state of knowledge in the late 1980s and early 1990s provided the most information for the lineages on the left and the least information for the pathways on the right. (This information is reviewed in [1–3])
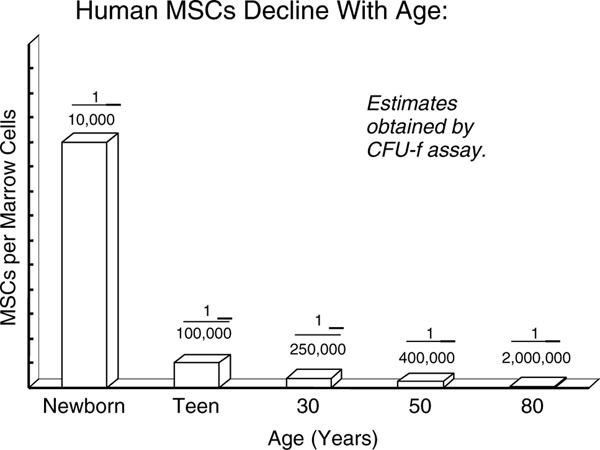
The replacement and turnover role for MSCs was also supported by the very crude data in Figure 2 [8]. We would seed 10–20 × 106 cells from the light-cell fraction of a bone marrow aspirate placed on a Percoll gradient into a 100 mm Petri dish, discard the unattached cells on day 3 and by days 10–12 colonies of fibroblastic cells (CFU-F) could be counted [9–11]. When grouped by decade, a dramatic decrease in MSCs per nucleated marrow cell could be observed, with a 10-fold decrease from birth to teens and another 10-fold decrease from teens to the elderly. These decreases paralleled the observed fracture healing rates: very rapid in the young and very slow in older patients. In comparison, the titres of HSCs in marrow remain constant throughout life at about one per 104 nucleated marrow cells.
Figure 2.
Marrow MSCs (CFU-F) and age. Bone marrow was obtained, dispersed, placed on a Percoll gradient and the light cell fraction seeded into culture [7]. After 7–10 days, colonies can be visualized as first described by Friedenstein and colleagues [8,9]. The CFU-F assay is an incomplete and crude estimate for the titre of MSCs in each marrow sample. Clearly, the number of MSCs in marrow decreases with age [8]
Bone marrow and the ceramic cube
At the very minimum, it has been shown that marrow MSCs contribute to the repair blastema (ie callus) formed at sites of bone breaks, and that both bone and cartilage forms at these sites, depending on its mechanical status — mechanically stable breaks form bone, unstable sites form cartilage that is endochondrally replaced [12]. Furthermore, culture-expanded human or animal MSCs from marrow that are seeded into the interstices of a porous calcium phosphate ceramic cube that is then implanted into a subcutaneous site of immuno-incompetent hosts form bone and/or cartilage, depending on the proximity of vasculature: the presence of vasculature orients osteoblasts to secrete osteoid onto the walls of the pores of the ceramic, while the absence of vasculature promotes cell replication, aggregation and subsequent chondrogenic differentiation [13–16]. Of direct importance are our early observations that marked MSCs in ceramic cubes that formed bone exhibited monolayers of marked secretory osteoblasts on newly formed bone that had labelled osteocytes within the calcified matrix [14,15]. Since human osteoblasts have half-lives of 8–10 days, the labelled cells were replaced after a few weeks by host-derived osteoblasts (and subsequently labelled osteocytes) that continued the bone formation process, eventually leading to vascularized marrow cavities of purely host origin [14,15]. These observations strengthen the supposition that MSCs naturally function to provide replacement cells for those that expire in highly differentiated tissues such as bone. Likewise, these observations document that the host vasculature brings in host MSCs that give rise to secretory osteoblasts that fabricate the segments of host-derived bone in the pores of the ceramic.
The other facts
Inconsistent with the idea that the key function of MSCs was to supply replacement parts for mesenchymal tissue were the observations that human marrow-derived MSCs could be activated to secrete cytokines/growth factors that inhibited an in vitro mixed lymphocyte assay [17,18]. These observations were used to suggest that MSCs could be used therapeutically as allogeneic cells, a so-called ‘universal cell’, a cell that could function in any host. To support this suggestion, it was further documented that culture-expanded MSCs did not have MHC class II cell surface markers, but rather only MHC class I and no co-stimulator molecules [19]. Thus, human MSCs could not be antigen-presenting cells and would be invisible to the host’s immune system [20,21].
Importantly, we first used human marrow-derived MSCs to supplement bone marrow transplantations [22–25] because we assumed that MSCs homed back to the bone marrow [26] and that the reconstituted stroma would more efficiently re-establish the microenvironment for HSC engraftment [27] and subsequent lineage translation of their progeny [25,26]. Our early successful clinical efforts for both safety and efficiency supported the idea that culture-expanded MSCs were indeed capable of promoting successful engraftment of haematopoietic progenitors and their efficient production of circulating mature blood cells [22–24]. In retrospect, it may be that haematopoietic stem cell engraftment is enhanced by the constitutive secretion of various bioactive agents by the MSCs [28], not the engraftment and stromal differentiation of the transplanted MSCs.
Again, based on our bone marrow homing hypothesis and the fact MSCs were able to differentiate into osteoblasts, we and others embarked on clinical efforts to cure gene defects by allogeneic transplantations, using normal marrows that did not exhibit the genetic defect [23,25,29]. In these cases, culture-expanded MSCs from the allo-donor were used to supplement the bone marrow transplantation, with the assumption that the MSCs homed to marrow and re-established the stroma, to enhance allogeneic engraftment and to ensure that ‘normal’ MSCs in the marrow could differentiate into normal differentiated mesenchymal phenotypes. A clear example of these efforts is documented in the publications of Horwitz and colleagues, in which they describe their attempts to cure osteogenesis imperfecta (OI) by allogeneic bone marrow transplantation, including a ‘booster’ shot of MSCs intravenously 18 months after the original allogeneic bone marrow transplantation [29,30]. The results in six children with OI were mixed, with a few regaining impressive skeletal growth rates while others showed no positive effects. The question was: did the MSCs engraft? If yes, how many and where? And, how long did the MSCs remain active and did they form osteoblasts to cure the bone problems of these afflicted patients? Or did the MSCs supply bioactive factors that directly or indirectly enhanced the growth rates?
It is important to note that during these early stages of MSC commercialization and bone marrow transplantation, clinical trials with MSCs by Mosca and his colleagues at Osiris Therapeutics Inc. documented that MSCs secreted bioactive molecules with immunomodulatory effects [31]. Further, in all clinical use by ourselves or others of human adult marrow-derived, culture-expanded MSCs, whether autologous or allogeneic, no adverse events were recorded. This established that isolation and culture expansion is safe and, indeed, clinical benefit from the intravenous delivery of hMSCs could be observed [24] (Osiris Therapeutics Inc. website: http://www.osiris.com).
The new era
Based on its expanded dataset and the detailed measurements of others, Osiris Therapeutics Inc. and a few independent investigators, especially LeBlanc et al [32], used culture-expanded human MSCs and their immunomodulatory capacity to combat steroid-resistant graft-versus-host-disease (GvHD). These results were quite striking in that the MSCs very effectively shut down the GvHD processes. The most striking observation comes from the compassionate use of adult marrow-derived culture-expanded allogeneic MSCs by Osiris Therapeutics in 12 children, aged 5 months to 15 years, who were considered completely resistant to any therapies for GvHD and who were expected to die. These children were given 3–21 intravenous infusions of hMSCs. As reported on the Osiris website, 7/12 had complete remission of GvHD at 1 month and 95% were alive at 6 months compared to a maximum survival of 25% for patients given additional immunosuppressive treatments. Also of importance is the fact that 9/12 had complete recovery from their gastrointestinal (GI) GvHD and the remaining three had their severity reduced to Grade 1 GI GvHD. Based on these last observations and others, Osiris has embarked on a clinical trial to treat Crohn’s (inflammatory bowel) disease with hMSC infusions and have documented an impressive, dose-dependent improvement in the clinical Crohn’s score.
Quite striking is the fact that, to date, in all of the infusions of allogeneic MSCs, even the multiple infusions discussed above, no adverse events have been reported. The question can be raised as to why, if MSCs can differentiate into osteoblasts, have there not been reports of ectopic osteogenesis in the infusion sites or in lung and liver, where we know infused cells first dock [33]? Perhaps the answer is that the natural function of MSCs is not to provide replacement units for the turnover of mesenchymal tissues such as bone, and their primary and most important function is to inhibit immunosurveillance and to establish a regenerative microenvironment!
The trophic effects of MSCs
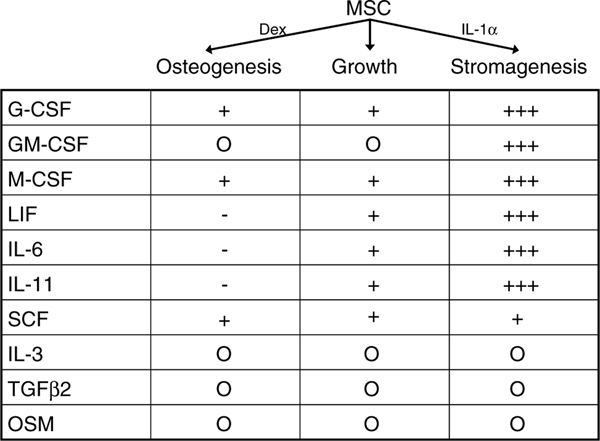
We long ago understood that MSCs in culture secrete a variety of molecules, both bioactive and extracellular matrix [27,28]. In 1996 we published our study of the hMSC secretion of molecules into the medium in log-growth, as they entered osteogenesis and as they entered stromagenesis [28]. In Figure 3, I summarize these published observations; the interpretation is that each vertical column documents a secretory signature of the activity state of the cells of each pathway in a 24-h period. We noted that the constitutive secretory quantities varied considerably between individual donors, but that percentage change as hMSCs went from growth to osteogenesis or stromagenesis was tightly clustered. Obvious from our ELISA quantitation of these bioactive molecules was that substantial levels of these molecules were fabricated every 24 h. This turns out to be the key observation and we now understand that one of the primary and substantial functions of MSCs is to secrete large quantities of bioactive molecules in response to their local environment and their activity status.
Figure 3.
Bioactive factor secretion by MSCs. Culture-expanded MSCs were put into osteogenic, growth or stromagenic conditions and the 24 h media were assayed by ELISA for specific bioactive molecules, as listed. MSCs from six donors were separately analysed. The quantities relative to growth conditions are represented by + or −signs. Although the absolute quantities varied greatly from donor to donor, the percentage change in osteogenic or stromagenic conditions clustered tightly for all donors [28]
The assumption that MSCs could differentiate into any number of mesenchymal phenotypes led others to the assumption that MSCs could differentiate into cardiac myocytes [34,35]. Such cardiac myocytes could be therapeutically useful in sites of cardiac ischaemia (infarct) where a large number of cardiac myocytes expire (apoptosis) due to the ischaemia; the replacement of these expired cells would be expected to provide clinical benefit. This would be especially useful if the replacement cells were allogeneic and an ‘off-the-shelf’ product could be available for the treatment of acute myocardial infarcts. Studies using skeletal myoblasts in experimental animals seem to support this supposition [35,36]. Moreover, we had long before shown that normal marrow MSCs could differentiate into myoblasts and integrate into functional myotubes of MDX mice and ‘cure’ those genetically defective myotubes by producing functional dystrophin that was dispersed throughout the length of the myotubes [37,38]. In experiments in mice, rats and pigs, Osiris Therapeutics and others showed that intravenous infusion of culture-expanded allogeneic MSCs could have significant and positive benefits in myocardial infarct models [39]. The signalling of the ischaemic tissue to the marrow MSCs, as recently reported, involves a number of secreted factors, including SDF-1 [40], MCP-3A [41] and others. Based on these positive preclinical results, Osiris Therapeutics has conducted an FDA-approved Phase I clinical trial whose results include a four-fold reduction in arrhythmias, fewer premature ventricular contractions at all time points studied and prompt return of heart rate after a 6 min walk, and the unexpected result was a substantial improvement in lung function. Presumably, infarct patients have other organ ailments and the infused MSCs seem to go to these injured or affected tissues, such as lung, and provide therapeutic improvement. It seems clear that the infused MSCs do not exert their therapeutic effects by differentiating into cardiac myocytes.
In addition, in a goat model of severe osteoarthritis, MSCs injected into knee joints in a hyaluronan delivery vehicle showed the regeneration of the surgically amputated meniscus [42]. Last, Michael Chopp and others have reported that both direct injection or intravenous infusion of human marrow-derived and culture-expanded MSCs into rodent stroke models 1 week after interrupting blood flow to the brain results in the recovery of coordinate function by 6–8 weeks [43,44].
In all of the above cases, it appears that MSCs do not differentiate into cardiac myocytes, meniscal cells or neurons. Rather, the MSCs exert their influence by the secretion of massive amounts of growth factors and cytokines to effect a therapeutic outcome. We call these effects ‘trophic’ activity [45], named after this term that was related to neural effects [46]. I interpret all of these models to have a common trophic mode of action of the MSCs: the MSCs secrete bioactive molecules that: (a) inhibit apoptosis and limit the field of damage or injury; (b) inhibit fibrosis or scarring at sites of injury; (c) stimulate angiogenesis and bring in a new blood supply; and (d) stimulate the mitosis of tissue-specific and tissue-intrinsic progenitors, such as cardiac or neural stem cells [45]. The infused MSCs also secrete immunomodulatory agents that turn off T cell surveillance and chronic inflammatory processes; thus, allogeneic or, in the case of rodent models, xenogenic hMSCs can be therapeutically effective.
The MSC niche: the pericyte
In the early era of MSC technology, we tried to identify the MSC niche. For example, we showed that the hMSC antibody SH-2 was associated with blood vessels in skin of very young, but not older, patients [47]. However, we never followed up on these preliminary observations, although sections of human bone marrow showed rare SH-2-positive cells. The key question was never addressed: where was the MSC normally located and what was its natural function at specific anatomical sites? With recent investigations into aspects of the control of vasculature in the human dermis [48,49] and the substantial observations of others [50–52], we now understand that MSCs can function as vascular pericytes [53]. We have recently proposed that the pericyte is released from its position on a vascular tube in the case of a focal injury and, as such [53], it functions as an immunomodulatory and trophic MSC. This immune modulation turns off T cell surveillance of the injured tissue and thus provides a block to autoimmune reactions, while its trophic activity ensures that the field of damage is limited, that scarring does not occur and that tissue-intrinsic progenitors replace the expired cells. Angiogenesis is brought about by MSC secretion of bioactive factors, such as VEGF, and by the stabilization of newly forming vessels by the repositioning of the MSC into its pericyte role as a perivascular cell [49,54]. Thus, the activated pericyte that is displaced from its natural position on blood vessels functions as a secretory MSC that is both trophic and immunoregulatory.
Further, we noted (Figure 2) that the quantity of MSCs (CFU-F) per nucleated marrow cell decreases with age from one MSC in 104 marrow cells in newborns, to one MSC in 105 cells in teenagers, to one MSC in 106 cells in older individuals. This dramatic decrease also mirrors the decrease in vascular density with age and strongly argues that MSCs are in coordinate contact with blood vessels. The factors that govern vascular density with age are not known.
The new era of cell-mediated therapy
Given the above, we have reconsidered our MSC logic for their therapeutic use. Surely, MSCs can still be of great value for use in tissue engineering therapies by virtue of their ability to differentiate into distinctive and specialized cells. The use of inductive or instructive delivery vehicles, or the jump-starting of MSCs down specific lineage pathways, would seem to be necessary.
However, the use of MSCs as site-regulated multidrug dispensers, especially allogeneic MSCs, opens avenues of therapy unimagined 5–10 years ago. For example, uses of MSCs in asthma [55], radiation exposure [56], neurological disorders [57], etc. are now being explored. The next technical hurdle is how to deliver a precise dose of effective MSCs to their site of action. Indeed, where is their main site of action? Is it the ischaemic heart or brain, is it in a central or systemic location, or is it the understudied lymph system? We have recently described a cell targeting technology for such site-directed therapeutics [58]. The issue is: where do we target these therapeutic cells?
Also clear is that since all blood vessels are not the same, all pericytes are not the same. Thus, the MSCs from fat must be different from the MSCs of marrow with regard to the details that govern their responses and activities; some publications now document these differences [59,60]. Are the MSCs from marrow ‘better’ than the MSCs from fat, muscle or liver for a specific site relative to trophic activity or immunomodulation? Only time and a great amount of careful, quantitative data will help us answer these questions.
Supplementary Material
Acknowledgements
Supported by the David and Virginia Baldwin Fund and by grants from NIH.
Footnotes
Teaching Materials
Power Point slides of the figures from this Review may be found in the supporting information
Conflict of interest statement: my colleagues and I started Osiris Therapeutics Inc. in late 1992. I no longer own stock in Osiris, neither do I have direct contact with the company.
References
- 1.Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol 2007;213:341–347. [DOI] [PubMed] [Google Scholar]
- 2.Caplan AI. Mesenchymal stem cells. J Orthop Res 1991;9:641–650. [DOI] [PubMed] [Google Scholar]
- 3.Caplan AI. Mesenchymal stem cell: cell-based reconstructive therapy in orthopaedics. TissEng 2005;11:1198–1211. [DOI] [PubMed] [Google Scholar]
- 4.Ed Thomas, Blume KG. Historical markers in the development of allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transpl 1999;5:341–346. [DOI] [PubMed] [Google Scholar]
- 5.Weiss L. The hematopoietic microenvironment of the bone marrow: an ultrastructural study of the stroma in rats. Anat Rec 1976;186:161–184. [DOI] [PubMed] [Google Scholar]
- 6.Bruder SP and Caplan AI. Osteogenic cell lineage analysis is facilitated by organ culture of embryonic chick periosteum. Dev Biol 1990;141:319–329. [DOI] [PubMed] [Google Scholar]
- 7.Caplan AI and Boyan BD. Endochondral bone formation: the lineage cascade. In Bone, vol 8, Hall B (ed.). CRC Press: Boca Raton, FL, 1994; 1–46. [Google Scholar]
- 8.Haynesworth SE, Goldberg VM, Caplan AI. Diminution of the number of mesenchymal stem cells as a cause for skeletal aging. In Musculoskeletal Soft-tissue Aging: Impact on Mobility, section 1, Buckwalter JA, Goldberg VM, Y Woo SL-Y (eds). American Academy of Orthopaedic Surgeons: Rosemont, IL, USA, 1994; 79–87. [Google Scholar]
- 9.Lennon DP, Haynesworth SE, Bruder SP, Jaiswall N, Caplan AI. Human and animal mesenchymal progenitor cells from bone marrow: identification of serum for optimal selection and proliferation. In vitro Cell Dev Biol 1996;32:602–611. [Google Scholar]
- 10.Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation 1968;6:230–247. [PubMed] [Google Scholar]
- 11.Owen M, Friedenstein AJ. Stromal stem cells: marrow-derived osteogenic precursors. Ciba Found Symp 1988;136:42–60. [DOI] [PubMed] [Google Scholar]
- 12.Caplan AI. Stem cell delivery vehicle. Biomaterials 1990;11:44–46. [PubMed] [Google Scholar]
- 13.Ohgushi H, Goldberg VM and Caplan AI. Heterotopic osteogenesis in porous ceramics induced by marrow cells. J Orthop Res 1989;7:568–578. [DOI] [PubMed] [Google Scholar]
- 14.Goshima J, Goldberg VM, Caplan AI. The origin of bone formed in composite grafts of porous calcium phosphate ceramic loaded with marrow cells. Clin OrthopRel Res 1991;269:274–283. [PubMed] [Google Scholar]
- 15.Allay JA, Dennis JE, Haynesworth SE, Majumdar M, Clapp DW, Caplan AI, et al. LacZ and IL-3 expression in vivo after retroviral transduction of marrow-derived human osteogenic mesenchymal progenitors. Human Gene Therapy 1997;8:1417–1427. [DOI] [PubMed] [Google Scholar]
- 16.Dennis JE, Caplan AI. Porous ceramic vehicles for rat-marrow-derived (Rattus norvegicus) osteogenic cell delivery: effects of pre-treatment with fibronectin or laminin. J Oral Implant 1993;19:106–115. [PubMed] [Google Scholar]
- 17.Le Blanc K, Tammik L, Sundberg B, Haynesworth SE, Ringden O. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol 2003;57:11–20. [DOI] [PubMed] [Google Scholar]
- 18.Maitra B, Szekely E, Gjini K, Laughlin MJ, Dennis J, Haynesworth SE, et al. Human mesenchymal stem cells support unrelated donor hematopoietic stem cells and suppress T cell activation Bone Marrow Transpl 2004;33:597–604. [DOI] [PubMed] [Google Scholar]
- 19.Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringden O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol 2003;31:890. [DOI] [PubMed] [Google Scholar]
- 20.Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC. Suppression of allogeneic T cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation 2003;75:389. [DOI] [PubMed] [Google Scholar]
- 21.Krampera M, Glennie S, Dyson J, Scott D, Laylor R, Simpson E, et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood 2003;101:3722. [DOI] [PubMed] [Google Scholar]
- 22.Lazarus HM, Haynesworth SE, Gerson SL, Rosenthal N, Caplan AI. Ex vivo expansion and subsequent infusion of human bone marrow-derived stromal progenitor cells (mesenchymal progenitor cells; MPCs): implications for therapeutic use. Bone Marrow Transpl 1995;16:557–564. [PubMed] [Google Scholar]
- 23.Koc ON, Peters C, Aubourg P, Raghavan S, Dyhouse S, DeGasperi R, et al. Bone marrow derived mesenchymal stem cells remain host-derived despite successful hematopoietic engraftment after allogeneic transplantation in patients with lysosomal and peroxisomal storage diseases. Exp Hemtaol 1999;27:1675–1681. [DOI] [PubMed] [Google Scholar]
- 24.Koc ON, Gerson SL, Cooper BW, Dyhouse SM, Haynesworth SE, Caplan AI, et al. Rapid hematopoietic recovery after co-infusion of autologous blood stem cells and culture expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high dose chemotherapy. J Clin Oncol 2000;18:307–316. [DOI] [PubMed] [Google Scholar]
- 25.Koc ON, Day J, Nieder M, Gerson SL, Lazarus HM, Krivit W. Allogeneic mesenchymal stem cell infusion for treatment of metachromatic leukodystrophy (MLD) and Hurler syndrome (MPS-IH). Bone Marrow Transpl 2002;30:215–22. [DOI] [PubMed] [Google Scholar]
- 26.Devine SM, Bartholomew AM, Mahmud N, Nelson M, Patil S, Hardy W, et al. Mesenchymal stem cells are capable of homing to the bone marrow of non-human primates following systemic infusion. Exp Hematol 2001;29:244–255. [DOI] [PubMed] [Google Scholar]
- 27.Majumdar MK, Thiede MA, Mosca JD, Moorman M, Gerson SL. Phenotypic and functional comparison of cultures of marrow-derived mesenchymal stem cells (MSCs) and stromal cells. J Cell Physiol 1998;176:57–66. [DOI] [PubMed] [Google Scholar]
- 28.Haynesworth SE, Baber MA, Caplan AI. Cytokine expression by human marrow-derived mesenchymal progenitor cells in vitro: effects of dexamethasone and IL-1α. J Cell Physiol 1996;166:585–592. [DOI] [PubMed] [Google Scholar]
- 29.Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WWK, Gordon PL, Neel M, et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med 1999;5:309–313. [DOI] [PubMed] [Google Scholar]
- 30.Horwitz EM, Gordon PL, Koo WK, Marx JC, Neel MD, McNall RY, et al. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: implications for cell therapy of bone. Proc Natl Acad Sci USA 2002;99:8932–8937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pittenger MD, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science 1999;284:143–147. [DOI] [PubMed] [Google Scholar]
- 32.Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet 2008;371:1579–1586. [DOI] [PubMed] [Google Scholar]
- 33.Gao J, Dennis JE, Muzic RF, Lundberg M and Caplan AI. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs 2001;169:12–20. [DOI] [PubMed] [Google Scholar]
- 34.Mangi AA, Noiseux N, Kong D, He H, Rezvani M, Ingwall JS, et al. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med 2003;10:10. [DOI] [PubMed] [Google Scholar]
- 35.Taylor DA, Atkins BZ, Hungspreugs P, Jones TR, Reedy MC, Hutcheson KA, et al. Regenerating functional myocardium: improved performance after skeletal myoblast transplantation. Nat Med 1998;4:929–933. [DOI] [PubMed] [Google Scholar]
- 36.Murry CE, Wiseman RW, Schwartz SM, Hauschka SD. Skeletal myoblast transplantation for repair of myocardial necrosis. J Clin Invest 1996;98:2512–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saito T, Dennis JE, Lennon DP, Young RG, Caplan AI. Myogenic expression of mesenchymal stem cells within myotubes of MDX mice in vitro and in vivo. Tissue Eng 1996;1:327–344. [DOI] [PubMed] [Google Scholar]
- 38.Wakitani S, Saito T, Caplan AI. Myogenic cells derived from rat bone marrow mesenchymal stem cells exposed to 5-azacytidine. Muscle Nerve 1995;18:1417–1426. [DOI] [PubMed] [Google Scholar]
- 39.Caparrelli DJ, Cattaneo SM, Shake JG, Flynn EC, Meyers J, Baumgartner WA, et al. Cellular myoplasty with mesenchymal stem cells results in improved cardiac performance in a swine model of myocardial infarction. Circulation 2001;104:II–599 (Abstr). [Google Scholar]
- 40.Askari A, Unzek S, Popovic ZB, et al. Effect of stromal-cell-derived factor-1 on stem cell homing and tissue regeneration in ischemic cardiomyopathy. Lancet 2003;362:697–703. [DOI] [PubMed] [Google Scholar]
- 41.Schenk S, Mal N, Finan A, Zhang M, Kiedrowski M, Popovic Z, et al. Monocyte chemotactic protein-3 is a myocardial mesenchymal stem cell homing factor. Stem Cells 2007;25:245–251. [DOI] [PubMed] [Google Scholar]
- 42.Murphy J, Fink D, Hunsiker E, Barry F. Stem cell therapy in a caprine model of osteoarthritis. Arthrit Rheum 2003;48:3464–3474. [DOI] [PubMed] [Google Scholar]
- 43.Li Y, Chen J, Chen XG Wang L, Gautam SC, Zu YX, et al. Human marrow stromal cell therapy for stroke in rat: neurotrophic and functional recovery. Neurology 2002;59:514–523. [DOI] [PubMed] [Google Scholar]
- 44.Mahmood A, Lu D, Lu M, Chopp M. Treatment of traumatic brain injury in adult rats with intravenous administration of human bone marrow stromal cells. Neurosurgery 2003;53:697–703. [DOI] [PubMed] [Google Scholar]
- 45.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem 2006;98:1076–1084. [DOI] [PubMed] [Google Scholar]
- 46.Singer M. The trophic quality of the neuron: some theoretical considerations. Prog Brain Res 1964;13:228–232. [DOI] [PubMed] [Google Scholar]
- 47.Fleming JE Jr., Cassiede P, Baber M, Haynesworth SE, Caplan AI. A Monoclonal antibody against adult marrow-derived mesenchymal cells recognizes developing vasculature in embryonic human skin. Dev Dyn 1998;212:119–132. [DOI] [PubMed] [Google Scholar]
- 48.Sorrell JM, Baber MA, Caplan AI. A self-assembled fibroblast–endothelial cell co-culture system that supports in vitro vasculogenesis by both human umbilical vein endothelial cells and human dermal microvascular endothelial cells. Cells Tissues Organs 2007;186:157–168. [DOI] [PubMed] [Google Scholar]
- 49.Sorrell JM, Baber MA, Caplan AI. Influence of adult mesenchymal stem cells on in vitro vascular formation. Tiss Eng A 2008;(in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Crisan M, Deasy B, Favina M, Zheng B, Huard J, Lazzari L, et al. Purification and long-term culture of multipotent progenitor cells affiliated with the walls of human blood vessels: myoendothelial cells and pericytes. Methods Cell Biol 2008;86:295–309. [DOI] [PubMed] [Google Scholar]
- 51.Sacchetti B, Funari A, Michienzi S, DiCesare S, Piersanti S, Saggio I, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell 2007;131:324–336. [DOI] [PubMed] [Google Scholar]
- 52.Brighton CT, Lorich DG, Kupcha R, Reilly TM, Jones AR, Woodbury RA. The pericyte as a possible osteoblast progenitor cell. Clin Orthop Relat Res 1992;275:287–99. [PubMed] [Google Scholar]
- 53.Caplan AI. All MSCs are pericytes? Cell Stem Cell 2008;3(3):229–230. [DOI] [PubMed] [Google Scholar]
- 54.da Silva Meirelles L, Caplan AI, Nardi NB. In search of the in vivo identity of mesenchymal stem cells. Stem Cells 2008;26:2287–2299. [DOI] [PubMed] [Google Scholar]
- 55.Sinsicalco D, Sullo N, Maione S, Rossi F, D’Agostino B. Review. Stem cell therapy: the great promise in lung disease. Therap Adv Resp Dis 2008;2:173–177. [DOI] [PubMed] [Google Scholar]
- 56.Chen MF, Lin CT, Chen WC, Yang CT, Chen CC, Lia SK, et al. The sensitivity of human mesenchymal stem cells to ionizing radiation. Int J Radiat Oncol Biol Phys 2006;66(1): 244–253. [DOI] [PubMed] [Google Scholar]
- 57.Bai L, Caplan AI, Lennon DL, Miller RH. Human mesenchymal stem cell signals regulate neural stem cell fate. Neurochem Res 2007;32:353–362. [DOI] [PubMed] [Google Scholar]
- 58.Dennis JE, Cohen N, Goldberg VM, Caplan AI. Targeted delivery of progenitor cells for cartilage repair. J Orthop Res 2004;22:735–741. [DOI] [PubMed] [Google Scholar]
- 59.Estes BT, Wu AW, Guilak F. Potent induction of chondrocytic differential of human adipose-derived adult stem cells by bone morphogenetic protein 6. Arthrit Rheum 2006; 54(4):1222–1232. [DOI] [PubMed] [Google Scholar]
- 60.da Silva Meirelles L, Sand TT, Harman RJ, Lennon DP, Caplan AI. MSC frequency correlates with blood vessel density in equine adipose tissue. Tiss Eng A 2008;14:(in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.