Abstract
Background
This retrospective study investigated biomarkers that can reflect coagulation, inflammation, and lipid abnormalities: platelet-to-albumin ratio (PAR), platelet-to lymphocyte ratio (PLR), low-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio (LDL-C/HDL-C), apolipoprotein B-to-apolipoprotein ratio (ApoB/ApoA1) whether may be viable prognostic predictors in children and adolescents with osteosarcoma.
Methods
The retrospective review has enrolled a total of 118 children and adolescent patients diagnosed with osteosarcoma. Analyses with a receiver operating characteristic (ROC) curve were performed to evaluate the optimal cut-off values and to compare the area under curves (AUC). Kaplan–Meier curves were used to visualize survival outcome and a Cox proportional hazards model were used to confirm independent prognostic factors.
Results
Osteosarcoma patients in high PAR group (> 4.41) and high ApoB/ApoA1 group (> 0.82) experienced significantly shorter overall survival compared with those in low PAR group (≤ 4.41) and low ApoB/ApoA1 group (≤ 0.82). In univariate and multivariable analyses, preoperative PAR and ApoB/ApoA1 were identified as independent prognostic factors for OS in children and adolescents with osteosarcoma.
Conclusion
Preoperative PAR and ApoB/ApoA1 can be used as promising predictors in children and adolescents with osteosarcoma to help clinicians recognize patients with an increased risk of poor prognosis.
Keywords: Platelet-to-albumin ratio, Apolipoprotein B-to-apolipoprotein A1 ratio, Osteosarcoma, Children and adolescents, Prognosis
Background
Osteosarcoma is the most prevalent primary malignancy of bone [1], with an average global annual incidence of 3.1 per million for all ages [2]. Adolescence is defined as the age period from 12 (early adolescence) to 25 years old (late adolescence) [3], while osteosarcoma has the highest incidence among adolescents aged 15–19 [4]. The 5-year overall survival (OS) of patients with osteosarcoma has increased from < 20% when surgery was the only therapy before the 1980s to nearly 65% after receiving combined treatment today [5, 6]. Due to the biological characteristics of osteosarcoma and the need for systemic treatment, it is strongly recommended that close follow-up, and meanwhile an accurate prediction of prognosis is related to deciding the optimal treatment plan for individual patient [2, 7]. Enneking surgical criteria, alkaline phosphatase as well as other widely-used prognostic indicators often observe heterogeneous clinical results in the same tumor stage, indicating a lack of accuracy [8, 9]. Therefore, finding accurate, practical and easy-to-detect pre-treatment indicators to assess osteosarcoma prognosis would be of great clinical significance. However, few studies have attempted to find promising potential biomarkers for predicting the prognosis of osteosarcoma, especially in children and adolescent patients.
Previous studies have shown that tumorigenesis and progression are linked with the activation of the coagulation system [10, 11], inflammation [12], and abnormalities in serum lipids and lipoproteins [13]. Platelets (PLT) are essential components of hemostasis. Recent years PLT have also been reported to be involved in the progression and metastasis of tumor cells by affecting coagulation, promoting inflammatory response and angiogenesis [14–16]. Albumin (ALB) is the most abundant plasma protein synthesized by the liver, with additional functions related to anticoagulation and anti-inflammatory, thereby inhibiting the growth of tumor cells [17, 18]. Moreover, the growing evidence revealed that lymphocyte, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), apolipoprotein A1 (ApoA1), and apolipoprotein B (ApoB) that reflect inflammation and lipid abnormalities are closely related to the progression and metastasis of tumors [19–22]. In recent years, some studies have indicated that preoperative PLT to ALB ratio (PAR), PLT to lymphocyte ratio (PLR), LDL-C to HDL-C ratio (LDL-C/HDL-C), and ApoB to ApoA1 ratio (ApoB/ApoA1) were negatively correlated with the prognosis of cholangiocarcinoma [23], lung cancer [24], colorectal cancer [25], and gastric cancer [26], respectively. Whereas, there are rarely retrospective studies concerning above indicators and the prognosis of osteosarcoma, particularly in children and adolescent patients.
Herein, this retrospective study investigated the prognostic value of preoperative PAR, PLR, LDL-C/HDL-C, and ApoB/ApoA1 in children and adolescents with osteosarcoma.
Methods
Study population
The present study enrolled a total of 118 children and adolescent patients with osteosarcoma who had been treated at Tongji Hospital of Tongji Medical College of Huazhong University of Science and Technology, and First Affiliated Hospital of Nanchang University from April 2012 to December 2018. The conditions for patients to enter the group included: osteosarcoma diagnosed by an experienced pathologist and without any previous anti-cancer treatment. Patients having received anticoagulant therapy or infused albumin before blood collection, used drugs affecting lipid metabolism or diagnosed with other coexisting malignancies, severe inflammation or infection in the past month, familial coagulopathy, haematology or autoimmune disease associated with elevated blood lipids (diabetes, hyperlipidemia or metabolic syndrome), blood transfusion within 4 months prior to admission or with incomplete data were excluded. Demographic information and clinical and laboratory parameters of all enrolled patients were collected, evaluated and recorded after admission. The treatment protocols of all patients were formulated and performed in accordance with the guidelines of the National Comprehensive Cancer Network. The clinical records were reviewed with the informed consent of the patients or their legal guardians.
Clinical parameters and laboratory results
The patient's baseline characteristics as well as clinical parameters and laboratory results including age, gender, tumor size, tumor site, histological type, Enneking stage, pathological fracture, neoadjuvant chemotherapy, local recurrence, metastasis, and laboratory data (PLT, ALB, lymphocyte count, HDL-C, LDL-C, ApoA1, ApoB) were collected from the electronic medical records. In order to perfect the preoperative preparation, all patients were required to quit smoking and drinking 2 weeks before the operation and switched to light meals. Pre-meal fasting blood specimens were collected within 10 days before surgery.
Follow-up
All the patients who were discharged after treatment were followed up every 3 months for the first 2 years, and then every 6 months for the next 3–5 years, and annually thereafter. The endpoint event is the death of the patient and the censoring time of the experiment is until March 2020. OS was calculated from the date of surgery to death or the last follow-up, and was obtained mainly through hospital records or telephone surveys.
Statistical analyses
IBM software SPSS version 23.0 (SPSS, Chicago, IL, USA) and GraphPad Prism version 7.00 (GraphPad Software, La Jolla, CA, USA) were used to perform statistical calculations. The distribution of data was utilized using the Kolmogorov–Smirnov test. Continuous variables were presented as mean ± standard deviation (SD) and discontinuous variables were expressed as median (range). Categorical variables were presented as percentages and evaluated by chi-squared test. A receiver operative characteristic (ROC) curve was calculated to determine evaluate the optimal cut-off values and to compare area under curves (AUC). Kaplan–Meier curves were used to visualize survival outcome and a Cox proportional hazards model were used to confirm independent prognostic factors. The results were pooled using two-sided P-value of < 0.05 for each outcome.
Results
Clinical characteristics of the subjects
The patient’s baseline characteristics were shown in Table 1. 118 children and adolescent patients with osteosarcoma included 72 (61.0%) men and 46 (39.0%) women. The mean age was 16.4 ± 3.9 years, and the median maximum tumor diameter was 4.8 cm. According to Enneking surgical staging criteria, patients of stages I–II and III was 85 (72.0%) and 33 (28.0%), respectively. Pathological fracture was presented in 27 patients (22.9%). Moreover, local recurrence and metastasis occurred in 25 (21.2%) and 45 (38.1%) patients, respectively. The neoadjuvant chemotherapy was given to 71 patients (60.2%).
Table 1.
The clinical characteristics of all patients
| Characteristics | Patients | % |
|---|---|---|
| Age (years) | 16.4 ± 3.9 | |
| Gender | ||
| Male | 72 | 61.0 |
| Female | 46 | 39.0 |
| Tumor size (cm) | ||
| ≤ 5 | 77 | 65.3 |
| > 5 | 41 | 34.7 |
| Tumor site | ||
| Extremities | 85 | 72.0 |
| Non-extremities | 33 | 28.0 |
| Histological type | ||
| Well-differentiated | 68 | 57.6 |
| Poorly differentiated | 50 | 42.4 |
| Enneking stage | ||
| I | 49 | 41.5 |
| II | 36 | 30.5 |
| III | 33 | 28.0 |
| Pathological fracture | ||
| Yes | 27 | 22.9 |
| No | 91 | 77.1 |
| Neoadjuvant chemotherapy | ||
| Yes | 71 | 60.2 |
| No | 47 | 39.8 |
| Local recurrence | ||
| Yes | 25 | 21.2 |
| No | 93 | 78.8 |
| Metastasis | ||
| Yes | 45 | 38.1 |
| No | 73 | 61.9 |
Continuous variables with normality were presented as mean ± standard deviation
Categorical variables were shown as percentages
Optimal cut-off value for PAR, PLR, LDL-C/HDL-C, and ApoB/ApoA1
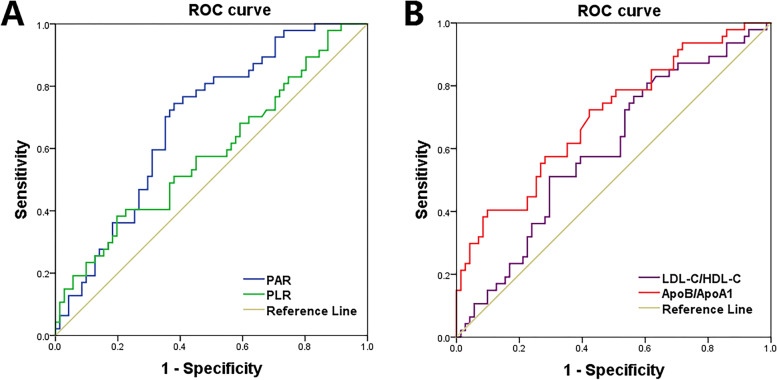
As shown in Table 2 and Fig. 1, ROC curve analysis was calculated for OS in children and adolescent patients with osteosarcoma. The cut-off value of joint maximum sensitivity and specificity for PAR was 4.41 (P = 0.001), PLR was 209.54 (P = 0.036), LDL-C/HDL-C was 2.04 (P = 0.017), and ApoB/ApoA1 was 0.82 (P < 0.001). According to each cut-off value, the patients were divided into high PAR or low PAR group (> 4.41 or ≤ 4.41, respectively), high PLR or low PLR group (> 209.54 or ≤ 209.54, respectively), high LDL-C/HDL-C or low LDL-C/HDL-C group (> 2.04 or ≤ 2.04, respectively), and high ApoB/ApoA1 or low ApoB/ApoA1 group (> 0.82 or ≤ 0.82, respectively).
Table 2.
Identification of optimal cut-off values for different prognostic factors based on the ROC curve
| Factor | Optimal cut-off value | Sensitivity | Specificity | Youden index | Positive predictive value | Negative predictive value | Overall accuracy rate | AUC | 95% CI | P value |
|---|---|---|---|---|---|---|---|---|---|---|
| PAR | 4.41 | 0.745 | 0.620 | 0.365 | 0.565 | 0.786 | 0.669 | 0.686 | 0.591–0.781 | 0.001 |
| PLR | 209.54 | 0.393 | 0.803 | 0.196 | 0.563 | 0.663 | 0.636 | 0.593 | 0.477–0.690 | 0.036 |
| LDL-C/HDL-C | 2.04 | 0.511 | 0.704 | 0.215 | 0.533 | 0.685 | 0.627 | 0.606 | 0.493–0.700 | 0.017 |
| ApoB/ApoA1 | 0.82 | 0.723 | 0.582 | 0.305 | 0.630 | 0.641 | 0.636 | 0.699 | 0.603–0.796 | < 0.001 |
Data were analyzed by the ROC curve. P < 0.05 was considered significant
Fig. 1.
ROC curves of preoperative (A) PAR and PLR, and preoperative (B) LDL-C/HDL-C and ApoB/ApoA1 for predicting OS in children and adolescents with osteosarcoma
Association of clinical characteristics with preoperative PAR, PLR, LDL-C/HDL-C, and ApoB/ApoA1
Table 3 showed the relationship between PAR, PLR, LDL-C/HDL-C, ApoB/ApoA1, and clinical characteristics. The results presented that PAR was significantly associated with gender (P = 0.020), Enneking stage (P = 0.020), pathological fracture (P = 0.011), local recurrence (P = 0.008). Besides, PLR was significantly correlated with Enneking stage (P = 0.020). In addition, ApoB/ApoA1 was significantly related with gender (P = 0.027), and pathological fracture (P = 0.032). There was no significant relationship between preoperative high or low groups of PAR, PLR, LDL-C/HDL-C, ApoB/ApoA1, and other clinical characteristics except the above (P > 0.05 for all).
Table 3.
Associations of clinical characteristics with preoperative PAR, PLR, LDL-C/HDL-C, and ApoB/ApoA1
| Characteristics | PAR | PLR | LDL-C/HDL-C | ApoB/ApoA1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High | Low | P value | High | Low | P value | High | Low | P value | High | Low | P value | |
| Age (years) | 0.985 | 0.850 | 0.780 | 0.804 | ||||||||
| ≤ 16 | 30 | 27 | 15 | 42 | 21 | 36 | 12 | 45 | ||||
| > 16 | 32 | 29 | 17 | 44 | 24 | 37 | 14 | 47 | ||||
| Gender | 0.020 | 0.057 | 0.179 | 0.027 | ||||||||
| Male | 44 | 28 | 24 | 48 | 24 | 48 | 11 | 61 | ||||
| Female | 18 | 28 | 8 | 38 | 21 | 25 | 15 | 31 | ||||
| Tumor size (cm) | 0.859 | 0.702 | 0.347 | 0.987 | ||||||||
| ≤ 5 | 40 | 37 | 20 | 57 | 27 | 50 | 17 | 60 | ||||
| > 5 | 22 | 19 | 12 | 29 | 18 | 23 | 9 | 32 | ||||
| Tumor site | 0.133 | 0.062 | 0.149 | 0.392 | ||||||||
| Extremities | 41 | 44 | 19 | 66 | 29 | 58 | 17 | 68 | ||||
| Non-extremities | 21 | 12 | 13 | 20 | 16 | 17 | 9 | 24 | ||||
| Histological type | 0.786 | 0.306 | 0.979 | 0.994 | ||||||||
| Well-differentiated | 35 | 33 | 16 | 52 | 26 | 42 | 15 | 53 | ||||
| Poorly differentiated | 27 | 23 | 16 | 34 | 19 | 31 | 11 | 39 | ||||
| Enneking stage | 0.020 | 0.020 | 0.550 | 0.529 | ||||||||
| I/II | 39 | 46 | 18 | 67 | 31 | 54 | 20 | 65 | ||||
| III | 23 | 10 | 14 | 19 | 14 | 19 | 6 | 27 | ||||
| Pathological fracture | 0.011 | 0.187 | 0.095 | 0.032 | ||||||||
| Yes | 20 | 7 | 10 | 17 | 14 | 13 | 10 | 17 | ||||
| No | 42 | 49 | 22 | 69 | 31 | 60 | 16 | 75 | ||||
| Neoadjuvant chemotherapy | 0.105 | 0.752 | 0.976 | 0.872 | ||||||||
| Yes | 33 | 38 | 20 | 51 | 27 | 44 | 16 | 55 | ||||
| No | 29 | 18 | 12 | 35 | 18 | 29 | 10 | 37 | ||||
| Local recurrence | 0.008 | 0.261 | 0.108 | 0.418 | ||||||||
| Yes | 19 | 6 | 9 | 16 | 13 | 12 | 7 | 18 | ||||
| No | 43 | 50 | 23 | 70 | 32 | 61 | 19 | 74 | ||||
| Metastasis | 0.203 | 0.734 | 0.651 | 0.340 | ||||||||
| Yes | 27 | 18 | 13 | 32 | 16 | 29 | 12 | 33 | ||||
| No | 35 | 38 | 19 | 54 | 29 | 44 | 14 | 59 | ||||
Data were present with Chi-square test. P < 0.05 was considered significant
Prognostic value of preoperative PAR, PLR, LDL-C/HDL-C, and ApoB/ApoA1
Kaplan–Meier curves were conducted to perform the survival analysis (Fig. 2). Compared with patients in the low groups of PAR, PLR, LDL-C/HDL-C, and ApoB/ApoA1, those in the high groups of PAR (P < 0.001, log-rank test; Fig. 2A), PLR (P = 0.016, log-rank test; Fig. 2B), LDL-C/HDL-C (P = 0.029, log-rank test; Fig. 2C), and ApoB/ApoA1 (P < 0.001, log-rank test; Fig. 2D) had shorter OS.
Fig. 2.
Kaplan–Meier curves for OS in children and adolescents with osteosarcoma according to (A) PAR, (B) PLR, (C) LDL-C/HDL-C and (D) ApoB/ApoA1
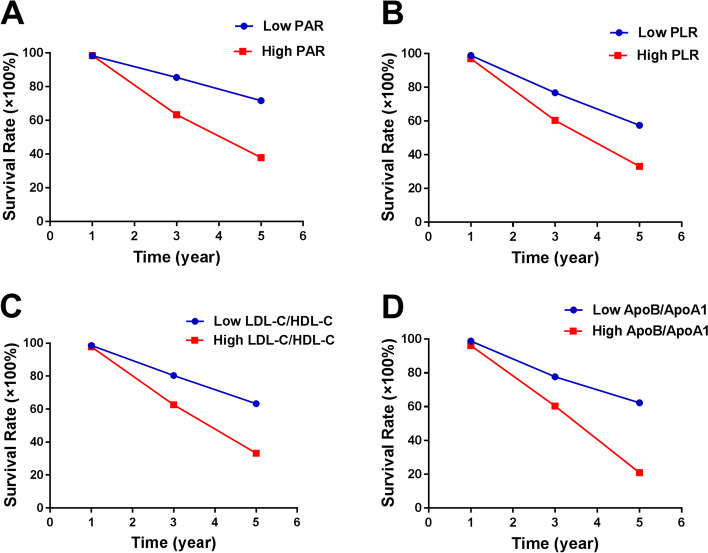
The 5-year OS in low groups of PAR, PLR, LDL-C/HDL-C, and ApoB/ApoA1 were 71.6%, 57.4%, 63.3%, and 62.3%, respectively (Fig. 3). While in high groups, the 5-year OS of PAR, PLR, LDL-C/HDL-C, and ApoB/ApoA1 were 37.9%, 33.0%, 33.2% and 20.9%, respectively (Fig. 3).
Fig. 3.
The 1-, 3- and 5-year rates of OS in all patients with osteosarcoma according to (A) PAR, (B) PLR, (C) LDL-C/HDL-C and (D) ApoB/ApoA1
Further subgroup analysis was performed to investigate the prognostic value of PAR, PLR, LDL-C/HDL-C, and ApoB/ApoA1 in children and adolescent patients with osteosarcoma stratified by Enneking stage. The results suggested that only ApoB/ApoA1 can identify the OS differences in both stage I-II (P < 0.001, Fig. 4D), and III (P = 0.026, Fig. 4H). Additionally, Poorer OS was observed in patients with high groups of PAR (P = 0.007, Fig. 4A), PLR (P = 0.040, Fig. 4B), LDL-C/HDL-C (P = 0.040, Fig. 4C) in subgroup of stage I-II. However, there was no significance OS difference between the high and low groups of PAR (P = 0.055, Fig. 4E), PLR (P = 0.904, Fig. 4F), LDL-C/HDL-C (P = 0.628, Fig. 4G) in stage III.
Fig. 4.

Kaplan–Meier curves of OS for PAR, PLR, LDL-C/HDL-C, and ApoB/ApoA1 in all patients stratified by Enneking stage. A-D Kaplan–Meier curves of OS for (A) PAR, (B) PLR, (C) LDL-C/HDL-C and (D) ApoB/ApoA1 in osteosarcoma patients with stage I-II. E–H Kaplan–Meier curves of OS for (E) PAR, (F) PLR, (G) LDL-C/HDL-C and (H) ApoB/ApoA1 in osteosarcoma patients with stage III
Univariate and multivariate Cox proportional hazards model.
The univariate Cox proportional hazards model showed that tumor size (P = 0.017), Enneking stage (P < 0.001), pathological fracture (P < 0.001), local recurrence (P = 0.001), metastasis (P < 0.001), PAR (P < 0.001), PLR (P = 0.019), LDL-C/HDL-C (P = 0.033), and ApoB/ApoA1 (P < 0.001), were significantly associated with OS (Table 4). In the multivariate Cox proportional hazards model, we identified Enneking stage (P = 0.007), local recurrence (P = 0.029), metastasis (P < 0.001), PAR (P = 0.033), and ApoB/ApoA1 (P = 0.001) as independent prognostic factors for OS.
Table 4.
Univariate and multivariate Cox proportional hazards model analysis for overall survival
| Characteristics | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Age (years) | 0.299 | |||||
| ≤ 16 | 1.000 | Reference | ||||
| > 16 | 1.358 | 0.762–2.420 | ||||
| Gender | 0.910 | |||||
| Male | 1.000 | Reference | ||||
| Female | 0.983 | 0.734–1.317 | ||||
| Tumor size (cm) | 0.017 | 0.349 | ||||
| ≤ 5 | 1.000 | Reference | 1.000 | Reference | ||
| > 5 | 2.006 | 1.130–3.563 | 1.408 | 0.688–2.883 | ||
| Tumor site | 0.946 | |||||
| Extremities | 1.000 | Reference | ||||
| Non-extremities | 0.979 | 0.525–1.824 | ||||
| Histological type | 0.415 | |||||
| Well-differentiated | 1.000 | Reference | ||||
| Poorly differentiated | 1.272 | 0.714–2.266 | ||||
| Enneking stage | < 0.001 | 0.007 | ||||
| I/II | 1.000 | Reference | 1.000 | Reference | ||
| III | 5.695 | 2.910–11.147 | 3.213 | 1.373–7.520 | ||
| Pathological fracture | < 0.001 | 0.162 | ||||
| Yes | 1.000 | Reference | 1.000 | Reference | ||
| No | 0.267 | 0.144–0.494 | 0.589 | 0.281–1.236 | ||
| Neoadjuvant chemotherapy | 0.329 | |||||
| Yes | 1.000 | Reference | ||||
| No | 1.331 | 0.749–2.365 | ||||
| Local recurrence | 0.001 | 0.029 | ||||
| Yes | 1.000 | Reference | 1.000 | Reference | ||
| No | 0.384 | 0.213–0.693 | 0.420 | 0.193–0.917 | ||
| Metastasis | < 0.001 | < 0.001 | ||||
| Yes | 1.000 | Reference | 1.000 | Reference | ||
| No | 0.286 | 0.158–0.516 | 0.252 | 0.123–0.518 | ||
| PAR | < 0.001 | 0.033 | ||||
| High | 1.000 | Reference | 1.000 | Reference | ||
| Low | 0.274 | 0.141–0.533 | 0.428 | 0.196–0.934 | ||
| PLR | 0.019 | 0.438 | ||||
| High | 1.000 | Reference | 1.000 | Reference | ||
| Low | 0.494 | 0.274–0.892 | 0.766 | 0.390–1.504 | ||
| LDL-C/HDL-C | 0.033 | 0.115 | ||||
| High | 1.000 | Reference | 1.000 | Reference | ||
| Low | 0.535 | 0.302–0.950 | 0.572 | 0.285–1.145 | ||
| ApoB/ApoA1 | < 0.001 | 0.001 | ||||
| High | 1.000 | Reference | 1.000 | Reference | ||
| Low | 0.336 | 0.186–0.606 | 0.268 | 0.135–0.530 | ||
Data were analyzed by Cox proportional hazards model. P < 0.05 was considered significant
Discussion
Tumorigenesis and progression are complex processes with many contributing factors. In this retrospective study, it was found that patients with relatively high preoperative PAR (> 4.41) and high preoperative ApoB/ApoA1 (> 0.82) were associated with shorter OS. More significantly, we confirmed for the first time that preoperative PAR and ApoB/ApoA1 can be identified as independent prognostic factors for OS in children and adolescents with osteosarcoma.
The clinical value of our current findings cannot be ignored. First of all, our findings indicate that activation of the coagulation system, the enhancement of inflammatory cell response and the abnormality of blood lipid may be involved in the progression of osteosarcoma. Based on this, interventions or drugs aimed at reducing the ratio of PAR and ApoB/ApoA1 might be a potentially effective strategy to improve the prognosis of osteosarcoma. In addition, these findings may help clinicians recognize patients with increased risk of poor prognosis in osteosarcoma and establish a framework for individualized treatment of children and adolescents with osteosarcoma in the future.
Furthermore, our findings are also consistent with other previous studies. Saito et al. [23], Shirai et al. [27], and Li et al. [28] have identified that PAR can be used as an independent factor for prognosis in cholangiocarcinoma, pancreatic ductal adenocarcinoma, and hepatocellular carcinoma, respectively. Meanwhile, the results of Yang et al. [29], Ma et al. [26], and Zhang et al. [30] have shown that high preoperative ApoB/ApoA1 is a significant association with poor cancer prognosis of colorectal, gastric, and renal cell carcinoma. Whereas, as new indicators for evaluating OS, the potential mechanism of serum PAR and ApoB/ApoA1 to predict the survival outcomes of osteosarcoma remains unclear. We could only try to give a preliminary explanation by reviewing previous studies.
Falanga et al. [10, 31] reported that platelets are activated by tumor to adhere heavily to peripheral blood tumor cells, aiding proliferation by protecting the tumor cells from immune cells. As the number of activated platelets increase and aggregate, the released inflammatory factors promote angiogenesis and tumor progression [32–34]; hematogenous metastasis is promoted as tumor cell emboli are blocked in microcirculation [35–37]. The elevation of tissue factors in tumor cells, and increased release of tissue factors in platelets, further activate coagulation and fibrinolysis to promote tumor progression and metastasis [11, 38, 39]. Besides, cisplatin is a crucial chemotherapeutic drug for osteosarcoma [2], while the study of Wang et al. [40] showed that high platelet levels can activate Akt and Erk signaling, thereby saving cisplatin-induced apoptosis, thus reducing the efficacy of platinum-based therapy, leading to poor prognosis. In addition, inflammatory cytokines released from cancer cells and platelets inhibit albumin synthesis in hepatocytes [41]. Albumin can inhibit the activation and aggregation of platelets induced by histone H4, and exerts an anticoagulant effect by binding arachidonic acid [18, 42]. When the albumin level of the body decreases, these functions are weakened, and tumor progression is then promoted. Meanwhile, Low albumin level is also the manifestation of the heightened systemic inflammatory reaction and malnutrition, which also may affect the prognosis of osteosarcoma [17, 43, 44]. ApoA1, a major protein component of HDL-C and a key medium for cholesterol homeostasis, plays an anti-tumor role via affecting the immune system and inhibiting new angiogenesis [45, 46]. The decrease of ApoA1 level in circulation can transform macrophages of anti-tumor M1 phenotype into M2-macrophages that promote tumor, and reduce cytotoxic CD8+ T cells to enhance the inflammatory response of tumor, and promote angiogenesis and increasing the activity of MMP-9, thereby causing poor OS of tumor [47–49]. Moreover, ApoB, the main structural protein of LDL-C, can promote lipoproteins to enter the blood vessel wall, stimulate the phagocytosis of macrophages, thereby enhancing the inflammatory response to further promote tumor progression [22, 50]. Hence, the mechanisms described above may explain, at least partially, the associations between relatively high preoperative PAR and ApoB/ApoA1, and poor prognosis of children and adolescents with osteosarcoma observed in the present study.
Indeed, the current study is limited by its retrospective nature, so selection bias and inaccuracies may exist. Additionally, given that osteosarcoma is a sporadic malignant tumor, our current research is limited by the small sample size, especially concerning the patients at stage III, so that patients at this stage might not be adequate to represent the whole population. Besides, the diagnosis of osteosarcoma relies on imaging and histopathological examinations, so that it is difficult to obtain accurate progression-free survival rates for patients. Last, our findings still warrant further investigations by large sample size and multi-center studies.
Conclusion
In summary, this study found that osteosarcoma patients with relatively high preoperative PAR and ApoB/ApoA1were associated with shorter OS. More significantly, we confirmed that preoperative PAR and ApoB/ApoA1 can be identified as independent prognostic factors for OS in children and adolescents with osteosarcoma. These findings may help clinicians recognize patients with an increased risk of poor prognosis in osteosarcoma and establish a framework for individualized treatment of children and adolescents with osteosarcoma in the future.
Acknowledgements
None.
Abbreviations
- PLT
Platelet
- ALB
Albumin
- HDL-C
High-density lipoprotein cholesterol
- LDL-C
Low-density lipoprotein cholesterol
- ApoA1
Apolipoprotein A1
- ApoB
Apolipoprotein B
- PAR
Platelet-to-albumin ratio
- PLR
Platelet-to-lymphocyte ratio
- LDL-C/HDL-C
LDL-C to HDL-C ratio
- ApoB/ApoA1
ApoB to ApoA1 ratio
- OS
Overall survival
- ROC
Receiver operative characteristic
- AUC
Area under curve
- HR
Hazard ratio
- CI
Confidence interval
Authors’ contributions
Jianfeng Guo designed the current study, reviewed the paper, and made critical revisions to the manuscript. Cong Ma was the primary writer of the paper, tested the entire index for all samples, and was responsible for the statistical analysis. Ruizhen Li created all tables and figures and provided suggestions for important intellectual content. Ronghui Yu, Jingjing Guo, Jianyun Xu, and Xuhui Yuan performed the literature search, data collection, and follow-up. The author(s) read and approved the final manuscript.
Funding
None.
Availability data and materials
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Declarations
Ethics and consent to participate
This study was approved by the ethics committee of Tongji Hospital of Tongji Medical College of Huazhong University of Science and Technology and First Affiliated Hospital of Nanchang University ratified the current study, conforming to the Declaration of Helsinki. Before enrollment in this current study, all participants handed in informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declared no conflict of interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Biermann JS, Adkins DR, Agulnik M, Benjamin RS, Brigman B, Butrynski JE, Cheong D, Chow W, Curry WT, Frassica DA, et al. Bone cancer. Journal of the National Comprehensive Cancer Network: JNCCN. 2013;11(6):688–723. doi: 10.6004/jnccn.2013.0088. [DOI] [PubMed] [Google Scholar]
- 2.Casali PG, Bielack S, Abecassis N, Aro HT, Bauer S, Biagini R, Bonvalot S, Boukovinas I, Bovee J, Brennan B, et al. Bone sarcomas: ESMO-PaedCan-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of oncology: official journal of the European Society for Medical Oncology. 2018;29(Suppl 4):iv79–iv95. doi: 10.1093/annonc/mdy310. [DOI] [PubMed] [Google Scholar]
- 3.Enstad F, Evans-Whipp T, Kjeldsen A, Toumbourou JW, von Soest T. Predicting hazardous drinking in late adolescence/young adulthood from early and excessive adolescent drinking - a longitudinal cross-national study of Norwegian and Australian adolescents. BMC Public Health. 2019;19(1):790. doi: 10.1186/s12889-019-7099-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coccia PF, Pappo AS, Beaupin L, Borges VF, Borinstein SC, Chugh R, Dinner S, Folbrecht J, Frazier AL, Goldsby R, et al. Adolescent and Young Adult Oncology, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16(1):66–97. doi: 10.6004/jnccn.2018.0001. [DOI] [PubMed] [Google Scholar]
- 5.Gianferante DM, Mirabello L, Savage SA. Germline and somatic genetics of osteosarcoma - connecting aetiology, biology and therapy. Nat Rev Endocrinol. 2017;13(8):480–491. doi: 10.1038/nrendo.2017.16. [DOI] [PubMed] [Google Scholar]
- 6.Luetke A, Meyers PA, Lewis I, Juergens H. Osteosarcoma treatment - where do we stand? A state of the art review. Cancer Treat Rev. 2014;40(4):523–532. doi: 10.1016/j.ctrv.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 7.Smeland S, Bielack SS, Whelan J, Bernstein M, Hogendoorn P, Krailo MD, Gorlick R, Janeway KA, Ingleby FC, Anninga J, et al. Survival and prognosis with osteosarcoma: outcomes in more than 2000 patients in the EURAMOS-1 (European and American Osteosarcoma Study) cohort. Eur J Cancer. 2019;109:36–50. doi: 10.1016/j.ejca.2018.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gou B, Cao H, Cheng X, Shang W, Xu M, Qian W. Prognostic value of mean platelet volume to plateletcrit ratio in patients with osteosarcoma. Cancer management and research. 2019;11:1615–1621. doi: 10.2147/CMAR.S193949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu B, Huang Y, Sun Y, Zhang J, Yao Y, Shen Z, Xiang D, He A. Prognostic value of inflammation-based scores in patients with osteosarcoma. Sci Rep. 2016;6:39862. doi: 10.1038/srep39862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Falanga A, Russo L, Milesi V, Vignoli A. Mechanisms and risk factors of thrombosis in cancer. Crit Rev Oncol Hematol. 2017;118:79–83. doi: 10.1016/j.critrevonc.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Lima LG, Monteiro RQ. Activation of blood coagulation in cancer: implications for tumour progression. Bioscience reports. 2013;33(5):e00064. doi: 10.1042/BSR20130057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radical Biol Med. 2010;49(11):1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borgquist S, Butt T, Almgren P, Shiffman D, Stocks T, Orho-Melander M, Manjer J, Melander O. Apolipoproteins, lipids and risk of cancer. Int J Cancer. 2016;138(11):2648–2656. doi: 10.1002/ijc.30013. [DOI] [PubMed] [Google Scholar]
- 14.Franco AT, Corken A, Ware J. Platelets at the interface of thrombosis, inflammation, and cancer. Blood. 2015;126(5):582–588. doi: 10.1182/blood-2014-08-531582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kubes P. The versatile platelet contributes to inflammation, infection, hemostasis, coagulation and cancer. Semin Immunol. 2016;28(6):535. doi: 10.1016/j.smim.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Haemmerle M, Stone RL, Menter DG, Afshar-Kharghan V, Sood AK. The Platelet Lifeline to Cancer: Challenges and Opportunities. Cancer Cell. 2018;33(6):965–983. doi: 10.1016/j.ccell.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Don BR, Kaysen G. Serum albumin: relationship to inflammation and nutrition. Semin Dial. 2004;17(6):432–437. doi: 10.1111/j.0894-0959.2004.17603.x. [DOI] [PubMed] [Google Scholar]
- 18.Paar M, Rossmann C, Nusshold C, Wagner T, Schlagenhauf A, Leschnik B, Oettl K, Koestenberger M, Cvirn G, Hallstrom S. Anticoagulant action of low, physiologic, and high albumin levels in whole blood. PloS one. 2017;12(8):e0182997. doi: 10.1371/journal.pone.0182997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Black CC, Turk MJ, Dragnev K, Rigas JR. Adenocarcinoma contains more immune tolerance regulatory t-cell lymphocytes (versus squamous carcinoma) in non-small-cell lung cancer. Lung. 2013;191(3):265–270. doi: 10.1007/s00408-013-9455-7. [DOI] [PubMed] [Google Scholar]
- 20.dos Santos CR, Domingues G, Matias I, Matos J, Fonseca I, de Almeida JM, Dias S. LDL-cholesterol signaling induces breast cancer proliferation and invasion. Lipids Health Dis. 2014;13:16. doi: 10.1186/1476-511X-13-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gordon EM, Figueroa DM, Barochia AV, Yao X, Levine SJ. High-density Lipoproteins and Apolipoprotein A-I: Potential New Players in the Prevention and Treatment of Lung Disease. Front Pharmacol. 2016;7:323. doi: 10.3389/fphar.2016.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi J, Yang H, Duan X, Li L, Sun L, Li Q, Zhang J. Apolipoproteins as Differentiating and Predictive Markers for Assessing Clinical Outcomes in Patients with Small Cell Lung Cancer. Yonsei Med J. 2016;57(3):549–556. doi: 10.3349/ymj.2016.57.3.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saito N, Shirai Y, Horiuchi T, Sugano H, Shiba H, Sakamoto T, Uwagawa T, Yanaga K. Preoperative Platelet to Albumin Ratio Predicts Outcome of Patients with Cholangiocarcinoma. Anticancer Res. 2018;38(2):987–992. doi: 10.21873/anticanres.12313. [DOI] [PubMed] [Google Scholar]
- 24.Han Y, Wang J, Hong L, Sun L, Zhuang H, Sun B, Wang H, Zhang X, Ren X. Platelet-lymphocyte ratio is an independent prognostic factor in patients with ALK-positive non-small-cell lung cancer. Future Oncol. 2017;13(1):51–61. doi: 10.2217/fon-2016-0317. [DOI] [PubMed] [Google Scholar]
- 25.Liao F, He W, Jiang C, Yin C, Guo G, Chen X, Qiu H, Rong Y, Zhang B, Xu D, et al. A high LDL-C to HDL-C ratio predicts poor prognosis for initially metastatic colorectal cancer patients with elevations in LDL-C. Onco Targets Ther. 2015;8:3135–3142. doi: 10.2147/OTT.S90479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma MZ, Yuan SQ, Chen YM, Zhou ZW. Preoperative apolipoprotein B/apolipoprotein A1 ratio: a novel prognostic factor for gastric cancer. Onco Targets Ther. 2018;11:2169–2176. doi: 10.2147/OTT.S156690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shirai Y, Shiba H, Haruki K, Horiuchi T, Saito N, Fujiwara Y, Sakamoto T, Uwagawa T, Yanaga K. Preoperative Platelet-to-Albumin Ratio Predicts Prognosis of Patients with Pancreatic Ductal Adenocarcinoma After Pancreatic Resection. Anticancer Res. 2017;37(2):787–793. doi: 10.21873/anticanres.11378. [DOI] [PubMed] [Google Scholar]
- 28.Li C, Peng W, Zhang XY, Wen TF, Chen LP. The preoperative platelet to albumin ratio predicts the prognosis of hepatocellular carcinoma patients without portal hypertension after liver resection. Medicine. 2019;98(45):e17920. doi: 10.1097/MD.0000000000017920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang DD, Chen ZH, Wang DS, Yu HE, Lu JH, Xu RH, Zeng ZL. Prognostic value of the serum apolipoprotein B to apolipoprotein A-I ratio in metastatic colorectal cancer patients. J Cancer. 2020;11(5):1063–1074. doi: 10.7150/jca.35659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang F, Xie Y, Ma X, Gu L, Li H, Li X, Guo G, Zhang X. Preoperative apolipoprotein B/A1 ratio is an independent prognostic factor in metastatic renal cell carcinoma. Urologic oncology. 2019;37(3):184–e189. doi: 10.1016/j.urolonc.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 31.Falanga A, Schieppati F, Russo D. Cancer Tissue Procoagulant Mechanisms and the Hypercoagulable State of Patients with Cancer. Semin Thromb Hemost. 2015;41(7):756–764. doi: 10.1055/s-0035-1564040. [DOI] [PubMed] [Google Scholar]
- 32.Sierko E, Wojtukiewicz MZ. Platelets and angiogenesis in malignancy. Semin Thromb Hemost. 2004;30(1):95–108. doi: 10.1055/s-2004-822974. [DOI] [PubMed] [Google Scholar]
- 33.Bambace NM, Holmes CE. The platelet contribution to cancer progression. Journal of thrombosis and haemostasis: JTH. 2011;9(2):237–249. doi: 10.1111/j.1538-7836.2010.04131.x. [DOI] [PubMed] [Google Scholar]
- 34.Schlesinger M. Role of platelets and platelet receptors in cancer metastasis. J Hematol Oncol. 2018;11(1):125. doi: 10.1186/s13045-018-0669-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stegner D, Dütting S, Nieswandt B. Mechanistic explanation for platelet contribution to cancer metastasis. Thromb Res. 2014;133:S149–S157. doi: 10.1016/S0049-3848(14)50025-4. [DOI] [PubMed] [Google Scholar]
- 36.Miyashita T, Tajima H, Makino I, Nakagawara H, Kitagawa H, Fushida S, Harmon JW, Ohta T. Metastasis-promoting role of extravasated platelet activation in tumor. J Surg Res. 2015;193(1):289–294. doi: 10.1016/j.jss.2014.07.037. [DOI] [PubMed] [Google Scholar]
- 37.Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer. 2011;11(2):123–134. doi: 10.1038/nrc3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lal I, Dittus K, Holmes CE. Platelets, coagulation and fibrinolysis in breast cancer progression. Breast cancer research: BCR. 2013;15(4):207. doi: 10.1186/bcr3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sato T, Tsujino I, Ikeda D, Ieko M, Nishimura M. Trousseau’s syndrome associated with tissue factor produced by pulmonary adenocarcinoma. Thorax. 2006;61(11):1009–1010. doi: 10.1136/thx.2004.031492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Z, Fang M, Li J, Yang R, Du J, Luo Y. High Platelet Levels Attenuate the Efficacy of Platinum-Based Treatment in Non-Small Cell Lung Cancer. Cellular physiology and biochemistry: international journal of experimental cellular physiology, biochemistry, and pharmacology. 2018;48(6):2456–2469. doi: 10.1159/000492683. [DOI] [PubMed] [Google Scholar]
- 41.Chojkier M. Inhibition of albumin synthesis in chronic diseases: molecular mechanisms. J Clin Gastroenterol. 2005;39(4 Suppl 2):S143–146. doi: 10.1097/01.mcg.0000155514.17715.39. [DOI] [PubMed] [Google Scholar]
- 42.Lam FW, Cruz MA, Leung HC, Parikh KS, Smith CW, Rumbaut RE. Histone induced platelet aggregation is inhibited by normal albumin. Thromb Res. 2013;132(1):69–76. doi: 10.1016/j.thromres.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 43.Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J. 2010;9:69. doi: 10.1186/1475-2891-9-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen S, Yan H, Du J, Li J, Shen B, Ying H, Zhang Y, Chen S. Prognostic significance of pre-resection albumin/fibrinogen ratio in patients with non-small cell lung cancer: A propensity score matching analysis. Clin chim acta. 2018;482:203–208. doi: 10.1016/j.cca.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 45.Mangaraj M, Nanda R, Panda S. Apolipoprotein A-I: A Molecule of Diverse Function. Indian journal of clinical biochemistry: IJCB. 2016;31(3):253–259. doi: 10.1007/s12291-015-0513-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zamanian-Daryoush M, Lindner D, Tallant TC, Wang Z, Buffa J, Klipfell E, Parker Y, Hatala D, Parsons-Wingerter P, Rayman P, et al. The cardioprotective protein apolipoprotein A1 promotes potent anti-tumorigenic effects. J Biol Chem. 2013;288(29):21237–21252. doi: 10.1074/jbc.M113.468967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yao X, Gordon EM, Figueroa DM, Barochia AV, Levine SJ. Emerging Roles of Apolipoprotein E and Apolipoprotein A-I in the Pathogenesis and Treatment of Lung Disease. Am J Respir Cell Mol Biol. 2016;55(2):159–169. doi: 10.1165/rcmb.2016-0060TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cine N, Baykal AT, Sunnetci D, Canturk Z, Serhatli M, Savli H. Identification of ApoA1, HPX and POTEE genes by omic analysis in breast cancer. Oncol Rep. 2014;32(3):1078–1086. doi: 10.3892/or.2014.3277. [DOI] [PubMed] [Google Scholar]
- 49.Chong PK, Lee H, Zhou J, Liu SC, Loh MC, So JB, Lim KH, Yeoh KG, Lim YP. Reduced plasma APOA1 level is associated with gastric tumor growth in MKN45 mouse xenograft model. J Proteomics. 2010;73(8):1632–1640. doi: 10.1016/j.jprot.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 50.Wu J, Wang Y, Li H, Tan W, Chen X, Ye S. Serum apolipoprotein B-to-apolipoprotein A1 ratio is independently associated with disease severity in patients with acute pancreatitis. Sci Rep. 2019;9(1):7764. doi: 10.1038/s41598-019-44244-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.