Abstract
Background
In response to the US opioid epidemic, the Centers for Disease Control and Prevention updated their guideline on prescription opioids for chronic pain management in March 2016. The aim of this study was to provide detailed analysis of trends in opioid claims among cancer patients in the United States during 2013-2018.
Methods
We analyzed pharmaceutical dispensing data from Symphony Health’s Integrated Dataverse database, which covers approximately 80% of the US population. We examined annual trends in dispensed opioids in cancer patients during 2013-2018. We examined quarterly trends of the prevalence, mean number of days, and dose (stated as morphine milligram equivalents) of opioid dispensing in cancer patients.
Results
Dispensing records of an average of over 3.7 million cancer patients contributed to the study annually in 2013-2018. The annual prevalence of opioid dispensing claims declined from 40.2% in 2013 to 34.5% in 2018. Annual declines occurred across cancer sites, and particularly among patients with metastatic cancer (decline of 19.8%), breast cancer (18.2%), and lung cancer (13.8%). By quarter, the prevalence of opioid claims declined statistically significantly from 26.6% in Q1 2013 to 21.2% in Q4 2018; this decline was more pronounced after Q3 2016 (2-sided P = .004). Both quarterly trends in mean days and morphine milligram equivalents of opioids supplied showed a gradual decline from 2013 to 2018, with a slightly larger decline after 2016.
Conclusions
We observed a decline in opioid use among cancer patients, particularly after 2016, coinciding with the publication of the Centers for Disease Control and Prevention’s guideline on prescription opioids for chronic pain management.
Prescription opioid misuse has become an increasing concern in recent years due to rising overdose deaths in the United States (1-3). In 2017, more than 47 000 Americans died from opioid overdoses, including overdose due to prescription opioids, heroin, and illicitly manufactured fentanyl (4). To chart a safer and more effective use of pharmaceutical opioids, the Centers for Disease Control and Prevention (CDC) updated their guidelines on opioid prescribing for chronic pain management in March 2016 (5). These guidelines were intended to improve safety and effectiveness of pain treatment and reduce risks related to long-term opioid therapy (5). For example, when opioids are used, clinicians are recommended to prescribe the lowest effective dosage and carefully reassess benefits and risks when considering increasing dosage to 50 morphine milligram equivalents (MMEs) or more per day (5).
Yet, opioids are also essential medications in the management of certain forms of pain, particularly in the context of cancer care. A recent review found that pain prevalence was 39% among cancer patients after curative treatment, 55% during anticancer treatment, and 66% in advanced, metastatic, or terminal stage (6). Reflecting the importance of opioid medications in cancer care, the CDC’s guidelines were originally specifically noted as not applicable to the use of opioids for the active treatment of cancer, palliative care, or end-of-life. Here, we used Symphony Health Solutions’ large integrated database to identify cancer patients using medical provider diagnosis claims, and to examine dispensed opioid prescriptions within this group using pharmacy claims. The aim of our study was to describe patterns and trends of opioid prescription claims among cancer patients in the United States from 2013 to 2018.
Methods
Data Source
This study was a descriptive analysis of patients contributing pharmaceutical dispensing data from 2013 to 2018. The data source was Symphony Health’s Integrated Dataverse (IDV) database. This large database collates retail pharmacy dispensing and medical provider claims (7) and cross-sectionally covers over 80% of the US population (approximately 280 million people) annually (7). The database includes commercial plans, Medicare Part D, cash, assistance programs, and Medicaid. These claims are open unadjudicated claims. For pharmacy claims, it captures approximately 92% of the retail and 65% specialty pharmacy claims and approximately 68% of mail orders. For medical claims, it covers approximately 60% of professional claims in an outpatient setting. For this analysis, data were deidentified according to Health Insurance Portability and Accountability Act’s deidentification rule (8).
Inclusion Criteria
We included individuals aged 18 years and older who had at least 1 pharmaceutical dispensing record from quarter (Q)1 2013 to Q4 2018 inclusive. To ensure proper trending, only pharmacies that consistently reported data for all months in the study period were included.
Definition of Study Participants and Outcome of Interest
In each calendar year between 2013 and 2018, the date of the first cancer diagnosis claim was identified as the index date and the corresponding quarter as index quarter. Cancer patients were identified as those having an initial cancer diagnosis claim (identified using the International Classification of Diseases criteria; Supplementary Table 1, available online) in a specific year and another diagnosis claim for the same cancer either within the preceding 4 quarters or following 2 quarters of the index quarter. Using the index quarters, we then identified opioid dispensing claims that occurred within the same quarter as the index date or occurred in the quarter following thereafter; this approach was adopted because pain medications and treatments often can occur over a number of months. Cancer patients as defined by our main definition therefore included both newly diagnosed cancer patients and cancer survivors receiving follow-up cancer care. Patients were flagged as metastatic in a particular calendar year if the index cancer claim was a secondary malignant neoplasm International Classification of Diseases code (Supplementary Table 1, available online). For a subgroup analysis, we identified patients who were likely to represent newly diagnosed cancer patients; this was performed using a criterion whereby patients were required to have had no prior diagnostic cancer claims in the year (4 quarters, Q1-Q4) before their index quarter. Supplementary Table 2 (available online) documents a list of opioids analyzed in this study.
Statistical Analysis
We first estimated the annual prevalence of opioid dispensing claims in the entire Symphony Health’s IDV database and compared this with the annual prevalence among cancer patients. Subsequently, we estimated quarterly opioid claims among cancer patients. To do so, we estimated the quarterly prevalence of opioid claims and the mean days of opioid supplied per cancer patient who received the opioid prescription, expressed per patient per quarter, for each quarter from 2013 to 2018 inclusive. We also estimated the dose of opioids per patient per quarter by calculating the daily dose, as quantified using MMEs (9). To determine dosage in MMEs, we multiplied the dose for each opioid claim by the conversion factor provided by the CDC (10). For example, tablets containing oxycodone at a strength of 10 mg for administration 4 times a day would equate to 60 MMEs daily.
We then conducted a joinpoint analysis to estimate the average percent change in the quarterly opioid claim prevalence using Joinpoint software (11). We used a weighted average of the quarterly percentage of change, such that the weights equated to the length of each segment over the fixed interval. To describe the trends of opioid use before and after the 2016 CDC guideline, we stratified the analyses using 2 segments: Q1 2013 to Q3 2016 and Q4 2016 to Q4 2018. Analyses are presented for all opioids combined and for each of 4 commonly prescribed opioid medications: hydrocodone, oxycodone, morphine, and tramadol. A threshold of P less than .05 was used to determine statistical significance using the permutation test. All tests were 2-sided.
Results
Opioid Use in the Overall Symphony Health’s IDV
For each calendar year from 2013 to 2018, dispensing records of approximately 156 million individuals were included. For the entire cohort, the percent change of the overall annual prevalence of dispensed opioid prescriptions declined by 29.2% from 28.4% in 2013 to 20.1% in 2018.
Opioid Use Among Cancer Patients
Characteristics of Cancer Patients and Annual Opioid Use. From 2013 to 2018, for each calendar year we identified an average of 3.7 million individuals with at least 2 records of cancer diagnosis claims. The mean age of identified cancer patients was 66 years, and 47.1% of patients were male (Table 1).
Table 1.
Characteristics of cancer patients, Symphony Health data (2013-2018)
| Characteristics | Year |
|||||
|---|---|---|---|---|---|---|
| 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | |
| (n = 3 730 402) | (n = 3 836 125) | (n = 3 857 693) | (n = 3 681 232) | (n = 3 565 016) | (n = 3 634 356) | |
| Mean age (SD), y | 67 (13) | 65 (13) | 65 (13) | 65 (13) | 66 (13) | 67 (13) |
| Sex, No. (%) | ||||||
| Female | 1 940 622 (52.0) | 2 008 776 (52.4) | 2 046 496 (53.0) | 1 966 363 (53.4) | 1 907 380 (53.5) | 1 930 243 (53.1) |
| Male | 1 789 780 (48.0) | 1 827 349 (47.6) | 1 811 197 (47.0) | 1 714 869 (46.6) | 1 657 636 (46.5) | 1 704 113 (46.9) |
| Cancer site, No. (%) | ||||||
| Breast | 773 415 (20.7) | 807 523 (21.1) | 840 040 (21.8) | 816 686 (22.2) | 797 362 (22.4) | 805 133 (22.2) |
| Prostate | 579 759 (15.5) | 591 142 (15.4) | 579 398 (15.0) | 545 462 (14.8) | 533 496 (15.0) | 560 664 (15.4) |
| Colorectal | 227 925 (6.1) | 233 207 (6.1) | 239 425 (6.2) | 231 686 (6.3) | 224 059 (6.3) | 224 513 (6.2) |
| Lung | 233 857 (6.3) | 235 261 (6.1) | 238 126 (6.2) | 230 713 (6.3) | 225 692 (6.3) | 230 468 (6.3) |
| Leukemia | 138 442 (3.7) | 145 096 (3.8) | 153 946 (4.0) | 152 065 (4.1) | 152 710 (4.3) | 156 808 (4.3) |
| Bladder | 137 031 (3.7) | 142 093 (3.7) | 137 164 (3.6) | 123 455 (3.4) | 116 509 (3.3) | 119 361 (3.3) |
| Disease stage, No. (%) | ||||||
| Metastasis present | 482 400 (12.9) | 543 153 (14.2) | 601 781 (15.6) | 635 310 (17.3) | 644 239 (18.1) | 677 405 (18.6) |
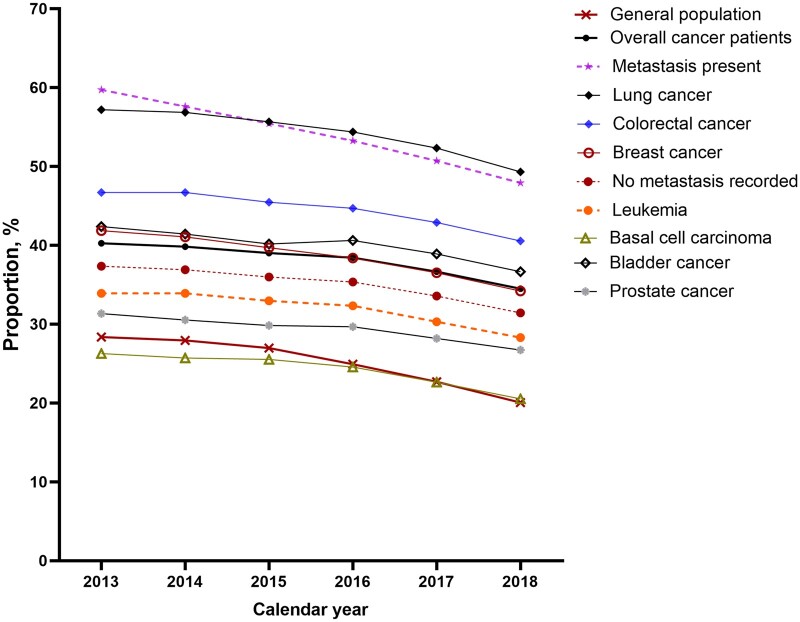
Among cancer patients, the prevalence of opioid claims was highest among those recorded as having metastasis. When considered according to cancer site, the prevalence of opioid claims was highest among patients with lung cancer, followed by bladder cancer and breast cancer, with the lowest prevalence occurring in patients with basal cell carcinoma. From 2013 to 2018, the percent change of the annual prevalence of opioid claims declined for overall cancer by 14.2% from 40.2% in 2013 to 34.5% in 2018 (Figure 1). Declines occurred across cancer sites, with the largest declines observed among patients with metastatic cancer (decline of 19.8% from 59.7% in 2013 to 47.9% in 2018) and breast cancer (decline of 18.2% from 41.8% in 2013 to 34.2% in 2018). For lung cancer, the prevalence of opioid claims declined by 13.8% from 57.2% in 2013 to 49.3% in 2018 (Figure 1).
Figure 1.
Annual prescription rate of opioids by groups, 2013 to 2018.
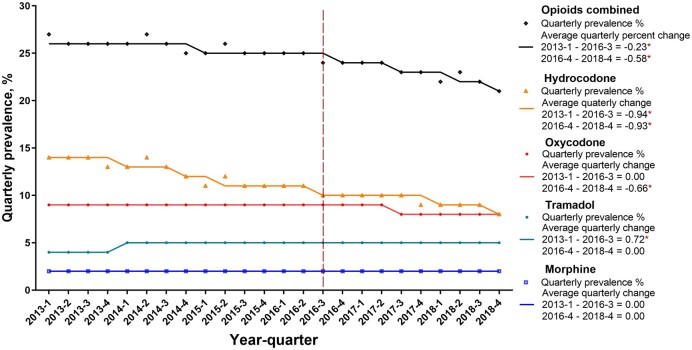
Trends of Quarterly Opioid Use By Rates, Mean Days of Supplied, and Dose of Supplied. Next, we examined trends in opioids dispensed to cancer patients considering the calendar quarter of the cancer claim plus the following calendar quarter. From Q1 2013 to Q4 2018, the prevalence of opioid claims declined statistically significantly from 26.6% in Q1 2013 to 21.2% in Q4 2018, with a more pronounced decline occurring after Q3 2016 (average quarterly percent change = −22.5% in Q1 2013 to Q3 2016, P = .001; and −57.8% in Q4 2016 to Q4 2018, P = .004) (Figure 2). Compared with other types of opioids, hydrocodone was associated with the largest decline in the quarterly prevalence; this fell from 14.0% in Q1 2013 to 8.4% in Q4 2018 (−93.9% in Q1 2013 to Q3 2016, P < .001; −92.9% in Q4 2016 to Q3 2018, P = .007). Oxycodone, the second-most frequently dispensed opioid, also showed statistically significant declines in the prevalence of claims; the prevalence dropped from 8.9% in Q1 2013 to 7.5% in Q4 2018, though this decline occurred after 2016 (0% in Q1 2013 to Q3 2016; −66.3% in Q4 2016 to Q4 2018, P = .02). By contrast, morphine remained relatively stable at a quarterly claim rate of 1.7% during the study period (Figure 2).
Figure 2.
Quarterly prescription rate of opioids in cancer patients, 2013-2018. An asterisk (*) indicates statistical significance. A threshold of P less than .05 was used to determine statistical significance using the permutation test. All tests were 2-sided.
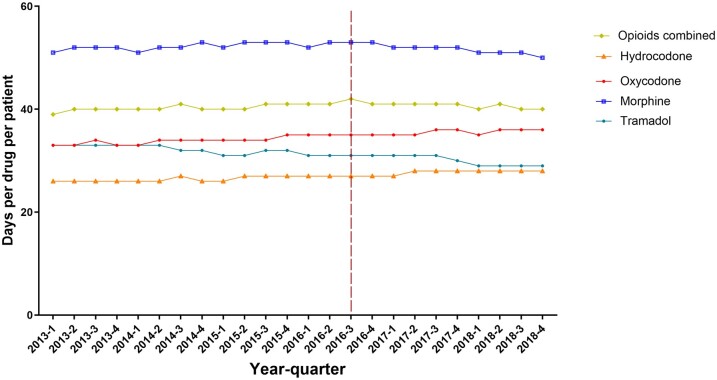
Trends in the mean number of days of opioids supplied per claim showed a gradual decline from 2013 to 2018, with a slightly larger decline after 2016. The mean number of days supplied per claim increased from 39 days per patient in Q1 2013 to 42 days in Q3 2016 and then declined to 40 days per patient in Q4 2018 (Figure 3). Compared with other type of opioids, morphine claims displayed the highest mean days of supply (52 days per patient per quarter, SD = 0.83). However, this declined from 53 days per patient per quarter in Q4 2016 to 50 days in Q4 2018. Tramadol had a similar decline from 31 days per patient per quarter in Q4 2016 to 29 days per patient per quarter in Q4 2018. By contrast, the mean days supplied increased for hydrocodone from 26 days per patient per quarter in Q1 2013 to 28 days per patient per quarter in Q4 2018 and oxycodone from 33 days per patient per quarter in Q1 2013 to 36 days per patient per quarter in Q4 2018 (Figure 3).
Figure 3.
Cancer patients, 2013-2018: total days of supply per patient per quarter overall and for individual opioid drugs.
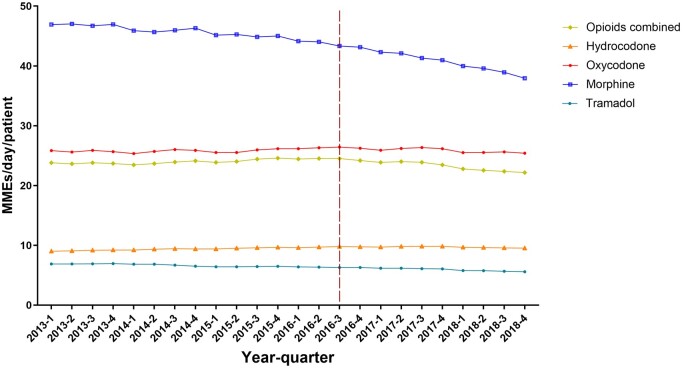
The average MMEs per dispensing claim per patient also showed a gradual decline from 2013 to 2018, with a slightly larger decline after 2016. For all opioids combined, mean MMEs dispensed increased from 23.8 per patient per quarter in Q1 2013 to 24.5 per patient per quarter in Q3 2016 and then declined to 22.2 per patient per quarter in Q4 2018 (Figure 4). Claims for morphine had the highest MMEs (mean = 43.7, SD = 2.75) per patient per quarter in the overall period 2013-2018. However, claims for morphine showed a decline particularly after the Q3 2016, falling to 37.9 per patient per quarter in Q4 2018. By contrast, MMEs for oxycodone increased from 25.8 per patient per quarter in Q1 2013 to 26.4 per patient per quarter in Q3 2016 and then declined to 25.4 per patient per quarter in Q4 2018. A similar trend was observed for hydrocodone (Figure 4).
Figure 4.
Cancer patients, 2013-2018: average morphine milligram equivalents (MMEs) supplied per day per patient for each quarter overall and for individual opioid drugs.
Opioid Use Among Newly Diagnosed Cancer Patients
For each calendar year from 2013 to 2018, we identified an average of 1 590 205 newly diagnosed cancer patients. We observed similar trends and patterns of opioid dispensing among newly diagnosed cancer patients compared with those observed in cancer patients overall, with slight variations in the mean days of supply of opioids and in the MMEs per patient per quarter. From 2013 to 2018, the prevalence of opioid claims declined by 21.6% among newly diagnosed cancer patients, from 26.4% in Q1 2013 to 20.7% in Q4 2018 (data not shown). This rate of decline tripled after Q3 2016 (average quarterly percent change: −18.1% in Q1 2013 to Q3 2016, P = .01; and −62.9% in Q4 2016 to Q4 2018, P = .04) (Supplementary Figure 1, available online). The mean days of opioids supplied was 32.6 (SD = 0.75) per patient per quarter, which was slightly shorter than that of the overall cancer patients (data not shown). The days of opioid supplied per patient per quarter remained stable between 2013 and Q3 2016 but declined from 33 days per patient per quarter in Q4 2016 to 32 days in Q4 2018 (Supplementary Figure 2, available online). Between 2013 and 2018, the mean MMEs of opioid dispensing was 50.4 (SD = 2.5) per patient per quarter among newly diagnosed cancer patients, and the MMEs declined dramatically after the Q3 2016 from 54.4 per patient per quarter in Q4 2016 to 48.8 in Q4 2018 (Supplementary Figure 3, available online). In addition, the mean MMEs of morphine dispensing declined by 15% from 118.9 per patient per quarter in Q3 2016 to 101.6 per patient per quarter in Q4 2018 (Supplementary Figure 3, available online).
Discussion
This analysis of dispensing records considered approximately 156 million individuals and 3.7 million cancer patients each year between 2013 and 2018. Among individuals overall, we found a 29% decline from 2013 to 2018 in the prevalence of annual opioid dispensing claims, and among cancer patients we found a 14% decline in the prevalence of annual opioid dispensing claims. Among cancer patients, the largest decline occurred after 2016, coinciding with the publication of the CDC’s guideline on prescription opioids for chronic pain management. Declines occurred across the different cancer sites considered but were largest among patients with metastatic status and breast cancer.
In the United States, opioid regulations have become increasingly stringent due to the opioid epidemic (12). In line with our results, a recent study of physicians using US Medicare and Medicaid Part D prescriber data found a 21% decline in opioid prescribing among oncologists between 2013 and 2017 (13). Moreover, we observed differing trends in the prescription by opioid types. Despite a relatively low and stable prevalence for morphine claims, there were small declines after 2016 in the days and dosage supplied for this drug. Similarly, the prevalence and dosage supplied per dispensing claim for oxycodone also declined statistically significantly among cancer patients during the same study period. It is plausible that these declines are related to concerns regarding unintended overdose from long-acting opioid use (14).
Although declines in dispensing claims were predominantly observed after 2016, we observed a consistent decline in claims for hydrocodone after 2014. This decline may be related to the introduction of the 2014 federal policy restricting hydrocodone combination products (HCP) (15,16). At the time, the Drug Enforcement Administration reclassified all HCP from schedule III to schedule II, thereby limiting all new HCP prescriptions to a maximum of a 30-day supply with no refills (16). Following this policy, reductions in hydrocodone use have been reported across various settings in the United States (17-19). A recent study using data from a US national commercial health insurance program showed a 26% decline in HCP prescriptions from June 2013 to June 2015 (20). This study also reported larger absolute decreases in HCP prescribing rates in patients being treated for cancer (20).
Our study benefits from the data source providing wide population coverage, which allowed for nationwide estimates of opioid use among cancer patients in the United States. However, our study has several limitations. First, we did not have data on severity of pain, nor were we able to comment on the appropriateness of the prescription of individual claims. Second, analysis was based on dispensing claims data and although such data better approximate usage than prescription data, some patients may have elected not to use a dispensed medication that they have received; as such, true uptake of opioids at the patient level is not known. Third, we examined opioid claims occurring among cancer patients. However, we do not know that these claims were specifically to manage patient’s cancer pain as opposed to use for other conditions. Nevertheless, we did observe similar patterns when we restricted our analysis from opioids dispensed in the same calendar year of cancer claim to opioids dispensed to the quarter and adjacent quarter of a cancer treatment claim. Fourth, because we used diagnosis claims data to identify cancer patients, this may have resulted in underreporting of data for cancer patients (21). However, claims-based definitions of cancers have been reported as demonstrating high specificity (22). We also note that in our study, we were unable to distinguish newly diagnosed cancer patients from patients who were in long-term remission and may have had a renewed need for opioid treatment that may or may not have been associated with cancer. Finally, information on race and ethnicity and socioeconomic status was not available in our analysis. Further studies are needed to confirm previously reported associations between racial-ethic and sociodemographic factors and opioid prescription (23).
In conclusion, we observed a large decline in opioid use among cancer patients, particularly after the introduction of guidelines on prescription opioids for chronic pain management in 2016.
Funding
This study was funded by the Intramural Research Program of the National Cancer Institute.
Notes
Role of the funder: The study funder had no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
Disclosures: The authors have no conflict of interest to disclose.
Author contributions: Conception and design: YC, SS, MSS, LY, DQ, ABG, NDF; Development of methodology: YC, SS, MSS, LY, DQ, ABG, NDF; Acquisition of data: SS, LY, DQ, NDF; Analysis: YC; Interpretation of data: YC, SS, MSS, LY, DQ, ABG, NDF; Drafting of the manuscript: YC; Review, and/or revision of the manuscript: YC, SS, MSS, LY, DQ, ABG, NDF; Study supervision: NDF.
Data Availability
Data are available upon request to the corresponding author at yingxi.chen@nih.gov.
Supplementary Material
References
- 1. Shiels MS, Tatalovich Z, Chen Y, et al. Trends in mortality from drug poisonings, suicide, and alcohol-induced deaths in the United States from 2000 to 2017. JAMA Netw Open. 2020;3(9):e2016217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen Y, Shiels MS, Thomas D, et al. Premature mortality from drug overdoses: a comparative analysis of 13 organisation for economic co-operation and development member countries with high-quality death certificate data, 2001 to 2015. Ann Intern Med. 2019;170(5):352–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dart RC, Surratt HL, Cicero TJ, et al. Trends in opioid analgesic abuse and mortality in the United States. N Engl J Med. 2015;372(3):241–248. [DOI] [PubMed] [Google Scholar]
- 4. National Vital Statistics System, Mortality. CDC WONDER, Atlanta, GA: US Department of Health and Human Services, CDC; 2020. https://wonder.cdc.gov. Accessed March 17, 2020.
- 5. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65(RR-1):1–49. [DOI] [PubMed] [Google Scholar]
- 6. van den Beuken-van Everdingen MH, Hochstenbach LM, Joosten EA, et al. Update on prevalence of pain in patients with cancer: systematic review and meta-analysis. J Pain Symptom Manage. 2016;51(6):1070–1090.e9. [DOI] [PubMed] [Google Scholar]
- 7. Deep insight into the pharmaceutical market. Integrated Dataverse; 2016. https://symphonyhealth.com/product/idv/. Accessed July 3, 2019.
- 8.Centers for Disease Control and Prevention. Health Insurance Portability and Accountability Act of 1996 (HIPAA). https://www.cdc.gov/phlp/publications/topic/hipaa.html. Accessed April 13, 2020.
- 9.Centers for Disease Control and Prevention. Analyzing prescription data and morphine milligram equivalents (MME). https://www.cdc.gov/opioids/data-resources/index.html. Accessed April 13, 2020.
- 10. CDC Guideline for Prescribing Opioids for Chronic Pain. https://www.cdc.gov/drugoverdose/prescribing/guideline.html. Accessed April 13, 2020.
- 11. National Cancer Institute. Joinpoint Trend Analysis Software. https://surveillance.cancer.gov/joinpoint/. Accessed April 17, 2019.
- 12. Bernard SA, Chelminski PR, Ives TJ, et al. Management of pain in the United States-a brief history and implications for the opioid epidemic. Health Serv Insights. 2018;11:1178632918819440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jairam V, , YangDX, , Pasha S,. et al. Temporal trends in opioid prescribing patterns among oncologists in the Medicare population. J Natl Cancer Inst. 2021;113(3):274–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Haider A, Zhukovsky DS, Meng YC, et al. Opioid prescription trends among patients with cancer referred to outpatient palliative care over a 6-year period. JOP. 2017;13(12):e972–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Office of Diversion Control DCES. Hydrocodone. Drug Enforcement Administration; 2014. https://www.deadiversion.usdoj.gov/drug_chem_info/hydrocodone.pdf. Accessed April 13, 2020.
- 16. U.S. Department of Justice. Drug Enforcement Administration [Docket No. DEA 389] Final Rule. Schedules of controlled substances: rescheduling of hydrocodone combination products from schedule III to schedule II. 21 CFR part 1308. Fed Regist. 2014;79(163):49661–49682. https://www.deadiversion.usdoj.gov/fed_regs/rules/2014/fr0822.htm. [PubMed] [Google Scholar]
- 17. Seago S, Hayek A, Pruszynski J, et al. Change in prescription habits after federal rescheduling of hydrocodone combination products. Proc (Bayl Univ Med Cent). 2016;29(3):268–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schultz S, Chamberlain C, Vulcan M, et al. Analgesic utilization before and after rescheduling of hydrocodone in a large academic level 1 trauma center. J Opioid Manag. 2016;12(2):119–122. [DOI] [PubMed] [Google Scholar]
- 19. Jones CM, Lurie PG, Throckmorton DC.. Effect of US Drug Enforcement Administration's rescheduling of hydrocodone combination analgesic products on opioid analgesic prescribing. JAMA Intern Med. 2016;176(3):399–402. [DOI] [PubMed] [Google Scholar]
- 20. Raji MA, Kuo YF, Adhikari D, et al. Decline in opioid prescribing after federal rescheduling of hydrocodone products. Pharmacoepidemiol Drug Saf. 2018;27(5):513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McClish DK, Penberthy L, Whittemore M, et al. Ability of Medicare claims data and cancer registries to identify cancer cases and treatment. Am J Epidemiol. 1997;145(3):227–233. [DOI] [PubMed] [Google Scholar]
- 22. Setoguchi S, Solomon DH, Glynn RJ, et al. Agreement of diagnosis and its date for hematologic malignancies and solid tumors between Medicare claims and cancer registry data. Cancer Causes Control. 2007;18(5):561–569. [DOI] [PubMed] [Google Scholar]
- 23. Pletcher MJ, Kertesz SG, Kohn MA, et al. Trends in opioid prescribing by race/ethnicity for patients seeking care in US emergency departments. JAMA. 2008;299(1):70–78. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon request to the corresponding author at yingxi.chen@nih.gov.