Abstract
Background
We aimed to assess the utility of HbA1c in the early detection of gestational diabetes (GDM) in the first trimester.
Methods
This prospective study was performed on 700 pregnant women in the perinatology clinic at a tertiary university hospital from March 2018 to March 2020. For all pregnant women, HbA1c and fasting blood glucose (FBG) levels were examined during the first trimester. Then, a GDM screening test was done within 24–28 weeks of pregnancy using a 100 g oral glucose tolerance test (OGTT) as the gold standard test. The GDM diagnosis was made according to the American Diabetes Association (ADA) criteria. Sensitivity, specificity, positive (PPV), and negative predictive value (NPV) of HbA1c and FBG were calculated using the receiver operating characteristic (ROC) curve.
Results
Of 700 participants, one hundred and fifteen (16.4%) women had GDM. The GDM patients were significantly older and had a higher pre-gestational body mass index and pregnancy weight gain compared to the non-GDM participants. The sensitivity and specificity for ruling out GDM at an HbA1c cut-off value of 4.85% was 92.2 and 32.8%, respectively, with a 95.5% NPV and a 21.2% PPV. Furthermore, sensitivity and specificity for diagnosing GDM at an HbA1c cut-off value of 5.45% was 54.8 and 96.8%, respectively, with a 91.5% NPV and a 76.8% PPV. Using HbA1c could decline OGTT in 40.4% of the pregnant women (28.7% with HbA1c < 4.85 and 11.7% with HbA1c ≥ 5.45%).
Conclusion
It seems that the first-trimester HbA1c cannot replace OGTT for the diagnosis of GDM because of its insufficient sensitivity and specificity. However, women with higher first-trimester HbA1c had a high risk for GDM incidence.
Keywords: Gestational diabetes mellitus, OGTT, HbA1c, Screening
Introduction
Gestational diabetes mellitus (GDM) or diabetes mellitus in pregnancy is the most prevalent metabolic abnormality during pregnancy and is defined as diabetes first detected at any time during pregnancy [1, 2].
The prevalence of GDM is on the rise since the past decades, with an overall frequency of 17.8% (range 9.3–25.5%) [3]. The possible causes for this enhancement are the rise in maternal age and body mass index (BMI), access to the prenatal screening test, and changes in diabetes diagnostic thresholds [2].
GDM can cause severe obstetrics complications, which affect both mothers and their offspring. A higher risk of preeclampsia and cesarean section rate, fetal growth abnormalities, macrosomia prematurity, neonatal hypoglycemia, as well as maternal and neonatal trauma are some common examples of GDM [4, 5].
Former evidence indicated some important risk factors for GDM in pregnant women, including BMI > 38.6 kg/m2, fasting glucose > 4.5 mmol/L (about 81 mg/dL), abdominal circumference > 91.5 cm, and presence of polycystic ovary syndrome (PCOS). Moreover, there is a significant increase in the GDM incidence if some of these risk factors coexist simultaneously [6].
Among the abovementioned risk factors, fasting glucose (FG) of 5.6 to 7 mmol/L, at the first prenatal visit was strongly associated with some adverse pregnancy outcomes such as shoulder dystocia/birth injury (OR 24.5; 95% CI: 2.8–214.8), and preeclampsia (OR 2.7; 95% CI: 1.2–5.9) even after adjustment for maternal age and BMI [7].
The oral glucose tolerance test (OGTT) is a well-known screening test for the diagnosis of GDM in pregnant women. However, the deficiencies of this test include the need for eight-hour fasting, obtaining at least two blood samples, vomiting, and its high variability. Hence, about 10% of pregnant women fail to complete their OGTT process [8–11].
Glycosylated hemoglobin (HbA1c) is mainly used to detect and manage type 2 diabetes mellitus. Compared to FBS and OGTT, the HbA1c test shows the two to three previous months’ mean glucose concentration. Fasting is not required for this measurement and has a lower intra-individual variability, which causes the test to be more acceptable for the patients [12, 13].
Although it seems HbA1c is an easy, acceptable, and helpful lab test in GDM diagnosis, first-trimester HbA1c usefulness to detect women who will develop GDM has not been evaluated sufficiently, and it has not been mentioned yet in any of the current guidelines for GDM [5, 14, 15].
Therefore, we aimed to assess the diagnostic profile of the first-trimester HbA1c in the early detection of GDM by determining the cut-off levels for ruling out and diagnosing first-trimester GDM.
Method
This prospective study was included 760 pregnant women who presented for their regular pregnancy care to the perinatology clinic at a tertiary university hospital, Yas hospital, from March 2018 to March 2020.
All singleton pregnant women older than 18 years, referred to our clinic within their first trimester of pregnancy (by the end of the 12th week of their gestational age), were included in this study. Pregnant women with type I or II diabetes mellitus, multiple pregnancies, spontaneous abortions, and elective termination of pregnancies were excluded. Furthermore, the patients who withdrew at any phase during the study were excluded. Forty women were excluded because of type II diabetes mellitus and 20 women withdrew to participate in the study.
A detailed history of all the patients was recorded at baseline. The first trimester HbA1c and fasting blood glucose (FBG) levels were requested for all participants. For measuring FBG, a venous blood sample was obtained from every participant in the morning and after 9 h of fasting. FBG was determined using commercially available laboratory kits via enzymatic methods and spectrophotometry techniques.
Between the 24th and 28th week of gestation, a Glucose Challenge Test (GCT) (blood glucose one hour post-50-g glucose without fasting) was requested for all participants. All tests were performed in the laboratory of Yas Hospital. In participants with abnormal blood glucose levels (GCT ≥140), OGTT with 100 g glucose after 8–10 h of overnight fasting was requested, and blood glucose levels were measured before and 1, 2, and 3 h after administration of 100 g of glucose.
The GDM diagnosis was made according to American Diabetes Association (ADA) criteria (having two or more plasma glucose levels higher than these cut-offs), FBS ≥ 95 mg/dl, BS one hour after 100 g glucose ≥180 mg/dl, BS two hours after 100 g glucose≥155 mg/dl, BS three hours after 100 g glucose ≥140 mg/dl [13].
Women diagnosed with GDM, overt diabetes (HbA1c ≥ 6.5% or/and FBS ≥126 mg /dl) at any time of our study were referred to an endocrinologist for immediate counseling and treatment. The women with an HbA1c < 6.5% received no extra treatment or additional testing. In addition, anemia was defined as a hemoglobin level less than 11 g/dl.
Statistical analysis
The data were analyzed with the statistical software package IBM SPSS Statistic version 24.0. The quantitative variables were presented as mean ± standard deviation and the categorical variables as frequency (percentages). The quantitative variables with normal distribution were compared between the groups using an independent T-test, and the Chi-square test was used to compare the categorical variables. Receiver operating characteristic (ROC) curve was applied for sensitivity, specificity, positive (PPV), and negative predictive value (NPV) calculation of distinct first-trimester HbA1c cut-off.
Results
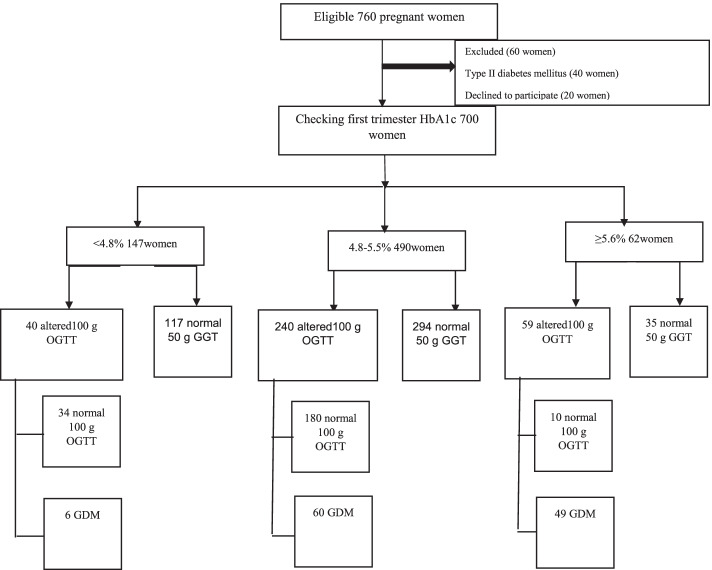
Of 700 participants, 115 (16.4%) women were diagnosed with GDM during the study (Fig. 1). Women with GDM had significantly older age, higher pre-gestational body mass index (BMI), and pregnancy weight gain compared to the non-GDM pregnant women. All the baseline characteristics of the pregnant women are summarized in Table 1.
Fig. 1.
Flow chart of the study protocol. OGTT: Oral Glucose Tolerance Test. GDM: Gestational Diabetes Mellitus
Table 1.
The study population’s information
| Variables | GDM | No GDM | P value |
|---|---|---|---|
| (n = 115) | (n = 585) | ||
| Age, years | 32.64 ± 5.49 | 30.64 ± 5.17 | < 0.001 |
| Gestational age, weeks | 10.2 ± 2.05 | 10.64 ± 1.66 | 0.033 |
| Pre-pregnancy BMI, kg/m2 | 27.23 ± 3.93 | 24.89 ± 2.93 | < 0.001 |
| Pregnancy weight gain, kg | 10.28 ± 2.3 | 9.64 ± 1.53 | 0.005 |
| 1st trimester FBG, mg/dl | 92.01 ± 7.79 | 82.61 ± 6.46 | < 0.001 |
| 1st trimester HbA1c, % | 5.45 ± 0.39 | 4.96 ± 0.30 | < 0.001 |
| Hemoglobin, g/dl | 13.05 ± 0.85 | 13.06 ± 0.96 | 0.938 |
| MCV, fl | 84.11 ± 4.53 | 84.40 ± 3.41 | 0.516 |
| Previous GDM | 14 (12.1) | 8 (1.3) | < 0.001 |
| Family history of DM | 53 (46.1) | 122 (20.8) | < 0.001 |
| Previous macrosomia | 6 (5.2) | 5 (0.8) | 0.001 |
| Physical activity | |||
| Bed rest | 0 (0) | 4 (0.7) | 0.003 |
| Inter home activity | 73 (63.5) | 274 (46.8) | |
| Extra home activity | 42 (36.5) | 307 (52.5) | |
| PCO/Metabolic syn. | 32 (27.8) | 38 (6.4) | < 0.001 |
| Anemia | 12 (10.4) | 59 (10.1) | 0.910 |
| Birth weight, g | 3519.13 ± 301.91 | 3307.06 ± 192.46 | < 0.001 |
GDM gestational diabetes mellitus, BMI body mass index, FBG fasting blood glucose, HbA1c glycosylated hemoglobin, MCV mean corpuscular volume, PCO Polycystic ovary syndrome
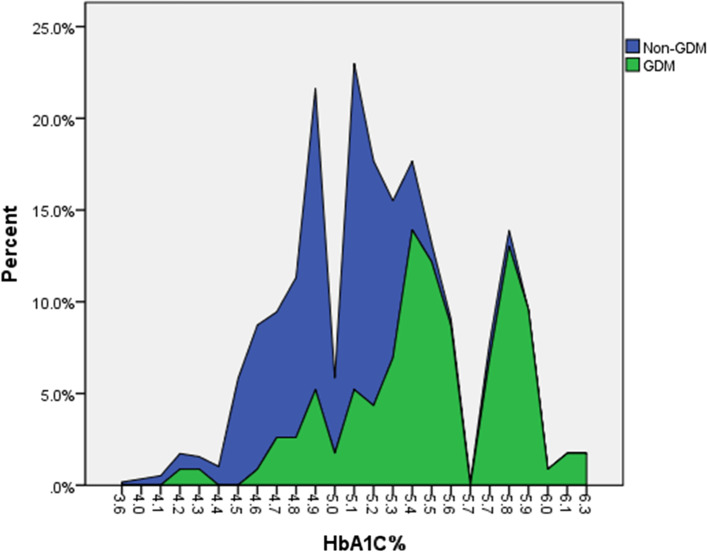
In pregnant women with GDM, the average HbA1c level was 5.45 ± 0.39% compared to 4.96 ± 0.30% in the women without GDM (P < 0.001). In addition, HbA1c overlap in women with and without GDM.s is depicted in Fig. 2. Use of HbA1c could decrease requesting OGTT in 40.4% of the pregnant women (28.70% with HbA1c < 4.85 and 11.7% with HbA1c ≥ 5.45%).
Fig. 2.
HbA1c distribution in women with and without gestational diabetes (GDM)
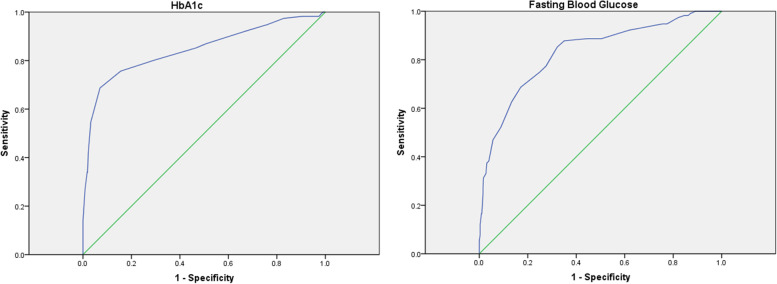
The area under the ROC curve for diagnosing GDM by HbA1c was 0.84 (95% CI: 0.79–0.89; P < 0.001) (Fig. 3). The sensitivity and specificity for ruling out GDM at an HbA1c cut-off value of 4.85% was 92.2 and 32.8%, respectively, with a 95.5% NPV and a 21.2% PPV. Furthermore, sensitivity and specificity for diagnosing GDM at an HbA1c cut-off value of 5.45% was 54.8 and 96.8%, respectively, with a 91.5% NPV and a 76.8% PPV. The diagnostic profile of HbA1c is shown in Table 2.
Fig. 3.
Receiver operating characteristic (ROC) curve for sensitivity and specificity according to different HbA1c and fasting blood glucose thresholds
Table 2.
The diagnostic profile of HbA1c
| HbA1c (%) | Sensivity | Specifity | NPV | PPV |
|---|---|---|---|---|
| 2.60 | 100 | 0 | – | 16.4 |
| 3.80 | 100 | 0.2 | 100 | 16.4 |
| 4.05 | 100 | 0.5 | 100 | 16.4 |
| 4.15 | 100 | 1.0 | 100 | 16.5 |
| 4.25 | 99.1 | 1.9 | 91.6 | 16.5 |
| 4.35 | 98.3 | 2.6 | 88.2 | 16.5 |
| 4.45 | 98.3 | 3.6 | 91.3 | 16.6 |
| 4.55 | 98.3 | 9.4 | 96.4 | 17.5 |
| 4.65 | 97.4 | 17.3 | 97.1 | 18.7 |
| 4.75 | 94.8 | 24.1 | 95.9 | 19.7 |
| 4.85 | 92.2 | 32.8 | 95.5 | 21.2 |
| 4.95 | 87.0 | 49.2 | 95 | 25.1 |
| 5.05 | 85.2 | 53.3 | 94.8 | 26.4 |
| 5.15 | 80.0 | 71.1 | 94.7 | 35.2 |
| 5.25 | 75.7 | 84.4 | 94.6 | 48.8 |
| 5.35 | 68.7 | 93.0 | 93.7 | 65.8 |
| 5.45 | 54.8 | 96.8 | 91.5 | 76.8 |
| 5.55 | 42.6 | 97.8 | 89.6 | 79 |
| 5.63 | 33.9 | 98.1 | 88.3 | 78 |
| 5.68 | 33.9 | 98.3 | 88.3 | 79.5 |
| 5.75 | 27.0 | 99.1 | 87.3 | 86.1 |
| 5.85 | 13.9 | 100 | 85.5 | 100 |
| 5.95 | 4.3 | 100 | 84.1 | 100 |
| 6.05 | 3.5 | 100 | 84 | 100 |
| 6.20 | 1.7 | 100 | 83.8 | 100 |
| 7.30 | 0 | 100 | 83.5 | – |
HbA1c glycosylated hemoglobin, NPV negative predictive value, PPV positive predictive value
The area under the ROC curve for diagnosing GDM by FBG was 0.83 (95% CI: 0.78–0.87; P < 0.001) (Fig. 3). The diagnostic profile of FBG is shown in Table 3.
Table 3.
The diagnostic profile of fasting blood glucose
| Fasting blood glucose (mg/dl) | Sensivity | Specifity |
|---|---|---|
| 58.00 | 100.00 | 0.00 |
| 62.00 | 100.00 | 0.17 |
| 65.50 | 100.00 | 0.34 |
| 66.50 | 100.00 | 0.68 |
| 67.50 | 100.00 | 0.85 |
| 68.50 | 100.00 | 1.03 |
| 69.50 | 100.00 | 2.22 |
| 70.50 | 100.00 | 5.64 |
| 71.50 | 100.00 | 6.32 |
| 72.50 | 100.00 | 9.23 |
| 73.50 | 100.00 | 10.77 |
| 74.50 | 99.13 | 12.82 |
| 75.50 | 98.26 | 13.85 |
| 76.50 | 98.26 | 15.38 |
| 77.50 | 97.39 | 17.95 |
| 78.50 | 94.78 | 22.56 |
| 79.50 | 94.78 | 24.44 |
| 80.50 | 93.04 | 33.68 |
| 81.50 | 92.17 | 38.29 |
| 82.50 | 88.70 | 49.57 |
| 83.50 | 88.70 | 55.56 |
| 84.50 | 87.83 | 64.96 |
| 85.50 | 85.22 | 67.86 |
| 86.50 | 77.39 | 72.48 |
| 87.50 | 74.78 | 75.21 |
| 88.50 | 68.70 | 82.91 |
| 89.50 | 62.61 | 86.67 |
| 90.50 | 52.17 | 91.11 |
| 91.50 | 46.96 | 94.36 |
| 92.50 | 38.26 | 96.07 |
| 93.50 | 37.39 | 96.92 |
| 94.50 | 33.04 | 97.26 |
| 95.50 | 31.30 | 98.29 |
| 96.50 | 26.96 | 98.46 |
| 97.50 | 25.22 | 98.46 |
| 98.50 | 21.74 | 98.63 |
| 99.50 | 16.52 | 98.97 |
| 100.50 | 16.52 | 99.15 |
| 101.50 | 12.17 | 99.66 |
| 102.50 | 7.83 | 99.66 |
| 104.00 | 5.22 | 100.00 |
| 106.00 | 2.61 | 100.00 |
| 112.00 | 1.74 | 100.00 |
| 118.00 | 0.00 | 100.00 |
Discussion
The prevalence of GDM in our study was 16.4%, which was in reported ranges of HAPO Study Cooperative Research Group [3], but it was higher compared to some former studies [5, 16]. This variation is because of referring high-risk pregnant women to our hospital, using different GDM diagnostic thresholds, and different screening OGTTs in studies. For instance, we used the Carpenter-Coustan threshold, which has a lower threshold for the GDM detection compared to the National diabetes data group that applied in Benaiges et al. study [5].
In this study, we found that the average first-trimester FBG and HbA1c of GDM women were significantly higher compared with normoglycaemic women, which was similar to previous studies [5, 17].
The women with HbA1c greater than 6% were at a higher risk for GDM, and those with HbA1c less than 4.5% were not complicated by GDM. Although in our study, HbA1c in GDM women fell within the range of former studies, HbA1c in normoglycaemic women was lower the average HbA1c concentration reported in Asian Indian pregnant women, 5.36 ± 0.36% [18, 19]..
This study has shown excellent reliability of HbA1c for the GDM diagnosis with an AUC of 0.84. This finding was in line with some previous studies [20–22] such as a recent meta-analysis evaluating 6406 pregnant women [23] in which the AUC values ranged from 0.80 to 0.93 and in contrast with the other ones [12, 24–28].
GDM has public health implications and the early detection of it is clinically essential. Women with GDM are at risk for developing type 2 diabetes mellitus and impaired glucose tolerance and have a higher risk for cardiovascular diseases along with their life [16].
The appropriate test for GDM diagnosis is a test with a high sensitivity to diagnose the patients and high specificity, but this could not be observed in the HbA1c test. As found in our study, by increasing HbA1c, the sensitivity decreased, and the specificity increased. Although using the higher 5.45% and lower 4.96% cut-off values for HbA1c could help, distinguish the pregnant women with a higher and lower risk for GDM, respectively [29].
Despite the acceptance of HbA1c among pregnant women and its advantages over other GDM diagnostic methods, such as its less intra-individual coefficient of variation of 1.9 to 4.2% [30], as our study demonstrated, HbA1c had a significant overlap in both normal and GDM groups.
Another challenge with HbA1c is its significantly decreasing in pregnancy, a decline of the upper normal level of HbA1c from 6.3 to 5.7% in early pregnancy and to 5.6% in the third trimester of pregnancy, indicating a reduction of HbA1c during normal pregnancy that is of clinical importance when defining the goal for HbA1c during pregnancy complicated with diabetes [31].
Furthermore, physiological hydremia during pregnancy, anemia, slower intestinal transition, increased red cell turnover, and nutritional alternations are factors that can considerably affect the HbA1c value [32]. For these reasons, there is no guideline using HbA1c for the diagnosis of GDM.
The advantage of our research was the large sample. Furthermore, all information was gathered from the same lab and the same clinic. However, our study had some limitations. For instance, we did not evaluate the cost-saving potentials while using HbA1c for screening GDM. In addition, there are possible variations in the degree of HbA1c, independent of glycemia, which could be associated with family history or genetics. In this study, we did not include these factors.
Conclusion
It seems that the first-trimester HbA1c, because of its insufficient sensitivity or specificity, cannot replace OGTT for the diagnosis of GDM. However, women with higher first-trimester HbA1c have a high risk for GDM.
Acknowledgements
Not applicable.
Authors’ contributions
“M.V. Project development, Z.B. Data collection and management, E.F. Data analysis and Manuscript writing and F.G. Manuscript editing. All authors reviewed the manuscript.” The author(s) read and approved the final manuscript.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed in this study will be made attainable from the corresponding author upon request.
Declarations
Ethics approval and consent to participate
All methods were performed in accordance with the relevant guidelines and regulations (Declaration of Helsinki). Ethical approval was received by the Imam Khomeini hospital ethics board (IR.TUMS.IKHC.REC. 1398.717). The patients’ data were used anonymously and confidentially and all participants signed informed consent.
Consent for publication
The study patients filled informed consent for the publication of their data anonymously.
Competing interests
The authors declare they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hod M, Kapur A, Sacks DA, Hadar E, Agarwal M, Di Renzo GC, Cabero Roura L, McIntyre HD, Morris JL, Divakar H. The International Federation of Gynecology and Obstetrics (FIGO) initiative on gestational diabetes mellitus: a pragmatic guide for diagnosis, management, and care. Int J Gynaecol Obstet. 2015;131(Suppl 3):S173–S211. doi: 10.1016/S0020-7292(15)30033-3. [DOI] [PubMed] [Google Scholar]
- 2.Yuen L, Saeedi P, Riaz M, Karuranga S, Divakar H, Levitt N, Yang X, Simmons D. Projections of the prevalence of hyperglycaemia in pregnancy in 2019 and beyond: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107841. doi: 10.1016/j.diabres.2019.107841. [DOI] [PubMed] [Google Scholar]
- 3.Sacks DA, Hadden DR, Maresh M, Deerochanawong C, Dyer AR, Metzger BE, Lowe LP, Coustan DR, Hod M, Oats JJ, Persson B, Trimble ER. HAPO study cooperative research group. Frequency of gestational diabetes mellitus at collaborating centers based on IADPSG consensus panel-recommended criteria: the hyperglycemia and adverse pregnancy outcome (HAPO) study. Diabetes Care. 2012;35(3):526–528. doi: 10.2337/dc11-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.HAPO Study Cooperative Research Group. Metzger BE, Lowe LP, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358(19):1991–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- 5.Benaiges D, Flores-Le Roux JA, Marcelo I, et al. Is first-trimester HbA1c useful in the diagnosis of gestational diabetes? Diabetes Res Clin Pract. 2017;133:85–91. doi: 10.1016/j.diabres.2017.08.019. [DOI] [PubMed] [Google Scholar]
- 6.Popova PV, Grineva EN, Gerasimov AS, Kravchuk EN, Ryazantseva EM, Shelepova ES. The new combination of risk factors determining a high risk of gestational diabetes mellitus. Minerva Endocrinol. 2015;40(4):239–247. [PubMed] [Google Scholar]
- 7.Popova P, Tkachuk A, Dronova A, Gerasimov A, Kravchuk E, Bolshakova M, Rozdestvenskaya O, Demidova K, Nikolaeva A, Grineva E. Fasting glycemia at the first prenatal visit and pregnancy outcomes in Russian women. Minerva Endocrinol. 2016;41(4):477–485. [PubMed] [Google Scholar]
- 8.Zhu WW, Fan L, Yang HX, et al. Fasting plasma glucose at 24-28 weeks to screen for gestational diabetes mellitus: new evidence from China. Diabetes Care. 2013;36(7):2038–2040. doi: 10.2337/dc12-2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hughes RC, Rowan J, Florkowski CM. Is there a role for HbA1c in pregnancy? Curr Diab Rep. 2016;16(1):5. doi: 10.1007/s11892-015-0698-y. [DOI] [PubMed] [Google Scholar]
- 10.Agarwal MM, Punnose J, Dhatt GS. Gestational diabetes: problems associated with the oral glucose tolerance test. Diabetes Res Clin Pract. 2004;63(1):73–74. doi: 10.1016/j.diabres.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Selvin E, Crainiceanu CM, Brancati FL, Coresh J. Short-term variability in measures of glycemia and implications for the classification of diabetes. Arch Intern Med. 2007;167(14):1545–1551. doi: 10.1001/archinte.167.14.1545. [DOI] [PubMed] [Google Scholar]
- 12.Ye M, Liu Y, Cao X, et al. The utility of HbA1c for screening gestational diabetes mellitus and its relationship with adverse pregnancy outcomes. Diabetes Res Clin Pract. 2016;114:43–49. doi: 10.1016/j.diabres.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 13.American Diabetes Association (2) classification and diagnosis of diabetes. Diabetes Care. 2015;38(Suppl):S8–S16. doi: 10.2337/dc15-S005. [DOI] [PubMed] [Google Scholar]
- 14.Hughes RCE, Moore MP, Gullam JE, Mohamed K, Rowan J. An early pregnancy HbA1c >5.9% (41 mmol/Mol) is optimal for detecting diabetes and identifies women at increased risk of adverse pregnancy outcomes. Diabetes Care. 2014;37:2953–2959. doi: 10.2337/dc14-1312. [DOI] [PubMed] [Google Scholar]
- 15.Mañé L, Flores-Le Roux JA, Benaiges D, Rodríguez M, Marcelo I, Chillarón JJ, Pedro-Botet J, Llauradó G, Gortazar L, Carreras R, Payà A. Role of first-trimester HbA1c as a predictor of adverse obstetric outcomes in a multiethnic cohort. J Clin Endocrinol Metab. 2017;102(2):390–397. doi: 10.1210/jc.2016-2581. [DOI] [PubMed] [Google Scholar]
- 16.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2007;30(Suppl 1):S42–S47. doi: 10.2337/dc07-S042. [DOI] [PubMed] [Google Scholar]
- 17.Ngala RA, Fondjo LA, Gmagna P, Ghartey FN, Awe MA. Placental peptides metabolism and maternal factors as predictors of risk of gestational diabetes in pregnant women. A case-control study. PLoS One. 2017;12(7):e0181613. doi: 10.1371/journal.pone.0181613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balaji V, Madhuri BS, Ashalatha S, et al. A1C in gestational diabetes mellitus in Asian Indian women. Diabetes Care. 2007;30:1865–1867. doi: 10.2337/dc06-2329. [DOI] [PubMed] [Google Scholar]
- 19.Rajput R, Yadav Y, Rajput M, Nanda S. Utility of HbA1c for diagnosis of gestational diabetes mellitus. Diabetes Res Clin Pract. 2012;98(1):104–107. doi: 10.1016/j.diabres.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 20.Kwon SS, Kwon JY, Park YW, Kim YH, Lim JB. HbA1c for diagnosis and prognosis of gestational diabetes mellitus. Diabetes Res Clin Pract. 2015;110(1):38–43. doi: 10.1016/j.diabres.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 21.Dubey D, Kunwar S, Gupta U. Mid-trimester glycosylated hemoglobin levels (HbA1c) and its correlation with oral glucose tolerance test (World Health Organization 1999) J Obstet Gynaecol Res. 2019;45(4):817–823. doi: 10.1111/jog.13916. [DOI] [PubMed] [Google Scholar]
- 22.Ryu AJ, Moon HJ, Na JO, et al. The usefulness of the glycosylated hemoglobin level for the diagnosis of gestational diabetes mellitus in the Korean population. Diabetes Metab J. 2015;39(6):507–511. doi: 10.4093/dmj.2015.39.6.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Renz PB, Chume FC, Timm JRT, Pimentel AL, Camargo JL. Diagnostic accuracy of glycated hemoglobin for gestational diabetes mellitus: a systematic review and meta-analysis. Clin Chem Lab Med. 2019;57(10):1435–1449. doi: 10.1515/cclm-2018-1191. [DOI] [PubMed] [Google Scholar]
- 24.Rayis DA, Ahmed ABA, Sharif ME, ElSouli A, Adam I. Reliability of glycosylated hemoglobin in the diagnosis of gestational diabetes mellitus. J Clin Lab Anal. 2020;34(10):e23435. doi: 10.1002/jcla.23435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Renz PB, Cavagnolli G, Weinert LS, Silveiro SP, Camargo JL. HbA1c test as a tool in the diagnosis of gestational diabetes mellitus. PLoS One. 2015;10(8):e0135989. doi: 10.1371/journal.pone.0135989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sevket O, Sevket A, Ozel A, Dansuk R, Kelekci S. The use of HbA1c as an aid in the diagnosis of gestational diabetes mellitus. J Obstet Gynaecol (Lahore) 2014;34(8):690–692. doi: 10.3109/01443615.2014.925855. [DOI] [PubMed] [Google Scholar]
- 27.Maesa J-M, Fernandez-Riejos P, Gonzalez-Rodriguez C, Sanchez-Margalet V. Screening for gestational diabetes mellitus by measuring glycated hemoglobin can reduce the use of the glucose challenge test. Ann Lab Med. 2019;39(6):524. doi: 10.3343/alm.2019.39.6.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rajput R, YogeshYadav RM, Nanda S. Utility of HbA 1c for diagnosis of gestational diabetes mellitus. Diabetes Res Clin Pract. 2012;98(1):104–107. doi: 10.1016/j.diabres.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 29.Amylidi S, Mosimann B, Stettler C, Fiedler GM, Surbek D, Raio L. First-trimester glycosylated hemoglobin in women at high risk for gestational diabetes. Acta Obstet Gynecol Scand. 2016;95(1):93–97. doi: 10.1111/aogs.12784. [DOI] [PubMed] [Google Scholar]
- 30.Barr RG, Nathan DM, Meigs JB, Singer DE. Tests of glycemia for the diagnosis of type II diabetes mellitus. Ann Intern Med. 2002;137:263–272. doi: 10.7326/0003-4819-137-4-200208200-00011. [DOI] [PubMed] [Google Scholar]
- 31.Nielsen LR, Ekbom P, Damm P, Glümer C, Frandsen MM, Jensen DM, Mathiesen ER. HbA1c levels are significantly lower in early and late pregnancy. Diabetes Care. 2004;27(5):1200–1201. doi: 10.2337/diacare.27.5.1200. [DOI] [PubMed] [Google Scholar]
- 32.Lippi G, Targher G. Glycated hemoglobin (HbA1c): old dogmas, a new perspective? Clin Chem Lab Med. 2010;48:609–614. doi: 10.1515/cclm.2010.144. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed in this study will be made attainable from the corresponding author upon request.