Abstract
Background
Frailty predicts adverse post-kidney transplant (KT) outcomes, yet the impact of frailty assessment on center-level outcomes remains unclear. We sought to test whether transplant centers assessing frailty as part of clinical practice have better pre- and post-KT outcomes in all adult patients (≥18 years) and older patients (≥65 years).
Methods
In a survey of US transplant centers (11/2017–4/2018), 132 (response rate = 65.3%) centers reported their frailty assessment practices (frequency and specific tool) at KT evaluation and admission. Assessment frequency was categorized as never, sometime, and always; type of assessment tool was categorized as none, validated (for post-KT risk prediction), and any other tool. Center characteristics and clinical outcomes for adult patients during 2017–2019 were gleaned from the transplant national registry (Scientific Registry of Transplant Recipients). Poisson regression was used to estimate incidence rate ratios (IRRs) of waitlist outcomes (waitlist mortality, transplantation) in candidates and IRRs of post-KT outcomes (all-cause mortality, death-censored graft loss) in recipients by frailty assessment frequency. We also estimated IRRs of waitlist outcomes by type of assessment tool at evaluation. All models were adjusted for case mix and center characteristics.
Results
Assessing frailty at evaluation was associated with lower waitlist mortality rate (always IRR = 0.91,95%CI:0.84–0.99; sometimes = 0.89,95%CI:0.83–0.96) and KT rate (always = 0.94,95%CI:0.91–0.97; sometimes = 0.88,95%CI:0.85–0.90); the associations with waitlist mortality rate (always = 0.86,95%CI:0.74–0.99; sometimes = 0.83,95%CI:0.73–0.94) and KT rate (always = 0.82,95%CI:0.77–0.88; sometimes = 0.92,95%CI:0.87–0.98) were stronger in older patients. Furthermore, using validated (IRR = 0.90,95%CI:0.88–0.92) or any other tool (IRR = 0.90,95%CI:0.87–0.93) at evaluation was associated lower KT rate, while only using a validated tool was associated with lower waitlist mortality rate (IRR = 0.89,95%CI:0.83–0.96), especially in older patients (IRR = 0.82,95%CI:0.72–0.93). At admission for KT, always assessing frailty was associated with a lower graft loss rate (IRR = 0.71,95%CI:0.54–0.92) but not with mortality (IRR = 0.93,95%CI:0.76–1.13).
Conclusions
Assessing frailty at evaluation is associated with lower KT rate, while only using a validated frailty assessment tool is associated with better survival, particularly in older candidates. Centers always assessing frailty at admission are likely to have better graft survival rates. Transplant centers may utilize validated frailty assessment tools to secure KT access for appropriate candidates and to better allocate health care resources for patients identified as frail, particularly for older patients.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12877-022-02777-2.
Keywords: Frailty, Kidney Transplant, Mortality, Graft Loss, Clinical Practice
Background
Frailty, originally characterized in community-dwelling older adults, is a clinical phenotype of decreased physiologic reserve and resistance to stressors [1]. Accumulating evidence has suggested that frailty may be a more meaningful predictor of mortality and hospitalizations than age among dialysis patients [2]. While a number of tools have been developed for frailty assessment over the past decades, the most commonly studied tool among end-stage kidney disease (ESKD) patients is the Physical Frailty Phenotype (PFP) developed by Fried et al. [3]. Frailty measured by the PFP is common in kidney transplant (KT) populations; 16% of KT candidates and 14% of KT recipients are frail in the United States [4]. Recently, frailty has gained the attention of renal healthcare providers for its ability to predict adverse outcomes among adult individuals with ESKD and KT patients [2, 5–10]. Among KT candidates, frailty is associated with lower chance of listing, higher waitlist mortality, and reduced access to KT [11–14]; among KT recipients, frailty is associated with surgical complications, delayed graft function, postoperative delirium, early hospital readmission, immunosuppression intolerance, and mortality [15–21]. Other surrogates of frailty that focus on physical function, such as the Short Physical Performance Battery (SPPB), functional status, Kidney Disease Quality of Life Short Form Physical Component Subscale (SF-12 PCS), gait speed, timed up and go, have also been found to predict adverse post-KT outcomes among ESKD and KT patients [22–28]. Another major frailty framework is the deficit accumulation model (Frailty Index) developed by Mitnitski et al., who viewed frailty as a state of accelerated deficit accumulation [29]. The frailty index has shown similar predictive value to PFP for clinical outcomes [30] but has yet to be applied to KT populations.
The American Society of Transplantation (AST) Kidney/Pancreas Community of Practice Workgroup conducted a survey of US KT programs in 2017–2018 to assess the landscape of frailty assessments at transplant centers in the United States. According to the survey, frailty is recognized as a clinically relevant construct in candidacy evaluation due to its potential in risk stratification for pre- and post-KT outcomes [6]. Particularly, measuring frailty is useful for identifying which older candidates are robust despite their age. In practice, frailty assessments are used in approximately 70% of transplant centers during candidacy evaluation and in approximately 30% of centers at KT admission [6].
Despite the use of frailty in the clinical practice of some transplant centers, it is unclear whether assessing frailty at evaluation and/or admission impacts pre- and post-KT outcomes of transplant centers. We hypothesized that transplant centers may take the advantage of frailty assessment results to better allocate health care resources for patients identified as frail, to prioritize robust older candidates, and to identify candidates whose physiologic reserve may improve after KT, which may in turn contribute to better patient outcomes at these centers. In this study, we sought to test whether transplant centers that measure frailty as part of clinical practice have better pre- and post-KT clinical outcomes and whether the associations differ among older patients (≥65 years).
Methods
Study Design
During 11/2017–4/2018, the AST Kidney Pancreas Community of Practice Workgroup administered a survey about practices related to frailty among all transplant centers that performed adult KT in the United States in 2017. The adult transplant centers were identified from the Scientific Registry of Transplant Recipients (SRTR) external release. The SRTR data system includes data on all donors, waitlisted candidates, and transplant recipients in the United States submitted by members of the Organ Procurement and Transplantation Network (OPTN). The Health Resources and Services Administration, United States Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors. The survey was exempt by the Johns Hopkins School of Medicine Institutional Review Board, and this research is in adherence with the Declaration of Helsinki and the Declaration of Istanbul. Further details about this survey were described elsewhere [6].
Among the 202 adult KT centers, a total of 132 centers (response rate = 65.3%) reported their frequencies of frailty assessment administration at evaluation and at KT. We gleaned center characteristics and clinical outcomes of the responding centers on waitlisted candidates and transplant recipients in the United States during 2017–2019. Center-level mean characteristics of waitlist candidates included adult KT listing volume per year (i.e., number of unique adult patients waitlisted in the study period), mean age of candidates at the time of KT listing, percentage of candidates who were older (aged ≥65 years), percentage of female candidates, percentage of Hispanic candidates, percentage of Black candidates, percentage of candidates who had a highest education level of high school or less, percentage of candidates currently working for income, percentage of candidates with diabetes, and percentage of candidates on dialysis. Center-level mean characteristics of recipients included adult KT volume per year (i.e., number of unique adult KT recipients in the study period), mean age of recipients at the time of KT, percentage of recipients who were older, percentage of female recipients, percentage of Hispanic recipients, percentage of Black recipients, percentage of recipients who had a highest education level of high school or less, percentage of recipients currently working for income, percentage of recipients with diabetes, percentage of recipients on dialysis, and percentage of recipients who received a living donor KT.
To assess the center performance at the time of the survey, we linked the dataset with the biannual SRTR Program-Specific Report (PSR) released in 10/2018 to obtain center-specific observed to expected (O/E) ratio for each adverse outcome rate: waitlist mortality rate and transplantation rate for waitlist candidates; 1-year all-cause mortality race and 1-year graft loss rate for recipients. The reference population of the waitlist estimates was all KT patients on the waiting list at any time during 12/31/2015–12/30/2017. The reference population of the post-transplant estimates was all KT patients undergoing transplant during the same 2-year period. We also examined whether the responding transplant centers had a geriatrics programs gleaned from the official website of each center.
Frailty Assessment Practice
The frequency of frailty assessment at candidacy evaluation and at KT admission were assessed by two questions: “Do you currently perform a standardized frailty assessment as part of evaluation for kidney transplant candidacy in your practice?” and “Do you currently perform a standardized frailty assessment for kidney transplant recipients at the time of transplantation in your practice?” The possible responses to either question included “always,” “sometimes,” and “never.” If the participants answered “always” or “sometimes” to either question, they were further asked “What tool for the assessment of frailty do you currently use routinely?” and were able to select all that applied from a list of common frailty assessment tools. The full list of the standardized tools and responses to this question were recently reported elsewhere [6]. We categorized frailty assessment tool as: none (i.e., never assessing frailty), validated tool, and any other tool. Based on the literature, we identified 5 validated frailty assessment tools for post-KT risk prediction, including PFP, SPPB, functional status (i.e., SF-12 PCS), timed walk (i.e., 6-min walk test), and timed up and go.
Waitlist Outcomes
The center-level waitlist outcomes for candidates included waitlist mortality (i.e., death while awaiting a KT) rate and transplantation rate. Waitlisted candidates were followed from listing to removal from waitlist (due to death or KT) or administrative censoring at 8/2020. Total numbers of deaths and KTs were counted for each center. For the follow-up period of each candidate, person-years were calculated as the number of days that the candidate was on the waitlist and converted to a fraction of a year. Total person-years for each transplant center were calculated from the sum of person-years of each candidate waitlisted in 2017–2019 at the center. Waitlist mortality rate per 100 person-years on the waitlist was calculated by dividing the number of removals due to death at each center by the total number of person-years on the waitlist at the center, multiplied by 100. Transplantation rate per 100 person-years on the waitlist was calculated by dividing the number of removals due to KT at each center by the total number of person-years on the waitlist at the center, multiplied by 100.
Post-KT Outcomes
The post-KT outcomes of interest included patient survival represented by all-cause mortality rate and graft survival represented by death-censored graft loss rate. KT recipients were followed for death and graft loss until administrative censoring at 8/2020. Total numbers of all-cause deaths and death-censored graft losses were counted for each center. Person-years were calculated as the number of days that the recipients spent until the outcome event (all-cause death or death-censored graft loss) and converted to a fraction of a year. Total person-years of each outcome event for each transplant center were calculated from the sum of person-years of each recipient in 2017–2019 at the center. All-cause mortality rate and death-censored graft loss rate per 100 person-years were calculated, respectively, by dividing the number of events at each center by the total number of person-years at the center, multiplied by 100.
Primary Analysis
For candidates who had multiple listing records during 2017–2019 at each center, only the first listing record of each candidate was included for analysis; similarly, only the first transplant record of each recipient over the same period at each center was included. We used patient-level data from SRTR to estimate center-level mean characteristics. We generated means with standard deviations (SDs) for center-mean characteristics, medians with interquartile ranges (IQRs) for non-normally distributed continuous variables, and percentages for categorical variables by frailty assessment frequency. Differences in the distributions were tested using analysis of variance (ANOVA) for normally distributed variables, Kruskal-Wallis test for non-normally distributed variables, and Fisher’s exact test for categorical variables.
Unadjusted waitlist mortality rate and transplantation rate were calculated by frequency of frailty assessment at evaluation; unadjusted rates of all-cause mortality and death-censored graft loss were calculated by frequency of frailty assessment at KT. Crude and adjusted incidence rate ratios (cIRRs and aIRRs) of each event (waitlist mortality, transplantation, all-cause mortality, and death-censored graft loss) by frequency of frailty assessment were estimated using three Poisson regression models: crude models generated cIRRs; demographic and health factor models adjusted for center-mean demographic (percentage of older, percentage of female, percentage of Black, percentage of Hispanic) and health characteristics (percentage of patients with diabetes, percentage of undergoing dialysis, and percentage of recipients who received a living donor KT); demographic, health, and social factor models further adjusted for center-mean socio-economic characteristics (percentage of having high school or less education, and percentage of working for income). As a secondary analysis, we further examined the impact of the type of frailty assessment tool by estimating cIRRs and aIRRs of waitlist outcomes (waitlist mortality, transplantation) by frailty assessment tool at evaluation.
All analyses were performed using Stata version 15 (StataCorp, College Station, TX). Two-sided p-values <0.05 were considered statistically significant.
Subgroup Analysis in Older Patients
We performed a subgroup analysis to evaluate the associations of frailty assessment practice with pre- and post-KT outcomes in older patients, specifically estimating 1) IRRs of waitlist mortality and transplantation by frailty assessment frequency and assessment tool at evaluation in older candidates, and 2) IRRs of all-cause mortality and death-censored graft loss by frailty assessment frequency at KT in older recipients.
Results
Center Characteristics of Candidates at Evaluation
During 2017–2019, the 132 responding centers listed 75.1% of the total adult KT candidates. These centers had a mean volume of adult KT listing of 206.0 (SD = 148.7) candidates per year during 2017–2019 and the center-mean age at listing was 52.9 years (SD = 2.0). The candidates listed at these centers were on average 21.2% older adults, 37.9% female, 16.5% Hispanic, 27.7% Black; 43.5% having high school or less education, and 37.5% working for income; 43.9% of candidates had diabetes and 70.1% were on dialysis at the time of listing. Center characteristics did not differ by frequency of frailty assessment at evaluation (all p > 0.05) (Table 1). There was no difference in median O/E ratios of waitlist mortality (never = 1.05, sometimes = 0.93, always = 0.98, p = 0.35) or transplantation (never = 1.06, sometimes = 0.92, always = 0.89, p = 0.22) by frailty assessment frequency (Table 1 and Supplementary Fig. S1). The proportion of centers having a geriatrics program was also similar by frailty assessment frequency (never = 66.7%, sometimes = 57.1%, always = 72.4%, p = 0.36) (Table 1).
Table 1.
Characteristics of kidney transplant centers by frequency of frailty assessment at kidney transplant evaluation (N = 132)
| Center characteristics | Frequency of frailty assessment at evaluation | |||
|---|---|---|---|---|
| Never (N = 54) | Sometimes (N = 49) | Always (N = 29) | P-value | |
| Adult KT listing volume per year | 211.1 (140.9) | 205.0 (173.4) | 198.4 (119.3) | 0.93 |
| Age (years) | 53.3 (2.0) | 52.6 (1.5) | 52.8 (2.4) | 0.27 |
| % Older (≥65 years) | 22.1 (6.0) | 20.2 (5.2) | 21.3 (6.4) | 0.26 |
| % Female | 37.6 (7.2) | 38.9 (3.5) | 36.7 (9.2) | 0.34 |
| % Hispanic | 16.2 (16.6) | 17.7 (20.5) | 14.7 (18.6) | 0.78 |
| % Black | 32.1 (19.0) | 23.2 (16.6) | 26.9 (21.1) | 0.06 |
| % High school or less | 44.3 (10.8) | 44.2 (9.9) | 40.9 (9.0) | 0.29 |
| % Working for income | 35.3 (9.4) | 39.3 (7.6) | 38.7 (9.0) | 0.05 |
| % Diabetes | 45.3 (6.0) | 43.0 (7.8) | 42.9 (7.1) | 0.19 |
| % Undergoing dialysis | 71.6 (11.0) | 68.1 (11.4) | 70.7 (9.2) | 0.26 |
| O/E ratio of waitlist mortality, median (IQR) | 1.05 (0.84, 1.13) | 0.93 (0.82, 1.10) | 0.98 (0.85, 1.13) | 0.35 |
| O/E ratio of transplantation, median (IQR) | 1.06 (0.77, 1.49) | 0.92 (0.75, 1.24) | 0.89 (0.68, 1.31) | 0.22 |
| Geriatrics program, n (%) | 36 (66.7%) | 28 (57.1%) | 21 (72.4%) | 0.36 |
| Use of a validated frailty assessment tool, n (%) | – | 33 (67.3%) | 18 (62.1%) | 0.64 |
Center characteristics were gleaned from SRTR data on adult KT candidates in 2017–2019. The table shows the mean center characteristics, including characteristics that are percentages. Values are presented as mean (SD) across transplant centers unless otherwise indicated. Abbreviations: KT Kidney transplantation, SD Standard deviation, IQR Interquartile range, O/E ratio Observed to expected ratio
Frailty Assessment at Evaluation and Waitlist Outcomes
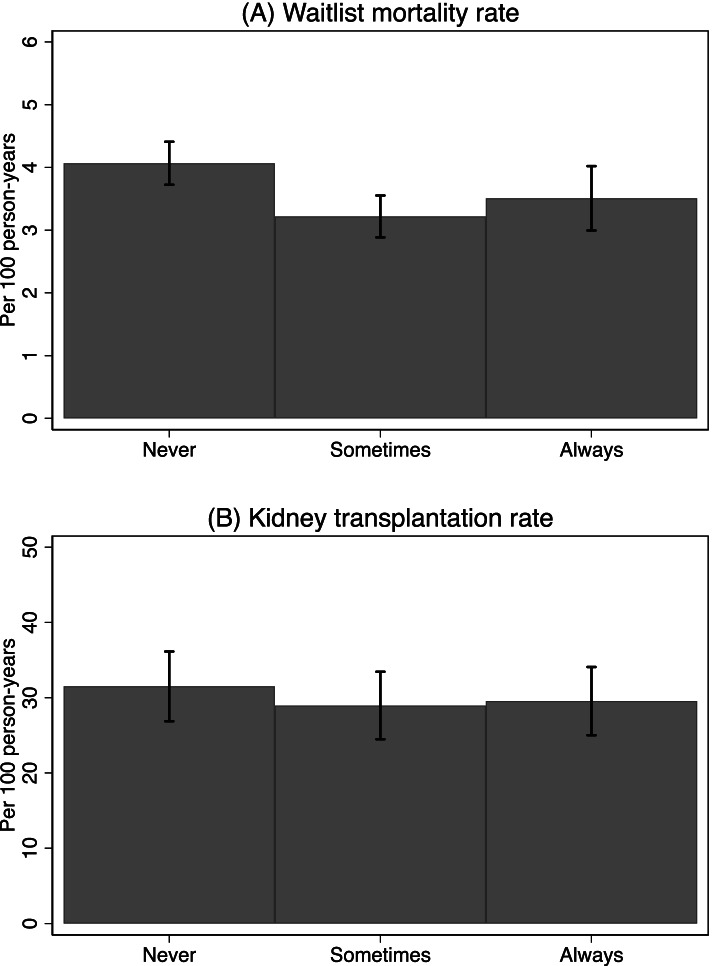
Of the 132 KT centers, 22.0% (n = 29) always, 37.1% (n = 49) sometimes, and 40.9% (n = 54) never assessed frailty at KT candidacy evaluation. Among centers assessing frailty at evaluation, 65.4% (n = 51) used a validated frailty assessment tool. The average of center median wait time for a KT was 1.3 years (SD = 0.3); there was no difference in wait time by frequency of frailty assessment (p = 0.38). The centers had a mean waitlist mortality rate of 3.6 deaths per 100 person-years (SD = 1.3), with 4.1 deaths (SD = 1.3), 3.2 deaths (SD = 1.2), and 3.5 deaths (SD = 1.3) per 100 person-years for centers never, sometimes, and always assessing frailty at evaluation, respectively (p = 0.003) (Fig. 1A). The center-mean transplantation rate was 30.1 KTs per 100 person-years (SD = 15.4), and there was no difference in transplantation rates by frequency of frailty assessment (p = 0.69) (Fig. 1B).
Fig. 1.
Unadjusted center-specific incidence rates among kidney transplant candidates by frequency of frailty assessment (N = 132). Patient survival data was gleaned from SRTR data on adult KT candidates in 2017–2019. Error bars represent the corresponding 95% confidence intervals
After the adjustment for demographic, health, and social factors, centers always assessing frailty had a 9% lower incidence rate of waitlist mortality (aIRR = 0.91, 95% confidence interval [CI]: 0.84–0.99), and centers sometimes assessing frailty had a 11% lower incidence rate of waitlist mortality (aIRR = 0.89, 95% CI: 0.83–0.96). Furthermore, centers always assessing frailty had a 6% lower incidence rate of KT (aIRR = 0.94, 95% CI: 0.91–0.97), and centers sometimes assessing frailty had a 12% lower incidence rate of KT (aIRR = 0.88, 95% CI: 0.85–0.90) (Table 2).
Table 2.
Frailty assessment at kidney transplant evaluation and center-specific waitlist mortality and transplantation rates (N = 132)
| Crude model | Demographic + health factor model | Demographic + health + social factor model | |
|---|---|---|---|
| cIRR (95% CI) | aIRR (95% CI) | aIRR (95% CI) | |
| Waitlist mortality rate | |||
| Never | reference | reference | reference |
| Sometimes | 0.84 (0.78, 0.90) | 0.89 (0.82, 0.95) | 0.89 (0.83, 0.96) |
| Always | 0.86 (0.79, 0.93) | 0.90 (0.83, 0.98) | 0.91 (0.84, 0.99) |
| Transplantation rate | |||
| Never | reference | reference | reference |
| Sometimes | 0.89 (0.87, 0.92) | 0.87 (0.85, 0.90) | 0.88 (0.85, 0.90) |
| Always | 1.07 (1.04, 1.10) | 0.94 (0.91, 0.97) | 0.94 (0.91, 0.97) |
Crude and adjusted incidence rate ratios (cIRR and aIRR) with 95% confidence intervals (CI) are presented from Poisson regression models. Demographic + health factor models adjusted for center-mean demographic (% older, % female, % Black, % Hispanic) and health factors (% with diabetes, % undergoing dialysis); demographic + health + social factor models additionally adjusted for center-mean social factors (% low education, % working for income). Associations that are statistically significant at p < 0.05 are bolded
Furthermore, independent of demographic, health, and social factors, using a validated frailty assessment tool at evaluation was associated with lower waitlist mortality (IRR = 0.89, 95% CI: 0.83–0.96) and lower KT rate (IRR = 0.90, 95% CI: 0.88–0.92), while using any other assessment tool was associated with lower KT rate (IRR = 0.90, 95% CI: 0.87–0.93) but not with waitlist mortality (IRR = 0.92, 95% CI: 0.84–1.00) (Table 3).
Table 3.
Type of frailty assessment tool at kidney transplant evaluation and center-specific waitlist mortality and transplantation rates (n = 132)
| Crude model | Demographic + health factor model | Demographic + health + social factor model | |
|---|---|---|---|
| cIRR (95% CI) | aIRR (95% CI) | aIRR (95% CI) | |
| Waitlist mortality rate | |||
| None | reference | reference | reference |
| Validated tool | 0.82 (0.77, 0.88) | 0.87 (0.82, 0.94) | 0.89 (0.83, 0.96) |
| Any other tool | 0.89 (0.82, 0.97) | 0.93 (0.85, 1.01) | 0.92 (0.84, 1.00) |
| Transplantation rate | |||
| None | reference | reference | reference |
| Validated tool | 0.96 (0.93, 0.98) | 0.90 (0.88, 0.93) | 0.90 (0.88, 0.92) |
| Any other tool | 0.95 (0.92, 0.98) | 0.89 (0.86, 0.92) | 0.90 (0.87, 0.93) |
Crude and adjusted incidence rate ratios (cIRR and aIRR) with 95% confidence intervals (CI) are presented from Poisson regression models. Demographic + health factor models adjusted for center-mean demographic (% older, % female, % Black, % Hispanic) and health factors (% with diabetes, % undergoing dialysis); demographic + health + social factor models additionally adjusted for center-mean social factors (% low education, % working for income). Associations that are statistically significant at p < 0.05 are bolded
Center Characteristics of Recipients at KT
During 2017–2019, the 132 responding centers performed 76.2% of the total adult KTs in the United States. At these centers, the mean volume of adult KTs was 119.3 (SD = 81.5) per year during 2017–2019 and the center-mean age at KT was 52.6 years (SD = 2.2). KT recipients at these centers were on average 21.3% older, 38.6% female, 16.1% Hispanic, 26.5% Black, 44.4% having high school or less education, 33.1% working for income; 34.4% had diabetes, 80.5% underwent pre-KT dialysis, and 28.8% were living donation recipients. Center characteristics did not differ by frequency of frailty assessment at KT (p > 0.1), except that centers that always assessed frailty at KT had a lower proportion of employed recipients (p = 0.03) (Table 4). There were no differences in the median O/E ratios of 1-year graft loss (never = 0.99, sometimes = 1.01, always = 0.93, p = 0.85) and 1-year mortality (never = 0.97, sometimes = 0.93, always = 0.96, p = 0.53) by frailty assessment frequency (Table 4 and Supplementary Fig. S2). The proportion of centers having geriatrics program was also similar by frailty assessment frequency at KT (never = 64.4%, sometimes = 57.1%, always = 80.0%, p = 0.46) (Table 4).
Table 4.
Characteristics of kidney transplant centers by frequency of frailty assessment at kidney transplantation (N = 132)
| Center characteristics | Frequency of frailty assessment administration | |||
|---|---|---|---|---|
| Never (N = 101) | Sometimes (N = 21) | Always (N = 10) | P-value | |
| Adult KT volume per year | 118.2 (80.4) | 140.4 (88.5) | 85.9 (71.1) | 0.21 |
| Age (years) | 52.6 (2.2) | 52.5 (1.9) | 52.6 (3.5) | 0.99 |
| % Older (≥65 years) | 21.2 (5.5) | 21.3 (4.7) | 22.0 (8.3) | 0.90 |
| % Female | 38.6 (7.0) | 38.9 (3.1) | 37.1 (12.0) | 0.79 |
| % Hispanic | 16.3 (18.4) | 11.1 (11.5) | 24.7 (30.2) | 0.16 |
| % Black | 26.7 (18.4) | 22.9 (15.2) | 32.1 (25.9) | 0.43 |
| % High school or less | 44.4 (10.1) | 42.9 (9.8) | 47.4 (13.5) | 0.54 |
| % Working for income | 33.0 (11.9) | 37.2 (7.8) | 25.3 (13.7) | 0.03 |
| % Diabetes | 34.7 (7.3) | 32.4 (5.0) | 35.5 (7.4) | 0.34 |
| % Undergoing dialysis | 80.3 (8.1) | 79.8 (8.2) | 84.8 (8.1) | 0.23 |
| % Living donor transplant | 29.0 (12.2) | 31.4 (13.5) | 21.4 (8.9) | 0.11 |
| O/E ratio of 1-year graft loss rate, median (IQR) | 0.99 (0.81, 1.17) | 1.01 (0.71, 1.16) | 0.93 (0.77, 1.27) | 0.85 |
| O/E ratio of 1-year mortality rate, median (IQR) | 0.97 (0.73, 1.29) | 0.93 (0.77, 1.03) | 0.96 (0.87, 1.39) | 0.53 |
| Geriatrics program, n (%) | 65 (64.4%) | 12 (57.1%) | 8 (80.0%) | 0.46 |
| Use of a validated frailty assessment tool, n (%) | – | 14 (66.7%) | 8 (80.0%) | 0.44 |
Center characteristics were gleaned from SRTR data on adult KT recipients in 2017–2019. The table shows the mean center characteristics, including characteristics that are percentages. Values are presented as mean (SD) across transplant centers unless otherwise indicated. Abbreviations: KT Kidney transplantation, SD Standard deviation, IQR Interquartile range, SRTR Scientific Registry of Transplant Recipients
Frailty Assessment at KT and Post-KT Outcomes
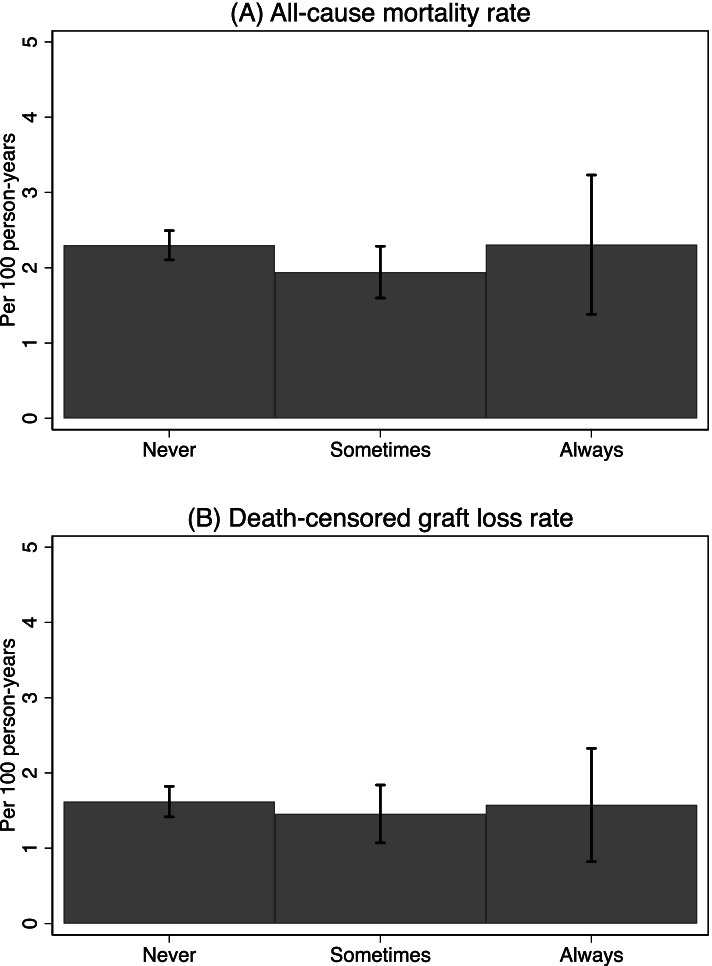
Of the 132 centers, 7.6% (n = 10) always, 15.9% (n = 21) sometimes, and 76.5% (n = 101) never assessed frailty at admission for KT. Among centers assessing frailty at KT, 71.0% (n = 22) used a validated frailty assessment tool. The average of center median follow-up time for patient survival was 2.0 years (SD = 0.2) post-KT, with no difference by frailty assessment frequency (p = 0.63). These centers had a mean all-cause mortality rate of 2.2 deaths per 100 person-years (SD = 1.0); centers assessing frailty at a different frequency had similar all-cause mortality rates (p = 0.31) (Fig. 2A). Similarly, the average of center median follow-up time for graft survival was 1.9 years (SD = 0.2) post-KT, with no difference by frailty assessment frequency (p = 0.61). On average, centers had a death-censored graft loss rate of 1.6 graft losses per 100 person-years (SD = 1.0), and no difference was observed by frequency of frailty assessment at admission for KT (p = 0.79) (Fig. 2B).
Fig. 2.
Unadjusted center-specific incidence rates among kidney transplant recipients by frequency of frailty assessment (N = 132). Patient survival data was gleaned from SRTR data on adult KT candidates in 2017–2019. Error bars represent the corresponding 95% confidence intervals
After the adjustment for demographic, health, and social factors, centers that always (aIRR = 0.93, 95% CI: 0.76–1.13) or sometimes (aIRR = 0.93, 95% CI: 0.82–1.04) assessed frailty at KT did not differ from centers never assessing frailty in terms of post-KT patient survival rate. Yet, always assessing frailty at KT was associated with a 29% lower death-censored graft loss rate (aIRR = 0.71, 95% CI: 0.54–0.92) compared to no assessment. Centers sometimes assessing frailty had similar death-censored graft loss rates to those never assessing frailty (aIRR = 0.98, 95% CI: 0.86–1.13) (Table 5).
Table 5.
Frailty assessment at kidney transplantation and center-specific all-cause mortality and death-censored graft loss rates (N = 132)
| Crude model | Demographic + health factor model | Demographic + health + social factor model | |
|---|---|---|---|
| cIRR (95% CI) | aIRR (95% CI) | aIRR (95% CI) | |
| All-cause mortality rate | |||
| Never | reference | reference | reference |
| Sometimes | 0.89 (0.80, 1.00) | 0.92 (0.82, 1.04) | 0.93 (0.82, 1.04) |
| Always | 1.01 (0.83, 1.22) | 0.94 (0.78, 1.15) | 0.93 (0.76, 1.13) |
| Death-censored graft loss rate | |||
| Never | reference | reference | reference |
| Sometimes | 0.95 (0.83, 1.08) | 0.98 (0.86, 1.13) | 0.98 (0.86, 1.13) |
| Always | 0.74 (0.57, 0.96) | 0.71 (0.54, 0.92) | 0.71 (0.54, 0.92) |
Crude and adjusted incidence rate ratios (cIRR and aIRR) with 95% confidence intervals (CI) are presented from Poisson regression models. Demographic + health factor models adjusted for center-mean demographic (% older, % female, % Black, % Hispanic) and health factors (% with diabetes, % undergoing dialysis, % living donor transplant); demographic + health + social factor models additionally adjusted for center-mean social factors (% low education, % working for income). Associations that are statistically significant at p < 0.05 are bolded
Subgroup Analysis among Older Candidates and Recipients
Among older candidates, there was a strong association between frequency of frailty assessment and waitlist mortality rate, with a 17% lower waitlist mortality rate in centers sometimes assessing frailty at evaluation (aIRR = 0.83, 95% CI: 0.73–0.94) and a 14% lower waitlist mortality rate in centers always assessing frailty (aIRR = 0.86, 95% CI: 0.74–0.99). Compared to centers never assessing frailty at evaluation, transplantation rates in older patients were 8% lower in centers sometimes (IRR = 0.92, 95% CI: 0.87–0.98) and 18% lower in centers always (IRR = 0.82, 95% CI: 0.77–0.88) assessing frailty, respectively (Supplementary Table S1). The associations of waitlist outcomes with type of frailty assessment tool used at evaluation were strengthened among older patients: using validated frailty assessment tool was associated with 18% lower waitlist mortality rate (IRR = 0.82, 95% CI: 0.72–0.93) and 11% lower KT rate (IRR = 0.89, 95% CI: 0.84–0.95), while using any other tool was associated with 15% lower KT rate (IRR = 0.85, 95% CI: 0.79–0.92) but had no association with waitlist mortality (IRR = 0.89, 95% CI: 0.76–1.04) (Supplementary Table S2). Among older recipients, the associations of frailty assessment at admission for KT with post-KT outcomes were similar to the patterns observed among older candidates but not significant (Supplementary Table S3).
Discussion
In this study of 132 transplant centers, representing 75.1% of all adult KT candidates and 76.2% of all adult KTs performed in the United States, we found that frailty assessments in routine clinical practices impacted pre- and post-KT outcomes. Specifically, centers always and sometimes assessing frailty at evaluation had a 9% and 11% lower waitlist mortality rate while a 6% and 12% lower transplantation rate, respectively. Yet, only using validated frailty assessment tool at evaluation was associated with lower waitlist mortality rate. Furthermore, the associations between frailty assessment practice at evaluation and waitlist outcomes were robust and had greater magnitudes among older candidates. Among recipients, centers always assessing frailty at KT had a 29% lower rate of death-censored graft loss, yet no difference in all-cause mortality rates was observed by frequency of frailty assessment at KT. However, there were no associations between frailty assessment frequency at KT and post-KT outcomes in older recipients.
Our results in KT candidates suggest that transplant centers always or sometimes assessing frailty at KT candidacy evaluation as part of clinical practice are likely to have better waitlist survival but lower transplantation rates, particularly for older candidates. This finding is consistent with prior findings from the national survey. It was reported that when a patient was identified as being frail, KT centers tend to determine the amount of social or home support before listing and prescribe prehabilitation for frail patients [6]. Thus, the better waitlist survival rate at these centers might be achieved by delivering effective interventions, such as prehabilitation [31], to frail candidates on the waitlist, by prioritizing robust older candidates, and by identifying candidates who likely improve their physiologic reserve after KT. Additionally, given the high frailty burden in KT candidates, especially in older candidates [4, 32], and the lower likelihood of receiving a KT among frail candidates [11], the lower transplantation rate at centers assessing frailty might partially result from the limited access to KT among frail candidates. However, the underlying mechanisms for this disparity are unclear.
Importantly, we found that the better waitlist survival was only associated with the use of validated frailty assessment tool; centers using any other frailty assessment tool at evaluation not only showed similar waitlist survival rates as centers not assessing frailty at all, but also had significantly lower transplantation rates, in particular among older candidates. This finding underscored that despite the demonstrated usefulness of frailty assessment in risk prediction for KT patients, the type of assessment instrument matters. The high heterogeneity of instruments to measure frailty in ESKD and KT populations has been demonstrated in several systematic reviews, and the prevalence of frailty measured by different instruments varied dramatically, ranging from 14% to 73% [33, 34]. Apart from the difference in the ability to discriminate frail patients of these assessment tools, different tools were developed on the basis of distinct frailty frameworks and are likely capturing distinct aspects of frailty. Therefore, transplant centers using frailty assessment tools that are not validated in the KT populations might not identify the most vulnerable patients and thus missed the opportunity for allocating necessary health care resources for frail patients, yet these centers could still limit the access to KT among patients being identified as “frail” by a non-validated tool. In this case, the most vulnerable older patients, suffering from both chronological aging and potentially accelerated physiological aging, are more likely to be affected. It is vital to carefully consider the ethical, financial, and medical implications of offering KT to the older population because the upper limit of age and extent of functional impairment at which the risk of transplantation outweigh the benefits are still unknown [35]. There has been an emerging need for a multidimensional approach to select ideal candidates despite age; yet routine frailty assessment based on validated instruments could potentially serve as a useful tool assisting in the process, though a standardized assessment tool tailored for KT evaluation is needed.
In KT recipients, we found a lower rate of graft loss, but not mortality, among centers always assessing frailty at admission for KT. Frailty assessments at KT admission could make healthcare providers aware of which patients need a tailored, patient-centered care for prevention of adverse transplant outcomes. Moreover, the lower graft loss rate might be a combined result from practices pre- and post-KT, as all the 10 centers always assessing frailty at KT also reported assessing frailty at candidacy evaluation (3 sometimes and 7 always). There has been evidence showing that frailty status, as measured by the PFP, is dynamic between evaluation and KT and transitions to more frail states are associated with poor post-KT outcomes [36]. By assessing frailty at both critical time points, these centers had better opportunities to identify patients who most likely benefit from pre-KT prehabilitation [31] and post-KT rehabilitation [37].
Our findings arise from the practices and outcomes of transplant centers that impact 75% of KT candidates and 76% of recipients in the United States. To our knowledge, this is the first study to date exploring the association of frailty assessments in clinical practices with center-level pre- and post-KT outcomes. There are a number of notable strengths of this study, including the novel analytic approach to explore the center-level associations, the ability to identify type of frailty assessment tool and to further study the association with use of a validated tool, separate analyses among older patients, geographic diversity of the responding centers, and the high percentages of KT candidates and recipients reflected by the results of this study.
This study is limited by the transplant centers included. For example, we were unable to examine the outcomes of centers that assessed frailty solely at KT admission, since no such centers were included in the survey. However, it is likely that few transplant centers assess frailty only at KT, since only 61% of centers agreed that frailty assessments should be used in decisions for the timing of KT, while 96% of centers agreed on the use in decisions regarding candidate selection [6]. Another limitation is the cross-sectional design; our findings do not support causal inferences between frailty assessment practices and clinical outcomes of transplant centers. In addition, although we accounted for all available center-level characteristics and tested the center performances (O/E ratios) and the presence of a geriatrics program at each center, there may be residual confounding effects. This may result from unmeasurable factors, such as how frailty assessment results were used in transplant care, the extent to which a negative result may affect clinical decision-making for the patient, and what additional healthcare services will be triggered by negative results. However, our results suggested that better quality-controlled centers are not the only centers that assess frailty for transplant decision-making. Furthermore, there were a wide variety of tools to measure frailty for KT patients in clinical practice, and the most commonly used tools were a timed walk test and measuring body mass index [6]. Although we examined the effects of using a validated frailty assessment tool at evaluation, the sample size did not allow the analysis of using a validated tool at KT, nor further subgroup analysis of specific frailty assessment tools or further adjustment for type of tools in the model of frailty assessment frequency. Thus, future studies should identify which measure is best suited for KT patients.
Conclusions
In summary, our findings suggest that, regardless of center characteristics, assessing frailty as part of KT candidacy evaluation is associated with a lower transplantation rate, but using a validated frailty assessment tool is also associated with better waitlist survival, in particular among older candidates. Centers always assessing frailty at admission for KT are likely to have a better graft survival, while it is unclear whether the lower graft loss rate resulted from frailty assessments at a single time point at admission or at both evaluation and admission for KT. This study presents the potential usefulness of using validated frailty assessments in clinical practice for KT patients. Based on our findings, we recommend that transplant centers may utilize frailty assessment instruments that have been validated in KT populations as a tool to secure KT access for appropriate candidates despite age and to better allocate health care resources for patients identified as frail, particularly for older patients. For example, the transplant team may adapt the well-developed geriatric care frameworks to the setting of KT care, developing conversations with the family members of KT patients to achieve a more favorable transplant outcome. Also, healthcare providers in other clinical specialties should be aware of the potential utility of frailty assessments having been validated in the target populations, even among younger patients. Further research should explore the optimal subpopulations and procedures for assessing frailty in clinical practice.
Supplementary Information
Acknowledgements
We would like to acknowledge the role of the Kidney/Pancreas Community of Practice Frailty Working Group Members for their contribution to the survey.
Abbreviations
- 95% CI
95% confidence interval
- aIRR
Adjusted incidence rate ratio
- ANOVA
Analysis of variance
- AST
American Society of Transplantation
- cIRR
Crude incidence rate ratio
- ESKD
End-stage kidney disease
- IRR
Incidence rate ratio.
- KT
Kidney transplant
- O/E ratio
Observed to expected ratio
- OPTN
Organ Procurement and Transplantation Network
- PFP
Physical Frailty Phenotype
- SD
Standard deviation
- SPPB
Short Physical Performance Battery
- SRTR
Scientific Registry of Transplant Recipients
Authors’ contributions
All authors contributed significantly to this work. Design and concept of the study: XC, MMD; Data analysis and interpretation: XC, YL, NMC, MMD; Writing and critical revisions for intellectual content and final approval of the article: All authors.
Funding
This study was supported by National Institute on Aging grant R01AG055781 (Principal Investigator: Mara A. McAdams-DeMarco), and National Institute of Diabetes and Digestive and Kidney Diseases grant R01DK114074 (Principal Investigator: McAdams-DeMarco). The data reported here have been supplied by the Hennepin Healthcare Research Institute as the contractor for the SRTR. The interpretation and reporting of the data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the SRTR, OPTN/UNOS, or the US government. The funding sources had no role in the design, methods, subject recruitment, data collections, analysis, and preparation of paper.
Dorry Segev was supported by grant K24AI144954 from the National Institute of Allergy and Infectious Diseases (NIAD).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The survey was reviewed and acknowledged to be exempt by the Johns Hopkins School of Medicine Institutional Review Board (September 2017), according to the Revised Common Rule; informed consent was waived. All methods performed in this research is in adherence with the Declaration of Helsinki and the Declaration of Istanbul.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fried LP, Xue QL, Cappola AR, Ferrucci L, Chaves P, Varadhan R, Guralnik JM, Leng SX, Semba RD, Walston JD, et al. Nonlinear multisystem physiological dysregulation associated with frailty in older women: implications for etiology and treatment. J Gerontol A Biol Sci Med Sci. 2009;64(10):1049–1057. doi: 10.1093/gerona/glp076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown EA, Johansson L. Old age and frailty in the dialysis population. J Nephrol. 2010;23(5):502–507. [PubMed] [Google Scholar]
- 3.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M156. doi: 10.1093/gerona/56.3.M146. [DOI] [PubMed] [Google Scholar]
- 4.Haugen CE, Thomas AG, Chu NM, Shaffer AA, Norman SP, Bingaman AW, Segev DL, McAdams-DeMarco M. Prevalence of frailty among kidney transplant candidates and recipients in the United States: Estimates from a National Registry and Multicenter Cohort Study. Am J Transplant. 2020;20(4):1170–1180. doi: 10.1111/ajt.15709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kallenberg MH, Kleinveld HA, Dekker FW, van Munster BC, Rabelink TJ, van Buren M, Mooijaart SP. Functional and Cognitive Impairment, Frailty, and Adverse Health Outcomes in Older Patients Reaching ESRD-A Systematic Review. Clin J Am Soc Nephrol. 2016;11(9):1624–1639. doi: 10.2215/CJN.13611215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McAdams-DeMarco MA, Van Pilsum Rasmussen SE, Chu NM, Agoons D, Parsons RF, Alhamad T, Johansen KL, Tullius SG, Lynch R, Harhay MN, et al. Perceptions and practices regarding frailty in kidney transplantation: results of a national survey. Transplantation. 2020;104(2):349–356. doi: 10.1097/TP.0000000000002779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kobashigawa J, Dadhania D, Bhorade S, Adey D, Berger J, Bhat G, Budev M, Duarte-Rojo A, Dunn M, Hall S, et al. Report from the American society of transplantation on frailty in solid organ transplantation. Am J Transplant. 2019;19(4):984–994. doi: 10.1111/ajt.15198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng XS, Lentine KL, Koraishy FM, Myers J, Tan JC. Implications of frailty for peritransplant outcomes in kidney transplant recipients. Curr Transplant Rep. 2019;6(1):16–25. doi: 10.1007/s40472-019-0227-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Exterkate L, Slegtenhorst BR, Kelm M, Seyda M, Schuitenmaker JM, Quante M, Uehara H, El Khal A, Tullius SG. Frailty and Transplantation. Transplantation. 2016;100(4):727–733. doi: 10.1097/TP.0000000000001003. [DOI] [PubMed] [Google Scholar]
- 10.Quint EE, Zogaj D, Banning LBD, Benjamens S, Annema C, Bakker SJL, Nieuwenhuijs-Moeke GJ, Segev DL, McAdams-DeMarco MA, Pol RA. Frailty and Kidney Transplantation: A Systematic Review and Meta-analysis. Transplant Direct. 2021;7(6):e701. doi: 10.1097/TXD.0000000000001156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haugen CE, Chu NM, Ying H, Warsame F, Holscher CM, Desai NM, Jones MR, Norman SP, Brennan DC, Garonzik-Wang J, et al. Frailty and Access to Kidney Transplantation. Clin J Am Soc Nephrol. 2019;14(4):576–582. doi: 10.2215/CJN.12921118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McAdams-DeMarco MA, Ying H, Thomas AG, Warsame F, Shaffer AA, Haugen CE, Garonzik-Wang JM, Desai NM, Varadhan R, Walston J, et al. Frailty, inflammatory markers, and waitlist mortality among patients with end-stage renal disease in a prospective cohort study. Transplantation. 2018;102(10):1740–1746. doi: 10.1097/TP.0000000000002213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lorenz EC, Cosio FG, Bernard SL, Bogard SD, Bjerke BR, Geissler EN, Hanna SW, Kremers WK, Cheng Y, Stegall MD, et al. The relationship between frailty and decreased physical performance with death on the kidney transplant waiting list. Prog Transplant. 2019;29(2):108–114. doi: 10.1177/1526924819835803. [DOI] [PubMed] [Google Scholar]
- 14.Pérez Fernández M, Martínez Miguel P, Ying H, Haugen CE, Chu NM, Rodríguez Puyol DM, Rodríguez-Mañas L, Norman SP, Walston JD, Segev DL, et al. Comorbidity, frailty, and waitlist mortality among kidney transplant candidates of all ages. Am J Nephrol. 2019;49(2):103–110. doi: 10.1159/000496061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garonzik-Wang JM, Govindan P, Grinnan JW, Liu M, Ali HM, Chakraborty A, Jain V, Ros RL, James NT, Kucirka LM, et al. Frailty and delayed graft function in kidney transplant recipients. Arch Surg. 2012;147(2):190–193. doi: 10.1001/archsurg.2011.1229. [DOI] [PubMed] [Google Scholar]
- 16.Haugen CE, Mountford A, Warsame F, Berkowitz R, Bae S, Thomas AG, CHT B, Brennan DC, Neufeld KJ, Carlson MC, et al. Incidence, risk factors, and sequelae of post-kidney transplant delirium. J Am Soc Nephrol. 2018;29(6):1752–1759. doi: 10.1681/ASN.2018010064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McAdams-DeMarco MA, Law A, Salter ML, Chow E, Grams M, Walston J, Segev DL. Frailty and early hospital readmission after kidney transplantation. Am J Transplant. 2013;13(8):2091–2095. doi: 10.1111/ajt.12300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McAdams-DeMarco MA, Law A, Tan J, Delp C, King EA, Orandi B, Salter M, Alachkar N, Desai N, Grams M, et al. Frailty, mycophenolate reduction, and graft loss in kidney transplant recipients. Transplantation. 2015;99(4):805–810. doi: 10.1097/TP.0000000000000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McAdams-DeMarco MA, Law A, King E, Orandi B, Salter M, Gupta N, Chow E, Alachkar N, Desai N, Varadhan R, et al. Frailty and mortality in kidney transplant recipients. Am J Transplant. 2015;15(1):149–154. doi: 10.1111/ajt.12992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dos Santos MM, Coelho de Carvalho N, Archangelo TE, Modelli de Andrade LG, Pires Ferreira Filho S, de Souza CR, Kawano PR, Papini SJ, Costa NA, de Barros M, Almeida RA. Frailty predicts surgical complications after kidney transplantation. A propensity score matched study. PLoS One. 2020;15(2):e0229531. doi: 10.1371/journal.pone.0229531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McAdams-DeMarco MA, King EA, Luo X, Haugen C, DiBrito S, Shaffer A, Kucirka LM, Desai NM, Dagher NN, Lonze BE, et al. Frailty, Length of Stay, and Mortality in Kidney Transplant Recipients: A National Registry and Prospective Cohort Study. Ann Surg. 2017;266(6):1084–1090. doi: 10.1097/SLA.0000000000002025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang SF, Yang RS, Lin TC, Chiu SC, Chen ML, Lee HC. The discrimination of using the short physical performance battery to screen frailty for community-dwelling elderly people. J Nurs Scholarsh. 2014;46(3):207–215. doi: 10.1111/jnu.12068. [DOI] [PubMed] [Google Scholar]
- 23.Chu NM, Chen X, Bae S, Brennan DC, Segev DL, McAdams-DeMarco MA. Changes in Functional Status Among Kidney Transplant Recipients: Data From the Scientific Registry of Transplant Recipients. Transplantation. 2021;105(9):2104–2111. doi: 10.1097/TP.0000000000003608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harhay MN, Hill AS, Wang W, Even-Shoshan O, Mussell AS, Bloom RD, Feldman HI, Karlawish JH, Silber JH, Reese PP. Measures of Global Health Status on Dialysis Signal Early Rehospitalization Risk after Kidney Transplantation. PLoS One. 2016;11(6):e0156532. doi: 10.1371/journal.pone.0156532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nastasi AJ, Bryant TS, Le JT, Schrack J, Ying H, Haugen CE, González Fernández M, Segev DL, McAdams-DeMarco MA. Pre-kidney transplant lower extremity impairment and transplant length of stay: a time-to-discharge analysis of a prospective cohort study. BMC Geriatr. 2018;18(1):246. doi: 10.1186/s12877-018-0940-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nastasi AJ, McAdams-DeMarco MA, Schrack J, Ying H, Olorundare I, Warsame F, Mountford A, Haugen CE, González Fernández M, Norman SP, et al. Pre-Kidney Transplant Lower Extremity Impairment and Post-Kidney Transplant Mortality. Am J Transplant. 2018;18(1):189–196. doi: 10.1111/ajt.14430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michelson AT, Tsapepas DS, Husain SA, Brennan C, Chiles MC, Runge B, Lione J, Kil BH, Cohen DJ, Ratner LE, et al. Association between the "Timed Up and Go Test" at transplant evaluation and outcomes after kidney transplantation. Clin Transpl. 2018;32(11):e13410. doi: 10.1111/ctr.13410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kutner NG, Zhang R, Huang Y, Painter P. Gait Speed and Mortality, Hospitalization, and Functional Status Change Among Hemodialysis Patients: A US Renal Data System Special Study. Am J Kidney Dis. 2015;66(2):297–304. doi: 10.1053/j.ajkd.2015.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal. 2001;1:323–336. doi: 10.1100/tsw.2001.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guaraldi G, Malagoli A, Theou O, Brothers TD, Wallace L, Torelli R, Mussini C, Sartini S, Kirkland SA, Rockwood K. Correlates of frailty phenotype and frailty index and their associations with clinical outcomes. HIV Med. 2017;18(10):764–771. doi: 10.1111/hiv.12527. [DOI] [PubMed] [Google Scholar]
- 31.McAdams-DeMarco MA, Ying H, Van Pilsum RS, Schrack J, Haugen CE, Chu NM, González Fernández M, Desai N, Walston JD, Segev DL. Prehabilitation prior to kidney transplantation: Results from a pilot study. Clin Transpl. 2019;33(1):e13450. doi: 10.1111/ctr.13450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chu NM, Chen X, Norman SP, Fitzpatrick J, Sozio SM, Jaar BG, Frey A, Estrella MM, Xue QL, Parekh RS, et al. Frailty Prevalence in Younger End-Stage Kidney Disease Patients Undergoing Dialysis and Transplantation. Am J Nephrol. 2020;51(7):501–510. doi: 10.1159/000508576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chowdhury R, Peel NM, Krosch M, Hubbard RE. Frailty and chronic kidney disease: A systematic review. Arch Gerontol Geriatr. 2017;68:135–142. doi: 10.1016/j.archger.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 34.Harhay MN, Rao MK, Woodside KJ, Johansen KL, Lentine KL, Tullius SG, Parsons RF, Alhamad T, Berger J, Cheng XS, et al. An overview of frailty in kidney transplantation: measurement, management and future considerations. Nephrol Dial Transplant. 2020;35(7):1099–1112. doi: 10.1093/ndt/gfaa016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Basu A. Role of physical performance assessments and need for a standardized protocol for selection of older kidney transplant candidates. Kidney Int Rep. 2019;4(12):1666–1676. doi: 10.1016/j.ekir.2019.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chu NM, Deng A, Ying H, Haugen CE, Garonzik Wang JM, Segev DL, McAdams-DeMarco MA. Dynamic frailty before kidney transplantation: time of measurement matters. Transplantation. 2019;103(8):1700–1704. doi: 10.1097/TP.0000000000002563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heiwe S, Jacobson SH. Exercise training in adults with CKD: a systematic review and meta-analysis. Am J Kidney Dis. 2014;64(3):383–393. doi: 10.1053/j.ajkd.2014.03.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.