Abstract
Interoception, the ability to detect internal bodily signals, is vital for an individual’s well-being and is increasingly connected to mental health disorders. Research investigating relationships between individual differences in interoception and personality types is limited, and mixed results are reported across a variety of interoceptive tasks, measures, and conceptualisations. Guided by biological theories, this study contributed further to the understanding of the relationship between interoception and personality by utilising two interoceptive measures. A sample of adults (N = 114) completed three questionnaires online questionnaire, two assessing interoceptive sensibility (Body Perception Questionnaire, BPQ; and the Multidimensional Assessment of Interoceptive Awareness, MAIA) and one that assessed personality (Eysenck Personality Inventory, EPI). Multiple regression and correlational analyses showed no significant relationship between interoceptive sensibility and introversion, whereas a predictive relationship was demonstrated between interoceptive sensibility and neuroticism. Furthermore, the BPQ and subscales of the MAIA predicted neuroticism in different directions suggesting the two measures assess different constructs and thus strengthened support for a multidimensional consideration of interoception. The results have clinical implications, including the targeting of contemplative training approaches for individuals demonstrating high interoceptive sensibility and neurotic traits to improve the mental well-being of healthy individuals and clinical populations.
Keywords: interoception, personality, extraversion, introversion, neuroticism
Introduction
Interoception describes the ability to perceive internal bodily signals. Examples include the feeling of gastro-intestinal functioning and heart rate. The term was introduced by Sherrington (1906) but has more recently been redefined by Craig (2002; p.1) as “the sense of the physiological condition of the entire body.” The receipt, recognition, and evaluation of bodily signals influence behaviour; thus, an individual self-regulates in order to address bodily needs and to maintain homeostasis (Farb et al., 2015). Interoception is also understood to underpin the experience of emotions. James’ (1884) theory of emotion proposes that emotions are experienced because of the perception of bodily reactions, or visceral arousal, elicited by emotional stimuli. Individuals differ in interoceptive sensitivity and the theory suggests that individuals with enhanced interoceptive sensitivity should experience emotions more intensely. In support of the theory an example study by Wiens et al. (2000) used the heartbeat detection task as an objective measure of interoceptive sensitivity, considered to reflect accuracy in perception of the autonomic nervous system. Findings showed that participants who were good at detecting their heartbeats reported more intense emotions toward positively as well as negatively arousing film clips. Emotion-related bodily signals also affect general mood states that contribute toward emotional well-being (Farb et al., 2015), and interoceptive dysfunction is increasingly connected to mental health disorders (Khalsa et al., 2018). Interoception is, therefore, vital for an individual’s well-being.
Concerning the neurobiology of interoception, afferent neurons relay information from internal organs to the central and peripheral nervous system (Garfinkel & Critchley, 2016; Quadt et al., 2018). Areas in the brainstem including the reticular formation (Garfinkel & Critchley, 2016) and nucleus of the solitary tract (Craig, 2002) are the first recipients of visceral signals, which process and then relay this information on to the thalamus and other areas of the brain, including the insula and somatosensory cortices (Farb et al., 2015).
Previous literature reveals ambiguous definitions and wide variations in the methods used to measure interoception. Garfinkel et al. (2015) have proposed a three-dimensional construct using the following definitions of interoception. “Interoceptive accuracy” is one of the three distinct components of interoception, described as objective accuracy in identifying internal body sensations. It is typically assessed through heartbeat detection procedures including tracking the count of heartbeats in a specified time period (Schandry, 1981) or through discriminating the timing of heartbeats from external auditory stimuli (Katkin et al., 1982). Both procedures are understood to involve different processes, with tracking being reliant on internal monitoring, and discrimination dependent upon the coordination of external and internal information (Garfinkel et al., 2015). “Interoceptive awareness” is defined as the metacognitive awareness of interoceptive accuracy. This dimension incorporates confidence ratings of one’s own perceived performance on heartbeat tracking tasks and measures the relationship between objective (actual) interoceptive and metacognitive (perceived) ability (Garfinkel et al., 2015). The third dimension of interoception is “interoceptive sensibility”, which is defined as the self-perceived sensitivity to internal bodily sensations and is usually assessed using self-report questionnaires.
The present study focuses on interoceptive sensibility utilising self-report questionnaires. This interoception dimension has been selected for a variety of reasons: it provides the opportunity to appropriately investigate the research question amongst a larger sample of participants outside the laboratory setting and therefore offering generalisability of results; it allows for speed and ease of data collection through the use of structured, standardised and widely used measurement tools. Most importantly, the dimension enables assessment across a wide range of different bodily sensations, rather than restricted to the measurement of one aspect of an individual’s complex experience e.g., heartbeat detection, as is typically the case with the interoceptive accuracy dimension (although see Murphy et al., 2020).
There is still a paucity of information on interoception and its level of concordance across different bodily axes. This issue has been emphasised by Garfinkel et al. (2015), who reported that questionnaire measures of interoceptive sensibility, such as the Body Perception Questionnaire (BPQ; Porges, 1993), are independent of objective measures of interoception. The current study extends the application of questionnaires by assessing and comparing the relationship of two measures of interoceptive sensibility with personality. The BPQ is a unidimensional biological trait measure of an individual’s awareness of adverse, anxiety-related bodily sensations (Mehling, 2016), often used in biological and neuroscientific research. For example, Wiebking et al.’s (2010) fMRI study demonstrated a positive relationship between BPQ scores and neural activity in the insular cortex, while Critchley et al. (2004) reported a correlation with cortical gray matter volume in the right anterior insula of healthy participants. By contrast, the Multidimensional Assessment of Interoceptive Awareness (MAIA; Mehling et al., 2012) is a multidimensional measure of interoception and has been developed to help broaden and improve interoceptive assessment. It has been selected here, alongside the BPQ, for its potential scope in discriminating between maladaptive and adaptive aspects of body awareness, e.g., attention styles, essential for clinical application. It is a widely used self-report measure, explicitly developed to cover the multidimensionality of interoception. Specifically, five dimensions were identified, covering Awareness of body sensations, Emotional reaction and attentional response to sensations, Capacity to regulate attention, Trusting body sensations, and Mind-body integration.
Personality is a broad psychological construct that is generally understood to shape behaviour (Maltby et al., 2017). Eysenck’s biological theory of personality (Eysenck, 1967) assumes that behaviour results from relatively constant, heritable individual characteristics (traits). The theory originally classified traits into two personality types with individuals being located on a continuum of each, that is, extraversion-introversion and neuroticism-emotional stability. The extraversion-introversion type is characterised by extravert traits at one end of the continuum, including being lively and sensation-seeking. Whereas individuals toward the other end of the continuum display introvert traits including being reserved and sensation-avoiding. The neuroticism-stability type is characterised by traits of emotional instability and worry at one end, as opposed to emotional stability and rationality at the other end (Maltby et al., 2017). According to Eysenck, personality differences are attributed to biology and are expressed by the balance of neural inhibitory and excitatory mechanisms. The theory proposes that this balance of stimulation, or arousal, is managed by the ascending reticular activating system (ARAS), positioned in the brainstem’s reticular formation. The ARAS regulates arousal, via two neural circuits corresponding to each personality type. Extraversion – introversion is related to the arousal of the reticulo-cortical circuit by incoming stimuli. An introvert’s ARAS generates high arousal, causing an over-arousal and leading to introverted behaviours that avoid stimulation. In contrast, extravert’s ARAS generates low levels of arousal, causing the individual to be under aroused, and pursuing and displaying extraverted behaviours (Maltby et al., 2017; Matthews & Gilliland, 1999). Neuroticism-stability relates to reticulo-limbic circuit arousal caused by emotional stimuli. Individuals scoring high for neuroticism will reveal high arousal to emotional stimuli, whereas more emotionally stable individuals will demonstrate low arousal to such stimuli leading to contrasting behavioural reactions (Maltby et al., 2017; Matthews & Gilliland, 1999). A recent neuroimaging review supports Eysenck’s assertion for the relationship between personality traits and the structure and functioning of particular brain regions (Mitchell & Kumari, 2016). Therefore, based on theory and research, individuals scoring high for both introversion and neuroticism should show heightened autonomic activity compared with those scoring high for extraversion and emotional stability.
Cardiovascular and electrodermal activity are the most common measures used to demonstrate arousal differences between personality types (Matthews & Gilliland, 1999). Supporting Eysenck’s theory, Richards and Eves (1991) used the Eysenck Personality Questionnaire (EPQ; Eysenck & Eysenck, 1975) to measure personality traits and reported that introverts had increased heart rate responses to auditory arousal stimuli. Matthews and Gilliland’s (1999) review also reports evidence for greater increases in heart rate in response to stimuli for introverts as opposed to extraverts. Similarly, in earlier research Harvey and Hirschmann (1980) demonstrated event-related increases in heart rate in participants displaying greater introversion and higher neuroticism.
Wilson (1990) used the EPQ (Eysenck & Eysenck, 1975) to measure personality in an electrodermal activity study and reported higher daytime skin conductance levels in introverts than extraverts. However, no significant effects were established for neuroticism. Correspondingly, Matthews and Gilliland’s (1999) review revealed a consistent demonstration of higher phasic electrodermal activity in introverts than in extraverts at low arousal levels, adding further support for Eysenck’s theory. Norris et al. (2007) used the Big 5 Personality Dimension scale (Goldberg, 1992) and found that higher neuroticism, but not extraversion, predicted greater skin conductance reactivity to aversive pictorial stimuli, which may be facilitated through the greater reactivity of visceral brain regions. Similarly, Reynaud et al. (2012) reported that participants higher in neuroticism, measured using the NEO-PI-R scale (Costa & McGrae, 1992), showed greater skin conductance response to fear-evoking film stimuli.
Based on the previous literature and on the arousal-based trait characteristics of extraversion-introversion and neuroticism-stability, it appears plausible that there may be a relationship between personality type and the sensitivity to perceive visceral arousal i.e., interoception. Based on the biological personality theory one might expect a close relationship, specifically between the sensory-based aspect of interoception and arousal-related personality types. Sensory-behavioural measures such as heartbeat detection tasks would thus appear to be the most direct measures to examine the relationship between actual arousal (expressed by personality type) and perceived arousal (interoception). Indeed, there is some supporting evidence for this notion, which we review below. Notably however, existing studies have largely focused on utilising heartbeat detection tasks and occasionally employed unidimensional, sensory-based questionnaire measures such as the BPQ (e.g. Garfinkel et al., 2014), thus making it impossible to draw conclusions regarding the potential link between arousal-based personality types and higher-order dimensions of interoception. For example, using Eysenck’s theory, it is plausible to conceive a relationship between neuroticism-stability and higher-order interoceptive dimensions such as, e.g., “Trusting” one’s bodily sensations, as assessed with the MAIA. Neuroticism-stability is controlled by the reticulo-limbic circuit that regulates arousal-driven emotional reactivity and subsequent emotional experiences (Mitchell & Kumari, 2016). Characterised by heightened reactivity to emotional arousal and negative affectivity, neurotic individuals might thus not only be more sensitive to internal bodily signals, but also experience greater worry following their perception. Such experiences are captured by the “Trusting” dimension of the MAIA with questions such as: “I trust my body sensations”, which neurotic individuals may find difficult to answer in the affirmative.
While it is too early in the research process to formulate predictions concerning specific interoceptive dimensions and personality types, it is timely to examine different interoceptive dimensions and their relationship with personality to inform future investigations. Existing research considered personality types such as sensation seeking (Kruschwitz et al., 2014), psychopathy, narcissism and Machiavellianism (Lyons & Hughes, 2015) in the context of interoception. However there has been little investigation into extraversion-introversion and neuroticism-stability, with most studies to date focusing on trait anxiety. For instance, a positive relationship between trait anxiety and interoceptive awareness (measured using a heartbeat perception score) was reported by Pollatos et al. (2007). The association was explained through the heightened reactivity of the autonomic system sensitising the anxious participant to their bodily signals. Similarly, Critchley et al. (2004) reported a positive relationship between interoceptive awareness and anxiety measures in healthy participants in their fMRI study, as did Dunn et al. (2010) who reported a positive association between interoceptive accuracy and anxiety-specific arousal symptoms. More recently, Ewing et al. (2017) found poor sleep quality to be associated with higher interoceptive sensibility, as measured by the BPQ (Porges, 1993), in participants with diagnoses of anxiety and/or depression. The relationship in clinical populations was also supported by Ehlers et al. (2000) who reported higher interoceptive awareness in participants with panic disorder. Moreover, Joint hypormobility syndrome, a constitutional trait that is closely associated with anxiety, shows biological markers of hyper-active interoceptive brain structures including the insular cortex (Eccles et al., 2016). In a sub-clinical population of hypermobile and non-hypermobile volunteers, Mallorquí-Bagué et al. (2014) found state anxiety to be positively related to interoceptive sensitivity, and negatively associated with the attention regulation subscale (ability to control attention to body sensations) and the trusting (body sensations) subscale of the MAIA (Mehling et al., 2012). This relationship was greatest amongst the hypermobile group who scored higher on state anxiety than the non-hypermobile group. Taken together, these findings provide converging evidence for a largely positive relationship between various measures of interoception and anxiety as well as constitutional traits associated with anxiety.
Neuroticism has been shown to predict trait anxiety (Zinbarg et al., 2016) and is equally associated with heightened states of autonomic arousal (Eysenck, 1967). It is thus reasonable to further examine interoception and its relationship to other arousal-related personality traits such as neuroticism-stability and extraversion-introversion (Pfeifer et al., 2017). Ferentzi et al. (2017) highlighted the sparsity of empirical studies on connections between personality and interoception constructs. Their study of students went on to report no correlation between either extraversion-introversion or neuroticism-stability, measured using the Big Five Inventory (John et al., 1991), and the interoceptive sensibility measure of body awareness, measured using the Body Awareness Questionnaire (Shields et al., 1989). However, they did find a correlation with somatosensory amplification, measured using the Somatosensory Amplification Scale (Barsky et al., 2002), which includes anxiety and negative affect belonging to the personality dimension of neuroticism-stability. By contrast, Lyyra and Parviainen (2018) found a positive association between interoceptive accuracy, using a heartbeat discrimination task, and introversion, measured using both the Karolinska Scales of Personality (KSP; Schalling et al., 1983) and the Adult Temperament Questionnaire (ATQ; Evans & Rothbart, 2007).
The narrow range of existing literature examining relationships between interoception and personality traits offers a mixed understanding across a variety of measures and tasks which operationally define the constructs of interest. Selective sampling using university student samples has also been noted, which is important owing to the reported effects of age (Khalsa et al., 2009) and gender (Franzoi et al., 1989; Grabauskaite et al., 2017; Kruschwitz et al., 2014) on interoception. Therefore, the aim of this research study is to contribute further to the understanding of the relationship between interoceptive sensibility and personality by extending previous studies and using different measures of both, interoceptive sensibility and personality in a more diverse sample of United Kingdom adults. The present study seeks to answer the following research question: What relationship is there between interoceptive sensibility and the personality traits of extraversion-introversion and neuroticism-stability in United Kingdom adults?
The theoretical and empirical evidence outlined above suggest the neural underpinnings of interoception functionally overlap with those in extraversion-introversion and neuroticism-stability. On these bases, the following hypotheses are considered: (1) greater interoceptive sensibility will predict greater degrees of introversion; and (2) greater interoceptive sensibility will predict greater degrees of neuroticism.
Materials and methods
Participants
Participants (N = 120) completed a computerized questionnaire, distributed via an online data collection platform (Qualtrics, Provo, UT; http://www.qualtrics.com). Six cases were excluded due to partial completion of the questionnaire, leaving 114 as the final dataset. Of the final data set, 90% of participants completed all questionnaire items; and 10% of participants completed a minimum of 94% of items. In our final sample of N = 114, participants were a combination of a non-probability snowball sample of 84 professionals (74.3%) and an opportunity sample of 29 University students (25.7%). One participant did not disclose their occupational status. Regarding gender, 85 identified as women (74.6%) and 29 identified as men (25.4%). The breakdown of participant age and occupational status can be found in Table 1. Sample size was determined from previous studies using self-report measures of interoception (Brewer et al., 2016; Ewing et al., 2017). Based on the results of these studies, we estimated interoceptive sensibility in our study to be of a medium effect, f2 = .15. A priori power calculations (using G*-Power software, version 3.1.6; Faul et al., 2007) indicated a required sample size of (N = 107) to detect such an effect with a power of 0.95, using two predictors of interoceptive sensibility. The research was approved by the Research Ethics Committee at Leeds Beckett University (United Kingdom) and complied with The British Psychological Society’s (2018) Code of Ethics and Conduct. All participants completed an informed consent form before filling in the questionnaire.
Table 1.
Participant demographics.
| N | % | |
|---|---|---|
| Age | ||
| 18–24 years | 22 | 19.3 |
| 25–34 years | 25 | 21.9 |
| 35–44 years | 39 | 34.2 |
| 45–54 years | 12 | 10.5 |
| 55–64 years | 9 | 7.9 |
| 65–74 years | 7 | 6.1 |
| 75+ years | – | – |
| Gender | ||
| Female | 85 | 74.6 |
| Male | 29 | 25.4 |
| Occupational status | ||
| Managers, directors, senior officials | 14 | 12.4 |
| Professionals | 45 | 39.8 |
| Associate professionals and technical | 7 | 6.2 |
| Admin and secretarial | 8 | 7.1 |
| Skilled trades | 2 | 1.8 |
| Caring, leisure and other services | 4 | 3.5 |
| Sales and customer services | 4 | 3.5 |
| Process, plant and machine operatives | – | – |
| Elementary | – | – |
| Student | 29 | 25.7 |
| United Kingdom nation | ||
| England | 109 | 95.6 |
| Scotland | 5 | 4.4 |
| Northern Ireland | – | – |
| Wales | – | – |
Questionnaires
Interoceptive sensibility
One measure was the Awareness subscale of the Porges Body Perception Questionnaire (BPQ) (Porges, 1993). The Awareness subscale measures an individual’s body perception awareness on a continuum of internal bodily sensations, thereby relating specifically to the measure of interoceptive sensibility. Questions include, e.g. “During most situations I am aware of how fast I am breathing”, or “During most situations I am aware of noises associated with my digestion”. The other subscales of the BPQ are associated with stress-based sensations which assess autonomic nervous system reactivity during stressful situations, specific stress responses and stress styles. For this reason, and in keeping with previous studies that have employed the BPQ (Betka et al., 2018; Garfinkel et al., 2014, 2015), we decided that the Awareness subscale was the most appropriate to use. The subscale consists of forty-five items which are rated on a 5-point scale of experience from “Never”(0) – “Always”(4), with higher scores representing higher levels of body awareness. The BPQ, which has been translated into several different languages, is a widely used measure that has been included in over 25 peer-reviewed papers since its inception (Cabrera et al., 2018). The psychometric properties of the BPQ Awareness subscale have recently been shown to demonstrate high test-retest reliability (r = .99), high internal consistency (categorical omega = .92) and strong convergent validity (r = .67) (Cabrera et al., 2018).
The second measure of interoceptive sensibility constituted the Multidimensional Assessment of Interoceptive Awareness (MAIA) questionnaire (Mehling et al., 2012). The MAIA measures an individual’s interoceptive body awareness across thirty-two items composed of eight scales (reflecting five dimensions). Each scale is comprised of between three and 7 items. The questionnaire asks participants to indicate how often each item applies to them generally in daily life on a 6-point scale from “Never” (0) – “Always” (5). Question examples include (i) Noticing: “When I am tense I notice where the tension is located in my body,” (ii) Not-distracting: “I do not notice (I ignore) physical tension or discomfort until they become more severe” (reverse scoring), (iii) Not-worrying: “When I feel physical pain, I become upset” (reverse scoring), (iv) Attention regulation: “I can pay attention to my breath without being distracted by things happening around me,” (v) Emotional awareness: “notice how my body changes when I am angry,” (vi) Self-regulation: “When I feel overwhelmed I can find a calm place inside,” (vii) Body listening: “listen for information from my body about my emotional state,” and (viii) Trusting: “I am at home in my body.” Five items, over two scales, are reverse scored. The original study (Mehling et al., 2012) demonstrated adequate psychometric properties of internal consistency reliability; Cronbach alphas ranged from 0.66 to 0.87 and were greater than 0.70 for five of the eight scales (0.69 for noticing; 0.66 for not-distracting; 0.67 for not-worrying; 0.87 for attention regulation; 0.82 for emotional awareness; 0.83 for self-regulation; 0.82 for body listening; 0.79 for trusting) and scale-scale correlations were low, suggesting independence. Evidence of construct validity was provided and confirmatory factor analysis revealed a good model fit.
Personality
The Eysenck Personality Inventory (EPI) (Eysenck & Eysenck, 1964) is a biologically based measure of personality comprising a fifty-seven-item scale requiring binary “yes” (1) or “no” (0) responses to questions regarding the way one behaves, feels, and acts. It measures a participant’s personality across the two dimensions of extraversion-introversion and neuroticism-stability, each assessed via twenty-four items. The former dimension scale contains nine items which are reverse scored. Questions include: “Do you often long for excitement?” and “Do you often worry about things you should have done or said?” An additional nine items comprise a lie scale that can be utilised to predict response bias, however these items were not considered in the present study. The extent of extraversion-introversion and neuroticism-stability are measured on score continuums of 0–24, with higher scores indicating greater extraversion or neuroticism, respectively. Good validity was reported in the original study (Eysenck & Eysenck, 1964) and extensive use in previous research has demonstrated largely sound psychometrics; construct validity for males (r = .58) and females (r = 0.63) has been supported (Platt et al., 1971) and the neuroticism-stability scale has been found to be orthogonal to the extraversion-introversion scale, showing weak correlations between the two scales for males (r = .06) and females (r = .19) (Howarth, 1976).
Data analysis
Using Cronbach’s alpha, reliability analyses were computed for the EPI, BPQ, and MAIA on our sample. The mean score for the BPQ and the mean scores for each subscale of the MAIA were used in data analyses, along with the total scores for the two dimensions of the EPI scale. Data were analysed using IBM SPSS Statistics v24.0 software. Linear regression analyses were performed across all measures to examine the specific predictions that (i) greater interoceptive sensibility would predict greater degrees of introversion, and (ii) greater interoceptive sensibility would predict greater degrees of neuroticism. To this end, two forced entry multiple regression analyses were performed with the eight subscales of interoceptive sensibility comprising the MAIA as the predictor variables, and with extraversion-introversion and neuroticism-stability as the criterion variables, respectively. Forced entry is a method in which the predictors are simultaneously forced into the regression model and is deemed an appropriate method for selection when there is no evidence-based reason to specify the order of the predictors entered into the model (Field, 2013). To examine whether the BPQ’s unidimensional measure of interoceptive sensibility could predict personality types, two simple regression analyses were computed, one with extraversion-introversion and one with neuroticism-stability as the criterion variable.
Pearson’s correlations were performed alongside regression analyses to explore relationships between all measures. In addition, and for completeness, correlational analyses were performed between each of the eight MAIA subscales (using the mean scores for each subscale): Noticing, Not-distracting, Not-worrying, Attention regulation, Emotional awareness, Self-regulation, Body listening, and Trusting. The correlation matrix of these analyses can be found in Table 5. Key assumptions of linearity, homoscedasticity, and independence of residuals were met for each variable and there was no evidence of multicollinearity or influential outliers apparent. There was concern over the violation of the normality of residuals assumption, revealed through P-P plots (Field, 2013). Therefore, a robust bootstrapping regression method was employed to generate confidence intervals (95% bias corrected and accelerated) and significance tests of the model parameters.
Table 5.
Correlation matrix illustrating the relationship between the two measures of interoception (BPQ and subscales of the MAIA), and the two personality dimensions (extraversion-introversion and neuroticism-stability).
| EPI – extraversion-introversion | EPI – neuroticism-stability | BPQ-1 (awareness) | MAIA | MAIA | MAIA | MAIA | MAIA | MAIA | MAIA | MAIA | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1) Noticing | 2) Not- distracting | 3) Not- worrying | 4) Attention regulation | 5) Emotional awareness | 6) Self-regulation | 7) Body listening | 8) Trusting | |||||
| EPI – extraversion-introversion | 1 | |||||||||||
| EPI – neuroticism-stability | −.061.521 | 1 | ||||||||||
| BPQ-1 (awareness) | .070.230 | .348* <.001 |
1 | |||||||||
|
−.003.978 | .215* .022 |
.340* <.001 |
1 | ||||||||
| MAIA2) Not-distracting | −.048 .614 |
−.153 .104 |
−.018 .847 |
−.043 .651 |
1 | |||||||
|
.056 .554 |
−.168 .073 |
−.133 .159 |
−.099 .295 |
−.304* .001 |
1 | ||||||
| MAIA4) Attention regulation | −.071 .452 |
−.202* .031 |
.038 .687 |
.436* <.001 |
.045 .637 |
.089 .349 |
1 | |||||
| MAIA5) Emotional awareness | −.128 .174 |
.111 .240 |
.264* .005 |
.596* <.001 |
.099 .296 |
−.156 .098 |
.416* <.001 |
1 | ||||
| MAIA6) Self-regulation | −.064 .498 |
−.363* <.001 |
−.070 .461 |
.239* .010 |
.079 .405 |
.014 .884 |
.571* <.001 |
.396* <.001 |
1 | |||
| MAIA7) Body listening | −.051 .588 |
−.015 .874 |
.167 .076 |
.456* <.001 |
.140 .137 |
−.176 .061 |
.455* <.001 |
.637* <.001 |
.526* <.001 |
1 | ||
| MAIA8) Trusting | .092 .328 |
−.525* <.001 |
−.247* .008 |
−.088 .354 |
.081 .394 |
.104 .272 |
.277* .003 |
.177 .060 |
.478* <.001 |
.312* .001 |
1 | |
Note: In each cell, the upper number corresponds to the r value, and the lower number denotes the p value (Significant results are shown in italics; *p =/< 0.05, 2-tailed).Scale-scale correlations are also presented.
Results
Descriptive statistics for all questionnaire results are shown in Table 2.
Table 2.
Descriptive statistics showing sample mean scores (SD) (N = 114) for all questionnaires and subscales.
| Measure | Range | Mean and (SD) |
|---|---|---|
| MAIA subscales | ||
| Noticing | 0–5 | 3.01 (0.92) |
| Not distracting | 0.33–4.67 | 2.08 (0.79) |
| Not worrying | 0.33–5 | 2.75 (0.83) |
| Attention regulation | 0.43–4.29 | 2.65 (0.79) |
| Emotional awareness | 0.6–5 | 3.08 (0.94) |
| Self-regulation | 0–4.25 | 2.56 (0.91) |
| Body listening | 0–4 | 1.97 (0.97) |
| Trusting | 0–5 | 3.34 (1.05) |
| BPQ | 0.09–3.42 | 1.49 (0.85) |
| EPI introversion-extraversion | 1–21 | 11.05 (4.81) |
| EPI stability-neuroticism | 2–24 | 12.30 (5.18) |
Reliability measures
The internal consistency reliability (Cronbach’s alpha) of the EPI was .823 for the extraversion-introversion dimension and .838 for the neuroticism-stability dimension. The BPQ yielded a high internal consistency reliability score of .975. Cronbach’s alphas for the MAIA ranged from 0.44 to 0.85 and were greater than 0.70 for six of the eight scales (0.75 for “Noticing”; 0.58 for “Not-distracting”; 0.44 for “Not-worrying”; 0.84 for “Attention regulation”; 0.85 for “Emotional awareness”; 0.83 for “Self-regulation”; 0.79 for “Body listening”; 0.84 for “Trusting”).
Regressions
Linear regression analyses were performed to assess if the two interoceptive measures, the eight subscales of the MAIA and the BPQ, could be used to predict the following criterion variables; (i) extraversion-introversion scores and (ii) neuroticism-stability scores, as measured using the EPI.
Extraversion-introversion dimension
Multiple regression results revealed that the 8 subscales of the MAIA explained a non-significant proportion (6%) of the variance in extraversion-introversion scores (F(8, 113) = 0.83, p = .580, R2 = .06). Similarly, the BPQ explained a non-significant proportion (0.5%) of the variance in extraversion-introversion scores (F(1, 113) = 0.55, p = .460, R2 = .005). Table 3 shows the beta coefficients for each model, including standard errors, standardised betas and significance values for each predictor. As can be seen in Table 3, both measures of interoceptive sensibility were non-significant predictors of extraversion-introversion.
Table 3.
Linear models showing predictors of extraversion-introversion (EPI).
| B | SE B | Beta | p | |
|---|---|---|---|---|
| BPQ score | 0.395 | 0.533 | .070 | .460 |
| MAIA subscale scores | ||||
| Noticing | 1.02 | .688 | .194 | .143 |
| Not-distracting | −.115 | .616 | −.019 | .852 |
| Not worrying | .161 | .604 | .028 | .791 |
| Attention regulation | −.527 | .779 | −.086 | .501 |
| Emotional awareness | −1.15 | .712 | −.224 | .110 |
| Self-regulation | −.45 | .706 | −.085 | .526 |
| Body listening | .151 | .683 | .031 | .825 |
| Trusting | .923 | .524 | .202 | .081 |
Note: Values are 95% bias corrected and accelerated confidence intervals and standard errors based on 1000 bootstrap samples.
Neuroticism-stability dimension
Multiple regression results showed that the 8 subscales of the MAIA explained a significant proportion (41%) of the variance in neuroticism-stability scores (F(8, 113) = 9.28, p = < .001, R2 = .41). Moreover, the BPQ explained a significant proportion (12%) of the variance in neuroticism-stability scores (F(1, 113) = 15.41, p < .001, R2 = .12). Table 4 shows the beta coefficients for each model, including standard errors, standardised betas, and significance values for each predictor. As can be seen in Table 4, both measures of interoceptive sensibility were significant predictors of neuroticism-stability, and the “Trusting” subscale of the MAIA was the best predictor of neuroticism-stability.
Table 4.
Linear models showing predictors of neuroticism-stability (EPI).
| B | SE B | Beta | p | |
|---|---|---|---|---|
| BPQ score | 2.12 | .539 | .348 | <.001* |
| MAIA subscale scores | ||||
| Noticing | .529 | .585 | .094 | .368 |
| Not-distracting | −1.04 | .524 | −.158 | .051 |
| Not worrying | −.604 | .513 | −.097 | .242 |
| Attention regulation | −.693 | .662 | −.105 | .298 |
| Emotional awareness | 1.01 | .605 | .184 | .097 |
| Self-regulation | −1.49 | .600 | −.260 | .015* |
| Body listening | .773 | .581 | .145 | .186 |
| Trusting | −2.05 | .445 | −.418 | <.001* |
Note: Values are 95% bias corrected and accelerated confidence intervals and standard errors based on 1000 bootstrap samples. (Significant results are shown in italics; *p =/< 0.05, 2-tailed).
Correlations
Pearson’s correlations (r) were calculated to determine the degree of linear association between participants’ mean scores for the interoceptive sensibility predictor variables (MAIA and BPQ) on the two dimensions of personality (EPI) criterion variables. The full matrix of correlations is included in Table 5.
Interoceptive sensibility and extraversion-introversion
There was a non-significant relationship between participant’s MAIA scores and their EPI extraversion-introversion scale scores (all subscales: r < .10, p > .17, N = 114, R2 = 0.06). Similarly, the relationship between participant’s BPQ scores and their EPI extraversion-introversion scale scores was non-significant (r = .07, p = .230, N = 114, R2 = 0.005).
Interoceptive sensibility and neuroticism-stability
Several significant negative relationships were found between participant’s MAIA scores and their EPI neuroticism-stability scale scores, such that participants who scored higher on subscales of the MAIA scored lower on the neuroticism-stability scale (i.e., more stable). These relationships were found for the subscales “Attention regulation”, “Self-regulation”, and “Trusting” of body sensations (all susbcales r > −.20, p < .02, N = 114, R2 = 0.041). Only one subscale of the MAIA, “Noticing” of body sensations, was significantly positively correlated with neuroticism (see Table 5 for further details).
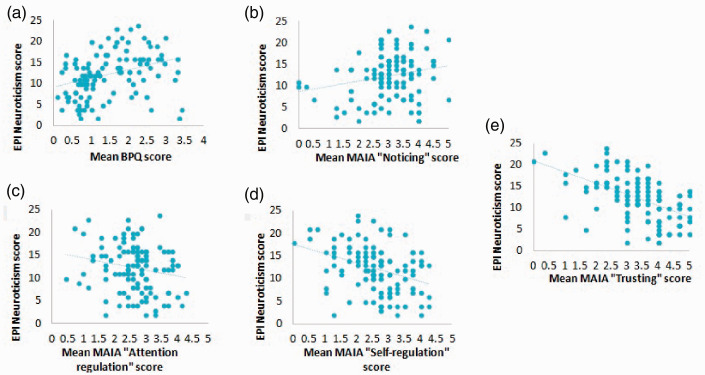
However, there was a significant, medium positive relationship between participant’s BPQ scores and their EPI neuroticism-stability scale scores, such that participants who scored higher on the BPQ measure of interoceptive sensibility scored higher for EPI neuroticism (i.e. more neurotic) (r = .35, p = <.001, N = 114, R2 = 0.121). These relationships are illustrated in Figure 1.
Figure 1.
Correlations between two measures of interoceptive sensibility (BPQ and MAIA subscales) and neuroticism-stability. (a and b) Scatterplots showing that higher scores on the BPQ and the MAIA subscale “Noticing” were associated with significantly lower scores for neuroticism. (c to e) Scatterplots showing that lower scores on the MAIA subscales “Attention regulation”, “Self-regulation” and “Trusting” were associated with significantly higher scores for neuroticism. MAIA: Multidimensional Assessment of Interoceptive Awareness; BPQ: Body Perception Questionnaire; EPI: Eysenck Personality Inventory.
Discussion
The current study was inspired by the narrow range of existing research investigating relationships between interoception and personality traits and the mixed results reported across a variety of interoceptive tasks, measures and conceptualisations. The research was guided by biological trait theories of interoception and personality and aimed to examine the relationship between interoceptive sensibility and extraversion-introversion and neuroticism-stability personality traits in a cross-sectional, questionnaire-based online questionnaire of United Kingdom adults.
This study identified a significant predictive relationship between interoceptive sensibility and neuroticism, consistent with the hypothesis and the findings of previous studies (Critchley et al., 2004; Dunn et al., 2010; Ehlers et al., 2000; Ewing et al., 2017; Mallorquí-Bagué et al., 2014; Pollatos et al., 2007). The relationship may be explained through the heightened autonomic reactivity of neurotic participants, which increases their sensitivity to body sensations. Specifically, neuroticism was predicted by the BPQ and correlated positively with the “Noticing” subscale of the MAIA. Both scales assess the subjective awareness of bodily (physiological) sensations, an interoceptive dimension which individuals scoring high in neuroticism might be particularly sensitive to. The high overlap in the type of interoception assessed by the two scales was further corroborated by the significant positive scale-scale correlation between the BPQ and the “Noticing” subscale of the MAIA.
Notably, the BPQ and two subscales of the MAIA, “Self regulation” and “Trusting”, predicted neuroticism in different directions. The BPQ’s positive relationship with neuroticism corroborates the findings from aforementioned studies (e.g. Zinbarg et al., 2016), which is unsurprising given the measure’s unidimensional biological focus on negative, anxiety-related bodily sensations (Mehling, 2016); whereas the MAIA’s negative relationship may reflect its multidimensional assessment of interoception. In contrast with the BPQ, the MAIA explores adaptive, beneficial aspects of body awareness as well as those which are maladaptive and negative. Therefore, we would expect participants displaying more emotional stability and lower neuroticism to better relate to positive body awareness questions posed by the MAIA. This was evidenced by the “Trusting” dimension that turned out to be the best predictor for low neuroticism, suggesting increased levels of trust in, and use of, bodily sensations for decision making (Mehling et al., 2012) in emotionally stable individuals. Similarly, the second predictor for low neuroticism, “Self regulation”, signifies an ability of emotionally stable individuals to regulate emotions and sensations following their awareness. Consistent with findings by Mallorquí-Bagué et al. (2014) this is further evidenced through the significant negative correlations demonstrated between neuroticism and the “Attention regulation”, “Self-regulation”, and “Trusting” subscales.
Contrary to expectations and previous studies (Harvey & Hirschmann, 1980; Lyyra & Parviainen, 2018; Matthews & Gilliland, 1999; Richards & Eves, 1991; Wilson, 1990), we found no significant relationship between interoceptive sensibility and introversion. A feasible explanation for this finding is a possible weaker link between cortical arousal (underpinning introversion) and interoception, compared with a stronger link between autonomic arousal (underpinning neuroticism) and interoception (see e.g. Ferentzi et al., 2017). Specifically, based on Eysenck’s (1967) theory, introversion is related to the arousal of the reticulo-cortical circuit whereas neuroticism is related to the arousal of the reticulo-limbic circuit. Therefore, these results support Eysenck’s model of arousal.
The findings from the current study could have important clinical implications based on the well-established association between heightened autonomic arousal and anxiety (Ehlers et al., 2000; Pollatos et al., 2007), as well as anxiety-related constitutional traits such as Joint hypermobility syndrome (Eccles et al., 2016; Mallorquí-Bagué et al., 2014). The close relationship between heightened autonomic arousal states and anxiety give rise to further constitutional vulnerabilities and traits to develop anxiety, including neuroticism. Neuroticism has been linked to increased psychological reactivity to stressors (Norris et al., 2007), while interoception (the sensitivity to changes in bodily arousal states) is considered to be critical to an individual’s mental health and well-being (Farb et al., 2015). Thus, individuals demonstrating high interoceptive sensibility and neurotic traits may benefit from targeted psychotherapeutic interventions that focus on resilience-enhancing attention styles, improving the ability to regulate attention towards interoceptive bodily sensations; self-regulate distress by attention to bodily signals, and trust body sensations e.g., using mindfulness approaches. Such contemplative training appears beneficial for the mental well-being of healthy individuals as well as in clinical populations including those experiencing anxiety-disorders (Bornemann et al., 2014). Future research could replicate this present study before and after such a contemplative training intervention, with healthy and clinical populations, to assess any change in the constructs of interest.
Notable limitations of this study are linked to the reliance upon subjective self-report questionnaires, rather than objective behavioural measures, which is important because individual participants do not necessarily have a good understanding of their interoceptive ability (Ewing et al., 2017). However, the use of questionnaires has enabled a broader assessment of the constructs of interest than would have been the case with objective measures. Findings need to be considered in light of the MAIA’s provision of low internal consistency for two if its subscales (“Not-distracting”, “Not-worrying”), both in Mehling et al.’s (2012) original study and in our sample, raising potential concerns over the validity of the measure and the findings reported herein. Future research could examine whether the findings from this study are consistent when investigated with the new MAIA-2 measure (Mehling et al., 2018), and with objective behavioural measures of interoception and personality. For example, an emerging gamification approach to personality testing could address some key self-report limitations (McCord et al., 2019).
Our correlational results (Table 5) need to be considered in the light of the uncorrected multiple comparisons that might have led to Type-1 errors. Yet, application of even a more stringent correction such as Bonferroni (Field, 2013) yielded significant correlations at a significance level of p < 0.001 that converged with the significant findings of our regression results.
A further limitation concerns the over-representation of women (74.6%) in the final sample. Previous research (Franzoi et al., 1989; Grabauskaite et al., 2017) suggests the existence of gender differences in interoceptive sensibility, with females reporting significantly higher scores. Therefore, the over-representation of women in the sample could have impacted the results. Future studies could look to achieve a more balanced sample. However, this study should be recognised for its achievement of a large sample of a more diverse range of adult participants than previous studies. The use of two interoceptive measures (BPQ and MAIA) in a within-subject design provides a valuable comparison, as well as a targeted guide for future application. Specifically, the predictive relationship between interoceptive sensibility and neuroticism in our adult sample reveals a divergence in findings within subjective measures of interoception. This suggests that the BPQ and MAIA do in fact measure different constructs and supports a multidimensional consideration of interoception that might find relevance in clinical applications.
Author Biographies
Alison Pearson MSc, is a Master of Psychology, Leeds School of Social Sciences, Psychology Department, Leeds Beckett University
Gaby Pfeifer PhD, is a Lecturer in Psychology, Leeds School of Social Sciences, Psychology Department, Leeds Beckett University.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Gaby Pfeifer https://orcid.org/0000-0002-8737-1255
References
- Barsky A. J., Saintfort R., Rogers M. P., Borus J. F. (2002). Nonspecific medication side effects and the nocebo phenomenon. Journal of the American Medical Association, 287(5), 622–627. [DOI] [PubMed] [Google Scholar]
- Betka S., Pfeifer G., Garfinkel S., Prins H., Bond R., Sequeira H., Duka D., Critchley H. (2018). How do self-assessment of alexithymia and sensitivity to bodily sensations relate to alcohol consumption? Alcoholism, Clinical and Experimental Research, 42(1), 81–88. 10.1111/acer.13542. [DOI] [PubMed] [Google Scholar]
- Bornemann B., Herbert B. E., Mehling W. E., Singer T. (2014). Differential changes in self-reported aspects of interoceptive awareness through 3 months of contemplative training. Frontiers in Psychology, 5, 1504–1513. 10.3389/fpsyg.2014.01504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer R., Cook R., Bird G. (2016). Alexithymia: A general deficit of interoception. Royal Society Open Science, 3(10), 150664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera A., Kolacz J., Pailhez G., Bulbena-Cabre A., Bulbena A., Porges S. W. (2018). Assessing body awareness and autonomic reactivity: Factor structure and psychometric properties of the body perception Questionnaire-Short form (BPQ-SF). International Journal of Methods in Psychiatric Research, 27(2), e1596. 10.1002/mpr.1596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa P. T., McGrae R. R. (1992). Professional manual of the revised NEO personality inventory (NEO-PI-R) and NEO five-factor inventory (NEO-FFI). Psychological Assessment Resources. [Google Scholar]
- Craig A. D. (2002). How do you feel? Interoception: The sense of the physiological condition of the body. Nature Reviews. Neuroscience, 3(8), 655–666. 10.1038/nrn894 [DOI] [PubMed] [Google Scholar]
- Critchley H. D., Wiens S., Rotshtein P., Ohman A., Dolan R. J. (2004). Neural systems supporting interoceptive awareness. Nature Neuroscience, 7(2), 189–195. 10.1038/nn1176 [DOI] [PubMed] [Google Scholar]
- Dunn B. D., Stefanovitch I., Evans D., Oliver C., Hawkins A., Dalgleish T. (2010). Can you feel the beat? Interoceptive awareness is an interactive function of anxiety – and depression-specific symptom dimensions. Behaviour Research and Therapy, 48(11), 1133–1138. 10.1016/j.brat.2010.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles J., Owens A., Harrison N., Grahame R., Critchley H. (2016). Joint hypermobility an autonomic hyperactivity: An autonomic and functional neuroimaging study. The Lancet, 387, S40. 10.1016/S0140-6736(16)00427-X [DOI] [Google Scholar]
- Ehlers A., Mayou R. A., Sprigings D. C., Birkhead J. (2000). Psychological and perceptual factors associated with arrhythmias and benign palpitations. Psychosomatic Medicine, 62(5), 693–702. 10.1097/00006842-200009000-00014 [DOI] [PubMed] [Google Scholar]
- Evans D. E., Rothbart M. K. (2007). Development of a model for adult temperament. Journal of Research in Personality, 41(4), 868–888. 10.1016/j.jrp.2006.11.002 [DOI] [Google Scholar]
- Ewing D. L., Manassei M., Gould van Praag C., Philippides A. O., Critchley H. D., Garfinkel S. N. (2017). Sleep and the heart: Interoceptive differences linked to poor experiential sleep quality in anxiety and depression. Biological Psychology, 127, 163–172. 10.1016/j.biopsycho.2017.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysenck H. J. (1967). The biological basis of personality. Thomas. [DOI] [PubMed] [Google Scholar]
- Eysenck H. J., Eysenck S. B. G. (1964). Manual of the eysenck personality inventory. University of London Press. [Google Scholar]
- Eysenck H. J., Eysenck S. B. G. (1975). Manual of the eysenck personality questionnaire. Hodder and Stoughton. [Google Scholar]
- Farb N., Daubenmier J., Price C. J., Gard T., Kerr C., Dunn B. D., Klein A. C., Paulus M. P., Mehling W. E. (2015). Interoception, contemplative practice, and health. Frontiers in Psychology, 6, 763–726. 10.3389/fpsyg.2015.00763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul F., Erdfelder E., Lang A.-G., Buchner A. (2007). G_power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39(2), 175–191. [DOI] [PubMed] [Google Scholar]
- Ferentzi E., Koteles F., Csala B., Drew R., Tihanyi B. T., Pulay-Kottlar G., Doering B. K. (2017). What makes sense in our body? Personality and sensory correlates of body awareness and somatosensory amplification. Personality and Individual Differences, 104, 75–81. 10.1016/j.paid.2016.07.034 [DOI] [Google Scholar]
- Field A. (2013). Discovering statistics using IBM SPSS statistics. SAGE Publications Ltd. [Google Scholar]
- Franzoi S. L., Kessenich J. J., Sugrue P. A. (1989). Gender differences in the experience of body awareness: An experiential sampling study. Sex Roles, 21(7-8), 499–515. 10.1007/BF00289100 [DOI] [Google Scholar]
- Garfinkel S. N., Critchley H. D. (2016). Threat and the body: How the heart supports fear processing. Trends in Cognitive Sciences, 20(1), 34–46. 10.1016/j.tics.2015.10.005 [DOI] [PubMed] [Google Scholar]
- Garfinkel S. N., Minati L., Gray M. A., Seth A. K., Dolan R. J., Critchley H. D. (2014). Fear from the heart: Sensitivity to fear stimuli depends on individual heartbeats. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 34(19), 6573–6582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfinkel S. N., Seth A., Barrett A., Suzuki K., Critchley H. (2015). Knowing your own heart: Distinguishing interoceptive accuracy from interoceptive awareness. Biological Psychology, 104, 65–74. 10.1016/j.biopsycho.2014.11.004 [DOI] [PubMed] [Google Scholar]
- Goldberg L. R. (1992). The development of markers for the Big-Five factor structure. Psychological Assessment, 4(1), 26–42. 10.1037/1040-3590.4.1.26 [DOI] [Google Scholar]
- Grabauskaite A., Baranauskas M., Griškova-Bulanova I. (2017). Interoception and gender: What aspects should we pay attention to? Consciousness and Cognition, 48, 129–137. 10.1016/j.concog.2016.11.002 [DOI] [PubMed] [Google Scholar]
- Harvey F., Hirschmann R. (1980). The influence of extraversion and neuroticism on heart rate responses to aversive visual stimuli. Personality and Individual Differences, 1(1), 97–100. 10.1016/0191-8869(80)90011-2 [DOI] [Google Scholar]
- Howarth E. (1976). A psychometric investigation of Eysenck’s personality inventory. Journal of Personality Assessment, 40(2), 173–185. 10.1207/s15327752jpa4002_9 [DOI] [PubMed] [Google Scholar]
- James W. (1884). What is an emotion? Mind, os-IX(34), 188–205. [Google Scholar]
- John O. P., Donahue E. M., Kentle R. L. (1991). The big five inventory-versions 4a and 54. University of California, Berkeley, Institute of Personality and Social Research.
- Katkin E. S., Morell M. A., Goldband S., Bernstein G. L., Wise J. A. (1982). Individual differences in heartbeat discrimination. Psychophysiology, 19(2), 160–166. [DOI] [PubMed] [Google Scholar]
- Khalsa S. S., Adolphs R., Cameron O. G., Critchley H. D., Davenport P. W., Feinstein J. S., Feusner J. D., Garfinkel S. N., Lane R. D., Mehling W. E., Meuret A. E., Nemeroff C. B., Oppenheimer S., Petzschner F. H., Pollatos O., Rhudy J. L., Schramm L. P., Simmons W. K., Stein M. B., … Paulus M. P, Interoception Summit 2016 participants. (2018). Interoception and mental health: A roadmap. Biological Psychiatry. Cognitive Neuroscience and Neuroimaging, 3(6), 501–513. 10.1016/j.bpsc.2017.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalsa S. S., Rudrauf D., Tranel D. (2009). Interoceptive awareness declines with age. Psychophysiology, 46(6), 1130–1136. 10.1111/j.1469-8986.2009.00859.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruschwitz J. D., Lueken U., Wold A., Walter H., Paulus M. P. (2014). High thrill and adventure seeking is associated with reduced interoceptive sensitivity: Evidence for an altered sex-specific homeostatic processing in high sensation seekers. European Journal of Personality, 28(5), 472–481. 10.1002/per.1946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons M., Hughes S. (2015). Feeling me feeling you: Links between the dark triad and internal body awareness. Personality and Individual Differences, 86, 308–311. [Google Scholar]
- Lyyra P., Parviainen T. (2018). Behavioral inhibition underlies the link between interoceptive sensitivity and anxiety-related temperamental traits. Frontiers in Psychology, 9, 1026–1028. 10.3389/fpsyg.2018.01026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallorquí-Bagué N., Garfinkel S. N., Engels M., Eccles J. A., Pailhez G., Bulbena A., Critchley H. D. (2014). Neuroimaging and psychophysiological investigation of the link between anxiety, enhanced affective reactivity and interoception in people with joint mobility. Frontiers in Psychology, 5, 1–8. 10.3389/fpsyg.2014.01162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltby J., Day L., Macaskill A. (2017). Personality, individual differences and intelligence. Pearson Education Limited. [Google Scholar]
- Matthews G., Gilliland K. (1999). The personality theories of H.J. Eysenck and J.A. Gray: A comparative review. Personality and Individual Differences, 26(4), 583–626. 10.1016/S0191-8869(98)00158-5 [DOI] [Google Scholar]
- McCord J., Harman J. L., Purl J. (2019). Game-like personality testing: An emerging mode of personality assessment. Personality and Individual Differences, 143, 95–102. 10.1016/j.paid.2019.02.017 [DOI] [Google Scholar]
- Mehling W. E. (2016). Differentiating attention styles and regulatory aspects of self-reported interoceptive sensibility. Philosophical Transactions of the Royal Society B: Biological Sciences, 371(1708), 20160013. 10.1098/rstb.2016.0013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehling W. E., Acree M., Stewart A., Silas J., Jones A. (2018). The multidimensional assessment of interoceptive awareness, version 2 (MAIA-2). PLos One, 13(12), e0208034. 10.1371/journal.pone.0208034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehling W. E., Price C., Daubenmier J. J., Acree M., Bartmess E., Stewart A. (2012). The multidimensional assessment of interoceptive awareness (MAIA). PLoS One, 7(11), e48230. 10.1371/journal.pone.0048230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell R. L. C., Kumari V. (2016). Hans Eysenck’s interface between the brain and personality: Modern evidence on the cognitive neuroscience of personality. Personality and Individual Differences, 103, 74–81. 10.1016/j.paid.2016.04.009 [DOI] [Google Scholar]
- Murphy J., Brewer R., Plans D., Khalsa S., Catmur C., Bird G. (2020). Testing the independence of self-reported interoceptive accuracy and attention. Quarterly Journal of Experimental Psychology, 73(1), 115–133. [DOI] [PubMed] [Google Scholar]
- Norris C. J., Larsen J. T., Cacioppo J. T. (2007). Neuroticism is associated with larger and more prolonged electrodermal responses to emotionally evocative pictures. Psychophysiology, 44(5), 823–826. 10.1111/j.1469-8986.2006.00551 [DOI] [PubMed] [Google Scholar]
- Pfeifer G., Garfinkel S. N., Gould van Praag C. D., Sahota K., Betka S., Critchley H. D. (2017). Feedback from the heart: Emotional learning and memory is controlled by cardiac cycle, interoceptive accuracy and personality. Biological Psychology, 126, 19–29. [DOI] [PubMed] [Google Scholar]
- Platt J. J., Pomeranz D., Eisenman R. (1971). Validation of the Eysenck personality inventory by the MMPI and Internal-External control scale. Journal of Clinical Psychology, 27(1), 104–105. [DOI] [PubMed] [Google Scholar]
- Pollatos O., Traut-Mattausch E., Schroeder H., Schandry R. (2007). Interoceptive awareness mediates the relationship between anxiety and the intensity of unpleasant feelings. Journal of Anxiety Disorders, 21(7), 931–943. 10.1016/j.janxdis.2006.12.004 [DOI] [PubMed] [Google Scholar]
- Porges S. W. (1993). Body perception questionnaire: Laboratory of development assessment. University of Maryland. [Google Scholar]
- Quadt L., Critchley H. D., Garfinkel S. N. (2018). The neurobiology of interoception in health and disease. Annals of the New York Academy of Sciences, 1428(1), 112–128. 10.1111/nyas.13915 [DOI] [PubMed] [Google Scholar]
- Reynaud E., El Khoury-Malhame M., Rossier J., Blin O., Khalfa S. (2012). Neuroticism modifies psychophysiological responses to fearful films. PLoS One, 7(3), e32413. 10.1371/journal.pone.0032413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards M., Eves F. (1991). Personality, temperament and the cardiac defense response. Personality and Individual Differences, 12(10), 999–1004. 10.1016/0191-8869(91)90030-F [DOI] [Google Scholar]
- Schalling D., Edman G., Asberg M. (1983). Biological basis of sensation seeking, impulsivity and anxiety. In Zuckerman M. (Ed.), Impulsive cognitive style and inability to tolerate boredom (pp. 123–145). Earlbaum. [Google Scholar]
- Schandry R. (1981). Heart beat perception and emotional experience. Psychophysiology, 18(4), 483–488. 10.1111/j.1469-8986.1981.tb02486.x [DOI] [PubMed] [Google Scholar]
- Sherrington C. S. (1906). The integrative action of the nervous system. Yale University Press. [Google Scholar]
- Shields S. A., Mallory M. E., Simon A. (1989). The body awareness questionnaire: Reliability and validity. Journal of Personality Assessment, 53(4), 802–815. 10.1207/s15327752jpa5304_16 [DOI] [Google Scholar]
- The British Psychological Society. (2018). Code of ethics and conduct.
- Wiebking C., Bauer A., de Greck M., Duncan N. W., Tempelmann C., Northoff G. (2010). Abnormal body perception and neural activity in the insula in depression: An fMRI study of the depressed “material me.” The World Journal of Biological Psychiatry: The Official Journal of the World Federation of Societies of Biological Psychiatry, 11(3), 538–549. 10.3109/15622970903563794 [DOI] [PubMed] [Google Scholar]
- Wiens S., Mezzacappa E. S., Katkin E. S. (2000). Heartbeat detection and the experience of emotions. Cognition & Emotion, 14(3), 417–427. 10.1080/026999300378905 [DOI] [Google Scholar]
- Wilson G. D. (1990). Personality, time of day and arousal. Personality and Individual Differences, 11(2), 153–168. 10.1016/0191-8869(90)90008-F [DOI] [Google Scholar]
- Zinbarg R. E., Mineka S., Bobova L., Craske M. G., Vrshek-Schallhorn S., Griffith J. W., Wolitzky-Taylor K., Waters A. M., Sumner J. A., Anand D. (2016). Testing a hierarchical model of neuroticism and its cognitive facets: Latent structure and prospective prediction of first onsets of anxiety and unipolar mood disorders during 3 years in late adolescence. Clinical Psychological Science, 4(5), 805–824. 10.1177/2167702615618162 [DOI] [Google Scholar]