Abstract
Purpose:
To evaluate the effect of sulindac, a non-selective anti-inflammatory drug, for activity to reduce breast density (BD), a risk factor for breast cancer.
Experimental Design:
An open-label Phase 2 study was conducted to test the effect of 12 months’ daily sulindac at 150 mg bid on change in percent BD in postmenopausal hormone receptor-positive breast cancer patients on aromatase inhibitor (AI) therapy. Change in percent BD in the contralateral, unaffected breast was measured by non-contrast MRI and reported as change in MRI percent BD (MRPD). A non-randomized patient population on AI therapy (observation group) with comparable baseline BD was also followed for 12-months. Changes in tissue collagen after 6 months of sulindac treatment were explored using second-harmonic generated (SHG) microscopy in a subset of women in the sulindac group who agreed to repeat breast biopsy.
Results:
In 43 women who completed 1 year of sulindac (86% of those accrued), relative MRPD significantly decreased by 9.8% (95% CI, −14.6 to −4.7) at 12 months; an absolute decrease of −1.4% (95% CI, −2.5 to −0.3). A significant decrease in mean breast tissue collagen fiber straightness (p=0.032), an investigational biomarker of tissue inflammation, was also observed. MRPD (relative or absolute) did not change in the AI only observation group (N = 40).
Conclusions.
This is the first study to indicate that the NSAID sulindac may reduce BD. Additional studies are needed to verify these findings and determine if prostaglandin E2 inhibition by NSAIDs is important for BD or collagen modulation.
INTRODUCTION
Non-steroidal anti-inflammatory drugs (NSAIDs) including aspirin have been extensively studied for their inhibition of cyclooxygenase 2 (COX2) and its pro-inflammatory/pro-tumorigenic metabolite, prostaglandin E2 (PGE2).(1) Early demonstration that PGE2 increases estrogen synthesis via alternate promoter use in CYP19, the aromatase gene, provided initial linking of PGE2 to estrogen in breast cancer risk. (2–6) In 1996, PGE2 was described as ‘one of the most potent factors stimulating aromatase expression’ in tissues.(7) Evidence that PGE2 promoted mammary tumors in mice, along with lower breast cancer incidence among regular aspirin/NSAID users, advanced earlier interest in NSAIDs for cancer chemoprevention.(8) However, success of the endocrine therapies, including the aromatase inhibitors (AI) for hormone-responsive breast cancers, and rare, but serious, toxicities of NSAIDs diminished enthusiasm for their use in prevention despite strong mechanistic evidence.(9)
After two decades, unpleasant and common side effects of endocrine therapies and inactivity toward aggressive, non-hormone-responsive cancers have resulted in poor uptake for primary breast cancer prevention.(10,11) In contrast, additional evidence of NSAID benefit in the breast has renewed interest in the prevention potential of anti-inflammatory agents in high-risk individuals. Such evidence includes lower breast cancer incidence in patients at genetic risk who regularly take NSAIDs.(12,13) In addition, experimental models link inflammation in breast tissue stroma and adipose with diffuse and focal desmoplastic-like changes that have been observed early in tumorigenesis and in patients with more dense breast tissue on mammography, an independent risk factor for breast cancer.(14–16) Mammographically dense breast tissue is a radiologic feature correlated with stromal features including a predominant contribution of fibrillary collagens.(17) Experimentally, inflammation affects the local extracellular matrix (ECM) including promoting physical changes in collagen (e.g., stiffness) and altering the permissiveness of the tissue to tumor growth.(18) Unclear is the extent to which localized tissue inflammation in the adipose or in the stroma underlie associations between percent mammographic BD (PMD) and breast cancer risk.
Despite strong preclinical data, whether or not the inhibition of PGE2 modifies breast cancer risk through effects on breast tissue stroma and adipose is unknown. Initial efforts to examine this question by evaluating the effect of PGE2 inhibition using celecoxib, a selective COX2 inhibitor, on tissue biomarkers of proliferation (Ki-67) were terminated due to toxicity concerns. Revisiting this, a recently completed study of women at increased risk for breast cancer treated with 400 mg celecoxib twice daily for 6 months using random periareolar fine needle aspiration sampling of the breast support favorable changes in cytology but no change in tissue Ki-67 or ER expression.(19) Observational studies of NSAID use for effects on BD using mammographic measurements have also been largely null though for women followed prospectively, those who continued NSAID use were more likely to remain low density than those who discontinued use. (20–23) Separately, Wood et al., reported a dose and duration dependent inverse relationship between BD and NSAID use.(24)In both positive studies, these associations were greater in younger women who on average have higher baseline BD. In the only randomized placebo-controlled trial to assess the effect of NSAIDs on mammographic BD in postmenopausal women at increased risk for breast cancer, a single daily dose of 325 mg aspirin for 6 months showed no effect on BD or on serum estradiol, estrone or free estradiol concentrations when compared to placebo. (25,26)
To examine the effect of NSAID use on BD, we conducted an open-label, single arm study of sulindac, a non-selective NSAID, using a non-contrast, breast magnetic resonance imaging (MRI) method measuring percent BD. (27) Sulindac was selected for its pleotropic anti-cancer activity attributed to both COX-dependent and COX-independent mechanisms.(28) A dose of 150 mg twice daily (bid) was selected based on our prior observation that GDF15, a biomarker of sulindac’s non-COX activity(29), was significantly increased in the nipple aspirate fluid of women who received 6 weeks of 150 mg twice daily sulindac compared to single daily dosing.(30) We targeted postmenopausal women on AIs with an intact unaffected, contralateral breast as an ‘at-risk’ group with homogeneously low estrogen levels and for whom NSAID may confer additional benefit on AI-associated arthralgia symptoms. Since no prior studies have assessed the effect of AI on BD by MRI, a non-randomized control arm of postmenopausal patients on AI (observation group) was followed in parallel.
METHODS
Study Design, Patient Eligibility and Enrollment
This open-label Phase 2 study was designed to test the effect of 12 months’ daily sulindac at 150 mg bid on MRI-based percent BD (MRPD) and to assess the feasibility of obtaining paired breast biopsies for tissue studies. Further, while most studies report no AI-specific effect on BD using mammography (31), no prior studies used quantitative fat water decomposition MRI to measure change in BD. Thus, a non-randomized observation arm from the same patient population as the sulindac group was included to obtain an estimate of the effect of 12 months’ AI therapy on MRPD (i.e., AI only observation group). Enrollment for both study groups targeted postmenopausal women with a history of stage 0-III hormone-receptor positive breast cancer on AI therapy. The main inclusion criteria were presence of an intact, non-irradiated, non-involved contralateral breast and BIRADs score of 2, 3 or 4 or presence of scattered, heterogenous or dense fibroglandular tissue on mammogram in the past 12 months, intent to remain on AI therapy for the duration of the study, and willingness to undergo breast MRI at 3 time points. For sulindac treatment, participants were asked to refrain from NSAID use except low dose aspirin (≤ 81 mg/day) throughout the study. Prior to starting sulindac, participants had to demonstrate adequate renal function, normal or controlled blood pressure, and have no contraindications to NSAIDs. Further, to washout any NSAID effects on BD, participants enrolled to sulindac were asked to stop NSAID use for 4 months prior to their baseline MRI. For sulindac, an optional core needle biopsy procedure at baseline and after 6 months sulindac therapy was included to explore sulindac effects on breast tissue. In recruiting eligible subjects to the sulindac study, eligible women not interested in participating or excluded during screening for any reason (e.g., NSAID intolerance) were offered participation in the observation only study. All participants were asked to undergo three MR breast imaging studies (baseline, 6, and 12 months). No breast biopsies were obtained from the observation group.
At the initiation of the study, the prespecified enrollments were 75 and 60 subjects to sulindac and observation groups, respectively. Following slow accrual and limited resources, accrual targets were reduced in year 5 to 50 subjects initiating sulindac with the expectation that at least 40 would complete 12 months of intervention and the prespecified futility analysis was treated as the primary analysis. There was no plan to test differences between the non-randomized groups, and no breast biopsy or adverse event (AE) data were obtained for the observation arm. All patients were enrolled at the University of Arizona Cancer Center and at the Stony Brook Cancer Center. The protocol was approved by the Institutional Review Board at each site, and all enrolled patients provided written informed consent.
Study End Points
Magnetic Resonance Percent Breast Density (MRPD).
The primary endpoint was relative change from baseline in MRPD at 12 months. Absolute change at 12 months and relative and absolute change at 6 months are included as additional endpoints. MRPD was obtained using a previously validated measure of the amount of fibroglandular tissue in the breast.(27,32) Briefly, the volumetric fraction of fibroglandular tissue in the breast was obtained and converted to MRPD as a validated comparator to percent mammographic density. During the trial, three MRI scanners were used for collecting MR breast images. The same scanner was used in collecting all breast images for each individual patient (Supplemental Methods). IDEAL fat-water separation was performed for all data sets with multi-peak fat spectrum and R2* correction. The fat-water separation was performed with in-house software written in Matlab (MathWorks, Natick, MA) using the complex echo images to ensure long-term stability. Automated breast segmentation was used to generate masks for the whole breast. (28) To assess effects of AI and sulindac on the fat and water compartments of the breast, change over time in each fraction was explored separately.
Second-Harmonic Generation (SHG) Microscopy.
On evidence that inflammation influences collagen physical properties in breast tissue including straightness, we explored collagen fiber length, width, and straightness in normal breast biopsy tissue and change after 6 months treatment with sulindac using second-harmonic resonance (SHG) microscopy. As described,(33) SHG occurs when a microscope laser field undergoes a nonlinear, second-order polarization as it passes through noncentrosymmetric ordered structures like fibrillary collagen. Briefly,5 μm H&E-stained normal breast tissue section pairs from 32 subjects blinded to baseline or 6-month status underwent SHG microscopy. Three randomly selected regions of denser stroma (226.32 μm x 226.32 μm) were identified and imaged using a Zeiss LSM 510 META NLO Two-Photon laser scanning confocal microscope system with a 40×/1.3 oil immersion Zeiss Plan-Neofluar objective. An excitation wavelength of 890 nm, with an approximately 100-fs width pulse at an 80 MHz repetition rate, was provided by a Coherent Ti:Sapphire laser XR (Coherent Inc., Santa Clara, CA, USA). Laser scan speed was per pixel at 3.20 μs, and acquisition time of each image (averaged by 8 scans) was around 60s. All images were processed using the computer-assisted image feature extraction software, CT-FIRE V2.0 BETA (12/2017) supported by MATLAB. CT-FIRE is an automated tracking algorithm for collagen fiber feature extraction from the SHG image that yields descriptive statistics for length, width, and straightness. Here, straightness is defined as the ratio of the end-to-end length to the total length of the fiber. (34) Feature extraction was conducted using default settings i.e., all fibers with minimum fiber length of 30 pixels, image resolution of 300 dpi, and max fiber width of 15 pixels.
Toxicity Endpoints.
All patients who received at least one dose of sulindac were included for toxicity analysis and AE reporting. AEs were graded using CTCAE v4. Because earlier studies suggested that the cardiac toxicity of NSAIDs may be related to elevations in blood pressure, repeated blood pressure measures were obtained in both the sulindac and observation arms. Summary findings are reported.
Statistical Methods
Continuous measures were compared using Wilcoxon rank-sum tests, and categorical variables were compared using Chi-square tests with exact P-values based on Monte Carlo simulation. Linear mixed effects models for longitudinal data were used to assess change in MRPD at 6 and 12 months in each of the study arms. For the primary endpoint, relative change in BD at 12 months was defined as (MRPD at 12 months – MRPD at baseline)/MRPD at baseline. To improve efficiency and power, log transformed MRPD was used as the dependent variable in the linear mixed model so that a log fold change could be estimated through a linear combination of estimated coefficients from the fitted model. The corresponding relative change was estimated using a back-transformed log fold change minus 1. The fixed effects of the model included study arm, visit, and an interaction between arm and visit. Factors that differed between the study arms and that may influence the primary endpoint were included as covariates: body mass index (BMI) at every time point, time on AI, and study site. The dependence structure for longitudinal data from the same subject was selected using Akaike Information Criterion, and the final selected one was Toeplitz. Only subjects who started sulindac intervention and had baseline MRPD were included in the primary analysis (n=48 for the sulindac arm). As a secondary endpoint, absolute change was defined as the difference between MRPD at 6 and 12 months from MRPD at baseline. Absolute change was analyzed in a separate linear mixed effect model with adjustment for baseline MRPD. Analysis of the residual indicated that no data transformation on the absolute change was needed. Other covariates in the model were the same as those in model for log-transformed MRPD. Compound symmetry structure was used to model correlation among longitudinal measurement from the same patient. The choice of covariance structure for reporting of absolute or relative change in MRD was considered separately. All analyses for primary and secondary MRPD endpoints were repeated for sulindac patients who were adherent to the protocol (completed ≥ 80% of study agent). Pill counts were recorded for intervention adherence at each clinic visit. Thirty-nine of the 50 evaluable subjects (78%) were adherent as defined by having taken ≥80% of the planned sulindac dose for the 12-month duration.
To explore if change in MRPD over 12 months differed in our study for women at higher risk of developing breast cancer based on baseline mammographic density pattern, we first examined the relationship between baseline MRPD in our study population and mammography density categories (Supplemental Figure 1). Women with heterogeneously or extremely dense breast patterns on mammography have a two-to-three-fold greater risk of developing breast cancer than women with predominantly fatty or scattered fibroglandular mammography patterns. (31) Based on the mean and standard deviation of MRPD in each category, we selected an MRPD cut-point of 25% as it best separated women with a scattered fibroglandular pattern (mean baseline MRPD 16.7% ± SD 7.5) from women with heterogeneously and extremely dense patterns (mean baseline MRPD 26.1% ± SD 13.1 and 55.5% ± SD 16.9, respectively).
Sensitivity to missing data was assessed by multiple imputation for MRPD and BMI measurements using each patient’s baseline age, race/ethnicity, BMI, time on AI, study site, mean arterial pressure, and prior NSAID use. Ten imputed datasets were generated, and all estimates of MRPD change constructed using Rubin’s rule.(34)
The effect of sulindac on breast tissue collagen features included analyses of collagen fiber length, width and angle using linear mixed models as described for the primary analysis to estimate change in these measures. The linear correlation between the change in MRPD and the change in each measure from tissue biopsy imaging was assessed using Pearson’s correlation coefficient or Spearman’s rank correlation coefficient if more appropriate.
RESULTS
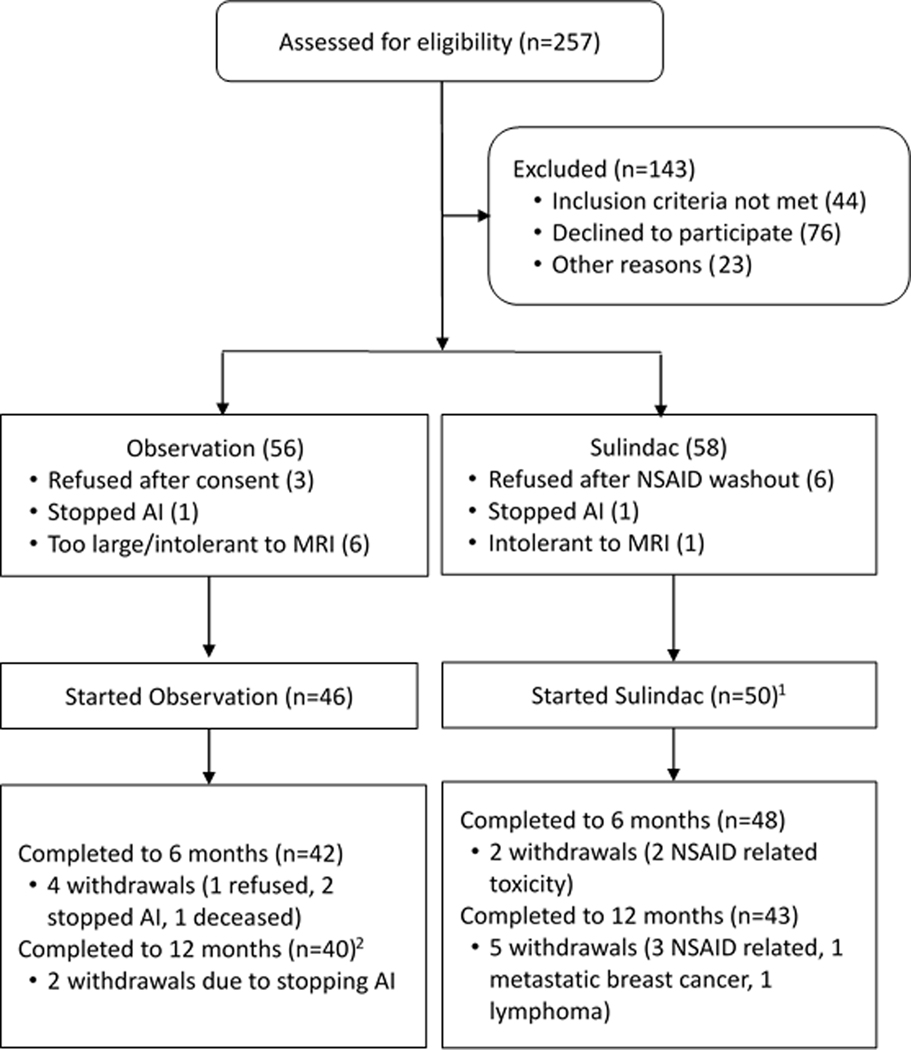
Between November 28, 2012 and February 19, 2018, 257 women were screened for eligibility: 58 enrolled to sulindac and 56 to observation (Figure 1). Of the 58 consented to sulindac, 50 started sulindac after an NSAID washout. Of these 50, 48 had baseline breast MR images (one was lost in IT security change on instrument, and one was corrupted by image artifact). Of the 50 that started sulindac, 48 (96%) completed to 6 months, and 43 (86%) to 12 months. Of 56 who consented to observation, only 46 (82%) underwent the baseline MRI procedure. Of these, 42 (91%) and 40 (87%) completed the study to 6- and 12-months MR imaging, respectively. Of the 40 who completed to 12 months, 39 had breast MR (one subject did not complete end of study MRI but completed end of study pain and quality of life questionnaires).
Figure 1.
Consort for Two Study Groups.
Baseline characteristics are summarized for the sulindac arm and non-random observation control arm (Table 1). Both sulindac and observation arms were majority non-Hispanic White of similar age and BMI. At baseline, the two study groups had similar distributions of MRPD (Table 1 and Supplemental Figure 2). Imbalances between the study arms for time on AI at baseline reflects the run-in/NSAID washout for sulindac participants.After adjusting for baseline MRPD, BMI at every time point, time on AI, and study site, the relative MRPD non-significantly decreased by 3.6% (95% CI, −8.9 to 2.0) over 12 months in the AI-only observation group (Table 2). The corresponding absolute reduction in MRPD was −0.65% (95% CI, −1.76 to 0.47) (Table 3). Additional adjustments for age, MRI scanner, low-dose aspirin use, or scan date did not change the estimates (Supplemental Table S1). No significant change in either relative or absolute MRPD was observed at 6 months (Tables 2 and 3). Further, small relative and absolute decreases in MRPD over 12 months did not differ significantly by baseline BD strata of ≤ versus >25% (Table 2 and 3).
Table 1.
Baseline Patient Characteristics.
| Variable | AI Only N=46 | Sulindac plus AI1 N=50 | P-value2,3 |
|---|---|---|---|
| Age (median years, ±IQR) | 63.0±10.8 | 62.6±8.0 | 0.232 |
| Time on AI (median months, ±IQR)4 | 12.6±17.2 | 16.9±26.5 | 0.005 |
| BMI (median kg/m2, ±IQR) | 28.0±7.1 | 27.1±5.8 | 0.512 |
| MRPD5 (median %, ±IQR) | 17.8±11.8 | 17.6±10.6 | 0.589 |
| Race/Ethnicity | 0.598 | ||
| White, Non-Hispanic | 44 (95.7%) | 44 (88.0%) | |
| Black, Non-Hispanic | 0 (0.0%) | 1 (2.0%) | |
| Hispanic | 2 (4.3%) | 3 (6.0%) | |
| Asian, Non-Hispanic | 0 (0%) | 2 (4.0%) | |
| Study Site | 0.025 | ||
| AZ | 21 (45.7%) | 35 (70.0%) | |
| SB | 25 (54.3%) | 15 (30.0%) | |
| Stage | 0.9225 | ||
| 0-I | 31 (67.39%) | 32 (64.00%) | |
| II-III | 15 (32.61%) | 18 (36.00%) | |
| Radiation | 38 (82.61%) | 40 (80.00%) | 0.7436 |
| Chemotherapy | 17 (36.96%) | 17 (34.00%) | 0.7622 |
| Low Dose Aspirin7 | 0.477 | ||
| No | 41 (89.1%) | 47 (94.0%) | |
| Yes | 5 (10.9%) | 3 (6.0%) |
Baseline percent BD unavailable for 2 subjects who started sulindac (one image artifact, one image lost during IT security change on clinical instrument).
For continuous variables p-value based on Wilcoxon rank sum test.
For categorical variables, p-value based on Chi-squared test with exact p-value from Monte Carlo simulation.
Time on AI is months on AI at time of baseline breast MRI.
Percent BD from magnetic resonance imaging described in methods.
There was no use of targeted therapy for HER2 breast cancer on study and only one subject was on a GnRH agonist, she was in the sulindac arm and completed to 6 months.
Use of 81 mg low dose aspirin for cardioprotection allowed on study
Table 2.
Adjusted estimates of relative change1 in percent BD by MRI at 6 and 12 months in AI Only and Sulindac Groups (All and stratified on baseline BD ≤ or >25%).
| Relative % Change in BD (95% CI) | |||||||
|---|---|---|---|---|---|---|---|
| AI Only (n=46) | |||||||
| N4 | 6- month | p-value | N5 | 12-month | p-value | ||
| All2 | 39 | +1.6 (−2.8, +6.1) | 0.486 | 39 | −3.6 (−8.9, +2.0) | 0.201 | |
| Adjust baseline BD category3 | BD ≤ 25% | 29 | +2.3 (−2.8, 7.7) | 0.388 | 28 | −4.0 (−9.8, +2.2) | 0.202 |
| BD > 25% | 10 | +1.0 (−7.4, +10.1) | 0.829 | 11 | −2.8 (−12.2, +7.6) | 0.582 | |
| Sulindac plus AI (n=50)6 | |||||||
| N4 | 6- month | p-value | N5 | 12-month | p-value | ||
| All2 | 44 | −4.2 (−8.1, −0.1) | 0.046 | 43 | −9.8 (−14.6, −4.7) | <0.001 | |
| Adjust baseline BD category3 | BD ≤ 25% | 34 | −3.8 (−8.2, +0.9) | 0.109 | 34 | −8.1 (−13.2, −2.7) | 0.004 |
| BD >25% | 9 | −6.2 (−14.5, +3.0) | 0.178 | 8 | −14.6 (−24.1, −3.9) | 0.009 | |
Estimated relative change in BD at 12 months is defined as BD at 12 months – BD at baseline/BD at baseline. Summary values are back-transformed estimates and 95% CI.
Analysis based on pooled data from both groups (n=96) and P-values were based on t-test from linear mixed model adjusted for log-transformed baseline BD, time on aromatase inhibitor, BMI at each time point and study site.
Analysis based on pooled data from both groups (n=96) and P-values were based on t-test from linear mixed model adjusted for baseline BD category, time on aromatase inhibitor, BMI at each time point and study site.
Sample size with baseline and 6-month percent BD by MRI
Sample size with baseline and 12-month percent BD by MRI
Baseline BD was unavailable for 2 subjects who started sulindac (one image artifact, one image lost during IT security change on clinical instrument).
Table 3.
Adjusted estimates of absolute change in percent BD by MRI at 6 and 12 months in AI Only and Sulindac Groups (All and stratified on baseline BD ≤ or >25%).
| Absolute % Change in BD (95% CI) | |||||||
|---|---|---|---|---|---|---|---|
| AI Only (n=46) | |||||||
| Group | N3 | 6- month | p-value | N4 | 12-month | p-value | |
| All1 | 39 | 0.3 (−.8, +1.4) | 0.561 | 39 | −0.7 (−1.8, +0.5) | 0.252 | |
| Adjust baseline BD category2 | BD ≤ 25% | 29 | +0.2 (−1.1, +1.5) | 0.809 | 28 | −0.6 (−1.9, +0.7) | 0.364 |
| BD > 25% | 10 | +0.6 (−1.7, +2.9) | 0.611 | 11 | −1.0 (−3.2, +1.3) | 0.386 | |
| Sulindac plus AI (n=50)5 | |||||||
| Group | N3 | 6- month | p-value | N4 | 12-month | p-value | |
| All1 | 44 | −0.3 (−1.4, +0.8) | 0.563 | 43 | −1.4 (−2.5, −0.3) | 0.014 | |
| Adjust baseline BD category2 | BD ≤ 25% | 34 | +0.1 (−1.1, 1.4) | 0.840 | 34 | −0.5 (−1.8, 0.7) | 0.398 |
| BD >25% | 9 | −1.9 (−4.3, 0.5) | 0.119 | 8 | −4.7 (−7.2, −2.3) | <0.001 | |
Analysis based on pooled data from both groups (n=96) and P-values were based on t-test from linear mixed model adjusted for baseline BD, time on aromatase inhibitor, BMI at each time point and study site.
Analysis based on pooled data from both groups (n=96) and P-values were based on t-test from linear mixed model adjusted for baseline BD category, time on aromatase inhibitor, BMI at each time point and study site.
Sample size with baseline and 6-month percent BD by MRI
Sample size with baseline and 12-month percent BD by MRI
Baseline BD was unavailable for 2 subjects who started sulindac (one image artifact, one image lost during IT security change on clinical instrument).
In the sulindac group, the adjusted relative MRPD significantly decreased by 9.8% (95% CI, −14.6 to −4.7) over 12 months (Table 2). The corresponding absolute reduction in MRPD was −1.4% (95% CI, −2.51 to −0.29) (Table 3). Further adjustments for MRI scanner, age, scan dates, and low dose aspirin use did not change the estimates. At 6 months, a small 4.2% decrease in relative MRPD was observed (95% CI, −8.1 to −0.10). Absolute change in MRPD at 6 months was not significant. Larger reductions in MRPD were seen in women enrolled to sulindac with baseline MRPD > 25% (−14.6%) versus those ≤ 25% (−8.1%) (Table 2 and 3). The difference between strata was not significant (p value = 0.269).
Preplanned sensitivity analyses on the main outcome of relative change in MRPD after 12 months included effects of adherence to sulindac and missing data for MRPD and BMI. Most participants who stopped study agent also withdrew early (Figure 1). Of 43 who completed to 12 months, 39 (91%) were adherent at >80% sulindac dose. For missing data, imputed adjusted results using a pooled estimate of change in relative MRPD for the sulindac arm for 6 and 12 months were −5.6% (95% CI, −10.2 to −0.9) and −13.3% (95% CI, −19.2 to −7.3), respectively. Results for the observation arm were unchanged from Table 3.
Because MRPD is derived from fat and water (i.e., fibroglandular) signals in the breast, we conducted exploratory analyses of the relative change in the fat and water fraction of the breast separately. Neither the water nor the fat volume changed significantly from baseline to 12 months in the AI-only observation arm (Table 4). In the sulindac arm, the estimated relative breast water volume significantly decreased by 7.6% (95% CI, −13.3 to −1.5) at 12-months with a non-significant increase in mean fat volume of 5.8% (95% CI, −0.4 to 12.3).
Table 4.
Adjusted Estimate of Relative Change in Breast MRI Water and Fat Volume from Baseline at 6 and 12 months in Sulindac and Observation Control.
| Group | n | Change % Water Volume1 (95% CI) | P-value | Change % Fat Volume2 (95% CI) | P- value |
|---|---|---|---|---|---|
| AI Only | |||||
| 0 v 6 | 39 | −0.2 (−5.4, 5.3) | 0.945 | −2.6 (−7.2, 2.2) | 0.277 |
| 0 v 12 | 39 | −3.7 (−9.8, 2.8) | 0.255 | 1.0 (−5.1, 7.4) | 0.755 |
| Sulindac plus AI | |||||
| 0 v 6 | 44 | −4.6 (−9.5, 0.4) | 0.072 | 0.5 (−4.0, 5.3) | 0.820 |
| 0 v 12 | 43 | −7.6 (−13.3, −1.5) | 0.016 | 5.8 (−0.4, 12.3) | 0.067 |
Pooled estimates using all participant data (n=96) adjusted for log-transformed baseline water volume, time on aromatase inhibitor, BMI at each time point on study and study site.
Pooled estimates using all participant data (n=96) adjusted for log-transformed baseline fat volume, time on aromatase inhibitor, BMI at each time point on study and study site.
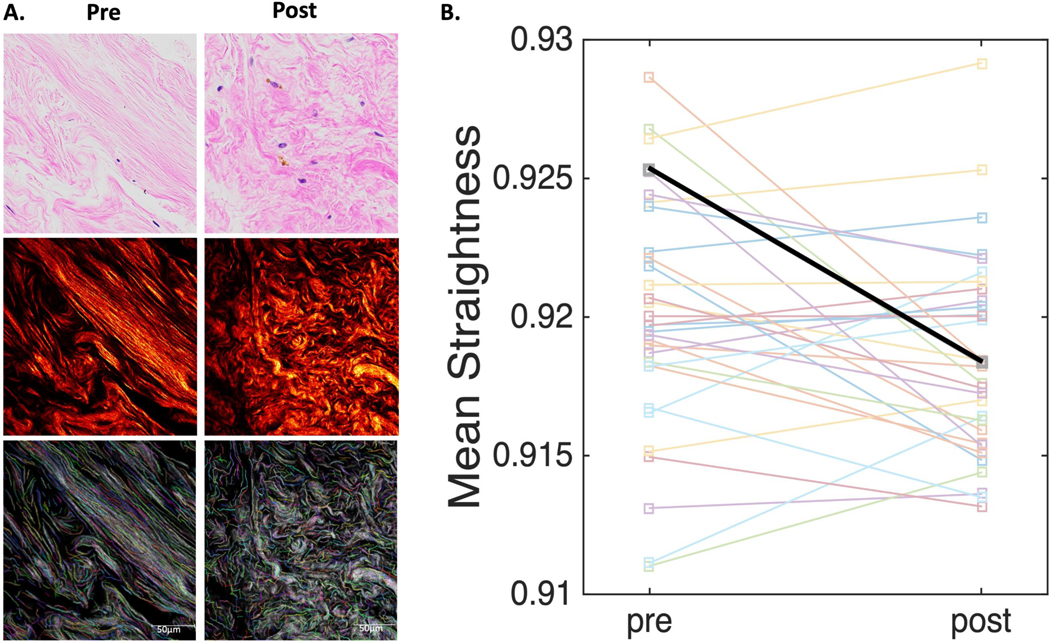
At 6 months, a total of 36 of 50 (72%) participants who started sulindac agreed to optional core needle biopsy at baseline and 33 to repeat the procedure at 6 months. For the 36 subjects at baseline, 34 underwent a research biopsy and 2 consented to tissue biopsy from the contralateral breast during a planned surgery. At 6 months, 31 underwent research biopsy and 2 provided tissue biopsy during planned surgery. Of the 33 available pairs, 32 had adequate tissue for exploratory studies of change in breast tissue collagen fibers using SHG microscopy. With SHG microscopy, breast tissue collagen fibers appear wavy or straight with thick bundles and thin strands (Figure 2). Descriptive statistics for three collagen features (length, width, and straightness) and within subject variance at the baseline measure are shown in Supplemental Tables S2 and S3. Unadjusted, mean straightness declined at 6 months (p=0.032), but attenuated slightly after adjusting for baseline BD, months on AI, BMI, and study site (p=0.053, Figure 2). No significant change in width or length were detected from baseline to 6 months (all p values >0.2). All 50 subjects who started sulindac were included in the toxicity analysis. The most common toxicities were attributed to AI therapy and included musculoskeletal symptoms in 11 patients (22%), insomnia in 2 patients, and increased depression in 1 patient (Supplemental Table S4). Grade 2 or higher AEs possibly, probably, or definitely attributed to sulindac included 7 (14%) patients with gastrointestinal side effects: abdominal pain (3), heartburn/indigestion (2), diarrhea (1), and nausea (1). An additional 3 patients complained of grade 2 rash or pruritis of unclear etiology (6). Seven patients had grade 2 or 3 hypertension (14%; 95% CI, 6.6 to 26.7). One of the 2 patients with grade 3 hypertension had the event during an intracranial hemorrhage serious AE. Two patients initiated new antihypertensive medications on study whereas the remainder identified during a clinic visit resolved following home blood pressure monitoring. Because earlier studies suggested that sulindac was more renal sparing than other NSAIDs (36, 37) clinical blood pressure was examined at three time points during the study. No change was observed in either arm for mean arterial pressure, diastolic, or systolic blood pressure over 12 months (Supplemental Figure S3).
Figure 2. Second-Harmonics Generated (SHG) Microscopy of Breast Tissue Biopsy Samples at Baseline and 6 months after Sulindac Treatment.
Panel A shows a representative breast biopsy tissue section and region of interest from a single patient and mean straightness value before sulindac (left) and 6 months after sulindac (right) stained with hematoxylin and eosin (H&E) under light microscopy (top row), fibrillary collagen SHG image using two-photon laser scanning confocal microscopy with an excitation wavelength of 890 nm (middle) and colorized (middle) the same region of interest post processing using the computer assisted image feature extraction software CT-FIRE. Panel B shows change in mean collagen straightness between baseline and 6 months in 32 pre/post paired sulindac tissue biopsy specimens (unadjusted p value = 0.032). The referenced case highlighted in panel A is represented in the thicker black line in panel B with mean straightness values of 0.925 and 0.918 at baseline and post sulindac, respectively.
Of 4 patients who experienced a serious AE, 2 were possibly related to sulindac, including a patient with an underlying cerebral amyloid angiopathy who developed an intraparenchymal hemorrhage, and a patient who developed acute pancreatitis requiring hospitalization. Both, as well as 2 patients with GI pain, discontinued sulindac therapy.
DISCUSSION
In this open-label study of 150 mg sulindac twice daily for 12 months, we found that postmenopausal women with a history of hormone receptor-positive breast cancer on AI therapy experienced a significant decrease at 12 months in relative and absolute MRPD. In a non-randomized but similar population of women on AI therapy only, no significant change in MRPD was observed after 12 months. This is the first study to evaluate the effect of 12 months’ use of an NSAID on BD and the first to support a possible effect of sulindac to decrease BD in postmenopausal women on AI therapy.
Only one randomized placebo-controlled trial has examined the effects of an NSAID on BD. In a 6-month study of 325 mg/day of aspirin or placebo, McTiernan et al., (25) found no effect of aspirin on absolute change in PMD at 6 months in a sample size nearly twice the size of the current study. Like the aspirin study, we observed no effect of sulindac on absolute change in MRPD at 6 months, with evidence for a small change in relative MRPD (4.2%). Both studies enrolled postmenopausal women with similar mean baseline BD (i.e., 18.3% aspirin study, 21.8% this study). In contrast, the current study enrolled only women on AI therapy, a group excluded from the aspirin study and the treatment duration was longer at 12 months. The current study also used a non-contrast MRI BD measure (MRPD) shown to demonstrate high reproducibility and low variability between measures. (27)
Unlike the aspirin study, a limitation of the current study is that it was not randomized, nor placebo controlled. And while a ‘placebo effect’ is unlikely with a quantitative imaging endpoint, the significant decrease with sulindac could be due to chance alone. Further, while we routinely monitored AI use in both arms and considered time on AI with baseline BD and any change in BMI as potential confounders, we did not conduct AI pill counts for dose adherence. As such, we cannot rule out the possibility that differences between the two groups may be due to imbalances in adherence to AI dose.
Alternatively, longer exposure to sulindac (12 months) and the high dose may explain differences between this and the aspirin study. Also dissimilar to the aspirin study, which reported no effect on circulating estrogen levels (26), sulindac was combined in this study with an AI. Whether sulindac acts independent of AI to reduce BD or by enhancing suppression of PGE2 mediated aromatase gene expression in the adipose of this predominantly overweight population is unknown. Greater activity of NSAIDs for breast cancers occurring in overweight/obese women is supported by observational findings though whether the benefit is greater for hormone dependent cancers remains unclear.(35) The potential for an effect of NSAIDs on BD in overweight women is further supported by findings that aromatase is upregulated in inflammatory foci in tissues of overweight women and that stromal adipocytes from overweight/obese women promote focal desmoplastic-like changes to the ECM.(16) Whether or not these changes link BD to increased breast cancer risk is however unknown. In addition to effects on PGE2, sulindac is pharmacologically distinct from aspirin (36,37) including COX (PGE2) independent activity (36,37) that may account for differences in the two studies.
It is appreciated that the ECM is functionally important in breast tumorigenesis including evidence that more aligned and stiffer collagen fibers correlate with increased COX-2 expression, promote tumor growth and invasion and predict worse patient outcomes. (18,38,39) Poorly understood however are the biochemical and physical ECM properties of ‘at risk’ breast tissue and how inflammation influences the ECM, relates to BD, and BD related cancer risk. Our finding that collagen fiber straightness measured by SHG decreased after 6 months with sulindac is promising for the potential ECM modulating effects of NSAIDs. However, the lack of similar paired biopsy specimens from the observation group and small sample size to relate changes to BD are clear limitations. Recognized insensitivity of CT-FIRE to differentiate wavy from straight collagen fibers present measurements constraints. For example, while we observed a statistically significant decrease in straightness after 6 months, the absolute change is quite small. This is despite what appears visually as large differences (Figure 2). Anecdotally, on unblinding, the appearance of the stroma by H&E light microscopy and by SHG were noticeably ‘more disorganized’ in the post sulindac samples. It is worth commenting that the magnitude of the differences in collagen fiber straightness observed here, while very small, is consistent with other studies that have shown for example that while mean collagen straightness is significantly higher in ductal carcinoma in situ (DCIS) with inflammation or with central necrosis compared to DCIS without inflammation or central necrosis, the absolute differences are very small and of the same magnitude to what we observe for pre and post sulindac.(40)
Unlike endocrine therapies, NSAIDS are widely used medications in adults and are generally well tolerated. Recognizing common GI intolerance and rare, but serious adverse events with chronic use, the need to understand the relative benefit of NSAIDs for breast cancer prevention remains. Currently, we are unable to address if a small decrease in BD with sulindac after 12 months would relate to a reduction in breast cancer events or breast cancer mortality. There is limited evidence on which to draw a relationship between the magnitude of a reduction in BD by any intervention and breast cancer risk reduction. The only randomized controlled trial evidence indicating that a reduction in BD correlates with a reduction in breast cancer incidence are from secondary analyses of the International Breast Cancer Intervention Study (IBIS-I) that compared tamoxifen to placebo in high-risk women for breast cancer chemoprevention.(41) Findings from IBIS-I were the first to show tamoxifen use reduces BD.(42) In follow-up analyses, Cuzick et al., explored change in BD using change in PMD intervals of 5% in participants with >10% baseline BD who received tamoxifen or placebo. (43) By 18-months, PMD decreased with tamoxifen by 7.9% (95% CI, 6.9, 8.9) compared to a 3.5% reduction (95% CI, 2.7, 4.3) with placebo revealing a significant difference between the two groups (p<0.001). Subsequently, the group found that women who received tamoxifen and who had a 10% or greater absolute reduction in BD by 18 months experienced significantly lower odds of developing breast cancer (OR=0.32; 95% CI, 0.14–0.72) when compared to women who received tamoxifen but had no change in BD. For women with ‘5%’ reduction, a non-significant reduced odds ratio of 0.90 and a wide confidence interval (0.40 to 2.04) was observed compared to women with no PMD change. These results support that PMD can serve as a predictor of response to tamoxifen (biomarker) in the prevention setting. However, in an accompanying editorial (44), Dr. Normal Boyd raised important points about interpreting these findings as well as for relating a reduction in PMD to a cancer prevention benefit. These are important points to reconsider here.
First, while the findings from IBIS-1 support an effect of tamoxifen to reduce PMD, the qualitative PMD measurement used in the study was recognized as highly subjective and prone to error. As noted by Boyd, measurement error combined with a small sample size could result in an underestimation of the effect of tamoxifen on PMD and also in evaluating relationships between the reduction in PMD with tamoxifen and a decrease in breast cancer incidence. In this context, it is worth noting the magnitude of BD change with tamoxifen is strongly dependent on two highly correlated factors, menopausal status and baseline BD. To illustrate this point, participants in IBIS-I (42) who were 55 years or older (postmenopausal) had an average baseline PMD of 28.53% and experienced an absolute net reduction in BD after 54 months of tamoxifen of 1.1%. Despite an equivalent sample size, women who were ≤45 years had an average baseline PMD of 48.81% and experienced a 13.4% absolute net reduction in BD. The null to small change with tamoxifen for effect on PMD in the postmenopausal setting are similar to what is reported for AIs used exclusively in postmenopausal women. While it is certainly plausible that the underlying factors that influence BD in pre- and post-menopausal women differ, we suspect that a more likely explanation for failure to relate endocrine therapy effects on BD in the postmenopausal setting is the low sensitivity of mammography to small changes in women with lower baseline BD. This motivated our efforts to develop and validate a quantitative MRI measure of BD.(27,32) Our results here support non-contrast breast MRI as a sensitive measure to detect small changes in BD.
Secondly, while we are encouraged by these results including the larger declines in MRPD in women with heterogenous or extremely dense breast (high-risk group), as discussed by Boyd, we cannot conclude that simply reducing BD will reduce the risk of breast cancer absent understanding of how sulindac or other NSAID affect the biology that links BD to breast cancer risk. The significance of our results depends on replicating findings that sulindac reduces BD and on relating reductions in BD with tissue changes in the factors that link BD to cancer risk. From our perspective, this includes gaining better understanding on hypothesized effects of PGE2 on breast tissue including ‘proestrogenic’ activity via aromatase gene upregulation in adipose and pro-desmoplasia effects on the ECM and their contribution to BD and to cancer risk.
In summary, this study provides the first evidence for an effect of the NSAID sulindac to reduce percent BD after 12 months of use. Improvements in AI-associated stiffness with sulindac (to be reported elsewhere) and findings of a decrease in breast tissue collagen straightness, a suspect tissue risk biomarker, add evidence for the potential benefits of sulindac for breast chemoprevention. However, while we are encouraged by the results and high adherence to sulindac in this breast cancer patient population (i.e., a high-risk and highly motivated patient group), NSAID related toxicities, including the more common GI side effects observed in this study remain a challenge. These results support continued efforts to investigate NSAIDs for their effects on BD and to understanding how BD and modulation of BD relates to cancer risk.
Supplementary Material
STATEMENT OF TRANSLATIONAL RELEVANCE.
Experimental evidence indicates that inflammation directly affects breast tissue stroma including desmoplastic changes associated with tumor permissiveness and may serve as a biological link between breast density (BD) and breast cancer risk. Whether non-steroidal anti-inflammatory drugs (NSAIDs) have activity to reduce BD remains unclear. Results from this study provide the first evidence for activity of NSAIDs to reduce BD in postmenopausal women following 12 months of use and the first to support potential effects of NSAIDs to reduce breast tissue collagen straightness, an emerging biomarker of tissue susceptibility to tumor development.
Acknowledgement
The study was supported by funding from the National Cancer Institute CA1615301. We acknowledge the biostatistical consultation and support provided by the Biostatistics and Bioinformatics Shared Resource at the Stony Brook Cancer Center. We also thank Dr. Matthew Conklin at University of Wisconsin at Madison for his assistance in establishing the Second Harmonics Generation Microscopy and implementation of CT-FIRE.
Financial Support
The work was supported by funding from the National Cancer Institute (grant number R01CA161534).
Abbreviations
- BD
Breast Density
- MRI
Magnetic Resonance Imaging
- MRPD
Magnetic Resonance Percent Density
- NSAID
Non-steroidal anti-inflammatory drug
- AI
aromatase inhibitor
- PGE2
prostaglandin E2
- BID
twice daily dosing
Footnotes
Author disclosures.
None
Trial Registration. ClinicalTrials.gov Identifier: NCT00245024
REFERENCES
- 1.Umar A, Steele VE, Menter DG, Hawk ET. Mechanisms of nonsteroidal anti-inflammatory drugs in cancer prevention. Seminars In Oncology 2016;43:65–77 [DOI] [PubMed] [Google Scholar]
- 2.Davies G, Martin LA, Sacks N, Dowsett M. Cyclooxygenase-2 (COX-2), aromatase and breast cancer: a possible role for COX-2 inhibitors in breast cancer chemoprevention. Annals Of Oncology : Official Journal of The European Society For Medical Oncology 2002;13:669–78 [DOI] [PubMed] [Google Scholar]
- 3.Bulun SE, Simpson ER. Regulation of aromatase expression in human tissues. Breast Cancer Research and Treatment 1994;30:19–29 [DOI] [PubMed] [Google Scholar]
- 4.Arun B, Goss P. The role of COX-2 inhibition in breast cancer treatment and prevention. Seminars In Oncology 2004;31:22–9 [DOI] [PubMed] [Google Scholar]
- 5.Wang D, Dubois RN. Cyclooxygenase-2: a potential target in breast cancer. Seminars In Oncology 2004;31:64–73 [DOI] [PubMed] [Google Scholar]
- 6.Harris RE, Casto BC, Harris ZM. Cyclooxygenase-2 and the inflammogenesis of breast cancer. World Journal of Clinical Oncology 2014;5:677–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao Y, Agarwal VR, Mendelson CR, Simpson ER. Estrogen biosynthesis proximal to a breast tumor is stimulated by PGE2 via cyclic AMP, leading to activation of promoter II of the CYP19 (aromatase) gene. Endocrinology 1996;137:5739–42 [DOI] [PubMed] [Google Scholar]
- 8.Uray IP, Brown PH. Prevention of breast cancer: current state of the science and future opportunities. Expert Opinion On Investigational Drugs 2006;15:1583–600 [DOI] [PubMed] [Google Scholar]
- 9.Nelson HD, Fu R, Zakher B, Pappas M, McDonagh M. Medication Use for the Risk Reduction of Primary Breast Cancer in Women: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2019;322:868–86 [DOI] [PubMed] [Google Scholar]
- 10.Condorelli R, Vaz-Luis I. Managing side effects in adjuvant endocrine therapy for breast cancer. Expert Review of Anticancer Therapy 2018;18:1101–12 [DOI] [PubMed] [Google Scholar]
- 11.Flanagan MR, Zabor EC, Stempel M, Mangino DA, Morrow M, Pilewskie ML. Chemoprevention Uptake for Breast Cancer Risk Reduction Varies by Risk Factor. Annals of Surgical Oncology 2019;26:2127–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bardia A, Keenan TE, Ebbert JO, Lazovich D, Wang AH, Vierkant RA, et al. Personalizing Aspirin Use for Targeted Breast Cancer Chemoprevention in Postmenopausal Women. Mayo Clinic Proceedings 2016;91:71–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kehm RD, Hopper JL, John EM, Phillips KA, MacInnis RJ, Dite GS, et al. Regular use of aspirin and other non-steroidal anti-inflammatory drugs and breast cancer risk for women at familial or genetic risk: a cohort study. Breast Cancer Research 2019;21:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeFilippis RA, Chang H, Dumont N, Rabban JT, Chen YY, Fontenay GV, et al. CD36 repression activates a multicellular stromal program shared by high mammographic density and tumor tissues. Cancer Discovery 2012;2:826–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeFilippis RA, Fordyce C, Patten K, Chang H, Zhao J, Fontenay GV, et al. Stress signaling from human mammary epithelial cells contributes to phenotypes of mammographic density. Cancer Research 2014;74:5032–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Subbaramaiah K, Morris PG, Zhou XK, Morrow M, Du B, Giri D, et al. Increased levels of COX-2 and prostaglandin E2 contribute to elevated aromatase expression in inflamed breast tissue of obese women. Cancer Discovery 2012;2:356–65 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Brower V. Homing In on Mechanisms Linking Breast Density to Breast Cancer Risk. Journal of the National Cancer Institute 2010;102:843–5 [DOI] [PubMed] [Google Scholar]
- 18.Keely PJ. Mechanisms by which the extracellular matrix and integrin signaling act to regulate the switch between tumor suppression and tumor promotion. Journal of Mammary Gland Biology and Neoplasia 2011;16:205–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bayraktar S, Baghaki S, Wu J, Liu DD, Gutierrez-Barrera AM, Bevers TB, et al. Biomarker Modulation Study of Celecoxib for Chemoprevention in Women at Increased Risk for Breast Cancer: A Phase II Pilot Study. Cancer Prevention Research. 2020;13:795–802 [DOI] [PubMed] [Google Scholar]
- 20.Yaghjyan L, Wijayabahu A, Eliassen AH, Colditz G, Rosner B, Tamimi RM. Associations of aspirin and other anti-inflammatory medications with mammographic breast density and breast cancer risk. Cancer Causes & Control : CCC 2020;31:827–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stone J, Willenberg L, Apicella C, Treloar S, Hopper J. The association between mammographic density measures and aspirin or other NSAID use. Breast Cancer Research And Treatment 2012;132:259–66 [DOI] [PubMed] [Google Scholar]
- 22.Maskarinec G, Urano Y, Gill J, Kolonel LN. Nonsteroidal anti-inflammatory drugs (NSAIDs) and mammographic density. Breast Cancer Research And Treatment 2008;112:133–9 [DOI] [PubMed] [Google Scholar]
- 23.Terry MB, Buist DS, Trentham-Dietz A, James-Todd TM, Liao Y. Nonsteroidal anti-inflammatory drugs and change in mammographic density: a cohort study using pharmacy records on over 29,000 postmenopausal women. Cancer Epidemiology, Biomarkers & Prevention 2008;17:1088–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wood ME, Sprague BL, Oustimov A, Synnstvedt MB, Cuke M, Conant EF, et al. Aspirin use is associated with lower mammographic density in a large screening cohort. Breast Cancer Research and Treatment 2017;162:419–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McTiernan A, Wang CY, Sorensen B, Xiao L, Buist DS, Aiello Bowles EJ, et al. No effect of aspirin on mammographic density in a randomized controlled clinical trial. Cancer Epidemiology, Biomarkers & Prevention 2009;18:1524–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duggan C, Wang CY, Xiao L, McTiernan A. Aspirin and serum estrogens in postmenopausal women: a randomized controlled clinical trial. Cancer Prevention Research 2014;7:906–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ding J, Stopeck AT, Gao Y, Marron MT, Wertheim BC, Altbach MI, et al. Reproducible automated breast density measure with no ionizing radiation using fat-water decomposition MRI. J Magn Reson Imaging 2018;48:971–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raz A. Is inhibition of cyclooxygenase required for the anti-tumorigenic effects of nonsteroidal, anti-inflammatory drugs (NSAIDs)? In vitro versus in vivo results and the relevance for the prevention and treatment of cancer. Biochemical Pharmacology 2002;63:343–7 [DOI] [PubMed] [Google Scholar]
- 29.Kim KS, Baek SJ, Flake GP, Loftin CD, Calvo BF, Eling TE. Expression and regulation of nonsteroidal anti-inflammatory drug-activated gene (NAG-1) in human and mouse tissue. Gastroenterology 2002;122:1388–98 [DOI] [PubMed] [Google Scholar]
- 30.Thompson PA, Hsu CH, Green S, Stopeck AT, Johnson K, Alberts DS, et al. Sulindac and sulindac metabolites in nipple aspirate fluid and effect on drug targets in a phase I trial. Cancer Prevention Research 2010;3:101–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ekpo EU, Brennan PC, Mello-Thoms C, McEntee MF. Relationship Between Breast Density and Selective Estrogen-Receptor Modulators, Aromatase Inhibitors, Physical Activity, and Diet: A Systematic Review. Integr Cancer Ther 2016;15:127–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosado-Toro JA, Barr T, Galons JP, Marron MT, Stopeck A, Thomson C, et al. Automated breast segmentation of fat and water MR images using dynamic programming. Acad Radiol 2015;22:139–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Y, Keikhosravi A, Mehta GS, Drifka CR, Eliceiri KW. Methods for Quantifying Fibrillar Collagen Alignment. Methods Mol Biol 2017;1627:429–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rubin DB, Schenker N. Multiple imputation in health-are databases: An overview and some applications. Statistics in Medicine 1991;10:585–98 [DOI] [PubMed] [Google Scholar]
- 35.Cui Y, Deming-Halverson SL, Shrubsole MJ, Beeghly-Fadiel A, Cai H, Fair AM, et al. Use of nonsteroidal anti-inflammatory drugs and reduced breast cancer risk among overweight women. Breast Cancer Research and Treatment 2014;146:439–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rocca J, Manin S, Hulin A, Aissat A, Verbecq-Morlot W, Prulière-Escabasse V, et al. New use for an old drug: COX-independent anti-inflammatory effects of sulindac in models of cystic fibrosis. British Journal of Pharmacology 2016;173:1728–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Piazza GA, Keeton AB, Tinsley HN, Gary BD, Whitt JD, Mathew B, et al. A novel sulindac derivative that does not inhibit cyclooxygenases but potently inhibits colon tumor cell growth and induces apoptosis with antitumor activity. Cancer Prevention Research 2009;2:572–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaushik S, Pickup MW, Weaver VM. From transformation to metastasis: deconstructing the extracellular matrix in breast cancer. Cancer Metastasis Reviews 2016;35:655–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Conklin MW, Eickhoff JC, Riching KM, Pehlke CA, Eliceiri KW, Provenzano PP, et al. Aligned collagen is a prognostic signature for survival in human breast carcinoma. Am J Pathol 2011;178:1221–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sprague BL, Vacek PM, Mulrow SE, Evans MF, Trentham-Dietz A, Herschorn SD, et al. Collagen Organization in Relation to Ductal Carcinoma In Situ Pathology and Outcomes. 2021;30:80–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cuzick J, Forbes J, Edwards R, Baum M, Cawthorn S, Coates A, et al. First results from the International Breast Cancer Intervention Study (IBIS-I): a randomised prevention trial. Lancet 2002;360:817–24 [DOI] [PubMed] [Google Scholar]
- 42.Cuzick J, Warwick J, Pinney E, Warren RM, Duffy SW. Tamoxifen and breast density in women at increased risk of breast cancer. Journal of the National Cancer Institute 2004;96:621–8 [DOI] [PubMed] [Google Scholar]
- 43.Cuzick J, Warwick J, Pinney E, Duffy SW, Cawthorn S, Howell A, et al. Tamoxifen-induced reduction in mammographic density and breast cancer risk reduction: a nested case-control study. Journal of the National Cancer Institute 2011;103:744–52 [DOI] [PubMed] [Google Scholar]
- 44.Boyd NF. Tamoxifen, Mammographic Density, and Breast Cancer Prevention. Journal of the National Cancer Institute 2011;103:704–5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.