Abstract
The Coronavirus Disease 2019 (COVID-19) pandemic has resulted in significant morbidity and mortality worldwide. Although initial reports concentrated on severe respiratory illness, emerging literature has indicated a substantially elevated risk of thromboembolic events in patients with COVID-19 disease. Pro-inflammatory cytokine release has been linked to endothelial dysfunction and activation of coagulation pathways, as evident by elevated D-dimer levels and deranged coagulation parameters. Both macrovascular and microvascular thromboses have been described in observational cohort and post-mortem studies. Concurrently, preliminary data have suggested the role of therapeutic anticoagulation in preventing major thromboembolic complications in moderately but not critically ill patients. However, pending results from randomized controlled trials, clear guidance is lacking regarding the intensity and duration of anticoagulation in such patients. Herein, we review the existing evidence on incidence and pathophysiology of COVID-19 related thromboembolic complications and guide anticoagulation therapy based on current literature and societal consensus statements.
Keywords: SARS-CoV2, COVID-19, thrombosis, thromboembolism, prophylactic anticoagulation, antithrombotic therapy
Introduction
Since the end of 2019, the spread of a novel coronavirus SARS-CoV2 has been reported, first in the Chinese mainland, followed by several European countries, and since early March 2020 in the USA. Shortly after, the World health organization (WHO) declared the fast spread of SARS-CoV2 infection as a pandemic. 1 Despite the high likelihood of a mild or asymptomatic clinical course, SARS-CoV2 infection called “COVID-19” can be associated with more severe manifestations such as severe pneumonia, acute respiratory distress syndrome (ARDS), hepatic, cardiac, and renal injury.2–4 Fortunately, vaccinations for SARS-CoV2 are underway throughout most of the world. Unfortunately, slow public participation and emerging variants with increased transmissibility such as the “Delta Variant” keep this serious disease at the forefront of worldwide attention, thus, necessitating focus on understanding the full pathophysiology of its disease process. 5
COVID-19 has a known correlation with the disruption of the coagulation cascade consequently resulting in cases of pulmonary microthrombi, arterial and/or venous thrombotic related events. 6 These events can result in the aggravation of one's hospital course leading to prolonged inpatient hospitalizations, exaggerated stays in intensive care units (ICU) or even death. Concerningly, these factors may result in exhaustion or even a collapse of health care systems. A high proportion of COVID-19 patients, especially if treated in ICUs, suffer from thrombotic or thromboembolic complications in addition to more traditional associated illnesses such as pneumonia.7–14 Initial reports hypothesized a disseminated intravascular like coagulopathy (DIC) in patients with severe COVID-19 infection.10,15 Those studies displayed consistently elevated D-dimer and fibrin degradation products (FDP). Prolongation of prothrombin time (PT) activated partial thromboplastin time (aPTT), and thrombocytopenia were also noted.15–17
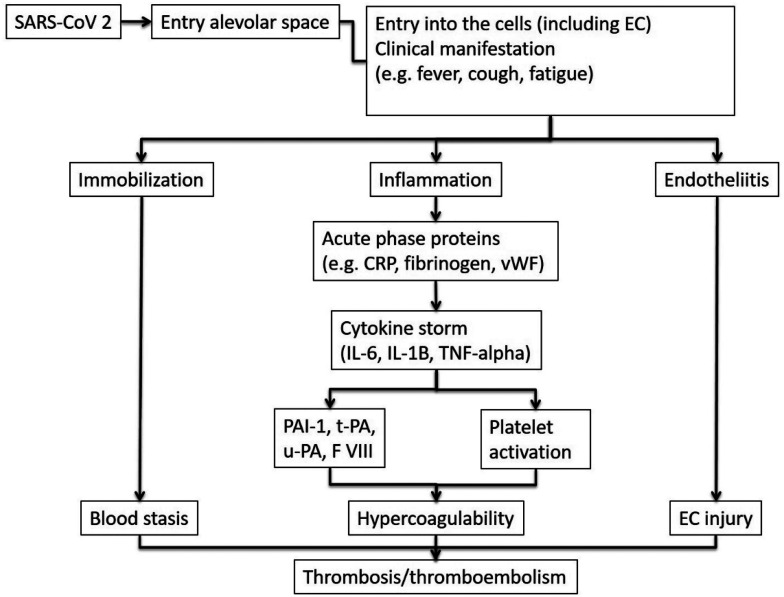
Although more recent studies have indicated the coagulopathy associated with COVID-19 infection is distinct from classic DIC,10,18 other studies, have not shown ample difference between COVID-19 and other pathogens inducing DIC.9,14,19,20 Recent publications for a pro-coagulant mechanism include the proof of a generalized coagulopathy21,22 which may include variables such as increased Factor V, 23 lupus anticoagulant, 24 or reduction of the von Willebrand factor inhibitor ADAMST13. 25 Elevation of these markers is associated with amplification of thrombin generation and thereby thrombus formation (Figure 1).26–29 Furthermore, increased levels of plasminogen activator inhibitor type 1 (PAI-1), as well as tissue type plasminogen activator (t-PA) were also found to be elevated in severe COVID-19 infection adding traction to the theory of an overall disrupted coagulation cascade similarly seen in DIC.28,30,31
Figure 1.
Graphical illustration of SARS-CoV 2 entry into human cells and development of thrombosis on the basis of the Virchow triad. CRP: C-reactive protein; EC: endothelial cell; FVIII: Factor VIII; IL-6 and IL-1B: Interleukin 6 and 1B; PAI-1: plasminogen activator inhibitor type 1; SARS-CoV 2: Coronavirus 19; t-PA: tissue type plasminogen activator; TNF-alpha: tumor necrosis factor alpha; vWF: von Willenbrand factor; u-PA: urikinase type plasminogen activator.
Epidemiology of Thrombosis in COVID-19
Venous Thromboses and Emboli:
Venous thromboembolism (VTE), including deep vein thrombosis (DVT) and/or pulmonary embolism (PE), frequently occur in patients with severe COVID-19 infection and as with most venous thrombotic events, is related to Virchow's triad of immobility, endothelial infection, and immune dysfunction resulting in hypercoagulability (Figure 1)3,32,33 Initial reports show an incidence of VTE between 20 to 43% mainly in the form of PE (Table 1). Among 184 consecutively investigated severe COVID-19 patients, VTE's cumulative incidence was 27%, again mostly from PE. 10 In another report of 150 patients with severe COVID-19 infection, VTE occurred in 43% of patients. Also reported was a higher frequency of extra corporal circuit clotting (hemodialysis and extra corporal membrane oxygenator lines). 9 In another study from France, 21% of severe COVID-19 patients were diagnosed with PE. 34 Similar rates were observed in smaller studies.11,13 Interestingly, in all reported studies, almost all patients were at least on prophylactic dose AC therapy.9–11,13
Table 1.
Studies investigating thrombotic and thromboembolic complication of patients with COVID-19 infection.
| Study name | Study description and findings | Antithrombotic management at time of diagnosis | VTE/PE | Arterial thromboses/emboli |
|---|---|---|---|---|
| Piazza et al. 8 | Cohort study of 1114 Covid-19 patients (ICU 170, admitted non-ICU 229, outpatient 715) | 89.4% ICU patients on prophylactic AC, 84.7% in admitted non-ICU | Arterial or venous thromboembolic event (35.3% vs 2.6% vs 0%) | |
| Klok et al. 10 | Observational study, 184 severe COVID-19 patients in ICU | All patients were on standard prophylactic dose of AC | Cumulative Incidence of VTE/PE 27% | Cumulative Incidence of strokes 3.7% |
| Helms et al. 9 | Observational study, 150 severe COVID-19 patients in ICU | All patients were on AC (70% prophylactic and 30% therapeutic dose) | Incidence of VTE/PE 43% | Incidence of stroke 2% |
| Poissy et al. 34 | Observational study, 107 COVID-19 patients in ICU | All patients were on prophylactic or therapeutic dose of AC | Incidence of VTE/PE 20.6% | Not reported |
| Study name | Study description and findings | Antithrombotic management at time of diagnosis | VTE/PE | Arterial thromboses/emboli |
| Middeldorp et al. 11 | Observational study, 74 COVID-19 patients in ICU | All patients were on prophylactic or therapeutic dose of AC | Incidence of VTE/PE 39% | Not reported |
| Cui et al. 13 | Observational study, 48 Covid-19 patients in ICU | No AC was administered | Incidence of DVT 85% (isolated distal 75%, proximal 10%) | Not reported |
| Llitjos et al. 12 | Observational study, 26 severe COVID-19 patients in ICU | 27% were on AC prior to admission | Incidence of VTE/PE 69% | Not reported |
| Ren et al. 35 | Observational study, 48 Covid-19 patients in ICU | All except 1 patient on prophylactic AC | Incidence of DVT 85% (isolated distal 75%, proximal 10%) | Not reported |
| Al-Samkari et al. 36 | Multicenter retrospective, 400 admitted COVID-19 patients (144 critically ill) | All on standard-dose prophylactic anticoagulation | 6% VTE rate (3.5% in non-critical, 10.4% in critically ill) | 2.8% arterial thrombosis rate (1.2% in non-critical, 5.6% in critically ill) |
| Study name | Study description and findings | Antithrombotic management at time of diagnosis | VTE/PE | Arterial thromboses/emboli |
| Mao et al. 37 | Observational study, 214 consecutive COVID-19 (mild to severe) patients |
Not reported | Incidence of VTE/PE was not reported | Neurologic manifestation 36.4% (dizziness, headache, loss of sensory function) Stroke was documented in 5.7% |
| Lodigiani et al. 14 | Observational study, 388 Consecutive COVID-19 patients | 100% and 75% of ICU and general ward patients respectively were on prophylactic dose of AC | Incidence of VTE/PE was 21% | Incidence of stroke was 2.5% and ACS/MI was 1.1% |
| Bellosta et al. 38 | Case series, 20 COVID-19 patients with ALI | Only 25% were on chronic AC due to atrial fibrillation | Not reported | Incidence of ALI 16.3% versus 1.8% comparing ALI admissions between January-March of the year 2020 versus 2019 |
| Oxley et al. 39 | Case series, 5 patients with COVID-19 | Only one patient was ASA 81 mg daily | Not reported | 5 COVID-19 patients presenting with CVA Large vessel strokes of the ICA, posterior and mid cerebral artery |
| Study name | Study description and findings | Antithrombotic management at time of diagnosis | VTE/PE | Arterial thromboses/emboli |
| Ilonzo et al. 40 | Case series, 21 COVID-19 (mild to severe) patients with acute thrombotic events | 52.4% and 19.1% were on ASA and AC respectively | 23.8% had VTE | 76.2% had arterial thrombosis |
| Perini et al. 41 | Case series | AC was administered in 2 cases | Not reported | 4 COVID-19 patients with acute arterial thrombosis |
| Fraisse et al. 42 | Observational study, 92 Covid-19 patients admitted to ICU | All patients were on prophylactic or therapeutic AC | 8 (21%) arterial thrombotic events | Not reported |
| Morassi et al. 43 | Case series | Not reported | Not reported | 6 Covid-19 patients with CVA (4 ischemic, 2 hemorrhagic) 4/6 patients had bilateral and multiple lesions |
| Bilaloglu et al. 7 | Observational study, 3334 consecutive patients with COVID-19 | Prophylactic AC dose was used in most patients | PE 106 (3.2%) and VTE 129 (3.9%) | Stroke 54 (1.6%), MI 298 (8.9%). |
| Study name | Study description and findings | Antithrombotic management at time of diagnosis | VTE/PE | Arterial thromboses/emboli |
| Leonard-Lorant et al. 44 | Observational study, 106 consecutive patient | 39.6% on prophylaxis dose AC and 6.6% on therapeutic AC at time of admission |
PE in 32 (30%) | Not reported |
| Menter et al. 45 | Post-mortem autopsy of 21 patients with COVID-19 | Not reported | 19% had PE 45% showed microthrombi in alveolar capillaries |
Not reported |
| Wichmann et al. 46 | Post-mortem autopsy of 12 patients with COVID-19 | Not reported | 58% had VTE | Not reported |
| Lax et al. 47 | Post-mortem investigation of 10 randomly selected COVID-19 patients | Not reported | DAD and thrombosis of small and mid-sized pulmonary arteries in all patients | Not reported |
| Study name | Study description and findings | Antithrombotic management at time of diagnosis | VTE/PE | Arterial thromboses/emboli |
| Ackermann et al. 48 | Post-mortem investigation of 7 COVID-19 patients | Not reported | DAD with perivascular T-cell infiltration. Severe endothelial injury (intracellular virus, cell membrane disruption), widespread alveolar capillary thrombosis neo-angiogenesis. | Not reported |
| Pellegrini et al. 49 | Post-mortem investigation of 40 hearts from hospitalized patients dying from COVID-19 | Not reported | Not reported | myocyte necrosis in 14 (35%), 3 of them with MI and 11 with focal myocyte necrosis. Cardiac thrombi in 11/14 (78.6%), 2/14 (14.2%) with epicardial coronary artery thrombi and 9/14 (64.3%) had microthrombi in myocardial capillaries, arterioles, and small muscular arteries. |
AC: anticoagulation; ACS acute coronary syndrome; ALI acute limb ischemia, ASA: aspirin; CVA cerebrovascular accident; DAD diffuse alveolar damage; ICA: internal carotid artery, ICU intensive care unit; MI: myocardial infarction; PE: pulmonary embolism; VTE: venous thromboembolism
Another concerning phenomenon involving the pulmonary circulation in COVID-19 patients has been observed in the form of microvascular thombi. These thrombi were found more frequently than large-vessel clots indicating a local pulmonary hypercoagulable process leading to the observed clinical presentation. 46–45 A post-mortem autopsy study revealed small to medium-sized pulmonary artery thrombi in all investigated patients. 47 The authors hypothesized that COVID-19 infection results in a direct pulmonary thrombosis rather than thromboembolism. 47 Such a manifestation can cause hemorrhagic necrosis, fibrosis, cessation of the pulmonary circulation, acute pulmonary hypertension, and ultimately death. 47 Another post-mortem autopsy study revealed severe endothelial injury with disruption of cell membranes, widespread vascular thrombosis, alveolar-capillary occlusion, and significant angiogenesis. 48 Such microthrombi were found in other organs but at a lower frequency.49,50
Arterial Thromboses and Emboli:
Arterial thromboses were less frequently reported (Table 1) in patients diagnosed with COVID-19. In an early report from Klok et al. the cumulative incidence of arterial thromboses was 3.7%. 10 Similarly, Lodigiani et al. found stroke present in 2.5% of cases while acute coronary syndromes and myocardial infarctions were present in 1.1% of cases among 388 COVID-19 infected patients. 14 A study from Italy showed an increase of patients presenting with acute limb ischemia (ALI) by comparing the first three months of 2020 with those of 2019 (16.3% vs 1.8%, respectively). 38 The patients’ mean age was 75 years, and 25% and 20% had a history of atrial fibrillation and peripheral arterial disease, respectively. 38 Alarmingly, the presentation of arterial thrombosis does not seem to be isolated to patients at higher age and advanced comorbidities. Recent case series have raised concerns about the presentation of young and generally healthy people with large-vessel strokes or ALI.39–51 In a single-center study from Spain, the incidence of arterial thromboses among 1419 admitted patients with COVID-19 was 14 (1%), including acute coronary syndromes, acute ischemic stroke, transient ischemic attack, and limb thrombotic events, respectively. 52
Furthermore, an increased risk of stroke and acute myocardial infarctions (MI), especially stent thromboses, were reported.39,53 Nevertheless, in recently published data from large health care systems involving a large sample size, the incidence of arterial thromboses/emboli was much lower than reported in earlier studies.7,8,35 A report from a large health care system in New York City identified a stroke incidence of 0.9% (32/3556). 51 However, compared to contemporary stroke patients, those with COVID-19 infection were younger, had higher D-dimer levels, were more frequently of the cryptogenic subtype, and had higher inpatient mortality. 51 Similarly, in a retrospective cohort of 1114 patients with COVID-19 diagnosed through Massachusetts General Brigham's integrated health network with independently adjudicated thrombotic/embolic events, stroke and MI incidence were 0.1% (1/1114) and 1.3% (14/1114). 8 Several reasons were postulated for the lower incidence of stroke compared to Chinese patients. 37 Besides the difference in patient characteristics, stroke detection in severely ill COVID-19 patients is challenging, especially in those intubated and sedated. 51 Of note, strokes in patients with COVID-19 more frequently affected large-vessels in younger patients compared to strokes in non-COVID patients. 39 Finally, an independent adjudication of events might have also contributed to the lower rates of arterial thromboses.8,35
Authors of the same health care system in New York presented a series of patients with COVID-19 infection presenting with ST-segment elevation MI (STEMI). 54 Those patients were also characterized by elevated D-dimer and abysmal prognosis (mortality of 72%). 54 Half of those patients underwent angiography, and two-thirds had obstructive disease. 54 Interestingly, in MI patients without COVID-19, the infection has declined in several countries during the first pandemic peak.55,56 Such a decline may have reflected fear of COVID-19 exposure while being evaluated for MI in the hospital. Additionally, delays in the management of STEMI patients during the pandemic's early days were reported.57,58 Such delays were associated with higher rates of short-term complications (eg, cardiogenic shock, congestive heart failure, sustained ventricular tachycardia/fibrillation, use of mechanical circulatory devices, and in-hospital death).57–59 In general, COVID-19 seems to be associated with a myocardial injury related to coronary thrombosis and other non-coronary pathologic mechanisms.49,60,61
Pathophysiology of Thrombosis in COVID-19 Patients
The pathophysiology of COVID-19 associated thrombotic/embolic complications, as stated before, can be highlighted based on Virchow's triad: stasis of blood flow, vessel injury, and hypercoagulability (Figure 1).
SARS-CoV-2 enters the human cells via angiotensin-converting enzyme receptor type 2 (ACE2).62–64 This receptor type is expressed on many human cell types, eg, alveolar cells, cardiac myocytes, podocytes, and endothelial cells (EC).63,65 Consequently, the direct impact of SARS-CoV-2 on the vascular system including veins and arteries is not surprising. EC injury results in tissue factor release, stimulation of the coagulation cascade, triggering of an inflammatory response called “cytokine storm” and activation of the complement system.66,67 Cytokines such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) rise dramatically in patients with severe COVID-19 infection. A similar pattern is seen in severe infections caused by other pathogens and are known to be associated with activation of the coagulation system.68–70 Both cytokines promote their prothrombotic features via interaction with EC.68,70,71
Interleukin-6 is an interesting inflammatory marker in its relationship with COVID-19. Several studies suggest elevated IL-6 serum levels positively correlate with worsened disease severity.72–74 A retrospective study by Ruan et al. showed an almost two-fold increase in serum IL-6 level in patients dying from COVID-19 versus those who were discharged from the hospital. 75 Whether this represents a correlation with an overall sicker patient population leading to a more general inflammatory state, versus correlation with a unique inflammatory cascade, is still not fully understood.
Additionally, TNF-α activates the complement system, which stimulates the coagulation system.69,76,77 The enhanced thrombosis is partially mediated by an increase in plasminogen activator inhibitor type 1 (PAI-1), a natural inhibitor of fibrinolysis.78,79 The imbalance between coagulation and fibrinolysis in patients with severe COVID-19 infection “COVID-19 associated coagulopathy (CAC)” is reflected by several markers. A systemic EC insult at the respiratory system level, the gateway of COVID-19 infection, results in dysfunctional haemostasis, fibrinolysis, and vessel permeability,47,48 leading to pulmonary intravascular coagulopathy with prominent capillary thrombosis, vessel wall edema, and hemorrhagic infarction.47,48,79 Once the virus affects EC in other organs, more systemic thromboses and emboli can emerge, including large arterial thromboses.38,39,54
CAC shares features of DIC as well as thrombotic microangiopathy.18,47 In early observations from China, over 71.4% of COVID-19 non-survivors developed DIC according to the international society of thrombosis and haemostasis (ISTH). 15 However, such an observation was not reported in subsequent studies. One must make several distinctions between DIC and CAC. While DIC is primarily characterized by bleeding due to coagulation markers and thrombocytes’ consumption, CAC's hallmark is thrombosis reflected by elevated D-dimer and fibrinogen, a prolonged aPTT, and mild thrombocytopenia.10,13,15,19 Nevertheless, in some late-stage severe COVID-19 infections, overt DIC might occur. 79
Biomarkers of Thrombosis, Risk Stratification and Monitoring
D-dimer and fibrin degradation products (FDP) have emerged as the most robust clinical applied marker to distinguish the risk of thromboembolic complications. They correlate with disease severity and predict outcome.13,15 D-dimer has a high negative predictive value and is recommended to rule out VTE in the general population. 80 In Patients with COVID-19 infection, D-dimer was independently associated with poor prognosis with D-dimer levels > 1 μg/mL being associated with an 18-times increased risk for mortality. 81 More importantly, COVID-19 infection non-survivors showed a progressive increase in D-dimer and FDP compared to survivors.15,81 Furthermore, a continuous elevation of fibrinogen, ferritin, and C-reactive protein may reflect a more deleterious clinical course.79,81,82 PT, aPTT, and thrombocytopenia are also found in patients with COVID-19 infection but do not correlate with disease severity or outcome.13,15,81 Of note, the mild increase in aPTT might be influenced by the development of antiphospholipid antibodies in some patients with severe COVID-19 infection. 9 Although less investigated, von Willebrand factor (vWF) and factor VIII may evolve as markers of activated endothelium.9,34
Like in sepsis and septic shock, thrombocytopenia, and dynamic changes in platelet count in patients with COVID-19 are predictive of outcomes.83,84 The pathology responsible for thrombocytopenia in COVID-19 is not fully understood. However, the alveolar inflammation and thrombosis result in platelet activation and consumption and, more importantly, extramedullary megakaryocytes, leading to exhaustion of the platelet system.83,85 Extramedullary “pulmonary or intravascular” megakaryocytes were found in all major organs of patients with COVID-19 infection, which actively produce platelets. 61 Additionally, direct infection of the bone marrow by the coronavirus with subsequent inhibition of hematopoiesis has been postulated as causative of thrombocytopenia.86,87
Even with the absence of high-quality evidence, the use of these biomarkers’ to monitor coagulation is recommended but should not be used to guide treatment with anticoagulants or antiplatelet agents. 2 Frankly, elevated D-dimer levels without other clinical correlates should not trigger the utilization of therapeutic anticoagulation.2,21,88,89
There is emerging evidence evaluating the role of the neutrophile-to-lymphocyte ratio (NLR) and the platelet-to-lymphocyte ratio (PLR) in predicting COVID-19 infections with a more aggressive course. Existing data retrospectively reviewed hospitalized patients with COVID-19 and found an elevated NLR or PLR at admission correlated with increased morbidity and mortality.90–92 Further data is needed to better understand the predictive accuracy of these markers, as well as understand their relationship to the pathophysiology of COVID-19 infections.
Antithrombotic Therapy in COVID-19 Positive Patients
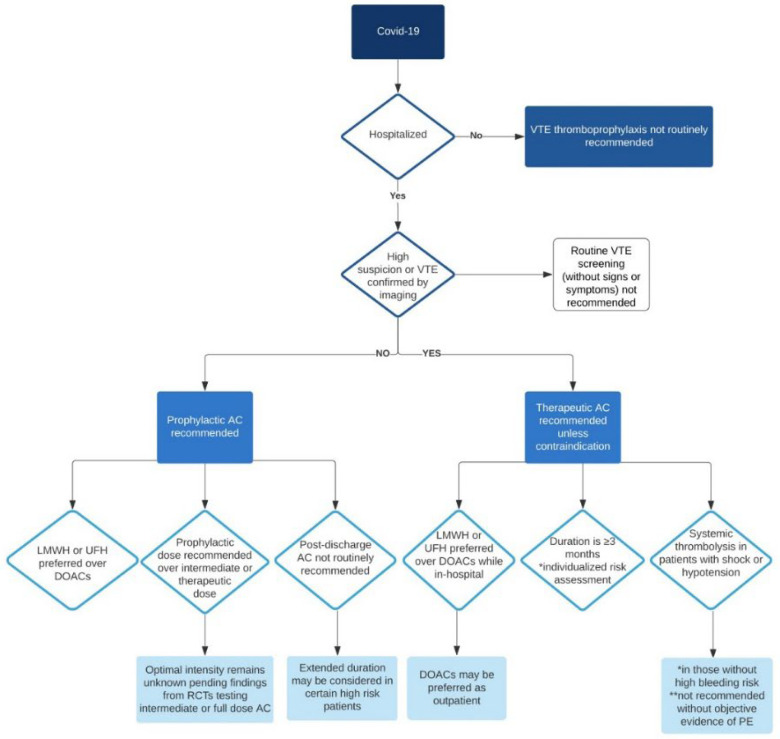
Antithrombotic therapy in COVID-19 patients can be challenging. Over the last year, several societies and medical associations published recommendations for antithrombotic management of patients with COVID-19.2,21,88,89,93 Currently, the National Institute of Health (NIH) does not recommend routine intermediate or full dose anticoagulation but does recommend all hospitalized non-pregnant adults with COVID-19 be placed on prophylactic dose anticoagulation. 2 Detailed dosing parameters for these various strengths among commonly used inpatient anticoagulants can be found in Table 2. Screening for DVT can be neither recommended nor discouraged based on the current evidence. 2 However, according to the recommendation of the American Society of Echocardiography (ASE), in critically ill patients with COVID-19 admitted to the ICU, DVT assessment may be reasonable by using a two-point compression point of care ultrasound technique. 94 While the treatment of arterial or venous thrombotic or thromboembolic events underlies recent international guidelines95–97 and is summarized in antithrombotic recommendations of institutions and associations,2,21,88,89,93 venous or arterial thromboprophylaxis is still the aim of ongoing studies and clinical experience (Table 3 and Figure 2).
Table 2.
Commonly used doses of anticoagulation medications in the inpatient setting. 98
| Prophylactic dose | |
| Enoxaparin | 30 to 40 mg SC / <0.7 mg/Kg/24hours 30 to 40 mg SC / <0.4 mg/Kg/12hours |
| Unfractionated heparin | 5000 IU SC TID 5000 to 7500 IU SC TID |
| Fondaparinux | 2.5 mg SC once daily for 5 to 10 days |
| Intermediate dose | |
| Enoxaparin | ≥0.4 but ≤0.7 mg/Kg/12hours |
| Unfractionated heparin | 5000 IU SC TID 5000 to 7500 IU SC TID |
| Therapeutic dose | |
| Enoxaparin | ≥0.7 mg/Kg/12 hours ≥0.7 mg/Kg/24 h for patients with CKD >1.4 mg/Kg/24 hours |
| Therapeutic Dose Cont. | |
| Unfractionated heparin | IV bolus followed by drip, with aPTT guided dose adjustments |
| Bivalirudin | IV bolus followed by drip, with ACT guided dose adjustments |
aPTT: activated partial thromboplastin time; ACT: activated clotting time; CKD: chronic kidney disease; IV intravenous; SC: subcutaneous; TID: three time per day.
Table 3.
Recommendations of international societies for thrombosis prophylaxis in patients with COVID-19 infection.
| Patients/setting | ACCP | ISTH | NIH | ASH |
|---|---|---|---|---|
| Critically ill | LMWH in prophylactic-dose |
|
AC in prophylactic-dose | Prophylactic-intensity over intermediate-intensity or therapeutic intensity AC |
| Non–critically ill | Prophylactic-dose LMWH or fondaparinux | LMWH in prophylactic-dose | AC in prophylactic-dose | Prophylactic-intensity over intermediate-intensity or therapeutic intensity AC |
| After discharge | Extended prophylaxis not routinely recommended | LMWH/DOAC for up to 30 d can be considered if high thrombosis risk and low bleeding risk | Extended prophylaxis not routinely recommended | Not addressed |
| Non-hospitalized | Routine prophylaxis not recommended | Routine prophylaxis not recommended | Routine prophylaxis not recommended | Not addressed |
AC: anticoagulation, ACCP: American college of chest physicians; ASH American Society of Hematology; DOAC: direct oral anticoagulant; ISTH: international society of thrombosis and haemostasis; LMWH: low-molecular-weight heparin; NIH: national institute of health
Figure 2.
Algorithm for the utilization of antithrombotic prophylaxis and therapy in patients with coronavirus 19 infection.
Management of Venous Thromboprophylaxis
The NIH COVID-19 treatment guideline suggests treating hospitalized COVID-19 patients with VTE prophylaxis per the standard of care for other hospitalized patients. 2 However, anecdotally some centers are using doubled antithrombotic dose (ie, intermediate dose) or even therapeutic dose (in high-risk patients or those admitted to the ICU) strategies given the higher incidence of thrombotic complications in COVID-patients requiring mechanical ventilation. 99 Table 2 depicts commonly used doses of unfractionated heparin (UFH) and low molecular weight heparin (LMWH).
Lemos et al. published the first randomized study comparing therapeutic versus prophylactic dose of either LMWH or UFH in COVID-19 patients requiring mechanical ventilation. 100 The primary endpoint was a variation of gas exchange over time (between baseline, 7, and 14 days after randomization) and the secondary endpoint was time to liberation from mechanical ventilation. 100 The authors reported a significant improvement in the gas exchange and higher ratio of liberation from mechanical ventilation and more ventilator-free days in the group treated by therapeutic dose than those treated by a prophylactic dose of anticoagulants. 100 Despite adding reassurance for clinicians, one must stress this study had a low sample size, used surrogate rather than hard clinical endpoints, and was conducted in a single center, reducing the reported findings’ generalizability.
In a retrospective study of 449 individuals with COVID-19, the administration of enoxaparin 40 or 60 mg once daily was associated with a lower mortality rate in patients with high sepsis-induced coagulopathy (SIC) score or elevated D-dimer, but not in the whole cohort, when compared with nonusers. 101 More recently, another retrospective large-sample size study of 4389 patients with proven COVID-19 infection, prophylactic and therapeutic anticoagulation were both associated with lower in-hospital mortality and a lower intubation rate in comparison to patients who did not receive anticoagulation. 35 In adjusted analyzes, a trend towards reducing in-hospital mortality was detected for therapeutic anticoagulation compared to prophylactic anticoagulation; however, this must be interpreted with caution as they are largely hypothesis generating given the inherit biases that exist within observational studies.
Several larger scale randomized trials are currently awaited to shed more light on the risks and benefits of prophylactic, intermediate, or therapeutic dose AC for patients with COVID-19 infection. 102 On December 22nd, 2020, the NIH announced a pause on enrollment of patients with critically ill COVID-19 in the Anti-thrombotics for Adults Hospitalized With COVID-19 (ACTIV-4) trial1 103 due to potential harm and futility of the therapeutic AC regimen as described by the independent overseeing board. Nevertheless, enrollment of moderately ill COVID-19 patients continued. 104
Additional trials are currently ongoing to investigate the ideal antithrombotic management for patients with COVID-19. An interim analysis of a multiplatform randomized controlled trials (ACTIV-4, REMAP-CAP and ATTACC) has been published online on January 28th 2021. 105 The analysis indicates that in patients with moderate COVID-19 (hospitalized but not initially requiring ICU therapies/level of care) therapeutic AC with UFH or LMWH is superior to prophylactic dose AC regarding the reduction of the primary endpoint of organ support-free days (to day 21). 105 The superiority of such strategy was evident regardless of D-dimer levels at randomization. Nevertheless, the very same strategy was shown to be futile and potentially harmful when applied on patients with severe COVID-19 infection (receiving organ support/ICU level care). Several issues remain to be discussed regarding the interpretation of this analysis. First, most of the data was provided by one trial, the Randomised, Embedded, Multi-factorial, Adaptive Platform Trial for Community-Acquired Pneumoniatrial (REMAP-CAP) (84%). Second, the majority of the enrolled patients were from the United Kingdom where intermediate dose was applied as standard of care for thrombosis prophylaxis. 106
More evidence suggests little benefit with potential harm in therapeutic-dose versus prophylactic-dose anticoagulation. A randomized controlled trial by Lopes et al. compared these two arms in patients hospitalized with COVID-19 symptoms. The authors found a statistically non-significant difference in the primary endpoint, a composite of death, duration of hospitalization, or duration of supplemental oxygen use through 30 days when comparing therapeutic with prophylactic anticoagulation (34.8% vs 41.3%; P = .40). 107 However, more aggressive anticoagulation was associated with higher risk of major bleeding and/or clinically significant non-major bleeding was statistically worse in the therapeutic dose anticoagulation arm (8% vs 2%; P = .001). 107
Another randomized clinical trial, Intermediate-dose versus Standard Prophylactic Anticoagulation and Statin versus Placebo in ICU Patients With COVID-19 (INSPIRATION) showed no difference in the primary endpoint of composite of venous or arterial thrombosis, treatment with extracorporeal membrane oxygenation, or mortality within 30 days comparing intermediate with standard prophylactic dose of AC in patients with COVID-19 infection admitted to ICU (45.7% vs 44.1%; P = .70). 108 The findings from those randomized control trials are also supported by reports from observational studies. 109 It appears that patients with moderate or severe COVID-19 infection do not derive benefits from dose escalation of antithrombotic therapy. However, it is not clear how to change thromboprophylaxis in a patient with moderate COVID-19 who progresses to severe illness..105,106
The evidence is sparse but there is recent data to help guide anticoagulation management in outpatients suffering from COVID-19. Conners at al recently published a paper in JAMA evaluating outcomes in symptomatic COVID-19 outpatients randomized to aspirin, apixaban 2.5 mg twice daily, apixaban 5 mg twice daily, and placebo. They found no significant difference in composite outcomes after 45 days of therapy between the active groups and the placebo group. 110 It should be noted the trial was stopped early due to an unanticipated overall low event rate.
Alternative AC for Patients with Heparin Induced Thrombocytopenia (HIT):
Generally there is an overall low incidence of HIT with UFH and LMWH, 3% and 0.2% respectively. 111 Nevertheless, fondaparinux, bivalirudin or argatroban might be a considerable option in patients with heparin induced thrombocytopenia. 112 Two clinical trials are being conducted to investigate the safety and efficacy of these drugs in patients with COVID-19.113,114 The use of direct oral anticoagulants (DOAC) in patients with COVID-19 is a matter of great debate. While proponents advocate its use in moderate COVID-19 patients to protect health care worker form exposure to the patients one must stress that using DOACs in critically ill patients in ICU settings cannot be recommended as the bioavailability and gastrointestinal absorption of these drugs are uncertain. 115 Additionally, several drug-drug interactions between anticoagulants and antiviral medicines have been reported.89,115 A study in 32 patients on chronic DOACs and COVID-19 infection treated with antiviral drugs showed alarmingly high plasma levels of the anticoagulant. 115 Several trials are currently being conducted to elucidate the role of DOAC in patients with moderate COVID-19 infection. 113 Furthermore the role of DOAC in the post-discharge period is also an important niche. 113 Currently, data on the antithrombotic management of patients with COVID-19 after hospital discharge is scarce. A study investigating the rate of post-discharge VTE in patients after COVID-19 admission showed no increased risk of VTE compared to discharges of other medicine patients. 116 Most experts recommend using thromboprophylaxis if immobilization persists after discharge and stopping it if the patient returns to daily life (Table 3) (Figure 2). Nevertheless, several randomized clinical trials are currently being conducted to investigate the impact of AC in the post-discharge phase. 113 However, in the absence of clinical evidence of VTE or a thrombotic event, therapeutic anticoagulation cannot be recommended outside the setting of clinical trials. 2
Monitoring AC in Patients with COVID-19 Receiving Thromboprophylaxis:
In general monitoring anticoagulation in patients undergoing thromboprophylaxis dose of AC is not necessary. Nevertheless, in the elderly, patients with chronic kidney disease, and those with the extremes of body weight (especially those who are underweight) might profit from monitoring AC levels. In patients with COVID-19, increased plasma levels of fibrinogen and Factor VIII can result in shortening of the aPTT. 117 Alternatively, Anti-Factor Xa activity measured 4 h after the third dose of UFH/LMWH can be considered.
Antiplatelet Agents for Thromboprophylaxis
A recent in-vitro study elucidated the fundamental role of platelet activation in the process of cytokine storm in COVID-19 infection. Platelet activation and tissue factor release by COVID-19 could be blunted by the interleukin-6 inhibitor Tocilizumab or aspirin. 118 Furthermore experimental data in mice indicate that aspirin can reduce intravascular thrombin activity and microvascular occlusion in staphylococcus aureus-induced sepsis. 119 This finding is in line with the results of a contemporary meta-analysis of cohort studies that found that antiplatelet therapy, particularly aspirin, was associated with decreased mortality in patients with sepsis. 120 However, it remains unclear whether this applies to patients suffering from COVID-19. Retrospective studies have indicated that patients on chronic aspirin treatment were associated with lower risks of mechanical ventilation, ICU admission, and in-hospital mortality. 121
There is limited information about the effect of antiplatelet drugs in critically ill patients with COVID-19 patients. A small-sample study showed that a combination of anticoagulant and antiplatelet therapy could reduce the pro-coagulant pattern of the blood measured by viscoelastic tests (clot stiffness and clot time). 26 Recently Meizlish et al. performed a propensity-matched analysis of patients with COVID-19. 122 The authors found a mortality benefit for intermediate-dose anticoagulation and aspirin compared to prophylactic-dose anticoagulation and no aspirin. 122 Another small sample-size randomized study investigated the clinical outcome of COVID-19 patients treated with dipyridamole versus placebo. 123 Patients in the dipyridamole arm had a significant reduction of D-dimer levels, as well as increased lymphocyte and platelet counts, as well as an overall beneficial clinical outcome. 123
Ticagrelor has been shown to reduce circulating platelet-leucocyte aggregates, interleukin-6 levels and improve oxygen requirements in patients admitted with pneumonia. 124 The effect of ticagrelor in patients with severe COVID-19 infection with or without thrombosis has not been investigated yet.
The CHEST Guideline, Expert Panel Report and NIH COVID-19 treatment guidelines discourage using antiplatelet agents to prevent arterial thromboses.2,88 Additionally, it is essential to avoid antiplatelet therapy 24 h after thrombolysis or mechanical thrombectomy in stroke patients. 125 It is critical to mention the risks and benefits of thrombolysis or mechanical thrombectomy. Several randomized controlled trials (RCTs) are currently underway to elucidate the role of antiplatelet therapy alone or in combination with other antithrombotic agents in patients with COVID-19 and are summarized by Talasaz et al.. 102
Considerations for Vulnerable Patients and Subgroups:
Several vulnerable populations and subgroups such as children, pregnant women, and the immune compromised should be mentioned. The amount of evidence for might be limited since these patients are mostly excluded from clinical trials. In children with COVID-19 infection, the same thromboprophylaxis should be applied as for those without COVID-19 infection according to the NIH. 2 Similarly pregnant women with severe COVID-19 infection should receive prophylactic dose of AC. 2 Anticoagulation therapy use during labor and delivery requires specialized care and planning and should be managed in pregnant patients with COVID-19 in a similar way as in pregnant patients with other conditions that require anticoagulation in pregnancy. 2 COVID-19 patients may be placed in an immune compromised state as suggested by an increased risk of mucormycosis infection identified in a recent met-analysis. 126 This relationship and its association with the morbidity and mortality of COVID-19 infected patients will require further study.
Conclusion
In summary, infection with SARS-Cov2 may result in serious illness requiring admission to ICU. Those patients are at risk of developing ARDS and thrombotic complications associated with a higher risk of mortality. Several biomarkers have been introduced and investigated, which may support more aggressive medical management of the infection, including anticoagulation therapy. Prophylactic antithrombotic strategies to avoid venous or arterial thrombotic/thromboembolic events are still in discussion and/or evaluation.
Prophylactic dose anticoagulation appears to be associated with the best efficacy and safety ratio as compared to regimen using intermediate or therapeutic doses of anticoagulation in patients hospitalized with COVID-19.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
Ethical Approval: Our institution does not require ethical approval for reporting individual cases or case series.
Informed Consent: Informed consent for patient information to be published in this article was not obtained because there is no patient protected information in the article.
ORCID iD: Michael Joseph Cryer https://orcid.org/0000-0001-8703-7284
References
- 1.Clinical Management of Severe Acute Respiratory Infection (SARI)When COVID-19 Disease is Suspected—Interim Guidance. World Health Organization; 2020. [Google Scholar]
- 2.COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health; 2021. [PubMed] [Google Scholar]
- 3.Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417‐1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farinholt T, Doddapaneni H, Qin X, et al. Transmission event of SARS-CoV-2 delta variant reveals multiple vaccine breakthrough infections. medRxiv. 2021;2021.06.28.21(258780). Published 2021 Jul 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thachil J, Agarwal S. Understanding the COVID-19 coagulopathy spectrum. Anaesthesia. 2020;75(11):1432‐1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bilaloglu S, Aphinyanaphongs Y, Jones S, Iturrate E, Hochman J, Berger JS. Thrombosis in hospitalized patients With COVID-19 in a New York city health system. JAMA. 2020;324(8):799‐801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piazza G, Campia U, Hurwitz S, et al. Registry of arterial and venous thromboembolic complications in patients With COVID-19. J Am Coll Cardiol. 2020;76(18):2060‐2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089‐1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klok FA, Kruip M, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145‐147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Middeldorp S, Coppens M, van Haaps TF, et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. Journal of thrombosis and haemostasis : JTH. 2020;18(8):1995‐2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Llitjos JF, Leclerc M, Chochois C, et al. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. Journal of thrombosis and haemostasis : JTH. 2020;18(7):1743‐1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cui S, Chen S, Li X, Liu S, Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. Journal of thrombosis and haemostasis : JTH. 2020;18(6):1421‐1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lodigiani C, Iapichino G, Carenzo L, et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in milan, Italy. Thromb Res. 2020;191:9‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. Journal of thrombosis and haemostasis : JTH. 2020;18(4):844‐847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barrett CD, Moore HB, Yaffe MB, Moore EE. ISTH Interim guidance on recognition and management of coagulopathy in COVID-19: a comment. Journal of thrombosis and haemostasis : JTH. 2020;18(8):2060‐2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York city: a prospective cohort study. Lancet. 2020;395(10239):1763‐1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goshua G, Pine AB, Meizlish ML, et al. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020;7(8):e575‐e582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thachil J, Tang N, Gando S, et al. ISTH Interim guidance on recognition and management of coagulopathy in COVID-19. Journal of thrombosis and haemostasis : JTH. 2020;18(5):1023‐1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mortus JR, Manek SE, Brubaker LS, et al. Thromboelastographic results and hypercoagulability syndrome in patients With coronavirus disease 2019 Who Are critically Ill. JAMA network open. 2020;3(6):e2011192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stefely JA, Christensen BB, Gogakos T, et al. Marked factor V activity elevation in severe COVID-19 is associated with venous thromboembolism. Am J Hematol. 2020;95(12):1522‐1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bowles L, Platton S, Yartey N, et al. Lupus anticoagulant and abnormal coagulation tests in patients with covid-19. N Engl J Med. 2020;383(3):288‐290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tiscia GL, Favuzzi G, De Laurenzo A, et al. Reduction of ADAMTS13 levels predicts mortality in SARS-CoV-2 patients. TH open : companion journal to thrombosis and haemostasis. 2020;4(3):e203‐e206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ranucci MA-O, Ballotta A, Di Dedda U, et al. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. Journal of thrombosis and haemostasis : JTH. 2020;18(7):1747‐1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Panigada M, Bottino N, Tagliabue P, et al. Hypercoagulability of COVID-19 patients in intensive care unit: a report of thromboelastography findings and other parameters of hemostasis. Journal of thrombosis and haemostasis : JTH. 2020;18(7):1738‐1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nougier C, Benoit R, Simon M, et al. Hypofibrinolytic state and high thrombin generation may play a major role in SARS-COV2 associated thrombosis. Journal of thrombosis and haemostasis : JTH. 2020;18(9):2215‐2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spiezia L, Boscolo A, Poletto F, et al. COVID-19-Related severe hypercoagulability in patients admitted to intensive care unit for acute respiratory failure. Thromb Haemost. 2020;120(6):998‐1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Masi P, Hékimian G, Lejeune M, et al. Systemic inflammatory response syndrome Is a major contributor to COVID-19-associated coagulopathy: insights From a prospective, single-center cohort study. Circulation. 2020;142(6):611‐614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang WH, Martin KF, Hwa J, Hwa J. Aldose reductase, oxidative stress, and diabetic mellitus. Front Pharmacol. 2012;3:87. doi: 10.3389/fphar.2012.00087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duan Q, Gong Z, Song H, et al. Symptomatic venous thromboembolism is a disease related to infection and immune dysfunction. Int J Med Sci. 2012;9(6):453‐461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cordon-Cardo C, Pujadas E, Wajnberg A, et al. COVID-19: staging of a New disease. Cancer Cell. 2020;38(5):594‐597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poissy J, Goutay J, Caplan M, et al. Pulmonary embolism in patients With COVID-19: awareness of an increased prevalence. Circulation. 2020;142(2):184‐186. [DOI] [PubMed] [Google Scholar]
- 35.Nadkarni GN, Lala A, Bagiella E, et al. Anticoagulation, bleeding, mortality, and pathology in hospitalized patients With COVID-19. J Am Coll Cardiol. 2020;76(16):1815‐1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Al-Samkari H, Karp Leaf RS, Dzik WH, et al. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood. 2020;136(4):489‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients With coronavirus disease 2019 in wuhan, China. JAMA Neurol. 2020;77(6):683‐690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bellosta R, Luzzani L, Natalini G, et al. Acute limb ischemia in patients with COVID-19 pneumonia. J Vasc Surg. 2020;72(6):1864‐1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oxley TJ, Mocco J, Majidi S, et al. Large-Vessel stroke as a presenting feature of covid-19 in the young. N Engl J Med. 2020;382(20):e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ilonzo N, Rao A, Safir S, et al. Acute thrombotic manifestations of coronavirus disease 2019 infection: experience at a large New York city health care system. J Vasc Surg. 2021;73(3):789‐796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perini P, Nabulsi B, Massoni CB, Azzarone M, Freyrie A. Acute limb ischaemia in two young, non-atherosclerotic patients with COVID-19. Lancet. 2020;395(10236):1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fraissé M, Logre E, Pajot O, Mentec H, Plantefève G, Contou D. Thrombotic and hemorrhagic events in critically ill COVID-19 patients: a French monocenter retrospective study. Crit Care. 2020(1);24:275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morassi MA-O, Bagatto D, Cobelli M, et al. Stroke in patients with SARS-CoV-2 infection: case series. J Neurol. 2020;267:2185‐2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Léonard-Lorant IA-O, Delabranche XA-O, Séverac FA-O, et al. Acute pulmonary embolism in patients with COVID-19 at CT angiography and relationship to d-dimer levels. Radiology. 2020;296:E189‐E191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Menter T, Haslbauer JD, Nienhold R, et al. Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology. 2020;77(2):198‐209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wichmann D, Sperhake JP, Lutgehetmann M, et al. Autopsy findings and venous thromboembolism in patients With COVID-19: a prospective cohort study. Ann Intern Med. 2020;173(4):268‐277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lax SF, Skok K, Zechner P, et al. Pulmonary arterial thrombosis in COVID-19 With fatal outcome : results From a prospective, single-center, clinicopathologic case series. Ann Intern Med. 2020;173(5):350‐361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in covid-19. N Engl J Med. 2020;383(2):120‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pellegrini D, Kawakami R, Guagliumi G, et al. Microthrombi as a major cause of cardiac injury in COVID-19: a pathologic study. Circulation. 2021;143(10):1031‐1042. [DOI] [PubMed] [Google Scholar]
- 50.Jhaveri KD, Meir LR, Flores Chang BS, et al. Thrombotic microangiopathy in a patient with COVID-19. Kidney Int. 2020;98(2):509‐512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yaghi S, Ishida K, Torres J, et al. SARS-CoV-2 and stroke in a New York healthcare system. Stroke. 2020;51(7):2002‐2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cantador E, Nunez A, Sobrino P, et al. Incidence and consequences of systemic arterial thrombotic events in COVID-19 patients. J Thromb Thrombolysis. 2020;50(3):543‐547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Prieto-Lobato A, Ramos-Martinez R, Vallejo-Calcerrada N, et al. A case series of stent thrombosis during the COVID-19 pandemic. JACC Case reports. 2020;2(9):1291‐1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bangalore S, Sharma A, Slotwiner A, et al. ST-Segment Elevation in patients with covid-19 - A case series. N Engl J Med. 2020;382(25):2478‐2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Metzler B, Siostrzonek P, Binder RK, Bauer A, Reinstadler SJ. Decline of acute coronary syndrome admissions in Austria since the outbreak of COVID-19: the pandemic response causes cardiac collateral damage. Eur Heart J. 2020;41(19):1852‐1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.De Rosa S, Spaccarotella C, Basso C, et al. Reduction of hospitalizations for myocardial infarction in Italy in the COVID-19 era. Eur Heart J. 2020;41(22):2083‐2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tam CF, Cheung KS, Lam S, et al. Impact of coronavirus disease 2019 (COVID-19) outbreak on outcome of myocardial infarction in Hong Kong, China. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions. 2021;97(2):E194‐E197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huber K, Goldstein P. Covid-19: implications for prehospital, emergency and hospital care in patients with acute coronary syndromes. European heart journal Acute cardiovascular care. 2020;9(3):222‐228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hassager C, Price S, Huber K. Cardiac arrest in the COVID-19 Era. European heart journal Acute cardiovascular care. 2020;9(3):239‐240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Knight DS, Kotecha T, Razvi Y, et al. COVID-19: myocardial injury in survivors. Circulation. 2020;142(11):1120‐1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fox SE, Akmatbekov A, Harbert JL, Li G, Quincy Brown J, Vander Heide RS. Pulmonary and cardiac pathology in african American patients with COVID-19: an autopsy series from New orleans. The Lancet Respiratory medicine. 2020;8(7):681‐686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yan R, Zhang Y, Li Y, Xia L, Guo Y, Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367(6485):1444‐1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Larsen CP, Bourne TD, Wilson JD, Saqqa O, Sharshir MA. Collapsing glomerulopathy in a patient With COVID-19. Kidney international reports. 2020;5(6):935‐939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and Is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271‐280 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Song WC, FitzGerald GA. COVID-19, microangiopathy, hemostatic activation, and complement. J Clin Invest. 2020;130(8):3950‐3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Translational research : the journal of laboratory and clinical medicine. 2020;220:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kerr R, Stirling D, Ludlam CA. Interleukin 6 and haemostasis. Br J Haematol. 2001;115(1):3‐12. [DOI] [PubMed] [Google Scholar]
- 69.Smith SA, Mutch NJ, Baskar D, Rohloff P, Docampo R, Morrissey JH. Polyphosphate modulates blood coagulation and fibrinolysis. Proc Natl Acad Sci U S A. 2006;103(4):903‐908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Page MJ, Bester J, Pretorius E. The inflammatory effects of TNF-alpha and complement component 3 on coagulation. Sci Rep. 2018;8(1):1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Swystun LL, Liaw PC. The role of leukocytes in thrombosis. Blood. 2016;128(6):753‐762. [DOI] [PubMed] [Google Scholar]
- 72.Bhaskar S, Sinha A, Banach M, et al. Cytokine storm in COVID-19-immunopathological mechanisms, clinical considerations, and therapeutic approaches: the REPROGRAM consortium position paper. Front Immunol. 2020;11:1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Castelli V, Cimini A, Ferri C. Cytokine storm in COVID-19: “when You come Out of the storm, You won’t Be the same person Who walked in”. Front Immunol. 2020;11:2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tang Y, Liu J, Zhang D, Xu Z, Ji J, Wen C. Cytokine storm in COVID-19: the current evidence and treatment strategies. Front Immunol. 2020;11:1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from wuhan, China. Intensive Care Med. 2020;46(5):846‐848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033‐2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Subramaniam S, Jurk K, Hobohm L, et al. Distinct contributions of complement factors to platelet activation and fibrin formation in venous thrombus development. Blood. 2017;129(16):2291‐2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hofstra JJ, Haitsma JJ, Juffermans NP, Levi M, Schultz MJ. The role of bronchoalveolar hemostasis in the pathogenesis of acute lung injury. Semin Thromb Hemost. 2008;34(5):475‐484. [DOI] [PubMed] [Google Scholar]
- 79.McGonagle D, O’Donnell JS, Sharif K, Emery P, Bridgewood C. Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. The Lancet Rheumatology. 2020;2(7):e437‐e445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Crawford F, Andras A, Welch K, Sheares K, Keeling D, Chappell FM. D-dimer test for excluding the diagnosis of pulmonary embolism. Cochrane Database Syst Rev;2016(8):CD010864. Published 2016 Aug 5. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang Y, Xiao M, Zhang S, et al. Coagulopathy and antiphospholipid antibodies in patients with covid-19. N Engl J Med. 2020;382(17):e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shoenfeld Y. Corona (COVID-19) time musings: our involvement in COVID-19 pathogenesis, diagnosis, treatment and vaccine planning. Autoimmun Rev. 2020;19(6):102538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu Y, Sun W, Guo Y, et al. Association between platelet parameters and mortality in coronavirus disease 2019: retrospective cohort study. Platelets. 2020;31(4):490‐496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lippi G, Plebani M, Henry BM. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin Chim Acta. 2020;506:145‐148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lefrancais E, Ortiz-Munoz G, Caudrillier A, et al. The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature. 2017;544(7648):105‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xu P, Zhou Q, Xu J. Mechanism of thrombocytopenia in COVID-19 patients. Ann Hematol. 2020;99(6):1205‐1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang M, Ng MH, Li CK. Thrombocytopenia in patients with severe acute respiratory syndrome (review). Hematology. 2005;10(4):101‐105. [DOI] [PubMed] [Google Scholar]
- 88.Moores LK, Tritschler T, Brosnahan S, et al. Prevention, diagnosis, and treatment of VTE in patients With coronavirus disease 2019: cHEST guideline and expert panel report. Chest. 2020;158(3):1143‐1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bikdeli B, Madhavan MV, Gupta A, et al. Pharmacological agents targeting thromboinflammation in COVID-19: review and implications for future research. Thromb Haemost. 2020;120(7):1004‐1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Basbus L, Lapidus MI, Martingano I, Puga MC, Pollán J. [Neutrophil to lymphocyte ratio as a prognostic marker in COVID-19]. Medicina (B Aires). 2020;80(Suppl 3):31‐36. [PubMed] [Google Scholar]
- 91.Sarkar S, Kannan S, Khanna P, Singh AK. Role of platelet-to-lymphocyte count ratio (PLR), as a prognostic indicator in COVID-19: a systematic review and meta-analysis. J Med Virol. 2022;94(1):211‐221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang AP, Liu JP, Tao WQ, Li HM. The diagnostic and predictive role of NLR, d-NLR and PLR in COVID-19 patients. Int Immunopharmacol. 2020;84:106504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bikdeli B, Madhavan MV, Jimenez D, et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-Up: jACC state-of-the-Art review. J Am Coll Cardiol. 2020;75(23):2950‐2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Johri AM, Galen B, Kirkpatrick JN, Lanspa M, Mulvagh S, Thamman R. ASE Statement on point-of-care ultrasound during the 2019 novel coronavirus pandemic. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2020;33(6):670‐673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC guideline on the management of patients With lower extremity peripheral artery disease: executive summary: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2017;69(11):1465‐1508. [DOI] [PubMed] [Google Scholar]
- 96.Welt FGP, Shah PB, Aronow HD, et al. Catheterization laboratory considerations during the coronavirus (COVID-19) pandemic: from the ACC’s interventional council and SCAI. J Am Coll Cardiol. 2020;75(18):2372‐2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: cHEST guideline and expert panel report. Chest. 2016;149(2):315‐352. [DOI] [PubMed] [Google Scholar]
- 98.Cuker A, Tseng EK, Nieuwlaat R, et al. American Society of hematology 2021 guidelines on the use of anticoagulation for thromboprophylaxis in patients with COVID-19. Blood Adv. 2021;5(3):872‐888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vandenbriele C, Aelst LV, Balthazar T, et al. Anticoagulant therapy in COVID-19 critically ill: should we go for more? Austrian Journal of Cardiology. 2020;27(5):156‐158. [Google Scholar]
- 100.Lemos ACB, do Espirito Santo DA, Salvetti MC, et al. Therapeutic versus prophylactic anticoagulation for severe COVID-19: a randomized phase II clinical trial (HESACOVID). Thromb Res. 2020;196:359‐366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. Journal of thrombosis and haemostasis : JTH. 2020;18(5):1094‐1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Talasaz AH, Sadeghipour P, Kakavand H, et al. Recent Randomized Trials of Antithrombotic Therapy for Patients With COVID-19: JACC State-of-the-Art Review. J Am Coll Cardiol. 2021;77(15):1903-1921. doi: 10.1016/j.jacc.2021.02.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.NIH ACTIV Trial of Blood Thinners Pauses Enrollment of Critically ill COVID-19 Patients. National Institute of Health; 2020. [Google Scholar]
- 104.Full-dose Blood Thinners Decreased Need for Life Support and Improved Outcome in Hospitalized COVID-19 Patients. National Heart, Lung and Blood Institute; 2021. [Google Scholar]
- 105.ATTAC, ACTIV-4a & REMAP-CAP Multiplatform RCT; Results of Interim Analysis. 2021.
- 106.Fan BE, Umapathi T, Chua K, et al. Delayed catastrophic thrombotic events in young and asymptomatic post COVID-19 patients. J Thromb Thrombolysis. 2021;51(4):971-977. doi:10.1007/s11239-020-02332-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lopes RD, de Barros ESPGM, Furtado RHM, et al. Therapeutic versus prophylactic anticoagulation for patients admitted to hospital with COVID-19 and elevated D-dimer concentration (ACTION): an open-label, multicentre, randomised, controlled trial. Lancet. 2021;397(10291):2253‐2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sadeghipour P, Talasaz AH, Rashidi F, et al. Effect of Intermediate-Dose versus Standard-Dose Prophylactic Anticoagulation on Thrombotic Events, Extracorporeal Membrane Oxygenation Treatment, or Mortality Among Patients With COVID-19 Admitted to the Intensive Care Unit: The INSPIRATION Randomized Clinical Trial. LID - 10.1001/jama.2021.4152 [doi] LID - e214152. Jama 2021;Online ahead of print. [DOI] [PMC free article] [PubMed]
- 109.Vaughn VM, Yost M, Abshire C, et al. Trends in venous thromboembolism anticoagulation in patients hospitalized With COVID-19. JAMA network open. 2021;4:e2111788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Connors JM, Brooks MM, Sciurba FC, et al. Effect of antithrombotic therapy on clinical outcomes in outpatients With clinically stable symptomatic COVID-19: the ACTIV-4B randomized clinical trial. Jama. 2021;326(17):1703‐1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Martel N, Fau LJ, Wells PS, Wells PS. Risk for heparin-induced thrombocytopenia with unfractionated and low-molecular-weight heparin thromboprophylaxis: a meta-analysis. Blood. 2005;106(8):2710‐2715. [DOI] [PubMed] [Google Scholar]
- 112.Marongiu F, Barcellona D. Fondaparinux: should It Be studied in patients with COVID-19 disease? TH open : companion journal to thrombosis and haemostasis. 2020;4:e300‐e302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Talasaz AH, Sadeghipour P, Kakavand H, et al. Recent randomized trials of antithrombotic therapy for patients With COVID-19: jACC state-of-the-Art review. J Am Coll Cardiol. 2021;77(15):1903‐1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kharma N, Roehrig S, Shible AA, et al. Anticoagulation in critically ill patients on mechanical ventilation suffering from COVID-19 disease, The ANTI-CO trial: a structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21(1):769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Testa S, Prandoni P, Paoletti O, et al. Direct oral anticoagulant plasma levels’ striking increase in severe COVID-19 respiratory syndrome patients treated with antiviral agents: the cremona experience. Journal of thrombosis and haemostasis : JTH. 2020;18(6):1320‐1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Roberts LN, Whyte MB, Georgiou L, et al. Postdischarge venous thromboembolism following hospital admission with COVID-19. Blood. 2020;136(11):1347‐1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lawlor M, Gupta A, Ranard LS, et al. Discordance in activated partial thromboplastin time and anti-factor Xa levels in COVID-19 patients on heparin therapy. Thromb Res. 2021;198:79‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Canzano P, Brambilla M, Porro B, et al. Platelet and endothelial activation as potential mechanisms behind the thrombotic complications of COVID-19 patients. JACC Basic Transl Sci. 2021;6(3):202‐218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Carestia A, Davis RP, Grosjean H, Lau MW, Jenne CN. Acetylsalicylic acid inhibits intravascular coagulation during staphylococcus aureus-induced sepsis in mice. Blood. 2020;135(15):1281‐1286. [DOI] [PubMed] [Google Scholar]
- 120.Ouyang Y, Wang Y, Liu B, Ma X, Ding R. Effects of antiplatelet therapy on the mortality rate of patients with sepsis: a meta-analysis. J Crit Care. 2019;50:162‐168. [DOI] [PubMed] [Google Scholar]
- 121.Chow JH, Khanna AK, Kethireddy S, et al. Aspirin Use Is associated With decreased mechanical ventilation, intensive care unit admission, and In-hospital mortality in hospitalized patients With coronavirus disease 2019. Anesth Analg. 2021;132(4):930‐941. [DOI] [PubMed] [Google Scholar]
- 122.Meizlish ML, Goshua G, Liu Y, et al. Intermediate-dose anticoagulation, aspirin, and in-hospital mortality in COVID-19: a propensity score-matched analysis. medRxiv : the preprint server for health sciences. 2021;2021.01.12.21249577. Published 2021 Jan 15. doi: 10.1101/2021.01.12.21249577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liu X, Li Z, Liu S, et al. Potential therapeutic effects of dipyridamole in the severely ill patients with COVID-19. Acta pharmaceutica Sinica B. 2020;10(7):1205‐1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sexton TR, Zhang G, Macaulay TE, et al. Ticagrelor reduces thromboinflammatory markers in patients With pneumonia. JACC Basic Transl Sci. 2018;3(4):435‐449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Qureshi AI, Abd-Allah F, Al-Senani F, et al. Management of acute ischemic stroke in patients with COVID-19 infection: report of an international panel. International journal of stroke : official journal of the International Stroke Society. 2020;15(5):540‐554. [DOI] [PubMed] [Google Scholar]
- 126.Sinha A, Bhaskar SMM. In-hospital prevalence of mucormycosis among coronavirus disease 2019 (COVID-19) patients and COVID-19 in mucormycosis: a systematic review and meta-analysis. [published online ahead of print, 2021 Oct 11]. Int Forum Allergy Rhinol. 2021;10.1002/alr.22906. doi: 10.1002/alr.22906. [DOI] [PMC free article] [PubMed] [Google Scholar]