Abstract
Purpose:
Active hexose-correlated compound (AHCC), a standardized extract of cultured Lentinula edodes mycelia, exerts antitumor effects through anti-inflammatory and immune-modulatory functions. Adjuvant therapy for patients with hepatocellular carcinoma (HCC) who have undergone curative hepatectomy has not been established. The purpose of this study was to evaluate the efficacy and safety of AHCC as adjuvant therapy in patients with advanced HCC after curative hepatectomy.
Patients and methods:
The study design was single-armed, non-randomized, open (no one was blinded), and uncontrolled. Patients with HCC who underwent curative hepatectomy were treated with AHCC (1 g) 3 times daily orally for 2 years. The inclusion criteria were HCC diagnosed preoperatively as stages A and B of the Barcelona clinic liver cancer (BCLC) classification and alpha-fetoprotein × protein induced by vitamin K absence or antagonist II (PIVKA-II) ≥ 105 for stage A.
Results:
A total of 29 patients were treated with AHCC, of which 25 (4 patients discontinued) were followed up. The 2-year recurrence-free survival rate after resection was 48% for those without discontinuations and 55.2% for all patients with a history of treatment. Serum albumin levels decreased to a minimum in the first postoperative month and gradually recovered to the preoperative level at 6 months. Almost no change in lymphocyte percentage was observed during follow-up. Inflammation-based prognostic scores were maintained at favorable levels after hepatectomy. Toxicity and adverse events were not observed in any patient.
Conclusion:
AHCC may be safe and effective in preventing HCC recurrence after curative hepatectomy, and further randomized trials of AHCC for its use in this setting are warranted.
This clinical trial was registered in UMIN Clinical Trials Registry (ID UMIN000024396).
Keywords: AHCC, hepatocellular carcinoma, hepatectomy, recurrence, adjuvant
Introduction
Liver cancer is the third leading cause of cancer-related deaths worldwide. 1 Hepatocellular carcinoma (HCC) has a poor prognosis and accounts for 70% to 85% of primary liver cancers. 2 Hepatectomy for HCC yields the highest local control of all local treatments, with a good survival rate. 3 However, according to the Barcelona clinic liver cancer (BCLC) staging classification, hepatectomy is contraindicated in intermediate-stage patients (stage B HCC) and transarterial chemoembolization (TACE) or systemic therapy (Atezolizumab plus bevacizumab) are suggested. 4 In contrast to the BCLC staging guidelines, hepatectomy is suggested in some patients with stage B HCC according to a Japanese evidence-based treatment algorithm 5 and clinical practice guidelines for HCC proposed by the Japan Society of Hepatology (JSH algorithm), 6 with acceptable prognoses.7,8 However, hepatectomy is associated with a high risk of HCC recurrence. 9 The use of adjuvant therapy for patients with HCC who have undergone curative hepatectomy has not been established, although new therapies, such as molecular targeted therapy and immune checkpoint inhibition, are available.10,11
Active hexose-correlated compound (AHCC), produced by Amino Up Co., Ltd. (Sapporo, Japan), is a standardized extract of cultured Lentinula edodes (Shiitake) mycelia obtained through the liquid culture of shiitake mycelia. 12 AHCC contains various nutrients including oligosaccharides, amino acids, and minerals. The effect of AHCC on the immune system for malignancy by natural killer (NK) and T cell, its usefulness in clinical cancer therapy13-15 and its anti-inflammatory and anti-oxidant effects16-18 have been reported. Only 1 study reported that the intake of AHCC improved the prognosis for patients with HCC who underwent curative hepatectomy. 19 However, in this study, patients in early to advanced stages were included, and the host-inflammatory response was not analyzed, although this response has been previously reported to be associated with cancer progression and patient survival. 20
The purpose of this study was the preliminary assessment of the efficacy, safety, and outcome of AHCC as adjuvant therapy in patients with advanced HCC after curative hepatectomy and the evaluation of the host-inflammatory response.
Methods
Study Design
The study design was single-armed, non-randomized, open (no one was blinded), and uncontrolled. The study period was from November 19, 2016 to March 31, 2021. The patients who had undergone curative hepatectomy at Hokkaido University Hospital during the same period were recruited by the physician co-investigators of the study based on their written informed consent. Patients with curative hepatectomy were administered 1 g AHCC orally, 3 times daily for 2 years. We used commercially available AHCC that was provided to us multiple times. AHCC was stored at room temperature (23°C) and used before its expiry date based on Food Labeling Guideline in Japan. Inclusion criteria for this study were: HCC diagnosed using computed tomography (CT) and/or magnetic resonance imaging (MRI) within stages A and B of BCLC classification; without pretreatment for HCC; alpha-fetoprotein (AFP) × protein induced by vitamin K absence or antagonist II (PIVKA-II) ≥ 105 for stage A; without distant metastasis and macroscopic tumor thrombus; age 20 to 85; ECOG performance status 0 to 2; Child–Pugh score ≤ 7; functional reserves of bone marrow, liver, and kidney; WBC ≥ 2000/mm3 and ≤10 000/mm3; platelets ≥ 50 000/mm3; Hb ≥ 8.0 g/dL; total bilirubin ≤ 2.0 mg/dL; prothrombin time activity ≥ 50%; creatinine ≤ 1.5 mg/dL; BUN ≤ 35 mg/dL; and acceptance of informed consent. Exclusion criteria were: other cancers, severe cardiac and/or pulmonary failure, renal failure contraindication of enhanced CT and/or MRI, mental illness, pregnancy, previous intake of AHCC, and contraindications as decided by the physician co-investigators of the study. Clinical, laboratory, surgical, and pathological data of enrolled patients were prospectively collected and retrospectively analyzed.
Toxicity was assessed according to the Common Terminology Criteria for Adverse Events (CTCAE) ver. 4.0. This assessment was performed once every 3 months to 2 years along with the physical examination and blood tests after hepatectomy by a physician coinvestigator in an outpatient setting. The drug intake was delayed if toxicity ≥ grade 3 was observed and stopped if the patients no longer met the eligibility criteria or if tumors recurred.
All analyses in this study were performed in accordance with the ethical guidelines of Hokkaido University Hospital and according to the Declaration of Helsinki. This study was approved by the Institutional Review Board of Hokkaido University (自016-0100) ( November 9, 2016).
Hepatectomy
We used our algorithm, which incorporates indocyanine green retention rate at 15 minutes (ICGR15) and remnant liver volume, to determine the optimal operative procedure, as described. 21 Anatomical resection is defined as complete anatomical removal of lesion(s) based on Couinaud’s classification (segmentectomy, sectionectomy, and hemihepatectomy or extended hemihepatectomy) in patients with sufficient functional reserve. Non-anatomical partial but complete resection was achieved in all patients. R0 resections were performed in all patients, and all resection surfaces were histologically free of HCC.
HCC Recurrence
In the first postoperative month after hepatectomy, patients underwent follow-up evaluations comprising liver function tests, measurements of AFP and PIVKA-II, ultrasonography, and dynamic CT. In the first postoperative month after hepatectomy, the same follow-up study was performed once every 3 months to 2 years after hepatectomy. If recurrence was suspected, CT and MRI were performed; if necessary, CT angiography and bone scintigraphy were performed. This enabled precise determination of the site, number, size, and extent of invasion of recurrent lesions.
Statistical Analysis
Overall survival (OS) and disease-free survival (DFS) rates were calculated using the Kaplan–Meier method. Potential prognostic factors were identified through a univariate analysis using the log-rank test. Independent prognostic factors were evaluated using a multivariable logistic regression analysis. Statistical significance was set at P < .05. Statistical analyses were performed using the JMP software (version 14 for Windows; SAS Institute, Cary, NC, USA).
Results
A total of 31 patients were included in this study, of which 29 were treated with AHCC. Two patients were not treated with AHCC because of procedural inconvenience. Due to other malignant diseases, such as malignant lymphoma, prostate cancer, and tongue cancer, the administration of AHCC was stopped in 3 cases after 2.4, 13.4, and 16.3 months. In 1 case, the administration of AHCC was stopped because of the patient’s refusal after 2 weeks. The mean period of administration of these 4 patients was 8.2 months. A prognostic analysis was performed for 25 patients and safety analysis for 29 patients who were treated with AHCC. Table 1 shows the data of the 29 treated patients, with the mean age of 68 (36-84). The number of males and females was 27 and 2, respectively. 11. The average length of follow-up of the patients in the study was 22 months.
Table 1.
Patient Characteristics.
| Variable | (n) or (mean) | Range |
|---|---|---|
| Age (year) | 68 | 36-84 |
| Sex | ||
| male:female | 27:2 | |
| Hepatic virus status | ||
| HBV | 10 | |
| HCV | 4 | |
| NBNC | 15 | |
| Albumin (g/dL) | 4.2 | 3.6-4.8 |
| Total bilirubin (mg/dL) | 0.6 | 0.3-0.9 |
| ICGR15 (%) | 10.8 | 1.4-23.5 |
| NLR | 3 | 0.8-12.7 |
| SII | 625 | 150.7-3242.1 |
| PNI | 50 | 39.7-58.7 |
| Size (mm) | 79.4 | 32-170 |
| Number | ||
| Solitary | 21 | |
| Multiple | 8 | |
| Stage | ||
| II | 18 | |
| III | 10 | |
| Iva | 1 | |
| BCLC stage | ||
| A | 1 | |
| B | 28 | |
| AFP (ng/mL) | 26 676 | 1.8-471 728 |
| PIVKA-II (mAU/mL) | 9632.4 | 17-140 350 |
| AP-factor | ||
| Low | 16 | |
| High | 13 | |
| Child–Pugh | ||
| A | 29 | |
| Differentiation | ||
| Well | 1 | |
| Moderate | 16 | |
| Poor | 12 | |
| Vascular invasion | ||
| vp+ | 2 | |
| vp− | 22 | |
| vv+ | 10 | |
| vv− | 19 | |
| Fibrosis 22 | ||
| F0 | 5 | |
| F1 | 9 | |
| F2 | 12 | |
| F3 | 3 | |
Abbreviations: HBV, hepatitis B virus surface antigen-positive, hepatitis C virus antibody-negative; HCV, hepatitis B virus surface antigen negative, hepatitis C virus antibody-positive; NBNC, hepatitis B virus surface antigen negative, hepatitis C virus antibody-negative; ICGR15, indocyanine green retention rate at 15 minutes; NLR, neutrophil-to-lymphocyte ratio; SII, systemic immune-inflammation index; PNI, prognostic nutritional index; BCLC stage, Barcelona clinic liver cancer staging classification; AFP, alpha-fetoprotein; PIVKA-II, protein induced by vitamin K absence or antagonist II; AP-factor, AFP × PIVKA-II; vp, microscopic tumor thrombus in the portal vein; vv, microscopic tumor thrombus in the hepatic vein; F0, absence of fibrosis; F1, portal fibrosis with no septa; F2, portal fibrosis with infrequent septa; F3, numerous septa but no cirrhosis.
Adverse Effects of AHCC
Fever of 38.0°C to 39.0°C for 4 days was observed in 1 case and this patient recovered with symptomatic treatment. This fever occurred 2 weeks after the operation and was related to the operation and not to the side effects of AHCC. No AHCC-related toxicity of ≥ grade 3 or adverse events were observed in all 29 patients.
Recurrence and Death
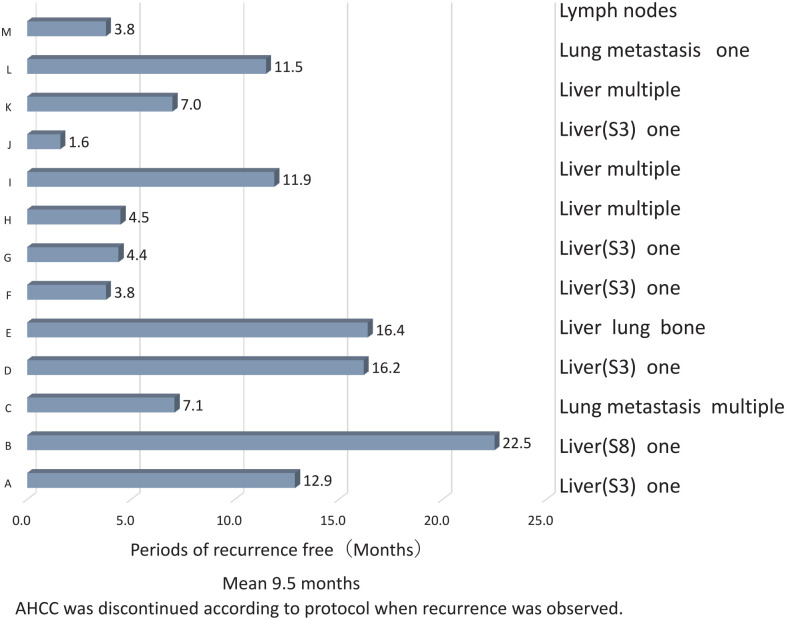
Recurrence occurred in 13 patients (13/25, 52%). Recurrent sites are shown in Figure 1. Remnant liver with multiple nodules was present in 3 cases, with solitary nodules in 6 cases, and with extrahepatic metastasis (lung and bone) in 1 case. Three cases with only distant metastasis without liver recurrence were observed, and the site of recurrence was the lymph node in 1 case and the lung in 2 cases (solitary and multiple). The mean duration from hepatectomy to recurrence was 9.5 months.
Figure 1.
Recurrence occurred in 13/15 (52%) patients. Sites of recurrence included remnant liver with multiple nodules in 3 cases, solitary nodules in 6 cases, and extrahepatic metastasis (lung and bone) in 1 case. Extrahepatic metastasis was observed only in the lymph nodes and lungs (multiple and solitary). The mean duration from hepatectomy to recurrence was 9.5 months.
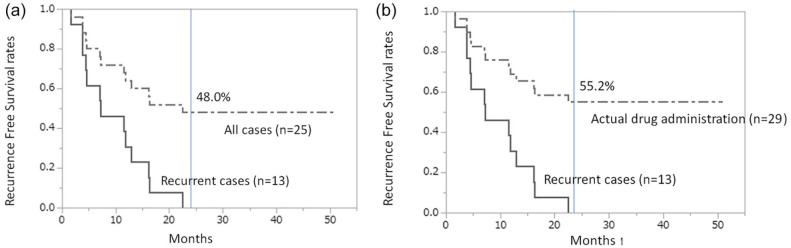
The recurrence-free survival rate at 2 years in 25 patients (excluding 4 cases who discontinued) was 48% (Figure 2a), whereas that of all 29 treated cases was 55.2% (Figure 2b). Death occurred in 1 patient with recurrence in the hilar region of the liver and periaortic lymph nodes of the abdomen, followed by obstructive jaundice with increasing size, liver failure, and death. In this patient, AHCC was discontinued according to the protocol when lymph node recurrence was observed.
Figure 2.
Kaplan–Meier curves for recurrence-free survival. (A) Recurrence-free survival rate at 2 years in 25 patients (excluding 4 cases who discontinued) was 48%. (B) Recurrence-free survival rate at 2 years in 29 treated cases was 55.2%.
For patients with recurrence within 2 years, a significant increase in preoperative total bilirubin level (P = .01), AP-factor value (P = .027), and hepatic vein invasion (P = .0148) was observed compared with that in patients without recurrence (Table 2).
Table 2.
Comparison Between Recurrent and No Recurrent Cases.
| Variable | Recurrence (+) (n = 13) | Recurrence (−) (n = 12) | P-value |
|---|---|---|---|
| Age (year) | |||
| Mean (SD) | 64.8 ± 12.4 | 69.7 ± 9.2 | .2754 |
| Sex | |||
| Male | 13 | 11 | .2881 |
| Female | 0 | 1 | |
| Hepatic virus | |||
| HBV | 7 | 3 | .3237 |
| HCV | 1 | 1 | |
| NBNC | 5 | 8 | |
| Albumin (g/dL) | |||
| Mean (SD) | 4.1 ± 0.4 | 4.3 ± 0.3 | .2626 |
| Total bilirubin (mg/dL) | |||
| Mean (SD) | 0.6 ± 0.1 | 0.7 ± 0.1 | .01 |
| ICGR15 (%) | |||
| Mean (SD) | 11.5 ± 5.7 | 9.8 ± 6.0 | .4752 |
| NLR | |||
| Mean (SD) | 2.8 ± 1.5 | 3.3 ± 3.2 | .5923 |
| SII | |||
| Mean (SD) | 539.6 ± 318.3 | 734.1 ± 840.7 | .445 |
| PNI | |||
| Mean (SD) | 48.9 ± 6.2 | 50.8 ± 4.4 | .3921 |
| Size (mm) | |||
| Mean (SD) | 95.1 ± 35.6 | 75.4 ± 8.8 | .1182 |
| Number | |||
| Solitary | 9 | 11 | .1612 |
| Multiple | 4 | 1 | |
| Stage | |||
| II | 7 | 10 | .1143 |
| III | 6 | 2 | |
| BCLC stage | |||
| B | 13 | 12 | NA |
| AFP (ng/mL) | |||
| Mean (SD) | 59 278.3 ± 136 068.7 | 75.4 ± 177.4 | .146 |
| PIVKA-II (mAU/mL) | |||
| Mean (SD) | 20 515.1 ± 40 087.3 | 983.0 ± 1784.1 | .1058 |
| AP-factor | |||
| Low | 4 | 9 | .027 |
| High | 9 | 3 | |
| Child–Pugh | |||
| A | 13 | 12 | NA |
| Differentiation | |||
| Well | 7 | 7 | .5061 |
| Moderate | 6 | 4 | |
| Poor | 0 | 1 | |
| Vascular invasion | |||
| vp+ | 11 | 9 | .5482 |
| vp− | 2 | 3 | |
| vv+ | 6 | 11 | .0148 |
| vv− | 7 | 1 | |
| Fibrosis 22 | |||
| F0 | 2 | 3 | .052 |
| F1 | 2 | 7 | |
| F2 | 7 | 2 | |
| F3 | 2 | 0 | |
HBV, hepatitis B virus surface antigen-positive, hepatitis C virus antibody-negative; HCV, hepatitis B virus surface antigen negative, hepatitis C virus antibody-positive; NBNC, hepatitis B virus surface antigen negative, hepatitis C virus antibody-negative; ICGR15, indocyanine green retention rate at 15 minutes; NLR, neutrophil-to-lymphocyte ratio; SII, systemic immune-inflammation index; PNI, prognostic nutritional index; BCLC stage, Barcelona clinic liver cancer staging classification; AFP, alpha-fetoprotein; PIVKA-II, protein induced by vitamin K absence or antagonist II; AP-factor, AFP × PIVKA-II; vp, microscopic tumor thrombus in the portal vein; vv, microscopic tumor thrombus in the hepatic vein; F0, absence of fibrosis; F1, portal fibrosis with no septa; F2, portal fibrosis with infrequent septa; F3, numerous septa but no cirrhosis; SD, standard deviation; NA, not applicable.
The logistic regression analysis of recurrence at 2 years after hepatectomy revealed that hepatic vein invasion was an independent prognostic factor (Table 3).
Table 3.
Logistic Regression Analysis Focused on Recurrence at 2 years After Hepatectomy.
| Variable | P-value | Odds ratio | 95% CI low | 95% CI high |
|---|---|---|---|---|
| AP-factor | ||||
| High | .4755 | 2.1709 | 0.2453 | 19.09 |
| Pre T. Bil | ||||
| High | .1151 | 0.1700 | 0.0076 | 1.4964 |
| Hepatic vein invasion | ||||
| Positive | .0325 | 19.25 | 1.2582 | 860.72 |
AFP, alpha-fetoprotein; PIVKA-II, protein induced by vitamin K absence or antagonist II; AP-factor, AFP × PIVKA-II; Pre T. Bil, preoperative total bilirubin; CI, confidence interval.
Change of Clinical Parameters and Inflammation-based Parameters
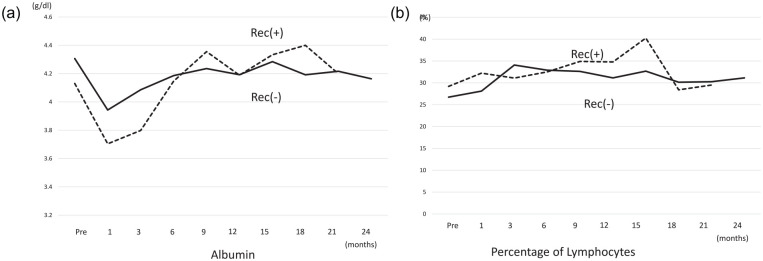
In both recurrence (+) and no recurrence (−) groups, serum albumin levels decreased to a minimum in the first postoperative month. Serum albumin levels in the first postoperative month in the recurrence (+) group were lower than those in the recurrence (−) group, although the difference was not significant. At postoperative 6 months, levels in both groups gradually recovered to preoperative levels (Figure 3a). Almost no change in lymphocyte percentage in either group was observed during the follow-up (Figure 3b).
Figure 3.
Mean profiles of clinical parameters in all 29 administered cases. (A) Albumin: Serum albumin levels decreased to a minimum in the first postoperative month. Serum levels were lower in the recurrence (+) group at the first postoperative month than in the recurrence (−) group, although the difference was not significant. At postoperative 6 months, the levels in both groups gradually recovered to the preoperative levels. (B) Lymphocyte percentage: Almost no change in lymphocyte percentage in both groups during the follow-up.
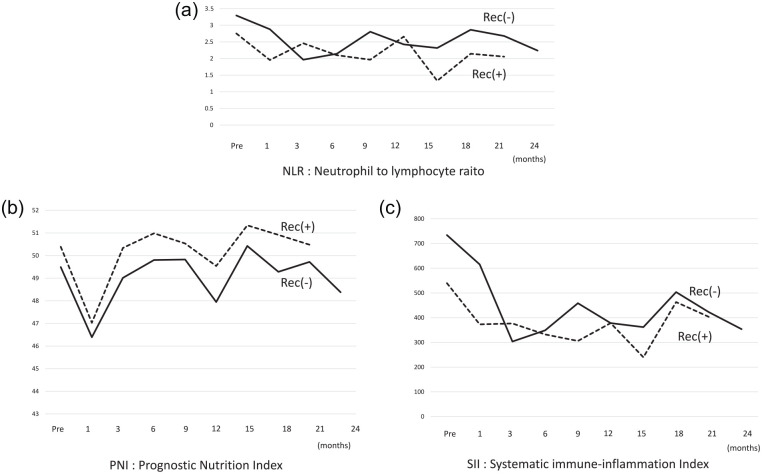
Regarding inflammation-based scores, changes in the neutrophil-to-lymphocyte ratio (NLR) were not different between the groups, and levels of NLR were maintained at <3.5 which were the cut-off points given in the report by Wang et al 23 (Figure 4a). Prognostic nutritional index (PNI) score = albumin (g/dL) + 0.005 × total lymphocyte count (mm3) decreased to a minimum in the first postoperative month and gradually recovered, although the minimum levels were not <46 which were also the cut-off points given in the report by Wang et al 23 (Figure 4b). Systemic immune-inflammation index (SII) score = Platelet (×109/L) × NLR of all patients decreased up to 3 months, and then maintained values of around 305 which were also the cut-off points given in the report by Wang et al 23 (Figure 4c). These values were not significantly different at the same time-points of NLR, PNI, and SII.
Figure 4.
The mean profiles of inflammation-based scores in all 29 administered cases. These values were not significantly different at the same time-points. (A) The change in the neutrophil-to-lymphocyte (NLR) ratio was not different between the groups, and NLR was maintained at <3.5. (B) Prognostic nutritional index (PNI) score decreased to a minimum in the first postoperative month and gradually recovered. (C) Systemic immune-inflammation index (SII) = Plt (×109/L) × NLR decreased up to 3 months and then maintained the same value.
Discussion
In this study, the 2-year recurrence-free survival rate for resected BCLC-B cases was 48% for those who completed 2 years of treatment and 55.2% for all patients with a history of treatment. These values are better than those previously reported.8,24 The inflammation-based prognostic scores of patients with AHCC, namely LNR, PNI, and SII, were maintained at favorable levels after hepatectomy. AHCC-related toxicity and adverse events were not observed in any patient treated with AHCC. In this study, AHCC has been found to be a promising adjuvant therapy for patients with advanced hepatocellular carcinoma after curative hepatectomy, and this should be verified in randomized trials.
In our study, the 2-year recurrence-free survival rates were 48.0% for complete two-year intake and 55.2% for all intakes. Hence, AHCC showed superior efficacy in preventing recurrence of HCC after curative hepatectomy compared with those reported in the previous reports.8,24,25 Although hepatectomy for HCC has the highest local controllability, HCC in BCLC-B HCC is contraindicated for hepatectomy and TACE or systemic therapy are recommended. 4 By contrast, the superiority of hepatic resection to TACE for patients with BCLC-B HCC was suggested on the basis of the survival analysis of the retrospective study population and propensity-matched pairs. 26 Moreover, patients with BCLC-B HCC who underwent hepatectomy were reported to have acceptable prognoses.8,24,25 The 2-year recurrence-free survival rates in these 2 reports were about 40% though the specific value of the 2-year recurrence-free survival rate was not given in these articles; hence, it was estimated from the survival curve. The 2 year-disease free survival rates of HCC in Eastern and Western Experience Study Groups were presumed to be more than 45% from the disease-free survival curve. 25 Therefore, although further studies are needed, the prevention of recurrence of BCLC-B hepatocellular carcinoma by AHCC after hepatic resection seems to be promising.
The analysis of the host-inflammatory response was not performed in the previous study. 19 However, it was recently reported to be associated with cancer progression and patient survival. 20 Although the independent prognostic factor for recurrence in this study was hepatic vein invasion positive, and SII, PNI, and NLR scores were not significant factors, the maintenance of these scores at favorable levels during follow-ups might contribute to preventing HCC recurrence. In patients with HCC, severe hepatitis and active inflammation are associated with recurrence after hepatectomy through the NF-κB pathway-mediated epithelial-mesenchymal transition. 27 Recently, the prognostic value of many inflammation-based prognostic scores constructed on systemic inflammatory responses has been reported, in particular, SII. 23 Hence, we evaluated inflammation-based prognostic scores, including SII, PNI, and NLR. No difference was observed in these parameters between patients with and without recurrence within 2 years. However, these parameters were maintained at the favorable levels of prognostic value after hepatectomy, as Wang et al 23 reported that SII, PNI, and NLR were useful indicators of post recurrence survival of HCC patients. These results might be supported by the antioxidant and anti-inflammatory effects of AHCC.16-18,28-30 These findings should be validated in a randomized trial setting.
We also evaluated albumin levels and lymphocyte percentages. Albumin levels were recovered after 6 months of hepatectomy, and lymphocyte percentages were maintained at almost the same levels during follow-up. The early recovery of albumin and maintenance of lymphocyte percentages maybe because of AHCC intake because Roman et al 31 reported that AHCC supplementation improved lymphocyte percentages. Moreover, AHCC intake could also prolong survival and improve the prognosis of patients with advanced liver cancer, including HCC and cholangiocarcinoma, and delay the gradual decline of their physiological status. No significant difference was observed in albumin levels and lymphocyte percentages, which did not deteriorate after AHCC treatment between the 2 groups with and without AHCC treatment. 32 In a previous clinical study of patients with HCC, the prognosis of 113 patients with HCC who underwent curative hepatectomy using AHCC was retrospectively compared to 109 patients who did not receive AHCC. 19 The results indicated that postoperative AHCC intake had a statistically significant effect on several parameters, including improvement of biochemical parameters (cholinesterase activity which is related to protein synthesis by the liver) and hepatitis, prevention of recurrence, and survival rates. The multivariate analysis revealed that AHCC intake was selected as an independent factor for no recurrence or OS.
In our study, recurrence after curative hepatectomy might have been prevented by the immune function of AHCC as described below. AHCC has been reported to exert immune-protective effects against many types of cancer and the effect of AHCC on the components of the immune system, such as NK cells, dendritic cells (DCs), and T cells, including CD4 and CD8 positive T cells. 15 After AHCC treatment, NK cell activity, which was depressed by an anticancer drug, was reportedly restored and peritoneal macrophage cytotoxicity, nitric oxide production, and cytokine production were stimulated. 12 In patients with breast cancer, who were scheduled to receive postoperative adjuvant anthracycline-based chemotherapy, administration of L. edodes mycelia extract maintained patients’ quality of life and immune function by inhibiting the increase in the proportion of regulatory T cells to peripheral CD4+ cells. 33 Intake of AHCC increased CD8+ T cell lymphocyte levels after 6 cycles of chemotherapy. 34 The antitumor effects of low-dose 5-fluorouracil were potentiated by AHCC in hepatoma 22 tumor-bearing mice through immunomodulation, such as increasing NK cell percentage. 14 AHCC treatment significantly delayed tumor development after B16-F0 melanoma inoculation through an increase in the number of NK cells and tumor antigen-specific CD8+ T cells producing interferon-γ and gammadelta T cells. 13 AHCC intake in healthy volunteers resulted in an increase in the number of DCs and function of CD11c+DC1s (22).
This study has some limitations. First, this was a single-armed, non-randomized, open, and uncontrolled study. Second, the number of patients was small. Third, the parameters did not include immune-related markers. In the future, a randomized, controlled study with a large number of patients should be performed for further validation of these results.
Conclusion
Oral intake of AHCC in patients with advanced HCC after curative hepatectomy was safe and maintained lymphocyte percentages and inflammatory markers such as NLR, PNI, and SII at favorable or normal levels accompanying the early recovery of albumin. This study indicated the potential for improved recurrence-free survival compared with that of previously published studies, and these results should be validated in randomized trials.
Acknowledgments
The authors acknowledge Amino Up Co., Ltd. (Sapporo, Japan) for providing samples of AHCC, which is a trademark of Amino Up Co., Ltd.
Footnotes
Author Contributions: Toshiya Kamiyama: conceptualization, data collection, data analysis, investigation, project administration, visualization, supervision, and writing; Tatsuya Orimo: data collection, data analysis, methodology; Kenji Wakayama: data collection, data analysis, methodology; Tatsuhiko Kakisaka: data collection, data analysis; Shingo Shimada: data collection, data analysis; Akihisa Nagatsu: data collection; Yoh Asahi: data collection; Takeshi Aiyama: data collection; Hirofumi Kamachi: data collection and supervision; Akinobu Taketomi: conceptualization and supervision
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Amino Up Co., Ltd. (Sapporo, Japan) (PC63160007).
Ethics Approval: All analyses in this study were performed in accordance with the ethical guidelines of Hokkaido University Hospital and according to the Declaration of Helsinki. This study was approved by the Institutional Review Board of Hokkaido University (CRB1180001).
Trial Registration: This clinical trial was registered in the UMIN Clinical Trials Registry. The trial registry: Prevention of recurrence after curative hepatectomy for hepatocellular carcinoma by active hexose correlated compound and URL; https://upload.umin.ac.jp/cgi-open-bin/ctr/ctr_view.cgi?recptno=R000028079, and ID; UMIN000024396.
ORCID iDs: Toshiya Kamiyama  https://orcid.org/0000-0002-9157-7811
https://orcid.org/0000-0002-9157-7811
Yoh Asahi  https://orcid.org/0000-0002-0985-7874
https://orcid.org/0000-0002-0985-7874
Takeshi Aiyama  https://orcid.org/0000-0001-5658-2730
https://orcid.org/0000-0001-5658-2730
Data Accessibility Statement: The data that support the findings of this study are openly available in the UMIN Clinical Trials Registry.
References
- 1. Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941-1953. [DOI] [PubMed] [Google Scholar]
- 2. Ahmed F, Perz JF, Kwong S, Jamison PM, Friedman C, Bell BP. National trends and disparities in the incidence of hepatocellular carcinoma, 1998-2003. Prev Chronic Dis. 2008;5:A74. [PMC free article] [PubMed] [Google Scholar]
- 3. Hasegawa K, Kokudo N, Imamura H, et al. Prognostic impact of anatomic resection for hepatocellular carcinoma. Ann Surg. 2005;242:252-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Llovet JM, Villanueva A, Marrero JA, et al. Trial design and endpoints in hepatocellular carcinoma: AASLD consensus conference. Hepatology. 2021;73:158-191. [DOI] [PubMed] [Google Scholar]
- 5. Kokudo N, Takemura N, Hasegawa K, et al. Clinical practice guidelines for hepatocellular carcinoma: The Japan Society of Hepatology 2017 (4th JSH-HCC guidelines) 2019 update. Hepatol Res. 2019;49:1109-1113. [DOI] [PubMed] [Google Scholar]
- 6. Kudo M, Izumi N, Kokudo N, et al. Management of hepatocellular carcinoma in Japan: Consensus-Based Clinical Practice Guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated version. Dig Dis. 2011;29:339-364. [DOI] [PubMed] [Google Scholar]
- 7. Arii S, Yamaoka Y, Futagawa S, et al. Results of surgical and nonsurgical treatment for small-sized hepatocellular carcinomas: a retrospective and nationwide survey in Japan. Hepatology. 2000;32:1224-1229. [DOI] [PubMed] [Google Scholar]
- 8. Kamiyama T, Orimo T, Wakayama K, et al. Survival outcomes of hepatectomy for stage B Hepatocellular carcinoma in the BCLC classification. World J Surg Oncol. 2017;15:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kamiyama T, Nakanishi K, Yokoo H, et al. Recurrence patterns after hepatectomy of hepatocellular carcinoma: implication of Milan criteria utilization. Ann Surg Oncol. 2009;16:1560-1571. [DOI] [PubMed] [Google Scholar]
- 10. Bruix J, Takayama T, Mazzaferro V, et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2015;16:1344-1354. [DOI] [PubMed] [Google Scholar]
- 11. Zhong JH, Li H, Li LQ, et al. Adjuvant therapy options following curative treatment of hepatocellular carcinoma: a systematic review of randomized trials. Eur J Surg Oncol. 2012;38:286-295. [DOI] [PubMed] [Google Scholar]
- 12. Matsushita K, Kuramitsu Y, Ohiro Y, et al. Combination therapy of active hexose correlated compound plus UFT significantly reduces the metastasis of rat mammary adenocarcinoma. Anticancer Drugs. 1998;9:343-350. [DOI] [PubMed] [Google Scholar]
- 13. Gao Y, Zhang D, Sun B, Fujii H, Kosuna K, Yin Z. Active hexose correlated compound enhances tumor surveillance through regulating both innate and adaptive immune responses. Cancer Immunol Immunother. 2006;55:1258-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cao Z, Chen X, Lan L, Zhang Z, Du J, Liao L. Active hexose correlated compound potentiates the antitumor effects of low-dose 5-fluorouracil through modulation of immune function in hepatoma 22 tumor-bearing mice. Nutr Res Pract. 2015;9:129-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shin MS, Park HJ, Maeda T, Nishioka H, Fujii H, Kang I. The effects of AHCC®, a standardized extract of cultured lentinura edodes mycelia, on natural killer and T cells in health and disease: reviews on human and animal studies. J Immunol Res. 2019;2019:3758576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ritz BW. Supplementation with active hexose correlated compound increases survival following infectious challenge in mice. Nutr Rev. 2008;66:526-531. [DOI] [PubMed] [Google Scholar]
- 17. Tanaka Y, Ohashi S, Ohtsuki A, et al. Adenosine, a hepato-protective component in active hexose correlated compound: its identification and iNOS suppression mechanism. Nitric Oxide. 2014;40:75-86. [DOI] [PubMed] [Google Scholar]
- 18. Doursout MF, Liang Y, Sundaresan A, et al. Active hexose correlated compound modulates LPS-induced hypotension and gut injury in rats. Int Immunopharmacol. 2016;39:280-286. [DOI] [PubMed] [Google Scholar]
- 19. Matsui Y, Uhara J, Satoi S, et al. Improved prognosis of postoperative hepatocellular carcinoma patients when treated with functional foods: a prospective cohort study. J Hepatol. 2002;37:78-86. [DOI] [PubMed] [Google Scholar]
- 20. Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15:e493-e503. [DOI] [PubMed] [Google Scholar]
- 21. Kamiyama T, Nakanishi K, Yokoo H, et al. Perioperative management of hepatic resection toward zero mortality and morbidity: analysis of 793 consecutive cases in a single institution. J Am Coll Surg. 2010;211:443-449. [DOI] [PubMed] [Google Scholar]
- 22. Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. Hepatology. 1996;24:289-293. [DOI] [PubMed] [Google Scholar]
- 23. Wang C, He W, Yuan Y, et al. Comparison of the prognostic value of inflammation-based scores in early recurrent hepatocellular carcinoma after hepatectomy. Liver Int. 2020;40:229-239. [DOI] [PubMed] [Google Scholar]
- 24. Wada H, Eguchi H, Noda T, et al. Selection criteria for hepatic resection in intermediate-stage (BCLC stage B) multiple hepatocellular carcinoma. Surgery. 2016;160:1227-1235. [DOI] [PubMed] [Google Scholar]
- 25. Torzilli G, Belghiti J, Kokudo N, et al. A snapshot of the effective indications and results of surgery for hepatocellular carcinoma in Tertiary Referral Centers. Ann Surg. 2013;257:929-937. [DOI] [PubMed] [Google Scholar]
- 26. Zhong JH, Ke Y, Gong WF, et al. Hepatic resection associated with good survival for selected patients with intermediate and advanced-stage hepatocellular carcinoma. Ann Surg. 2014;260:329-340. [DOI] [PubMed] [Google Scholar]
- 27. Wu TJ, Chang SS, Li CW, et al. Severe hepatitis promotes hepatocellular carcinoma recurrence via NF-κB pathway-mediated epithelial-mesenchymal transition after resection. Clin Cancer Res. 2016;22:1800-1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Daddaoua A, Martínez-Plata E, Ortega-González M, et al. The nutritional supplement active hexose correlated compound (AHCC) has direct immunomodulatory actions on intestinal epithelial cells and macrophages involving TLR/MyD88 and NF-κB/MAPK activation. Food Chem. 2013;136:1288-1295. [DOI] [PubMed] [Google Scholar]
- 29. Daddaoua A, Martínez-Plata E, López-Posadas R, et al. Active hexose correlated compound acts as a prebiotic and is antiinflammatory in rats with hapten-induced colitis. J Nutr. 2007;137:1222-1228. [DOI] [PubMed] [Google Scholar]
- 30. Olamigoke L, Mansoor E, Mann V, et al. AHCC activation and selection of human lymphocytes via genotypic and phenotypic changes to an adherent cell type: a possible novel mechanism of T cell activation. Evid Based Complement Alternat Med. 2015;2015:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Roman BE, Beli E, Duriancik DM, Gardner EM. Short-term supplementation with active hexose correlated compound improves the antibody response to influenza B vaccine. Nutr Res. 2013;33:12-17. [DOI] [PubMed] [Google Scholar]
- 32. Cowawintaweewat S, Manoromana S, Sriplung H, et al. Prognostic improvement of patients with advanced liver cancer after active hexose correlated compound (AHCC) treatment. Asian Pac J Allergy Immunol. 2006;24:33-45. [PubMed] [Google Scholar]
- 33. Nagashima Y, Yoshino S, Yamamoto S, et al. Lentinula edodes mycelia extract plus adjuvant chemotherapy for breast cancer patients: Results of a randomized study on host quality of life and immune function improvement. Mol Clin Oncol. 2017;7:359-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Suknikhom W, Lertkhachonsuk R, Manchana T. The effects of active hexose correlated compound (AHCC) on levels of CD4+ and CD8+ in patients with epithelial ovarian cancer or peritoneal cancer receiving platinum based chemotherapy. Asian Pac J Cancer Prev. 2017;18:633-638. [DOI] [PMC free article] [PubMed] [Google Scholar]