Abstract
Background:
BEAR (bridge-enhanced anterior cruciate ligament [ACL] restoration), a paradigm-shifting technology to heal midsubstance ACL tears, has been demonstrated to be effective in a single-center 2:1 randomized controlled trial (RCT) versus hamstring ACL reconstruction. Widespread dissemination of BEAR into clinical practice should also be informed by a multicenter RCT to demonstrate exportability and compare efficacy with bone--patellar tendon–bone (BPTB) ACL reconstruction, another clinically standard treatment.
Purpose:
To present the design and initial preparation of a multicenter RCT of BEAR versus BPTB ACL reconstruction (the BEAR: Multicenter Orthopaedic Outcomes Network [BEAR-MOON] trial). Design and analytic issues in planning the complex BEAR-MOON trial, involving the US National Institute of Arthritis and Musculoskeletal and Skin Diseases, the US Food and Drug Administration, the BEAR implant manufacturer, a data and safety monitoring board, and institutional review boards, can usefully inform both clinicians on the trial’s strengths and limitations and future investigators on planning of complex orthopaedic studies.
Study Design:
Clinical trial.
Methods:
We describe the distinctive clinical, methodological, and operational challenges of comparing the innovative BEAR procedure with the well-established BPTB operation, and we outline the clinical motivation, experimental setting, study design, surgical challenges, rehabilitation, outcome measures, and planned analysis of the BEAR-MOON trial.
Results:
BEAR-MOON is a 6-center, 12-surgeon, 200-patient randomized, partially blinded, noninferiority RCT comparing BEAR with BPTB ACL reconstruction for treating first-time midsubstance ACL tears. Noninferiority of BEAR relative to BPTB will be claimed if the total score on the International Knee Documentation Committee (IKDC) subjective knee evaluation form and the knee arthrometer 30-lb (13.61-kg) side-to-side laxity difference are both within respective margins of 16 points for the IKDC and 2.5 mm for knee laxity.
Conclusion:
Major issues include patient selection, need for intraoperative randomization and treatment-specific postoperative physical therapy regimens (because of fundamental differences in surgical technique, initial stability construct, and healing), and choice of noninferiority margins for short-term efficacy outcomes of a novel intervention with evident short-term advantages and theoretical, but unverified, long-term benefits on other dimensions.
Keywords: ACL reconstruction, ACL repair, IKDC, instrumented knee laxity, RCT
This article describes key design features of the bridge-enhanced anterior cruciate ligament (ACL) restoration: Multicenter Orthopaedic Outcomes Network (BEAR-MOON) study and their underlying rationales. BEAR-MOON is a 6-center, partially blinded, noninferiority, randomized controlled trial (RCT) comparing the BEAR technique for treating first-time midsubstance ACL tears with ACL reconstruction (ACLR) using a bone-patellar tendon-bone (BPTB) autograft. The design and execution of the trial present a distinctive set of clinical, methodological, and operational challenges in comparing the innovative BEAR procedure with the well-established BPTB operation. These include patient selection, intraoperative randomization, different postoperative physical therapy regimens because of fundamental differences in surgical technique and healing, and choice of noninferiority margins for short-term efficacy outcomes for a novel intervention with evident short-term advantages and theoretical, but unverified, long-term benefits on other dimensions. We discuss these and other issues.
CLINICAL MOTIVATION
ACL tears are the most common ligament injuries of the knee requiring surgical intervention. Patients, and especially athletes, with these injuries frequently undergo ACLR using autologous hamstring or patellar tendon tissue. The Knee Anterior Cruciate Ligament, Nonsurgical versus Surgical Treatment (KANON) RCT demonstrated that ACLR is similarly efficacious when performed early after the injury or delayed after attempted conservative rehabilitation via physical therapy, which fails in 37% of patients at 2 years and 55% by 5 years. 14,15 In an economic analysis with probabilities from the KANON trial and utilities (Short Form 6-Dimension) derived from the MOON cohort, the MOON Knee Group found that early ACLR yields higher quality of life at a lower cost than does a strategy of prolonged rehabilitation. 7,34 However, despite the plethora of trials and cohorts documenting the very good outcomes of ACLR, there are 3 principle unresolved problems. Patients experience autograft harvest pain with some long-term morbidity; many do not return to their previous sports, and, for those who do, the duration of participation is often attenuated compared with those with intact ligaments. 8,35 In addition, patients with and without ACLR frequently develop significant posttraumatic osteoarthritis (PTOA) 10 to 20 years after their injuries. 29,30,60 We briefly consider each in turn.
All patients have early postoperative pain after ACLR from harvest of hamstring tendons or the central one-third of the patellar tendon. Each technique also has negative longer-term consequences, specifically sacrifice of some hamstring strength due to missing semitendinosus and gracilis tendons 1,26,45 or persistent quadriceps weakness when the patellar tendon is used. 28,48,49 The recent increase in autograft harvest of quadriceps tendons seeks to mitigate these consequences, but there has been no RCT of quadriceps tendon versus hamstring or patellar tendon to evaluate the entire breadth of clinically relevant outcomes. Allografts confer a 3-fold higher failure rate than do autografts 24,35 and hence are not a viable option for high school and collegiate athletes. If the torn ACL could heal without requiring autologous harvest for reconstruction, both short-term pain and longer-term morbidity from autograft harvest would be avoided.
Moreover, presumably because the complex geometry of both the tibial and the femoral footprints cannot be fully reconstructed, residual kinematic deficits are observed after recovery from ACLR and some patients, including almost one-third of football and soccer players, do not return to their sports. 8,35
In addition, PTOA after an ACL tear, with or without ACLR, has long been recognized. 29,30,60 PTOA incidence, which varies depending on whether, and how precisely, it is defined by symptoms and/or radiography, can be as high as 50% at 10 years. Approximately one-third of patients of the 17 surgeons in the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS)-funded MOON Knee Group are aged ≤ 18 years. A significant percentage are thus expected to develop PTOA before the age of 30 years.
EXPERIMENTAL INTERVENTION
The proprietary BEAR process, developed by Martha M. Murray, MD, promotes intra-articular healing of torn ACL where healing does not otherwise occur. The failure to heal was shown to be due to a premature loss of the blood clot bridging at the wound site. 36 The BEAR device is a cylindrical hydrophilic, uncrosslinked, low-DNA, collagen-based sponge formed by processing tissue from a closed herd of cows in New Zealand. This sterile, porous, and fully resorbable device is inserted into the notch between the torn ligament ends and saturated with autologous blood, forming a viscoelastic substance that fills the notch and provides a stable intra-articular bridging sponge for healing to occur in the knee synovial cavity. The device retains the blood in the wound site, where the clot releases wound-healing growth factors and proteins that stimulate ingrowth of cells into the implant. This environment allows the torn ACL tibial stump fibers to connect via this implant to the ACL femoral stump. The process was initially demonstrated in a canine model of partial ACL tear, where the implant mixed with autologous blood demonstrated significant histologic and biomechanical healing. 44,56 Multiple studies of the current technique were then performed in a clinically relevant porcine model. 23,31,41,42 The results have documented intra-articular healing via magnetic resonance imaging (MRI) scans, 6,43 histological studies, 23,50 and biomechanical testing. 23,32,37,42,43,61 Moreover, in a porcine model of ACL tear, PTOA was reduced by BEAR as compared with ACLR and untreated ACL transection. 37
These animal studies justified translation to human research by the developer’s team at Boston Children’s Hospital. In an initial feasibility and safety cohort study, 10 patients treated with BEAR were compared with 10 patients receiving 4-stranded hamstring autograft, without safety signal and showing that the BEAR procedure was safe at 24 months (ClinicalTrials.gov identifier: NCT02292004). 39 Subsequently, 100 patients were randomized 2:1 in a single-center, noninferiority trial comparing BEAR with ACLR (65 BEAR, 33 hamstring autograft reconstructions, and 2 BPTB autograft reconstructions; ClinicalTrials.gov identifier: NCT02664545). 38 Both human studies demonstrated comparable patient-reported outcome measures (International Knee Documentation Committee [IKDC] subjective knee evaluation score) (see Table 1) and knee stability (side-to-side comparison as measured via a knee arthrometer) from BEAR and (predominantly) hamstring reconstruction. 38,39 A 250-patient cohort study funded by the implant manufacturer (Miach Orthopedics), comparing the efficacy of the BEAR procedure for patients in different age ranges (<14, 14-17, 18-25, and >25 years), with IKDC as the primary outcome, is ongoing at Boston Children’s Hospital and Rhode Island Hospital (ClinicalTrials.gov identifier: NCT03348995). When BEAR is performed in patients in whom the complex tibial footprint is preserved, another potential advantage is that the healed ACL tear retains its complex geometry that cannot be duplicated with a graft. In addition, a native proprioceptive nerve supply could potentially be preserved using BEAR as compared with ACLR.
Table 1.
Subjective IKDC Scores of the 2 BEAR Clinical Studies a
| Subjective IKDC Score | BEAR 38,39 | ACLR |
|---|---|---|
| 3 months 39 | 54.3 ± 6.4 | 60.7 ± 10.2 |
| 2 years 38 | 88.9 ± 13.2 | 84.8 ± 13.2 |
a Data are reported as mean ± SD. ACLR, anterior cruciate ligament reconstruction; BEAR, bridge-enhanced ACL restoration; IKDC, International Knee Documentation Committee.
STUDY DESIGN AND SETTING
The BEAR-MOON noninferiority RCT will include patients with midsubstance ACL tears of 12 fellowship-trained sports medicine surgeons at 6 sites. The patients will be randomized to receive BEAR or BPTB, with 2-year IKDC scores and anteroposterior (AP) knee laxity results as coprimary outcomes. The plan is to demonstrate scalability and generalizability of BEAR from the site and team of its developer to other expert orthopaedic surgeons at multiple referral centers. We also intend to establish comparability of results of BEAR with those of BPTB autograft reconstruction, for which knee stability may be superior to that achieved using hamstring reconstruction. For these purposes, we chose an RCT rather than a prospective cohort comparing BEAR and BPTB. RCTs have all the methodological advantages of a prospective cohort design. Beyond these, randomization provides additional protection against selection bias and confounding by both known and unknown risk factors for the outcome. In BEAR-MOON, differences in the rehabilitation regimens for BEAR and ACLR make blinding of the patient difficult. However, surgical incisions will not vary between procedures, and patients will wear sleeves on both knees to rehabilitation sessions and for evaluations by examiners who are blinded to the identity of the injured knee and the surgical treatment.
While BEAR-MOON builds on 2 prior studies 38,39 and is informed by the ongoing cohort study performed at the inventor’s and 1 other institution, BEAR-MOON is investigator initiated, funded by a NIAMS independent investigator award (R01-AR074131), and governed independently of the inventor and current corporate developer. It is a traditional explanatory RCT, designed to compare its treatments administered under ideal circumstances, with the understanding that after BEAR is established in this manner, the attractions of a tendon-sparing healing procedure over an autograft reconstructive approach will lead to wider adoption and pragmatic trials will become relevant. BEAR-MOON will be conducted in academic referral centers (Cleveland Clinic, The Ohio State University, TRIA/University of Minnesota, the University of Colorado, Rhode Island Hospital, and Vanderbilt University), of which 4 are founding members of the NIAMS-funded MOON Knee Group established in 2002. The participating surgeons are considered leaders in their fields, have performed ACLR regularly, and have published on all the outcome assessments utilized in this study. However, the majority of surgeons in this study are new to the BEAR procedure and “repairs” of ACL tears. Thus, we will model appropriate surgical training for the procedure. We believe that this training and the results of the trial will be generalizable to fellowship-trained sports medicine surgeons.
Considerations for Patient Enrollment and Intraoperative Randomization
Establishing the diagnosis of an ACL tear is relatively easy via physical examination (PE) and MRI scan. However, both patients and surgeons in an RCT must be comfortable with either ACL surgery type and with randomization, which requires a degree of impartiality known as “clinical equipoise.” 13,25 BEAR-MOON poses some challenges in ensuring clinical equipoise, specifically in defining and choosing inclusion/exclusion criteria to select “repairable” midsubstance ACL tears. Moreover, the developer’s studies suggest that surgery must be completed sufficiently early, within approximately 50 days of injury, for healing to occur. 38,40,42
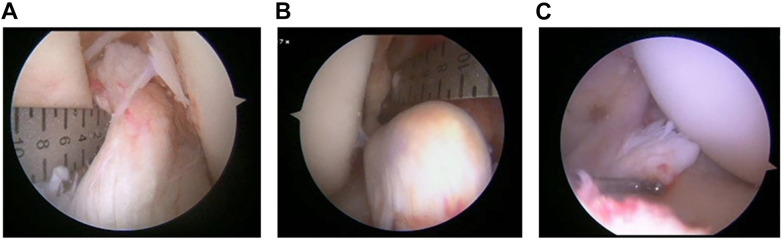
In this regard, all surgeons and other key clinical personnel participated in face-to-face discussions regarding the appropriate inclusion and exclusion criteria that ensured that the clinical team would be comfortable having all eligible participants treated via either intervention (clinical equipoise). A guiding principle for the MOON Knee Group, as a test for equipoise in any trial, has been whether the surgeon is willing to enroll family members or other loved ones into the study. If not, the surgeon lacks equipoise. The basic inclusion criteria (Table 2) are a primary midsubstance tear in patients aged 18 to 40 years, with surgery performable within 50 days of the injury. The exclusion criteria include a repairable bucket-handle tear of medial meniscus, articular injury requiring more treatment than chondroplasty or microfracture, and a “nonrepairable” ACL tibial stump. The surgeons recognized that a formal study would be useful to arrive at an acceptable operational definition of nonrepairable ACL tear. We thus conducted a training session and then an interrater agreement study using 75 short video clips of torn ACLs, for which surgeons were required to complete a questionnaire covering assessments of repairability and contributory factors, including tibial stump length, percentage of tibial footprint intact, and “quality” of tissue to suture. 5 Figure 1 demonstrates 3 ACL tears assessed by consensus as repairable, potentially repairable, and not repairable, respectively.
Table 2.
Inclusion and Exclusion Criteria a
| Inclusion Criteria (partial) | Exclusion Criteria (partial) |
|---|---|
| • Patient age 18-40 y • Complete ACL tear confirmed via MRI scan • Time from injury to surgery ≤50 d |
• Grade 3 MCL injury requiring surgical treatment • Full-thickness chondral defect • Bucket-handle tear of the medial meniscus • History of anaphylaxis • ACL tibial stump length <1 cm • ACL tibial footprint attachment <50% intact |
a ACL, anterior cruciate ligament; MCL, medial collateral ligament.
Figure 1.
Which is a repairable ACL stump? (A) Repairable; (B) potentially repairable; (C) not repairable. ACL, anterior cruciate ligament.
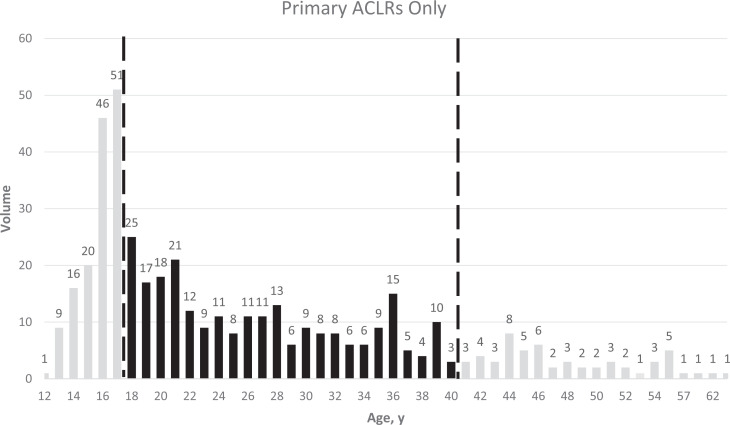
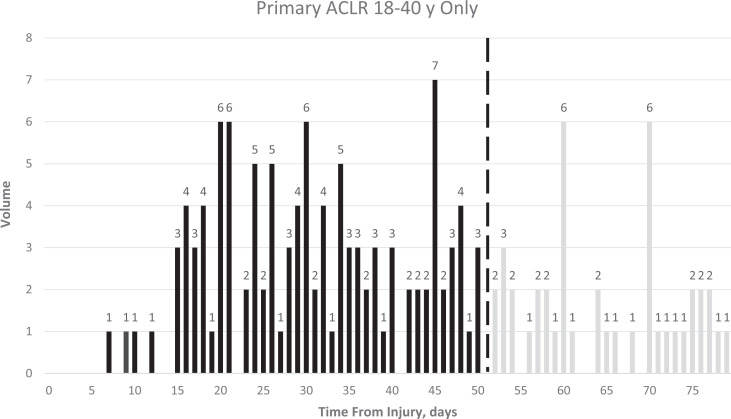
Enrollment is often the rate-limiting factor in completing an RCT in any field. To estimate the size of the pool of potentially eligible patients, we also created a prospective, deidentified Research Electronic Data Capture (REDCap; Vanderbilt University) database of every ACLR performed for 6 months by 10 surgeons at 5 sites and examined the distributions of ACLR by patient age (Figure 2) and by time from injury (Figure 3). These surgeons performed 510 ACLRs in 6 months, including 87% (444/510) primary surgeries, of which 55% (245/444) were in patients between 18 and 40 years old. In this age range, 46% (112/245) of ACLRs were performed before 50 days, and another 9% (23/245) could have been scheduled within 50 days of injury, amounting overall to 27 candidate surgeries per surgeon-year or, with 12 surgeons, 648 surgeries over 2 years. From this REDCap database and the interrater agreement study, 5 we estimated that 70% to 80% of midsubstance ACL tears would be repairable. If, conservatively, only 70% are repairable, we will need 44% of such initially eligible patients to consent to participate in the study in order to meet the study’s enrollment schedule, assuming no direct referrals for the BEAR trial.
Figure 2.
Prospectively-assessed distribution of ages of patients undergoing anterior cruciate ligament (ACL) reconstructions. The dashed lines indicate the boundaries of the age range chosen for eligibility to participate in BEAR-MOON. ACLR, anterior cruciate ligament reconstruction; BEAR-MOON, bridge-enhanced ACL restoration: Multicenter Orthopaedic Outcomes Network.
Figure 3.
Prospectively-assessed distribution of times from injury to anterior cruciate ligament (ACL) reconstructions. The dashed line indicates the maximal duration from injury to surgery chosen for eligibility to participate in BEAR-MOON. ACLR, anterior cruciate ligament reconstruction; BEAR-MOON, bridge-enhanced ACL restoration: Multicenter Orthopaedic Outcomes Network.
We also recognized during planning that maintaining equipoise and minimizing surgeon bias between BEAR and ACLR require that eligibility be determined after a diagnostic arthroscopic inspection. Thus, final eligibility of the ACL stump requires a tactile feel of the quality of torn ACL tissue and its ability to hold a stitch, which can only be done intraoperatively. This posed a design choice between preoperative randomization versus intraoperative randomization after intraoperative exclusions of ineligible patients. We chose the latter because it allows surgeons to achieve equipoise and, since they are not obligated to repair ACL tears failing intraoperative assessment criteria including ability to hold a stitch well, to better ensure patient safety. This strategy also limits treatment crossovers only to patients with sufficient tibial footprint, stump length, and tendon quality to justify attempted BEAR repair, as judged by the surgeon, but for whom an adequate repair procedure cannot actually be accomplished and thus ACLR rescue becomes necessary.
Thus, relative to conventional preoperative randomization, this strategy greatly reduces the extent to which selection biases manifesting in treatment crossovers can compromise study integrity. Because repairability is determined before randomization, the characteristics of tears undergoing BEAR and ACLR should be very similar, controlling potential confounding. However, the strategy complicates logistics because patients must be prepared in the consent process for the 20% to 30% probability of not meeting intraoperative randomization criteria and thus undergoing an ACLR without continuation in BEAR-MOON follow-up. It also requires initial real-time data capture by research coordinators and immediate electronic randomization in the operating room, with backup personnel available to provide each required randomization to the surgical team if any of computer hardware, software, or telecommunications fails when a treatment is requested.
Surgical Comparators
BEAR-MOON requires training a multicenter group of 12 surgeons at 6 sites to perform the BEAR technique, which has been pioneered by the 3 surgeons at Boston Children’s Hospital who conducted and published the first 2 clinical studies. 38,39 Traditionally, cadavers have been used for such training and, in some cases, preliminary practice, and such cadaveric sessions have been completed or scheduled for all participating surgeons. We believed an anatomically correct model that allowed repetitive performance on all the unique steps of BEAR would also be beneficial. Thus, we designed and built a 3-dimensional printed knee model, based on a knee of a study team member, that allows practice of surgical techniques unique to BEAR, utilizing actual implants, sutures, and instruments, although, to save on cost and waste, without the BEAR device. Practice steps include simulating arthroscopic suture of the ACL stump, assembling implants and sutures for the suture bridge, passing the sutures, and fixing the knee in extension using the suture bridge. Each surgeon will have a 3-dimensional knee model to use for practice as often as desired, including review and practice the night preceding and/or day of surgery, and can bring the model into the operating room.
The autologous BPTB ACLR is the standard performed by each surgeon, who is enjoined from changing any technique or implant during the trial. Because the final step in placing the BEAR implant requires a minimedial arthrotomy, the surgical incisions can be matched between the BEAR and ACLR arms, which allows blinding of much of clinical care and patient rehabilitation to the treatment arm. Patients in both groups have the same postoperative icing device and long-leg brace locked in extension for the first 24 hours. Except for wound checks, patients will present to clinic or therapy with sleeves covering both knees.
Rehabilitation
Ideally, in an RCT comparing 2 procedures, postoperative rehabilitation would be identical for both groups. This would not be a problem when performing 2 different ACLR techniques because fixation of graft and initial graft strength are comparable among ACLR procedures. However, early postoperative healing of the ACL repair in BEAR has limited strength, and the suture bridge is the only source of initial stability. In the prior BEAR safety study and RCT, subsequent MRI estimates of the healing ACL repair strength in patients who underwent BEAR were greater in patients with slower rehabilitation, posing a dilemma. If patients undergoing BEAR were to follow a MOON ACLR rehabilitation protocol for increasing range of motion (ROM), healing might be compromised and ultimately insufficient. This could result in functional instability in return to activities after BEAR. Conversely, as learned from research in the late 1980s and early 1990s, overly restricting motion after ACLR promotes poor outcomes: loss of motion (especially extension) and development of arthrofibrosis. 57
Therefore, the BEAR-MOON team of surgeons, physical therapists, and trialists has followed the recommendation of the inventor to emphasize initial “construct” strength and so developed a BEAR-specific rehabilitation protocol incorporating a slower progression than that for ACLR. This protocol is designed to protect the early stages of ACL healing from postoperative stress (Table 3) by restricting weightbearing in the brace for the first 6 weeks and restricting ROM to between 0° and 30° for postoperative weeks 0 to 2, between 0° and 60° for weeks 2 to 4, and between 0° and 90° for weeks 4 to 6. Patients in the BEAR-MOON trial who undergo ACLR follow the MOON ACLR rehabilitation guidelines, 62 which allow initial weightbearing and do not restrict ROM. Patients are not informed of the choice between randomized treatments, for which surgical incisions are indistinguishable. As noted, they all use the same ice device, postoperative long-leg brace, functional knee brace, and knee sleeves. In addition, all are restricted from returning to sports for 9 months. The postoperative nursing staff, physical therapists, and clinical personnel are instructed not to tell the patient which technique was performed. BEAR-MOON thus compares surgery-rehabilitation regimen packages: BEAR surgery coupled with a specific rehabilitation protocol designed for BEAR and ACLR coupled with the MOON ACLR rehabilitation protocol.
Table 3.
Major Differences in the Rehabilitation Protocols Between BEAR and ACLR a
| Parameter | BEAR | ACLR |
|---|---|---|
| Locking hinge knee brace settings | • 0°-30° for 2 wk • 0°-60° for 2 wk • 0°-90° for 2 wk • Lock at 0° for ambulation for 6 wk |
• 0°-90° for 2 wk • 0°-120° starting week 3 • Brace unlocked for ambulation after quadriceps control returns (approximately 1-2 wk) |
| Weightbearing status | • PWB for 6 wk • Wean crutches after 6 wk |
• PWB for 2 wk, WBAT with crutches for additional 2 wk • Wean crutches after 4 wk |
| Start quadriceps strengthening | 3-4 wk postoperatively | 0-2 wk postoperatively |
| Start jogging | 3-6 mo postoperatively | 3-6 mo postoperatively |
a ACL, anterior cruciate ligament; ACLR, ACL reconstruction; BEAR, bridge-enhanced ACL restoration; PWB, partial weightbearing; WBAT, weightbearing as tolerated.
OUTCOME MEASURES
Coprimary Outcomes
The goal of treating ACL tears is to restore functional stability of the knee. Because outcomes of ACL surgery are multidimensional, we chose a patient-reported outcome measure (PROM) and a stability measure as coprimary outcomes.
Two dominant PROMs, the IKDC and the Knee injury and Osteoarthritis Outcome Score (KOOS), measure sports function. 18 –20,52,53 In the National Institutes of Health (NIH) funded MOON ACLR prospective cohort study, both forms were solicited from all 3500 patients at enrollment and at 2-, 6-, and 10-year follow-up. 10,11,17,54,55 We found that they were highly correlated and identified similar risk factors for outcomes (especially knee-related quality of life). 10,11,17,54,55 The KOOSglobal, an 11-question amalgamation of the KOOS for joint replacement with 4 KOOS knee-related quality-of-life questions, is highly and significantly correlated with IKDC (r = 0.91; P < .001) and accounts for 83% of its variability with <15% combined floor and ceiling responses. 21 To decide which outcome measure to use (the IKDC 19-question single measure or the 43-question, 5-subscale KOOS), we reviewed the outcomes and degree of variation in ACLR studies and found SDs tended to be lower for the IKDC. 10,54 Because lower variability translates into higher statistical power and lower sample size requirements, we chose the IKDC for use in BEAR-MOON.
A fundamentally new technique such as BEAR requires an instrumented measure of AP stability of the knee. Stability is assessed most commonly using a knee arthrometer. An RCT of ACLR with high versus low tension at MOON sites used the arthrometer successfully, 2,12 observing the expected side-to-side difference and variation (SD). The knee arthrometer has also been used to compare the stability of BPTB with that of hamstring ACLRs and will be used here as a coprimary outcome at 2 years. 38
The timing of the coprimary outcomes (IKDC and knee arthrometer laxity measurement) is based on the need to demonstrate that BEAR remains stable for at least 1 year after return to sports, which should occur no earlier than 9 months postoperatively. This suggests a final assessment at 2 years. Noninferiority of BEAR to BPTB ACLR will be claimed only if statistical criteria for noninferiority are met for both IKDC and AP knee laxity.
Ideally, BEAR would delay the onset of PTOA, the assessment of which might have also been included as a third coprimary outcome. However, BEAR-MOON follow-up is too short to evaluate PTOA via traditional gold standards (radiographs and PROMs [IKDC, KOOS Pain and Symptoms subscales]). There is a potential to use a novel quantitative MRI to evaluate articular cartilage changes consistent with PTOA at 2 years, although MRI is not currently included in the RCT, and we believe that fixed-flexion radiographs 5 to 6 years postoperatively will enable us to evaluate PTOA. 9,22,45 We hope to pursue additional funding to include these as exploratory assessments.
Secondary Outcomes
Secondary outcome measures collected at varying schedules are KOOS, 52,53 quadriceps strength, 48 ROM, Lachman test, 58 Kujala Anterior Knee Pain Score (AKPS), 27 Marx activity level, 33 functional hop testing, 4,46 and fixed-flexion radiograph metrics. 9,22,47 The Marx activity level is captured to evaluate the resumption of cutting and pivoting activities at 1 to 2 years after surgery. The quadriceps strength manual dynamometer testing and functional hop testing are also collected semiannually, as are standardized standing bilateral fixed-flexion radiographs at baseline and 2 years. Finally, “failures” of BEAR and ACLR are monitored as described below under Surveillance for Failure and Infection, although the study is not powered to detect differences at the expected relatively low failure rate of ACL in 18- to 40-year-old patients.
The timing of BEAR-MOON follow-up visits adheres to the surgical standard-of-care clinical schedule, with the addition of 1- and 2-year research assessments (Table 4). Patients return to see the surgeon at approximately 1 to 3 weeks (5- to 24-day window), 6 weeks (4.5- to 8-week window), 3 months (10- to 17-week window), and 6 months (22- to 34-week window), at which time the surgical standard-of-care PE and history to assess for adverse events (AEs) are completed. The standard PE consists of ROM and effusion, with the Lachman portion of the PE performed initially at 3 months. 58 The history screens particularly for increasing pain, signs of infection, and declining knee function.
TABLE 4.
Timing of Baseline, Surgery, and Follow-up Outcomes a
| Postoperative | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Measure | Preoperative Baseline | Intraoperative Surgery | 1-3 Wk (Days 5-24) |
6 Wk (Weeks 4.5-8) |
3 Mo (Weeks 10-17) |
6 Mo (Weeks 22-34) |
9 Mo (Months 8-10) |
1 Y (Months 11-15) |
2 Y (Months 23-30) |
Unscheduled Visit/Early Discharge Visit |
| Screening and eligibility | X | |||||||||
| Informed consent | X | |||||||||
| MRI | X b | |||||||||
| PE | X c | X | X | X | X | X | X | |||
| CBC draw | X d | |||||||||
| Blinded assessment | X e | X e | X e | |||||||
| Return-to-sports clearance | X f | X f | ||||||||
| Knee fixed-flexion radiograph 9,22,47 | X | X | X g | |||||||
| Pivot-shift | X | X | X | X | X | X g | ||||
| Knee laxity (arthrometer) 3 | X | X | X | X g | ||||||
| IKDC 18 –20 | X | X | X | X | X | X | ||||
| Marx activity level 33 | X | X | X | X | ||||||
| KOOS 52,53 | X | X | X | X | X | X g | ||||
| AKPS 27 | X | X | X | X | X | X g | ||||
| Hop tests 4,46 | X | X | X | X g | ||||||
| Quadriceps strength 48 | X | X | X | X g | ||||||
| AE query | X | X | X | X | X | X | X | X | ||
a Onsite BEAR-MOON components: fixed-flexion radiograph, patient-reported outcomes measures (IKDC, Marx activity level, KOOS, AKPS). An independent blinded assessor then performs instrumented laxity arthrometer, PE (Lachman, pivot shift, ROM), clinical evaluation of muscle strength, quadriceps strength measurement using a dynamometer, effusion and gait, and hop testing. ACL, anterior cruciate ligament; AE, adverse event; AKPS, Kujala Anterior Knee Pain Score; BEAR, bridge-enhanced ACL restoration; CBC, complete blood count; IKDC, International Knee Documentation Committee; KOOS, Knee injury and Osteoarthritis Outcome Score; MRI, magnetic resonance imaging; PE, physical examination; ROM, range of motion.
b Standard of care MRI confirms ACL rupture and provides signal of sufficient tibial stump ACL.
c Surgeon standard PE and history to assess for AEs. The history evaluates increasing pain, signs of infection, and declining function. The standard PE consists of ROM and effusion. The Lachman portion of the examination is performed initially after 3 months, and the pivot shift is performed after 6 months.
d Only patients undergoing BEAR.
e Research assessments of study endpoints and safety by independent blinded assessor.
f At 6 months, the operating surgeon will clear, or not clear, the patient for return to sports training in a structured rehabilitation program. Return to unrestricted/unsupervised sports will not occur until a minimum of 9 months after surgery.
g Assessment only if the visit is ≥6 months postoperatively.
Research functional evaluations take place at 6 months, then at 1 and 2 years. Research examinations include fixed-flexion radiographs and PROMs (IKDC, Marx, KOOS, AKPS). An independent blinded examiner then performs instrumented laxity (knee arthrometer), PE (Lachman, pivot shift, ROM, muscle strength, and gait), and quadriceps strength and hop testing. Only the PE findings recorded by the blinded examiner at 6 months, 1 year, and 2 years will be used in outcome analyses.
To minimize potential measurement bias in patients, differences between the postoperative rehabilitation programs are not discussed during the consent process, allowing patients and their families, as well as study team members who perform postprocedure outcome evaluations (independent blinded assessors), to be masked to treatment group. The principal investigator, operating surgeon, operating room team, research coordinators, and research nurses cannot be masked to the study treatment group; they fulfill their trial responsibilities but are prohibited from revealing treatment to the patient. We cannot exclude the possibility of some patients learning of the difference in rehabilitation regimens and inferring their treatments from that, but this possibility is an unavoidable consequence of the decision to tailor rehabilitation to the surgical procedure. Regardless, patients are instructed not to discuss or otherwise volunteer any information at any time to personnel performing study assessments. Testing on the ACL is conducted with sleeves covering both knees of the patient and by research personnel without access to the patient’s treatment arm (blinded), as in the NIH-funded MOON protocol for ACLR follow-up examinations.
Patients will be unmasked after the 2-year research follow-up examination and may be unmasked earlier only in the case of a medical emergency requiring this information or if an investigator deems it clinically necessary to provide this information in order to better treat the patient, such as if a subsequent surgery is being considered to stabilize a failed ACL surgery. When unmasking occurs, the study coordinator will note this in the medical record, and the patient will continue in the trial.
Surveillance for Failure and Infection
Two surgical complications of special interest are deep joint infection and failure of the BEAR implant or ACLR with autograft, as both are strongly associated with poor clinical outcomes.
Joint infections have occurred in 0.68% of patients in 16 prior ACLR clinical trials. Assuming this incidence will be experienced by both treatment arms, we would expect, on average, 1 to 2 such infections and 0 to 1 in each treatment arm during the course of the study.
Because experience with BEAR is so limited, however, we will pause accrual and surgeries, pending consideration of early stopping in conjunction with the Data and Safety Monitoring Board (DSMB) and NIAMS, if 2 of the first 20, 3 of the first 40, or 4 of the enrolled subcohort of patients in the experimental arm who actually undergo BEAR experience a deep joint infection. Recommendations and decisions on early termination, trial continuation with the protocol unchanged, or trial continuation after protocol modification can then be made based on a global review of BEAR-MOON methods and conduct as well as the input based on clinical expertise and judgment from the investigators and oversight personnel. Based on 1 million trial simulations, this rule has an approximately 1.2% chance of a false-positive halt sometime during the trial when the underlying infection rate is 0.68% but a >90% probability of halting when the true infection rate is ≥ 7.0%.
Later reoperation (revision ACLR) for treatment failure is performed for 6% of patients with autograft ACLR with a mean age of ∼18 years. 24 All participating surgeons are highly experienced with ACLR, so their ACLR failure rate will likely be consistent with this surgery’s established safety profile. Because younger athletes (age, 14-17 years) are excluded and the revision rate for ACLR failure decreases with increasing age, to <1% in those ≥40 years, 24 the BEAR-MOON reoperation rate should be low. MRI-proven retear and knee laxity >6 mm without reinjury were not included in the above-referenced statistics but will also be considered treatment failures even without reoperation. In view of the variation in follow-up times during which later treatment failures may occur and varying implications of failures of different types, BEAR-MOON does not employ a formal statistical guideline for pausing for treatment failures. We will, however, monitor failures closely and consider, in consultation with the DSMB, any accumulating evidence of failures in substantially >10% of patients over 2 years.
DATA MANAGEMENT
The BEAR-MOON research plan was developed, funding initiated, and approved by institutional review boards, the US Food and Drug Administration (FDA), NIH, and a DSMB under an FDA investigational device exemption for the BEAR implant. Cognizant of this and aware that BEAR-MOON data could be relevant to ultimate FDA deliberations on approval of the device, we initially chose the REDCap Cloud platform (nPhase Inc) for the BEAR-MOON trial. 16 REDCap is an academically developed, secure, web-based application designed to support data capture for research studies, providing (1) an intuitive interface for validated data entry, (2) audit trails for tracking data manipulation and export procedures, (3) automated export procedures for seamless data downloads to common statistical packages, and (4) procedures for importing data from external sources. REDCap Cloud is a commercially vended online software-as-a-service data collection system that is built around REDCap and incorporates these features and others that further facilitate data collection in FDA 21 Code of Federal Regulations Part 11-compliant environments. REDCap Cloud, however, does not support the BEAR-MOON custom randomization, for which BEAR-MOON requires a small auxiliary REDCap database, and in other ways the parent REDCap is somewhat more flexible and user-friendly.
After development of the BEAR-MOON database using REDCap Cloud but during a delay in initial enrollment due to coronavirus 2019 (COVID-19) considerations at participating sites, the BEAR implant received FDA approval based on prior trial results for use for patients and under the conditions of this trial. Implications of this for BEAR-MOON data monitoring and data management are now being explored, with a possible adjustment being redeployment of the database from REDCap Cloud in REDCap. The features below of the current REDCap Cloud deployment will be maintained in any redeployment to REDCap.
Data collection is the responsibility of the clinical trial staff at each site. The study database is designed not only to store all data (patient information, study visits, treatments, measurements, imaging, outcomes, and AEs) but also to streamline the data entry process and simplify various decision-making aspects. The complex enrollment screening required by the study design is programmed into the data collection process via branching logic so eligibility of patients is assessed automatically and consistently and documented across all sites without requiring arduous decisions by local coordinators. Where appropriate, animated visual guides are included in electronic case report forms to guide coordinators through particularly tricky data entry tasks. Automated triggers alert necessary parties to take various assigned actions based on events such as new participant enrollment and AEs requiring timely reporting to internal and external regulatory boards.
Access to the study database follows strict adherence to the “principle of least privilege,” which specifies that users have access only to those areas and features of the database that are absolutely necessary to perform their given tasks. The database administrator ensures that each user’s password-protected account is assigned to a role that grants only the minimum necessary permissions to correctly and accurately collect study data at each appropriate site.
Each site is provided a unique account to the study database so that all data are securely entered and attributable to that site. Each site investigator is responsible for ensuring the accuracy, completeness, intelligibility, and timeliness of the data reported, which will be regularly audited with the help of the database administrator to verify that data quality is being maintained.
Analytical Approach
Noninferiority testing of the coprimary outcomes and other statistical inference will be embedded within, and use fitted values from, longitudinal mixed models for the repeated observations, using maximum likelihood estimation if possible. This will account for missing outcomes due to dropout or other causes if the missing data mechanism is independent of the unobserved outcome (missing at random).
An as-treated (AT) analysis will compare patients by whether they receive BEAR or an ACLR, whether randomized or as a rescue procedure, and an intention-to-treat (ITT) analysis will compare patients by their randomized treatments. The only difference between these will be the analytic placement of any patients who are intraoperatively judged suitable for and randomized to the BEAR procedure but for whom the surgeon cannot successfully complete the BEAR procedure or belatedly realizes it is so unlikely to be successful that rescue ACLR is required. In the AT analysis, these patients will be grouped with those randomized to ACLR, while in the ITT analysis, they will be grouped with others randomized to BEAR. While bias in an ITT comparison is generally regarded as conservative because any bias is toward the null equality hypothesis in a superiority trial and most appropriate for superiority comparisons on that basis, in noninferiority trials, the ITT comparison can easily be biased toward the noninferiority hypothesis to be demonstrated and thus is anticonservative and no longer the preferable approach.
Specifically, in BEAR-MOON, the patients at issue would be those most likely to heal poorly and have poorer outcomes compared with others randomized to BEAR but likely to experience comparable outcomes with those randomized to ACLR, the surgery they will actually have completed. If BEAR is truly inferior to ACLR, retaining those patients in the BEAR group with their presumably normal ACLR outcomes will bring performance of the nominal BEAR and ACLR groups closer than they would have been if those patients had not been converted to rescue ACLR, thus biasing the comparison toward noninferiority. In contrast, placing them in the ACLR group will leave that group’s outcomes representative of patients undergoing ACLR and the BEAR group’s outcomes representative of those for whom BEAR appears suitable and is completed successfully. Consequently, consistent with page 8 of the “Non-Inferiority Clinical Trials to Establish Effectiveness: Guidance for Industry,” 59 we will treat the AT comparison as primary and conduct ITT comparisons as secondary analyses of the coprimary outcomes.
Noninferiority of BEAR relative to BPTB will be claimed if the respective IKDC subjective knee evaluation form total score and knee arthrometer 30-lb (13.61-kg) side-to-side laxity difference hypotheses of inferiority of BEAR by at least 16 points for IKDC and 2.5 mm for knee laxity are rejected using 1-sided 2.5%-level significance tests. While the choice of coprimary outcomes is identical to that of the initial and recently published BEAR RCT 38,39 and the chosen noninferiority margins for both BEAR clinical trials are based on the study of Irrgang et al 20 for IKDC and the review of Arneja and Leith 3 for AP knee laxity, our interpretation of those references differs from that of Murray et al, 38 who performed a smaller trial with more stringent margins of 11.5 points for IKDC and 2.0 mm for knee laxity.
Arneja and Leith 3 reviewed 3 studies, each with serious methodological limitations. Of these, only the largest, with 138 patients, compared arthrometer-measured knee laxity in patients with and without acute ACL injuries, analogous to the patients in BEAR-MOON. That study associated laxity of >3.0 mm with ACL disruption. Two smaller, uncontrolled studies, a 68-patient study with no stated enrollment criteria or description of the acuteness of injuries and only history and clinical examination as the diagnostic gold standard and a 38-patient study with arthroscopically confirmed ACL injury, together led to Arneja and Leith's summary that “using the knee arthrometer score may be more appropriate as a dichotomous test with a threshold of 2 or 3 mm.” Arneja and Leith examined only diagnostic accuracy, with no consideration of clinical importance. Murray, Fleming et al. 38 chose Arneja and Leith's3lower limit as their margin, presumably based on its stringency and higher diagnostic sensitivity without consideration of diagnostic specificity.
Irrgang et al 20 related patients’ self-perceptions as unchanged or slightly, somewhat, or greatly better or worse to changes in their IKDC scores. They suggested that “a change in the IKDC Subjective Knee Form score of 11.5 is necessary to distinguish between those who have improved and those who have not improved.” They based this on dichotomizing self-perceptions as improved (somewhat or greatly better) or not and picking the IKDC threshold with highest sensitivity for detecting improvement at the minimum of a range of thresholds from 11.5 to 20.5 yielding similar sums of sensitivity and specificity, noting similarity of this threshold to a 12.8-point estimated IKDC minimal detectable change. However, the minimal detectable change is a measurement error parameter that is conceptually unrelated to clinical importance, and the reported data from Irrgang et al 20 showed no ability of the IKDC score to discriminate patients who self-report as somewhat better from those who report themselves to be slightly better, unchanged, or slightly worse. Thus, there is no principled basis to prefer an 11.5 threshold as noninferiority margin over the 20.5 threshold that Irrgang et al 20 indicated “should be used to maximize the specificity of change” or any other threshold within the 11.5 to 20.5 range.
While Murray et al 38 selected the most stringent noninferiority margins from among the ranges suggested by these source papers, less-restrictive margins of 2.5 mm for knee laxity and 16 points for IKDC score were selected for BEAR-MOON because they are midway in ranges similarly justified by the data in these source papers and sufficiently tight to be considered reasonable trade-offs by a substantial fraction of knee surgeons for the BEAR approach’s reduction in surgical morbidity and potential though unverified reduction in osteoarthritis. This is also reasonable in light of data suggesting that the BPTB BEAR comparator may yield as much as a 0.5-mm decrease in knee laxity as compared with the primarily hamstring comparator arm in the initial BEAR RCT. Note that statistical significance will not be achievable without observed BEAR performance well within both noninferiority margins. Consequently, even with these more relaxed noninferiority margins, we have designed BEAR-MOON to include twice the number of patients as in the initial BEAR trial in order to have reasonable power in the event of true inferiority in both coprimary outcomes in the lower half of the noninferiority range.
It is noteworthy that whereas prespecified noninferiority margins are important in planning noninferiority trials, after the trial is completed, interpretation of its results by the clinical community will hinge on the reported point estimate and upper CI bound for the degree of inferiority. We will provide 95% CIs for treatment effects for all outcomes, interpretable as ranges of treatment effects that remain plausible in light of the data from the trial.
Statistical Power
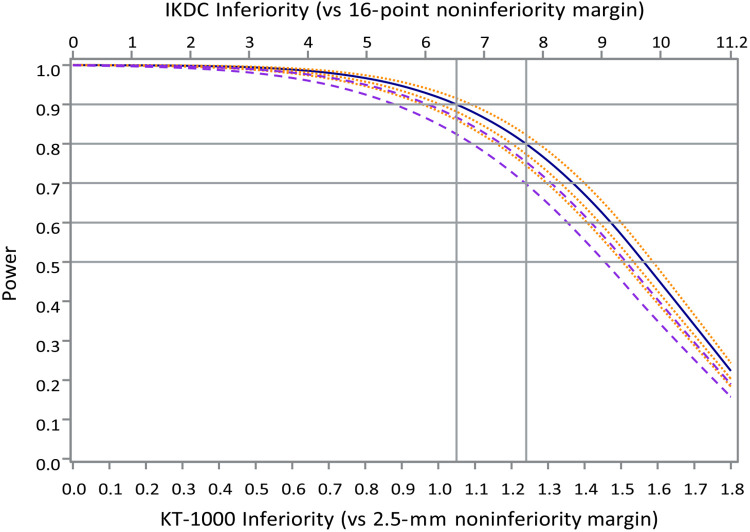
Following the most relevant FDA guidance cited earlier, we will test noninferiority by computing the upper bounds of 95% CIs for the degree of BEAR inferiority on each endpoint and claiming noninferiority only if both are below their respective noninferiority margins. This approach carries a type I (false positive) error probability for mistakenly claiming noninferiority for an inferior surgical approach of no more than 2.5% when BEAR is actually inferior by ≥16 points on the IKDC and/or by 2.5 mm of side-to-side knee laxity using the knee arthrometer. The statistical power of this approach was calculated conservatively based on separate Student t tests of each outcome. Power of the combined outcome is obtained as the products of the powers of the individual tests, as would be the case if errors of the 2 tests were statistically independent. This is a conservative approach because observed treatment benefits in knee laxity and patient-perceived knee function cannot plausibly be negatively correlated and any positive correlation between them will tend to induce positive correlation in test errors, thus increasing the probability that both noninferiority tests will be positive together over that assuming independence. We used SDs from relevant prior studies, 12,51 inflated by 6% to 7% to protect against sampling variability in the prior study estimates, and we assumed that 8% of patients randomized to BEAR will actually receive ACLR and 15% of all patients would be unavailable for 2-year follow-up. Power is a function of the combination of true treatment differences for IKDC and knee laxity. As a simple summary of this function of 2 variables, Figure 4 plots statistical power to affirm noninferiority for circumstances when the true degree of inferiority is below and equal to fractions of the noninferiority margins for both endpoints, for the “base case” assumptions stated in the preceding sentence (solid blue curve), or with both variances increased by 10% and 20% (dashed violet curves), or with dropout fraction decreased by 5% or increased by 5% or 10% (dotted orange curves). Power under the nominal assumptions (solid blue curves) exceeds 90% for levels of noninferiority up to 42% of the noninferiority range, exceeds 80% for noninferiority levels up to 49% of the noninferiority range, and exceeds 50% up to 60% of the noninferiority range, past which power declines almost linearly. Thus, the design allows demonstration of noninferiority not just when both treatments are equivalent but at modest levels of BEAR inferiority on IKDC and knee laxity that we believe to be consistent with clinical acceptability, and even preferability, of the BEAR procedure because of its inherent protection from the short-term, and even potentially the long-term, morbidity associated with ACLR.
Figure 4.
Power, depicted over the lower 70% of both noninferiority ranges, for the selected sample size of 200 patients (solid blue curve) under the stated assumptions as well as power curves for recruitment shortfalls or, equivalently, variance increases of 10% and 20% and thus SD increases of 4.8% and 9.5% above the 80th percentiles used for the nominal case (dashed violet curves); or a decrease of 5% or increases of 5% or 10% in the anticipated dropout fraction (dotted orange curves). Plausible increases in the crossover fraction would have substantially smaller effect and are omitted. IKDC, International Knee Documentation Committee; MM, millimeters; NI, noninferiority.
CONCLUSION
We have described the basic components and some distinctive characteristics of the BEAR-MOON 6-center, randomized, partially blinded, noninferiority trial (RCT) comparing a new BEAR technique for treating first-time midsubstance ACL tears and BPTB ACLR on dual primary and numerous secondary outcomes. Design and execution of this complex trial involves navigating requirements of the FDA, NIAMS, and manufacturer.
AUTHORS
BEAR-MOON Design Group comprises the following authors:
Kurt P. Spindler, MD (Cleveland Clinic, Cleveland, Ohio, USA); Peter B. Imrey, PhD (Cleveland Clinic, Cleveland, Ohio, USA); Sercan Yalcin, MD (Cleveland Clinic, Cleveland, Ohio, USA); Gerald J. Beck, PhD (Cleveland Clinic, Cleveland, Ohio, USA); Gary Calbrese, DPT (Cleveland Clinic, Cleveland, Ohio, USA); Charles L. Cox, MD (Vanderbilt Medical Center, Nashville, Tennessee, USA); Paul D. Fadale, MD (Rhode Island Hospital, Providence, Rhode Island, USA); Lutul Farrow, MD (Cleveland Clinic, Cleveland, Ohio, USA); Robert Fitch, MD (Vanderbilt Medical Center, Nashville, Tennessee, USA); David Flanigan, MD (The Ohio State University, Columbus, Ohio, USA); Braden C. Fleming, PhD (Rhode Island Hospital, Providence, Rhode Island, USA); Michael J. Hulstyn, MD (Rhode Island Hospital, Providence, Rhode Island, USA); Morgan H. Jones, MD (Brigham and Women’s Hospital, Boston, Massachusetts, USA); Christopher Kaeding, MD (The Ohio State University, Columbus, Ohio, USA); Jeffrey N. Katz, MD, MSc (Brigham and Women’s Hospital, Boston, Massachusetts, USA); Peter Kriz, MD (Rhode Island Hospital, Providence, Rhode Island, USA); Robert Magnussen, MD (The Ohio State University, Columbus, Ohio, USA); Ellen McErlean, MSN (Cleveland Clinic, Cleveland, Ohio, USA); Carrie Melgaard, MS (Cleveland Clinic, Cleveland, Ohio, USA); Brett D. Owens, MD (Rhode Island Hospital, Providence, Rhode Island, USA); Paul Saluan, MD (Cleveland Clinic, Cleveland, Ohio, USA); Greg Strnad, MS (Cleveland Clinic, Cleveland, Ohio, USA); Carl S. Winalski, MD (Cleveland Clinic, Cleveland, Ohio, USA); and Rick Wright, MD (Vanderbilt Medical Center, Nashville, Tennessee, USA).
Supplemental material for this article is available at http://journals.sagepub.com/doi/suppl/10.1177/23259671211065447.
Supplemental Material
Supplemental Material, sj-pdf-1-ojs-10.1177_23259671211065447 for Design Features and Rationale of the BEAR-MOON (Bridge-Enhanced ACL Restoration Multicenter Orthopaedic Outcomes Network) Randomized Clinical Trial by BEAR-MOON Design Group, Kurt P. Spindler, Peter B. Imrey, Sercan Yalcin, Gerald J. Beck, Gary Calbrese, Charles L. Cox, Paul D. Fadale, Lutul Farrow, Robert Fitch, David Flanigan, Braden C. Fleming, Michael J. Hulstyn, Morgan H. Jones, Christopher Kaeding, Jeffrey N. Katz, Peter Kriz, Robert Magnussen, Ellen McErlean, Carrie Melgaard, Brett D. Owens, Paul Saluan, Greg Strnad, Carl S. Winalski and Rick Wright in Orthopaedic Journal of Sports Medicine
Acknowledgment
The authors thank Brittany Stojsavljevic, Cleveland Clinic Foundation, for editorial management.
Footnotes
Final revision submitted June 8, 2021; accepted August 24, 2021.
One or more of the authors has declared the following potential conflict of interest or source of funding: see Supplemental Material for details. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
References
- 1. Abourezk MN, Ithurburn MP, McNally MP, et al. Hamstring strength asymmetry at 3 years after anterior cruciate ligament reconstruction alters knee mechanics during gait and jogging. Am J Sports Med. 2017;45(1):97–105. [DOI] [PubMed] [Google Scholar]
- 2. Akelman MR, Fadale PD, Hulstyn MJ, et al. Effect of matching or overconstraining knee laxity during anterior cruciate ligament reconstruction on knee osteoarthritis and clinical outcomes: a randomized controlled trial with 84-month follow-up. Am J Sports Med. 2016;44(7):1660–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arneja S, Leith J. Review article: validity of the KT-1000 knee ligament arthrometer. J Orthop Surg (Hong Kong). 2009;17(1):77–79. [DOI] [PubMed] [Google Scholar]
- 4. Barber SD, Noyes FR, Mangine RE, Hartman W. Quantitative assessment of functional limitations in normal and anterior cruciate ligament-deficient knees. Clin Orthop Relat Res. 1990;255:204–214. [PubMed] [Google Scholar]
- 5. BEAR-MOON, Vega JF, Strnad GJ, et al. Interrater agreement of an arthroscopic anterior cruciate ligament tear classification system. Orthop J Sports Med. 2020;8(12):2325967120966323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Biercevicz AM, Miranda DL, Machan JT, Murray MM, Fleming BC. In situ, noninvasive, T2*-weighted MRI-derived parameters predict ex vivo structural properties of an anterior cruciate ligament reconstruction or bioenhanced primary repair in a porcine model. Am J Sports Med. 2013;41(3):560–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ. 2002;21(2):271–292. [DOI] [PubMed] [Google Scholar]
- 8. Brophy RH, Schmitz L, Wright RW, et al. Return to play and future ACL injury risk after ACL reconstruction in soccer athletes from the Multicenter Orthopaedic Outcomes Network (MOON) group. Am J Sports Med. 2012;40(11):2517–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Buckland-Wright JC, Ward RJ, Peterfy C, Mojcik CF, Leff RL. Reproducibility of the semiflexed (metatarsophalangeal) radiographic knee position and automated measurements of medial tibiofemoral joint space width in a multicenter clinical trial of knee osteoarthritis. J Rheumatol. 2004;31(8):1588–1597. [PubMed] [Google Scholar]
- 10. Cox CL, Huston LJ, Dunn WR, et al. Are articular cartilage lesions and meniscus tears predictive of IKDC, KOOS, and Marx activity level outcomes after anterior cruciate ligament reconstruction? A 6-year multicenter cohort study. Am J Sports Med. 2014;42(5):1058–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dunn WR, Spindler KP. Predictors of activity level 2 years after anterior cruciate ligament reconstruction (ACLR): a Multicenter Orthopaedic Outcomes Network (MOON) ACLR cohort study. Am J Sports Med. 2010;38(10):2040–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fleming BC, Fadale PD, Hulstyn MJ, et al. The effect of initial graft tension after anterior cruciate ligament reconstruction: a randomized clinical trial with 36-month follow-up. Am J Sports Med. 2013;41(1):25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Freedman B. Equipoise and the ethics of clinical research. N Engl J Med. 1987;317(3):141–145. [DOI] [PubMed] [Google Scholar]
- 14. Frobell RB, Roos EM, Roos HP, Ranstam J, Lohmander LS. A randomized trial of treatment for acute anterior cruciate ligament tears. N Engl J Med. 2010;363(4):331–342. [DOI] [PubMed] [Google Scholar]
- 15. Frobell RB, Roos HP, Roos EM, et al. Treatment for acute anterior cruciate ligament tear: five year outcome of randomised trial. BMJ. 2013;346:F232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hettrich CM, Dunn WR, Reinke EK, Spindler KP. The rate of subsequent surgery and predictors after anterior cruciate ligament reconstruction: two- and 6-year follow-up results from a multicenter cohort. Am J Sports Med. 2013;41(7):1534–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Irrgang JJ, Anderson AF. Development and validation of health-related quality of life measures for the knee. Clin Orthop Relat Res. 2002;402:95–109. [DOI] [PubMed] [Google Scholar]
- 19. Irrgang JJ, Anderson AF, Boland AL, et al. Development and validation of the International Knee Documentation Committee Subjective Knee Form. Am J Sports Med. 2001;29(5):600–613. [DOI] [PubMed] [Google Scholar]
- 20. Irrgang JJ, Anderson AF, Boland AL, et al. Responsiveness of the International Knee Documentation Committee Subjective Knee Form. Am J Sports Med. 2006;34(10):1567–1573. [DOI] [PubMed] [Google Scholar]
- 21. Jacobs CA, Peabody MR, Lattermann C, et al. Development of the KOOSglobal platform to measure patient-reported outcomes after anterior cruciate ligament reconstruction. Am J Sports Med. 2018;46(12):2915–2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jones MH, Oak SR, Andrish JT, et al. Predictors of radiographic osteoarthritis 2 to 3 years after anterior cruciate ligament reconstruction: data from the MOON on-site nested cohort. Orthop J Sports Med. 2019;7(8):2325967119867085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Joshi SM, Mastrangelo AN, Magarian EM, Fleming BC, Murray MM. Collagen-platelet composite enhances biomechanical and histologic healing of the porcine anterior cruciate ligament. Am J Sports Med. 2009;37(12):2401–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kaeding CC, Pedroza AD, Reinke EK, Huston LJ, Spindler KP. Risk factors and predictors of subsequent ACL injury in either knee after ACL reconstruction: prospective analysis of 2488 primary ACL reconstructions from the MOON cohort. Am J Sports Med. 2015;43(7):1583–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Katz JN, Wright JG, Losina E. Clinical trials in orthopaedics research, part II: prioritization for randomized controlled clinical trials. J Bone Joint Surg Am. 2011;93(7):e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Konrath JM, Vertullo CJ, Kennedy BA, et al. Morphologic characteristics and strength of the hamstring muscles remain altered at 2 years after use of a hamstring tendon graft in anterior cruciate ligament reconstruction. Am J Sports Med. 2016;44(10):2589–2598. [DOI] [PubMed] [Google Scholar]
- 27. Kujala UM, Jaakkola LH, Koskinen SK, et al. Scoring of patellofemoral disorders. Arthroscopy. 1993;9(2):159–163. [DOI] [PubMed] [Google Scholar]
- 28. Li S, Chen Y, Lin Z, et al. A systematic review of randomized controlled clinical trials comparing hamstring autografts versus bone-patellar tendon-bone autografts for the reconstruction of the anterior cruciate ligament. Arch Orthop Trauma Surg. 2012;132(9):1287–1297. [DOI] [PubMed] [Google Scholar]
- 29. Lie MM, Risberg MA, Storheim K, Engebretsen L, Øiestad BE. What’s the rate of knee osteoarthritis 10 years after anterior cruciate ligament injury? An updated systematic review. Br J Sports Med. 2019;53(18):1162. [DOI] [PubMed] [Google Scholar]
- 30. Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35(10):1756–1769. [DOI] [PubMed] [Google Scholar]
- 31. Magarian EM, Fleming BC, Harrison SL, et al. Delay of 2 or 6 weeks adversely affects the functional outcome of augmented primary repair of the porcine anterior cruciate ligament. Am J Sports Med. 2010;38(12):2528–2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mankovich NJ, Cheeseman AM, Stoker NG. The display of three-dimensional anatomy with stereolithographic models. J Digit Imaging. 1990;3(3):200–203. [DOI] [PubMed] [Google Scholar]
- 33. Marx RG, Stump TJ, Jones EC, Wickiewicz TL, Warren RF. Development and evaluation of an activity rating scale for disorders of the knee. Am J Sports Med. 2001;29(2):213–218. [DOI] [PubMed] [Google Scholar]
- 34. Mather RC III, Koenig L, Kocher MS, et al. Societal and economic impact of anterior cruciate ligament tears. J Bone Joint Surg Am. 2013;95(19):1751–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McCullough KA, Phelps KD, Spindler KP, et al. Return to high school— and college-level football after anterior cruciate ligament reconstruction: a Multicenter Orthopaedic Outcomes Network (MOON) cohort study. Am J Sports Med. 2012;40(11):2523–2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Murray MM, Fleming BC. Biology of anterior cruciate ligament injury and repair: Kappa Delta Ann Doner Vaughn award paper 2013. J Orthop Res. 2013;31(10):1501–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Murray MM, Fleming BC. Use of a bioactive scaffold to stimulate anterior cruciate ligament healing also minimizes posttraumatic osteoarthritis after surgery. Am J Sports Med. 2013;41(8):1762–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Murray MM, Fleming BC, Badger GJ, et al. Bridge-enhanced anterior cruciate ligament repair is not inferior to autograft anterior cruciate ligament reconstruction at 2 years: results of a prospective randomized clinical trial. Am J Sports Med. 2020;48(6):1305–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Murray MM, Flutie BM, Kalish LA, et al. The bridge-enhanced anterior cruciate ligament repair (BEAR) procedure: an early feasibility cohort study. Orthop J Sports Med. 2016;4(11):2325967116672176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Murray MM, Kalish LA, Fleming BC, et al. Bridge-enhanced anterior cruciate ligament repair: two-year results of a first-in-human study. Orthop J Sports Med. 2019;7(3):2325967118824356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Murray MM, Magarian E, Zurakowski D, Fleming BC. Bone-to-bone fixation enhances functional healing of the porcine anterior cruciate ligament using a collagen-platelet composite. Arthroscopy. 2010;26(9):S49–S57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Murray MM, Magarian EM, Harrison SL, et al. The effect of skeletal maturity on functional healing of the anterior cruciate ligament. J Bone Joint Surg Am. 2010;92(11):2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Murray MM, Spindler KP, Abreu E, et al. Collagen-platelet rich plasma hydrogel enhances primary repair of the porcine anterior cruciate ligament. J Orthop Res. 2007;25(1):81–91. [DOI] [PubMed] [Google Scholar]
- 44. Murray MM, Spindler KP, Ballard P, et al. Enhanced histologic repair in a central wound in the anterior cruciate ligament with a collagen-platelet-rich plasma scaffold. J Orthop Res. 2007;25(8):1007–1017. [DOI] [PubMed] [Google Scholar]
- 45. Nomura Y, Kuramochi R, Fukubayashi T. Evaluation of hamstring muscle strength and morphology after anterior cruciate ligament reconstruction. Scand J Med Sci Sports. 2015;25(3):301–307. [DOI] [PubMed] [Google Scholar]
- 46. Noyes FR, Barber SD, Mangine RE. Abnormal lower limb symmetry determined by function hop tests after anterior cruciate ligament rupture. Am J Sports Med. 1991;19(5):513–518. [DOI] [PubMed] [Google Scholar]
- 47. Oksendahl HL, Gomez N, Thomas CS, et al. Digital radiographic assessment of tibiofemoral joint space width: a variance component analysis. J Knee Surg. 2009;22(3):205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Palmieri-Smith RM, Thomas AC, Wojtys EM. Maximizing quadriceps strength after ACL reconstruction. Clin Sports Med. 2008;27(3):405–424. [DOI] [PubMed] [Google Scholar]
- 49. Poehling-Monaghan KL, Salem H, Ross KE, et al. Long-term outcomes in anterior cruciate ligament reconstruction: a systematic review of patellar tendon versus hamstring autografts. Orthop J Sports Med. 2017;5(6):2325967117709735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Proffen BL, Fleming BC, Murray MM. Histologic predictors of maximum failure loads differ between the healing ACL and ACL grafts after 6 and 12 months in vivo. Orthop J Sports Med. 2013;1(6):2325967113512457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Reinke EK, Spindler KP, Lorring D, et al. Hop tests correlate with IKDC and KOOS at minimum of 2 years after primary ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2011;19(11):1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee Injury and Osteoarthritis Outcome Score (KOOS)—development of a self-administered outcome measure. J Orthop Sports Phys Ther. 1998;28(2):88–96. [DOI] [PubMed] [Google Scholar]
- 53. Roos EM, Toksvig-Larsen S. Knee injury and Osteoarthritis Outcome Score (KOOS)---validation and comparison to the WOMAC in total knee replacement. Health Qual Life Outcomes. 2003;1:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Spindler KP, Huston LJ, Chagin KM, et al. Ten-year outcomes and risk factors after anterior cruciate ligament reconstruction: a MOON longitudinal prospective cohort study. Am J Sports Med. 2018;46(4):815–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Spindler KP, Huston LJ, Wright RW, et al. The prognosis and predictors of sports function and activity at minimum 6 years after anterior cruciate ligament reconstruction: a population cohort study. Am J Sports Med. 2011;39(2):348–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Spindler KP, Murray M, Devin C, Nanney L, Davidson J. The central ACL defect as a model for failure of intra-articular healing. J Orthop Res. 2006;24(3):401–406. [DOI] [PubMed] [Google Scholar]
- 57. Spindler KP, Wright RW. Clinical Practice. Anterior cruciate ligament tear. New Engl J Med. 2008;359(20):2135–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Torg JS, Conrad W, Kalen V. Clinical diagnosis of anterior cruciate ligament instability in the athlete. Am J Sports Med. 1976;4(2):84–93. [DOI] [PubMed] [Google Scholar]
- 59. US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER). Non-inferiority clinical trials to establish effectiveness: guidance for industry. FDA. Published November 2016. https://www.fda.gov/media/78504/download
- 60. van Yperen DT, Reijman M, van Es EM, Bierma-Zeinstra SMA, Meuffels DE. Twenty-year follow-up study comparing operative versus nonoperative treatment of anterior cruciate ligament ruptures in high-level athletes. Am J Sports Med. 2018;46(5):1129–1136. [DOI] [PubMed] [Google Scholar]
- 61. Vavken P, Fleming BC, Mastrangelo AN, Machan JT, Murray MM. Biomechanical outcomes after bioenhanced anterior cruciate ligament repair and anterior cruciate ligament reconstruction are equal in a porcine model. Arthroscopy. 2012;28(5):672–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wright RW, Haas AK, Anderson J, et al. Anterior cruciate ligament reconstruction rehabilitation: MOON guidelines. Sports Health. 2015;7(3):239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-pdf-1-ojs-10.1177_23259671211065447 for Design Features and Rationale of the BEAR-MOON (Bridge-Enhanced ACL Restoration Multicenter Orthopaedic Outcomes Network) Randomized Clinical Trial by BEAR-MOON Design Group, Kurt P. Spindler, Peter B. Imrey, Sercan Yalcin, Gerald J. Beck, Gary Calbrese, Charles L. Cox, Paul D. Fadale, Lutul Farrow, Robert Fitch, David Flanigan, Braden C. Fleming, Michael J. Hulstyn, Morgan H. Jones, Christopher Kaeding, Jeffrey N. Katz, Peter Kriz, Robert Magnussen, Ellen McErlean, Carrie Melgaard, Brett D. Owens, Paul Saluan, Greg Strnad, Carl S. Winalski and Rick Wright in Orthopaedic Journal of Sports Medicine