Abstract
Objective:
Participant recruitment is a challenge for any clinical trial but is especially complex in cancer specifically due to the need to initiate treatment urgently. Most participants enrolled in oncology clinical trials are identified as potential participants by the oncologist or other referring provider. Optimal clinical care for patients with cancer includes consideration of participation in a clinical trial. However, the process of finding a clinical trial that is appropriate the patient can be cumbersome and time consuming.
Material and Methods:
The University of Kansas Cancer Center has developed a mobile application (app) which streamlines the clinical trial search process for physicians, patients, and caregivers by cohesively integrating all clinical trials currently recruiting in the center and making them easy to browse.
Results:
Key aspects of the app include simple filtering options, the ability to search for trials by name, easily accessible assistance, and in-app referral by phone or email. Initial feedback on the app has been very positive, with several suggestions already being implemented in future development. The app was designed to be used both by physicians to find trials, as well as patients in collaboration with their physicians.
Conclusion:
While long-term results will be crucial to understanding how the app can best serve our patient population, our initial results suggest that health system specific clinical trial apps can address a currently unmet need in the clinical trial recruitment process.
Keywords: Patient recruitment, clinical trials, screening, patient referral
Objective
Engagement of both of patients and physicians is a central aspect of clinical trial recruitment. Without sufficient engagement and recruitment, clinical trials are frequently terminated early due to poor accrual or are unable to achieve results that are statistically significant. An estimated 19% of phase 2 and phase 3 clinical trials in Canada are terminated due to inadequate enrollment. 1 Significant factors associated with research centers that suffer from poor recruitment include low physician referral rates, 2 lack of awareness of clinical trials in patients, 3 and a lack of available information regarding clinical trials.3,4
Cancer Clinical Trials (CCTs) face a particular challenge in engaging and recruiting patients. Only 55% of cancer trials in the UK were able to reach their originally specified recruitment goals. 5 Among cancer patients CCT participation is as low as 3% to 5%, 6 and only 10% of cancer survivors reported being aware that CCT participation was a possibility during their treatment. 7 Additionally, among cancer patients made aware of potential CCT participation, 73% were made aware by their physician. This suggests that measures to foster physician and patient engagement and raise awareness of ongoing CCTs could provide access to a previous untapped source of participants in CCTs.
The University of Kansas Cancer Center (KUCC) at The University of Kansas Medical Center sought to accomplish this by developing a clinical trial finder application (app) that could be used by both patients and physicians. The design of the app centered on ease of use, a fluid referral process, and quick access to technical support. After researching features in clinical trial navigators that were commonly requested by physicians, the KUMC team landed on 3 such features to focus on. Firstly, physicians desired for clinical trial information to be easily accessible. 4 This desire was addressed through designing our in-house clinical trial navigator as a mobile application. The second desire was an efficient means for physicians to filter trials by treatment circumstance as well as a simple means to weigh risks and benefits. 8 This was addressed by having clinical trials be filtered in the application design from disease group all the way to treatment modality within cancer types. Lastly, physicians wanted to be able to easily find clinical trials by location. 9 This last point led the KUCC team to restrict displayed trials to our health system. This decision was made because traditional clinical trial navigation systems typically include trials from all over the country and require extensive time or experience to navigate. A clinical trial application limited to trials from a local health system streamlines that process for local and regional physicians. The ability to use the application on a portable device further encourages engagement in physicians by allowed them to browse clinical trials while on the move.
Surveys of cancer patients revealed that one-third of cancer patients are first presented with the possibility of CCT participation at diagnosis. 10 However, these patients often felt unqualified to search for trials and overwhelmed by the breadth of information surrounding their diagnosis. A mobile application that displays local trials would allow physicians to encourage patients and families to download it at the bedside and navigate through trial finding together, resulting in a list of possible trials that is accessible by the family for consideration.
Materials and Methods
In order to encourage physician engagement in clinical trials, our team focused primarily on addressing physician concerns regarding the process of making a referral. As previously mentioned, physicians desired information on clinical trials that were local, could be easily assessed for risks and benefits, and was internet accessible.
We included several secondary aims in development based on physician feedback. The first of these was to allow physicians to access clinical trial information on the go or at the bedside, as many physicians expressed that they often did not have time to search for clinical trials at a desk. The second of these was allowing physicians to find trials without manual searching. This aim ties in to the first, where usability without manual searching would allow physicians to search for trials while on the move. The third of these was allowing physicians to differentiate between first line and second line treatment trials. The last of these was building a tool that would promote discussions of clinical trials at the patient bedside.
Very few universities have built a trial searching application, among those applications most of them are web-based and restricted to just a few research personnel. Others are specific to health systems such as Stanford’s SCI Cancer Clinical Trials app. 11 However, most of these apps and other trial search functions are designed for both patient and physician use.12,13 When designed this way, these systems typically do not provide much benefit to healthcare professionals for their medical expertise and lead to physicians spending similar amounts of time to laymen searching for trials before finding the information they need.
In designing the Clinical Trial application for physician referral use, The University of Kansas Cancer Center’s aim was to capitalize on physician expertise to lead them more quickly to trials and minimize manual text searching.
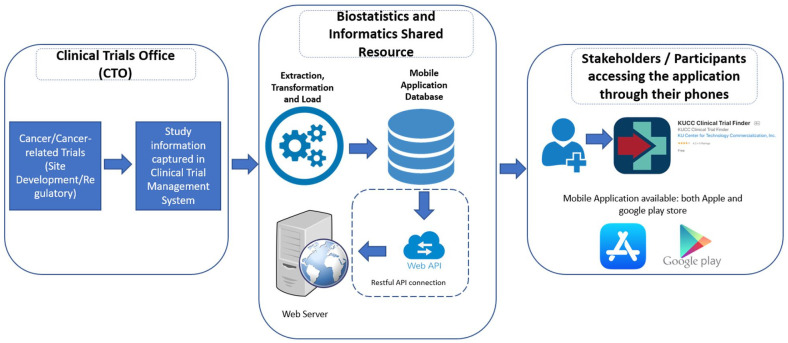
CT finder data flow
To protect patient privacy, data on patient characteristics is not processed or stored within the app. Instead, the primary data being captured and utilized for the app is current data on available cancer clinical trials being held within the University of Kansas Health Systems at its various locations.
Incorporating this information into the mobile application didn’t require capturing new information as most of the information on current cancer trials had already been captured under the KUMC Clinical Trial Management System (CTMS), powered by Velos inc. Given that this information was already regularly captured by our in-house staff for the CTMS, our primary focus for app development was efficient filtering of study information. We did this by capturing the first line and second line of treatment for all therapeutic trials in our system. This study information is subsequently pushed into the mobile application database once every day via the standard extraction, transformation, and load process (ETL). Secure RESTFUL Application Programing Interface (APIs) were then built to fetch the data from the CT Finder database, and act as a communication layer between the database and the application. This process is depicted below in Figure 1.
Figure 1.
Clinical trial finder application data flow.
The User Interface (UI), has been designed using Angular version 5 (v5) framework and implementing Ionic framework. The API (middleware) has been designed using the dot net core 2.1 framework. Physician’s inputs were solicitated at every step during the user interface design. This input included but is not limited to the selection options, the amount of information displayed, and the color schema for the user interface. The application was designed with the intention to be made available to the broad Physician and research user base. This meant that the application had to be made available for both Apple and Android users. The Ionic Cordova plugin has been used to convert Angular type script code to Swift-UI (apple) and java (android) code. Ionic supports this cross-platform framework to release the app in both Apple and Android ecosystems.
As a supplement file (Data Dictionary) we have attached the fields that are being utilized by the CT Finder application. The hierarchy is built while the data is captured with in the clinical trial management system. Additionally, a video (Supplemental Video) has also been attached showing how to navigate through the application.
Results
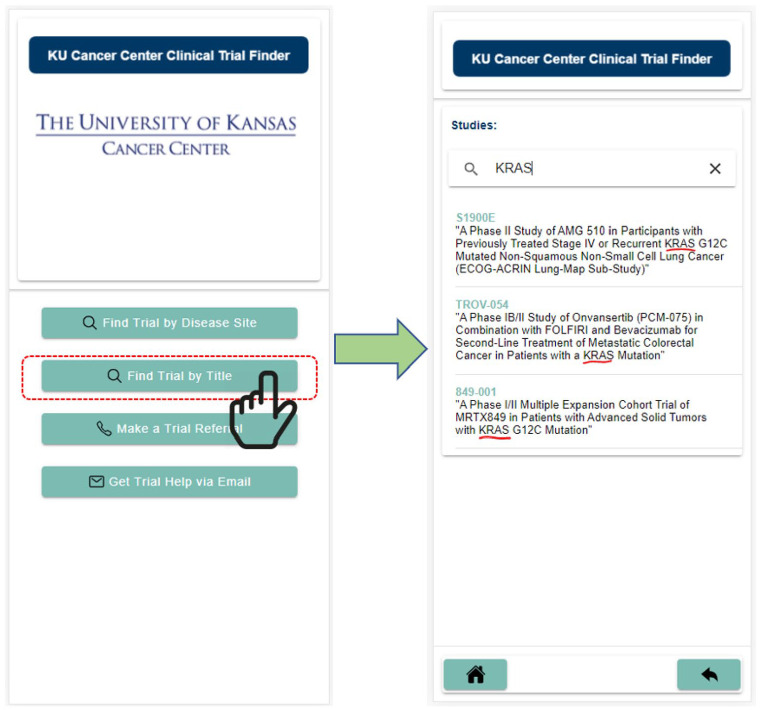
The figure below (Figure 2) demonstrates a sample search process which narrows available trials by disease working groups, then further by cancer type in that system, and lastly by first-line or second-line treatment. These options follow a line of logic that is consistent with physician considerations in researching potential clinical trials, while still being accessible to laypeople.
Figure 2.
A sample of the navigation process for finding a clinical trial with windows for narrowing down from disease working group, cancer type, and first line or second line treatment: (a) disease working groups in menu navigation can be selected to further narrow, and (b) number of trials under selection displayed.
These options were also implemented according to the secondary aims of development. To allow physicians to access information on the go, options were laid out in a clear progression with full utility available through single button presses. A filtering system was implemented to allow physicians to find trials without searching by name. The first- and second-line filtering after trial type selection allows physicians to make those differentiations. This filtering process allows users to filter the available trials for display by broad characteristics such as cancer type as well as more specific characteristics like whether the treatment is first line or second line. Lastly, the broad availability of the app would allow physicians to guide patients and families through the process at the bedside to provide a tailored list of available trials. All trials present on the app are currently open to recruitment at the University of Kansas Cancer Center. With these features a physician will no longer need to find a workstation and manually search through trials to narrow results using national-level web-based trial finders. Instead, they can use the mobile application at the bedside or in a free moment to find trials quickly and easily. Additionally, because the information contained within the app is derived from clinical trial data that is captured daily, the application will consistently be updated with new trials or the removal of trials which have ceased recruitment.
There were several additional features that were included for additional utility after feedback. Additional information was included in the trial viewer under the selection menu which includes study contact information as well as information on the principal investigator. Under these trial descriptions, users can select “Refer Patient to this Study.” This option links the user automatically the physician’s work email on outlook with the subject link auto filled with the Protocol ID and the PI name as shown in Figure 3. In this way, the referring physician can simply insert the high-level information of the patient who needs to be followed up, to determine eligibility for the trial. Because this option redirects the user to outlook email, the patient information being communicated is not processed or stored within the app.
Figure 3.
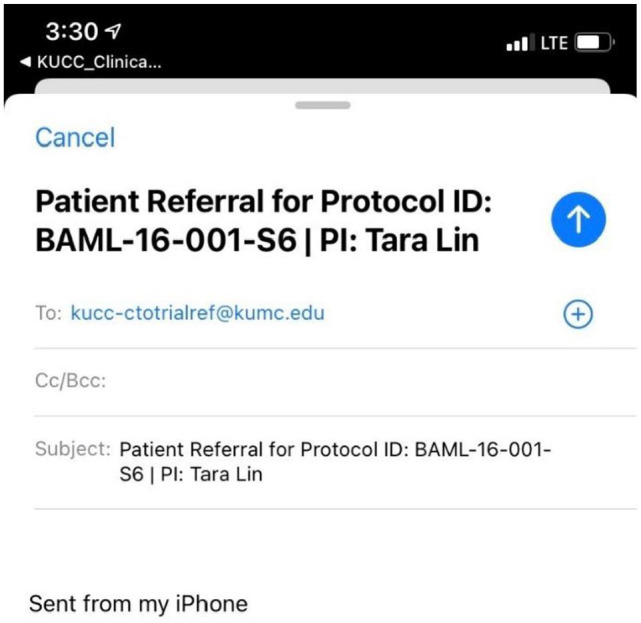
Refer patient to this study feature—physician could refer a patient through email.
Physicians can also click on the “Make a Trial Referral” to directly call and leave a voice message for the nurse navigator. Additionally, they may select “Get Trial Help via Email” button which is found on the home screen of the application. Get Trial Help will route their email directly to the central nurse navigator inbox that is monitored by a team of centralized screeners. The default method of searching for trials by title was included, and additionally allows physicians to search using keywords such as biomarkers, drugs, and treatment therapies. This is useful when physicians already have trials in mind, have specific patient needs, or prefer traditional clinical trial navigation. This system can be seen below in Figure 4.
Figure 4.
Searching using the study title or keywords.
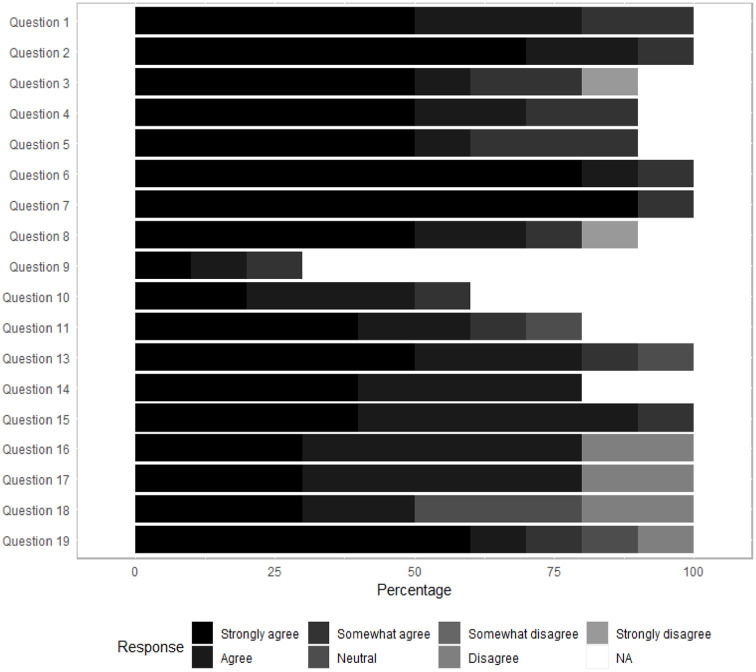
Ten of our end users which included physicians and clinical staff responded to the usability survey administered immediately after the app roll out at the beginning of 2021. The usability survey was developed by the Biostatistics and Informatics Shared Resource (BISR) team at the University of Kansas Cancer Center based on the IBM Computer Usability Satisfaction Questionnaire (https://garyperlman.com/quest/quest.cgi) developed by Lewis to solicitate constructive feedback from both Physician and the research teams. Responses were exceptionally positive, with some great suggestions to further enhance the user interface as shown in Figure 5. The validated survey included 19 categorical questions as well as 2 open ended questions regarding the positives and negatives of the application.
Figure 5.
Results from the usability survey. Responses are based on the n = 10.
Positive responses to the 2 open-ended questions expressed that the CT finder application was easy to use, effective, and helped easily find the clinical trial that fit each participant’s portfolio. The respondents described the app as “Easy to use,” “It has ease of access and allows the physician to make referrals in app,” “The interface is pleasant, and the information provided is laid out incredibly well. Very easy to use and navigate.,” “Well Organized,” “Great design and layout.”
Discussion
The KUMC Clinical trial finder app streamlines the physician referral process and can accelerate the process of enrolling eligible patients in the correct study for them. The simplification of this first step in patient enrollment can lead smoothly into the next steps such as pre-screening tests. The trust that these patients have in their physicians can make a collaborative discussion tool such as this particularly beneficial. Considering that 73% of oncology recruitment is done through physician referral, it is crucial to develop clinical trial engagement tools that facilitate this process. 7
Currently over 300 users have downloaded the CT finder app since its launch in December 2020. The informatics team continues to work closely with physicians and the clinical teams across the KU Cancer Center to solicit feedback that would help further optimize the application and streamline the data flow. Some of the feedback from the usability survey suggested that we should allow users to search using the study titles as these contain key words which are familiar to clinicians. The informatics team was able to incorporate this feature in only a few weeks and is currently available in the version that is deployed in the app store.
Our initial release was limited to Apple users only since our majority of our target audience were Apple users. With the use of a cross platform compiler (Ionic Framework) our informatics team was able to build a version of android without the need to recode much of the app from scratch. 14 The Android version of the application was released in the market mid-January of 2021, almost a month after the initial launch date of the CT Finder.
The primary benefits of a mobile clinical trial application over similar options include location restriction, ease of use, an in-application referral process, and broad accessibility. Location restriction, which arises from the fact that only KU Cancer Center trials are featured on the application, eliminates the part of the clinical trial search process where families and physicians must decide if it is feasible to travel for their clinical trials of interest. Ease of use comes as a benefit from the fact that the applications user interface was designed for a mobile phone, which makes navigation significantly easier than using a web browser on a mobile phone. The in-app referral process provides physicians with a template for making trial referrals via email, as opposed to having to find contact information and format their referral manually. Lastly, broad accessibility means that patients and families can use the app during discussions with their physicians to see the same potential trials that their physicians do.
Future developments to the app would include the option to prioritize studies within the CT Finder. Through this feature Primary Investigators would be able to easily collaborate and refer or recruit patients for prioritized studies. The other feature we anticipate including in the near future is to index studies based on current accrual rates. These indexes could then be displayed for Primary Investigators to track the progression of their studies through the app.
Information surrounding the launch of the app spread quickly and led to higher-than-expected download rates from patients of the cancer center. We plan to meet with our Patient and Investigator Voices Organizing Together (PIVOT) team to gather feedback on how a similar application with more accessibility to non-clinicians could be built specifically for patients and families of patients. 15 Recent studies have indicated that lay navigation tools for patients to find clinical trials can increase enrollment significantly, and further emphasize the need for a development of this kind. 16
Departments across the KU Medical Center have expressed interest in incorporating clinical trials from specialties beyond oncology. The CTSA grant has committed to invest in the CT Finder application to be expanded to at least 3 other non-oncology groups which potentially include Cardiology, Neurology and Internal Medicine. With similar data being available for other departments as well, we anticipate this should be an easy expansion of the CT Finder application.
Limitations
One of the main limitations of the application is its reliance on the KUMC clinical trial management system. As the app pulls its data directly from the management system, clinical trials will not appear on the CT Finder if not first entered into the system. As described previously, the application is currently limited to oncology trials. As trials from other specialties are incorporated, design decisions will need to be made on how to easily differentiate between trials by specialty.
Conclusions
Patient recruitment in cancer clinical trials is a process with several challenges including complex eligibility criteria, interactions between trial treatment and cancer treatment course, variable physician referral rates, and a lack of accessible information. While the KUMC data science team cannot solve all these issues, we can attempt to make information on clinical trials in our health system easily accessible and streamline the referral process. With a short period of familiarization, physicians and researchers can quickly use the CT Finder app to find the correct study for their patient, discuss that study with their patient, and make a referral. Through removing barriers in information availability, informatics may facilitate the development of treatment modalities to improve the quality of life and prognosis of patients of not only cancer patients, but all patients.
Supplementary Material
Acknowledgments
We would like to extend our gratitude to Dr Roy Jensen, Cancer Center Physicians, clinical and administrative staff for their indispensable guidance and advice they provided on the design and development of Clinical Trial Finder Application.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Development of the Clinical Trial Finder Application was supported by the National Cancer Institute (NCI) Cancer Center Support Grant P30 CA168524 and used the Biostatistics and Informatics Shared Resource (BISR).
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Contribution: D.P.M. and V.M is the architect and developer of the application. D.P.M and A.M.A. oversaw all aspects of drafting, revision, and final approval of the manuscript. B.G. contributed to the C3OD usability survey and participated in the drafting and revising of the manuscript. B.G. and J.T participated in the drafting and revising of the manuscript. T.L, B.G, M.S.M. provided advice on design, development, and operational issues related to C3OD implementation and participated in the drafting and revising of the manuscript.
ORCID iD: Dinesh Pal Mudaranthakam  https://orcid.org/0000-0001-9767-1158
https://orcid.org/0000-0001-9767-1158
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Fogel DB. Factors associated with clinical trials that fail and opportunities for improving the likelihood of success: a review. Contemp Clin Trials Commun. 2018;11:156-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Levett KM, Roberts CL, Simpson JM, Morris JM. Site-specific predictors of successful recruitment to a perinatal clinical trial. Clin Trials. 2014;11:584-589. [DOI] [PubMed] [Google Scholar]
- 3. Kadam RA, Borde SU, Madas SA, Salvi SS, Limaye SS. Challenges in recruitment and retention of clinical trial subjects. Perspect Clin Res. 2016;7:137-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Galvin JE, Meuser TM, Boise L, Connell CM. Predictors of physician referral for patient recruitment to Alzheimer disease clinical trials. Alzheimer Dis Assoc Disord. 2009;23:352-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sygna K, Johansen S, Ruland CM. Recruitment challenges in clinical research including cancer patients and their caregivers. A randomized controlled trial study and lessons learned. Trials. 2016;17:428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Uveges MK, Lansey DG, Mbah O, Gray T, Sherden L, Wenzel J. Patient navigation and clinical trial participation: a randomized controlled trial design. Contemp Clin Trials Commun. 2018;12:98-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Comis RL, Miller JD, Colaizzi DD, Kimmel LG. Physician-related factors involved in patient decisions to enroll onto cancer clinical trials. J Oncol Pract. 2009;5:50-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Taft T, Weir C, Kramer H, Facelli JC. Primary care perspectives on implementation of clinical trial recruitment. J Clin Transl Sci. 2020;4:61-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Institute of Medicine (US) Committee on Cancer Clinical Trials and the NCI Cooperative Group Program, Nass SJ, Moses HL, Mendelsohn J. A National Cancer Clinical Trials System for the 21st Century: Reinvigorating the NCI Cooperative Group Program. National Academies Press; 2010. [PubMed] [Google Scholar]
- 10. Ridgeway JL, Asiedu GB, Carroll K, Tenney M, Jatoi A, Radecki Breitkopf C. Patient and family member perspectives on searching for cancer clinical trials: a qualitative interview study. Patient Educ Couns. 2017;100:349-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Morain SR, Largent EA. Recruitment and trial-finding apps-time for rules of the road. J Natl Cancer Inst. 2019;111:882-886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Atkinson NL, Saperstein SL, Massett HA, Leonard CR, Grama L, Manrow R. Using the Internet to search for cancer clinical trials: a comparative audit of clinical trial search tools. Contemp Clin Trials. 2008;29:555-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jiang SY, Weng C. Cross-system evaluation of clinical trial search engines. AMIA Summits Transl Sci Proc. 2014;2014:223-229. [PMC free article] [PubMed] [Google Scholar]
- 14. Carter L, Rogith D, Franklin A, Myneni S. NewCope: a theory-linked mobile application for stress education and management. Stud Health Technol Inform. 2019;264:1150-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. “Patient Research Advocacy: PIVOT: The University of Kansas Cancer Center.” Patient Research Advocacy | PIVOT | The University of Kansas Cancer Center. 2019. www.kucancercenter.org/research/give-back/patient-research-advocacy
- 16. Cartmell KB, Bonilha HS, Matson T, et al. Patient participation in cancer clinical trials: A pilot test of lay navigation. Contemp Clin Trials Commun. 2016;3:86-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.