Abstract
Objective:
To investigate the effects of systemically given propolis on the expanded premaxillary suture in a rat study model.
Materials and Methods:
The 24 rats were randomly divided into three groups—only expansion (OE), expansion plus propolis (PRO), and nonexpansion (control) groups. After the 5-day expansion period was completed, the OE and PRO groups underwent 12 days of mechanical retention. At the end of this period, the animals were euthanatized and their pre-maxillae were dissected and fixed. Histomorphometric examination was performed to determine the number of osteoclasts, osteoblasts, and capillaries as well as the intensity of inflammatory cells and amount of new bone formation.
Results:
Statistical analysis showed that the intensities of inflammatory cells, number of osteoblasts, and amount of new bone formation were greater in the PRO group than in the other groups. The PRO group also had more osteoclasts and new capillaries.
Conclusion:
Systemic use of propolis may hasten new bone formation at the expanded suture in rats.
Keywords: Rapid maxillary expansion, Antioxidants, Propolis, Bone formation
INTRODUCTION
Expansion of the midpalatal suture with rapid maxillary expansion (RME), a common procedure in orthodontic practice, leads to stretching of collagenous fibers as well as new bone formation, producing a net increase in the transverse width of the maxillary basal bone.1 However, the tendency for relapse is still formidable.2–5 Although the actual causes of relapse are not yet fully understood, regulation of bone metabolism and retention period duration were among some of the causes suggested by researchers.2,3 It has also been stated that the velocity and the quality of bone formation at the suture might influence early postexpansion relapse.
Nowadays many treatment approaches and new materials are focused on accelerating and enhancing new bone formation at the expanded suture, and some researchers3,4,6–9 have attested that these approaches are effective.
Living organisms have developed many defense mechanisms in which various endogenous antioxidants take part so as to maintain their integrity in response to the free oxygen radicals of endogenous or exogenous origin.10 Recently, antioxidant therapies have been widely researched by investigators4,8,11 as a result of their effect on bone metabolism via inhibition of osteoclastic activity and promotion of osteoblastic activity.
Propolis, a substance made by the honeybee, is an effective antioxidant, antimicrobial, and anti-inflammatory agent.12 Honeybees collect the resin from cracks in the bark of trees and leaf buds. This resin is masticated, salivary enzymes are added, and the partially digested material is mixed with bee wax and used by bees to seal holes in their honeycombs, smooth out the internal walls of the honeycomb, and protect the entrance against intruders.13 In general, propolis is composed of 50% resin and vegetable balsam, 30% wax, 10% essential and aromatic oils, 5% pollen, and 5% various other substances, including organic debris.14 Since ancient times propolis has been used for the treatment of many diseases.12
In the literature, propolis has been reported to possess various biological activities, including antibacterial15 and anti-inflammatory16 properties. Therefore, propolis is widely used in food and beverages to restore health and to prevent disorders such as inflammation, diabetes, and cancer.17,18
In a recent study, Guney et al.12 investigated the effects of propolis on the antioxidant system and fracture healing in an experiment. The results revealed that the bone mineral density was higher and that radiological and histological evaluation scores were better in the rats that received oral propolis treatment.
In light of the above information, it could be advantageous to use propolis, a purely natural, inexpensive agent, one also supports the immune system, in order to enhance bone formation at the expanded suture without any adverse effect. Consequently, the aim of this study was to evaluate the effects of propolis on new bone formation following RME in a rat study model.
MATERIALS AND METHODS
An animal cohort of 12-week-old adult male Wistar albino rats weighing 200 g (±10 g) was provided by the Animal Laboratory at the Cumhuriyet University Faculty of Medicine. The animals were restricted to plastic cages under artificial lighting, with a 12-hour light:dark photoperiod. The room temperature was maintained at 25°C, and food and water were provided ad libitum. The Animal Ethics Committee at Cumhuriyet University approved all study procedures.
Preparation of Propolis Extract
Propolis samples were collected from Trabzon, Turkey. The samples were obtained from plastic nets and subsequently frozen to promote propolis removal. Hand-collected propolis was kept in the dark until processing. All extracts of propolis were weighed under aseptic conditions in sterile volumetric flasks. A 25% ethanolic extract of propolis (EEP) was prepared (3 g of propolis; the volume was completed to 12 mL with 99% ethyl alcohol) and protected from bright light; the volume was shaken moderately by magnetic mixer and maintained at room temperature.19 After 2 days, the extract was filtered under a vacuum and final concentrations were calculated; we obtained the dry weight of the solutions (250 mg/mL). Specific dilutions of this solution were prepared in the appropriate culture medium.
The Expansion Appliance
To prepare for insertion of the expansion appliance, the rats were anesthetized using a xylazine (3 mg/kg; Rompun, Bayer, Leverkusen, Germany) and ketamine (90 mg/kg; Ketalar, Pfizer, New York, NY, USA) combination. A helical spring fabricated from an 0.012-inch piece of stainless-steel wire was used to perform the expansion of the midpalatal suture. The springs were placed on a grid and activated using pliers. The spring force was measured with a gauge and was found to be 30 g. To obtain retention, a stainless-steel disc was used to prepare a groove at the level of the gingival papilla on the distal sides of the maxillary incisor teeth. Next, an 0.009-inch stainless-steel ligature wire was used to fix the spring to the maxillary incisors.
Twenty-four rats were randomly divided into three equally sized groups (n = 8): the only expansion group (OE), the expansion plus propolis group (PRO), and the nonexpansion group (NE; the control group, which received no procedure). In the PRO group, PRO was administered systemically via orogastric tubes after the expansion period at a rate of 100 mg/kg/d.
The rats were observed for signs of weight loss, infection, and appliance failure throughout the duration of the study. In the event of the appearance of infection, a rapid decrease in rat body weight, or appliance failure the animals were excluded from study and replaced with a new one.
The consolidation period was initiated after an expansion period of 5 days when at least 1.5 mm of distance was provided between maxillary incisors; this amount of distance was reported20 to be sufficient to induce the maximum rate of midpalatal suture expansion (Figure 1). After a consolidation period of 12 days, the animals were euthanatized via injection 200 mg/kg of sodium pentothal (Pentothal; Abbot, North Chicago, Ill), and the maxillary bone containing the midpalatal suture cartilage was surgically removed and subjected to further fixation in a 10% neutral buffered formalin solution at room temperature for 24–48 hours.
Figure 1. .

After expansion.
Histological Preparation
After fixation, the springs were removed and the samples were demineralized in an aqueous 10% formic acid solution. These specimens were then dehydrated and embedded in paraffin. The maxillary incisor acted as the primary guide for orienting the sections. The section was cut perpendicular to the sagittal plane and was determined by two points, one located at the alveolar crest and the other located 4 mm apical to the crest. This plane passed through the center of the incisor crown at its gingival portion. The paraffin blocks were sliced into 5-µm-thick sections and prepared for hematoxylin and eosin staining prior to optical microscope examination. Measurement for bone histomorphometry was performed centered around the premaxillary suture and 175–250 µm (35th–50th sections) below the surface of the osseous palate facing the oral cavity because bone formation of the surface area was sometimes irregular and unsuitable for quantitative measurement.
A single examiner in a blind study carried out the histomorphometric evaluation, and the results were taken as an average of the counts. Three histological sections from each animal were analyzed. The study and control groups were compared in order to establish the number of osteoclasts, osteoblasts, and capillaries as well as the number and intensities of inflammatory cells and the amount of new bone formation. The intensities were rated as mild (+, score = 1), moderate (++, score = 2), or strong (+++, score = 3).
Statistical Analysis
Data were analyzed using SPSS version 14.0 for Windows (SPSS Inc, Chicago, Ill). Differences among the three groups with regard to the number of osteoclasts and the number of capillaries were evaluated using the Kruskal-Wallis test. The parameters belonging to the number of osteoblasts, inflammatory cell infiltration, and new bone formation were represented as the scores indicating intensities. The scores of the groups were compared with the Fisher's exact test. A value of P < .05 was considered statistically significant.
RESULTS
During the experimental period, serious weight loss was not encountered in members of either the treated or the control group, nor was there any infection noted in any group during the study. Deep mucosal infection, dehiscence, and other adverse effects were not observed in any animals. However, two animals were excluded from the study as a result of appliance failure. They were replaced with two other rats. The midpalatal suture was successfully expanded following application of the activated helical spring, as determined via measurement of the distance between the maxillary incisors with a digital caliper.
Histological Findings
Number of osteoclasts
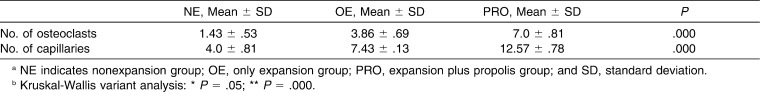
Osteoclast cells were found in all four groups, as shown in Table 1. The number of osteoclasts in the PRO group was significantly higher than that in the other groups (P < .05) (Table 1; Figure 2).
Table 1. .
Effects of Propolis on the Number of Osteoclasts and Capillaries at the End of the 12th Day of Experimental Periodab
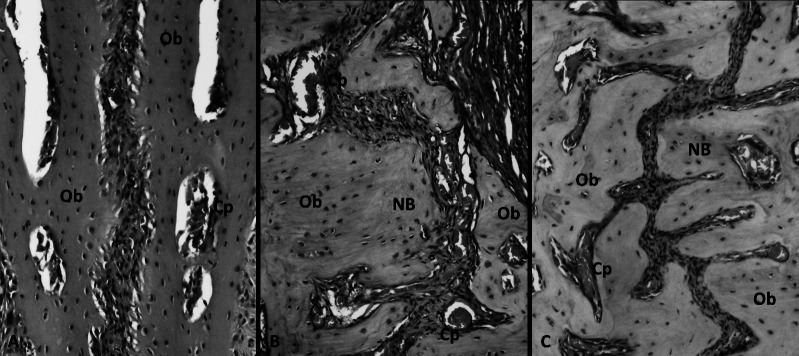
Figure 2. .
Hematoxylin and eosin (H&E) staining photomicrographs from the study groups. (A) Nonexpansion (NE) group; (B) only expansion (OE) group; (C) expansion plus propolis (PRO) group. Ob indicates osteoblast; NB, new bone; Cp, capillary; and S, midpalatal suture (200× original magnification).
Number of capillaries
The PRO group showed a significant increase in the number of capillaries relative to the other groups. The number of capillaries was also higher in the OE group than in the NE group (Table 1; Figure 2).
Number of osteoblasts
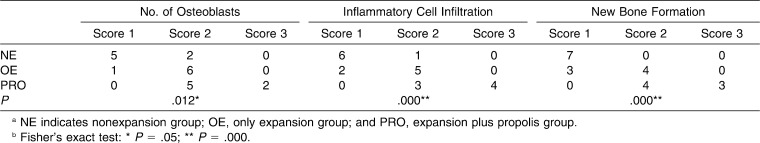
The histologic findings revealed that the number of osteoblasts was significantly higher in the PRO group than in the other groups (P < .05), indicating the increased activity. The lowest number of osteoblastic cells was found in the NE group, which represented the physiological situation (Table 2; Figure 2).
Table 2. .
Effects of Propolis on the Number of Osteoblasts, Inflammatory Cell Infiltration, and New Bone Formation. Scores Indicate the Number of Subject Animals Representing that Scoreab
New Bone Formation
The groups were compared based on new bone formation, and considerable differences were found among the groups. The results showed that there was an increase in new bone formation in the PRO group that was significantly greater than that observed in the other groups. The OE group also showed increased growth exceeding that of the NE group (Table 2; Figure 2).
Inflammatory Cell Infiltration
Intensities of inflammatory cells in the PRO group were significantly higher than those found in the OE and NE groups, respectively. The intensity of inflammatory cells was also found to be higher in the OE group relative to the NE group (Table 2; Figure 2).
DISCUSSION
The aim of this study was to investigate the possible effects of propolis on new bone formation in the expanded midpalatal suture. To the best of our knowledge, this study is the first to report the positive effects of propolis intake.
For experimental RME, the rat model of maxillary expansion is a well-established model. Although the maxillary sutures of cats and monkeys resemble those of humans more closely, rats and rabbits are considered to be suitable for animal models designed to achieve a clear picture of bony and sutural changes under stress.6
Several authors have measured the distance between the mesial corners of the maxillary incisors at the beginning and on the fifth day of the expansion with a caliper.3 Since it has been clearly defined that the 5-day expansion period is enough to allow sutural expansion,3 the distance between the maxillary incisors was not measured in our study, but the researchers did confirm the required space between the incisor teeth of all subjects. Furthermore, it was also confirmed by a histologist during histomorphometric evaluation that the required amount of expansion was observed in the palatal sutures of all animals.
In the literature, numerous researchers and studies have demonstrated a correlation between oxidative stress and bone metabolism. Oxidative stress caused by excessive generation of intracellular reactive oxygen species (ROS) can exert adverse biological effects on bone through inhibition of bone cell differentiation in the preosteoblastic cell line and in the marrow of the stromal cell line. ROS can also directly promote osteoclast formation and activity.21 Moreover, ROS and tumor necrosis factor–α also suppress osteoblastic differentiation.22 Thus, considering the detrimental effect of oxidants, various host-modulatory agents, including antioxidants, have been widely investigated for their ability to cope with the oxidant-related breakdown of hard tissues and for their possible role in promotion of bone healing. Enhanced new bone formation with antioxidants such as vitamin D analog,3 thymoquinone,4 and boron8 has been demonstrated in the literature.
Propolis and its constituents have been widely investigated as a result of their antioxidant effects. Guney et al.12 investigated the effects of propolis on the oxidant-antioxidant system and found that the plasma levels of endogenous antioxidants decreased in association with propolis administration. The authors explained that this situation represents the reduced need for endogenous antioxidants due to the effect of propolis, an exogenous antioxidant.
Although Kumazawa et al.23 and Scheller et al.24 related the powerful antioxidant properties of propolis to its kemferol and phenyl cafeate content, flavonoids are known to be the most effective and abundant antioxidant substances in propolis. Moreno et al.25 showed a significant association between antioxidant activity and flavonoid content for Argentinean propolis. On the other hand, substances other than flavonoids have been reported to be the contributors to the antioxidant properties of propolis. Stojko et al.26 evaluated the influence of EEP on regeneration of bone tissue at a defected area and observed that EEP accelerated the rate of ossification.
The composition of propolis, which contains a variety of chemical compounds, depends on the vegetation at the site of collection. Propolis samples from Europe, South America, and Asia have different chemical compositions depending on the geographical differences that lead to the display of different biological activities.27 Compared to Brazilian propolis, which contains 2% to 4% flavonoids, European propolis contains an average of 20% to 25% of this substance.28 Kumazawa et al.23 investigated the in vitro antioxidant activity of various propolis samples from various geographic origins and concluded that it was difficult to evaluate the quality of propolis without the knowledge of the constituents and quantitative values in propolis.
Several studies examined the effects of Anatolian propolis on various tissues, including the bone.12 Uzel et al.29 investigated the chemical components of four different Anatolian propolis samples; the one from the Trabzon region (European-Siberian, phytogeographic region) had been found to contain the highest concentrations of pinocembrin (a flavonoid compound).
According to the literature, adult patients would benefit from an antioxidant-rich diet prior to and after surgery. It has been reported30 that negative effects of free oxygen radicals seem to be more prominent during the early phases of the healing period, since antioxidants have been found to be most effective during this phase. In our study, the systemic application of propolis extract after the expansion period was similar to the methods of studies8 that surveyed bone healing after expansion. Our results indicated a significant increase in bone formation in the PRO group relative to the control group.
Propolis is considered to be safe at low dosages. However, adverse effects are common at dosages >15 g/d in human and animal studies.31,32 The most common adverse effects are allergic reactions and skin or mucous membrane irritations.32 In our study we used propolis at a dose of 100 mg/kg and found no adverse effects.
In this study, the effect of propolis on bone formation was investigated histomorphologically. Additionally, it is also possible to evaluate the bone mineral density reflecting the quality of the new callus tissue via the dual-energy x-ray absorptiometry (DEXA) method. In the study of Guney et al.,12 DEXA and x-ray findings showed significantly better fracture healing with propolis treatment, and these findings were confirmed by the results of histopathological examination. DEXA might also have provided a better option in the present study in terms of evaluating the quality of bone as well. Nevertheless, we would likely obtain findings that are correlated with the histomorphological results.
Our results indicated that the number of osteoblasts and, accordingly, the amount of new bone formation were increased in the PRO group relative to the controls. This corroborates the results of the study of Guney et al.12 We found that the number of osteoclasts was also higher in the PRO group. This elevation in the number of osteoclasts is most likely related to the acceleration of bone turnover. These results demonstrated that systemic administration of 100 mg/kg of PRO can promote bone formation and may be effective in the prevention of relapse following the RME procedure.
Today various forms of propolis are available, including tablets, capsules, chewing granules, soluble granules, throat pastilles, and chewing gums. Orthodontic patients and their parents may be more willing to use propolis than the other bone-inducing materials or methods because propolis is an entirely natural and inexpensive product and has no known side effects, other than mild allergic reactions.
CONCLUSION
Systemic use of propolis may hasten new bone formation at the expanded suture in rats.
REFERENCES
- 1.McNamara JA, Brudon WL. Orthodontics and Dentofacial Orthopedics. Ann Arbor, Mich: Needham Press; 1995. pp. 211–212. [Google Scholar]
- 2.Iseri H, Ozsoy S. Semirapid maxillary expansion—a study of long-term transverse effects in older adolescents and adults. Angle Orthod. 2004;74:71–78. doi: 10.1043/0003-3219(2004)074<0071:SMESOL>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 3.Uysal T, Amasyali M, Enhos S, Sonmez MF, Sagdic D. Effect of ED-71, a new active vitamin D analog, on bone formation in an orthopedically expanded suture in rats. A histomorphometric study. Eur J Dent. 2009;3:165–172. [PMC free article] [PubMed] [Google Scholar]
- 4.Kara IM, Erciyes K, Altan AB, Ozkut M, Ay S, Inan S. Thymoquinone accelerates new bone formation in the rapid maxillary expansion procedure. Arch Oral Biol. 2012;57(4):357–363. doi: 10.1016/j.archoralbio.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 5.Joondeph DR. Stability, retention and relapse. In: Graber LW, Vanarsdall RL, Vig KWL, editors. Orthodontics—Current Principles and Techniques 5th ed. Philadelphia, Pa: Mosby; 2012. pp. 1002–1003. [Google Scholar]
- 6.Saito S, Shimizu N. Stimulatory effects of low-power laser irradiation on bone regeneration in midpalatal suture during expansion in the rat. Am J Orthod Dentofacial Orthop. 1997;111:525–532. doi: 10.1016/s0889-5406(97)70152-5. [DOI] [PubMed] [Google Scholar]
- 7.Liu SS, Opperman LA, Buschang PH. Effects of recombinant human bone morphogenetic protein-2 on midsagittal sutural bone formation during expansion. Am J Orthod Dentofacial Orthop. 2009;136:768.e1–768.e8. doi: 10.1016/j.ajodo.2009.03.035. [DOI] [PubMed] [Google Scholar]
- 8.Uysal T, Ustdal A, Sonmez MF, Ozturk F. Stimulation of bone formation by dietary boron in an orthopedically expanded suture in rabbits. Angle Orthod. 2009;79:984–990. doi: 10.2319/112708-604.1. [DOI] [PubMed] [Google Scholar]
- 9.Ozturk F, Babacan H, Gümüş C. Effects of zoledronic acid on sutural bone formation: a computed tomography study. Eur J Orthod. 2012;34:141–146. doi: 10.1093/ejo/cjq160. [DOI] [PubMed] [Google Scholar]
- 10.Carson DA, Ribeiro JM. Apoptosis and disease. Lancet. 1993;341:1251–1254. doi: 10.1016/0140-6736(93)91154-e. [DOI] [PubMed] [Google Scholar]
- 11.Ozdemir H, Kara MI, Erciyas K, Ozer H, Ay S. Preventive effects of thymoquinone in a rat periodontitis model: a morphometric and histopathological study. J Periodont Res. 2012;47:74–80. doi: 10.1111/j.1600-0765.2011.01406.x. [DOI] [PubMed] [Google Scholar]
- 12.Guney A, Karaman I, Mithat Oner M, Yerer MB. Effects of propolis on fracture healing: an experimental study. Phytother Res. 2011;25:1648–1652. doi: 10.1002/ptr.3470. [DOI] [PubMed] [Google Scholar]
- 13.Molan P. Why honey is effective as a medicine. Part 2. The scientific explanation of its effects. Bee World. 2001;82:22–40. [Google Scholar]
- 14.Neiva Moreno MI, Isla MI, Cudmani NG, Vattuone MA, Sampietro AR. Screening of antibacterial activity of Amaicha del Valle (Tucuman, Argentina) propolis. J Ethnopharmacol. 2009;68:97–102. doi: 10.1016/s0378-8741(99)00051-3. [DOI] [PubMed] [Google Scholar]
- 15.Kujumgiev A, Tsvetkova I, Serkedjieva Y, Bankova V, Christov R, Popov S. Antibacterial, anti-fungal and antiviral activity of propolis of different geographic origin. J Ethnopharmacol. 1999;64:235–240. doi: 10.1016/s0378-8741(98)00131-7. [DOI] [PubMed] [Google Scholar]
- 16.Wang L, Mineshita S, Ga I, Shigematsu T, Matsuno T. Anti-inflammatory effect of propolis. Jpn J Pharmacol Ther. 1993;24:223–224. [Google Scholar]
- 17.Banskota AH, Tezuka Y, Kadota S. Recent progress in pharmacological research of propolis. Phytother Res. 2001;15:561–571. doi: 10.1002/ptr.1029. [DOI] [PubMed] [Google Scholar]
- 18.Ahn MR, Kumazawa S, Usui Y, Nakamura J, Matsuka M, Zhu F, Nakayama T. Antioxidant activity and constituents of propolis collected in various areas of China. Food Chem. 2007;101:1383–1392. [Google Scholar]
- 19.Sa-Nunes A, Faccioli LH, Sforcin JM. Propolis: lymphocyte proliferation and IFN-gamma production. J Ethnopharmacol. 2003;87:93–97. doi: 10.1016/s0378-8741(03)00121-1. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi O. Histological investigations on the effect of interrupted expansion force applied to the midpalatal suture in the rat. Nihon Univ J Oral Sci. 1990;16:212–236. [PubMed] [Google Scholar]
- 21.Bai XC, Lu D, Bai J, Zheng H, Ke ZY, Li XM. Oxidative stress inhibits osteoblastic differentiation of bone cells by ERK and NF-kappaB. Biochem Biophys Res Commun. 2004;314:197–207. doi: 10.1016/j.bbrc.2003.12.073. [DOI] [PubMed] [Google Scholar]
- 22.Lean JM, Davies JT, Fuller K, Jagger CJ, Kirstein B, Partington GA. A crucial role for thiol antioxidants in estrogen-deficiency bone loss. J Clin Invest. 2003;112:915–923. doi: 10.1172/JCI18859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumazawa S, Hamasaka T, Nakayama T. Antioxidant activity of propolis of various geographic origin. Food Chem. 2004;84:329–339. [Google Scholar]
- 24.Scheller S, Wilczok T, Imielski S, et al. Free radical scavenging by ethanol extract of propolis. Int J Radiat Biol. 1990;57:461–465. doi: 10.1080/09553009014552601. [DOI] [PubMed] [Google Scholar]
- 25.Moreno MI, Isla MI, Sampietro AR, Vattuone MA. Comparison of the free radical-scavenging activity of propolis from several regions of Argentina. J Ethnopharmacol. 2000;71:109–114. doi: 10.1016/s0378-8741(99)00189-0. [DOI] [PubMed] [Google Scholar]
- 26.Stojko A, Scheller S, Szwarnowiecka I, Tustanowski J, Ostach H, Obuszko Z. Biological properties and clinical application of propolis. VIII. Experimental observation on the influence of ethanol extract of propolis (EEP) on the regeneration of bone tissue. Arzneimittelforschung. 1978;28:35–37. [PubMed] [Google Scholar]
- 27.Marcucci MC. Propolis: chemical composition, biological properties and therapeutic activity. Apidologie. 1995;26:83–99. [Google Scholar]
- 28.Aliyazicioglu Y, Deger O, Ovali E, Barlak Y, Hosver I, Tekelioglu Y, Karahan SC. Effects of Turkish pollen and propolis extracts on respiratory burst for K-562 cell lines. Int Immunopharmacol. 2005;5:1652–1657. doi: 10.1016/j.intimp.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 29.Uzel A, Sorkun K, Onçağ O, Cogŭlu D, Gençay O, Salih B. Chemical compositions and antimicrobial activities of four different Anatolian propolis samples. Microbiol Res. 2005;160:189–195. doi: 10.1016/j.micres.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 30.Gokturk E, Turgut A, Baycu C, et al. Oxygen-free radicals impair fracture healing in rats. Acta Orthop Scand. 1995;66:473–475. doi: 10.3109/17453679508995590. [DOI] [PubMed] [Google Scholar]
- 31.Castaldo S, Capasso F. Propolis, an old remedy used in modern medicine. Fitoterapia. 2003;73(suppl 1):S1–S6. doi: 10.1016/s0367-326x(02)00185-5. [DOI] [PubMed] [Google Scholar]
- 32.Toker H, Ozan F, Ozer H, Ozdemir H, Eren K, Yeler H. A morphometric and histopathologic evaluation of the effects of propolis on alveolar bone loss in experimental periodontitis in rats. J Periodontol. 2008;79:1089–1094. doi: 10.1902/jop.2008.070462. [DOI] [PubMed] [Google Scholar]