Abstract
Two methods of inoculum preparation for filamentous fungi were compared: counting with a hematocytometer and spectrophotometric adjustment. One hundred eighty-two filamentous fungi pathogenic for humans were used. Colony counts were done for all inoculum preparations. The agreement between the hematocytometer counts and the colony counts (CFU per milliliter) was 97.2%. The reproducibility between the hematocytometer counts and the colony counts by means of an intraclass correlation coefficient was 0.70. Pearson's correlation index for hematocytometer counts versus colony counts was 0.56, whereas that for optical density versus colony counts was 0.008. Both methods can be used for inoculum size adjustment. However, the use of the spectrophotometric method requires that each species be standardized separately.
An increasing prevalence of infections caused by filamentous fungi has been detected in humans in recent years (1). This increasing prevalence has stimulated interest in a reliable and reproducible antifungal susceptibility testing method for molds. Recently, the National Committee for Clinical Laboratory Standards (NCCLS) proposed a reference method (the NCCLS M38-P method) for the susceptibility testing of conidium-forming filamentous fungi (7). The NCCLS method recommends inoculum preparation by a spectrophotometric procedure (2–4, 7). However, it is well known that larger objects such as spores or filaments do not obey the rule that established a proportional relationship between dry weight concentration and optical density (6). In addition, the inoculum size has a great influence on the MICs for filamentous fungi (3–5, 7). Other variables such as the color of the spores can also influence the optical density value.
In the work described here, two procedures for inoculum size adjustment were evaluated, and the results were compared with those obtained by colony counting.
MATERIALS AND METHODS
Isolates.
A set of clinical isolates was tested. Each isolate was obtained from a different patient and was sent to the laboratory for identification or antifungal susceptibility testing.
The following species were included: 31 Aspergillus fumigatus, 28 Aspergillus flavus, 25 Aspergillus terreus, 16 Aspergillus niger, 18 Scedosporium apiospermum, 27 Scedosporium prolificans, 24 Fusarium solani, and 13 Fusarium oxysporum isolates. The isolates were maintained as a suspension in sterile distilled water at 4°C until testing was performed.
Inoculum preparation.
The isolates were subcultured from the stock water suspensions on Sabouraud agar and on potato dextrose agar. Isolates of all species except members of the genus Fusarium were incubated at 35°C; members of the genus Fusarium were incubated at 30°C. Inoculum suspensions were prepared from fresh, mature (3- to 5-day-old) cultures grown on Sabouraud agar or potato dextrose agar slants. The colonies were covered with 5 ml of distilled sterile water. Tween 20 (5%) was added to facilitate the preparation of Aspergillus inocula. For Aspergillus spp., the inocula were achieved by carefully rubbing the colonies with a sterile loop; the isolates were then shaken vigorously for 15 s with a Vortex mixer and then transferred to a sterile tube. For slowly sporulating fungi, such as Scedosporium and Fusarium, the suspensions were obtained by exhaustive scraping of the surface with a sterile loop. Then, the inoculum was transferred to a sterile syringe attached to a sterile filter with a pore diameter of 11 μm (Millipore, Madrid, Spain). The suspension was filtered and collected in a sterile tube. This procedure removed the majority of the hyphae, producing a inoculum mainly composed of spores.
Inoculum adjustment.
The inoculum size was adjusted to between 1.0 × 106 and 5.0 × 106 spores/ml by microscopic enumeration with a cell-counting hematocytometer (Neubauer chamber; Merck, S.A., Madrid, Spain). Then, the optical density at 530 nm (OD530) of the suspensions was measured in a spectrophotometer (Bausch & Lomb, Pacisa, Madrid, Spain). All adjusted suspensions were quantified by plating on Sabouraud agar plates. The inocula were agitated for 15 s with a Vortex mixer and were diluted 1:1,000. Aliquots of 100, 50, and 25 μl were spread onto the Sabouraud agar plates with a glass hockey stick. The plates were incubated at 35°C (Aspergillus and Scedosporium spp.) or 30°C (Fusarium spp.) and were observed daily for the presence of growth. The colonies were counted as soon as possible after the observation of visible growth.
Statistical analysis.
The proper inoculum size range, according to previous work (2), was established to be between 1.0 × 106 and 5.0 × 106 CFU/ml. The percent agreement between inoculum sizes determined by counting with a hematocytometer and colony counting was calculated by taking into account the colony counts in a range between 1.0 × 106 and 5.0 × 106 CFU/ml.
The reproducibility of the results obtained by counting with a hematocytometer and colony counting was evaluated by using the intraclass correlation coefficient (ICC). Reproducibility was calculated by means of a scales analysis in which reliability was the extent to which the counting with a hematocytometer yielded an equivalent colony count. The ICC assesses reliability as an internal consistency statistic by means of interitem correlations. A two-way mixed-effect model was used to calculate the ICC, which was expressed to a maximum value of 1 and with a confidence interval (CI) of 95%. When appropriate, the variables were transformed to log10 data. In addition, the correlation among the hematocytometer count; the colony count, and the OD530 was also determined with Pearson's coefficient (r), which was also expressed to a maximum value of 1. All statistical analyses were done with the Statistical Package for the Social Sciences (version 10.0; SPSS, S.L., Madrid, Spain).
RESULTS AND DISCUSSION
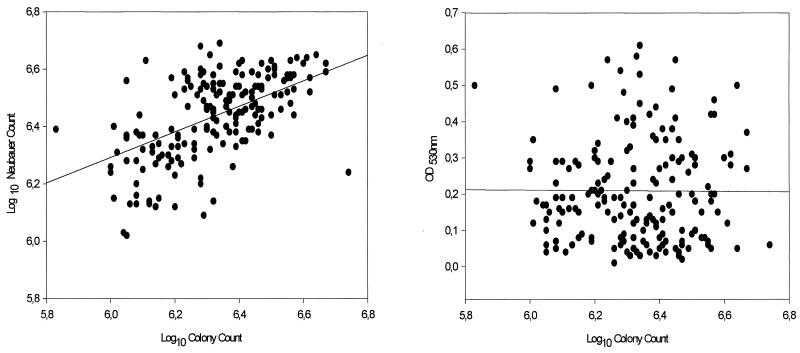
Table 1 shows the range, 95% CI, ICC, and Pearson's correlation index (r) of the colony counts versus the hematocytometer counts and OD530s. The percent agreement between the hematocytometer counts and the colony counts was 97.2%. As expected, some values for the colony counts were out of the established range (1.0 × 106 to 5.0 × 106 CFU/ml). The colony count for one A. fumigatus isolate was above 5.0 × 106 CFU/ml. The colony counts for one A. niger isolate, one S. prolificans isolate, and two F. solani isolates were below 1.0 × 106 CFU/ml. Nevertheless, the 95% CIs of the colony counts were between 1.0 × 106 and 5.0 × 106 CFU/ml for all isolates tested (Table 1). Pearson's correlation indices between hematocytometer counts and colony counts were low for A. flavus (r = 0.24) and A. fumigatus (r = 0.26). For the remainder of the filamentous fungi, Pearson's correlation indices were above 0.57 (Table 1). This fact can be explained by the hydrophobic nature of Aspergillus species, which causes more dilution mistakes with aspergillus species than with hydrophilic filamentous fungi. The exceptions were A. niger (r = 0.71) and A. terreus (r = 0.58). The reproducibility, analyzed by determination of ICC, between the hematocytometer counts and the colony counts was adequate for all species tested. Thus, an ICC of 0.70 and 95% CIs between 0.60 and 0.78 were obtained (Table 1 and Fig. 1). On the other hand, Pearson's correlation index for hematocytometer counts versus colony counts was 0.56 (Fig. 1 and Table 1). Pearson's correlation indices between the OD530s and the colony counts were lower than those obtained by enumeration with a hematocytometer. The exceptions were A. flavus (r = 0.49) and A. fumigatus (r = 0.38). A larger range of OD530s was obtained for each different species. As expected, the range of OD530s was species dependent. Thus, among Aspergillus species, A. fumigatus had an OD530 range between 0.03 and 0.13 absorbance units. On the other hand, the range for A. niger was between 0.20 and 0.50 absorbance units. The size of the spores (A. fumigatus, 2.5 to 3.0 μm; A. niger, 3.5 to 5.0 μm) and the color of the species (green versus black, respectively) can explain the differences in the OD530. In addition, each species of filamentous fungi does not have a uniform color. These differences in color tonalities can influence the OD530 and can explain the large range of values obtained for each species (Table 1). However, the 95% CI showed a narrow range, sustaining the possibility that the inoculum can be prepared by this procedure. Nevertheless, a correlation index of 0.008 was obtained when all strains were analyzed together, suggesting that each species must be standardized independently (Table 1 and Fig. 1). NCCLS document M38-P (7) recommends the spectrophotometric adjustment of the inoculum. The original article (2) showed that at certain OD530s the size of inoculum was between 1.0 × 106 and 5.0 × 106 CFU/ml (2). Unfortunately, the number of strains used was small, precluding an adequate statistical analysis (2). Further work showed that only 90% of the inocula prepared by the spectrophotometric procedure had between 4.0 × 105 and 3.3 × 106 CFU/ml (4). Observations described in a subsequent article (3) sustained the previous observation. Thus, only 87% of the inoculum preparations for S. apiospermum had between 4.0 × 105 and 3.2 × 106 CFU/ml. For Aspergillus, Fusarium, and Rhizopus species, more than 92% of the the inoculum suspensions had between 4.0 × 105 and 5.0 × 106 CFU/ml (3).
TABLE 1.
Range, 95% CI, correlation index, and ICC among colony counts, hematocytometer counts, and OD530s
| Species | No. of isolates tested | Colony count (CFU/ml [106])
|
Hematocytometer count (CFU/ml [106])
|
ra | ICC (95% CI)b | OD530
|
rc | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Range | 95% CI | Range | 95% CI | Range | 95% CI | |||||
| A. fumigatus | 31 | 1.11–5.55 | 1.97–2.67 | 1.40–4.40 | 2.75–3.42 | 0.26 | 0.44 (−0.15–0.73) | 0.03–0.13 | 0.06–0.08 | 0.38 |
| A. flavus | 28 | 1.02–3.69 | 1.70–2.26 | 1.85–3.92 | 2.48–2.94 | 0.24 | 0.35 (−0.40–0.69) | 0.07–0.27 | 0.16–0.19 | 0.49 |
| A. terreus | 25 | 1.21–4.32 | 2.39–2.94 | 1.81–4.48 | 2.78–3.41 | 0.58 | 0.73 (0.40–0.88) | 0.01–0.15 | 0.05–0.08 | 0.18 |
| A. niger | 16 | 0.45–4.36 | 1.72–2.86 | 1.31–4.46 | 2.19–3.27 | 0.71 | 0.80 (0.44–0.93) | 0.20–0.50 | 0.29–0.39 | 0.42 |
| S. apiospermum | 18 | 1.01–2.73 | 1.43–1.94 | 1.04–4.10 | 1.97–2.81 | 0.76 | 0.85 (0.60–0.94) | 0.13–0.50 | 0.25–0.36 | 0.44 |
| S. prolificans | 27 | 0.61–4.70 | 1.93–2.67 | 1.07–4.90 | 2.38–3.14 | 0.61 | 0.75 (0.47–0.89) | 0.15–0.53 | 0.26–0.34 | 0.42 |
| F. solani | 24 | 0.55–3.84 | 1.60–2.42 | 1.22–4.79 | 2.50–3.32 | 0.57 | 0.70 (0.31–0.87) | 0.13–0.61 | 0.23–0.36 | 0.01 |
| F. oxysporum | 13 | 1.45–4.71 | 2.24–3.42 | 1.65–3.98 | 2.43–3.40 | 0.90 | 0.94 (0.81–0.98) | 0.09–0.57 | 0.13–0.28 | 0.42 |
| Total | 182 | 0.45–5.55 | 2.11–2.37 | 1.04–4.90 | 2.72–2.97 | 0.56 | 0.70 (0.60–0.78) | 0.01–0.61 | 0.18–0.22 | 0.008 |
Pearson's correlation index for colony counts versus hematocytometer counts.
ICC and 95% CI for colony counts versus hematocytometer counts.
Correlation index for colony counts versus OD530.
FIG. 1.
Scatter plots of log10 colony counts versus log10 hemacytometer (Neubauer) counts (left) (r = 0.56; ICC = 0.70) and log10 colony counts versus OD530 (r = 0.008) (right) for 182 strains of filamentous fungi pathogenic for humans.
By the spectrophotometric method for inoculum preparation, the best scenario is retrieval of an inoculum that varies between 4 × 105 and 5 × 106 spores per ml. This wide range of sizes of inoculum suspensions could have a significant influence on MICs. Nevertheless, the level of agreement between laboratories that use NCCLS document M38-P (7) is adequate (3).
In any case, the range of OD530 obtained in the present work included the OD530 recommended by NCCLS document M38-P (7). However, proper identification of the filamentous fungi should be done by using the spectrophotometric method for inoculum preparation. Although many laboratories can identify usual pathogens like A. fumigatus, very experienced staff is needed for the identification of less common species.
In summary, two methodologies for inoculum adjustment have been compared by using 182 strains of fungi pathogenic for humans. The results indicate that both methodologies can be used. However, counting with a hematocytometer is a universal procedure that is independent of the color of the strain and the size of the spores. Use of this procedure could save a lot of time in the process of standardization of antifungal susceptibility testing of filamentous fungi.
ACKNOWLEDGMENTS
This work was partially supported by a grant from the EC-TMR-EUROFUNG network (grant ERBFMXR-CT970145) and by a grant from the Fondo de Investigaciones Sanitarias, Instituto de Salud Carlos III (grant 99/0198). Alicia Gómez is a fellow of the Instituto de Salud Carlos III (grant 99/0198).
REFERENCES
- 1.Denning D W. Invasive aspergillosis. Clin Infect Dis. 1998;26:781–803. doi: 10.1086/513943. [DOI] [PubMed] [Google Scholar]
- 2.Espinel-Ingroff A, Kerkering T M. Spectrophotometric method of inoculum preparation for the in vitro susceptibility testing of filamentous fungi. J Clin Microbiol. 1991;29:393–394. doi: 10.1128/jcm.29.2.393-394.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Espinel-Ingroff A, Bartlett M, Bowden R, Chin N X, Cooper C, Jr, Fothergill A, McGinnis M R, Menezes P, Messer S A, Nelson P W, Odds F C, Pasarell L, Peter J, Pfaller M A, Rex J H, Rinaldi M G, Shankland G S, Walsh T J, Weitzman I. Multicenter evaluation of proposed standardized procedure for antifungal susceptibility testing of filamentous fungi. J Clin Microbiol. 1997;35:139–143. doi: 10.1128/jcm.35.1.139-143.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Espinel-Ingroff A, Dawson K, Pfaller M, Anaissie E, Breslin B, Dixon D, Fothergill A, Paetznick V, Peter J, Rinaldi M, Walsh T. Comparative and collaborative evaluation of standardization of antifungal susceptibility testing for filamentous fungi. Antimicrob Agents Chemother. 1995;39:314–319. doi: 10.1128/aac.39.2.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gehrt A, Peter J, Pizzo P A, Walsh T J. Effect of increasing inoculum sizes of pathogenic filamentous fungi on MICs of antifungal agents by broth microdilution method. J Clin Microbiol. 1995;33:1302–1307. doi: 10.1128/jcm.33.5.1302-1307.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koch A L. Growth measurement. In: Gerhardt P, Murray G E, Costilow R N, Nester E W, Wood W A, Krieg N R, Phillips G B, editors. Manual of methods for general bacteriology. Washington, D.C.: American Society for Microbiology; 1981. pp. 179–207. [Google Scholar]
- 7.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of conidium-forming filamentous fungi. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1998. [Google Scholar]