Abstract
Introduction:
Implantable Doppler devices are reliable adjuncts used for free flap monitoring. Occasionally, the probe/wire is not removed and remains in the soft tissues. The clinical safety of the retained probes and safety and compatibility with magnetic resonance imaging (MRI) have not been studied. We present a series of retained implantable Doppler probes examining clinic outcomes, safety and compatibility with MRI, and effect on MRI image quality.
Methods:
A retrospective review was conducted of patients who had an implantable Doppler device for free flap monitoring between July 2007 and August 2018. Routine post-operative imaging was reviewed for all patients to identify incidental findings of a retained probe. A subset of patients with retained implantable Doppler probes who underwent MRI was identified. Magnetic resonance images were reviewed to detect any degradation of image quality.
Results:
A total of 323 patients who had an implantable Doppler device placed were reviewed 18 (5.6%) patients were identified with a retained probe and were included in this study. Mean age was 49 years with mean follow-up of 34.4 months. One potential device-related complication occurred in 1 (5.6%) patient. A total of 32 MRI scans were performed in 8 patients with retained devices, including 6 patients who underwent a total of 21 MRIs of the surgical site. There were no complications related to the MRI scans, and we found no significant degradation of image quality.
Conclusion:
Retained implantable Doppler probes were not associated with substantial adverse clinical outcomes nor affected MRI image quality of the surgical site.
Keywords: microsurgery, free flap, implantable Doppler, Cook-Swartz, flap monitoring
Abstract
Introduction:
Les dispositifs de Doppler implantables sont fiables pour compléter la surveillance des lambeaux libres. Il arrive que la sonde ou le fil ne soit pas retiré et demeure dans les tissus mous. La sécurité clinique de ces sondes et leur compatibilité avec l’imagerie par résonance magnétique n’ont pas fait l’objet d’études. Les auteurs examinent les résultats cliniques d’une série de sondes de Doppler implantables laissées dans les tissus, de même que leur sécurité, leur compatibilité avec l’IRM et leur effet sur la qualité de l’image d’IRM.
Méthodologie:
Les chercheurs ont effectué une analyse rétrospective des patients à qui on avait implanté un dispositif de Doppler pour surveiller un lambeau libre entre juillet 2007 et août 2018. Ils ont analysé l’imagerie postopératoire systématique de tous les patients pour trouver les observations fortuites de sonde laissée dans les tissus. Ils ont extrait un sous-groupe de patients qui présentaient une sonde de Doppler implantable laissée dans les tissus et ont examiné l’IRM pour déceler toute dégradation de la qualité de l’image.
Résultats:
Sur un total de 323 patients à qui on avait implanté un dispositif de Doppler, 18 (5,6%) présentaient une sonde laissée dans les tissus et ont été inclus dans l’étude. D’un âge moyen de 49 ans, ils avaient reçu un suivi moyen de 34,4 mois. Un patient (5,6%) a subi une complication susceptible d’avoir été causée par le dispositif. Au total, les chercheurs ont effectué 32 IRM chez huit patients dont une partie du dispositif avait été laissée dans les tissus, y compris six patients qui ont subi un total de 21 IRM au foyer chirurgical. Ils n’ont constaté aucune complication liée à l’IRM et aucune dégradation importante de la qualité de l’image.
Conclusion:
Les sondes de Doppler implantable laissées dans les tissus n’entraînaient pas de résultats cliniques indésirables importants ni ne nuisaient à la qualité de l’IRM au foyer chirurgical.
Introduction
Rigorous and close monitoring of free flaps is crucial for early identification of microvascular complications allowing prompt intervention and potentially higher salvage rates. 1 -4 Although clinical evaluation remains the gold standard for post-operative free flap monitoring, multiple devices are available for adjunctive monitoring. In 1988, Swartz et al first presented an implantable Doppler device for flap monitoring. 2,5 The Cook-Swartz (Cook Medical LLC) is a removable 20 MHz ultrasonic Doppler crystal probe within a nonremovable 8 × 5 mm silicone cuff film, which is wrapped around the vessel to be monitored. The probe is connected to a thin metallic wire, which exits the wound and is connected to a portable monitor at the patient bedside. 6 The device provides continuous blood flow information, and when vascular monitoring is no longer necessary, the implantable Doppler is removed by simply applying gentle traction. The probe is designed to detach from the cuff with 50 g of tension. 5 Removing the Cook-Swartz Doppler probe occasionally requires the use of more force, and in these cases, the metallic filament may be cut at the skin level and a portion of the wire is retained in the soft tissues. However, there is a concern regarding the foreign material left inside the wound. 5,7 A significant number of patients undergoing free tissue transfer may need magnetic resonance imaging (MRI) surveillance for recurrent malignancy or osteomyelitis, and the presence of retained metal probes may raise some level of concern for MRI safety and image quality due to a retained metallic body at the surgical site.
We present a series of retained implantable Doppler devices examining clinical outcomes, MRI safety, and image quality. To the best of our knowledge, no previous study has addressed post-operative clinical follow-up of patients with implantable Doppler device retained or MRI safety and changes in imaging quality.
Methods
Following institutional review board approval, a retrospective chart review was conducted of patients who underwent microvascular free tissue transfer and placement of an implantable Doppler probe from July 2007 to August 2018. Any available post-operative imaging of the surgical site was reviewed for all patients to identify incidental findings of a retained metal probe. Data related to patient demographics, post-operative complications, and surgical site complications. All subsequent MRI scans performed with a retained probe in place were reviewed for documentation of adverse events. The images were reviewed by a senior radiologist (M.F.) to detect any degradation of image quality. Descriptive statistics was reported in mean (range) and frequency (percentage).
Results
During the study period, implantable Doppler probes were placed in 323 free tissue transfer patients. Eighteen (5.6%) patients were identified with an incidental radiographic finding of a retained probe and were subsequently included in the analysis. Ten (55.5%) patients were male, 8 (45.5%) were female. The mean age at surgery 48.8 (range 25-67) years with a mean follow-up duration of 34.4 (range 2-122) months. The retained device was detected on various imaging modalities, including plain radiograph, mammogram, and computed tomography scans (Figures 1 and 2). The mean time from surgery to the earliest radiographic appearance was 20.8 (range 1.2-65.3) months. Of the patients included in this study, free tissue transfer was performed for esophageal reconstruction (n = 5), breast reconstruction (n = 5), extremity reconstruction (n = 5), and facial reconstruction (n = 3). Reconstruction was performed following tumor extirpation (n = 14) or reconstruction of traumatic defects (n = 4). Patient characteristics and post-operative complications are described in Table 1.
Figure 1.

A 56-year-old male patient underwent free jejunal flap for esophageal reconstruction. The surgical team attempted to remove the implantable Doppler device on the 10th post-operative day. An esophagram performed on the 35th post-operative day showed the implantable Doppler device retained (arrow).
Figure 2.

A 31-year-old female patient underwent sarcoma resection in the right ankle followed by free latissimus dorsi flap and split thickness skin graft. The surgical team attempted to remove the implantable Doppler device on the 49th post-operative day; however, the wire fractured just below the skin level. An x-ray performed at 3.6 months showed the retained wire (arrow).
Table 1.
Patient Characteristics and Post-Operative Complications.
| Characteristic | |
|---|---|
| Total number of patients with retained device | 18 (100%) |
| Gender | |
| Male | 10 (55.5%) |
| Female | 8 (44.5%) |
| Mean age (years old) | 48.8 (25-67) |
| Follow-up after surgery (months) | 34.4 (2-122.4) |
| Type of reconstruction | |
| Esophageal reconstruction | 5 (27.7%) |
| Breast reconstruction | 5 (27.7%) |
| Extremity reconstruction | 5 (27.7%) |
| Facial reconstruction | 3 (16.6%) |
| Indication for free tissue transfer | |
| Oncologic resection | 14 (77.8%) |
| Trauma/osteomyelitis | 4 (22.2%) |
| Mean time to radiological identification of the device after surgery (months) | 20.8 (1-65) |
| Post-op interval until attempted removal (days) | 36 (5-165) |
| Site of post-operative MRI | |
| Surgical site (retained probe) | 21 (0-9) |
| Other site | 11 (0-5) |
| Post-operative complications | |
| Related to probe | 1 |
| Unrelated to retained probe | 5 |
Abbreviation: MRI, magnetic resonance imaging.
Clinical Outcomes
In this series, removal of the device was attempted at a mean of 36 (range, 5-165) days following surgery. Potential device–related complications occurred in only 1 (5.5%) patient, who underwent lower extremity reconstruction after oncologic resection and subsequently developed chronic draining sinus 14 months after surgery. The etiology of the draining sinus was attributed to the retained filament and was successfully treated with removal of the retained filament. Five (27.7%) patients developed post-operative complication presumed unrelated to the Cook-Swartz device, such as minor superficial wound dehiscence, bloodstream infection, post-operative bleeding, arterial line infection, and osteomyelitis 10 years after a total knee arthroplasty. One (5.5%) patient who underwent breast reconstruction presented with shoulder pain 8 months the initial surgery, and an MRI was requested as part of her workup. Before the MRI was performed, the retained implantable Doppler device was localized with mammogram and surgically removed due to concerns about MRI safety and potential effects on imaging quality (Figure 3).
Figure 3.
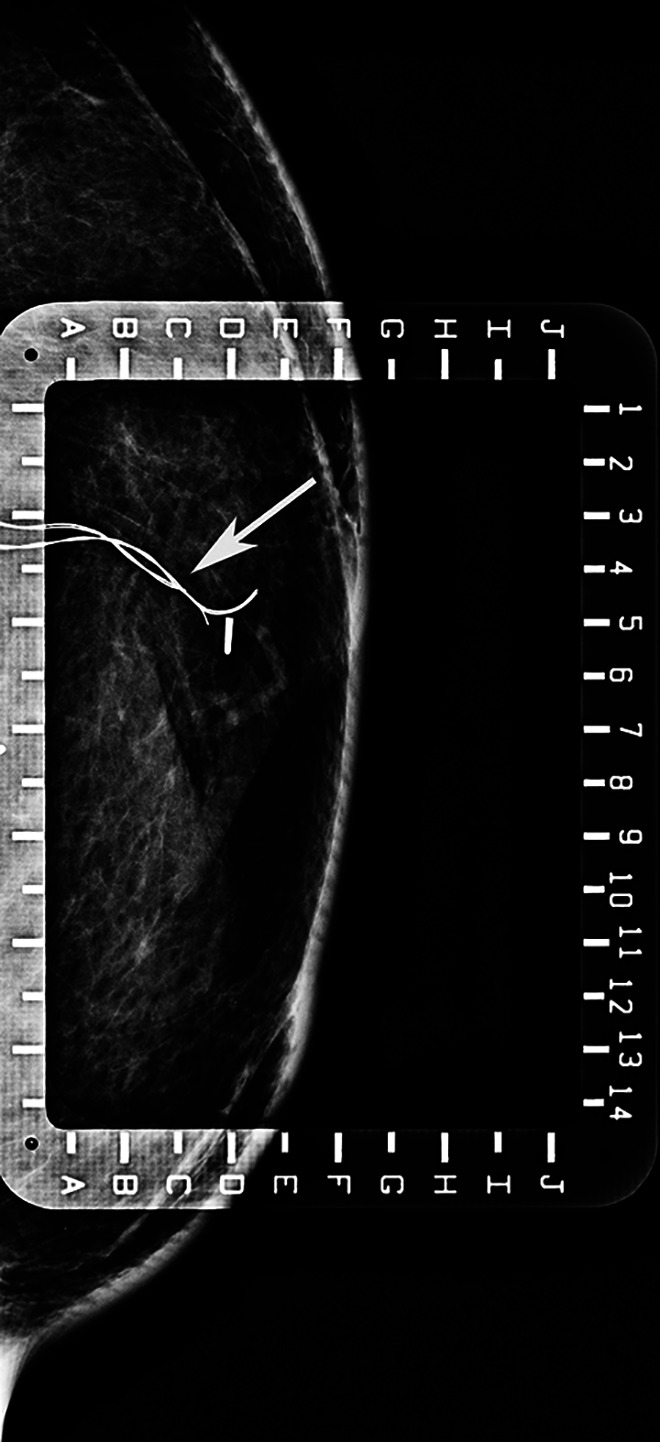
A 44-year-old woman had a bilateral DIEP flap for breast reconstruction. Post-operatively, a mammogram and an ultrasound demonstrated the presence of the implantable Doppler wires (arrow). The device was surgically removed due to concerns about MRI safety and potential effects on imaging quality. MRI indicates magnetic resonance imaging.
Radiologic Outcomes
A total of 32 MRIs were performed in 8 patients (44.4%) with retained devices. Six of 8 patients underwent a total of 21 MRIs of the surgical site. In these 8 patients, the number of MRI exams per patient ranged from 1 to 9 exams per patient. One patient initially had one MRI postponed due to concerns related to the retained device but later underwent the exam with no reported MRI-related complications. No patient who underwent MRI with a retained probe experienced any known MRI-related complications due to device heating or motion. Upon independent review of the quality of MRIs images by a senior musculoskeletal radiologist (M.F.), no significant image quality degradation was noted. In some patients, the retained device was not even detected on the MRI but was evident on other imaging studies such as x-ray, computed tomography scan, or mammogram.
Discussion
In this study, we present a series of 32 patients with a retained component of an implantable Doppler device with only 1 required intervention and without adverse events related to the MRI scan. When MRI was performed, the quality of the obtained images did not seem to be affected by the retained device as evident by lack of image quality degradation.
Reviewing the Manufacturer and User Facility Device Experience available on Food and Drug Administration (FDA) website, the authors identified a total of 28 reports related with Cook-Swartz complications. 8 The most frequent report was related to Cook-Swartz early detachment (13 reports), all requiring reoperation to inspect the flap and place another implantable Doppler. The second most common complication was malfunctioning device (8 reports), in which 6 of these cases required reoperation. The third most frequent complication was retained wire (5 reports), in which 1 patient developed subsequently infection requiring reoperation to remove the retained filament. The least frequent complication was failure to disconnect the implantable Doppler resulting in injury to the anastomosed vessels (2 reports), both cases required reoperation. 8 Based on our small series, the retained component of the implantable Doppler device does not seem to pose major clinical or radiological risks. We are not aware of other studies evaluating the clinical safety of retained implantable Doppler devices and a larger series is necessary to challenge or validate our findings.
The compatibility of the retained metal component with MRI is a concern, and the clinical consequences of probe/wire heating are largely unknown. The presence of metallic objects may limit MRI diagnostic utility as well as its safety. In our study, the majority of patients in whom part of the implantable Doppler device was retained are patients who underwent reconstructions following oncologic resections and tumor extirpation. Magnetic resonance imaging is a crucial surveillance method, and the inability to obtain MRI may negatively impact and hinder the required cancer surveillance. 9 Therefore, evaluating the safety of MRI and the impact of retained implantable Doppler devices on MRI imaging quality may help to address some of these concerns.
Some implanted devices, such as the implantable Doppler studied in this paper, are considered unsafe for MRI. 10,11 The major recognized mechanical risk associated with MRI scanner is the presence of ferromagnetic devices, including biomedical implants. The static field produces attractive and rotational forces on these devices, whose magnitude is related to their mass and distance from the bore entrance. 9,12,13 Another concerning aspect is that some implants, such as guide wires, can produce heating due to their interaction with the radiofrequency field. 9,14,15 Even though no major complication was reported in patients with the implantable Doppler who underwent MRI in our study, the retained implantable Doppler eventually may heat during the MRI exam. We did not find studies reporting the implantable Doppler’s magnetic field interactions. Other implantable devices, such as coils, stents, filters, and vascular grafts have demonstrated magnetic field interactions; however, 6 weeks after implantation, these devices are incorporated in tissue due to tissue ingrowth and other mechanisms. 11 According to Shellock, it is unlikely that implants will move or dislodge due to MRI exposure, and there is no reported case of these types of implants heating excessively due to MRI exposure. 11,16
Regarding MRI quality, metallic devices can produce artefact, which consists of a feature appearing in an image which actually is not present in the real object. 17 This occurs due to magnetic differences between the metal device and the adjacent tissue. 17,18 Multiple factors may influence the artefact formation such as size of the implant itself, the MRI field strength (1.5 Tesla vs 3 Tesla), the MRI protocol, and sequence parameters. 18,19 In the present study, no significant degradation of MRI quality was perceived by a senior radiologist. The small size of the implantable Doppler and the round symmetric cross-sectional area of the metal implant likely limits the magnitude of artefact formation. 9
The present study has several limitations. Identification of retained probes was based on their presence on post-operative imaging; however, many patients who underwent free flap reconstruction with implantable Doppler monitoring did not have any imaging exams of the surgical site performed post-operatively. The prevalence of retained devices reported in our study (5.6%) is therefore likely an underestimate. Additionally, there is a relatively small sample size of patients who underwent MRI scans with a retained device in place and our study lacks a control cohort. Further studies of implantable Doppler properties, such as the radiofrequency field and heating characteristics should be performed before the implantable Doppler device can be considered absolutely safe in patients undergoing MRI scans with a retained device. Despite these limitations, this study highlights critical clinical and radiological aspects of retained implantable Doppler devices that have not been addressed in previous studies.
Conclusion
Retained implantable Doppler devices were not associated with substantial adverse clinical outcomes following free tissue transfer for extremity, face, breast, and esophageal reconstruction. Retained filaments did not affect MRI image quality of the surgical site. Additionally, no patient who underwent MRI with a retained probe experienced any MRI-related complications due to heating or motion. Nevertheless, the implantable Doppler device may heat during MRI, and if this imaging exam is to be considered in situations with a known retained probe, we recommend that patients should be awake and communicative for the study in order to be able to report any complication.
Footnotes
Authors’ Note: Approval was obtained from the local ethics committee. Informed consent was obtained from all patients included in this study. This manuscript was presented (podium presentation) at the Plastic Surgery Research Council (PSRC), in May 2019, Baltimore, USA.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Lucas Kreutz-Rodrigues, MD https://orcid.org/0000-0003-2954-0628
References
- 1. Wong AK, Joanna Nguyen T, Peric M, et al. Analysis of risk factors associated with microvascular free flap failure using a multi-institutional database. Microsurgery. 2014;35(1):6–12. [DOI] [PubMed] [Google Scholar]
- 2. Agha RA, Gundogan B, Fowler AJ, Bragg TWH, Orgill DP. The efficacy of the cook-Swartz implantable Doppler in the detection of free-flap compromise: a systematic review protocol. BMJ Open. 2014;4(3):e004253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schmulder A, Gur E, Zaretski A. Eight-year experience of the cook-Swartz Doppler in free-flap operations: microsurgical and reexploration results with regard to a wide spectrum of surgeries. Microsurgery. 2011;31(1):1–6. [DOI] [PubMed] [Google Scholar]
- 4. Frost MW, Niumsawatt V, Rozen WM, Eschen GE, Damsgaard TE, Kiil BJ. Direct comparison of postoperative monitoring of free flaps with microdialysis, implantable cook-Swartz Doppler probe, and clinical monitoring in 20 consecutive patients. Microsurgery. 2015;35(4):262–271. [DOI] [PubMed] [Google Scholar]
- 5. Kim JT, Kim YH, Kim SW. Fixation of implantable Doppler probe with fibrin sealant after detachment of the silicone cuff. J Plast Reconstr Aesthet Surg. 2015;68(12):e207–208. [DOI] [PubMed] [Google Scholar]
- 6. Oliver DW, Whitaker IS, Giele H, Critchley P, Cassell O. The cook-Swartz venous Doppler probe for the post-operative monitoring of free tissue transfers in the United Kingdom: a preliminary report. Br J Plast Surg. 2005;58(3):366–370. [DOI] [PubMed] [Google Scholar]
- 7. Kreutz-Rodrigues L, Frick MA, Gorny KR, Carlsen BT, Mardini S, Bakri K. Abstract QS35: clinical and radiological safety of retained implantable Doppler devices after free flaps. Plast Reconstr Surg Glob Open. 2019;7(4 suppl):125–126. [Google Scholar]
- 8. Food and Drug Administration FaDA. MAUDE—Manufacturer and User Facility Device Experience—Cook-Swartz. Published May 31, 2020. Accessed June 01, 2020. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfMAUDE/textResults.cfm?q=Q29vay1Td2FydHo=&sc=&pf=&pn=500
- 9. Hargreaves BA, Worters PW, Pauly KB, Pauly JM, Koch KM, Gold GE. Metal-induced artifacts in MRI. AJR Am J Roentgenol. 2011;197(3):547–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cook. Cook-Swartz Doppler Probe. Published. 2018. Accessed December 19, 2018. https://www.cookmedical.com/products/vas_dpsdp_webds/
- 11. Frank G, Shellock PD, FACR, FISMRM, FACC MRI safety. Published 2018. Accessed December 19, 2018. http://www.mrisafety.com/
- 12. Kanal E, Shellock FG, Talagala L. Safety considerations in MR imaging. Radiology. 1990;176(3):593–606. [DOI] [PubMed] [Google Scholar]
- 13. Hartwig V, Giovannetti G, Vanello N, Lombardi M, Landini L, Simi S. Biological effects and safety in magnetic resonance imaging: a review. Int J Environ Res Public Health. 2009;6(6):1778–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wildermuth S, Dumoulin CL, Pfammatter T, Maier SE, Hofmann E, Debatin JF. MR-guided percutaneous angioplasty: assessment of tracking safety, catheter handling and functionality. Cardiovasc Intervent Radiol. 1998;21(5):404–410. [DOI] [PubMed] [Google Scholar]
- 15. Nitz WR, Oppelt A, Renz W, Manke C, Lenhart M, Link J. On the heating of linear conductive structures as guide wires and catheters in interventional MRI. J Magn Reson Imaging. 2001;13(1):105–114. [DOI] [PubMed] [Google Scholar]
- 16. Shellock FG. Magnetic resonance safety update 2002: implants and devices. J Magn Reson Imaging. 2002;16(5):485–496. [DOI] [PubMed] [Google Scholar]
- 17. Erasmus LJ, Hurter D, Naude M, Kritzinger HG, Acho S. A short overview of MRI artefacts. South Afri J Radiol. 2004;8(2):a127. doi:104102/sajrv8i2127 [Google Scholar]
- 18. Jungmann PM, Agten CA, Pfirrmann CW, Sutter R. Advances in MRI around metal. J Magn Reson Imaging. 2017;46(4):972–991. [DOI] [PubMed] [Google Scholar]
- 19. Dillenseger JP, Moliere S, Choquet P, Goetz C, Ehlinger M, Bierry G. An illustrative review to understand and manage metal-induced artifacts in musculoskeletal MRI: a primer and updates. Skeletal Radiol. 2016;45(5):677–688. [DOI] [PubMed] [Google Scholar]


