Abstract
APVO436 is a recombinant bispecific antibody designed to direct host cytotoxic T-cells to CD123-expressing blast cells in patients with hematologic malignancies. APVO436 showed promising tolerability and single-agent activity in relapsed or refractory (R/R) acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS). The primary purpose of this post-hoc analysis was to evaluate the therapeutic and pharmacodynamic effects of APVO436 in 14 R/R AML/MDS patients who had failed treatment with hypomethylating agents (HMA) or venetoclax plus HMA prior to being enrolled in the APVO436 Phase 1 dose-escalation study that was recently completed. Eight of these 14 patients had R/R AML and had failed treatment with HMA (N=2) or venetoclax plus HMA (N=6). The remaining 6 patients had R/R MDS and had also failed treatment with HMA (N=5) or venetoclax plus HMA (N=1). They were treated with APVO436 at submicrogram dose levels >0.08 mcg/kg that were active in preclinical NOD/SCID mouse xenograft models of AML. APVO436 activated patients’ T-cells as evidenced by reduced numbers of circulating CD123+CD34+ and CD33+CD34+ peripheral blasts. Single-agent activity was observed at dose levels ranging from 0.1 mcg/kg to 0.7 mcg/kg in 4 R/R AML patients (50%), including 3 patients with prolonged stable disease (SD) and one patient with complete remission (CR). Likewise, 3 MDS patients had SD (50%) and 3 additional MDS patients (50%) had a marrow CR at dose levels ranging from 0.1 mcg/kg to 0.8 mcg/kg. The median survival for the combined group of 14 R/R AML/MDS patients was 282 days. This early evidence of single-agent activity of APVO436 in R/R AML/MDS patients who failed HMA with or without venetoclax provides proof of concept supporting its in vivo immunomodulatory and anti-leukemic activity and warrants further investigation of its clinical impact potential.
Keywords: AML – acute myeloid leukaemia, CD123 expression, venetoclax (BCL2 inhibitor), bispecific antibody (bsAb), myelodysplastic and myeloproliferative syndromes
Introduction
AML is a common hematologic malignancy in adults with more than 10 thousand anticipated deaths in the US alone for 2021 (SEER Program, www.seer.cancer.gov). Notably, older patients with newly diagnosed AML respond poorly to standard induction chemotherapy. Therefore, there is an urgent and unmet need for effective new treatment modalities for elderly patients with newly diagnosed AML (1–6). In recent years, a number of promising treatment regimens have been identified for this high-risk AML patient population, such as the combination of venetoclax with hypomethylating agents (HMA) (1, 7–9). By comparison, no effective salvage treatment strategies exist for elderly patients with relapsed AML who often suffer cumulative organ toxicity from previous chemotherapy representing an additional hurdle to identifying effective options for their reinduction therapy (10, 11). Not surprisingly, these patients have a dismal prognosis and are in urgent need of new strategies for their chemo-resistant leukemia.
As a member of the BCL-2 homology 3 (BH3)-mimetic class of compounds, Venetoclax can disrupt the association of the proapoptotic BH3-only proteins such as BIM and BID with the antiapoptotic protein B-cell lymphoma 2 (BCL-2) (12, 13). In addition, BCL-2 inhibition by Venetoclax has the potential to selectively damage chemotherapy-resistant leukemic stem cell populations (LSCs) by inhibition of amino acid metabolism and reduction of oxidative phosphorylation (14, 15). Notably, the combined use of venetoclax and a hypomethylating agent (HMA) such as azacitidine (AZA) reduced oxidative phosphorylation in AML cells and killed primary LSC populations obtained from AML patients (15).
AML-associated CD123 antigen is the α-chain of IL-3 receptor, and it is broadly expressed on AML cells, including the LSC populations (16–18). Its expression is associated with chemotherapy resistance and poor prognosis (16, 19, 20). Several biotherapeutic agents targeting CD123 have been developed as biotherapeutic agents against AML, including the CD123-directed recombinant human IL3 fusion toxin Tagraxofusb (SL-401) and bispecific antibodies targeting CD123 antigen, such as bispecific T-cell engagers, dual affinity retargeting antibodies, bispecific killer cell engagers, and trispecific killer cell engagers (21–25). APVO436 is a humanized bispecific antibody designed to direct host cytotoxic T-cells (CTLs) to CD123-expressing blast cells from patients with hematologic malignancies (25–28).
APVO436 showed promising tolerability and single-agent activity in relapsed or refractory (R/R) AML and MDS (28, 29). The primary purpose of this post-hoc analysis was to evaluate the therapeutic and pharmacodynamic effects of APVO436 in 14 R/R AML/MDS patients who had failed treatment with HMA or venetoclax plus HMA prior to being enrolled in the APVO436 Phase 1 study.
Materials and Methods
APVO436
APVO436 is a humanized bispecific antibody (BiAB) that targets CD123 and CD3ϵ (25–27).
Clinical Study
The primary study was a multi-institutional Phase 1B clinical dose-escalation trial of APVO436 in patients with relapsed/refractory AML and higher-risk myelodysplastic syndrome (MDS) (ClinicalTrials.gov identifier: NCT03647800). APVO436 exhibited a promising tolerability and manageable treatment-emergent AEs (28, 29). The weekly target dose levels for cohorts 2–10 were 1 mcg for Cohort 2, 3 mcg for Cohort 3, 9 mcg for Cohort 4, 18 mcg for Cohort 6A, 12 mcg for Cohort 6B, 24 mcg for Cohort 7, 36 mcg for Cohort 8, 48 mcg for Cohort 9, and 60 mcg for Cohort 10 (28). The exploratory studies included pre-planned evaluation of the pharmacodynamic effects of APVO436 on flow cytometrically quantitated CD123+ target cells in peripheral blood samples in relationship to clinical responses. The analysis of the single-agent activity of APVO436 in R/R AML/MDS patients who failed HMA with or without venetoclax was a post-hoc analysis that was not planned as the success of the Venetoclax + HMA regimen could not be predicted in 2018 when the Phase 1B trial of APVO436 was initiated. Likewise, the enrollment of an adequate number of patients for such an analysis could not be anticipated or estimated in advance.
Patient Characteristics
Eight patients, including 3 males and 5 females with a median age of 66 years (Mean ± SE = 65 ± 6 years) of whom 7 were Caucasian and 1 was African American, had R/R AML ( Table 1 ). 4 males and 2 females had R/R MDS. They had a median age of 75 years (Mean ± SE = 75 ± 2 years) of whom 5 were Caucasian and 1 was Asian, had R/R MDS. 5 of 8 AML patients and all 6 MDS patients were ≥60 years of age ( Table 1 ). Of the 8 AML patients, 4 had AML with MDS-related features – one of these patients also had FLT3-ITD gene mutation -, 1 had AML with recurrent genetic abnormalities, 2 had AML with gene mutations and 1 had AML-NOS (M0-AML) ( Table 1 ). All 6 MDS patients had MDS with excess blasts according to WHO classification (MDS-EB-1 or MDS-EB-2). Five of these patients had IPSS prognosis scores consistent with an intermediate-1 (IM-1) or intermediate-2 (IM-2) risk group and one had high-risk MDS ( Table 1 ). Patient characteristics and treatment outcome data are shown in Table 1 .
Table 1.
Patient Characteristics and Treatment Outcomes.
| Parameter | Number of patients |
|---|---|
| Age | |
| ≥60 years | AML: 5; MDS: 6 |
| <60 years | AML: 3; MDS: 0 |
| Gender | |
| Male | AML: 3; MDS: 4 |
| Female | AML: 5; MDS: 2 |
| Race | |
| Caucasian | AML: 7; MDS: 5 |
| Black | AML: 1; MDS: 0 |
| Asian | AML: 0; MDS: 1 |
| Adverse Events | |
| DLT | AML: 0; MDS: 0 |
| Grade ≥3 AE | AML: 2***; MDS: 1$ |
| CRS | AML: 2 (Grade 1-2); MDS: 1 (Grade 1) |
| AML** | 8 (UPN01-UPN08) |
| AML with MDS features | 4 |
| AML with recurrent genetic abnormalities |
1 |
| AML-NOS | 1 |
| AML with gene mutations | 2 |
| MDS&& | 6 (UPN09-UPN14) |
| MDS-EB-1 | 5 |
| MDS-EB-2 | 1 |
| Previous Therapy | |
| Failed HMA without Venetoclax | 7 (AML: 2; MDS: 5) |
| Failed HMA plus Venetoclax | 7 (AML:6; MDS: 1) |
| Best Overall Response (BOR) | |
| AML (N=8) | |
| SD | 5 |
| CR | 1 |
| PD | 2 |
| TTP ≥90 days | 5 (Median: 211 days, Range: 38->224 days) |
| MDS (N=6) | |
| SD (includes marrow CR) | 6 |
| Marrow CR | 3 |
| TTP (≥90 days | 6 (Median: 189 days, Range: 104-321 days) |
**The karyotypes/genetic mutations were: UPN01: -7, del(5q)/TP53; UPN02: -7, del(5q),del(17p)/TP53; UPN03: inv(16)/ND;UPN04: t(1;15),t(5;10),11q23/FLT-ITD; UPN05: 46,XX,del(20)(q11.2q13.1)/ND; UPN06: 46,XX/ND; UPN07: t(2;15)/NF1,RUNX1,GATA2,IKZF1; UPN08: -Y/FLT3-ITD.
&&UPN09: IPSS Score: 1.5; Risk classification: Intermediate-2; Anemia (Hgb 9.7 g/dL), Thrombocytopenia (Plt 30,000/µL), Interrmediate risk karyotype, 47,XX+8, Multiple genetic mutations (NRAS, ASXL-1, PTPNII, RUNX1, STAG2), progressed to CMML-MPN with an absolute monocyte count 17,600/µL around Cycle 10
UPN10: IPSS score: 1.0; Risk classification: Intermediate-1; Anemia (Hgb 8.0 g/dL), thrombocytopenia (Plt: 9,000/µL), severe neutropenia (ANC: 0.29x103/µL), 7.7% blasts in bone marrow, cytogenetics not available. Achieved a marrow CR with bone marrow myeloblast count of 2.4%.
UPN11: IPSS score: 2.5; Risk classification: High Anemia (Hgb 9.3 g/dL, thrombocytopenia (Plt: 23,000/µL) (- non-severe neutropenia with ANC 1.3 x103/µL), intermediate risk cytogenetics with MDS related aberrations 11q- and -7, 11.3% blasts in the bone marrow. Achieved a marrow CR with bone marrow myeloblast countdown to 0% at C2D1.
UPN12: IPSS score: 0.5; Risk classification: Intermediate-1No pancytopenia (Hgb 11 g/dL, Plt: 100,000/µL, ANC: 1.1 x103/µL). Favorable karyotype: 46, XY, 8.2% blasts in bone marrow. Achieved a marrow CR with bone marrow myeloblast count reduced to 2% at C2D1.
UPN13: IPSS score: 2.0; IPSS score: 1.0; Risk classification: Intermediate-1; Anemia (Hgb 7.2 g/dL), thrombocytopenia (Plt: 8,000/µL), severe neutropenia (ANC: 0.5x103/µL), 5% blasts in bone marrow, complex karyotype (poor risk category): 46,XY,der(12;19)(q10;p10), +mar[8]/46,XY, del(20)(q11.2q13.1). Intermediate risk karyotype with 12(p) aberration on cytogenetics. 5% blasts in bone marrow.
UPN14: IPSS score: 1.0, Risk classification: Intermediate-1. Anemia (Hgb 9.3/dL), Intermediate risk karyotype with t(1;2)(p36.3;p21) on cytogenetics, 6% blasts in bone marrow.
***Grade ≥3 Adverse events and Any Grade CRS/Neurotoxicity: One AML patient, UPN05, had Grade 3 sepsis, Grade 3 diarrhea, Grade 3 vomiting, and Grade 1 CRS and Grade 1 neurotoxicity. Another AML patient, UPN06, had Grade 3 confusion as well as Grade 2 CRS.
$One MDS patient, UPN09, had multiple transient Grade 3-4 AEs that have resolved; She had tumor lysis syndrome (TLS) Grade 3 on C1D2, lasting 2 days; TLS Grade 3 on C4D1 lasting 2 days; Anemia, Grade 3 on C5D1 lasting 8 days, Anemia Grade 3 on C7D22 lasting 8 days, Anemia Grade 3 on C6D22 lasting 12 days, Anemia Grade 3 on C7D8 lasting 4 days; decreased platelet count on C4D15 Grade 3 lasting 7 days, decreased platelet count Grade 4 on C4D22 lasting 50 days, decreased platelet count Grade 3 on C6D15 lasting 2 days, decreased platelet count Grade 4 on C6D22 lasting 33 days; hyperglycemia Grade 3 on C5D5 lasting 2 days.
IPSS, International Prognosis Scoring System; 2016 WHO myelodysplastic syndrome subtypes, MDS with excess blasts (MDS-EB).
No DLT or Grade 5 AE was observed in any of the 14 cases analyzed. Among the 8 AML patients, 6 had no CRS, one patient had Grade 1 CRS lasting 2 days, and one patient had transient Grade 2 CRS lasting 2 days ( Table 1 ). There were 2 SAEs: One SAE was reported for UPN05 who developed sepsis, diarrhea and vomiting on C6D5 showing full recovery within 5 days. Grade 2 CRS of UPN06 was also reported as an SAE due to hospitalization but lasted only 2 days and fully resolved ( Table 1 ). No SAEs were reported for any of the 6 MDS patients. One MDS patient experienced Grade 1 CRS on C2D1 lasting one day and another MDS patient experienced transient several Grade 3-4 AEs, including tumor lysis syndrome (TLS), Anemia, decreased platelet count, and hyperglycemia ( Table 1 ).
Ethics Statement and Study Approval
The study protocol was approved by a Central IRB (WCG; IRB00000533) as well as the local IRBs of the investigative sites. Each patient provided a written informed consent (ICF) prior to enrollment.
Flow Cytometry
Immunophenotyping was performed on cryopreserved peripheral blood mononuclear cells from patients by standard flow cytometry using a BD LSR II flow cytometer and FACSDiva Software Version 8.0.2 fluorochrome-labeled monoclonal antibodies reactive with CD5 (anti-human CD5, clone REA782 [PE-Vio770), CD45 (anti-human CD45, Clone H130, V500, BD Biosciences #560777), CD34 (anti-human CD34, Clone REA1164, VioBright 515, Miltenyl Biotech #130-120-517), CD38 (anti-human CD38, clone HIT-2, BV605, Biolegend#303532), and CD123 (anti-human CD123, Clone 9F5, AF647, BD Biosciences #563599) antigens.
Statistical Analyses
Standard statistical methods and the GraphPad Prism 9 statistical program (GraphPad Software, LLC, San Diego, CA) were used in data analysis. Survival data was analyzed by the Kaplan-Meier method (28–30).
Results
Pharmacodynamic Effects of APVO436
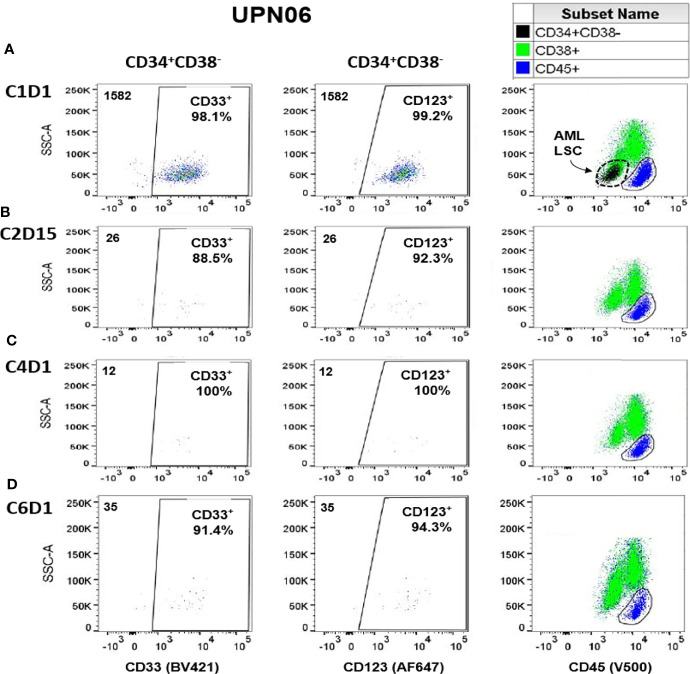
We used immunophenotyping by multiparameter flow cytometry to examine the effects of APVO436 on tumor burden reflected by CD123+CD34+CD38- target blast cells (19). Most of these cells co-express CD33 antigen (i.e., they are CD33+CD34+CD38-) (19). Flow cytometric analysis of post-treatment blood samples from 3 of 4 AML patients (UPN01, UPN03, UPN06) showed decreased numbers of the circulating CD123+CD34+CD38- and CD33+CD34+CD38- cells ( Table 2 ). The maximum reduction in the numbers of the CD123+CD34+CD38- cells were 76.1% in UPN01 (C6D1 sample), 57.8% in UPN03 (C1D30 sample), and 99.2% in UPN06 (C4D1 sample). Figure 1 depicts the multicolor dot profiles from the multi-parameter flow cytometry test that illustrates the rapid and marked depletion of the CD123+CD34+CD38- and CD33+CD34+CD38- cells by APVO436 monotherapy in UPN06. Virtually all the CD34+CD38- cells were CD123+ and CD33+ consistent with AML. The size of this CD123+CD33+CD34+CD38- AML blast population indicated with the arrow in Panel A, 3rd column, was significantly reduced by APVO436 monotherapy. In contrast to these 3 AML cases, Table 2 and Figure S1 illustrate that the CD34+CD38-CD123+ cells from the 4th AML patient, UPN05, were not depleted to APVO436 treatments, although their expansion was prevented which was associated with stable disease with a time to progression of 238 days. As shown in Table 2 , reductions of CD123+CD34+CD38- and CD33+CD34+ CD38- cells were also observed in post-treatment samples of two MDS patients.
Table 2.
Pharmacodynamic Effects of APVO436 on Circulating CD123+CD34+CD38- and CD33+CD34+CD38- AML Blast Cells.
| UPN | CD123+CD34+CD38-Cells Number (% of CD45+)/% Depletion | CD33+CD34+CD38-Cells Number (% of CD45+)/% Depletion | CD45+ |
|---|---|---|---|
| UPN01/AML | |||
| Baseline/C1D1 | 155 (0.16%) | 156 (0.17%) | 94415 |
| C2D15 | 74/52.3% | 73/53.2% | 86266 |
| C4D1 | 71/54.2% | 72/53.8% | 89848 |
| C6D1 | 37 (0.04%)/76.1% | 36 (0.04%)/76.9% | 84337 |
| UPN03/AML | |||
| Baseline/C1D1 | 2757 (2.8%) | 2732 (2.8%) | 97928 |
| EOT/C1D30 | 1163 (1.2%)/57.8% | 1153 (1.2%)/57.8% | 93827 |
| UPN05/AML | |||
| Baseline/C1D1 | 13782 (15.4%) | 13147 (14.7%) | 89598 |
| C2D15 | 21115/0% | 17185/0% | 94131 |
| C4D1 | 22787/0% | 18565/0% | 96053 |
| C6D1 | 23653 (24.3%)/0% | 15001 (15.4%)/0% | 97346 |
| UPN06/AML | |||
| Baseline/C1D1 | 1569 (1.7%) | 1552 (1.7%) | 92694 |
| C2D15 | 24/98.5% | 23/98.5% | 92219 |
| C4D1 | 12 (0.01%)/99.2% | 12 (0.01%)/99.2% | 92694 |
| C6D1 | 33 (0.01%)/97.9% | 32 (0.01%)/97.9% | 93863 |
| UPN09/MDS | |||
| Baseline/C1D1 | 1441 (1.52%) | 907 (0.96% | 94715 |
| C2D1 | 1489/0% | 1191/0% | 94300 |
| C4D1 | 947/34.3% | 575/36.6% | 95129 |
| C6D1 | 1419/4.7% | 1421/0% | 94893 |
| UPN10/MDS | |||
| Baseline/C1D1 | 833 (0.9%) | 836 (0.9%) | 972155 |
| C2D15 | 833/0% | 775/7.3% | 101335 |
| C4D1 | 477 (0.48%)/42.7% | 457/45.8% | 99979 |
| EOT/C5D20 | 2567 (2.5%) /3.1-fold expansion) |
2296 (2.3%)/ 2.7-fold expansion |
100884 |
UPN03 had 40% bone marrow blasts at screening and 40% at C1D22. EOT was on C1D30.
UPN10 has 7.5% bone marrow blasts at screening and 20% bone marrow blasts on C5D1. EOT was on C5D20.
Figure 1.
Depletion of Circulating CD123+CD34+CD38- and CD33+CD34+CD38- Cells in VENAZA-resistant Relapsed AML Patient Receiving APVO436 Monotherapy. The numbers in the left upper corner in the first 2 columns represent the total CD34+CD38- cell numbers of which the vast majority co-expressed both CD123 and CD33. Panels (A–D) show the results at specific study time points: (A) C1D1 = Cycle 1, Day 1; (B) C2D15 = Cycle 2, Day 15; (C) C4D1 = Cycle 4, Day 1; (D) C6D1 = Cycle 6, Day 1. Virtually all the CD34+CD38- cells were CD123+ and CD33+ consistent with AML. The size of this CD123+CD33+CD34+CD38- AML blast population indicated with the arrow in Panel (A), 3rd column, was significantly reduced by APVO436 monotherapy. See also Table 2 .
These preliminary pharmacodynamic results provided early proof of concept that the CD3xCD123 BiAB APVO436 can activate T-cells and causing depletion of targeted CD123+ blast cells in patients with MDS and AML. Notably, at the time of progression, there was a significant expansion of the CD123+ population in UPN10 and the cells in UPN03 that were not depleted were CD123+. These results illustrate that APVO436 monotherapy failures can occur independent of CD123 expression as the anti-leukemic activity of this BiAB is dependent on the cytotoxic T-cell (CTL) activity of the CD3-expressing T-cell populations that are redirected to CD123+ AML/MDS cells.
Efficacy
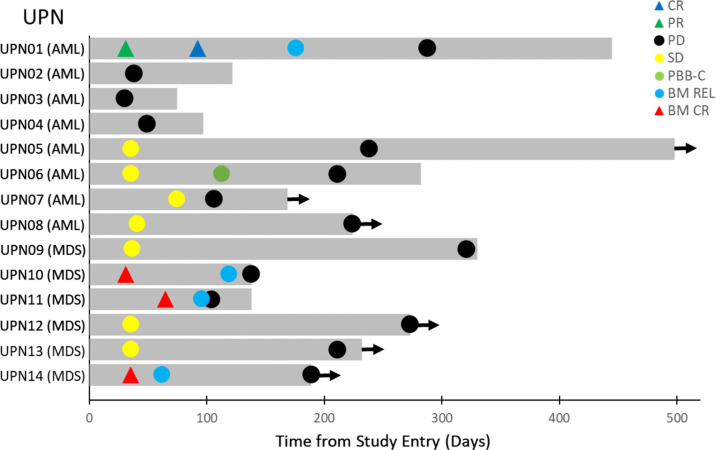
Of the 8 R/R AML patients, 2 patients (UPN2 and UPN4) had progressive disease (PD) and one had a stable disease (SD)/resistant disease (RES) (UPN3) as best overall response (BOR) and died of leukemia between 75-122 days ( Table 1 ). Three patients (UPN05, UPN06, UPN07) had prolonged SD. Their time to progression ranged from 211 days to 238 days. UPN06 from Cohort 7 (Dose level: 0.4 mcg/kg), an AML patient with MDS related features, had a complete clearance of peripheral blasts along with a >50% reduction of the percentage of bone marrow blasts, followed by sustained SD with a time-to-progression of 211 days ( Figure 2 ). This patient also had evidence of depletion of target CD123+CD34+CD38- AML cells ( Table 2 and Figure 1 ). Another AML patient with MDS-related features (UPN01 from Cohort 6B, Dose level: 0.1 mcg/kg), who had 29% bone marrow blasts, unfavorable cytogenetics (del 5q and monosomy 7) and TP53 mutation, whose disease had previously progressed on Venetoclax + Decitabine therapy, achieved a PR at 31 days and CR at 92 days following APVO436 monotherapy with full hematologic recovery as BOR ( Table 1 and Figure S1 ). This patient also had evidence of depletion of target CD123+CD34+CD38- blast cells ( Table 2 ). The onset and duration of the SD, peripheral blood blast count clearance (PBBC-C), PR, or CR in these patients is illustrated by the Swimmer plot depicted in Figure 2 .
Figure 2.
Swimmer Plot of Best Overall Responses. The onset and duration of stable disease (SD, PR, CR, clearance of peripheral blasts, and onset of PD are indicated with specific symbols. Arrow: Alive. The disease progression and survival data were updated since the initial report of the primary study (28).
Likewise, 3 MDS patients had SD (50%) and 3 additional MDS patients (50%) had a bone marrow CR at dose levels ranging from 0.1 mcg/kg to 0.4 mcg/kg ( Table 1 ): One MDS patient (UPN10 from Cohort 6A, Dose level: 0.2 mcg/kg) had a pretreatment BM blast percentage of 7.5% with 10% cellularity and a C2D1 posttreatment BM blast percentage of 2.4% with 20% cellularity. Another MDS patient (UPN11 from Cohort 7, Dose level: 0.3 mcg/kg) had baseline BM blasts of 11.3% with 20-30% cellularity and a C2D1 posttreatment BM blast percentage of 0% with 50% cellularity. A third MDS patient (UPN12 from Cohort 9, Dose level: 0.4 mcg/kg) had a pretreatment BM blast percentage of 8.2% with 15% marrow cellularity and a C2D1 posttreatment BM showing 2% blasts with 20% cellularity. One MDS patient, UPN09 in Cohort 4, with ASXL-1 mutation showed a decrease in the percentage of myeloblasts in the bone marrow, but this was part of the transformation of the case into a high-risk chronic myelomonocytic leukemia-myeloproliferative neoplasm (CMMN-MPN) with an absolute monocyte count 17,600/µL around Cycle 10 ( Table 1 ). The onset of marrow CR and time to progression in these MDS patients is illustrated by the Swimmer plot depicted in Figure 2 . The median overall survival of the 14 HMA/Venetoclax-resistant AML/MDS patients was 282 days ( Figure S2 ).
Discussion
Therapy-naïve patients with AML who are elderly (>=75) or unfit to receive intensive chemotherapy have high response rates and improved survival when treated with Venetoclax and HMA compared to HMA alone (7, 31). This combination therapy was therefore approved by the Food and Drug Administration (FDA) (15). However, with increasing clinical experience involving the use of Venetoclax, several challenges and limitations of Venetoclax-based therapy have emerged, as emphasized in recent publications (13, 32).
Venetoclax-induced remissions in therapy-naïve older/unfit AML patients are short-lived lasting less than 12 months (median) even after combined use of Venetoclax and HMAs. Furthermore, patients with secondary AML as well as AML patients previously treated with HMAs are less responsive to Venetoclax-based treatment regimens with substantially worse CR rates and <6-month overall survival times (13). Likewise, some AML patient populations in the adverse risk category, such as patients with TP53 mutations, or those with RTK mutations, may exhibit inherent Venetoclax-resistance (13). Unlike its remarkable activity in therapy-naïve AML patients, Venetoclax is not as effective in relapsed AML patients. The reported overall response rate was 21% for relapsed or refractory AML patients treated with venetoclax in combination with HMAs, LDAC, or other agents such as cladribine or midostaurin (33). Therefore, new agents that can potentially be combined with and improve the clinical efficacy of Venetoclax-based treatment regimens for elderly AML patients in the upfront setting are urgently needed.
This analysis provides clinical proof of concept evidence supporting the mechanism of action of APVO436 using the primary data from a recently completed Phase 1B study in R/R AML and MDS patients (28). Reponses in patients resistant to HMA or venetoclax plus HMA included CR in an AML patient with P53/monosomal karyotype and marrow CR in 3 MDS patients, along with 5 AML patients and 6 MDS patients with prolonged stable disease. Median overall survival for all 14 patients was 282 days. Our results should be interpreted with due caution due to the inherent limitations associated with a small size and heterogeneity of the patient population and the fact that prior treatment regimens were not uniform across all patients. One further weakness in our study is the lack of characterization of the patients’ immune repertoire or immunosuppressive bone marrow microenvironment in relationship to the observed clinical effects. In this regard, it will be important to determine if abundance of myeloid-derived suppressor cells (MDSCs) will correlate with resistance to APVO436 as MDSCs have been shown to inhibit the cytotoxic T-cell (CTL) activation by CD3-engaging BiAB (34).
Here we have demonstrated promising early single-agent activity and immunomodulatory effects of APVO436 (given as a weekly IV infusion) in patients who have failed prior treatment with Venetoclax plus HMA for AML or HMA alone for MDS. If confirmed in future studies of APVO436, our observations will add to the growing clinical evidence that BiAB against AML may overcome chemotherapy resistance and thereby improve treatment outcome of patients with adverse cytogenetic features (22, 28). Although single-agent APVO436 has not been associated with dose-limiting myelosuppression, we are planning to formally evaluate in a Phase IB study the tolerability and efficacy of APVO436 in combination with a Venetoclax plus Azacitidine backbone in adverse-risk fit AML patients (NCT03647800, Expansion phase, Cohort 2). There will be a 2-week lead-in phase of Venetoclax plus Azacitidine to mitigate risk of TLS and cytokine release syndrome (CRS). We hypothesize that the addition of APVO436 will eradicate residual CD123+ blasts as well as leukemic stem cells that are resistant to Venetoclax, leading to more durable responses. Likewise, Venetoclax is currently being studied in combination with other CD123-targeted therapies such as SL-401 and IMGN632 [ClinicalTrials.gov identifiers: NCT03113643, NCT04086264]. It is noteworthy that Venetoclax augments T-cell effector function by increased production of reactive oxygen species (35). Likewise, Azacitidine enhances sensitivity of AML cells to cytotoxic T-cells via activation of the STING pathway (35). Therefore, a combination of T-cell redirecting BiAb such as APVO436 with Venetoclax or Azacitidine may have clinical potential.
The results reported here informed the design of currently accruing Cohort 2 of the Expansion phase of the Phase 1B study (NCT03647800). In future studies of APVO436, it will be important to prospectively evaluate the dynamic changes of the pharmacodynamic parameters, including target AML blast populations as well as levels of T-cell derived cytokines and correlate such changes with induction of remission as well as treatment-emergent adverse events (e.g. CRS, infusion related reactions, neurotoxicity).
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Central IRB (WCG; IRB00000533). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
All authors have equally contributed to this manuscript. TL, AM, PP, PS, EC, CRC, EW, and JW assisted in study design, served as site investigators, screened and recruited participants, administered treatments, assessed adverse events and disease responses, collected safety and efficacy data, met regularly to review study data during the study, and reviewed the manuscript. FU designed the evaluations reported in this paper, directed the data compilation and analysis, and prepared the initial draft of the manuscript. FU, AS, and CL analyzed and validated data, performed statistical analyses. Each author reviewed and revised the manuscript, and provided final approval for submission of the final version.
Funding
This study received funding from Aptevo Therapeutics. The funder had the following involvement with the study: 1. Provided funding to a clinical research organization for the operationalization of the study by clinical monitoring, medical monitoring, pharmacovigilance, and data management; 2. Provided site awards to the investigative sites to compensate the sites for their clinical research expenses; and 3. Sponsored the IND, funded the regulatory affairs and quality assurance activities to ensure ICH/GCP compliance. The sponsor did not participate in the safety or efficacy assessments of the investigators.
Conflict of Interest
Author FU is employed by Ares Pharmaceuticals, he served as a consultant for Aptevo Therapeutics and he serves as a consultant to Reven Pharmaceuticals. Author CL is employed by Oncotelic Therapeutics, she served as a consultant to Aptevo Therapeutics and she serves as a consultant to Reven Pharmaceuticals. Author AS also served as a consultant to Aptevo.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank the patients who participated in this trial and their families, the coinvestigators, nurses, and study coordinators at each of the participating sites. The study was performed at the following 10 centers in the US as an open-label study sponsored by Aptevo Therapeutics: (1) Greenville Health System, Institute for Translational Oncology Research, Greenville, South Carolina, United States, 29605; (2) The University of Kansas Clinical Research Center, Westwood, Kansas, United States, 66205; (3) Sylvester Comprehensive Cancer Center, University of Miami, Miami, Florida, United States, 33136; (4) University of Utah, Huntsman Cancer Institute, Salt Lake City, Utah, United States, 84112; (5) The Ohio State University Wexner Medical Center/James Cancer Hospital; Columbus, Ohio, United States, 43210; (6) Roswell Park Cancer Institute; Buffalo, New York, United States, 14263; (7) Fred Hutchinson Cancer Research Center; Seattle, Washington, United States, 98109; (8) University of Texas Southwestern Medical Center, Dallas, Texas, United States, 75390; (9) University of Florida College of Medicine, Gainesville, Florida, United States, 32610; (10) University of California, San Francisco Medical Center, San Francisco, California, United States, 94143.
This study was sponsored by Aptevo Therapeutics, which provided APVO436 and worked with investigators to design the study, as well as collect, analyze, and interpret the data. We thank our preferred provider for Clinical Research Organization (CRO) services, Lab Corp Drug Development Team for the medical monitoring, clinical monitoring, program management, data management, and pharmacovigilance services. We thank the Aptevo Lab Personnel for centralizes laboratory services for immunophenotyping by flow cytometry. We thank the research coordinators from the participating clinical sites for their assistance with study coordination and data management.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.806243/full#supplementary-material
Circulating AML-LSC Cells in VENAZA-resistant Relapsed AML Patient Receiving APVO436 Monotherapy. Virtually all the CD34+CD38- cells were CD123+ and CD33+ consistent with AML. The size of this CD123+CD33+CD34+CD38- AML LSC population indicated with the arrow in the 3rd column, did not significantly change during APVO436 monotherapy. See also Table 2 in the main manuscript.
Survival Outcome of R/R AML/MDS Patients According to Prior Therapy. Depicted is the overall survival curve of the 14 HMA/Venetoclax-resistant AML/MDS patients shown in Figure 2 in the main manuscript. All patients were treated with APVO436 monotherapy.
References
- 1. Ferrara F, Lessi F, Vitagliano O, Birkenghi E, Rossi G. Current Therapeutic Results and Treatment Options for Older Patients With Relapsed Acute Myeloid Leukemia. Cancers (Basel) (2019) 11(2):E224. doi: 10.3390/cancers11020224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. DiNardo CD, Wei AH. How I Treat Acute Myeloid Leukemia in the Era of New Drugs. Blood (2020) 135(2):85–96. doi: 10.1182/blood.2019001239 [DOI] [PubMed] [Google Scholar]
- 3. Blum WG, Mims AS. Treating Acute Myeloid Leukemia in the Modern Era: A Primer. Cancer (2020) 126(21):4668–77. doi: 10.1002/cncr.32904 [DOI] [PubMed] [Google Scholar]
- 4. Short NJ, Konopleva M, Kadia TM, Borthakur G, Ravandi F, DiNardo CD, et al. Advances in the Treatment of Acute Myeloid Leukemia: New Drugs and New Challenges. Cancer Discov (2020) 10(4):506–25. doi: 10.1158/2159-8290.CD-19-1011 [DOI] [PubMed] [Google Scholar]
- 5. Daver N, Wei AH, Pollyea DA, Fathi AT, Vyas P, DiNardo CD. New Directions for Emerging Therapies in Acute Myeloid Leukemia: The Next Chapter. Blood Cancer J (2020) 10:107. doi: 10.1038/s41408-020-00376-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Döhner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Büchner T, et al. Diagnosis and Management of AML in Adults: 2017 ELN Recommendations From an International Expert Panel. Blood (2017) 129:424–47. doi: 10.1182/blood-2016-08-733196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. DiNardo CD, Pratz K, Pullarkat V, Jonas BA, Arellano M, Becker PS, et al. Venetoclax Combined With Decitabine or Azacitidine in Treatment-Naive, Elderly Patients With Acute Myeloid Leukemia. Blood (2019) 133:7–17. doi: 10.1182/blood-2018-08-868752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Othman TA, Azenkot T, Moskoff BN, Tenold ME, Jonas BA. Venetoclax-Based Combinations for the Treatment of Newly Diagnosed Acute Myeloid Leukemia. Future Oncol 2021;17(23):2989–3005. doi: 10.2217/fon-2021-0262 [DOI] [PubMed] [Google Scholar]
- 9. DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N Engl J Med (2020) 383(7):617–29. doi: 10.1056/NEJMoa2012971 [DOI] [PubMed] [Google Scholar]
- 10. Mims AS, Blum W. Progress in the Problem of Relapsed or Refractory Acute Myeloid Leukemia. Curr Opin Hematol (2019) 26(2):88–95. doi: 10.1097/MOH.0000000000000490 [DOI] [PubMed] [Google Scholar]
- 11. Schlenk RF, Muller-Tidow C, Benner A, Kieser M. Relapsed/refractory Acute Myeloid Leukemia: Any Progress? Curr Opin Oncol (2017) 29:467–73. doi: 10.1097/CCO.0000000000000404 [DOI] [PubMed] [Google Scholar]
- 12. Kapoor I, Bodo J, Hill BT, Hsi ED, Almasan A. Targeting BCL-2 in B-Cell Malignancies and Overcoming Therapeutic Resistance. Cell Death Dis (2020) 11:941. doi: 10.1038/s41419-020-03144-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pollyea DA, Stevens BM, Jones CL, Winters A, Pei S, Minhajuddin M, et al. Venetoclax With Azacitidine Disrupts Energy Metabolism and Targets Leukemia Stem Cells in Acute Myeloid Leukemia Patients. Nat Med (2018) 24:1859–66. doi: 10.1038/s41591-018-0233-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lagadinou ED, Sach A, Callahan K, Rossi RM, Neering SJ, Minhajuddin M, et al. BCL-2 Inhibition Targets Oxidative Phosphorylation and Selectively Eradicates Quiescent Human Leukemia Stem Cells. Cell Stem Cell (2013) 12(3):329–41. doi: 10.1016/j.stem.2012.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jones CL, Stevens BM, D’Alessandro A, Reisz JA, Culp-Hill R, Nemkov T, et al. Inhibition of Amino Acid Metabolism Selectively Targets Human Leukemia Stem Cells. Cancer Cell (2018) 34(5):724–40.e4. doi: 10.1016/j.ccell.2018.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Testa U, iccion ,, Coccia E, Stellacci E, Samoggia P, Latagliata R, et al. Elevated Expression of IL-3Ralpha in Acute Myelogenous Leukemia Is Associated With Enhanced Blast Proliferation, Increased Cellularity, and Poor Prognosis. Blood (2002) 100(8):2980–8. doi: 10.1182/blood-2002-03-0852 [DOI] [PubMed] [Google Scholar]
- 17. Hwang K, Park CJ, Jang S, Chi HS, Kim DY, Lee JH, et al. Flow Cytometric Quantification and Immunophenotyping of Leukemic Stem Cells in Acute Myeloid Leukemia. Ann Hematol (2012) 91(10):1541–6. doi: 10.1007/s00277-012-1501-7 [DOI] [PubMed] [Google Scholar]
- 18. Jin L, Lee EM, Ramshaw HS, Busfiled SJ, Peoppl AG, Wilkinson L, et al. Monoclonal-Antibody Mediated Targeting of CD123, IL-3 Receptor Alpha Chain, Eliminates Human Acute Myeloid Leukemia Stem Cells. Cell Stem Cell (2009) 5(1):31–42. doi: 10.1016/j.stem.2009.04.018 [DOI] [PubMed] [Google Scholar]
- 19. Al-Mawali A, Pinto AD, Al-Zadjali S. CD34+CD38-CD123+ Cells Are Present in Virtually All Acute Myeloid Leukaemia Blasts: A Promising Single Unique Phenotype for Minimal Residual Disease Detection. Acta Haematol (2017) 138(3):175–81. doi: 10.1159/000480448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vergez F, Green AS, Tamburini J, Sarry JE, Gaillard B, Cornillet-Lefebvre P, et al. High Levels of CD34+CD38low/-CD123+ Blasts Are Predictive of an Adverse Outcome in Acute Myeloid Leukemia: A Groupe Ouest-Est Des Leucemies Aigues Et Maladies Du Sang (GOELAMS) Study. Haematologica (2011) 96(12):1792–8. doi: 10.3324/haematol.2011.047894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hussaini MH AL, Ritchey J, Rettig MP, Eissenberg L, Uy GL, Chichili G, et al. Targeting CD123 in Leukemic Stem Cells Using Dual Affinity Re-Targeting Molecules (Darts®). Blood (2013) 122:360–0. doi: 10.1182/blood.V122.21.360.360 [DOI] [Google Scholar]
- 22. Uy GL, Aldoss I, Foster MC, Sayre PH, Wieduwilt MJ, Advani AS, et al. Flotetuzumab as Salvage Immunotherapy for Refractory Acute Myeloid Leukemia. Blood (2021) 137(6):751–62. doi: 10.1182/blood.2020007732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Daver N, Alotaibi AS, Bücklein V, Subklewe M. T-Cell-Based Immunotherapy of Acute Myeloid Leukemia: Current Concepts and Future Developments. Leukemia (2021) 35(7):1843–63. doi: 10.1038/s41375-021-01253-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Isidori A, Cerchione C, Daver N, DiNardo C, Garcia-Manero G, Konopleva M, et al. Immunotherapy in Acute Myeloid Leukemia: Where We Stand. Front Oncol (2021) 11:656218. doi: 10.3389/fonc.2021.656218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Comeau MR, Gottschalk R, Daugherty M, Sewell T, Sewell T, Misher L, et al. APVO436, a Bispecific Anti-CD123 X Anti-CD3 ADAPTIR™ Molecule for Redirected T-Cell Cytotoxicity With Limited Cytokine Release, Is Well Tolerated in Repeat Dose Toxicology Studies in Cynomolgus Macaques [Abstract]. In: Proceedings of the American Association for Cancer Research Annual Meeting 2019, 2019 Mar 29-Apr 3, Vol. 79(13 Suppl). pp. Abstract nr LB–199. Atlanta, GA. Philadelphia (PA): AACR; Cancer Res; (2019). [Google Scholar]
- 26. Comeau MR, Miller RE, Bannink J, Johnson S, Bader R, Gottschalk R, et al. Characterization of APVO436, a Bispecific Anti-CD123 X Anti-CD3 ADAPTIR™ Molecule for Redirected T-Cell Cytotoxicity, in Preclinical Models of AML and Nonhuman Primates [Abstract]. In: Proceedings of the AACR-NCI-EORTC International Conference: Molecular Targets and Cancer Therapeutics, 2017 Oct 26-30, Vol. 17(1 Suppl). p. Abstract nr B111. Philadelphia, PA. Philadelphia (PA): AACR; Mol Cancer Ther; (2018). [Google Scholar]
- 27. Comeau MR, Miller RE, Bannink J, Johnson S, Bader R, Gottschalk R, et al. APVO436, a Bispecific Anti-CD123 X Anti-CD3 ADAPTIR™ Molecule for Redirected T-Cell Cytotoxicity, Induces Potent T-Cell Activation, Proliferation and Cytotoxicity With Limited Cytokine Release [Abstract]. In: Proceedings of the American Association for Cancer Research Annual Meeting 2018, 2018 Apr 14-18, Vol. 78(13 Suppl). p. Abstract nr 1786. Chicago, IL. Philadelphia (PA): AACR; Cancer Res; (2018). [Google Scholar]
- 28. Uckun FM, Lin TL, Mims A, Patel P, Lee C, Shahidzadeh A, et al. Clinical Phase 1b Study of the CD3xCD123 Bispecific Antibody APVO436 in Patients With Relapsed/Refractory Acute Myeloid Leukemia or Myelodysplasia. Cancers (2021) 13(16):4113. doi: 10.3390/cancers13164113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Uckun FM, Watts J, Mims AS, Patel P, Wang E, Shami PJ, et al. Risk, Characteristics and Biomarkers of Cytokine Release Syndrome in Patients With Relapsed/Refractory AML or MDS Treated With CD3xCD123 Bispecific Antibody Apvo436. Cancers (2021) 13(21):5287. doi: 10.3390/cancers13215287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Uckun FM, Cogle CR, Lin TL, Qazi S, Trieu VN, Schiller G, et al. A Phase 1b Clinical Study of Combretastatin A1 Diphosphate (OXi4503) and Cytarabine (ARA-C) in Combination (OXA) for Patients With Relapsed or Refractory Acute Myeloid Leukemia. Cancers (Basel) (2019) 12(1):74. doi: 10.3390/cancers12010074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. DiNardo CD, Pratz KW, Letai A, Jonas BA, Wei AH, Thirman M, et al. Safety and Preliminary Efficacy of Venetoclax With Decitabine or Azacitidine in Elderly Patients With Previously Untreated Acute Myeloid Leukaemia: A non-Randomised, Open-Label, Phase 1b Study. Lancet Oncol (2018) 19(2):216–28. doi: 10.1016/S1470-2045(18)30010-X [DOI] [PubMed] [Google Scholar]
- 32. Feld J, Tremblay D, Dougherty M, Czaplinska T, Sanchez G, Brady C, et al. Safety and Efficacy: Clinical Experience of Venetoclax in Combination With Hypomethylating Agents in Both Newly Diagnosed and Relapsed/Refractory Advanced Myeloid Malignancies. HemaSphere (2021) 5(4):e549. doi: 10.1097/HS9.0000000000000549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. DiNardo CD, Rausch CR, Benton C, Kadia T, Jain N, Pemmaraju N, et al. Clinical Experience With the BCL2-Inhibitor Venetoclax in Combination Therapy for Relapsed and Refractory Acute Myeloid Leukemia and Related Myeloid Malignancies. Am J Hematol (2018) 93(3):401–7. doi: 10.1002/ajh.25000 [DOI] [PubMed] [Google Scholar]
- 34. Perez C, Botta C, Zabaleta A, Puig N, Cedena MT, Goicoechea I, et al. Immunogenomic Identification and Characterization of Granulocytic Myeloid-Derived Suppressor Cells in Multiple Myeloma. Blood (2020) 136(2):199–209. doi: 10.1182/blood.2019004537 [DOI] [PubMed] [Google Scholar]
- 35. Lee JB, Khan DH, Hurren R, Xu M, Na Y, Kang H, et al. Venetoclax Enhances T Cell-Mediated Antileukemic Activity by Increasing ROS Production. Blood (2021) 138(3):234–45. doi: 10.1182/blood.2020009081 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Circulating AML-LSC Cells in VENAZA-resistant Relapsed AML Patient Receiving APVO436 Monotherapy. Virtually all the CD34+CD38- cells were CD123+ and CD33+ consistent with AML. The size of this CD123+CD33+CD34+CD38- AML LSC population indicated with the arrow in the 3rd column, did not significantly change during APVO436 monotherapy. See also Table 2 in the main manuscript.
Survival Outcome of R/R AML/MDS Patients According to Prior Therapy. Depicted is the overall survival curve of the 14 HMA/Venetoclax-resistant AML/MDS patients shown in Figure 2 in the main manuscript. All patients were treated with APVO436 monotherapy.
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.