ABSTRACT
Farnesoid X receptor (FXR) is a nuclear receptor that has emerged as a key regulator in the maintenance of hepatic steatosis, inflammation, and fibrosis. However, the role of FXR in renal fibrosis remains to be established. Here, we investigate the effects of the FXR agonist EDP‐305 in a mouse model of tubulointerstitial fibrosis via unilateral ureteral obstruction (UUO). Male C57Bl/6 mice received a UUO on their left kidney. On postoperative d 4, mice received daily treatment by oral gavage with either vehicle control (0.5% methylcellulose) or 10 or 30 mg/kg EDP‐305. All animals were euthanized on postoperative d 12. EDP‐305 dose‐dependently decreased macrophage infiltration as measured by the F4/80 staining area and proinflammatory cytokine gene expression. EDP‐305 also dose‐dependently reduced interstitial fibrosis as assessed by morphometric quantification of the collagen proportional area and kidney hydroxyproline levels. Finally, yes‐associated protein (YAP) activation, a major driver of fibrosis, increased after UUO injury and was diminished by EDP‐305 treatment. Consistently, EDP‐305 decreased TGF‐β1—induced YAP nuclear localization in human kidney 2 cells by increasing inhibitory YAP phosphorylation. YAP inhibition may be a novel antifibrotic mechanism of FXR agonism, and EDP‐305 could be used to treat renal fibrosis.—Li, S., Ghoshal, S., Sojoodi, M., Arora, G., Masia, R., Erstad, D. J., Ferriera, D. S., Li, Y., Wang, G., Lanuti, M., Caravan, P., Or, Y. S., Jiang, L.‐J., Tanabe, K. K., Fuchs, B. C. The farnesoid X receptor agonist EDP‐305 reduces interstitial renal fibrosis in a mouse model of unilateral ureteral obstruction. FASEB J. 33, 7103–7112 (2019). www.fasebj.org
Keywords: FXR, kidney, injury, UUO, YAP1
ABBREVIATIONS
- Acta2
smooth muscle aortic α‐actin
- Areg
amphiregulin
- BUN
blood urea nitrogen
- Ccl2
chemokine ligand 2
- CKD
chronic kidney disease
- Collai
collagen type 1 α1
- Col3a1
collagen type 3 α1
- CPA
collagen proportional area
- Ctgf
connective tissue growth factor
- Cyr61
cysteine‐rich angiogenic inducer 61
- ECM
extracellular matrix
- EpCAM
epithelial cell adhesion molecule
- ESRD
end‐stage renal disease
- Fn1
fibronectin 1
- FXR
farnesoid X receptor
- H&E
hematoxylin and eosin
- HK‐2
human kidney 2
- IF/TA
interstitial fibrosis and tubular atrophy
- RQ
relative quantification
- SHP
small heterodimer partner
- Timp1
TIMP metallopeptidase inhibitor 1
- UUO
unilateral ureteral obstruction
- YAP
yes‐associated protein
End‐stage renal disease (ESRD) is the common clinical end point for all progressive renal diseases. Common etiologies include untreated diabetes, hypertension, glomerulonephritis, and chronic pyelonephritis. ESRD is a major burden to the health care system, and most patients are inevitably placed on life‐long dialysis and ultimately require transplantation (1, 2). The progression of chronic kidney disease (CKD) to ESRD is clinically measured by the deterioration of renal function, including proteinuria and decreased glomerular filtration rate. Histologic hallmarks of ESRD are fibrotic lesions in the form of glomerulosclerosis, vascular sclerosis, and interstitial fibrosis (3). Pathologic analysis has revealed that the impairment in renal function is more closely associated with interstitial fibrosis and tubular atrophy compared with glomerular or vascular injuries (3, 4).
Macrophage infiltration, derived from circulating monocytes, is observed early after renal injury and plays a critical role in the initial inflammatory response and induction of fibrogenesis. The degree of inflammatory proliferation within the renal interstitium correlates to the induction of injury, degree of fibrosis, and fulminant renal injury (5). Activated macrophages have been shown to directly promote interstitial fibrosis via the production of profibrotic factors such as TGF‐β (6). Native interstitial fibroblasts, which are essential for the maintenance of normal tissue architecture, differentiate to myofibroblasts through a high level of mesenchymal activation and are responsible for the synthesis and the pathologic accumulation of interstitial extracellular matrix (7). It has also been suggested that tubular epithelial cells undergo transition to a mesenchymal phenotype as a result of epithelial to mesenchymal transition, but this remains controversial (8). During epithelial to mesenchymal transition, tubules lose epithelial characteristics including polarity while inducing fibroblast markers such as vimentin and smooth muscle aortic α‐actin (ACTA2) (8, 9). However, the mechanisms of renal fibrosis are still not completely understood, and thus effective anti‐fibrotic therapies are still lacking.
For many years, scientists have used animal models of renal injury to mimic the diseased glomerulus. However, recent attention has turned to murine models of tubular and interstitial injuries, most commonly unilateral ureteral obstruction (UUO) (10). In this model, the ureter is temporarily or permanently ligated, leading to hydronephrosis and interstitial inflammatory infiltration followed by interstitial matrix accumulation, ultimately predisposing the injured kidney to peritubular capillary loss and tubular atrophy (11–13). Thus, the UUO model is a readily available and facile model for antifibrotic drug discovery.
Unfortunately, little success has been made over the past decade in developing antifibrotic therapies to slow the progression of CKD to ESRD (14). Currently, angiotensin‐converting enzyme inhibitors and angiotensin II receptor blockers are used to reduce proteinuria and slow CKD progression (15). However, these drugs have incomplete efficacy, and trials targeting other mechanisms have failed, including the nuclear factor E2‐related factor 2 inducer bardoxolone (16) and the endothelin receptor blocker avosentan (17). By comparison, attempts to test drugs that have been effective in other fibrotic diseases, such as pirfenidone in interstitial pulmonary fibrosis, have had more success (18, 19).
Along these same lines, farnesoid X receptor (FXR) is a nuclear hormone receptor expressed in the liver, intestines, and kidneys (12). FXR helps to maintain bile acid homeostasis by regulating bile acid synthesis, secretion, and intestinal bile acid uptake (20). Targeting FXR has been implicated in the management of cholestasis, hepatic fibrosis, and inflammatory bowel disease (21). FXR agonism has also been shown to modulate renal lipid metabolism and renal expression of inflammatory cytokines and fibrotic markers (22). In fact, Zhao et al. (23) demonstrated that FXR activation suppressed renal fibrosis via down‐regulating the TGF‐β/Smad3 pathway. However, the relationship between the effects of FXR agonism and renal fibrosis is still relatively unknown. Here, we examine the effects of EDP‐305, a steroidal, non‐carboxylic acid FXR agonist currently in clinical trials for nonalcoholic steatohepatitis and primary biliary cholangitis, on the development of interstitial fibrosis and loss of renal function in a mouse UUO model.
MATERIALS AND METHODS
Chemicals
EDP‐305 (Enanta Pharmaceutical, Watertown, MA, USA) was dissolved in 0.5% methylcellulose before treatment.
Animal experiments
C57BL/6 mice underwent a permanent UUO via a midline laparotomy and suture ligation of the left ureter (Charles River Laboratories, Wilmington, MA, USA). Sham‐treated mice received only a midline laparotomy. On postoperative d 4, animals were received and housed in accordance with the guidelines of the Massachusetts General Hospital Institutional Care and Use Committee and received humane care according to the criteria outlined in the Guide for the Care and Use of Laboratory Animals [National Institutes of Health (NIH), Bethesda, MD, USA]. On postoperative d 5, UUO‐injured mice received either vehicle control (0.5% methylcellulose), 10 mg/kg EDP‐305, or 30 mg/kg EDP‐305 over the course of 8 d (n = 8 for each group). On postoperative d 12, mice were anesthetized and sedated, and a terminal cardiac puncture was performed. Bilateral kidneys were removed for measurement of weights, snap frozen for further analysis, or fixed in formalin for histology.
Serum laboratory analysis and hydroxyproline quantification
A terminal cardiac puncture was performed at the time of euthanasia. Blood was allowed to clot at room temperature before centrifugation for 5 min. Serum was isolated and stored at −80°C prior to use. Serum levels of several biochemical markers, including blood urea nitrogen (BUN), creatinine, albumin, and total protein were measured with a chemical analyzer (Dri‐Chem 4000 Analyzer; Heska, Fribourg, Switzerland). The BUN‐to‐creatinine ratio was also calculated.
Hydroxyproline in tissue was quantified by HPLC analysis as previously described in Hutson et al. (24).
Histology, immunohistochemistry, and immunofluorescence
Formalin‐fixed samples were embedded in paraffin, cut into 5‐µm—thick sections, and stained with hematoxylin and eosin (H&E) and Sirius Red according to standard procedures. Sirius Red was used to quantify the collagen proportional area (CPA) with image‐processing software (ImageJ; NIH). Additional sections were stained with antibodies specific for F4/80 (Bio‐Rad, Hercules, CA, USA) and yes‐associated protein (YAP). For immunofluorescence, sections were stained for epithelial cell adhesion molecule (EpCAM), vimentin (Cell Signaling Technology, Danvers, MA, USA), α‐SMA, and CD31 (Abcam, Cambridge, MA, USA), with detection by appropriate secondary antibodies labeled with either Cy3 or Alexa488 according to the manufacturer's instructions. All slides were reviewed blindly by a renal pathologist.
Cell culture
Human proximal tubular epithelial cells immortalized by transduction with human papilloma virus (human kidney 2 (HK‐2) cells; American Type Culture Collection, Manassas, VA, USA) were maintained in 50% DMEM containing 10% fetal bovine serum, 1% penicillin and streptomycin, and 50% keratinocyte serum‐free medium with bovine pituitary extract and human recombinant epidermal growth factor at 37°C in a 5% CO2 incubator. Fresh growth medium was added to the cells every 3–4 d until confluent. Cells were serum starved overnight before experimental manipulation. HK‐2 cells were stimulated overnight with 5 ng/ml recombinant human TGF‐β1 (R&D Systems, Minneapolis, MN, USA).
Western blot
HK‐2 cells were homogenized, and protein was extracted in RIPA buffer (Boston Bio Products, Ashland, MA, USA) containing protease and phosphatase inhibitors (MilliporeSigma, Burlington, MA, USA). Protein concentrations were normalized to 100 µg. Samples were separated by SDS‐PAGE and transferred to a PVDF membrane. Antibodies used were phosphorylated (Ser127) YAP (p‐YAP), YAP (both from Cell Signaling Technology), and β‐actin (Abcam). Blots were incubated with species‐appropriate secondary antibodies conjugated to horseradish peroxidase (GE Healthcare, Waukesha, WI, USA) and were developed with chemiluminescent horseradish peroxidase substrate (PerkinElmer, Waltham, MA, USA). Western blot was repeated twice to verify data reproducibility.
RNA isolation and real‐time PCR
RNA was isolated from kidney tissue using Trizol (Thermo Fisher Scientific, Waltham, MA, USA) and subsequently treated with DNAse I (Promega, Madison, WI, USA). Total RNA (1 µg) from each sample was used to synthesize cDNA by single‐strand reverse transcription (Superscript III First Strand Synthesis SuperMix; Thermo Fisher Scientific). Expression of Acia2, amphiregulin (Areg), chemokine ligand 2 (Ccl2), Cd68, collagen type 1 αl (Col1a1), collagen type 3 αl (Col3a1), connective tissue growth factor (Ctgf), cysteine‐rich angiogenic inducer 61 (Cyr61), fibronectin 1 (Fn1), Fxr, Il‐6, nuclear receptor subfamily 0 group b member 2 or small heterodimer partner (Shp), Tgf‐β1, TIMP metallopeptidase inhibitor 1 (Timp1), and Tnf‐α in the affected kidney were analyzed by real‐time quantitative PCR using TaqMan gene expression assays on an Applied Bioscience 7900HT Fast Real Time PCR System and 384‐well plates with a reaction volume of 10 µl (Thermo Fisher Scientific). The 2(−2Ct ) method was used for relative quantification of mRNA with normalization to 18S. TaqMan probe sets used were as follows: 18S (Hs03003631_g1), Acta2 (Mm00725412_g1), Areg (Mm01354339_m1), Ccl2 (Mm00441242_m1), Cd68 (Mm03047343_m1), Col1a1 (Mm00801666_g1), Col3a1 (Mm01254454_g1), Ctgf (Mm01192933_g1), Cyr61 (Mm00487498_m1), Fn1 (Mm01256744_m1), Fxr (Mm00484523_m1), Il‐6 (Mm00446190_m1), Shp (Mm00442278_m1), Fgf‐β1 (Mm01178820_m1), Timp1 (Mm01341361_m1), and Tnf‐α (Mm00441883_g1).
Statistics
Results are expressed as the mean ± sd unless otherwise noted. One‐way ANOVA followed by post hoc Tukey's tests with 2‐tailed distribution were performed to analyze data among groups of ≥3. A Student's t test compared data between control and 1 experimental group. A value of P < 0.05 was considered significant.
RESULTS
Fxr and Shp expression is reduced with UUO
FXR is ubiquitously expressed in the body, specifically the liver, intestines, and kidney. The kidney has been shown to represent the highest concentration of FXR expression (25). Zhang et al. (26) demonstrated that FXR is expressed in almost all of the renal tubules, with higher concentrations in the proximal tubule as well as the thick ascending limb. In the current UUO model, the left ureter was permanently ligated in C57BL/6 mice for 12 d. We observed that Fxr mRNA expression was significantly reduced in UUO‐injured mice compared with sham‐treated animals [relative quantification (RQ) = 0.22 ± 0.06 vs. 1.1 ± 0.15, P < 0.01] (Fig. 1A ). Activated FXR induces the expression of the nuclear receptor subfamily 0 group b member 2 (also known as SHP). Loss of SHP has been shown to accelerate the development of renal fibrosis (27). We similarly observed that Shp mRNA expression was significantly reduced with UUO injury compared with sham (RQ = 0.17 ± 0.05 vs. 1.16 ± 0.20, P < 0.01) (Fig. 1B ). Treatment with EDP‐305 dose‐dependently increased both Fxr and Shp expression compared with the untreated UUO animals (RQ = 0.38 ± 0.03 for EDP‐305 30 mg/kg vs. 0.155 ± 0.06 for UUO, P < 0.05; and 0.38 ± 0.07 for EDP‐305 30 mg/kg vs. 0.10 ± 0.04 for UUO, P < 0.05) (Fig. 1C, D ).
Figure 1.
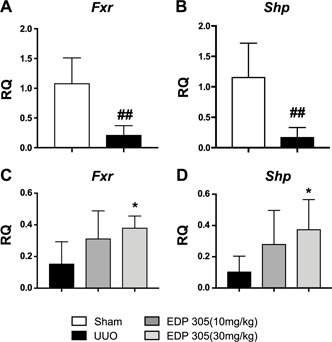
UUO results in reduction of Fxr and Shp expression in the ligated kidney. C57BL/6 mice underwent an irreversible UUO procedure on the left kidney for 12 d. A, B) Fxr (A) and Shp (B) expression are significantly reduced after UUO (n = 8/group). C, D) Fxr (C) and Shp (D) dose‐dependently increased with EDP‐305 10 and 30 mg/kg (n = 8/group). **P < 0.01 compared with sham, *P < 0.05 compared with UUO.
EDP‐305 reduces inflammatory and mesenchymal activation
Within hours of the UUO procedure, macrophages secrete proinflammatory cytokines and chemokines, predisposing the kidney to early tubular injury. In addition, epithelial, mesangial, and endothelial cells can also secrete cytokines upon activation (28, 29). In the UUO model, we observed accumulation of inflammatory cells within the interstitium spread uniformly across the cortex and the medulla (Fig. 2A ). Notably, on immunohistochemistry, we saw a significant elevation in macrophage‐positive F4/80 staining in UUO‐injured mice compared with sham‐treated animals (area quantification, 4.5 ± 0.5 vs. 0.74 ± 0.11, P < 0.01) (Fig. 2B, D ). After 8 d of treatment with the FXR agonist EDP‐305 via oral gavage, we saw a significant dose‐dependent reduction in inflammatory infiltration evident on both H&E as well as F4/80 quantification (4.5 ± 0.5 for UUO vs. 3.2 ± 0.52 with 10 mg/kg EDP‐305 vs. 1.4 ± 0.49 with 30 mg/kg EDP‐305, P < 0.01) (Fig. 2C ). Consistently, we observed a decrease in the mRNA expression of the macrophage marker Cd68 (RQ = 23.6 ± 5.7 for UUO vs. 9.2 ± 3.1 for EDP‐305 30 mg/kg, P < 0.05) as well as a dose‐dependent reduction in several proinflammatory cytokines, including Il‐6 (RQ = 208.3 ± 59.5 for UUO vs. 32.5 ± 3.3 for EDP‐305 30 mg/kg, P < 0.01), Tnf‐α (RQ = 34.2 ± 10.4 for UUO vs. 13.4 ± 2.8 for EDP‐305 30 mg/kg, P < 0.05), and Ccl2 (RQ = 208.3 ± 59.5 for UUO vs. 32.53 ± 3.3 for EDP‐305 30 mg/kg, P < 0.05) (Fig. 2E–G ).
Figure 2.
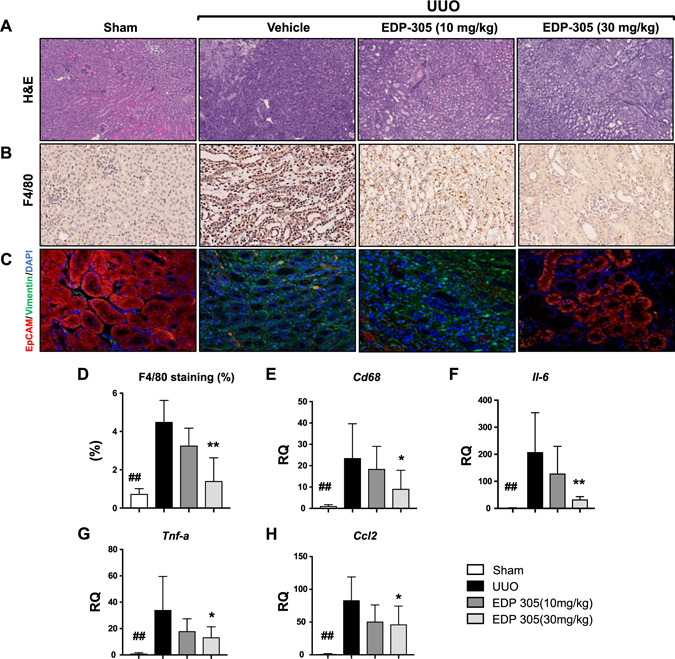
EDP‐305 reduces inflammatory and mesenchymal activation. A–C) Representative kidney sections of sham‐treated mice, UUO mice treated with vehicle control (0.5% methylcellulose), EDP‐305 10 mg/kg, or EDP‐305 30 mg/kg that were stained for H&E (A) (original magnification, ×10), F4/80 (B) (original magnification, ×10), or EpCAM and vimentin (C) (original magnification, ×20). D) F4/80 staining was quantified (n = 8/group). E–H) Expression of Cd68 (E), Il‐6 (F), Tnf‐α (G), and Ccl‐2 (H) was assessed by real‐time PCR using TaqMan primers (n = 8/group). ## P < 0.01 compared with sham, *P < 0.05, **P < 0.01 compared with UUO.
Mesenchymal activation plays a crucial role in the transition from fibroblasts to activated myofibroblasts. We performed immunofluorescence staining of paraffin‐embedded kidney sections with both an epithelial marker, EpCAM, and a mesenchymal marker, vimentin. In the sham‐treated mice, renal tubules stained positively for EpCAM, whereas no vimentin staining was detected. In the UUO‐injured mice, tubules lost EpCAM staining, but an up‐regulation of vimentin expression was seen within the interstitium. Interestingly, vimentin staining decreased in a dose‐dependent manner after treatment with EDP‐305, and EpCAM expression within the renal tubule was again regained (Fig. 2C ).
EDP‐305 reduces interstitial renal fibrosis
Complete UUO initiates a rapid sequence of events that leads to progressive fibrosis and, ultimately, renal tubular death over 1–2 wk. The UUO model accurately recapitulates a pattern of tubulointerstitial fibrosis similar to that seen in humans, and there is a direct relationship between the degree of fibrosis and the degree of renal injury (30).
To confirm extracellular matrix deposition within the interstitium, we first morphometrically analyzed disease by quantifying the CPA in Sirius Red—stained sections. We observed an increase in collagen staining within the interstitium (6.9 ± 0.95 vs. 0.59 ± 0.09, P < 0.01) but no changes within the glomerulus (11 ± 0.84 vs. 12 ± 0.69) or perivasculature (11.6 ± 1.9 vs. 14.4 ± 2.2) in the UUO‐injured mice compared with sham‐treated controls (Fig. 3A–D ). Treatment with EDP‐305 (either 10 or 30 mg/kg) for 8 d inhibited interstitial fibrosis as indicated by Sirius Red staining (6.9 ± 0.95 vs. 3.2 ± 0.38, P < 0.01 for EDP‐305 10 mg/kg; 2.6 ± 0.39, P < 0.01 for EDP‐305 30 mg/kg) but did not affect glomerular or vascular collagen deposition. EDP‐305 was further shown to reduce collagen deposition in a dose‐dependent manner by hydroxyproline analysis with a significant 26% reduction seen after treatment with EDP‐305 30 mg/kg (1504 ± 140 vs. 1089 ± 53.9, P < 0.05) (Fig. 3E ). Finally, a modified interstitial fibrosis and tubular atrophy (IF/TA) score was also calculated by a renal pathologist by determining the percent of cortical area involved by interstitial fibrosis and tubular atrophy. A significant (P < 0.05) reduction in the IF/TA score was observed after treatment with EDP‐305 30 mg/kg (Fig. 3F ).
Figure 3.
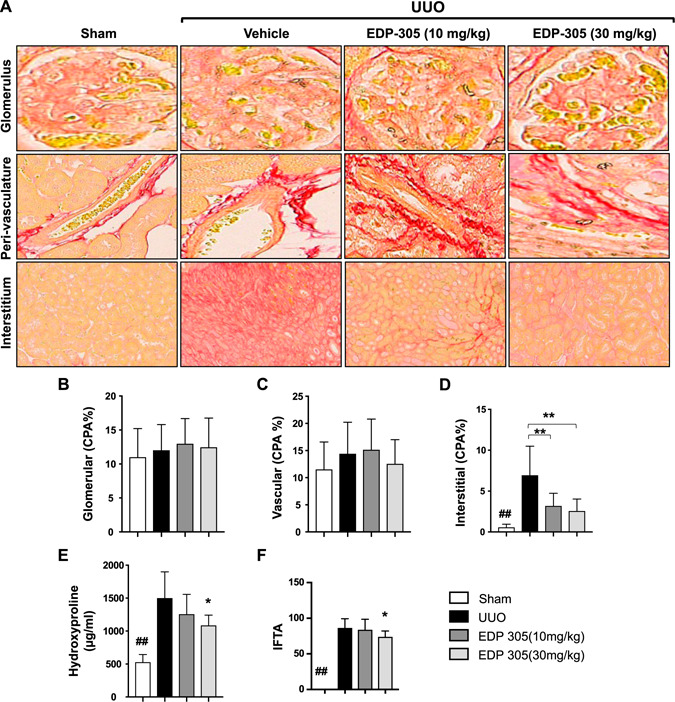
EDP‐305 attenuates interstitial renal fibrosis. A) Representative Sirius Red staining of the glomerulus (top panel; original magnification, ×40), vasculature (middle panel; original magnification, ×40), and the interstitium (lower panel; original magnification, ×20). B–D) CPA was calculated in the glomerulus (B) and perivascular (C) and within the intersititum (D) (n = 8/group). E) Hydroxyproline content was quantified in the whole kidney (n = 8/group). F) A modified IF/TA score was determined by a renal pathologist as a percent of the affected area (n = 8/group). ## P < 0.01 compared with sham, *P < 0.05, **P < 0.01 compared with UUO.
We further assessed the effects of EDP‐305 on renal fibrosis by measuring the expression of several profibrotic markers, including Acta2 (RQ = 9.4 ± 1.03 for UUO vs. 5.4 ± 0.92 for EDP‐305 30 mg/kg), Col1a1 (RQ = 53.5 ± 8.7 for UUO vs. 29.08 ± 5.94 for EDP‐305 30 mg/kg, P < 0.01), Col3a1 (RQ = 29.2 ± 2.7 for UUO vs. 14.2 ± 2.69 for EDP‐305 30 mg/kg, P < 0.01), Fn1 (19.95 ± 2.39 for UUO vs. 12.57 ± 1.52 for EDP‐305 30 mg/kg, P < 0.05), Timp1 (RQ = 572 ± 151 for UUO vs. 241.1 ± 44 for EDP‐305 30 mg/kg, P < 0.05), and Tgf‐β (RQ = 9.5 ± 1.4 for UUO vs. 5.5 ± 1.1 for EDP‐305 30 mg/kg). For the majority of these profibrotic genes, we observed a dose‐dependent reduction after EDP‐305 treatment, with significant reductions observed with the 30‐mg/kg dose (Fig. 4A–F ).
Figure 4.
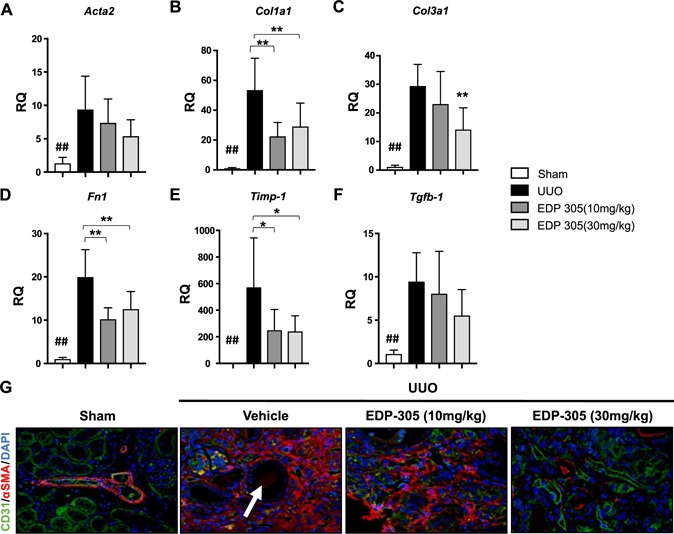
EDP‐305 reduces renal fibrosis and protects against peritubular capillary loss. A–F) Expression of Acta2 (A), Collai (B), Col3a1 (C), Fn1 (D), Timp1 (E), and Tgf‐β1 (F) was assessed with real‐time PCR with TaqMan primers (n = 8/group). G) Representative kidney sections of UUO mice treated with EDP305 10 or 30 mg/kg or vehicle control that were stained for CD31 or α‐SMA (original magnification, ×20) are shown. White arrow denotes proteinaceous debris within tubule. ## P < 0.01 compared with sham, *P < 0.05, **P < 0.01 compared with UUO.
EDP‐305 reduces peritubular capillary loss
Peritubular capillary rarefaction and interstitial fibrosis are often observed in concordance with tubular epithelial injury. Mesenchymal activation, microvascular regression, and tubular atrophy often perpetuate a vicious cycle that culminates in the eventual deterioration of renal function (31). We evaluated the effects of targeting FXR with EDP‐305 on peritubular capillary loss in the UUO model through dual immunofluorescence with the well‐established fibrotic marker, α‐SMA, and a vascular marker, CD31. Though very little α‐SMA was observed in the sham‐treated animals, except for the normal amount of deposition within the vascular basement membrane, the level of α‐SMA increased in UUO animals and colocalized with CD31. Tubules in the UUO model were dilated and filled with proteinaceous debris resembling tubular atrophy, and there was also less CD31 staining around the tubules. EDP‐305 treatment at 10 mg/kg resulted in less α‐SMA—positive staining, whereas treatment with 30 mg/kg dramatically reduced the α‐SMA—positive area and increased areas of CD31+ vasculature (Fig. 4G ).
EDP‐305 improves renal function
With the perpetuation of tubular damage caused by ongoing inflammation and interstitial fibrosis, there is a decrease in the number of functioning nephrons leading to atubular atrophy. The remaining nephrons become more vulnerable to injury, subsequently leading to filtration leaks, proteinuria, and decreased glomerular filtration rate (32). After observing a reduction in inflammatory infiltration and interstitial fibrosis with EDP‐305 treatment, we next set out to determine whether FXR agonism improved renal function in mice subjected to UUO. EDP‐305 had no effect on body weight or the weight of the liga ted or contralateral kidney. After the UUO procedure, serum protein decreased compared with sham, which is likely due to injury‐related nephropathy, and EDP‐305 treatment at both doses returned these levels back to baseline (4.16 ± 0.08 for UUO vs. 4.62 ± 0.13 for EDP‐30510 mg/kg and 4.64 ± 0.12 for EDP‐305 30 mg/kg). Similar findings were seen with serum albumin levels. In addition, renal function declined after the UUO procedure as indicated by an increased serum BUN:creatinine ratio, and EDP‐305 treatment dose‐dependently lowered the BUN: creatinine ratio (135.8 ± 9.46 for UUO vs. 84.08 ± 4.58 for EDP‐305 30 mg/kg; P < 0.01) (Supplemental Fig. S1A–F).
EDP‐305 treatment decreased YAP activation in UUO‐injured mice, and TGF‐B stimulated HK‐2 cells
The signaling mechanisms involved in renal fibrosis remain poorly understood. One proposed mechanism is the activation of the Hippo pathway, culminating in nuclear localization of the transcriptional coactivator YAP (33). YAP1 expression is predominately found in fibrotic tissue and regulates fibroblast activation and extracellular matrix (ECM) synthesis (34). We assessed YAP signaling in UUO‐injured mice through irnmunohistochemistry. Very little nuclear YAP was observed in the sham‐treated animals, but constitutive staining of cytoplasmic YAP was observed in the distal tubules. After UUO injury, we observed an increase in the expression of nuclear YAP within the interstitium, and EDP‐305 treatment resulted in less YAP activation, especially at the higher 30‐mg/kg dose (Fig. 5A ). We further assessed the role of EDP‐305 on YAP activation by measuring the mRNA expression of several YAP transcriptional targets, including Areg, Ctgf, and Cyr61. EDP‐305 treatment decreased the expression of all of these known YAP transcriptional targets in a dose‐dependent fashion (Fig. 5B–D ).
Figure 5.
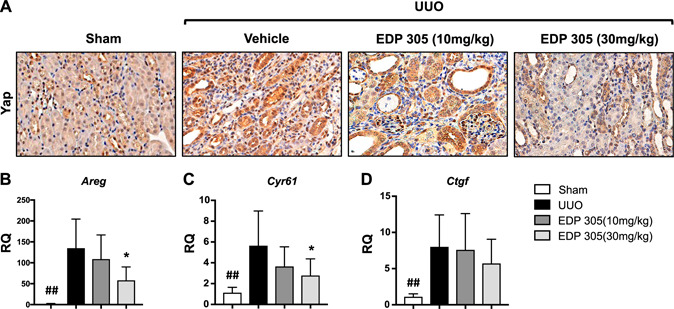
EDP‐305 reduces nuclear activation of YAP. A) Representative kidney sections from sham‐treated mice or UUO mice treated with EDP 305 10 or 30 mg/kg or vehicle control were stained for YAP (original magnification, ×40). B–D) Expression of Areg (B), Cyr61 (C), and Ctgf (D) was assessed with real‐time PCR with TaqMan probes (n = 8/group). ## P < 0.01 compared with sham, *P < 0.05 compared with UUO.
HK‐2 cells respond in a similar fashion to primary cultures of human proximal tubular cells and are thus a reliable and accurate way to study renal tubular biology. HK‐2 express Exr, and treatment with 5 µM EDP‐305 can further increase Exr expression (RQ = 1 ± 0.001 vs. 1.41 ± 0.08, P < 0.01). Interestingly, though Shp expression was not expressed at baseline in HK‐2 cells, treatment with EDP‐305 induced Shp expression (Fig. 6A, B ).
Figure 6.
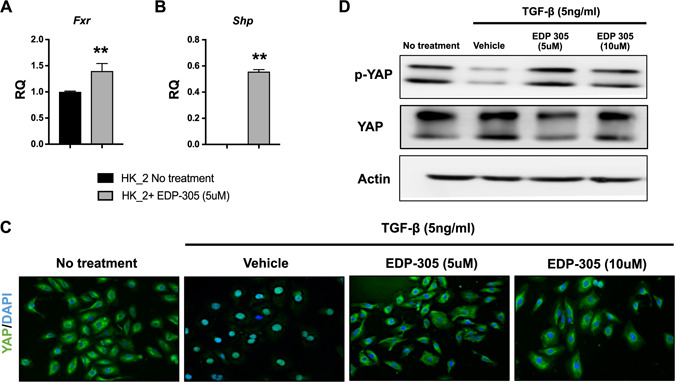
EDP‐305 reduces nuclear activation of YAP in TGF‐β stimulated human proximal tubule epithelial cells (HK‐2 cells). A, B) Expression of Fxr (A) and Shp (B) was measured after EDP‐305 stimulation. **P < 0.01 compared with untreated HK‐2 cells. HK‐2 cells were serum starved for 24 h and treated with 5 ng/ml of TGF‐β for 30 min in the presence or absence of FXR agonist. C) Immunofluorescence staining for YAP (original magnification, ×20). D) Western blot analysis was performed for the same study for phosphorylated YAP.
When serum‐starved HK‐2 cells were treated with 5 ng/ml TGF‐β1, we observed increased nuclear localization of YAP, a known driver of fibrosis. Next, we cotreated HK‐2 cells with TGF‐β1 and EDP‐305 for 30 min. Treatment with EDP‐305 decreased TGF‐β1—induced YAP activation as evidenced by increased cytoplasmic YAP staining (Fig. 6C ). We further investigated the effects of EDP‐305 on YAP activation by analyzing the expression of phosphorylated YAP. In the absence of phosphorylation, YAP enters the nucleus, where it stimulates genes involved in proliferation and migration (35). We observed that TGF‐β1 treatment alone decreased YAP phosphorylation, whereas treatment with EDP‐305 increased phosphorylated YAP (Fig. 6D ).
DISCUSSION
The degree of renal fibrosis, specifically tubulointerstitial fibrosis, reliably predicts the extent of renal injury and insufficiency. Interstitial collagen in a nonpathologic state maintains the structural integrity of the extracellular matrix. However, upon stress activation, large fibrous scars are formed accompanied by tubular loss, eventually leading to severe renal injury and parenchymal destruction.
The results of our investigation tie together several key observations. First, we observed mesenchymal activation within the interstitium after UUO. Markers for epithelium, such as EpCAM, were expressed in the renal tubules in uninjured animals, but UUO injury resulted in epithelial loss within the tubules and increased accumulation of mesenchymal cells. The ongoing deposition of ECM by these mesenchymal cells within the interstitium contributes to peritubular capillary loss and, ultimately, atubular atrophy. Furthermore, as the ECM accumulated, we observed a decrease in CD31‐positive vasculature. Studies have postulated that subendothelial pericytes can detach from the vascular basement membrane, leading to vascular loss. In addition, these migrated pericytes can contribute to interstitial activation in renal fibrosis (36), ultimately resulting in complete loss of renal function.
FXR is highly expressed within the kidney, and studies have demonstrated that FXR activation can mitigate renal injury. FXR activation using obeticholic acid has been shown to decrease inflammation antioxidative stress as well as tubulointerstitial fibrosis in an ischemic reperfusion model of renal injury (37). Consistently, we demonstrated the expression of FXR is decreased during chronic injury after ureteral ligation. EDP‐305 treatment reduced mesenchymal expression and restored epithelial expression within the renal tubules. In addition, treatment with EDP‐305 decreased inflammatory infiltration and subsequent renal fibrosis, which was associated with improvements in renal function as evidenced by the normalization of the BUN:creatinine ratio.
The detailed mechanisms by which FXR agonists reduce renal injury, fibrogenesis, and eventual tubular atrophy remain to be worked out. FXR also activates SHP, an atypical orphan nuclear receptor that binds to the cytochrome P450 family 7 subfamily A member 1 promotor to repress bile acid synthesis (38). In our study, we saw a significant up‐regulation of SHP expression in the UUO‐injured animal treated with EDP‐305. Studies have demonstrated that FXR is highly up‐regulated within the proximal renal tubule. We similarly found that treating a proximal tubule cell line with EDP‐305 resulted in an up‐regulation of both FXR and SHP.
Recently, YAP1 has also been shown to mediate the effects of TGF‐β1 signaling; however, the effects of FXR agonism on YAP activation has not been explored. Our results demonstrate that there is increased nuclear localization of YAP within the interstitium in UUO‐injured mice. We confirmed this finding by demonstrating that the transcriptional targets of YAP, including Areg, Ctgf, and Cyr61, are up‐regulated in UUO‐injured mice. After EDP‐305 treatment, YAP phosphorylation is increased, and nuclear YAP localization is decreased. Interestingly, Fxr and Shp knockout mice have increased YAP activation, resulting in the development of spontaneous liver tumors in mice (39). Thus, suppression of YAP nuclear localization may be a major mediator of the antifibrotic effects of FXR agonists in multiple organs.
CONCLUSIONS
Our study provides further evidence that FXR is renal protective. The results reported here have immediate and important clinical translational implications. Renal fibrosis ultimately leads to ESRD, and therefore FXR agonism could have clinical implications for patients with recurrent acute kidney injuries or CKD. FXR agonists that are under development for primary biliary cholangitis and non‐alcoholic steatohepatitis should also be explored in patients with renal fibrosis.
AUTHOR CONTRIBUTIONS
S. Li, Y. Li, G. Wang, M. Lanuti, P. Caravan, Y. S. Or, L.‐J. Jiang, K. K. Tanabe, and B. C. Fuchs designed the research; S. Li, S. Ghoshal, M. Sojoodi, G. Arora, and D. J. Erstad performed the research; S. Li, M. Sojoodi, R. Masia, and D. S. Ferriera contributed new reagents or analytic tools; S. Li, S. Ghoshal, M. Sojoodi, G. Arora, and D. J. Erstad analyzed the data; and S. Li wrote the manuscript.
Supporting information
Supplementary Material 1
Supplementary Material 2
Li, S. , Ghoshal, S. , Sojoodi, M. , Arora, G. , Masia, R. , Erstad, D. J. , Ferriera, D. S. , Li, Y. , Wang, G. , Lanuti, M. , Caravan, P. , Or, Y. S. , Jiang, L.-J. , Tanabe, K. K. , Fuchs, B. C. The farnesoid X receptor agonist EDP‐305 reduces interstitial renal fibrosis in a mouse model of unilateral ureteral obstruction. FASEB J. 33, 7103–7112 (2019). www.fasebj.org
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1. Liu, Y. (2006) Renal fibrosis: new insights into the pathogenesis and therapeutics. Kidney Int. 69, 213–217 [DOI] [PubMed] [Google Scholar]
- 2. Collins, A. J. , Foley, R. N. , Gilbertson, D. T. , and Chen, S. C. (2009) The state of chronic kidney disease, ESRD, and morbidity and mortality in the first year of dialysis. Clin. J. Am. Soc. Nephrol. 4 (Suppl 1), S5–S11 [DOI] [PubMed] [Google Scholar]
- 3. Liu, Y. (2011) Cellular and molecular mechanisms of renal fibrosis. Nat. Rev. Nephrol. 7, 684–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nath, K. A. (1992) Tubulointerstitial changes as a major determinant in the progression of renal damage. Am. J. Kidney Dis. 20, 1–17 [DOI] [PubMed] [Google Scholar]
- 5. Eardley, K. S. , Kubal, C. , Zehnder, D. , Quinkler, M. , Lepenies, J. , Savage, C. O. , Howie, A. J. , Kaur, K. , Cooper, M. S. , Adu, D. , and Cockwell, P. (2008) The role of capillary density, macrophage infiltration and interstitial scarring in the pathogenesis of human chronic kidney disease. Kidney Int. 74, 495–504 [DOI] [PubMed] [Google Scholar]
- 6. Huen, S. C. , Moeckel, G. W. , and Cantley, L. G. (2013) Macrophage-specific deletion of transforming growth factor-β1 does not prevent renal fibrosis after severe ischemia-reperfusion or obstructive injury. Am. J. Physiol. Renal Physiol. 305, F477–F484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gabbiani, G. , Ryan, G. B. , and Majne, G. (1971) Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia 27, 549–550 [DOI] [PubMed] [Google Scholar]
- 8. Burns, W. C. , Kantharidis, P. , and Thomas, M. C. (2007) The role of tubular epithelial-mesenchymal transition in progressive kidney disease. Cells Tissues Organs (Print) 185, 222–231 [DOI] [PubMed] [Google Scholar]
- 9. Masszi, A. , Fan, L. , Rosivall, L. , McCulloch, C. A. , Rotstein, O. D. , Mucsi, I. , and Kapus, A. (2004) Integrity of cell-cell contacts is a critical regulator of TGF-β 1-induced epithelial-to-myofibroblast transition: role for β-catenin. Am. J. Pathol. 165, 1955–1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chevalier, R. L. , Forbes, M. S. , and Thornhill, B. A. (2009) Ureteral obstruction as a model of renal interstitial fibrosis and obstructive nephropathy. Kidney Int. 75, 1145–1152 [DOI] [PubMed] [Google Scholar]
- 11. Verbeke, L. , Mannaerts, I. , Schierwagen, R. , Govaere, O. , Klein, S. , Vander Elst, I. , Windmolders, P. , Farre, R. , Wenes, M. , Mazzone, M. , Nevens, F. , van Grunsven, L. A. , Trebicka, J. , and Laleman, W. (2016) FXR agonist obeticholic acid reduces hepatic inflammation and fibrosis in a rat model of toxic cirrhosis. Sci. Rep. 6, 33453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gadaleta, R. M. , van Erpecum, K. J. , Oldenburg, B. , Willemsen, E. C. , Renooij, W. , Murzilli, S. , Klomp, L. W. , Siersema, P. D. , Schipper, M. E. , Danese, S. , Penna, G. , Laverny, G. , Adorini, L. , Moschetta, A. , and van Mil, S. W. (2011) Farnesoid X receptor activation inhibits inflammation and preserves the intestinal barrier in inflammatory bowel disease. Gut 60, 463–472 [DOI] [PubMed] [Google Scholar]
- 13. Nagle, R. B. , Bulger, R. E. , Cutler, R. E. , Jervis, H. R. , and Benditt, E. P. (1973) Unilateral obstructive nephropathy in the rabbit. I. Early morphologic, physiologic, and histochemical changes. Lab. Invest. 28, 456–467 [PubMed] [Google Scholar]
- 14. Zhong, J. , Yang, H. C. , and Fogo, A. B. (2017) A perspective on chronic kidney disease progression. Am. J. Physiol. Renal Physiol. 312, F375–F384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fried, L. F. , Emanuele, N. , Zhang, J. H. , Brophy, M. , Conner, T. A. , Duckworth, W. , Leehey, D. J. , McCullough, P. A. , O'Connor, T. , Palevsky, P. M. , Reilly, R. F. , Seliger, S. L. , Warren, S. R. , Watnick, S. , Peduzzi, P. , and Guarino, P. ; VA NEPHRON-D Investigators . (2013) Combined angiotensin inhibition for the treatment of diabetic nephropathy. N. Engl. J. Med. 369, 1892–1903; erratum: 158, A7255 [DOI] [PubMed] [Google Scholar]
- 16. De Zeeuw, D. , Akizawa, T. , Audhya, P. , Bakris, G. L. , Chin, M. , Christ-Schmidt, H. , Goldsberry, A. , Houser, M. , Krauth, M. , Lambers Heerspink, H. J. , McMurray, J. J. , Meyer, C. J. , Parving, H. H. , Remuzzi, G. , Toto, R. D. , Vaziri, N. D. , Wanner, C. , Wittes, J. , Wrolstad, D. , and Chertow, G. M. ; BEACON Trial Investigators . (2013) Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N. Engl. J. Med. 369, 2492–2503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mann, J. F. , Green, D. , Jamerson, K. , Ruilope, L. M. , Kuranoff, S. J. , Littke, T. , and Viberti, G. ; ASCEND Study Group . (2010) Avosentan for overt diabetic nephropathy. J. Am. Soc. Nephrol. 21, 527–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cho, M. E. , Smith, D. C. , Branton, M. H. , Penzak, S. R. , and Kopp, J. B. (2007) Pirfenidone slows renal function decline in patients with focal segmental glomerulosclerosis. Clin. J. Am. Soc. Nephrol. 2, 906–913 [DOI] [PubMed] [Google Scholar]
- 19. Sharma, K. , Ix, J. H. , Mathew, A. V. , Cho, M. , Pflueger, A. , Dunn, S. R. , Francos, B. , Sharma, S. , Falkner, B. , McGowan, T. A. , Donohue, M. , Ramachandrarao, S. , Xu, R. , Fervenza, F. C. , and Kopp, J. B. (2011) Pirfenidone for diabetic nephropathy. J. Am. Soc. Nephrol. 22, 1144–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Alikhan, M. A. , and Ricardo, S. D. (2013) Mononuclear phagocyte system in kidney disease and repair. Nephrology (Carlton) 18, 81–91 [DOI] [PubMed] [Google Scholar]
- 21. Verma, S. K. , and Molitoris, B. A. (2015) Renal endothelial injury and microvascular dysfunction in acute kidney injury. Semin. Nephrol. 35, 96–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jiang, T. , Wang, X. X. , Scherzer, P. , Wilson, P. , Tallman, J. , Takahashi, H. , Li, J. , Iwahashi, M. , Sutherland, E. , Arend, L. , and Levi, M. (2007) Farnesoid X receptor modulates renal lipid metabolism, fibrosis, and diabetic nephropathy. Diabetes 56, 2485–2493 [DOI] [PubMed] [Google Scholar]
- 23. Zhao, K. , He, J. , Zhang, Y. , Xu, Z. , Xiong, H. , Gong, R. , Li, S. , Chen, S. , and He, F. (2016) Activation of FXR protects against renal fibrosis via suppressing Smad3 expression. Sci. Rep. 6, 37234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hutson, P. R. , Crawford, M. E. , and Sorkness, R. L. (2003) Liquid chromatographic determination of hydroxyproline in tissue samples. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 791, 427–430 [DOI] [PubMed] [Google Scholar]
- 25. Ding, L. , Yang, L. , Wang, Z. , and Huang W. (2015) Bile acid nuclear receptor FXR and digestive system diseases. Acta Pharm. Sin. B5, 135–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang, Y. , Lee, F. Y. , Barrera, G. , Lee, H. , Vales, C. , Gonzalez, F. J. , Willson, T. M. , and Edwards, P. A. (2006) Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. Proc. Natl. Acad. Sci. USA 103, 1006–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jung, G. S. , Kim, M. K. , Choe, M. S. , Lee, K. M. , Kim, H. S. , Park, Y. J. , Choi, H. S. , Lee, K. U. , Park, K. G. , and Lee, I. K. (2009) The orphan nuclear receptor SHP attenuates renal fibrosis. J. Am. Soc. Nephrol. 20, 2162–2170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zeisberg, M. , and Neilson, E. G. (2010) Mechanisms of tubulointerstitial fibrosis. J. Am. Soc. Nephrol. 21, 1819–1834 [DOI] [PubMed] [Google Scholar]
- 29. Hodgkins, K. S. , and Schnaper, H. W. (2012) Tubulointerstitial injury and the progression of chronic kidney disease. Pediatr. Nephrol. 27, 901–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ma, L. J. , Yang, H. , Gaspert, A. , Carlesso, G. , Barty, M. M. , Davidson, J. M. , Sheppard, D. , and Fogo, A. B. (2003) Transforming growth factor-β-dependent and -independent pathways of induction of tubulointerstitial fibrosis in β6(-/-) mice. Am. J. Pathol. 163, 1261–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li, X. , and Zhuang, S. (2014) Recent advances in renal interstitial fibrosis and tubular atrophy after kidney transplantation. Fibrogenesis Tissue Repair 7, 15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schnaper, H. W. (2017) The tubulointerstitial pathophysiology of progressive kidney disease. Adv. Chronic Kidney Dis. 24, 107–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang, J. , Ji, J. Y. , Yu, M. , Overholtzer, M. , Smolen, G. A. , Wang, R. , Brugge, J. S. , Dyson, N. J. , and Haber, D. A. (2009) YAP-dependent induction of amphiregulin identifies a non-cell-autonomous component of the Hippo pathway. Nat. Cell Biol. 11, 1444–1450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liu, F. , Lagares, D. , Choi, K. M. , Stopfer, L. , Marinković, A. , Vrbanac, V. , Probst, C. K. , Hiemer, S. E. , Sisson, T. H. , Horowitz, J. C. , Rosas, I. O. , Fredenburgh, L. E. , Feghali-Bostwick, C. , Varelas, X. , Tager, A. M. , and Tschumperlin, D. J. (2015) Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 308, L344–L357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Das, A. , Fischer, R. S. , Pan, D. , and Waterman, C. M. (2016). YAP nuclear localization in the absence of cell-cell contact is mediated by a filamentous actin-dependent, myosin II- and phospho-YAP-independent pathway during extracellular matrix mechanosensing. J. Biol. Chem. 291, 6096–6110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fiorucci, S. , Rizzo, G. , Donini, A. , Distrutti, E. , and Santucci, L. (2007) Targeting farnesoid X receptor for liver and metabolic disorders. Trends Mol. Med. 13, 298–309 [DOI] [PubMed] [Google Scholar]
- 37. Gai, Z. , Chu, L. , Xu, Z. , Song, X. , Sun, D. , and Kullak-Ublick, G. A. (2017) Farnesoid X receptor activation protects the kidney from ischemia-reperfusion damage. Sci. Rep. 7, 9815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Goodwin, B. , Jones, S. A. , Price, R. R. , Watson, M. A. , McKee, D. D. , Moore, L. B. , Galardi, C. , Wilson, J. G. , Lewis, M. C. , Roth, M. E. , Maloney, P. R. , Willson, T. M. , and Kliewer, S. A. (2000) A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol. Cell 6, 517–526 [DOI] [PubMed] [Google Scholar]
- 39. Anakk, S. , Bhosale, M. , Schmidt, V. A. , Johnson, R. L. , Finegold, M. J. , and Moore, D. D. (2013) Bile acids activate YAP to promote liver carcinogenesis. Cell Rep. 5, 1060–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1
Supplementary Material 2


