ABSTRACT
Osteoblast differentiation and proliferation are regulated by several modulators, among which are adenosine A2a receptors (A2ARs) and Wingless/Integrated‐β‐catenin pathways. Cytosolic β‐catenin stabilization promotes its nuclear translocation and transcriptional activity. In the present study, we seek to determine whether there is a connection between A2AR stimulation and cellular β‐catenin levels in osteoblasts. Osteoblast precursor cell line (MC3T3‐E1) and primary murine osteoblasts were treated with CGS21680, a highly selective A2AR agonist. We analyzed cellular content and nuclear translocation of phosphorylated (p)‐serine 552 (S552) β‐catenin in response to A2AR stimulation in MC3T3‐E1 cells, in both wild‐type and A2AR knockout (A2AKO) mice. Moreover, we measured cellular β‐catenin levels in MC3T3‐E1 cells transfected with scrambled or protein kinase B (Akt) small interfering RNA following A2AR activation. CGS21680 (1 µM) stimulated an increase in both the cellular content and nuclear translocation of p‐S552 β‐catenin after 15 min of incubation. A2AR activation had no tangible effect on the cellular β‐catenin level either in A2AKO mice or in osteoblasts with diminished Akt content. Our findings demonstrate an interaction between A2AR, β‐catenin, and Akt signaling in osteoblasts. The existence of such a crosstalk has significant repercussions in the development of novel therapeutic approaches targeting medical conditions associated with reduced bone density.—Borhani, S., Corciulo, C., Larranaga‐Vera, A., Cronstein, B. N. Adenosine A2a receptor (A2AR) activation triggers Akt signaling and enhances nuclear localization of β‐catenin in osteoblasts. FASEB J. 33, 7555–7562 (2019). www.fasebj.org
Keywords: canonical Wnt pathway, bone regeneration, CGS21680
ABBREVIATIONS
- A2AKO
A2AR knockout
- A2AR
adenosine A2A receptor
- Akt
protein kinase B
- BS A.
bovine serum albumin
- FBS
fetal bovine serum
- Fz
frizzled
- GSK3
glycogen synthase kinase 3
- IF
immunofluorescence
- LRP
LDL receptor related protein
- S552
serine 552
- siRNA
small interfering RNA
- Wnt
Wingless/Integrated
- WT
wild type
Osteoblast differentiation, proliferation, and bone formation are regulated by a number of local and systemic factors, including the adenosine‐mediated signaling and Wingless/Integrated (Wnt)‐β‐catenin pathway. The adenosine A2a receptor (A2AR) is linked to Gs proteins, which activate adenylyl cyclase and augment cellular cAMP levels. cAMP in turn induces a series of kinases, which participate in downstream signaling pathways. A2AR stimulation promotes bone regeneration while diminishing osteoclast differentiation in vitro and in a variety of models (1–4).
Wnt‐β‐catenin, also referred to as canonical Wnt signaling, affects bone mass through several mechanisms, including: 1) mediating renewal of stem cells, 2) promoting mesenchymal precursor differentiation to osteoblasts, 3) inducing proliferation of osteoblasts, and 4) inhibiting bone cell apoptosis (5–7). The dual signaling and adhesion β‐catenin protein transduces Wnt signals through interacting with the T‐cell factor and lymphocyte enhancer factor to promote transcriptional activities. Cytoplasmic β‐catenin is constantly degraded by a complex of proteins [i.e., the scaffolding protein Axin 1/2, adenomatous polyposis coli, glycogen synthase kinase 3 (GSK3), casein kinase 1, and protein phosphatase 2A]. The Wnt‐β‐catenin pathway is activated upon binding a Wnt ligand to the transmembrane domain‐spanning frizzled (Fz) receptor and LDL receptor related proteins (LRPs) 5 and 6 at the cell surface. The Wnt‐activated Fz receptor and LRPs inhibit β‐catenin destruction complex, thus allowing for β‐catenin protein stabilization and nuclear translocation (8).
β‐Catenin protein is modified by regulatory phosphorylation at multiple sites. In particular, it has been demonstrated that phosphorylation at serine 552 (S552) by protein kinase B (Akt) accelerates β‐catenin nuclear translocation and T‐cell factor‐β‐catenin transactivation, thus promoting its transcriptional activity (9–14). In prior studies, we showed that A2AR stimulation (15) leads to enhanced nuclear translocation of β‐catenin in primary human fibroblasts, whereas A2AR blockade (16) diminishes its nuclear localization in fibroblasts in vivo. Here, we investigate the impact of A2AR activation on cellular β‐catenin level in osteoblasts both in vitro and in vivo, as well as the role of Akt signaling as a potential intermediate pathway.
MATERIALS AND METHODS
The A2AR agonist used in this study, CGS21680, was purchased from Tocris Bioscience (Bristol, United Kingdom). The protease and phosphatase inhibitor cocktail was purchased from MilliporeSigma (Burlington, MA, USA). The NE‐PER Nuclear and Cytoplasmic Extraction Kit and the BCA protein assay were obtained from Thermo Fisher Scientific (Waltham, MA, USA). The list of antibodies used in this study includes anti β‐catenin antibody (610154; BD Biosciences, San Jose, CA, USA), anti‐phospho‐β‐catenin antibody S552 (9566; Cell Signaling Technology, Danvers, MA, USA), anti‐phosphorylated (p)‐Akt antibody S473 (Cell Signaling Technology), anti β‐actin antibody (Abcam, Cambridge, United Kingdom), mAb to nuclear matrix protein p84 (Abcam), and anti‐osteopontin antibody (Santa Cruz Biotechnology, Dallas, TX, USA). The secondary antibodies (IRDye R 800 CW, goat anti‐rabbit and IRDye R 600 CW, goat anti‐mouse) were purchased from Li‐Cor Biosciences (Lincoln, NE, USA).
Cell culture and cellular extract preparation
C57BL/6 mice were purchased from Taconic Bioscience (Albany, NY, USA) and maintained in the New York University School of Medicine Medical Center animal facility. C57BL/6 mice lacking A2AR, previously described in Mediero et al. (3), were maintained in the New York University School of Medicine Medical Center animal facility. They were kept under regular lighting conditions (12‐h light/dark cycles) and provided food pellets and water ad libitum. The mice used in this study were male aged between 10 and 12 wk. All experimental procedures were approved by the New York University School of Medicine Institutional Animal Care and Use Committee. Mice were euthanized by CO2 narcosis and femoral bone marrow was obtained after flushing. Bone marrow cells from aspirates were cultured (5% CO2 at 37°C) in α‐minimum essential medium containing 10% of fetal bovine serum (FBS), 100 µg/ml streptomycin, and 100 U/ml penicillin. The nonadherent cells were removed, and primary marrow cells were cultured in DMEM for a period of 10–14 d to differentiate into osteoblasts. The medium was supplemented with 2% FBS, 1% penicillin‐streptomycin, 5 µM ascorbic acid, 50 µM glycerophosphate, and 0.1 µM dexamethasone. Osteoblasts were subcultured into 6‐well plates and further treated with the A2AR agonist. Following incubation, the cells were lysed with RIPA buffer and a mixture of protease inhibitor and phosphatase inhibitor cocktails. Nuclear and cytoplasmic proteins were extracted using NE‐PER nuclear and cytoplasmic extraction reagent kit in accordance with the manufacturer's instructions. BCA protein assay was used to quantify protein concentration following the manufacturers' instructions. All experiments on primary osteoblasts were conducted using osteogenic medium.
The murine osteoblast precursor cell line (MC3T3‐E1) was obtained from American Type Culture Collection Manassas, VA, USA. Osteoblastoid cells were cultured (5% CO2 at 37°C) in α‐minimum essential medium containing 10% FBS, 100 µg/ml streptomycin, and 100 U/ml penicillin. Cellular extraction preparation methods were performed as previously described.
Western blot assays and immunoprecipitations
Cell lysates were loaded on 10% SDS‐PAGE gels (Bio‐Rad, Hercules, CA, USA) and transferred onto nitrocellulose membranes (GE Healthcare, Waukesha, WI, USA). Transblotting buffer contained Tris (20 mM), glycine (150 mM), and 20% of methanol. The membranes were blocked in Tris‐buffered saline with 0.1% Tween 20 and 3% bovine serum albumin (BSA) (MilliporeSigma) for a period of 1 h at room temperature and incubated overnight with primary antibodies at 4°C Afterwards, membranes were washed in Trisbuffered saline with 0.1% Tween 20 and incubated with immunofluorescent antibodies for another hour. The immunoblots were identified using Li‐Cor Odyssey equipment (Li‐Cor Biosciences). Band density quantification was normalized to actin or p84.
Immunocytochemistry
We analyzed nuclear translocation of p‐S552 β‐catenin in MC3T3‐E1 cells, as well as both primary wild‐type (WT) and A2AR knockout (A2AKO) osteoblasts cultures through immunofluorescence (IF) staining techniques. To perform the immunocytochemistry method, osteoblastoid cells and primary osteoblasts were plated on Lab‐Tek II chamber slides (Thermo Fisher Scientific) and fixed in paraformaldehyde (4%). After washing with PBS, the samples were incubated with 0.25% Triton X‐100. Cell cultures were blocked in a mixture of PBS‐Tween 20 and BSA (1%) for 30 min and then incubated overnight with anti‐phospho‐β‐catenin antibody S552 (dilution of 1:200) in a humidified chamber at 4°C. The samples were washed in PBS and exposed to conjugated antibodies against rabbit for 1 h at room temperature. We used Fluorcshield with DAPI (MilliporeSigma) as a counterstain, and ultimately, the IF signals were detected by a Nikon Eclipse fluorescence microscope (Nikon, Tokyo, Japan).
In vivo studies
We examined male C57B1/6 WT mice aged 6–8 wk. Male mice were used because their bone mass is greater, and they are not subject to hormonal variation in bone production. The mice were anesthetized by intraperitoneal injection of 100 mg/kg ketamine and 10 mg/kg xylazine, and a full‐thickness midline incision was made, extending from the nasofrontal to occipital region. The calvaria was trepanned to create a 3‐mm defect with a punch biopsy apparatus and then covered with a collagen sponge (DuraGen Plus; Integra LifeScienes, Plainsboro, NJ, USA). The sponges contained either 1 µM CGS21680 or 0.9% saline in the control group. Additional treatment included daily local injection of 1 µM CGS21680 or 0.9% saline, beginning immediately after incision closure and continuing every day until euthanization. Animals were euthanized after 8 wk following defect formation in a CO2 chamber. The calvariae were removed and fixed in 4% paraformaldehyde for 48 h followed by decalcification in 10% EDTA for 4 wk and paraffin embedding (3).
Immunohistochemistry
The fixed paraffin‐embedded bone sections were deparaffinized in xylene for a total of 30 min and subsequently hydrated with a descending concentration of ethanol (i.e., 100–70%) and, eventually, distilled water. To retrieve the antigen, slides were incubated in a mixture of proteinase K. and glycerol in Tris and ethylenediaminetetra‐acetic acid buffer (pH = 8.0) for 20 min. After washing with PBS, the specimens were blocked in PBS‐BSA (3%), Triton X‐100 (1%), and FBS (5%) for 1 h and incubated with anti–phospho–β‐catenin antibody S552 (dilution of 1:200) and anti‐osteopontin antibody (dilution of 1:200) overnight in a humidified chamber. We used conjugated antibodies against rabbit and mouse as the secondary antibodies. The remaining steps are identical to those described in the immunocytochemistry section.
RNA interference and gene silencing
The MC3T3‐E1 cells were transfected with silencer small interfering RNA (siRNA) standard for Akt‐1 or negative control (Thermo Fisher Scientific) at a concentration of 5 nM. We used Lipofectamine 2000 reagent and Block‐iT fluorescent control (Thermo Fisher Scientific) to transfect the cells. The cells were examined 4 h after transfection using Evos fluorescence cell imaging system (Thermo Fisher Scientific). The transfected cells were treated with 1 µM CGS21680 for 15 min, and the cell lysates were collected for Western blot analysis. We used anti–Akt‐1 (Cell Signaling Technology) for immunoblot detection.
Statistical analysis
All data reported in this study are presented as difference between mean ± se, computed using a Student's t test for observations involving 2 groups and 1‐way ANOVA test with repeated observations and post hoc Bonferroni test for experiments with observations involving more than 2 groups. In either case, a value of P < 0.05 is used to indicate statistical significance. All statistical analyses were performed using Prism Software v.7.05 (GraphPad Software, La Jolla, CA, USA).
RESULTS
A2AR stimulation is associated with increased β‐catenin nuclear translocation
To explore intracellular signaling via A2AR in osteoblasts, we examined the effect of CGS21680, a selective A2AR agonist, on S552 phosphorylation of β‐catenin. Treatment of MC3T3C‐E1 cells with CGS21680 (1 µM) stimulated an increase in cellular content of p‐S552 β‐catenin (Fig. 1A ) and total β‐catenin (Fig. 1B ) following 15 min of incubation, an increase which was maintained for up to 4 h. Moreover, there was a dose‐dependent increase in cellular content of p‐S552 β‐catenin (Fig. 2A ), as well as total β‐catenin after 15 min of incubation (Fig. 2B ).
Figure 1.
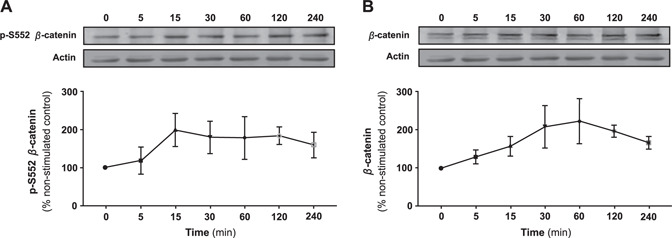
A2AR stimulation promotes phosphorylation of β‐catenin and increases total cellular β‐catenin levels in MC3T3C‐E1 cells in a time‐dependent fashion. The effect of CGS21680 treatment (1 µM) on the cellular content of p‐S552 β‐catenin (A) and total β‐catenin (B) in MC3T3C‐E1 cells over time. Data represents mean percentage nonstimulated control ± sem of 5 independent experiments. Levels increased of both p‐S552 β‐catenin and total cellular β‐catenin over time.
Figure 2.
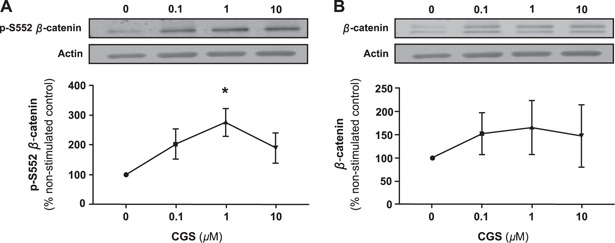
A2AR stimulation increases p‐S552 β‐catenin and total β‐catenin in MC3T3C‐E1 cells in a dose‐dependent fashion. The effect of exposure to different concentrations of CGS21680 for 15 min on the cellular content of p‐S552 β‐catenin (A) and total β‐catenin (B) in MC3T3C‐E1 cells. Data represents mean change ± sem of 3 independent experiments. *P < 0.05, 1‐way ANOVA, repeated measures.
Next, we examined the effect of A2AR stimulation on nuclear localization of p‐S522 β‐catenin by semiquantitative analysis of Western blots on nuclear and cytosolic extracts. We observed a statistically significant increase in p‐S552 β‐catenin level in the nuclear fraction of (Fig. 3A ), as well as the cytosolic extracts (Fig. 3B ) in MC3T3C‐E1 cells after treatment with CGS21680 (1 µM) for 15 min. Examining primary murine osteoblasts in the same experimental settings, we found a significant increase in p‐S552 β‐catenin level in both the nuclear fractions (Fig. 3C ) and cytosolic extracts (Fig. 3D ).
Figure 3.
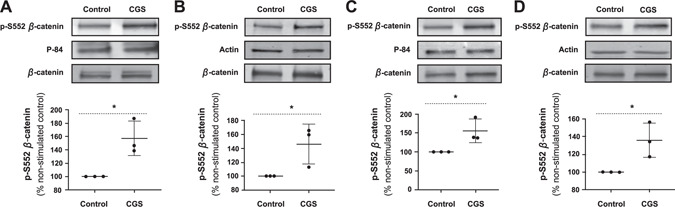
A2AR stimulation increases nuclear translocation of p‐S552 β‐catenin in MC3T3C‐E1 and primary murine osteoblasts detected by Western blot assays. The p‐S552 β‐catenin levels after treatment with CGS21680 (1 µM) for 15 min compared with control in the nuclear fractions in MC3T3C‐E1 cells (A) and the associated cytosolic extracts (B), as well as the nuclear fractions in primary murine osteoblasts (C) and the associated cytosolic extracts (D). *P < 0.05, Student's t test.
To confirm the nuclear translocation of p‐S552 β‐catenin through IF staining, we examined cultured osteoblastoid cells and primary osteoblasts after treatment with CGS21680 (1 µM) for 15 min. A2AR activation in MC3T3C‐E1 cells led to an increase in nuclear translocation of p‐S522 β‐catenin (Fig. 4A ). Similarly, CGS21680 raised nuclear localization of p‐S522 β‐catenin in primary murine osteoblasts (Fig. 4B ).
Figure 4.
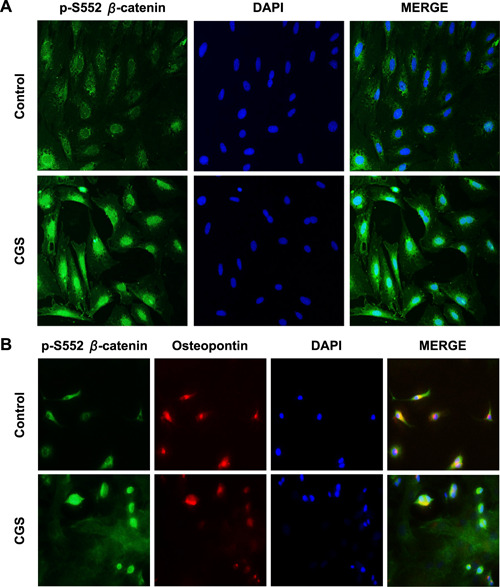
A2AR stimulation promotes nuclear translocation of P‐S552 β‐catenin in MC3T3C‐E1 and primary murine osteoblasts detected by IF staining. Nuclear translocation of p‐S522 β‐catenin after CGS21680 treatment for 15 min in cultured MC3T3C‐E1 cells (A) and primary murine osteoblasts (B). The merge panel is formed by superimposing p‐S522 β‐catenin, osteopontin, and DAPI images. Original magnification, ×40.
A2AR stimulation promotes Akt kinase activity and β‐catenin phosphorylation
We had previously observed that A2AR signaling stimulated Akt activation in fibroblasts (15, 17). To investigate whether A2AR activation leads to increased Akt kinase activity in osteoblasts, we measured both the total and activated Akt isoform in response to CGS21680 treatment, revealing a statistically significant increase in p‐S473 Akt, with no detectable change in the total Akt level following A2AR activation (Fig. 5 ).
Figure 5.
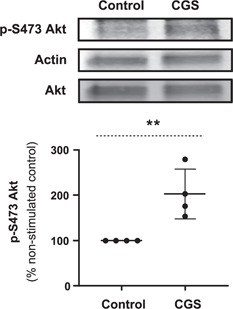
A2AR stimulation increases cellular levels of activated Akt. The P‐S473 Akt level remarkably increased in MC3T3C‐E1 cells after treatment with CGS21680 (1 µM) for 15 min. Total cellular Akt level did not change following A2AR activation. **P < 0.01, Student's t test.
To determine whether activation of Akt is required for observing the previously stated results, we knocked down Akt‐1 in MC3T3C‐E1 cells by liposomal transfection of siRNA (4 h, 37°C). Transfection (Fig. 6A, B ) of siRNA for Akt‐1 but not scrambled siRNA caused reduction in cellular p‐S473 Akt levels (Fig. 6C ). We found that whereas A2AR activation with CGS21680 (1 µM) increases cellular p‐S552 β‐catenin levels in cells transfected with scrambled siRNA, it has no effect on p‐S552 β‐catenin levels in the Akt‐1 siRNA‐transfected cells (Fig. 6D ).
Figure 6.
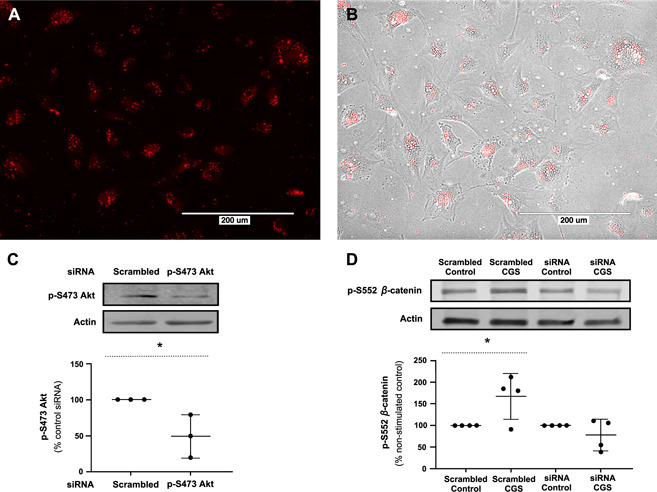
A2AR stimulation does not increase p‐S552 β‐catenin levels after Akt knockdown. A) Liposomal transfection of siRNA in MC3T3C‐E1 cells (4 h). Scale bar, 200 µm. B) Superimposition of bright‐field and fluorescent images. Scale bar, 200 µm. C) Transfection of siRNA for Akt‐1 reduced cellular p‐S473 Akt levels. *P < 0.05, Student's t test. D) Stimulation of A2AR with CGS21680 (1 µM) for 15 min increased cellular p‐S552 β‐catenin levels in the cells transfected with scrambled siRNA but had no effect on p‐S552 β‐catenin levels in the Akt‐1 siRNA‐transfected cells. *P < 0.05, Student's t test.
A2AR activation increases β‐catenin and promotes bone regeneration in vivo
To determine whether the effect of in vitro observations was relevant to A2AR‐stimulated bone growth in vivo, we examined cellular p‐S552 β‐catenin levels at sites of bone regeneration stimulated by local application of CGS21680. We studied decalcified sections of calvaria taken from the edges of trephination sites in the skulls of mice treated with CGS21680, the same A2AR agonist used in the in vitro studies. Compared with the control groups treated with 0.9% saline, we observed increased cellular p‐S552 β‐catenin levels in the regenerating bone in CGS21680‐treated mice (Fig. 7 ), which is consistent with the in vitro observations.
Figure 7.
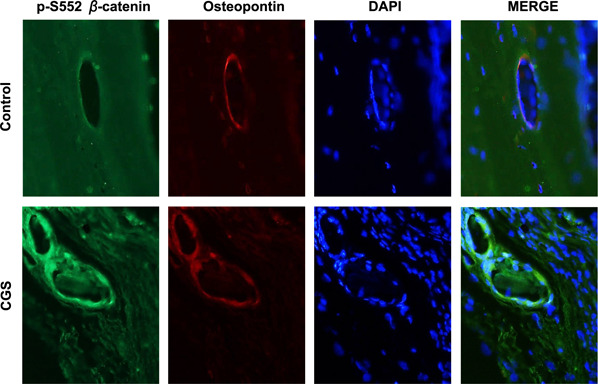
A2AR stimulation increases cellular p‐S552 β‐catenin level and osteoblast proliferation in vivo. A 3‐mm defect was created in the calvaria of mice as described in Materials and Methods, following which the defect was covered with a collagen sponge and either saline or CGS21680 (1 µM) in saline was applied daily to the sponge for 8 wk, at which time animals were euthanized and calvaria was collected, decalcified, and embedded in paraffin. IF staining of osteopontin and p‐S552 β‐catenin and DAPI staining of nuclei was carried out as described. Shown are representative images of 3 replicates from different mice taken from the regenerating bone at the edge of the trephination defect. The merge panels are images that are generated by the superimposition of p‐S552 β‐catenin, osteopontin, and DAPI images. Original magnification, ×40.
CGS21680 does not increase cellular β‐catenin level in A2AKO mice
To determine whether CGS21680 works exclusively through A2AR in osteoblasts, we examined the effect of CGS21680 treatment (1 µM) for 15 min on p‐S552 β‐catenin in primary osteoblasts derived from WT and A2AKO mice. Treatment with CGS21680 increased total cellular and nuclear localization of p‐S552 β‐catenin in WT osteoblasts but not in osteoblasts from A2AKO mice (Fig. 8 ).
Figure 8.
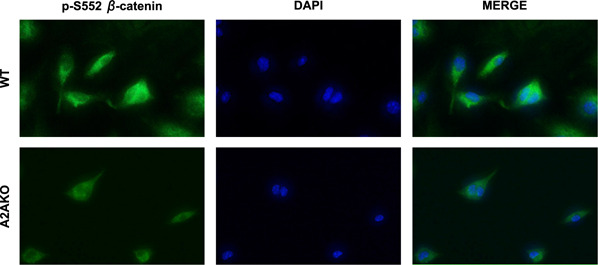
CGS21680 treatment does not increase p‐S552 β‐catenin following A2AKO. Treatment with CGS21680 (1 µM) for 15 min increased cellular p‐S552 β‐catenin level in WT primary murine osteoblasts but had no effect on p‐S552 β‐catenin level in A2AKO mice. The merge panel is formed by superimposing p‐S522 β‐catenin and DAPI images. Original magnification, ×40.
DISCUSSION
Wnt‐β‐catenin signaling is critical to bone generation, remodeling, and mechanotransduction (18). Similarly, adenosine receptors have been shown to play a critical role in increasing bone formation (1–4). In this study, we explored the interaction between A2AR and β‐catenin in osteoblasts and found strong evidence that stimulating A2AR leads to activation of Akt, which in turn enhances phosphorylation of β‐catenin and its nuclear translocation, signals previously shown to be critical for bone generation.
We observed through Western blot and IF assays that p‐S552 β‐catenin is markedly increased in the nuclei of both an osteoblastoid cell line and primary murine osteoblasts following A2AR activation, whereas CGS21680 treatment does not impact cellular β‐catenin level in A2AKO mice. There is a strong parallel between these findings in osteoblasts and the results of adenosine A2AR activation in human dermal fibroblasts, in which the nuclear localization of p‐S552 β‐catenin has been shown to increase following CGS21680 treatment (15), and also with the observation that treatment of skin undergoing fibrosis with an adenosine A2AR antagonist leads to diminished nuclear translocation of β‐catenin (16). When we examined A2AR‐stimulated regenerating murine calvarial bone, we found that there was a similar increase in cellular level of p‐S552 β‐catenin. Our experiments also indicate that A2AR activation increases osteoblast proliferation and bone regeneration in vivo.
β‐Catenin is modified at different sites. For example, phosphorylation at S33, S37, S45, and T41 amino terminal regions by casein kinase 1 and GSK3 leads to proteolytic degradation of the β‐catenin protein (19). Additionally, phosphorylation at serine 675 by PKA promotes β‐catenin binding to the transcription factor cAMP response element‐binding protein (20). Given that the present study was exclusively focused on discovering the link between A2AR activation and phosphorylation of β‐catenin at serine 552, the existence of a similar link at other β‐catenin phosphorylation sites remains a direction for future investigation.
In the present work, we showed not only that A2AR activation increases both the cellular content and nuclear localization of p‐S552 β‐catenin but also that adenosine‐mediated Akt kinase activity is the most likely signaling mechanism involved. There are numerous reports indicating that adenosine receptor ligation enhances Akt kinase activity downstream from the secondary messenger cAMP in various cell types, including microvascular endothelial cells (14), neurons (21, 22), adipocytes (23), hepatocytes (24), and fibroblasts (15, 17). Moreover, the interaction between Akt kinase and Wnt‐β‐catenin signaling is well established (25, 26). The Akt pathway modulates canonical Wnt signaling through distinct mechanisms: phosphorylation and inactivation of GSK3, which enhances β‐catenin stability (27), and phosphorylation of β‐catenin protein at serine 552 residue, which leads to an increase in β‐catenin transcriptional activity (9–14) (Fig. 9 ).
Figure 9.
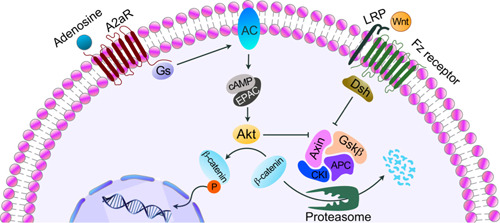
Stimulation of A2AR activates Gs protein, which in turn activates adenylyl cyclase (AC), leading to an increase in cellular cAMP levels, which triggers activation of downstream signaling molecules including Akt kinase. Activated Akt isoform has a dual function: inhibiting β‐catenin destruction complex as well as phosphorylation of β‐catenin at serine 552. The Wnt‐β‐catenin pathway is activated upon binding a Wnt ligand to the Fz receptor and LRPs. The Wnt‐activated Fz receptor and LRPs inhibit β‐catenin destruction complex. APC, adenomatous polyposis coli; CK1, casein kinase 1; Dsh, dishevelled; EPAC, exchange factor directly activated by cAMP.
Taken together, our findings indicate that A2AR activation modulates cellular content and nuclear translocation of β‐catenin in osteoblasts at least in part through the Akt kinase. Moreover, our results parallel those that reported CGS21680 treatment promotes bone regeneration (3, 4, 28, 29).
AUTHOR CONTRIBUTIONS
S. Borhani, C. Corciulo, and B. N. Cronstein designed the study; S. Borhani, C. Corciulo, and A. Larranaga‐Vera analyzed the data; S. Borhani performed the experiments; S. Borhani wrote the manuscript; and B. N. Cronstein supervised the project.
ACKNOWLEDGMENTS
This work was supported by grants from the Arthritis Foundation and the U.S. National Institutes of Health (NIH), National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01 AR056672 and R01 AR068593), and by the New York University (NYU) Health and Hospitals Corp. (HHC) Clinical and Translational Science Institute (ULI TR000038), and the Translational Research Laboratory Instruments at NYU‐Langone Health. Dr. Aranzazu Mediero (Fundacion Jimenez Diaz, Madrid, Spain) originally generated the calvarial sections used in this work as part of a previously published work. B.N.C. has patents on the use of adenosine A2a receptor agonists for the promotion of bone growth and regeneration. B.N.C. has a grant from AstraZeneca. B.N.C. has consulted for Eli Lilly & Co. and AstraZeneca. B.N.C. has significant equity in Regenosine and CanFite Biopharmaceuticals and lesser equity in Amgen, Novartis, Baxter, Abbott, Gilead, and Bristol‐Myers, Squibb. The remaining authors declare no conflicts of interest.
Borhani, S. , Corciulo, C. , Larranaga‐Vera, A. , Cronstein, B. N. Adenosine A2a receptor (A2AR) activation triggers Akt signaling and enhances nuclear localization of β‐catenin in osteoblasts. FASEB J. 33, 7555–7562 (2019). www.fasebj.org
REFERENCES
- 1. He, W. , Mazumder, A. , Wilder, T. , and Cronstein, B. N. (2013) Adenosine regulates bone metabolism via A1, A2A, and A2B receptors in bone marrow cells from normal humans and patients with multiple myeloma. FASEB J. 27, 3446–3454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mediero, A. , Perez-Aso, M. , and Cronstein, B. N. (2013) Activation of adenosine A(2A) receptor reduces osteoclast formation via PKA- and ERK1/2-mediated suppression of nfkB nuclear translocation. Br. J. Pharmacol. 169, 1372–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mediero, A. , Wilder, T. , Perez-Aso, M. , and Cronstein, B. N. (2015) Direct or indirect stimulation of adenosine A2A receptors enhances bone regeneration as well as bone morphogenetic protein-2. FASEB J. 29, 1577–1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mediero, A. , Wilder, T. , Shah, L. , and Cronstein, B. N. (2018) Adenosine a2a receptor (A2AR) stimulation modulates expression of semaphorins 4D and 3A, regulators of bone homeostasis. FASEB J. 32, 3487–3501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Van Camp, J. R. , Beckers, S. , Zegers, D. , and Van Hul, W. (2014) Wnt signaling and the control of human stem cell fate. Stem Cell Rev. 10, 207–229 [DOI] [PubMed] [Google Scholar]
- 6. Wang, Y. , Li, Y. P. , Paulson, C. , Shao, J. Z. , Zhang, X. , Wu, M. , and Chen, W. (2014) Wnt and the Wnt signaling pathway in bone development and disease. Front. Biosci. 19, 379–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bodine, P. V. (2008) Wnt signaling control of bone cell apoptosis. Cell Res. 18, 248–253 [DOI] [PubMed] [Google Scholar]
- 8. Stamos, J. L. , and Weis, W. I. (2013) The β-catenin destruction complex. Cold Spring Harb. Perspect. Biol. 5, a007898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fang, D. , Hawke, D. , Zheng, Y. , Xia, Y. , Meisenhelder, J. , Nika, H. , Mills, G. B. , Kobayashi, R. , Hunter, T. , and Lu, Z. (2007) Phosphorylation of beta-catenin by AKT promotes beta-catenin transcriptional activity. J. Biol. Chem. 282, 11221–11229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang, D. , Ma, W. , Wang, F. , Dong, J. , Wang, D. , Sun, B. , and Wang, B. (2015) Stimulation of Wnt/β-catenin signaling to improve bone development by naringin via interacting with AMPK and Akt. Cell. Physiol. Biochem. 36, 1563–1576 [DOI] [PubMed] [Google Scholar]
- 11. Tian, Q. , Feetham, M. C. , Tao, W. A. , He, X. C. , Li, L. , Aebersold, R. , and Hood, L. (2004) Proteomic analysis identifies that 14-3-3zeta interacts with beta-catenin and facilitates its activation by Akt. Proc. Natl. Acad. Sci. USA 101, 15370–15375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goretsky, T. , Bradford, E. M. , Ye, Q. , Lamping, O. F. , Vanagunas, T. , Moyer, M. P. , Keller, P. C. , Sinh, P. , Llovet, J. M. , Gao, T. , She, Q. B. , Li, L. , and Barrett, T. A. (2018) Beta-catenin cleavage enhances transcriptional activation. Sci. Rep. 8, 671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. He, X. C. , Yin, T. , Grindley, J. C. , Tian, Q. , Sato, T. , Tao, W. A. , Dirisina, R. , Porter-Westpfahl, K. S. , Hembree, M. , Johnson, T. , Wiedemann, L. M. , Barrett, T. A. , Hood, L. , Wu, H. , and Li, L. (2007) PTEN-deficient intestinal stem cells initiate intestinal polyposis. Nat. Genet. 39, 189–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ahmad, A. , Schaack, J. B. , White, C. W. , and Ahmad, S. (2013) Adenosine A2A receptor-dependent proliferation of pulmonary endothelial cells is mediated through calcium mobilization, PI3-kinase and ERK1/2 pathways. Biochem. Biophys. Res. Commun. 434, 566–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shaikh, G. , Zhang, J. , Perez-Aso, M. , Mediero, A. , and Cronstein, B. (2016) Adenosine a2a receptor promotes collagen type III synthesis via β-catenin activation in human dermal fibroblasts. Br. J. Pharmacol. 173, 3279–3291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang, J. , Corciulo, C. , Liu, H. , Wilder, T. , Ito, M. , and Cronstein, B. (2017) Adenosine A2a receptor blockade diminishes Wnt/β-catenin signaling in a murine model of bleomycin-induced dermal fibrosis. Am. J. Pathol. 187, 1935–1944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Perez-Aso, M. , Fernandez, P. , Mediero, A. , Chan, E. S. , and Cronstein, B. N. (2014) Adenosine 2A receptor promotes collagen production by human fibroblasts via pathways involving cyclic AMP and AKT but independent of Smad2/3. FASEB J. 28, 802–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Regard, J. B. , Zhong, Z. , Williams, B. O. , and Yang, Y. (2012) Wnt signaling in bone development and disease: making stronger bone with Wnts. Cold Spring Harb. Perspect. Biol. 4, a007997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu, C. , Li, Y. , Semenov, M. , Han, C. , Baeg, G. H. , Tan, Y. , Zhang, Z. , Lin, X. , and He, X. (2002) Control of beta-catenin phosphorylation/ degradation by a dual-kinase mechanism. Cell 108, 837–847 [DOI] [PubMed] [Google Scholar]
- 20. Hino, S. , Tanji, C. , Nakayama, K. I. , and Kikuchi, A. (2005) Phosphorylation of beta-catenin by cyclic AMP-dependent protein kinase stabilizes beta-catenin through inhibition of its ubiquitination. Mol. Cell. Biol. 25, 9063–9072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gervitz, L. M. , Nalbant, D. , Williams, S. C. , and Fowler, J. C. (2002) Adenosine-mediated activation of Akt/protein kinase B in the rat hippocampus in vitro and in vivo. Neurosci. Lett. 328, 175–179 [DOI] [PubMed] [Google Scholar]
- 22. Ouyang, Y. B. , Tan, Y. , Comb, M. , Iiu, C. L. , Martone, M. E. , Siesjö, B. K. , and Hu, B. R. (1999) Survival- and death-promoting events after transient cerebral ischemia: phosphorylation of Akt, release of cytochrome C and Activation of caspase-like proteases. J. Cereb. Blood Now Metab. 19, 1126–1135 [DOI] [PubMed] [Google Scholar]
- 23. Takasuga, S. , Katada, T. , Ui, M. , and Hazeki, O. (1999) Enhancement by adenosine of insulin-induced activation of phosphoinositide 3-kinase and protein kinase B in rat adipocytes. J. Biol. Chem. 274, 19545–19550 [DOI] [PubMed] [Google Scholar]
- 24. Zhang, B. , Li, S. , and Harbrecht, B. G. (2011) Akt-mediated signaling is induced by cytokines and cyclic adenosine monophosphate and suppresses hepatocyte inducible nitric oxide synthase expression independent of MAPK P44/42. Biochim. Biophys. Acta 1813, 73–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Anderson, E. C. , and Wong, M. H. (2010) Caught in the Akt: regulation of Wnt signaling in the intestine. Gastroenterology 139, 718–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bellacosa, A. , Kumar, C. C. , Di Cristofano, A. , and Testa, J. R. (2005) Activation of AKT kinases in cancer: implications for therapeutic targeting. Adv. Cancer Res. 94, 29–86 [DOI] [PubMed] [Google Scholar]
- 27. Fukumoto, S. , Hsieh, C. M. , Maemura, K. , Layne, M. D. , Yet, S. F , Lee, K. H. , Matsui, T. , Rosenzweig, A. , Taylor, W. G. , Rubin, J. S. , Perrella, M. A. , and Lee, M. E. (2001) Akt participation in the Wnt signaling pathway through Dishevelled. J. Biol. Chem. 276, 17479–17483 [DOI] [PubMed] [Google Scholar]
- 28. Vincenzi, F. , Targa, M. , Corciulo, C. , Gessi, S. , Merighi, S. , Setti, S. , Cadossi, R. , Goldring, M. B. , Borea, P. A. , and Varani, K. (2013) Pulsed electromagnetic fields increased the anti-inflammatory effect of A2A and A3 adenosine receptors in human T/G28a2 chondrocytes and hFOB 1.19 osteoblasts. PLoS One 8, e65561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mediero, A. , Kara, F. M. , Wilder, T. , and Cronstein, B. N. (2012) Adenosine A(2A) receptor ligation inhibits osteoclast formation. Am. J. Pathol. 180, 775–786 [DOI] [PMC free article] [PubMed] [Google Scholar]


