Keywords: cystic fibrosis, enteroid, goblet cell, small intestine, Toll-like receptor
Abstract
Goblet cell hyperplasia is an important manifestation of cystic fibrosis (CF) disease in epithelial-lined organs. Explants of CF airway epithelium show normalization of goblet cell numbers; therefore, we hypothesized that small intestinal enteroids from Cftr knockout (KO) mice would not exhibit goblet cell hyperplasia. Toll-like receptors 2 and 4 (Tlr2 and Tlr4) were investigated as markers of inflammation and influence on goblet cell differentiation. Ex vivo studies found goblet cell hyperplasia in Cftr KO jejunum compared with wild-type (WT) mice. IL-13, SAM pointed domain-containing ETS transcription factor (Spdef), Tlr2, and Tlr4 protein expression were increased in Cftr KO intestine relative to WT. In contrast, WT and Cftr KO enteroids did not exhibit differences in basal or IL-13-stimulated goblet cell numbers, or differences in expression of Tlr2, Tlr4, and Spdef. Ileal goblet cell numbers in Cftr KO/Tlr4 KO and Cftr KO/Tlr2 KO mice were not different from Cftr KO mice, but enumeration was confounded by altered mucosal morphology. Treatment with Tlr4 agonist LPS did not affect goblet cell numbers in WT or Cftr KO enteroids, whereas the Tlr2 agonist Pam3Csk4 stimulated goblet cell hyperplasia in both genotypes. Pam3Csk4 stimulation of goblet cell numbers was associated with suppression of Notch1 and Neurog3 expression and upregulated determinants of goblet cell differentiation. We conclude that goblet cell hyperplasia and inflammation of the Cftr KO small intestine are not exhibited by enteroids, indicating that this manifestation of CF intestinal disease is not epithelial-automatous but secondary to the altered CF intestinal environment.
NEW & NOTEWORTHY Studies of small intestinal organoids from cystic fibrosis (CF) mice show that goblet cell hyperplasia and increased Toll-like receptor 2/4 expression are not primary manifestations of the CF intestine. Intestinal goblet cell hyperplasia in the CF mice was not strongly altered by genetic ablation of Tlr2 and Tlr 4, but could be induced in both wild-type and CF intestinal organoids by a Tlr2-dependent suppression of Notch signaling.
INTRODUCTION
Cystic fibrosis (CF) is caused by loss-of-activity mutations of the protein product of the CFTR gene. Loss of this cyclic nucleotide-regulated anion channel leads to the pathological condition of mucoviscidosis, i.e., tenacious, concentrated mucus on epithelial surfaces (1). Mucoviscidosis of CF airway epithelia is most life-threatening by impairing mucociliary clearance with the consequence of opportunistic bacterial colonization of the lungs. Other organs affected by mucoviscidosis include the nasal sinuses, pancreatic ducts, biliary ducts, reproductive tract, and gastrointestinal tract. The manifestation of CF in the intestine involves obstructive disease, dysbiosis, inflammation, compromised barrier function, and an increased risk of gastrointestinal cancer (2). Cftr knockout (KO) and mutated Cftr mouse models recapitulate CF intestinal disease without confounding comorbidities of lung or pancreatic disease (3).
Intestinal mucoviscidosis involves goblet cell hyperplasia, defective degranulation by goblet cells, and elaboration of viscid mucus on the epithelial surface (1, 4, 5). Of these, the pathogenic factors resulting in intestinal goblet cell hyperplasia have not been well defined. Intestinal dysbiosis is present in persons with CF, even at neonatal stages (6), and in Cftr KO mouse models (7, 8), which likely provides an intestinal environment for inflammation and goblet cell hyperplasia. The idea that goblet cell hyperplasia is a secondary consequence of CF disease and not primary to the loss of CFTR from the epithelium has been demonstrated in past studies using CF human airway epithelial cells seeded into denuded rat tracheas and implanted subcutaneously in athymic mice (9, 10). Although these studies primarily concentrated on the sulfation defects of secreted mucus and ion transport by the CF epithelium, it was observed that the number of goblet cells between non-CF and CF xenografted epithelium was not different. This observation was further supported by studies of well-differentiated airway epithelial cultures that increase MUC5B and MUC5AC expression only when exposed to mucopurulent material from CF lungs (1). In contrast to airways and to the best of our knowledge, the premise that goblet cell hyperplasia in the CF intestine is a direct consequence of the CFTR-null epithelia has not been tested.
In the present study, we evaluate goblet cell hyperplasia in the Cftr KO mouse intestine in vivo and in well-differentiated small intestinal organoid cultures (enteroids). Toll-like receptors (Tlrs), especially Tlr2 and Tlr4, have been implicated in goblet cell differentiation (11), therefore the hypothesis that Tlr2 and Tlr4 impact goblet cell hyperplasia in the CF intestine was tested using Tlr/Cftr double-KO mice and treatment of wild-type (WT) and Cftr KO enteroids with Tlr2 and Tlr4 agonists.
MATERIALS AND METHODS
Animals
Mice with gene-targeted disruptions of the murine homolog of Cftr (Abcc7, Cftrtm1Unc, and Cftr KO) and sex-matched wild-type (WT, Cftr+/+, or Cftr+/−) littermates were used (3). Mice were outbred to Black Swiss (Charles River, Wilmington, MA) mice at three generational intervals and resultant F1 heterozygotes were crossed to generate F2 offspring for experimentation. The Cftr KO mouse line was crossed with C.129(B6)-Tlr2tm1Kir/J (Jackson Laboratories, Bar Harbor, ME) mice to generate Tlr2 KO/Cftr KO double-knockout mice or with C57BL/10ScNJ (Jackson Laboratories, Bar Harbor, ME) to generate Tlr4 KO/Cftr KO double-knockout mice. Double-KO mice with at least one allele of Cftr were considered wild-type with regard to Cftr activity. Genotypes were identified by polymerase chain reaction analysis of tail-snip DNA, as previously described for mutant Cftr (12). All mice used in experiments were maintained ad libitum on standard laboratory chow (Formulab 5008, Rodent Chow; Nestle Purina, St. Louis, MO) and distilled drinking water containing polyethylene glycol (PEG) (Schwartz Pharma, Mequon, WI) laxative to prevent intestinal obstruction in the Cftr KO mice. In some experiments, mice were switched for 2 wk to a nutritionally complete liquid diet Peptamen (Nestle), which also prevents intestinal impaction in Cftr KO mice but increases microbiota burden and inflammation (13). Mice were housed individually in a temperature- and light-controlled room (22°C–26°C; 12-h light:12-h dark cycle) in the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC)-accredited animal facility at the Dalton Cardiovascular Research Center at the University of Missouri. Mouse experiments were performed in accordance with guidelines outlined in the Guide for the Care and Use of Laboratory Animals prepared by the National Academy of Sciences and published by the National Institutes of Health and with approval from the University of Missouri Institutional Animal Care and Use Committee.
Enteroid Culture
Culture of isolated crypt epithelium from the proximal jejunum has been described previously in detail (14). Cultures were overlaid with growth medium containing Ham’s F-12 medium with 5% fetal bovine serum, 50 mg/mL gentamicin, 125 ng/mL R-spondin1, 25 ng/mL noggin, and 12.5 ng/mL epidermal growth factor. Growth medium was changed every 3 days and enteroids were passaged at 7–10 days using Cell Recovery Solution (BD Sciences, San Jose, CA). Passage 1 enteroids were fed fresh growth medium on days 3–4 and used for experimentation on days 5–7.
Immunoblotting
Mice were euthanized by CO2 asphyxiation followed by cervical dislocation. A laparotomy was used for the removal of the entire small intestine, which was flushed with ice-cold PBS. Epithelial cells were isolated using an EDTA technique, as previously described (15), suspended in ice-cold, radioimmunoprecipitation assay buffer (Cell Signaling, Danvers, MA) containing Halt Protease inhibitor (Thermo Fisher Scientific) and lysed at 4°C by supersonication. Total lysate protein was loaded in sodium dodecyl sulfate-polyacrylamide gels for electrophoresis followed by membrane transfer and immunoblotting. Antibodies for immunoblotting were rabbit polyclonal to TLR4/MD-2 complex (Abcam Cat. No. ab13867, 3 µg/mL RRID:AB_300696), rabbit polyclonal to TLR2 (Santa Cruz Biotechnology, sc-10739, 1:1,000, RRID:AB_2303458), and rabbit polyclonal to SAM pointed domain-containing erythroblast transcription specific (ETS) transcription factor (Spdef, Santa Cruz Biotechnology Cat. No. sc-67022, 1:1,000, RRID:AB_2195411). Secondary antibody was goat anti-rabbit horseradish peroxidase (Cell Signaling Cat. No. 7074, 1:2,000, RRID:AB_2099233). Anti-β-actin (Santa Cruz Biotechnology Cat. No. sc-130656; 1:2,000 dilution, RRID:AB_2223228) or anti-glyceraldehyde-3 phosphate dehydrogenase (Santa Cruz Biotechnology Cat. No. sc-25778; 1:2,000 dilution, RRID:AB_10167668) were used as loading controls. Densitometry was performed using ImageLab Software (version 5.2.1; Bio-Rad).
Quantitative Real-Time PCR
Passage 1 enteroids (7 days old) were removed from Matrigel using Cell Recovery Solution (BD Sciences), washed with ice-cold PBS, and processed for total RNA extraction and reverse transcription, as previously described (14). cDNA was mixed with TaqMan Gene Expression Master Mix (Applied Biosystems), according to the manufacturer’s protocol, and loaded onto customized mini-array 96-well plates containing TaqMan assays for the genes of interest (Supplemental Table S1; see https://doi.org/10.6084/m9.figshare.16613470.v1). A Mastercycler EP RealPlex thermocycler (Eppendorf) was used for quantitative PCR. cDNA from untreated and treated WT enteroids were run simultaneously on one plate. For measurement of IL-13, fresh EDTA-isolated crypts from the proximal jejunum were placed in RNALater and stored at −80°C until processing, as above. cDNA was used with individual TaqMan Gene Expression Assays (Thermo Fisher), according to the manufacturer’s protocol, for interleukin 13 (Assay ID: Mm00434204_m1), three housekeeping genes β-glucuronidase (Assay ID Mm00446953_m1), hypoxanthine-guanine phosphoribosyltransferase 1 (Assay ID Mm00446968_m1), mitochondrial ribosomal protein L19 (Assay ID Mm00452754_m1), and the negative RT sample actin β (Assay ID Mm00607939_s1). The threshold cycle (Ct) of a gene of interest was subtracted from the geometric mean Ct of the three housekeeping genes to yield ΔCt. mRNA expression of Cftr KO relative to WT enteroids was calculated using the ΔΔCt method (16). Data presented as fold change of the gene of interest in Cftr KO relative to WT.
Histology
Mouse ileum was removed after euthanasia via a laparotomy, rinsed in ice-cold PBS, and fixed in 10% buffered formalin (Thermo Fisher Scientific) overnight. Fixed ileum was embedded in paraffin, sectioned at 8 µm, and stained with periodic acid-Schiff. Enteroids (5–7 days, passage 1) were isolated from Matrigel using Cell Recovery Solution (BD Sciences), washed with PBS, fixed overnight in 10% buffered formalin, placed in HistoGel (Thermo Fisher Scientific), and embedded in paraffin for sectioning. Sections were examined by light microscopy using an Olympus BX50WI upright scope, imaged using a Sensicam CCD camera (Cooke, Auburn Hills, MI) fitted with a microcolor filter (Cambridge Research and Instrumentation, Boston, MA) and acquired with Slidebook 5.0 software (Intelligent Imaging Innovations, Denver, CO). Some images were captured using an Olympus IMT-2 with an Infinity1-3C camera (Teledyne Lumenera, Ottawa, Canada) and acquired with Image-Pro Plus software (Media Cybernetics, Rockville, MD). Goblet cell and epithelial nuclear counts of jejunum, ileum, and enteroids sections were performed by a blinded observer and recorded as number of goblet cells per 100 nuclei on villi (ileum) or in the villus-like region between crypts of enteroids (17). Goblet cells with distinct PAS staining were counted in these regions when goblet cells were completely or partially sectioned in the longitudinal axis of a contiguous epithelial layer.
Materials
The Tlr4 agonist lipopolysaccharide (LPS) from Escherichia coli (TLR grade, Cat. No. ALX-581-007-L002) was purchased from Enzo Life Sciences. The Tlr2/Tlr1 agonist Pam3Csk4 (Cat. No. tlrl-pms) was purchased from InvivoGen. Caffeic acid phenethyl ester (CAPE, Cat. No. sc-200800A) was purchased from Santa Cruz Biotechnology. The p38 MAPK inhibitor SB202190 (Cat. No. 1264/10) was purchased from R&D Systems. Other supplies and reagents were purchased from Thermo Fisher Scientific.
Statistics
Cumulative data are reported as the means ± SE. Data between two groups were compared using a two-tailed unpaired Student’s t test or, if not normally distributed with equal variances, by Mann–Whitney rank sum test. Data from multiple treatment groups were compared using a one-way ANOVA with a post hoc Holm–Sidak pairwise comparisons or versus control tests. A probability value of P < 0.05 was considered statistically significant.
RESULTS
Investigation of Goblet Cell Hyperplasia in Cftr KO Mice and Cftr KO Enteroids
Cftr KO intestine exhibits goblet cell hyperplasia in vivo.
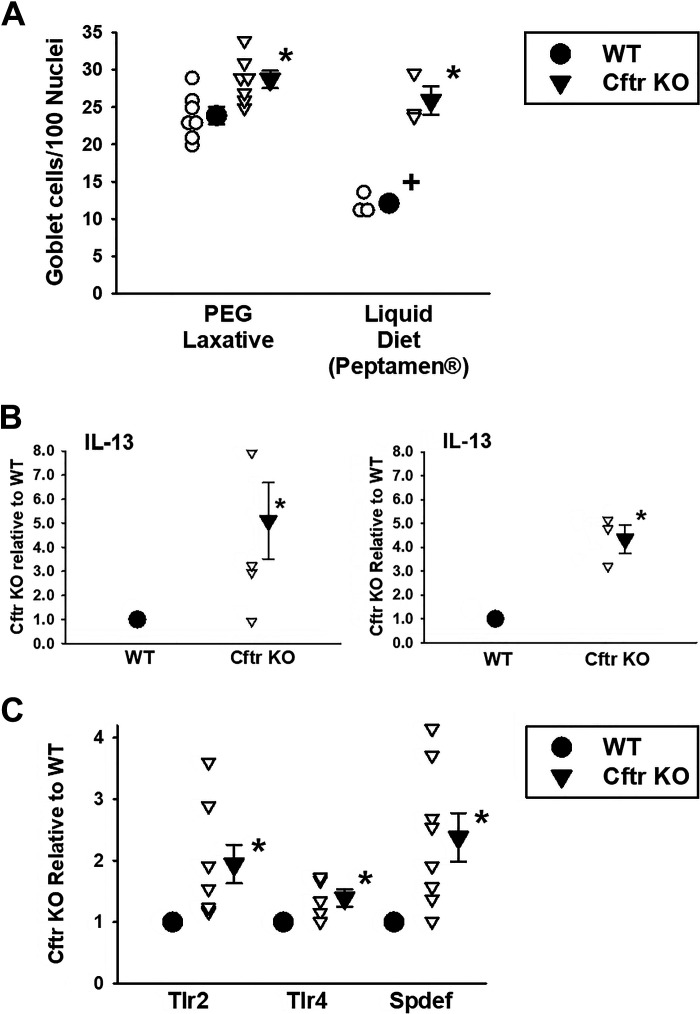
Goblet cell hyperplasia of airway and intestinal epithelia is considered to be a major component of mucoviscidosis, the pathological entity that defines cystic fibrosis disease. As shown in Fig. 1A (left) and Supplemental Fig. S1; see https://doi.org/10.6084/m9.figshare.16613464.v1, Cftr KO mice maintained on standard laboratory chow and PEG-treated drinking water (to avoid obstructive bowel disease) showed a significantly greater number of PAS-stained goblet cells as compared with WT sex-matched littermate mice, as we and others have shown (4, 18). A dietary measure to prevent intestinal obstruction in Cftr KO mice is also accomplished by providing a complete liquid diet, i.e., Peptamen, instead of providing laxative in the drinking water (19). Previous studies have shown that PEG-treated Cftr KO intestine, compared with Peptamen-treated mice, have reduced intestinal mucus accumulation (as measured by crypt impaction), reduced bacterial load in the small intestine, and a reduced expression of innate immune response-related genes (13). We asked whether provision of a Peptamen diet rather than PEG-treated drinking water would alter the degree of goblet cell hyperplasia in the Cftr KO intestine. As shown in Fig. 1A (right), the difference in PAS-stained goblet cell numbers between Cftr KO versus WT intestine on mice given Peptamen was much greater in magnitude relative to the Colyte-treated mice (ΔGCs: Colyte = 7.8 ± 2.3/100 nuclei s. Peptamen = 13.8 ± 1.1/100 nuclei). Interestingly, provision of Peptamen did not increase goblet cell numbers in the Cftr KO intestine compared with PEG-treated Cftr KO intestine, but rather reduced goblet cell numbers in the WT intestine. The intestinal goblet cell numbers of WT mice consuming Peptamen were also significantly lower compared with WT mice on the PEG laxative. We speculate that WT goblet cells do not show the same propensity as CF goblet cells to retain mucus (20, 21) on the Peptamen diet, but further studies will be necessary to compare mucus retention and release by WT and Cftr KO intestinal goblet cells under the two conditions (PEG laxative vs. Peptamen).
Figure 1.
Goblet cell hyperplasia and markers of inflammation in Cftr KO mouse jejunum. A: mean goblet cell numbers per 100 cell nuclei in villous epithelium of proximal jejunum from WT and Cftr KO sex-matched littermate mouse pairs. Individual data points are shown. PEG laxative: mice consumed a mouse chow diet and were provided 3,350 m. wt. polyethylene glycol in their drinking water to prevent intestinal impaction in the Cftr KO mice. Liquid diet: mice consumed a nutritionally complete liquid diet (Peptamen) for 2 wk to prevent intestinal impaction in the Cftr KO mice. *Significantly different from WT, P = 0.0123 (PEG laxative), P = 0.0026 (liquid diet) by unpaired t test. +Significantly different from PEG laxative, P = 0.00026 by unpaired t test; n = 7 WT-Cftr KO mouse pairs (3 males, 4 females) and three WT-Cftr KO mouse pairs (1 male, 2 females), respectively (see Supplemental Fig. S1; https://doi.org/10.6084/m9.figshare.16613464.v1, for images of PAS-stained images of WT and Cftr KO intestine under the two conditions). B: mRNA expression of IL-13 in freshly isolated jejunal crypts from WT and Cftr KO sex-matched littermate mouse pairs consuming PEG osmotic laxative since weaning (left) or after switching to Peptamen liquid diet for 2 wk (right). Individual data points are shown. *Significantly different from WT, P = 0.029 by Mann–Whitney rank sum test; n = 4 WT-Cftr KO mouse pairs. *Significantly different from WT, P < 0.005 by unpaired t test; n = 3 WT-Cftr KO mouse pairs. C: densitometry of immunoblots of Toll-like receptor Tlr2, Tlr4, and SAM pointed domain-containing ETS transcription factor (Spdef). WT densitometry is set at 1.0. Individual data points are shown. *Significantly different from WT by Mann–Whitney rank sum test, P = 0.001 for Tlr2, n = 8 WT-Cftr KO mouse pairs (4 males and 4 females); P = 0.0252 for Tlr4, n = 5 WT-Cftr KO mouse pairs (3 males and 2 females); P = 0.001 for Spdef, n = 8 WT-Cftr KO mouse pairs (4 males and 4 females). KO, knockout; PAS, periodic acid-Schiff; PEG, polyethylene glycol; Tlr, Toll-like receptor; WT, wild-type.
Cftr KO intestine exhibits increased signaling for goblet cell differentiation in vivo.
Previous studies of airway epithelium and studies of intestinal epithelium have shown a central role of IL-13 as a proinflammatory regulator of goblet cell differentiation (22, 23). Regulation of IL-13 secretion from innate lymphoid cells (ILCs) that are enriched at mucosal sites is an area of active investigation and primarily involves cytokine stimuli such as IL-25, IL-33, thymic stromal lymphopoietin (TSLP) (22, 24), as well as both direct and indirect effects of Toll-like receptor (TLR) activation (25, 26). TLR2 is expressed on ILC2s isolated from the lung and induces IL-13 secretion upon activation. Similarly, TLR activation of dendritic cells/monocytes elaborates the tumor necrosis factor-like factor TL1A (TNFSF15), which stimulates IL-13 production and goblet cell hyperplasia in the small intestine. Based on these studies, we asked whether IL-13 expression was increased in the intestine of Cftr KO mice. Using a freshly isolated mucosal preparation, we measured the mRNA expression of IL-13. As shown in Fig. 1B, increased transcriptional activity of IL-13 was present in the Cftr KO intestinal mucosa as compared with WT intestine under both PEG-treated and Peptamen-treated conditions. Studies of airway epithelia have shown that IL-13 induces goblet cell hyperplasia by increasing expression of the SAM pointed domain-containing ETS transcription factor (Spdef) by a STAT6-dependent pathway involving delayed phosphorylation of p38 MAPK (27, 28). Spdef is well expressed in the intestine (29), therefore, immunoblots were used to examine the level of expression of Spdef in isolated intestinal epithelium of sex-matched WT and Cftr KO littermates. We also measured the epithelial protein expression of Tlr2 and Tlr4, which have been associated with goblet cell differentiation and serve as markers of inflammatory states (11, 25, 26, 30, 31). Previous immunohistochemistry studies of Tlr4 in the Cftr KO intestine have shown greater numbers of Tlr4-expressing cells relative to WT (18). As shown in Fig. 1C, Spdef, Tlr2, and Tlr4 protein expression were significantly increased in isolated Cftr KO intestinal epithelium compared with WT intestinal epithelium.
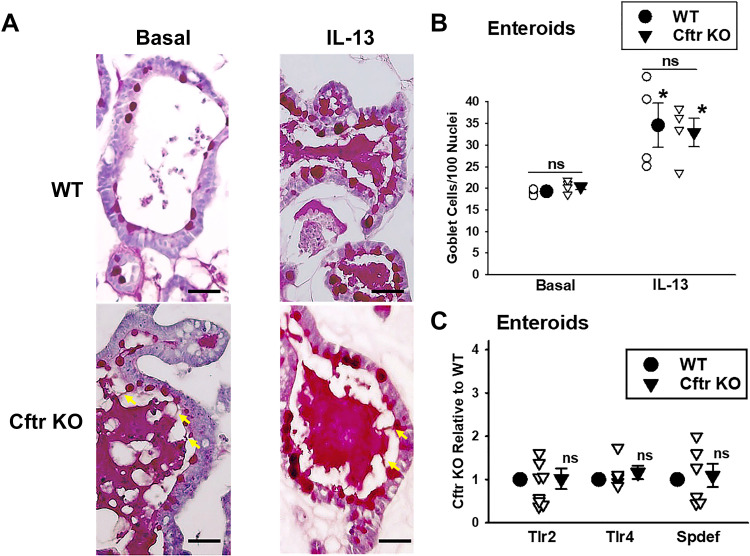
Goblet cell hyperplasia, markers of goblet cell differentiation, and inflammation are normalized in primary cftr KO intestinal epithelial organoids.
The development of primary three-dimensional (3-D) intestinal culture has provided the opportunity to investigate a well-differentiated, self-renewing intestinal epithelium in the absence of other cell types (neural, immune), the microbiota, or humoral agents (17), features that are known to be altered in the Cftr KO intestinal environment (7, 13, 32, 33). Our previous studies have shown that Cftr is expressed and functional in WT murine intestinal organoids but not in Cftr KO organoids (14). Therefore, we asked whether goblet cell hyperplasia is an intrinsic property of Cftr KO intestinal epithelium using primary small intestinal organoids (enteroids). Freshly isolated jejunal enteroids were cultured in Matrigel for ∼7 day, passaged once, and cultured to days 5–7 before sectioning and PAS staining. WT and Cftr KO enteroid goblet cell numbers were counted in the “villus-like” domain (i.e., intercrypt) to ensure mature goblet cells and to avoid confusion with granule-bearing Paneth or intermediate cells (34). Goblet cell counts were performed under basal (untreated) conditions and following treatment with recombinant IL-13 (50 ng/mL) for 72 h. As shown in Fig. 2, A and B, goblet cell numbers in the enteroids were not significantly different between WT and Cftr KO enteroids either under basal conditions or following stimulation with exogenous IL-13. Interestingly, as shown in Fig. 2A, unstimulated and, to a degree, stimulated Cftr KO goblet cells demonstrated mucus retention as indicated by the attachment of secreted mucus to individual goblet cells, consistent with previous observations of inefficient goblet cell degranulation in the Cftr KO intestine (4).
Figure 2.
Goblet cell hyperplasia and intestinal inflammation are normalized in Cftr KO enteroids. A: photomicrographs of PAS-stained enteroids under basal (left) and IL-13-treated (right) conditions. Yellow arrows, mucus anchored to goblet cells in Cftr KO enteroids. Scale bars = 150 µm. B: goblet cell count/100 nuclei in the villus-like region (between crypts) in jejunal enteroids from WT and Cftr KO sex-matched littermate mice under basal and IL-13-treated conditions. Individual data points are shown. ns, not significant WT vs. Cftr KO; *Significantly different from basal by unpaired t test. WT basal vs. WT IL-13, P = 0.024, Cftr KO basal vs. Cftr KO IL-13, P = 0.009; n = 4 WT-Cftr KO mouse pairs (3 males and 1 female). Average from 5 to 15 enteroids counted per mouse. C: densitometry of immunoblots of Toll-like receptor Tlr2, Tlr4, and SAM pointed domain-containing ETS transcription factor (Spdef) in passage 1, days 5–7 enteroids from WT, and Cftr KO sex-matched littermate mice. WT densitometry is set to 1. Individual data points are shown. ns, not significant from WT by Mann–Whitney rank sum test for Tlr2 (8 mouse pairs, 4 males and 4 females), Tlr4 (5 mouse pairs, 2 males, 3 female), and Spdef (6 mouse pairs, 3 maless and 3 female). KO, knockout; PAS, periodic acid-Schiff; WT, wild-type.
Markers of goblet cell differentiation and inflammation are normalized in Cftr KO enteroids.
Goblet cell hyperplasia was not present in Cftr KO enteroids, therefore, we examined the protein expression of regulators of goblet cell differentiation. As shown in Fig. 2C, it was found that the expression of Spdef, Tlr2, and Tlr4 were not significantly different between WT and Cftr KO enteroids cultured to the same time point as used for the goblet cell counts (passage 1, days 5–7).
Investigation of Inflammatory Pathways and Goblet Cell Hyperplasia
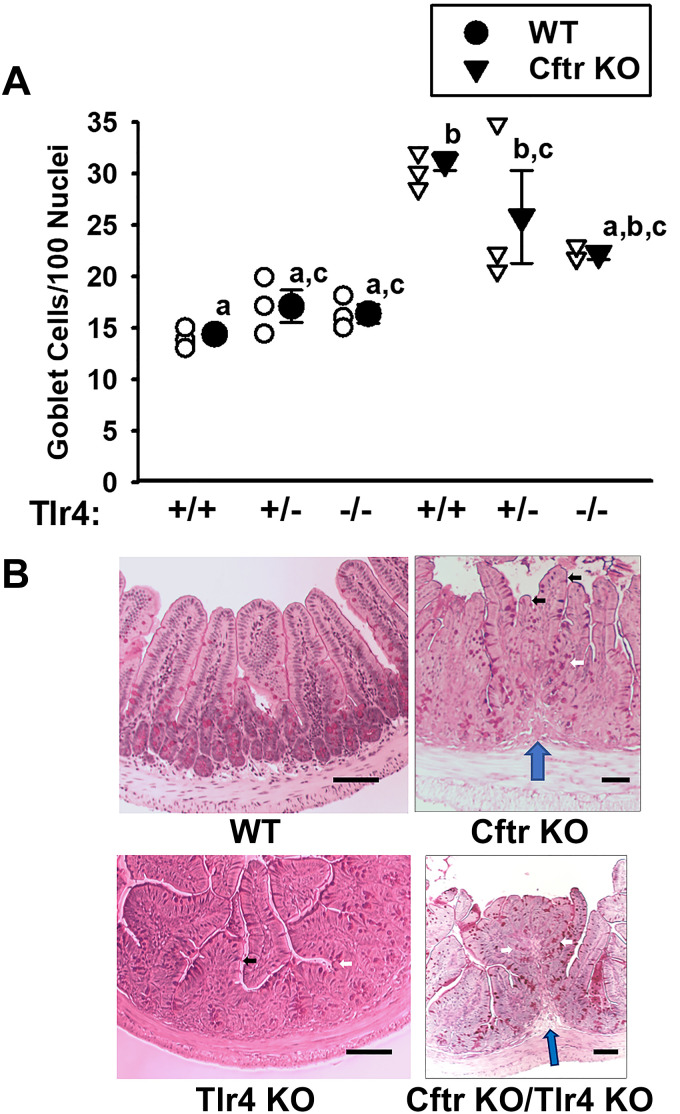
Tlr2 and Tlr4 knockout alters intestinal morphology in the Cftr KO ileum.
The expression of both Tlr2 and Tlr4 in the intestine has been shown to be altered by the presence of the microbiota (35). Cftr KO mice exhibit small intestinal bacterial overgrowth (SIBO) and eradication by antibiotic therapy results in reduced small intestinal mucus accumulation (36, 37). Based on these findings and the fact that intestinal impaction in Cftr KO mice usually initiates at the ileum, we crossbred Cftr KO mice with global Tlr2 and Tlr4 knockout mice (Tlr2 KO, Tlr4 KO) to determine the impact on goblet cell counts in ileal sections. As shown in Fig. 3A, the goblet cell numbers in Cftr-replete (WT) ileum were not significantly affected by either Tlr4+/− or Tlr4−/− status, although a slight upward trend was noted in the WT/Tlr4+/− and WT/Tlr4−/− ileum. In the Cftr KO mice, goblet cell hyperplasia was not significantly affected in either the Cftr KO/Tlr4+/− or Cftr KO/Tlr4−/− ileum, although a downward trend was apparent. Most notably, the Cftr KO/Tlr4+/+ ileum had significantly more goblet cell hyperplasia as compared with WT/Tlr4+/+, WT/Tlr4+/−, or WT/Tlr4−/−. Because of the downward trend in goblet cells/100 nuclei, the Cftr KO/Tlr4+/− was only different from the WT/Tlr4+/+, and the Cftr KO/Tlr4−/− double knockout counts were not different from the WT/Tlr4+/+, WT/Tlr4+/−, or WT/Tlr4−/− ileal counts. Importantly, as shown in Fig. 3B, the goblet cell counts in the epithelium of the villi were confounded by morphological changes of the mucosa, especially in the Cftr/Tlr4 double knockouts. Specifically, Cftr/Tlr4 double-KO mice demonstrated evidence of colonic metaplasia (for low power images of WT and Cftr/Tlr4 double KO ileum, see Supplemental Fig. S2; https://doi.org/10.6084/m9.figshare.16958785.v1). As compared with the WT ileum, shown in Fig. 3B (top left), Cftr KO ileum (Fig. 3B, top right) displayed short villi (black arrows), elongated crypts (white arrows), and the apparent development of mucosal folds with lamina propria extensions into some microsections (blue arrow). However, these changes were sporadic and could be avoided by performing counts on villi with a typical WT appearance. The Tlr4 KO (Fig. 3B, bottom left) also displayed sporadic morphological changes (short villi, lengthened crypts). In contrast to these genotypes, the Cftr/Tlr4 double knockout ileum (Fig. 3B, bottom right) showed overt colonic metaplasia with structures reminiscent of longitudinal colonic folds with tightly packed elongated crypts (white arrows), lack of villi, and a lamina propria core (blue arrow). The morphological changes were more pervasive and villi were difficult to distinguish, thereby confounding enumeration of “villous goblet cells.”
Figure 3.
Goblet cell number and morphological changes in ileum from WT, Cftr KO, and Tlr4 KO crossbred mice. A: goblet cell counts/100 nuclei in villous epithelium of PAS-stained sections of ileum from WT/Tlr4+/+, WT/Tlr4+/−, WT/Tlr4−/−, Cftr KO/Tlr4+/+, Cftr KO/Tlr4+/−, and Cftr KO/Tlr4−/− mice. Individual data points are shown. a,b,cMeans with the same letter are not significantly different by one-way ANOVA and Holm–Sidak pairwise comparisons. Cftr KO/Tlr4+/+ significantly different from all WT genotypes, P < 0.001 vs. WT/Tlr4+/+, P = 0.004 vs. WT/Tlr4+/−, and P = 0.003 vs. WT/Tlr4−/−. Cftr KO/Tlr4+/− significantly different from WT/Tlr4+/+, P = 0.021; n = 4 WT/Tlr4+/+ (2 males, 2 females), 3 WT/Tlr4+/− (3 males), 3 WT/Tlr4−/− (3 males), 4 Cftr KO/Tlr4+/+ (2 females, 2 males), 3 Cftr KO/Tlr4+/− (2 males, 1 female), and 2 Cftr KO/Tlr4−/− mice (2 males). B: photomicrographs of PAS-stained ileum from WT (top left), Cftr KO (top right), Tlr4 KO (bottom left), and Cftr KO/Tlr4 KO double knockout (bottom right). White arrows, elongated crypts. Black arrows, shortened villi. Blue arrow, lamina propria upfolding. Scale bars = 50 µm. KO, knockout; PAS, periodic acid-Schiff; Tlr, Toll-like receptor; WT, wild-type.
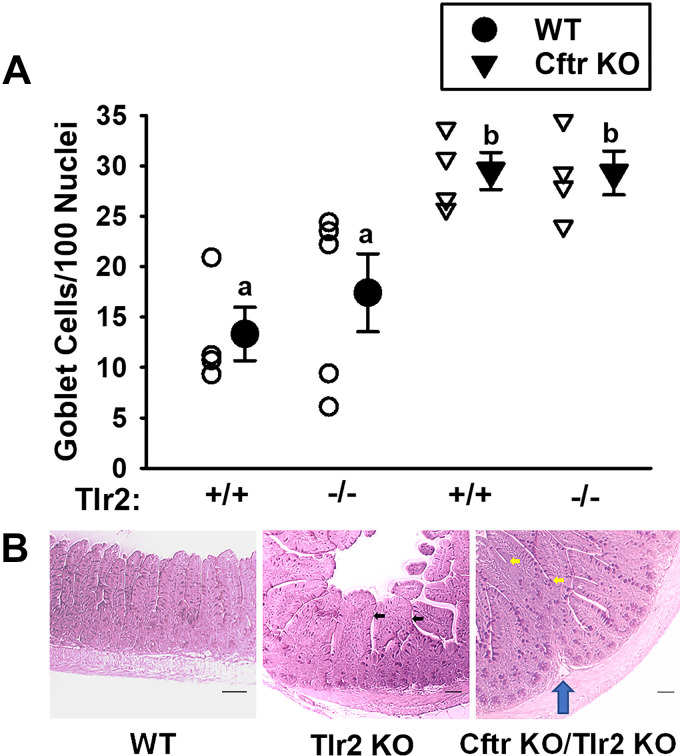
In studies of Tlr2/Cftr double-mutant mice, shown in Fig. 4A, the absence of Tlr2 did not significantly affect the degree of goblet cell hyperplasia resulting from Cftr KO alone. As compared with WT ileum (Fig. 4B, left), the Tlr2 KO ileum (Fig. 4B, middle) showed morphological changes of the ileal mucosa with villi that were increased in diameter (black arrows). The Cftr KO/Tlr2 double-KO ileum (Fig. 4B, right) had more pervasive morphological changes that were found in most microscopic fields. There was evidence of mucosal folding (blue arrow) and numerous fused villi (yellow arrows), which also confounded enumeration of villous goblet cells.
Figure 4.
Goblet cell number and morphological changes in ileum from WT, Cftr KO, and Tlr2 KO crossbred mice. A: goblet cell counts/100 nuclei in villous epithelium of PAS-stained sections of ileum from WT/Tlr2+/+, WT/Tlr2−/−, Cftr KO/Tlr2+/+, and Cftr KO/Tlr2−/−, and WT/Tlr2−/− vs. individual data points shown. a,bMeans with the same letter are not significantly different by one-way ANOVA and Holm–Sidak pairwise comparisons. P = 0.004; WT/Tlr2+/+ vs. Cftr KO/Tlr2+/+, P = 0.014; WT/Tlr2+/+ vs. Cftr KO/Tlr2−/−, P = 0.014; WT/Tlr−/− vs. Cftr KO/Tlr2+/+, P = 0.044; WT/Tlr2−/− vs. Cftr KO/Tlr2−/−, P = 0.038; n = 4 WT/Tlr2+/+ (1 male, 3 females), 5 WT/Tlr2−/− (3 males, 2 females), 4 Cftr KO/Tlr2+/+ (1 male, 3 females), and 3 Cftr KO/Tlr4−/− mice (2 males, 1 female). B: photomicrographs of PAS-stained ileum from WT (left), Tlr2 KO (middle), and Cftr KO/Tlr2 KO (right) mice. Black arrows, widened villi. Yellow arrows, villus fusion. Blue arrow, lamina propria upfolding. Scale bars = 50 µm. KO, knockout; PAS, periodic acid-Schiff; Tlr, Toll-like receptor; WT, wild-type.
Stimulation of Tlr2 but not Tlr4 induces goblet cell hyperplasia in WT enteroids.
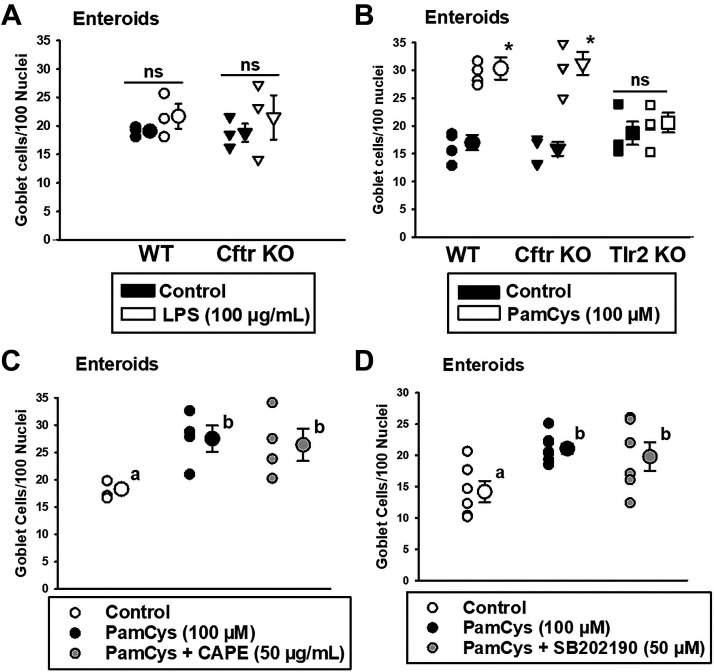
Intestinal epithelium expresses both Tlr2 and Tlr4, which can affect goblet cell differentiation (25, 26, 38). To determine if agonists of Tlr2 and Tlr4 directly regulate the goblet cell population of the small intestinal epithelium, we exposed WT and Cftr KO enteroids (passage 1, day 4) to either the Tlr4-specific agonist LPS or the Tlr2/Tlr1-specific agonist Pam3Csk4 for 72 h. Initial studies with typical concentrations of the agonists (10 µg/mL) did not affect the number goblet cells in WT enteroids with either LPS or Pam3Csk4 (data not shown), which may have resulted from binding to the Matrigel encasing the enteroids. Therefore, the concentrations of both LPS and Pam3Csk4 were increased to 100 µg/mL or 100 µM, respectively, for 72 h. As shown in Fig. 5A, 100 µg/mL LPS treatment did not significantly affect the number of goblet cells/100 nuclei in either WT or Cftr KO enteroids, consistent with evidence that the role of Tlr4 in goblet cell differentiation may be separate from its role in host defense (11). Surprisingly, as shown in Fig. 5B, treatment with the Tlr2/Tlr1 agonist Pam3Csk4 (100 µg/mL) stimulated a significant increase in goblet cell numbers/100 nuclei equally in both WT and Cftr KO enteroids. Pam3Csk4 treatment of Tlr2 KO enteroids had no effect on goblet cell number, indicating a Tlr2/Tlr1-dependent event. Previous studies of Tlr2 signaling in monocytes have shown involvement of the transcription factor nuclear factor-κB (NF-κB) (39). Therefore, studies of Pam3Csk4 stimulation of goblet cell hyperplasia in WT enteroids were repeated but included vehicle (DMSO) or NF-κB antagonist caffeic acid phenethyl ester (CAPE, 50 µg/mL) (40) throughout the stimulatory period. As shown in Fig. 5C, CAPE did not affect the increased goblet cell number resulting from Pam3Csk4 treatment. Previous studies have also shown TLR2 signaling involves p38 mitogen-activated protein kinases (p38 MAPK), which can activate a number of cellular transcription factors (41–43). However, as shown in Fig. 5D, cotreatment of WT enteroids with vehicle (DMSO) or the p38 MAPK inhibitor SB202190 (50 µM) (44) also did not affect Pam3Csk4-stimulated goblet cell hyperplasia.
Figure 5.
Goblet cell numbers in LPS and Pam3Csk4-treated enteroids from WT and Cftr KO mice. A: Passage 1, 7-day-old WT and Cftr KO small intestinal enteroids that were untreated (control) or treated with LPS (100 µg/mL) for 72 h. Individual data points shown. ns, not significantly different by unpaired t test; n = 3 WT and Cftr KO sex-matched littermate mouse pairs (2 males, 1 female). Average from 4 to 18 enteroids counted per mouse. B: Passage 1, 7-day-old WT, Cftr KO, and Tlr2 KO enteroids that were untreated (control) or treated with Pam3Csk4 (100 µM) for 72 h. Individual data points shown. *Signficantly different from Control by unpaired t test, P < 0.0002 for WT and P < 0.0007 for Cftr KO. n = 4 WT and Cftr KO sex-matched littermate mouse pairs (3 males, 1 female). Average from 5 to 23 enteroids counted per mouse. C: Passage 1, 7-day-old WT that were treated with DMSO vehicle (control) or treated with vehicle + Pam3Csk4 (100 µM) or Pam3Csk4 (100 µM) + CAPE (50 µg/mL) for 72 h. Individual data points shown. a,bMeans with the same letter are not significantly different by one-way ANOVA and Holm–Sidak vs. control comparisons. P = 0.035; n = 4 WT mice (3 males, 1 female). Average from 4 to 22 enteroids counted per mouse. D: Passage 1, 7-day-old WT that were treated with DMSO vehicle (control) or treated with vehicle + Pam3Csk4 (100 µM) or Pam3Csk4 (100 µM) + SB202190 (50 µM) for 72 h. Individual data points shown. a,bMeans with the same letter are not significantly different by one-way ANOVA and Holm–Sidak vs. control comparisons. P = 0.022; n = 4 WT mice (3 males, 1 female). Average from 3 to 11 enteroids counted per mouse. KO, knockout; LPS, lipopolysaccharide; PAS, periodic acid-Schiff; PEG, polyethylene glycol; Tlr, Toll-like receptor; WT, wild-type.
Pam3Csk4 (100 µg/mL) treatment decreases Notch1 expression in WT enteroids and increases genes of goblet cell differentiation/mucus production.
Unable to identify the signaling pathway of Pam3Csk4 stimulation using inhibitors, we measured mRNA expression by qRT-PCR using a panel of 46 genes that are known to be involved in goblet cell differentiation and mucus production (TaqMan Assays, Applied Biosciences). As shown in Table 1, Pam3Csk4 treatment for 72 h, caused a significant decrease in Notch1 expression in enteroids from six different WT mice. Correspondingly, there was increased expression of Atoh1, the master regulator of the intestinal secretory lineage, and Spdef, the amplifier of Atoh1-dependent transcription (45). Increased expression of the transcriptional repressors Gfi1 and Creb3l4 is also consistent with selective differentiation of Paneth cell and goblet cell lineages (31, 46), whereas, downregulation of Neurog3 transcription factor would be predicted to impair differentiation of secretory precursors toward enteroendocrine cell lineages (47). In terms of mucus production, increased expression of Muc1 and Muc2 were noted as well as the protein disulfide isomerase AGR2, an enzyme in the endoplasmic reticulum that is required for Muc2 production (48).
Table 1.
Transcriptomic changes consistent with reduced Notch signaling and increased goblet cell differentiation in WT enteroids treated with 100 µM Pam3Csk4 for 72 h
| Gene | Means | ± SE | P Value |
|---|---|---|---|
| Increased mRNA expression | |||
| Relative to untreated enteroids | |||
| Muc1 | 3.61 | ± 0.78 | 0.0055 |
| Muc2 | 1.32 | ± 0.33 | 0.0097 |
| Spdef | 0.67 | ± 0.18 | 0.0064 |
| Agr2 | 0.65 | ± 0.13 | 0.0046 |
| c-Fos | 0.55 | ± 0.09 | 0.0055 |
| Creb3l1 | 0.51 | ± 0.20 | 0.0356 |
| Atoh1 | 0.33 | ± 0.14 | 0.0099 |
| Gfi1 | 0.26 | ± 0.18 | 0.0055 |
| Decreased mRNA expression | |||
| Relative to untreated enteroids | |||
| Notch1 | −1.10 | ± 0.36 | 0.0340 |
| Sox9 | −0.88 | ± 0.30 | 0.0340 |
| Neurog3 | −0.57 | ± 0.12 | 0.0340 |
| Foxa2 | −0.48 | ± 0.16 | 0.0340 |
Effect of Pam3Csk4 treatment on WT enteroids on mRNA expression of epithelial differentiation markers. Enteroids were treated for 72 h with 100 µg/mL of Pam3Csk4 before collection for mRNA measurement by qRT-PCR; n = 6 WT mice (4 males, 2 females). Genes with unchanged expression are shown in Supplemental Table S2. WT, wild-type.
DISCUSSION
Mucoviscidosis is one of the primary pathogenic processes leading to the organ manifestations of CF disease. Goblet cell hyperplasia is typical in CF epithelia and contributes to mucoviscidosis with pathological consequences in the airways and intestines. With the use of the enteroid culture system, this study found that goblet cell hyperplasia is not an intrinsic disease property of the Cftr-null intestinal epithelium, either under basal or IL-13 stimulated conditions. These data extend to the intestine the novel observations by Zhang et al. (9) regarding the lack of goblet cell hyperplasia in CF human bronchial epithelial xenografts in denuded rat tracheas grown subcutaneously in athymic mice. The conclusion drawn from these studies is that goblet cell hyperplasia in CF-diseased organs is likely a consequence of the secondary effects of the CF epithelial environment, which includes inflammatory milieu, neurohumoral changes, altered immunity, and effects of the luminal microbiota (dysbiosis).
Evidence of an altered microenvironment in this investigation of CF mouse intestinal goblet cell hyperplasia was indicated by increased expression of IL-13, Spdef, Tlr2, and Tlr4. Increased IL-13 mRNA expression in freshly isolated small intestinal mucosa is likely sourced to epithelial-adjacent group 2 innate lymphoid cells (ILC2) (22, 49, 50). Stimulation of IL-13 production by ILC2 cells may result from alarmins [IL-25, IL-33, and thymic stromal lymphopoietin (TSLP)] elaborated by intestinal epithelial cells in response to damage, inflammation, or perhaps as a consequence of intestinal dysbiosis (7, 8, 51–54). As shown by increased protein expression of Spdef in this study, IL-13 treatment of murine enteroids is known to stimulate Atoh1 and Spdef mRNA expression (22), which are essential to intestinal goblet cell differentiation (45). Increased Tlr2 and Tlr4 protein expression were investigated because both of these pathogens and commensal-associated molecular pattern receptors have previously been shown to differ in CF epithelial cells (18, 33, 55, 56). Tlr signals are involved in a variety of intestinal functions including maintenance of tight junctions, Paneth cell antimicrobial secretion, and epithelial proliferation, and they play important roles in diseased states such as inflammation (as reviewed in Ref. 57). Consistent with the findings of others, we found both Tlr2 and Tlr4 expression to be increased in the isolated epithelium of the Cftr KO small intestine.
Cftr KO mice were bred to mice with global knockout of either Tlr2 or Tlr4 for the purposes of assessing the goblet cell populations in the CF ileum in the absence of each receptor. Previous studies using inbred BALB/c Cftr KO mice crossbred to inbred Balb/c C.C3-Tlr4Lps-d/J mice, which have a Tlr4 point mutation that produces a nonfunctional gene product (58), showed very poor survivability of the Cftr KO/Tlr4 KO double mutants that prevented analysis of the adult intestinal phenotype (18). However, the double-KO mice lived for ∼4 days, which allowed assessment of goblet cell numbers in the neonatal intestine. In that study, the proximal half of the intestine of Cftr KO mice showed goblet cell hyperplasia as compared with WT that was independent of Tlr4 genotype (+/+, +/−, −/−), whereas the goblet cell counts in the distal half of the intestine were similar between WT and Cftr KO (18). In the present study, Cftr KO and Tlr4 (and Tlr2) KO were crossbred to Black Swiss mice every third generation, a husbandry protocol that we have found increases the survivability of several gene knockout disease mouse models. This strategy provided a few adult Cftr KO/Tlr4 KO mice to permit analysis of goblet cell counts. Goblet cell counts in the ileum of adult Cftr KO/Tlr4 KO mice and Cftr KO/Tlr2 KO mice did not appear to be significantly altered from the goblet cell hyperplasia induced by Cftr KO alone, i.e., Cftr KO/Tlr4+/+ or Cftr KO/Tlr2+/+ compared with WT. These findings are consistent with the neonatal Cftr KO/Tlr4 KO intestinal goblet cell numbers (18) but differ from findings using intestinal epithelial-specific Tlr4 KO mice where goblet cell numbers were increased (11). However, the goblet cell counts in adult Cftr KO/Tlr4 KO and Cftr KO/Tlr2 KO mice in our study were confounded by morphological changes in the mucosal architecture of the distal ileum, which is reminiscent of colonic metaplasia as described in the ileal pouches of patients with colectomy (59, 60). Although we occasionally saw evidence of mucosal dysplasia in Cftr KO ileum, the changes occurring in the mucosa of both Cftr KO/Tlr4 KO and Cftr KO/Tlr2 KO mice were more apparent. Shorter and thicker villi, i.e., where we enumerated goblet cells, made difficult the direct comparison of the double Cftr KO/Tlr KO intestine with WT or Cftr KO goblet cell numbers. The crypts in the double KO mice, especially the Tlr4 KO/Cftr KO mice, were extended in length and exhibited sections with a mucosal architecture similar to a colonic mucosal fold. Studies of mice with MyD88 knockout, an adaptor molecular required for TLR induction of inflammatory cytokines, have shown that the interaction of TLRs with commensal bacteria provide a protective function to assure intestinal homeostasis (31). As part of that study, colonic hyperplasia with increased susceptibility to injury was demonstrated in the MyD88 KO mice. In the present study, mice had global KO of Tlr4, so further investigation will be necessary to determine whether the intestinal dysplasia is epithelial- or myeloid-mediated.
An interesting finding in our investigation was that the TLR2 agonist Pam3Csk4 in WT mouse enteroids induced goblet cell hyperplasia, apparently by suppression of Notch1 expression and increased expression of the goblet cell lineage determinants. Previous in vivo studies in which mice were gavaged with Pam3Csk4 show induction of goblet cell hyperplasia in the colon and have indicated that a similar phenomenon occurred in the small intestine (data not shown) (38). Our studies extend these findings with evidence that goblet cell hyperplasia induced by Tlr2/Tlr1 signaling is an epithelial-specific response, which does not occur in Tlr2 KO enteroids, indicating a Tlr2-dependent effect. Inhibitor studies of known pathways for Tlr2 signaling, i.e., p38 (28, 61, 62) and NF-κB (25, 39, 57, 63), did not affect the increased goblet cell numbers during enteroid treatment with Pam3Csk4. Therefore, we screened transcripts of genes involved in intestinal proliferation and differentiation. The transcripts of Pam3Csk4-treated WT enteroids revealed an almost canonical pattern for goblet cell differentiation as compared with untreated WT enteroids. Decreased expression of Notch1 and increased expression of Atoh1 that provides the master transcriptional regulator of the intestinal secretory cell lineage is consistent with increased goblet cell differentiation (64). Transcripts of Hes1 and Hes5 were not significantly changed but were highly variable (Supplemental Table S2; see https://doi.org/10.6084/m9.figshare.16613479.v1). Coincident with increased Atoh1 were increases in the Atoh1 target gene Spdef, which is a transcription coregulator with Atoh1 that has binding motifs in ∼11% of the Atoh1 target genes (45). These target genes have been associated with terminal differentiation of goblet cells by regulating the expression of genes such as Muc2, Agr2, and the transcription factor Creb3l4, a bispecific marker of Paneth and goblet cells (65) (Table 1). Atoh1 target gene Gfi1 was also increased, indicating a majority of secretory cell progenitors were destined for the Paneth cell/goblet cell fate (46), whereas Neurog3, the transcription factor that is necessary and sufficient for enteroendocrine cell differentiation was downregulated in Pam3Csk4-treated WT enteroids (Table 1).
A caveat to the Pam3Csk4 experiments on the enteroids was the use of a high concentration of the Tlr2-specific agonist Pam3Csk4. It is known that there is cross talk between Toll-like receptors (66) and we did not monitor all Tlrs in this study. However, the lack of goblet cell hyperplasia in the Pam3Csk4-treated Tlr2 KO enteroids indicated a specific response. Recent studies of zebrafish intestine have shown that the intestinal microbiota can promote epithelial secretory cell determination by inhibiting Notch1 signaling using a MyD88-specific pathway (67). That discovery is at odds with innate myeloid cell lineages wherein TLR ligands increase Notch signaling and its cognate ligands (see review in Ref. 68). However, some evidence indicates that commensal bacteria such as Lactobacillus spp. inhibit Notch signaling (69), which is associated with increased goblet cell differentiation (64, 69). Our finding that Pam3Csk4 induces goblet cell hyperplasia in enteroids suggests the hypothesis that an intestinal epithelial response to intestinal microbiota alters goblet cell differentiation through stimulation of a Tlr2-specific pathway (70–75). Consistent with the present observations are previous studies showing Tlr2 in Cftr KO mice is specifically upregulated in airway epithelial exposed to bacteria (55).
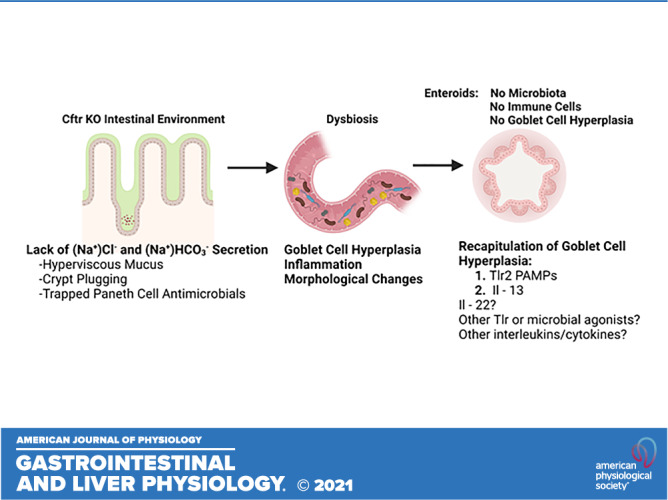
In summary, this study provides evidence that goblet cell hyperplasia in the Cftr-deficient small intestine is not a direct consequence of deficient Cftr activity in the epithelium, but likely secondary to the CF intestinal environment. In this environment, upregulation of Tlr2 and Tlr4 likely play important roles in inflammatory modulation and intestinal homeostasis. The absence of these Tlr2 and Tlr4 (i.e., Cftr KO/Tlr KO) resulted in morphological changes of the mucosa in the Cftr KO ileum consistent with colonic metaplasia. Although we did not find a direct role of Tlr4 using the agonist LPS in altering goblet cell numbers in WT enteroids, stimulation of Tlr2-dependent signaling initiated goblet cell hyperplasia that appeared secondary to decreased Notch signaling and consistent with a terminal goblet cell differentiation program of transcription factors involving increased Atoh1, Spdef, Gfi1, and decreased Neurog3 expression. Finally, the enteroid model may be useful to recapitulate goblet cell hyperplasia using factors external to the epithelium (see Graphical Abstract). In the present study, we found that Tlr2 stimulation and IL-13 exposure can induce goblet cell hyperplasia in mouse enteroids. Another potential contributor is IL-22, which has been shown to enhance goblet cell differentiation in mouse intestine (76, 77). More broadly, the model could serve for testing a panel of microbial agonists or interleukins/cytokines to evaluate their effectiveness in inducing goblet cell hyperplasia of the intestine in CF and other inflammatory bowel disorders.
SUPPLEMENTAL DATA
Supplemental Table S1: https://doi.org/10.6084/m9.figshare.16613470.v1.
Supplemental Table S2: https://doi.org/10.6084/m9.figshare.16613479.v1.
Supplemental Fig. S1: https://doi.org/10.6084/m9.figshare.16613464.v1.
Supplemental Fig. S2: https://doi.org/10.6084/m9.figshare.16958785.v1.
GRANTS
This work was supported by the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases Grant R01 DK48816 (to L. L. Clarke) and the Cystic Fibrosis Foundation Grants CLARKE15G0, CLARKE16P0, GAWENI16I0, and CLARKE19XX0.
DISCLOSURES
L.L.C. serves as an outside consultant to Entrinsic Health Solutions, Inc. and TranslateBIO MA, Inc. but does not receive compensation or travel/personal expenses. None of the other authors has conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
N.M.W., J.L., and L.L.C. conceived and designed research; N.M.W., J.L., S.M.Y., R.A.W., and L.L.C. performed experiments; N.M.W., J.L., R.A.W., and L.L.C. analyzed data; N.M.W., J.L., R.A.W., and L.L.C. interpreted results of experiments; N.M.W., J.L., R.A.W., and L.L.C. prepared figures; L.L.C. drafted manuscript; N.M.W., J.L., S.M.Y., R.A.W., and L.L.C. edited and revised manuscript; N.M.W., J.L., S.M.Y., R.A.W., and L.L.C. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank John Logan for expert technical assistance. Graphical abstract was created with BioRender.com
Present address of S. M. Young: AmplifyBio, 1425 Plain-City Georgesville Rd, Bldg. JM 10, West Jefferson, OH 43162, Ohio (SYoung@amplify-bio.com).
REFERENCES
- 1.Henderson AG, Ehre C, Button B, Abdullah LH, Cai L-H, Leigh MW, DeMaria GC, Matsui H, Donaldson SH, Davis CW, Sheehan JK, Boucher RC, Kesimer M. Cystic fibrosis airway secretions exhibit mucin hyperconcentration and increased osmotic pressure. J Clin Invest 124: 3047–3060, 2014. doi: 10.1172/JCI73469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borowitz D, Durie PR, Clarke LL, Werlin SL, Taylor CJ, Semler J, De Lisle RC, Lewindon P, Lichtman SM, Sinaasappel M, Baker RD, Baker SS, Verkade HJ, Lowe ME, Stallings VA, Janghorbani M, Butler R, Heubi J. Gastrointestinal outcomes and confounders in cystic fibrosis. J Pediatr Gastroenterol Nutr 41: 273–285, 2005. doi: 10.1097/01.mpg.0000178439.64675.8d. [DOI] [PubMed] [Google Scholar]
- 3.Snouwaert JN, Brigman KK, Latour AM, Malouf NN, Boucher RC, Smithies O, Koller BH. An animal model for cystic fibrosis made by gene targeting. Science 257: 1083–1088, 1992. doi: 10.1126/science.257.5073.1083. [DOI] [PubMed] [Google Scholar]
- 4.Liu J, Walker NM, Ootani A, Strubberg AM, Clarke LL. Defective goblet cell exocytosis contributes to murine cystic fibrosis-associated intestinal disease. J Clin Invest 125: 1056–1068, 2015. doi: 10.1172/JCI73193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gustafsson JK, Ermund A, Ambort D, Johansson MEV, Nilsson HE, Thorell K, Hebert H, Sjövall H, Hansson GC. Bicarbonate and functional CFTR channel are required for proper mucin secretion and link cystic fibrosis with its mucus phenotype. J Exp Med 209: 1263–1272, 2012. doi: 10.1084/jem.20120562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoen AG, Li J, Moulton LA, O'Toole GA, Housman ML, Koestler DC, Guill MF, Moore JH, Hibberd PL, Morrison HG, Sogin ML, Karagas MR, Madan JC. Associations between gut microbial colonization in early life and respiratory outcomes in cystic fibrosis. J Pediatr 167: 138–147.e3, 2015. doi: 10.1016/j.jpeds.2015.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lynch SV, Goldfarb KC, Wild YK, Kong W, De Lisle RC, Brodie EL. Cystic fibrosis transmembrane conductance regulator knockout mice exhibit aberrant gastrointestinal microbiota. Gut Microbes 4: 41–47, 2013. doi: 10.4161/gmic.22430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bazett M, Honeyman L, Stefanov AN, Pope CE, Hoffman LR, Haston CK. Cystic fibrosis mouse model-dependent intestinal structure and gut microbiome. Mamm Genome 26: 222–234, 2015. doi: 10.1007/s00335-015-9560-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y, Yankaskas J, Wilson J, Engelhardt JF. In vivo analysis of fluid transport in cystic fibrosis airway epithelia of bronchial xenografts. Am J Physiol Cell Physiol 270: C1326–C1335, 1996. doi: 10.1152/ajpcell.1996.270.5.C1326. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Doranz B, Yankaskas JR, Engelhardt JF. Genotypic analysis of respiratory mucous sulfation defects in cystic fibrosis. J Clin Invest 96: 2997–3004, 1995. doi: 10.1172/JCI118372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sodhi CP, Neal MD, Siggers R, Sho S, Ma C, Branca MF, Prindle T Jr, Russo AM, Afrazi A, Good M, Brower-Sinning R, Firek B, Morowitz MJ, Ozolek JA, Gittes GK, Billiar TR, Hackam DJ. Intestinal epithelial toll-like receptor 4 regulates goblet cell development and is required for necrotizing enterocolitis in mice. Gastroenterology 143: 708–718.e5, 2012. doi: 10.1053/j.gastro.2012.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clarke LL, Gawenis LR, Franklin CL, Harline MC. Increased survival of CFTR knockout mice with an oral osmotic laxative. Lab Anim Sci 46: 612–618, 1996. [PubMed] [Google Scholar]
- 13.De Lisle RC, Roach E, Jansson K. Effects of laxative and N-acetylcysteine on mucus accumulation, bacterial load, transit, and inflammation in the cystic fibrosis mouse small intestine. Am J Physiol Gastrointest Liver Physiol 293: G577–G584, 2007. doi: 10.1152/ajpgi.00195.2007. [DOI] [PubMed] [Google Scholar]
- 14.Liu J, Walker NM, Cook MT, Ootani A, Clarke LL. Functional Cftr in crypt epithelium of organotypic enteroid cultures from murine small intestine. Am J Physiol Cell Physiol 302: C1492–C1503, 2012. doi: 10.1152/ajpcell.00392.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gawenis LR, Hut H, Bot AGM, Shull GE, de Jonge HR, Stien X, Miller ML, Clarke LL. Electroneutral sodium absorption and electrogenic anion secretion across murine small intestine are regulated in parallel. Am J Physiol Gastrointest Liver Physiol 287: G1140–G1149, 2004. doi: 10.1152/ajpgi.00177.2004. [DOI] [PubMed] [Google Scholar]
- 16.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25: 402–408, 2001. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 17.Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459: 262–265, 2009. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 18.Canale-Zambrano JC, Auger ML, Haston CK. Toll-like receptor-4 genotype influences the survival of cystic fibrosis mice. Am J Physiol Gastrointest Liver Physiol 299: G381–G390, 2010. doi: 10.1152/ajpgi.00003.2010. [DOI] [PubMed] [Google Scholar]
- 19.Eckman EA, Cotton CU, Kube DM, Davis PB. Dietary changes improve survival of CFTR S489X homozygous mutant mouse. Am J Physiol Lung Cell Mol Physiol 269: L625–L630, 1995. doi: 10.1152/ajplung.1995.269.5.L625. [DOI] [PubMed] [Google Scholar]
- 20.Gawenis LR, Hodges CA, McHugh DR, Valerio DM, Miron A, Cotton CU, Liu J, Walker NM, Strubberg AM, Gillen AE, Mutolo MJ, Kotzamanis G, Bosch J, Harris A, Drumm ML, Clarke LL. A BAC transgene expressing human CFTR under control of its regulatory elements rescues Cftr knockout mice. Sci Rep 9: 11828, 2019. doi: 10.1038/s41598-019-48105-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hays SR, Fahy JV. Characterizing mucous cell remodeling in cystic fibrosis: relationship to neutrophils. Am J Respir Crit Care Med 174: 1018–1024, 2006. doi: 10.1164/rccm.200603-310OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Waddell A, Vallance JE, Hummel A, Alenghat T, Rosen MJ. IL-33 induces murine intestinal goblet cell differentiation indirectly via innate lymphoid cell IL-13 secretion. J Immunol 202: 598–607, 2019. doi: 10.4049/jimmunol.1800292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu D, Ahrens R, Osterfeld H, Noah TK, Groschwitz K, Foster PS, Steinbrecher KA, Rothenberg ME, Shroyer NF, Matthaei KI, Finkelman FD, Hogan SP. Interleukin-13 (IL-13)/IL-13 receptor α1 (IL-13Rα1) signaling regulates intestinal epithelial cystic fibrosis transmembrane conductance regulator channel-dependent Cl− secretion. J Biol Chem 286: 13357–13369, 2011. doi: 10.1074/jbc.M110.214965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.von Moltke J, Ji M, Liang HE, Locksley RM. Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature 529: 221–225, 2016. doi: 10.1038/nature16161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishii T, Muroi M, Horiguchi K, Tanamoto KI, Nagase T, Yamashita N. Activation through toll-like receptor 2 on group 2 innate lymphoid cells can induce asthmatic characteristics. Clin Exp Allergy 49: 1624–1632, 2019. doi: 10.1111/cea.13490. [DOI] [PubMed] [Google Scholar]
- 26.Taraban VY, Slebioda TJ, Willoughby JE, Buchan SL, James S, Sheth B, Smyth NR, Thomas GJ, Wang EC, Al-Shamkhani A. Sustained TL1A expression modulates effector and regulatory T-cell responses and drives intestinal goblet cell hyperplasia. Mucosal Immunol 4: 186–196, 2011. doi: 10.1038/mi.2010.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park K-S, Korfhagen TR, Bruno MD, Kitzmiller JA, Wan H, Wert SE, Khurana Hershey GK, Chen G, Whitsett JA. SPDEF regulates goblet cell hyperplasia in the airway epithelium. J Clin Invest 117: 978–988, 2007. doi: 10.1172/JCI29176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fujisawa T, Ide K, Holtzman MJ, Suda T, Suzuki K, Kuroishi S, Chida K, Nakamura H. Involvement of the p38 MAPK pathway in IL-13-induced mucous cell metaplasia in mouse tracheal epithelial cells. Respirology 13: 191–202, 2008. doi: 10.1111/j.1440-1843.2008.01237.x. [DOI] [PubMed] [Google Scholar]
- 29.Yamada N, Tamai Y, Miyamoto H, Nozaki M. Cloning and expression of the mouse Pse gene encoding a novel Ets family member. Gene 241: 267–274, 2000. doi: 10.1016/S0378-1119(99)00484-9. [DOI] [PubMed] [Google Scholar]
- 30.Erridge C, Duncan SH, Bereswill S, Heimesaat MM. The induction of colitis and ileitis in mice is associated with marked increases in intestinal concentrations of stimulants of TLRs 2, 4, and 5. PLoS One 5: e9125, 2010. doi: 10.1371/journal.pone.0009125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 118: 229–241, 2004. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 32.De Lisle RC. Altered transit and bacterial overgrowth in the cystic fibrosis mouse small intestine. Am J Physiol Gastrointest Liver Physiol 293: G104–G111, 2007. doi: 10.1152/ajpgi.00548.2006. [DOI] [PubMed] [Google Scholar]
- 33.Canale-Zambrano JC, Poffenberger MC, Cory SM, Humes DG, Haston CK. Intestinal phenotype of variable-weight cystic fibrosis knockout mice. Am J Physiol Gastrointest Liver Physiol 293: G222–G229, 2007. doi: 10.1152/ajpgi.00405.2006. [DOI] [PubMed] [Google Scholar]
- 34.King SL, Mohiuddin JJ, Dekaney CM. Paneth cells expand from newly created and preexisting cells during repair after doxorubicin-induced damage. Am J Physiol Gastrointest Liver Physiol 305: G151–G162, 2013. doi: 10.1152/ajpgi.00441.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hörmann N, Brandão I, Jäckel S, Ens N, Lillich M, Walter U, Reinhardt C. Gut microbial colonization orchestrates TLR2 expression, signaling and epithelial proliferation in the small intestinal mucosa. PLoS One 9: e113080, 2014. doi: 10.1371/journal.pone.0113080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Lisle RC, Roach EA, Norkina O. Eradication of small intestinal bacterial overgrowth in the cystic fibrosis mouse reduces mucus accumulation. J Pediatr Gastroenterol Nutr 42: 46–52, 2006. doi: 10.1097/01.mpg.0000189322.34582.3e. [DOI] [PubMed] [Google Scholar]
- 37.Norkina O, Burnett TG, De Lisle RC. Bacterial overgrowth in the cystic fibrosis transmembrane conductance regulator null mouse small intestine. Infect Immun 72: 6040–6049, 2004. doi: 10.1128/IAI.72.10.6040-6049.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Podolsky DK, Gerken G, Eyking A, Cario E. Colitis-associated variant of TLR2 causes impaired mucosal repair because of TTF3 deficiency. Gastroenterology 137: 209–220, 2009. doi: 10.1053/j.gastro.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brandt KJ, Fickentscher C, Kruithof EK, de Moerloose P. TLR2 ligands induce NF-κB activation from endosomal compartments of human monocytes. PLoS One 8: e80743, 2013. doi: 10.1371/journal.pone.0080743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Natarajan K, Singh S, Burke TR Jr, Grunberger D, Aggarwal BB. Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-κB. Proc Natl Acad Sci USA 93: 9090–9095, 1996. doi: 10.1073/pnas.93.17.9090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang L, Zuo M, Chen H, Liu S, Wu X, Cui Z, Yang H, Liu H, Ge B. Mycobacterium tuberculosis lipoprotein MPT83 induces apoptosis of infected macrophages by activating the TLR2/p38/COX-2 signaling pathway. J Immunol 198: 4772–4780, 2017. doi: 10.4049/jimmunol.1700030. [DOI] [PubMed] [Google Scholar]
- 42.Li D, Lei H, Li Z, Li H, Wang Y, Lai Y. A novel lipopeptide from skin commensal activates TLR2/CD36-p38 MAPK signaling to increase antibacterial defense against bacterial infection. PLoS One 8: e58288, 2013. doi: 10.1371/journal.pone.0058288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oeztuerk-Winder F, Ventura JJ. The many faces of p38 mitogen-activated protein kinase in progenitor/stem cell differentiation. Biochem J 445: 1–10, 2012. doi: 10.1042/BJ20120401. [DOI] [PubMed] [Google Scholar]
- 44.Düzgün ŞA, Yerlikaya A, Zeren S, Bayhan Z, Okur E, Boyaci I. Differential effects of p38 MAP kinase inhibitors SB203580 and SB202190 on growth and migration of human MDA-MB-231 cancer cell line. Cytotechnology 69: 711–724, 2017. doi: 10.1007/s10616-017-0079-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lo Y-H, Chung E, Li Z, Wan Y-W, Mahe MM, Chen M-S, Noah TK, Bell KN, Yalamanchili HK, Klisch TJ, Liu Z, Park J-S, Shroyer NF. Transcriptional regulation by ATOH1 and its target SPDEF in the intestine. Cell Mol Gastroenterol Hepatol 3: 51–71, 2017. doi: 10.1016/j.jcmgh.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shroyer NF, Wallis D, Venken KJ, Bellen HJ, Zoghbi HY. Gfi1 functions downstream of Math1 to control intestinal secretory cell subtype allocation and differentiation. Genes Dev 19: 2412–2417, 2005. doi: 10.1101/gad.1353905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.López-Díaz L, Jain RN, Keeley TM, VanDussen KL, Brunkan CS, Gumucio DL, Samuelson LC. Intestinal neurogenin 3 directs differentiation of a bipotential secretory progenitor to endocrine cell rather than goblet cell fate. Dev Biol 309: 298–305, 2007. doi: 10.1016/j.ydbio.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park S-W, Zhen G, Verhaeghe C, Nakagami Y, Nguyenvu LT, Barczak AJ, Killeen N, Erle DJ. The protein disulfide isomerase AGR2 is essential for production of intestinal mucus. Proc Natl Acad Sci USA 106: 6950–6955, 2009. doi: 10.1073/pnas.0808722106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Herbert DR, Douglas B, Zullo K. Group 2 innate lymphoid cells (ILC2): type 2 immunity and helminth immunity. Int J Mol Sci 20: 2276, 2019. doi: 10.3390/ijms20092276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu P, Zhu X, Wu J, He L, Lu T, Wang Y, Liu B, Ye B, Sun L, Fan D, Wang J, Yang L, Qin X, Du Y, Li C, He L, Ren W, Wu X, Tian Y, Fan Z. IL-13 secreted by ILC2s promotes the self-renewal of intestinal stem cells through circular RNA circPan3. Nat Immunol 20: 183–194, 2019. doi: 10.1038/s41590-018-0297-6. [DOI] [PubMed] [Google Scholar]
- 51.Ganal-Vonarburg SC, Duerr CU. The interaction of intestinal microbiota and innate lymphoid cells in health and disease throughout life. Immunology 159: 39–51, 2020. doi: 10.1111/imm.13138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roan F, Obata-Ninomiya K, Ziegler SF. Epithelial cell-derived cytokines: more than just signaling the alarm. J Clin Invest 129: 1441–1451, 2019. doi: 10.1172/JCI124606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Van Biervliet S, Eggermont E, Mariën P, Hoffman I, Veereman G. Combined impact of mucosal damage and of cystic fibrosis on the small intestinal brush border enzyme activities. Acta Clin Belg 58: 220–224, 2003. doi: 10.1179/acb.2003.58.4.002. [DOI] [PubMed] [Google Scholar]
- 54.Coffey MJ, Nielsen S, Wemheuer B, Kaakoush NO, Garg M, Needham B, Pickford R, Jaffe A, Thomas T, Ooi CY. Gut microbiota in children with cystic fibrosis: a taxonomic and functional dysbiosis. Sci Rep 9: 18593, 2019. doi: 10.1038/s41598-019-55028-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Muir A, Soong G, Sokol S, Reddy B, Gomez MI, Van Heeckeren A, Prince A. Toll-like receptors in normal and cystic fibrosis airway epithelial cells. Am J Respir Cell Mol Biol 30: 777–783, 2004. doi: 10.1165/rcmb.2003-0329OC. [DOI] [PubMed] [Google Scholar]
- 56.Shuto T, Furuta T, Oba M, Xu H, Li J-D, Cheung J, Gruenert DC, Uehara A, Suico MA, Okiyoneda T, Kai H. Promoter hypomethylation of Toll-like receptor-2 gene is associated with increased proinflammatory response toward bacterial peptidoglycan in cystic fibrosis bronchial epithelial cells. FASEB J 20: 782–784, 2006. doi: 10.1096/fj.05-4934fje. [DOI] [PubMed] [Google Scholar]
- 57.Burgueño JF, Abreu MT. Epithelial Toll-like receptors and their role in gut homeostasis and disease. Nat Rev Gastroenterol Hepatol 17: 263–278, 2020. doi: 10.1038/s41575-019-0261-4. [DOI] [PubMed] [Google Scholar]
- 58.Poltorak A, He X, Smirnova I, Liu M-Y, Huffel CV, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282: 2085–2088, 1998. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 59.de Silva HJ, Millard PR, Kettlewell M, Mortensen NJ, Prince C, Jewell DP. Mucosal characteristics of pelvic ileal pouches. Gut 32: 61–65, 1991. doi: 10.1136/gut.32.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fruin AB, El-Zammer O, Stucchi AF, O'Brien M, Becker JM. Colonic metaplasia in the ileal pouch is associated with inflammation and is not the result of long-term adaptation. J Gastrointest Surg 7: 246–253, 2003. doi: 10.1016/S1091-255X(02)00191-9. [DOI] [PubMed] [Google Scholar]
- 61.Chen R, Lim JH, Jono H, Gu X-X, Kim YS, Basbaum CB, Murphy TF, Li J-D. Nontypeable Haemophilus influenzae lipoprotein P6 induces MUC5AC mucin transcription via TLR2-TAK1-dependent p38 MAPK-AP1 and IKKβ-IκBα-NF-κB signaling pathways. Biochem Biophys Res Commun 324: 1087–1094, 2004. doi: 10.1016/j.bbrc.2004.09.157. [DOI] [PubMed] [Google Scholar]
- 62.Latorre E, Layunta E, Grasa L, Castro M, Pardo J, Gomollón F, Alcalde AI, Mesonero JE. Intestinal serotonin transporter inhibition by Toll-Like receptor 2 activation. A feedback modulation. PLoS One 11: e0169303, 2016. doi: 10.1371/journal.pone.0169303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mukherjee S, Sinha D, Ghosh AK, Biswas T. Bacterial ligand stimulates TLR2-dependent chemokines of colon cell. Immunobiology 219: 350–356, 2014. doi: 10.1016/j.imbio.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 64.van Es JH, van Gijn ME, Riccio O, van den Born M, Vooijs M, Begthel H, Cozijnsen M, Robine S, Winton DJ, Radtke F, Clevers H. Notch/γ-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature 435: 959–963, 2005. doi: 10.1038/nature03659. [DOI] [PubMed] [Google Scholar]
- 65.Gregorieff A, Stange DE, Kujala P, Begthel H, Van Den Born M, Korving J, Peters PJ, Clevers H. The Ets-domain transcription factor Spdef promotes maturation of goblet and paneth cells in the intestinal epithelium. Gastroenterology 137: 1333–1345.e3, 2009. doi: 10.1053/j.gastro.2009.06.044. [DOI] [PubMed] [Google Scholar]
- 66.van Aubel RAMH, Keestra AM, Krooshoop DJEB, van Eden W, van Putten JPM. Ligand-induced differential cross-regulation of Toll-like receptors 2, 4 and 5 in intestinal epithelial cells. Mol Immunol 44: 3702–3714, 2007. doi: 10.1016/j.molimm.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 67.Troll JV, Hamilton MK, Abel ML, Ganz J, Bates JM, Stephens WZ, Melancon E, van der Vaart M, Meijer AH, Distel M, Eisen JS, Guillemin K. Microbiota promote secretory cell determination in the intestinal epithelium by modulating host Notch signaling. Development 145: dev155317, 2018. doi: 10.1242/dev.155317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shang Y, Smith S, Hu X. Role of Notch signaling in regulating innate immunity and inflammation in health and disease. Protein Cell 7: 159–174, 2016. doi: 10.1007/s13238-016-0250-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ghanavati R, Asadollahi P, Shapourabadi MB, Razavi S, Talebi M, Rohani M. Inhibitory effects of Lactobacilli cocktail on HT-29 colon carcinoma cells growth and modulation of the Notch and Wnt/β-catenin signaling pathways. Microb Pathog 139: 103829, 2020. doi: 10.1016/j.micpath.2019.103829. [DOI] [PubMed] [Google Scholar]
- 70.Engevik MA, Luk B, Chang-Graham AL, Hall A, Herrmann B, Ruan W, Endres BT, Shi Z, Garey KW, Hyser JM, Versalovic J. Bifidobacterium dentium fortifies the intestinal mucus layer via autophagy and calcium signaling pathways. mBio 10: e01087-19, 2019. doi: 10.1128/mBio.01087-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kaur K, Saxena A, Debnath I, O'Brien JL, Ajami NJ, Auchtung TA, Petrosino JF, Sougiannis AJ, Depaep S, Chumanevich A, Gummadidala PM, Omebeyinje MH, Banerjee S, Chatzistamou I, Chakraborty P, Fayad R, Berger FG, Carson JA, Chanda A. Antibiotic-mediated bacteriome depletion in ApcMin/+ mice is associated with reduction in mucus-producing goblet cells and increased colorectal cancer progression. Cancer Med 7: 2003–2012, 2018. doi: 10.1002/cam4.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Genda T, Kondo T, Sugiura S, Hino S, Shimamoto S, Nakamura T, Ukita S, Morita T. Bacterial fermentation of water-soluble cellulose acetate raises large-bowel acetate and propionate and decreases plasma cholesterol concentrations in rats. J Agric Food Chem 66: 11909–11916, 2018. doi: 10.1021/acs.jafc.8b04093. [DOI] [PubMed] [Google Scholar]
- 73.Ganesh BP, Nelson JW, Eskew JR, Ganesan A, Ajami NJ, Petrosino JF, Bryan RM Jr, Durgan DJ. Prebiotics, probiotics, and acetate supplementation prevent hypertension in a model of obstructive sleep apnea. Hypertension 72: 1141–1150, 2018. doi: 10.1161/HYPERTENSIONAHA.118.11695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Khailova L, Baird CH, Rush AA, Barnes C, Wischmeyer PE. Lactobacillus rhamnosus GG treatment improves intestinal permeability and modulates inflammatory response and homeostasis of spleen and colon in experimental model of Pseudomonas aeruginosa pneumonia. Clin Nutr 36: 1549–1557, 2017. doi: 10.1016/j.clnu.2016.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.de Moreno de LeBlanc A, Dogi CA, Galdeano CM, Carmuega E, Weill R, Perdigón G. Effect of the administration of a fermented milk containing Lactobacillus casei DN-114001 on intestinal microbiota and gut associated immune cells of nursing mice and after weaning until immune maturity. BMC Immunol 9: 27, 2008. doi: 10.1186/1471-2172-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sugimoto K, Ogawa A, Mizoguchi E, Shimomura Y, Andoh A, Bhan AK, Blumberg RS, Xavier RJ, Mizoguchi A. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J Clin Invest 118: 534–544, 2008. doi: 10.1172/JCI33194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim JH, Ahn JB, Kim DH, Kim S, Ma HW, Che X, Seo DH, Kim TI, Kim WH, Cheon JH, Kim SW. Glutathione S-transferase theta 1 protects against colitis through goblet cell differentiation via interleukin-22. FASEB J 34: 3289–3304, 2020. doi: 10.1096/fj.201902421R. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table S1: https://doi.org/10.6084/m9.figshare.16613470.v1.
Supplemental Table S2: https://doi.org/10.6084/m9.figshare.16613479.v1.
Supplemental Fig. S1: https://doi.org/10.6084/m9.figshare.16613464.v1.
Supplemental Fig. S2: https://doi.org/10.6084/m9.figshare.16958785.v1.