Keywords: enterohepatic bile acid cycling, FGF19, intestinal adaptation, short gut syndrome
Abstract
Loss of functional small bowel surface area causes short bowel syndrome (SBS), intestinal failure, and parenteral nutrition (PN) dependence. The gut adaptive response following resection may be difficult to predict, and it may take up to 2 yr to determine which patients will wean from PN. Here, we examined features of gut microbiota and bile acid (BA) metabolism in determining adaptation and ability to wean from PN. Stool and sera were collected from healthy controls and from patients with SBS (n = 52) with ileostomy, jejunostomy, ileocolonic, and jejunocolonic anastomoses fed with PN plus enteral nutrition or who were exclusively enterally fed. We undertook 16S rRNA gene sequencing, BA profiling, and 7α-hydroxy-4-cholesten-3-one (C4) quantitation with LC-MS/MS and serum amino acid analyses. Patients with SBS exhibited altered gut microbiota with reduced gut microbial diversity compared with healthy controls. We observed differences in the microbiomes of patients with SBS with ileostomy versus jejunostomy, jejunocolonic versus ileocolonic anastomoses, and PN dependence compared with those who weaned from PN. Stool and serum BA composition and C4 concentrations were also altered in patients with SBS, reflecting adaptive changes in enterohepatic BA cycling. Stools from patients who were weaned from PN were enriched in secondary BAs including deoxycholic acid and lithocholic aicd. Shifts in gut microbiota and BA metabolites may generate a favorable luminal environment in select patients with SBS, promoting the ability to wean from PN. Proadaptive microbial species and select BA may provide novel targets for patient-specific therapies for SBS.
NEW & NOTEWORTHY Loss of intestinal surface area causes short bowel syndrome, intestinal failure, and parenteral nutrition dependence. We analyzed the gut microbiota and bile acid metabolome of a large cohort of short bowel syndrome adult patients with different postsurgical anatomies. We report a novel analysis of the microbiome of patients with ileostomy and jejunostomy. Enrichment of specific microbial and bile acid species may be associated with the ability to wean from parenteral nutrition.
INTRODUCTION
The loss of functional small bowel surface area following surgical resection for Crohn’s disease, ischemia, and trauma, necrotizing enterocolitis, or other disorders may result in short bowel syndrome (SBS). In the United States, ∼10,000–20,000 patients have intestinal failure due to SBS and are parenteral nutrition (PN) dependent. SBS is a major cause of morbidity and mortality and obligates extensive health care costs, because patients depend on parenteral nutrition to meet their nutritional requirements. Intestinal failure with long-term PN dependence is most likely to occur in patients with less than 100 cm of remaining small bowel without residual colon or with less than 50 cm anastomosed to residual colon (1, 2).
Following intestinal resection, the remaining small bowel epithelium mounts an adaptive response that includes morphologic and functional modifications that enhance nutrient and electrolyte absorption (1, 3). Presence of a colon, remaining small bowel length, and underlying disease activity affect the ability to wean from PN. However, this adaptive response may be difficult to predict, and it may require 2 yr or more (4) to determine which patients will wean from parenteral nutrition (PN), even with an accurate assessment of remnant small bowel length. Little is known about what role the composition of the intestinal microbiome and metabolome plays in adaptation in adult patients with SBS.
Among potential mechanisms influencing adaptation, we hypothesized that distinct gut microbial communities might be associated with the ability to wean PN support in adult patients with SBS and might reflect more nuanced outcomes in those SBS patients with jejunostomy or ileostomy, as well as in a subset of SBS patients with colon in continuity. Alterations in microbial composition in SBS are of particular interest, because taxonomic shifts impact not only the ability of the host to harvest energy but also regulate elements of bile acid (BA) metabolism and signaling. Alterations in BA species composition have been shown in turn to modulate gut epithelial proliferation and apoptosis (5) and reduce gut inflammation and injury by signaling via farnesoid X receptor (FXR) pathway (6–8) as well as promote adaptive signaling via G-coupled BA receptor1 (TGR5) pathways. These data prompt us to interrogate in humans the possible role of BA signaling pathways in SBS adaptation, specifically the role of conjugated versus deconjugated and dehydroxylated BA species in SBS patients with versus without a colon, as well as the adaptations in fibroblast growth factor 19 (FGF19)/enterohepatic signaling and BA synthesis.
To address these questions, we collected stool and blood from adult SBS patients with ileostomy, jejunostomy, ileocolonic, and jejunocolonic anastomoses, who consumed an oral diet only or who received PN and an oral diet, as well as normal patient controls. We compared the microbiome and examined homeostatic BA regulation and composition in patients with SBS compared with control subjects, in patients with SBS who were either PN dependent or independent, and compared subsets of SBS patients with different surgical anatomies (ileostomy vs. jejunostomy, ileocolonic vs. jejunocolonic anastomoses, and colon present in continuity vs. colon absent). We found reduced microbial diversity in patients with SBS compared with controls and observed microbial shifts in patients with different postsurgical anatomies. We found enrichment in Lachnospiraceae and Clostridium cluster XIVa, which play important roles in short-chain fatty acid metabolism/metabolic adaptation and bile acid metabolism, respectively, in patients with jejunocolonic anastomoses who weaned from PN compared with those who were PN dependent. We also observed differences in fecal BA profiles between PN-dependent patients compared with those who weaned from PN.
MATERIALS AND METHODS
Human Studies
All human studies reported in this paper were approved by the Institutional Review Board of Washington University in St. Louis, as indicated in Human Studies protocol IRB# 201611003. Written informed consent was received from participants before inclusion in the study. All studies were performed in compliance with the Declaration of Helsinki ethical principles for medical research involving human subjects.
Patient Population
We recruited adults with short bowel syndrome (SBS; n = 54) defined as patients with the clinical syndrome of SBS (diarrhea, weight loss, malabsorption) and ≤200 cm small bowel remaining after surgical resection, patients who had complete colectomy and ileostomy for treatment of familial adenomatous polyposis (FAP) but had normal small bowel length (n = 3), and normal controls (n = 7). The patients with SBS were recruited from the Intestinal Rehabilitation Gastroenterology clinic at Washington University in St. Louis, which focuses primarily on the management of patients with SBS. Normal control patients, defined as individuals without gastrointestinal (GI)-related illnesses (n = 7), and patients with FAP after colectomy with ileostomy and normal small bowel length (n = 3) were recruited by the Biobank of the Digestive Diseases Research Core Center. Exclusion criteria for the patients with SBS included active Crohn’s disease as defined by evidence of clinical or mucosal disease activity, previous liver or small bowel transplantation, previous stool transplant, and patients with <20 cm of remaining small bowel, because the extreme loss of small bowel eliminates the possibility of weaning from PN. Adult patients with SBS were either completely enterally nourished or receiving partial (<7 days/wk) or complete (daily) PN infusions. All patients with SBS fed with partial or complete PN were encouraged to ingest oral calories as desired and to maximize oral calorie ingestion as part of the ongoing goal of achieving independence from PN, per clinical protocols. Dietary counseling and specific diets were tailored to each individual’s postresection anatomy (with or without a colon in continuity) to attain optimal nutrient absorption and to control diarrhea.
The clinical and demographic characteristics of the subjects are supplied in Table 1 and Supplemental Table S1 (all Supplemental material is available at https://doi.org/10.6084/m9.figshare.17036441), respectively.
Table 1.
Clinical characteristics of healthy control and adult patients with short bowel syndrome
| Variables | Healthy Controls (n = 7) | PN-Independent Patients with SBS (n = 30) | PN-Dependent Patients with SBS (n = 22) | P Valuea |
|---|---|---|---|---|
| Age, yr | 46 (28) | 58 (22) | 62 (30) | 0.69 |
| Women (n) | 5 | 20 | 13 | |
| Males (n) | 2 | 10 | 9 | |
| Etiology of SBS (n) | ||||
| Ischemic bowel | 2 | 8 | ||
| Crohn’s disease | 13 | 4 | ||
| Radiation enteritis | 0 | 2 | ||
| Malignancy | 3 | 2 | ||
| Congenital | 4 | 1 | ||
| Adhesions/EC fistula | 3 | 3 | ||
| Small bowel obstruction or incarcerated hernia | 4 | 2 | ||
| Sclerosing mesenteritis | 1 | 0 | ||
| SBS duration, yr | 2.5 (10) | 14.5 (19) | 0.002 | |
| Remaining small bowel length, cm | 120 (82.5) | 100 (82.5) | 0.09 | |
| Ileocolonic anastomosis (n) | 15 | 9 | ||
| Jejunocolonic anastomosis (n) | 4 | 4 | ||
| Ileostomy (n) | 10 | 6 | ||
| Jejunostomy (n) | 1 | 3 |
aP value comparing PN-independent to PN-dependent patients with SBS (unpaired two-tailed Student’s t test). Age, SBS duration, and remaining small bowel length are reported as median and interquartile range (IQR). EC, enterocutaneous; PN, parenteral nutrition; SBS, short bowel syndrome.
Specimens
Stool was collected from patients at enrollment (n = 54 patients; 52 samples were used for microbiome analysis; 2 were discarded because of an inadequate number of sequence reads). Normal control patients (n = 7) and patients who had colectomy for treatment of familial adenomatous polyposis coli but had normal small bowel (FAP, n = 3) had stools and blood collected at enrollment. Blood was collected simultaneously with the stool samples in a subset of patients with SBS (n = 32 patients at enrollment; additional timed blood samples were collected from a subset of these patients over a 24-mo time period). Plasma was separated from blood by centrifugation, aliquoted, and frozen in bar-coded tubes. Stool aliquots (5 aliquots of 0.2 g each) were weighed into 2-mL Omni prefilled bead homogenization tubes, then frozen until use. The remainder of the bulk specimens were also stored at −80°C. Patients came to clinic for blood draws; participants with a colon in continuity were asked to either provide a stool specimen at blood draw or bring a fresh morning stool collection on ice. Participants with ostomies had stool collected at the time of blood draw. Blood was drawn from PN patients’ central venous catheters, coordinated with routine blood draws. patients with PN were off PN infusions when blood was drawn. All materials were frozen at −80°C until analyzed.
16S rRNA Gene Sequencing
DNA was extracted from stool using the Fecal DNA Extraction kit (MoBio Laboratories), followed by lysozyme and staphylolysin treatment, which improved the extraction of many difficult-to-lyse Gram-positive species. Stools were analyzed by sequencing of the V4 region of the 16S rRNA gene by MR DNA (Shallowater, TX, http://www.mrdnalab.com/) using the MiSeq platform (Illlumina). The 16S rRNA gene V4 variable region PCR primers 515/806 with barcode on the forward primer were used in a 28-cycle PCR (5 cycles used on PCR products) using the HotStarTaq Plus Master Mix Kit (Qiagen) under the following conditions: 94°C for 3 min, followed by 28 cycles of 94°C for 30 s, 53°C for 40 s, and 72°C for 1 min, after which a final elongation step at 72°C for 5 min was performed. After amplification, PCR products were checked in 2% agarose gel to determine the success of amplification and the relative intensity of bands. Barcoded samples were pooled together in equal proportions based on their molecular weight and DNA concentrations. Pooled samples were purified using calibrated Ampure XP beads. Then the pooled and purified PCR product was used to prepare an Illumina DNA library. Sequencing was performed at MR DNA (www.mrdnalab.com, Shallowater, TX) on a MiSeq following the manufacturer’s guidelines.
16S rRNA Gene Sequence Analysis
Nucleotide sequences provided by MR DNA were demultiplexed into paired FASTQ files based on sample-to-barcode id associations. Microbiome sequence analysis was performed with Mothur using the methods described by Kozich et al. (9), whereas the UCHIME (10) package was used to filter-out chimeric sequence reads. Sequence reads were assigned to operational taxonomic units (OTUs) using Mothur’s opticlust method (11) and clustering required a threshold 97% similarity. Subsequently, a naïve Bayesian classifier (12) was used with the Ribosomal Database Project training set (v.16) to classify sequences and assign taxonomy to the lowest taxonomic level at which 80% confidence was satisfied. Differential representation of specific taxa was evaluated by logarithmic linear discriminate analysis (LDA) using the LEfSe (13) package (α value for the factorial Kruskal–Wallis test among classes = 0.05; α value for the pairwise Wilcoxon test between subclasses = 0.05). An LDA score of 2.0 (log 10) or greater was considered significantly differential when comparing bacteria abundances for given conditions. Sequence data will be deposited into NCBI Sequence Read Archive (SRA) and accession numbers will be supplied before publication.
Bile Acid Species and 7α-Hydroxy-3-Keto-4-Cholestene Determination
We simultaneously collected blood samples from a subset of these patients (n = 32). Plasma was analyzed for the bile acid (BA) metabolome. In addition, stool (n = 54 samples from 41 patients with SBS of whom a subset contributed one or more additional timed samples) was analyzed for the fecal bile acid metabolome. BAs were individually determined by LC-MS/MS, including cholic acid (CA), glycocholic acid (GCA), taurocholic acid (TCA), ursodeoxycholic acid (UDCA), glycoursodeoxycholic acid (GUDCA), tauroursodeoxycholic acid (TUDCA), lithocholic acid (LCA), glycolithocholic acid (GLCA), taurolithocholic acid (TLCA), chenodeoxycholic acid (CDCA), glycochenodeoxycholic acid (GCDCA), taurochenodeoxycholic acid (TCDCA), deoxycholic acid (DCA), glycodeoxycholic acid (GDCA), and taurodeoxycholic acid (TDCA).
Briefly, participants in clinic with ostomy bags or who were able to provide a stool sample were asked to submit samples directly, which were immediately transported to the laboratory. Stool samples were collected from some participants using a stool collection kit that was couriered to the laboratory within 1.5 h after the patient contacted the courier service. On receipt, samples were either processed immediately or frozen at −80°C. For processing, stool samples (∼0.2 g) were transferred into tared vials for accurate weight determination and then homogenized in water (12 mL/g stool). Those initial stool homogenates were then further diluted 1:10 with water and vigorously vortexed. A protein precipitation procedure was used to extract BAs from the stool homogenate with/without dilution (depending on the starting material, either solid or liquid) in the presence of deuterated internal standards. The BA values determined from diluted stool samples were reported if BA values in the initial homogenate samples were above the upper limit of quantification (ULOQ). The BA concentrations in stool homogenates (ng/mL) were also converted into BA content in the original stool sample (ng/g) by multiplying BA concentration in the stool homogenate concentrations by a factor of 13 based on the assumption that the density of this stool homogenate is ∼1 g/mL. Deuterated internal standards included d4-CA, d4-GCDCA, d4-GCA, d4-CDCA, d4-DCA, d4-LCA, d4-UDCA, d4-GUDCA, d4-GDCA, d4-GLCA, d4-TUDCA, d4-TLCA, d4-TLDCA, d4-TCA, d4-TCDCA, and d6-TDCA. The extracts were separated by column-switching high-performance liquid chromatography (HPLC) on a SecurityGuard Gemini C18 (4 × 3 mm) and ACE Excel column, then analyzed by Applied Biosystems Sciex 4000QTRAP tandem mass spectrometer (MS/MS) equipped with an electrospray ion source in the negative ion mode and multiple-reaction monitoring (MRM) detection. The 11-point calibration curve in surrogate matrix was prepared in duplicate. The calibrators were injected at the beginning and end of the run. The calibrators deviated by more than 15% of nominal concentrations were excluded from the construction of calibration curve, except that deviation of 20% was acceptable for the lower limit of quantification (LLOQ), which is the low end of calibration curve. The bile acid concentrations in plasma were expressed as ng/mL.
7α-Fydroxy-3-keto-4-cholestene (C4) in plasma was also determined by LC-MS/MS (n = 50 normal healthy subjects and n = 33 samples from 27 patients with SBS). A protein precipitation procedure was used to extract C4 from 0.05 mL plasma in the presence of d7-C4. The C4 was separated on a SecurityGuard C18 (4 × 3 mm) and ACE C8 column (3 µm, 100 × 4.6 mm). C4 was detected with positive MRM on an Applied Biosystems Sciex 4000QTRAP tandem mass spectrometer. The 8-point calibration curve for C4 was prepared in surrogate matrix. The calibrators were prepared in duplicate and injected at the beginning and end of the run. The calibrators deviated by more than 15% of nominal concentrations were excluded from construction of calibration curve, except that deviation of 20% was acceptable for the lower limit of quantification (LLOQ), which is the low end of calibration curve. The C4 concentrations below the LLOQ were reported as <LLOQ. The C4 concentration in plasma was expressed as nM.
Plasma FGF19 Measurements
Plasma FGF19 level was determined by ELISA using R&D Systems Quantikine ELISA kit (DF1900), following the manufacturer’s instructions (n = 50 normal healthy subjects and n = 33 samples from 27 patients with SBS). The FGF19 concentration was expressed as pg/mL.
Serum GLP-1 and GLP-2 Measurements
Serum glucagon-like peptide 1 (GLP-1) was determined by ELISA using Invitrogen ELISA kit (Cat. No. BMS2194) following the manufacturer’s instructions. Serum GLP2 was determined by ELISA using Millipore ELISA Kit (Cat. No. EZGLP2-37K), following the manufacturer’s instructions. GLP-1 and GLP-2 measurements were performed on a subset of samples that had an adequate amount for analysis; SBS, n = 21 and healthy control, n = 12.
Plasma Amino Acid Levels
Blood was collected from SBS (n = 38 patients who provided 46 samples including 8 timed samples) and normal healthy control subjects (n = 7), and plasma was analyzed for amino acid levels by high performance liquid chromatography-ultraviolet detection in the Boston Children’s Hospital, Boston MA (Department of Laboratory Medicine, Kellogg R&D Laboratory Mark Kellogg, PhD).
General Statistical Analysis
Two-group demographic comparisons, comparisons of remaining small bowel length, and serum amino acid levels were analyzed by unpaired two-tailed Student’s t test using SPSS software; P < 0.05 was considered significant.
BA data were analyzed using GraphPad Prism 8.4.0. Two-group comparison was by two-tailed unpaired Student’s t test or Mann–Whitney test. For multigroup comparisons, statistical differences were determined by one-way analysis of variance, with Tukey’s multiple comparisons test. The correlation between plasma C4 and Fgf19 was analyzed by Spearman’s correlation. The level of significance was set at P < 0.05.
For 16S sequences, α (Shannon diversity and richness) and β (Bray–Curtis dissimilarity) diversity measures were calculated using the vegan R package (https://CRAN.R-project.org/package=vegan). The taxonomic summary bar plots were used to visualize abundance at various taxonomic levels.
RESULTS
Patient with SBS and Normal Subject Demographics
Fifty-four patients with SBS were recruited (Table 1), of whom 52 (33 females, 19 males) were analyzed for the fecal microbiome. Two samples were eliminated from the analysis due to an inadequate number of sequence reads. Complete demographic details are found in Supplemental Table S1. Twenty-two were PN dependent (13 females, 9 males), and 30 were PN independent (20 females, 10 males). All patients ate an oral diet, including PN-dependent patients who were encouraged to eat as much as tolerated to try to reduce PN requirements, and all were provided dietary instructions that were appropriate for their postsurgical anatomy. Causes of SBS in the PN-dependent cohort included ischemic bowel (n = 8), congenital (n = 1), malignancy (n = 2), small bowel obstruction or incarcerated hernia (n = 2), enterocutaneous fistulae or adhesions (n = 3), radiation enteritis (n = 2), and Crohn’s disease (n = 4). Causes of SBS in the PN-independent cohort were congenital (n = 4), ischemia (n = 2) Crohn’s disease (n = 13), sclerosing mesenteritis (n = 1), malignancy (n = 3), small bowel obstruction or incarcerated hernia (n = 4), adhesions, or enterocutaneous fistulae (n = 3).
Patients with Short Bowel Syndrome Have Reduced Microbial Diversity and an Altered Microbiome Compared with Healthy Controls
Figure 1 shows the taxonomic analysis of the microbiome of healthy controls compared with all patients with SBS grouped by PN status. Patients with SBS had increased relative abundance of Enterobacteriaceae and Lactobacillus compared with normal control participants. Shannon diversity analysis showed reduced α diversity in all patients with SBS compared with healthy controls (P = 0.002 for the entire group, Fig. 2). Subgroup analysis demonstrated reduced α diversity in patients with ileostomy versus controls (P = 0.003), in patients with ileocolonic anastomosis versus controls (P = 0.003), similar to previously published data (14) and a trend in patients with jejunostomy versus controls (P = 0.054) but the jejunocolonic anastomosis subgroup showed no significant change in diversity compared with controls (P = 0.122).
Figure 1.
The proportion of genera within the microbiota of healthy controls (NormCrtl, normal control, n = 7), patients with familial adenomatous polyposis coli who have had a total colectomy (NoColon, n = 3), and patients who were fed enterally only (OffTPN, n = 30) or were dependent on parenteral nutrition (OnTPN, n = 22). Stacked bar charts represent the relative abundances of taxa in each sample, with each color representing a different taxon (key on right).
Figure 2.
Shannon diversity indices for healthy control subjects compared with ileostomy (I, n = 16), ileocolonic anastomosis (IC Anast, n = 24), jejunostomy (J, n = 4), and jejunocolonic anastomosis groups (JC Anast, n = 8). **P = 0.002; control vs. whole group (I + IC Anast + J + JC Anast, n = 52). Subgroup analysis revealed reduced α diversity in patients with ileostomy vs. controls (I vs. controls, *P = 0.003) and in patients with ileocolonic anastomosis vs. controls (IC Anast vs. controls *P = 0.003). Patients with jejunostomy vs. controls, P = 0.054. Jejunocolonic anastomosis subgroup showed no significant change in diversity compared with controls (P = 0.122). Horizontal bars represent the median, and box and whiskers show quartiles and the minimum and maximum, respectively.
To determine which changes in the microbiome were significant among these groups, linear discriminant analysis (LDA) was performed, with an LDA score of 2.0 (log 10) or greater indicating significantly abundant bacteria for the condition (Fig. 3). There were significant differences in relative abundance of numerous bacteria in control compared with patients with SBS. Bacteroidetes is enriched in healthy controls versus patients with SBS, and Proteobacteria is enriched in patients with SBS compared with controls (Fig. 3A), consistent with published data, reviewed in Neelis et al. (15). Similarly, we found that Gammaproteobacteria and Bacilli were increased in patients with SBS at the class level (Fig. 3B), Enterobacteriales and Lactobacilliales at the order level, and Veillonellaceae and Enterobacteriaceae at the family level (Fig. 3, C and D). At the genus level (Fig. 3E), controls were enriched for Bacteroides, Faecalibacterium, Ruminococcaceae, Blautia, Lachnospiraceae, Roseburia, Clostridiales, and Eggerthia compared with all patients with SBS. In contrast, Fusobacterium, Veillonella, and Enterobacteriaceae were enriched in patients with SBS compared with healthy controls.
Figure 3.
LDA analysis of microbiota of normal controls (n = 7) compared with all patients with SBS (n = 52). A: phylum level. B: class level. C: order level. D: family level. E: genus level. Ctrl, control; LDA, linear discriminate analysis; SBS, short bowel syndrome.
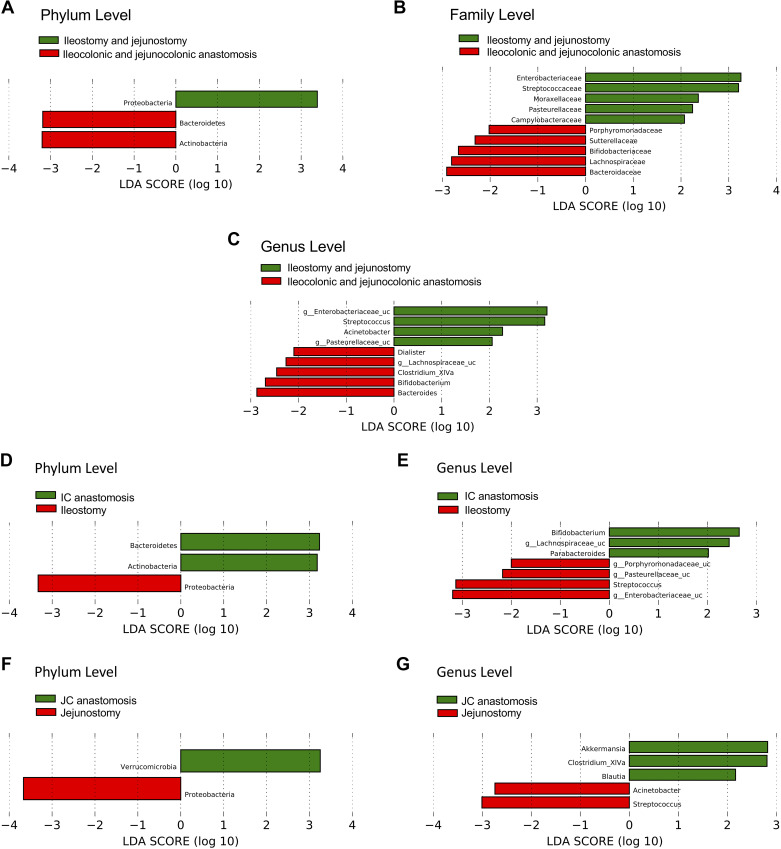
LDA analysis also showed changes in relative bacterial abundances comparing a subset of patients with SBS whose colons were in continuity to patients with a jejunostomy or ileostomy (Fig. 4). The microbiome of SBS patients with a colon in continuity was enriched with Bacteroidetes and Actinobacter, whereas the microbiome of SBS patients with ileostomy or jejunostomy was enriched with Proteobacteria (Fig. 4A). At the family level (Fig. 4B), patients with ileostomy and jejunostomy were enriched with Enterobacteriaceae, Streptococcaceae, Moraxellaceae, Pasteurellaceae, and Campylobacteraceae, whereas those with a colon in continuity had increased Porphyromonadaceae, Sutterellaceae, Bifidobacteriaceae, Lachnospiraceae, and Bacteroidaceae. At the genus level, Streptococcus and Acinetobacter were enriched in patients with ostomy (Enterobacteriaceae and Pasteurellaceae were unclassified at the genus level); in contrast, Dialister, Clostridium_XIVa, Bifidobacterium, and Bacteroides were enriched in SBS patients with a colon in continuity and Lachnospiraceae was unclassified (Fig. 4C).
Figure 4.
LDA analysis to compare the microbiota of SBS patients with a small bowel ostomy without colon in continuity (n = 20) vs. those with a colon in continuity (n = 32). A–C: SBS ostomy patients (ileostomy + jejunostomy) compared with patients with SBS who have a colon in continuity (ileocolonic and jejunocolonic anastomoses). A: phylum level. B: family level. C: genus level. D and E: comparison of microbiota of SBS patients with ileostomy (n = 16) vs. ileocolonic (IC) anastomosis (n = 24) at phylum (D) and genus (E) levels. F and G: comparison of microbiota of SBS patients with jejunostomy (n = 4) vs. jejunocolonic (JC) anastomosis (n = 8) at phylum (F) and genus (G) levels. LDA, linear discriminate analysis; SBS, short bowel syndrome.
Subgroup analysis of SBS patients with an ileostomy versus ileocolonic anastomosis showed enrichment of phylum Proteobacteria in patients with ileostomy and enrichment of Bacteroidetes and Actinobacteria in SBS patients with a colon in continuity (Fig. 4, D and E). Comparison of the microbiome of SBS patients with a jejunostomy versus jejunocolonic anastomosis also showed enrichment of Proteobacteria in the jejunostomy cohort, but the two intestinal segments generally demonstrated region-specific microbial identities (Fig. 4, D–G).
Furthermore, SBS patients with jejunocolonic compared with ileocolonic anastomoses showed enrichment in Verrucomicrobia including Akkermansia, and genus Dialister, the Firmicutes (Fig. 5A). SBS patients with jejunostomy had enrichment of Acinetobacter (Proteobacter) and Prevotella (Bacteroidetes) compared with patients with ileostomy (Fig. 5B).
Figure 5.
LDA analysis of the microbiota of SBS patients with jejunocolonic (n = 8) compared with ileocolonic anastomoses (n = 24; A), or jejunostomy (n = 4) vs. ileostomy (n = 16; B), at genus level. LDA, linear discriminate analysis; SBS, short bowel syndrome.
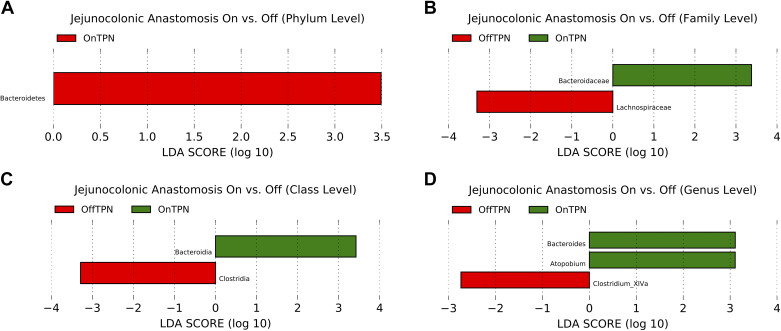
Altered Microbiome in PN-Independent Compared with PN-Dependent Patients Who Have Jejunocolonic Anastomoses
LDA analysis of the microbiome of patients with jejunocolonic anastomoses who are PN dependent versus those who are PN independent revealed enrichment of the phylum Bacteroidetes, class Bacteroidia, family Bacteroidaceae, order Bacteroidales, and genera Bacteroides and Atopobium in the PN-dependent group (Fig. 6, A–D). PN-independent patients showed enrichment in class Clostridia, order Clostridiales, which identified as the family Lachnospiraceae and genus Clostridia XIVa, both of which play a critical role in short-chain fatty acid production and bile acid metabolism (16, 17).
Figure 6.
LDA analysis of the microbiota of SBS patients with jejunocolonic anastomoses and who are fed with parenteral nutrition (OnTPN, n = 4) vs. patients with SBS who have weaned from PN (OffTPN, n = 4). A: phylum level. B: family level. C: class level. D: genus level. LDA, linear discriminate analysis; PN, parenteral nutrition; SBS, short bowel syndrome.
Patients with SBS Have Altered Enterohepatic BA Metabolism
We collected simultaneous blood samples from a subset of the patients with SBS who provided fecal samples. Because of the importance of enterohepatic BA cycling in the homeostatic regulation of BA metabolism, we examined stool and serum BA composition, along with surrogate measures of homeostatic signaling and BA production [C4, which reflects hepatic Cyp7A1 activity (18)] and plasma FGF19, which reflects ileal BA uptake and enterohepatic cycling. We compared patients with SBS who are fed with PN versus patients with SBS who are PN independent, and we examined subsets of SBS patients with jejunostomy, ileostomy, jejunocolonic, and ileocolonic anastomoses.
We observed a significant negative correlation between plasma C4 and FGF19 concentrations across the entire cohort (Fig. 7A) and verified that relationship in both healthy controls (Fig. 7B) and patients with SBS (Fig. 7C). In particular, we demonstrated a significant negative inverse correlation between fasting serum C4 concentrations and serum FGF19 concentrations. C4 levels were increased and FGF19 levels decreased in SBS compared with normal healthy subjects reflecting decreased ileal production (Fig. 7, D and E), findings in agreement with other studies showing compensatory upregulation of hepatic BA synthesis and attenuated production of FGF19 in SBS patients (19). We compared plasma FGF19 levels in normal subjects and SBS patients with ileostomy, ileocolonic, or jejunocolonic anastomoses (Fig. 7F) and observed a significant reduction in FGF19 levels in patients with SBS compared with normal controls, independent of anatomy. However, FGF19 levels were not significantly different among SBS patients with differing anatomies. Patients with jejunocolonic anastomoses had similar FGF19 levels compared with patients with residual ileum, suggesting an adaptive jejunal response.
Figure 7.
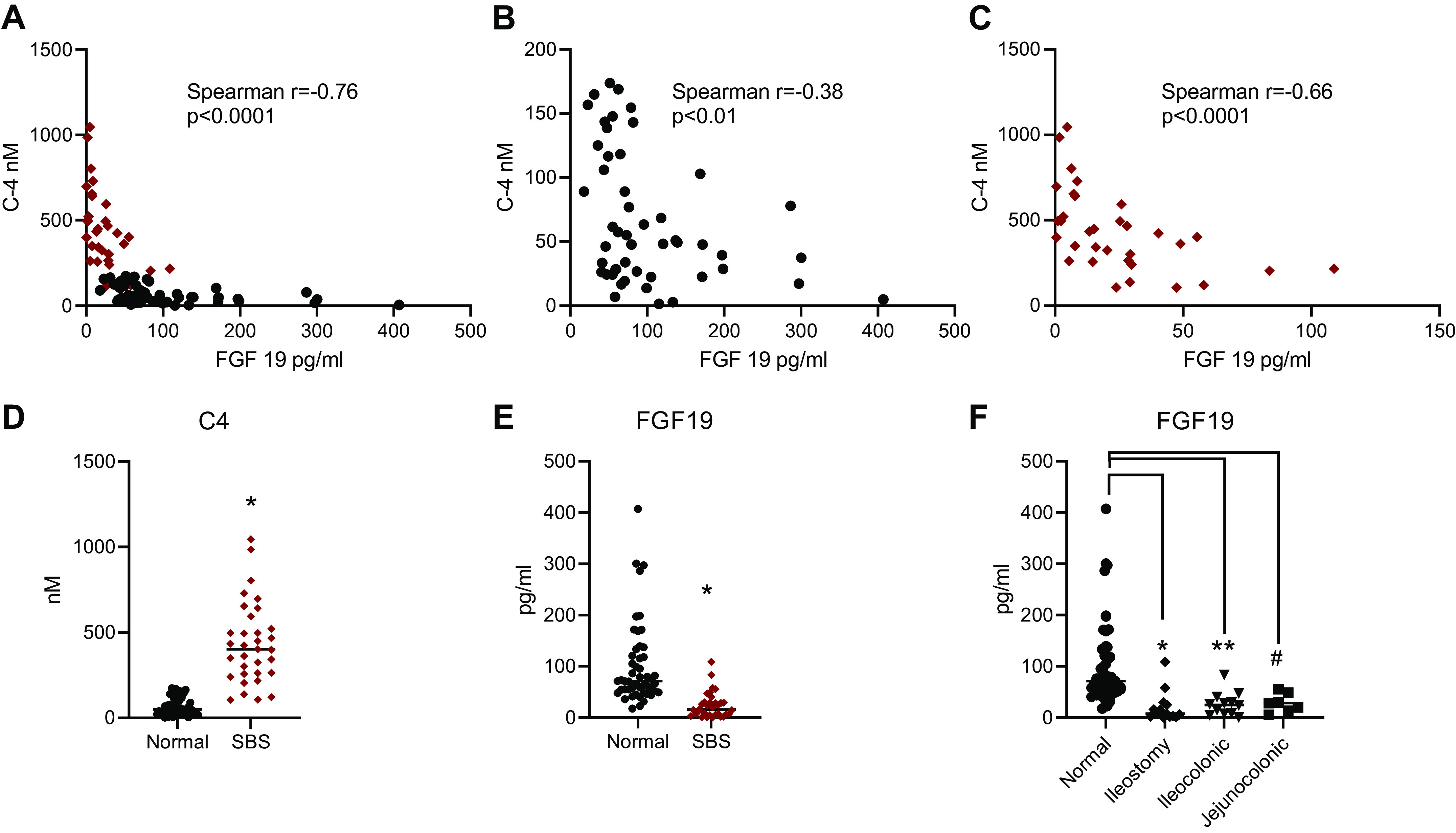
Plasma C4 and FGF19 levels in normal healthy subjects (n = 50) and in patients with SBS (n = 33). A: C4 vs. FGF19 correlation in patients with SBS (red dots) and normal subjects (black dots). B: normal subjects. C: patients with SBS. Comparison of mean C4 plasma levels (D) and FGF19 levels (E) in normal subjects vs. patients with SBS. F: FGF19 levels in normal subjects compared with patients with SBS with ileostomy, ileocolonic, or jejunocolonic anastomoses. For normal compared with ileostomy, *P = 0.014; normal compared with ileocolonic, **P = 0.028; normal compared with jejunocolonic, #P = 0.032. P = NS for all other comparisons. C4, 7α-hydroxy-4-cholesten-3-one; FGF19, fibroblast growth factor 19; NS, not significant; SBS, short bowel syndrome.
We also compared serum total, conjugated, and unconjugated bile acid concentrations in healthy versus patients with SBS and found no significant differences (Supplemental Fig. S1, A and C). There was no significant correlation of serum total BA concentration with C4 concentration (Supplemental Fig. S1D), but the ratio of serum conjugated to unconjugated bile acids was positively correlated with C4 concentrations. (Supplemental Fig. S1E; P = 0.007).
We next profiled stool and serum bile acid composition in SBS patients with and without a colon in continuity. Compared with healthy control participants, the concentration of the primary BA cholic acid (CA) and chenodeoxycholic acid (CDCA) was elevated in the stools of patients with SBS whose colons were in continuity (Fig. 8, A and B), consistent with the loss of ileum and reduced BA reabsorptive capacity. Conjugated BA species including GCA, TCA, TCDCA, and GCDCA were more abundant in SBS patients without a colon compared with controls (Fig. 8, C–F) and also when compared with SBS patients with a colon in continuity, consistent with the known role of colonic microbial taxa in mediating BA deconjugation. In addition, total fecal BA content was increased in patients with SBS compared with controls, and the ratios of conjugated to unconjugated BA and primary to secondary BA were significantly higher in SBS patients with jejunostomy/ileostomy than in SBS patients with a colon in continuity (Fig. 9, A–C).
Figure 8.
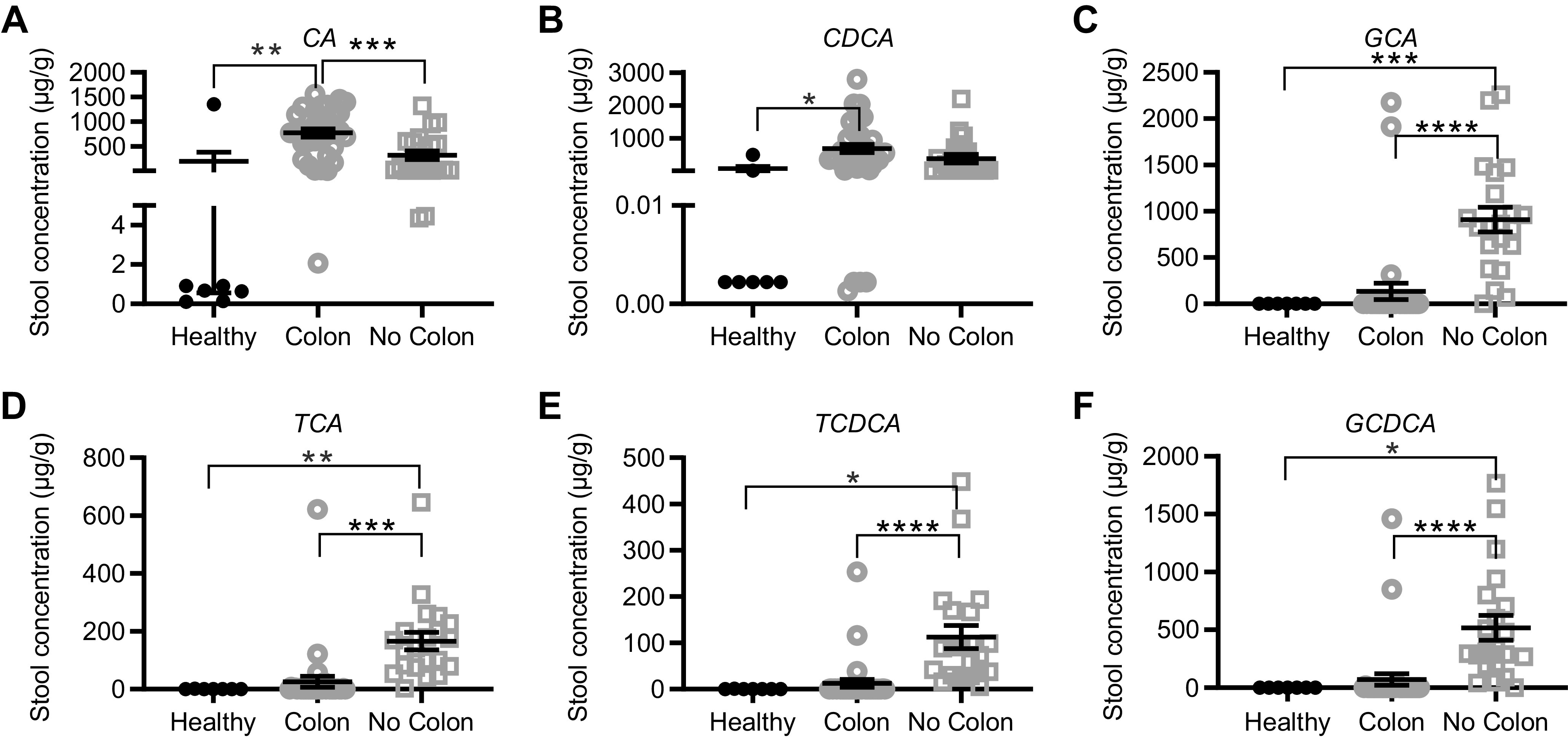
Stool bile acid concentrations in normal healthy subjects (n = 7) compared with patients with SBS who had jejunocolonic or ileocolonic anastomosis (colon, n = 33 samples) or who had ileostomy or jejunostomy and no colon in continuity (no colon, n = 21 samples). A: Cholic acid (CA), **P = 0.013; ***P = 0.026; B: chenodeoxycholic acid (CDCA), *P = 0.030; C: glycocholic acid (GCA), ***P = 0.004, ****P < 0.00001; D: taurocholic acid (TCA), **P = 0.012, ***P < 0.0001; E: taurochenodeoxycholic acid (TCDCA), *P = 0.03, ****P = 0.0002; F: glycochenodeoxycholic acid (GCDCA), *P = 0.030, ****P = 0.0004.
Figure 9.

Stool bile acid concentrations in healthy subjects (n = 7) compared with SBS patients with residual colon in continuity (colon, n = 33 samples) and to patients with small bowel ostomy (no colon, n = 21 samples). A: total bile acid stool concentration, *P = 0.049 healthy vs. colon; *P = 0.03 healthy vs. no colon. B: conjugated/unconjugated ratio: colon vs. no colon, ***P = 0.0007. C: primary bile acid/secondary bile acid ratio: healthy vs. no colon, ****P < 0.0001; colon vs. no colon, ****P < 0.0001. SBS, short bowel syndrome.
LCA Enrichment in PN-Independent Fecal Bile Acids
Total bile acids were increased in patients with SBS compared with healthy controls (Fig. 10A); SBS patients with a jejunocolonic anastomosis and who were PN independent showed significantly increased fecal LCA and a trend toward increased DCA (P = 0.054) compared with those who were PN dependent (Fig. 10, B and C). In contrast, GCA, GCDCA, and the ratio of conjugated versus unconjugated BA were increased in patients with ileocolonic anastomoses who were PN dependent compared with those who had weaned off PN (Fig. 10, D–F). These findings suggest that enrichment in fecal secondary BA (LCA, DCA) is associated with the ability to wean, whereas enrichment in primary BA (GCA and GCDCA) was associated with PN dependence.
Figure 10.

Stool bile acid concentrations in normal healthy subjects and in patients with SBS who are fed with parenteral nutrition (“on”) vs. patients with SBS who are fed with enteral nutrition (“off”). A: total bile acids in normal healthy subjects (n = 7) compared with patients with SBS on (n = 12; *P = 0.04) or off (n = 20;*P = 0.028) PN. B: LCA concentration in healthy subjects (n = 7) vs. patients with jejunocolonic anastomosis who are on PN (n = 6) or off PN (n = 6); LCA, **P = 0.003; *P = 0.015. C: DCA concentration in healthy subjects (n = 7) or patients with jejunocolonic anastomoses who are on PN (n = 6) or off PN (n = 6); DCA, **P = 0.004. D–F: GCA, GCDCA, and conjugated/unconjugated ratio in healthy subjects (n = 7) vs. SBS patients with ileocolonic anastomosis on PN (n = 7) or off PN (n = 14). GCA, *P = 0.037; GCDCA, *P = 0.045; conjugated/unconjugated, *P = 0.040. DCA, deoxycholic acid; GCA, glycocholic acid; GCDCA, glycochenodeoxycholic acid; LCA, lithocholic acid; PN, parenteral nutrition; SBS, short bowel syndrome.
Because LCA and DCA are TGR5 agonists, which in turn increase GLP-2 secretion, and GLP-2 has a key role in gut adaptation, we measured serum GLP-2 as well as cosecreted GLP-1 levels in our patients with SBS and correlated them with stool DCA and LCA concentrations. No significant correlation was noted between DCA or LCA and GLP-1 or GLP-2 serum levels in patients with a colon in continuity or in patients with ileostomy (data not shown).
Serum Bile Acids Are Altered in SBS
Although serum bile acids were not significantly increased in patients with SBS compared with healthy controls (Supplemental Fig. S1), the ratio of serum primary to secondary BA was higher in all SBS groups (on or off PN) than in healthy controls (P = 0.02, data not shown). Primary BAs were increased in SBS patients with a colon versus SBS patients with no colon, and the ratio of conjugated to unconjugated serum BA was higher in SBS patients without a colon compared with those with a colon (Supplemental Fig. S2). Total serum BA concentrations were significantly higher in SBS patients with versus without a colon.
Patients with SBS overall exhibited a trend toward increased serum CA and CDCA levels compared with healthy controls (Supplemental Fig. S3; P = 0.09 and 0.095, respectively). This difference was attributable to the patients with SBS who had a colon in continuity (Supplemental Fig. S3, C and D), because patients with SBS who had a colon versus those with a small bowel ostomy (i.e., no colon) had significantly higher serum CA and CDCA concentrations. These data suggest increased colonic absorption of malabsorbed BA in SBS. In contrast, serum concentrations of GCA and TCA were significantly greater in SBS patients without a colon than in those with a colon (Supplemental Fig. S3, E and F) but were not significantly different compared with normal participants. These data also suggest passive absorption of GCA and TCA through the small bowel. In contrast, the presence of a colon was associated with reduced serum GCA and TCA levels because these BAs undergo microbial degradation. Serum TCDCA levels were significantly lower in patients with SBS compared with controls (Supplemental Fig. S3G), and in SBS patients with a colon in continuity compared with those without a colon (Supplemental Fig. S3H), and GCDCA showed a trend to be lower in SBS patients with a colon (P = 0.09; data not shown).
Microbiota That Metabolize Bile Acids: Listeria, Lactobacillus, Bifidobacterium, Peptostreptococcus, Akkermansia, and Escherichia
To further explore the differences in the microbiome and BA composition of patients with SBS compared with controls and because of the importance of the microbiome in bile acid metabolism, we analyzed our microbial data for changes in the relative abundance of Listeria, Lactobacillus, Bifidobacterium, Peptostreptococcus, Akkermansia, and Escherichia/Shigella, which in addition to Clostridia XIVa and XVI clusters play a well-recognized role in BA metabolism (7, 17). We found no Listeria in our samples. Bifidobacterium was significantly reduced in SBS patients with jejunostomy/ileostomy compared with normal controls (P = 0.035) and Bifidobacterium was significantly reduced in SBS patients with ileostomy compared with those with ileocolonic anastomoses (P = 0.0065). Lactobacillus trended toward a significant increase in SBS patients with jejunocolonic and ileocolonic anastomoses compared with normal controls (P = 0.08). There were no significant differences for other bacteria in our patients with SBS versus control or PN-dependent versus -independent SBS cohorts.
Amino Acid Profiles in Patients with SBS
To further explore the differences in the stool concentration of glycine-conjugated BA among patients with SBS, we profiled serum amino acid levels and compared PN-dependent and -independent patients and normal controls (Supplemental Table S2). There were no significant differences in glycine, proline, glutamate, alanine, or ornithine levels between patients with SBS and controls. Serum taurine levels were lower in patients with SBS compared with controls (48.1 and 63.7 µM/L, respectively; P = 0.0008) and were lower in PN-dependent versus PN-independent patients (43 vs. 51.8 µM/L; P = 0.019). In our SBS patient population, the PN solutions provided did not contain taurine. Of the essential amino acids, there were no differences in lysine, valine, methionine, threonine, or tryptophan levels between normal control and patients with SBS or between PN-dependent and PN-independent patients with SBS. Leucine levels were lower in PN-dependent versus PN-independent patients (96.2 vs. 113.4 µM/L; P = 0.05). Histidine levels were lower in patients with SBS versus controls (67.6 vs. 76.5 µM/L; P = 0.049) but there was no difference in PN-dependent versus -independent patients. In contrast, phenylalanine levels were higher in patients with SBS versus controls (55 vs. 46 µM/L; P = 0.015), and cystine levels were higher in patients on PN compared with those who had weaned off (43 vs. 35 µM/L; P = 0.049) but were not significantly different from normal (all SBS = 37 vs. 38 µM/L).
Of additional amino acids that were not added to PN, aspartate levels were not different from normal compared with patients with SBS but were lower in PN-dependent versus PN-independent patients with SBS (4.42 vs. 5.75 µM/L; P = 0.005). Glutamine was lower in PN-dependent versus -independent patients (523.4 vs. 591.1 µM/L; P = 0.028), as was hydroxyproline (8.9 vs. 11.9 µM/L; P = 0.002) and asparagine (37.6 vs. 47.9 µM/L; P = 0.009).
DISCUSSION
Here, we report an analysis of the fecal microbiome and bile acid metabolome of 52 patients with SBS. Our study is among the largest SBS adult cohorts to date (14, 20, 21) and includes significant numbers of patients with ileostomy and jejunostomy in addition to those with a residual colon in continuity. Among the key findings, we demonstrate convergent alterations in both microbial taxa and enterohepatic BA composition in patients with SBS. Several of these findings merit expanded discussion.
In agreement with several studies of adult and pediatric SBS (14, 15, 20–24), we observed that the microbiome of patients with SBS shows overall reduced diversity compared with normal participants. Budinska et al. (14) noted decreased α-diversity in SBS patients with jejunostomy and jejunocolonic anastomosis. Our data show a significant decrease in microbial diversity in our overall SBS patient population. Subgroup analysis demonstrated reduced diversity in SBS patients with ileostomy and ileocolonic anastomosis as compared with controls. We observed a trend toward decreased microbial diversity in SBS patients with jejunostomy but no reduction in SBS patients with jejunocolonic anastomoses (Fig. 2). In attempting to account for these variations in postsurgical anatomy, we examined average residual small bowel length as a predictor of microbial diversity (14), but we found no significant difference in small bowel length comparing SBS patients with ileocolonic anastomosis to SBS patients with jejunocolonic anastomosis [mean remaining small bowel length for patients with ileocolonic anastomosis = 119.9 cm; mean remaining small bowel length for patients with jejunocolonic anastomosis = 83.8 cm; P = NS (not significant), two-tailed Student’s t test]. Accordingly, our interpretation of the microbial diversity analysis suggests that the presence of residual jejunum in our patients is important for preserving diversity in patients with SBS, a function that is independent of remnant bowel length. Of note, findings in a mouse model of SBS with resection of 50% of the proximal small bowel showed differences in bacterial composition but no change in microbial diversity compared with the control group (25). Conversely, a porcine model of SBS with resection of 75% of small bowel showed significantly decreased diversity of piglet colon microbiota (26). A potential reason for the differences in the two models is that the baseline diversity of the mouse microbiome was much higher than that of the piglet, masking the effect of smaller changes. Nevertheless, those findings in preclinical models when considered in the context of the current findings begin to suggest that specific loss of ileal function may contribute to the decreased microbial diversity observed in SBS patients with jejunostomy or jejunocolonic anastomosis. In addition, loss of ileum results in increased bile acid flow into the colon and loss of bile acid-sensitive species.
In the present analysis, the SBS microbiome showed enrichment in Proteobacteria in agreement with several published studies (20, 23, 24, 27). We also noted reduced Bacteroidetes, consistent with previous studies of adult and pediatric SBS (14). Our analysis showed enrichment of Akkermansia in patients with jejunocolonic compared with ileocolonic anastomosis anatomy. All of our patients with a colon in continuity received specific, identical dietary guidelines, which include dietary enrichment with soluble fiber. Therefore, we speculate that the differences in Akkermansia may not reflect differences in diet, but detailed food diary collection and analysis will be required to rule this out. A previous analysis showed that patients with ileocolonic and jejunocolonic anastomoses had enrichment in Akkermansia compared with patients with ileostomy and jejunostomy (14). These findings are relevant in considering the metabolic adaptations in patients with SBS, since enrichment with Akkermansia is negatively correlated with obesity and other adverse metabolic markers such as serum cholesterol levels (28–30). Our recent study of patients with abetalipoproteinemia showed enrichment of Akkermansia in their stools, associated with increased fecal neutral sterol extraction, and reduced hepatic lipid content (31). Taken together, these findings permit us to speculate that decreased Akkermansia in a subset of patients with SBS may confer a risk for adverse metabolic effects. This speculation is consistent with the observation that patients with SBS have increased percent body fat and reduced fat-free mass, putting them at higher risk for metabolic syndrome (32). Akkermansia metabolizes host mucin and thus may affect goblet cell function, and fecal abundance of Akkermansia has also been implicated in enhancing host immune function by inducing immunoglobulin and antigen-specific T-cell responses in mice (33).
Comparing PN-independent to PN-dependent SBS patients with jejunocolonic anastomosis, we observed that PN-dependent patients’ stools are enriched in Bacteroidetes, whereas the PN-independent cohort showed increased Firmicutes, more specifically Clostridium cluster XIVa of the Lachnospiraceae family. Oral diet composition clearly regulates microbiome composition; of note, our PN-dependent and -independent patients are encouraged to eat an oral diet as tolerated and are provided with identical dietary instructions that are tailored to their postoperative (in this subset, jejunocolonic) anatomy. Our previous study (32) analyzed body composition, dietary composition, and calorie consumption of a cohort of PN-dependent and -independent patients. We observed that PN-dependent patients ingested significant numbers of oral calories per day (as high as 50%–70% of total daily calories including PN).
Clostridium cluster XIVa includes Clostridium, Eubacterium, Ruminococcus, Coprococcus, Dorea, Lachnospira, Roseburia, and Butyrivibrio genera and is related to the XVIa cluster, which is enriched in colonic species with 7-dehydroxylating activity that are responsible for generating secondary BAs (16, 34). Clostridium cluster XIVa also regulates production of short-chain fatty acids (16), specifically butyrate, which is involved in many pathways of gut homeostasis including energy utilization and mucosal integrity (35). Butyrate and other short-chain fatty acids are absorbed by the colon and are an important source of additional calories in patients with SBS who had a colon in continuity; diets high in complex carbohydrates are recommended to enhance short chain fatty acids (SCFAs) in the colon lumen from which they are readily absorbed (1, 36). Other beneficial effects of butyrate include decreased TNF and proinflammatory cytokine production from lamina propria immune cells in Crohn’s disease (37) and antitumor effects. In rodent models (38) and in human colon cancer cells (39), exposure to butyrate inhibited tumor growth and cell proliferation. Also, loss of the Clostridium cluster XIVa from antibiotic use is associated with expansion of enteric pathogens such as Salmonella (40). Patients with Clostridioides difficile infection who have undergone successful fecal microbiota transplantation (FMT) harbor a post-FMT microbiome enriched in Lachnospiraceae and reduced in Enterobacteriaceae (41). Lachnospiraceae has also been shown to be decreased in relative abundance in ulcerative colitis compared with healthy controls (42).
The small bowel microbiome is much less well described chiefly because of difficulties in sampling and confounding effects of enteral nutrition and pancreatic and biliary secretions (6, 43). The small bowel microbiome is normally enriched in Streptococcus, Veillonella, Prevotella, Rothia, Haemophilus, Actinobacter, Escherichia, and Fusobacterium (43). We showed that stools from SBS patients with ileostomy or jejunostomy compared with those with a colon in continuity were enriched in Streptococcus but also were enriched in Acinetobacter, Enterobacteriaceae, Pasteurellaceae, and Porphyomodaceae. Thus, the small bowel microbiome is also altered in SBS and as predicted, differs in patients with or without a colon in continuity. Environmental factors such as increased exposure of microbial contents to oxygen in patients with ileostomy or jejunostomy compared with patients with a colon in continuity would be expected to play a significant role in changing the microbiome and may in fact be the initiating event.
Patients with SBS had elevated C4 and reduced FGF19 concentrations compared with controls, consistent with ileal resection leading to reduced bile acid absorption, reduced enterohepatic recirculation, and increased bile acid synthesis (5, 8). FGF19 and C4 concentrations correlated negatively as expected, indicating disturbances in enterohepatic BA cycling and adaptive increases in hepatic BA production in patients with SBS.
SBS patients with jejunocolonic anastomoses who were PN dependent exhibited reduced fecal LCA and DCA abundance compared with control participants, yet patients with SBS who were PN independent with jejunocolonic anastomoses had significantly increased fecal LCA content and a trend toward increased DCA compared with those who were PN dependent (Fig. 10, B and C). Those observations correlate well with the enrichment of fecal Clostridium cluster XIVA in weaned versus PN-dependent patients (Fig. 6) consistent with observations that members of this cluster exhibit bile salt hydrolase activity (17, 44) and are closely related to 7-α dehydroxylating species, which play an important role in producing LCA and DCA (16, 34). Deep sequencing of these samples is planned to attempt to identify a key commensal bacteria species to fit this profile.
In contrast, GCA, GCDCA, and the ratio of conjugated versus unconjugated BA were increased in patients with ileocolonic anastomoses who were PN dependent compared with those who had weaned off PN (Fig. 10, D–F). These findings together suggest that enrichment in secondary BA (LCA, DCA) in patients with SBS was associated with the ability to wean, whereas enrichment in primary BA (GCA and GCDCA) was associated with PN dependence.
LCA and DCA are the major colonic bile acids species (7, 17). Both LCA and DCA affect colonic epithelial proliferation and inflammation, with the majority of studies indicating proproliferative, proinflammatory, and procarcinogenic effects (7, 45, 46). Our previous studies have shown stem cell expansion and increased crypt cell proliferation in enteroids of patients with SBS (47). High-fat diets increase LCA and DCA concentrations in the colon, resulting in increased inflammation and tumorigenesis (45, 46). Other studies show an anti-inflammatory effect, for example, LCA incubation with T84 cells treated with polyIC reduces TNFα secretion (48). Studies of effects of bile acids in the small intestinal epithelium demonstrate pleiotropic roles for LCA and DCA. LCA and DCA regulate glucagon-like peptide-1, neurotensin, and peptide-YY secretion from small intestinal L cells (49). It is worth noting that LCA and DCA are more potent activators of TGR5 than CDCA or CA, and these effects were dependent on intestinal absorption and activation of the L-cell basolateral membrane Takeda G-protein receptor 5 (TGR5) (46). Bile acids are vitamin D receptor ligands (50), and LCA interacts with this receptor in the ileum to induce expression of downstream target genes (51). Multiple studies have shown that DCA increases intestinal permeability (52, 53) but paradoxically DCA increased transepithelial resistance via an enteric neural mechanism, as measured in Ussing chamber studies of mouse jejunum (54). These findings raise the possibility that BAs signal through multiple pathways to influence adaptive pathways in patients with SBS, depending on which regions of the intestine are left in continuity.
Plasma amino acid profiling demonstrated that despite reduced fecal GCDCA and GCA in patients with SBS who wean from PN compared with PN-dependent patients, glycine concentrations were not significantly different, and plasma glycine levels were unchanged in SBS compared with controls. Patients with SBS had reduced taurine concentrations compared with normal participants and taurine concentrations are lower in PN-dependent versus -independent patients; this reduction can be explained at least, in part, by the lack of taurine in PN formulations. However, taurine is also synthesized from cysteine and methionine, and cystine levels were higher in PN-dependent versus weaned patients and were the same as normal healthy subjects. Neither cysteine nor cystine are added to our patients’ PN formulations; thus, it is possible that cysteine conversion to taurine is reduced in SBS. Alternatively, taurine may be highly utilized in SBS due to bile acid wasting and increased synthesis, resulting in reduced plasma levels compared with normal controls.
In summary, we have shown that patients with SBS have an altered microbiome, which likely contributes to the observed distinct fecal bile acid profile changes. Our study is among the first to provide a detailed study of the small bowel microbiome in patients without a residual colon in continuity. Also, we report novel observations that Clostridium cluster XIVa and increased secondary fecal bile acid concentrations (DCA and LCA) are associated with weaning from PN. These may confer metabolic benefits based on enhanced short-chain fatty acid production or other as yet unidentified mechanisms, which are the subject of future study. Ongoing prospective analyses will provide further information regarding the role of these microbial and bile acid changes in weaning from PN, as well as potentially identify additional candidates that confer a proadaptive advantage for weaning.
SUPPLEMENTAL DATA
Supplemental Tables S1 and S2 and Supplemental Figs. S1–S3: https://doi.org/10.6084/m9.figshare.17036441.
GRANTS
These studies were supported by NIH National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) R01 112378 (to D.C.R., N.O.D., B.W.W., M.S.L.), NIDDK RO1 106382 and NCI CA 230282 (to D.C.R., M.S.L.); NIDDK R01 119437 (to N.O.D.); NIDDK T32 07130 (to H.J.B.J., J.C.); and the Washington University Digestive Diseases Research Core Center NIDDK P30 DK052574 (to D.C.R., N.O.D., P.I.T.).
DISCLOSURES
P. Tarr has an equity interest in, and is a member of the Scientific Advisory Board of, and a consultant to, MediBeacon Inc., which is developing a noninvasive technique to measure intestinal permeability in humans. P. Tarr is also a coinventor on a patent related to this technology, which might generate royalty payments. None of the other authors has any conflicts of interest, financial or otherwise, to disclose. Writing of this paper and data analysis were not funded by additional organizations, and there was no writing support provided by any additional individuals, organizations, or companies.
AUTHOR CONTRIBUTIONS
H.J.B.J., P.I.T., M.S.L., B.W.W., N.O.D., and D.C.R. conceived and designed research; H.J.B.J., Y.X., and A.P. performed experiments; J.C., T.N.W., K.M.W., Y.X., M.G., A.P., V.G., N.O.D., and D.C.R., analyzed data; J.C., T.N.W., K.M.W., M.G., V.G., M.S.L., N.O.D., and D.C.R., interpreted results of experiments; D.C.R. prepared figures; H.J.B.J. and J.C. drafted manuscript; T.N.W., K.M.W., P.I.T. M.S.L., B.W.W., N.O.D., and D.C.R. edited and revised manuscript; H.J.B.J., J.C., T.N.W., K.M.W., Y.X., M.G., A.P., V.G., P.I.T., M.S.L., B.W.W., N.O.D., and D.C.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank B. Darren Nix, Kelly Monroe, Latoya Evans, Sewuese Akuse, and Rodney Newberry of the Biobank Core of the Digestive Diseases Research Core Center, Washington University School of Medicine; David Scherrer and Dan Ory of the Mass Spectroscopy Core, Washington University School of Medicine; and Nadim Ajami of the Alkek Center for Metagenomics and Microbiome Research, Baylor College of Medicine.
REFERENCES
- 1.Buchman AL. Short-bowel syndrome. Clin Gastroenterol Hepatol 3: 1066–1070, 2005. doi: 10.1016/S1542-3565(05)00856-6. [DOI] [PubMed] [Google Scholar]
- 2.Kelly DG, Tappenden KA, Winkler MF. Short bowel syndrome: highlights of patient management, quality of life, and survival. JPEN J Parenter Enteral Nutr 38: 427–437, 2014. doi: 10.1177/0148607113512678. [DOI] [PubMed] [Google Scholar]
- 3.Levin MS, Rubin DC. Intestinal adaptation: the biology of the intestinal response to resection and disease. In: Intestinal Failure: Diagnosis Management and Transplantation, edited by Langnas AN, Quigley EMM, Tappenden KA.. Malden, MA: Blackwell Publishing, 2008, p. 45–54. [Google Scholar]
- 4.Amiot A, Messing B, Corcos O, Panis Y, Joly F. Determinants of home parenteral nutrition dependence and survival of 268 patients with non-malignant short bowel syndrome. Clin Nutr 32: 368–374, 2013. doi: 10.1016/j.clnu.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 5.Hegyi P, Maléth J, Walters JR, Hofmann AF, Keely SJ. Guts and gall: Bile acids in regulation of intestinal epithelial function in health and disease. Physiol Rev 98: 1983–2023, 2018. doi: 10.1152/physrev.00054.2017. [DOI] [PubMed] [Google Scholar]
- 6.Friedman ES, Li Y, Shen TD, Jiang J, Chau L, Adorini L, Babakhani F, Edwards J, Shapiro D, Zhao C, Carr RM, Bittinger K, Li H, Wu GD. FXR-dependent modulation of the human small intestinal microbiome by the bile acid derivative obeticholic acid. Gastroenterology 155: 1741–1752.e5, 2018. doi: 10.1053/j.gastro.2018.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jia W, Xie G, Jia W. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat Rev Gastroenterol Hepatol 15: 111–128, 2018. doi: 10.1038/nrgastro.2017.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kliewer SA, Mangelsdorf DJ. Bile acids as hormones: the FXR-FGF15/19 pathway. Dig Dis 33: 327–331, 2015. doi: 10.1159/000371670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kozich JJ, Westcott SL, Baxter NT, Highlander SK, Schloss PD. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl Environ Microbiol 79: 5112–5120, 2013. doi: 10.1128/AEM.01043-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27: 2194–2200, 2011. doi: 10.1093/bioinformatics/btr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Westcott SL, Schloss PD. OptiClust, an improved method for assigning amplicon-based sequence data to operational taxonomic units. mSphere 2: e00073-17, 2017. doi: 10.1128/mSphereDirect.00073-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 73: 5261–5267, 2007. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C. Metagenomic biomarker discovery and explanation. Genome Biol 12: R60, 2011. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Budinska E, Gojda J, Heczkova M, Bratova M, Dankova H, Wohl P, Bastova H, Lanska V, Kostovcik M, Dastych M, Senkyrik M, Krizova J, Mraz M, Hradecky J, Hajslova J, Lenicek M, Podzimkova K, Chalupsky K, Sedlacek R, Cahova M. Microbiome and metabolome profiles associated with different types of short bowel syndrome: implications for treatment. JPEN J Parenter Enteral Nutr 44: 105–118, 2020. doi: 10.1002/jpen.1595. [DOI] [PubMed] [Google Scholar]
- 15.Neelis E, de Koning B, Rings E, Wijnen R, Nichols B, Hulst J, Gerasimidis K. The gut microbiome in patients with intestinal failure: current evidence and implications for clinical practice. JPEN J Parenter Enteral Nutr 43: 194–205, 2019. doi: 10.1002/jpen.1423. [DOI] [PubMed] [Google Scholar]
- 16.Lopetuso LR, Scaldaferri F, Petito V, Gasbarrini A. Commensal clostridia: leading players in the maintenance of gut homeostasis. Gut Pathog 5: 23, 2013. doi: 10.1186/1757-4749-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ridlon JM, Harris SC, Bhowmik S, Kang DJ, Hylemon PB. Consequences of bile salt biotransformations by intestinal bacteria. Gut Microbes 7: 22–39, 2016. [Erratum in Gut Microbes 7: 262, 2016] doi: 10.1080/19490976.2015.1127483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gälman C, Arvidsson I, Angelin B, Rudling M. Monitoring hepatic cholesterol 7α-hydroxylase activity by assay of the stable bile acid intermediate 7α-hydroxy-4-cholesten-3-one in peripheral blood. J Lipid Res 44: 859–866, 2003. doi: 10.1194/jlr.D200043-JLR200. [DOI] [PubMed] [Google Scholar]
- 19.Pattni SS, Brydon WG, Dew T, Walters JR. Fibroblast growth factor 19 and 7α-Hydroxy-4-cholesten-3-one in the diagnosis of patients with possible bile acid diarrhea. Clin Transl Gastroenterol 3: e18, 2012. doi: 10.1038/ctg.2012.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang Y, Guo F, Li Y, Wang J, Li J. Fecal microbiota signatures of adult patients with different types of short bowel syndrome. J Gastroenterol Hepatol 32: 1949–1957, 2017. doi: 10.1111/jgh.13806. [DOI] [PubMed] [Google Scholar]
- 21.Joly F, Mayeur C, Bruneau A, Noordine ML, Meylheuc T, Langella P, Messing B, Duée PH, Cherbuy C, Thomas M. Drastic changes in fecal and mucosa-associated microbiota in adult patients with short bowel syndrome. Biochimie 92: 753–761, 2010. doi: 10.1016/j.biochi.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 22.Engstrand Lilja H, Wefer H, Nyström N, Finkel Y, Engstrand L. Intestinal dysbiosis in children with short bowel syndrome is associated with impaired outcome. Microbiome 3: 18, 2015. doi: 10.1186/s40168-015-0084-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Korpela K, Mutanen A, Salonen A, Savilahti E, de Vos WM, Pakarinen MP. Intestinal microbiota signatures associated with histological liver steatosis in pediatric-onset intestinal failure. JPEN J Parenter Enteral Nutr 41: 238–248, 2017. doi: 10.1177/0148607115584388. [DOI] [PubMed] [Google Scholar]
- 24.Wang P, Wang Y, Lu L, Yan W, Tao Y, Zhou K, Jia J, Cai W. Alterations in intestinal microbiota relate to intestinal failure-associated liver disease and central line infections. J Pediatr Surg 52: 1318–1326, 2017. doi: 10.1016/j.jpedsurg.2017.04.020. [DOI] [PubMed] [Google Scholar]
- 25.Sommovilla J, Zhou Y, Sun RC, Choi PM, Diaz-Miron J, Shaikh N, Sodergren E, Warner BB, Weinstock GM, Tarr PI, Warner BW. Small bowel resection induces long-term changes in the enteric microbiota of mice. J Gastrointest Surg 19: 56–64, 2015. doi: 10.1007/s11605-014-2631-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lapthorne S, Pereira-Fantini PM, Fouhy F, Wilson G, Thomas SL, Dellios NL, Scurr M, O'Sullivan O, Ross RP, Stanton C, Fitzgerald GF, Cotter PD, Bines JE. Gut microbial diversity is reduced and is associated with colonic inflammation in a piglet model of short bowel syndrome. Gut Microbes 4: 212–221, 2013. doi: 10.4161/gmic.24372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davidovics ZH, Carter BA, Luna RA, Hollister EB, Shulman RJ, Versalovic J. The fecal microbiome in pediatric patients with short bowel syndrome. JPEN J Parenter Enteral Nutr 40: 1106–1113, 2016. doi: 10.1177/0148607115591216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karlsson CL, Onnerfält J, Xu J, Molin G, Ahrné S, Thorngren-Jerneck K. The microbiota of the gut in preschool children with normal and excessive body weight. Obesity (Silver Spring) 20: 2257–2261, 2012. doi: 10.1038/oby.2012.110. [DOI] [PubMed] [Google Scholar]
- 29.Derrien M, Belzer C, de Vos WM. Akkermansia muciniphila and its role in regulating host functions. Microb Pathog 106: 171–181, 2017. doi: 10.1016/j.micpath.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 30.Brahe LK, Le Chatelier E, Prifti E, Pons N, Kennedy S, Hansen T, Pedersen O, Astrup A, Ehrlich SD, Larsen LH. Specific gut microbiota features and metabolic markers in postmenopausal women with obesity. Nutr Diabetes 5: e159–e159, 2015. doi: 10.1038/nutd.2015.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xie Y, Matsumoto H, Kennedy S, Newberry EP, Moritz W, DeBosch BJ, Moley KH, Rubin DC, Warner BW, Kau AL, Tarr PI, Wylie TN, Wylie KM, Davidson NO. Impaired chylomicron assembly modifies hepatic metabolism through bile acid-dependent and transmissible microbial adaptations. Hepatology 70: 1168–1184, 2019. doi: 10.1002/hep.30669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chiplunker AJ, Chen L, Levin MS, Warner BW, Davidson NO, Rubin DC. Increased adiposity and reduced lean body mass in patients with short bowel syndrome. Dig Dis Sci 65: 3271–3279, 2020. doi: 10.1007/s10620-019-06032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ansaldo E, Slayden LC, Ching KL, Koch MA, Wolf NK, Plichta DR, Brown EM, Graham DB, Xavier RJ, Moon JJ, Barton GM. Akkermansia muciniphila induces intestinal adaptive immune responses during homeostasis. Science 364: 1179–1184, 2019. doi: 10.1126/science.aaw7479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ridlon JM, Kang DJ, Hylemon PB, Bajaj JS. Bile acids and the gut microbiome. Curr Opin Gastroenterol 30: 332–338, 2014. doi: 10.1097/MOG.0000000000000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lloyd-Price J, Arze C, Ananthakrishnan AN, Schirmer M, Avila-Pacheco J, Poon TW, et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature 569: 655–662, 2019. doi: 10.1038/s41586-019-1237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parrish CR, DiBaise JK. Managing the adult patient with short bowel syndrome. Gastroenterol Hepatol (N Y) 13: 600–608, 2017. [PMC free article] [PubMed] [Google Scholar]
- 37.Segain JP, Raingeard de la Blétière D, Bourreille A, Leray V, Gervois N, Rosales C, Ferrier L, Bonnet C, Blottière HM, Galmiche JP. Butyrate inhibits inflammatory responses through NFκB inhibition: implications for Crohn’s disease. Gut 47: 397–403, 2000. doi: 10.1136/gut.47.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McIntyre A, Gibson PR, Young GP. Butyrate production from dietary fibre and protection against large bowel cancer in a rat model. Gut 34: 386–391, 1993. doi: 10.1136/gut.34.3.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Archer S, Meng S, Wu J, Johnson J, Tang R, Hodin R. Butyrate inhibits colon carcinoma cell growth through two distinct pathways. Surgery 124: 248–253, 1998. doi: 10.1016/S0039-6060(98)70127-8. [DOI] [PubMed] [Google Scholar]
- 40.Rivera-Chávez F, Zhang LF, Faber F, Lopez CA, Byndloss MX, Olsan EE, Xu G, Velazquez EM, Lebrilla CB, Winter SE, Bäumler AJ. Depletion of butyrate-producing Clostridia from the gut microbiota drives an aerobic luminal expansion of Salmonella. Cell Host Microbe 19: 443–454, 2016. doi: 10.1016/j.chom.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dutta SK, Girotra M, Garg S, Dutta A, von Rosenvinge EC, Maddox C, Song Y, Bartlett JG, Vinayek R, Fricke WF. Efficacy of combined jejunal and colonic fecal microbiota transplantation for recurrent Clostridium difficile infection. Clin Gastroenterol Hepatol 12: 1572–1576, 2014. doi: 10.1016/j.cgh.2013.12.032. [DOI] [PubMed] [Google Scholar]
- 42.Takeshita K, Mizuno S, Mikami Y, Sujino T, Saigusa K, Matsuoka K, Naganuma M, Sato T, Takada T, Tsuji H, Kushiro A, Nomoto K, Kanai T. A single species of Clostridium subcluster XIVa decreased in ulcerative colitis patients. Inflamm Bowel Dis 22: 2802–2810, 2016. doi: 10.1097/MIB.0000000000000972. [DOI] [PubMed] [Google Scholar]
- 43.Kastl AJ Jr, Terry NA, Wu GD, Albenberg LG. The structure and function of the human small intestinal microbiota: current understanding and future directions. Cell Mol Gastroenterol Hepatol 9: 33–45, 2020. doi: 10.1016/j.jcmgh.2019.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pathak P, Xie C, Nichols RG, Ferrell JM, Boehme S, Krausz KW, Patterson AD, Gonzalez FJ, Chiang JYL. Intestine farnesoid X receptor agonist and the gut microbiota activate G-protein bile acid receptor-1 signaling to improve metabolism. Hepatology 68: 1574–1588, 2018. doi: 10.1002/hep.29857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zeng H, Umar S, Rust B, Lazarova D, Bordonaro M. Secondary bile acids and short chain fatty acids in the colon: a focus on colonic microbiome, cell proliferation, inflammation, and cancer. Int J Mol Sci 20: 1214, 2019. doi: 10.3390/ijms20051214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ocvirk S, O'Keefe SJD. Dietary fat, bile acid metabolism and colorectal cancer. Semin Cancer Biol 73: 347–355, 2021. doi: 10.1016/j.semcancer.2020.10.003. [DOI] [PubMed] [Google Scholar]
- 47.Gazit VA, Swietlicki EA, Liang MU, Surti A, McDaniel R, Geisman M, Alvarado DM, Ciorba MA, Bochicchio G, Ilahi O, Kirby J, Symons WJ, Davidson NO, Levin MS, Rubin DC. Stem cell and niche regulation in human short bowel syndrome. JCI Insight 5: e137905, 2020.doi: 10.1172/jci.insight.137905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ward JBJ, Lajczak NK, Kelly OB, O'Dwyer AM, Giddam AK, Ní Gabhann J, Franco P, Tambuwala MM, Jefferies CA, Keely S, Roda A, Keely SJ. Ursodeoxycholic acid and lithocholic acid exert anti-inflammatory actions in the colon. Am J Physiol Gastrointest Liver Physiol 312: G550–G558, 2017. doi: 10.1152/ajpgi.00256.2016. [DOI] [PubMed] [Google Scholar]
- 49.Kuhre RE, Wewer Albrechtsen NJ, Larsen O, Jepsen SL, Balk-Møller E, Andersen DB, Deacon CF, Schoonjans K, Reimann F, Gribble FM, Albrechtsen R, Hartmann B, Rosenkilde MM, Holst JJ. Bile acids are important direct and indirect regulators of the secretion of appetite and metabolism-regulating hormones from the gut and pancreas. Mol Metab 11: 84–95, 2018. doi: 10.1016/j.molmet.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Makishima M, Lu TT, Xie W, Whitfield GK, Domoto H, Evans RM, Haussler MR, Mangelsdorf DJ. Vitamin D receptor as an intestinal bile acid sensor. Science 296: 1313–1316, 2002. doi: 10.1126/science.1070477. [DOI] [PubMed] [Google Scholar]
- 51.Ishizawa M, Akagi D, Makishima M. Lithocholic acid is a vitamin D receptor ligand that acts preferentially in the ileum. Int J Mol Sci 19: 1975, 2018. doi: 10.3390/ijms19071975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stenman LK, Holma R, Eggert A, Korpela R. A novel mechanism for gut barrier dysfunction by dietary fat: epithelial disruption by hydrophobic bile acids. Am J Physiol Gastrointest Liver Physiol 304: G227–G234, 2013. doi: 10.1152/ajpgi.00267.2012. [DOI] [PubMed] [Google Scholar]
- 53.Camilleri M. Leaky gut: mechanisms, measurement and clinical implications in humans. Gut 68: 1516–1526, 2019. doi: 10.1136/gutjnl-2019-318427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Forsgård RA, Korpela R, Stenman LK, Osterlund P, Holma R. Deoxycholic acid induced changes in electrophysiological parameters and macromolecular permeability in murine small intestine with and without functional enteric nervous system plexuses. Neurogastroenterol Motil 26: 1179–1187, 2014. doi: 10.1111/nmo.12383. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Tables S1 and S2 and Supplemental Figs. S1–S3: https://doi.org/10.6084/m9.figshare.17036441.