Sorghum is a multipurpose crop resilient to suboptimal growth conditions. We highlight what genetic, genomic, and biotechnological resources are available for sorghum, with an emphasis on transformation technologies.
Keywords: Agrobacterium, biofuels, bioinformatic resources, genetic engineering, genetic resources, sorghum transformation
Abstract
Sorghum [Sorghum bicolor (L.) Moench] is the fifth most important cereal crop globally by harvested area and production. Its drought and heat tolerance allow high yields with minimal input. It is a promising biomass crop for the production of biofuels and bioproducts. In addition, as an annual diploid with a relatively small genome compared with other C4 grasses, and excellent germplasm diversity, sorghum is an excellent research species for other C4 crops such as maize. As a result, an increasing number of researchers are looking to test the transferability of findings from other organisms such as Arabidopsis thaliana and Brachypodium distachyon to sorghum, as well as to engineer new biomass sorghum varieties. Here, we provide an overview of sorghum as a multipurpose feedstock crop which can support the growing bioeconomy, and as a monocot research model system. We review what makes sorghum such a successful crop and identify some key traits for future improvement. We assess recent progress in sorghum transformation and highlight how transformation limitations still restrict its widespread adoption. Finally, we summarize available sorghum genetic, genomic, and bioinformatics resources. This review is intended for researchers new to sorghum research, as well as those wishing to include non-food and forage applications in their research.
Introduction
Sorghum [Sorghum bicolor (L.) Moench] is the world’s fifth largest cereal crop by acreage and production (FAOSTAT, https://www.fao.org/faostat/en/#data). It is an important staple food in the semi-arid tropics of Asia and Africa. Globally, sorghum is used for animal feed, fodder, and high-value products such as syrup and bioethanol. Harboring traits such as tolerance to drought, waterlogging, and salinity make it a highly productive crop in environmental conditions that restrict the cultivation of other cereals (Hadebe et al., 2017; Huang, 2018). Sorghum has also been the source of exciting advances in fundamental biology such as the discovery of a metabolon for dhurrin biosynthesis (Laursen et al., 2016) and a new gene and chemistry involved in conferring Striga resistance (Gobena et al., 2017). Although sorghum holds great promise, it is still underutilized. In this review, we will present the current state of research employing sorghum as a multipurpose feedstock for the bioeconomy, summarize available research tools with a focus on transformation and genetic engineering, and identify promising areas for future research.
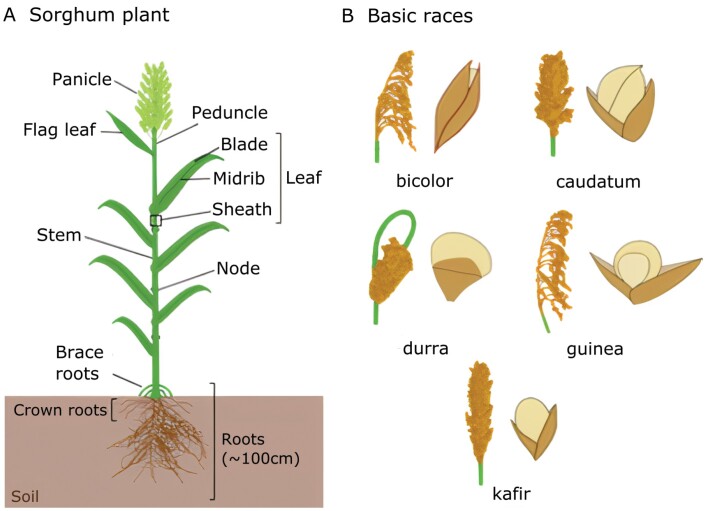
Cultivated sorghum (Fig. 1A) can be classified into five basic races: bicolor, guinea, caudatum, kafir, and durra, which are differentiated by the phenotype of their mature panicles and spikelets (Harlan and Wet, 1972) (Fig. 1B). Sorghum bicolor (L.) Moench subsp. bicolor contains all the cultivated sorghum varieties (Dahlberg, 2000). Sorghum can also be classified based on its agronomic characteristics into forage, biomass, sweet, and grain types (Table 1). Forage sorghum is tall, and the biomass is used to feed livestock. Important traits of forage sorghum include digestibility, nutrient content, and palatability. Biomass sorghum is bred to maximize vegetative yields, with reports of up to 61 Mg ha–1 (Snider et al., 2012), but, unlike forage sorghum, palatability is not a concern. Some of the original biomass breeding stock was derived from forage sorghum, so high-biomass sorghums can also be produced for forage (Venuto and Kindiger, 2008). Dedicated biomass sorghum is used to produce biofuels and chemicals from the lignocellulosic biomass (cell wall), fibers for biomaterials, and biogas via anaerobic digestion (Reddy and Yang, 2005; Wannasek et al., 2017; Silva and Vermerris, 2020). Sweet sorghum accumulates large amounts of soluble sugars (sucrose, glucose, and fructose) in its stems and was initially identified as an alternative sugar source in areas unsuitable for sugarcane production. Besides its use for syrup production, it can also be used for biofuel production and high-sugar forage (Rooney et al., 2007). Sorghum is typically a photoperiod-sensitive plant, requiring short days (8h/16h light/dark) to transition from the vegetative to the reproductive stage. Hybrid grain sorghum is photoperiod insensitive, meaning it can flower rapidly even in the summer in temperate regions, and therefore has shorter stature and reaches maturity earlier (Smith and Frederiksen, 2000). Grain sorghum is grown for its seeds and is used as a staple food mainly in the semi-arid tropics of Asia and Africa, as animal and poultry feed, as well as a sugar source for distillation into alcohol. Recently, grain sorghum has become more popular in other countries because of its health benefits, such as reducing rates of cardiovascular disease, obesity, and certain types of cancer (reviewed in Awika and Rooney, 2004). Certain genotypes contain 3–4 times more anthocyanin, a plant pigment which has antioxidant properties, compared with other grains (Awika et al., 2004). It is also a gluten-free alternative for people with celiac disease. However, in countries such as the USA, grain sorghum is primarily used to feed livestock and produce pet food, with approximately one-third of its production being directed to produce biofuels (United Sorghum Checkoff Program, https://www.sorghumcheckoff.com/).
Fig. 1.
Sorghum plant morphology (A) and panicle and spikelet phenotypes of the five basic races (B). The race bicolor is the most primitive of the cultivated races and has upright semi-open panicles, with long and clasping glumes. Commercially cultivated sorghum tends to be a mixture of these major races. The race guinea originated in humid regions of West Africa and has open, elongated panicles, which helps decrease mold infection. Caudatum originated in eastern Africa and has panicles ranging from compact to open, with shorter, asymmetric, glumes that expose the grain. On the other hand, kafir, which originated in southern Africa, has tighter and longer panicles. Durras have compact panicles and originated in southern Sahara.
Table 1.
Characteristics of sorghum groups
| Forage | Sweet | Grain | Biomass | |
|---|---|---|---|---|
| Height (m) | 1.8–3.6 | >3 | 0.6–1.2 | 3.5–6 |
| Traits | Single or multicut harvest, digestibility, nutrient content, palatability | Large amount of soluble sugars in stems | Photoperiod sensitive and insensitive, high grain yield | Photoperiod sensitive, dual-purpose, high lignocellulosic biomass |
| Uses | Livestock feed | Syrup and biofuel production, high-sugar forage | Seed as staple food in some regions, livestock feed and biofuel production | Biofuel, biogas, and biomaterial production |
Independent of the usage type, sorghum is an attractive crop for cultivation in a wide range of environments (tropical, subtropical, and temperate regions) and in soils that are considered marginal for other food crops such as maize (Fu et al., 2016; Ameen et al., 2017). Sorghum can grow in mineral-rich soils with pH values that limit profitable cultivation of other crops (Smith and Frederiksen, 2000). It also requires less water and exhibits drought and waterlogging tolerance (Rosenow et al., 1983; Promkhambu et al., 2010; Varoquaux et al., 2019). As an adaptive mechanism, sorghum becomes dormant during severe drought conditions and resumes growth when re-exposed to water (reviewed by Assefa et al., 2010). Another post-flowering drought adaptation is known as non-senescence or stay-green (Borrell et al., 1999, 2000; Borrell and Hammer, 2000). The stay-green trait allows delayed remobilization of nitrogen in the leaves, maintaining photosynthetic activity and carbohydrate supply to the developing grain, which results in higher biomass and grain yield (Borrell et al., 1999, 2000; Borrell and Hammer, 2000). For instance, stay-green sorghum hybrids can produce 47% more post-anthesis biomass under drought conditions (Borrell et al., 2000). Along with drought tolerance, sorghum is also heat tolerant (Craufurd and Peacock, 1993; Nguyen et al., 2013), which is particularly relevant, considering climate change predictions that include reductions in rainfall and increases in temperature in many cereal-growing regions.
Sorghum as a multipurpose feedstock for the bioeconomy
While sorghum is an important staple food and forage crop globally, it has potential as a feedstock for renewable fuel and bioproducts (U.S. Department of Energy, 2016, https://www.energy.gov/eere/bioenergy/2016-billion-ton-report). For it to be a viable feedstock, agronomic and biomass compositional traits will likely need to be further developed to make the economics of the manufacturing processes comparable with those for fossil fuel-derived products (Baral et al., 2019; Yang et al., 2020, 2021). This is exemplified by the US Department of Energy Bioenergy Research Centers, which have been funded since 2007 to investigate all aspects of advanced biofuel production process (https://genomicscience.energy.gov/centers/). Sorghum is one of three DOE flagship biomass crops, and open research questions include biomass improvement and co-production of valuable chemicals. Sorghum’s versatility in multiple processing configurations is one of its key appeals (Stamenković et al., 2020). For example, biodiesel can be produced from sorghum grains after pressing and transesterifying lipids (Ved and Padam, 2013). Starch from the grain or the sucrose-rich juice from the stems of sweet sorghum can be used for fermentation into biofuels and bioproducts. Beyond this, sorghum, especially the photoperiod-sensitive varieties, can produce large amounts of aerial lignocellulosic biomass that can also be used as a sustainable and economically feasible feedstock for conversion. Because of sorghum’s versatility, designing an ideal sorghum ideotype is challenging (Yang et al., 2021). Instead, it is more likely that a range of sorghum varieties will continue to be developed, with their phenotype tuned to the desired downstream market.
Target traits for biomass improvement: the cell wall
The cell wall is a crucial organelle for cell structure and protection, and is made up mainly of cellulose, hemicelluloses, and lignin. Cellulose, which constitutes 25–35% of the sorghum biomass, is made of β-1,4-glucose chains, which in turn form crystalline fibrils via hydrogen bonding (Ioelovich, 2008; Polko and Kieber, 2019). Hemicelluloses are a collection of branched hetero-polysaccharides (Ebringerová et al., 2005), but in the sorghum cell wall, glucuronoarabinoxylans dominate, making up ~35% of the total biomass (Anglani, 1998; Xu et al., 2018). Lignins are complex branched polyphenolics, made up of monolignol subunits derived from phenylalanine and tyrosine metabolism, and are found only in some secondary cell walls (Boerjan et al., 2003). Sorghum lignin content varies between ~2% and 11% of dry matter depending on the cultivar (Brenton et al., 2016), and is a key factor affecting forage palatability and biorefinery efficiency.
During biomass processing in a biorefinery, cellulose, hemicelluloses, and soluble sugars can be converted to monosaccharides, which can then be utilized as a carbon source by microbes. Most microbes preferentially use hexose sugars (such as the glucose in cellulose) over pentose sugars (such as the xylose and arabinose in xylan), so biomass with a high hexose:pentose ratio, namely reduced xylan, is preferable (Brandon et al., 2020). Branched hemicelluloses such as xylan require multiple enzymes to hydrolyze them to monosaccharides, so hemicelluloses with fewer branches, or altered branch frequency, may also be preferable (Gao et al., 2020). However, the cell wall should not be weakened so much that the plant lodges in the field or is more susceptible to pathogens and pests. Susceptibility to lodging and diseases due to cell wall modifications have proved difficult to predict, with some plants with engineered walls being more resistant to pathogens (reviewed by Miedes et al., 2014).
In addition to sugar engineering, lignin can be modified for biomass improvement. An ideal biomass feedstock would have low lignin, since it both physically shields polysaccharides from polysaccharide-degrading enzymes and reduces enzymatic efficiency via non-specific binding. With the advent of designer lignins and use of microbes that can consume phenolics as a carbon source, monolignols are increasingly considered high-value intermediates for the production of important biochemicals (Eudes et al., 2014; Karlen et al., 2016; Baral et al., 2019). Therefore, the desired biomass phenotypes (the sorghum biomass ideotype) will vary depending on the final target product. As a plant breeding problem, this variability highlights the need for seed producers to be able to respond rapidly to needs in the supply chain beyond their direct market (farmers), as the bioeconomy develops.
The isolation of naturally occurring lignin mutants has already proved beneficial for commercial sorghum cultivars. Nineteen Brown midrib (Bmr) mutant loci have been identified in sorghum, though only 3–4 loci are considered of agronomic interest due to their lower lignin content and higher potential for biomass conversion (Porter et al., 1978; da Silva et al., 2018). Engineering approaches that re-route the lignin biosynthetic pathway have been demonstrated in a number of plant species (Fu et al., 2011; Eudes et al., 2014; Wilkerson et al., 2014; Yan et al., 2018). Restricting engineering to specific cell types has been successful in reducing lignin while avoiding stem weakness (Yan et al., 2018).
Beyond biomass: oils, bioproducts, and novel materials
In addition to being a source of starch and lignocellulose to produce biofuels, sorghum has the potential to function as a factory for other bioproducts or their precursors, and this will be important for the economic success of advanced biofuels. Compared with microbial production systems, in planta production of chemical compounds can reduce inputs, costs of post-production conversion steps, and the amount of pathway engineering needed (Yang et al., 2020). Proposed examples include pharmaceuticals (artemisinin and cannabidiol), materials (e.g. latex), insecticides (e.g. limonene), and plastic precursors [e.g. polyhydroxybutyrate (PHB)]. Modeling has shown that the added value of bioproducts can lower biofuel production costs to prices competitive with fossil fuels, as well as providing a better farmgate price for growers (Yang et al., 2020). Another promising route for sorghum metabolic engineering is to target triacylglycerol (TAG) accumulation in leaves for oil production, which can be used for biodiesel production. Although vegetative organs represent most of the above-ground biomass, leaves accumulate <1% lipids (Yang and Ohlrogge, 2009), so plant oil production relies on seeds rich in TAG. However, up to an 8.4% increase of TAG in leaf tissues has been achieved in sorghum by simultaneous overexpression of the genes encoding the maize transcription factor WRINKLED1, Umbelopsis ramanniana acyltransferase UrDGAT2a, and Sesamum indicum oil body protein OLEOSIN-L, providing a basis for further improvements in levels of extractable oil for commercial purposes (Vanhercke et al., 2019).
Lignin valorization is another attractive option to add value to compounds from waste products in a biorefinery (Mottiar et al., 2016). Potential high-value applications of lignin range from synthesis of lignin nanotubes for gene delivery (Ten et al., 2014) to development of lignin-based antibacterial products for pharmaceutical and biomedical industries, demonstrating the wide range of properties that can be exploited (Grossman et al., 2020). Lignin precursors have been re-routed in tobacco to produce intermediates that can be converted by an engineered microbial chassis to produce high-value compounds pyrogallol and cis,cis-muconic acid (Wu et al., 2017). Similar approaches could be applied to re-route higher levels of valuable intermediates in sorghum, although it will require better understanding of the regulation of cell wall biosynthesis pathways. Lignin valorization into phenolic compounds such as eugenol is also of great interest. Eugenol can be used in food, cosmetics, and pharmaceutical industries, and its high demand can lead to high market value (Martinez-Hernandez et al., 2019). Techno-economic analysis (TEA) and life cycle assessments (LCA) have shown that lignin valorization into eugenol and other methoxyphenols can reduce the cost of ethanol production by up to 23% and reduce greenhouse gas emissions by up to 78% compared with the petrochemical industry (Martinez-Hernandez et al., 2019). As demonstrated by this and other examples (Yang et al., 2020), TEA and LCA are important resources to guide decisions on which compounds should be targeted for genetic engineering, based on their economic value.
Finally, novel materials can be produced from biomass. For example, cellulose derived from lignocellulosic material can be broken into nanofibers, which have nanostructure favorable to high mechanical performance of nanofiber networks and composite materials (Sehaqui et al., 2010). Cellulose nanofibers are a great renewable material for the manufacturing of ultrafiltration membranes and can also be used as barrier layers in packaging material, among other useful applications (Forde et al., 2016). Additionally, both hemicelluloses and pectins have been suggested for use in a range of materials which include medical devices (Zheng et al., 2020), superconductors (Di Giacomo et al., 2015), and biodegradable packaging (Gouveia et al., 2019; Mendes et al., 2020). Collaborations between sorghum researchers and material scientists to develop new uses for biomass components or to engineer improvements are likely to be fruitful.
Barriers to using sorghum in biotechnology applications
There are three major barriers to the use of engineered sorghum: technical challenges around sorghum transformation, general societal concerns about engineered crops, and specific concerns about sorghum gene flow to weedy relatives. We will not dwell on the GMO issue here because it is reviewed in depth in the literature.(McHughen and Wager, 2010; National Academies of Sciences, Engineering, and Medicine, 2016; Wolt, 2017; Callaway, 2018; Spicer and Molnar, 2018; Waltz, 2018; Zhang et al., 2020).
Though we describe many examples of existing transgenic sorghum technology, to our knowledge, there is no transgenic sorghum grown commercially. One main reason for the limited use of transgenic sorghum in the USA is concerns about gene flow to its sexually compatible wild weedy relatives such as Johnsongrass (S. halepense), S. bicolor subsp. drummondii, and S. bicolor subsp. verticilliflorum via pollen dispersal and subsequent cross-pollination and hybridization. These wild relatives can easily hybridize with the cultivated sorghum to produce the noxious weed shattercane (Ejeta and Grenier, 2005). Strategies to limit gene flow, such as male sterility, could be implemented, as could agronomic strategies which monitor for compatible weedy species within the range of pollen flow. For example, in sorghum, it has been estimated that after 700 m, very little, if any, outcrossing would be expected (Schmidt and Bothma, 2006). It is also important to note that most of the discussed modifications would likely be considered ‘null’; that is, they would not be expected to give weedy relatives a selective advantage. This makes regulation more straightforward than traits such as herbicide tolerance.
The rapid development of transgenic sorghum varieties will be necessary to complement gains from traditional sorghum breeding, as humanity faces increasing challenges from climate change, degraded soils, and increased population. In the next section, we will give an overview of the transformation methods adopted for sorghum biotechnology thus far and discuss the main bottlenecks that need to be addressed to have efficiencies comparable with other grasses and move the field forward.
Sorghum transformation
The limited ability to transform sorghum is the major barrier to the widespread adoption of sorghum as a research model and as feedstock for the growing bioeconomy. Sorghum transformation is technically challenging, comparatively costly and time-consuming, and limited to a few genotypes. Sorghum is highly recalcitrant to tissue culture and transformation, mainly because of genotype-dependent responses, production of phenolic compounds, short-term plant regeneration ability, and acclimatization issues (the ability of plants to survive the transfer from in vitro culture to soil) (Maheswari et al., 2006; Altpeter et al., 2016). Here, we describe the achievements so far, and outline research questions that would help resolve existing barriers to sorghum engineering.
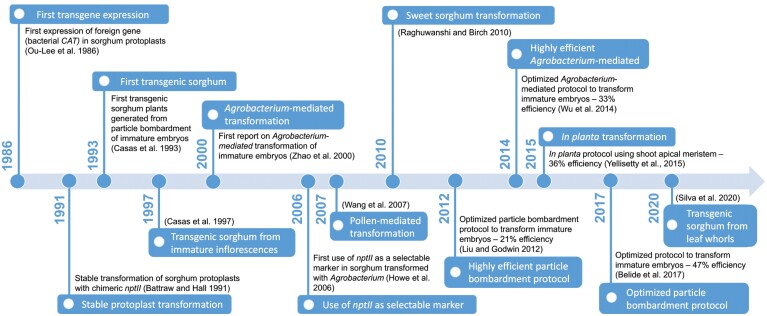
Since transgenic sorghum was first described (Casas et al., 1993), many improvements have been reported (Fig. 2). Casas and colleagues used immature embryos from the genotype P898012 to induce callus formation for particle bombardment, and obtained a transformation efficiency of 0.3% (Casas et al., 1993). Since then, the process has been improved using the genotype Tx430, and reached efficiencies of up to 46.6% (Belide et al., 2017). Using Agrobacterium tumefaciens to introduce the transgene via infection, transformation efficiency has increased from 9.7% in the initial studies (Zhao et al., 2000) to 33.2% (Wu et al., 2014). An important factor for tissue culture and, consequently, transformation success, is genotype selection. For the past 10 years, the grain sorghum inbred line Tx430 has been routinely used due to its consistently high callus induction and regeneration frequencies (Howe et al., 2006; Gurel et al., 2009; Liu and Godwin, 2012; Wu et al., 2014; Liu et al., 2015; Belide et al., 2017). However, Tx430 was directly compared with seven bioenergy parental sorghum lines using the protocols from Liu and Godwin (2012) and Wu et al. (2014). While Tx430 had high callus proliferation accompanied by low phenolic release, lines PI329311 and Rio had the best regeneration rates (Flinn et al., 2020).
Fig. 2.
Timeline of advances in sorghum transformation. Cat, chloramphenicol acetyltransferase; NptII, neomycin phosphotransferase II.
The explant source also plays a role in transformation efficiency. A variety of explants, such as immature and mature embryos, immature inflorescences, leaf discs, leaf whorls, and shoot meristems, have been used (Tables 2, 3). The most successful studies have used immature embryos due to their high embryogenic and regeneration competence (Tables 2, 3). However, the plant needs to reach the reproductive stage, which is limited to specific seasons or periods of time, and the narrow time window of 10–15 d in which the immature seeds need to be collected. To overcome these drawbacks, Silva et al. (2020) tested leaf whorls from the genotypes Tx430 and P898012, since this material can be collected throughout the year. The protocol also saves at least 4 weeks, as the explants can be collected around 30 d after emergence, compared with 70 d needed to collect immature embryos. Furthermore, the excision of leaf whorls is more technically straightforward than embryo isolation, allowing higher throughput.
Table 2.
Relevant literature regarding sorghum transformations and their use in research articles
Citation count checked on 12 November 20, based on CrossRef (source indicated when CrossRef was not available).
Citation count based on Google Scholar metrics
Tested both particle bombardment and Agrobacterium-mediated transformation
Table 3.
Main sorghum transformation methods, explants, genotypes, selectable markers, optimizations, and Agrobacterium strains, when appropriate, adopted for improvements in tissue culture and transformation efficiency
| References | Explantsa | Genotypesa | Agrobacterium straina | SMa | Max. TE | Optimizations |
|---|---|---|---|---|---|---|
| Particle bombardment | ||||||
| Casas et al., (1993) | Immature embryos | P898012 | – | Bar | 0.29% | Genotypes (IS4225, CS3541, M91051, Tx430, P898012, P954035, SRN39, and Shanqui red) |
| Able et al., (2001) | Immature embryos | SA281 | – | Bar | 3 out of 4 tested events | Genotypes (M35-1, SA281, QL41, and P898012), explant (immature embryos and leaf segments), promoters (Act1, CaMV35S, and Ubi) and biolistic parameters (acceleration pressure, distance to target tissue from expulsion point, aperture of helium inlet valve) |
| Tadesse et al., (2003) | Immature embryos | Ethiopian accession ‘214856’ | – | Npt | 1.30% | Explants (immature and mature embryos, shoot tips, calli), promoters (Act1D, Adh1, CaMV35S, and Ubi1), selectable markers (Bar and Npt) and biolistics parameters (acceleration pressure, target distance, gap width and travel distance) |
| Casas et al., (1997) | Immature inflorescences | SRN39 | – | Bar | 2.61% | Genotypes (M91051, P898012, P954035, PP290, and SRN39), panicle length and biolistic parameters (particle size and material, DNA amount, acceleration pressure and target distance) |
| Grootbroom et al., (2008) | Immature embryos | P898012 | – | Pmi | 0.77% | Selectable markers (Bar and Pmi) |
| Raghuwanshi and Birch, (2010) | Immature embryos | Ramada | – | Hpt | 0.09% | Genotypes (32 sweet sorghum), tissue culture media composition (increase of cytokinin), selectable markers (Hpt and NptII) |
| Liu and Godwin, (2012) | Immature embryos | Tx430 | – | NptII | 20.70% | Tissue culture media composition and biolistics parameters |
| Brandão et al., (2012) | Immature inflorescences | CMSXS102B | – | Bar | 3.33% | Genotypes (nine accessions from Embrapa Maize and Sorghum National Research Center, Brazil), explant developmental stages (3–5cm in length), biolistics parameters (in osmotic medium, acceleration pressure, microcarriers flying distance) |
| Visarada et al., (2014) | Immature embryos | CS3541 and 296B | – | Bar | 0.25% | Delivery method (Agrobacterium and particle bombardment), explant size, post-bombardment treatments |
| Belide et al., (2017) | Immature embryos | Tx430 | – | NptII | 46.60% | Tissue culture media composition (addition of lipoic acid), explant size, selectable markers (Bar and NptII), method of subculture post-bombardment |
| Silva et al., (2020) | Leaf whorls | Tx430 | – | NptII | All 7 tested events | Genotypes (Tx430 and P898012) and tissue culture media composition (addition of activated charcoal and polyvinylpyrrolidone) |
| Agrobacterium-mediated transformation | ||||||
| Zhao et al., (2000) | Immature embryos | P898012 | LBA4404 | Bar | 10.10% | Genotypes (P898012 and PHI391), source of explant (grown in the field or greenhouse), tissue culture conditions and media composition |
| Carvalho et al., (2004) | Immature embryos | P898012 | LBA4404 | Hpt | 3.50% | Genotypes (Feterita Gesish, P898012, P967083, IS2329, Rio, Sugar drip, B-Wheatland, RTx430, and Candystripe), embryo selection, tissue culture media composition, tissue culture conditions, Agrobacterium inoculation methods |
| Gao et al., (2005) | Immature embryos | C401 | EHA101 | Pmi | 3.30% | Genotypes (C401 and Pioneer 8505), tissue culture media composition |
| Howe et al. (2006) | Immature embryos | C2-97 | NTL4 | NptII | 4.50% | Genotypes (Tx430 and C2-97), Agrobacterium strains (C58C1, LBA4404, EHA101, C58, and NTL4), selection agent (geneticin and paromomycin) |
| Nguyen et al., (2007) | Immature embryos | Sensako 85/1191 | LBA4404 | Hpt | 5.00% | Explant pre-treatment, tissue culture conditions and media composition |
| Gurel et al., (2009) | Immature embryos | P898012 | LBA4404 | Pmi | 8.30% | Genotypes (P898012, Tx430, 296B, and C401), explant pre-treatmemt, Agrobacterium strains (EHA101 and LBA4404), tissue culture media composition |
| Visarada et al., (2014) | Immature embryos and shoot buds | CS3541 and 296B | EHA105 | Bar | 0.23% | Delivery method (Agrobacterium and particle bombardment), explant type (shoot buds and immature embryos), decontamination treatments for removal of Agrobacterium |
| Wu et al., (2014) | Immature embryos | Tx430 | AGL1 | Pmi | 33.20% | Agrobacterium strains (AGL1 and LBA4404), selectable markers (PAT and Pmi), tissue culture media composition (increased copper sulfate and plant hormone BAP) |
| Yellisetty et al., (2015) | Shoot apical meristem | SPV462 | LBA4404 | Hpt | 36%b | In planta method development |
| Do et al., (2016) | Immature embryos | P898012 | AGL1 | Bar | 14.20% | Genotypes (P898012, TBx623, Tx2737, Tx430, and Wheatland), Agrobacterium strains (AGL1, EHA101, and GV3101), promoters (CaMV35S, MAS, and Ubi), tissue culture conditions |
| Sato-Izawa et al., (2018) | Immature embryos | Tx430 | GV2260 | Hpt | 1.90% | Explant pre-treatment and size |
Selectable markers (SM): Bar, Bialaphos resistance; Hpt, Hygromycin phosphotransferase; Npt, Neomycin phosphotransferase; PAT, Phosphinothricin acetyltransferase; Pmi, Phosphomannose isomerase. Promoters: Act1D, Actin 1D; Adh1, Alcohol dehydrogenase isozyme 1; CaMV35S, Cauliflower mosaic virus 35S; MAS, Mannopine synthase; Ubi, Ubiquitin. Max. TE: maximum transformation efficiency. TE is generally defined as the total number of independent events regenerated divided by the total number of transformed explants, although it can be omitted or vary depending on the publication.
Results from the most successful transformations or optimized conditions.
Results from T1 from selected positive T0 plants.
Current transformation methods
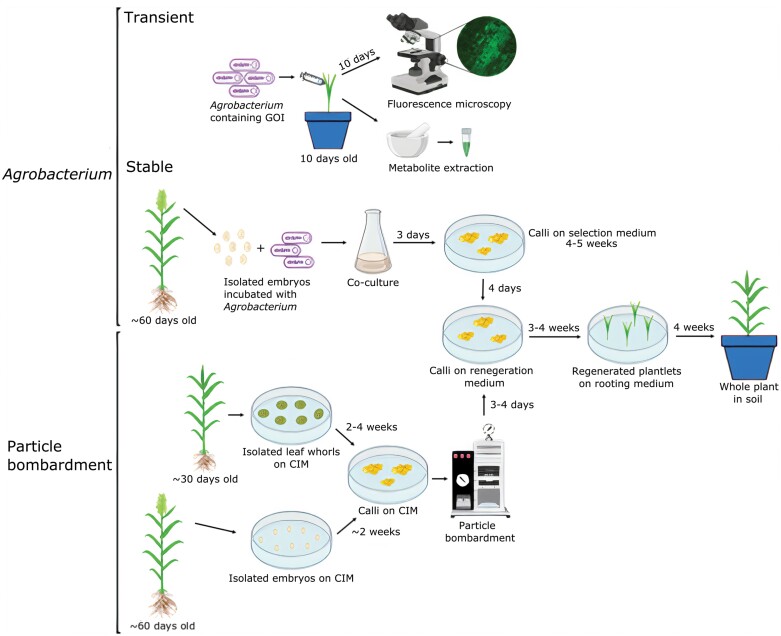
To improve sorghum tissue culture and transformation, different genotypes, transformation methods, and explant sources have been tested over the years. The improvements resulted in reported increases in transformation efficiency from 0.3% to 46.6%, but these remain restricted to select genotypes, and hampered by the seasonality of explant availability. Thus far, four transformation methods have been reported for stable and transient gene expression in sorghum: electroporation; pollen-mediated transformation; particle bombardment; and the Agrobacterium-mediated method. Of these, particle bombardment and Agrobacterium-mediated transformation have been widely tested (Fig. 3). In this section, we summarize these methods, including the extent of their published usage following the initial report, which we use as a proxy for robustness, in Tables 2, 3. We will focus here on the two most commonly used methods: particle bombardment and Agrobacterium-mediated transformation.
Fig. 3.
Representation of transformation methods adopted for sorghum. GOI, gene of interest; CIM, callus induction media.
Particle bombardment
Particle bombardment, also called biolistics or the gene gun method, physically delivers DNA into intact cells or tissues. It is based on high-speed acceleration of DNA-coated gold or tungsten particles (Sanford et al., 1987). The method overcomes the host range restrictions faced when using Agrobacterium and viral vectors. Furthermore, since it does not introduce additional non-plant-derived DNA elements into the plant (as with Agrobacterium-mediated methods), it can simplify transgenic crop regulation. Particle bombardment has also been used for plastid transformation. Since plastids are maternally regulated, this can also aid control of gene flow (Svab et al., 1990; Svab and Maliga, 1993; Kumar et al., 2004; Dufourmantel et al., 2007; Lu et al., 2013; Li et al., 2016). The method of Liu and Godwin (2012), currently the most widely prescribed for sorghum transformation using particle bombardment (as judged by citation count in peer-reviewed literature outlined in Table 2), obtained a transformation efficiency of 20.7% with an optimized protocol using Tx430 immature embryos. Moreover, >90% of the transgenic plants exhibited normal growth and fertility. Adding lipoic acid to the medium and splitting the calli further enhanced the callus induction rate (Belide et al. 2017) (Table 3).
A major drawback of particle bombardment is the random integration of multiple copies of the transgene into the genome, which can lead to transgene rearrangements and silencing (reviewed by Kohli et al., 2003). However, optimization of the procedure can result in single or a low number of transgene copies (Yao et al., 2006). Random integration can also be mitigated by using approaches such as genomic safe harbors: sites in the genome that accommodate transgenes without unwanted interactions (Papapetrou and Schambach, 2016). For example, (Dong et al., 2020) have achieved targeted insertion of a 5.2kb carotenoid biosynthesis cassette at two pre-determined genomic safe harbors in rice. Therefore, this approach could potentially be applied to any crop species. Another issue with particle bombardment is the variable transformation efficiency among genotypes. For example, the elite parental lines CS3541 and 296B were transformed to increase stem borer resistance, but the highest transformation efficiency obtained was 0.25% (Visarada et al., 2014). Traditionally, the most extensively studied genotypes belong to the grain sorghum category, so reported advances are mostly applicable to that type of sorghum. For example, a comparison of 32 sweet sorghum varieties reported a transformation efficiency maximum of 0.09% (Raghuwanshi and Birch, 2010). To fully exploit sorghum as a multipurpose crop that supports the growing bioeconomy, it will be necessary to easily transform many sorghum types, including biomass and forage varieties. Finally, a recent study showed, using whole-genome sequencing, that particle bombardment can frequently induce large-scale genome damage and rearrangement (J. Liu et al., 2019). This can be problematic both for researchers, as this may impact phenotype, and potentially for regulators, as it may increase the risk of the crop being a food safety hazard. Therefore, Agrobacterium-mediated transformation, despite its drawbacks as discussed below, is still considered the preferred method of transformation by most.
Agrobacterium-mediated transformation
Agrobacterium tumefaciens mediated-transformation (reviewed by Gelvin, 2003) was initially used in eudicotyledonous plants, since monocotyledons are not natural hosts of A. tumefaciens. However, successful transformations of many monocots, such as barley, maize, rice, sorghum, and wheat, have now been achieved (Hiei et al., 1994; Ishida et al., 1996; Cheng et al., 1997; Zhao et al., 2000). Agrobacterium-mediated transformation is generally preferred when the goal is to produce plants with single- or low-copy inserts. This approach also has the advantage of resulting in minimal rearrangement of the integrated transgene.
The first reported use of Agrobacterium for stable sorghum transformation was from Zhao et al. (2000). Wu et al. (2014) optimized the resting and selection media by adding increased levels of copper sulfate and the plant hormone 6-benzylaminopurine (BAP) to generate high-quality, fast-growing, and regenerable transgenic calli. They also tested different Agrobacterium strains and selectable markers. Tx430 immature embryos infected by the Agrobacterium strain LBA4404 resulted in transformation efficiencies of up to 12.4% when the selectable marker adopted was Phosphomannose isomerase (Pmi), and 13.4% when Phosphinothricin acetyltransferase (PAT) was used. Using the strain AGL1 and Pmi selection, efficiencies of up to 33.2% were obtained, which is the most effective Agrobacterium protocol reported so far. The authors also point out that the size of T-DNA impacts the quality event frequency, as lower frequency was obtained when larger T-DNA was used (16.3kb versus 7.9kb). Quality events are defined as transformants with intact single copies of T-DNA integrated in the genome without the presence of a vector backbone.
Another optimization of transformation and regeneration with Agrobacterium was achieved by using standard binary vectors containing the Bar gene as the selectable marker under the control of a Mannopine synthase (MAS) promoter and the Agrobacterium strain AGL1 to transform immature embryos from P898012 (Do et al., 2016). Activities of modified Cauliflower mosaic virus 35S (CaMV35S), maize Ubiquitin (Zm-Ubi), and MAS promoters were evaluated, and the highest transformation efficiency was achieved using MAS. Additionally, transformation efficiency was significantly improved using a standard binary vector, while studies that achieved higher efficiencies, such as that of Wu et al. (2014), adopted superbinary vectors. Superbinary vectors have additional virulence genes from a Ti plasmid, which is beneficial for recalcitrant plants (Komari et al., 2006), but are challenging for vector construction, cloning, and transformation. The authors achieved a regeneration time frame of 7–12 weeks and an overall transformation efficiency of 14% (Do et al., 2016).
Improving Agrobacterium transformation efficiency with morphogenic regulators
To increase efficiency of transformation and expand the range of genotypes amenable to transformation, growth-stimulating morphogenic regulators have been used to induce somatic embryogenesis in monocots (Lowe et al., 2016; Mookkan et al., 2017; Nelson-Vasilchik et al., 2018). Morphogenic regulators are genes involved in developmental processes that control morphogenesis such as embryo and meristem development. Lowe et al. (2016) successfully introduced the morphogenic regulators Baby boom (Bbm) and Wuschel2 (Wu2) in maize, sorghum, and rice using the Agrobacterium strain LBA4404 and in sugarcane using the strain AGL1. Although morphogenic regulators promote the induction of somatic embryogenesis, they also cause calli necrosis, preventing the regeneration of transgenic plants (Lowe et al., 2016). To overcome this, a CRE/lox recombination system under the control of a desiccation-induced gene (Rab17) was used to remove the region of the expression cassette containing Bbm and Wu2. Transgenic calli are then subjected to desiccation prior to regeneration, allowing production of healthy transgenic plants. In sorghum, using Tx430 immature embryos as the starting material, the transformation efficiency improved from 1.9% to 18.3% when Bbm and Wu2 were introduced simultaneously (Lowe et al., 2016).
Although morphogenic regulators represented a significant improvement, higher transformation efficiencies of 33.2% (Wu et al., 2014) and 46.6% (Belide et al., 2017) were obtained with the genotype Tx430 using traditional methods. The most compelling argument for the morphogenic regulator method is the possibility of transforming genotypes that are currently recalcitrant to transformation. However, to date in sorghum, this approach has been reported mostly in transformable cultivars (Lowe et al., 2016; Mookkan et al., 2017). Mookkan et al. (2017) used the Agrobacterium strain EHA101 to transform immature embryos from sorghum genotype P898012 with a vector containing Bbm, Wu2, and the desiccation-inducible CRE/lox recombination system. They observed that calli transformed with Bbm and Wu2 reached up to 54.5% of green fluorescent protein (GFP) expression, while calli transformed with vectors without them did not show any GFP expression. Additionally, Nelson-Vasilchik et al. (2018) published a protocol using the genotype BTx623, besides the previously reported P898012, for Agrobacterium-mediated transformation with the same morphogenic regulators, the Rab17pro:CRE/lox-inducible system, and Agrobacterium strains AGL1 and EHA101, and showed a regeneration rate of ~15%.
In planta transformation
Agrobacterium tumefaciens has also been used for in planta transformation in sorghum, which allows the introduction of DNA directly into intact plant tissue, removing the dependence on tissue culture and regeneration protocols. Yellisetty et al. (2015) reported an in planta transformation method where Agrobacterium strain LBA4404 was inoculated onto the shoot apical meristems of germinating sorghum seedlings, with transformation efficiencies of up to 36%. Despite these high reported efficiencies, the method has not been applied to further studies (Table 2). Haploid egg cell transformation by floral dipping is widely used for A. thaliana and has been applied to other Brassicaceae species, flax, and even a grass Setaria viridis (Liu et al., 2012; Martins et al., 2015). However, in planta transformation has not been established as a standard protocol for many species due to a lack of reproducibility (Hamada et al., 2017). Fundamental understanding of why some plants, such as A. thaliana and Camelina sativa, are susceptible to Agrobacterium haploid egg cell transformation would be an important step forward in plant science, as this could lead to application of this method to other species, such as sorghum.
Transient expression
The methods discussed above are mainly used for stable transformation, in which the genes are integrated into the host chromosomes and are inherited through subsequent generations. Stable transformation is particularly interesting if the goal is to engineer traits in the long term. However, for studies aiming at gene characterization, vector validation, or protein subcellular localization, especially in recalcitrant species such as sorghum, transient expression is a valuable and time-saving tool. It allows temporary expression of the introduced genes, which do not integrate into the host genome, but uses its transcriptional and translational machinery to synthesize the desired proteins. Transient expression generally reaches its maximum level between 18-48h after transformation and persists for a few days (Abel and Theologis, 1994). Agrobacterium-mediated transformation can also be successfully applied to transient expression. For example, Sharma et al. (2020) developed an in planta method using Agrobacterium for infiltration in leaves of 3- to 4-week-old sorghum, in which GFP expression was detected 3–4 d after infiltration. The method was also used to demonstrate clustered regularly interspaced short palindromic repeats (CRISPR)-mediated genome editing as a promising approach to test single-guide RNA (sgRNA) efficiencies in vivo (Liang et al., 2019).
Future needs for sorghum transformation
Although there has been progress, the technical challenges associated with sorghum tissue culture and transformation mean that efficiencies still lag behind most other monocot crops such as rice, which routinely reaches efficiencies of up to 90% (Hiei and Komari, 2008). To move the field forward, the main bottlenecks that need to be addressed are genotype dependence, prevention of transgene flow to wild relatives, and achieving higher transformation efficiency reproducibly. Overcoming these bottlenecks will allow the efficient application of synthetic biology principles, and the direct engineering of elite germplasm. In particular, this will enable the routine use of gene editing tools, including CRISPR/Cas systems and successful metabolic engineering for high-value traits. Here, we highlight some key areas for future research.
Genotype independence
Currently, the inbred lines Tx430 and P898012 are the most used genotypes for transformation due to their higher embryogenic capacity. This is limiting, particularly for commercial purposes, where the engineering of elite cultivars would be beneficial. What underpins these genotypic differences in transformability is not known. However, overexpression of morphogenic regulators such as Bbm and Wus2 has the potential not only to induce somatic embryogenesis in an expanded range of genotypes, but also to bypass or accelerate tissue culture via de novo meristem formation as demonstrated in eudicots (Maher et al., 2020). Assessment of other morphogenic regulators such as Leafy cotyledon1 (Lec1), Lec2, Monopteros (MP), Shoot meristemless (STM), hormone biosynthetic genes such as Isopentenyl transferase (Ipt), and their combinations are all promising strategies.
Besides using morphogenic regulators, genotype independence can be achieved by using tissues other than embryos, which has been achieved in barley, cotton, and rice (Dey et al., 2012; Ma et al., 2013; Han et al., 2021). For example, Han et al. 2021)successfully used microspores from barley anthers to induce callus formation for transformation and CRISPR/Cas gene editing. The diverse genetic backgrounds of the tested varieties indicated that the method was genotype independent and could be expanded to other species with established anther culture protocols. Additionally, shoot apices from 3- to 5-day-old seedlings have been used for Agrobacterium infection of cotton and rice for development of genotype-independent regeneration protocols (Dey et al., 2012; Ma et al., 2013).
Another approach for achieving genotype independence is to identify specific genes associated with tissue culture responses. Quantitative trait loci (QTL) mapping studies to identify genomic regions associated with callus induction and plant regeneration have been carried out in grasses such as barley, maize, and wheat (Amer et al., 1997; Mano and Komatsuda, 2002; Salvo et al., 2018). Although these studies concluded that tissue culture response is a complex polygenic trait, further investigation of specific candidate genes is needed, especially in sorghum, to identify genetic mechanisms that control somatic embryogenesis and efficient regeneration response.
Improved transformation efficiency
Successful introduction of a wide range of genes of interest into sorghum will depend on efficient tissue culture and transformation protocols. Currently, sorghum transformation typically uses indirect somatic embryogenesis, which goes through the callus stage. The maintenance of callus cultures is labor intensive and a lengthy process that can induce somaclonal variation. Direct somatic embryogenesis is an alternative that has been achieved in maize and sugarcane (Taparia et al., 2012; Lowe et al., 2018), and could be applied to sorghum to shorten tissue culture time and increase efficiency. As shown in maize, introduction of morphogenic regulators enables immature embryos to transition into somatic embryos in a few days and allows bypassing the callus stage (Lowe et al., 2018). Alternatively, the tissue culture method using leaf whorls reported by Silva et al. (2020) could be adapted to induce direct somatic embryogenesis as previously demonstrated in sugarcane (Desai et al., 2004; Taparia et al., 2012).
A promising alternative to somatic embryos is using embryogenic cell suspension cultures, which have been used to transform switchgrass, with high efficiency of 85% (Ondzighi-Assoume et al., 2019), and cotton, reaching transformation efficiency of ~19% (Ke et al., 2012). Efficient methods for maintaining sorghum cell cultures have potential to improve transformation efficiency by reducing somaclonal variation, decreasing false positives, and increasing the survival rate of transgenics. Additionally, cells with a synchronized cell cycle could be obtained, which may benefit CRISPR/Cas genome editing studies aiming for homology-directed repair (HDR). Cells have different abilities to repair double-stranded breaks using the non-homologous end joining (NHEJ) or HDR pathways, and the phase of the cell cycle plays a major role in the choice of the pathway (Heyer et al., 2010). The HDR pathway activity is restricted to the late S and G2 phases of the cell cycle, while NHEJ occurs during the entire cell cycle . Therefore, cell suspension cultures can be a valuable tool not only to improve transformation efficiency, but also to increase genome editing efficiencies for targeting gene insertions, replacements, or stacking.
Other approaches to improve transformation efficiency involve the development of more efficient DNA delivery methods. Although progress has been made in Agrobacterium-mediated transformation, engineering strains with increased virulence and a wider host range will be necessary to boost efficiency. Optimizations to avoid overgrowth of Agrobacterium in the tissue culture selection media will also be relevant (Ahmed et al., 2018). Another promising strategy is the use of nanoparticles to deliver DNA, which has already been demonstrated in wheat and cotton leaves, resulting in strong protein expression (Demirer et al., 2019).
Genome editing
CRISPR/Cas-mediated genome editing can be applied broadly, including creating mutant collections of specific genes that have not been well characterized, creating variations for breeding purposes, and altering regulatory elements. CRISPR/Cas9-mediated gene editing in sorghum was first reported using Agrobacterium-mediated transformation to restore the function of an out-of-frame red fluorescence protein (DsRED2) through NHEJ (Jiang et al., 2013). Since then, CRISPR/Cas9 delivery by Agrobacterium has been adopted to mutate several sorghum genes (A. Li et al., 2018; Che et al., 2018; Char et al., 2020). To date, only one protocol for CRISPR/Cas9 genome editing using particle bombardment has been published (G. Liu et al., 2019).
Cas9 requires a 5ʹ-NGG-3ʹ protospacer adjacent motif (PAM) site upstream of the sgRNA-binding region in the genome. Other endonucleases, such as Cpf1 that targets T-rich regions (Zetsche et al., 2015), have not yet been exploited in sorghum. These alternative endonucleases broaden the range of sequences that can be targeted. In cases where the goal is generating precise point mutations, an alternative to the low-efficiency HDR pathway is using the CRISPR base editors (Komor et al., 2016). CRISPR base editors allow cytosine to thymine or adenine to guanine base editing, and have been widely adopted to introduce targeted substitutions in other crops such as rice and wheat to improve important agricultural traits, such as flowering time and herbicide resistance (C. Li et al., 2018; Kang et al., 2018; Zhang et al., 2019; Li et al., 2020).
Prevention of transgene flow
Valid concerns about transgene flow to sorghum’s sexually compatible wild weedy relatives have dampened commercial interest in engineered cultivars. Therefore, techniques that prevent transgene introgression or propagation through pollen should be prioritized. Alternatively, transgene-free methods for genome editing such as the delivery of a pre-assembled ribonucleoprotein (RNP) complex, which is done via protoplast transfection or particle bombardment, can be used (Woo et al., 2015; Svitashev et al., 2016; Liang et al., 2017). Particle bombardment would be the most suitable method for sorghum as it does not require plant regeneration from protoplasts, an ongoing challenge for sorghum tissue culture. Distinct methods adopted in other species also have potential in sorghum. For example, Zhang et al. (2016) generated transgene-free and homozygous wheat mutants in the T1 generation by transiently expressing Cas9 in callus cells.
Another promising strategy to impede transgene flow into the wild would be the delivery of transgenes into chloroplasts to take advantage of their maternal inheritance. This avoids transgene transmission via pollen, closing a potential escape route into the environment (Daniell, 2002). Thus, chloroplast transformation would allow stable introduction of Cas9 into sorghum’s chloroplast genome to generate Cas9 lines that would not propagate the transgene via pollen.
Current genetic, genomic, and bioinformatic resources
Sorghum has several characteristics that make it an excellent potential model species for grass research. It is a diploid (2n=20), which makes it more amenable to genetic and genomic studies compared with polyploid bioenergy crops such as sugarcane. It also has a small genome size (~730 Mbp) compared with maize (2.5 Gbp), sugarcane (~10 Gbp), and wheat (~17 Gbp) (Paterson et al., 2009). Extensive variations across cultivated and wild species have been identified, suggesting a rich genetic source for adaptation and engineering (Tao et al., 2021). Additionally, sorghum is a C4 grass with high nitrogen and water use efficiency (Ghannoum et al., 2011) and complements other grass models such as rice and Brachypodium, which are C3 grasses. The wide genetic variation found within and among sorghum cultivars is also attractive as it can be exploited to improve the crop through breeding, population genetic, and quantitative genetic approaches (Satish et al., 2016). To support the adoption of a plant species as a research system, it is critical to have accessible resources, including germplasm collections, reference genome sequences with good quality functional annotations, and easy-to-use informatics tools that collate existing data. While sorghum does have some of these resources, there are still many gaps.
Genetic resources
The largest sorghum germplasm collection is maintained by the USDA Agricultural Research Service (ARS) National Plant Germplasm System and consists of >40 000 accessions from 114 countries, of which many regional specific subsets have been genetically characterized (Cuevas et al., 2017, 2018; Olatoye et al., 2018; Cuevas and Prom, 2020; Faye et al., 2021). The International Crops Research Institute for the Semi-Arid Tropics (ICRISAT) in India also has a large collection of 37 904 accessions (Morris et al., 2013; Cuevas et al., 2017). A third collection with >16 000 accessions is kept by the National Crop Genebank of China. Information and sources of seeds can be identified via databases such as USDA-ARS GRIN (https://npgsweb.ars-grin.gov/gringlobal/search.aspx), Eurisco (http://eurisco.ecpgr.org/), and Genesys (https://www.genesys-pgr.org/). Additional collections with particular relevance to the use of sorghum as a biomass crop include the biomass association panel (Brenton et al., 2016) and the nested association mapping population (Bouchet et al., 2017). These collections contain immense genetic diversity, which is essential for breeding programs that aim to develop cultivars better adapted to different conditions worldwide and also an important resource to elucidate molecular machineries that lead to traits of interest.
Furthermore, alleles not found in nature can be generated through mutagenesis (e.g. genotoxic chemicals or γ-irradiation) (Xin et al., 2008; Jiao et al., 2016; Chen et al., 2019) or, more recently, through genome editing. Mutant lines are being added to these germplasm collections to create an even more diverse community resource. Increasingly, these mutant populations are accompanied by whole-genome sequences, allowing researchers to take a reverse genetics approach to identifying gene function (Addo-Quaye et al., 2018).
Genomic resources
The first sorghum reference genome (from the grain sorghum BTx623) was generated using whole-genome shotgun sequencing in 2009 (Paterson et al., 2009), and placed ~98% of the genes in their chromosomal context. More recently, BTx623 version 3.1.1 was released with improved assembly and annotation (McCormick et al., 2018). The high-quality reference genome of the sweet sorghum ‘Rio’ was also recently released using Pacific Biosciences long-read sequencing (Cooper et al., 2019). The authors used it to explore the possible genomic differences between sorghum types, and revealed a high rate of non-synonymous and potential loss-of-function mutations in sweet sorghum. However, few changes in gene content and overall genome structure were observed (Cooper et al., 2019). Two additional genomes, BTx642 and RTx430, are also available on Phytozome (see below). An ongoing sorghum pan-genome project at the DOE Joint Genome Institute (JGI) will explore this information further (Mockler, 2016).
Bioinformatic resources
Several bioinformatic resources host sorghum data (links and references described in Table 4). Sorghum breeders and researchers can rely on bioinformatic resources such as Phytozome, the Plant Comparative Genomics portal of the DOE Joint Genome Institute (JGI) (Goodstein et al., 2012). This includes the latest sorghum reference genome (McCormick et al., 2018). Additionally, the Sorghum genome SNP database (SorGSD), a database with 62 million single nucleotide polymorphisms (SNPs) from 48 sorghum accessions, allows the user to search for synonymous and non-synonymous SNPs, their annotation, geographic origin, and breeding information (Luo et al., 2016). A valuable resource for sorghum improvement is the Sorghum Genomics Functional Gene Discovery Platform, which enables the identification of sorghum lines containing natural and chemical-induced variations in coding sequences (https://www.purdue.edu/sorghumgenomics/)(REF). The Sorghum Functional Genomics Database (SorghumFDB) also has a search feature with orthologous pairs in A. thaliana, rice, and maize, in addition to gene family classifications, gene annotations, loci conversions, miRNA and target gene information, and a genome browser (Tian et al., 2016). The PlantGDB, a resource for comparative plant genomics, has a section on sorghum (SbGDB), which includes gene structure annotation, sequence analysis tools, and annotated protein alignments. Also, sorghum metabolic network data can be found in SorghumbicolorCyc at the Plant Metabolic Network (PMN), a curated source of metabolic information from the literature and computational analyses (Schläpfer et al., 2017). Lastly, UniProt has sorghum protein sequences from genome sequencing projects (Saski et al., 2007; Paterson et al., 2009; Hawkins et al., 2021). These resources can assist researchers who are new to sorghum research to understand sorghum genome architecture and its variations, and to draw comparisons with other extensively studied species.
Table 4.
Bioinformatics resources available for sorghum research
| Bioinformatic tool | Purpose | Website | Source | Reference |
|---|---|---|---|---|
| Phytozome | Reference genome and alignment searches | https://phytozome.jgi.doe.gov/pz/portal.html#!info?alias=Org_Sbicolor | Joint Genome Institute (JGI) | McCormick et al., (2018) |
| Plant Metabolic Network | Network of metabolic pathway data | https://plantcyc.org/content/sorghumbicolorcyc-7.0.1 | Carnegie Institution for Science | Hawkins et al., (2021) |
| Gramene sorghum | All sorghum resources as statistics, germplasm resources, metabolic pathways | https://archive.gramene.org/species/sorghum/sorghum_intro.html | Cold Spring Harbor Laboratory and Cornell University | Tello-Ruiz et al., (2018) |
| Sorghum FDB - Functional Genomics Database | Integrated search for gene family classifications, gene annotations, miRNA and target gene information, orthologous pairs in Arabidopsis, rice, and maize, gene loci conversions and a genome browser | http://structuralbiology.cau.edu.cn/sorghum | Zhen Su’s group at China Agricultural University | Tian et al., (2016) |
| SbGDB | Sequence-centered genome view with focus on gene structure annotation | http://www.plantgdb.org/SbGDB/ | Brendel group at Indiana University | – |
| Uniprot | Proteomic data | https://www.uniprot.org/proteomes/UP000000768 | UniProt Consortium | – |
| Sorghum genomics - Functional Gene Discovery Platform | Search for lines containing natural and ems-induced variations in coding sequences | https://www.purdue.edu/sorghumgenomics# | Purdue University | – |
Conclusion
Sorghum has a bright future as a multipurpose crop that is suited to the challenging growth conditions that climate change will bring. Its extensive genetic diversity combined with relatively recent and limited domestication means that it also has an excellent potential for further improvement. Sorghum can become a model system for other grass species, particularly in areas such as abiotic and biotic stress responses, plant–microbiome interactions, and evolution. We see transformation challenges as a major bottleneck to the development of sorghum as both a widely adopted research system and a key feedstock for the bioeconomy, and contend that research tackling this problem is a high priority.
Acknowledgements
We thank members of the Sorghum Metabolic Atlas team (https://www.sorghummetabolicatlas.org/) for helpful and inspiring discussions about sorghum transformation and two anonymous reviewers for their helpful comments and insights, which have improved the manuscript. This work was done, in part, on the ancestral land of the Muwekma Ohlone Tribe, which was and continues to be of great importance to the Ohlone people.
Author contributions
TNS, JBT, JCM, and SYR: conceptualization; TNS and JCM: data curation; JCM, SYR, and JBT: funding acquisition; JCM and SYR: project administration; TNS, JBT, and JCM: writing—original draft; TNS, JBT, JD, SYR, and JCM: writing—review and editing.
Funding
This research was supported, in part, by the U.S. Department of Energy, Office of Science, Office of Biological and Environmental Research, Genomic Science Program grant no. DE-DE-SC0020366 (SYR and JCM) and DE-SC0018277 (SYR), the DOE Joint BioEnergy Institute (http://www.jbei.org) supported by the U.S. Department of Energy, Office of Science, Office of Biological and Environmental Research, through contract DE-AC02-05CH11231 between the Lawrence Berkeley National Laboratory (JCM) and the U.S. Department of Energy, and the U.S. National Science Foundation grants IOS-1546838 (SYR) and MCB-1617020 (SYR).
References
- Able JA, Rathus C, Godwin ID.. 2001. The investigation of optimal bombardment parameters for transient and stable transgene expression in Sorghum. In Vitro Cellular & Developmental Biology. Plant 37, 341–348. [Google Scholar]
- Abel S, Theologis A.. 1994. Transient transformation of Arabidopsis leaf protoplasts: a versatile experimental system to study gene expression. The Plant Journal 5, 421–427. [DOI] [PubMed] [Google Scholar]
- Addo-Quaye C, Tuinstra M, Carraro N, Weil C, Dilkes BP.. 2018. Whole-genome sequence accuracy is improved by replication in a population of mutagenized sorghum. G3 8, 1079–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed RI, Ding A, Xie M, Kong Y.. 2018. Progress in optimization of Agrobacterium-mediated transformation in sorghum (Sorghum bicolor). International Journal of Molecular Sciences 19, 2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altpeter F, Springer NM, Bartley LE, et al. 2016. Advancing crop transformation in the era of genome editing. The Plant Cell 28, 1510–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameen A, Yang X, Chen F, Tang C, Du F, Fahad S, Xie GH.. 2017. Biomass yield and nutrient uptake of energy sorghum in response to nitrogen fertilizer rate on marginal land in a semi-arid region. BioEnergy Research 10, 363–376. [Google Scholar]
- Amer IMB, Worland AJ, Korzun V, Börner A.. 1997. Genetic mapping of QTL controlling tissue-culture response on chromosome 2B of wheat (Triticum aestivum L.) in relation to major genes and RFLP markers. Theoretical and Applied Genetics 94, 1047–1052. [Google Scholar]
- Anglani C. 1998. Sorghum carbohydrates—a review. Plant Foods for Human Nutrition 52, 77–83. [DOI] [PubMed] [Google Scholar]
- Aregawi K, Shen J, Pierroz G, Bucheli C, Sharma M, Dahlberg J, Owiti J, Lemaux PG.. 2020. Pathway to validate gene function in key bioenergy crop, Sorghum bicolor. Bioxiv [Preprint]. [Google Scholar]
- Assefa Y, Staggenborg SA, Prasad VPV.. 2010. Grain sorghum water requirement and responses to drought stress: a review. Crop Management 9, 1–11. [Google Scholar]
- Assem SK, Zamzam MM, Saad ME, Hussein BA, Hussein EHA.. 2017. The impact of over-expression of NPK1 gene on growth and yield of sorghum under drought stress. African Journal of Biotechnology 16, 2267–2277. [Google Scholar]
- Awika JM, Rooney LW.. 2004. Sorghum phytochemicals and their potential impact on human health. Phytochemistry 65, 1199–1221. [DOI] [PubMed] [Google Scholar]
- Awika JM, Rooney LW, Waniska RD.. 2004. Properties of 3-deoxyanthocyanins from sorghum. Journal of Agricultural and Food Chemistry 52, 4388–4394. [DOI] [PubMed] [Google Scholar]
- Baral NR, Sundstrom ER, Das L, Gladden JM, Eudes A, Mortimer J, Singer SW, Mukhopadhyay A, Scown CD.. 2019. Approaches for more efficient biological conversion of lignocellulosic feedstocks to biofuels and bioproducts. ACS Sustainable Chemistry & Engineering 7, 9062–9079. [Google Scholar]
- Battraw M, Hall TC.. 1991. Stable transformation of Sorghum bicolor protoplasts with chimeric neomycin phosphotransferase II and β-glucuronidase genes. Theoretical and Applied Genetics 82, 161–168. [DOI] [PubMed] [Google Scholar]
- Belide S, Vanhercke T, Petrie JR, Singh SP.. 2017. Robust genetic transformation of sorghum (Sorghum bicolor L.) using differentiating embryogenic callus induced from immature embryos. Plant Methods 13, 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerjan W, Ralph J, Baucher M.. 2003. Lignin biosynthesis. Annual Review of Plant Biology 54, 519–546. [DOI] [PubMed] [Google Scholar]
- Borrell AK, Bidinger FR, Sunitha K.. 1999. Stay-green trait associated with yield in recombinant inbred sorghum lines varying in rate of leaf senescence. International Sorghum and Millets Newsletter 40, 31–34. [Google Scholar]
- Borrell AK, Hammer GL.. 2000. Nitrogen dynamics and the physiological basis of stay-green in sorghum. Crop Science 40, 1295–1307. [Google Scholar]
- Borrell AK, Hammer GL, Douglas ACL.. 2000. Does maintaining green leaf area in sorghum improve yield under drought? I. Leaf growth and senescence. Crop Science 40, 1026–1037. [Google Scholar]
- Bouchet S, Olatoye MO, Marla SR, Perumal R, Tesso T, Yu J, Tuinstra M, Morris GP.. 2017. Increased power to dissect adaptive traits in global sorghum diversity using a nested association mapping population. Genetics 206, 573–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandao RL, Carneiro NP, de Oliveira AC, Coelho GTCP, Carneiro AA.. 2012. Genetic transformation of immature sorghum inflorescence via microprojectile bombardment. In: Ozdenifti Y, ed. Transgenic plants—advances and limitations. InTech. [Google Scholar]
- Brandon AG, Birdseye DS, Scheller HV.. 2020. A dominant negative approach to reduce xylan in plants. Plant Biotechnology Journal 18, 5–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenton ZW, Cooper EA, Myers MT, Boyles RE, Shakoor N, Zielinski KJ, Rauh BL, Bridges WC, Morris GP, Kresovich S.. 2016. A genomic resource for the development, improvement, and exploitation of sorghum for bioenergy. Genetics 204, 21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway E. 2018. CRISPR plants now subject to tough GM laws in European Union. Nature 560, 16. [DOI] [PubMed] [Google Scholar]
- Cardinal M-J, Kaur R, Singh J.. 2016. Genetic transformation of Hordeum vulgare ssp. spontaneum for the development of a transposon-based insertional mutagenesis system. Molecular Biotechnology 58, 672–683. [DOI] [PubMed] [Google Scholar]
- Carvalho CHS, Zehr UB, Gunaratna N, Anderson J, Kononowicz HH, Hodges TK, Axtell JD.. 2004. Agrobacterium-mediated transformation of sorghum: factors that affect transformation efficiency. Genetics and Molecular Biology 27, 259–269. [Google Scholar]
- Casas AM, Kononowicz AK, Haan TG, Zhang L, Tomes DT, Bressan RA, Hasegawa PM.. 1997. Transgenic sorghum plants obtained after microprojectile bombardment of immature inflorescences. In Vitro Cellular & Developmental Biology. Plant 33, 92–100. [Google Scholar]
- Casas AM, Kononowicz AK, Zehr UB, Tomes DT, Axtell JD, Butler LG, Bressan RA, Hasegawa PM.. 1993. Transgenic sorghum plants via microprojectile bombardment. Proceedings of the National Academy of Sciences, USA 90, 11212–11216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Char SN, Wei J, Mu Q, Li X, Zhang ZJ, Yu J, Yang B.. 2020. An Agrobacterium-delivered CRISPR/Cas9 system for targeted mutagenesis in sorghum. Plant Biotechnology Journal 18, 319–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che P, Anand A, Wu E, et al. 2018. Developing a flexible, high-efficiency Agrobacterium-mediated sorghum transformation system with broad application. Plant Biotechnology Journal 16, 1388–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Zou G, Xin Z.. 2019. Development of a pedigreed sorghum mutant library. Methods in Molecular Biology 1931, 61–73. [DOI] [PubMed] [Google Scholar]
- Cheng M, Fry JE, Pang S, Zhou H, Hironaka CM, Duncan DR, Conner TW, Wan Y.. 1997. Genetic transformation of wheat mediated by Agrobacterium tumefaciens. Plant Physiology 115, 971–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho M-J, Wu E, Kwan J, et al. 2014. Agrobacterium-mediated high-frequency transformation of an elite commercial maize (Zea mays L.) inbred line. Plant Cell Reports 33, 1767–1777. [DOI] [PubMed] [Google Scholar]
- Chou J, Huang J, Huang Y.. 2020. Simple and efficient genetic transformation of sorghum using immature inflorescences. Acta Physiologiae 42, 41. [Google Scholar]
- Cooper EA, Brenton ZW, Flinn BS, et al. 2019. A new reference genome for Sorghum bicolor reveals high levels of sequence similarity between sweet and grain genotypes: implications for the genetics of sugar metabolism. BMC Genomics 20, 420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craufurd PQ, Peacock JM.. 1993. Effect of heat and drought stress on sorghum (Sorghum bicolor). II. Grain yield. Experimental Agriculture 29, 77–86. [Google Scholar]
- Cuevas HE, Prom LK.. 2020. Evaluation of genetic diversity, agronomic traits, and anthracnose resistance in the NPGS Sudan Sorghum Core collection. BMC Genomics 21, 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas HE, Prom LK, Rosa-Valentin G.. 2018. Population structure of the NPGS Senegalese sorghum collection and its evaluation to identify new disease resistant genes. Plos One 13, e0191877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas HE, Rosa-Valentin G, Hayes CM, Rooney WL, Hoffmann L.. 2017. Genomic characterization of a core set of the USDA-NPGS Ethiopian sorghum germplasm collection: implications for germplasm conservation, evaluation, and utilization in crop improvement. BMC Genomics 18, 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas HE, Zhou C, Tang H, et al. 2016. The evolution of photoperiod-insensitive flowering in sorghum, a genomic model for panicoid grasses. Molecular Biology and Evolution 33, 2417–2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlberg JA. 2000. Classification and characterization of sorghum. In: Smith CW, Frederiksen RA, eds. Sorghum: origin, history, technology, and production. New York: John Wiley & Sons, 99–130. [Google Scholar]
- Daniell H. 2002. Molecular strategies for gene containment in transgenic crops. Nature Biotechnology 20, 581–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva MJ, Carneiro PCS, de Souza Carneiro JE, Damasceno CMB, Parrella NNLD, Pastina MM, Simeone MLF, Schaffert RE, da Costa Parrella RA.. 2018. Evaluation of the potential of lines and hybrids of biomass sorghum. Industrial Crops and Products 125, 379–385. [Google Scholar]
- Demirer GS, Zhang H, Matos JL, et al. 2019. High aspect ratio nanomaterials enable delivery of functional genetic material without DNA integration in mature plants. Nature Nanotechnology 14, 456–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai NS, Suprasanna P, Bapat VA.. 2004. Simple and reproducible protocol for direct somatic embryogenesis from cultured immature inflorescence segments of sugarcane (Saccharum spp.). Current Science 87, 764. [Google Scholar]
- Dey M, Bakshi S, Galiba G, Sahoo L, Panda SK.. 2012. Development of a genotype independent and transformation amenable regeneration system from shoot apex in rice (Oryza sativa spp. indica) using TDZ. 3 Biotech 2, 233–240. [Google Scholar]
- Di Giacomo R, Daraio C, Maresca B.. 2015. Plant nanobionic materials with a giant temperature response mediated by pectin–Ca2+. Proceedings of the National Academy of Sciences, USA 112, 4541–4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do PT, Lee H, Mookkan M, Folk WR, Zhang ZJ.. 2016. Rapid and efficient Agrobacterium-mediated transformation of sorghum (Sorghum bicolor) employing standard binary vectors and bar gene as a selectable marker. Plant Cell Reports 35, 2065–2076. [DOI] [PubMed] [Google Scholar]
- Do PT, Lee H, Nelson-Vasilchik K, Kausch A, Zhang ZJ.. 2018. Rapid and efficient genetic transformation of Sorghum via Agrobacterium-mediated method. Current Protocols in Plant Biology 3, e20077. [DOI] [PubMed] [Google Scholar]
- Dong OX, Yu S, Jain R, et al. 2020. Marker-free carotenoid-enriched rice generated through targeted gene insertion using CRISPR–Cas9. Nature Communications 11, 1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufourmantel N, Dubald M, Matringe M, et al. 2007. Generation and characterization of soybean and marker-free tobacco plastid transformants over-expressing a bacterial 4-hydroxyphenylpyruvate dioxygenase which provides strong herbicide tolerance. Plant Biotechnology Journal 5, 118–133. [DOI] [PubMed] [Google Scholar]
- Dwivedi KK, Roche DJ, Clemente TE, Ge Z, Carman JG.. 2014. The OCL3 promoter from Sorghum bicolor directs gene expression to abscission and nutrient-transfer zones at the bases of floral organs. Annals of Botany 114, 489–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebringerová A, Hromádková Z, Heinze T.. 2005. Hemicellulose. In: Heinze T, ed. Polysaccharides I. Berlin/Heidelberg: Springer-Verlag, 1–67. [Google Scholar]
- Ejeta G, Grenier C.. 2005. Sorghum and its weedy hybrids. In: Gressel J, ed. Crop ferality and volunteerism. Boca Raton, FL: CRC Press, Taylor and Francis Group, 123–135. [Google Scholar]
- Elkonin LA, Italianskaya JV, Domanina IV, Selivanov NY, Rakitin AL, Ravin NV.. 2016. Transgenic sorghum with improved digestibility of storage proteins obtained by Agrobacterium-mediated transformation. Russian Journal of Plant Physiology 63, 678–689. [Google Scholar]
- Emani C, Sunilkumar G, Rathore KS.. 2002. Transgene silencing and reactivation in sorghum. Plant Science 162, 181–192. [Google Scholar]
- Eudes A, Liang Y, Mitra P, Loqué D.. 2014. Lignin bioengineering. Current Opinion in Biotechnology 26, 189–198. [DOI] [PubMed] [Google Scholar]
- Faye JM, Maina F, Akata EA, et al. 2021. A genomics resource for genetics, physiology, and breeding of West African sorghum. The Plant Genome 14, e20075. [DOI] [PubMed] [Google Scholar]
- Flinn B, Dale S, Disharoon A, Kresovich S.. 2020. Comparative analysis of in vitro responses and regeneration between diverse bioenergy sorghum genotypes. Plants 9, 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forde GM, Rainey TJ, Speight R, Batchelor W, Pattenden LK.. 2016. Matching the biomass to the bioproduct. In: Xu CP, Luque R, eds. Biomaterials: biological production of fuels and chemicals. Germany: Walter de Gruyter, 1–44. [Google Scholar]
- Fu C, Mielenz JR, Xiao X, et al. 2011. Genetic manipulation of lignin reduces recalcitrance and improves ethanol production from switchgrass. Proceedings of the National Academy of Sciences, USA 108, 3803–3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu HM, Meng FY, Molatudi RL, Zhang BG.. 2016. Sorghum and switchgrass as biofuel feedstocks on marginal lands in northern china. BioEnergy Research 9, 633–642. [Google Scholar]
- Gao Y, Lipton AS, Wittmer Y, Murray DT, Mortimer JC.. 2020. A grass-specific cellulose–xylan interaction dominates in sorghum secondary cell walls. Nature Communications 11, 6081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Xie X, Ling Y, Muthukrishnan S, Liang GH.. 2005. Agrobacterium tumefaciens-mediated sorghum transformation using a mannose selection system. Plant Biotechnology Journal 3, 591–599. [DOI] [PubMed] [Google Scholar]
- Gelvin SB. 2003. Agrobacterium-mediated plant transformation: the biology behind the ‘gene-jockeying’ tool. Microbiology and Molecular Biology Reviews 67, 16–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghannoum O, Evans JR, von Caemmerer S.. 2011. Nitrogen and water use efficiency of C4 plants. In: Raghavendra AS, Sage RF, eds. Advances in photosynthesis and respiration. C4 photosynthesis and related CO2 concentrating mechanisms. Dordrecht: Springer Netherlands, 129–146. [Google Scholar]
- Gobena D, Shimels M, Rich PJ, Ruyter-Spira C, Bouwmeester H, Kanuganti S, Mengiste T, Ejeta G.. 2017. Mutation in sorghum LOW GERMINATION STIMULANT 1 alters strigolactones and causes Striga resistance. Proceedings of the National Academy of Sciences, USA 114, 4471–4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodstein DM, Shu S, Howson R, et al. 2012. Phytozome: a comparative platform for green plant genomics. Nucleic Acids Research 40, D1178–D1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouveia TIA, Biernacki K, Castro MCR, Gonçalves MP, Souza HKS.. 2019. A new approach to develop biodegradable films based on thermoplastic pectin. Food Hydrocolloids 97, 105175. [Google Scholar]
- Grootboom AW, Mkhonza NL, Mbambo Z, O’Kennedy MM, da Silva LS, Taylor J, Taylor JRN, Chikwamba R, Mehlo L.. 2014. Co-suppression of synthesis of major α-kafirin sub-class together with γ-kafirin-1 and γ-kafirin-2 required for substantially improved protein digestibility in transgenic sorghum. Plant Cell Reports 33, 521–537. [DOI] [PubMed] [Google Scholar]
- Grootboom AW, Mkhonza NL, O’Kennedy MM, Chakauya E, Kunert K, Chikwamba RK.. 2010. Biolistic mediated sorghum (Sorghum bicolor L. Moench) transformation via mannose and bialaphos based selection systems. International Journal of Botany 6, 89–94. [Google Scholar]
- Grootboom AW, O’Kennedy MM, Mkhonza NL, Kunert K, Chakauya E, Chikwamba KR.. 2008. In vitro culture and plant regeneration of sorghum genotypes using immature zygotic embryos as explant source. International Journal of Botany 4, 450–455. [Google Scholar]
- Grossman AB, Rice KC, Vermerris W.. 2020. Lignin solvated in zwitterionic Good’s buffers displays antibacterial synergy against. Journal of Applied Polymer Science 137, 49107. [Google Scholar]
- Gurel S, Gurel E, Kaur R, Wong J, Meng L, Tan HQ, Lemaux PG.. 2009. Efficient, reproducible Agrobacterium-mediated transformation of sorghum using heat treatment of immature embryos. Plant Cell Reports 28, 429–444. [DOI] [PubMed] [Google Scholar]
- Hadebe ST, Modi AT, Mabhaudhi T.. 2017. Drought tolerance and water use of cereal crops: a focus on sorghum as a food security crop in sub-saharan Africa. Journal of Agronomy and Crop Science 203, 177–191. [Google Scholar]
- Hamada H, Linghu Q, Nagira Y, Miki R, Taoka N, Imai R.. 2017. An in planta biolistic method for stable wheat transformation. Scientific Reports 7, 11443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Broughton S, Liu L, Zhang XQ, Zeng J, He X, Li C.. 2021. Highly efficient and genotype-independent barley gene editing based on anther culture. Plant Communications 2, 100082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlan JR, Wet JMJ.. 1972. A simplified classification of cultivated sorghum. Crop Science 12, 172–176. [Google Scholar]
- Hawkins C, Ginzburg D, Zhao K, et al. 2021. Plant Metabolic Network 15: a resource of genome-wide metabolism databases for 126 plants and algae. Journal of Integrative Plant Biology doi: 10.1111/jipb.13163. [DOI] [PubMed] [Google Scholar]
- Hayta S, Smedley MA, Demir SU, Blundell R, Hinchliffe A, Atkinson N, Harwood WA.. 2019. An efficient and reproducible Agrobacterium-mediated transformation method for hexaploid wheat (Triticum aestivum L.). Plant Methods 15, 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyer WD, Ehmsen KT, Liu J.. 2010. Regulation of homologous recombination in eukaryotes. Annual Review of Genetics 44, 113–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiei Y, Komari T.. 2008. Agrobacterium-mediated transformation of rice using immature embryos or calli induced from mature seed. Nature Protocols 3, 824–834. [DOI] [PubMed] [Google Scholar]
- Hiei Y, Ohta S, Komari T, Kumashiro T.. 1994. Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. The Plant Journal 6, 271–282. [DOI] [PubMed] [Google Scholar]
- Howe A, Sato S, Dweikat I, Fromm M, Clemente T.. 2006. Rapid and reproducible Agrobacterium-mediated transformation of sorghum. Plant Cell Reports 25, 784–791. [DOI] [PubMed] [Google Scholar]
- Huang R. 2018. Research progress on plant tolerance to soil salinity and alkalinity in sorghum. Journal of Integrative Agriculture 17, 739–746. [Google Scholar]
- Ioelovich M. 2008. Cellulose as a nanostructured polymer: a short review. Bioresources 3, 1403–1418. [Google Scholar]
- Ishida Y, Saito H, Ohta S, Hiei Y, Komari T, Kumashiro T.. 1996. High efficiency transformation of maize (Zea mays L.) mediated by Agrobacterium tumefaciens. Nature Biotechnology 14, 745–750. [DOI] [PubMed] [Google Scholar]
- Jeoung JM, Krishnaveni S, Muthukrishnan S, Trick HN, Liang GH.. 2002. Optimization of sorghum transformation parameters using genes for green fluorescent protein and beta-glucuronidase as visual markers. Hereditas 137, 20–28. [DOI] [PubMed] [Google Scholar]
- Jiang W, Zhou H, Bi H, Fromm M, Yang B, Weeks DP.. 2013. Demonstration of CRISPR/Cas9/sgRNA-mediated targeted gene modification in Arabidopsis, tobacco, sorghum and rice. Nucleic Acids Research 41, e188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y, Burke J, Chopra R, Burow G, Chen J, Wang B, Hayes C, Emendack Y, Ware D, Xin Z.. 2016. A Sorghum mutant resource as an efficient platform for gene discovery in grasses. The Plant Cell 28, 1551–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang BC, Yun JY, Kim ST, Shin Y, Ryu J, Choi M, Woo JW, Kim JS.. 2018. Precision genome engineering through adenine base editing in plants. Nature Plants 4, 427–431. [DOI] [PubMed] [Google Scholar]
- Karlen SD, Zhang C, Peck ML, et al. 2016. Monolignol ferulate conjugates are naturally incorporated into plant lignins. Science Advances 2, e1600393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke L, Liu R, Chu B, et al. 2012. Cell suspension culture-mediated incorporation of the rice bel gene into transgenic cotton. PLoS One 7, e39974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komor AC, Kim YB, Packer MS, Zuris JA, Liu DR.. 2016. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 533, 420–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempinski C, Jiang Z, Zinck G, Sato SJ, Ge Z, Clemente TE, Chappell J.. 2019. Engineering linear, branched-chain triterpene metabolism in monocots. Plant Biotechnology Journal 17, 373–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohli A, Twyman RM, Abranches R, Wegel E, Stoger E, Christou P.. 2003. Transgene integration, organization and interaction in plants. Plant Molecular Biology 52, 247–258. [DOI] [PubMed] [Google Scholar]
- Komari T, Takakura Y, Ueki J, Kato N, Ishida Y, Hiei Y.. 2006. Binary vectors and super-binary vectors. Methods in Molecular Biology 343, 15–41. [DOI] [PubMed] [Google Scholar]
- Kumar S, Dhingra A, Daniell H.. 2004. Plastid-expressed betaine aldehyde dehydrogenase gene in carrot cultured cells, roots, and leaves confers enhanced salt tolerance. Plant Physiology 136, 2843–2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar T, Dweikat I, Sato S, et al. 2012. Modulation of kernel storage proteins in grain sorghum (Sorghum bicolor (L.) Moench). Plant Biotechnology Journal 10, 533–544. [DOI] [PubMed] [Google Scholar]
- Kumar V, Campbell LM, Rathore KS.. 2011. Rapid recovery- and characterization of transformants following Agrobacterium-mediated T-DNA transfer to sorghum. Plant Cell, Tissue and Organ Culture 104, 137–146. [Google Scholar]
- Kuriyama T, Shimada S, Matsui M.. 2019. Improvement of Agrobacterium-mediated transformation for tannin-producing sorghum. Plant Biotechnology 36, 43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamont KC, Mudge SR, Liu G, Godwin ID.. 2017. Expression patterns of the native Shrunken-2 promoter in Sorghum bicolor visualised through use of the GFP reporter gene. Plant Cell Reports 36, 1689–1700. [DOI] [PubMed] [Google Scholar]
- Laursen T, Borch J, Knudsen C, et al. 2016. Characterization of a dynamic metabolon producing the defense compound dhurrin in sorghum. Science 354, 890–893. [DOI] [PubMed] [Google Scholar]
- Li A, Jia S, Yobi A, Ge Z, Sato SJ, Zhang C, Angelovici R, Clemente TE, Holding DR.. 2018. Editing of an alpha-kafirin gene family increases, digestibility and protein quality in sorghum. Plant Physiology 177, 1425–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Zong Y, Wang Y, Jin S, Zhang D, Song Q, Zhang R, Gao C.. 2018. Expanded base editing in rice and wheat using a Cas9–adenosine deaminase fusion. Genome Biology 19, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Tang N, Fang Z, Xia Y, Cao M.. 2016. Co-transfer of TALENs construct targeted for chloroplast genome and chloroplast transformation vector into rice using particle bombardment. Journal of Nanoscience and Nanotechnology 16, 12194–12201. [Google Scholar]
- Li Y, Zhu J, Wu H, Liu C, Huang C, Lan J, Zhao Y, Xie C.. 2020. Precise base editing of non-allelic acetolactate synthase genes confers sulfonylurea herbicide resistance in maize. The Crop Journal 8, 449–456. [Google Scholar]
- Liang Y, Eudes A, Yogiswara S, et al. 2019. A screening method to identify efficient sgRNAs in Arabidopsis, used in conjunction with cell-specific lignin reduction. Biotechnology for Biofuels 12, 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Z, Chen K, Li T, et al. 2017. Efficient DNA-free genome editing of bread wheat using CRISPR/Cas9 ribonucleoprotein complexes. Nature Communications 8, 14261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipkie TE, De Moura FF, Zhao Z-Y, Albertsen MC, Che P, Glassman K, Ferruzzi MG.. 2013. Bioaccessibility of carotenoids from transgenic provitamin A biofortified sorghum. Journal of Agricultural and Food Chemistry 61, 5764–5771. [DOI] [PubMed] [Google Scholar]
- Liu G, Gilding EK, Godwin ID.. 2013. Additive effects of three auxins and copper on sorghum in vitro root induction. In Vitro Cellular & Developmental Biology. Plant 49, 191–197. [Google Scholar]
- Liu G, Gilding EK, Godwin ID.. 2015. A robust tissue culture system for sorghum [Sorghum bicolor (L.) Moench]. South African Journal of Botany 98, 157–160. [Google Scholar]
- Liu G, Godwin ID.. 2012. Highly efficient sorghum transformation. Plant Cell Reports 31, 999–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Lamont KC, Ahmad N, Tomkins A, Mudge SR, Gilding EK, Godwin ID.. 2017. The functionality of α-kafirin promoter and α-kafirin signal peptide. Plant Cell, Tissue and Organ Culture 128, 133–143. [Google Scholar]
- Liu G, Li J, Godwin ID.. 2019. Genome editing by CRISPR/Cas9 in sorghum through biolistic bombardment. Methods in Molecular Biology 1931, 169–183. [DOI] [PubMed] [Google Scholar]
- Liu J, Nannas NJ, Fu FF, Shi J, Aspinwall B, Parrott WA, Dawe RK.. 2019. Genome-scale sequence disruption following biolistic transformation in rice and maize. The Plant Cell 31, 368–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Brost J, Hutcheon C, et al. 2012. Transformation of the oilseed crop Camelina sativa by Agrobacterium-mediated floral dip and simple large-scale screening of transformants. In Vitro Cellular and Devepmental Biology. Plant 48, 462–468. [Google Scholar]
- Lowe K, La Rota M, Hoerster G, Hastings C, Wang N, Chamberlin M, Wu E, Jones T, Gordon-Kamm W.. 2018. Rapid genotype ‘independent’ Zea mays L. (maize) transformation via direct somatic embryogenesis. In Vitro Cellular & Developmental Biology. Plant 54, 240–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe K, Wu E, Wang N, et al. 2016. Morphogenic regulators Baby boom and Wuschel improve monocot transformation. The Plant Cell 28, 1998–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Wu X, Yin X, Morrand J, Chen X, Folk WR, Zhang ZJ.. 2009. Development of marker-free transgenic sorghum [Sorghum bicolor (L.) Moench] using standard binary vectors with bar as a selectable marker. Plant Cell, Tissue and Organ Culture 99, 97–108. [Google Scholar]
- Lu Y, Rijzaani H, Karcher D, Ruf S, Bock R.. 2013. Efficient metabolic pathway engineering in transgenic tobacco and tomato plastids with synthetic multigene operons. Proceedings of the National Academy of Sciences, USA 110, E623–E632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H, Zhao W, Wang Y, et al. 2016. SorGSD: a sorghum genome SNP database. Biotechnology for Biofuels 9, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Liu J, Wang X.. 2013. Agrobacterium-mediated transformation of cotton (Gossypium hirsutum) shoot apex with a fungal phytase gene improves phosphorus acquisition. Methods in Molecular Biology 958, 211–222. [DOI] [PubMed] [Google Scholar]
- Magomere T, Obukosia S, Albertsen M, et al. 2016. Evaluation of fitness in F2 generations of Africa Biofortified Sorghum event 188 and weedy Sorghum bicolor ssp. drummondii. Electronic Journal of Biotechnology 22, 52–61. [Google Scholar]
- Maher MF, Nasti RA, Vollbrecht M, Starker CG, Clark MD, Voytas DF.. 2020. Plant gene editing through de novo induction of meristems. Nature Biotechnology 38, 84–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maheswari M, Jyothi Lakshmi N, Yadav SK, Varalaxmi Y, Vijaya Lakshmi A, Vanaja M, Venkateswarlu B.. 2006. Efficient plant regeneration from shoot apices of sorghum. Biologia Plantarum 50, 741–744. [Google Scholar]
- Mall TK, Dweikat I, Sato SJ, Neresian N, Xu K, Ge Z, Wang D, Elthon T, Clemente T.. 2011. Expression of the rice CDPK-7 in sorghum: molecular and phenotypic analyses. Plant Molecular Biology 75, 467–479. [DOI] [PubMed] [Google Scholar]
- Mano Y, Komatsuda T.. 2002. Identification of QTLs controlling tissue-culture traits in barley (Hordeum vulgare L.). Theoretical and Applied Genetics 105, 708–715. [DOI] [PubMed] [Google Scholar]
- Martinez Hernandez E, Cui X, Scown CD, Amezcua Allieri MA, Aburto J, Simmons BA.. 2019. Techno economic and greenhouse gas analyses of lignin valorization to eugenol and phenolic products in integrated ethanol biorefineries. Biofuels, Bioproducts & Biorefining 13, 978–993. [Google Scholar]
- Martins PK, Nakayama TJ, Ribeiro AP, Cunha BADBD, Nepomuceno AL, Harmon FG, Kobayashi AK, Molinari HBC.. 2015. Setaria viridis floral-dip: a simple and rapid Agrobacterium-mediated transformation method. Biotechnology Reports 6, 61–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick RF, Truong SK, Sreedasyam A, et al. 2018. The Sorghum bicolor reference genome: improved assembly, gene annotations, a transcriptome atlas, and signatures of genome organization. The Plant Journal 93, 338–354. [DOI] [PubMed] [Google Scholar]
- McHughen A, Wager R.. 2010. Popular misconceptions: agricultural biotechnology. New Biotechnology 27, 724–728. [DOI] [PubMed] [Google Scholar]
- Mendes JF, Norcino LB, Martins HHA, Manrich A, Otoni CG, Carvalho EEN, Piccoli RH, Oliveira JE, Pinheiro ACM, Mattoso LHC.. 2020. Correlating emulsion characteristics with the properties of active starch films loaded with lemongrass essential oil. Food Hydrocolloids 100, 105428. [Google Scholar]
- Miedes E, Vanholme R, Boerjan W, Molina A.. 2014. The role of the secondary cell wall in plant resistance to pathogens. Frontiers in Plant Science 5, 358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mockler T. 2016. A complete-sequence population for pan-genome analysis of Sorghum. https://www.osti.gov/dataexplorer/biblio/dataset/1488180. [Google Scholar]
- Mookkan M, Nelson-Vasilchik K, Hague J, Zhang ZJ, Kausch AP.. 2017. Selectable marker independent transformation of recalcitrant maize inbred B73 and sorghum P898012 mediated by morphogenic regulators BABY BOOM and WUSCHEL2. Plant Cell Reports 36, 1477–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris GP, Ramu P, Deshpande SP, et al. 2013. Population genomic and genome-wide association studies of agroclimatic traits in sorghum. Proceedings of the National Academy of Sciences, USA 110, 453–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottiar Y, Vanholme R, Boerjan W, Ralph J, Mansfield SD.. 2016. Designer lignins: harnessing the plasticity of lignification. Current Opinion in Biotechnology 37, 190–200. [DOI] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine. 2016. Genetically engineered crops: experiences and prospects. Washington, DC: The National Academies Press. [PubMed] [Google Scholar]
- Nelson-Vasilchik K, Hague J, Mookkan M, Zhang ZJ, Kausch A.. 2018. Transformation of recalcitrant sorghum varieties facilitated by baby boom and wuschel2. Current Protocols in Plant Biology 3, e20076. [DOI] [PubMed] [Google Scholar]
- Nguyen CT, Singh V, van Oosterom EJ, Chapman SC, Jordan DR, Hammer GL.. 2013. Genetic variability in high temperature effects on seed-set in sorghum. Functional Plant Biology 40, 439–448. [DOI] [PubMed] [Google Scholar]
- Nguyen T-V, Thanh Thu T, Claeys M, Angenon G.. 2007. Agrobacterium-mediated transformation of sorghum (Sorghum bicolor (L.) Moench) using an improved invitro regeneration system. Plant Cell, Tissue and Organ Culture 91, 155–164. [Google Scholar]
- Olatoye MO, Hu Z, Maina F, Morris GP.. 2018. Genomic signatures of adaptation to a precipitation gradient in Nigerian sorghum. G3 8, 3269–3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ondzighi-Assoume CA, Willis JD, Ouma WK, Allen SM, King Z, Parrott WA, Liu W, Burris JN, Lenaghan SC, Stewart CN Jr. 2019. Embryogenic cell suspensions for high-capacity genetic transformation and regeneration of switchgrass (Panicum virgatum L.). Biotechnology for Biofuels 12, 290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou-Lee T-M, Turgeon R, Wutt R.. 1986. Expression of a foreign gene linked to either a plant-virus or a Drosophila promoter, after electroporation of protoplasts of rice, wheat, and sorghum. . Proceedings of the National Academy of Sciences, USA 83, 6815–6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Z, Baerson SR, Wang M, et al. 2018. A cytochrome P450 CYP71 enzyme expressed in Sorghum bicolor root hair cells participates in the biosynthesis of the benzoquinone allelochemical sorgoleone. New Phytologist 218, 616–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papapetrou EP, Schambach A.. 2016. Gene insertion into genomic safe harbors for human gene therapy. Molecular Therapy 24, 678–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson AH, Bowers JE, Bruggmann R, et al. 2009. The Sorghum bicolor genome and the diversification of grasses. Nature 457, 551–556. [DOI] [PubMed] [Google Scholar]
- Peña PA, Quach T, Sato S, Ge Z, Nersesian N, Changa T, Dweikat I, Soundararajan M, Clemente TE.. 2017a. Expression of the maize dof1 transcription factor in wheat and sorghum. Frontiers in Plant Science 8, 434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña PA, Quach T, Sato S, Ge Z, Nersesian N, Dweikat IM, Soundararajan M, Clemente T.. 2017b. Molecular and phenotypic characterization of transgenic wheat and sorghum events expressing the barley alanine aminotransferase. Planta 246, 1097–1107. [DOI] [PubMed] [Google Scholar]
- Polko JK, Kieber JJ.. 2019. The regulation of cellulose biosynthesis in plants. The Plant Cell 31, 282–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter KS, Axtell JD, Lechtenberg VL, Colenbrander VF.. 1978. Phenotype, fiber composition, and in vitro dry matter disappearance of chemically induced brown midrib (bmr) mutants of Sorghum. Crop Science 18, 205. [Google Scholar]
- Promkhambu A, Younger A, Polthanee A, Akkasaeng C.. 2010. Morphological and physiological responses of sorghum (Sorghum bicolor L. Moench) to waterlogging. Asian Journal of Plant Sciences 9, 183–193. [Google Scholar]
- Raghuwanshi A, Birch RG.. 2010. Genetic transformation of sweet sorghum. Plant Cell Reports 29, 997–1005. [DOI] [PubMed] [Google Scholar]
- Reddy N, Yang Y.. 2005. Biofibers from agricultural byproducts for industrial applications. Trends in Biotechnology 23, 22–27. [DOI] [PubMed] [Google Scholar]
- Rooney WL, Blumenthal J, Bean B, Mullet JE.. 2007. Designing sorghum as a dedicated bioenergy feedstock. Biofuels, Bioproducts & Biorefining 1, 147–157. [Google Scholar]
- Rosenow DT, Quisenberry JE, Wendt CW, Clark LE.. 1983. Drought tolerant sorghum and cotton germplasm. Agricultural Water Management 7, 207–222. [Google Scholar]
- Salvo S, Cook J, Carlson AR, Hirsch CN, Kaeppler SM, Kaeppler HF.. 2018. Genetic fine-mapping of a quantitative trait locus (QTL) associated with embryogenic tissue culture response and plant regeneration ability in maize (Zea mays L.). The Plant Genome 11, 170111. [DOI] [PubMed] [Google Scholar]
- Sanford JC, Klein TM, Wolf ED, Allen N.. 1987. Delivery of substances into cells and tissues using a particle bombardment process. Particulate Science and Technology 5, 27–37. [Google Scholar]
- Saski C, Lee SB, Fjellheim S, Guda C, Jansen RK, Luo H, Tomkins J, Rognli OA, Daniell H, Clarke JL.. 2007. Complete chloroplast genome sequences of Hordeum vulgare, Sorghum bicolor and Agrostis stolonifera, and comparative analyses with other grass genomes. Theoretical and Applied Genetics 115, 591. [DOI] [PubMed] [Google Scholar]
- Satish L, Shilpha J, Pandian S, Rency AS, Rathinapriya P, Ceasar SA, Largia MJ, Kumar AA, Ramesh M.. 2016. Analysis of genetic variation in sorghum (Sorghum bicolor (L.) Moench) genotypes with various agronomical traits using SPAR methods. Gene 576, 581–585. [DOI] [PubMed] [Google Scholar]
- Sato S, Clemente T, Dweikat I.. 2004. Identification of an elite sorghum genotype with high in vitro performance capacity. In Vitro Cellular & Developmental Biology. Plant 40, 57–60. [Google Scholar]
- Sato-Izawa K, Tokue K, Ezura H.. 2018. Development of a stable Agrobacterium-mediated transformation protocol for Sorghum bicolor Tx430. Plant Biotechnology 35, 181–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schläpfer P, Zhang P, Wang C, et al. 2017. Genome-wide prediction of metabolic enzymes, pathways, and gene clusters in plants. Plant Physiology 173, 2041–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M, Bothma G.. 2006. Risk assessment for transgenic sorghum in Africa: crop-to-crop gene flow in Sorghum bicolor (L.) Moench. Crop Science 46, 790. [Google Scholar]
- Schnippenkoetter W, Lo C, Liu G, et al. 2017. The wheat Lr34 multipathogen resistance gene confers resistance to anthracnose and rust in sorghum. Plant Biotechnology Journal 15, 1387–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scully ED, Gries T, Sarath G, et al. 2016. Overexpression of SbMyb60 impacts phenylpropanoid biosynthesis and alters secondary cell wall composition in Sorghum bicolor. The Plant Journal 85, 378–395. [DOI] [PubMed] [Google Scholar]
- Sehaqui H, Liu A, Zhou Q, Berglund LA.. 2010. Fast preparation procedure for large, flat cellulose and cellulose/inorganic nanopaper structures. Biomacromolecules 11, 2195–2198. [DOI] [PubMed] [Google Scholar]
- Sharma R, Liang Y, Lee MY, Pidatala VR, Mortimer JC, Scheller HV.. 2020. Agrobacterium-mediated transient transformation of sorghum leaves for accelerating functional genomics and genome editing studies. BMC Research Notes 13, 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva LS, Jung R, Zhao Z, Glassman K, Taylor J, Taylor JRN.. 2011. Effect of suppressing the synthesis of different kafirin sub-classes on grain endosperm texture, protein body structure and protein nutritional quality in improved sorghum lines. Journal of Cereal Science 54, 160–167. [Google Scholar]
- Silva TN, Kelly ME, Vermerris W.. 2020. Use of Sorghum bicolor leaf whorl explants to expedite regeneration and increase transformation throughput. Plant Cell, Tissue and Organ Culture 141, 243–255. [Google Scholar]
- Silva TN, Vermerris W.. 2020. High-biomass sorghums as a feedstock for renewable fuels and chemicals. In: Tonapi VA, Talwar HS, Are AK, Bhat BV, Reddy CR, Dalton TJ, eds. Sorghum in the 21st century: food – fodder – feed – fuel for a rapidly changing world. Singapore: Springer Singapore, 723–754. [Google Scholar]
- Singh S, Tan HQ, Singh J.. 2012. Mutagenesis of barley malting quality QTLs with Ds transposons. Functional & Integrative Genomics 12, 131–141. [DOI] [PubMed] [Google Scholar]
- Smith CW, Frederiksen RA.. 2000. Sorghum: origin, history, technology and production. New York: J. Wiley. [Google Scholar]
- Snider JL, Raper RL, Schwab EB.. 2012. The effect of row spacing and seeding rate on biomass production and plant stand characteristics of non-irrigated photoperiod-sensitive sorghum (Sorghum bicolor (L.) Moench). Industrial Crops and Products 37, 527–535. [Google Scholar]
- Spicer A, Molnar A.. 2018. Gene editing of microalgae: scientific progress and regulatory challenges in Europe. Biology 7, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamenković OS, Siliveru K, Veljković VB, Banković-Ilić IB, Tasić MB, Ciampitti IA, Đalović IG, Mitrović PM, Sikora VŠ, Prasad PVV.. 2020. Production of biofuels from sorghum. Renewable and Sustainable Energy Reviews 124, 109769. [Google Scholar]
- Svab Z, Hajdukiewicz P, Maliga P.. 1990. Stable transformation of plastids in higher plants. Proceedings of the National Academy of Sciences, USA 87, 8526–8530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svab Z, Maliga P.. 1993. High-frequency plastid transformation in tobacco by selection for a chimeric aadA gene. Proceedings of the National Academy of Sciences, USA 90, 913–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svitashev S, Schwartz C, Lenderts B, Young JK, Mark Cigan A.. 2016. Genome editing in maize directed by CRISPR–Cas9 ribonucleoprotein complexes. Nature Communications 7, 13274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadesse Y, Sági L, Swennen R, Jacobs M.. 2003. Optimisation of transformation conditions and production of transgenic sorghum (Sorghum bicolor) via microparticle bombardment. Plant Cell, Tissue and Organ Culture 75, 1–18. [Google Scholar]
- Tao Y, Luo H, Xu J, et al. 2021. Extensive variation within the pan-genome of cultivated and wild sorghum. Nature Plants 7, 766–773. [DOI] [PubMed] [Google Scholar]
- Taparia Y, Fouad WM, Gallo M, Altpeter F.. 2012. Rapid production of transgenic sugarcane with the introduction of simple loci following biolistic transfer of a minimal expression cassette and direct embryogenesis. In Vitro Cellular & Developmental Biology. Plant 48, 15–22. [Google Scholar]
- Tello-Ruiz MK, Naithani S, Stein JC, et al. 2018. Gramene 2018: unifying comparative genomics and pathway resources for plant research. Nucleic Acids Research 46, D1181–D1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ten E, Ling C, Wang Y, Srivastava A, Dempere LA, Vermerris W.. 2014. Lignin nanotubes as vehicles for gene delivery into human cells. Biomacromolecules 15, 327–338. [DOI] [PubMed] [Google Scholar]
- Tian T, You Q, Zhang L, Yi X, Yan H, Xu W, Su Z.. 2016. SorghumFDB: sorghum functional genomics database with multidimensional network analysis. Database 2016, baw099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Energy. 2016. 2016 Billion-ton report: advancing domestic resources for a thriving bioeconomy, Volume 1: Economic availability of feedstock. In: Langholtz MH, Stokes BJ, Eaton LM (Leads), ORNL/TM-2016/160. Oak Ridge National Laboratory, Oak Ridge, TN. p. 448. doi: 10.2172/1271651. http://www.energy.gov/eere/bioenergy/2016-billion-ton-report. [DOI] [Google Scholar]
- Vanhercke T, Belide S, Taylor MC, et al. 2019. Up-regulation of lipid biosynthesis increases the oil content in leaves of Sorghum bicolor. Plant Biotechnology Journal 17, 220–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varoquaux N, Cole B, Gao C, et al. 2019. Transcriptomic analysis of field-droughted sorghum from seedling to maturity reveals biotic and metabolic responses. Proceedings of the National Academy of Sciences, USA 116, 27124–27132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ved K, Padam K.. 2013. Study of physical and chemical properties of biodiesel from sorghum oil. Research Journal of Chemical Sciences 3, 64–68. [Google Scholar]
- Venuto B, Kindiger B.. 2008. Forage and biomass feedstock production from hybrid forage sorghum and sorghum–sudangrass hybrids. Grassland Science 54, 189–196. [Google Scholar]
- Visarada KBRS, Prasad GS, Royer M.. 2016. Genetic transformation and evaluation of two sweet sorghum genotypes for resistance to spotted stemborer, Chilo partellus (Swinhoe). Plant Biotechnology Reports 10, 277–289. [Google Scholar]
- Visarada KBRS, Padmaja PG, Saikishore N, Pashupatinath E, Royer M, Seetharama N, Patil JV.. 2014. Production and evaluation of transgenic sorghum for resistance to stem borer. In Vitro Cellular & Developmental Biology. Plant 50, 176–189. [Google Scholar]
- Waltz E. 2018. With a free pass, CRISPR-edited plants reach market in record time. Nature Biotechnology 36, 6–7. [DOI] [PubMed] [Google Scholar]
- Wang W, Wang J, Yang C, Li Y, Liu L, Xu J.. 2007. Pollen-mediated transformation of Sorghum bicolor plants. Biotechnology and Applied Biochemistry 48, 79–83. [DOI] [PubMed] [Google Scholar]
- Wannasek L, Ortner M, Amon B, Amon T.. 2017. Sorghum, a sustainable feedstock for biogas production? Impact of climate, variety and harvesting time on maturity and biomass yield. Biomass and Bioenergy 106, 137–145. [Google Scholar]
- Wilkerson CG, Mansfield SD, Lu F, et al. 2014. Monolignol ferulate transferase introduces chemically labile linkages into the lignin backbone. Science 344, 90–93. [DOI] [PubMed] [Google Scholar]
- Wolt JD. 2017. Safety, security, and policy considerations for plant genome editing. Progress in Molecular Biology and Translational Science 149, 215–241. [DOI] [PubMed] [Google Scholar]
- Woo JW, Kim J, Kwon SI, Corvalán C, Cho SW, Kim H, Kim SG, Kim ST, Choe S, Kim JS.. 2015. DNA-free genome editing in plants with preassembled CRISPR–Cas9 ribonucleoproteins. Nature Biotechnology 33, 1162–1164. [DOI] [PubMed] [Google Scholar]
- Wu E, Lenderts B, Glassman K, Berezowska-Kaniewska M, Christensen H, Asmus T, Zhen S, Chu U, Cho MJ, Zhao ZY.. 2014. Optimized Agrobacterium-mediated sorghum transformation protocol and molecular data of transgenic sorghum plants. In Vitro Cellular & Developmental Biology. Plant 50, 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Dutta T, Varman AM, Eudes A, Manalansan B, Loqué D, Singh S.. 2017. Lignin valorization: two hybrid biochemical routes for the conversion of polymeric lignin into value-added chemicals. Scientific Reports 7, 8420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin Z, Wang ML, Barkley NA, Burow G, Franks C, Pederson G, Burke J.. 2008. Applying genotyping (TILLING) and phenotyping analyses to elucidate gene function in a chemically induced sorghum mutant population. BMC Plant Biology 8, 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Li J, Moore C, Xin Z, Wang D.. 2018. Physico-chemical characterization of pedigreed sorghum mutant stalks for biofuel production. Industrial Crops and Products 124, 806–811. [Google Scholar]
- Yamaguchi M, Fujimoto H, Hirano K, et al. 2016. Sorghum Dw1, an agronomically important gene for lodging resistance, encodes a novel protein involved in cell proliferation. Scientific Reports 6, 28366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Aznar A, Chalvin C, Birdseye DS, Baidoo EEK, Eudes A, Shih PM, Loqué D, Zhang A, Scheller HV.. 2018. Increased drought tolerance in plants engineered for low lignin and low xylan content. Biotechnology for Biofuels 11, 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Baral NR, Simmons BA, Mortimer JC, Shih PM, Scown CD.. 2020. Accumulation of high-value bioproducts in planta can improve the economics of advanced biofuels. Proceedings of the National Academy of Sciences, USA 117, 8639–8648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Dahlberg J, Baral NR, Putnam D, Scown CD.. 2021. Identifying forage sorghum ideotypes for advanced biorefineries. ACS Sustainable Chemistry & Engineering 9, 7873–7881. [Google Scholar]
- Yang Z, Ohlrogge JB.. 2009. Turnover of fatty acids during natural senescence of Arabidopsis, Brachypodium, and switchgrass and in Arabidopsis beta-oxidation mutants. Plant Physiology 150, 1981–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Q, Cong L, Chang JL, Li KX, Yang GX, He GY.. 2006. Low copy number gene transfer and stable expression in a commercial wheat cultivar via particle bombardment. Journal of Experimental Botany 57, 3737–3746. [DOI] [PubMed] [Google Scholar]
- Yellisetty V, Reddy LA, Mandapaka M.. 2015. In planta transformation of sorghum (Sorghum bicolor (L.) Moench) using TPS1 gene for enhancing tolerance to abiotic stresses. Journal of Genetics 94, 425–434. [DOI] [PubMed] [Google Scholar]
- Zetsche B, Gootenberg JS, Abudayyeh OO, et al. 2015. Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR–Cas system. Cell 163, 759–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Hussain A, Manghwar H, Xie K, Xie S, Zhao S, Larkin RM, Qing P, Jin S, Ding F.. 2020. Genome editing with the CRISPR–Cas system: an art, ethics and global regulatory perspective. Plant Biotechnology Journal 18, 1651–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Liu J, Chai Z, Chen S, Bai Y, Zong Y, Chen K, Li J, Jiang L, Gao C.. 2019. Generation of herbicide tolerance traits and a new selectable marker in wheat using base editing. Nature Plants 5, 480–485. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Liang Z, Zong Y, Wang Y, Liu J, Chen K, Qiu JL, Gao C.. 2016. Efficient and transgene-free genome editing in wheat through transient expression of CRISPR/Cas9 DNA or RNA. Nature Communications 7, 12617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao ZY, Cai T, Tagliani L, et al. 2000. Agrobacterium-mediated sorghum transformation. Plant Molecular Biology 44, 789–798. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Pierce A, Wagner WL, Scheller HV, Mohnen D, Ackermann M, Mentzer SJ.. 2020. Water-dependent blending of pectin films: the mechanics of conjoined biopolymers. Molecules 25, 2108. [DOI] [PMC free article] [PubMed] [Google Scholar]