Abstract
The development of secondary vascular tissue enhances the transport capacity and mechanical strength of plant bodies, while contributing a huge proportion of the world’s biomass in the form of wood. Cell divisions in the cambium, which constitutes the vascular meristem, provide progenitors from which conductive xylem and phloem are derived. The cambium is a somewhat unusual stem cell population in two respects, making it an interesting subject for developmental research. Firstly, it arises post-germination, and thus represents a model for understanding stem cell initiation beyond embryogenesis. Secondly, xylem and phloem differentiate on opposing sides of cambial stem cells, making them bifacial in nature. Recent discoveries in Arabidopsis thaliana have provided insight into the molecular mechanisms that regulate the initiation, patterning, and maintenance of the cambium. In this review, the roles of intercellular signalling via mobile transcription factors, peptide–receptor modules, and phytohormones are described. Crosstalk between these regulatory pathways is becoming increasingly apparent, yet the underlying mechanisms are not fully understood. Future study of the interaction between multiple independently identified regulators, as well as the functions of their orthologues in trees, will deepen our understanding of radial growth in plants.
Keywords: Arabidopsis, auxin, cambium, cytokinin, phloem, procambium, stem cells, signalling, transcription factors, xylem
We review the literature describing the molecular mechanisms by which the vascular cambium is initiated and maintained in Arabidopsis.
Introduction
Plants exhibit an extraordinary capacity for growth and developmental plasticity, owing to their ability to maintain populations of continuously dividing stem cells in tissues called meristems. Two indeterminate meristems are established during embryogenesis. These are the root apical meristem (RAM), which gives rise to the subterranean root system, and the shoot apical meristem (SAM), from which all aerial tissues are derived. The elongation of plants along the apical–basal axis that results from RAM and SAM activity is termed primary growth. In addition, plants develop a variety of post-embryonic meristems, from which axillary branches, floral organs, and lateral roots are derived (Taiz and Zeiger, 2002). As the architectures of land plants (Embryophyta) became larger and more complex during the Devonian period ~400 million years ago, the development of specialized tissues for mechanical support and efficient long-distance transport of fluids became increasingly advantageous (Tonn and Greb, 2017). As a result, the ability to expand roots and stems along the radial axis (termed secondary growth) has evolved multiple times in the Embryophyte lineage, and can be observed in extant gymnosperms and the majority of dicotyledonous angiosperms (Spicer and Groover, 2010; Nieminen et al., 2015).
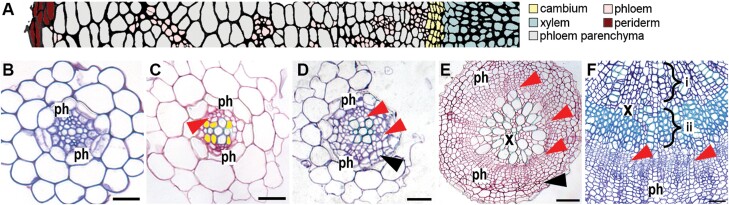
Secondary growth arises from tightly controlled cell divisions in post-embryonic meristems known as the vascular and cork cambia. The vascular cambium, which is the focus of this review, gives rise to a network of interconnected transport cells and their supporting tissues, which span the entire primary plant body and its lateral organs. Uniquely among plant meristems, the vascular cambium harbours a single, bifacial stem cell in each radial file, which divides periclinally (parallel to the surface of the organ) to drive the development of distinct specialized tissues on opposing sides (Bossinger and Spokevicius, 2018; Shi et al., 2019; Smetana et al., 2019). Stem cells in the vascular cambium give rise to xylem centripetally and phloem centrifugally. Together, these dividing cells and their undifferentiated daughters (xylem and phloem progenitors) form a ‘cambial zone’, which is especially visible in transverse sections of mature Arabidopsis hypocotyls (Fig. 1A).
Fig. 1.
Secondary growth in Arabidopsis root and hypocotyl. (A) Cell types and their distribution in the Arabidopsis hypocotyl. (B–E) Transverse sections of Arabidopsis roots at different stages of secondary development. Red arrowheads mark cambial cell divisions. (B) Primary diarch pattern, in 7-day-old root, characterized by a central row of xylem with two adjacent phloem poles. (C) Formation of xylem vessels adjacent to the central xylem axis and initial cambial cell divisions (14-day-old root). Differentiating secondary xylem (marked yellow) acts as a cambium organizer, promoting adjacent cells to divide, thus initiating secondary growth. (D) In 18-day-old root, cambial divisions surround the xylem. Divisions in the cork cambium are apparent (black arrows). Secondary growth pushes the vascular cylinder through the cortex and epidermis. The radial pattern is complete in a 30-day-old root (E). (F) Transverse section through a 36-day-old hypocotyl showing xylem formed during the proportional growth phase (i) and xylem expansion growth phase (ii). Scales are 20 µm in (B–D); 50 µm in (E) and (F). X, xylem; ph, phloem.
Xylem tissue is composed of conductive vessel elements that become hollow and vertically connected following programmed cell death (reviewed in Bollhöner et al., 2012). The resulting channel facilitates the transport of a continuous acropetal stream of water and dissolved minerals. Interspersed with vessel elements are dead xylem fibres and living parenchyma cells, the latter of which store starches, oils, and tanniniferous compounds (Esau, 1977). Vessel elements and fibres possess thick secondary cell walls of cellulose and hemicellulose, reinforced with lignin biopolymers to confer a high tensile strength. This allows xylem to mechanically support plant tissues as they elongate and withstand the negative pressures required for water transport (Taiz and Zeiger, 2002). On the opposing side of the vascular cambium, the phloem supports the bidirectional transport of sugars, proteins, amino acids, and other metabolites. Like xylem, phloem is composed of multiple specialized cell types, including conductive sieve elements (Fig. 1A). As phloem progenitors exit the cambial zone, organelles including the nucleus, vacuole, and cytoskeleton are degraded, and perforated sieve plates form to establish continuous connections between vertically adjoining cells (Furuta et al., 2014). Sieve elements have highly restricted metabolic activities and are thus supported by companion cells, to which they are connected via plasmodesmata. Other supporting cells include the phloem parenchyma and fibres, which fulfil storage roles and provide mechanical support, respectively (Esau, 1977). In addition to their roles in transporting photoassimilates and other nutrients, the importance of phloem highways for carrying long-distance RNA, phytohormone, and electrical signals is becoming increasingly apparent (Hilleary and Gilroy, 2018; Johns et al., 2021). This gives phloem tissue a central role in both plant growth and stress responses.
While the vascular cambium produces xylem and phloem, the outer cork cambium (also known as the phellogen) contributes to radial growth by producing protective tissue (Fig. 1A, D, E). In a layer known as the periderm, the cork cambium divides periclinally to produce phelloderm centripetally and phellem centrifugally. The dead cork cells that comprise the phellem have walls layered with suberin and lignin, making them difficult for insects and phytopathogens to penetrate (Wunderling et al., 2018; Campilho et al., 2020). Therefore, replacement of the epidermis with phellem in woody stems and roots protects the internal transport tissues from environmental stress by reducing water loss and susceptibility to biotic threats.
Arabidopsis thaliana as a secondary growth model
While secondary growth is evidently important for plant development, the position of cambial meristems deep within internal tissues has hampered efforts to study their activity. Nevertheless, advances in plant genetics, tissue processing, and microscopy have recently accelerated vascular development research (reviewed in Nieminen et al., 2015; Lehmann and Hardtke, 2016; Fischer et al., 2019; Wang, 2020). Despite its herbaceous nature (i.e. lack of a persistent woody stem), Arabidopsis exhibits secondary thickening of its roots, hypocotyls, and inflorescence stems. Comparative anatomical studies have revealed striking similarity between the concentric patterns of xylem–cambium–phloem in the hypocotyls of Arabidopsis, stems of angiosperm trees, and storage roots of numerous crop species (Chaffey et al., 2002; Campilho et al., 2020; Hoang et al., 2020b). However, notable differences include the inability of Arabidopsis to form annual growth rings and its lack of ‘ray’ systems, which facilitate the radial transport of resins and gums in mature trees (Esau, 1977; Chaffey et al., 2002). Even so, key genetic regulators of vascular development are conserved between Arabidopsis and its distant tree and root crop relatives (Wunderling et al., 2018; Hoang et al., 2020a, b), as discussed later in this review.
A robust primary pattern sets the stage
Before secondary growth commences in any plant, a precise primary vascular pattern is established. In Arabidopsis, the initiation of vascular stem cells and the specification of xylem and phloem in the embryo have been well characterized (reviewed in Miyashima et al., 2013; De Rybel et al., 2016). At the late globular stage of embryogenesis, vascular stem cells and surrounding pericycle tissue arise from the periclinal divisions of four preprocambial initials. Further division and differentiation of these cells during the subsequent heart stage give rise to a stereotypical radial pattern of cell types, which is mimicked in the post-embryonic root. This arrangement includes five or six xylem vessels in a single file, spanning the diameter of the stele (Baum et al., 2002). The central metaxylem has highly lignified, pitted cell walls, while their smaller protoxylem neighbours have spiral wall thickenings. Either side of these central xylem cells is a pool of procambium cells and a phloem pole containing sieve element precursors (Rodriguez-Villalon et al., 2014). Having two strands of protoxylem, this primary pattern is referred to as a diarch (Fig. 1B).
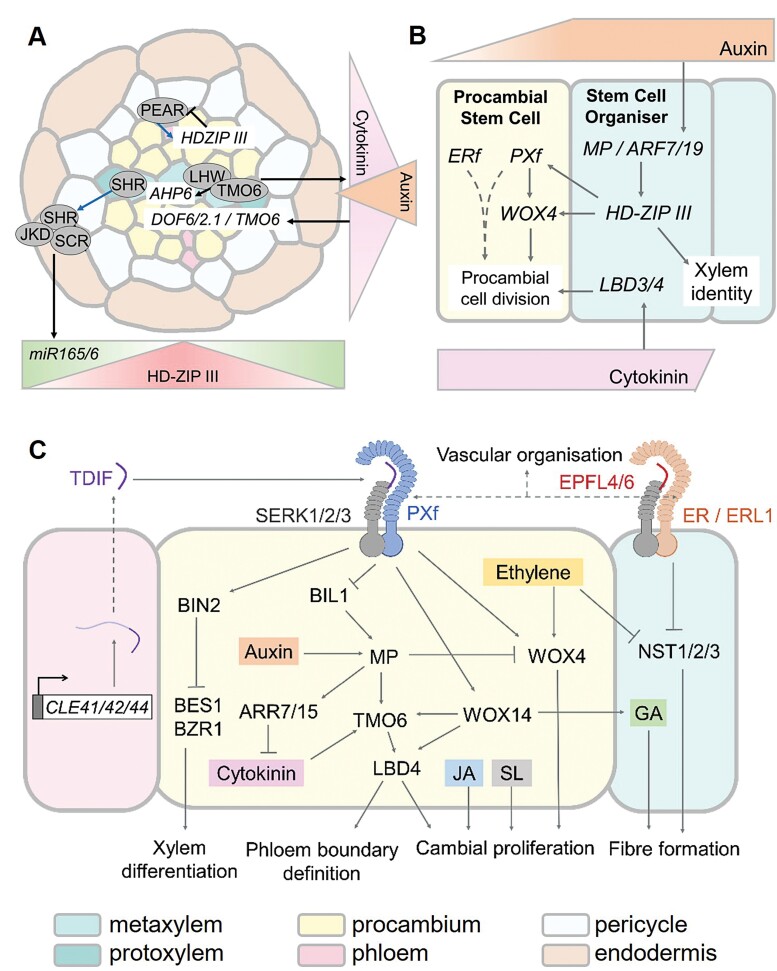
The formation of a diarch vascular cylinder is heavily dependent on intercellular signalling (Fig. 2A). Being one of the most important and extensively studied growth substances in plant development (Leyser, 2018), it is unsurprising that the phytohormone, auxin, has been implicated in this process. AUXIN RESPONSE FACTOR 5 (ARF5), also known as MONOPTEROS (MP), is released from its Aux/indole-3-acetic acid (IAA) inhibitor, BODENLOS (BDL), when cotyledon-derived auxin arrives at the vascular initials during embryogenesis (Weijers et al., 2006). MP is essential for early procambium formation, given that mp loss-of-function mutants were deficient in embryonic provascular cell divisions (Hardtke and Berleth, 1998). In addition, expression of a mutated, stabilized version of BDL conferred a rootless phenotype (Weijers et al., 2006), highlighting the importance of auxin signalling and MP function for root vascular development.
Fig. 2.
Mechanisms of secondary growth regulation in Arabidopsis. (A) Establishment of diarch vascular pattern in the primary root. (B) Initiation of secondary growth in roots, during which a stem cell organizer of xylem identity promotes division of neighbouring procambial cells. (C) Maintenance of the vascular cambium and organized development of secondary vasculature, involving integration of ligand–receptor pairs and phytohormone signalling. Phytohormones are represented by coloured boxes: JA, jasmonic acid; SL, strigolactone; GA, gibberellic acid. Triangles represent concentration gradients of phytohormones or gene products (A, B). Blue arrows represent protein movement in (A); black arrows show positive interactions; blunt lines show inhibition. Dashed lines represent unknown mechanism of interaction (B, C) or TDIF processing (C).
The cell to cell movement of transcription factors and miRNAs is known to play crucial roles in the establishment of root vascular patterns (De Rybel et al., 2016). The GRAS transcription factor SHORT ROOT (SHR) is expressed in the stele of developing roots and moves outwards to the endodermis, where it induces the expression of another GRAS protein, SCARECROW (SCR) (Nakajima et al., 2001). By binding SCR and the zinc finger BIRD transcription factors, JACKDAW (JKD) and BALDIBIS (BIB), SHR is sequestered in the nucleus of endodermal cells (Koizumi et al., 2012; Long et al., 2015, 2017). Here, SHR binds the promoters of MIR165 and MIR166, thereby inducing the expression of cell to cell diffusible miRNA, miR165/6 (Carlsbecker et al., 2010). The five vascular-expressed class III HOMEODOMAIN LEUCINE ZIPPER (HD-ZIP III) transcription factors, REVOLUTA (REV), PHABULOSA (PHB), PHAVOLUTA (PHV), ARABIDOPSIS THALIANA HOMEOBOX 8 (ATHB8), and CORONA (CNA/ATHB15), are known targets of miRNA165/6-mediated post-transcriptional silencing (Mallory et al., 2004; Smetana et al., 2019). Accordingly, HD-ZIP III protein concentrations across the stele exhibit a gradient opposing that of their miRNA inhibitors, with abundance peaking in the stele centre and decreasing towards the pericycle (Carlsbecker et al., 2010; Fig. 2A). phb-1d gain-of-function roots demonstrated ectopic development of metaxylem in place of protoxylem, while simultaneous knockout of four HD-ZIP III members triggered ectopic protoxylem marker gene expression in the centre of the xylem axis (Carlsbecker et al., 2010). Thus, in a wild-type root, it is predicted that high HD-ZIP III levels promote metaxylem differentiation, whereas low levels promote protoxylem differentiation. This mechanism of HD-ZIP III-mediated xylem patterning is predicted to be similarly reflected in the Arabidopsis embryo (De Rybel et al., 2016).
Beyond HD-ZIP IIIs, heterodimers of basic helix–loop–helix (bHLH) transcription factors were found to be necessary for the establishment of primary vasculature in Arabidopsis embryos (Schlereth et al., 2010). These heterodimers comprise LONESOME HIGHWAY (LHW), or LHW-LIKE 1 (LHL1) proteins, and MP-induced TARGET OF MONOPTEROS 5 (TMO5), or TMO5-LIKE (T5L) proteins (Schlereth et al., 2010; De Rybel et al., 2013). In a screen for mutants with compromised cell fate specification, lhw-deficient primary roots had a reduced number of vascular cells and displayed an abnormal monarch pattern with only one protoxylem strand (Ohashi-Ito and Bergmann, 2007). A similar phenotype was later observed in tmo5 t5l1 roots, and the formation of heterodimers between proteins of the LHW and TMO5 clades was confirmed in xylem precursor cells (De Rybel et al., 2013). In the embryo and primary root, the LWH–TMO5 module stabilizes growth and patterning of vascular tissue via regulation of cytokinin signalling. LHW–TMO5 up-regulates expression of cytokinin biosynthesis genes, LONELY GUY3 (LOG3) and LOG4, generating a cytokinin gradient that peaks in the xylem-adjacent procambium (De Rybel et al., 2014). Here, cytokinin promotes expression of the transcription factor gene, DNA-BINDING ONE FINGER2.1 (DOF2.1), which drives procambial cell division alongside its two homologues, TMO6 and DOF6 (Smet et al., 2019). At the same time, ARABIDOPSIS HISTIDINE PHOSPHOTRANSFER PROTEIN 6 (AHP6), a repressor of cytokinin signalling, is cell autonomously induced by LHW–TMO5 which act downstream of auxin signalling to maintain the primary xylem in a non-dividing state (Bishopp et al., 2011; Ohashi-Ito et al., 2014).
Recently, TMO6 and DOF6 were assigned to a group of cytokinin-inducible PHLOEM EARLY DOF (PEAR) proteins, which promote the division of protophloem sieve elements in young roots (Miyashima et al., 2019). PEARs move intercellularly and up-regulate the expression of HD-ZIP IIIs in neighbouring cells. In turn, these inhibit PEAR expression to form a negative feedback loop, inhibiting periclinal division in the phloem-adjacent internal region of the root (Miyashima et al., 2019). Overall, the interlinking of auxin and cytokinin signalling via HD-ZIP IIIs, LHW–TMO5, and DOF proteins facilitates the establishment of a robust primary vascular pattern. This is essential for defining domain boundaries for subsequent cell division.
Procambial cells divide at the onset of radial growth
Soon after the establishment of diarch vascular bundles, radial growth initiates in the procambium, and secondary xylem is formed opposite the phloem poles (Esau, 1977; Baum et al., 2002). Pioneering cell lineage tracing experiments in the Arabidopsis root have pin-pointed secondary growth initiation to the divisions of xylem-adjacent procambial cells that occur 5–6 d after germination (Smetana et al., 2019) and ~15–18mm from the root tip (Ye et al., 2021), although the exact timing of this transition is dependent on growth conditions (see, for example, Thamm et al., 2019; Fig. 1C, D). HD-ZIP III transcription factors are known to promote this early vascular proliferation. Expression of these genes, together with auxin signalling via MP, ARF7, and ARF19, defines a ‘stem cell organizer’ (yellow cells in Figs 1C and 2B) that is continually renewed as the procambium divides. In support of this model, suppression of HD-ZIP III function by chemical induction of MIR165, thus targeting HD-ZIP III transcripts for degradation, resulted in scattered cambial divisions, erratic xylem formation, and mitotic re-entry of previously quiescent xylem (Smetana et al., 2019). Following the initiation of procambial divisions, phloem-adjacent pericycle cells divide, pushing out the phloem region to generate an oblong-shaped stele (Fig. 1C; Baum et al., 2002). Divisions in the pericycle adjacent to the xylem poles subsequently contribute cells to generate a continuous cylinder of vascular cambium, a process that is similarly observed in young hypocotyls (Fig. 1D, E; Lehmann and Hardtke, 2016).
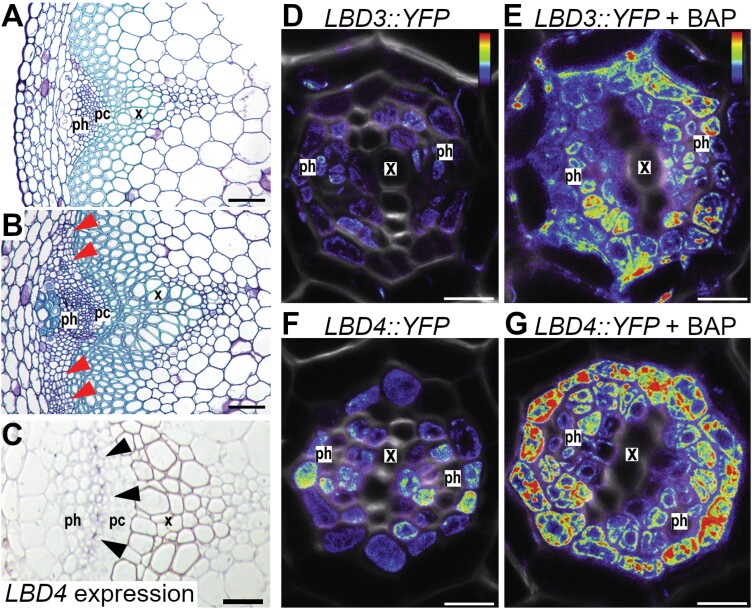
In contrast to the diarch patterns of immature roots and hypocotyls, primary vascular tissues in the Arabidopsis inflorescence stem are arranged in discrete vascular bundles (or fascicles). In a wild-type stem, 5–8 fascicles are derived from procambial cells below the SAM and arranged around a central pith (Fig. 3A), as is typical in dicots and gymnosperms (Fischer et al., 2019). Secondary growth initiates in the procambium of vascular bundles, forming fascicular cambium. Meristematic activity also extends to the interfascicular regions, where stem cells are formed de novo from differentiated parenchyma cells or those in the inner endodermal layer (known as the starch sheath) (Agusti et al., 2011b; Fig. 3B). The precise location of interfascicular cambium initiation is dependent on the stem’s developmental stage, with a gradual shift towards the cortex being observed as the stem apex is approached (Sehr et al., 2010). Clusters of fascicular cambia are subsequently connected to form a continuous cambial ring in mature stems, from which secondary xylem and phloem are derived.
Fig. 3.
Secondary growth in the stem, and contrasting LBD3/4 expression patterns in stems and roots. (A, B) Transverse sections of fascicles in Arabidopsis stems at 36 d. (A) Section from 2cm above the rosette showing a discrete vascular bundle with no secondary growth. (B) Section adjacent to the rosette showing secondary initiation, including cambial cell divisions (red arrowheads) adjacent to the fascicle. (C) In situ hybridization with the antisense LBD4 probe marking the phloem–procambium boundary (Smit et al., 2020). (D–G) LBD3:YFP and LBD4:YFP throughout the vascular cylinder in both the presence (E, G) and absence (D, F) of a 24h cytokinin treatment. Expression is higher in cytokinin-treated roots. Reprinted from Current Biology 31, Ye L, Wang X, Lyu M, Siligato R, Eswaran G, Vainio L, Blomster T, Zhang J, Mähönen AP. Cytokinins initiate secondary growth in the Arabidopsis root through a set of LBD genes. 3365–3373, Copyright (2021), with permission from Elsevier. Scales are 50 µm (A, B), 30 µm (C) and 10 µm (D–G). ph, phloem; pc, procambium; and x, xylem.
Intercellular signalling via TDIF–PXY organizes secondary growth
Following the initiation of secondary growth, cell to cell communication remains integral for maintaining the activity of the vascular cambium. Coordinated regulation of cambial cell division and differentiation requires integration of multiple external signals, including those from the extracellular matrix and adjacent cell layers (Busch et al., 2010; De Rybel et al., 2016; Trewavas, 2021). The molecular mechanisms governing cambial maintenance are topics of ongoing research (for reviews, see Fischer et al., 2019; Bagdassarian et al., 2020; Wang, 2020).
Peptide perception by transmembrane receptor(-like) kinases (RK/RLK) is a key signalling mechanism underpinning vascular cambium activity. The ligand–receptor pair comprising TRACHEARY ELEMENT DIFFERENTIATION INHIBITORY FACTOR (TDIF) and PHLOEM INTERCALATED WITH XYLEM (PXY), also known as TDIF RECEPTOR (TDR), is an extensively studied and multifunctional signalling module in this context (Fig. 2C). As part of the largest RLK family in Arabidopsis (Becraft, 2002), PXY possesses an extracellular ligand-binding domain with 21 leucine-rich repeats (LRRs), a single-helix transmembrane domain, and a cytoplasmic kinase domain that is activated upon TDIF perception (Fisher and Turner, 2007; Hirakawa et al., 2008). PXY has two close homologues in Arabidopsis, PXY-LIKE 1 (PXL1) and PXL2 (Fisher and Turner, 2007; Etchells et al., 2013), collectively referred to as the PXY family (PXf).
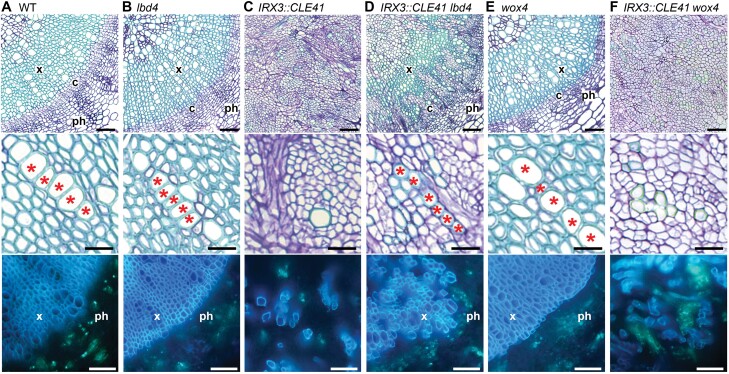
PXY is expressed in vascular tissues throughout the plant body, including in leaf veins, inflorescence stems, hypocotyls, and root steles. This expression specifically localizes to the xylem side of the vascular cambium (Hirakawa et al., 2008; Etchells and Turner, 2010a, b; Shi et al., 2019; Smetana et al., 2019; Wang et al., 2019). In contrast, the genes encoding the PXY ligand, CLAVATA3/ENDOSPERM SURROUNDING REGION 41 (CLE41), CLE42, and CLE44 (Ito et al., 2006), are expressed in the phloem (Hirakawa et al., 2008; Etchells and Turner, 2010a). CLE41/42/44 peptides are 88–101 amino acids in length and are cleaved by a currently unknown mechanism to yield the TDIF dodecapeptide (Ito et al., 2006). X-ray crystallography demonstrated that TDIF specifically bound PXY by interacting with the inner concave surface of the receptor’s LRR domain (Morita et al., 2016; Zhang et al., 2016a), following earlier identification of this interaction by photoaffinity labelling (Hirakawa et al., 2008). Further structural and genetic studies revealed that, like other RLKs, PXY activation required SOMATIC EMBRYOGENESIS RECEPTOR KINASE (SERK) co-receptors, which associated with PXY in a ligand-dependent manner at the plasma membrane (Zhang et al., 2016a, b). An identifying feature of pxy loss-of-function mutants is their lack of a continuous cambial zone and resulting intercalation of xylem and phloem (Fisher and Turner, 2007; Wang et al., 2019). Meanwhile, phloem-specific overproduction of TDIF in SUC2::CLE41 stems resulted in enhanced, yet organized, vascular proliferation (Etchells and Turner, 2010a). In the same study, overexpression of CLE41 via the 35S or xylem-specific IRREGULAR XYLEM 3 (IRX3) promoter triggered disorganized vascular development (Fig. 4A, C). Considering this, a phloem-derived TDIF signal is thought to convey positional information to PXY in order to maintain the activity and bifacial nature of cambial stem cells (Etchells and Turner, 2010a, b; Etchells et al., 2016). Presently, this is understood to occur via three distinct pathways.
Fig. 4.
wox4 and lbd4 suppression of IRX3::CLE41 phenotypes. (A) Wild type, (B) lbd4, (C) IRX3::CLE41, (D) IRX3::CLE41 lbd4, (E) wox4, and (F) IRX3::CLE41 wox4 hypocotyls. Upper and middle panels show thin sections, stained with toluidine blue; the lower panel shows aniline blue hand sections. In aniline blue-stained sections, callose in the sieve plates fluoresces green, marking phloem. Lignin in secondary cell walls autofluoresces blue, marking the xylem. Red asterisks in (D) (IRX3::CLE41 lbd4) denote a file of differentiated xylem absent from IRX3::CLE41 (C). Thus lbd4 suppresses the organization defects in IRX3::CLE41. IRX3::CLE41 wox4 lines (F) lack organization, but differentiated cell types are close together, suggesting a reduction in stem cells (lower panel). Thus wox4 suppresses stem cell overproliferation observed in IRX3::CLE41. Scale bars are 50 μm in upper and lower panels; 20 μm in middle panels. X, xylem; ph, phloem; c, cambium.
Firstly, PXY has an established role as a suppressor of cell differentiation. The TDIF peptide was originally identified as an inhibitor of xylem vessel differentiation when applied to Zinnia cell cultures (Ito et al., 2006), a finding that was later replicated in Arabidopsis hypocotyls (Whitford et al., 2008) and leaf disc cultures (Kondo et al., 2015). Consistently, 35S:CLE41 plants (in which TDIF–PXY signalling is enhanced) showed reduced expression of the xylem development marker, IRX3 (Etchells and Turner, 2010a). In addition, ectopic xylem differentiation and lignification of parenchyma cells were evident in pxy inflorescence stems (Etchells et al., 2016). In wild-type plants, activation of glycogen synthase kinase 3 (GSK3) proteins actively suppresses xylem differentiation downstream of TDIF–PXY. Förster resonance energy transfer (FRET)-based analyses revealed PXY association with the GSK3, BRASSINOSTEROID INSENSITIVE 2 (BIN2), at the plasma membrane (Kondo et al., 2014). In response to brassinosteroid perception, BIN2 is known to phosphorylate the transcription factors BRASSINAZOLE RESISTANT 1 (BZR1) and BRI1-EMS-SUPPRESSOR 1 (BES1), targeting them for proteolytic degradation (He et al., 2002). These transcription factors redundantly promote xylem and phloem differentiation in cell culture systems (Saito et al., 2018). PXY-mediated enhancement of BIN2 kinase activity is thus thought to promote phosphorylation and subsequent destabilization of BES1/BZR1, which is consistent with the reduced nuclear localization of BZR1–green fluorescent protein (GFP) observed in TDIF-treated seedlings (Kondo et al., 2014). Through this BIN2–BZR1/BES1 pathway, PXY signalling protects cambial stem cells from differentiation, thereby maintaining a pool of dividing cells for secondary growth.
Secondly, TDIF–PXY promotes vascular proliferation by targeting the cambium-expressed WUSCHEL-RELATED HOMEOBOX (WOX) transcription factor genes, WOX4 and WOX14 (Ji et al., 2010; Etchells et al., 2013). Such CLE–RLK–WOX signalling modules have been repeatedly observed in plants and are known to regulate SAM and RAM maintenance (Schoof et al., 2000; Stahl and Simon, 2009; Lee and Torii, 2012). Wild-type Arabidopsis seedlings incubated with TDIF exhibited an increase in procambial cell number and PXY-dependent up-regulation of WOX4 and WOX14 expression (Hirakawa et al., 2010; Etchells et al., 2013). Furthermore, WOX4-deficient plants had a reduced number of cells in their root and stem vascular bundles—a phenotype that was enhanced by simultaneous knockout of WOX14 (Etchells et al., 2013; Zhang et al., 2019). Given that wox4 and wox4 wox14 mutants showed no defects in vascular organization (Fig. 4E, F), the TDIF–PXY–WOX4/14 module for regulation of cambial cell division is suggested to act in parallel to those regulating xylem differentiation (Etchells et al., 2013).
Thirdly, a function for TDIF–PXY in defining the boundaries between vascular tissues was recently uncovered. Through loss- and gain-of-function approaches, LATERAL ORGAN BOUNDARIES DOMAIN 4 (LBD4) and WOX14 were identified as positive regulators of procambium activity in Arabidopsis roots (Zhang et al., 2019). Later, these transcription factors were found to be part of a PXY-regulated feedforward loop that mediated cell proliferation and vascular bundle shape in inflorescence stems (Smit et al., 2020). Strikingly, lbd4 was found to be required for IRX3:CLE41 phenotypes (Fig. 4C, D). Downstream of PXY, WOX14 functions with the auxin- and cytokinin-inducible DOF transcription factor, TMO6 (Schlereth et al., 2010; Miyashima et al., 2019) to promote the expression of LBD4 along the phloem–procambium boundary (Smit et al., 2020). Disruption of this pattern in lbd4 IRX3::LBD4 stems (in which LBD4 expression was confined to the xylem) resulted in a loss of the characteristic phloem arc and reduced expansion of fascicles along the radial axis (Smit et al., 2020). Through this indirect regulation of LBD4, PXY is thought to regulate vascular organization by defining boundaries and amplifying cell divisions on the phloem side of the procambium.
Cytokinin and the LBD family drive vascular development
Cytokinin is critical for cambium initiation. Indeed, simultaneous loss of four ATP/ADP ISOPENTENYLTRANSFERASE (IPT) enzymes, required for cytokinin biosynthesis (Miyawaki et al., 2006), abolished secondary growth initiation in primary roots (Matsumoto-Kitano et al., 2008). The vascular cambium was restored to ipt mutants by exogenous cytokinin application. This rescue was dependent on the presence of LBD3 and LBD4, the expression of which is rapidly induced upon cytokinin treatment (Ye et al., 2021; Fig. 3D–G). The expression of additional homologues, LBD1 and LBD11, increased subsequently. Induction of the former pair occurred in the presence of protein synthesis inhibitors, demonstrating that these genes were primary cytokinin targets as their induction did not rely on the synthesis of intermediates. Thus, LBD4 and its close relatives, LBD3, LBD1, and LBD11, act together to regulate radial growth by controlling cell division in the cambium. In support of this, loss of these four transcription factors resulted in considerable reductions in root secondary growth (Ye et al., 2021).
Evidence suggests that a subset of LBD genes also influence cell size. Induction of LBD1, LBD3, or LBD11 in young roots resulted in a marked increase in radial area of vascular cells, while their loss was characterized by a reduction (Ye et al., 2021). One explanation for this combination of phenotypes is that cellular growth is closely interlinked with cell cycle progression (Sablowski and Carnier Dornelas, 2014). Therefore, these three LBD genes may drive division by first promoting cell enlargement. While cytokinin is primarily associated with cell division, increases in cell size have yet to be reported following its application during secondary growth. Also, evidence from the stem suggesting that LBD4 expression marks the phloem–cambium boundary (Fig. 3C) in a PXY-mediated mechanism (Smit et al., 2020) does not hold for cambium initiation in the root vascular cylinder, where LBD3 and LBD4 promoter activity is widespread (Fig. 3D–G). Perhaps the simplest explanation for these contradictions is that LBD4 is multifunctional, with modified outputs depending on the developmental context.
ERECTA receptors regulate vascular proliferation and fibre formation
Since the characterization of PXY, further RLKs with roles in vascular development have been identified (Agusti et al., 2011b; Uchida and Tasaka, 2013; Wang et al., 2013; Gursanscky et al., 2016), although the components and interactions of their corresponding signalling pathways in vascular development are less well understood. Among these, ERECTA (ER) receptors are known to regulate cell division and xylem fibre formation during secondary growth (Ragni et al., 2011; Uchida and Tasaka, 2013; Ikematsu et al., 2017; Milhinhos et al., 2019). ER encodes an RLK with 20 LRRs in its extracellular domain (Torii et al., 1996), and possesses two close homologues, ER-LIKE 1 (ERL1) and ERL2 (Shpak et al., 2004). This trio is collectively referred to as the ER family (ERf). ER was first cloned over two decades ago (Torii et al., 1996), and has subsequently been implicated in a surprisingly diverse array of processes, including stomatal patterning (Shpak et al., 2005), elongation of aerial organs (Woodward et al., 2005; Chen et al., 2013), reproductive development (Shpak et al., 2004; Pillitteri et al., 2007), meristem maintenance (Uchida et al., 2012), leaf morphogenesis (Tameshige et al., 2016), and responses to necrotrophic pathogens (Llorente et al., 2005; Jordá et al., 2016; Cai et al., 2021). The ligands for ERf receptors reside within the family of EPIDERMAL PATTERNING FACTOR (EPF)/EPFL-LIKE (EPFL) peptides. These range from 45 to 75 amino acids in length and are encoded by 11 genes in Arabidopsis (Kondo et al., 2010; Sugano et al., 2010; Takata et al., 2013). However, only a few receptor–ligand combinations have been studied in depth.
The influence of ERf signalling on plant development is seemingly dependent on the organ and ER/ERL genes in question. In inflorescence stems, the ER promoter is active in the xylem, phloem, and endodermis (Uchida et al., 2012). erf triple mutants contained fewer cells within their stem vascular bundles, implicating ER/ERL receptors as positive regulators of vascular proliferation (Etchells et al., 2013). Similarly, er erl1 stems showed defects in procambium maintenance, a phenotype that was rescued by phloem-specific (but not xylem-specific) expression of ER (Uchida and Tasaka, 2013). The candidate ligands perceived by the ERf in this context are EPFL6 and EPFL4, also known as CHALLAH (CHAL) and CHAL-LIKE 2 (CLL2), respectively, which are highly expressed in inflorescence stems (Abrash et al., 2011; Uchida et al., 2012). The physical binding of ER to EPFL4/6 was confirmed by co-immunoprecipitation, while endodermis-specific expression of EPFL4 or EPFL6 rescued the er-like dwarf and compact inflorescence phenotypes of epfl4 epfl6 double mutants (Uchida et al., 2012). Thus, EPFL peptides secreted from the endodermis are hypothesized to signal to ERf receptors in the phloem to promote both inflorescence elongation and vascular development.
Conversely, a role for ERf receptors as suppressors of secondary growth has been uncovered in hypocotyls. ER, ERL1, and ERL2 expression is observed in most hypocotyl cell types and peaks in the cambium, xylem initials, and periderm, although ERL2 promoter activity is only evident in mature hypocotyls (Ikematsu et al., 2017; Wang et al., 2019). In contrast to the stem, EPFL4/6 expression in hypocotyls is greatest in the xylem parenchyma and differentiating xylem, suggesting that most active EPFL4/6–ERf complexes reside in xylem initials (Wang et al., 2019). While er erl2 hypocotyls were indistinguishable from those of the wild type (Wang et al., 2019) and those of erf triple mutants were reduced in diameter (Etchells et al., 2013), er erl1 hypocotyls exhibited a dramatic enhancement of secondary growth (Ikematsu et al., 2017). In wild-type Arabidopsis, radial growth in this organ proceeds in two distinct phases. During the initial ‘proportional phase’, the rate of xylem and phloem formation is roughly equal. The subsequent ‘xylem expansion’ (Fig. 1F) phase is induced by bolting (Sibout et al., 2008), which generates a shoot-derived gibberellic acid (GA) signal (Ragni et al., 2011). The arrival of GA triggers the release of BREVIPEDICELLUS (BP), ARF6, and ARF8 transcription factors from DELLA-mediated repression, shifting the balance of secondary growth to favour xylem production and differentiation of fibres (Ben-Targem et al., 2021). Interestingly, er erl1 mutation enhanced lignification and expansion of the xylem area to roughly three times that of the wild type (Ikematsu et al., 2017). This suggests that ER and ERL1 ordinarily suppress precocious xylem fibre differentiation during the proportional phase of secondary growth.
Currently, our understanding of signalling components acting downstream of ER in the vasculature is less complete in comparison with stomata. Stomatal clustering in epidermal tissues is regulated by ERf receptors binding to EPF1, EPF2, or EPFL9, in association with SERK co-receptors and the receptor-like protein, TOO MANY MOUTHS (TMM) (Shpak et al., 2005; Lee et al., 2012, 2015; Shpak, 2013). Following ligand perception in the epidermis, a MITOGEN-ACTIVATED PROTEIN KINASE (MAPK) cascade is initiated. This culminates in the phosphorylation and destabilization of the transcription factor, SPEECHLESS (SPCH), which specifies the initiation and proliferation of stomatal cells (Jewaria et al., 2013; Meng et al., 2015). In contrast, signalling via EPFL4/6–ERf does not require TMM (Lin et al., 2017). Genetic loss-of-function and expression analyses have highlighted likely transcription factors downstream of this module involved in the regulation of pathogen responses (Cai et al., 2021), inflorescence architecture (Uchida et al., 2012; Meng et al., 2013; Cai et al., 2017), and ovule development (Pillitteri et al., 2007). In hypocotyls, genes encoding the transcriptional regulators of xylem differentiation, NAC SECONDARY WALL THICKENING PROMOTING FACTOR 1 (NST1) and NST3, were up-regulated in er erl1 mutants (Ikematsu et al., 2017), making these likely downstream targets of ER/ERL1 signalling in the xylem. Beyond this suppression of NAC transcription factors, the molecular signalling events underlying ER-mediated inhibition of secondary growth remain elusive. Further targets acting downstream of EPFL4/6–ERf complexes may help explain, for example, the contrasting vascular phenotypes of erf and er erl1 hypocotyls.
The PXY and ERECTA families genetically interact
In addition to their individual characterization, understanding the crosstalk between RLK-mediated signalling pathways is an important focus of ongoing vascular development research (Fukuda and Hardtke, 2020). ER was recently found to physically interact with another LRR RLK, SUPPRESSOR OF BIR-1 (SOBIR1), and, together, these two receptors signal to suppress precocious fibre development in hypocotyls (Milhinhos et al., 2019). The EPFL4/6–ERf module is also known to genetically interact with TDIF–PXf to control cell proliferation, cell size, and organization within the plant vasculature (Etchells et al., 2013; Uchida and Tasaka, 2013; Wang et al., 2019). When the functions of PXY receptors were removed in pxy single or pxf triple mutants, hypocotyl vascular tissue developed in a disorganized manner, as exemplified by the occurrence of non-periclinal cell divisions (Etchells et al., 2013; Wang et al., 2019). In these same studies, the vasculature of er or erf hypocotyls exhibited wild-type organization. Interestingly, when both PXf and ERf function were simultaneously compromised, disruption to vascular organization became more pronounced and mean hypocotyl radius was reduced beyond that of pxf, to the extent that secondary growth initiation was absent in pxf erf sextuple mutants (Etchells et al., 2013; Wang et al., 2019). Similarly, pxf er stem vascular bundles carried fewer cells than the wild type and displayed a dramatically altered shape owing to reduced expansion along the radial axis—a phenotype that was absent in pxy and er mutants (Uchida and Tasaka, 2013; Wang et al., 2019). Given that the introduction of erf mutation(s) in a pxf background resulted in non-additive enhancement of organizational defects, this is indicative of a synergistic interaction between the two receptor families.
The PXf–ERf genetic interaction could be underpinned by multiple non-mutually exclusive phenomena at the molecular level. Firstly, PXf signalling may regulate the expression of ERf signalling components and vice versa, which could result in compensatory expression when receptors from one family are removed. In corroboration of this hypothesis, the expression of ERL1 and ERL2 was up-regulated in pxf er hypocotyls, suggesting that PXf and ER may ordinally interact to suppress ERL gene expression (Wang et al., 2019). In surprising contrast, evidence suggests that PXf and ER jointly promote EPFL6, EPFL4, ERL1, and ERL2 expression in inflorescence stems, highlighting the organ-specific nature of this interaction (Wang et al., 2019). Secondly, PXf and ERf receptors may form protein complexes at the plasma membrane, and the activity of these complexes may be unequally perturbed when different receptor combinations are removed. The expression patterns of ER and PXY consistently overlap on the xylem side of the cambium in hypocotyls (Hirakawa et al., 2008; Ikematsu et al., 2017; Wang et al., 2019). Furthermore, a recent high-throughput screen for in vitro interactions between RLK LRR domains supported the binding of ER to PXY and PXL1, as well as binding of PXL2 to ERL2 (Smakowska-Luzan et al., 2018; Mott et al., 2019).
Thirdly, it is possible that PXf and ERf interact via convergence of their signalling pathways on common genes or proteins. In this scenario, PXf- or ERf-mediated regulation of these hypothetical targets persists in the absence of one receptor family, yet removal of both families abolishes this regulation and gives rise to an enhanced phenotype. With the identification of SERK co-receptors (Zhang et al., 2016b) and GSK3 proteins (Kondo et al., 2014; Han et al., 2018), the molecular signalling components transducing PXY signalling to the nucleus are partially understood. Additionally, the NAC transcription factor XYLEM DIFFERENTIATION, DISRUPTION OF VASCULAR PATTERNING (XVP) was recently shown to negatively regulate TDIF–PXY outputs by binding BRI1-ASSOCIATED RECEPTOR KINASE1 (BAK1/SERK3) at the plasma membrane and thus the BAK1–PXY heterodimer (Yang et al., 2020a). In contrast, components acting downstream of ER to regulate vascular proliferation are unknown. It is therefore possible that PXY and ER could have shared targets within their signalling cascades, and/or at the level of transcription. The low number of genes known to play a role in the regulation of cambial morphogenesis and maintenance suggests that further regulatory components await discovery (Nieminen et al., 2015; Lehmann and Hardtke, 2016). Indeed, only a few genetic repressors of vascular development have been identified to date (Gursanscky et al., 2016; Ikematsu et al., 2017; Zhang et al., 2019; Wallner et al., 2020; Yang et al., 2020a). Given that interactions between the PXf, Erf, and phytohormones evidently make a significant contribution to cambial regulation (Qiang et al., 2013; Wang et al., 2019; Smit et al., 2020), identification of the molecular mechanisms linking these components will be an interesting focus for future research.
Signalling crosstalk in cambium development requires further study
Crosstalk and interactions between developmental regulators has emerged as a feature of cambium development (Fig. 2C). LBD4, described above, is a prime example of this, being regulated by both cytokinin and PXY signalling, as is the genetic interaction between PXf and ERf. Further examples include ethylene signalling converging on WOX4 (generally considered to act downstream of PXY) to regulate cambial proliferation, while simultaneously inhibiting xylem fibre development through suppression of NAC transcription factors (Yang et al., 2020b). In stems, overexpression of another PXY target, WOX14, promoted accumulation of bioactive GA, inducing strong lignification of secondary xylem (Denis et al., 2017). Crosstalk between TDIF–PXY and auxin is prevalent in the cambium, as auxin-induced promotion of cell division was reduced in wox4 stems (Suer et al., 2011). Expression analysis suggested that PXY and WOX4 were transcriptionally regulated by both auxin-responsive MP and HD-ZIP III transcription factors in the root procambium (Smetana et al., 2019). However, MP activity in stems ordinarily inhibits cytokinin biosynthesis and vascular proliferation via activation of ARABIDOPSIS RESPONSE REGULATOR 7 (ARR7) and ARR15 expression (Han et al., 2018), and suppression of WOX4 (Brackmann et al., 2018). PXY signalling is thought to remove this inhibition by repressing the kinase activity of the GSK3, BIN2-LIKE 1 (BIL1), a positive regulator of MP function (Han et al., 2018). Overall, it is evident that the coordinated action of multiple factors is crucial for regulating the rate and organization of secondary growth. In future, additional phytohormones such as strigolactone and jasmonic acid, which have been independently identified as positive regulators of secondary growth (Sehr et al., 2010; Agusti et al., 2011a), will probably be drawn in to complete the picture.
Mechanisms of cambial regulation are conserved
As previously discussed, some factors (e.g. MP and ERf) reportedly have organ-specific roles in the Arabidopsis stem and root. Nevertheless, key signalling components, such as PXY–TDIF and cytokinin, function similarly across the vascular system and may therefore represent conserved organizers and drivers of secondary growth. Indeed, there is increasing evidence that these components also regulate wood formation in divergent plant lineages. Tree species in the genus Populus and their hybrids (encompassing poplars, aspens, and cottonwoods) have been employed as models for understanding wood formation owing to their relatively rapid growth and availability of genomic resources (Tuskan et al., 2006). Through in vitro propagation and transformation of Populus spp. (for examples, see Takata and Eriksson, 2012; Maheshwari and Kovalchuk, 2016; Li et al., 2017), combined with tissue-specific transcriptomics and hormone profiling (Immanen et al., 2016; Sundell et al., 2017), researchers have dissected the roles of vascular development regulators in trees.
The three major phytohormone classes implicated in Arabidopsis secondary growth (auxin, cytokinin, and GAs), are also implicated in wood formation. For instance, GA signalling is crucial for triggering the xylem expansion phase of secondary growth of Arabidopsis hypocotyls (Ragni et al., 2011; Ben-Targem et al., 2021). Bioactive GA4 peaks in the developing xylem of Populus trichocarpa (Immanen et al., 2016), and expression of Pinus densiflora GA20-oxidase (PdGA20ox1) under constitutive or xylem-specific promoters triggered increased xylem width and cell number in hybrid poplar (Jeon et al., 2016). Together, this highlights a conserved role for GA in regulating secondary xylem expansion.
Reminiscent of models in the Arabidopsis root, auxin and HD-ZIP III transcription factors drive cambial proliferation in Populus. In cryosections of hybrid aspen stems (Populus tremula×tremuloides), a gradient of IAA (a major bioactive auxin) was detected in developing vascular tissue, with a peak in the cambium and decreasing concentration on either side (Tuominen et al., 1997). Disruption of auxin signalling by ectopic expression of IAA biosynthesis genes or constitutive reduction of auxin responsiveness led to reduced cambial cell division (Tuominen et al., 1997; Nilsson et al., 2008). In addition, tissue-specific transcriptomic analysis identified auxin-responsive genes whose expression patterns correlated with the phytohormone gradient (Nilsson et al., 2008; Immanen et al., 2016). Among these was PttHB8, an orthologue of the Arabidopsis HD-ZIP III transcription factor gene, ATHB8. Interestingly, popREVOLUTA (PRE), the Populus orthologue of Arabidopsis REV, was up-regulated following the transition of stems to secondary growth, while expression of an miRNA-resistant form of PRE resulted in aberrant vascular patterning, including polarity defects and ectopic cambium initiation (Robischon et al., 2011). This is consistent with the role of the HD-ZIP III class in cambial stem cell organization (Smetana et al., 2019). Auxin gradients with peak concentrations in the vascular cambium were similarly detected in Pinus sylvestris (Uggla et al., 1996), suggesting that auxin may be important for defining the zone of cambial cell division in both gymnosperms and angiosperms.
Unsurprisingly, auxin’s role in wood formation is interlinked with that of cytokinin. Hormonal profiling and RNA-sequencing of Populus stems revealed a peak in cytokinin concentration, biosynthesis, and signalling in the developing phloem (Immanen et al., 2016; Fu et al., 2021). Transgenic hybrid aspen overexpressing the Arabidopsis cytokinin biosynthesis gene, AtIPT7, in the cambium and developing xylem contained more cambial stem cells than the wild type and displayed an increased cambial auxin concentration (Immanen et al., 2016). Conversely, cytokinin catabolism was achieved in Populus vascular tissue by localizing expression of Arabidopsis CYTOKININ OXIDASE 2 (AtCKX2) to either the cambium or the phloem (Nieminen et al., 2008; Fu et al., 2021). In both cases, this resulted in a reduction in cambial cell division. Phloem- and cambium-localized cytokinin signalling are therefore thought to drive wood formation synergistically.
While cytokinin is a conserved positive regulator of secondary growth, the observation that cytokinin signalling peaks in the tree phloem does not align fully with Arabidopsis root models, in which the cytokinin response is greatest in the procambium (Bishopp et al., 2011). Indeed, the regulation of cytokinin signalling is hypothesized to be more complex in Populus spp. owing to the expansion of gene families associated with negative regulation of cytokinin responses (Tuskan et al., 2006). Downstream of cytokinin in Arabidopsis, LBD1, LBD3, LBD4, and LBD11 drive cambial proliferation in roots (Ye et al., 2021). LBD genes were independently identified as positive regulators of wood formation in trees, as P. tremula×alba trees overexpressing PtaLBD1 had thicker stems and enhanced secondary phloem production compared with the wild type (Yordanov et al., 2010). In fact, four PtaLBD family members are highly expressed in Populus stems undergoing secondary growth, and PtaLBD1 expression specifically localized to the phloem and adjacent region of the cambial zone (Yordanov et al., 2010), a pattern resembling that of AtLBD4 (Fig. 3C). Whether or not PtaLBD genes are cytokinin responsive or mediate cell growth like their Arabidopsis orthologues remains to be determined.
Alongside those of phytohormones, the roles of TDIF–PXY in promoting and organizing secondary growth are conserved between Arabidopsis and Populus spp. Within the P. trichocarpa genome, six genes encode putative TDIF-like peptides and four of these (encoded by PtCLE41a–d) are specifically expressed in secondary phloem (Kucukoglu et al., 2017). Genes encoding the LRR RLKs, PtPXYa and PtPXYb, and homeodomain transcription factors, PtWOX4a and PtWOX4b, were also identified as orthologues of AtPXY and AtWOX4, respectively. Promoter activity of these four genes peaked in the vascular cambium and positively correlated with cambial activity throughout cycles of growth and dormancy (Kucukoglu et al., 2017). Furthermore, overexpression of PttCLE41b and PttPXYa in the phloem and cambium, respectively, resulted in a dramatic increase in xylem cell numbers, stem thickening, and biomass formation in hybrid aspen (Etchells et al., 2015). In contrast, hybrid aspen expressing an RNAi construct targeting PttWOXa/b suffered severely compromised radial growth, to the extent that they failed to support their own weight (Kucukoglu et al., 2017). Combined with observations that 35S::PttPXYa constructs complemented Arabidopsis pxy mutants (Etchells et al., 2015), this suggests that TDIF–PXY signalling functions similarly in both Arabidopsis and trees to maintain the vascular cambium and promote secondary growth.
Beyond Populus, orthologues with similar spatial expression patterns to AtCLE41, AtPXY, and AtWOX4 were identified in the gymnosperm tree, Pinus abies (Kucukoglu et al., 2017). Additionally, tissue-specific transcriptomics in radish (Raphanus sativus), followed by in situ hybridization, revealed restricted expression of RsHB8, RsWOX4a, and RsPXY to a subset of root cambial cell layers (Hoang et al., 2020a). Further orthologues of established vascular development regulators, including RsMP, RsTMO5, RsLHWs, RsDOF2.1, and RsTMO6, showed cambium-enriched expression. It is thus possible that both TDIF–PXY and auxin-responsive regulatory pathways coordinate radial growth in herbaceous weeds, trees, and domesticated root crops.
Perspectives
In the last few decades, our understanding of plant radial growth has been greatly enhanced by pioneering studies that have revealed a complex web of interacting phytohormones and peptide signalling modules. However, several outstanding questions remain. What are the downstream targets of ERf signalling in the vasculature? What is the molecular basis of the PXf–ERf genetic interaction? How is TDIF processed and exported from phloem cells? How is vascular development regulated during times of stress? Interestingly, within their gene regulatory network of Arabidopsis and radish cambium-enriched transcription factors, Hoang et al. (2020a) noticed over-representation of several stress-responsive genes, including those in the WRKY and ETHYLENE RESPONSE FACTOR (ERF) family. As ethylene positively regulates secondary growth (Etchells et al., 2012; Yang et al., 2020b) and acts as an abiotic and biotic stress signal (Dubois et al., 2018; Sasidharan et al., 2018; Riyazuddin et al., 2020), the ethylene response represents a potential pathway whereby secondary growth regulation and stress signalling could be integrated. Future exploration of these open questions may lead to the identification of novel regulatory components and promising intervention points for targeted enhancement of plant secondary growth.
There is increasing evidence that studies of Arabidopsis secondary growth are informative when seeking to understand and manipulate wood formation in trees. However, gene family expansion has been a major contributor to Populus biology, and these plants therefore carry considerably more protein-encoding genes than Arabidopsis (Tuskan et al., 2006). For instance, there are 57 LBD family genes in P. trichocarpa (Zhu et al., 2007), while only 43 are present in Arabidopsis (Shuai et al., 2002). An example of genetic neofunctionalization following duplication in the Populus genome could be that of PtCLE47, which is a close relative of AtCLE25. In Arabidopsis, CLE25 peptides are expressed in phloem cell lineages and signal via CLAVATA 2 (CLV2) receptors to promote phloem initiation (Ren et al., 2019). Surprisingly, PtCLE47 is expressed in the cambium and drives the formation of secondary xylem (Kucukoglu et al., 2020). It is thus important to consider that both increased genetic redundancy and the evolution of novel regulators may underpin secondary growth in trees.
Collectively, plants contribute a huge proportion (~80%) of the world’s biomass, with stems and tree trunks alone thought to contribute 70% of this (Bar-On et al., 2018). The secondary vascular tissues of trees therefore represents a significant carbon sink and must play a major part in efforts to slow global warming (Bastin et al., 2019). Radial growth also underlies the swelling of root and tuber crops, which provide vital carbohydrates in human diets worldwide (Chandrasekara and Kumar, 2016). While this review has focused on a somewhat esoteric weed, studies in trees and root vegetables demonstrate that discoveries made in Arabidopsis may nevertheless contribute to the enhancement of forestry and agricultural outputs.
Funding
JPE gratefully acknowledges support from the Biotechnology and Biological Sciences Research Council (Grant: BB/V008129/1).
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- Abrash EB, Davies KA, Bergmann DC.. 2011. Generation of signaling specificity in Arabidopsis by spatially restricted buffering of ligand–receptor interactions. The Plant cell 23, 2864–2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agusti J, Herold S, Schwarz M, et al. 2011a. Strigolactone signaling is required for auxin-dependent stimulation of secondary growth in plants. Proceedings of the National Academy of Sciences, USA 108, 20242–20247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agusti J, Lichtenberger R, Schwarz M, Nehlin L, Greb T.. 2011b. Characterization of transcriptome remodeling during cambium formation identifies MOL1 and RUL1 as opposing regulators of secondary growth. PLoS Genetics 7, e1001312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdassarian KS, Brown CM, Jones ET, Etchells P.. 2020. Connections in the cambium, receptors in the ring. Current Opinion in Plant Biology 57, 96–103. [DOI] [PubMed] [Google Scholar]
- Bar-On YM, Phillips R, Milo R.. 2018. The biomass distribution on Earth. Proceedings of the National Academy of Sciences, USA 115, 6506–6511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastin JF, Finegold Y, Garcia C, Mollicone D, Rezende M, Routh D, Zohner CM, Crowther TW.. 2019. The global tree restoration potential. Science 365, 76–79. [DOI] [PubMed] [Google Scholar]
- Baum SF, Dubrovsky JG, Rost TL.. 2002. Apical organization and maturation of the cortex and vascular cylinder in Arabidopsis thaliana (Brassicaceae) roots. American Journal of Botany 89, 908–920. [DOI] [PubMed] [Google Scholar]
- Becraft PW. 2002. Receptor kinase signaling in plant development. Annual Review of Cell and Developmental Biology 18, 163–192. [DOI] [PubMed] [Google Scholar]
- Ben-Targem M, Ripper D, Bayer M, Ragni L.. 2021. Auxin and gibberellin signaling cross-talk promotes hypocotyl xylem expansion and cambium homeostasis. Journal of Experimental Botany 72, 3647–3660. [DOI] [PubMed] [Google Scholar]
- Bishopp A, Help H, El-Showk S, Weijers D, Scheres B, Friml J, Benková E, Mähönen AP, Helariutta Y.. 2011. A mutually inhibitory interaction between auxin and cytokinin specifies vascular pattern in roots. Current Biology 21, 917–926. [DOI] [PubMed] [Google Scholar]
- Bollhöner B, Prestele J, Tuominen H.. 2012. Xylem cell death: emerging understanding of regulation and function. Journal of Experimental Botany 63, 1081–1094. [DOI] [PubMed] [Google Scholar]
- Bossinger G, Spokevicius AV.. 2018. Sector analysis reveals patterns of cambium differentiation in poplar stems. Journal of Experimental Botany 69, 4339–4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackmann K, Qi J, Gebert M, et al. 2018. Spatial specificity of auxin responses coordinates wood formation. Nature Communications 9, 875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch W, Miotk A, Ariel FD, et al. 2010. Transcriptional control of a plant stem cell niche. Developmental Cell 18, 849–861. [DOI] [PubMed] [Google Scholar]
- Cai H, Huang Y, Chen F, Liu L, Chai M, Zhang M, Yan M, Aslam M, He Q, Qin Y.. 2021. ERECTA signaling regulates plant immune responses via chromatin-mediated promotion of WRKY33 binding to target genes. New Phytologist 230, 737–756. [DOI] [PubMed] [Google Scholar]
- Cai H, Zhao L, Wang L, Zhang M, Su Z, Cheng Y, Zhao H, Qin Y.. 2017. ERECTA signaling controls Arabidopsis inflorescence architecture through chromatin-mediated activation of PRE1 expression. New Phytologist 214, 1579–1596. [DOI] [PubMed] [Google Scholar]
- Campilho A, Nieminen K, Ragni L.. 2020. The development of the periderm: the final frontier between a plant and its environment. Current Opinion in Plant Biology 53, 10–14. [DOI] [PubMed] [Google Scholar]
- Carlsbecker A, Lee JY, Roberts CJ, et al. 2010. Cell signalling by microRNA165/6 directs gene dose-dependent root cell fate. Nature 465, 316–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaffey N, Cholewa E, Regan S, Sundberg B.. 2002. Secondary xylem development in Arabidopsis: a model for wood formation. Physiologia Plantarum 114, 594–600. [DOI] [PubMed] [Google Scholar]
- Chandrasekara A, Josheph Kumar T.. 2016. Roots and tuber crops as functional foods: a review on phytochemical constituents and their potential health benefits. International Journal of Food Science 2016, 3631647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MK, Wilson RL, Palme K, Ditengou FA, Shpak ED.. 2013. ERECTA family genes regulate auxin transport in the shoot apical meristem and forming leaf primordia. Plant Physiology 162, 1978–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rybel B, Adibi M, Breda AS, et al. 2014. Plant development. Integration of growth and patterning during vascular tissue formation in Arabidopsis. Science 345, 1255215. [DOI] [PubMed] [Google Scholar]
- De Rybel B, Mähönen AP, Helariutta Y, Weijers D.. 2016. Plant vascular development: from early specification to differentiation. Nature Reviews. Molecular Cell Biology 17, 30–40. [DOI] [PubMed] [Google Scholar]
- De Rybel B, Möller B, Yoshida S, Grabowicz I, Barbier de Reuille P, Boeren S, Smith RS, Borst JW, Weijers D.. 2013. A bHLH complex controls embryonic vascular tissue establishment and indeterminate growth in Arabidopsis. Developmental Cell 24, 426–437. [DOI] [PubMed] [Google Scholar]
- Denis E, Kbiri N, Mary V, Claisse G, Conde E Silva N, Kreis M, Deveaux Y.. 2017. WOX14 promotes bioactive gibberellin synthesis and vascular cell differentiation in Arabidopsis. The Plant Journal 90, 560–572. [DOI] [PubMed] [Google Scholar]
- Dubois M, Van den Broeck L, Inzé D.. 2018. The pivotal role of ethylene in plant growth. Trends in Plant Science 23, 311–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esau K. 1977. Anatomy of seed plants. New York: John Wiley & Sons, Ltd. [Google Scholar]
- Etchells JP, Mishra LS, Kumar M, Campbell L, Turner SR.. 2015. Wood formation in trees is increased by manipulating PXY-regulated cell division. Current Biology 25, 1050–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchells JP, Provost CM, Mishra L, Turner SR.. 2013. WOX4 and WOX14 act downstream of the PXY receptor kinase to regulate plant vascular proliferation independently of any role in vascular organisation. Development 140, 2224–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchells JP, Provost CM, Turner SR.. 2012. Plant vascular cell division is maintained by an interaction between PXY and ethylene signalling. PLoS Genetics 8, e1002997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchells JP, Smit ME, Gaudinier A, Williams CJ, Brady SM.. 2016. A brief history of the TDIF–PXY signalling module: balancing meristem identity and differentiation during vascular development. New Phytologist 209, 474–484. [DOI] [PubMed] [Google Scholar]
- Etchells JP, Turner SR.. 2010a. The PXY–CLE41 receptor ligand pair defines a multifunctional pathway that controls the rate and orientation of vascular cell division. Development 137, 767–774. [DOI] [PubMed] [Google Scholar]
- Etchells JP, Turner SR.. 2010b. Orientation of vascular cell divisions in Arabidopsis. Plant Signaling & Behavior 5, 730–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer U, Kucukoglu M, Helariutta Y, Bhalerao RP.. 2019. The dynamics of cambial stem cell activity. Annual Review of Plant Biology 70, 293–319. [DOI] [PubMed] [Google Scholar]
- Fisher K, Turner S.. 2007. PXY, a receptor-like kinase essential for maintaining polarity during plant vascular-tissue development. Current Biology 17, 1061–1066. [DOI] [PubMed] [Google Scholar]
- Fu X, Su H, Liu S, Du X, Xu C, Luo K.. 2021. Cytokinin signaling localized in phloem noncell-autonomously regulates cambial activity during secondary growth of Populus stems. New Phytologist 230, 1476–1488. [DOI] [PubMed] [Google Scholar]
- Fukuda H, Hardtke CS.. 2020. Peptide signaling pathways in vascular differentiation. Plant Physiology 182, 1636–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta KM, Yadav SR, Lehesranta S, et al. 2014. Plant development. Arabidopsis NAC45/86 direct sieve element morphogenesis culminating in enucleation. Science 345, 933–937. [DOI] [PubMed] [Google Scholar]
- Gursanscky NR, Jouannet V, Grünwald K, Sanchez P, Laaber-Schwarz M, Greb T.. 2016. MOL1 is required for cambium homeostasis in Arabidopsis. The Plant Journal 86, 210–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Cho H, Noh J, Qi J, Jung HJ, Nam H, Lee S, Hwang D, Greb T, Hwang I.. 2018. BIL1-mediated MP phosphorylation integrates PXY and cytokinin signalling in secondary growth. Nature Plants 4, 605–614. [DOI] [PubMed] [Google Scholar]
- Hardtke CS, Berleth T.. 1998. The Arabidopsis gene MONOPTEROS encodes a transcription factor mediating embryo axis formation and vascular development. The EMBO Journal 17, 1405–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He JX, Gendron JM, Yang Y, Li J, Wang ZY.. 2002. The GSK3-like kinase BIN2 phosphorylates and destabilizes BZR1, a positive regulator of the brassinosteroid signaling pathway in Arabidopsis. Proceedings of the National Academy of Sciences, USA 99, 10185–10190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilleary R, Gilroy S.. 2018. Systemic signaling in response to wounding and pathogens. Current Opinion in Plant Biology 43, 57–62. [DOI] [PubMed] [Google Scholar]
- Hirakawa Y, Kondo Y, Fukuda H.. 2010. TDIF peptide signaling regulates vascular stem cell proliferation via the WOX4 homeobox gene in Arabidopsis. The Plant Cell 22, 2618–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirakawa Y, Shinohara H, Kondo Y, Inoue A, Nakanomyo I, Ogawa M, Sawa S, Ohashi-Ito K, Matsubayashi Y, Fukuda H.. 2008. Non-cell-autonomous control of vascular stem cell fate by a CLE peptide/receptor system. Proceedings of the National Academy of Sciences, USA 105, 15208–15213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang NV, Choe G, Zheng Y, et al. 2020a. Identification of conserved gene-regulatory networks that integrate environmental sensing and growth in the root cambium. Current Biology 30, 2887–2900. [DOI] [PubMed] [Google Scholar]
- Hoang NV, Park C, Kamran M, Lee JY.. 2020b. Gene regulatory network guided investigations and engineering of storage root development in root crops. Frontiers in Plant Science 11, 762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikematsu S, Tasaka M, Torii KU, Uchida N.. 2017. ERECTA-family receptor kinase genes redundantly prevent premature progression of secondary growth in the Arabidopsis hypocotyl. New Phytologist 213, 1697–1709. [DOI] [PubMed] [Google Scholar]
- Immanen J, Nieminen K, Smolander OP, et al. 2016. Cytokinin and auxin display distinct but interconnected distribution and signaling profiles to stimulate cambial activity. Current Biology 26, 1990–1997. [DOI] [PubMed] [Google Scholar]
- Ito Y, Nakanomyo I, Motose H, Iwamoto K, Sawa S, Dohmae N, Fukuda H.. 2006. Dodeca-CLE peptides as suppressors of plant stem cell differentiation. Science 313, 842–845. [DOI] [PubMed] [Google Scholar]
- Jeon HW, Cho JS, Park EJ, Han KH, Choi YI, Ko JH.. 2016. Developing xylem-preferential expression of PdGA20ox1, a gibberellin 20-oxidase 1 from Pinus densiflora, improves woody biomass production in a hybrid poplar. Plant Biotechnology Journal 14, 1161–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewaria PK, Hara T, Tanaka H, Kondo T, Betsuyaku S, Sawa S, Sakagami Y, Aimoto S, Kakimoto T.. 2013. Differential effects of the peptides Stomagen, EPF1 and EPF2 on activation of MAP kinase MPK6 and the SPCH protein level. Plant & Cell Physiology 54, 1253–1262. [DOI] [PubMed] [Google Scholar]
- Ji J, Strable J, Shimizu R, Koenig D, Sinha N, Scanlon MJ.. 2010. WOX4 promotes procambial development. Plant Physiology 152, 1346–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns S, Hagihara T, Toyota M, Gilroy S.. 2021. The fast and the furious: rapid long-range signaling in plants. Plant Physiology 185, 694–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordá L, Sopeña-Torres S, Escudero V, Nuñez-Corcuera B, Delgado-Cerezo M, Torii KU, Molina A.. 2016. ERECTA and BAK1 receptor like kinases interact to regulate immune responses in Arabidopsis. Frontiers in Plant Science 7, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi K, Hayashi T, Gallagher KL.. 2012. SCARECROW reinforces SHORT-ROOT signaling and inhibits periclinal cell divisions in the ground tissue by maintaining SHR at high levels in the endodermis. Plant Signaling & Behavior 7, 1573–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo T, Kajita R, Miyazaki A, et al. 2010. Stomatal density is controlled by a mesophyll-derived signaling molecule. Plant & Cell Physiology 51, 1–8. [DOI] [PubMed] [Google Scholar]
- Kondo Y, Fujita T, Sugiyama M, Fukuda H.. 2015. A novel system for xylem cell differentiation in Arabidopsis thaliana. Molecular Plant 8, 612–621. [DOI] [PubMed] [Google Scholar]
- Kondo Y, Ito T, Nakagami H, Hirakawa Y, Saito M, Tamaki T, Shirasu K, Fukuda H.. 2014. Plant GSK3 proteins regulate xylem cell differentiation downstream of TDIF–TDR signalling. Nature Communications 5, 3504. [DOI] [PubMed] [Google Scholar]
- Kucukoglu M, Chaabouni S, Zheng B, Mähönen AP, Helariutta Y, Nilsson O.. 2020. Peptide encoding Populus CLV3/ESR-RELATED 47 (PttCLE47) promotes cambial development and secondary xylem formation in hybrid aspen. New Phytologist 226, 75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucukoglu M, Nilsson J, Zheng B, Chaabouni S, Nilsson O.. 2017. WUSCHEL-RELATED HOMEOBOX4 (WOX4)-like genes regulate cambial cell division activity and secondary growth in Populus trees. New Phytologist 215, 642–657. [DOI] [PubMed] [Google Scholar]
- Lee JS, Hnilova M, Maes M, Lin YC, Putarjunan A, Han SK, Avila J, Torii KU.. 2015. Competitive binding of antagonistic peptides fine-tunes stomatal patterning. Nature 522, 439–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Kuroha T, Hnilova M, Khatayevich D, Kanaoka MM, McAbee JM, Sarikaya M, Tamerler C, Torii KU.. 2012. Direct interaction of ligand–receptor pairs specifying stomatal patterning. Genes & Development 26, 126–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Torii KU.. 2012. A tale of two systems: peptide ligand–receptor pairs in plant development. Cold Spring Harbor Symposia on Quantitative Biology 77, 83–89. [DOI] [PubMed] [Google Scholar]
- Lehmann F, Hardtke CS.. 2016. Secondary growth of the Arabidopsis hypocotyl–vascular development in dimensions. Current Opinion in Plant Biology 29, 9–15. [DOI] [PubMed] [Google Scholar]
- Leyser O. 2018. Auxin signaling. Plant Physiology 176, 465–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Zhen C, Xu W, Wang C, Cheng Y.. 2017. Simple, rapid and efficient transformation of genotype Nisqually-1: a basic tool for the first sequenced model tree. Scientific Reports 7, 2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin G, Zhang L, Han Z, Yang X, Liu W, Li E, Chang J, Qi Y, Shpak ED, Chai J.. 2017. A receptor-like protein acts as a specificity switch for the regulation of stomatal development. Genes & Development 31, 927–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorente F, Alonso-Blanco C, Sánchez-Rodriguez C, Jorda L, Molina A.. 2005. ERECTA receptor-like kinase and heterotrimeric G protein from Arabidopsis are required for resistance to the necrotrophic fungus Plectosphaerella cucumerina. The Plant Journal 43, 165–180. [DOI] [PubMed] [Google Scholar]
- Long Y, Smet W, Cruz-Ramírez A, et al. 2015. Arabidopsis BIRD zinc finger proteins jointly stabilize tissue boundaries by confining the cell fate regulator SHORT-ROOT and contributing to fate specification. The Plant Cell 27, 1185–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long Y, Stahl Y, Weidtkamp-Peters S, et al. 2017. In vivo FRET-FLIM reveals cell-type-specific protein interactions in Arabidopsis roots. Nature 548, 97–102. [DOI] [PubMed] [Google Scholar]
- Maheshwari P, Kovalchuk I.. 2016. Agrobacterium-mediated stable genetic transformation of Populus angustifolia and Populus balsamifera. Frontiers in Plant Science 7, 296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallory AC, Reinhart BJ, Jones-Rhoades MW, Tang G, Zamore PD, Barton MK, Bartel DP.. 2004. MicroRNA control of PHABULOSA in leaf development: importance of pairing to the microRNA 5ʹ region. The EMBO Journal 23, 3356–3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto-Kitano M, Kusumoto T, Tarkowski P, Kinoshita-Tsujimura K, Václavíková K, Miyawaki K, Kakimoto T.. 2008. Cytokinins are central regulators of cambial activity. Proceedings of the National Academy of Sciences, USA 105, 20027–20031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Chen X, Mang H, Liu C, Yu X, Gao X, Torii KU, He P, Shan L.. 2015. Differential function of Arabidopsis SERK family receptor-like kinases in stomatal patterning. Current Biology 25, 2361–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Wang H, He Y, Liu Y, Walker JC, Torii KU, Zhang S.. 2013. A MAPK cascade downstream of ERECTA receptor-like protein kinase regulates Arabidopsis inflorescence architecture by promoting localized cell proliferation. The Plant Cell 24, 4948–4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milhinhos A, Vera-Sirera F, Blanco-Touriñán N, et al. 2019. SOBIR1/EVR prevents precocious initiation of fiber differentiation during wood development through a mechanism involving BP and ERECTA. Proceedings of the National Academy of Sciences, USA 116, 18710–18716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashima S, Roszak P, Sevilem I, et al. 2019. Mobile PEAR transcription factors integrate positional cues to prime cambial growth. Nature 565, 490–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashima S, Sebastian J, Lee JY, Helariutta Y.. 2013. Stem cell function during plant vascular development. The EMBO Journal 32, 178–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyawaki K, Tarkowski P, Matsumoto-Kitano M, Kato T, Sato S, Tarkowska D, Tabata S, Sandberg G, Kakimoto T.. 2006. Roles of Arabidopsis ATP/ADP isopentenyltransferases and tRNA isopentenyltransferases in cytokinin biosynthesis. Proceedings of the National Academy of Sciences, USA 103, 16598–16603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita J, Kato K, Nakane T, Kondo Y, Fukuda H, Nishimasu H, Ishitani R, Nureki O.. 2016. Crystal structure of the plant receptor-like kinase TDR in complex with the TDIF peptide. Nature Communications 7, 12383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott GA, Smakowska-Luzan E, Pasha A, et al. 2019. Map of physical interactions between extracellular domains of Arabidopsis leucine-rich repeat receptor kinases. Scientific Data 6, 190025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima K, Sena G, Nawy T, Benfey PN.. 2001. Intercellular movement of the putative transcription factor SHR in root patterning. Nature 413, 307–311. [DOI] [PubMed] [Google Scholar]
- Nieminen K, Blomster T, Helariutta Y, Mähönen AP.. 2015. Vascular cambium development. The Arabidopsis Book 13, e0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieminen K, Immanen J, Laxell M, et al. 2008. Cytokinin signaling regulates cambial development in poplar. Proceedings of the National Academy of Sciences, USA 105, 20032–20037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson J, Karlberg A, Antti H, Lopez-Vernaza M, Mellerowicz E, Perrot-Rechenmann C, Sandberg G, Bhalerao RP.. 2008. Dissecting the molecular basis of the regulation of wood formation by auxin in hybrid aspen. The Plant Cell 20, 843–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi-Ito K, Bergmann DC.. 2007. Regulation of the Arabidopsis root vascular initial population by LONESOME HIGHWAY. Development 134, 2959–2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi-Ito K, Saegusa M, Iwamoto K, Oda Y, Katayama H, Kojima M, Sakakibara H, Fukuda H.. 2014. A bHLH complex activates vascular cell division via cytokinin action in root apical meristem. Current Biology 24, 2053–2058. [DOI] [PubMed] [Google Scholar]
- Pillitteri LJ, Bemis SM, Shpak ED, Torii KU.. 2007. Haploinsufficiency after successive loss of signaling reveals a role for ERECTA-family genes in Arabidopsis ovule development. Development 134, 3099–3109. [DOI] [PubMed] [Google Scholar]
- Qiang Y, Wu J, Han H, Wang G.. 2013. CLE peptides in vascular development. Journal of Integrative Plant Biology 55, 389–394. [DOI] [PubMed] [Google Scholar]
- Ragni L, Nieminen K, Pacheco-Villalobos D, Sibout R, Schwechheimer C, Hardtke CS.. 2011. Mobile gibberellin directly stimulates Arabidopsis hypocotyl xylem expansion. The Plant cell 23, 1322–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren SC, Song XF, Chen WQ, Lu R, Lucas WJ, Liu CM.. 2019. CLE25 peptide regulates phloem initiation in Arabidopsis through a CLERK–CLV2 receptor complex. Journal of Integrative Plant Biology 61, 1043–1061. [DOI] [PubMed] [Google Scholar]
- Riyazuddin R, Verma R, Singh K, Nisha N, Keisham M, Bhati KK, Kim ST, Gupta R.. 2020. Ethylene: a master regulator of salinity stress tolerance in plants. Biomolecules 10, 959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robischon M, Du J, Miura E, Groover A.. 2011. The Populus class III HD ZIP, popREVOLUTA, influences cambium initiation and patterning of woody stems. Plant Physiology 155, 1214–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Villalon A, Gujas B, Kang YH, Breda AS, Cattaneo P, Depuydt S, Hardtke CS.. 2014. Molecular genetic framework for protophloem formation. Proceedings of the National Academy of Sciences, USA 111, 11551–11556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sablowski R, Carnier Dornelas M.. 2014. Interplay between cell growth and cell cycle in plants. Journal of Experimental Botany 65, 2703–2714. [DOI] [PubMed] [Google Scholar]
- Saito M, Kondo Y, Fukuda H.. 2018. BES1 and BZR1 redundantly promote phloem and xylem differentiation. Plant & Cell Physiology 59, 590–600. [DOI] [PubMed] [Google Scholar]
- Sasidharan R, Hartman S, Liu Z, Martopawiro S, Sajeev N, van Veen H, Yeung E, Voesenek LACJ.. 2018. Signal dynamics and interactions during flooding stress. Plant Physiology 176, 1106–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlereth A, Möller B, Liu W, Kientz M, Flipse J, Rademacher EH, Schmid M, Jürgens G, Weijers D.. 2010. MONOPTEROS controls embryonic root initiation by regulating a mobile transcription factor. Nature 464, 913–916. [DOI] [PubMed] [Google Scholar]
- Schoof H, Lenhard M, Haecker A, Mayer KF, Jürgens G, Laux T.. 2000. The stem cell population of Arabidopsis shoot meristems in maintained by a regulatory loop between the CLAVATA and WUSCHEL genes. Cell 100, 635–644. [DOI] [PubMed] [Google Scholar]
- Sehr EM, Agusti J, Lehner R, Farmer EE, Schwarz M, Greb T.. 2010. Analysis of secondary growth in the Arabidopsis shoot reveals a positive role of jasmonate signalling in cambium formation. The Plant Journal 63, 811–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi D, Lebovka I, Loṕez-Salmeroń V, Sanchez P, Greb T.. 2019. Bifacial cambium stem cells generate xylem and phloem during radial plant growth. Development 146, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shpak ED. 2013. Diverse roles of ERECTA family genes in plant development. Journal of Integrative Plant Biology 55, 1238–1250. [DOI] [PubMed] [Google Scholar]
- Shpak ED, Berthiaume CT, Hill EJ, Torii KU.. 2004. Synergistic interaction of three ERECTA-family receptor-like kinases controls Arabidopsis organ growth and flower development by promoting cell proliferation. Development 131, 1491–1501. [DOI] [PubMed] [Google Scholar]
- Shpak ED, McAbee JM, Pillitteri LJ, Torii KU.. 2005. Stomatal patterning and differentiation by synergistic interactions of receptor kinases. Science 309, 290–293. [DOI] [PubMed] [Google Scholar]
- Shuai B, Reynaga-Peña CG, Springer PS.. 2002. The lateral organ boundaries gene defines a novel, plant-specific gene family. Plant Physiology 129, 747–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibout R, Plantegenet S, Hardtke CS.. 2008. Flowering as a condition for xylem expansion in Arabidopsis hypocotyl and root. Current Biology 18, 458–463. [DOI] [PubMed] [Google Scholar]
- Smakowska-Luzan E, Mott GA, Parys K, et al. 2018. An extracellular network of Arabidopsis leucine-rich repeat receptor kinases. Nature 553, 342–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smet W, Sevilem I, de Luis Balaguer MA, et al. 2019. DOF2.1 controls cytokinin-dependent vascular cell proliferation downstream of TMO5/LHW. Current Biology 29, 520–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smetana O, Mäkilä R, Lyu M, et al. 2019. High levels of auxin signalling define the stem-cell organizer of the vascular cambium. Nature 565, 485–489. [DOI] [PubMed] [Google Scholar]
- Smit ME, McGregor SR, Sun H, et al. 2020. A PXY-mediated transcriptional network integrates signaling mechanisms to control vascular development in Arabidopsis. The Plant Cell 32, 319–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spicer R, Groover A.. 2010. Evolution of development of vascular cambia and secondary growth. New Phytologist 186, 577–592. [DOI] [PubMed] [Google Scholar]
- Stahl Y, Simon R.. 2009. Is the Arabidopsis root niche protected by sequestration of the CLE40 signal by its putative receptor ACR4? Plant Signaling & Behavior 4, 634–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suer S, Agusti J, Sanchez P, Schwarz M, Greb T.. 2011. WOX4 imparts auxin responsiveness to cambium cells in Arabidopsis. The Plant Cell 23, 3247–3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugano SS, Shimada T, Imai Y, Okawa K, Tamai A, Mori M, Hara-Nishimura I.. 2010. Stomagen positively regulates stomatal density in Arabidopsis. Nature 463, 241–244. [DOI] [PubMed] [Google Scholar]
- Sundell D, Street NR, Kumar M, et al. 2017. AspWood: high-spatial-resolution transcriptome profiles reveal uncharacterized modularity of wood formation in Populus tremula. The Plant Cell 29, 1585–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taiz L, Zeiger E.. 2002. Plant physiology. Sunderland, MA: Sinauer Associates Inc. [Google Scholar]
- Takata N, Eriksson ME.. 2012. A simple and efficient transient transformation for hybrid aspen (Populus tremula × P. tremuloides). Plant Methods 8, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata N, Yokota K, Ohki S, Mori M, Taniguchi T, Kurita M.. 2013. Evolutionary relationship and structural characterization of the EPF/EPFL gene family. PLoS One 8, e65183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tameshige T, Okamoto S, Lee JS, Aida M, Tasaka M, Torii KU, Uchida N.. 2016. A secreted peptide and its receptors shape the auxin response pattern and leaf margin morphogenesis. Current Biology 26, 2478–2485. [DOI] [PubMed] [Google Scholar]
- Thamm A, Sanegre-Sans S, Paisley J, Meader S, Milhinhos A, Contera S, Agusti J.. 2019. A simple mathematical model of allometric exponential growth describes the early three-dimensional growth dynamics of secondary xylem in Arabidopsis roots. Royal Society Open Science 6, 190126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonn N, Greb T.. 2017. Radial plant growth. Current Biology 27, R878–R882. [DOI] [PubMed] [Google Scholar]
- Torii KU, Mitsukawa N, Oosumi T, Matsuura Y, Yokoyama R, Whittier RF, Komeda Y.. 1996. The Arabidopsis ERECTA gene encodes a putative receptor protein kinase with extracellular leucine-rich repeats. The Plant Cell 8, 735–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trewavas A. 2021. Awareness and integrated information theory identify plant meristems as sites of conscious activity. Protoplasma 258, 673–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuominen H, Puech L, Fink S, Sundberg B.. 1997. A radial concentration gradient of indole-3-acetic acid is related to secondary xylem development in hybrid aspen. Plant Physiology 115, 577–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuskan GA, Difazio S, Jansson S, et al. 2006. The genome of black cottonwood, Populus trichocarpa (Torr. & Gray). Science 313, 1596–1604. [DOI] [PubMed] [Google Scholar]
- Uchida N, Lee JS, Horst RJ, Lai HH, Kajita R, Kakimoto T, Tasaka M, Torii KU.. 2012. Regulation of inflorescence architecture by intertissue layer ligand–receptor communication between endodermis and phloem. Proceedings of the National Academy of Sciences, USA 109, 6337–6342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida N, Tasaka M.. 2013. Regulation of plant vascular stem cells by endodermis-derived EPFL-family peptide hormones and phloem-expressed ERECTA-family receptor kinases. Journal of Experimental Botany 64, 5335–5343. [DOI] [PubMed] [Google Scholar]
- Uggla C, Moritz T, Sandberg G, Sundberg B.. 1996. Auxin as a positional signal in pattern formation in plants. Proceedings of the National Academy of Sciences, USA 93, 9282–9286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner ES, Tonn N, Shi D, Jouannet V, Greb T.. 2020. SUPPRESSOR OF MAX2 1-LIKE 5 promotes secondary phloem formation during radial stem growth. The Plant Journal 102, 903–915. [DOI] [PubMed] [Google Scholar]
- Wang H. 2020. Regulation of vascular cambium activity. Plant Science 291, 110322. [DOI] [PubMed] [Google Scholar]
- Wang J, Kucukoglu M, Zhang L, Chen P, Decker D, Nilsson O, Jones B, Sandberg G, Zheng B.. 2013. The Arabidopsis LRR-RLK, PXC1, is a regulator of secondary wall formation correlated with the TDIF–PXY/TDR–WOX4 signaling pathway. BMC Plant Biology 13, 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Bagdassarian KS, Doherty RE, Kroon JT, Connor KA, Wang XY, Wang W, Jermyn IH, Turner SR, Etchells JP.. 2019. Organ-specific genetic interactions between paralogues of the PXY and ER receptor kinases enforce radial patterning in Arabidopsis vascular tissue. Development 146, 177105. [DOI] [PubMed] [Google Scholar]
- Weijers D, Schlereth A, Ehrismann JS, Schwank G, Kientz M, Jürgens G.. 2006. Auxin triggers transient local signaling for cell specification in Arabidopsis embryogenesis. Developmental Cell 10, 265–270. [DOI] [PubMed] [Google Scholar]
- Whitford R, Fernandez A, De Groodt R, Ortega E, Hilson P.. 2008. Plant CLE peptides from two distinct functional classes synergistically induce division of vascular cells. Proceedings of the National Academy of Sciences, USA 105, 18625–18630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward C, Bemis SM, Hill EJ, Sawa S, Koshiba T, Torii KU.. 2005. Interaction of auxin and ERECTA in elaborating Arabidopsis inflorescence architecture revealed by the activation tagging of a new member of the YUCCA family putative flavin monooxygenases. Plant Physiology 139, 192–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunderling A, Ripper D, Barra-Jimenez A, Mahn S, Sajak K, Targem MB, Ragni L.. 2018. A molecular framework to study periderm formation in Arabidopsis. New Phytologist 219, 216–229. [DOI] [PubMed] [Google Scholar]
- Yang JH, Lee KH, Du Q, Yang S, Yuan B, Qi L, Wang H.. 2020a. A membrane-associated NAC domain transcription factor XVP interacts with TDIF co-receptor and regulates vascular meristem activity. New Phytologist 226, 59–74. [DOI] [PubMed] [Google Scholar]
- Yang S, Wang S, Li S, Du Q, Qi L, Wang W, Chen J, Wang H.. 2020b. Activation of ACS7 in Arabidopsis affects vascular development and demonstrates a link between ethylene synthesis and cambial activity. Journal of Experimental Botany 71, 7160–7170. [DOI] [PubMed] [Google Scholar]
- Ye L, Wang X, Lyu M, Siligato R, Eswaran G, Vainio L, Blomster T, Zhang J, Mähönen AP.. 2021. Cytokinins initiate secondary growth in the Arabidopsis root through a set of LBD genes. Current Biology 31, 3365–3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yordanov YS, Regan S, Busov V.. 2010. Members of the LATERAL ORGAN BOUNDARIES DOMAIN transcription factor family are involved in the regulation of secondary growth in Populus. The Plant Cell 22, 3662–3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Lin X, Han Z, Qu LJ, Chai J.. 2016a. Crystal structure of PXY–TDIF complex reveals a conserved recognition mechanism among CLE peptide–receptor pairs. Cell Research 26, 543–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Lin X, Han Z, Wang J, Qu LJ, Chai J.. 2016b. SERK family receptor-like kinases function as co-receptors with PXY for plant vascular development. Molecular Plant 9, 1406–1414. [DOI] [PubMed] [Google Scholar]
- Zhang J, Eswaran G, Alonso-Serra J, et al. 2019. Transcriptional regulatory framework for vascular cambium development in Arabidopsis roots. Nature Plants 5, 1033–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu QH, Guo AY, Gao G, Zhong YF, Xu M, Huang M, Luo J.. 2007. DPTF: a database of poplar transcription factors. Bioinformatics 23, 1307–1308. [DOI] [PubMed] [Google Scholar]