Abstract
In land plants and algae, cellulose is important for strengthening cell walls and preventing breakage due to physical forces. Though our understanding of cellulose production by cellulose synthases (CESAs) has seen significant advances for several land plant and bacterial species, functional characterization of this fundamental protein is absent in red algae. Here we identify CESA gene candidates in the calcifying red alga Calliarthron tuberculosum using sequence similarity-based approaches, and elucidate their phylogenetic relationship with other CESAs from diverse taxa. One gene candidate, CtCESA1, was closely related to other putative red algal CESA genes. To test if CtCESA1 encoded a true cellulose synthase, CtCESA1 protein was expressed and purified from insect and yeast expression systems. CtCESA1 showed glucan synthase activity in glucose tracer assays. CtCESA1 activity was relatively low when compared with plant and bacterial CESA activity. In an in vitro assay, a predicted N-terminal starch-binding domain from CtCESA1 bound red algal floridean starch extracts, representing a unique domain in red algal CESAs not present in CESAs from other lineages. When the CtCESA1 gene was introduced into Arabidopsis thaliana cesa mutants, the red algal CtCESA1 partially rescued the growth defects of the primary cell wall cesa6 mutant, but not cesa3 or secondary cell wall cesa7 mutants. A fluorescently tagged CtCESA1 localized to the plasma membrane in the Arabidopsis cesa6 mutant background. This study presents functional evidence validating the sequence annotation of red algal CESAs. The relatively low activity of CtCESA1, partial complementation in Arabidopsis, and presence of unique protein domains suggest that there are probably functional differences between the algal and land plant CESAs.
Keywords: Arabidopsis, Calliarthron, cellulose synthase, floridean starch, glucan synthase, red alga, Rhodophyta
Cellulose synthases (CESAs) within the red algal lineage share a close evolutionary relationship, but their function has not been demonstrated. This study functionally characterizes CESA from the coralline alga Calliarthron, including its floridean starch binding capacity.
Introduction
Coralline red algae are major contributors to the ecology of marine communities (Nelson, 2009; O’Leary et al., 2017). Their calcified thalli cement together reefs and promote invertebrate recruitment (Kitamura et al., 2007; Williams et al., 2008). In particular, coralline algae in the genus Calliarthron have garnered increased attention as joints along their calcified thalli help them bend, but not break, in response to wave impacts (Denny et al., 2013; Martone and Denny, 2008). Relative to fleshy macroalgae, joint tissue in Calliarthron exhibits high strength and extensibility in pull-to-break and bending tests. These properties are thought to be partly due to the ubiquitous presence of cellulose, a linear homopolymer of β-1,4-linked glucose, in their cell walls (Martone et al., 2019). In these joints, cellulose is deposited in random orientations, comprising ~8% of primary cell walls (PCWs) and ~22% of secondary cell walls (SCWs), laid down earlier and later in development respectively (Martone et al., 2019). Application of calcofluor white, a commonly used stain identifying cellulose in bacteria and land plants, over a period of several years demonstrated that Calliarthron continuously deposits cellulose (Martone, 2010). Despite the architectural and ecological importance of cellulose in the cell walls of Calliarthron and other red algae, we lack a functional understanding of how red algae produce cellulose.
Our understanding of cellulose production comes primarily from work done in bacteria and land plants. Cellulose is produced at the plasma membrane by cellulose synthases (CESAs) organized into multimeric cellulose synthase complexes (McFarlane et al., 2014; Anderson and Kieber, 2020). Enzymatic and structural studies of purified Rhodobacter sphaeroides cellulose synthase A/B complex (BCSA/BCSB) and land plant Populus tremula×tremuloides CESA8 (PttCESA8) have demonstrated the in vitro biochemical conditions for CESA glucan synthase activity (Omadjela et al., 2013; Purushotham et al., 2016; Cho et al., 2017). In the presence of divalent ions, Mg2+ or Mn2+, CESAs transfer glucose from UDP-glucose to the growing β-1,4-linked glucan chain while extruding it into the apoplastic space (Morgan et al., 2013; Omadjela et al., 2013). These emerging glucan chains aggregate into crystalline or para-crystalline cellulose through regularly patterned hydrogen bonding (Nishiyama et al., 2003).
Each CESA contains a glycosyltransferase (GT) domain, flanked by seven transmembrane helices (Purushotham et al., 2020). High sequence conservation exists between the GT domains of distantly related CESAs (Pear et al., 1996), making these regions of sequence homology useful for identifying new CESA sequences. This GT domain has several necessarily conserved motifs such as the QXXRW and D,D,D motifs that are required for coordination of the UDP-glucose substrate and the growing glucan chain (Morgan et al., 2013).
Cellulose production in vivo has been well characterized in Arabidopsis. The Arabidopsis genome encodes multiple non-redundant CESA genes expressed during distinct developmental stages of PCW synthesis (CESA1, -3, and the partially redundant CESA2, -5, -6, and -9) (McFarlane et al., 2014) or during SCW synthesis (CESA4, -7, and -8) in specialized cells such as fibers and vessels. Mutations in these CESA genes cause impaired cellulose production and lead to growth defects, demonstrating their essential roles in proper cell and tissue development in Arabidopsis. Mutations in CESA6, in a mutant allele called procuste1-1 (prc1-1), causes stunted hypocotyl growth in dark-grown seedlings (Fagard et al., 2000; MacKinnon et al., 2006), mutations in CESA3 (cesa3-2) are gametophytic lethal (Persson et al., 2007), and mutations in any SCW CESA4, -7, or -8 cause collapsed xylem, referred to as an irregular xylem (irx) phenotype, and dwarfed plant size (Turner and Somerville, 1997; Taylor et al., 2003; Brown et al., 2005).
Apart from the CESA machinery, there are accessory proteins that facilitate proper cellulose synthesis. In land plants such as Arabidopsis, CESA trafficking, CESA stabilization, CESA–microtubule interactions, and cellulose crystallization require the proteins TRANVIA (TVA), STELLO (STL), Cellulose Synthase Interacting 1/POM-POM2 (CSI), Cellulose Synthase-Microtubule Uncoupling Protein (CMU), Companion Of Cellulose Synthase (CC), KORRIGAN (KOR), Chitinase-Like1/POM-POM1 (CTL), and COBRA (COB) (Allen et al., 2021; Anderson and Kieber, 2020; Vellosillo et al., 2021). It is unclear whether some or all of these mechanisms are universally required for proper cellulose synthesis or are present in red algae such as Calliarthron. In a phylogenetic analysis, Lampugnani et al. (2019) did not recover any accessory proteins within the ‘red algal lineage’, but this was limited by the study’s reduced scope that only considered a single red algal genome. As the phylum Rhodophyta represents a morphologically and genetically diverse group of >6000 red algal species (Yoon et al., 2010), sampling from other red algal sequence datasets is necessary to elucidate whether these accessory proteins are present in other red algae.
Our understanding of cellulose synthesis in red algae is limited, but also presumably involves CESA enzymes. In freeze-fracture electron micrographs, CESA complexes from land plants appear as circular ‘rosette’-shaped complexes on the plasma membrane (Kimura et al., 1999; Nixon et al., 2016), and red algae CESA complexes appear as linear arrays, at times connected to cylindrical cellulose microfibrils (Tsekos et al., 1993; Okuda et al., 1994). Interestingly, red algal CESA sequences have a predicted carbohydrate-binding module (CBM48) at their N-terminus (Matthews et al., 2010), which may facilitate cytosolic binding of α-1,4- and α-1,6-linked glucans, such as starch. Red algal floridean starch is composed of amylopectin-like molecules (α-1,4-linked glucose with branched α-1,6-linked glucose) (Yu et al., 2002), unlike land plant starches composed of both amylose (linear α-1,4-linked glucose) and amylopectin (α-1,4-linked glucose with branched α-1,6-linked glucose) (Tester et al., 2004). Moreover, amylopectin’s frequency of α-1,6-linked glucose branching and branch lengths can vary, where some red algae have shorter branches and more frequent branching than potato starch (Yu et al., 2002). Given these differences, it is unclear if this CBM48 domain could be specific to floridean starchs or bind starches with α-1,4- and α-1,6-linked glucans more generally. Because red algae store floridean starch as free-floating granules in the cytosol (Viola et al., 2001), these granules may be able to interact with the red algal CBM48, although this interaction has not been tested and the functional significance is unknown.
While multiple CESAs with subspecializations have been identified in land plants, transcriptomic analyses have not identified any red algae species that carry more than one full-length CESA (Roberts and Roberts, 2009; Matthews et al., 2010; Brawley et al., 2017). However, the identification of distinct PCWs and SCWs in Calliarthron species (Martone et al., 2009, 2019) raised questions about whether Calliarthron might encode one CESA that acts in both PCWs and SCWs, or if Calliarthron might have subspecialized CESAs, as in land plants. This question has become more tractable as transcriptomic and genomic (Chan et al., 2011) datasets emerge for C. tuberculosum, hereafter referred to as Calliarthron. While the recent identification and phylogenetic analysis of putative red algal CESA genes is intriguing, to date there has been no functional evidence to demonstrate that they truly encode CESA proteins.
We identify sequences of CESA and CESA accessory proteins from the calcifying red alga C. tuberculosum (CtCESA) using sequence homology-based methods and compare them with CESAs from other organisms using phylogenetic analyses. One gene candidate, CtCESA1, encoded the predicted key CESA catalytic domains. Accordingly, its glucan synthase activity was tested and compared with the plant PttCESA8 and bacterial BCSA/BCSB in tracer assays for glucan polymer formation. The predicted CBM48 was tested for starch binding, and it bound floridean, but not potato, starch. An additional line of evidence that the CtCESA1 encodes a cellulose synthase came from introducing the red algal CtCESA1 into the Arabidopsis PCW and SCW cesa mutants. CtCESA1 was able to partially restore growth to the prc1-1/cesa6 mutant’s severely defective growth phenotype. In this mutant, fluorescently tagged CtCESA1 localized to the plasma membrane in Arabidopsis, which points towards some shared modalities of CESA trafficking across these evolutionarily distant lineages.
Materials and methods
Sequence identification
To create a database for sequence searches, the Calliarthron transcriptome dataset (European Nucleotide Archive project number PRJEB39919) was translated into six reading frames using EMBOSS Transeq (Rice et al., 2000). All sequence alignments were produced using MUSCLE (Edgar, 2004). HMM ‘profiles’ were produced (data available at Zenodo https://doi.org/10.5281/zenodo.5010240) by aligning CESA amino acid sequences (Finn et al., 2011). HMM profiles were queried against the Calliarthron transcriptome in HMMER searches to identify candidate sequences. Sequences <700 bp were removed. Sequences supported by Calliarthron genomic scaffolds were identified (BLASTN, E≤10–5).
Motif analysis
ORFs were identified with NCBI’s ORF finder under default settings (Rombel et al., 2002). Transmembrane domains were identified with TOPCONS (Tsirigos et al., 2015). General domains were identified with NCBI’s conserved domain search tool (Marchler-Bauer et al., 2015). Key residues were manually identified in a multiple sequence alignment (MSA) produced using MUSCLE under default settings (Edgar, 2004). Sites with <90% coverage were removed using trimAl (Capella-Gutiérrez et al., 2009).
Gene tree analysis
Maximum likelihood (ML) phylogenetic methods were applied to the recovered CESA sequences from Calliarthron, CESA annotated sequences retrieved from NCBI, and sequences with similarity to Calliarthron CESAs in BLASTP (E≤10–40) (for identifiers see data at Zenodo https://doi.org/10.5281/zenodo.5010240). Retrieved sequences were aligned, and sites with <80% coverage were trimmed. IQtree was used to search for the evolutionary model of the alignment under a Baysian information criterion (Nguyen et al., 2015) and then to search for an ML gene tree with branch support calculated under 1000 replicates of ultrafast-bootstrap approximation (Hoang et al., 2018). Interpretation of clade reliability is high when support is ≥95% under ultrafast bootstrap approximation.
Sample collection and cDNA production
Calliarthron tuberculosum samples were collected from Botanical Beach, Port Renfrew, BC (location 48°31′31.1N, 124°26′26.4W, BC Parks permit number 109636) on Pacheedhat First Nations lands. Samples were cleaned with 70% ethanol, flash-frozen in liquid nitrogen, and ground into a powder. RNA was extracted using the cetyltrimethylammonium bromide (CTAB) method (Barbier et al., 2019), treated with DNase (DNase I), and reverse transcribed (SuperScript™ III) with anchored oligo(dT)22 into cDNA. CtCESA1 was amplified from the cDNA by PCR and confirmed by Sanger sequencing.
CtCESA1 expression in SF9 insect cells
For expression in insect cell lines, CtCESA1 was PCR amplified with Phusion polymerase (NEB) and tagged with a 5′ 12×HIS and Tobacco etch virus (TEV) protease-cut site with flanking SacI- and HindIII-cut sites (Supplementary Table S1). The modified CtCESA1 was introduced into the pACEBac1 shuttle vector by restriction digest cloning to generate 12×HIS-CtCESA1-pACEBac1 and confirmed by sequencing. 12×HIS-CtCESA1-pACEBac1 was introduced into DH10MultiBac Escherichia coli cells to generate viral bacmid DNA and selected on kanamycin/gentamycin/tetracycline blue/white selection media blu-gal and isopropyl-β-d-thiogalactopyranoside (IPTG). Bacmid DNAs containing CtCESA1 were harvested from white colonies and used to transfect insect cell cultures, and large-scale cultures of CtCESA1-expressing insect cells were grown as described in Purushotham et al. (2020), pelleted, flash-frozen, and stored at –80°C.
CtCESA1 expression in Pichia yeast cells
For methanol-inducible expression in Pichia, CtCESA1 with a 5′ Strep (Schmidt and Skerra, 2007), 12×HIS, and TEV site was codon optimized for yeast expression and synthesized into pPICZA (ThermoFisher) to generate Strep-12×HIS-CtCESA1-pPICZA. A 30 μg aliquot of plasmid was linearized with PmeI (New England Biolabs) for genomic incorporation, and transformed into Pichia pastoris strain SMD1168H.
Initial starter cultures of CtCESA1-expressing Pichia were grown overnight in 1% yeast extract, 2% peptone, and 1% glycerol-containing medium (220 rpm shaking, 30°C). When the optical density (OD600) reached 4, the starter culture was pelleted and resuspended in the above medium containing yeast nitrogen base with ammonium sulfate and 0.5% methanol (YNBM) at 4000 rpm, 16 °C. Expression of CtCESA1 was induced in 0.5% methanol medium. Starter cultures were scaled up to 200–300 ml volumes of YNBM and grown for 2.5 d (30 °C, shaking 220 rpm) with 0.5% methanol after 24 h to induce protein expression of CtCESA1. Cells were pelleted by centrifugation (5000 g, 14 °C), flash-frozen in liquid nitrogen, and stored at –80 °C.
Detergent screen of membranes containing heterologously expressed CtCESA1
Membrane fractions, the pellet, isolated from the cell lysate by centrifugation (138 000 g, 1 h, 4 °C) were exposed to detergents [40 mM dodecyl maltoside (DDM); 40 mM lauryldimethylamine-N-oxide (LDAO); 40 mM sodium cholate (NaC); 2% Triton X-100; 2% lauryl maltose neopentyl glycol (LMNG)+0.4% cholesteryl hemisuccinate (CHS); 2% SDS (Purushotham et al., 2016). All detergents except for SDS were sol grade (Anatrace), resuspended in water, and rotated at 4 °C to resuspend. Briefly, membrane fractions and detergents were rotated for 1 h at 4 °C. Samples were centrifuged (228 000 g, 25 min, 4 °C) to separate solubilized protein in the supernatant from insoluble protein in the pellet and western blotted.
CtCESA1 purification from insect or yeast cell membranes
Pelleted cells expressing CtCESA1 were resuspended with cold membrane resuspension buffer (MRB; 20 mM Tris and 100 mM NaCl at pH 7.5), protease inhibitor cOmplete (Roche), and phenylmethylsulfonyl fluoride (PMSF) by douncing. Cells were lysed by microfluidizing at 30 000 psi three times (Microfluidics). The lysate was pelleted (10 000 g, 4 °C) and the subsequent supernatant was also pelleted (138 000 g, 4 °C) to obtain the membrane fraction. The pellet was resuspended in solubilization buffer (1% LMNG+0.2% CHS, 5 mM β-mercaptoethanol, and MRB to volume), rotated for 1 h at 4 °C, and centrifuged at 138 000 g, 4 °C. The supernatant was batch-bound with 2.5 ml of nickel beads (Thermofisher) with 20 mM imidazole by rocking for 1 h at 4 °C. Batch-bound CtCESA1 was passed through a gravity flow column, and washed with the following solutions: MRB with 20 mM imidazole (wash 1); MRB with 20 mM imidazole, 0.03% glyco-diosgenin (GDN), and 5 mM β-mercaptoethanol (wash 2); wash 2 with 1 M NaCl (wash 3); and wash 3 with 40 mM imidazole (wash 4). CtCESA1 was eluted using MRB with 400 mM imidazole, 0.03% GDN, and 5 mM β-mercaptoethanol.
CtCESA1 eluate was passed through a Superdex 200 Increase 10/300 (GE) column, in size exclusion chromatography (SEC), equilibrated with gel filtration buffer (GFB) as follows: 20 mM Tris pH 7.5, 100 mM NaCl, 0.03% GDN, and 5 mM β-mercaptoethanol. Protein purity of the collected fractions was determined through SDS–PAGE, and protein presence was detected by western blotting with anti-HIS and anti-Strep antibodies. Appropriate fractions containing CtCESA1 were concentrated (0.15 mg ml–1) for use in subsequent enzyme activity assays.
Reconstitution of CtCESA1 into proteoliposomes
Purified CtCESA1 was reconstituted into proteoliposomes by incubating with 4 mg ml–1E. coli total lipid extract (Avanti) solubilized with 40 mM DDM, addition of Bio-Beads (Bio-Rad), and rotation overnight at 4 °C. Visually turbid samples (indicating the formation of liposomes) were used for subsequent activity assays as described (Purushotham et al., 2016).
Glucosyltransferase activity assay of CtCESA1, PttCESA8, and BCSA/BCSB
To test for glucosyltransferase activity, 75 μg of CtCESA1 in 0.03% GDN or 75 μg of proteoliposome-reconstituted CtCESA1 was incubated for 10 h at 30 °C with 5 mM UDP-glucose, 0.25 μCi of radioactive 3H-labeled glucose (Perkin Elmer; [6-3H]uridine diphospho-d-glucose), 25 mM of supplied cation (Ca2+, Mg2+, Mn2+, or nothing added Ø), and brought to final volume with the GFB. Purified insect cell-expressed PttCESA8 at 5 μM and E. coli-expressed BCSA/BCSB protein at 100 nM were tested in the same conditions, with an addition of 30 μM cyclic di-GMP in BCSA/BCSB samples. Reactions were stopped by addition of 2% of SDS. Polymeric glucans and monomeric glucose were separated by paper chromatography. Samples were blotted onto chromatography paper (Whatman-2MM), developed in a mobile phase of 60% ethanol, dried, submerged in scintillation fluid, and incorporation of radioactive [3H]glucose into higher molecular weight polymers was quantified by scintillation counting as described (Purushotham et al., 2016). Activities were normalized by protein concentration.
CBM48 expression and purification
The entire N-terminal domain of CtCESA1 was amplified by PCR using Phusion polymerase (NEB) with the appropriate primers (Supplementary Table S1). NcoI and XhoI restriction sites were included in the forward and reverse primers for digestion and ligation into a modified pET28 with a C-terminal 12×His-tag. The pET28 containing only the CBM48 sequence (CtCBM48-pET28) used in this study was subsequently isolated by PCR-mediated plasmid DNA deletion (Hansson et al., 2008) with appropriate primers (Supplementary Table S1). Plasmids were verified by Sanger sequencing. CtCBM48-pET28 was introduced into chemically competent Rosetta-gami 2 (DE3) E. coli. Starter cultures were grown overnight (37 °C, 220 rpm shaking) in lysogeny broth (LB) containing 25 µg ml–1 chloramphenicol and 50 µg ml–1 kanamycin. Starter cultures at log growth phase (OD600 ~0.5) were scaled up in Terrific Broth (TB) supplemented with 20xM (50 mM NH4Cl, 25 mM KH2PO4, 25 mM Na2HPO4, 5 mM Na2SO4), 80 155 autoinduction medium (0.8% glycerol, 0.015% glucose, 0.5% alpha lactose), 0.37% aspartate, 2 mM MgSO4 (Blommel and Fox, 2007), and antibiotics. Large-scale cultures were grown overnight (28 °C, 220 rpm) then pelleted (4000 g, 4 °C), flash-frozen in liquid nitrogen, and stored at –80 °C until use.
CtCESA1-expressing E. coli were resuspended in cold resuspension buffer (RB; 25 mM HEPES pH 7.5, 300 mM NaCl, 5% glycerol, 40 mM imidazole pH 8) with lysozyme (Sigma) and PMSF. Samples were lysed by sonication (35% amplitude, 20 s on, 20 s off, for a total of 3 min of sonication on). Cell debris was pelleted by centrifugation (138 000 g, 4 °C), then CtCBM48-containing supernatant was batch-bound with Ni+2 beads (4 °C, 1 h rocking). Beads were washed three times with RB and eluted (25 mM HEPES pH 7.5, 150 mM NaCl, 5% glycerol, 350 mM imidazole pH 8). Samples were run through a Superdex 75 HiLoad 16/600 (GE) column in size exclusion buffer (SEB) 25 mM HEPES pH 7.5, 150 mM NaCl, 5% glycerol. Peak fractions containing CtCBM48 were pooled, concentrated to 2 mg ml–1, flash-frozen in liquid nitrogen, and stored at –80 °C until use. Protein purity was determined through SDS–PAGE and detected through western blotting.
Floridean starch extraction
Mazzaella splendens (Gigartinales, Rhodophyta) collected from Stanley Park, Vancouver, BC (49°180′10″N, 123°070′35″W) were cleaned with 70% ethanol and scrubbed. Samples were frozen with liquid nitrogen and ground to a powder. Samples were resuspended in SEB, sonicated (40% amplitude, 20 s on, 20 s off, for a total of 1 min of sonication on), and filtered through a 100 μm mesh, then a 50 μm mesh. Samples were centrifuged (1186 g). The starch-containing supernatant was collected by centrifugation (13 000 g) and the starch-containing pellet was sonicated (40% amplitude, 5 s on). The pellet was washed three times in deionized water by resuspension, once with 70% ethanol, and then floated on a 65% sucrose cushion and centrifuged (10 000 g). The pellet was washed three times in deionized water and once in 70% ethanol. Ethanol was evaporated off and the starch was used directly. Starch was visually inspected by bright field microscopy with iodine–potassium iodide (IKI) staining.
Carbohydrate co-sedimentation assay
Protein binding to the insoluble carbohydrates (potato starch, microcrystalline cellulose, and floridean starch extracts) was qualitatively assessed in co-sedimentation assays. BSA was used as a non-binding protein control. A 10 mg aliquot of carbohydrate and 100 μg of either CtCBM48 or BSA in 200 μl total volume with SEB was incubated overnight (4 °C, rotating). Samples were centrifuged (16 000 g, 5 min) and the supernatant containing unbound protein was collected. The pellet fraction containing the bound protein was washed three times with 200 μl of SEB and pipetting to resuspend. Bound protein was released by adding 200 μl of 4× reducing SDS loading buffer and heating (80 °C, 10 min). Equal volumes of both fractions were subjected to SDS–PAGE.
Western blotting
CESA proteins were run on 10% SDS–PAGE gels and CBM48 proteins on 18% SDS–PAGE gels. Samples were wet transferred onto nitrocellulose film at 100 V for 1 h. Blots were washed with TBS-T (10 mM Tris pH 7.4, 150 mM NaCl, 0.05% Tween-20), blocked in 5% milk, rinsed twice with TBST, and incubated with primary mouse anti-HIS or anti-Strep at 4 °C with rocking overnight (1:1000 in 5% BSA and 1× TBS-T). Blots were rinsed twice, incubated with secondary antibody for 1 h at room temperature (1:3000 dilution), and washed four times before imaging.
A. thaliana plant growth conditions
Seeds were sterilized with chlorine gas and sown on germination medium (GM) [1× Murashige and Skoog (MS), 1% sucrose, 1× Gamborg’s vitamin mix, 0.05% MES, 0.8% agar at pH 5.8] with antibiotic selection when appropriate. Seeds were cold and dark treated at 4 °C for 4 d, and grown vertically at 21 °C until the first true leaves grew. Seedlings were transplanted onto soil and grown in 18 h light and 6 h dark at 21 °C.
Generation of plant lines in Arabidopsis mutants
CtCESA1 was PCR amplified from Calliarthron cDNA and cloned into the Gateway entry vector pDONR/Zeo by a BP clonase reaction to generate the CtCESA1-pDONR/Zeo clone. For expression in PCW cesa mutants, CtCESA1 was cloned into the empty vectors proUBQ10 with and without green fluorescent protein (GFP) by an LR clonase reaction to produce the destination clones (proUBQ10::CtCESA1 and proUBQ10::GFP:CtCESA1; BASTA plant selection) (Grefen et al., 2010).
For expression in SCW cesa mutants, the promoter of Arabidopsis CESA7 (proAtCESA7) was used to enable expression in a secondary cell wall-specific spatio-temporal manner (Smith et al., 2013). 1500 bp upstream of AtCESA7 was cloned into the Gateway entry vector pDONR/P4-P1R by a BP reaction to generate the proAtCESA7-pDONR/P4-P1R construct. Multisite Gateway was used to combine the proAtCESA7-pDONR/P4-P1R, CtCESA1-pDONR/Zeo, and the binary vector pDEST501 by an LR reaction to generate the final destination clone (proAtCESA7::CtCESA1; hygromycin plant selection). Plasmids were verified by Sanger sequencing. Plasmids were transformed into Agrobacterium, strain GUV3101, and introduced into Arabidopsis plants using the floral dip method.
cesa6/prc1-1 etiolated hypocotyl growth assay
Hypocotyl lengths of dark-grown (etiolated) seedlings were measured. Arabidopsis wild type (Col), cesa6/prc1-1, and T2: proUBQ10::CtCESA1 in a cesa6/prc1-1 background were cold treated for 4 d, exposed to white light for 2 h to synchronize germination, wrapped in three layers of aluminum foil, and grown vertically at 21 °C for 5 d. Seedlings were imaged with a ruler for scale. Hypocotyl lengths from the shoot apical meristem to the start of root hairs were measured using Fiji.
Fluorescence imaging and analysis
Prior to fluorescence imaging, seedlings were light treated for 2 h then dark grown for 4 d on GM plates. Seedlings were submerged in 5 μg ml–1 FM 4-64 dye (with water) on ice for 3–5 min, removed, and immediately mounted onto a microscope slide with water and a 1.5×45×40 coverslip. Seedlings were imaged within 15 min of removal from ice. Fluorescence images were acquired on an Olympus FV1000 confocal laser-scanning microscope using a ×60 NA 1.2 water immersion objective and Olympus Fluoview software (FV10-ASW2). Each channel was imaged sequentially at 15% laser power. Yellow fluorescent protein (YFP) was imaged using a 515 nm laser and 530–545 nm emission filter, GFP using a 473 nm laser and 485–545 nm emission filter, and FM 4-64 stain with a 559/473 nm laser and 634–734 nm emission filter. Photomultiplier voltage was 600–700, offset was 7–12%, gain was 1, and pinhole was 135. False coloring, contrast/brightness adjustments, and overlays were performed using Fiji software. Manders overlap coefficients for co-localization analysis were identified with the Fiji plugin JACOP with a threshold to >450 (Bolte and Cordelières, 2006).
Results
Identification of cellulose synthase genes from the transcriptome of Calliarthron
To identify putative CESA sequences from Calliarthron, a CESA sequence homology model was built and searched against the Calliarthron transcriptomic dataset. Four candidate sequences were recovered and named CtCESA1, -2, -3, and -4 (Table 1A, HMM searches E≤10–40). Candidates CtCESA1, -2, and -4 had one, and CtCESA3 had two predicted isoforms. Each isoform represents a splice variant detected during transcriptome assembly. All four candidate CESA genes had genomic support when queried against the Calliarthron genomic dataset (Table 1A, BLASTN E≤10–5).
Table 1.
Candidate genes encoding (A) CESA and (B) CESA accessory proteins recovered from the transcriptome of C. tuberculosum
| Sequence transcript | Gene | Sequence length (no. of amino acids) | HMMER E-value | Identified in HMMER search | Genome support | |
|---|---|---|---|---|---|---|
| (A) | ||||||
| CESAs | c137075 | CtCESA1 | 877 | 6.60E-159 | Yes | Yes |
| c101319 | CtCESA2 | 797 | 3.40E-53 | Yes | Yes | |
| c142521 | CtCESA3 isoform 1 | 770 | 1.80E-40 | Yes | Yes | |
| c142521 | CtCESA3 isoform 2 | 770 | 2.30E-40 | Yes | Yes | |
| c140545 | CtCESA4 | 649 | 7.70E-48 | Yes | Yes | |
| (B) | ||||||
| CESAaccessory proteins | Not identified | KOR | – | – | No | – |
| accessory | COBRA | – | – | No | – | |
| proteins | CC | – | – | No | – | |
| Not identified | CSI | – | – | No | – | |
| c140937 | CtCMU1 | 601 | 4.50E-08 | Yes | Yes | |
| c141595 | CtCMU2 isoform 2 | 77 | 2.10E-07 | Yes | Yes | |
| c141595 | CtCMU2 isoform 1 | 117 | 4.40E-07 | Yes | No | |
| c141595 | CtCMU2 isoform 3 | 77 | 4.50E-07 | Yes | Yes | |
| c141292 | CtCMU3 isoform 3 | 132 | 1.30E-06 | Yes | No | |
| c139844 | CtCMU4 | 108 | 1.30E-06 | Yes | Yes | |
| c95453 | CtCMU5 | 103 | 2.70E-06 | Yes | Yes | |
| c142512 | CtSTELLO | 727 | 1.20E-32 | Yes | Yes | |
| c142519 | CtCTL1 isoform 1 | 330 | 4.50E-16 | Yes | Yes | |
| c142519 | CtCTL1 isoform 4 | 330 | 4.50E-16 | Yes | Yes | |
| c142519 | CtCTL1 isoform 5 | 335 | 1.00E-13 | Yes | Yes | |
| c142519 | CtCTL1 isoform 2 | 330 | 1.40E-13 | Yes | Yes | |
| c140381 | CtCTL2 | 182 | 1.40E-12 | Yes | Yes | |
| c142519 | CtCTL3 | 335 | 3.00E-12 | Yes | Yes |
Sequences with genomic support are indicated. Amino acid sequence length is based on in silico predictions of the longest ORFs.
CtCESA1 from Calliarthron shares a close relationship with other putative red algal CESAs
A maximum likelihood CESA tree was produced to examine the relationships between the identified CtCESA1, -2, -3, -4, and other known CESAs (Fig. 1). To place the red algal CESA sequences in a broad evolutionary context, the phylogenetic analysis contained amino acid sequences from 50 red algal species as well as diverse organisms (land plants, Embryophyta; red algae, Rhodophyta; Dinophyta; Chlorophyta; Glaucophyta; Phaeophyceae; Haptophyta; Animalia; fungi; Oomycota; bacteria; and cyanobacteria). As CESAs are thought to be a monophyletic group with a shared origin (Nobles and Brown, 2004), the tree was rooted with the closely related hyaluronan synthases (HASs), N-acetylglucosaminyltransferases (NODs), and chitin synthases (CHSs) (gray, Fig. 1).
Fig. 1.
Maximum likelihood CESA gene tree showing relationships of the sequences of Calliarthron (highlighted magenta star) to other taxa. Major groups are collapsed for brevity. Lineage identification is indicated by name and color, with CESAs forming either a linear or rosette terminal complex shape indicated by an L or R, respectively (Tsekos, 1999). Branch support of Ufboot (%) is shown. Sequences were aligned with MUSCLE and sites with <80% coverage were removed. For sequences used, see the bioinformatics data at Zenodo (https://doi.org/10.5281/zenodo.5010240). Outgroups were the closely related hyaluronan synthases, N-acetylglucosaminyltransferases, and chitin synthases. For the fully expanded tree, see Supplementary Fig. S1.
The CESAs from Calliarthron formed two distinct groups (starred in Fig. 1; Supplementary Fig. S1). CtCESA1 was found in the middle of the red algal CESA clade with high support [ultrafast bootstrap support (BS) >95%]. In contrast, CtCESA2, -3, and -4 formed a clade (BS >95%) that did not group with the other putative red algal CESAs. Their relationship with CESAs from other organismal lineages was unresolved due to the weak support in their backbone branches. By including putative CESAs from diverse taxa, this analysis demonstrates that CtCESA1 and other red algal sequences are a sister group to the oomycete and dinoflagellate CESA clade with high support (BS >97%). In contrast, the streptophyte and land plant CESAs formed their own derived clade with short branch lengths and high support (BS >97%), separated from the other major organismal lineages by their CESA-like (CSL) clades D, B, G, and E (Fig. 1). Mapping previous freeze-fracture data onto our phylogeny further differentiated the streptophyte and land plant CESA clade by their rosette-forming CESA terminal complexes (Fig. 1) (Tsekos, 1999). In contrast, CESAs outside this streptophyte and land plant clade had data for linear terminal complexes only. This suggests that the land plant CESAs have probably evolved separately from other organismal lineages, and all the red algal sequences, including CtCESA1, are equally distantly related to the land plant CESAs.
In silico translated ORFs of the four CtCESA candidates were examined for completeness by comparison with the bacterial Rhodobacter BCSA and Arabidopsis CESA6 (Fig. 2A), as these represent functional well-characterized CESAs. All sequences had a centrally located cytosolic GT domain (yellow, Fig. 2A). Upon manual inspection in a multiple sequence alignment, the glucose-coordinating motifs in the GT domain, DDX, DXD, TED, and the QXXRW motif, were highly conserved (Fig. 2B; Supplementary Fig. S2). CtCESA1, -2, and -3 had all seven transmembrane helices, while CtCESA4 had only five (blue, Fig. 2A). The absent transmembrane helices preceding the GT domain in CtCESA4 suggested it is likely a fragmented sequence and, therefore, a poor candidate for further experimental work. Bioinformatics analysis of these significant domains revealed that CtCESA1, -2, and -3 were viable CESA candidates while CtCESA4, which was missing key transmembrane helices, was not.
Fig. 2.
CESA sequence ORF topology comparison between Calliarthron tuberculosum, Griffithsia monilis, Arabidopsis thaliana, and Rhodobacter sphaeroides. (A) Predicted topology map aligned by their glucosyltransferase (GT) domains. Motif identities are indicated in the key. Broad sequence relationships are shown (left). Sequence length is scaled in the horizontal axis. (B) Multiple sequence alignment highlighting the glucose-coordinating residues QXXRW, DDX, DXD, and TED/XXD. Pi=Phytophthora infestans, Py=Pyropia yezoensis. Starred is CtCESA1 used in further experimental studies. Sites in red or blue indicate high sequence similarity. Sequences shown are trimmed for brevity.
In silico translated CESA sequences from linear terminal complex-forming groups (red algal CtCESA1, Pyropia, and oomycete Saprolegnia CESA) were further compared with those from rosette-forming groups (green algal Chlorokybus, and land plant Arabidopsis, Populus, and Physcomitrella CESAs) (Supplementary Fig. S2). Despite linear and rosette-forming CESAs showing conservation in their UDP-glucose-binding motifs and the glycosyltransferase core, the zinc-finger domain, class-specific region (CSR), Ala549 involved in rosette stabilization, and plant conserved region (PCR) are either largely absent or highly divergent in the linear complex-forming CESAs. Additionally, a putative CBM48 was identified in the N-terminus of the CtCESA1 sequence (pink, Fig. 2A; Supplementary Fig. S2). This putative CBM48 is unique to the red algal CESAs, as illustrated by the similar putative Griffithsia CESA (Fig. 2A), and not found in CESAs of other lineages. These differences highlight diversification between the red algal and land plant CESAs. Given the presence of key sequence motifs and close relationship with the other red algal CESAs, CtCESA1 presents a particularly interesting candidate to build on previous sequence identifications of other red algal CESAs.
Heterologous expression and purification of CtCESA1 from yeast and insect cells
To test for glucan synthase activity, CtCESA1 engineered with an N-terminal polyhistidine tag was expressed in eukaryotic heterologous protein expression systems, purified, reconstituted into lipid vesicles, and assayed. The engineered CtCESA1 was expressed in either Spodoptera frugiperda (SF9) insect cell or P. pastoris yeast cell expression systems. As CESAs are transmembrane proteins, embedded in the plasma membrane, detergents are required to extract and solubilize these proteins from their host membrane. To identify an optimal detergent, several common detergents were screened for their ability to solubilize CtCESA1 into solution from insect cell membranes (Supplementary Fig. S3A) and yeast cell membranes (Supplementary Fig. S3B). The previously published PttCESA8 was used as a positive control. CtCESA1, at a predicted size of 101.7 kDa, was partially isolated in 1% LMNG and 0.2% CHS (LMNG+CHS or L+C) (supernatant, Supplementary Fig. S3). LMNG+CHS was used for all further purifications as it was a relatively effective detergent to stabilize CtCESA1 in solution.
The polyhistidine-tagged CtCESA1 was purified first by nickel column affinity purification of solubilized protein from Pichia membranes (Elution; Fig. 3A) then further separated with SEC, with CtCESA1 found in fractions 2, 3, 4, and 5 (Fig. 3B, C). Fractions 4 and 5 represented monomeric proteins at ~100 kDa size and were used for subsequent assays. CtCESA1 isolated from insect cell membranes was purified similarly (Supplementary Fig. S4). From each host system, isolated fractions of CtCESA1 were combined and reconstituted in either GDN detergent or total E. coli lipid proteoliposomes prior to enzymatic assay.
Fig. 3.
Heterologous expression and purification of CtCESA1. (A) CtCESA1 expressed in Pichia and purified from column affinity purification (the arrow indicates the band at the CtCESA1 predicted molecular weight). Purification steps are shown (Pel=pellet; Sup=supernatant; FT=flow through; W=wash; L=ladder). (B) Protein profile of CtCESA1 isolated from the Superdex 200 Increase 10/300 column in size exclusion chromatography (SEC). Fractions of protein taken from SEC are shown above the x-axis. Fractions 4 and 5 corresponding to ~100 kDa were used for subsequent enzyme assays. Initial volumes on the x-axis have been truncated for brevity. (C) Selected protein fractions from (B) western blotting for the presence of CtCESA1 that also has an anti-Strep tag.
Glucan synthase activities of the red algal CtCESA1, land plant PttCESA8, and bacterial BCSA/BCSB complex
CtCESA1 proteins were then tested for glucosyltransferase activity in tracer assays with 3H-labeled UDP-glucose and either Mg2+, Mn2+, or Ca2+, or with no cation added (Ø) (Fig. 4). CESA activity requires an Mg2+ or Mn2+ cofactor. Ca2+ is known to activate callose synthase (β-1,3-glucan), a common contaminate in plant extracts, but not cellulose synthase (Cifuentes et al., 2010). Therefore, measured values in conditions with no cofactor (Ø) or Ca2+ represented background radioactivity.
Fig. 4.
Glucosyltransferase activity of purified CESAs (A) CtCESA1 isolated from heterologous expression in insect cell and yeast cells. (B) CtCESA1 activity in GDN detergent from (A) compared with PttCESA8 and BCSA/BCSB reconstituted proteins or E. coli total lipids, respectively, normalized by protein concentration. UDP-glucose 3H-labeled tracer activity assays was measured with either nothing (Ø), Ca2+ (ca), Mg2+ (mg), or Mn2+ (mn). DPM=disintegrations per minute. Significantly different values are indicated with letters. All measurements were performed in triplicate and represented as one average above each boxplot. Statistical differences determined with Welch’s ANOVA followed by Tukey post-hoc analysis (P<0.01).
CtCESA1 isolated from insect cell membranes only displayed activity in the presence of the Mn2+ cofactor (Fig. 4A). This trend was found in both lipid reconstitution systems (GDN or E. coli proteoliposomes). In the controls (no cofactor or Ca2+) and in the Mg2+ cofactor, there was no significant difference indicated by Welch’s ANOVA (red, Fig. 4A). In the presence of Mn2+, there was an increase of 0.5× radioactivity above background for both CtCESA1 in GDN and proteoliposomes (P≤0.01) (Fig. 4A). While the CtCESA1 isolated from insect cells displayed statistically significant activity in the presence of the Mn2+ cofactor, it was very low.
CtCESA1 isolated from Pichia cells displayed activity in the presence of the Mg2+ cofactor. In the presence of Mg2+, there was a difference in radioactivity 3.5× above background, for Pichia-expressed CtCESA1 in GDN (F3,7=33.81, P≤0.01) (Fig. 4A), indicative of glucan synthase activity. CtCESA1 in proteoliposomes also showed a significant difference in radioactivity in the presence of Mg2+ that was 2× above background (F3,8=63.96, P≤0.01) (Fig. 4A). As predicted, CtCESA1 exposed to either no cofactor (Ø) or Ca2+ showed background levels that were not significantly different in activity (Fig. 4A). When exposed to Mn2+, CtCESA1 in GDN and CtCESA1 in proteoliposomes showed no significant difference from background levels. Thus, CtCESA1 glucan synthase activity varied with experimental conditions, but it was highest when CtCESA1 was purified from Pichia cells in detergent in the presence of the Mg2+ cofactor.
Glucan synthase activities were compared in simultaneous assays between the red algal CtCESA1, land plant PttCESA8, and bacterial BCSA/BCSB complex under their optimal conditions (Fig. 4B). CtCESA1 in GDN detergent was isolated from a Pichia host, PttCESA8 in GDN detergent was isolated from an insect cell host, and the BCSA/BCSB complex in E. coli total lipid proteoliposomes was isolated from a bacterial host. For comparison, all activities were normalized by protein concentration. Relative to background radiation, CtCESA1 showed a 3.5× increase in activity in the presence of Mg2+ (blue, Fig. 4B). Relative to background radiation, PttCESA8 showed a 50× increase in activity in the presence of Mg2+ and a 100× increase in activity in the presence of Mn2+ (green, Fig. 4B). Relative to background radiation, the BCSA/BCSB complex showed a 230× increase in activity in the presence Mg2+ (purple, Fig. 4B). Here, differences were seen in the glucosyltransferase activities of cellulose synthases from different organismal origins, with the least to most active being CtCESA1, PttCESA8, and BCSA/BCSB complex.
CtCESA1’s N-terminal carbohydrate-binding module 48 domain binds floridean starch extracts
CtCESA1’s CBM48 domain (hereafter referred to as CtCBM48) was expressed and purified from E. coli (Fig. 5A; Supplementary Fig. S5). Floridean starch was extracted and verified by increased purple staining with IKI (iodine), similar to their land plant potato starch counterpart (Fig. 5B). Binding of CtCBM48 and BSA, the negative binding control, to floridean starch extracts, potato starch, and crystalline cellulose was tested in co-sedimentation assays. The protein and carbohydrate were incubated together then centrifuged to separate unbound protein in the supernatant from protein bound to the carbohydrate in the pellet. CtCBM48 was largely found in the pellet fractions when incubated with floridean starch, and only minor amounts of CtCBM48 were found in the pellet fractions of potato starch and cellulose (Fig. 5C). In contrast, the BSA negative binding control was consistently found in the supernatant fraction unable to bind the carbohydrates. This indicates that CtCESA1’s predicted CBM48 domain specifically binds floridean starch and not potato starch or cellulose.
Fig. 5.
CtCESA1’s putative CBM48 domain binds floridean starch extracts in co-sedimentation assays. (A) CtCBM48-specific region cloned from CtCESA1. (B) Floridean starch extracts and plant potato starch without and with starch IKI stain. Inset scale=5 μm. (C) Visualization of CtCBM48 and BSA protein fractions from co-sedimentation assays with floridean starch extracts, potato starch, and crystalline cellulose. Carbohydrate-bound protein in the pellet fraction (P) and unbound protein in the supernatant fraction (S) are diagrammed below. Experiments were performed in triplicate.
Identification of CESA accessory proteins in Calliarthron
With the same sequence identification approach described earlier for the CESAs, Calliarthron sequences were recovered for CMU, CTL, and STELLO, but not for CC, COB, CSI, and KOR (Table 1B, HMM searches, E≤10–6). Except for two CtCMU candidates, all recovered putative accessory proteins were supported by the Calliarthron genomic dataset (Table 1B, BLASTN E≤10–5). Based on homology with the Arabidopsis proteins, these results can drive new hypotheses, for example, that cellulose production in Calliarthron could include CESA processing in the Golgi by STELLO (Zhang et al., 2016), interactions with microtubules by CMU, and microfibril assembly by CTL (Anderson and Kieber, 2020).
CtCESA1 partially restores growth defects of the primary cell wall cesa6 mutant, but not cesa3 or cesa7 mutants
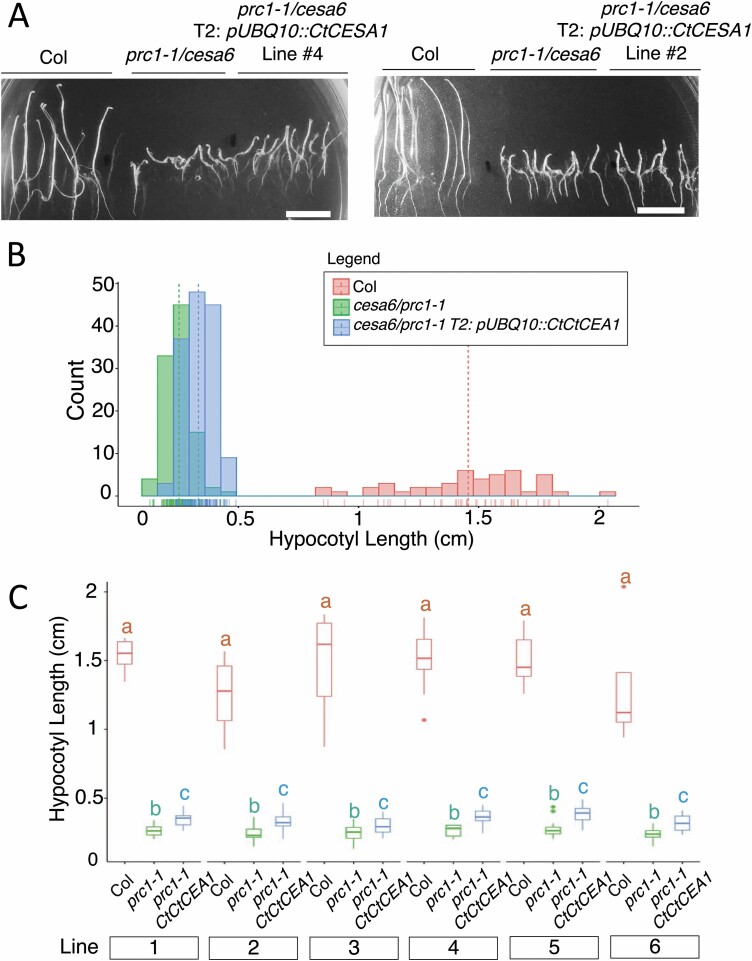
To assess if CtCESA1 is functionally comparable with the Arabidopsis CESA6 in planta, CtCESA1 driven by the constitutively active ubiquitin 10 promoter (pUBQ10::CtCESA1) was introduced into homozygous cesa6/prc1-1 mutant plants and tested for recovery of the hypocotyl growth defect phenotype. Mutations in the primary cell wall-specific CESA6 cause reduced hypocotyl lengths in dark-grown, etiolated, seedlings relative to their wild-type (Col) counterparts (Fig. 6), as previously reported (Fagard et al., 2000; MacKinnon et al., 2006). Six transformed lines of pUBQ10::CtCESA1 in the prc1-1/cesa6 background were recovered and grown to their T2 generation to test for differences in hypocotyl growth. Welch’s ANOVA indicated that there were significant differences in hypocotyl length between dark-grown seedlings of Col, cesa6/prc1-1, and T2: pUBQ10::CtCESA1 in the cesa6/prc1-1 background (Fig. 6A, B), consistent within six independently transformed lines (Fig. 6C). Post-hoc analysis with Games–Howell pairwise comparison tests indicated that hypocotyl lengths were different between all three genotypes, Col, cesa6/prc1-1, and T2: UBQ10::CtCESA1, in the cesa6/prc1-1 background for each transformant line tested. The consistent 0.1 cm difference in hypocotyl length between the cesa6/prc1-1 mutant and the independently produced lines of T2: UBQ10::CtCESA1 in the cesa6/prc1-1 background demonstrated replicable partial growth rescue (Fig. 6C). Taken together with the glucan synthase assays, these data suggest that CtCESA1 was able to produce cellulose in these cesa6 mutants to enhance their growth.
Fig. 6.
Partial rescue of the cesa6/prc1-1 mutant phenotype with the CtCESA1 gene. (A) 4-day-old dark-grown seedlings of Col, cesa6/prc1-1, and two lines of T2: UBQ10::CtCESA1 in the cesa6/prc1-1 background. Scale bars=1 cm. (B) All measured hypocotyl lengths by genotype, Col (n=99), cesa6/prc1-1 (n=155), and T2: UBQ10::CtCESA1 in the cesa6/prc1-1 background (n=229). The dotted line represents the mean value and individual samples are below. (C) Hypocotyl lengths of Col, cesa6/prc1-1, and T2: UBQ10::CtCESA1 in the cesa6/prc1-1 background are statistically different in six independently transformed lines. Statistical analysis was performed within each experimental line, determined with Welch’s ANOVA followed by Games–Howell post-hoc analysis (P<0.01), and significant differences are indicated with letters.
UBQ10::CtCESA1 was also introduced into the homozygous embryo-lethal PCW cesa3 mutant (cesa3-2 allele), and CtCESA1 driven by the CESA7 promoter was introduced into the dwarf SCW cesa7 mutant (irx3-4) allele. However, in both cases, CtCESA1 was unable to recover their growth defects (Supplementary Table S2; Supplementary Fig. S6).
As CESA function predominantly occurs at the plasma membrane, we generated a GFP-tagged CtCESA1 line (pUBQ10::GFP:CtCESA1) in the Arabidopsis cesa6/prc1-1 and cesa3-2 background to look for GFP–CtCESA1 localization in hypocotyl cells of 5-day-old etiolated seedlings. YFP–AtCESA6 in the Arabidopsis cesa6/prc1-1 background was a positive control for PCW CESA localization (Fujita et al., 2011). YFP–AtCESA6 localized predominantly to intracellular Golgi and the cell peripheries (Fig. 7A). GFP–CtCESA1 was also predominantly located at the cell periphery and Golgi in two transformed lines of cesa6/prc1-1 (Fig. 7B) and cesa3-2 (Fig. 7C). These samples were also stained with the sterol dye FM4-64 as a marker of the plasma membrane. As expected, YFP–AtCESA6 co-localized with the FM4-64-stained plasma membrane (Fig. 7A). GFP–CtCESA1 also co-localized with the FM4-64 stain (Fig. 7A, B), demonstrating GFP–CtCESA1 localization to the plasma membrane, probably via a Golgi-mediated pathway.
Fig. 7.
GFP–CtCESA1 localizes at the plasma membrane of cesa6 and cesa3 mutant hypocotyl cells. Dark-grown 4-day-old seedlings expressing either (A) YFP–AtCESA6 (green) in the prc1-1/cesa6 mutant, (B) GFP–CtCESA1 (green) in the prc1-1/cesa6 mutant, or (C) GFP–CtCESA1 (green) in the cesa3-2 mutant background. Seedlings were stained with the plasma membrane dye FM4-64 (red). Fluorescent images were taken sequentially. Merged images show co-localization (thresholded Mander’s coefficients >0.3). Scale bars=25 μm.
Discussion
Aside from plant and bacterial systems, cellulose synthesis in other major lineages remains relatively unexplored. Yet, cellulose is an important cell wall component of many organisms, including Calliarthron species, which require thick cellulosic walls to survive in the wave-swept intertidal zone. As no CESA from red algae had previously been functionally characterized, this work provides the first opportunity to link transcriptomic and genomic information to CESA protein biochemistry in red algae. While four promising CESA gene candidates were identified from the calcifying red alga Calliarthron, further domain and phylogenetic analyses highlighted CtCESA1 as it had a close relationship with other annotated red algal CESAs identified to date (Fig. 1) (Roberts and Roberts, 2009; Matthews et al., 2010; Brawley et al., 2017). These results suggested that the red algal CESAs share a common ancestry and were likely to have been acquired before the divergence of the two major red algal taxonomic classes (Florideophyceae and Bangiophyceae). The functional evidence presented for CESA1 glucan synthase activity in Calliarthron (Fig. 4A) therefore also provides insight into other red algal CESA sequences.
A floridean starch-binding CBM48 domain may regulate CtCESA1 substrate proximity
Previous sequence identification of the red algal CESAs predicted a starch-binding (CBM48) domain (Matthews et al., 2010). The CtCBM48 protein domain in Calliarthron was able to strongly bind red algal floridean starch extracts but not potato starch or crystalline cellulose in vitro. These red algal CESA CBM48 domains have substitutions in their carbohydrate-binding domain that differ from traditional CBM48 domains involved in binding glycogen or plant starches (Matthews et al., 2010). These modified residues may therefore represent substrate specificity for floridean starch, facilitating future identification of other floridean starch-related proteins.
As the N-terminal CBM48 tail of the plasma membrane-bound CtCESA1 and floridean starch are both cytosolically located, they could be interacting in vivo. If the CBM48 domain allows CtCESA1 to associate with starch granules, it could be within close enough proximity to amylose breakdown and UDP-glucose production to feed cellulose production. Additionally, the CBM48 domain is capable of interacting with other CBM48 domains through disulfide bonds (Supplementary Fig. S5). Perhaps the CBM48 domain facilitates intermolecular bonding with other CBM48 domains on starch metabolism proteins in multiprotein complexes. Alternatively, this domain could also facilitate CtCESA1 oligomer interactions. Future protein pull-down assays could help elucidate the functional role of this intriguing CESA–floridean starch interaction.
Variations in CESA activities may reflect in planta demands for cellulose production
The measured rate of glucosyltransferase activity differs among the in vitro purified CESAs, with the fastest to slowest activity being bacterial (BCSA), eudicot plant (PttCESA8), and red algal (CtCESA1), respectively. Granted, these differences in activity cannot be untangled from protein-specific experimental caveats that need to be considered, such as relative protein stability, degradation over time, or the absence of unknown additional secondary modifications or cofactors for protein function. Under the assumption that the measured differences in CESA activity could reflect native activities in vivo, these rates may reflect physiological requirements for cellulose to maintain organism-specific cell growth and biomass production. Cellulose is a structural component in most cell walls of Calliarthron and Populus. However, these organisms have drastically different cellulose deposition patterns and growth rates. In Calliarthron, cellulose is continuously deposited throughout the lifetime of the algae, comprising ~8% of the PCWs and ~22% of the SCWs (Martone et al., 2019), with growth rates of ~2–5 cm year–1 (Smith, 1972; Fisher and Martone, 2014). In contrast, trees in the genus Populus are composed of ~50% cellulose (reviewed in Balatinecz and Kretschmann, 2001) with growth rates of 1–2 m year–1 (Demeritt, 1981). Compared with Calliarthron, Populus species have a higher growth rate, are composed of more cellulose, and would therefore require higher levels of cellulose production to maintain their biomass. The more active PttCESA8 relative to CtCESA1 could reflect these differences in cellulose demand, provided that the activity levels are determined at the protein level.
The functional analysis of the CtCESA1 demonstrated glucose incorporation but not their specific linkage patterns. Cellulose polymers are composed solely of β-1,4-linked glucose. Previous samples of PttCESA8 heterologously expressed in P. pastoris (Purushotham et al., 2016) had variable batch-to-batch contamination of a putative Pichia 1,3-glucan synthase (GSL2). Physcomitrella patens CESA5 samples heterologously expressed under the same conditions also reported batch-to-batch variation of Pichia GSL2 protein presence. Therefore, without linkage analysis, we cannot exclude that some activity seen may be due to the presence of this protein for samples derived from Pichia host cells. However, the fact that background activity was not observed under Ca2+ conditions, which usually stimulates callose synthases (Cifuentes et al., 2010), argues against callose synthase being responsible for the glucan synthase activity observed. In addition, such endogenous callose synthase activity would not explain the above-background activity of the CtCESA1 produced in insect cells, which are not known to contain callose synthases (Fig. 4A).
Hints of conserved CESA trafficking and major enzymatic divergences between CESAs of red algal and land plant lineages
Transformation of Arabidopsis cellulose synthase mutants provides insights into both the putative role of CtCESA1 as a cellulose synthase and the differences between algal and land plant mechanisms of cellulose synthesis. The introduction of CtCESA1, driven by the appropriate promoter for comparable expression with their Arabidopsis host, could function in a land plant and mitigate some of the growth defects in the cesa6/prc1-1 mutant. While the fluorescently tagged protein could be observed at the plasma membrane, it is unclear if it was incorporated into the land plant style rosette cellulose synthase complex or operated independently.
In our phylogenetic analysis, rosette-forming CESA complexes are found solely within the monophyletic streptophyte and land plant CESA clade (Fig. 1). This suggests that rosette complexes are likely a derived architecture, with linear complexes better representing the ancestral state. Given this, it is unclear if linear type CESAs, such as rhodophyte CESAs and presumably CtCESA1, have the structural motifs to integrate into the Arabidopsis CESA rosette. Recent structural elucidation of the plant GhCESA7 and PttCESA8 speculated that the class-specific region at the periphery of the trimer complex is advantageously positioned to be involved in trimer–trimer interaction for rosette formation (Purushotham et al., 2020; Zhang et al., 2021), and Ala549 (arrow, Supplementary Fig. S2) probably stabilizes rosette formation (Arioli et al., 1998). These sites are conserved in rosette-forming but not linear-forming CESA sequences such as CtCESA1 (Supplementary Fig. S2). Given that CtCESA1 appears to lack these regions, it may not be capable of integrating into the Arabidopsis CESA rosettes. However, no large linear arrays were seen in the live-cell imaging, as were observed in freeze-fracture of other red algae (Tsekos et al., 1993; Okuda et al., 1994). Alternatively, it is possible that some Calliarthron homotrimers were able to form and provide the partial recovery observed. Given that cryo-EM of the PttCESA8 expressed in Pichia demonstrated that each subunit of PttCESA8 could produce a glucan chain and that trimers of PttCESA8 formed in this system (Purushotham et al., 2020), CtCESA1s could have conceivably formed their own functional trimers to produce cellulose.
The observation that CtCESA1 was unable to restore the PCW cesa3 and SCW cesa7 mutant phenotypes, and could only moderately restore the growth defects of the PCW cesa6 mutants suggests that the overall processes surrounding cellulose synthesis probably differ between the red alga Calliarthron and the land plant Arabidopsis. These results show that CtCESA1 is generally unable to compensate for the loss of the native CESA genes in Arabidopsis and that their functional compatibility is therefore limited. It is not surprising that the algal gene could not rescue both PCW and SCW mutants, as our phylogenetic analysis strongly supports that the specialization of the land plant PCW and SCW CESAs occurred independently from the evolution of red algal CESAs (Fig. 1). Moreover, the CESA accessory proteins present in land plants are mostly absent in red algae (Table 1B). Accessory protein sequence candidates in Calliarthron were identified for CMU, STELLO, and CTL proteins, but not for KOR, COBRA, CC, or CSI.
Given the partial rescue of the cesa6 background, we must assume that CtCESA1 can arrive at the plasma membrane through the trafficking pathway present in land plants. This is supported by functional observation of GFP-tagged CtCESA1 at the plasma membrane in Arabidopsis. Together this supports a hypothesis that there are deeply conserved CESA trafficking mechanisms to the plasma membrane between the two lineages. Moreover, the recovery of a putative STELLO in Calliarthron has interesting implications for cellulose biosynthesis in the red algae. Golgi-localized STELLO proteins have been suggested to function in proper cellulose synthase complex assembly and trafficking to the plasma membrane by an unknown mechanism (Zhang et al., 2016). Perhaps STELLO proteins in Arabidopsis may facilitate CtCESA1 cellulose synthase complex formation and trafficking to the plasma membrane in a similar fashion to putative CtSTELLOs.
This work provides validation for the annotation of CESA sequences from the red alga Calliarthron as bona fide cellulose synthases, expanding our knowledge from land plant models. Despite some similarities in trafficking, the major differences highlighted suggest that our understanding of cellulose synthesis in land plants should be applied with caution and limitation when looking at evolutionarily distant CESAs such as those in red algae, brown algae, oomycetes, tunicates, and others. These proposed lineage-specific differences further highlight the need for studies in these groups to properly assess their mechanisms of cellulose synthesis.
Supplementary data
The following supplementary data are available at JXB online.
Table S1. Cloning primers used in this study.
Table S2. Genotypes of cesa3-2 and independently transformed lines T2: UBQ10::CtCESA1 in a cesa3-2 background.
Fig. S1. Fully expanded maximum likelihood CESA gene tree showing relationships of Calliarthron’s sequences (magenta) to other taxa.
Fig. S2. Alignment of representative CESA sequences from rosette-forming (from Ca=Chlorokybus atmophyticus, Pt=Populus tremuloides, At=Arabidopsis thaliana, Pp=Physcomitrella patens) and linear-forming (from Sm=Saprolegnia monoica, Ct=Calliarthron tuberculosum, Py=Pyropia yezoensis) terminal complexes (Fugelstad et al., 2009; Mikkelsen et al., 2014).
Fig. S3. Detergent screen shows 1% LMNG+0.2% CHS is acceptable for isolation of heterologously expressed CtCESA1.
Fig. S4. CtCESA1 expressed and purified from SF9 insect cells.
Fig. S5. Heterologous expression and purification of CtCBM48, CtCESA1’s floridean starch-binding domain.
Fig. S6. CtCESA1 driven by the Arabidopsis CESA7 promoter is unable to recover the cesa7/irx3-4 growth defects.
Acknowledgements
We thank the University of British Columbia Bioimaging Facility for technical support; Irek Gorniak for BCSA/BCSB proteins; Alana Breitkreutz and Varoon Pornsinsiriruk for collection of Mazzaella and IKI stain; and Pacheedaht First Nations for allowing algal collections on their land. Dr. Miki Fujita and Professor Geoffery Wasteneys kindly provided the YFP–AtCESA6 in the Arabidopsis cesa6/prc1-1 background.
Author contributions
JX: conducted bioinformatics analysis, extracted floridean starch, conducted plant assays, and CtCESA1 localizations; JX, PP, and RH: expressed, purified, and tested CtCESA1 activity; JFA and JX: conceived and designed the CtCBM48 project; JX, JFA, and CM: expressed and purified CtCBM48; JZ, PVP, PM, and LS: funding acquisition; JX, PM, and LS: conceptualization, design, and writing. All authors approved the final manuscript.
Conflicts of interest
No conflicts of interest declared.
Funding
This work was supported by the Natural Sciences and Engineering Research Council (NSERC) Discovery Grants to ALS (RGPIN-2019-04592) and PTM (RGPIN-2019-06240); JX was supported by NSERC Canada Graduate Scholarships—Master’s, Michael Smith Foreign Study Supplement, Postgraduate Scholarship-D, and Gordan Campbell Scholarship. JZ, RH, and PP are supported by The Center for Lignocellulose Structure and Formation, an Energy Frontier Research Center funded by the US Department of Energy, Office of Science, Basic Energy Sciences (award no. DESC0001090). JFA was supported by National Institutes of Health grant no. 1F32GM126647-01.
Data availability
The CtCESA1 sequence can be found in GenBank (accession number MZ779117) and the transcriptome dataset is deposited in the European Nucleotide Archive (project number PRJEB39919). Sequence data for the bioinformatics analysis can be found at https://doi.org/10.5281/zenodo.5010240, (Xue et al., 2021).
References
- Allen H, Wei D, Gu Y, Li S. 2021. A historical perspective on the regulation of cellulose biosynthesis. Carbohydrate Polymers 252, 117022. [DOI] [PubMed] [Google Scholar]
- Anderson CT, Kieber JJ. 2020. Dynamic construction, perception, and remodeling of plant cell walls. Annual Review of Plant Biology 71, 39–69. [DOI] [PubMed] [Google Scholar]
- Arioli T, Peng L, Betzner AS, et al. 1998. Molecular analysis of cellulose biosynthesis in Arabidopsis. Science 279, 717–720. [DOI] [PubMed] [Google Scholar]
- Balatinecz JJ, Kretschmann DE. 2001. Properties and utilization of poplar wood. In: Dickmann DI, Isebrands JG, Eckenwalder JE, Richardson J, eds. Poplar culture in North America. Ottawa: NRC Research Press, 277–291. [Google Scholar]
- Barbier FF, Chabikwa TG, Ahsan MU, Cook SE, Powell R, Tanurdzic M, Beveridge CA. 2019. A phenol/chloroform-free method to extract nucleic acids from recalcitrant, woody tropical species for gene expression and sequencing. Plant Methods 15, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blommel PG, Fox BG.. 2007. A combined approach to improving large-scale production of tobacco etch virus protease. Protein Expression and Purification 55, 53–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolte S, Cordelières FP. 2006. A guided tour into subcellular colocalization analysis in light microscopy. Journal of Microscopy 224, 213–232. [DOI] [PubMed] [Google Scholar]
- Brawley SH, Blouin NA, Ficko-Blean E, et al. 2017. Insights into the red algae and eukaryotic evolution from the genome of Porphyra umbilicalis (Bangiophyceae, Rhodophyta). Proceedings of the National Academy of Sciences, USA 114, E6361–E6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DM, Zeef LA, Ellis J, Goodacre R, Turner SR. 2005. Identification of novel genes in Arabidopsis involved in secondary cell wall formation using expression profiling and reverse genetics. The Plant Cell 17, 2281–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capella-Gutiérrez S, Silla-Martínez JM, Gabaldón T. 2009. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 25, 1972–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CX, Yang EC, Banerjee T, Yoon HS, Martone PT, Estevez JM, Bhattacharya D. 2011. Red and green algal monophyly and extensive gene sharing found in a rich repertoire of red algal genes. Current Biology 21, 328–333. [DOI] [PubMed] [Google Scholar]
- Cho SH, Purushotham P, Fang C, Maranas C, Díaz-Moreno SM, Bulone V, Zimmer J, Kumar M, Nixon BT. 2017. Synthesis and self-assembly of cellulose microfibrils from reconstituted cellulose synthase. Plant Physiology 175, 146–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cifuentes C, Bulone V, Emons AM. 2010. Biosynthesis of callose and cellulose by detergent extracts of tobacco cell membranes and quantification of the polymers synthesized in vitro. Journal of Integrative Plant Biology 52, 221–233. [DOI] [PubMed] [Google Scholar]
- Demeritt ME. 1981. Growth of hybrid poplars in Pennsylvania and Maryland clonal tests. US Department of Agriculture, Forest Service. [Google Scholar]
- Denny M, Mach K, Tepler S, Martone P. 2013. Indefatigable: an erect coralline alga is highly resistant to fatigue. Journal of Experimental Biology 216, 3772–3780. [DOI] [PubMed] [Google Scholar]
- Edgar RC. 2004. MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics 5, 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagard M, Desnos T, Desprez T, Goubet F, Refregier G, Mouille G, McCann M, Rayon C, Vernhettes S, Höfte H. 2000. PROCUSTE1 encodes a cellulose synthase required for normal cell elongation specifically in roots and dark-grown hypocotyls of Arabidopsis. The Plant Cell 12, 2409–2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn RD, Clements J, Eddy SR. 2011. HMMER web server: interactive sequence similarity searching. Nucleic Acids Research 39, W29–W37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher K, Martone PT. 2014. Field study of growth and calcification rates of three species of articulated coralline algae in British Columbia, Canada. The Biological Bulletin 226, 121–130. [DOI] [PubMed] [Google Scholar]
- Fugelstad J, Bouzenzana J, Djerbi S, Guerriero G, Ezcurra I, Teeri TT, Arvestad L, Bulone V. 2009. Identification of the cellulose synthase genes from the Oomycete Saprolegnia monoica and effect of cellulose synthesis inhibitors on gene expression and enzyme activity. Fungal Genetics and Biology 46, 759–767. [DOI] [PubMed] [Google Scholar]
- Fujita M, Himmelspach R, Hocart CH, Williamson RE, Mansfield SD, Wasteneys GO. 2011. Cortical microtubules optimize cell‐wall crystallinity to drive unidirectional growth in Arabidopsis. The Plant Journal 66, 915–928. [DOI] [PubMed] [Google Scholar]
- Grefen C, Donald N, Hashimoto K, Kudla J, Schumacher K, Blatt MR. 2010. A ubiquitin-10 promoter-based vector set for fluorescent protein tagging facilitates temporal stability and native protein distribution in transient and stable expression studies. The Plant Journal 64, 355–365. [DOI] [PubMed] [Google Scholar]
- Hansson MD, Rzeznicka K, Rosenbäck M, Hansson M, Sirijovski N. 2008. PCR-mediated deletion of plasmid DNA. Analytical Biochemistry 375, 373–375. [DOI] [PubMed] [Google Scholar]
- Hoang DT, Chernomor O, von Haeseler A, Minh BQ, Vinh LS. 2018. UFBoot2: improving the ultrafast bootstrap approximation. Molecular Biology and Evolution 35, 518–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura S, Laosinchai W, Itoh T, Cui X, Linder CR, Brown RM Jr. 1999. Immunogold labeling of rosette terminal cellulose-synthesizing complexes in the vascular plant Vigna angularis. The Plant Cell 11, 2075–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura M, Koyama T, Nakano Y, Uemura D. 2007. Characterization of a natural inducer of coral larval metamorphosis. Journal of Experimental Marine Biology and Ecology 340, 96–102. [Google Scholar]
- Lampugnani ER, Flores-Sandoval E, Tan QW, Mutwil M, Bowman JL, Persson S. 2019. Cellulose synthesis—central components and their evolutionary relationships. Trends in Plant Science 24, 402–412. [DOI] [PubMed] [Google Scholar]
- MacKinnon IM, Sturcová A, Sugimoto-Shirasu K, His I, McCann MC, Jarvis MC. 2006. Cell-wall structure and anisotropy in procuste, a cellulose synthase mutant of Arabidopsis thaliana. Planta 224, 438–448. [DOI] [PubMed] [Google Scholar]
- Marchler-Bauer A, Derbyshire MK, Gonzales NR, et al. 2015. CDD: NCBI’s conserved domain database. Nucleic Acids Research 43, D222–D226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martone PT. 2010. Quantifying growth and calcium carbonate deposition of Calliarthron cheilosporioides (Corallinales, Rhodophyta) in the field using a persistent vital stain. Journal of Phycology 46, 13–17. [Google Scholar]
- Martone PT, Denny MW. 2008. To bend a coralline: effect of joint morphology on flexibility and stress amplification in an articulated calcified seaweed. Journal of Experimental Biology 211, 3421–3432. [DOI] [PubMed] [Google Scholar]
- Martone PT, Estevez JM, Lu F, Ruel K, Denny MW, Somerville C, Ralph J. 2009. Discovery of lignin in seaweed reveals convergent evolution of cell-wall architecture. Current Biology 19, 169–175. [DOI] [PubMed] [Google Scholar]
- Martone PT, Janot K, Fujita M, Wasteneys G, Ruel K, Joseleau JP, Estevez JM. 2019. Cellulose-rich secondary walls in wave-swept red macroalgae fortify flexible tissues. Planta 250, 1867–1879. [DOI] [PubMed] [Google Scholar]
- Matthews PR, Schindler M, Howles P, Arioli T, Williamson RE. 2010. A CESA from Griffithsia monilis (Rhodophyta, Florideophyceae) has a family 48 carbohydrate-binding module. Journal of Experimental Botany 61, 4461–4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane HE, Döring A, Persson S. 2014. The cell biology of cellulose synthesis. Annual Review of Plant Biology 65, 69–94. [DOI] [PubMed] [Google Scholar]
- Mikkelsen MD, Harholt J, Ulvskov P, Johansen IE, Fangel JU, Doblin MS, Bacic A, Willats WG. 2014. Evidence for land plant cell wall biosynthetic mechanisms in charophyte green algae. Annals of Botany 114, 1217–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JL, Strumillo J, Zimmer J. 2013. Crystallographic snapshot of cellulose synthesis and membrane translocation. Nature 493, 181–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson WA. 2009. Calcified macroalgae critical to coastal ecosystems and vulnerable to change: a review. Marine and Freshwater Research 60, 787–801. [Google Scholar]
- Nguyen LT, Schmidt HA, von Haeseler A, Minh BQ. 2015. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Molecular Biology and Evolution 32, 268–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama Y, Sugiyama J, Chanzy H, Langan P. 2003. Crystal structure and hydrogen bonding system in cellulose I(alpha) from synchrotron X-ray and neutron fiber diffraction. Journal of the American Chemical Society 125, 14300–14306. [DOI] [PubMed] [Google Scholar]
- Nixon BT, Mansouri K, Singh A, et al. 2016. Comparative structural and computational analysis supports eighteen cellulose synthases in the plant cellulose synthesis complex. Scientific Reports 6, 28696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobles DR, Brown RM. 2004. The pivotal role of cyanobacteria in the evolution of cellulose synthases and cellulose synthase-like proteins. Cellulose 11, 437–448. [Google Scholar]
- O’Leary JK, Barry JP, Gabrielson PW, Rogers-Bennett L, Potts DC, Palumbi SR, Micheli F. 2017. Calcifying algae maintain settlement cues to larval abalone following algal exposure to extreme ocean acidification. Scientific Reports 7, 5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda K, Tsekos L, Brown RM. 1994. Cellulose microfibril assembly in Erythrocladia subintegra Rosenv.: an ideal system for understanding the relationship between synthesizing complexes (TCs) and microfibril crystallization. Protoplasma 180, 49–58. [Google Scholar]
- Omadjela O, Narahari A, Strumillo J, Mélida H, Mazur O, Bulone V, Zimmer J. 2013. BcsA and BcsB form the catalytically active core of bacterial cellulose synthase sufficient for in vitro cellulose synthesis. Proceedings of the National Academy of Sciences, USA 110, 17856–17861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pear JR, Kawagoe Y, Schreckengost WE, Delmer DP, Stalker DM. 1996. Higher plants contain homologs of the bacterial celA genes encoding the catalytic subunit of cellulose synthase. Proceedings of the National Academy of Sciences, USA 93, 12637–12642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson S, Paredez A, Carroll A, Palsdottir H, Doblin M, Poindexter P, Khitrov N, Auer M, Somerville CR. 2007. Genetic evidence for three unique components in primary cell-wall cellulose synthase complexes in Arabidopsis. Proceedings of the National Academy of Sciences, USA 104, 15566–15571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purushotham P, Cho SH, Díaz-Moreno SM, Kumar M, Nixon BT, Bulone V, Zimmer J. 2016. A single heterologously expressed plant cellulose synthase isoform is sufficient for cellulose microfibril formation in vitro. Proceedings of the National Academy of Sciences, USA 113, 11360–11365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purushotham P, Ho R, Zimmer J. 2020. Architecture of a catalytically active homotrimeric plant cellulose synthase complex. Science 369, 1089–1094. [DOI] [PubMed] [Google Scholar]
- Rice P, Longden I, Bleasby A. 2000. EMBOSS: the European molecular biology open software suite. Trends in Genetics 16, 276–277. [DOI] [PubMed] [Google Scholar]
- Roberts E, Roberts AW. 2009. A cellulose synthase (CESA) gene from the red alga Porphyra yezoensis (rhodophyta). Journal of Phycology 45, 203–212. [DOI] [PubMed] [Google Scholar]
- Rombel IT, Sykes KF, Rayner S, Johnston SA. 2002. ORF-FINDER: a vector for high-throughput gene identification. Gene 282, 33–41. [DOI] [PubMed] [Google Scholar]
- Schmidt TGM, Skerra A.. 2007. The Strep-tag system for one-step purification and high-affinity detection or capturing of proteins. Nature Protocols 2, 1528–1535. [DOI] [PubMed] [Google Scholar]
- Smith SV. 1972. Production of calcium carbonate on the mainland shelf of Southern California. Limnology and Oceanography 17, 28–41. [Google Scholar]
- Smith RA, Schuetz M, Roach M, Mansfield SD, Ellis B, Samuels L. 2013. Neighboring parenchyma cells contribute to Arabidopsis xylem lignification, while lignification of interfascicular fibers is cell autonomous. The Plant Cell 25, 3988–3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor NG, Howells RM, Huttly AK, Vickers K, Turner SR. 2003. Interactions among three distinct CesA proteins essential for cellulose synthesis. Proceedings of the National Academy of Sciences, USA 100, 1450–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tester RF, Karkalas J, Qi X. 2004. Starch—composition, fine structure and architecture. Journal of Cereal Science 39, 151–165. [Google Scholar]
- Tsekos I. 1999. The sites of cellulose synthesis in algae: diversity and evolution of cellulose-synthesizing enzyme complexes. Journal of Phycology 35, 635–655. [Google Scholar]
- Tsekos I, Reiss H-D, Schnepf E. 1993. Cell-wall structure and supramolecular organization of the plasma membrane of marine red algae visualized by freeze-fracture. Acta Botanica Neerlandica 42, 119–132. [Google Scholar]
- Tsirigos KD, Peters C, Shu N, Käll L, Elofsson A. 2015. The TOPCONS web server for consensus prediction of membrane protein topology and signal peptides. Nucleic Acids Research 43, W401–W407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner SR, Somerville CR. 1997. Collapsed xylem phenotype of Arabidopsis identifies mutants deficient in cellulose deposition in the secondary cell wall. The Plant Cell 9, 689–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellosillo T, Dinneny JR, Somerville CR, Ehrhardt DW. 2021. TRANVIA (TVA) facilitates cellulose synthase trafficking and delivery to the plasma membrane. Proceedings of the National Academy of Sciences, USA 118, e2021790118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viola R, Nyvall P, Pedersén M. 2001. The unique features of starch metabolism in red algae. Proceedings of the Royal Society B: Biological Sciences 268, 1417–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams EA, Craigie A, Yeates A, Degnan SM. 2008. Articulated coralline algae of the genus Amphiroa are highly effective natural inducers of settlement in the tropical abalone Haliotis asinina. The Biological Bulletin 215, 98–107. [DOI] [PubMed] [Google Scholar]
- Xue J, Purushotham P, Acheson JF, Ho R, Zimmer J, McFarlane C, Van Petegem F, Martone PT, Samuels AL. 2021. Data from: Characterization of a cellulose synthase from the marine red alga Calliarthron tuberculosum (Corallinales). Zenodo doi: 10.5281/zenodo.5010240. [DOI] [PMC free article] [PubMed]
- Yoon HS, Zuccarello GC, Bhattacharya D. 2010. Evolutionary history and taxonomy of red algae. In: Seckbach J, Chapman DJ, eds. Red algae in the genomic age. Dordrecht: Springer Netherlands, 25–42. [Google Scholar]
- Yu S, Blennow A, Bojko M, Madsen F, Olsen CE, Engelsen SB. 2002. Physico-chemical characterization of floridean starch of red algae. Starch/Staerke 54, 66–74. [Google Scholar]
- Zhang Y, Nikolovski N, Sorieul M, et al. 2016. Golgi-localized STELLO proteins regulate the assembly and trafficking of cellulose synthase complexes in Arabidopsis. Nature Communications 7, 11656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Xue Y, Guan Z, et al. 2021. Structural insights into homotrimeric assembly of cellulose synthase CesA7 from Gossypium hirsutum. Plant Biotechnology Journal 19, 1579–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The CtCESA1 sequence can be found in GenBank (accession number MZ779117) and the transcriptome dataset is deposited in the European Nucleotide Archive (project number PRJEB39919). Sequence data for the bioinformatics analysis can be found at https://doi.org/10.5281/zenodo.5010240, (Xue et al., 2021).