Abstract
Cardiac voltage-gated sodium channel gain-of-function prolongs repolarization in the long-QT syndrome type 3 (LQT3). Previous studies suggest that narrowing the perinexus within the intercalated disc, leading to rapid sodium depletion, attenuates LQT3-associated action potential duration (APD) prolongation. However, it remains unknown whether extracellular sodium concentration modulates APD prolongation during sodium channel gain-of-function. We hypothesized that elevated extracellular sodium concentration and widened perinexus synergistically prolong APD in LQT3. LQT3 was induced with sea anemone toxin (ATXII) in Langendorff-perfused guinea pig hearts (n = 34). Sodium concentration was increased from 145 to 160 mM. Perinexal expansion was induced with mannitol or the sodium channel β1-subunit adhesion domain antagonist (βadp1). Epicardial ventricular action potentials were optically mapped. Individual and combined effects of varying clefts and sodium concentrations were simulated in a computational model. With ATXII, both mannitol and βadp1 significantly widened the perinexus and prolonged APD, respectively. The elevated sodium concentration alone significantly prolonged APD as well. Importantly, the combination of elevated sodium concentration and perinexal widening synergistically prolonged APD. Computational modeling results were consistent with animal experiments. Concurrently elevating extracellular sodium and increasing intercalated disc edema prolongs repolarization more than the individual interventions alone in LQT3. This synergistic effect suggests an important clinical implication that hypernatremia in the presence of cardiac edema can markedly increase LQT3-associated APD prolongation. Therefore, to our knowledge, this is the first study to provide evidence of a tractable and effective strategy to mitigate LQT3 phenotype by means of managing sodium levels and preventing cardiac edema in patients.
NEW & NOTEWORTHY This is the first study to demonstrate that the long-QT syndrome type 3 (LQT3) phenotype can be exacerbated or concealed by regulating extracellular sodium concentrations and/or the intercalated disc separation. The animal experiments and computational modeling in the current study reveal a critically important clinical implication: sodium dysregulation in the presence of edema within the intercalated disc can markedly increase the risk of arrhythmia in LQT3. These findings strongly suggest that maintaining extracellular sodium within normal physiological limits may be an effective and inexpensive therapeutic option for patients with congenital or acquired sodium channel gain-of-function diseases.
Keywords: action potential duration, gain-of-function, perinexus, sodium concentration, Nav1.5
INTRODUCTION
Congenital long-QT syndrome type 3 (LQT3) is associated with gain-of-function (GOF) mutations in the SCN5A gene that encodes the cardiac voltage-gated sodium channel α-subunit (Nav1.5) (1). These mutations result in incomplete inactivation of Nav1.5, leading to increased late sodium current (INaL) and prolonged ventricular repolarization, together forming an arrhythmogenic substrate (2, 3). Prolonged repolarization can manifest on the surface electrocardiogram (ECG) as a prolonged QT interval (1). However, some patients with confirmed LQT3-associated variants present with normal corrected QT intervals, which is referred to as a concealed LQT3 phenotype (4, 5). A clinical study investigating the incidence and prevalence of long-QT syndromes suggests that 28% of all patients with LQT3 have a concealed phenotype (6). Although the mechanism of the concealed LQT3 phenotype has not been clinically confirmed, our group has determined that this phenotype can be masked experimentally through changes in ephaptic coupling that are mediated by narrowing an intercalated disc (ID) nanodomain, named the perinexus (7–10). A fundamental prerequisite for this mechanism is that Nav1.5 is densely expressed within the ID (11, 12), specifically within the perinexus (13), and thereby the associated integrated current through Nav1.5 is limited by local extracellular sodium ion availability. Thus, the perinexus may serve as a key extracellular nanodomain modulating INaL in diseases such as LQT3 (7).
We previously proposed that changes in perinexal width (Wp) can modulate action potential repolarization in LQT3 through an ionic self-attenuation mechanism (7, 14, 15). This putative mechanism suggests that narrowing the perinexus shortens action potential duration (APD) via rapid depletion of local sodium ions within this Nav1.5-rich nanodomain. Briefly, activation of Nav1.5 causes a large and fast inward sodium current (INa), rapidly depleting sodium ions in the narrow extracellular perinexus. This depletion of local sodium ions within the perinexal nanodomain subsequently reduces the sodium reversal potential (ENa) via a negative feedback mechanism, thereby attenuating INaL, and ultimately resulting in normal APD. Conversely, expanding the perinexus would blunt the ability of INa to deplete local sodium ions and the associated reduction of ENa, thereby permitting increased INaL and prolonged APD in LQT3. However, under normal conditions with physiological Nav1.5 inactivation, perinexal widening has no effect on APD in ex vivo hearts (7, 16), which suggests that APD prolongation due to widened perinexi preferentially occurs under conditions of incomplete Nav1.5 inactivation. Therefore, perinexal expansion could play a role in APD prolongation in any condition associated with increased INaL, including heart failure, coronary artery disease, diabetes mellitus, and atrial fibrillation (17–20).
Currently, perinexal expansion in isolated hearts can be achieved by two approaches with different mechanisms. The first approach uses mannitol, a hyperosmotic agent inducing acute interstitial edema, which has been demonstrated to expand the perinexus (21). The second one uses βadp1, a novel inhibitor of homophilic adhesion by voltage-gated sodium channel β1-subunit, which is required for regulating perinexal intermembrane distance (16). Importantly, although previous studies and computational models suggest local sodium ion depletion may conceal LQT3, it remains unknown whether modulating extracellular sodium alone or in conjunction with perinexal expansion exacerbates APD prolongation secondary to sodium channel GOF. This gap in knowledge has important clinical implications if extracellular sodium concentrations can conceal Nav1.5 GOF.
We reason that for a fixed extracellular sodium concentration, widening the perinexus increases the number of sodium ions within this nanodomain, just as increasing extracellular sodium concentration should increase the number of ions for a given perinexal volume. Therefore, we hypothesize that increasing extracellular sodium concentration prolongs APD and, when perinexi are widened, exacerbates APD prolongation under conditions of sodium channel GOF. In this study, we investigate the effect of elevated extracellular sodium concentration on APD with and without perinexal expansion in both a drug-induced animal model and a computational model of LQT3. We determine that perinexal widening and elevated sodium concentration each prolong APD, and combined, they have a synergistic effect on APD prolongation. Finally, the results of this study imply that high levels of serum sodium could be a risk factor for APD prolongation in cardiac diseases associated with enhanced INaL.
METHODS
The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Publication No. 85-23, Revised 1996). All animal study protocols were approved by the Institutional Animal Care and Use Committee at the Virginia Polytechnic Institute and State University.
Optical Mapping
Optical mapping of Langendorff-perfused guinea pig hearts has been extensively described by our group (7, 22, 23). Briefly, male, 3-mo-old, albino, 500-g Hartley guinea pigs (n = 34, Hilltop Lab Animals, Scottsdale, PA) were anesthetized with isoflurane inhalation. Hearts were rapidly excised, cannulated (<5 min), and retrogradely perfused with a crystalloid perfusion solution, consisting of (in mM) 1.25 CaCl2, 139.5 NaCl, 4.56 KCl, 5.5. dextrose, 0.7 MgCl2, 10 HEPES, and 5.5 NaOH (pH = 7.4). A 145 mM sodium concentration (145Na) is a result of 139.5 mM NaCl and 5.5 mM NaOH, and a high-sodium concentration of 160 mM (160Na) is a result of 154.5 mM NaCl and 5.5 mM NaOH. Importantly, 160Na was chosen to model clinically relevant hypernatremia (24). Temperature was maintained at 36°C in a three-dimensional (3-D) printed polylactic acid bath (22). Atria were removed and ventricles electrically stimulated by a bipolar plunge electrode placed in the right ventricular septum near the atrioventricular node with a current set at ×1.5 the minimum stimulation threshold.
The voltage-sensitive fluorophore, di-4-ANEPPS (15 µM, Biotium, CA), was perfused into the heart, and the heart position was visually adjusted to move the left anterior descending artery in the center of the field of view so that the anterior epicardial surfaces of both ventricles were mapped during external pacing to record optical action potentials with a MiCAM HS02-CMOS camera (SciMedia: 92 × 80 pixels, field of view = 14.02 × 12.19 mm). The electromechanical uncoupler, blebbistatin (10 µM, ApexBio Tech, LLC), was added to the perfusate to reduce cardiac motion. An INaL agonist, sea anemone venom ATXII (7 nM, Alomone, Jerusalem, Israel) (7), was perfused into the heart to simulate the phenotype of long-QT syndrome type 3 (LQT3). Mannitol (26.1 g/L, Sigma Aldrich), which induces cardiac edema (7, 21), or βadp1 (1 µM, LifeTein, LLC), which is a sodium channel β1-subunit adhesion domain antagonist (16), were perfused to induce perinexal expansion.
Time control experiments with 145Na (baseline, n = 6 hearts) or ATXII (n = 6 hearts) over 60 min were performed to determine APD stability over time. 145Na + ATXII significantly prolonged APD relative to 145Na at either 30-min or 60-min perfusion, respectively, and there is no significant change in APD between 30 and 60 min with 145Na or 145Na + ATXII (Supplemental Material 1, Fig. S1, A and C, see https://doi.org/10.6084/m9.figshare.14825058). Therefore, for experiments involving ATXII and mannitol, a subset of hearts (n = 6) was perfused with ATXII for 60 min, whereas another subset of hearts (n = 5) was perfused with ATXII and mannitol for 30 min following 30 min of perfusion with ATXII.
Time control experiments with βadp1 were also performed to determine APD stability over 40 min. There is no significant change in APD between 20 and 40 min with 145Na + βadp1 (n = 4 hearts) or 145Na + ATXII + βadp1 (n = 4 hearts), respectively. As expected, ATXII still significantly prolonged APD in the presence of βadp1 (Supplemental Material 1, Fig. S1, B and D). Therefore, for βadp1 experiments, the hearts were perfused with βadp1 for 20 min after 20-min perfusion of ATXII (n = 5 hearts). For high-sodium concentration experiments, the hearts were perfused with 160Na for 20 min after 20-min perfusion of 145Na in the absence and presence of βadp1 (n = 8 hearts). Half of the experiments were performed in a blinded manner by a second experimentalist for the purpose of rigor and reproducibility. Since the interventions cannot be washed out over the time course of experiments, the solution order is not randomized.
Optical signals were recorded during steady-state pacing at progressively increasing cycle lengths (CL) of 200, 300, 400, 500, 600, 700, and 800 ms until intrinsic activity prevented 1:1 capture. Action potential alternans were not observed under any experimental condition (Supplemental Material 1, Fig. S2); therefore, action potential duration (APD) of only one optical action potential in each of 1,840 pixels was calculated after 2 × 2 spatial binning. The average APD across the array was considered a single data point for a heart at one pacing CL. APD was measured as the difference between the time of the maximum upstroke velocity and 90% or 70% repolarization (APD90 and APD70, respectively) of the optical action potential. APD90 and APD70 were only reported when the heart was successfully paced at the indicated CL. The data for APD70 can be found in the supplemental materials.
Arrhythmia Induction Protocol
An S1-S2 stimulation protocol (25, 26) was performed as follows. S1 and S2 were stimulated with a 2 ms pulse width through the same bipolar plunge electrode inserted from the right ventricular septum to evaluate the susceptibility to cardiac arrhythmia perfused in the following order: 145Na + ATXII, 145Na + ATXII + βadp1, and 160Na + ATXII + βadp1. The heart was paced with a train of 10 regular (S1) pulses followed by a premature extra stimulus (S2). All hearts were stimulated with an S1 of 700 ms, and the S2 was progressively reduced by as low as 1 ms until loss of 1:1 capture or arrhythmia induction.
Transmission Electron Microscopy
Left ventricular tissue was collected at the end of the optical mapping experiment and fixed with glutaraldehyde as previously described for transmission electron microscopy (TEM) (27). A minimum of 20 images of gap junctions and the associated perinexus were obtained at ×150,000 magnification for each heart. For each image, i.e., each perinexus, the mean perinexal width (Wp) was calculated over the distance of 30–105 nm from the edge of the gap junction plaque by a MATLAB algorithm developed in our laboratory (28). The average Wp for each heart was obtained by averaging the measured Wp from each image for a given heart. Wp from 145Na + ATXII, 145Na + ATXII + mannitol, and 145Na or 160Na + ATXII + βadp1 is reported.
Simulations
We simulated a 50-cell cable (1-dimensional tissue) comprised of individual myocytes coupled via gap junctions and ephaptic coupling, as in prior work by us and others (7–9, 29–31). We accounted for nonuniform sodium channel subcellular localization by spatially discretizing each cell into multiple (10) axial membrane patches along the length of the cell and two intercalated disc (ID) membrane patches at the ends of the cell. Unless otherwise stated, 90% of sodium channels were localized in the ID patches. A T-shaped network of cleft axial resistances and a radial resistance, proportional and inversely proportional, respectively, to the intercellular cleft width (w, proportional to experimentally measured Wp), governed extracellular potentials at the ID and cleft. ID sodium currents and diffusion with the bulk extracellular space governed the extracellular cleft sodium ion concentration () dynamics, which also depended on the cleft volume.
We used an established ventricular guinea pig myocyte ionic model (32) representing individual ion channel dynamics and incorporated a Markov model of an LQT3-associated Nav1.5 GOF mutant (33), which reproduces a pronounced INaL. Specifically, the mutant channel model exhibited two modes of gating, a “background mode” and a “burst mode,” the latter of which represented a population of channels that transiently fail to inactivate. Full model equations, parameters, and details of the numerical integration are reported in our prior work (7).
The cable was paced at one end with a specified CL. We performed a computational parameter study, varying values for CL, w, and bulk extracellular sodium concentration (Nao). For each simulation, APD was calculated by taking the difference between the action potential repolarization time (defined as the time Vm crossed below −60 mV) and activation time (above −60 mV) for each cell and averaging the values for cells 15 to 35. The simulation codes can be found in Supplemental Material 2, see https://doi.org/10.6084/m9.figshare.16624240.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism 8 (GraphPad Software Inc., San Diego, CA). The results are presented as means ± SD. The unpaired Student’s t test was performed for statistical analysis of Wp. Comparisons of APD90, APD70, and QT interval at a single pacing CL were analyzed with the unpaired Student’s t test or one-way ANOVA followed by Bonferroni correction for multiple comparisons. Statistical comparisons of APD90, APD70, ΔAPD90, and ΔQT interval as a function of CL were made using a two-way ANOVA followed by Bonferroni correction for multiple comparisons. Specific statistical methods are described in the figure legends. A post hoc power analysis reveals a power of greater than 80% for α = 0.05, and an n = 4 hearts based on means and standard deviations for all experimental interventions. Therefore, a P < 0.05 was considered statistically significant.
RESULTS
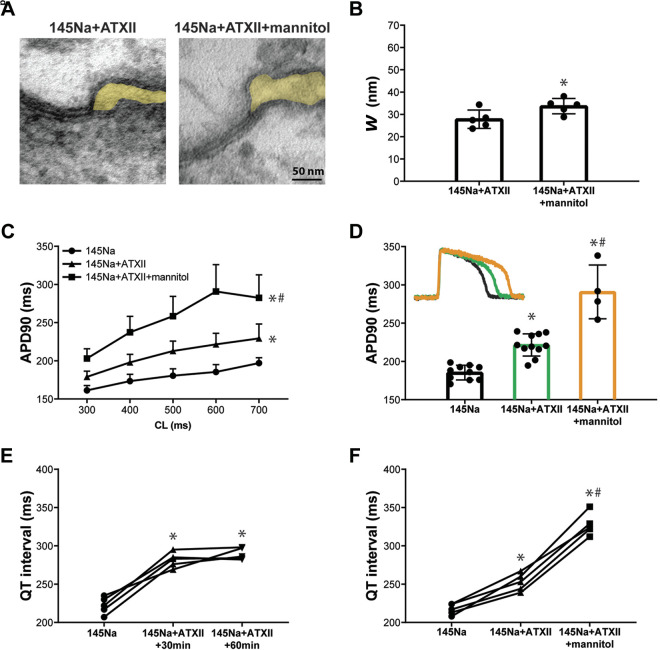
We previously demonstrated that widening the perinexus in the intercalated disc (ID) can prolong action potential duration (APD) in this drug-induced long-QT syndrome type 3 (LQT3) model in aged guinea pig hearts (15 mo old) (7). As a positive control, previous findings were replicated in the present study with adult animals (3 mo old). Representative perinexal images (Fig. 1A) acquired by transmission electron microscopy (TEM) and summary data for perinexal width (Wp) (Fig. 1B) show that mannitol in the presence of 145Na + ATXII significantly widens the perinexus relative to 145Na + ATXII, consistent with our previous study using older guinea pigs (7). Optical mapping experiments reveal that at 30 min of perfusion with 145Na + ATXII, APD is significantly increased (Fig. 1C) and the intrinsic heart rate is also significantly decreased (data not shown) relative to 145Na (baseline). Importantly, APD at 90% repolarization (APD90) is significantly prolonged with 145Na + ATXII + mannitol relative to 145Na + ATXII (Fig. 1C). The representative and summary data for APD reveal that ATXII + mannitol significantly increases APD relative to ATXII at a cycle length of 600 ms (bradycardia for a guinea pig, Fig. 1D). The findings for APD90 are similar to those found for APD at 70% repolarization (Supplemental Material 1, Fig. S3). Furthermore, early afterdepolarization (EAD) or R-on-T events in ECG were not observed in any experimental hearts.
Figure 1.
Perinexal widening induced by mannitol correlates with prolonged APD in the drug-induced LQT3 model. A: representative TEM images of the perinexus (shaded yellow) after perfusion of ATXII alone (145Na + ATXII) for 60 min or ATXII and mannitol (145Na + ATXII + mannitol) for 30 min. B: summary data for perinexal width (Wp) with perfusion of 145Na + ATXII or 145Na + ATXII + mannitol (145Na + ATXII: n = 5 hearts; 145Na + ATXII + mannitol: n = 5 hearts; *P < 0.05 by unpaired t test). C: action potential duration at 90% repolarization (APD90) as a function of cycle length (CL) for 145Na, 145Na + ATXII, or 145Na + ATXII + mannitol (145Na: n = 10 hearts; 145Na + ATXII: n = 11 hearts; 145Na + ATXII + mannitol: n = 5 hearts; *P < 0.05 relative to 145Na; #P < 0.05 relative to 145Na + ATXII; two-way ANOVA with mixed-effects model). Perfusion duration of each solution is 30 min. D: representative action potentials superimposed to demonstrate differences in repolarization (inset) and summary data for APD90 at CL = 600 ms with 145Na, 145Na + ATXII, and 145Na + ATXII + mannitol (*P < 0.05 relative to 145Na; #P < 0.05 relative to 145Na + ATXII; one-way ANOVA with mixed-effects model). E: QT interval during pacing at 500 ms in paired experiments (n = 5 hearts) with 145Na, 145Na + ATXII at 30 and 60 mins (*P < 0.05 relative to 145Na; one-way ANOVA with repeated measures). F: QT interval during pacing at 500 ms in paired experiments (n = 5 hearts) with 145Na, 145Na + ATXII, and 145Na + ATXII + mannitol. (*P < 0.05 relative to 145Na; #P < 0.05 relative to 145Na + ATXII; one-way ANOVA with repeated measures). Perfusion duration of each solution is 30 min. APD, action potential duration; ATXII, sea anemone toxin; LQT3, long-QT syndrome type 3; QT, TEM, transmission electron microscopy.
With ATXII, APD prolongation is reflected in the volume-conducted ECG obtained during pacing. 145Na + ATXII significantly prolongs QT interval at 30 and 60 min of perfusion relative to 145Na (Fig. 1E, CL = 500 ms), with no difference between 30 and 60 min. 145Na + ATXII + mannitol significantly increases QT interval when compared with 145Na and 145Na + ATXII (Fig. 1F, CL = 500 ms). Overall, these results, together with the data from our previous work (7, 8), demonstrate that the widened intercellular cleft correlates with prolonged APD in the drug-induced LQT3 model in both adult and aged guinea pig hearts.
Interestingly, Li et al. (34) demonstrated that mannitol alone can increase APD in rabbit ventricular myocytes, presumably by inhibiting outward potassium currents (35, 36), and we recently confirmed that mannitol prolongs APD in isolated guinea pig myocytes (8). In contrast, our previous optical mapping studies in the intact guinea pig whole heart preparation did not observe changes in APD in response to mannitol (7, 8, 37).
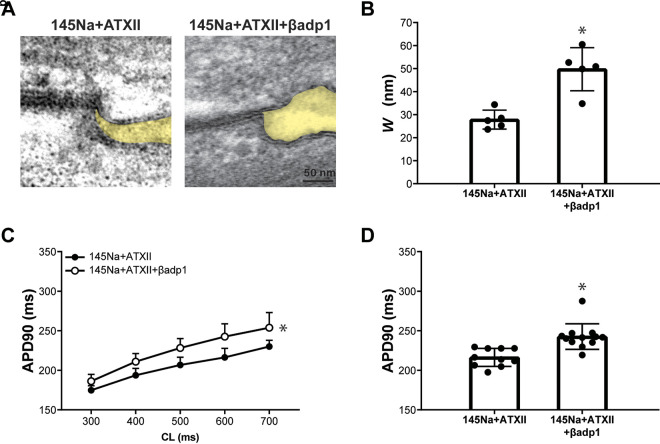
To determine whether perinexal expansion is a generalizable mechanism of APD prolongation with increased INaL, the perinexus was expanded with the sodium channel β1-subunit adhesion domain antagonist (βadp1) instead of mannitol in subsequent experiments. We have previously shown that, under normal conditions without ATXII, βadp1 significantly increases Wp without prolonging APD in either isolated cardiomyocytes or intact guinea pig heart preparations (16). In this drug-induced LQT3 model, representative perinexal images (Fig. 2A) and summary data for Wp (Fig. 2B) reveal that, in the presence of ATXII, βadp1 widens Wp relative to ATXII alone. With regards to effects on repolarization, perfusion of 145Na + ATXII + βadp1 significantly prolongs APD relative to 145Na + ATXII (Fig. 2, C and D). To confirm that βadp1 preferentially prolongs APD in the presence of ATXII and has little effect on APD in the absence of ATXII as reported previously (16), the effect of βadp1 on APD in the presence and absence of ATXII is evaluated in Supplemental Material 1, Fig. S4. In contrast with the minimal effect of βadp1 alone on APD, βadp1 in the presence of ATXII causes a significantly rate-dependent APD prolongation. Again, APD70 responds similarly to APD90 for all interventions in this experimental group (Supplemental Material 1, Fig. S5, A and B). Overall, these results demonstrate that βadp1 widens the perinexus and prolongs APD in the drug-induced LQT3 model, consistent with the effects observed with mannitol (7).
Figure 2.
Perinexal widening induced by βadp1 is associated with prolonged APD in the drug-induced LQT3 model. A: representative TEM images of the perinexus (shaded yellow) after perfusion of ATXII (145Na + ATXII) or ATXII and βadp1 (145Na + ATXII + βadp1). B: summary data for perinexal width (Wp) with perfusion of 145Na + ATXII or 145Na + ATXII + βadp1 (145Na + ATXII: n = 5 hearts; 145Na + ATXII + βadp1: n = 5 hearts; *P < 0.05 by unpaired t test). C: action potential duration at 90% repolarization (APD90) as a function of cycle length (CL) for 145Na + ATXII and 145Na + ATXII + βadp1 (145Na + ATXII: n = 11 hearts; 145Na + ATXII + βadp1: n = 13 hearts; *P < 0.05 by two-way ANOVA with mixed-effects model). Perfusion duration of each solution is 20 min. D: summary data for APD90 at CL = 600 ms with 145Na + ATXII and 145Na + ATXII + βadp1 (*P < 0.05 by unpaired t test). APD, action potential duration; ATXII, sea anemone toxin; βadp1, β1-subunit adhesion domain antagonist; LQT3, long-QT syndrome type 3; TEM, transmission electron microscopy.
Computational models suggest that one consequence of narrow intercellular cleft separation is the rapid depletion of sodium ions during depolarization of Nav1.5-rich ID nanodomains; this phenomenon is termed “self-attenuation” (7). For a fixed extracellular sodium concentration, widening the intercellular cleft (increased volume) would theoretically increase the total number of sodium ions in this nanodomain. With a wider cleft, the capacity for incomplete Nav1.5 inactivation to substantively deplete sodium levels in this nanodomain should be reduced, thereby preventing negative feedback (self-attenuation) and permitting enhanced INaL. However, it is unknown if an increased sodium ion concentration in the widened perinexus would further reduce self-attenuation in the drug-induced LQT3 model.
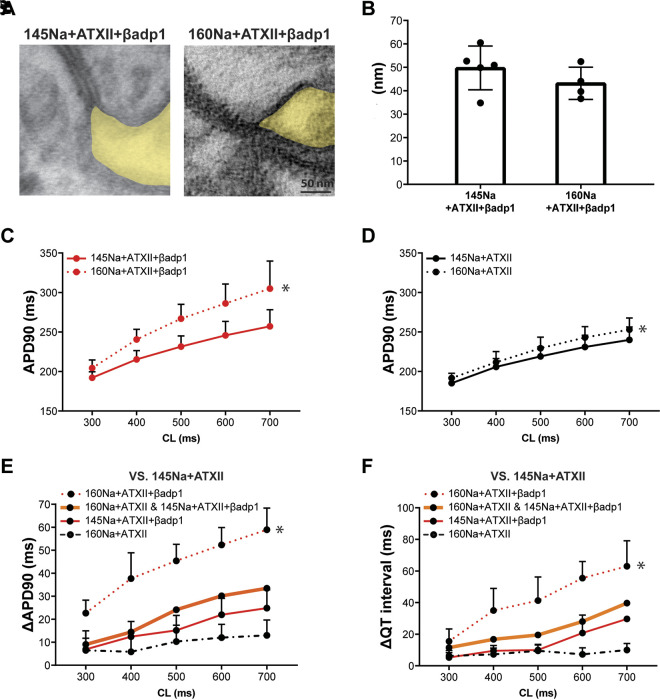
Since Nav1.5 is densely expressed in the ID (11, 12), and in particular within the perinexus (13), it is important to determine if an elevated extracellular sodium concentration can alter perinexal width and prolong APD in the drug-induced LQT3 model. Neither representative TEM images of perinexi (Fig. 3A) nor summary data (Fig. 3B) reveal significantly different perinexal widths in ATXII + βadp1-treated hearts perfused with either 145 mM sodium (145Na) or 160 mM sodium (160Na) concentrations. This suggests that the sodium concentration has little effect on Wp under these conditions. Despite a lack of difference in Wp, elevating sodium concentration to 160Na did significantly prolong APD relative to 145Na during treatment with βadp1 (Fig. 3C and Supplemental Material 1, Fig. S5C). To demonstrate whether elevated sodium concentration alone prolongs APD independent of perinexal expansion (i.e., in the absence of βadp1), APD as a function of CL is compared between 145Na and 160Na in the presence of ATXII. Figure 3D and Supplemental Material 1 Fig. S5D show that even without perinexal expansion, elevated sodium concentration alone significantly prolongs APD90. Furthermore, no EAD or R-on-T events were observed in hearts perfused with βadp1, even with an elevated sodium concentration.
Figure 3.
Perinexal widening and high-sodium concentration synergistically prolong APD in the drug-induced LQT3 model. A: representative TEM images of the perinexus (shaded yellow) after perfusion of 145 mM sodium concentration, ATXII and βadp1 (145Na + ATXII + βadp1), and 160 mM sodium concentration, ATXII and βadp1 (160Na + ATXII + βadp1). B: summary data for perinexal width (Wp) with perfusion of 145Na + ATXII + βadp1 (n = 5 hearts) and 160Na + ATXII + βadp1 (n = 4 hearts). C: action potential duration at 90% repolarization (APD90) as a function of cycle length (CL) for 145Na + ATXII + βadp1 and 160Na + ATXII + βadp1 (145Na + ATXII + βadp1: n = 9 hearts; 160Na + ATXII + βadp1: n = 8 hearts; *P < 0.05 by two-way ANOVA with mixed-effects model). Perfusion duration of each solution is 20 min. D: APD90 as a function of CL for 145Na + ATXII and 160Na + ATXII (145Na + ATXII: n = 4 hearts; 160Na + ATXII: n = 4 hearts; *P < 0.05 by two-way ANOVA with mixed-effects model). Perfusion duration of each solution is 20 min. E: the change in APD (relative to 145Na + ATXII) due to perfusion with 160Na + ATXII (n = 4 hearts; black dashed line), 145Na + ATXII + βadp1 (n = 7 hearts; red solid line), the summation of the individual effects of 160Na and βadp1 perfusion (160Na + ATXII and 145Na + ATXII + βadp1, thick orange solid line), and the effect of combined perfusion with 160Na and βadp1 (n = 6 hearts; 160Na + ATXII + βadp1, red dotted line) (*P < 0.05 relative to 160Na + ATXII and 145Na + ATXII + βadp1; two-way ANOVA with repeated measures). F: the change in QT interval (relative to 145Na + ATXII) due to perfusion with 160Na + ATXII, 145Na + ATXII + βadp1, the summation of the individual effects of 160Na and βadp1 perfusion (160Na + ATXII and 145Na + ATXII + βadp1), and the effect of combined perfusion with 160Na and βadp1 (160Na + ATXII + βadp1) (*P < 0.05 relative to 160Na + ATXII and 145Na + ATXII + βadp1; two-way ANOVA with repeated measures). APD, action potential duration; ATXII, sea anemone toxin; βadp1, β1-subunit adhesion domain antagonist; LQT3, long-QT syndrome type 3; TEM, transmission electron microscopy.
To investigate whether region-dependent APD heterogeneity exists across the ventricular epicardium, or if the interventions affect APD heterogeneity, quartile APDs in the base and apex of left and right ventricles are presented in Supplemental Material 1, Fig. S6. In short, neither mannitol, βadp1 nor elevated sodium in the presence of ATXII significantly modulates APD heterogeneity.
To investigate whether the combination of 160Na and βadp1 prolongs APD synergistically or additively relative to either 160Na or βadp1 separately, the change in APD with ATXII is plotted in Fig. 3E. The black dashed line represents the change in APD caused by switching from 145Na to 160Na (160Na + ATXII vs. 145Na + ATXII; effect of sodium alone). The red solid line represents the change in APD caused by the addition of βadp1 to 145Na (145Na + ATXII + βadp1 vs. 145Na + ATXII; effect of perinexal widening alone). Summing the individual effects on APD caused by elevating sodium alone and adding βadp1 alone produces the thick orange solid line (sum of the black dashed line and red solid line), revealing the APD prolongation predicted by the calculated additive effect.
The red dotted line indicates the measured change in APD in the presence of ATXII caused by perfusion of 160Na and βadp1 (160Na + ATXII + βadp1) relative to 145Na + ATXII. The comparison of these four lines reveals that perfusion with βadp1 and elevated sodium prolongs APD to a greater extent than the summation of the individual effects of increasing sodium from 145Na to 160Na or adding βadp1, i.e., the red dotted line (measured) is well above the thick orange line (predicted). Finally, the change in QT interval as a result of increasing sodium and perinexal width concurrently is greater than the individual effects, consistent with the changes observed with APD (Fig. 3F). Taken together, these results demonstrate that in this LQT3 model, elevating sodium concentration alone prolongs APD and when combined with widened intercellular clefts, the effect on APD prolongation is synergistic.
We also investigated the susceptibility to cardiac arrhythmia with the S1S2 stimulation protocol. Cardiac arrhythmias are rare and not significantly different from the different perfusates (Supplemental Material 1, Fig. S7). Specifically, one polymorphic and self-terminating arrhythmia is observed in one of four hearts perfused with 145Na + ATXII. In addition, no arrhythmias are induced in any heart perfused with either 145Na + ATXII + βadp1 or 160Na + ATXII + βadp1. Therefore, widened perinexi and elevated sodium prolong APD, but do not induce cardiac arrhythmias in this model of LQT3.
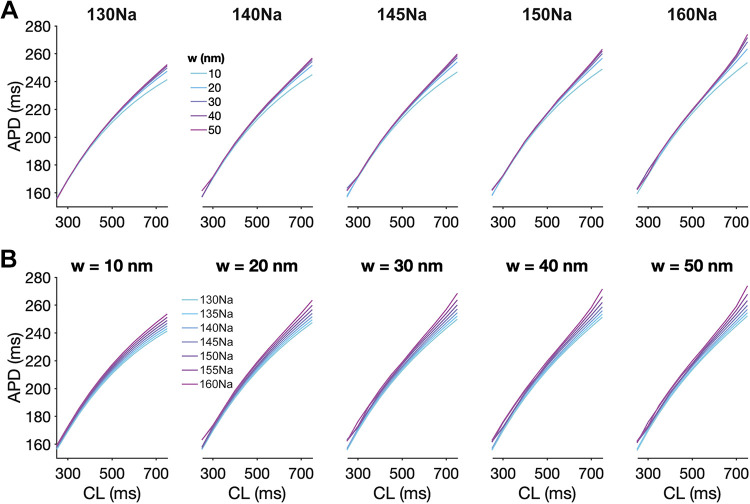
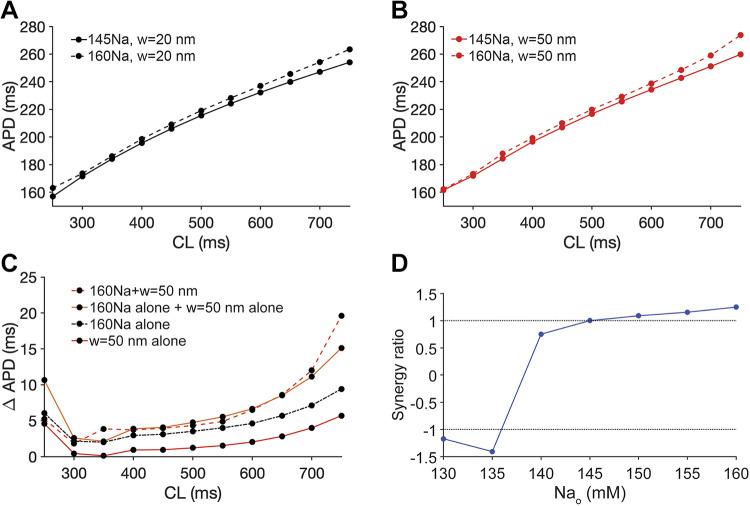
In our prior work (7), simulations predicted that preferential localization of Nav1.5 at the ID was necessary for perinexal cleft width regulation of Nav1.5 GOF and self-attenuation. Here, we performed new simulations of cardiac tissue with an LQT3-associated Nav1.5 GOF mutation (33) in the setting of variable extracellular sodium concentrations and Nav1.5 ID localization (Supplemental Material 1, Fig. S8). Simulations predict that both cleft expansion and elevated sodium concentration prolong APD, in agreement with the above experimental results in Figs. 1–3, only for conditions of preferential Nav1.5 localization at ID, consistent with our prior work (7, 8). We next broadly investigated the complex relationship between APD, cleft width, sodium concentration, and pacing rate in simulated cardiac tissue with the LQT3-associated Nav1.5 GOF mutation (Fig. 4). For all conditions, APD gradually increases with increasing CL, consistent with expected APD restitution. For a given sodium concentration, APD prolongs as cleft width increases (Fig. 4A), and similarly, for a given cleft width, APD prolongs as the sodium concentration increases (Fig. 4B). Furthermore, these effects are more pronounced for longer CLs (i.e., slower pacing rates). Thus, simulations predict that separately, both cleft expansion and elevated sodium concentration prolong APD, as demonstrated in Figs. 1–3. We next quantified the summative nature of these predictions in mutant cardiac tissue.
Figure 4.
Cleft expansion and elevated sodium concentration separately prolong APD in cardiac tissue with an LQT3-associated sodium channel gain-of-function mutation. Action potential duration (APD) is shown as a function of cycle length (CL) for different sodium concentrations and cleft widths (w) in simulations of tissue with LQT3-associated sodium channel gain-of-function. A: APD prolongation for increasing cleft width (w) and variable sodium concentration. B: APD prolongation for elevated sodium concentration and variable cleft widths (w). LQT3, long-QT syndrome type 3.
Performing parallel analyses to experiments in Fig. 3, C–E, we plot APD as a function of CL for 145Na (solid lines) and 160Na (dashed lines) for nominal (20 nm, black lines, Fig. 5A) and expanded (50 nm, red lines, Fig. 5B) cleft widths. As in Fig. 4, we found that elevated sodium concentration prolongs APD for both normal and wide cleft widths. Importantly, simulations predict that the observed combined effect (dashed red line) is greater than the calculated sum of the individual contributions to APD prolongation (Fig. 5C, solid orange line) of cleft expansion (solid red line) and elevated sodium (dashed black line), denoting a synergistic effect consistent with the experiments presented in Fig. 3E.
Figure 5.
Simulation predicts the synergistic effect of expanded intercellular clefts and elevated sodium concentration on action potential duration (APD), prolongation in cardiac tissue with an LQT3-associated sodium channel gain-of-function mutation. A: action potential duration (APD) as a function of cycle length (CL) for two different sodium concentrations (145Na and 160Na) and nominal cleft width (w) of 20 nm. B: APD as a function of CL for two different sodium concentrations and a widened cleft width (w) of 50 nm. C: the change in APD due to expanded cleft width 50 nm (w = 50 nm alone), elevated sodium concentration 160 mM (160Na alone), summation of expanded cleft width and elevated sodium concentration (160Na alone + w = 50 nm alone), and combination of expanded cleft width and elevated sodium concentration (160Na + w = 50 nm) relative to cleft width of 20 nm and sodium concentration of 145 mM. D: the synergy ratio with different sodium concentrations. For sodium concentration above 145 mM, a synergy ratio greater than 1 indicates that APD is prolonged synergistically, and for sodium concentration below 140 mM, the synergy ratio less than 1 indicates that APD is shortened synergistically. LQT3, long-QT syndrome type 3.
To expand on these experiments and simulations, we next investigated whether these synergistic effects are similarly predicted for the case in which sodium concentration is reduced. In Fig. S9 of Supplemental Material 1, we plot APD as a function of CL for 145Na (solid lines) and 130Na (dashed lines) for nominal (20 nm, Supplemental Material 1, Fig. S9A) and expanded (50 nm, Supplemental Material 1, Fig. S9B) cleft widths. As in Fig. 4, the reduced sodium concentration shortens APD for both cleft widths. In this case, simulations predict that the individual contributions of APD prolongation via cleft expansion (Supplemental Material 1, Fig. S9C, solid red line) and APD shortening via reduced sodium concentration (dashed black line) together sum (solid orange line) to shorten APD overall. However, the simulated combined effect (dashed red line) exhibits an even greater degree of APD shortening. That is, cleft expansion-mediated APD prolongation is dampened in a synergistic manner by reduced sodium concentration that is more than the individual contributions.
To quantify the comparison of the combined effects with the summed individual contributions, we define the “synergy ratio” as the ratio of the combination of cleft expansion and altered sodium concentration (dashed red lines in Fig. 5C and Supplemental Material 1, S9C) divided by the summed individual contributions (solid orange lines in Fig. 5C and Supplemental Material 1, S9C), with the ratio presented as positive for APD prolongation and negative for APD shortening. In Fig. 5D, we plot the synergy ratio measured for CL of 750 ms as a function of the sodium concentration. Consistent with Fig. 5C and Supplemental Material 1, S9C, the synergy ratio in the model is greater than 1 for sodium concentrations above 145 mM, whereas the ratio is less than −1 for sodium concentration below 140 mM. These measures thus predict that elevated sodium concentrations synergistically enhance the influence of cleft expansion, whereas reduced sodium concentrations synergistically mitigate the influence of cleft expansion.
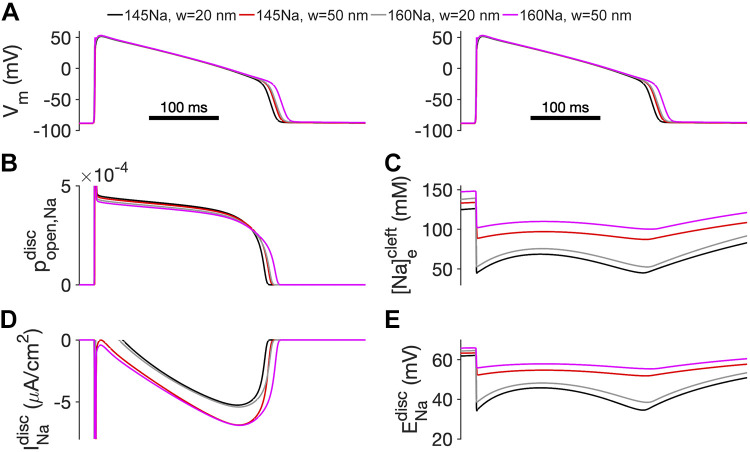
Finally, we investigated the mechanism of the synergistic effects of elevated sodium concentration and cleft expansion in the LQT3-associated Nav1.5 GOF cardiac tissue. Simulated cardiac tissue was paced at one end of the strand at a CL of 750 ms. In Fig. 6, we plot the time series for the transmembrane potential (Vm, Fig. 6A), Nav1.5 open probability (Fig. 6B), intercellular cleft sodium concentration (Fig. 6C), INa at the ID (Fig. 6D), and ID ENa (Fig. 6E) for different combinations of nominal (20 nm) and expanded cleft width (50 nm) and normal (145Na) and elevated (160Na) extracellular sodium. Note that, for clarity, traces are only shown for a single ID membrane or cleft (cell 25 out of 50). For nominal cleft width and normal sodium concentration (145Na, w = 20 nm; black lines), mutant Nav1.5 promotes an increased open probability during the cardiac action potential and a persistent INaL. However, both the initial sodium influx during the action potential upstroke and subsequent late current deplete the local cleft sodium concentration (Fig. 6C) and reduce the ID ENa (Fig. 6E). This reduced ENa, in turn, reduces the magnitude of INaL.
Figure 6.
Combination of expanded intercellular clefts and elevated sodium concentration prolongs APD by reducing cleft sodium depletion and maintaining INa driving force. A: transmembrane potential (Vm), B: Nav1.5 open probability, C: intercellular cleft sodium concentration, D: sodium current at the intercalated disc (ID), and E: ID sodium reversal potential (ENa) are shown as a function of time for different cleft widths and sodium concentrations for an action potential elicited during pacing at a cycle length (CL) = 750 ms in cardiac tissue with an LQT3-associated sodium channel gain-of-function mutation. APD, action potential duration; INa, sodium current; LQT3, long-QT syndrome type 3.
As discussed in our prior work (7), ID cleft sodium depletion represents a negative feedback mechanism that suppresses APD prolongation. However, in the setting of cleft expansion (145Na, w = 50 nm; red lines), cleft sodium depletion is mitigated, resulting in an enhanced INaL and prolonged APD. In the setting of elevated sodium concentration (160Na, w = 20 nm; gray lines), local cleft sodium is depleted to a greater extent than the setting of cleft expansion alone (red line). However, the larger extracellular sodium concentration results in rapid cleft refilling during the plateau, which, in turn, increases INaL and thus prolongs APD so much so that the red and gray lines in Fig. 6A are nearly indistinguishable. That is, cleft expansion and elevated sodium individually prolong APD to a similar extent. Finally, for the combination of both cleft expansion and elevated sodium (160Na, w = 50 nm; magenta lines), both individual effects are enhanced: cleft sodium level after rapid depletion is maintained at the level greater than that in the setting of either cleft expansion or elevated sodium concentration alone (Fig. 6C), which in turn promotes INaL and prolongs APD.
In our prior work (7), we demonstrated that the altered INaL driving force could also promote EADs, in particular, under bradycardic conditions. In Supplemental Material 1 (Figs. S10–S13), we demonstrate that for longer CLs the synergy between cleft expansion and altered sodium concentration is more pronounced, such that small individual contributions to APD prolongation combine to promote pronounced EADs (Supplemental Material 1, Fig. S10). Plots of APD restitution for longer CLs under the explored conditions further demonstrate the synergistic effects on EAD promotion (Supplemental Material 1, Figs. S11–S13). Furthermore, we find that the synergy ratio magnitude is generally greater than 1 for longer CLs, illustrating both synergistic APD prolongation and shortening for elevated and reduced extracellular sodium concentration, respectively (Supplemental Material 1, Fig. S14).
DISCUSSION
Previous experimental and simulation studies revealed that widening intercellular separation in the gap junction adjacent and Nav1.5 dense perinexus can unmask an LQT3 phenotype (7, 8). However, to our knowledge, this is the first study to elucidate how altering extracellular sodium concentration modulates action potential duration (APD) prolongation secondary to Nav1.5 gain-of-function (GOF). Specifically, we demonstrate the synergistic effects of increased perinexal width and elevated sodium concentration on APD prolongation. Both ex vivo and in-silico experiments consistently show that both perinexal widening and elevated sodium concentration alone prolong APD. Importantly, by simultaneously widening the perinexus and elevating sodium concentration, the singular effects are amplified in a synergistic manner. The results suggest that a high concentration of sodium in widened intercellular clefts greatly increases INaL and subsequently prolongs ventricular repolarization in response to Nav1.5 GOF.
Despite the synergistic effect of widened perinexus and high-sodium concentration on APD, neither spontaneous arrhythmia nor induced arrhythmia was observed in the same condition. In contrast with spontaneous arrhythmias observed in a previous aged animal study (7), the current study using younger animals suggests an age-related difference in the incidence of spontaneous arrhythmia. S1S2 is a well-established protocol to induce arrhythmias, but the premature stimulus is usually delivered on the epicardium (25, 26). In this study, S1S2 was stimulated from the septum. However, we do not know if the stimulation site could affect the arrhythmia induction. In addition, we do not know the three-dimensional distribution of APD gradients in the whole heart with these interventions. Therefore, future studies are required to address these specific and important questions.
It is well established that incomplete inactivation of Nav1.5 causes APD prolongation in a number of diseases including LQT3 (1, 17–20). Previous work suggested that decreasing the extracellular volume in nanodomains between cardiomyocytes such as the perinexus leads to extracellular sodium depletion and attenuation of APD prolongation despite incompletely inactivated and therefore conductive Nav1.5 (7–9). In effect, our previous studies explored how changing the total number of sodium ions available (fixed sodium concentration and altered volume) modulates the rate of extracellular sodium depletion. In contrast, this study explored how altering both the perinexal volume and sodium concentration individually and concurrently modulate APD prolongation during Nav1.5 GOF.
A proposed mechanism for increased sodium concentration modulating APD during Nav1.5 GOF is as follows. According to ephaptic coupling theory, rapid transactivation of Nav1.5 withdraws sodium ions from a shared restricted space and decreases extracellular sodium in that space with two electrophysiological consequences (7, 8). First, sodium charge withdrawal will reduce extracellular potentials and thereby increase transmembrane potential of both cells. Second, rapid extracellular sodium depletion will reduce ENa along those shared membrane boundaries. The net effect of reduced extracellular potential and ENa reduces the driving force for sodium through the sodium channel due to incomplete inactivation, so INaL is markedly decreased. However, the elevated extracellular sodium increases the number of ions available in the extracellular space, thereby increasing ENa during the diastolic interval. As a result, INa-induced sodium depletion of shared nanodomains such as within the perinexus is now insufficient to reduce ENa to the level required for INa-induced self-attenuation. As a result, Nav1.5 can continue to conduct, thereby sustaining INaL, which prolongs APD.
As demonstrated previously and explored in greater detail here, these effects are highly dependent on Nav1.5 localization within the sarcolemma and whether those channels localize to relatively narrow extracellular domains that can be self-regulated by the same currents. This suggests that enhancing INaL without self-attenuation would more frequently cause dramatic and potentially life-threatening APD prolongation during Nav1.5 GOF. This is important because mannitol is commonly used in the priming solution for patients undergoing cardiac surgery and traumatic brain injury due to its osmotic diuretic effect (38, 39). However, it has been reported that mannitol is associated with prolonged QT interval, which correlates with increased cardiac arrhythmia in patients with brain injury (40). QT prolongation could be either due to mannitol-induced human ether-a-go-go-related gene (hERG) inhibition (35, 36) or age-associated, increased Nav1.5 late current (41). Therefore, the mechanism of QT prolongation with mannitol requires additional research to separate the effects of hERG inhibition and unmasking Nav1.5 GOF.
Although it was shown that mannitol prolongs APD in the current LQT3 heart study due to widening the perinexus (7, 8), mannitol is also associated with hERG inhibition to prolong APD in the wild-type isolated cardiomyocytes (8, 34–36) However, mannitol did not prolong APD in the isolated wild-type whole heart preparations (7, 8, 37). Despite the unclear mechanism, the consistency of the mannitol data with βadp1 data in the current LQT3 model raises our confidence that APD prolongation in LQT3 hearts is not an effect of mannitol inhibiting hERG channels, but rather an effect of both agents expanding the perinexus.
Sodium channel subunit-β1 (SCN1B) facilitates cell-cell adhesive interaction, and Scn1b loss of function is associated with increased cardiac arrhythmias in Scn1b null mouse hearts (42) and hearts perfused with 10 µM βadp1 (16). In the current study, βadp1 widened the perinexus, but we did not observe spontaneous or inducible cardiac arrhythmias even in the presence of ATXII. Previously, 10 µM βadp1 was shown to increase arrhythmias in the isolated guinea pig heart (16), but this concentration is an order of magnitude greater than the 1 µM used in this study. Therefore, the degree of perinexal disruption in this manuscript is difficult to compare with genetically reduced Scn1b in mouse models or studies using larger βadp1 concentrations. Future studies are necessary to determine the degree of sodium channel β1-subunit loss-of-function that will be associated with APD prolongation and increased arrhythmia risk.
Synergy, as proposed in this study, suggests that the rate of change of sodium concentration in Nav1.5-rich nanodomains is modulated by both cleft volume and the bulk extracellular ionic concentration (through diffusive coupling between the cleft and bulk extracellular spaces). Importantly, the synergy ratio reveals that both a widened cleft and elevated sodium will dramatically prolong APD in this model, and in a manner that is greater than the sum of the individual effects. Equally important is the predicted negative synergy ratio. When clefts are small and extracellular sodium reduced, the study suggests that APD can be shortened as a result of a Nav1.5 GOF. Therefore, our study indicates that the electrophysiological phenotype of Nav1.5 GOF in LQT3 may be fully concealed by reducing extracellular sodium concentrations synergistically with narrowing perinexal width. Although these findings are highly provocative and tantalizing due to the translational potential of managing LQT3 by modulating plasma sodium concentration, the negative synergy ratio requires additional experimental evidence and careful consideration.
High-sodium intake may increase the risk of cardiac events in diseases associated with increased INaL. High-serum sodium is associated with prolonged QT interval in patients (43–46) suggesting that elevated plasma sodium may exacerbate QT interval prolongation in diseases associated with increased INaL (17–20). Importantly, prolonged QT interval and structural disruption of the intercalated disc (ID) are each associated with aging (41, 47) suggesting that elevated plasma sodium may greatly increase the incidence of cardiac events in aged patients. Interestingly, our recent simulation study of the age progression in LQT3 predicted that ID structural properties have minimal influence on arrhythmias for conditions associated with neonatal and early developmental stages, whereas cellular properties, specifically cell size and sodium channel density, are more critical (9). In contrast, for adult cardiac tissue, we found that ID structure is a key determinant of arrhythmias, consistent with our current study. Here, we have further identified elevated sodium concentration as an additional regulatory factor for patients with LQT3. Importantly, the synergistic effect observed in this study suggests that elevated sodium concentrations within the structurally disrupted intercalated disc may increase the propensity for cardiac events in aged patients with LQT3.
However, reducing serum sodium levels must be carefully considered in patients with LQT3. Current pharmacological therapies aimed to treat LQT3 may also cause an LQT3 overlap syndrome with Brugada syndrome (BrS) as a consequence of the therapy reducing both late and peak INa. Some Nav1.5 variants, including insD1795 (48) and E1784K (49), cause increased INaL and reduced peak INa, which are associated with the LQT3 and BrS overlap syndrome. Importantly, the R1193Q (50) and V2016M (51) variants are not associated with a change in peak current, but INaL is increased, and both variants are associated with the LQT3 and BrS overlap syndrome. The negative synergy ratio predicted by the model supports a hypothesis that even in the absence of reduced peak INa measured in isolated cells, Nav1.5 GOF may contribute to BrS because of enhanced INa self-attenuation that will ultimately reduce the peak current in tissue. Therefore, hyponatremia may increase arrhythmogenic risk in some patients with BrS. In fact, several case reports have shown that BrS ECG patterns were observed during hyponatremic episodes and became normal again after serum sodium concentrations were restored (52–54).
Limitations
The LQT3 model in the present study was established by perfusing isolated hearts with ATXII based on previous studies establishing that the compound prevents complete inactivation of Nav1.5. Like all pharmacological interventions, ATXII may have off-target effects. For example, ATXII may increase GOF of other sodium channels beyond the cardiac Nav1.5 isoform, and it may also enhance the inward rectifier potassium current (55, 56). Even if all sodium channels in the lateral membrane, t-tubules, or intercalated disc (57) respond similarly to changes in the extracellular ionic concentration or the βadp1 peptide, the model predicts only ID-localized Na channels underlie APD prolongation under the conditions explored in this study. In addition, we have previously shown that the mechanism of perinexal expansion-induced APD prolongation is independent of at least three different LQT3 models including one experimental drug-induced LQT3 guinea pig model and two simulated Nav1.5 LQT3 variants (7). Still, future studies are needed to demonstrate broader applicability based on other sodium channel-specific dynamics, different voltage-gated sodium channel isoforms, and the development of a computational model of sodium channel interactions with pharmacological agents.
Experiments were conducted in pharmacologically arrested hearts with the agent blebbistatin. All electromechanical uncouplers have effects that may limit interpretation of the present results. For example, blebbistatin could prolong APD in the isolated hearts (58, 59) by a proposed mechanism of altering intracellular calcium handling (60).
The effects of elevated sodium concentration on intracellular calcium homeostasis are not investigated in the current study. Nav1.5 GOF should increase intracellular sodium during the action potential plateau followed by increased intracellular calcium via reduced sodium-calcium exchanger (NCX) forward mode (61, 62). Altered calcium homeostasis is associated with the occurrence of EADs (63). However, elevating extracellular sodium should reduce intracellular calcium accumulation (64), by a mechanism thought to occur later in the action potential and diastolic intervals. Future studies that manipulate NCX and pacing rate are needed to understand the effect of increased extracellular sodium on intracellular calcium homeostasis. With self-attenuation though, sodium entry into myocytes during prolonged action potential repolarization will be reduced, and coupled with long diastolic intervals, should result in further intracellular calcium depletion. Thus, one might assume elevating extracellular sodium would be antiarrhythmic by a mechanism of reducing the likelihood of spontaneous calcium release events or delayed afterdepolarizations. Our data and models suggest that LQT3 is associated with EADs, and the prolonged action potentials are more likely associated with reactivation of calcium channels as the arrhythmic substrate rather than intracellular calcium overload. Importantly, the models in this study and our previous work incorporate NCX and demonstrate significant APD prolongation and EADs in the presence of widened clefts and increased extracellular sodium concentration.
We note that simulations predict smaller changes in APD due to cleft width expansion and altered extracellular sodium, compared with experiments, for shorter cycle lengths, whereas simulations predict larger APD changes for longer cycle lengths. We speculate that these quantitative discrepancies arise due to model sensitivity in the positive feedback mechanism in which INaL promotes EADs. Although our model incorporates significant structural details, specifically nonuniform Nav1.5 distribution and restricted intercellular cleft spaces, this formulation is nonetheless a simplified representation of complex cardiac tissue structure. Structural details not incorporated in our model may dampen this positive feedback and thus result in quantitative measurements closer to experiments. Although this limitation results in quantitative differences, the simulations qualitatively predict trends consistent with experiments and importantly identify the mechanism underlying the synergistic effect of cleft expansion and altered extracellular sodium.
All experiments were performed with adult male guinea pigs and the findings will need to be confirmed in female animals, particularly given the well-established differences in the expression of a variety of potassium channels between the sexes (65, 66).
Finally, caution should be exercised before directly translating these results into humans since Langendorff-perfused isolated hearts operate outside in vivo ionic control mechanisms that are provided by the renal, gastrointestinal, and pulmonary systems. It is, therefore, unclear if serum sodium concentrations are a mechanism of prolonged QT interval in patients with LQT3 with normal electrolyte homeostasis. Clinical studies have shown that hypernatremia is an important risk factor for QT interval prolongation (43–46). However, in vivo studies need to be conducted to evaluate the safety and efficacy of modulating serum sodium in conditions associated with Nav1.5 GOF.
Conclusions
In conclusion, Nav1.5 GOF can be exacerbated by either expanding cellularly regulated shared extracellular nanodomains densely expressing highly conductive sodium channels or elevating extracellular sodium. Importantly, the combination of widened extracellular nanodomains like the perinexus with elevated sodium may constitute a putative mechanism that synergistically exacerbates APD prolongation during Nav1.5 GOF.
Although several drugs have been used as relatively specific INaL inhibitors to treat LQT3 in patients (67), little progress has been made in recent years to treat enhanced INaL. Therefore, our findings potentially provide a new and cost-effective strategy to mitigate sodium channel GOF-related diseases like LQT3 by means of managing sodium levels and preventing cardiac edema in patients.
SUPPLEMENTAL DATA
Supplemental Material 1 (Supplemental Figs. S1–S14): https://doi.org/10.6084/m9.figshare.14825058.
Supplemental Material 2 (simulation code): https://doi.org/10.6084/m9.figshare.16624240.
GRANTS
This work was supported by the National Heart, Lung, and Blood Institute Grants F31HL160172 (to G.A.B.), F31HL147438 (to D.R.K.), R01HL138003 (to S.P. and S.H.W.), R01HL102298 (to S.P.), R01HL141855 (to R.G.G. and S.P.), and R01HL056728 (to R.G.G.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.P. conceived and designed research; X.W., G.B., and S.H.W. performed experiments; X.W., G.B., and S.H.W. analyzed data; X.W., G.B., S.H.W., and S.P. interpreted results of experiments; X.W., G.B., S.H.W., and S.P. prepared figures; X.W. and S.P. drafted manuscript; G.S.H., G.B., D.R.K., R.G.G., S.H.W., and S.P. edited and revised manuscript; X.W., G.S.H., G.B., D.R.K., R.G.G., S.H.W., and S.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Kathy J. Lowe for tissue processing and transmission electron microscopy.
REFERENCES
- 1.Song W, Shou W. Cardiac sodium channel Nav1.5 mutations and cardiac arrhythmia. Pediatr Cardiol 33: 943–949, 2012. doi: 10.1007/s00246-012-0303-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burashnikov A, Antzelevitch C. Acceleration-induced action potential prolongation and early afterdepolarizations. J Cardiovasc Electrophysiol 9: 934–948, 1998. doi: 10.1111/j.1540-8167.1998.tb00134.x. [DOI] [PubMed] [Google Scholar]
- 3.Lemoine MD, Duverger JE, Naud P, Chartier D, Qi XY, Comtois P, Fabritz L, Kirchhof P, Nattel S. Arrhythmogenic left atrial cellular electrophysiology in a murine genetic long QT syndrome model. Cardiovasc Res 92: 67–74, 2011. doi: 10.1093/cvr/cvr166. [DOI] [PubMed] [Google Scholar]
- 4.Vincent GM, Timothy KW, Leppert M, Keating M. The spectrum of symptoms and QT intervals in carriers of the gene for the long-QT syndrome. N Engl J Med 327: 846–852, 1992. doi: 10.1056/NEJM199209173271204. [DOI] [PubMed] [Google Scholar]
- 5.Viskin S. The QT interval: too long, too short or just right. Heart Rhythm 6: 711–715, 2009. doi: 10.1016/j.hrthm.2009.02.044. [DOI] [PubMed] [Google Scholar]
- 6.Goldenberg I, Horr S, Moss AJ, Lopes CM, Barsheshet A, McNitt S, Zareba W, Andrews ML, Robinson JL, Locati EH, Ackerman MJ, Benhorin J, Kaufman ES, Napolitano C, Platonov PG, Priori SG, Qi M, Schwartz PJ, Shimizu W, Towbin JA, Vincent GM, Wilde AA, Zhang L. Risk for life-threatening cardiac events in patients with genotype-confirmed long-QT syndrome and normal-range corrected QT intervals. J Am Coll Cardiol 57: 51–59, 2011. doi: 10.1016/j.jacc.2010.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greer-Short A, George SA, Poelzing S, Weinberg SH. Revealing the concealed nature of long-QT type 3 syndrome. Circ Arrhythm Electrophysiol 10: e004400, 2017. doi: 10.1161/CIRCEP.116.004400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nowak MB, Greer-Short A, Wan X, Wu X, Deschênes I, Weinberg SH, Poelzing S. Intercellular sodium regulates repolarization in cardiac tissue with sodium channel gain of function. Biophys J 118: 2829–2843, 2020. doi: 10.1016/j.bpj.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nowak MB, Poelzing S, Weinberg SH. Mechanisms underlying age-associated manifestation of cardiac sodium channel gain-of-function. J Mol Cell Cardiol 153: 60–71, 2021. doi: 10.1016/j.yjmcc.2020.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rhett JM, Jourdan J, Gourdie RG. Connexin 43 connexon to gap junction transition is regulated by zonula occludens-1. Mol Biol Cell 22: 1516–1528, 2011. doi: 10.1091/mbc.E10-06-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petitprez S, Zmoos AF, Ogrodnik J, Balse E, Raad N, El-Haou S, Albesa M, Bittihn P, Luther S, Lehnart SE, Hatem SN, Coulombe A, Abriel H. SAP97 and dystrophin macromolecular complexes determine two pools of cardiac sodium channels Nav1.5 in cardiomyocytes. Circ Res 108: 294–304, 2011. doi: 10.1161/CIRCRESAHA.110.228312. [DOI] [PubMed] [Google Scholar]
- 12.Vermij SH, Abriel H, van Veen TA. Refining the molecular organization of the cardiac intercalated disc. Cardiovasc Res 113: 259–275, 2017. doi: 10.1093/cvr/cvw259. [DOI] [PubMed] [Google Scholar]
- 13.Rhett JM, Ongstad EL, Jourdan J, Gourdie RG. Cx43 associates with Na(v)1.5 in the cardiomyocyte perinexus. J Membr Biol 245: 411–422, 2012. doi: 10.1007/s00232-012-9465-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.George SA, Bonakdar M, Zeitz M, Davalos RV, Smyth JW, Poelzing S. Extracellular sodium dependence of the conduction velocity-calcium relationship: evidence of ephaptic self-attenuation. Am J Physiol Heart Circ Physiol 310: H1129–H1139, 2016. doi: 10.1152/ajpheart.00857.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hichri E, Abriel H, Kucera JP. Distribution of cardiac sodium channels in clusters potentiates ephaptic interactions in the intercalated disc. J Physiol 596: 563–589, 2018. doi: 10.1113/JP275351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Veeraraghavan R, Hoeker GS, Alvarez-Laviada A, Hoagland D, Wan X, King DR, Sanchez-Alonso J, Chen C, Jourdan J, Isom LL, Deschenes I, Smyth JW, Gorelik J, Poelzing S, Gourdie RG. The adhesion function of the sodium channel beta subunit (β1) contributes to cardiac action potential propagation. eLife 7: e37610, 2018. doi: 10.7554/eLife.37610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valdivia CR, Chu WW, Pu J, Foell JD, Haworth RA, Wolff MR, Kamp TJ, Makielski JC. Increased late sodium current in myocytes from a canine heart failure model and from failing human heart. J Mol Cell Cardiol 38: 475–483, 2005. doi: 10.1016/j.yjmcc.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 18.Hale SL, Shryock JC, Belardinelli L, Sweeney M, Kloner RA. Late sodium current inhibition as a new cardioprotective approach. J Mol Cell Cardiol 44: 954–967, 2008. doi: 10.1016/j.yjmcc.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 19.Lu Z, Jiang YP, Wu CY, Ballou LM, Liu S, Carpenter ES, Rosen MR, Is C, Lin RZ. Increased persistent sodium current due to decreased PI3K signaling contributes to QT prolongation in the diabetic heart. Diabetes 62: 4257–4265, 2013. doi: 10.2337/db13-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olesen MS, Yuan L, Liang B, Holst AG, Nielsen N, Nielsen JB, Hedley PL, Christiansen M, Olesen SP, Haunsø S, Schmitt N, Jespersen T, Svendsen JH. High prevalence of long QT syndrome-associated SCN5A variants in patients with early-onset lone atrial fibrillation. Circ Cardiovasc Genet 5: 450–459, 2012. doi: 10.1161/CIRCGENETICS.111.962597. [DOI] [PubMed] [Google Scholar]
- 21.Veeraraghavan R, Lin J, Hoeker GS, Keener JP, Gourdie RG, Poelzing S. Sodium channels in the Cx43 gap junction perinexus may constitute a cardiac ephapse: an experimental and modeling study. Pflugers Arch 467: 2093–2105, 2015. doi: 10.1007/s00424-014-1675-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Entz M 2nd, King DR, Poelzing S. Design and validation of a tissue bath 3-D printed with PLA for optically mapping suspended whole heart preparations. Am J Physiol Heart Circ Physiol 313: H1190–H1198, 2017. doi: 10.1152/ajpheart.00150.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoeker GS, James CC, Tegge AN, Gourdie RG, Smyth JW, Poelzing S. Attenuating loss of cardiac conduction during no-flow ischemia through changes in perfusate sodium and calcium. Am J Physiol Heart Circ Physiol 319: H396–H409, 2020. doi: 10.1152/ajpheart.00112.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vyas S, Saini AG, Kaur A, Singh P, Jayashree M, Sundaram V, Mukhopadhyay K, Singh P. Neuroimaging spectrum of severe hypernatremia in infants with neurological manifestations. Neuropediatrics 52: 316–325, 2021. doi: 10.1055/s-0041-1730938. [DOI] [PubMed] [Google Scholar]
- 25.Osadchii OE. Effects of ventricular pacing protocol on electrical restitution assessments in guinea-pig heart. Exp Physiol 97: 807–821, 2012. doi: 10.1113/expphysiol.2012.065219. [DOI] [PubMed] [Google Scholar]
- 26.Akar FG, Rosenbaum DS. Transmural electrophysiological heterogeneities underlying arrhythmogenesis in heart failure. Circ Res 93: 638–645, 2003. doi: 10.1161/01.RES.0000092248.59479.AE. [DOI] [PubMed] [Google Scholar]
- 27.George SA, Sciuto KJ, Lin J, Salama ME, Keener JP, Gourdie RG, Poelzing S. Extracellular sodium and potassium levels modulate cardiac conduction in mice heterozygous null for the Connexin43 gene. Pflugers Arch 467: 2287–2297, 2015. doi: 10.1007/s00424-015-1698-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raisch T, Khan M, Poelzing S. Quantifying intermembrane distances with serial image dilations. J Vis Exp (139), e58311, 2018. doi: 10.3791/58311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kucera JP, Rohr S, Rudy Y. Localization of sodium channels in intercalated disks modulates cardiac conduction. Circ Res 91: 1176–1182, 2002. doi: 10.1161/01.res.0000046237.54156.0a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weinberg SH. Ephaptic coupling rescues conduction failure in weakly coupled cardiac tissue with voltage-gated gap junctions. Chaos 27: 093908, 2017. doi: 10.1063/1.4999602. [DOI] [PubMed] [Google Scholar]
- 31.Moise N, Struckman HL, Dagher C, Veeraraghavan R, Weinberg SH. Intercalated disk nanoscale structure regulates cardiac conduction. J Gen Physiol 153: e202112897, 2021. doi: 10.1085/jgp.202112897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Livshitz LM, Rudy Y. Regulation of Ca2+ and electrical alternans in cardiac myocytes: role of CAMKII and repolarizing currents. Am J Physiol Heart Circ Physiol 292: H2854–H2866, 2007. doi: 10.1152/ajpheart.01347.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clancy CE, Kass RS. Defective cardiac ion channels: from mutations to clinical syndromes. J Clin Invest 110: 1075–1077, 2002. doi: 10.1172/JCI16945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li GR, Zhang M, Satin LS, Baumgarten CM. Biphasic effects of cell volume on excitation-contraction coupling in rabbit ventricular myocytes. Am J Physiol Heart Circ Physiol 282: H1270–H1277, 2002. doi: 10.1152/ajpheart.00946.2001. [DOI] [PubMed] [Google Scholar]
- 35.Yabuuchi F, Beckmann R, Wettwer E, Hegele-Hartung C, Heubach JF. Reduction of hERG potassium currents by hyperosmolar solutions. Eur J Pharmacol 566: 222–225, 2007. doi: 10.1016/j.ejphar.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 36.Taglialatela M, Castaldo P, Iossa S, Pannaccione A, Fresi A, Ficker E, Annunziato L. Regulation of the human ether-a-gogo related gene (HERG) K+ channels by reactive oxygen species. Proc Natl Acad Sci USA 94: 11698–11703, 1997. doi: 10.1073/pnas.94.21.11698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Veeraraghavan R, Salama ME, Poelzing S. Interstitial volume modulates the conduction velocity-gap junction relationship. Am J Physiol Heart Circ Physiol 302: H278–H286, 2012. doi: 10.1152/ajpheart.00868.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poullis M. Mannitol and cardiac surgery. Thorac Cardiovasc Surg 47: 58–62, 1999. doi: 10.1055/s-2007-1013112. [DOI] [PubMed] [Google Scholar]
- 39.Wakai A, McCabe A, Roberts I, Schierhout G. Mannitol for acute traumatic brain injury. Cochrane Database Syst Rev 2013: CD001049, 2013. doi: 10.1002/14651858.CD001049.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dabrowski W, Siwicka-Gieroba D, Robba C, Badenes R, Bialy M, Iwaniuk P, Schlegel TT, Jaroszynski A. Plasma hyperosmolality prolongs QTc interval and increases risk for atrial fibrillation in traumatic brain injury patients. J Clin Med 9: 1293, 2020. doi: 10.3390/jcm9051293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Signore S, Sorrentino A, Borghetti G, Cannata A, Meo M, Zhou Y, Kannappan R, Pasqualini F, O'Malley H, Sundman M, Tsigkas N, Zhang E, Arranto C, Mangiaracina C, Isobe K, Sena BF, Kim J, Goichberg P, Nahrendorf M, Isom LL, Leri A, Anversa P, Rota M. Late Na(+) current and protracted electrical recovery are critical determinants of the aging myopathy. Nat Commun 6: 8803, 2015. doi: 10.1038/ncomms9803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin X, O'Malley H, Chen C, Auerbach D, Foster M, Shekhar A, Zhang M, Coetzee W, Jalife J, Fishman GI, Isom L, Delmar M. Scn1b deletion leads to increased tetrodotoxin-sensitive sodium current, altered intracellular calcium homeostasis and arrhythmias in murine hearts. J Physiol 593: 1389–1407, 2015. doi: 10.1113/jphysiol.2014.277699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nafrialdi Kurniawan TG, Setiawati A, Makmun LH. QT interval prolongation associated with amiodarone use in Cipto Mangunkusumo Hospital, Jakarta. Acta Med Indones 46: 292–297, 2014. [PubMed] [Google Scholar]
- 44.Michishita R, Ishikawa-Takata K, Yoshimura E, Mihara R, Ikenaga M, Morimura K, Takeda N, Yamada Y, Higaki Y, Tanaka H, Kiyonaga A, Study N; Nakagawa Study Group. Influence of dietary sodium and potassium intake on the heart rate corrected-QT interval in elderly subjects. J Nutr Sci Vitaminol (Tokyo) 61: 138–146, 2015. doi: 10.3177/jnsv.61.138. [DOI] [PubMed] [Google Scholar]
- 45.Kim S, Kwak CH, Jung J, Baek JH, Jung JH, Park KJ, Kim K, Kim SK, Kang D, Hahm JR. Changes in serum electrolytes, ECG, and baroreflex sensitivity during combined pituitary stimulation test. Biomed Res Int 2018: 8692078, 2018. doi: 10.1155/2018/8692078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cole NI, Suckling RJ, Swift PA, He FJ, MacGregor GA, Hinton W, van Vlymen J, Hayward N, Jones S, de Lusignan S. The association between serum sodium concentration, hypertension and primary cardiovascular events: a retrospective cohort study. J Hum Hypertens 33: 69–77, 2019. doi: 10.1038/s41371-018-0115-5. [DOI] [PubMed] [Google Scholar]
- 47.Raisch TB, Yanoff MS, Larsen TR, Farooqui MA, King DR, Veeraraghavan R, Gourdie RG, Baker JW, Arnold WS, St A, Poelzing S. Intercalated disk extracellular nanodomain expansion in patients with atrial fibrillation. Front Physiol 9: 398, 2018. doi: 10.3389/fphys.2018.00398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baroudi G, Chahine M. Biophysical phenotypes of SCN5A mutations causing long QT and Brugada syndromes. FEBS Lett 487: 224–228, 2000. doi: 10.1016/s0014-5793(00)02360-7. [DOI] [PubMed] [Google Scholar]
- 49.Makita N, Behr E, Shimizu W, Horie M, Sunami A, Crotti L, Schulze-Bahr E, Fukuhara S, Mochizuki N, Makiyama T, Itoh H, Christiansen M, McKeown P, Miyamoto K, Kamakura S, Tsutsui H, Schwartz PJ, George AL Jr, Roden DM. The E1784K mutation in SCN5A is associated with mixed clinical phenotype of type 3 long QT syndrome. J Clin Invest 118: 2219–2229, 2008. doi: 10.1172/JCI34057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang H, Zhao J, Barrane FZ, Champagne J, Chahine M. Nav1.5/R1193Q polymorphism is associated with both long QT and Brugada syndromes. Can J Cardiol 22: 309–313, 2006. doi: 10.1016/s0828-282x(06)70915-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen J, Makiyama T, Wuriyanghai Y, Ohno S, Sasaki K, Hayano M, Harita T, Nishiuchi S, Yuta Y, Ueyama T, Shimizu A, Horie M, Kimura T. Cardiac sodium channel mutation associated with epinephrine-induced QT prolongation and sinus node dysfunction. Heart Rhythm 13: 289–298, 2016. doi: 10.1016/j.hrthm.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 52.Ramsaroop K, Seecheran R, Seecheran V, Persad S, Giddings S, Mohammed B, Seecheran NA. Suspected hyponatremia-induced Brugada phenocopy. Int Med Case Rep J 12: 61–65, 2019. doi: 10.2147/IMCRJ.S200201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tamene A, Sattiraju S, Wang K, Benditt DG. Brugada-like electrocardiography pattern induced by severe hyponatraemia. Europace 12: 905–907, 2010. doi: 10.1093/europace/euq034. [DOI] [PubMed] [Google Scholar]
- 54.Alvarez PA, Vázquez Blanco M, Lerman J. Brugada type 1 electrocardiographic pattern induced by severe hyponatremia. Cardiology 118: 97–100, 2011. doi: 10.1159/000327089. [DOI] [PubMed] [Google Scholar]
- 55.Blechschmidt S, Haufe V, Benndorf K, Zimmer T. Prog Biophys Mol Biol 98: 309–318, 2008. Voltage-gated Na+ channel transcript patterns in the mammalian heart are species-dependent. doi: 10.1016/j.pbiomolbio.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 56.Isenberg G, Ravens U. The effects of the Anemonia sulcata toxin (ATX II) on membrane currents of isolated mammalian myocytes. J Physiol 357: 127–149, 1984. doi: 10.1113/jphysiol.1984.sp015493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Westenbroek RE, Bischoff S, Fu Y, Maier SKG, Catterall WA, Scheuer T. Localization of sodium channel subtypes in mouse ventricular myocytes using quantitative immunocytochemistry. J Mol Cell Cardiol 64: 69–78, 2013. doi: 10.1016/j.yjmcc.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brack KE, Narang R, Winter J, Ng GA. The mechanical uncoupler blebbistatin is associated with significant electrophysiological effects in the isolated rabbit heart. Exp Physiol 98: 1009–1027, 2013. doi: 10.1113/expphysiol.2012.069369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kappadan V, Telele S, Uzelac I, Fenton F, Parlitz U, Luther S, Christoph J. High-resolution optical measurement of cardiac restitution, contraction, and fibrillation dynamics in beating vs. blebbistatin-uncoupled isolated rabbit hearts. Front Physiol 11: 464, 2020. doi: 10.3389/fphys.2020.00464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fedorov VV, Lozinsky IT, Sosunov EA, Anyukhovsky EP, Rosen MR, Balke CW, Efimov IR. Application of blebbistatin as an excitation-contraction uncoupler for electrophysiologic study of rat and rabbit hearts. Heart Rhythm 4: 619–626, 2007. doi: 10.1016/j.hrthm.2006.12.047. [DOI] [PubMed] [Google Scholar]
- 61.Kornyeyev D, El-Bizri N, Hirakawa R, Nguyen S, Viatchenko-Karpinski S, Yao L, Rajamani S, Belardinelli L. Contribution of the late sodium current to intracellular sodium and calcium overload in rabbit ventricular myocytes treated by anemone toxin. Am J Physiol Heart Circ Physiol 310: H426–H435, 2016. doi: 10.1152/ajpheart.00520.2015. [DOI] [PubMed] [Google Scholar]
- 62.Belardinelli L, Shryock JC, Fraser H. Inhibition of the late sodium current as a potential cardioprotective principle: effects of the late sodium current inhibitor ranolazine. Heart 92, Suppl 4: iv6–iv14, 2006. doi: 10.1136/hrt.2005.078790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weiss JN, Garfinkel A, Karagueuzian HS, Chen P-S, Qu Z. Early afterdepolarizations and cardiac arrhythmias. Heart Rhythm 7: 1891–1899, 2010. doi: 10.1016/j.hrthm.2010.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.King DR, Padget RL, Perry J, Hoeker G, Smyth JW, Brown DA, Poelzing S. Elevated perfusate [Na(+)] increases contractile dysfunction during ischemia and reperfusion. Sci Rep 10: 17289, 2020. doi: 10.1038/s41598-020-74069-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gaborit N, Varro A, Bouter Szuts LS, Escande V, Nattel D, Demolombe S. S. Gender-related differences in ion-channel and transporter subunit expression in non-diseased human hearts. J Mol Cell Cardiol 49: 639–646, 2010. doi: 10.1016/j.yjmcc.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 66.Trépanier-Boulay V, St-Michel C, Tremblay A, Fiset C. Gender-based differences in cardiac repolarization in mouse ventricle. Circ Res 89: 437–444, 2001. doi: 10.1161/hh1701.095644. [DOI] [PubMed] [Google Scholar]
- 67.Pérez-Riera AR, Barbosa-Barros R, Daminello Raimundo R, da Costa de Rezende Barbosa MP, Sorpreso ICE, de Abreu LC. The congenital long QT syndrome type 3: an update. Indian Pacing Electrophysiol J 18: 25–35, 2018. doi: 10.1016/j.ipej.2017.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material 1 (Supplemental Figs. S1–S14): https://doi.org/10.6084/m9.figshare.14825058.
Supplemental Material 2 (simulation code): https://doi.org/10.6084/m9.figshare.16624240.