Abstract
Background and Objectives:
Several cross-sectional and prospective longitudinal studies have shown a progressive decline in Serum (S) Testosterone levels with an increase in age. The clinical consequence of this decline in S Testosterone is not clear from the prevailing data. Several ageing features like decreased libido, Osteo-sarcopenia, anemia, and depressed mood may be associated with reduced androgen levels in elderly males. This study was aimed to study the prevalence of androgen deficiency in elderly males more than 60 years of age presenting to the outpatient department of a tertiary care hospital and its association with frailty and mobility.
Methods:
A cross-sectional observational study was conducted over two years at a tertiary care hospital in Pune, India. The participants underwent a detailed history and physical examination. Biochemical tests and S total testosterone estimation was done. Mobility was estimated by calculating the time taken to perform the Timed Up and Go test (TUGT). Frailty was calculated by Fried's frailty index. Data are presented as mean ± standard deviation, and a comparison between the groups was made using Mann–Whitney U-test. The categorical variables are presented in frequencies along with respective percentages and were compared using the Chi-square or Fisher's exact test. The data was analyzed using SPSS version 22. A P <.05 was considered statistically significant in all the tests.
Results:
The mean age of the study participants was 68.37 ± 6.3 years, with a range of 60-88 years. The mean S total testosterone levels were 3.95 ± 2.06 ng/ml with a range of 0.04–25.36 ng/ml. As per the study definition, Ninety-two (21.67%) participants had testosterone deficiency. Three hundred and thirty-three (78.5%) participants had impaired motility represented by a TUGT time of more than 12 seconds. The Frailty index calculated revealed 94 (22.2%) of the study participants to be normal, 263 (62%) to be vulnerable, and 67 (15.8%) of the patients to be frail.
Conclusion:
The prevalence of testosterone deficiency in the elderly male population was 21.67%. However, there was no association of testosterone deficiency with frailty or impaired mobility. Furthermore, testosterone deficiency was not associated with BMI and hemoglobin levels. In the elderly, testosterone deficiency is associated with low bone mass and therefore imply an increased risk of osteoporotic fractures.
Keywords: Frailty, restricted mobility, testosterone deficiency in elderly
INTRODUCTION
Several cross-sectional and prospective longitudinal studies have shown a progressive decline in Serum (S) testosterone levels with an increase in age.[1,2,3,4] This decrease in S testosterone with age has also been referred to as Androgen Deficiency in Aging Male (ADAM). The clinical consequence of this decline in S testosterone is not clear from the prevailing data. Several ageing features like decreased libido, Osteo-sarcopenia, anemia, and depressed mood may be associated with reduced androgen levels in elderly males.[5] Aging is also associated with increased frailty, reduced mobility and an increase in fall risk.[6,7] The exact association of androgen deficiency in the ageing male and frailty, decreased mobility and increase in fall risk is not known. This study was aimed to study the prevalence of androgen deficiency in elderly males more than 60 years of age presenting to the outpatient department of a tertiary care hospital and its association with frailty and mobility.
MATERIALS AND METHODS
Study objectives
The study's primary objective was to study the prevalence of androgen deficiency in the elderly male population. The secondary objective was to examine the association between androgen deficiency in the elderly male and frailty, mobility and fall risk.
Study population
The study was an observational cross-sectional study conducted at a tertiary care hospital in Pune, India over a 2-year period from April 2017 to June 2019. Individuals aged 60 years or more were defined as the elderly population. All elderly males visiting the outpatient department were screened and invited to participate in the study. These elderly males were healthy, ambulant, independent. Exclusion criteria included participants with a rheumatological disease, previous history of fractures, chronic medical conditions like chronic kidney disease, diabetes mellitus, hypothyroidism, malignancy, or steroid use. In addition, participants taking androgen replacement or androgen depletion therapy were excluded from the study. The study protocol was approved by the institutional ethics committee (IEC), and written informed consent was obtained from the study participants.
Study measures
A detailed history and physical examination were carried out. 10 ml of blood was withdrawn in the morning, Serum separated and preserved at –70 degrees. Serum total testosterone was measured and evaluated commercially available radioimmunoassay kits from Beckman Coulter (Made in Czech Republic) on Startech SR-300 RIA analyzer. The normal range of morning basal cortisol was 4.30–22.40 μg/dL (7–9 AM). The range of measurement of S total testosterone was 0.04 – 20 ng/ml and the CV was 10.6-19%. The range of measurement of S estradiol was 2.2-750 pg/ml and the CV was 8.9-12.2%. A Sample giving a reading more than the highest caliberator were run in dilution. All patients underwent a Dual Energy X-Ray Absorptiometry (DEXA) scan using Hologic Discovery A device for BMD estimation. Mobility was estimated by calculating the time taken to perform the Timed Up and Go test (TUGT).[8] The TUGT time is the time that a person takes to rise from a chair, walk three meters, turn around 180 degrees, walk back to the chair and sit down. Q-TUGTM instrument and accompanying software were used to measure the TUGT time. Frailty was calculated by Fried's frailty index, which is based on five parameters.[9] The five parameters used to calculate the frailty index are weight loss, self-reported exhaustion, low physical activity, slowness and weakness. In the Baltimore Longitudinal Aging Study (BLSA), a prevalence of 19% for testosterone deficiency in elderly males more than 60 years was estimated. In the study population with 5% as confidence limit and 99% confidence level, the minimum sample size calculated was 409.[4]
Study definitions are given as per Table 1.[8,9,10,11]
Table 1.
| Testosterone levels | |
| Normal | ≥ 2.75 ng/ml |
| Deficient | < 2.75 ng/ml |
| Timed Up and Go Test Time (TUGT Time) | |
| Normal | ≤ 12 seconds |
| Impaired | >12 seconds |
| Bone mineral density (BMD) | |
| Normal | T score more than -1 |
| Osteopenia | T score between -2.4 and -1.1 |
| Osteoporosis | T Score - 2.5 or less |
| Frailty | |
| Normal | Fried’s frailty index 0 |
| At-risk | Fried’s frailty index 1-2 |
| Frail | Fried’s frailty index three or more |
Statistical analysis
Data are presented as mean ± standard deviation, and a comparison between the groups was made using Mann–Whitney U-test. The categorical variables are presented in frequencies along with respective percentages and were compared using the Chi-square or Fisher's exact test. The data was analyzed using SPSS version 22. A P < .05 was considered statistically significant in all the tests.
RESULTS
A total of 689 elderly male participants were screened for the study as they satisfied the inclusion criteria. Two hundred and seventeen (217) participants were excluded based on exclusion criteria. Out of 472 participants invited, 424 were enrolled in the study and included in the analysis as 48 participants were detected to have comorbidities or could not undergo the full evaluation. [Figure 1]
Figure 1.
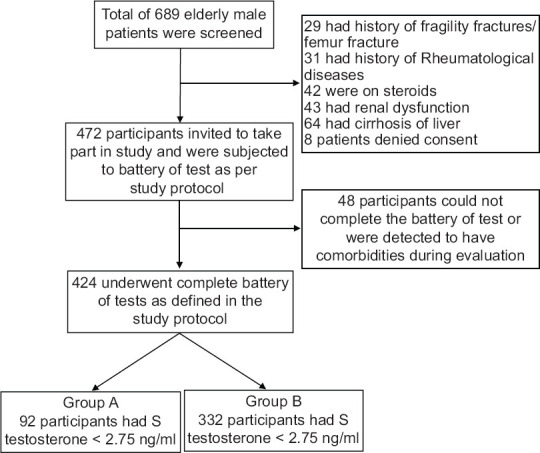
Initial enrolment and flow of the study
The mean age of the study participants was 68.37 ± 6.3 years, with a range of 60-88 years. The mean S total testosterone levels were 3.95 ± 2.06 ng/ml with a range of 0.04 – 25.36 ng/ml. As per the study definition, Ninety-two (21.67%) participants had testosterone deficiency with a mean total testosterone level of 1.68 ± 0.75 ng/ml (Group A) as compared to 332 participants that did not have testosterone deficiency with a mean total testosterone level of 4.58 ± 1.85 ng/ml (P= <.0001) (Group B). The S estrogen level in group A was 28.89 ± 21.20, and group B was 31.23 ± 25.70 pg/ml (P = .42). Three hundred and thirty-three (78.5%) participants had impaired motility represented by a TUGT time of more than 12 seconds. The Frailty index calculated revealed 94 (22.2%) of the study participants to be normal, 263 (62%) to be vulnerable, and 67 (15.8%) of the patients to be frail. [Table 2] There was no significant correlation between hemoglobin level and S total testosterone levels. (Pearson's coefficient 0.09 P. 61).
Table 2.
Comparison of the study parameters in two groups
| Parameters | Unit | Testosterone deficiency (n=92) | Normal Testosterone (n=332) | P |
|---|---|---|---|---|
|
| ||||
| Group A | Group B | |||
| Age Mean (SD) | Years | 68.86 (6.41) | 68.23 (6.28) | 0.4 |
| S Testosterone Mean (SD) | (ng/ml) | 1.68 (0.75) | 4.58 (1.85) | <.0001 |
| BMI Mean (SD) | Kg/m2 | 24.12 (4.09) | 24.06 (4.00) | 0.9 |
| Haemoglobin Mean (SD) | gm/dl | 13.23 (1.20) | 13.09 (1.26) | 0.9 |
| Fried’s frailty Score | No frailty (0) (n) | 14 | 80 | 0.186 |
| At risk of frailty (1-2) (n) | 63 | 200 | ||
| Frail (> 3) (n) | 15 | 52 | ||
| Weight Loss | n (Percentage) | 16 (17.39%) | 52 (15.66%) | 0.75 |
| Self-reported low physical activity | Low physical activity present (n) | 32 | 83 | 0.062 |
| Normal physical activity (n) | 60 | 249 | ||
| Self-reported Exhaustion | n (Percentage) | 22 (23.91%) | 65 (19.58%) | 0.38 |
| Hand Grip | Normal (n) | 43 | 154 | 0.95 |
| Weak (n) | 49 | 178 | ||
| Gait (Time taken to walk 4 mts) Mean (SD) |
Seconds | 7.56 (1.02) | 7.54 (0.84) | 0.87 |
| Mobility | n with TUGT Time ≤12 secs (Normal) | 18 | 73 | 0.67 |
| n with TUGT Time >12 secs (Impaired mobility) | 74 | 259 | ||
| Bone Density | Normal | 10 | 15 | 0.04 |
| Osteopenia | 45 | 155 | ||
| Osteoporosis | 37 | 162 | ||
There was no significant difference in BMI (P = .9) and Hemoglobin (P = .9) levels in the two groups, with and without testosterone deficiency. Testosterone deficiency in the elderly was not associated with frailty (P = .19) or impaired mobility (P = .67) [Figure 2]. The prevalence of osteoporosis in elderly men was higher in the testosterone deficiency group when compared to the group with normal S total testosterone and was bordering on significance (P = .04)
Figure 2.
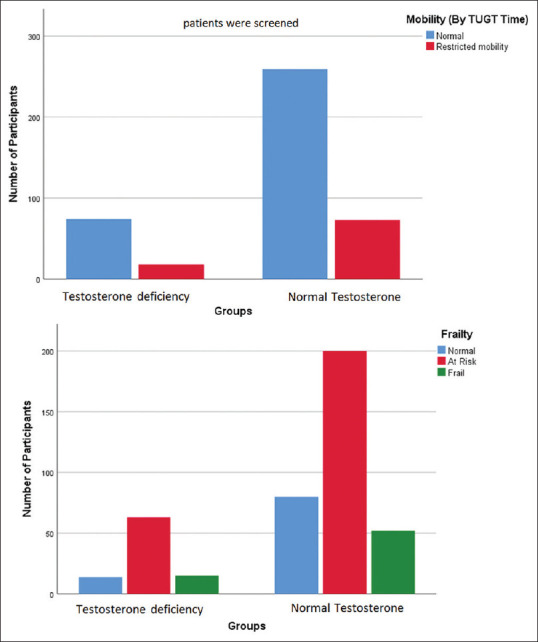
Mobility and Frailty in the study participants
DISCUSSION
We report the prevalence of testosterone deficiency as 21.67% in elderly males aged more than 60 years. As men age, their androgen levels decrease modestly in a gradual way. Wu et al.[1] have reported a decline of S total testosterone by 0.4 per cent per year between ages 40-79. The incidence of androgen deficiency in elderly males has been reported to vary from studies to study. This inconsistency is probably due to the different levels of S testosterone taken to define testosterone deficiency and the baseline population characteristics. Studies also have reported symptoms to be included in diagnostic criteria of hypogonadism. Erenpreiss et al.,[12] in their cohort of 1822 males aged 40-70, had reported a prevalence of 36.12% when a cut-off of 3.46 ng/ml was used to define testosterone deficiency. Araujo et al.[13] have reported a crude incidence rate of 12.3 per 1000 person-years in men aged 40-69. The European Male Aging Study in 3369 community-dwelling men aged 40-79 years have reported an incidence of 23.3% for hypogonadism which is either primary secondary or compensated.[5] Our study evaluated only the elderly population and took the total testosterone cut-off as 2.75 ng/ml to define testosterone deficiency. Therefore, our data may differ from other studies. The elderly population studied closely resembles a subset of the Baltimore Longitudinal ageing study, which reported an incidence of 19% based on S total testosterone level of <3.25 ng/ml.[4] However, to the best of our knowledge, this study remains the only study from India to define the magnitude of the problem.
Frailty is defined as an age-related decline in a physiological condition that may lead to adverse health outcomes. The incidence of frailty (Fried's index ≥3) in our group was 15.8%, and a further 62% of participants were at risk (Fried's index 1 or 2). There was no difference in the two groups (P = .9). The incidence of frailty varies in different studies based on the methods to assess the same. However, an incidence of 4-16% has been reported in community-dwelling men and women aged 65 or above. An additional 28-44% of the same population is at risk.[9,14,15] The prevalence of frailty in our group is similar to that in the prevailing literature. However, we do report a higher number of participants who are at-risk. This may be due to our selection criteria or the population dynamics of visitors to our centre. Declining levels of androgen with age may contribute to obesity, sarcopenia and ultimately frailty.[16] The evidence, however not convincing.[17,18,19,20] We did not find a significant association of testosterone deficiency in the elderly with ‘Frailty’ or ‘At risk of frailty'. Our finding is consistent with the findings of Mohr et al.[20] that report no association of total or free T and the frailty phenotype after adjusting for confounders. Low levels of bioavailable were independently associated with worse baseline frailty status.[14] Travison et al.[19] report age-related changes in blood androgens and estrogens may contribute to the development or progression of frailty in men. However, the measure of frailty was the cardiovascular health study (CHS) indices and could account for the difference in the findings.
Impairment of physical performance and mobility are age-related clinical problems with a significant negative impact on the quality of life of older adults. Several factors can cause restricted mobility in the elderly. Testosterone, by its anabolic effects, can increase muscle mass and strength. Testosterone deficiency is often associated with osteo-sarcopenia, which may restrict mobility in the elderly population.[16] However, the age-associated decline in testosterone on mobility in the elderly male population is not evident. We assessed mobility in our participants by estimating the TUGT time. We found restricted mobility in about 78.54% of the study participants based on a TUGT time of >12 seconds. There was no difference in the two groups (P = .67). However, normative data for TUGT time is not available in our country. The Indian population may have a higher cut-off for TUGT Time due to slower baseline mobility.[21] A community-based cross-sectional study conducted on elderly participants reported 53.6% of participants to have a functional disability based on Barthel's Index questions.[22] Though the index of functional disability is different from our study, we reported a higher incidence of restricted mobility. The gait velocity in elderly Indian population has been reported to be lower (0.6 m/sec) when compared to elderly western population (1.1-1.5 m/sec).[21] Therefore, the Indian population may have a higher cut-off for TUGT Time due to slower movement. Further, the population selected in our study was from OPD visits which may further bias our result. We, however, could demonstrate decreased mobility in most participants in our cohort. Positive association of indices of muscle strength with S total testosterone or free testosterone have been reported in several cohorts of the elderly population.[23,24,25] The BACH/Bone study demonstrated that total testosterone weakly correlates with walking speed, but not with grip strength, timed chair standing or a composite physical performance score.[26] In the longitudinal analysis of MrOS US cohort, Longitudinal Aging Study Amsterdam cohort and the Framingham offspring study cohort, Low free or Total S Testosterone was not associated with various mobility parameters.[27–29] In our cohort, there was no association of restricted mobility as assessed by TUGT time and testosterone deficiency in the elderly. We, however, did not evaluate the free testosterone level and the effect of SHBG on mobility parameters.
Hypogonadism is a risk factor for osteoporosis. We report a statistically increased incidence of osteoporosis in elderly men with low total testosterone compared to the elderly with normal total testosterone levels (P = .04). Kadam et al.[30] have reported that there is a decline in BMD with age. Our findings are consistent with Stanley et al.,[31] who conclude that hypogonadal elderly white men may be at increased risk for Minimal Trauma Hip Fracture due to low bone mass.
The strength of our study was the large sample size and the study population, which consisted of the most vulnerable people for effects of androgen deficiency. However, the study participants in our cohort were from OPD visits, and the findings of our study cannot be generalized in the community setting.
CONCLUSION
In our study, the prevalence of testosterone deficiency in the elderly male population was 21.67%. However, there was no association of testosterone deficiency with frailty or impaired mobility. Furthermore, testosterone deficiency was not associated with BMI and Hemoglobin levels. In the elderly, testosterone deficiency is associated with low bone mass and therefore imply an increased risk of osteoporotic fractures.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Wu FCW, Tajar A, Pye SR, Silman AJ, Finn JD, O'Neill TW, et al. Hypothalamic-pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: The European Male Aging Study. J Clin Endocrinol Metab. 2008;93:2737–45. doi: 10.1210/jc.2007-1972. [DOI] [PubMed] [Google Scholar]
- 2.Handelsman DJ, Yeap B, Flicker L, Martin S, Wittert GA, Ly LP. Age-specific population centiles for androgen status in men. Eur J Endocrinol. 2015;173:809–17. doi: 10.1530/EJE-15-0380. [DOI] [PubMed] [Google Scholar]
- 3.Travison TG, Araujo AB, Kupelian V, O'Donnell AB, McKinlay JB. The relative contributions of aging, health, and lifestyle factors to serum testosterone decline in men. J Clin Endocrinol Metab. 2007;92:549–55. doi: 10.1210/jc.2006-1859. [DOI] [PubMed] [Google Scholar]
- 4.Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Baltimore Longitudinal Study of Aging. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore longitudinal study of aging. J Clin Endocrinol Metab. 2001;86:724–31. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- 5.Tajar A, Huhtaniemi IT, O'Neill TW, Finn JD, Pye SR, Lee DM, et al. Characteristics of androgen deficiency in late-onset hypogonadism: Results from the European Male Aging Study (EMAS) J Clin Endocrinol Metab. 2012;97:1508–16. doi: 10.1210/jc.2011-2513. [DOI] [PubMed] [Google Scholar]
- 6.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381:752–62. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bradley SM. Falls in older adults. Mt Sinai J Med. 2011;78:590–5. doi: 10.1002/msj.20280. [DOI] [PubMed] [Google Scholar]
- 8.Bischoff HA, Stähelin HB, Monsch AU, Iversen MD, Weyh A, von Dechend M, et al. Identifying a cut-off point for normal mobility: A comparison of the timed ‘up and go’ test in community-dwelling and institutionalised elderly women. Age Ageing. 2003;32:315–20. doi: 10.1093/ageing/32.3.315. [DOI] [PubMed] [Google Scholar]
- 9.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–56. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 10.Snyder PJ, Bhasin S, Cunningham GR, Matsumoto AM, Stephens-Shields AJ, Cauley JA, et al. Effects of testosterone treatment in older men. N Engl J Med. 2016;374:611–24. doi: 10.1056/NEJMoa1506119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watts NB, Adler RA, Bilezikian JP, Drake MT, Eastell R, Orwoll ES, et al. Osteoporosis in men: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2012;97:1802–22. doi: 10.1210/jc.2011-3045. [DOI] [PubMed] [Google Scholar]
- 12.Erenpreiss J, Fodina V, Pozarska R, Zubkova K, Dudorova A, Pozarskis A. Prevalence of testosterone deficiency among aging men with and without morbidities. Aging Male. 2020;23:901–5. doi: 10.1080/13685538.2019.1621832. [DOI] [PubMed] [Google Scholar]
- 13.Araujo AB, O'Donnell AB, Brambilla DJ, Simpson WB, Longcope C, Matsumoto AM, et al. Prevalence and incidence of androgen deficiency in middle-aged and older men: Estimates from the Massachusetts male aging study. J Clin Endocrinol Metabo. 2004;89:5920–6. doi: 10.1210/jc.2003-031719. [DOI] [PubMed] [Google Scholar]
- 14.Cawthon PM, Marshall LM, Michael Y, Dam T-T, Ensrud KE, Barrett-Connor E, et al. Frailty in older men: Prevalence, progression, and relationship with mortality. J Am Geriatr Soc. 2007;55:1216–23. doi: 10.1111/j.1532-5415.2007.01259.x. [DOI] [PubMed] [Google Scholar]
- 15.Kiely DK, Cupples LA, Lipsitz LA. Validation and comparison of two frailty indexes: The MOBILIZE Boston study. J Am Geriatr Soc. 2009;57:1532–9. doi: 10.1111/j.1532-5415.2009.02394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saad F, Röhrig G, Haehling von S, Traish A. Testosterone deficiency and testosterone treatment in older men. Gerontology. 2017;63:144–56. doi: 10.1159/000452499. [DOI] [PubMed] [Google Scholar]
- 17.Poehlman ET, Toth MJ, Fishman PS, Vaitkevicius P, Gottlieb SS, Fisher ML, et al. Sarcopenia in aging humans: The impact of menopause and disease. J Gerontol A Biol Sci Med Sci. 1995;50:73–7. doi: 10.1093/gerona/50a.special_issue.73. [DOI] [PubMed] [Google Scholar]
- 18.O'Donnell AB, Araujo AB, McKinlay JB. The health of normally aging men: The Massachusetts male aging study (1987-2004) Exp Gerontol. 2004;39:975–84. doi: 10.1016/j.exger.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 19.Travison TG, Nguyen A-H, Naganathan V, Stanaway FF, Blyth FM, Cumming RG, et al. Changes in reproductive hormone concentrations predict the prevalence and progression of the frailty syndrome in older men: The concord health and ageing in men project. J Clin Endocrinol Metab. 2011;96:2464–74. doi: 10.1210/jc.2011-0143. [DOI] [PubMed] [Google Scholar]
- 20.Mohr BA, Bhasin S, Kupelian V, Araujo AB, O'Donnell AB, McKinlay JB. Testosterone, sex hormone-binding globulin, and frailty in older men. J Am Geriatr Soc. 2007;55:548–55. doi: 10.1111/j.1532-5415.2007.01121.x. [DOI] [PubMed] [Google Scholar]
- 21.Gunasekaran V, Banerjee J, Dwivedi SN, Upadhyay AD, Chatterjee P, Dey AB. Normal gait speed, grip strength and thirty seconds chair stand test among older Indians. Arch Gerontol Geriatr. 2016;67:171–8. doi: 10.1016/j.archger.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Keshari P, Shankar H. Prevalence and spectrum of functional disability of urban elderly subjects: A community-based study from Central India. J Family Community Med. 2017;24:86–90. doi: 10.4103/jfcm.JFCM_80_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsu B, Cumming RG, Naganathan V, Blyth FM, Le Couteur DG, Seibel MJ, et al. Longitudinal relationships of circulating reproductive hormone with functional disability, muscle mass, and strength in community-dwelling older men: The concord health and ageing in men project. J Clin Endocrinol Metab. 2014;99:3310–8. doi: 10.1210/jc.2014-1124. [DOI] [PubMed] [Google Scholar]
- 24.van den Beld AW, de Jong FH, Grobbee DE, Pols HA, Lamberts SW. Measures of bioavailable serum testosterone and estradiol and their relationships with muscle strength, bone density, and body composition in elderly men. J Clin Endocrinol Metab. 2000;85:3276–82. doi: 10.1210/jcem.85.9.6825. [DOI] [PubMed] [Google Scholar]
- 25.Schaap LA, Pluijm SMF, Smit JH, van Schoor NM, Visser M, Gooren LJG, et al. The association of sex hormone levels with poor mobility, low muscle strength and incidence of falls among older men and women. Clin Endocrinol (Oxf) 2005;63:152–60. doi: 10.1111/j.1365-2265.2005.02315.x. [DOI] [PubMed] [Google Scholar]
- 26.Araujo AB, Travison TG, Bhasin S, Esche GR, Williams RE, Clark RV, et al. Association between testosterone and estradiol and age-related decline in physical function in a diverse sample of men. J Am Geriatr Soc. 2008;56:2000–8. doi: 10.1111/j.1532-5415.2008.01965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.LeBlanc ES, Wang PY, Lee CG, Barrett-Connor E, Cauley JA, Hoffman AR, et al. Higher testosterone levels are associated with less loss of lean body mass in older men. J Clin Endocrinol Metab. 2011;96:3855–63. doi: 10.1210/jc.2011-0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krasnoff JB, Basaria S, Pencina MJ, Jasuja GK, Vasan RS, Ulloor J, et al. Free testosterone levels are associated with mobility limitation and physical performance in community-dwelling men: The Framingham Offspring Study. J Clin Endocrinol Metab. 2010;95:2790–9. doi: 10.1210/jc.2009-2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schaap LA, Pluijm SMF, Deeg DJH, Penninx BW, Nicklas BJ, Lips P, et al. Low testosterone levels and decline in physical performance and muscle strength in older men: Findings from two prospective cohort studies. Clin Endocrinol (Oxf) 2008;68:42–50. doi: 10.1111/j.1365-2265.2007.02997.x. [DOI] [PubMed] [Google Scholar]
- 30.Kadam NS, Chiplonkar SA, Khadilkar AV, Khadilkar VV. Prevalence of osteoporosis in apparently healthy adults above 40 years of age in Pune City, India. Indian J Endocrinol Metab. 2018;22:67–73. doi: 10.4103/ijem.IJEM_438_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stanley HL, Schmitt BP, Poses RM, Deiss WP. Does hypogonadism contribute to the occurrence of a minimal trauma hip fracture in elderly men? J Am Geriatr Soc. 1991;39:766–71. doi: 10.1111/j.1532-5415.1991.tb02698.x. [DOI] [PubMed] [Google Scholar]


