Sir,
In animal models, incretin-based therapy has been shown to be effective in protecting and preserving β-cell functions.[1] Farilla et al. 2002 and Li et al. 2003 have corroborated that DPP-4 inhibitors increase islet cell proliferation, and decrease cell apoptosis in vitro.[2]
However, as with any other drugs we need to be aware of the adverse effects of Sitagliptin so that it may be withdrawn at the very advent of any adverse reaction. The adverse skin manifestations of Sitagliptin are all the more important because reports have illustrated their appearance almost 6 months after initiation of therapy;[3] thus if the physician is unaware of these uncommon reactions then both the physician and the patient would be baffled and puzzled by these manifestations. Obviously, the drug will not be withdrawn because it will be the least suspected culprit and the patient would be left battling with these manifestations indefinitely.
A 60-year-old woman came to our outdoor department with the complaint of generalized skin lesion for the last 3 months. Distribution of lesions around umbilicus, lower back, lower extremities, and face is shown in Figures 1-5. She was diagnosed as a case of Type 2 diabetes mellitus about 1 year back and she was taking one tablet of Sitagliptin 50 mg + Metformin 500 mg twice a day since then as advised by her physician with proper follow up. Her blood sugar levels were well under controlled with this regime, HbA1c 6.8%, fasting blood sugar 98 mg/dL, postprandial blood sugar 165 mg/dL. She developed these skin lesions at 3 months of following this said regime. She has never had any skin disease in past nor history of any fixed drug eruption in past. Her vitals were stable and her general and systemic examinations were within normal limits. Her complete blood count, liver function test, kidney function test, lipid profile were within normal limits. Anti-HCV, HBsAg, HIV, and VDRL were non-reactive. Her chest X-ray, ultrasonography of abdomen, fundus examination were normal. On searching through web journals and case reports we found out that there exists association of such skin lesion with the use of Sitagliptin + Metformin regime. On consultation with our dermatology department, we made the diagnosis of drug-induced Papulonodular lesions in a patient of Diabetes Mellitus receiving Sitagliptin and Metformin. As both Sitagliptin and Metformin can cause papulonodular lesion as mentioned in literature[4] so we cannot discriminate whether it is due to which drug.
Figure 1.
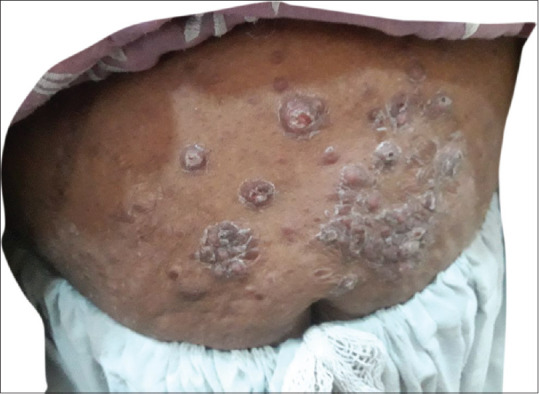
Papulonodular lesions around umbilicus
Figure 5.
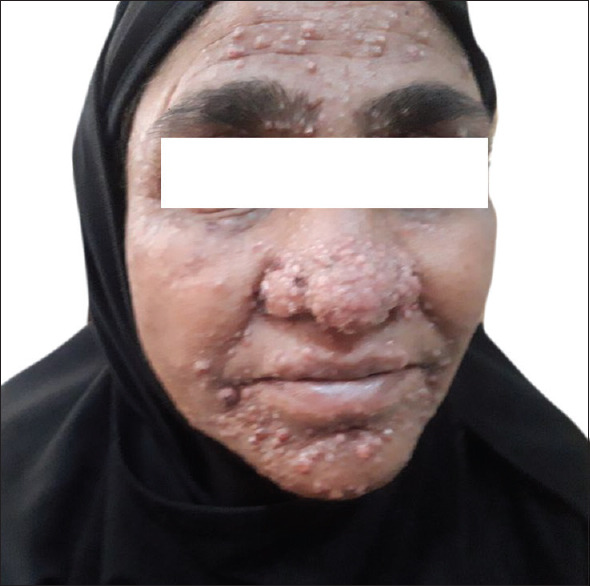
Papulonodular lesions around face
Figure 2.
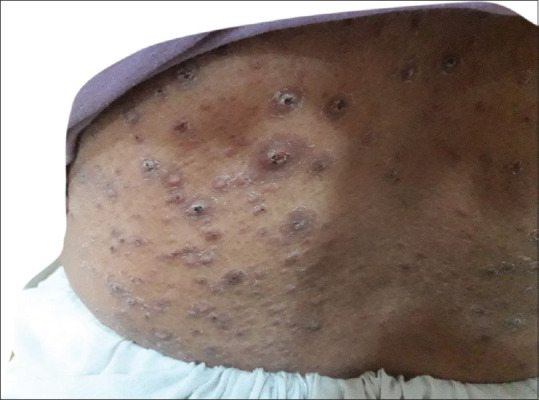
Papulonodular lesions around lower back
Figure 3.
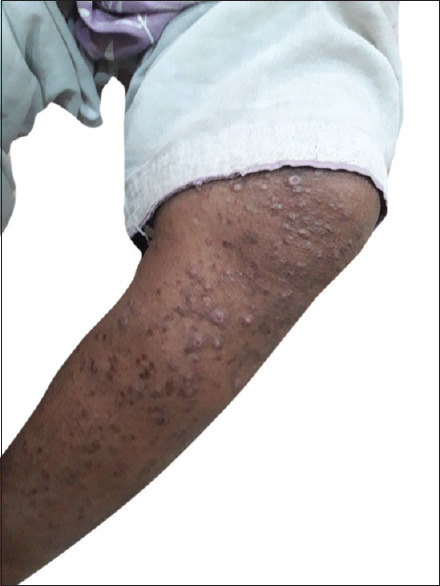
Papulonodular lesions around lower extremity
Figure 4.
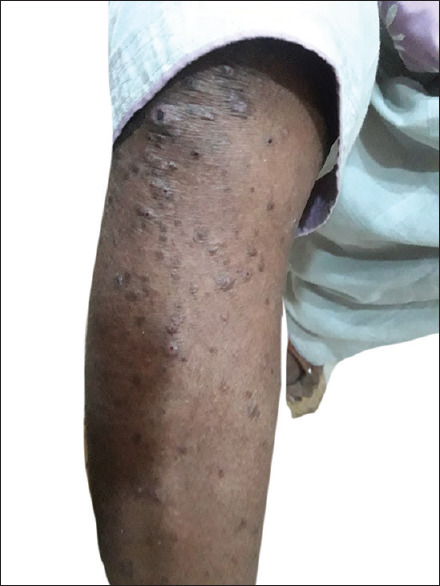
Papulonodular lesions around lower extremity
As a treatment, we stopped both of these drugs immediately and started subcutaneous insulin therapy, within a period of 1 month the lesions gradually regressed and within 3 months they totally regressed with only scarring left behind. So we conclude that papulonodular lesions are due to combination of Sitagliptin and metformin.
A wide array of cutaneous adverse effects have been reported with the use of Sitagliptins which include Psoriasiform eruption, Maculopapular rash, Stevens-Johnson syndrome, Toxic epidermal necrolysis, Anaphylaxis, Cutaneous vasculitis, Bullous pemphigoid, Photosensitivity and Angioedema on co-administration with ACE inhibitors.[5] There are also reports of fixed drug eruptions[5] and generalized skin eruptions.[3] Like all known photo-sensitizers, Sitagliptin has a phenyl ring, carbonyl group, and an absorption spectrum showing three absorption peaks (199.9, 265.0, and 400.1 nm) and its photosensitive mechanism could result in itchy edematous plaque. Although both the 199.0 and 265.0 nm wavelengths are within the UV-C spectrum, the 400.1 nm absorbance peak indicates that Sitagliptin also absorbs UV-A visible light. Thus, Sitagliptin could cause persistent photosensitive eruption after cessation of the drug even with protection from UV light by hapten formation with subcutaneous protein.[5]
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient (s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Eligar VS, Bain SC. A review of sitagliptin with special emphasis on its use in moderate to severe renal impairment. Drug Des Devel Ther. 2013;7:893–903. doi: 10.2147/DDDT.S32331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farilla L, Hui H, Bertolotto C, Kang E, Bulotta A, Di Mario U, et al. Gluca gon-like peptide-1 promotes islet cell growth and inhibits apoptosis in Zucker diabetic rats. Endocrinology. 2002;143:4397–408. doi: 10.1210/en.2002-220405. [DOI] [PubMed] [Google Scholar]
- 3.Nakatani K, Kurose T, Hyo T, Watanabe K, Yabe D, Kawamoto T, et al. Drug-induced generalized skin eruption in a diabetes mellitus patient receiving a dipeptidyl peptidase-4 inhibitor plus metformin. Diabetes Ther. 2012;3:14. doi: 10.1007/s13300-012-0014-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Badr D, Kurban M, Abbas O. Metformin in dermatology: An overview. J Eur Acad Dermatol Venereol. 2013;27:1329–35. doi: 10.1111/jdv.12116. [DOI] [PubMed] [Google Scholar]
- 5.Gupta M, Gupta A. Fixed drug eruption to sitagliptin. J Diabetes Metab Disord. 2015;14:18. doi: 10.1186/s40200-015-0145-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


