Abstract
Radiation therapy is one of the treatment methods for hepatocellular carcinoma. However, radiation tolerance of the liver is low, and the detailed effect of radiation on liver regeneration has not been clarified. C57BL/6J mice or hepatocyte‐specific p53 knockout (KO) mice (albumin [Alb]‐Cre Trp53flox/flox ) were irradiated with a single fraction of 10 Gy localized to the upper abdomen. We performed 70% partial hepatectomy (PHx) 24 hours after irradiation. Liver regeneration was assessed by proliferation cell nuclear antigen (PCNA)‐ and Ki‐67‐positive hepatocyte ratios and liver‐to‐body weight ratio after PHx. To establish a fibrosis model, CCl4 was orally administered for 8 weeks. The murine hepatocyte cell line BNL CL.2 (CL2) was irradiated with 10 Gy. Irradiation activated p53, induced downstream p21 in the liver, and delayed liver regeneration after PHx. While PHx increased hepatocyte growth factor (HGF) levels and activated Met with or without irradiation in the regenerative liver, it activated Akt and extracellular kinase 1 and 2 (Erk 1/2) less in irradiated mice than in nonirradiated mice. In CL2 cells cultured with HGF, irradiation suppressed cell growth by decreasing phosphorylated Akt and Erk 1/2 levels, which was abolished by small interfering RNA‐mediated p53 knockdown but not by p21 knockdown. Hepatocyte‐specific knockout of p53 in mice abolished the irradiation‐induced suppression of both liver regeneration and Akt and Erk 1/2 activation after PHx. In the fibrotic mouse model, the survival rate after PHx of irradiated p53 KO mice was higher than that of wild‐type mice. Conclusion: p53 but not p21 is involved in the impaired regenerative ability of the irradiated liver.
Abbreviations
- Alb
albumin
- ANOVA
analysis of variance
- AU
arbitrary units
- CL2
BNL CL.2
- Erk
extracellular signal‐regulated kinase
- fl
flox
- G1/G2
growth 1 and growth 2 phase
- Gab1
GRB2‐associated binding protein 1
- H&E
hematoxylin and eosin
- h
hours
- HGF
hepatocyte growth factor
- KO
knockout
- M
mitosis phase
- mRNA
messenger RNA
- NS
not significant
- p‐
phosphorylated
- PCNA
proliferation cell nuclear antigen
- phospho
phosphorylated
- PHx
partial hepatectomy
- RILD
radiation‐induced liver damage
- RT‐PCR
real‐time reverse transcription polymerase chain reaction
- siRNA
small interfering RNA
- WT
wild type
Radiation therapy is one of the treatment methods for hepatocellular carcinoma. Radiation therapy can be adjusted for patients who are elderly and those with a poor general condition who are unsuitable for resection, ablation, or transplantation.( 1 ) However, radiation therapy has the risk of radiation‐induced liver disease (RILD), which is sometimes life threatening and has no established treatment.( 2 , 3 ) Classic RILD is a complication of radiation that causes hepatomegaly, ascites, and elevated alkaline phosphatase levels after whole‐liver irradiation with 30‐35 Gy using a conventionally fractionated regimen.( 4 ) Classic RILD can be prevented by reducing the radiation dose.( 3 , 5 , 6 ) Recently, advances in computed tomography planning technology have made it possible to administer a high radiation dose locally, and classic RILD has become rare.( 7 , 8 ) However, even with localized irradiation, noncancerous liver lesions around the carcinoma are still irradiated with 10 Gy or more. Cases of increases in the Child‐Pugh score and transaminase level after radiation therapy are observed in 5%‐12% of patients treated with localized radiation therapy.( 9 , 10 ) Such cases of liver damage after irradiation are called nonclassic RILD. Patients with nonclassic RILD have high mortality due to fatal hepatic failure within 12 months of radiation therapy, even with control of hepatocellular carcinoma.( 10 ) The risk factor for nonclassic RILD has been reported to be cirrhosis in the background liver.( 9 ) The detailed mechanism of nonclassic RILD is unclear; however, it has been reported that the effect of radiation on regenerating hepatocytes may be related to nonclassic RILD.( 4 , 8 ) In the present study, we investigated the regenerative capacity of the irradiated liver.
Materials and Methods
Mice
We obtained 8‐10‐week‐old male C57BL/6J mice from Charles River Laboratories (Tokyo, Japan). Male C57BL/6J mice were introduced into the laboratory 1 week before the start of the experiment, and it was confirmed that there was no problem with their health condition. Male C57BL/6J mice were randomly assigned to each group. Trp53flox (fl)/fl mice with a C57BL/6J background and albumin (Alb)‐Cre transgenic mice were purchased from The Jackson Laboratory (Bar Harbor, ME). We generated 8‐11‐week‐old male hepatocyte‐specific p53 knockout (KO) mice (Trp53fl/fl Alb‐Cre) by mating Trp53fl/fl mice and Alb‐Cre transgenic mice. Male p53 KO and littermate wild‐type (WT) mice (Trp53fl/fl ) were randomly assigned to each group so that there was no difference in body weight between p53 KO and littermate WT mice. Liver irradiation was performed with the lower abdomen and chest of some mice shielded with lead, and a single fraction of 10 Gy was delivered at a dose rate of 177 Gy/minute. No mice died from irradiation alone during the experiment. Liver fibrosis was induced by intragastric administration of 5 mL/kg body weight of 25% CCl4 dissolved in corn oil 3 times a week for 8 weeks.( 11 ) Male p53 KO mice and littermate WT mice did not die during the CCl4 administration period. Mice were maintained in a pathogen‐free facility with a 12‐hour light/dark cycle and received humane treatment. All animal‐related procedures were approved by the Animal Care and Use Committee of Osaka University Medical School and performed according to Animal Research: Reporting of In Vivo Experiments guidelines.
Cell Culture
We used the nontransformed murine hepatocyte cell line BNL CL.2 (CL2), which was obtained from ATCC (Manassas, VA). Cells were incubated at 37°C under 5% CO2 in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum (26140‐079; Gibco, Grand Island, NY). The cell line was tested and shown to be free of mycoplasma contamination. For irradiation, a single fraction of 10 Gy was delivered. Cells were transfected with 5 nM small interfering RNA (siRNA) for 48 hours using Lipofectamine RNAiMAX Transfection Reagent (Invitrogen Corporation, Carlsbad, CA) as described.( 12 ) siRNA negative control; 4389843, siRNA p53; s75474 and siRNA p21; s63813 (Thermo Fisher Scientific, Waltham, MA) were purchased. Mouse recombinant hepatocyte growth factor (HGF) was purchased from R&D Systems (Minneapolis, MN). HGF (10 ng/mL) was supplied 2 hours after irradiation. Vanadate was purchased from Sigma‐Aldrich (St Louis, MO). Vanadate (200 μM) was supplied 0.5 hours before irradiation, and the final concentration of vanadate in the medium was administered to be 200 µM.
Partial Hepatectomy or Sham Operation in Mice
Male mice were subjected to a sham operation or 70% partial hepatectomy (PHx) as described.( 13 ) All mice were randomly assigned to each group, and there was no significant difference in body weight before surgery between the irradiated and nonirradiated groups. PHx was performed at 24 hours after irradiation. Mice were killed at the indicated time points after surgery. All mice were confirmed to have no thrombus formation in the regenerated liver by hematoxylin and eosin (H&E) staining of liver sections after PHx. Liver regeneration was assessed by proliferation cell nuclear antigen (PCNA)‐ and Ki‐67‐positive hepatocyte ratios and liver‐to‐body weight ratio after PHx.
Immunohistochemistry
Dissected livers were fixed in 10% formalin for 24 hours and embedded in paraffin. Sections were stained with H&E. We also stained the sections with sirius red (Sigma‐Aldrich) to assess liver fibrosis. The sirius red‐positive area was measured with BZ‐700 image analysis software (KEYENCE, Osaka, Japan). To assess liver proliferation, sections were stained with an anti‐PCNA antibody (#2586, 1:4,000; Cell Signaling Technology, Danvers, MA) and anti‐Ki‐67 antibody (#12202s, 1:400; Cell Signaling Technology). The numbers of PCNA‐ and Ki‐67‐positive nuclear cells were counted in 10 random periportal fields of view per liver section. Immunohistochemistry was performed as described.( 14 ) To assess progenitor cells, sections were stained with an anti‐A6 antibody (A6 BCM, 1:200; Developmental Studies Hybridoma Bank, Iowa City, IA) and anti‐pancytokeratin antibody (ab27988, 1:40; Abcam, Cambridge, United Kingdom). The numbers of A6‐positive cells were counted in 10 random fields of view per liver section.
Real‐Time Quantitative Polymerase Chain Reaction
Total RNA isolated from liver tissues using the RNeasy Mini Kit (Qiagen) was reverse transcribed. Messenger RNA (mRNA) was subjected to real‐time reverse transcription polymerase chain reaction (RT‐PCR) with quantification using the following TaqMan gene expression assays (Applied Biosystems, Foster City, CA): Trp53 (assay identification [ID], Mm01731290_g1), cyclin dependent kinase inhibitor 1A (Cdkn1a; assay ID, Mm00432448_m1), BCL2 binding component 3 (Bbc3; assay ID, Mm00519268_m1), and beta‐actin (β‐actin; assay ID, Mm00607939_s1). Transcript levels are presented as the fold change relative to controls.
Western Blot and Analysis
Western blotting was carried out as described.( 14 ) For immunodetection, antibodies against the following proteins were used: Met (Cell Signaling Technology, #3127) at 1:1,000; phosphorylated (phospho)‐Met (Tyr1234/1235) (Cell Signaling Technology, #3077) at 1:1,000; GRB2 associated binding protein 1 (Gab1; Cell Signaling Technology) at 1:1,000; phospho‐Gab1 (Cell Signaling Technology) at 1:1,000; Akt (Cell Signaling Technology, #4691) at 1:1,000; phospho‐Akt (Thr308) (Cell Signaling Technology, #4060) at 1:2,000; extracellular kinase 1 and 2 (Erk 1/2; Cell Signaling Technology, #4695) at 1:1,000; phospho‐Erk 1/2 (Thr202/Tyr204) (Cell Signaling Technology, #4370) at 1:2,000; p53 (Leica, Nussloch, Germany, #NCL‐L‐p53‐CM5p) at 1:2,000; phospho‐p53 (primary target, Serine 15 [Ser15]) (Cell Signaling Technology, #9284) at 1:1,000; HGF (Abcam, #ab83760) at 1:1,000; p21 (Abcam, #ab109199) at 1:1,000; and β‐actin (Sigma‐Aldrich, A5316) at 1:10,000.
Cell‐Counting Assay and Apoptosis Assay
Cell number was counted using a TC20 automated cell counter (Bio‐Rad, Hercules, CA). Caspase 3/7 activity in cell culture medium was measured by a luminescent substrate assay (Caspase Glo assay; Promega, Madison, WI) according to the manufacturer's instructions.
Cell‐Cycle Assay
Irradiated CL2 cells were incubated for 6 hours and collected with 0.05% trypsin followed by cell‐cycle evaluation using a Cycletest Plus DNA Reagent Kit (Becton, Dickinson, Franklin Lakes, NJ) according to the protocol. The number of cells in each phase was counted by a fluorescence‐activated cell sorting (FACS) Canto flow cytometer. At least 10,000 cells/group were collected for each measurement in triplicate.
Statistics
Results are shown as mean ± SD for the number of samples. Statistical analysis of two groups was performed with an unpaired Student t test. Multigroup comparisons were performed by applying one‐way analysis of variance (ANOVA), followed by the Tukey‐Kramer method, using JMP Pro 14 software (SAS Institute Inc., Cary, NC). Differences with P < 0.05 were considered statistically significant.
Results
Irradiation Activated p53 and Suppressed Liver Regeneration in C57BL6/J Mice
In the C57BL6/J mouse liver, irradiation induced p53 phosphorylation (Ser15), stabilized p53, and induced p21 transcription but did not induce transcription of puma, another downstream target of p53 (Fig. 1A). To examine the effect of radiation on liver regeneration, C57BL6/J mice were subjected to PHx or a sham operation at 24 hours after irradiation. H&E staining demonstrated no sign of inflammatory cell or necrotic changes in the liver of either irradiated mice or nonirradiated mice at 48 hours after PHx (Fig. 1B). The liver of the irradiated mice at 48 hours after PHx showed lower PCNA‐ and Ki‐67‐positive hepatocyte ratios than the liver of nonirradiated mice (Fig. 1B). PCNA‐ and Ki‐67‐positive hepatocyte rates in nonirradiated mice decreased from 48 hours after PHx to 72 hours after PHx and reached the same level as those in sham‐operated mice at 168 hours after PHx (Fig. 1B). In contrast, PCNA‐ and Ki‐67‐positive hepatocyte rates in irradiated mice increased from 48 hours after PHx to 72 hours after PHx and decreased to the same level as those in sham‐operated mice at 168 hours after PHx (Fig. 1B). The protein expression levels of p21 in the irradiated mouse liver were higher than those in the nonirradiated mouse liver at 48 hours after PHx (Fig. 1C). The protein expression levels of HGF and its downstream targets Met and Gab1 were increased by PHx regardless of irradiation, and there was no difference in those levels after PHx between the nonirradiated mouse liver and irradiated mouse liver (Fig. 1C). On the other hand, the expression of phospho‐Akt and phospho‐Erk 1/2 at 48 hours after PHx was suppressed in irradiated mice compared to nonirradiated mice (Fig. 1C). The liver‐to‐body weight ratio at 72 hours after PHx in the irradiated mice was significantly lower than that in the nonirradiated mice, although there was no difference in the liver‐to‐body weight ratio at 168 hours after PHx between them (Supporting Fig. S1A). There was no difference in the cell rate stained with an anti‐A6 antibody, which specifically stains hepatic oval cells in murine liver,( 15 ) between nonirradiated mice and irradiated mice 48, 72, and 168 hours after partial PHx (Supporting Fig. S1C). These cells were also stained with anti‐pancytokeratin antibody, a substitute marker for progenitor cells.( 15 ) This result suggested that hepatic progenitor activation after PHx was similar between irradiated and nonirradiated livers
FIG. 1.
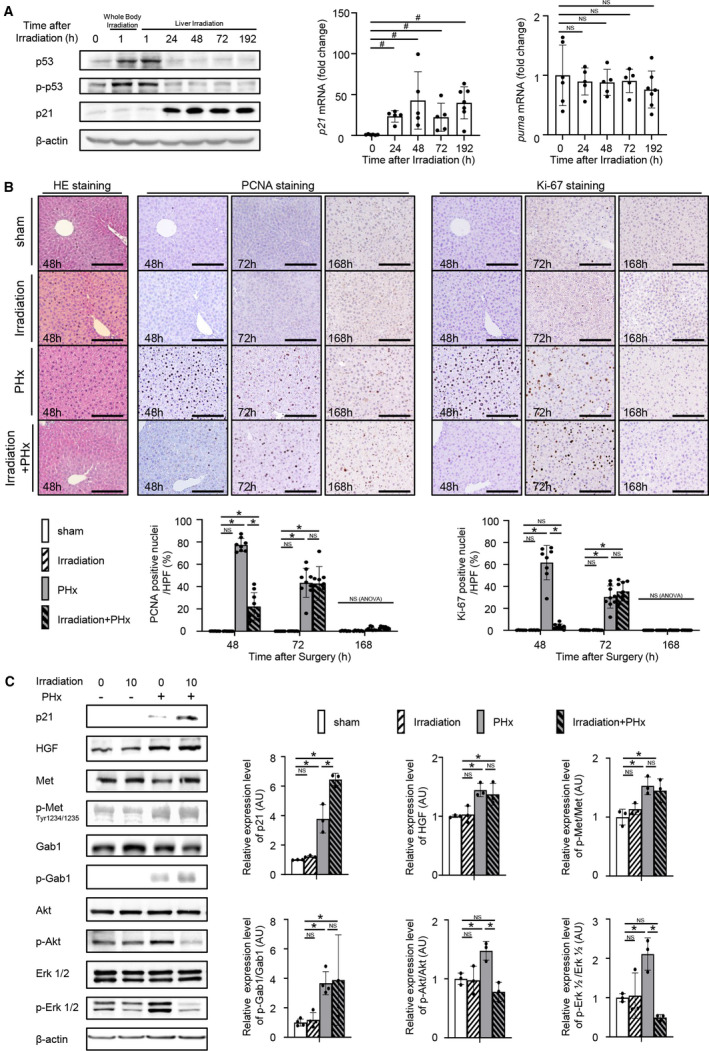
Irradiation suppresses hepatocyte proliferation after PHx. (A) Male C57BL/6J mice were irradiated with 10 Gy (n = 6, 5, 5, 5, 7 mice at 0, 24, 48, 72, 192 hours after irradiation, respectively). (A, left) Protein expression levels of p53, phospho‐p53 (Ser15), and p21 in the liver at the indicated times after irradiation were assessed by western blot analysis. (A, center and right) mRNA expression levels of p21 and puma in the livers at the indicated times after irradiation were quantified by quantitative RT‐PCR; # P < 0.05 based on the unpaired Student t test. (B,C) Male C57BL/6J mice were randomly assigned to the following four groups: nonirradiated mice subjected to a sham operation 24 hours later (sham group); irradiated mice with 10 Gy subjected to a sham operation 24 hours later (irradiation group); nonirradiated mice subjected to PHx 24 hours later (PHx group); and irradiated mice with 10 Gy subjected to PHx 24 hours later (irradiation+PHx group) (sham n = 7, 9, 7 mice; irradiation n = 7, 9, 7 mice; PHx n = 8, 9, 9 mice; irradiation+PHx n = 9, 9, 9 mice; at 48, 72, 168 hours after surgery, respectively). (B, upper) Representative images of liver sections stained with H&E at 48 hours after surgery. Representative images of liver sections stained with anti‐PCNA antibody or anti‐Ki‐67 antibody at the indicated times after surgery with or without irradiation; magnification ×200. (B, lower) Percentages of PCNA‐ and Ki‐67‐positive nuclei/HPFs are reported. Ten fields of view were counted in the liver sections of individual mice. (C) Expression levels of cell‐growth‐related proteins in the liver at 48 hours after surgery with or without irradiation were assessed by western blot analysis. Relative expression levels of proteins in the hepatectomized mice were normalized to those in the sham‐operated mice; *P < 0.05 based on the Tukey‐Kramer method. Data show mean (bar) ± SD. Abbreviation: HPF, high‐power field.
Irradiation Impaired HGF‐Induced Proliferation and Akt/Erk 1/2 Signaling in Cultured Hepatocytes
While HGF administration increased the number of nonirradiated CL2 cells, it did not increase the number of irradiated CL2 cells (Fig. 2A). Caspase 3/7 activity did not differ between irradiated and nonirradiated CL2 cells with or without HGF administration (Fig. 2A). Irradiation induced phospho‐p53 (Ser15) and p21 in CL2 cells (Fig. 2B). Although irradiation did not suppress the phosphorylation of Met and Gab1 in CL2 cells after HGF administration, it suppressed the phosphorylation of Akt and Erk 1/2 in CL2 cells after HGF administration (Fig. 2B).
FIG. 2.
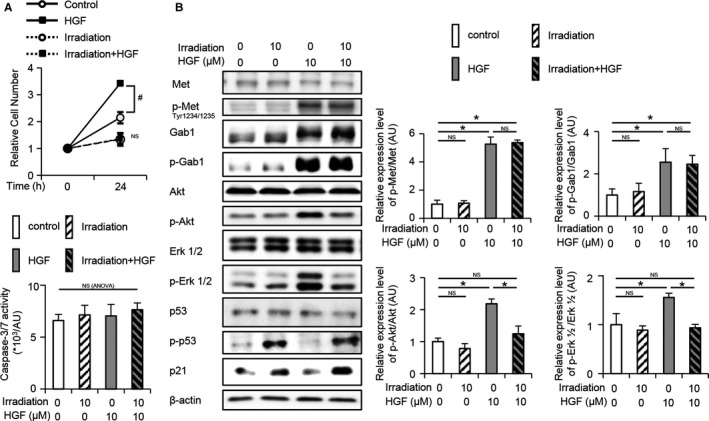
Irradiation suppresses cell proliferation. (A) Irradiated or nonirradiated control CL2 cells were administered HGF or control treatment at 2 hours after irradiation (n = 3 per group). Cell number and caspase 3/7 activity were assessed at 24 hours after HGF or control administration. Relative cell numbers were normalized to the number of cells seeded 6 hours before irradiation; # P < 0.05 based on the unpaired Student t test; NS based on one‐way ANOVA. (B) Expression levels of cell‐growth‐related proteins at 1 hour after HGF or control administration were assessed by western blotting analysis; *P < 0.05 based on the Tukey‐Kramer method. Data show mean (bar) ± SD.
p53 Knockdown But Not p21 Knockdown Restored the Irradiation‐Induced Suppression of Hepatocyte Proliferation and Phospho‐Akt/phospho‐Erk 1/2 Expression
To assess the effect of p53 and p21 on cell proliferation, p53 or p21 was knocked down, followed by HGF administration. The number of viable cells in the irradiated p53‐knockdown cell group was significantly higher than that in the irradiated control cell group after HGF administration (Fig. 3A). Irradiation did not affect phospho‐Met and phospho‐Gab1 expression in control and p53‐knockdown cells after HGF administration. While irradiation suppressed the phosphorylation of Akt and Erk 1/2 in control cells after HGF administration, it did not suppress the phosphorylation of Akt and Erk 1/2 in p53‐knockdown cells after HGF administration (Fig. 3B). Although irradiation increased the growth 2 (G2)/mitosis (M) phase rate without any alterations in the growth 1 (G1) phase rate in control cells, it did not change the G2/M phase rate or G1 phase rate in p53‐knockdown cells (Supporting Fig. S2A). Similar to p53‐knockdown cells, treatment with vanadate, which inhibits the DNA‐binding activity of p53,( 16 ) inhibited irradiation‐induced p21 (Supporting Fig. S2B) and irradiation‐induced cell number decrease (Supporting Fig. S2C). On the other hand, the number of viable irradiated p21‐knockdown cells did not show any difference compared with that of irradiated control cells after HGF administration (Fig. 3C). In irradiated p21‐knockdown cells, the phosphorylation of Met, Gab1, Akt, and Erk 1/2 induced by HGF was equivalent to that in irradiated control cells (Fig. 3D). Irradiation increased the G2/M phase rate in p21‐knockdown cells similar to irradiated control cells (Supporting Fig. S2A).
FIG. 3.
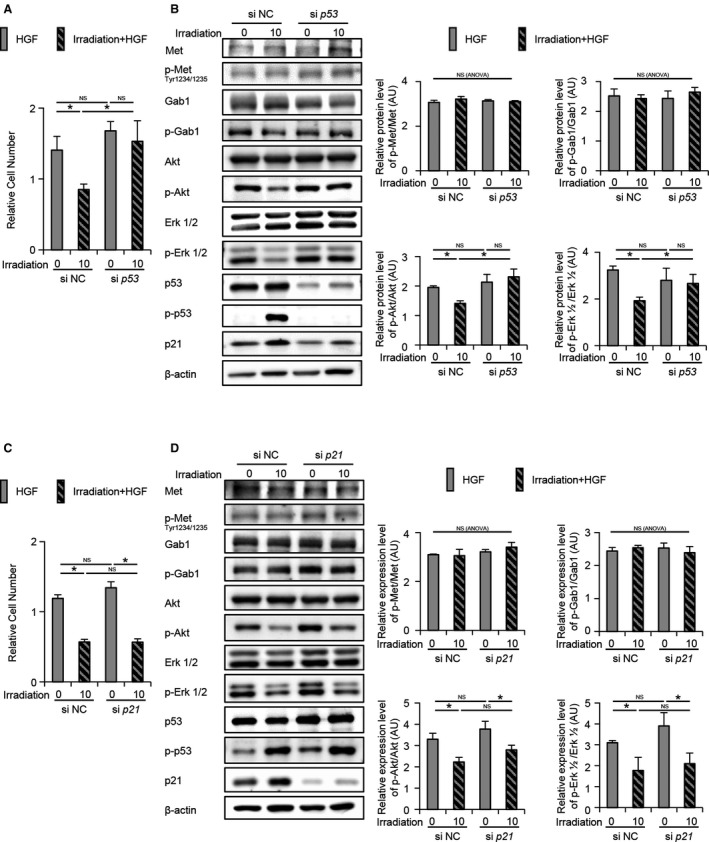
p53 not p21 is responsible for the irradiation‐induced cell proliferation delay. (A‐D) CL2 cells were transfected with siRNA against (A,B) p53, (C,D) p21, or a negative control for 48 hours before irradiation and with HGF 2 hours after irradiation. (A,C) The cell number at 24 hours after HGF administration was counted (n = 3 per each). (B,D) Expression levels of cell‐growth‐related proteins at 1 hour after HGF administration were assessed by western blot analysis. Relative expression levels of proteins were normalized to control levels; *P < 0.05 based on the Tukey‐Kramer method; NS based on one‐way ANOVA. Data show mean (bar) ± SD. Abbreviations: NC, negative control; si, small interfering.
Hepatocyte‐Specific p53 Deficiency Restored the Irradiation‐Induced Suppression of Liver Regeneration
In irradiated hepatocyte‐specific p53 KO mice, the irradiation‐induced phosphorylation of p53 (Ser15), stabilization of p53, and transcription of p21 were suppressed (Fig. 4A,B). To assess the effect of p53 induced by irradiation on hepatocyte regeneration in vivo, hepatocyte‐specific p53 KO mice and littermate WT mice were subjected to PHx 24 hours after irradiation. The PHx‐induced elevation in p21 expression in the irradiated WT mice at 48 hours after PHx was not detected in the irradiated p53 KO mice (Fig. 4D). In irradiated p53 KO mice, PCNA‐ and Ki‐67‐positive hepatocyte rates at 48 hours after PHx were higher than those in irradiated WT mice, and there was no difference in these rates between irradiated p53 KO mice and nonirradiated WT mice (Fig. 4C). The PCNA‐ and Ki‐67‐positive hepatocyte rates at 72 hours did not differ between irradiated p53 KO mice and irradiated WT mice (Fig. 4C). The protein expression of HGF and its downstream targets Met and Gab1 in the irradiated and nonirradiated mouse livers at 48 hours after PHx was higher than that in the sham‐operated mouse livers. PHx‐induced HGF, phospho‐Met, and phospho‐Gab1 in the liver showed no difference between the irradiated WT and p53 KO mice at 48 hours after PHx. PHx‐induced decreases in phospho‐Akt and phospho‐Erk 1/2 expression, which were detected in irradiated WT mice, were not detected in irradiated p53 KO mice (Fig. 4D). In the irradiated p53 KO mice, the liver‐to‐body weight ratio at 168 hours after PHx was significantly higher than that in the irradiated WT mice; there was no difference among irradiated p53 KO mice, nonirradiated WT mice, and nonirradiated p53 KO mice (Supporting Fig. S1B).
FIG. 4.
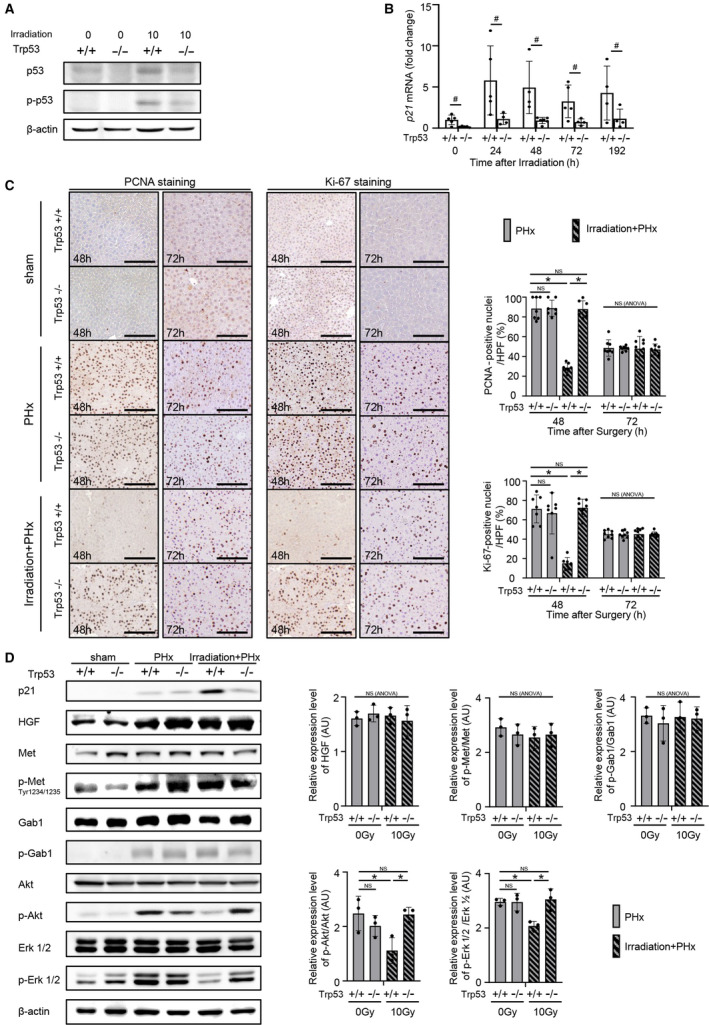
Hepatocyte‐specific p53 KO abolishes irradiation‐induced impairment in liver regeneration. (A,B) Male p53 KO and littermate WT mice were irradiated with 10 Gy. (A) Protein expression levels of p53 and phospho‐p53 (Ser15) in the liver at 1 hour after irradiation were assessed by western blot analysis. (B) mRNA expression levels of p21 in the liver at the indicated times after irradiation were quantified by quantitative RT‐PCR (n = 5, 5, 4, 5, and 4 WT mice [+/+] and 5, 4, 6, 4, and 4 KO mice [−/−] at 0, 24, 48, 72, and 192 hours after irradiation, respectively); # P < 0.05 based on the unpaired Student t test. (C,D) Male p53 KO and littermate WT mice were randomly assigned to the following three groups: nonirradiated mice subjected to sham operation 24 hours later (sham group), nonirradiated mice subjected to PHx 24 hours later (PHx group), irradiated mice with 10 Gy subjected to PHx 24 hours later (irradiation+PHx group) (n = 5, 6 WT mice and 5, 6 KO mice for sham; 7, 8 WT mice and 7, 8 KO mice for PHx; 7, 9 WT mice and 7, 8 KO mice for irradiation+PHx; at 48 and 72 hours after surgery, respectively). (C) Representative images of liver sections stained with anti‐PCNA and anti‐Ki‐67 antibodies at 48 and 72 hours after surgery with or without irradiation are shown; original magnification ×200. The percentages of PCNA‐ and Ki‐67‐positive nuclei/HPFs are reported. Ten fields of view were counted in the liver sections of individual mice. (D) Expression levels of cell‐growth‐related proteins in the liver at 48 hours after surgery were assessed by western blotting analysis. Relative expression levels of proteins in the hepatectomized mice were normalized to expression levels in sham‐operated WT mice; *P < 0.05 based on the Tukey‐Kramer method; NS based on one‐way ANOVA. Data show mean (bar) ± SD. Abbreviation: HPF, high‐power field.
Survival Rate After PHx of Irradiated Hepatocyte‐Specific p53 KO Mice With Severe Fibrosis Was Higher Than That of Irradiated WT Mice With Severe Fibrosis
CCl4 was orally administered to hepatocyte‐specific p53 KO mice and littermate WT mice for 8 weeks. There were no differences in the sirius red‐positive area or mRNA expression of collagen, type I, alpha 1 (Col1a1) and Col1a2 between the p53 KO and WT mice after 8 weeks of CCl4 administration (Fig. 5A,B). To assess the effect of radiation on the severely fibrotic liver, p53 KO and WT mice with a normal liver or severe fibrosis were irradiated with 10 Gy. In the WT mice with severe fibrosis, the expression levels of p53 and p21 were higher than those in WT mice with a normal liver at 1 hour after irradiation (Fig. 5C). When PHx was performed on irradiated p53 KO and WT mice with severe fibrosis, the survival rate of the fibrotic p53 KO mice was significantly higher than that of the WT mice with severe fibrosis (Fig. 5D).
FIG. 5.
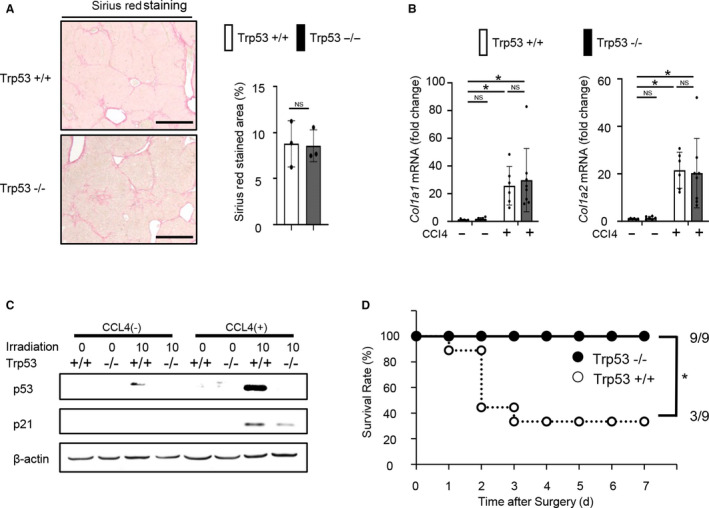
Survival rate after PHx of hepatocyte‐specific p53 KO mice with severe fibrosis was higher than that of WT mice with severe fibrosis. (A,B) Male p53 KO and littermate WT mice were orally administered CCl4 for 8 weeks. (A) Representative images of liver sections stained with sirius red (original magnification ×200) and analysis of the sirius red‐positive area are shown (n = 4 mice per group); NS based on the unpaired Student t test. (B) mRNA expression levels of Col1a1 and Col1a2 in the liver were quantified by quantitative RT‐PCR (n = 5 mice per group); *P < 0.05 based on the Tukey‐Kramer method. (C) Male p53 KO and littermate WT mice were administered CCl4 for 8 weeks and irradiated with 10 Gy. Irradiation was performed 48 hours after the final administration of CCl4. Protein expression levels of p53 and p21 in the liver at 1 hour after irradiation were assessed by western blot analysis. (D) Male p53 KO and littermate WT mice administered CCl4 for 8 weeks were subjected to PHx 24 hours after irradiation. Irradiation was performed 48 hours after the final administration of CCl4. Cumulative survival rates are shown (n = 9 mice per group); *P < 0.05 based on the chi‐squared test. Data show mean (bar) ± SD. Abbreviations: Col1a1/2, collagen, type I, alpha 1 and 2; d, days.
Discussion
In the present study, irradiation‐induced suppression of hepatocyte proliferation and Akt/Erk 1/2 activation in the presence of HGF was abolished by p53 knockdown but not by p21 knockdown (Fig. 3A‐D). It is known that irradiation activates p53 and promotes p21 transcription, resulting in cell‐cycle arrest.( 17 ) It has been reported that in the process of liver regeneration, p21 expression increases and HGF expression decreases in the irradiated liver, resulting in delayed liver regeneration.( 18 ) It has also been reported that p53 deficiency does not significantly affect liver regeneration ability while p21 deficiency increases the survival rate after extended hepatectomy.( 19 , 20 ) In our in vitro study, p53 knockdown or treatment with vanadate abolished the suppression of hepatocyte proliferation in irradiated CL2 cells; however, p21 knockdown did not. Cell‐cycle assays suggested that irradiation induces p53‐dependent but p21‐independent G2/M cell arrest rather than G1 arrest (Supporting Fig. S2A). Further study is needed to elucidate the mechanisms involving these molecules in the irradiation‐induced suppression of hepatocyte proliferation. In our in vivo study, knocking out p53 improved the suppression of liver regeneration due to irradiation. Irradiation‐induced p53 and its downstream p21 and puma induction are completely different for each organ. The effect of p53 deletion in irradiated organs is also diverse.( 21 , 22 , 23 ) p53 deficiency promotes cell death induced by irradiation in the small intestine.( 21 ) On the other hand, p53 deficiency protects hematopoietic cells from irradiation‐induced cell death.( 22 ) In the liver, irradiation‐induced proliferation disorder was protected by p53 deficiency in the present study.
The HGF/Met signaling pathway is induced in the process of liver regeneration.( 24 ) In HepG2 cells, which have activated p53, HGF administration did not alter phospho‐Met expression levels but it enhanced phospho‐Met expression levels in the presence of pifithrin‐α, an inhibitor of p53.( 25 ) These results suggest that HGF‐induced Met phosphorylation is inhibited by p53 in HepG2 cells. On the other hand, in the present study, HGF administration enhanced Met phosphorylation in irradiated CL2 cells, which have activated p53. P53 knockdown in irradiated CL2 cells did not alter the HGF‐induced enhancement of Met phosphorylation. These data suggest that irradiation‐induced p53 in CL2 cells does not suppress Met phosphorylation, in contrast to p53 activation in HepG2 cells. This difference might be due to differences in p53 downstream activation. Further study on this will be needed.
It has been shown that classic RILD is due to catastrophic damage to the liver caused by high doses of radiation, which is induced regardless of the presence of chronic liver disease. On the other hand, the most important risk factor for nonclassic RILD is the presence of liver cirrhosis, but the mechanism by which nonclassic RILD frequently occurs in patients with liver cirrhosis has not been investigated.( 4 , 10 ) We found that irradiation‐induced p53 activation was higher in fibrotic livers than in normal livers and that postoperative mortality was higher in irradiated fibrotic WT mice than in irradiated fibrotic p53 KO mice. Stronger induction of p53 by irradiation in fibrotic livers than in normal livers may be one reason why nonclassic RILD is more likely to occur in patients with cirrhosis.
In conclusion, irradiation‐induced activation of p53 resulted in suppression of liver regeneration. p53 suppression may be a treatment for the decrease in the regenerative ability of the liver caused by irradiation.
Supporting information
Fig S1
Fig S2
Supported in part by the Japan Society for the Promotion of Science (Grants‐in‐Aid for Scientific Research JP18H02795 to T.Tak., JP20K08307 to H.H., and JP16K15430 to T.Tat.).
Potential conflict of interest: Nothing to report.
References
- 1. Sanuki N, Takeda A, Kunieda E. Role of stereotactic body radiation therapy for hepatocellular carcinoma. World J Gastroenterol 2014;20:3100‐3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Reed GB Jr, Cox AJ Jr. The human liver after radiation injury. A form of veno‐occlusive disease. Am J Pathol 1966;48:597‐611. [PMC free article] [PubMed] [Google Scholar]
- 3. Emami B, Lyman J, Brown A, Coia L, Goitein M, Munzenrider JE, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys 1991;21:109‐122. [DOI] [PubMed] [Google Scholar]
- 4. Koay EJ, Owen D, Das P. Radiation‐induced liver disease and modern radiotherapy. Semin Radiat Oncol 2018;28:321‐331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Burman C, Kutcher GJ, Emami B, Goitein M. Fitting of normal tissue tolerance data to an analytic function. Int J Radiat Oncol Biol Phys 1991;21:123‐135. [DOI] [PubMed] [Google Scholar]
- 6. Cheng J‐H, Wu J‐K, Huang C‐M, Liu H‐S, Huang DY, Cheng SH, et al. Radiation‐induced liver disease after three‐dimensional conformal radiotherapy for patients with hepatocellular carcinoma: dosimetric analysis and implication. Int J Radiat Oncol Biol Phys 2002;54:156‐162. [DOI] [PubMed] [Google Scholar]
- 7. Lawrence TS, Robertson JM, Anscher MS, Jirtle RL, Ensminger WD, Fajardo LF. Hepatic toxicity resulting from cancer treatment. Int J Radiat Oncol Biol Phys 1995;31:1237‐1248. [DOI] [PubMed] [Google Scholar]
- 8. Guha C, Kavanagh BD. Hepatic radiation toxicity: avoidance and amelioration. Semin Radiat Oncol 2011;21:256‐263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pan CC, Kavanagh BD, Dawson LA, Li XA, Das SK, Miften M, et al. Radiation‐associated liver injury. Int J Radiat Oncol Biol Phys 2010;76(Suppl.):S94‐S100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sanuki N, Takeda A, Oku Y, Eriguchi T, Nishimura S, Aoki Y, et al. Influence of liver toxicities on prognosis after stereotactic body radiation therapy for hepatocellular carcinoma. Hepatol Res 2015;45:540‐547. [DOI] [PubMed] [Google Scholar]
- 11. Xue F, Takahara T, Yata Y, Kuwabara Y, Shinno E, Nonome K, et al. Hepatocyte growth factor gene therapy accelerates regeneration in cirrhotic mouse livers after hepatectomy. Gut 2003;52:694‐700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tanaka S, Hikita H, Tatsumi T, Sakamori R, Nozaki Y, Sakane S, et al. Rubicon inhibits autophagy and accelerates hepatocyte apoptosis and lipid accumulation in nonalcoholic fatty liver disease in mice. Hepatology 2016;64:1994‐2014. [DOI] [PubMed] [Google Scholar]
- 13. Mitchell C, Willenbring H. A reproducible and well‐tolerated method for 2/3 partial hepatectomy in mice. Nat Protoc 2008;3:1167‐1170. Erratum in: Nat Protoc 2014;9. [DOI] [PubMed] [Google Scholar]
- 14. Kawaguchi T, Kodama T, Hikita H, Tanaka S, Shigekawa M, Nawa T, et al. Carbamazepine promotes liver regeneration and survival in mice. J Hepatol 2013;59:1239‐1245. [DOI] [PubMed] [Google Scholar]
- 15. Kuramitsu K, Sverdlov DY, Liu SB, Csizmadia E, Burkly L, Schuppan D, et al. Failure of fibrotic liver regeneration in mice is linked to a severe fibrogenic response driven by hepatic progenitor cell activation. Am J Pathol 2013;183:182‐194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Morita A, Zhu J, Suzuki N, Enomoto A, Matsumoto Y, Tomita M, et al. Sodium orthovanadate suppresses DNA damage‐induced caspase activation and apoptosis by inactivating p53. Cell Death Differ 2006;13:499‐511. [DOI] [PubMed] [Google Scholar]
- 17. Gudkov AV, Komarova EA. The role of p53 in determining sensitivity to radiotherapy. Nat Rev Cancer 2003;3:117‐129. [DOI] [PubMed] [Google Scholar]
- 18. Du S‐S, Zeng Z‐C, Tang Z‐Y, Zhang Z‐Y, Shi L‐S, Wu Z, et al. Regenerative capacity of normal and irradiated liver following partial hepatectomy in rats. Int J Radiat Biol 2009;85:1114‐1125. [DOI] [PubMed] [Google Scholar]
- 19. Lehmann K, Tschuor C, Rickenbacher A, Jang J, Oberkofler CE, Tschopp O, et al. Liver failure after extended hepatectomy in mice is mediated by a p21‐dependent barrier to liver regeneration. Gastroenterology 2012;143:1609‐1619.e4. [DOI] [PubMed] [Google Scholar]
- 20. Kurinna S, Stratton SA, Coban Z, Schumacher JM, Grompe M, Duncan AW, et al. p53 regulates a mitotic transcription program and determines ploidy in normal mouse liver. Hepatology 2013;57:2004‐2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Komarova EA, Kondratov RV, Wang K, Christov K, Golovkina TV, Goldblum JR, et al. Dual effect of p53 on radiation sensitivity in vivo: p53 promotes hematopoietic injury, but protects from gastro‐intestinal syndrome in mice. Oncogene 2004;23:3265‐3271. [DOI] [PubMed] [Google Scholar]
- 22. Yu H, Shen H, Yuan Y, XuFeng R, Hu X, Garrison SP, et al. Deletion of Puma protects hematopoietic stem cells and confers long‐term survival in response to high‐dose gamma‐irradiation. Blood 2010;115:3472‐3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fei P, Bernhard EJ, El‐Deiry WS. Tissue‐specific induction of p53 targets in vivo. Cancer Res 2002;62:7316‐7327. [PubMed] [Google Scholar]
- 24. Cheng Z, Liu L, Zhang X‐J, Lu M, Wang Y, Assfalg V, et al. Peroxisome proliferator‐activated receptor gamma negatively regulates liver regeneration after partial hepatectomy via the HGF/c‐Met/ERK1/2 pathways. Sci Rep 2018;8:11894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu W‐T, Jing Y‐Y, Yu G‐F, Chen H, Han Z‐P, Yu D‐D, et al. Hepatic stellate cell promoted hepatoma cell invasion via the HGF/c‐Met signaling pathway regulated by p53. Cell Cycle 2016;15:886‐894. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2


