Abstract
Physiologic aging leads to attrition of telomeres and replicative senescence. An acceleration of this process has been hypothesized in the progression of chronic liver disease. We sought to examine the association of telomere length (TL) with liver disease and its impact on mortality risk. A cohort of 7,072 adults with leukocyte TL measurements from the National Health and Nutrition Examination Survey 1999‐2002 with mortality follow‐up through 2015 was analyzed. Liver disease was defined by aminotransferase levels and classified into etiology‐based and advanced fibrosis categories. Multivariable‐adjusted linear regression models estimated effect sizes, with 95% confidence intervals (CIs), of the presence of liver disease on TL. Cox regression models evaluated associations between TL and all‐cause mortality risk using adjusted hazard ratios (HRs). The cohort was representative of the US population with mean age 46.1 years and mean TL 5.79 kilobase pairs. No overall association between TL and liver disease was found; however, there was a significant negative association of TL and advanced liver fibrosis in individuals aged 65 and above. The liver disease cohort (HR 1.22, 95% CI 0.99‐1.51) and those with metabolic syndrome (HR 1.26, 95% CI 0.96‐1.67) had increased mortality risk with shorter TL. The relationship between TL and all‐cause mortality was stronger in women (HR 1.51, 95% CI 1.02‐2.23) and in non‐Hispanic Whites (HR 1.37, 95% CI 1.02‐1.84). Conclusion: Shortened leukocyte TL is independently associated with advanced liver disease at older ages, and with a higher risk of all‐cause mortality in those with liver disease. These associations reaffirm the need to better understand the role of telomeres in the progression of liver disease.
We examined the association of peripheral telomere length with liver disease and assessed its impact on mortality using data from the National Health and Nutrition Examination Survey (NHANES). We discovered that shortened leukocyte telomere length is independently associated with advanced liver disease at older ages, and also with a higher risk of all‐cause mortality in those with liver disease.

Abbreviations
- ALT
alanine aminotransferase
- APRI
aspartate aminotransferase–to‐platelet ratio index
- AST
aspartate aminotransferase
- CI
confidence interval
- CLD
chronic liver disease
- HCV
hepatitis C virus
- HR
hazard ratio
- MetS
metabolic syndrome
- NCHS
National Center of Health Statistics
- NDI
National Death Index
- NHANES
National Health and Nutrition Examination Survey
- TL
telomere length
Chronic liver disease (CLD) plays an increasingly relevant role in global morbidity and mortality. Due to the eventual development of cirrhosis and its complications such as hepatocellular carcinoma, CLD accounts for at least 2 million deaths each year, ranking as the 11th most common cause of death in the United States( 1 ) and globally.( 2 ) Independent of etiology, CLD results in progressive hepatic fibrosis as a consequence of chronic inflammation leading to replicative hepatocyte senescence. This mechanism has long linked telomere maintenance( 3 , 4 ) and telomere length (TL)( 5 , 6 ) to the development of cirrhosis.
Telomeres are DNA‐protein structures found at the end of all eukaryotic chromosomes that, along with the telomerase holoenzyme, allow organisms to mitigate loss of genetic information from incomplete lagging‐strand DNA replication.( 7 ) In humans, telomeres consist of hundreds to thousands of tandem repeats of a six‐nucleotide sequence (TTAGGGn) coated by numerous six‐protein complexes known as shelterin.( 8 , 9 , 10 ) Despite these protective and maintenance mechanisms, telomere attrition is a physiologic aging phenomenon; eventually telomeres reach a threshold length that signals entry into replicative senescence.( 11 , 12 )
Animal studies have formed the backbone of our understanding of the links between CLD and telomere biology. Not only do telomerase‐deficient mice have increased rates of cirrhosis development,( 3 ) but there is increasing evidence that functioning telomere machinery is essential for hepatocyte regeneration.( 13 , 14 ) Telomerase variants in humans have also shown an increased association with cirrhosis,( 4 , 15 ) while recent insights into metabolic syndrome (MetS) and human telomeres suggest a possible role of TL in the progression of nonalcoholic fatty liver disease (NAFLD).( 16 , 17 ) Additionally, alterations in telomere‐related proteins, most commonly dyskerin and others comprising the shelterin complex, have been found to result in clinical syndromes characterized by critically short TL. These rare, but increasingly recognized, short telomere syndromes often present with distinct liver disease phenotypes such as cryptogenic cirrhosis and nodular regenerative hyperplasia.( 18 , 19 , 20 )
In this study, we sought to leverage cross‐sectional data from the National Health and Nutrition Examination Survey (NHANES) to explore the relationship between telomeres and liver disease at the population level. These survey data have been used to examine associations between leukocyte TL, as a proxy for somatic TL, and a multitude of clinical states, including presumed NAFLD( 21 , 22 ); however, broader expansion to include other liver diseases and to assess the contribution of TL to long‐term survival in CLD remain unmet needs. Therefore, in this cohort representative of the US population, we aimed to explore the association between TL and presence of advanced liver disease, as well as the impact of TL on survival in those with liver disease.
Patients and Methods
Continuous NHANES is a survey conducted by the National Center of Health Statistic’s (NCHS) Division of Health and Nutrition Examination Surveys (DHANES) in the Centers for Disease Control and Prevention. The survey collects data about health, nutritional status, and health behaviors of participants using personal interviews as well as standardized physical examinations and laboratory tests. This iteration of the NHANES began in 1999 and since then has conducted annual surveys of approximately 5,000 people with 2‐year data‐release cycles. These participant cohorts are carefully constructed using a complex, multistage probability sampling design to provide estimates representative of the noninstitutionalized, civilian resident population of the United States.( 23 )
Our analyses are restricted to two cycles of Continuous NHANES collected from 1999 to 2002. During this period, all participants aged 20 or older were asked if they would be willing to provide whole‐blood DNA for future research. There were 7,827 participants, from a total of 21,004 (Fig. 1), who met these criteria, and their peripheral leukocyte telomeres were measured. We excluded all individuals aged 85 and above (n = 226) to reduce survivor bias. Furthermore, samples with telomere lengths longer than the 99th percentile were excluded (n = 76). The distribution of this sample of measured TLs is known to have an extreme right skew with biologically implausible lengths from possible assay errors.( 24 ) The final TL analysis cohort (n = 7,072) excluded pregnant individuals (n = 440) and any participants who lacked laboratory tests vital for defining liver disease (n = 13) such as platelet count, or alanine aminotransferase (ALT), and aspartate aminotransferase (AST) levels.
FIG. 1.
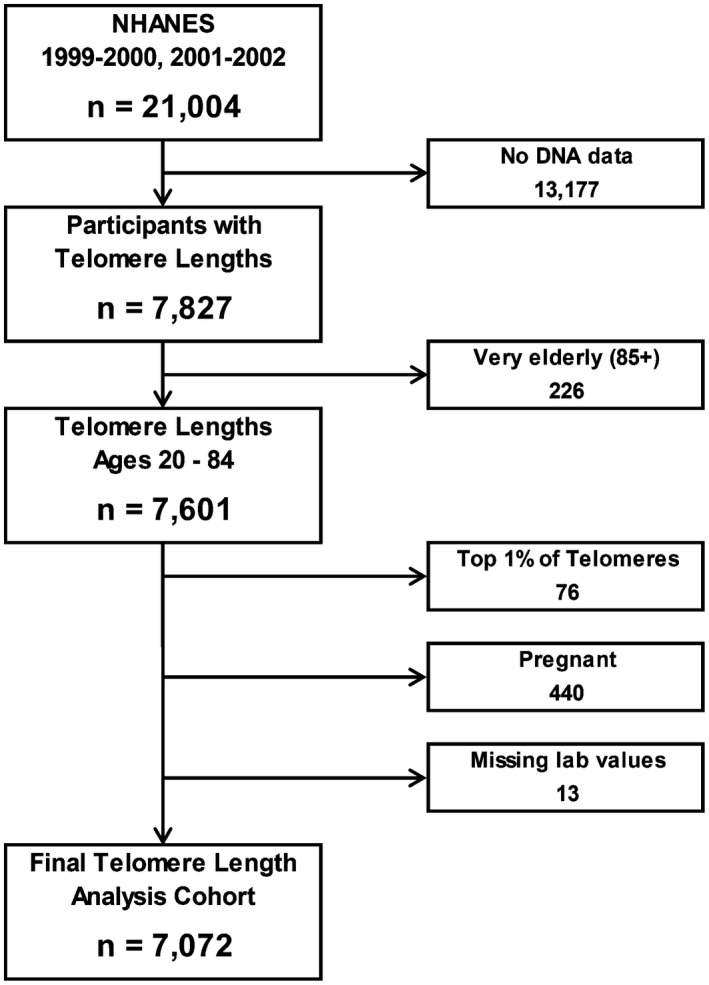
Flowchart of cohort selection for current analysis from NHANES 1999‐2002.
Every participant in the NHANES provided written informed consent, and the survey data collection was approved by the NCHS Research Ethics Review Board. The data used for these analyses were completely de‐identified and released as public‐use data, and therefore deemed exempt from review by our institutional review board.
TL Measurement
The DHANES provided aliquots of purified DNA to the laboratory of Dr. Elizabeth Blackburn at the University of California, San Francisco. Quantitative polymerase chain reaction, as described previously,( 25 , 26 ) was used to measure leukocyte TL relative to standard reference DNA (T/S ratio). The relationship between the T/S ratio to a standardized kilobase pairs (kbp) length was kbp = (3,274 + 2,413*[T/S])/1,000. The final TL values were validated and underwent a quality control review before being linked with the Continuous NHANES 1999‐2002 data.( 27 )
Definition of Liver Disease
The presence of liver disease in the TL analysis cohort was sequentially defined and stratified into three etiology‐based groups using a combination of laboratory values and responses to interview questionnaires (Supporting Fig. S1). Initially, any participant with evidence of active infection with either hepatitis B virus (positive for hepatitis B surface antigen), or hepatitis C virus (HCV; positive for HCV RNA or positive for antibodies to HCV with nonnegative HCV RNA) was labeled as having viral liver disease (n = 155). Next, possible alcohol‐associated liver disease (n = 714) was defined by the presence of elevated ALT or AST (>19 IU for females and >29 IU for males)( 28 ) and evidence of either (1) current heavy alcohol use (≥3 drinks per day for females, ≥4 drinks per day for males, or binge drinking [≥4 drinks on same occasion for females, ≥5 drinks on same occasion for males] on 5 or more days per month), (2) current moderate alcohol use (≥2 drinks per day for females, ≥3 drinks per day for males, or binge drinking ≥2 days per month), or (3) a history of daily binge drinking. From the remaining participants, MetS‐related liver disease (n = 1,203) was defined in the individuals with elevated ALT or AST (>19 IU for females and >29 IU for males) who also met the criteria for MetS. The presence of three or more of the following conditions defined MetS: (1) impaired glycemic control (hemoglobin A1c ≥ 5.7%, or fasting serum glucose ≥100 mg/dL, or use of diabetes medications), (2) either increased waist circumference (≥88 cm for females, ≥101 cm for males) or elevated body mass index (≥30 Kg/m2), (3) low high‐density lipoprotein cholesterol (<50 mg/dL in females, <40 mg/dL in males), (4) high triglyceride levels (≥150 mg/dL), or (5) hypertension (systolic blood pressure ≥130 mm Hg or diastolic blood pressure ≥85 mm Hg). Finally, a fourth group of uncharacterized liver disease (n = 955) consisted of individuals with abnormal ALT or AST (>25 IU for females and >33 IU for males) who did not meet any of the criteria for viral, alcohol‐associated, or MetS‐related liver disease. The remaining participants (n = 4,418) were presumed to not have liver disease. The liver disease cohorts were further classified into those with or without advanced fibrosis based on the AST‐to‐platelet ratio index (APRI; 100 [AST/AST upper limit of normal]/[platelet count]). As APRI is independent of age, it was preferred over other age‐dependent noninvasive indices for advanced fibrosis, such as the Fibrosis‐4 Index. A threshold of APRI greater than or equal to 0.7 was used to define advanced fibrosis.( 29 )
Covariates
Several self‐reported behaviors and sociodemographic characteristics are available in the NHANES, allowing us to use multiple covariates that have a potential association with leukocyte TL. The covariates chosen included the age and dummy variables indicating the presence of female sex, diabetes, MetS, smoking history, current excess alcohol use, as well as race/ethnicity.
The relationship with age was explored as a continuous variable in years, as well as approximately equal age groups: 20‐34, 35‐49, 50‐64, and over 65 years. Additionally, an age‐squared term was calculated and included to account for the potential nonlinear association between age and the outcomes. The presence of cardiovascular disease or malignancy was based on the participants’ responses on the NHANES medical conditions questionnaire. Smoking history was defined as having smoked at least 100 or more cigarettes in an individual’s lifetime. Individuals with heavy or moderate current use of alcohol were labeled with a current excess alcohol use dummy variable. Race/ethnicity were collected in five categories by Continuous NHANES: non‐Hispanic White, non‐Hispanic Black, Mexican American, other Hispanic, and other/multi‐racial. These categories were grouped into a dummy variable with non‐Hispanic White being the reference category and non‐Hispanic Black, all Hispanic (Mexican American and other Hispanic), and other/multi‐racial being the remaining categories. Where appropriate, these possible confounders were added as explanatory variables to create multivariable‐adjusted regression models.
Mortality Data
The National Death Index (NDI) is a database of all deaths in the United States maintained by the NCHS. Importantly, the NCHS has linked most of their surveys, including the Continuous NHANES, to the NDI to facilitate mortality follow‐up for a large proportion of the participants surveyed. The iteration of the NDI used in these analyses had follow‐up data for the NHANES 1999‐2002 participants from the date of survey participation through December 31, 2015. Survivors past this date were censored at this date. NDI data were not available for only 3 participants from our final TL analysis cohort, allowing us to analyze the relationship between TL, liver disease, and all‐cause mortality among a sample of 7,069 participants.
Statistical Analyses
The NCHS analytic guidelines requires that all statistical analyses using the Continuous NHANES account for the complex survey design to produce estimates and variances representative of the civilian noninstitutionalized US population. Therefore, sample weights as well as the geographical clustering indicators (primary sampling units and strata) were incorporated into the analyses. The descriptive statistics presented here summarize categorical variables with nationally representative proportions, while continuous variables are summarized with estimated means—both with an adjusted 95% confidence interval (CI).
The association between TL and the presence of liver disease was explored using multivariable‐adjusted linear regression models with TL as a continuous dependent variable. Models were created for both the presence of elevated liver tests and the presence of advanced fibrosis as explanatory variables of interest. These models were adjusted by age, an age‐squared term, as well as dummy variables for sex, diabetes, MetS, smoking, and excess alcohol use.
A set of Cox regression models was used to estimate the effect TL on the risk of mortality. The models were constructed with a time‐on‐study time scale measured in months. Data were censored at time of death or December 31, 2015, as provided in the linked NDI files. Three sets of models were created to explore the effects of TL on mortality risk: (1) in the full analysis cohort stratified by liver disease etiology, (2) in the liver disease subset stratified by race/ethnicity, and (3) in the liver disease subset stratified by sex. Further details regarding the construction of these models is contained in the Supporting Methods.
All analyses were performed using R version 3.6.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Cohort Characteristics and Liver Disease Severity
Among 7,072 eligible Continuous NHANES 1999‐2002 participants, 4,229 (60%) had normal aminotransferase levels, whereas 2,843 (40%) had elevations of these enzymes. The distribution of liver disease among characterized (viral hepatitis, MetS‐related, or alcohol‐associated) disease with no advanced fibrosis, characterized disease with advanced fibrosis, and uncharacterized disease was 68%, 4.4%, and 27%, respectively. In comparison to those without liver disease, the characterized liver disease cohort was found to be older and included higher proportions of individuals with MetS, obesity, diabetes, and self‐reported excess alcohol use. The proportion of self‐reported cardiovascular disease or a malignancy, however, did not differ among the cohorts. The advanced fibrosis cohort had significantly fewer women, and more individuals with a smoking history when compared to those without liver disease (Table 1). Within characterized liver disease, the MetS‐related cohort was not only older but also contained far more women than the viral hepatitis and alcohol‐associated cohorts. Additionally, those with viral hepatitis were more likely to identify as non‐Hispanic Black when compared with the total population and the other subsets of characterized liver disease. The average TL among the different etiologies, however, was not significantly different (Supporting Table S1).
TABLE 1.
Cohort Characteristics
| Characteristic | Complete Cohort (n = 7,072) | Normal Aminotransferase Levels (n = 4,229) | Liver Disease, No Advanced Fibrosis (n = 1,946) | Liver Disease, Advanced Fibrosis (n = 126) | Liver Disease, Uncharacterized (n = 771) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Prop./Mean | 95% CI | Prop./Mean | 95% CI | Prop./Mean | 95% CI | Prop./Mean | 95% CI | Prop./Mean | 95% CI | |
| Age | 46.1 | [45.3‐46.8] | 45.2 | [44.5‐45.8] | 49.0 | [47.9‐50.2] | 52.0 | [49.7‐54.3] | 43.1 | [41.3‐44.9] |
| Female | 0.501 | [0.491‐0.511] | 0.477 | [0.461‐0.494] | 0.535 | [0.507‐0.563] | 0.291 | [0.202‐0.380] | 0.572 | [0.527‐0.617] |
| Ethnicity | ||||||||||
| White (Non‐Hispanic) | 0.736 | [0.700‐0.773] | 0.746 | [0.707‐0.786] | 0.721 | [0.679‐0.762] | 0.708 | [0.610‐0.806] | 0.724 | [0.677‐0.772] |
| Black (Non‐Hispanic) | 0.0931 | [0.0712‐0.115] | 0.0978 | [0.0743‐0.121] | 0.0914 | [0.0675‐0.115] | 0.113 | [0.0517‐0.175] | 0.0698 | [0.0477‐0.0918] |
| Mexican American | 0.0696 | [0.0514‐0.0878] | 0.0621 | [0.0455‐0.0787] | 0.0760 | [0.0533‐0.0987] | 0.0678 | [0.0313‐0.104] | 0.0954 | [0.0643‐0.126] |
| Other Hispanic | 0.0679 | [0.0342‐0.102] | 0.0648 | [0.0306‐0.0990] | 0.0690 | [0.0366‐0.101] | 0.0760 | [0.00723‐0.145] | 0.0812 | [0.0362‐0.126] |
| Multi‐ethnic | 0.0328 | [0.0223‐0.0433] | 0.0289 | [0.0187‐0.0392] | 0.0430 | [0.0220‐0.0641] | 0.0350 | [0.000‐0.0878] | 0.0293 | [0.0179‐0.0406] |
| BMI | 28.1 | [27.8‐28.4] | 27.1 | [26.8‐27.4] | 30.6 | [30.0‐31.1] | 29.0 | [27.6‐30.5] | 27.5 | [26.9‐28.0] |
| MetS | 0.332 | [0.315‐0.349] | 0.221 | [0.208‐0.233] | 0.712 | [0.677‐0.748] | 0.660 | [0.554‐0.767] | 0.000 | [0.000‐0.000] |
| CVD | 0.0617 | [0.0541‐0.0693] | 0.0608 | [0.0518‐0.0698] | 0.0696 | [0.0568‐0.0823] | 0.104 | [0.0411‐0.166] | 0.0424 | [0.0231‐0.0617] |
| Malignancy | 0.0777 | [0.0680‐0.0874] | 0.0795 | [0.0668‐0.0923] | 0.0779 | [0.0625‐0.0932] | 0.117 | [0.0454‐0.188] | 0.0625 | [0.0395‐0.0855] |
| Obese (BMI) | 0.297 | [0.276‐0.318] | 0.234 | [0.212‐0.256] | 0.463 | [0.428‐0.498] | 0.326 | [0.194‐0.458] | 0.247 | [0.208‐0.286] |
| Obese (waist circumference) | 0.483 | [0.459‐0.507] | 0.391 | [0.364‐0.417] | 0.719 | [0.686‐0.753] | 0.626 | [0.527‐0.724] | 0.406 | [0.357‐0.455] |
| Diabetes | 0.295 | [0.277‐0.314] | 0.232 | [0.216‐0.247] | 0.510 | [0.477‐0.543] | 0.629 | [0.521‐0.737] | 0.0921 | [0.0676‐0.117] |
| Smoking history | 0.504 | [0.478‐0.530] | 0.496 | [0.464‐0.527] | 0.563 | [0.524‐0.602] | 0.666 | [0.564‐0.769] | 0.392 | [0.343‐0.441] |
| Excess alcohol use | 0.227 | [0.214‐0.239] | 0.179 | [0.166‐0.193] | 0.412 | [0.374‐0.450] | 0.581 | [0.488‐0.674] | 0.000 | [0.000‐0.000] |
| TL (kbp) | 5.79 | [5.72‐5.86] | 5.81 | [5.74‐5.88] | 5.75 | [5.67‐5.83] | 5.62 | [5.45‐5.79] | 5.82 | [5.75‐5.90] |
Abbreviations: BMI, body mass index; Prop, proportion.
Notably, decreasing mean TL was observed with increasing severity of liver disease. This directionality between TL and advanced fibrosis was also present in an unweighted analysis of the mean TL within participants grouped by age, sex, and presence of MetS (Supporting Table S2). The distribution of TL in the survey cohort was further examined by determining specific quantiles of TL grouped by decade of age (Fig. 2), which demonstrated a consistent downtrend in TL with increasing age. Additional exploratory analysis stratified by presence of advanced fibrosis revealed a widening gap in median TL at older ages (Fig. 3).
FIG. 2.
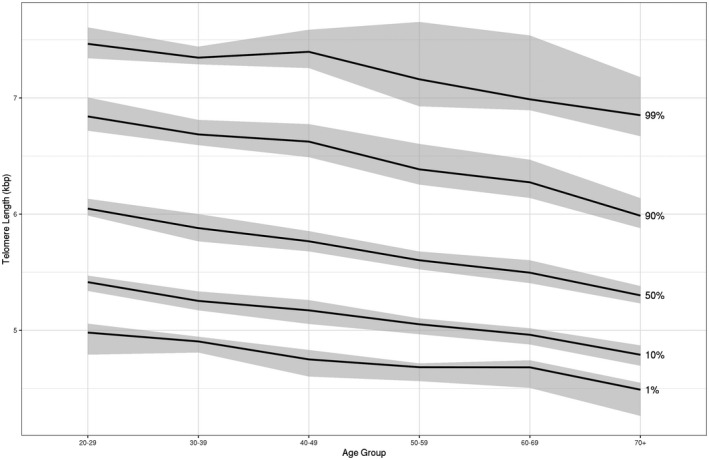
Leukocyte TL quantiles by age groups (20‐84) with 95% CIs in NHANES 1999‐2002.
FIG. 3.
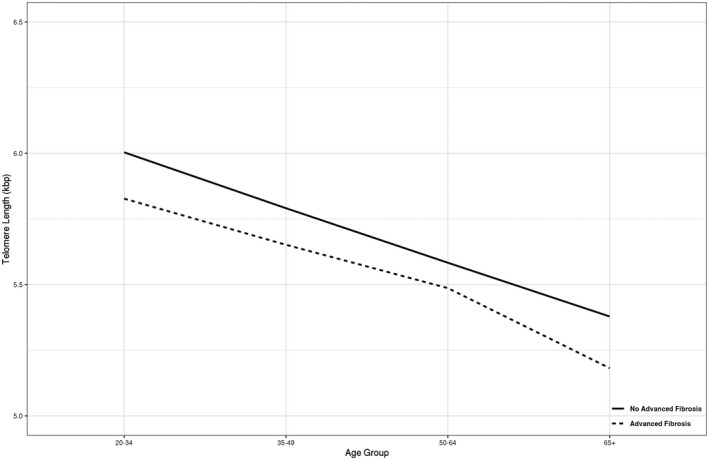
Median leukocyte TL across age groups: comparison of advanced fibrosis versus no advanced fibrosis.
Liver Disease and TL
The presence of liver disease, defined as either elevated aminotransferase levels or presence of advanced fibrosis, did not appear to have a significant effect on TL after adjusting for age, sex, MetS, smoking, and excess alcohol use (Fig. 4, top row). However, within the specified age groups, these models revealed a significant negative association between the presence of advanced fibrosis and TL in individuals aged 65 and older (Fig. 4, bottom row). Similar models restricted to either those with MetS or with excessive alcohol use (Supporting Figs. S2 and S3) showed a similar pattern; however, the sample sizes within each age group were too small for the estimates to be reliable based on the NCHS analytic guidelines.
FIG. 4.
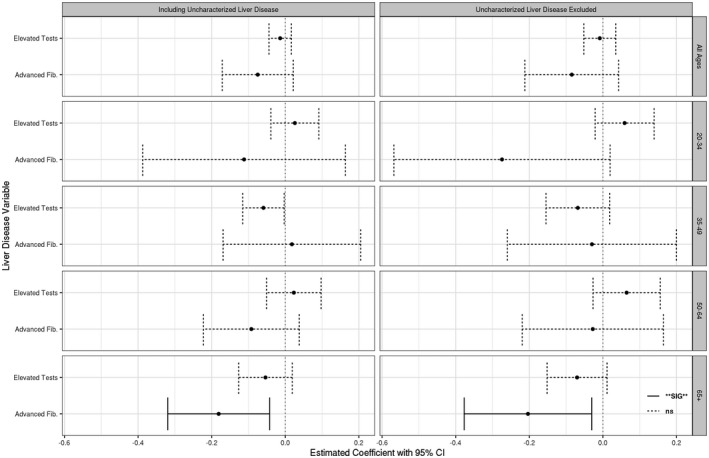
Estimated coefficient (effect size) of liver disease on TL by age groups using multivariable‐adjusted linear regression models. Models including uncharacterized liver disease (left panel) and models with only characterized (viral hepatitis, MetS‐related, alcohol‐associated) liver disease (right panel) are both present. Stratified by explanatory liver disease variable used (left axis) and age subset (right axis). All models adjusted by age and age‐squared, and dummy variables adjusted for the presence of female sex, diabetes, MetS, smoking history, and excess alcohol use.
All‐Cause Mortality and TLs in Liver Disease
The 7,069 participants from the TL analysis cohort with available mortality data had a median follow‐up time of 172 months (interquartile range, 160‐186). During this period, the all‐cause cumulative mortality was 23% (1,630 deaths). Table 2 lists the adjusted Cox regression models examining the relationship between TL and all‐cause mortality risk in the complete cohort and specified subsets of the characterized liver disease cohort. A decrease in TL was associated with increased risk of all‐cause mortality in the full cohort (Hazard Ratio [HR] 1.20; 95% CI, 1.04‐1.38), with similar trends observed in those with liver disease (HR 1.22; 95% CI, 0.99‐1.51) and a subset with MetS‐related liver disease (HR 1.26; 95% CI, 0.96‐1.67). Notably, this association was not observed in individuals with alcohol‐associated liver disease (HR 1.13; 95% CI 0.77‐1.68). Additionally, when TL was represented as decade‐specific quartiles, being in the lowest 25th percentile (first quartile) of TL conferred a 24% increased risk of all‐cause mortality when compared with the longest quartile (fourth quartile) in the full cohort.
TABLE 2.
Cox Regression Survival Model in Full Cohort and Liver Disease Etiology Subsets: Effect of TL (as continuous variable) and Between TL Quartiles (Compared With Longest Quartile) on All‐Cause Mortality
| TL Analysis Cohort (n = 7,069) | Characterized Liver Disease (n = 2,071) | MetS‐Related Liver Disease (n = 1,203) | Alcohol‐Associated Liver Disease (n = 713) | |||||
|---|---|---|---|---|---|---|---|---|
| Deaths | HR (95% CI) | Deaths | HR (95% CI) | Deaths | HR (95% CI) | Deaths | HR (95% CI) | |
| All‐Cause Mortality | ||||||||
| TL (kbp) | 1630 | 1.20 [1.04‐1.38] | 516 | 1.22 [0.99‐1.51] | 313 | 1.26 [0.96‐1.67] | 151 | 1.13 [0.77‐1.68] |
| TL quartiles | ||||||||
| First quartile | 468 | 1.24 [1.02‐1.50] | 157 | 1.22 [0.91‐1.63] | 91 | 1.21 [0.80‐1.81] | 46 | 1.28 [0.81‐2.04] |
| Second quartile | 386 | 1.07 [0.90‐1.27] | 116 | 1.00 [0.75‐1.35] | 68 | 0.95 [0.64‐1.42] | 36 | 1.08 [0.69‐1.68] |
| Third quartile | 414 | 1.11 [0.92‐1.35] | 117 | 0.99 [0.69‐1.43] | 74 | 1.02 [0.58‐1.78] | 34 | 1.10 [0.63‐1.92] |
| Fourth quartile (longest) | 362 | 1.00 | 126 | 1.00 | 80 | 1.00 | 35 | 1.00 |
All models adjusted for age, and dummy variables adjusted for female sex, race/ethnicity, smoking history, MetS, and presence of advanced fibrosis.
The relationship between all‐cause mortality risk and race/ethnicity within the characterized liver disease is given in Table 3. Non‐Hispanic White individuals demonstrated 37% higher risk of all‐cause mortality with decreasing TL (HR 1.37; CI, 1.02‐1.84), whereas combined Black or Hispanic ethnicity (Table 3; non‐White category) showed no association of TL with all‐cause mortality (HR 1.01; CI, 0.79‐1.31). Similar associations of TL and all‐cause mortality in these race/ethnicity groups were seen in the full cohort as well (Supporting Table S3).
TABLE 3.
Cox Regression Survival Model in Liver Disease Cohort and Race/Ethnicity Subsets: Effect of TL (as Continuous Variable) and Between TL Quartiles (Compared With Longest Quartile) on All‐Cause Mortality
| Characterized Liver Disease (n = 2,071) | White (Non‐Hispanic) (n = 978) | Non‐White (Excluding Multi‐ethnic) (n = 1,031) | Black (Non‐Hispanic) (n = 359) | Hispanic (n = 672) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Deaths | HR (95% CI) | Deaths | HR (95% CI) | Deaths | HR (95% CI) | Deaths | HR (95% CI) | Deaths | HR (95% CI) | |
| All‐Cause Mortality | ||||||||||
| TL (kbp) | 516 | 1.22 [0.99‐1.51] | 273 | 1.37 [1.02‐1.84] | 233 | 1.01 [0.79‐1.31] | 97 | 1.26 [0.88‐1.81] | 136 | 0.86 [0.58‐1.27] |
| TL quartiles | ||||||||||
| First quartile | 157 | 1.22 [0.91‐1.63] | 82 | 1.38 [0.95‐2.00] | 72 | 0.96 [0.65‐1.42] | 29 | N/A | 43 | N/A |
| Second quartile | 116 | 1.00 [0.75‐1.35] | 70 | 1.16 [0.80‐1.69] | 44 | 0.69 [0.42‐1.13] | 19 | N/A | 25 | N/A |
| Third quartile | 117 | 0.99 [0.69‐1.43] | 60 | 1.08 [0.69‐1.69] | 54 | 0.94 [0.62‐1.44] | 21 | N/A | 33 | N/A |
| Fourth quartile (longest) | 126 | 1.00 | 61 | 1.00 | 63 | 1.00 | 28 | N/A | 35 | N/A |
All models adjusted for age, and dummy variables adjusted for female sex, smoking history, MetS, and presence of advanced fibrosis. N/A, sample size (number of deaths) was too small to provide a reliable estimate.
In addition to the marked sex imbalance in the composition of the advanced fibrosis subset, a significantly protective effect of the female sex on the risk of mortality was seen in the full cohort (Supporting Table S5; HR 0.73; 95% CI, 0.65‐0.82). Therefore, another set of models was created to explore the role of sex in the relationship between TL and mortality risk in liver disease (Table 4). Although males showed no association between shortened TL and mortality risk (HR 0.91; 95% CI, 0.65‐1.28), females had almost a 50% increase in all‐cause mortality risk with a unit decrease in TL (HR 1.49; 95% CI, 1.15‐1.94). Being in the shortest quartile of TL also conferred an increased risk of all‐cause mortality, when compared with the longest quartile, in the female cohort (HR 1.51; 95% CI, 1.02‐2.23). Both of these patterns were also seen in the full TL analysis cohort but with a lower effect size in the female cohort (Supporting Table S4).
TABLE 4.
Cox Regression Survival Model in Liver Disease Cohort and Sex Subsets: Effect of TL (as Continuous Variable) and Between TL Quartiles (compared With Longest Quartile) on All‐Cause Mortality
| Characterized Liver Disease (n = 2,071) | Male (n = 943) | Female (n = 1,128) | ||||
|---|---|---|---|---|---|---|
| Deaths | HR (95% CI) | Deaths | HR (95% CI) | Deaths | HR (95% CI) | |
| All‐Cause Mortality | ||||||
| TL (kbp) | 516 | 1.22 [0.99‐1.51] | 218 | 0.91 [0.65‐1.28] | 298 | 1.49 [1.15‐1.94] |
| TL quartiles | ||||||
| First quartile | 157 | 1.22 [0.91‐1.63] | 72 | 0.89 [0.57‐1.39] | 85 | 1.51 [1.02‐2.23] |
| Second quartile | 116 | 1.00 [0.75‐1.35] | 47 | 0.73 [0.49‐1.08] | 69 | 1.25 [0.84‐1.86] |
| Third quartile | 117 | 0.99 [0.69‐1.43] | 47 | 0.77 [0.44‐1.33] | 70 | 1.22 [0.72‐2.06] |
| Fourth quartile (longest) | 126 | 1.00 | 52 | 1.00 | 74 | 1.00 |
All models adjusted for age, and dummy variables adjusted for race/ethnicity, smoking history, MetS, and presence of advanced fibrosis.
Discussion
This study, based on a representative sample of the US adult population with up to 16‐year survival follow‐up, revealed that shortened leukocyte TL is not only independently associated with advanced liver disease at older ages, but also with a significantly higher risk of mortality among those with liver disease. The observed negative impact of shorter TL on survival is particularly evident in women and non‐Hispanic White populations.
The earliest links between TL and CLD were elucidated in a series of small studies in which TL was measured in liver tissue from humans with various stages of liver disease.( 5 , 30 , 31 ) The more advanced stages of disease tended to have shorter TL, specifically in hepatocytes,( 6 ) and it was hypothesized that frequent replication in these disease states led to increased attrition of hepatocyte telomeres. Subsequently, animal studies have demonstrated the importance of functional telomere machinery for hepatocyte regeneration( 3 , 13 ) and the identification of specialized hepatocytes with increased telomerase activity that are necessary for tissue renewal.( 14 , 32 ) Even though humans with telomerase mutations have been found to have a higher risk of developing cirrhosis,( 4 ) large‐scale studies with regard to TL have not previously shown a clear causative relationship with the progression of liver diseases.( 16 , 17 , 33 ) In our study, the association between the possible presence of advanced liver disease and leukocyte TL is present only at older ages, but not in the population as a whole or in younger age cohorts. Complementing prior discoveries, our findings further suggest that advanced liver disease results in accelerated telomere attrition rates compared to those without liver disease; thus, the absolute difference only becoming apparent after many years of hepatic injury.
The shortening of telomeres with each cell replication has resulted in TL being considered a proxy for the biological age of the human body, and therefore intrinsically linked to its longevity.( 11 ) The seminal analysis exploring this link revealed that lower leukocyte TL was associated with a higher risk of all‐cause mortality (HR 1.86; CI, 1.22–2.83) in 143 Utah residents all above the age of 60.( 34 ) A subsequent study with 64,637 Danish participants similarly found a subtle yet significant signal with a higher risk of all‐cause mortality (HR 1.02, 95% CI 1.02‐1.03) for each 200‐bp decrease in TL.( 35 ) A recent meta‐analysis summarily confirmed this relationship, revealing a 9% increase in the risk of mortality (HR 1.09, 95% CI 1.06‐1.13) with each kilobase‐pair decrease in TL.( 36 ) Both the Danish study and meta‐analysis revealed a 26%‐28% increase in mortality risk for the individuals in the shortest TL quartile when compared with the longest quartile. Our results not only support these findings, but also expand to individuals with liver disease as well as MetS‐related liver disease. Interestingly, alcohol‐associated liver disease had a much weaker correlation between shorter TL and mortality risk. It is not clear whether this is indicative of a specific characteristic of this disease state or due to accumulation of non‐liver‐related mortality; thus, additional studies are required.
Yet another set of findings in our data revealed that within the characterized liver disease cohort, both non‐Hispanic White individuals and women had a significant association with a decrease in TL and increased mortality risk, whereas non‐White (specifically non‐Hispanic Black and all Hispanic) individuals and males did not. There has historically been a lot of variability in the reported relationship between race/ethnicity and TL; however, more recently there has been consensus that non‐White populations tend to have longer TL.( 37 , 38 ) Alternatively, it is well accepted that men have shorter TL in general when compared with women.( 38 , 39 ) These differences have not been previously described in populations with liver disease.
Interestingly, the sex differences in the TL and mortality risk are also present in the total population, but this effect is much more prominent in the presence of liver disease. It is notable that this finding persists, despite female sex being a protective feature with regard to mortality and that men tend to have shorter TL in comparison. Although not clearly demonstrated, this finding perhaps suggests that women have intrinsically lower telomere attrition rates, and a unit decrease in TL confers a much higher mortality risk among those with liver disease.
Using Continuous NHANES data to evaluate liver disease has several limitations that merit acknowledgement. The cross‐sectional nature of the data set limits not only the analyses that can be performed but also restricts us from attributing directionality to the discovered associations. These definitions of liver disease rely on one‐time laboratory measurements of aminotransferase levels and platelet counts, which could represent acute changes rather than being reflective of CLD. Additionally, the determination of alcohol use was dependent on self‐reported answers to a questionnaire and could not be verified. With regard to the TL, only peripheral leukocyte TL was available, and although leukocyte TL has been shown to correlate with various metabolomic markers,( 40 ) its relationship with hepatic parenchymal cells’ TL at all stages of liver disease is not clear. Finally, as TL data were only available for 1999‐2002 participants, there was limited power to discern differences among the individuals classified with advanced fibrosis, or to identify cohorts of rare diseases such as those individuals with short telomere syndromes.
Despite these limitations, quite a few strengths of the study design add weight to our findings. The construct of the survey data allowed us to formulate estimates, devoid of ascertainment bias, which were representative of the noninstitutionalized, civilian population of the United States. With respect to the TL values, all measurements were performed at Dr. Blackburn’s laboratory over the same time period, allowing processing with a consistent and reliable methodology. Additionally, the availability of well‐validated mortality follow‐up data for up to 16 years through the NDI formed an important aspect of this study design.
Collectively, these analyses provide further evidence that TL is related to the progression of liver disease. Not only do they suggest that accelerated telomere attrition in liver disease becomes apparent with aging, but also reveal an association between TL and all‐cause mortality in both liver disease and the general population. Furthermore, the race/ethnicity and sex‐related differences in mortality risk in those with liver disease represent especially intriguing findings, which in turn may reveal avenues of discovery in the study of TL and liver disease.
The establishment of such associations at a population level is a rudimentary, but important, step in addressing several questions related to telomeres and liver disease that remain unanswered. These include studying the role of TL in the development of liver fibrosis in various CLD, the progression to decompensation and acute‐on‐chronic liver failure, and even the use of TL as a prognostic tool in liver transplant. Further clinical investigation of the patterns uncovered here, combined with the rapidly evolving understanding of telomere machinery at the tissue level,( 14 , 32 ) will be critical in determining the role of telomeres in the individualized management of CLD.
Supporting information
Supplementary Material
Table S1‐S4
Supported by the National Institutes of Health (U01AA026886‐03).
Potential conflict of interest: Nothing to report.
References
- 1. Murphy SL, Xu J, Kochanek KD, Arias E, Tejada‐Vera B. Deaths: final data for 2018. Natl Vital Stat Rep 2021;69:1‐83. [PubMed] [Google Scholar]
- 2. Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol 2019;70:151‐171. [DOI] [PubMed] [Google Scholar]
- 3. Rudolph KL, Chang S, Millard M, Schreiber‐Agus N, DePinho RA. Inhibition of experimental liver cirrhosis in mice by telomerase gene delivery. Science 2000;287:1253‐1258. [DOI] [PubMed] [Google Scholar]
- 4. Calado RT, Brudno J, Mehta P, Kovacs JJ, Wu C, Zago MA, et al. Constitutional telomerase mutations are genetic risk factors for cirrhosis. Hepatology 2011;53:1600‐1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Urabe Y, Nouso K, Higashi T, Nakatsukasa H, Hino N, Ashida K, et al. Telomere length in human liver diseases. Liver 1996;16:293‐297. [DOI] [PubMed] [Google Scholar]
- 6. Wiemann SU, Satyanarayana A, Tsahuridu M, Tillmann HL, Zender L, Klempnauer J, et al. Hepatocyte telomere shortening and senescence are general markers of human liver cirrhosis. FASEB J 2002;16:935‐942. [DOI] [PubMed] [Google Scholar]
- 7. Blackburn EH. Switching and signaling at the telomere. Cell 2001;106:661‐673. [DOI] [PubMed] [Google Scholar]
- 8. de Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev 2005;19:2100‐2110. [DOI] [PubMed] [Google Scholar]
- 9. Armanios M, Blackburn EH. The telomere syndromes. Nat Rev Genet 2012;13:693‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shay JW, Wright WE. Telomeres and telomerase: three decades of progress. Nat Rev Genet 2019;20:299‐309. [DOI] [PubMed] [Google Scholar]
- 11. Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature 1990;345:458‐460. [DOI] [PubMed] [Google Scholar]
- 12. Zou Y, Sfeir A, Gryaznov SM, Shay JW, Wright WE. Does a sentinel or a subset of short telomeres determine replicative senescence? Mol Biol Cell 2004;15:3709‐3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sirma H, Kumar M, Meena JK, Witt B, Weise JM, Lechel A, et al. The promoter of human telomerase reverse transcriptase is activated during liver regeneration and hepatocyte proliferation. Gastroenterology 2011;141:326‐337.e3. [DOI] [PubMed] [Google Scholar]
- 14. Lin S, Nascimento EM, Gajera CR, Chen LU, Neuhöfer P, Garbuzov A, et al. Distributed hepatocytes expressing telomerase repopulate the liver in homeostasis and injury. Nature 2018;556:244‐248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hartmann D, Srivastava U, Thaler M, Kleinhans KN, N'Kontchou G, Scheffold A, et al. Telomerase gene mutations are associated with cirrhosis formation. Hepatology 2011;53:1608‐1617. [DOI] [PubMed] [Google Scholar]
- 16. Aravinthan A, Scarpini C, Tachtatzis P, Verma S, Penrhyn‐Lowe S, Harvey R, et al. Hepatocyte senescence predicts progression in non‐alcohol‐related fatty liver disease. J Hepatol 2013;58:549‐556. [DOI] [PubMed] [Google Scholar]
- 17. Laish I, Mannasse‐Green B, Hadary R, Biron‐Shental T, Konikoff FM, Amiel A, et al. Telomere dysfunction in nonalcoholic fatty liver disease and cryptogenic cirrhosis. Cytogenet Genome Res 2017;150:93‐99. [DOI] [PubMed] [Google Scholar]
- 18. Patnaik MM, Kamath PS, Simonetto DA. Hepatic manifestations of telomere biology disorders. J Hepatol 2018;69:736‐743. [DOI] [PubMed] [Google Scholar]
- 19. Kapuria D, Ben‐Yakov G, Ortolano R, Cho MH, Kalchiem‐Dekel OR, Takyar V, et al. The spectrum of hepatic involvement in patients with telomere disease. Hepatology 2019;69:2579‐2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Penrice DD, Simonetto DA. Short telomeres: cause and consequence in liver disease. Semin Liver Dis 2020;40:385‐391. [DOI] [PubMed] [Google Scholar]
- 21. Wojcicki JM, Rehkopf D, Epel E, Rosenthal P. Shorter leukocyte telomere length in relation to presumed nonalcoholic fatty liver disease in Mexican‐American men in NHANES 1999‐2002. Int J Hepatol 2017;2017:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim D, Li AA, Ahmed A. Leucocyte telomere shortening is associated with nonalcoholic fatty liver disease‐related advanced fibrosis. Liver Int 2018;38:1839‐1848. [DOI] [PubMed] [Google Scholar]
- 23. Zipf G, Chiappa M, Porter KS, Ostchega Y, Lewis BG, Dostal J. National health and nutrition examination survey: plan and operations, 1999‐2010. Vital Health Stat 2013;1:1‐37. [PubMed] [Google Scholar]
- 24. Rehkopf DH, Needham BL, Lin J, Blackburn EH, Zota AR, Wojcicki JM, et al. Leukocyte telomere length in relation to 17 biomarkers of cardiovascular disease risk: a cross‐sectional study of US adults. PLoS Med 2016;13:e1002188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res 2002;30:e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lin J, Epel E, Cheon J, Kroenke C, Sinclair E, Bigos M, et al. Analyses and comparisons of telomerase activity and telomere length in human T and B cells: insights for epidemiology of telomere maintenance. J Immunol Methods 2010;352:71‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Needham BL, Adler N, Gregorich S, Rehkopf D, Lin J, Blackburn EH, et al. Socioeconomic status, health behavior, and leukocyte telomere length in the National Health and Nutrition Examination Survey, 1999–2002. Soc Sci Med 2013;85:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kwo PY, Cohen SM, Lim JK. ACG clinical guideline: evaluation of abnormal liver chemistries. Am J Gastroenterol 2017;112:18‐35. [DOI] [PubMed] [Google Scholar]
- 29. Lin Z‐H, Xin Y‐N, Dong Q‐J, Wang Q, Jiang X‐J, Zhan S‐H, et al. Performance of the aspartate aminotransferase‐to‐platelet ratio index for the staging of hepatitis C‐related fibrosis: an updated meta‐analysis. Hepatology 2011;53:726‐736. [DOI] [PubMed] [Google Scholar]
- 30. Kitada T, Seki S, Kawakita N, Kuroki T, Monna T. Telomere shortening in chronic liver diseases. Biochem Biophys Res Commun 1995;211:33‐39. [DOI] [PubMed] [Google Scholar]
- 31. Miura N, Horikawa I, Nishimoto A, Ohmura H, Ito H, Hirohashi S, et al. Progressive telomere shortening and telomerase reactivation during hepatocellular carcinogenesis. Cancer Genet Cytogenet 1997;93:56‐62. [DOI] [PubMed] [Google Scholar]
- 32. Munroe M, Niero EL, Fok WC, Vessoni AT, Jeong H‐C, Brenner KA, et al. Telomere dysfunction activates p53 and represses HNF4α expression leading to impaired human hepatocyte development and function. Hepatology 2020;72:1412‐1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ping F, Li Z‐Y, Lv KE, Zhou M‐C, Dong Y‐X, Sun QI, et al. Deoxyribonucleic acid telomere length shortening can predict the incidence of non‐alcoholic fatty liver disease in patients with type 2 diabetes mellitus. J. Diabetes Investig 2017;8:174‐180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cawthon RM, Smith KR, O’Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet 2003;361:393‐395. [DOI] [PubMed] [Google Scholar]
- 35. Rode L, Nordestgaard BG, Bojesen SE. Peripheral blood leukocyte telomere length and mortality among 64 637 individuals from the general population. JNCI 2015;107:74. [DOI] [PubMed] [Google Scholar]
- 36. Wang Q, Zhan Y, Pedersen NL, Fang F, Hägg S. Telomere length and all‐cause mortality: a meta‐analysis. Ageing Res Rev 2018;48:11‐20. [DOI] [PubMed] [Google Scholar]
- 37. Lynch SM, Peek MK, Mitra N, Ravichandran K, Branas C, Spangler E, et al. Race, ethnicity, psychosocial factors, and telomere length in a multicenter setting. PLoS One 2016;11:e0146723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sanders JL, Newman AB. Telomere length in epidemiology: a biomarker of aging, age‐related disease, both, or neither? Epidemiol Rev 2013;35:112‐131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lapham K, Kvale MN, Lin J, Connell S, Croen LA, Dispensa BP, et al. Automated assay of telomere length measurement and informatics for 100,000 subjects in the genetic epidemiology research on adult health and aging (GERA) cohort. Genetics 2015;200:1061‐1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zierer J, Kastenmüller G, Suhre K, Gieger C, Codd V, Tsai PC, et al. Metabolomics profiling reveals novel markers for leukocyte telomere length. Aging (Albany, NY) 2016;8:77‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Table S1‐S4


