Abstract
The National Committee for Clinical Laboratory Standards (NCCLS) M38-P method describes standard parameters for testing the fungistatic antifungal activities (MICs) of established agents against filamentous fungi (molds). The present study evaluated the in vitro fungistatic activities of itraconazole and amphotericin B by the E-test and the NCCLS M38-P microdilution method against 186 common and emerging pathogenic molds (123 isolates of Aspergillus spp. [five species], 16 isolates of Fusarium spp. [two species], 4 Paecilomyces lilacinus isolates, 5 Rhizopus arrhizus isolates, 15 Scedosporium spp., 18 dematiaceous fungi, and 5 Trichoderma longibrachiatum isolates). The agreement between the methods for amphotericin B MICs ranged from 70% for Fusarium solani to ≥90% for most of the other species after the first reading; agreement was dependent on both the incubation time and the species being evaluated. Major discrepancies between the amphotericin B MICs determined by the E-test and the NCCLS M38-P method were demonstrated for three of the five species of Aspergillus tested and the two species of Fusarium tested. This discrepancy was more marked after 48 h of incubation; the geometric mean MICs determined by the E-test increased between 24 and 48 h from between 1.39 and 3.3 μg/ml to between 5.2 and >8 μg/ml for Aspergillus flavus, Aspergillus fumigatus, and Aspergillus nidulans. The agreement between the itraconazole MICs determined by the E-test and the NCCLS M38-P method ranged from 83.3% for A. nidulans to ≥90% for all the other species tested; the overall agreement was higher (92.7%) than that for amphotericin B (87.9%). The agreement was less dependent on the incubation time. Clinical trials need to be conducted to establish the role of the results of either the E-test or the NCCLS M38-P method in vitro for molds with the two agents as predictors of clinical outcome.
A higher incidence of fungal infections has been documented since the 1980s with the parallel emergence of either new fungal pathogens or fungi that were considered nonpathogenic as etiologic agents of systemic disease, especially in the immunocompromised host (2, 4, 8, 16, 27, 28, 31). Although the volume of disseminated infections caused by the filamentous fungi (molds) is lower than that caused by yeasts, their higher incidence and the increased resistance of molds to established antifungal agents (2, 4, 9, 10, 14–16, 18, 27, 31) warrant the evaluation of the in vitro susceptibilities of these fungi to both established and investigational agents. Aspergillus fumigatus is responsible for the majority (85 to 90%) of the different clinical manifestations of severe mold infections (9). However, other Aspergillus spp., Fusarium spp., Scedosporium apiospermum (Pseudallescheria boydii), Scedosporium prolificans, and less common molds have become important emerging pathogens (2, 4, 15, 18, 27, 28).
The National Committee for Clinical Laboratory Standards (NCCLS) Subcommittee on Antifungal Susceptibility Tests has proposed standard procedures for the antifungal susceptibility testing of molds (NCCLS M38-P document [22]). On the basis of data from several studies (12, 13), this document recommends the use of (i) standard RPMI 1640 broth; (ii) nongerminated conidial inoculum suspensions of approximately 104 CFU/m1; and (iii) incubation at 35°C for 24 h (Rhizopus spp.), 48 h (Aspergillus spp., Fusarium spp., and other opportunistic molds), and 72 h (S. apiospermum). The determination of MICs according to the instructions in the NCCLS M38-P document requires the visual examination of growth inhibition compared to the growth for the growth control (22). Some degree of correlation has been documented between the results of the M38-P method and treatment outcomes in experimental infections (24); however, the clinical value of the NCCLS methods for the testing of molds needs to be established.
The NCCLS methods are cumbersome and time-consuming, and alternative methods have been evaluated in recent years for testing of yeasts. Among these procedures, the E-test has been suggested as an alternative approach for the antifungal susceptibility testing of yeasts (11, 21, 25, 32), and more recently, this method has been evaluated for the antifungal susceptibility testing of certain molds (30). Good levels of agreement between the E-test and NCCLS methods have been demonstrated in these studies. The present study evaluated the activities of itraconazole and amphotericin B by the E-test against 186 common and emerging pathogenic molds recovered from clinical specimens during the last 5 years. The MICs obtained by the E-test were compared to those obtained by the proposed NCCLS M38-P broth microdilution method for filamentous fungi (22).
(This work was partially presented at the 96th General Meeting of the American Society for Microbiology 1996, 19 to 23 May 1996, New Orleans, La., and the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy, 24 to 27 September 1998, San Diego, Calif.)
MATERIALS AND METHODS
Isolates.
The set of 186 isolates evaluated included Aspergillus flavus (16 isolates), A. fumigatus (69 isolates), Aspergillus nidulans (12 isolates), Aspergillus niger (10 isolates), Aspergillus terreus (16 isolates), Fusarium oxysporum (6 isolates), Fusarium solani (10 isolates), Paecilomyces lilacinus (4 isolates), Rhizopus arrhizus (5 isolates), S. apiospermum (10 isolates), S. prolificans (5 isolates), Trichoderma longibrachiatum (5 isolates), and 18 dematiaceous molds (one to three isolates each of Bipolaris spp., Cladophialophora bantiana, Cladophialophora cladosporioides, Dactylaria constricta var. gallopava, Phaeoacremonium parasiticum [Phialophora parasitica], and Wangiella dermatitidis). Each isolate originated from a different patient and was received at the Medical Mycology Research Laboratory, Medical College of Virginia, Virginia Commonwealth University, for MIC testing during the last 5 years. Isolates were maintained at −70°C until testing was performed. The reference isolate A. flavus ATCC 204304 (22) and the quality control (QC) strain Candida parapsilosis ATCC 22019 (23) were included as control isolates for both the NCCLS and the E-test methods. For the latter strain, microdilution MIC ranges of the agents evaluated in the study as well as preliminary QC ranges for the E-test are well established (3, 11). Reference MIC ranges have also been established for A. flavus ATCC 204304 on the basis of repeated testing in a prior study (13), and these values are listed in the M38-P document (22). MIC ranges for the QC and reference isolates were within established values by both methods (3, 11, 22, 23).
Antifungal agents.
The E-test gradient strips of amphotericin B and itraconazole were provided by the manufacturer (AB BIODISK, Solna, Sweden). The concentration gradient for each drug ranged from 0.004 to 32 μg/ml. The strips were stored at −20°C until the day on which the test was performed. Amphotericin B (Bristol-Myers Squibb Pharmaceutical Research Institute, Wallingford, Conn.) and itraconazole (Janssen Pharmaceutica, Titusville, N.J.) were provided by the manufacturers as assay powders. As described in the NCCLS M38-P document (22), additive drug dilutions were prepared at 100 times the final concentrations in 100% dimethyl sulfoxide, followed by further dilutions (1:50) in the NCCLS standard RPMI 1640 medium to yield two times the final strength required for the test. The drugs were frozen at −70°C at their final concentrations (8 to 0.0078 μg/ml) for testing by the M38-P method until they were needed.
Inoculum preparations.
Stock inoculum suspensions were prepared as described in the NCCLS M38-P document (22) from 7-day-old cultures grown on potato dextrose agar slants at 35°C, and the suspensions were adjusted spectrophotometrically to optical densities that ranged from 0.09 to 0.3 at 530 nm (82 to 60% transmittance); the stock suspensions contained mostly conidia. The final sizes of the stock inoculum suspensions of most of the isolates tested ranged from 0.5 × 106 to 4.5 × 106 CFU/ml, as demonstrated by quantitative colony counts on Sabouraud dextrose agar. The densities of Bipolaris sp. stock inoculum suspensions were lower (2 × 105 to 7 × 105 CFU/ml). The nongerminated conidial inoculum suspensions were diluted 1:50 in medium for testing by the M38-P method.
E-test pilot study.
During an earlier investigation for testing of yeasts (11), several medium formulations were evaluated in order to identify the optimal medium for MIC determination by the E-test. In the present study, a pilot study was conducted with three media with a small sample of the 186 mold isolates evaluated. MICs were obtained by the E-test for the 30 isolates listed in Table 1 (single test runs) with three medium formulations: (i) solidified (1.5%) RPMI 1640 medium with 2% dextrose (RPMI-agar), (ii) solidified antibiotic medium 3 (M-3 agar), and (iii) Casitone agar (Remel Inc., Lenexa, Kans.). Since the MICs obtained with the three media were comparable for the 30 isolates and both antifungal agents tested (see Table 1), the remaining 156 molds were tested only with the RPMI-agar. The latter medium is a more chemically defined medium than the other two and is less subject to lot-to-lot variation.
TABLE 1.
E-test pilot study resultsa
| Fungus (no. of isolates tested) | Antifungal agent | Incubation time (h) | E-test MIC range (μg/ml) with:
|
NCCLS MIC range (μg/ml) | ||
|---|---|---|---|---|---|---|
| R | C | M-3 | ||||
| A. flavus (5) | A | 24 | 0.25–2 | 0.5–2 | 0.25–1.0 | |
| 48 | 0.5–>8 | 0.5–>8 | 0.5–>8 | 1.0–2 | ||
| I | 24 | 0.12–1.0 | 0.06–0.5 | 0.12–0.5 | 0.06–0.5 | |
| 48 | 0.25–1.0 | 0.06–0.5 | 0.12–1.0 | |||
| A. fumigatus (10) | A | 24 | 0.12–0.5 | 0.25–0.5 | 0.25–1.0 | |
| 48 | 0.25–2 | 0.25–1.0 | 0.5–8 | 0.5–1.0 | ||
| I | 24 | 0.06–>8 | 0.12–>8 | 0.12–>8 | 0.06–>8 | |
| 48 | 0.12–>8 | 0.12–>8 | 0.25–>8 | |||
| F. solani (5) | A | 48 | 1.0–>8 | 2–>8 | 1.0–>8 | 1.0–2 |
| I | 24 | >8 | >8 | >8 | ||
| 48 | >8 | >8 | >8 | 1.0–>8 | ||
| S. apiospermum (5) | A | 48 | 2–4 | 2–4 | 1.0–4 | 1.0–8 |
| 72 | 4–>8 | 4–>8 | 4–>8 | 2–8 | ||
| I | 48 | 0.5–2 | 0.25–1.0 | 0.5–2 | 0.25–1.0 | |
| 72 | 0.5–2 | 0.25–1.0 | 0.5–2 | |||
| R. arrhizus (5) | A | 24 | 0.06–0.25 | 0.03–0.5 | 0.5–2 | 0.25–0.5 |
| 48 | 0.06–>8 | 0.03–4 | 0.5–4 | 0.25–2 | ||
| I | 24 | 2–>8 | 1.0–>8 | 2–>8 | 0.12–0.25 | |
| 48 | 4–>8 | 2–>8 | >8 | 0.25–1.0 | ||
Abbreviations: NCCLS MICs, MICs obtained by the NCCLS M38-P microdilution method; E-test MICs, MICs obtained with three solidified media, RPMI with 2% dextrose (R), Casitone agar (C), and antibiotic medium 3 (M-3); A, amphotericin B; I, itraconazole.
E-test procedure.
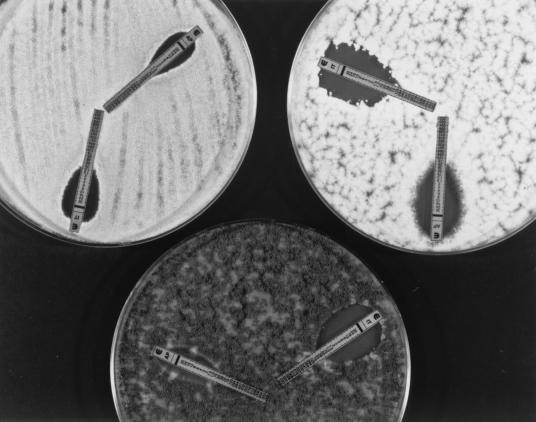
The E-test was performed by following the manufacturer's instructions. Each solidified medium was inoculated by dipping a nontoxic (latex-free) sterile swab into the respective undiluted stock inoculum suspension and evenly streaking it in three directions over the entire surface of a 150-mm petri plate containing 60 ml of medium; the swab was dipped into the inoculum suspension each time that the agar surface was streaked when isolates of Bipolaris spp. were tested. The agar surface was allowed to dry for 15 min, and the strips were placed onto the inoculated agar. As described above, C. parapsilosis ATCC 22019 and A. flavus ATCC 204304 were tested each time that a set of isolates was evaluated. The plates were incubated at 35°C, and the MICs were determined following incubation for 24 h to 4 days. The MICs determined by the E-test were the lowest drug concentrations at which the border of the elliptical inhibition intercepted the scale on the antifungal strip (see Fig. 1 and 2).
FIG. 1.
Itraconazole and amphotericin B MICs at 48 h for A. fumigatus (top left), A. terreus (top right), and A. flavus (bottom), as determined by the E-test.
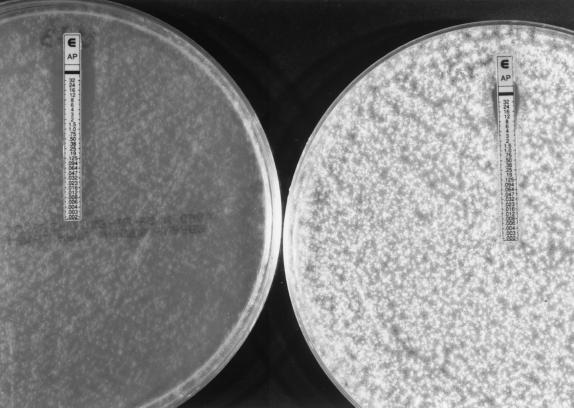
FIG. 2.
Amphotericin B MICs for one isolate of Aspergillus flavus: MIC at 24 h, 0.5 μg/ml (left); MIC at 48 h, 8 μg/ml (right).
NCCLS broth microdilution method (M38-P document).
On the day of the test, each microdilution well that contained 100 μl of the diluted (two times) drug concentrations was inoculated with 100 μl of the diluted (two times) conidial inoculum suspensions (final volume in each well, 200 μl). Growth and sterility controls were included for each isolate tested. As described above, C. parapsilosis ATCC 22019 and A. flavus ATCC 204304 were tested each time that a set of isolates was evaluated. Microdilution trays were incubated at 35°C and examined at 48 h for MIC determination; MICs for S. apiospermum were determined after 72 h of incubation, and those for C. bantiana were determined on day 4. The MICs were determined by the visual inspection of growth inhibition as described in the NCCLS M38-P document (22) and corresponded to complete growth inhibition.
Data analyses.
Because the E-test strips contain a continuous gradient instead of the established twofold drug dilution schema, MICs determined by the E-test were elevated to the next twofold dilution concentration, which matched the drug dilution schema of the NCCLS M38-P method (8 to 0.0078 μg/ml). This elevation of MICs for the E-test facilitated the comparisons and presentation of results. Both on-scale and off-scale MICs were included in the analysis. As analyzed previously (11, 13), discrepancies between the MIC endpoints of no more than 3 dilutions (e.g., 0.5, 1.0, and 2 μg/ml) were used for calculation of the percent agreement. The MICs and MIC ranges determined by the E-test and the NCCLS M38-P method and the corresponding geometric mean values were obtained for each species-drug combination tested. For both the E-test and the NCCLS M38-P method, the MICs for 90% of the isolates tested (MIC90s) were determined for species for which ≥10 isolates were available; MICs for 50% of the isolates tested were obtained for species represented by less than 10 isolates.
RESULTS
E-test pilot study.
Because optimal medium conditions had not been investigated for susceptibility testing of molds by the E-test when the present study was initiated, three medium formulations were evaluated with the first 30 mold isolates that were tested. The results for amphotericin B and itraconazole by the E-test with the three media were the same or no more than 2 dilutions different for the representative isolates of each species listed in Table 1. However, the inhibition ellipses were narrower with RPMI-agar than with M-3 agar, especially for A. flavus. The trailing effect was a major problem only for testing of some R. arrhizus isolates with itraconazole. Therefore, the MIC data obtained with the RPMI-agar for 25 of the 30 molds tested in the pilot study were incorporated into the data presented in Table 2, and the comparative evaluation was continued by using only RPMI-agar for the E-test. Only one other isolate of R. arrhizus was received in the Medical Mycology Research Laboratory for MIC testing during the last 3 years, and the results obtained by both procedures were similar to those presented in Table 1.
TABLE 2.
M38-P MICs for 181 opportunistic molds determined by the E-test and the NCCLS M38-P methoda
| Fungus (no. of isolates tested) | Antifungal Agentb | Incubation time (h) | MIC (μg/ml) determined by:
|
|||||
|---|---|---|---|---|---|---|---|---|
| E-test
|
NCCLS M38-P method
|
|||||||
| Range | Geometric mean | 90% | Range | Geometric Mean | 90% | |||
| A. flavus (16) | A | 24 | 0.25–>8 | 3.3 | >8 | |||
| 48 | 0.5–>8 | >8 | >8 | 0.5–4 | 1.26 | 2 | ||
| I | 24 | 0.06–0.25 | 0.14 | 0.25 | ||||
| 48 | 0.12–1.0 | 0.25 | 0.5 | 0.03–0.25 | 0.11 | 0.25 | ||
| A. fumigatus (69) | A | 24 | 0.25–8 | 1.39 | 2 | |||
| 48 | 0.25–>8 | 5.2 | >8 | 0.25–4 | 1.2 | 1.0 | ||
| I | 24 | 0.06–>8 | 0.84 | 2 | ||||
| 48 | 0.12–>8 | 1.57 | 4 | 0.03–>8 | 0.71 | 0.5 | ||
| A. nidulans (12) | A | 24 | 0.25–>8 | 1.5 | 2 | |||
| 48 | 0.5–>8 | 6.1 | >8 | 0.5–4 | 0.88 | 2 | ||
| I | 24 | 0.06–0.25 | 0.12 | 0.25 | ||||
| 48 | 0.12–1.0 | 0.15 | 0.5 | 0.06–0.25 | 0.16 | 0.25 | ||
| A. niger (10) | A | 24 | 0.5–1.0 | 0.75 | 1.0 | |||
| 48 | 1.0 | 1.0 | 1.0 | 0.5–1.0 | 0.7 | 1.0 | ||
| I | 24 | 0.5 | 0.5 | 0.5 | ||||
| 48 | 1.0 | 1.0 | 1.0 | 0.12–1.0 | 0.48 | 1.0 | ||
| A. terreus (16) | A | 24 | 0.25–4 | 2.13 | 4 | |||
| 48 | 0.25–8 | 2.85 | 4 | 0.5–4 | 1.4 | 4 | ||
| I | 24 | 0.01–0.5 | 0.18 | 0.5 | ||||
| 48 | 0.03–0.5 | 0.19 | 0.5 | 0.03–0.5 | 0.14 | 0.25 | ||
| F. oxysporum (6) | A | 24 | 1.0–>8 | >8 | >8 | |||
| 48 | 1.0–>8 | >8 | >8 | 1.0 | 1.0 | 1.0 | ||
| I | 24 | 4–>8 | >8 | >8 | ||||
| 48 | >8 | 1.0–>8 | >8 | >8 | ||||
| F. solani (10) | A | 24 | 0.12–>8 | 2.08 | 8 | |||
| 48 | 0.5–>8 | >8 | >8 | 0.5–4 | 1.62 | 4 | ||
| I | 24 | >8 | ||||||
| 48 | >8 | 8–>8 | >8 | >8 | ||||
| P. lilacinus (4) | A | 48–72 | >8 | >8 | ||||
| I | 48–72 | 2–>8 | 7 | >8 | 2–>8 | 5.7 | >8 | |
| S. apiospermum (10) | A | 48 | 4–8 | 6.7 | 8 | |||
| 72 | 4–8 | 6.7 | 8 | 1–8 | 3.17 | 4 | ||
| I | 48 | 0.25–0.5 | 0.4 | 0.5 | ||||
| 72 | 0.25–0.5 | 0.4 | 0.5 | 0.25–1.0 | 0.85 | 1.0 | ||
| S. prolificans (5) | A | 48 | >8 | 8–>8 | >8 | >8 | ||
| I | 48 | >8 | 4–>8 | >8 | >8 | |||
| T. longibrachiatum (5) | A | 24 | 0.5–1.0 | 0.83 | 1.0 | |||
| 48 | 1.0–4 | 2.2 | 2 | 0.5–2 | 0.87 | 0.5 | ||
| I | 24 | >8 | ||||||
| 48 | >8 | >8 | ||||||
| Dematiaceous molds (18)c | A | 48 | 0.06–4 | 1.0 | 2 | |||
| 72 | 0.12–>8 | 2.74 | 8 | 0.12–4 | 0.85 | 2 | ||
| I | 48 | 0.01–0.12 | 0.06 | 0.12 | ||||
| 72 | 0.01–0.5 | 0.13 | 0.25 | 0.06–2 | 0.37 | 0.5 | ||
The NCCLS M38-P broth microdilution method has been described previously (22).
A, amphotericin B; I, itraconazole
This group included one to three isolates each of Bipolaris spp., C. bantiana, C. cladosporioides, D. constricta var. gallopava, P. parasiticum (P. parasitica), and W. dermatitidis.
Comparison of amphotericin B MICs obtained by the E-test and the NCCLS M38-P method.
Although the inhibition ellipses for amphotericin B were clear and well defined, they were usually narrower than those for itraconazole for most isolates (Fig. 1 and 2), especially after the second reading. The agreement between the methods for amphotericin B MICs ranged from 70% for F. solani to ≥90% for most of the other species after the first reading; agreement was dependent on both the incubation time and the species being evaluated (Table 3). Major discrepancies between the amphotericin B MICs determined by the E-test and the NCCLS M38-P method were demonstrated for three of the five species of Aspergillus tested and the two species of Fusarium tested, for which wider and higher MICs were obtained by the E-test (Table 2). This discrepancy was more marked after 48 h of incubation; the geometric mean MICs obtained by the E-test increased between 24 and 48 h from between 1.39 and 3.3 μg/ml to between 5.2 and >8 μg/ml for A. flavus, A. fumigatus, A. nidulans, and F. oxysporum. The percent agreements were also substantially lower after 48 h of incubation than after 24 h of incubation for four of the five species of Aspergillus tested and F. solani (Table 3). The agreement between the amphotericin B MICs obtained by the two methods was good for the dematiaceous molds and other species evaluated.
TABLE 3.
Distribution of differences in MICs for 181 molds and percent agreement within 3 dilutions for the E-test and the NCCLS M38-P method
| Fungus (no. of isolates tested) | Antifungal agenta | Incubation time (h) or reading no. | No. of isolates for which MICs determined by E-test differed from MICs determined by method by the following dilution:
|
% Agreementb | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| >+2 | >+2 | +1 | 0 | −1 | −2 | >−2 | ||||
| A. flavus (16) | A | 24 | 4 | 3 | 3 | 3 | 1 | 2 | 75 | |
| 48 | 11 | 3 | 1 | 1 | 31.2 | |||||
| I | 24 | 3 | 5 | 5 | 3 | 100 | ||||
| 48 | 7 | 4 | 5 | 100 | ||||||
| A. fumigatus (69) | A | 24 | 4 | 14 | 16 | 22 | 8 | 2 | 97 | |
| 48 | 25 | 15 | 8 | 21 | 63.8 | |||||
| I | 24 | 5 | 11 | 17 | 23 | 7 | 2 | 1 | 90 | |
| 48 | 18 | 12 | 20 | 17 | 2 | 74 | ||||
| A. nidulans (12) | A | 24 | 1 | 2 | 2 | 4 | 1 | 1 | 83.3 | |
| 48 | 5 | 2 | 4 | 1 | 58.3 | |||||
| I | 24 | 1 | 2 | 1 | 5 | 2 | 83.3 | |||
| 48 | 2 | 1 | 1 | 6 | 2 | 83.3 | ||||
| A. niger (10) | A | 24 | 2 | 5 | 3 | 100 | ||||
| 48 | 3 | 7 | 100 | |||||||
| I | 24 | 1 | 9 | 100 | ||||||
| 48 | 1 | 9 | 100 | |||||||
| A. terreus (16) | A | 24 | 2 | 1 | 5 | 3 | 1 | 1 | 77 | |
| 48 | 3 | 4 | 4 | 4 | 1 | 75 | ||||
| I | 24 | 1 | 4 | 3 | 1 | 4 | 92 | |||
| 48 | 2 | 4 | 3 | 2 | 1 | 4 | 88 | |||
| F. oxysporum (6) | A | 24 | 5 | 1 | NAc | |||||
| 48 | 6 | NA | ||||||||
| I | 24 | 2 | 3 | 1 | NA | |||||
| 48 | 6 | NA | ||||||||
| F. solani (10) | A | 24 | 1 | 1 | 2 | 2 | 2 | 2 | 70 | |
| 48 | 6 | 1 | 1 | 2 | 40 | |||||
| I | 24 | 10 | 100 | |||||||
| 48 | 10 | 100 | ||||||||
| P. lilacinus (4) | A | 48–72 | 4 | NA | ||||||
| I | 48–72 | 1 | 2 | 1 | NA | |||||
| S. apiospermum (10) | A | 48 | 3 | 3 | 4 | 100 | ||||
| 72 | 3 | 3 | 4 | 100 | ||||||
| I | 48 | 3 | 4 | 3 | 100 | |||||
| 72 | 3 | 4 | 3 | 100 | ||||||
| S. prolificans (5) | A | 24 | ||||||||
| 48 | 1 | 4 | NA | |||||||
| I | 24 | |||||||||
| 48 | 1 | 4 | NA | |||||||
| T. longibrachiatum (5) | A | 24 | 3 | 2 | NA | |||||
| 48 | 2 | 3 | NA | |||||||
| I | 24 | 5 | NA | |||||||
| 48 | 5 | NA | ||||||||
| Dematiaceous molds (18)d | A | 48 | 1 | 3 | 3 | 5 | 2 | 2 | 93.7 | |
| 72 | 1 | 3 | 4 | 5 | 93.3 | |||||
| I | 48 | 1 | 1 | 3 | 2 | 7 | 7 | 81.2 | ||
| 72 | 1 | 3 | 7 | 2 | 2 | 2 | 83.3 | |||
| All isolates (181) | A | Reading 1 | 14 | 17 | 36 | 42 | 33 | 15 | 6 | 87.7 |
| Reading 2 | 57 | 27 | 22 | 40 | 26 | 8 | 1 | 67.9 | ||
| I | Reading 1 | 7 | 21 | 29 | 63 | 21 | 17 | 5 | 92.7 | |
| Reading 2 | 21 | 26 | 41 | 63 | 15 | 11 | 4 | 86 | ||
A, amphotericin B; I, itraconazole.
Percent agreement is the percentage of the MIC results obtained by the two methods that were within 3 dilutions.
NA, not applicable (too few isolates).
Isolates of C. bantiana needed 4 days of incubation.
Comparison of itraconazole MICs obtained by the E-test and the NCCLS M38-P method.
A major problem with antifungal susceptibility testing of yeasts with azole compounds is the trailing effect, which is due to the concentration-dependent partial inhibition of fungal growth. When one is performing the E-test procedure, microcolonies around or inside the entire inhibition ellipse are observed with trailing MICs for certain yeasts. It has been demonstrated that the trailing effect can be minimized in the testing of yeasts by the use of either RPMI-agar or Casitone agar (11). In the present study trailing was not a major problem, as it was when Candida spp. and other yeasts were tested. In addition, there was no apparent medium dependence, as demonstrated by the results for the 30 isolates that were tested on the three agars. E-test inhibition ellipses for itraconazole were as clear and well-defined as those for amphotericin B for most of the isolates tested (Fig. 1). Small colonies were observed inside the inhibition ellipses (trailing effect) for some isolates of F. oxysporum, P. lilacinus, and R. arrhizus; ellipses for some of these isolates were also narrow. The agreement between the itraconazole MICs obtained by the E-test and the NCCLS M38-P method ranged from 83.3% for A. nidulans and the dematiaceous fungi to ≥90% for all the other species tested; the overall agreement was higher (92.7%) than that for amphotericin B (87.9%) (Table 3). In contrast to the MICs of amphotericin B, the itraconazole MICs determined by the E-test either had no change or shifted very little between the two incubation times evaluated for most of the species (Table 2). The exception was A. fumigatus, because more discrepant MICs (>2 dilutions) were found between the two methods at 48 h (18 isolates) than at 24 h (18 isolates) (Table 3).
DISCUSSION
In the present study, the percent agreement between the results of the two methods for amphotericin B was lower (<80%) for A. flavus, A. terreus, and the two Fusarium spp. Low levels of agreement (60 to 80%) between the results of the E-test and a microdilution method have previously been reported for A. flavus and F. solani (30). However, the investigators did not evaluate isolates of either A. nidulans or F. oxysporum. Also, MICs determined by the E-test were read only after 48 h of incubation in that study. On the basis of the level of agreement between the two methods in the present study, it appears that amphotericin B MICs for Aspergillus spp. should be determined by the E-test as soon as sufficient growth allows it (before 48 h). However, which of the two incubation times is providing the most clinically relevant MICs by the E-test? Although the conventional form of amphotericin B remains the drug of choice for the treatment of life-threatening fungal infections, its clinical efficacy is suboptimal in the immunocompromised host (10). In several studies (5, 17, 20, 26), death was attributed to disseminated aspergillosis in 27 of 62 patients, despite adequate treatment with amphotericin B (10). In the report of one of those studies (17), six of the seven patients whose cause of death was attributed to disseminated aspergillosis were infected with A. flavus. In the present study, the disagreement between the two methods was higher for A. flavus than for the other Aspergillus spp. tested (Table 3). The reason was that the MIC determined by the E-test shifted from ≤1.0 to ≥8 μg/ml (Fig. 2) for 4 of the 16 isolates of A. flavus tested and MICs were 2 to >8 μg/ml for the other isolates tested. Clinical resistance of this species to amphotericin B has been reported; unfortunately, the MICs for the infecting isolates were not reported (5, 17, 20, 26). It is noteworthy that the E-test appeared to be superior to the NCCLS method for yeasts (23) in its ability to detect amphotericin B-resistant Candida spp. and Cryptococcus neoformans strains (6, 21, 32). Further studies are needed to evaluate the correlation of the MICs determined by the E-test and those determined by the NCCLS M38-P method with in vivo outcome in experimental infections or clinical trials.
It has been suggested that amphotericin B MICs are not good predictors of the clinical response to treatment with this agent in patients with disseminated fusarial infections. In 56 of 73 patients who failed amphotericin B therapy for disseminated fusarial infection, the MICs for the infecting isolates were indiscriminately low (≤1.0 μg/ml) or high (≥2 μg/ml) (2, 16). However, in vitro testing was performed in those studies by using nonstandardized procedures. In addition, the status of the host and the degree of tissue involvement are also important in predicting the clinical outcome of therapy in patients with fusarial and other opportunistic infections. The amphotericin B MIC90s for F. solani determined by the E-test in the present evaluation and in the study of Szekely et al. (30) were ≥8 μg/ml. Again, the clinical relevance of the MICs for molds determined by both methods need to be established in clinical trials with standardized methods for in vitro testing.
The most important role of antifungal susceptibility testing is detection of isolates potentially resistant to the agent being evaluated. High amphotericin B MICs were obtained by both methods for most isolates of P. lilacinus and Scedosporium spp. (MIC90s, 4 to >8 μg/ml) and some isolates of A. terreus. The agreement between the two methods was excellent for the first three species and low for A. terreus (Table 3). In contrast, Szekely et al. (30) reported a 20% agreement between the two methods for Scedosporium spp. and an excellent agreement for A. terreus by following a procedure similar to the M38-P method (22). In their study, they prepared drug dilutions directly in RPMI 1640 broth instead of the solvent. The recent published literature regarding the results of in vitro and clinical studies indicates that the amphotericin B MICs for these four species are usually high and that infections caused by these molds are refractory to treatment with amphotericin B (1, 7, 8, 15, 18, 19, 29).
Although the NCCLS M38-P document states that azole MICs are the lowest concentrations that show a 50% inhibition of growth compared to the growth for the control, recent data developed by an NCCLS subcommittee suggest that the conventional criterion of MIC determination (100% or complete growth inhibition) could more clearly and reliably detect azole resistance (A. Espinel-Ingroff, M. S. Bartlett, V. Chaturvedi, K. Hazen, M. A. Ghannoum, M. A. Pfaller, M. G. Rinaldi, and T. J. Walsh, unpublished data). Because of that, the values depicted in Table 2 were obtained by using the 100% inhibition criterion for MIC determination. Furthermore, most itraconazole concentrations resulting in 50% inhibition (not shown in Table 2) were only 1 to 2 dilutions lower than the corresponding concentrations causing 100% inhibition.
In the present study, the overall agreement between the two methods was higher for itraconazole (92.7%) than for amphotericin B (87.9%). Similar but lower levels of agreement (71% for amphotericin B versus 88% for itraconazole) were reported in another evaluation of the E-test for susceptibility testing of Aspergillus spp., F. solani, Scedosporium spp., and W. dermatitidis (30). The set of A. fumigatus isolates evaluated included four isolates for which high itraconazole MICs (≥8 μg/ml) have been obtained by the NCCLS and other methods by using the 100% criterion (9; Espinel-Ingroff et al., unpublished data). In the present study, the itraconazole MICs for these isolates determined by the E-test were 4 to ≥8 μg/ml at both incubation times. As reported by Denning et al. (9), two of the four isolates were recovered from patients who failed appropriate itraconazole therapy. These results suggest that both methods can identify itraconazole-resistant isolates of Aspergillus. Both methods also yielded high itraconazole MICs for Fusarium spp., P. lilacinus, S. prolificans, and T. longibrachiatum (Table 2); and these results are comparable to those determined by other procedures for these species (1, 2, 7, 8, 27). E-test results have not been published for P. lilacinus, R. arrhizus, or T. longibrachiatum, and the dematiaceous species (except W. dermatitidis) evaluated in the study.
In conclusion, on the basis of data from this and another study (30), the E-test has potential value for use for the antifungal susceptibility testing of mold pathogens. Although the E-test is easier to perform, it is important to consider that, as for any test for antimicrobial susceptibility testing, the medium formulation and, in this case, the depth of the agar can influence the MIC. Therefore, the manufacturer's recommendations should be followed when attempting to obtain MICs by the E-test. Also, E-test strips are available only for investigational purposes. The wider amphotericin B MICs obtained by the E-test for some Aspergillus spp. also suggest that this method could be more useful than the NCCLS M38-P method in detecting Aspergillus isolates potentially resistant to this agent or that the latter method may not be a suitable procedure for these species. Future studies with the new triazoles and echinocandins, which are undergoing phase II and III clinical trials, will assess the value of the E-test for measurement of their in vitro activities against molds. However, in vivo-in vitro evaluations of drug efficacy also are needed to provide a better assessment of the utilities of both methods for use in the clinical laboratory as predictors of antifungal resistance in patients with mold infections.
ACKNOWLEDGMENTS
Many thanks go to AB Biodisk and Remel Inc. for providing the E-test strips and the solified agars for the E-test.
REFERENCES
- 1.Aguilar C, Pujol I, Sala J, Guarro J. Antifungal susceptibilities of Paecilomyces species. Antimicrob Agents Chemother. 1998;42:1601–1604. doi: 10.1128/aac.42.7.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anaissie E, Kantarjian H, Ro H, Hopfer R, Rolston R K, Fainstein V, Bodey G. The emerging role of Fusarium infections in patients with cancer. Medicine. 1988;67:77–83. doi: 10.1097/00005792-198803000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Barry A L, Pfaller M A, Brown S D, Espinel-Ingroff A, Ghannoum M A, Knapp C, Rennie R P, Rex J H, Rinaldi M G. Quality control limits for broth microdilution susceptibility tests of ten antifungal agents. J Clin Microbiol. 2000;38:3457–3459. doi: 10.1128/jcm.38.9.3457-3459.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berenguer J, Rodriguez-Tudela J L, Richard C, Alvarez M, Sanz M A, Guztelurrutia L the Scedosporium prolificans Spanish Study Group. Deep infections caused by Scedosporium prolificans. A report on 16 cases in Spain and a review of the literature. Medicine. 1997;76:256–265. doi: 10.1097/00005792-199707000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Chumpitazi B F F, Pinel C, Lebeau B, Ambroise-Thomas P, Grillot R. Aspergillus fumigatus antigen detection in sera from patients at risk for invasive aspergillosis. J Clin Microbiol. 1999;38:438–443. doi: 10.1128/jcm.38.1.438-443.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clancy C, Nguyen M H. Correlation between in vitro susceptibility determined by E test and response to therapy with amphotericin B: results from a multicenter prospective study of candidemia. Antimicrob Agents Chemother. 1999;43:1289–1290. doi: 10.1128/aac.43.5.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cuenca-Estrella M, Ruiz-Diez B, Martinez-Suarez V, Monzon A, Rodriguez-Tudela J L. Comparative in vitro activity of voriconazole (UK-109,496) and six other antifungal agents against clinical isolates of Scedosporium prolificans and Scedosporium apiospermum. J Antimicrob Chemother. 1999;43:149–151. doi: 10.1093/jac/43.1.149. [DOI] [PubMed] [Google Scholar]
- 8.De Batlle J, Motje M, Balanza R, Guardia R, Ortiz R. Disseminated infection caused by Scedosporium prolificans in a patient with acute multilineal leukemia. J Clin Microbiol. 2000;38:1694–1695. doi: 10.1128/jcm.38.4.1694-1695.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Denning D W, Venkateswarlu K, Oakley K L, Anderson M J, Manning N J, Stevens D A, Warnock D W, Kelly S L. Itraconazole resistance in Aspergillus fumigatus. Antimicrob Agents Chemother. 1997;41:1364–1368. doi: 10.1128/aac.41.6.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dismukes W E. Introduction to antifungal drugs. Clin Infect Dis. 2000;30:653–657. doi: 10.1086/313748. [DOI] [PubMed] [Google Scholar]
- 11.Espinel-Ingroff A, Pfaller M, Erwin M E, Jones R N. Interlaboratory evaluation of Etest method for testing antifungal susceptibilities of pathogenic yeasts to five antifungal agents by using Casitone agar and solidified RPMI 1640 medium with 2% glucose. J Clin Microbiol. 1996;34:848–852. doi: 10.1128/jcm.34.4.848-852.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Espinel-Ingroff A, Dawson K, Pfaller M, Anaissie E, Breslin B, Dixon D, Fothergill A, Paetznick V, Peter J, Rinaldi M, Walsh T. Comparative and collaborative evaluation of standardization of antifungal susceptibility testing for filamentous fungi. Antimicrob Agents Chemother. 1995;39:314–319. doi: 10.1128/aac.39.2.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Espinel-Ingroff A, Bartlett M, Bowden R, Chin N X, Cooper C, Jr, Fothergill A, McGinnis M R, Menezes P, Messer S A, Nelson P W, Odds F C, Pasarell L, Peter J, Pfaller M A, Rex J H, Rinaldi M G, Shankland G S, Walsh T J, Weitzman I. Multicenter evaluation of proposed standardized procedure for antifungal susceptibility testing of filamentous fungi. J Clin Microbiol. 1997;35:139–143. doi: 10.1128/jcm.35.1.139-143.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallis H A, Drew R H, Pickard W W. Amphotericin B: 30 years of clinical use. Rev Infect Dis. 1990;12:308–329. doi: 10.1093/clinids/12.2.308. [DOI] [PubMed] [Google Scholar]
- 15.Girmenia C, Luzi G, Monaco M, Martino P. Use of voriconazole in treatment of Scedosporium apiospermum infection: case report. J Clin Microbiol. 1998;36:1436–1438. doi: 10.1128/jcm.36.5.1436-1438.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guarro J, Gene J. Opportunistic fusarial infections in humans. Eur J Clin Microbiol Infect Dis. 1995;14:741–754. doi: 10.1007/BF01690988. [DOI] [PubMed] [Google Scholar]
- 17.Iwen P C, Rupp M E, Hinrichs S H. Invasive mold sinusitis: 17 cases in immunocompromised patients and review of the literature. Clin Infect Dis. 1997;24:1178–1184. doi: 10.1086/513662. [DOI] [PubMed] [Google Scholar]
- 18.Iwen P C, Rupp M E, Langnas L N, Reed E C, Hinrichs S H. Invasive pulmonary aspergillosis due to Aspergillus terreus: 12-year experience and review of literature. Clin Infect Dis. 1998;26:1092–1097. doi: 10.1086/520297. [DOI] [PubMed] [Google Scholar]
- 19.Lass-Florl C, Kofler G, Kropshofer G, Hermans J, Kreczy A, Dierich M P, Niederwieser D. In vitro testing of susceptibility to amphotericin B is a reliable predictor of clinical outcome in invasive aspergillosis. J Antimicrob Chemother. 1998;42:497–502. doi: 10.1093/jac/42.4.497. [DOI] [PubMed] [Google Scholar]
- 20.Lortholary O, Meyohas M-C, Dupont B, Cadranel J, Salmon-Ceron D, Peyramond D, Simonin D Centre d'Informations et de Soins de I'Immunodeficience Humaine de l'Est Parisien. Invasive aspergillosis in patients with acquired immunodeficiency syndrome: report of 33 cases. Am J Med. 1992;95:177–186. doi: 10.1016/0002-9343(93)90258-q. [DOI] [PubMed] [Google Scholar]
- 21.Lozano-Chiu M, Paetznick V L, Ghannoum M A, Rex J H. Detection of resistance to amphotericin B among Cryptococcus neoformans clinical isolates: performances of three different media assessed by using E-test and National Committee for Clinical Laboratory Standards M27-A methodologies. J Clin Microbiol. 1998;36:2817–2822. doi: 10.1128/jcm.36.10.2817-2822.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of conidium-forming filamentous fungi. Proposed standard M38-P. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1998. [Google Scholar]
- 23.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 24.Odds F C, Gerven F V, Espinel-Ingroff A, Bartlett M S, Ghannoum M A, Lancaster M V, Pfaller M A, Rex J H, Rinaldi M G, Walsh T J. Evaluation of possible correlations between antifungal susceptibilities of filamentous fungi in vitro and antifungal treatment outcomes in animal infection models. Antimicrob Agents Chemother. 1998;42:282–288. doi: 10.1128/aac.42.2.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pfaller M A, Messer S A, Karlsson A, Bolmstrom A. Evaluation of the Etest method for determining fluconazole susceptibilities of 402 clinical yeast isolates by using three different agar media. J Clin Microbiol. 1998;36:2586–2589. doi: 10.1128/jcm.36.9.2586-2589.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ribaud, Chastang P C, Latge J-P, Baffroy-Lafitte L, Parquet N, Devergie A, Esperou H, Selimi F, Rocha V, Derouin F, Socie G, Gluckman E. Survival and prognostic factors of invasive aspergillosis after allogeneic bone marrow transplantation. Clin Infect Dis. 1998;28:322–330. doi: 10.1086/515116. [DOI] [PubMed] [Google Scholar]
- 27.Richter S, Cormican M G, Pfaller M A, Lee C K, Gingrich R, Rinaldi M G, Sutton D A. Fatal disseminated Trichoderma longibrachiatum infection in an adult bone marrow transplant patient: species identification and review of the literature. J Clin Microbiol. 1999;37:1154–1160. doi: 10.1128/jcm.37.4.1154-1160.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh N, Chang F Y, Gayowski T, Marino I R. Infections due to dematiaceous fungi in organ transplant recipients: case report and review. Clin Infect Dis. 1996;24:369–374. doi: 10.1093/clinids/24.3.369. [DOI] [PubMed] [Google Scholar]
- 29.Sutton A A, Sanche S E, Revankar S G, Fothergill A W, Rinaldi M G. In vitro amphotericin B resistance in clinical isolates of Aspergillus terreus, with a head-to-head comparison to voriconazole. J Clin Microbiol. 1999;37:2343–2345. doi: 10.1128/jcm.37.7.2343-2345.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szekely A, Johnson E M, Warnock D W. Comparison of E-test and broth microdilution methods for antifungal drug susceptibility testing of molds. J Clin Microbiol. 1999;37:1480–1483. doi: 10.1128/jcm.37.5.1480-1483.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verweij P E, van den Bergh M F Q, Rath P M, dePauw B E, Voss A, Meis J F G M. Invasive aspergillosis caused by Aspergillus ustus: case report and review. J Clin Microbiol. 1999;37:1606–1609. doi: 10.1128/jcm.37.5.1606-1609.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wanger A, Mills K, Nelson P W, Rex J H. Comparison of Etest and National Committee for Clinical Laboratory Standards broth macrodilution method for antifungal susceptibility testing: enhanced ability to detect amphotericin B-resistant Candida isolates. Antimicrob Agents Chemother. 1995;39:2520–2522. doi: 10.1128/aac.39.11.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]