Abstract
Mechanosensation is the ability to detect dynamic mechanical stimuli (e.g., pressure, stretch, and shear stress) and is essential for a wide variety of processes, including our sense of touch on the skin. How touch is detected and transduced at the molecular level has proved to be one of the great mysteries of sensory biology. A major breakthrough occurred in 2010 with the discovery of a family of mechanically gated ion channels that were coined PIEZOs. The last 10 years of investigation have provided a wealth of information about the functional roles and mechanisms of these molecules. Here we focus on PIEZO2, one of the two PIEZO proteins found in humans and other mammals. We review how work at the molecular, cellular, and systems levels over the past decade has transformed our understanding of touch and led to unexpected insights into other types of mechanosensation beyond the skin.
Keywords: PIEZO2, mechanosensation, mechanotransduction, somatosensation, touch, proprioception, ion channel
1. DISCOVERY OF PIEZO2
1.1. The Search for the Touch Receptor
As we move through the world, we sense, respond, and adapt to many types of mechanical forces. These processes, broadly referred to as mechanosensation, include the conscious perception of touch (an aspect of somatosensation), the effortless control of our posture (proprioception), and the unconscious regulation of physiological functions such as breathing and heart rate (interoception). In each of these cases, force activates specialized cells called mechanoreceptors that generate and transmit signals to the nervous system and body. At the mechanistic level, the key step in mechanosensation is the conformational change of molecules expressed in mechanoreceptors. Conformational changes of these molecules are caused by stress, shear stress, pressure, and/or tension and convert force into electrochemical signals, a process called mechanotransduction.
Of the many types of mechanosensation, touch is perhaps the most integral to our daily activities and therefore the most relatable. This sensory system is exquisitely sensitive, remarkably accurate, and extremely fast, allowing us to localize miniscule forces such as the movement of a single hair within a fraction of a second. Uncovering the mechanisms by which the touch system achieves these feats has been a major effort of scientists for over a century. But it is really in only the past decade that we have begun to understand how this type of mechanosensation functions at the molecular level.
Research dating back to the mid-to-late-1800s revealed beautiful details about the structural basis for mechanosensation in the skin. In the nineteenth century, anatomists such as von Frey, Pacini, and Merkel described the structural specializations of mechanoreceptors and developed theories describing how their physical forms influence sensory function (1). Physiologists, beginning with Sherrington (2), followed by pioneering studies from Iggo, Burgess, Perl, Loewenstein (3–6) and others, used electrical recordings to characterize peripheral neuron responses and the signals they send to the central nervous system. These studies laid out principles for the organization of the somatosensory system that serve as the foundation of our understanding of touch today. However, the question remained as to how forces activate these specialized cells in the first place.
The search for mechanotransduction mechanisms can be traced back to the mid-twentieth century, when neurophysiological observations indicated that force sensing might be an intrinsic feature of touch fibers (7). A turning point in the search for the molecular basis of mechanotransduction came in 1979, when Corey and Hudspeth (8) showed that mechanotransduction in hair cells, the principal mechanoreceptors for hearing, is extremely fast (<40 μs). They concluded that only the physical opening, or gating, of ion channels could convert force to electrical current with that temporal resolution. However, it was not until the invention of the patch-clamp recording technique in the early 1980s (9) that direct evidence for mechanotransduction involving ion channels could be demonstrated (10, 11). Since then, mechanosensitive ion channels have been proposed to function as receptors in a diverse array of cells, tissues, and sensory systems from many organisms (12). However, these hypotheses proved difficult to test. In many cases, the site of mechanotransduction (e.g., skin, muscle, or organs) is embedded in thick protective tissue, making it inaccessible to direct physical characterization. Assays that reliably and quantitatively apply force to cell membranes have also proved difficult to implement. But, perhaps the biggest challenge of all has been uncovering the identity of the molecules involved.
Major advances in identifying transduction molecules in the somatosensory system came in the late 1990s and early 2000s with the cloning of the capsaicin receptor TRPV1 (which also responds to heat, protons, toxins, and irritants) (13), followed by those of other TRP channels that sense temperature and chemical irritants (14, 15). At the same time, mechanosensitive ion channels were identified in bacteria using electrophysiological and biochemical approaches (16–19) and in invertebrates using genetic screens [notably in Caenorhabditis elegans (20, 21) and Drosophila melanogaster (22)]. Disappointingly, however, orthologs for these ion channels in vertebrates were either not found or had seemingly nonmechanosensory functions. More promisingly, candidate molecules in hearing were identified during this period by mapping the genes involved in hereditary deafness (23). Unfortunately, hereditary mutations selectively affecting touch had not yet been observed (24), and consequently, the transduction mechanisms for touch remained a mystery. While candidate approaches turned up several potential mechanosensitive ion channels (25), none of the proposed molecules proved to be essential for touch responses.
Despite these discouraging results, by the early 2000s, enough evidence had accumulated from new methods to indicate that the touch receptor was a force-gated ion channel. One method, the pressure-clamp technique, allowed screening to be performed both quickly and accurately. In this approach, positive or negative pressure can be applied to a membrane patch while simultaneously recording electrical activity (26). Another approach poked cells with a blunt glass probe using a piezo actuator during whole-cell recording (27). The use of piezo positioning allowed membrane indentation to be triggered at millisecond speeds, with micrometer resolutions, and in a reproducible manner. Notably, using a piezo-driven probe to mechanically stimulate sensory neuron cultures isolated from the sensory ganglia of rodents evoked large and distinctive currents whose biophysical properties could be rigorously characterized (28, 29). Together, these data provided key signatures of mechanosensitive ion channels in sensory neurons and the technical means to uncover their identity.
1.2. The Identification of PIEZO2
The challenges of finding the touch receptor gene(s) were several-fold. Unlike with thermosensation, there were no natural products like capsaicin or menthol that could be used as pharmacological probes. As candidate approaches continued to fail, the best guess was that the touch receptor would be a molecule with multiple membrane-spanning domains that would be enriched in sensory neurons. The prevailing view from the touch system in C. elegans and vertebrate hair cells was that mechanotransduction involved the assembly of multimeric complexes, making the prospect of expression cloning daunting. As it turned out, instead of a direct approach using sensory neurons, the touch receptor was ultimately found by screening a neural crest cell–derived cancer cell line (N2a cells).
In their landmark study, Coste et al. (30) reasoned that if they identified immortalized cell lines that had robust responses to membrane stretch measured using patch-clamp recording, they could perform a loss-of-function screen to discover mechanotransducers. Sure enough, while many cell lines had no mechanically evoked currents, mouse neuroblastoma N2a cells exhibited characteristic responses in the pressure-clamp and poking assays. Microarray gene expression profiling was used to create a list of genes with transmembrane domains found in N2a cells but not in nonresponsive cell lines. The necessity of each candidate molecule for the observed pressure-clamp responses was then laboriously tested one at a time using a loss-of-function knockdown strategy. Screening this way revealed that knockdown of a gene called Fam38a significantly reduced pressure-clamp responses. It turned out that Fam38a was a very unusual molecule that did not resemble anything previously studied in the context of mechanosensation (31). The gene had an enormous open reading frame (over 9 kb) that encoded a molecule predicted to span the membrane dozens of times. As predicted for a bona fide mechanotransducer, expression of the Fam38a cDNA in a nonresponsive cell line endowed those cells with large mechanically evoked currents that matched the kinetics of the endogenous responses seen in N2a cells. Intriguingly, a homology search found that Fam38a had a single homologous family member in the mouse and human genomes, called Fam38b, that also made normally nonresponsive cells mechanosensitive when it was expressed heterologously. Examining the expression patterns of these genes revealed that while Fam38a transcripts were found in many tissues, Fam38b appeared to be more selectively expressed and particularly enriched in somatosensory neurons. Importantly, siRNA knockdown of Fam38b reduced mechanically evoked currents in somatosensory neurons cultured from mouse tissue.
Based on their ability to respond to force, the mouse proteins Fam38a and Fam38b were renamed Piezo1 and Piezo2 respectively, after the Greek word for pressure. The human homologs are highly similar at the amino acid level (95% for PIEZO2) (24). Correspondingly, the human versions of these proteins are termed PIEZO1 and PIEZO2. In the decade since their discovery, PIEZOs have been shown to be evolutionarily conserved force-gated ion channels present in plants, protists and worms (30), insects (32), and multiple vertebrate species including fish (33), birds (34), rodents (35), and humans (24, 36). In vertebrates, PIEZO2 has a specialized sensory role. Knockout studies in mice (35, 37–40) and the study of human individuals with rare loss-of-function mutations (24, 38) have revealed that PIEZO2 is indeed an essential mechanotransducer for touch, proprioception, and interoception. In the following sections, we summarize what we have learned in the past 10 years about the structure and function of PIEZO2, with an emphasis on its role as a mechanotransducer in vertebrate sensory systems, and we highlight some of the key questions that remain unanswered.
2. MECHANOTRANSDUCTION BY PIEZO2 CHANNELS
2.1. Channel Structure and Mechanogating
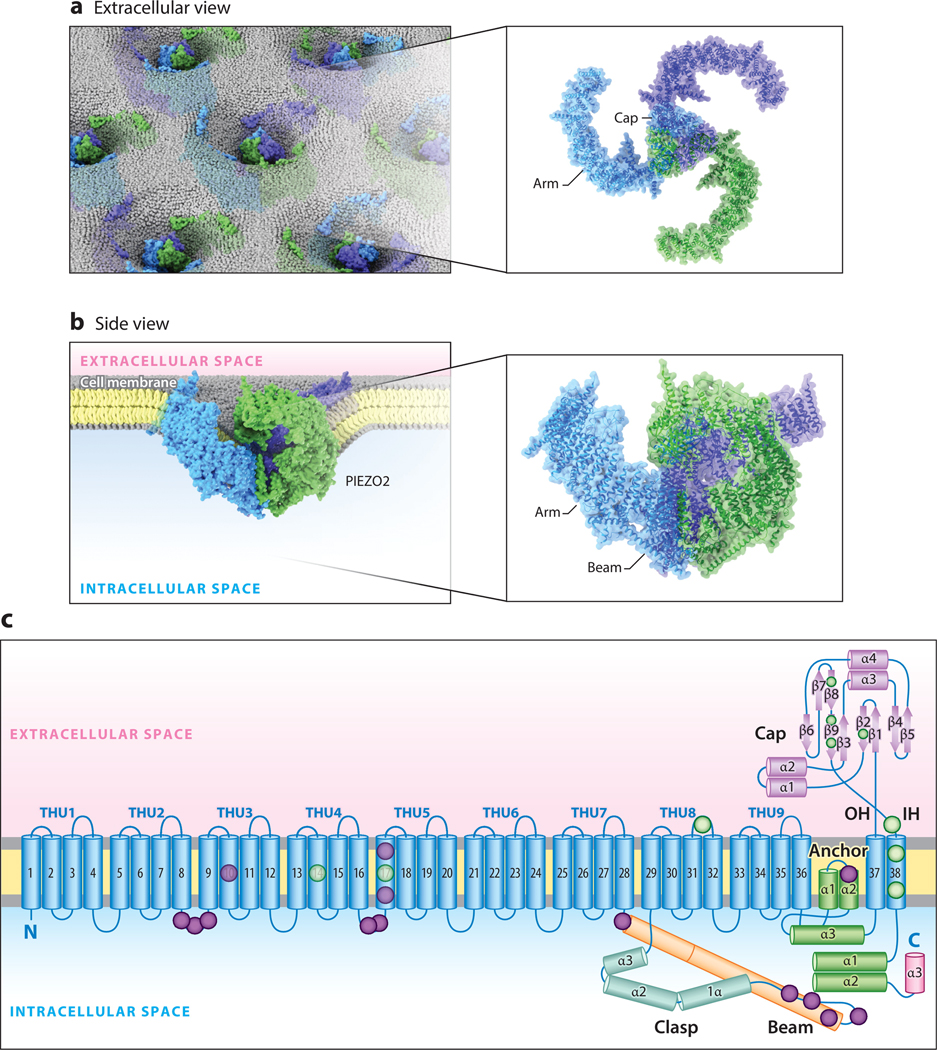
Initially, mutagenesis and epitope mapping (41, 42) were used to investigate the transmembrane topology of PIEZO1 and PIEZO2. From these and other studies, it was clear that the ion permeation pathway is localized to the C-terminal region and that the channels contain a very large intracellular loop of likely functional significance (43). A major advance came with the development of cryo–electron microscopy as a tool to study ion-channel structure (44). First, three research groups were able to obtain high-resolution structures of mouse PIEZO1, showing that the functional ion channel is a trimer assembled from three PIEZO1 monomers and arrayed in an unusual triple-bladed propeller conformation (45–47). Recently, this arrangement was confirmed for mouse PIEZO2, whose structure further revealed that each monomer contains an unprecedented 38 transmembrane segments, the most of any known membrane protein (48). The pore of the channel, which conducts an excitatory nonselective cation current that slightly favors Ca2+ (30), is located at the center of the propeller shape, where the three monomers meet, and spans the plasma membrane (48). Several additional structural elements—such as the beam and clasp—lie below the plane of the membrane in the cytoplasm, and another component—the cap—lies above the pore in the extracellular space (Figure 1a–c). Based on structure-function studies, these modules were shown to critically regulate or directly facilitate channel opening in response to mechanical force (42, 48–50).
Figure 1.
The structure of mouse PIEZO2. (a) A top-down illustration of mouse PIEZO2 channels in the membrane as viewed from outside the cell. (b) A side-view illustration of a PIEZO2 channel curving the plasma membrane. (c) Ribbon diagram of one blade of PIEZO2 highlighting key functional domains. Lavender and green circles indicate approximate reported locations of human loss-of-function and gain-of-function variants, respectively. Structure adapted with permission from Reference 48. Abbreviations: α, α-helix; β, β-pleated sheet; IH, inner helix of the ion-conducting pore; OH, outer helix of the ion-conducting pore; THU, transmembrane helical unit.
A potential clue to the mechanogating mechanism of PIEZO2 comes from an unexpected structural feature: When viewed from the side, the channel’s propeller blades are curved upward and away from the plane of the plasma membrane in a convex arrangement (Figure 1b) (48). A similar conformation is found in PIEZO1 when reconstituted in synthetic liposomes, and this arrangement has been proposed to deform the local membrane into a cup shape while the channel is in the closed state (45–47, 51). If a mechanical stimulus were to laterally stretch the channel, the cup shape could hypothetically be pulled flat; the propeller blades would consequently straighten, providing the energy needed for channel opening (51). It remains unknown whether this mechanogating mechanism occurs in native plasma membranes, as the aforementioned experiments were conducted in cell-free systems (52). Nonetheless, such detailed views of mouse PIEZO2 architecture enable a mechanistic understanding of how RNA alternative splicing could alter channel structure (42, 53), and they explain how clinically relevant mutations in human PIEZO2 might affect channel function (Figure 1c). For a comprehensive review on PIEZO structures, refer to a recent article by Xiao (43).
The full repertoire of mechanical stimuli that PIEZO2 can transduce, as well as the means by which force is coupled to channel gating, remains unclear. One of the most prominent ideas for how mechanogating might occur is the force-from-lipid model (54). If the gating of PIEZO2 conforms to this model, a sudden increase in lipid bilayer tension would be sufficient to open the channel without any auxiliary proteins present. The force-from-lipid hypothesis can be tested by mechanically stretching the membrane of liposomes that contain purified PIEZO2 while recording the channel’s conductance. Although this result has not yet been achieved for PIEZO2, data from other mechanosensitive ion channels—including mouse PIEZO1—indicate they can be gated in response to membrane stretch alone (55, 56). Based on the high degree of structural relatedness between PIEZO1 and PIEZO2 (46–48), it seems likely that PIEZO2 would be similarly receptive to membrane stretch. However, some studies have indicated that PIEZO2 might preferentially respond to indentation of the plasma membrane, while PIEZO1 is sensitive to all forms of mechanical perturbation (57, 58). Furthermore, lipid bilayer stiffness has a lesser effect on the mechanosensitivity of PIEZO2 compared to PIEZO1 (50, 59). Interestingly, chemical destruction of the cytoskeleton impairs PIEZO2 mechanogating more than PIEZO1 (50, 59, 60), and enzymatic digestion of the extracellular matrix also putatively interferes with PIEZO2 transduction (61). These data indicate that PIEZO2 may be influenced by both intra- and extracellular protein tethers as well as lipid bilayer curvature. Future studies are needed to address the specific lipid, water, and protein interactions that underlie PIEZO2 mechanogating. For a more detailed discussion of mechanogating mechanisms, see the review by Grandl and colleagues (62).
2.2. Lipid and Protein Interactome
In addition to the factors discussed in Section 2.1, the fatty acid composition of the lipid bilayer may affect PIEZO2 function directly, through lipid-protein binding, or indirectly, through changes to membrane fluidity and lipid raft organization (63, 64). Recent discoveries indicate that PIEZO2 mechanogating is modulated by several classes of membrane lipids. Margaric acid, a dietary saturated fatty acid, incorporates into cell membranes and increases their structural order and stiffness at micromolar concentrations in vitro (59). Margaric acid treatment inhibits PIEZO2 currents in a concentration-dependent manner, indicating that PIEZO2 mechanosensitivity is influenced by lipid bilayer rigidity (50). PIEZO2 function has also been shown to be potentiated by the presence of cholesterol-rich lipid rafts and the cholesterol-binding protein Stoml3 (stomatin-like 3) (65, 66). In addition, phosphoinositides—specifically phosphatidylinositol 3,5-bisphosphate [PI(3,5)P2], as well as PI(4,5)P2 and perhaps PI(4)P2—can bind directly to PIEZO2 and facilitate mechanotransduction (67, 68). The importance of phosphoinositides was further suggested by studies showing that PIEZO2 function can be modulated by expression of the proteins Mtmr2 and Tentonin3, which are proposed to be involved in phospholipid homeostasis (68, 69). Overall, the mechanisms for lipid-based control of PIEZO2 are not fully understood, and it is still unknown whether many of these lipids act directly or indirectly on the channel. In the future, it will be interesting to examine whether PIEZO2 localizes to specific membrane microdomains, similar to other sensory channels such as Trpa1 (70), where its function may be dynamically modified based on the local lipid environment.
The extracellular matrix and intracellular cytoskeleton have also been implicated in affecting PIEZO2 function. They are essential anchors for tissue and cell structures that could transmit mechanical force to PIEZO2, either by indirect linkage to the plasma membrane or by a direct tether to PIEZO2. A tether mechanism has already been demonstrated for the NOMPC mechanotransduction channel, which mediates touch sensation in Drosophila (71–73). Although some evidence suggests that an extracellular matrix linker can mediate force transfer in mammalian mechanosensory neurons (61, 74), a direct interaction between PIEZO2 and the extracellular matrix has not yet been found. So far, there is also no biochemical proof of a direct linkage between PIEZO2 and the cytoskeleton, despite several lines of functional evidence. Treatment with latrunculin A, an actin-depolymerizing toxin, strongly blunts PIEZO2 mechanosensitivity in vitro (60). By contrast, the mechanogating function of PIEZO1 is much less affected by latrunculin A treatment (59). Interestingly, a chimeric PIEZO2 in which the intracellular beam domain is replaced by the PIEZO1 version of the same structure loses much of its sensitivity to latrunculin A actin disruption (50). This points to a functional link between the PIEZO2 beam domain and the cytoskeleton that may mediate a form of mechanogating distinct from that of PIEZO1.
Immunoaffinity purification followed by mass spectrometry has been used as an exploratory method to identify proteins that potentially interact with PIEZO2 in mouse sensory ganglia. So far, this approach has uncovered pericentrin, SERCA, and Mtmr2, among others (68, 75, 76). Interestingly, expression of each of these proteins is correlated with suppression of PIEZO2 mechanogating. Mtmr2, as mentioned briefly above, functions as a negative regulator of the PIEZO2-potentiating lipid PI(3,5)P2 (68). The biochemical mechanisms by which PIEZO2 is regulated by pericentrin and SERCA, as well as by other proteins identified by mass spectrometry, remain to be determined. Future work is needed to explore whether PIEZO2 can respond to mechanical stimuli when reconstituted in lipid bilayers in the absence of other proteins.
2.3. Intracellular Signaling and Regulation
As a sensory transduction channel, PIEZO2 represents the first step in a bioelectrical cascade that translates mechanical inputs into cellular responses. Mechanical force on the cell membrane permits the passage of cations through the PIEZO2 channel (30). For neurons, this influx of cations to the cytosol is excitatory, acting to depolarize the membrane and trigger the firing of action potentials. Several studies have shown that genetic deletion of PIEZO2 in certain skin cells and mechanosensory neurons ablates all mechanically evoked electrical activity in those cells, leading to profound deficits in mechanosensation (39, 77–79). These results, combined with sensory testing of human patients with PIEZO2 loss of function, demonstrate the importance of this receptor for initiating the electrochemical relays underlying our perception of various mechanical stimuli (24, 38). The physiological roles of PIEZO2 in human and animal models are covered in Section 3.
Ca2+ entry into cells is the starting point for many biochemical signals, including regulation of gene expression, cytoskeletal remodeling, and protein trafficking (80). Thus far, the Ca2+ permeability of PIEZO2 has been implicated in the mechanical regulation of axon pathfinding, cell motility, differentiation, and cancer metastasis (74, 81–83). PIEZO2 is also thought to interact with various intracellular Ca2+-response proteins (76), which can drive specific outcomes including actin polymerization or the activation of the NFAT, Yap1, and β-catenin transcription factors (81–83). In tissues that express both PIEZO1 and PIEZO2, it is still unclear how both channels are used and whether there are distinct intracellular pathways linked to each channel (40, 83).
In addition to regulating various signal cascades, PIEZO2 itself is regulated. There are at least two broad intracellular mechanisms that either suppress or potentiate PIEZO2 activity; they involve phosphoinositide or cyclic adenosine monophosphate (cAMP) second messengers, respectively. As discussed in Section 2.2, depletion of plasma membrane phosphoinositides such as PI(3,5)P2, PI(4,5)P2, and PI(5)P2 inhibits PIEZO2 mechanotransduction (67, 68). The major known mechanism involves Ca2+ influx through either the heat-sensing channel Trpv1 or the electrophile-sensing channel Trpa1; this activates phospholipase C, which in turn enzymatically depletes PI(4,5)P2, leading to blockade of PIEZO2 activity (67). In contrast, the cAMP pathway can boost PIEZO2 mechanosensitivity via activation of PKA and PKC—a process that can be initiated by the inflammatory peptide bradykinin, which signals through Gαq-coupled receptors on the surface of sensory neurons (84). Similarly, expression of Epac1 potentiates PIEZO2 sensitivity and is linked to the development of inflammatory pain, perhaps through Gαs-coupled prostaglandin receptors (60, 85). Distinct from these Gαq and Gαs pathways, PIEZO2 is also potentiated by activation of Gβγ-coupled receptors, which can include γ-aminobutyric acid, serotonin, or opioid receptors (86). Many of these signaling molecules, including PKA, PKC, and Epac1, are targets of cAMP signaling in pain-sensing neurons (87), suggesting a concerted inflammatory pathway that impinges on PIEZO2.
Another source of intracellular regulation of PIEZO2 is through alternative RNA splicing. In the somatosensory system of mice, PIEZO2 can exist in as many as 12 mRNA isoforms (42). The resulting structural diversity affects functional properties of the protein. Isoforms differ in deactivation kinetics and their relative permeability to Ca2+, which may be a mechanism for regulating downstream signaling cascades, as described above. The mechanical sensitivity of PIEZO2 splice variants can also be differentially modulated by intracellular Ca2+, indicating a potential for self-regulation during prolonged or repeated activity.
At present, many elements of PIEZO2 biochemistry are still unknown, including the exact inflammatory molecules, intracellular signaling networks, and posttranslational modifications that influence PIEZO2 mechanotransduction. Identifying these factors may have particular clinical relevance for understanding and treating mechanical allodynia and hyperalgesia—conditions thought to be caused in part by injury-induced enhancement of mechanotransduction (37, 38, 88). For a more in-depth discussion, Borbiro and Rohacs (89) have recently reviewed PIEZO2 regulatory pathways.
3. PIEZO2 IN MECHANOSENSATION
3.1. PIEZO2 Clinical Significance
Several years ago, the Chesler and Bönnemann labs identified two unrelated individuals with an unusual constellation of symptoms, including severe motor and skeletal phenotypes such as hip dysplasia, finger contractures, progressive scoliosis, and hypotonia (24). Family reports indicated that both subjects were born by cesarean section due to being in a breech position and required immediate oxygen support and food supplementation as infants. Both individuals were delayed in reaching motor milestones, learning to crawl only by age four and walk (with assistance) by age eight. These subjects have ongoing difficulties with routine activities, such as dressing and eating, that significantly affect their daily lives. In addition, they display abnormal interoceptive phenotypes, including lack of breath support (i.e., shallow breathing), urinary urgency, and nocturnal enuresis.
Exome sequencing revealed that each subject carried two inactivating mutations in the PIEZO2 gene in compound heterozygosity. Complete loss of PIEZO2 function was confirmed using heterologous in vitro models (24) and later with patient-derived sensory neurons generated using stem cell technologies (for details, see Section 4) (90). The parents of both individuals were healthy carriers, indicating that PIEZO2 haploinsufficiency is apparently asymptomatic. Medical and quantitative sensory assessment showed that loss of function of PIEZO2 results in a profound lack of the sense of proprioception: Patients exhibited pseudoathetosis (involuntary limb movements) when closing their eyes, a lack of awareness of limb and joint positions, and an absence of tendon reflexes. Both subjects also had severe hyposensitivity to gentle touch, including an inability to perform simple touch discrimination tasks and a complete loss of vibration sensation. These deficits were highly selective, with temperature detection and pain thresholds in these individuals matching those of healthy volunteers. Notably, since the discovery of the first patients with PIEZO2 null mutations, several other groups have identified individuals that lack functional PIEZO2 genes, and subjects across the studies share highly conserved motor and skeletal phenotypes (91–94). In each new case, both PIEZO2 alleles have base changes that cause premature stop mutations, truncating protein translation such that the ion channel permeation pathway found in the protein’s C terminus is missing (Figure 1c). Together, these studies form the foundation for the diagnosis of the rare disease we now call PIEZO2 deficiency syndrome.
In parallel studies, the Patapoutian group described distinct PIEZO2 mutations linked to a condition called distal arthrogryposis type 5 (DA5). Similar to people with PIEZO2 deficiency syndrome, individuals with DA5 also have joint contractures and skeletal abnormalities, including short stature (36). However, detailed sensory evaluations have not yet been carried out for individuals with DA5, and the phenotypes of these patients include ophthalmoplegia (restriction of eye movement) and hypomimia (reduced facial expression) not seen with loss of PIEZO2 function. Notably, in vitro characterization of the biophysical properties of PIEZO2 channels affected by the point mutations seen in DA5 patients showed that these channels are slower to inactivate, likely leading to hyperexcitability or aberrant signaling of PIEZO2-expressing cells. These findings indicate that at least one cause of DA5 may be PIEZO2 gain of function. Interestingly, whole-exome sequencing has found potential gain-of-function mutations in PIEZO2 linked to other types of distal arthrogryposis (type 3 or DA3) (95–97). As of today, dozens of potential disease-causing, gain-of-function mutations in PIEZO2 have been identified (Figure 1c).
One of the most remarkable aspects of the human studies described above is how well they match conclusions drawn from animal studies, particularly those from mouse models. When considered together, a compelling picture has emerged of a conserved and essential role for PIEZO2 as a principal mechanotransduction channel governing several key aspects of mechanosensation including touch, proprioception, and interoception. Next, we examine each sensory modality where PIEZO2 function has been demonstrated to play an important role in mouse models and compare those results to the known clinical data.
3.2. Expression
Although a comprehensive survey has not been completed, it is clear that PIEZO2 is predominantly expressed in sensory ganglia and limited types of epithelial cells. In mice, high levels of PIEZO2 transcripts are found in the dorsal root and trigeminal ganglia (DRG and TG, respectively). These ganglia are comprised of the cell bodies of a heterogeneous group of peripheral sensory neurons that include multiple types of touch neurons, proprioceptors, thermoreceptors, and nociceptors (Figure 2a,b). Each type of sensory neuron has a unique transcriptomic profile, and often the expression of one or a few genes is enough to genetically identify them. This type of genetic classification has been useful in outlining which functional subtypes of sensory neurons express PIEZO2. Multicolor fluorescent in situ RNA hybridization and single-cell RNA sequencing experiments have demonstrated that within the DRG and TG, PIEZO2 is detectable in all known types of low-threshold mechanoreceptors (LTMRs), consistent with a broad role in touch (see Section 3.3) (42, 98–101). These touch neurons can be differentiated by their physiological properties: fast-conducting, thickly myelinated, slowly adapting Aβ fibers (SA-LTMRs); fast-conducting, thickly myelinated, rapidly adapting Aβ fibers (RA-LTMRs); medium-conducting, thinly myelinated Aδ fibers (Aδ-LTMRs); and slow-conducting, unmyelinated C fibers (c-LTMRs) (102). PIEZO2 is also found in select types of nociceptors including fast conducting Aδ nociceptors and slowly conducting nonpeptidergic C nociceptors, indicating a potential role in pain. Notably, however, PIEZO2 expression is not found in thermoreceptors or neurons believed to detect itch, consistent with the highly selective touch deficits seen in individuals with PIEZO2 deficiency syndrome (however, for more details, see Section 3.5). Also, as expected from the clinical phenotypes, PIEZO2 transcripts are particularly abundant in proprioceptors (see Section 3.6).
Figure 2.
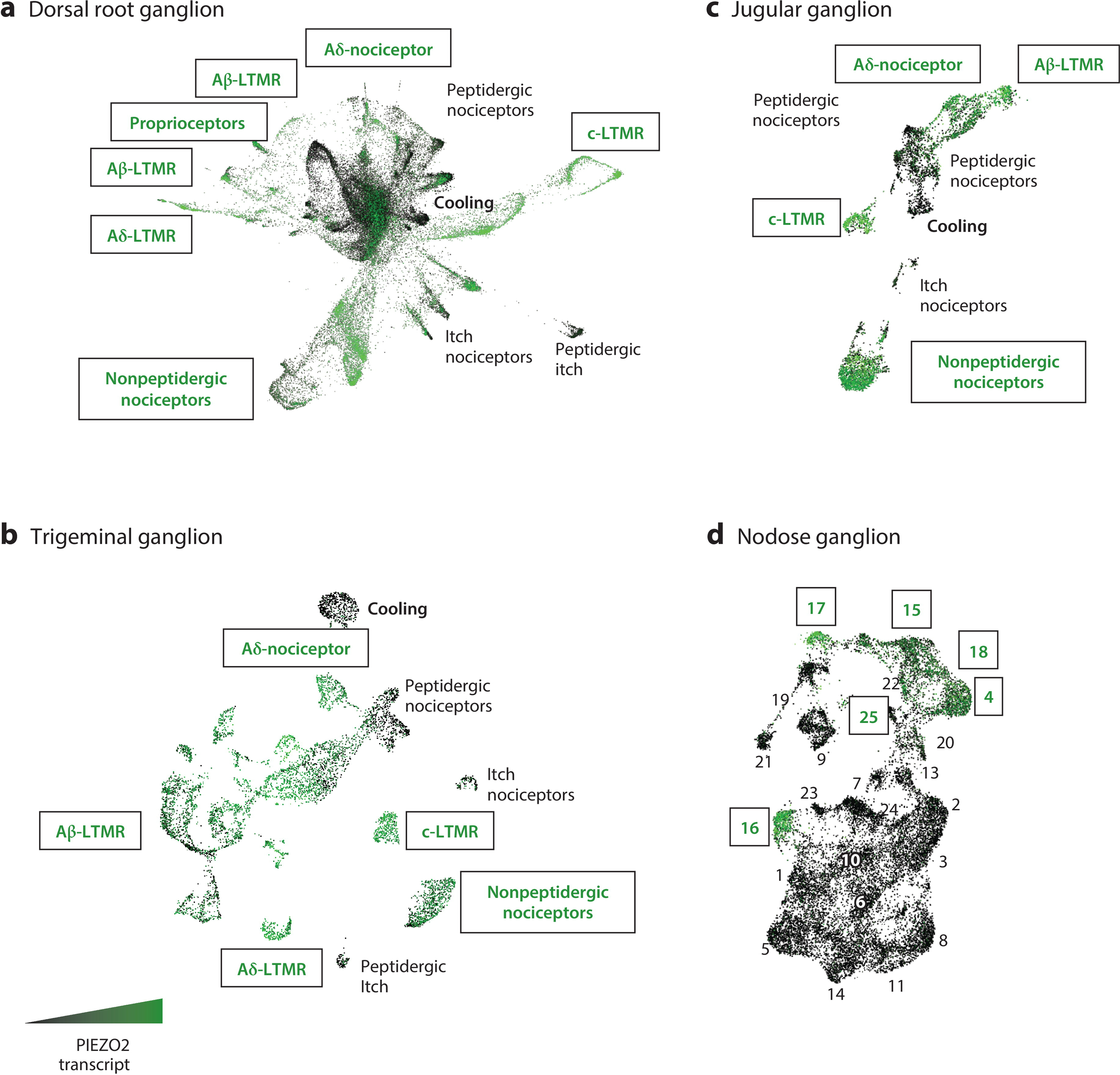
The expression of PIEZO2 in mouse sensory neuron classes. Single-cell RNA sequencing data from four distinct peripheral ganglia are shown: dorsal root, trigeminal, jugular, and nodose. For each ganglion, transcriptomic cell classes are given in the UMAP plots with PIEZO2 expression level visualized and color coded by high expression (green) or no expression (black). (a) Single-cell sequencing from isolated dorsal root ganglia neurons across development, with the earliest embryonic time points at the center of the UMAP and adult neurons at the outer edges (100). (b) Single-nucleus sequencing of trigeminal ganglia. Note that the more prominent representation of Aβ-LTMRs is likely to be the result of the nuclear isolation method (106), which better captures large-diameter neuron subtypes that are normally lost during single-cell isolation. (c) Single-cell sequencing of the vagal complex shows that cell classes in the jugular ganglia are transcriptomically analogous to those found in the dorsal root and trigeminal ganglia. (d) The nodose ganglion, on the other hand, is comprised of transcriptomically and functionally unique sensory neurons (105). The labels for classes of neurons with high PIEZO2 expression are boxed, while those with low or no expression are in italics. Panel a adapted with permission from Reference 100, panels b and d adapted with permission from Reference 105, and panel c adapted from Reference 106. Abbreviations: LTMR, low-threshold mechanoreceptor; UMAP, uniform manifold approximation and projection.
It is becoming increasing clear that PIEZO2 is very important for interoception as well as somatosensation (103–105). Single-cell sequencing of the mouse vagal complex revealed selective expression of PIEZO2 in several classes of jugular and nodose ganglia neurons ( JG and NG, respectively) (105). The JG contains the cell bodies of neurons that innervate somatic and visceral structures in the cranial and cervical regions. Transcriptomically, JG neurons closely resemble those found in other neural crest–derived ganglia (the DRG and TG) and likely perform analogous functions (Figure 2c). On the other hand, placode-derived NG neurons are quite distinct in terms of transcriptomic profile (Figure 2d) and functional roles. Deciphering the distinct functions of the different types of NG neurons remains an ongoing effort, but interestingly, PIEZO2 is very selectively expressed in approximately half a dozen of these cell classes. Since many NG neurons innervate organs that contain tissues in highly dynamic mechanical environments (e.g., lungs, stomach, intestines, and bladder), it is not surprising that mechanotransduction through PIEZO2 is important for aspects of breathing (see Section 3.7), bladder control (see Section 3.9), blood pressure regulation (see Section 3.10), and likely many other interoceptive processes.
3.3. Touch
Complete loss of function of PIEZO2 in mice results in mortality within hours after birth due to a breathing deficit (107, 108). Therefore, strategies to examine PIEZO2 function have relied on conditional gene knockout (PIEZO2fl/−), whereby cell-type-specific promoters driving Cre recombinase allow for the elimination of PIEZO2 in subsets of cells. Early studies used the Advillin gene (Adv) to drive a drug-inducible version of Cre (CreERT2) that allowed for postnatal PIEZO2 knockout in peripheral neurons (109). AdvCreERT2;PIEZO2fl/− mice treated with tamoxifen as adults were found to have reduced brush sensitivity, elevated withdrawal thresholds to punctate touch (von Frey assay), and several touch-related behavioral defects (i.e., failed to remove small pieces of adhesive tape from their backs or avoid mildly aversive vibrating floors) (35). While these phenotypes were consistent with PIEZO2 having a broad role in gentle touch sensation, the animals still responded to many types of mechanical stimuli, albeit with reduced sensitivity. Moreover, electrical recordings from an ex vivo preparation revealed a reduction in evoked action potentials to mechanical stimuli in only a portion of neurons. Notably, the touch deficits of AdvCreERT2;PIEZO2fl/− mice were less severe than those that were subsequently reported for human subjects with PIEZO2 loss of function. PIEZO2 deficiency syndrome patients required orders of magnitude more force compared to healthy volunteers before detecting a von Frey filament and were unable to feel brushing on the palm of the hand or detect a vibrating probe (24, 38).
Recently developed approaches to knock out murine PIEZO2 expression cause more severe mechanosensory phenotypes in mice that better match those reported for humans, suggesting that the prior conditional strategy was incompletely effective. Crossing PIEZO2fl/− mice with HoxB8Cre animals results in loss of expression of PIEZO2 in the majority of sensory neurons from the cervical region of the spinal cord and more caudal regions (37). These mice survive to adulthood, presumably because neurons including those in the jugular complex and rostral DRG do not lose PIEZO2 expression (see Section 3.7), allowing for behavioral and functional characterization. HoxB8Cre;PIEZO2fl/− mice are completely insensitive to von Frey stimulation of their paws. Additionally, skin-nerve recordings from these mutant mice show that the majority of Aβ and Aδ fibers require PIEZO2 for mechanosensitivity to touch stimuli, whereas C fibers were less affected (37). PIEZO2 can also be knocked out through systemic injection of viral vectors (38). Injection of AAV vectors encoding Cre into newborn PIEZO2fl/fl mice results in recombination in peripheral neurons throughout the body with 50–90% efficiency. As seen in the HoxB8Cre animals, high-efficiency knockout of PIEZO2 using viral vectors (>80% of sensory neurons) results in profound behavioral deficits to punctate and dynamic touch stimuli. Importantly, in vivo Ca2+ imaging revealed that PIEZO2 is absolutely required for responses to gentle brush and vibration across a range of frequencies (38, 79); responses to high-intensity mechanical stimuli such as pinch, however, remain intact. Together these studies show that PIEZO2 is the principal touch receptor for many types of dynamic, punctate, and repetitive mechanical stimuli in the innocuous range (Figure 3).
Figure 3.
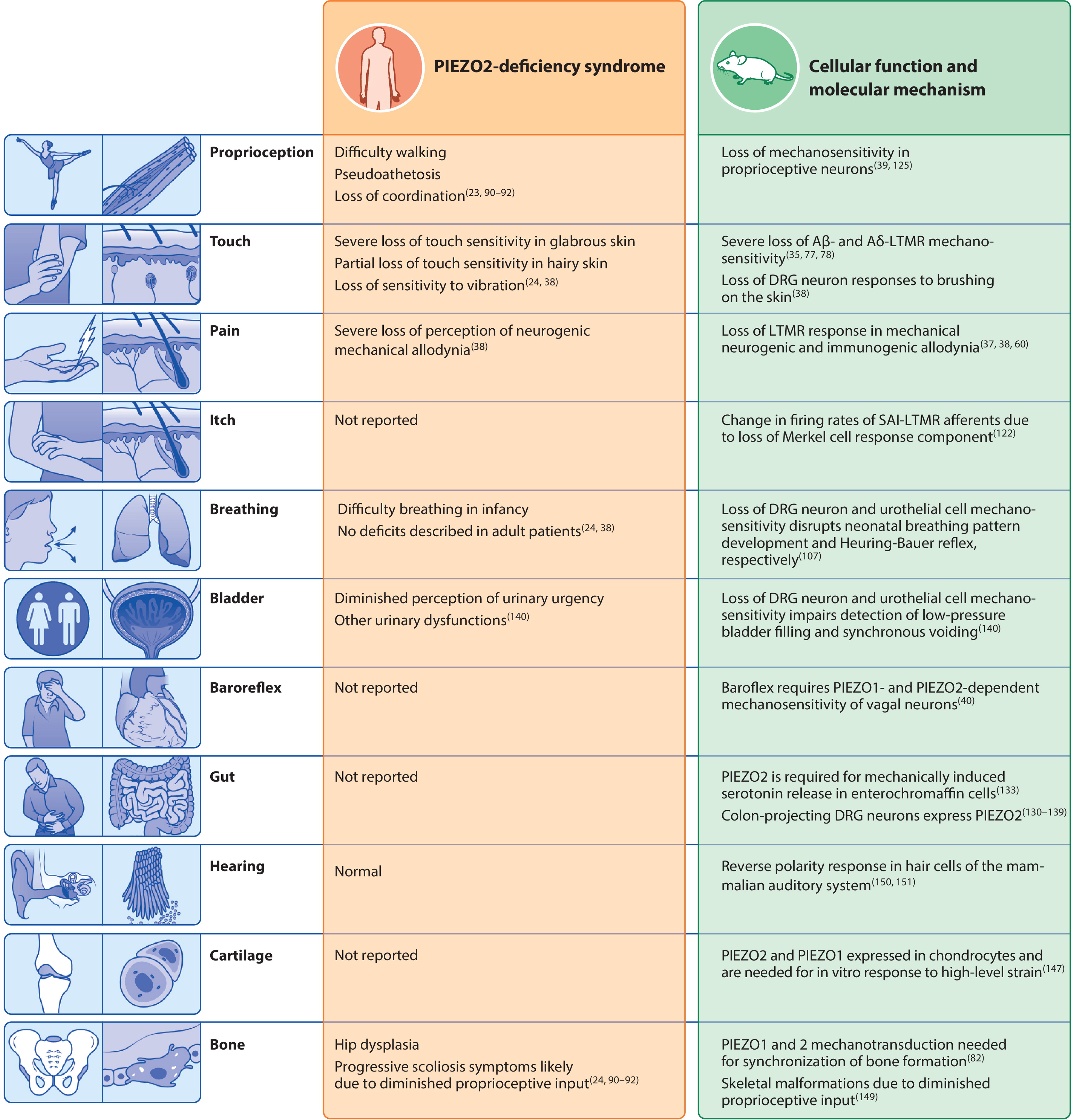
Biological systems in which PIEZO2 transduction is involved. The left column summarizes the clinical phenotypes reported in PIEZO2 deficiency syndrome. The right column describes the physiological roles of PIEZO2 in mouse models that may underlie each phenotype. Abbreviations: DRG, dorsal root ganglion; LTMR, low-threshold mechanoreceptor; SA1-LTMR, slowly adapting type I Aβ-LTMR.
It has become well appreciated that some nonneuronal cells in the skin can express PIEZO2 and play important roles in touch. Particularly important are the interactions between slowly adapting type I Aβ neurons (SA1-LTMRs) and specialized epithelial (Merkel) cells. The function of Merkel cells in touch was debated (110–113), but studies from the Lumpkin group and others have provided clear evidence that they have a primary sensory role by showing that these cells are required for normal touch responses (114, 115). Mouse Merkel cells were demonstrated to be intrinsically mechanosensitive, relying on PIEZO2 for mechanotransduction; additionally, PIEZO2 expression in these cells was shown to be necessary for normal Aβ SA1-LTMR responses (77, 116). A two-part mechanism for how the Merkel cell–neurite complex transduces touch stimuli has been proposed, whereby both the primary sensory neuron and Merkel cells use PIEZO2 for mechanotransduction and contribute to the responses generated by different phases of sustained touch (117).
While it is clear that PIEZO2 is broadly required for touch transduction, many questions remain to be answered. Sensory neurons are functionally and anatomically heterogeneous, and the exact contribution of PIEZO2 to each class has yet to be determined. At the subcellular level, knowing where PIEZO2 molecules localize within afferent terminals in skin is important for determining how force is transmitted through tissue and gates these molecules with such exquisite sensitivity.
3.4. Mechanical Pain
In addition to being expressed in LTMRs, PIEZO2 is also present in several classes of pain-sensing somatosensory neurons defined as nociceptors (Figure 2a,b), raising the possibility that this receptor plays a role in the sensation of mechanical pain. Optogenetic activation of all PIEZO2-expressing neurons in mice evokes nocifensive behaviors such as flinching, guarding, and licking (37). HoxB8Cre;PIEZO2fl/− mice also need over four times the mechanical force as wild type controls to induce paw withdrawal and fail to properly respond to punctate touch stimulation using forces in the noxious range (37). However, cells lacking PIEZO2 still exhibit robust Ca2+ responses in vivo to pinching during functional imaging studies (38). Individuals with PIEZO2 deficiency syndrome also display normal perceptual thresholds to acute mechanical nociceptive stimuli (38). Therefore, from both studies, it appears PIEZO2 may be important for some aspects of acute mechanical pain detection, but it is not the primary mechanotransducer for this modality. As-yet-undiscovered and recently identified ion channels such as Tmem63 (118) or TACAN (119) are potential candidate high-threshold mechanotransducers. Furthermore, nonneuronal cells, notably terminal Schwann cells (120), have been implicated as having an important role in the transduction of noxious stimuli.
Following tissue injury and inflammation, normally innocuous mechanical stimuli, such as gentle touch, become painful. This sensory transformation, called mechanical allodynia, is a leading symptom in clinical pain and is often therapeutically intractable. Notably, mice lacking PIEZO2 and human individuals with PIEZO2 deficiency syndrome do not become oversensitive to touch stimuli, showing that PIEZO2 is essential for mechanical allodynia. Specifically, in vivo Ca2+ imaging showed that neural responses to gentle touch and vibration required PIEZO2 expression, even during conditions known to produce profound touch hypersensitivity (38). Furthermore, behavioral responses to gentle mechanical stimuli are not sensitized by tissue inflammation or nerve injury in HoxB8Cre;PIEZO2fl/− mice (37). Most importantly, tactile allodynia was evaluated in human subjects experimentally using a capsaicin neurogenic inflammation model. Healthy volunteers invariably found the area surrounding inflammation to be more painful to touch than reference sites, whereas individuals with PIEZO2 deficiency syndrome failed to report altered touch-evoked sensations, painful or otherwise (38). Together, these studies provided compelling evidence that mechanical allodynia is mediated by mechanotransduction through PIEZO2 (Figure 3).
The detailed mechanisms through which PIEZO2 contributes to mechanical allodynia remain uncertain. In the periphery, PIEZO2 channels have been shown to be potentiated downstream of proinflammatory compounds like bradykinin (84) and second messenger proteins such as Epac1 (60). These biophysical changes can sensitize sensory neurons, making them easier to activate or making their responses to mechanical stimuli last longer. In the central nervous system, tissue damage and inflammation cause alterations in information processing in the dorsal spinal cord. Under normal conditions, touch and pain sensations result from activation of distinct ascending pathways. The prevalent gate-control model states that mechanical allodynia arises when neurons carrying information about gentle mechanical stimulation of skin gain access to the ascending nociceptive pathways. In this scenario, the absence of PIEZO2 blocks LTMR touch responses; consequently, mechanical allodynia simply cannot occur. Recently, a specific Aδ-LTMR subtype that expresses high levels of PIEZO2 has been proposed to be particularly important for driving mechanical allodynia (121). Together these findings indicate that PIEZO2 may prove an advantageous pharmacological target with widespread clinical relevance, as its loss of function does not impair biologically useful acute pain sensing but instead abolishes hypersensitivity and aberrant pain perception.
3.5. Itch
Itch is yet another complex human experience where advances have been made in recent years by correlating human clinical experiences with experiments in animal models (122). A recent rodent study has linked PIEZO2 to a pathological form of mechanical itch known as alloknesis, or excessive itching evoked by light touch, through an indirect pathway (123). The authors found that in aged mice, where the activity of Aβ SA1-LTMRs decreases because of Merkel-cell loss, alloknesis is much more prominent. Further experiments revealed that loss of Merkel cell activity is necessary and sufficient for alloknesis. Interestingly, the same result can be achieved by knocking out PIEZO2 expression only in Merkel cells. Together, these findings indicate that PIEZO2 in the Merkel cell afferent pathway may not only act as a touch detector but also help maintain the tone of Aβ SA1-LTMRs and suppress itch circuitry in the spinal cord (Figure 3) (124).
To date, no differences in itch sensitivity have been reported in PIEZO2 deficiency syndrome. This may be a result of the relatively young patient demographic, as alloknesis is a typically late-onset condition. Alternatively, the global absence of PIEZO2 in both sensory neurons and Merkel cells in these individuals may result in much more dramatic silencing of Aβ SA1-LTMRs such that itch is less likely to occur. Further clinical assays focused on addressing the role of PIEZO2 in itch and potential translational implications of the findings in mice are important.
3.6. Proprioception
Based on clinical data, perhaps one of the most profound phenotypes seen in the absence of PIEZO2 function is the complete lack of proprioception. This underappreciated and poorly understood sense provides the brain with feedback about body position and posture (125) through proprioceptive neurons that innervate muscle spindles (which signal change in the length of muscles) and Golgi tendon organs (GTOs; which react to muscle tone). Individuals with PIEZO2 deficiency syndrome are uncoordinated, unable to sense joint positions, lack tendon reflexes, display pseudoathetosis, and have difficulty performing even simple motor tasks when deprived of visual inputs (24). Experiments in mice demonstrate that these phenotypes almost certainly arise from a failure of proprioceptive neurons to be activated by changing mechanical forces. Conditional deletion of PIEZO2 in mouse proprioceptors (39) or across many classes of sensory neurons (37, 38) results in mice with severe ataxia, irregular gait, and atypical posture. Anatomical examination of muscle spindles and GTOs confirmed that loss of PIEZO2 does not cause morphological changes to these end structures. Physiologically, however, PIEZO2 expression was required for stretch-evoked nerve responses from muscles ex vivo as well as mechanical responses from isolated proprioceptive neurons in culture (39). Interestingly, PIEZO2 is also functionally expressed in a small group of sensory neurons, located within the mesencephalic trigeminal nucleus in the brainstem, that are thought to perform proprioceptive functions for the head and require this receptor for mechanosensitivity (126). Collectively, these experiments demonstrate an essential function of PIEZO2 for proprioception in both mice and humans (Figure 3).
Although the identification of PIEZO2 as the primary transducer of proprioception provided a molecular understanding of the sense, many questions about PIEZO2’s functional and temporal roles remain unanswered. For example, all mouse proprioceptive fibers fire slowly adapting action potentials that persist over the course of seconds; however, the vast majority of PIEZO2 responses recorded in proprioceptive neurons adapt rapidly on the order of milliseconds (39). How PIEZO2 mediates sustained firing in these cells remains unknown. An even more intriguing question stems from clinical observations showing that, despite significant impairments, individuals with PIEZO2 deficiency syndrome develop a number of compensatory strategies to perform motor tasks. The central mechanisms underlying these adaptations may reveal new features governing plasticity and flexibility of the neural circuits controlling proprioception.
3.7. Breathing
Breathing is an essential physiological process that requires interoceptive sensing of pressure in the upper and lower respiratory tract. When PIEZO2 is globally ablated in mice, newborn pups suffer respiratory distress and die within a day of birth (108). Similarly, human infants born with PIEZO2 deficiency syndrome require emergency oxygen support at birth and continue to exhibit diminished breath support with shallow breathing throughout life (24). Together, these results indicate that PIEZO2 is required for newborn mammals to make the transition from umbilical oxygen supply to air breathing.
The lungs are exposed to continuous mechanical stresses and need to sequentially compress and decompress; not surprisingly, many of the sensory neurons innervating them are mechanosensitive (127, 128). PIEZO2 is prevalently expressed in several classes of lung-innervating NG, TG, and DRG neurons (Figure 2a–d) (105, 129, 130). Lung neuroepithelial cells also express PIEZO2 (42, 108). PIEZO2 knockout from all of the JG, TG, and DRG results in similar lethality from respiratory distress as seen in germline PIEZO2 knockouts. By contrast, mice lacking PIEZO2 expression only in lung epithelial cells have normal breathing, and mice with selective loss of PIEZO2 in NG neurons survive but inhale more air per breath and exhibit a diminished Hering–Breuer reflex. The phenotype of mice subjected to PIEZO2 knockout induced during adulthood is also less severe, involving lower vagal nerve firing and increased respiration activity during lung inflation. Together, these results indicate that mechanosensory neurons in the vagal complex and DRG use PIEZO2 to sense and control respiration throughout development and into adulthood (Figure 3) (108).
3.8. Gastrointestinal Tract
Another organ system in which mechanosensation is critically important is the gut. Many of the tasks for which the gastrointestinal (GI) tract is responsible require mechanosensory information, including peristalsis, digestion, hunger, and satiety signaling. Innervation of the GI tract is correspondingly complex, and PIEZO2 is expressed in endothelial cells of the gut as well as various myenteric and sensory neurons (131, 132). Therefore, recent studies have sought to define the role of PIEZO2 in the gut, since discoveries in this area may have relevance to several GI pathologies.
Enteroendocrine cells (ECs) within the gut can autonomously regulate aspects of GI function via sensory-stimulated hormone and neurotransmitter release (133). Recently, PIEZO2 was shown to be expressed in ECs; these cells respond to mechanical stimulation in vitro and signal slow intestinal stretch in vivo (109). In a subset of ECs known as enterochromaffin cells, PIEZO2 activity is partly responsible for neurotransmitter release (134), a process thought to contribute to a host of symptoms in inflammatory bowel diseases when disrupted (135). A series of parallel and elegant tracing studies showed that another subpopulation of ECs participates in volume sensing in the gut and forms a glutamatergic synapse with sensory neurons (136, 137). Through this mechanism, ECs can send fast signals to vagal afferents about intestinal content (138). While PIEZO2 expression was not evaluated in these tracing studies, it is tempting to speculate that mechanical stimuli may also trigger these important physiological responses in a PIEZO2-dependent mechanism.
Compared to that in ECs, the role of PIEZO2 in sensory neurons targeting the gut is less well studied. In mice, transcriptomic profiling of colon-projecting DRG neurons has revealed that PIEZO2 is widely expressed among the diverse sensory neuron populations targeting the lower intestine, but the specific functions of each remain to be tested (132). Additionally, the function of PIEZO2 in the NG neurons that innervate the upper intestine is currently unknown. Recent evidence suggests that one subset of these neurons is likely to be a class of silent nociceptors previously shown to prevalently innervate internal organs (139). These nociceptors express PIEZO2 but have very limited mechanical sensitivity, except after exposure to proinflammatory molecules (140). Future studies should clarify whether this mechanism is clinically relevant in humans (Figure 3).
3.9. Bladder
Early transcriptomic data in mice pointed to the bladder as an organ outside of the peripheral nervous system that contains PIEZO2-expressing cells (30, 38), but it was completely unknown how PIEZO2 influences bladder physiology. Recently, PIEZO2 was demonstrated to be required for normal urinary system function (141). When patients with PIEZO2 deficiency syndrome are questioned about urinary routines and deficiencies, multiple manifestations of bladder dysfunction are evident (24, 141). Their symptoms include decreased voiding frequency, difficulty urinating, and urinal urgency.
Genetic mouse models reveal that PIEZO2 is the principal mechanotransduction channel for low-pressure mechanical activation of bladder-innervating neurons, but high-pressure sensing by a subset of DRG neurons is PIEZO2 independent (141). Careful investigation demonstrated that PIEZO2 is required for synchronization and triggering of micturition reflexes, and PIEZO2 knockout animals show signs of bladder pathology (bladder wall hypertrophy). Interestingly, it appears that PIEZO2 expression in both urothelial cells and bladder afferent neurons is required for proper micturition (141). These findings open a new field of study investigating the role of PIEZOs in bladder function (PIEZO1 is also expressed in bladder tissue). Future work is needed for the molecular identification of specific mechanosensitive neurons and epithelial cells in the bladder, with a goal of elucidating their exact roles in precisely tuning pressure detection in the urinary system.
3.10. Baroreflex
The baroreflex plays a vital role in maintaining homeostasis of blood pressure. For instance, when blood pressure increases, the baroreflex causes heart rate to drop and return pressure to baseline levels. Specific sensory neurons (baroreceptors) innervate the carotid sinus and aortic arch, which are stretch-susceptible tissues that may facilitate this pressure sensing (142). In 2018, Zeng et al. (40) provided evidence that baroreceptors rely on both PIEZO1 and PIEZO2 for mechanotransduction (143). The double conditional knockout of PIEZO1 and PIEZO2 from the vagal sensory neurons abolishes the baroreflex, with the resulting mutant mice exhibiting unstable blood pressure. Additionally, when PIEZO2-expressing carotid sinus and superior laryngeal nerves were optogenetically stimulated, the heart rate and blood pressure of mice fluctuated by over 50%. While some vagal neurons express both PIEZO1 and PIEZO2, most neurons express only one of these channels, suggesting diversity within the baroreceptor population (40).
Recently, Min et al. (104) provided further structural insight into the organization of the baroreceptor by showing that PIEZO2-expressing neurons form a distinct macroscopic claw-like terminal in the walls of the aorta. When these neurons were ablated from the vagus and glossopharyngeal nerves, heart rate was severely affected. These results further underscore the fact that PIEZO2 is involved with interoceptive processes as well as somatosensation (Figure 3).
3.11. Additional Biological Roles
In addition to the mechanosensory functions described in Sections 3.1–3.10, PIEZO2 may have clinically relevant roles in additional tissues. For example, PIEZO2 is found in neurons innervating the dental pulp and tongue of mice (144–146) and may contribute to mouthfeel or toothache. PIEZO2 is also expressed in mouse bone stem cells, where it may be involved in mechanical signaling during bone formation and growth (83), as well as in chondrocytes, a component of cartilage that undergoes constant mechanical stress (147–149). The presence of PIEZO2 in cells related to bone development may explain the abnormal skeletal phenotypes found in individuals with PIEZO2 deficiency syndrome, although recent evidence supports the hypothesis that many of these deficits arise from lack of proprioception (150). PIEZO2 is also functionally expressed in hair cells of the mouse auditory system. While the bulk of mechanotransduction in the ear is carried out by other channels, the reverse-polarity current resulting from nonphysiological bending of stereocilia is dependent on PIEZO2 (151, 152). It is apparent from these studies that our knowledge of PIEZO2 in various aspects of mechanosensation is still far from complete.
4. NEW MODEL SYSTEMS AND PHARMACOLOGICAL INTERVENTIONS
4.1. Human Stem Cell–Based Models
PIEZO2 has been difficult to study in humans due to the limited accessibility of the tissues in which it resides and the rarity of PIEZO2 deficiency syndrome. To circumvent this problem, induced pluripotent stem cells (iPSCs) can be developed from patients and healthy subjects; iPSCs can theoretically produce any human cell type in vitro, providing an exciting new platform to examine PIEZO2 function in human cells. In this section, we focus on the use of iPSC technology to study human peripheral sensory neurons, since these are cells in which PIEZO2 exerts many of its physiological effects.
When exposed to a specific and empirically determined cocktail of five small molecules, iPSCs can be directed to differentiate into peripheral sensory neurons over the course of a few weeks (153). Neurons produced via this method express PIEZO2 (154); however, several research groups have reported different results with the protocol, ranging from the exclusive generation of nociceptors to a mixture of sensory neuron subtypes with uncertain identities (153, 155–158). To study PIEZO2 in individual cellular subtypes, iPSCs can be more selectively differentiated into just a single category of sensory neuron by forcing overexpression of transcription factors that specify distinct sensory neuron subtypes. A brief pulse of NGN2 expression in iPSC-derived neural crest cells produces large-diameter, PIEZO2-positive touch neurons (78). The yield of this neuronal subtype can be greatly enhanced by additionally expressing the prosensory neuron factor BRN3A (90). Long-term coexpression of NGN2 and BRN3A converts iPSCs into a subtype of cold-sensing mechanoreceptor that also relies on PIEZO2 for mechanosensitivity (90). Overexpression of NGN1 instead generates various populations of heat-sensing nociceptors (159).
Moving forward, the creation of a single-cell RNA sequencing atlas of human sensory ganglia should be an urgent goal (160). Such a resource would reveal key differences between humans and commonly used animal models and also identify which human sensory neuron subtypes express PIEZO2 and other markers. The sequencing atlas could then be used as a template for determining how closely iPSC-derived sensory neurons generated through different protocols mimic in vivo sensory neurons—this is a crucial step toward developing naturalistic in vitro models to examine PIEZO2 structure, biochemistry, physiology, and pharmacology in specific human cell types.
4.2. PIEZO2 Drug Discovery
Exploring the pharmacology of PIEZO2 represents a new and exciting avenue of research, since the ability to chemically control PIEZO2 could have a profound impact on basic research and treatment for diseases of mechanosensation. There are currently no known drugs or endogenous ligands that specifically bind PIEZO2. However, high-throughput screens on PIEZO1 have already identified several small-molecule agonists, indicating that this family of ion channels can be chemically modulated (161, 162). In this section, we discuss potential strategies for PIEZO2 drug discovery in light of prior studies on PIEZO1 and other mechanosensitive proteins.
PIEZO2 agonists would undoubtedly serve as useful research tools. Currently, to study PIEZO2 transduction, a physical stimulus must be used to deflect the cell body or neurite membrane (58, 66), and this greatly constrains the types of experiments that can be used to investigate PIEZO2-related physiology. Chemical activation of PIEZO2 presents a more selective and less invasive way to trigger channel gating, which would be amenable to bulk or high-throughput in vitro transduction assays, as well as in vivo applications. PIEZO2 agonists could also have clinical utility in treating mechanosensory disorders. For example, certain forms of chronic itch have been linked to a reduction of PIEZO2-expressing cells in the skin (163). In such conditions, drug-induced activation of PIEZO2 might suppress pathological itch signals.
In the case of PIEZO1, agonists were found by high-throughput chemical screening on HEK293T cells overexpressing mouse PIEZO1 (160, 162). Taking advantage of the Ca2+ current mediated by PIEZO1 activation, intracellular Ca2+ levels were imaged in a fluorescent imaging plate reader (FLIPR). This approach identified the validated PIEZO1 agonists Yoda1, Jedi1, and Jedi2. The same strategy was used in an attempt to identify PIEZO2 agonists, but no agonists were found (160, 162). One possible explanation for this failure is that, because PIEZO2 currents inactivate more rapidly than PIEZO1 (30), the Ca2+ influx and fluorescent signal are below the thresholds required by the FLIPR to detect channel opening.
In contrast to agonists, there are no known antagonists that are specific to either PIEZO1 or PIEZO2. Existing molecules such as GsMTx4 and margaric acid nonspecifically affect mechanotransduction by changing the properties of the plasma membrane (50, 59, 164). A PIEZO2-selective antagonist would be a valuable substitute for using genetic knockout approaches to decipher the causal physiological effects of PIEZO2. Also, given that PIEZO2 loss of function prevents mechanical allodynia, PIEZO2 antagonists have been suggested as a treatment for this painful skin condition (37, 38).
Screening for antagonists of PIEZO2 will likely be even more challenging than the search for agonists. Such a screen would presumably require a mechanical stimulus to activate PIEZO2 in a high-throughput cell culture format, and antagonists would be identified based on their ability to block the mechanically evoked PIEZO2 current (Figure 4). Membrane stretch, pull, indentation, fluid shear stress, and substrate deformation are examples of stimuli that have been classically applied in a low-throughput setting (30, 42, 66, 165, 166). However, some of these techniques are now being scaled up and may be amenable for mechanical screening of PIEZO2 (167–172).
Figure 4.
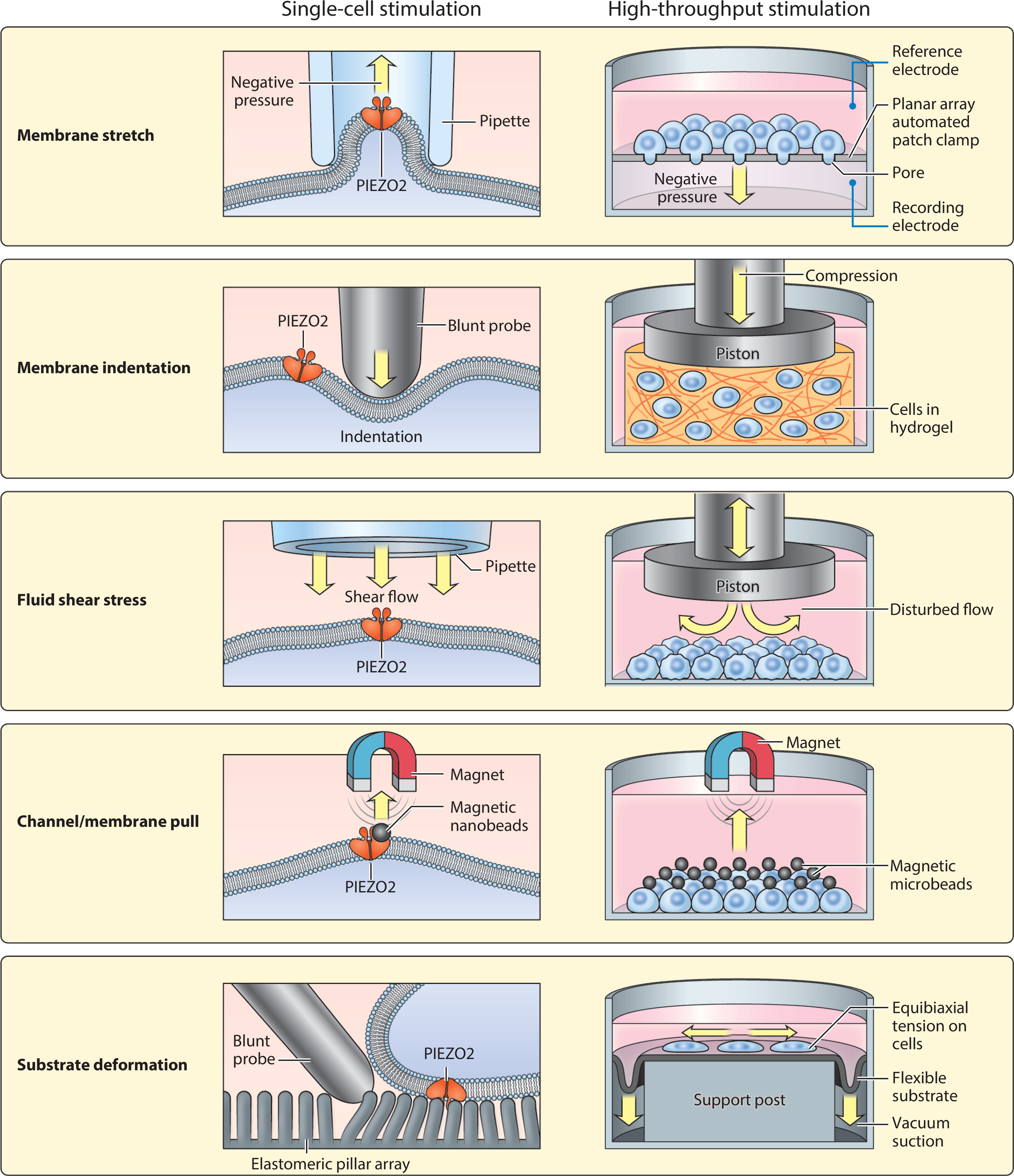
Adapting single-cell mechanical stimulation methods to high-throughput formats. (Left) Existing methods for applying mechanical force to the cell membrane. For reference, a graphic depiction of a single PIEZO2 channel is shown in dark orange spanning the cell membrane. (Right) Potential strategies for adapting each method for use in high-throughput screening platforms. Yellow arrows indicate the direction of mechanical force in each assay.
Given that PIEZO2 has several physiological roles, even if selective agonists and/or antagonists are identified, systemic drug delivery methods may not safely treat a single function in isolation. Incorporation of PIEZO2 drugs into a topical ointment is one strategy for targeting channel function in the skin without affecting internal organs, as has been suggested for treating mechanical allodynia (37, 38). It is important to test various drug vehicles and delivery routes to ensure that essential PIEZO2 functions, such as respiration and proprioception, are not affected.
5. CONCLUDING REMARKS AND FUTURE PERSPECTIVES
The year 2020 marked a decade since the discovery of PIEZO2. Thanks to mechanistic studies in animal models and humans, multiple milestones have been achieved in the last decade: PIEZO2 is now established as the principal mechanotransducer in several organ systems, a structural basis for PIEZO2 mechanosensitivity has been outlined, and clinically relevant roles for PIEZO2 have been identified in humans. Now, the journey to pinpoint the cellular and molecular underpinnings of PIEZO2 mechanotransduction is entering a new phase with different challenges and opportunities ahead.
Pioneering studies have demonstrated that PIEZO2 is evolutionarily repurposed across multiple seemingly unrelated mechanical senses, ranging from touch and proprioception to respiration. One of the major efforts at hand is to elucidate PIEZO2 function in all sensory cell types that express it. Very little is known about the physiological tuning, modulation, and molecular anatomy of PIEZO2 in various cells in the peripheral nervous system. Progress in sophisticated mechanical stimulation assays and loss-of-function behavioral models offers new tools to investigate these topics (166, 173, 174).
Another subject of great interest centers around the molecular basis of PIEZO2-independent mechanotransduction. Several mechanosensory processes, notably mechanical pain, persist in the absence of PIEZO2 (24, 37, 38). Definitively identifying the proteins that mediate these specific modalities remains an area of active research (118, 119). Given the broad expression of PIEZO2 in sensory ganglia (Figure 2), further work is needed to understand the specific roles that different mechanotransducer proteins play with respect to one another.
The discovery of PIEZO2 deficiency syndrome was a defining moment for translational research on mechanosensation. Being able to talk with these patients has added a dimension simply unachievable in animal studies. Most importantly, the mouse and human data complement each other to provide a broad and consistent view of PIEZO2 function. Together, these data have put a spotlight on how targeting this molecule pharmacologically may be useful for treating a range of clinical conditions involving mechanotransduction. In just 10 years since the discovery of PIEZO2, we have gone from not even knowing what the touch receptor was to having an extensive understanding of its form and function as well as its clinical relevance. This review set out to highlight how much we have learned and, in doing so, has hopefully also made clear that there are many exciting things left to learn about this remarkable protein.
ACKNOWLEDGMENTS
We would like to thank Mark Hoon, Claire Le Pichon, Valeria Vasquez, Ardem Patapoutian, Kara Marshall, Jennifer Osborne, and Sarah Shnayder for feedback on the manuscript. We would like to thank David Ginty, Stephen Liberles, Nicholas Ryba and Bailong Xiao for generously providing data and permission to adapt their data into Figures 1 and 2. This research was supported by the National Center for Complementary and Integrative Health Intramural Research Program and the National Center for Advancing Translational Sciences through the National Institutes of Health Helping to End Addiction Long-term℠ (HEAL) Initiative. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or its HEAL Initiative.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Iggo A, Andres KH. 1982. Morphology of cutaneous receptors. Annu. Rev. Neurosci. 5:1–31 [DOI] [PubMed] [Google Scholar]
- 2.Sherrington C 1906. The Integrative Action of the Nervous System. New Haven: Yale Univ. Press [Google Scholar]
- 3.Perl E 1968. Myelinated afferent fibres innervating the primate skin and their response to noxious stimuli. J. Physiol. 197:593–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burgess P, Petit D, Warren RM. 1968. Receptor types in cat hairy skin supplied by myelinated fibers. J. Neurophysiol. 31:833–48 [DOI] [PubMed] [Google Scholar]
- 5.Brown A, Iggo A. 1967. A quantitative study of cutaneous receptors and afferent fibres in the cat and rabbit. J. Physiol. 193:707–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loewenstein WR, Rathkamp R. 1958. Localization of generator structures of electric activity in a Pacinian corpuscle. Science 127:341. [DOI] [PubMed] [Google Scholar]
- 7.Alvarez-Buylla R, De Arellano JR. 1952. Local responses in Pacinian corpuscles. Am. J. Physiol. 172:237–44 [DOI] [PubMed] [Google Scholar]
- 8.Corey D, Hudspeth A. 1979. Ionic basis of the receptor potential in a vertebrate hair cell. Nature 281:675–77 [DOI] [PubMed] [Google Scholar]
- 9.Neher E, Sakmann B. 1976. Single-channel currents recorded from membrane of denervated frog muscle fibres. Nature 260:799–802 [DOI] [PubMed] [Google Scholar]
- 10.Guharay F, Sachs F. 1984. Stretch-activated single ion channel currents in tissue-cultured embryonic chick skeletal muscle. J. Physiol. 352:685–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakmann B, Bormann J, Hamill OP. 1983. Ion transport by single receptor channels. Cold Spring Harb. Symp. Quant. Biol. 48(Part 1):247–57 [DOI] [PubMed] [Google Scholar]
- 12.Chalfie M 2009. Neurosensory mechanotransduction. Nat. Rev. Mol. Cell Biol. 10:44–52 [DOI] [PubMed] [Google Scholar]
- 13.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. 1997. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389:816–24 [DOI] [PubMed] [Google Scholar]
- 14.Julius D 2013. TRP channels and pain. Annu. Rev. Cell Dev. Biol. 29:355–84 [DOI] [PubMed] [Google Scholar]
- 15.Dhaka A, Viswanath V, Patapoutian A. 2006. Trp ion channels and temperature sensation. Annu. Rev. Neurosci. 29:135–61 [DOI] [PubMed] [Google Scholar]
- 16.Martinac B, Buechner M, Delcour AH, Adler J, Kung C. 1987. Pressure-sensitive ion channel in Escherichia coli. PNAS 84:2297–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sukharev SI, Blount P, Martinac B, Blattner FR, Kung C. 1994. A large-conductance mechanosensitive channel in E. coli encoded by mscL alone. Nature 368:265–68 [DOI] [PubMed] [Google Scholar]
- 18.Gustin MC, Zhou X-L, Martinac B, Kung C. 1988. A mechanosensitive ion channel in the yeast plasma membrane. Science 242:762–65 [DOI] [PubMed] [Google Scholar]
- 19.Martinac B, Adler J, Kung C. 1990. Mechanosensitive ion channels of E. coli activated by amphipaths. Nature 348:261–63 [DOI] [PubMed] [Google Scholar]
- 20.Chalfie M, Au M. 1989. Genetic control of differentiation of the Caenorhabditis elegans touch receptor neurons. Science 243:1027–33 [DOI] [PubMed] [Google Scholar]
- 21.O’Hagan R, Chalfie M, Goodman MB. 2005. The MEC-4 DEG/ENaC channel of Caenorhabditis elegans touch receptor neurons transduces mechanical signals. Nat. Neurosci. 8:43–50 [DOI] [PubMed] [Google Scholar]
- 22.Walker RG, Willingham AT, Zuker CS. 2000. A Drosophila mechanosensory transduction channel. Science 287:2229–34 [DOI] [PubMed] [Google Scholar]
- 23.Petit C 2006. From deafness genes to hearing mechanisms: harmony and counterpoint. Trends Mol. Med. 12:57–64 [DOI] [PubMed] [Google Scholar]
- 24.Chesler AT, Szczot M, Bharucha-Goebel D, Čeko M, Donkervoort S, et al. 2016. The role of PIEZO2 in human mechanosensation. N. Engl. J. Med. 375:1355–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lumpkin EA, Bautista DM. 2005. Feeling the pressure in mammalian somatosensation. Curr. Opin. Neurobiol. 15:382–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McBride DW Jr., Hamill OP. 1993. Pressure-clamp technique for measurement of the relaxation kinetics of mechanosensitive channels. Trends Neurosci. 16:341–45 [DOI] [PubMed] [Google Scholar]
- 27.McCarter GC, Reichling DB, Levine JD. 1999. Mechanical transduction by rat dorsal root ganglion neurons in vitro. Neurosci. Lett. 273:179–82 [DOI] [PubMed] [Google Scholar]
- 28.Delmas P, Hao J, Rodat-Despoix L. 2011. Molecular mechanisms of mechanotransduction in mammalian sensory neurons. Nat. Rev. Neurosci. 12:139–53 [DOI] [PubMed] [Google Scholar]
- 29.Drew LJ, Wood JN, Cesare P. 2002. Distinct mechanosensitive properties of capsaicin-sensitive and -insensitive sensory neurons. J. Neurosci. 22:Rc228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, et al. 2010. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 330:55–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McHugh BJ, Buttery R, Lad Y, Banks S, Haslett C, Sethi T. 2010. Integrin activation by Fam38A uses a novel mechanism of R-Ras targeting to the endoplasmic reticulum. J. Cell Sci. 123:51–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim SE, Coste B, Chadha A, Cook B, Patapoutian A. 2012. The role of Drosophila Piezo in mechanical nociception. Nature 483:209–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Faucherre A, Nargeot J, Mangoni ME, Jopling C. 2013. piezo2b regulates vertebrate light touch response. J. Neurosci. 33:17089–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schneider ER, Mastrotto M, Laursen WJ, Schulz VP, Goodman JB, et al. 2014. Neuronal mechanism for acute mechanosensitivity in tactile-foraging waterfowl. PNAS 111:14941–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ranade SS, Woo S-H, Dubin AE, Moshourab RA, Wetzel C, et al. 2014. Piezo2 is the major transducer of mechanical forces for touch sensation in mice. Nature 516:121–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coste B, Houge G, Murray MF, Stitziel N, Bandell M, et al. 2013. Gain-of-function mutations in the mechanically activated ion channel PIEZO2 cause a subtype of Distal Arthrogryposis. PNAS 110:4667–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murthy SE, Loud MC, Daou I, Marshall KL, Schwaller F, et al. 2018. The mechanosensitive ion channel Piezo2 mediates sensitivity to mechanical pain in mice. Sci. Transl. Med. 10: eaat9897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Szczot M, Liljencrantz J, Ghitani N, Barik A, Lam R, et al. 2018. PIEZO2 mediates injury-induced tactile pain in mice and humans. Sci. Transl. Med. 10:eaat9892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woo S-H, Lukacs V, De Nooij JC, Zaytseva D, Criddle CR, et al. 2015. Piezo2 is the principal mechanotransduction channel for proprioception. Nat. Neurosci. 18:1756–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zeng WZ, Marshall KL, Min S, Daou I, Chapleau MW, et al. 2018. PIEZOs mediate neuronal sensing of blood pressure and the baroreceptor reflex. Science 362:464–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coste B, Murthy SE, Mathur J, Schmidt M, Mechioukhi Y, et al. 2015. Piezo1 ion channel pore properties are dictated by C-terminal region. Nat. Commun. 6:7223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Szczot M, Pogorzala LA, Solinski HJ, Young L, Yee P, et al. 2017. Cell-type-specific splicing of Piezo2 regulates mechanotransduction. Cell Rep. 21:2760–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xiao B 2020. Levering mechanically activated Piezo channels for potential pharmacological intervention. Annu. Rev. Pharmacol. Toxicol. 60:195–218 [DOI] [PubMed] [Google Scholar]
- 44.Liao M, Cao E, Julius D, Cheng Y. 2013. Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature 504:107–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guo YR, MacKinnon R. 2017. Structure-based membrane dome mechanism for Piezo mechanosensitivity. eLife 6:e33660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saotome K, Murthy SE, Kefauver JM, Whitwam T, Patapoutian A, Ward AB. 2018. Structure of the mechanically activated ion channel Piezo1. Nature 554:481–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao Q, Zhou H, Chi S, Wang Y, Wang J, et al. 2018. Structure and mechanogating mechanism of the Piezo1 channel. Nature 554:487–92 [DOI] [PubMed] [Google Scholar]
- 48.Wang L, Zhou H, Zhang M, Liu W, Deng T, et al. 2019. Structure and mechanogating of the mammalian tactile channel PIEZO2. Nature 573:225–29 [DOI] [PubMed] [Google Scholar]
- 49.Taberner FJ, Prato V, Schaefer I, Schrenk-Siemens K, Heppenstall PA, Lechner SG. 2019. Structure-guided examination of the mechanogating mechanism of PIEZO2. PNAS 116:14260–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Romero LO, Caires R, Nickolls AR, Chesler AT, Cordero-Morales JF, Vásquez V. 2020. A dietary fatty acid counteracts neuronal mechanical sensitization. Nat. Commun. 11:2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lin Y-C, Guo YR, Miyagi A, Levring J, MacKinnon R, Scheuring S. 2019. Force-induced conformational changes in PIEZO1. Nature 573:230–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chesler AT, Szczot M. 2018. Piezo ion channels: portraits of a pressure sensor. eLife 7:e34396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Geng J, Liu W, Zhou H, Zhang T, Wang L, et al. 2020. A plug-and-latch mechanism for gating the mechanosensitive Piezo channel. Neuron 106:438–51.e6 [DOI] [PubMed] [Google Scholar]
- 54.Kung C 2005. A possible unifying principle for mechanosensation. Nature 436:647–54 [DOI] [PubMed] [Google Scholar]
- 55.Cox CD, Bae C, Ziegler L, Hartley S, Nikolova-Krstevski V, et al. 2016. Removal of the mechanoprotective influence of the cytoskeleton reveals PIEZO1 is gated by bilayer tension. Nat. Commun. 7:10366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Syeda R, Florendo MN, Cox CD, Kefauver JM, Santos JS, et al. 2016. Piezo1 channels are inherently mechanosensitive. Cell Rep. 17:1739–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moroni M, Servin-Vences MR, Fleischer R, Sánchez-Carranza O, Lewin GR. 2018. Voltage gating of mechanosensitive PIEZO channels. Nat. Commun. 9:1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shin KC, Park HJ, Kim JG, Lee IH, Cho H, et al. 2019. The Piezo2 ion channel is mechanically activated by low-threshold positive pressure. Sci. Rep. 9:6446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Romero LO, Massey AE, Mata-Daboin AD, Sierra-Valdez FJ, Chauhan SC, et al. 2019. Dietary fatty acids fine-tune Piezo1 mechanical response. Nat. Commun. 10:1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eijkelkamp N, Linley J, Torres J, Bee L, Dickenson A, et al. 2013. A role for Piezo2 in EPAC1-dependent mechanical allodynia. Nat. Commun. 4:1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hu J, Chiang LY, Koch M, Lewin GR. 2010. Evidence for a protein tether involved in somatic touch. EMBO J. 29:855–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu J, Lewis AH, Grandl J. 2017. Touch, tension, and transduction—the function and regulation of Piezo ion channels. Trends Biochem. Sci. 42:57–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cordero-Morales JF, Vásquez V. 2018. How lipids contribute to ion channel function, a fat perspective on direct and indirect interactions. Curr. Opin. Struct. Biol. 51:92–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weinrich M, Worcester DL, Bezrukov SM. 2017. Lipid nanodomains change ion channel function. Nanoscale 9:13291–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Qi Y, Andolfi L, Frattini F, Mayer F, Lazzarino M, Hu J. 2015. Membrane stiffening by STOML3 facilitates mechanosensation in sensory neurons. Nat. Commun. 6:8512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Poole K, Herget R, Lapatsina L, Ngo HD, Lewin GR. 2014. Tuning Piezo ion channels to detect molecular-scale movements relevant for fine touch. Nat. Commun. 5:3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Borbiro I, Badheka D, Rohacs T. 2015. Activation of TRPV1 channels inhibits mechanosensitive Piezo channel activity by depleting membrane phosphoinositides. Sci. Signal. 8:ra15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Narayanan P, Hütte M, Kudryasheva G, Taberner FJ, Lechner SG, et al. 2018. Myotubularin related protein-2 and its phospholipid substrate PIP2 control Piezo2-mediated mechanotransduction in peripheral sensory neurons. eLife 7:e32346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Anderson EO, Schneider ER, Matson JD, Gracheva EO, Bagriantsev SN. 2018. TMEM150C/Tentonin3 is a regulator of mechano-gated ion channels. Cell Rep. 23:701–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Startek JB, Boonen B, López-Requena A, Talavera A, Alpizar YA, et al. 2019. Mouse TRPA1 function and membrane localization are modulated by direct interactions with cholesterol. eLife 8:e46084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang W, Cheng LE, Kittelmann M, Li J, Petkovic M, et al. 2015. Ankyrin repeats convey force to gate the NOMPC mechanotransduction channel. Cell 162:1391–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yan Z, Zhang W, He Y, Gorczyca D, Xiang Y, et al. 2013. Drosophila NOMPC is a mechanotransduction channel subunit for gentle-touch sensation. Nature 493:221–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Walker RG, Willingham AT, Zuker CS. 2000. A Drosophila mechanosensory transduction channel. Science 287:2229–34 [DOI] [PubMed] [Google Scholar]
- 74.Chiang L-Y, Poole K, Oliveira BE, Duarte N, Sierra YAB, et al. 2011. Laminin-332 coordinates mechanotransduction and growth cone bifurcation in sensory neurons. Nat. Neurosci. 14:993–1000 [DOI] [PubMed] [Google Scholar]
- 75.Zhang T, Chi S, Jiang F, Zhao Q, Xiao B. 2017. A protein interaction mechanism for suppressing the mechanosensitive Piezo channels. Nat. Commun. 8:1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Narayanan P, Sondermann J, Rouwette T, Karaca S, Urlaub H, et al. 2016. Native Piezo2 interactomics identifies pericentrin as a novel regulator of Piezo2 in somatosensory neurons. J. Proteome Res. 15:2676–87 [DOI] [PubMed] [Google Scholar]
- 77.Woo S-H, Ranade S, Weyer AD, Dubin AE, Baba Y, et al. 2014. Piezo2 is required for Merkel-cell mechanotransduction. Nature 509:622–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schrenk-Siemens K, Wende H, Prato V, Song K, Rostock C, et al. 2015. PIEZO2 is required for mechanotransduction in human stem cell-derived touch receptors. Nat. Neurosci. 18:10–16 [DOI] [PubMed] [Google Scholar]
- 79.von Buchholtz LJ, Ghitani N, Lam RM, Licholai JA, Chesler AT, Ryba NJ. 2021. Decoding cellular mechanisms for mechanosensory discrimination. Neuron 109:285–98.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hook SS, Means AR. 2001. Ca2+/CaM-dependent kinases: from activation to function. Annu. Rev. Pharmacol. Toxicol. 41:471–505 [DOI] [PubMed] [Google Scholar]
- 81.Song Y, Li D, Farrelly O, Miles L, Li F, et al. 2019. The mechanosensitive ion channel piezo inhibits axon regeneration. Neuron 102:373–89.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pardo-Pastor C, Rubio-Moscardo F, Vogel-González M, Serra SA, Afthinos A, et al. 2018. Piezo2 channel regulates RhoA and actin cytoskeleton to promote cell mechanobiological responses. PNAS 115:1925–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhou T, Gao B, Fan Y, Liu Y, Feng S, et al. 2020. Piezo1/2 mediate mechanotransduction essential for bone formation through concerted activation of NFAT-YAP1-ß-catenin. eLife 9:e52779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dubin AE, Schmidt M, Mathur J, Petrus MJ, Xiao B, et al. 2012. Inflammatory signals enhance piezo2-mediated mechanosensitive currents. Cell Rep. 2:511–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Singhmar P, Huo X, Eijkelkamp N, Berciano SR, Baameur F, et al. 2016. Critical role for Epac1 in inflammatory pain controlled by GRK2-mediated phosphorylation of Epac1. PNAS 113:3036–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Del Rosario JS, Yudin Y, Su S, Hartle CM, Mirshahi T, Rohacs T. 2020. Gi-coupled receptor activation potentiates Piezo2 currents via Gβγ. EMBO Rep. 21:e49124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hucho TB, Dina OA, Levine JD. 2005. Epac mediates a cAMP-to-PKC signaling in inflammatory pain: an isolectin B4(+) neuron-specific mechanism. J. Neurosci. 25:6119–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lechner SG, Lewin GR. 2009. Peripheral sensitisation of nociceptors via G-protein-dependent potentiation of mechanotransduction currents. J. Physiol. 587:3493–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Borbiro I, Rohacs T. 2017. Regulation of Piezo channels by cellular signaling pathways. Curr. Top. Membr. 79:245–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nickolls AR, Lee MM, Espinoza DF, Szczot M, Lam RM, et al. 2020. Transcriptional programming of human mechanosensory neuron subtypes from pluripotent stem cells. Cell Rep. 30:932–46.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Delle Vedove A, Storbeck M, Heller R, Hölker I, Hebbar M, et al. 2016. Biallelic loss of proprioception-related PIEZO2 causes muscular atrophy with perinatal respiratory distress, arthrogryposis, and scoliosis. Am. J. Hum. Genet. 99:1206–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Haliloglu G, Becker K, Temucin C, Talim B, Küçükşahin N, et al. 2017. Recessive PIEZO2 stop mutation causes distal arthrogryposis with distal muscle weakness, scoliosis and proprioception defects. J. Hum. Genet. 62:497–501 [DOI] [PubMed] [Google Scholar]
- 93.Mahmud A, Nahid N, Nassif C, Sayeed M, Ahmed M, et al. 2017. Loss of the proprioception and touch sensation channel PIEZO2 in siblings with a progressive form of contractures. Clin. Genet. 91:470–75 [DOI] [PubMed] [Google Scholar]
- 94.Case LK, Liljencrantz J, Madian N, Necaise A, Tubbs J, et al. 2021. Innocuous pressure sensation requires A-type afferents but not functional PIEZO2 channels in humans. Nat. Commun. 12(1):657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.McMillin MJ, Beck AE, Chong JX, Shively KM, Buckingham KJ, et al. 2014. Mutations in PIEZO2 cause Gordon syndrome, Marden-Walker syndrome, and distal arthrogryposis type 5. Am. J. Hum. Genet. 94:734–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Okubo M, Fujita A, Saito Y, Komaki H, Ishiyama A, et al. 2015. A family of distal arthrogryposis type 5 due to a novel PIEZO2 mutation. Am. J. Med. Genet. Part A 167:1100–6 [DOI] [PubMed] [Google Scholar]
- 97.Alisch F, Weichert A, Kalache K, Paradiso V, Longardt AC, et al. 2017. Familial Gordon syndrome associated with a PIEZO2 mutation. Am. J. Med. Genet. Part A 173:254–59 [DOI] [PubMed] [Google Scholar]
- 98.Nguyen MQ, Wu Y, Bonilla LS, von Buchholtz LJ, Ryba NJ. 2017. Diversity amongst trigeminal neurons revealed by high throughput single cell sequencing. PLOS ONE 12:e0185543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zheng Y, Liu P, Bai L, Trimmer JS, Bean BP, Ginty DD. 2019. Deep sequencing of somatosensory neurons reveals molecular determinants of intrinsic physiological properties. Neuron 103:598–616.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sharma N, Flaherty K, Lezgiyeva K, Wagner DE, Klein AM, Ginty DD. 2020. The emergence of transcriptional identity in somatosensory neurons. Nature 577:392–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.von Buchholtz LJ, Lam RM, Emrick JJ, Chesler AT, Ryba NJ. 2020. Assigning transcriptomic class in the trigeminal ganglion using multiplex in situ hybridization and machine learning. Pain 161:2212–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kandel ER, Jessell TM, Schwartz JH, Siegelbaum SA, Hudspeth AJ, Mack S. 2013. Principles of Neural Science. New York: McGraw-Hill Educ. 5th ed. [Google Scholar]
- 103.Umans BD, Liberles SD. 2018. Neural sensing of organ volume. Trends Neurosci. 41:911–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Min S, Chang RB, Prescott SL, Beeler B, Joshi NR, et al. 2019. Arterial baroreceptors sense blood pressure through decorated aortic claws. Cell Rep. 29:2192–201.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Prescott SL, Umans BD, Williams EK, Brust RD, Liberles SD. 2020. An airway protection program revealed by sweeping genetic control of vagal afferents. Cell 181:574–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nguyen MQ, Le Pichon CE, Ryba N. 2019. Stereotyped transcriptomic transformation of somatosensory neurons in response to injury. eLife 8:e49679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chang R, Strochlic D, Nonomura K, Patapoutian A, Liberles S. 2018. Airway mechanoreceptors that control breathing. FASEB J. 32:893.3 [Google Scholar]
- 108.Nonomura K, Woo SH, Chang RB, Gillich A, Qiu Z, et al. 2017. Piezo2 senses airway stretch and mediates lung inflation-induced apnoea. Nature 541:176–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang F, Knutson K, Alcaino C, Linden DR, Gibbons SJ, et al. 2017. Mechanosensitive ion channel Piezo2 is important for enterochromaffin cell response to mechanical forces. J. Physiol. 595:79–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Iggo A, Muir AR. 1969. The structure and function of a slowly adapting touch corpuscle in hairy skin. J. Physiol. 200:763–796.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Diamond J, Mills L, Mearow K. 1988. Evidence that the Merkel cell is not the transducer in the mechanosensory Merkel cell–neurite complex. Prog. Brain Res. 74:51–56 [DOI] [PubMed] [Google Scholar]
- 112.Mills L, Diamond J. 1995. Merkel cells are not the mechanosensory transducers in the touch dome of the rat. J. Neurocytol. 24:117–34 [DOI] [PubMed] [Google Scholar]
- 113.Anand A, Iggo A, Paintal AS. 1979. Lability of granular vesicles in Merkel cells of the type I slowly-adapting cutaneous receptors of the cat [proceedings]. J. Physiol. 296:19P–20P [PubMed] [Google Scholar]
- 114.Maksimovic S, Baba Y, Lumpkin EA. 2013. Neurotransmitters and synaptic components in the Merkel cell–neurite complex, a gentle touch receptor. Ann. N. Y. Acad. Sci. 1279:13–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Maricich SM, Wellnitz SA, Nelson AM, Lesniak DR, Gerling GJ, et al. 2009. Merkel cells are essential for light-touch responses. Science 324:1580–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Maksimovic S, Nakatani M, Baba Y, Nelson AM, Marshall KL, et al. 2014. Epidermal Merkel cells are mechanosensory cells that tune mammalian touch receptors. Nature 509:617–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Woo S-H, Lumpkin EA, Patapoutian A. 2015. Merkel cells and neurons keep in touch. Trends Cell Biol. 25:74–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Murthy SE, Dubin AE, Whitwam T, Jojoa-Cruz S, Cahalan SM, et al. 2018. OSCA/TMEM63 are an evolutionarily conserved family of mechanically activated ion channels. eLife 7:e41844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Beaulieu-Laroche L, Christin M, Donoghue A, Agosti F, Yousefpour N, et al. 2020. TACAN is an ion channel involved in sensing mechanical pain. Cell 180:956–67.e17 [DOI] [PubMed] [Google Scholar]
- 120.Abdo H, Calvo-Enrique L, Lopez JM, Song J, Zhang M-D, et al. 2019. Specialized cutaneous Schwann cells initiate pain sensation. Science 365:695–99 [DOI] [PubMed] [Google Scholar]
- 121.Dhandapani R, Arokiaraj CM, Taberner FJ, Pacifico P, Raja S, et al. 2018. Control of mechanical pain hypersensitivity in mice through ligand-targeted photoablation of TrkB-positive sensory neurons. Nat. Commun. 9:1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bautista DM, Wilson SR, Hoon MA. 2014. Why we scratch an itch: the molecules, cells and circuits of itch. Nat. Neurosci. 17:175–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Feng J, Luo J, Yang P, Du J, Kim BS, Hu H. 2018. Piezo2 channel–Merkel cell signaling modulates the conversion of touch to itch. Science 360:530–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Feng J, Hu H. 2019. A novel player in the field: Merkel disc in touch, itch and pain. Exp. Dermatol. 28:1412–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sherrington CS. 1907. On the proprio-ceptive system, especially in its reflex aspect. Brain 29:467–82 [Google Scholar]
- 126.Florez-Paz D, Bali KK, Kuner R, Gomis A. 2016. A critical role for Piezo2 channels in the mechanotransduction of mouse proprioceptive neurons. Sci. Rep. 6:25923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhang J, Walker JF, Guardiola J, Yu J. 2006. Pulmonary sensory and reflex responses in the mouse. J. Appl. Physiol. 101:986–92 [DOI] [PubMed] [Google Scholar]
- 128.Schelegle ES, Green JF. 2001. An overview of the anatomy and physiology of slowly adapting pulmonary stretch receptors. Respir. Physiol. 125:17–31 [DOI] [PubMed] [Google Scholar]
- 129.Bai L, Mesgarzadeh S, Ramesh KS, Huey EL, Liu Y, et al. 2019. Genetic identification of vagal sensory neurons that control feeding. Cell 179:1129–43.e23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chang RB, Strochlic DE, Williams EK, Umans BD, Liberles SD. 2015. Vagal sensory neuron subtypes that differentially control breathing. Cell 161:622–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kupari J, Häring M, Agirre E, Castelo-Branco G, Ernfors P. 2019. An atlas of vagal sensory neurons and their molecular specialization. Cell Rep. 27:2508–23.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hockley JR, Taylor TS, Callejo G, Wilbrey AL, Gutteridge A, et al. 2019. Single-cell RNAseq reveals seven classes of colonic sensory neuron. Gut 68:633–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bülbring E, Crema A. 1959. The action of 5-hydroxytryptamine, 5-hydroxytryptophan and reserpine on intestinal peristalsis in anaesthetized guinea-pigs. J. Physiol. 146:29–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Alcaino C, Knutson KR, Treichel AJ, Yildiz G, Strege PR, et al. 2018. A population of gut epithelial enterochromaffin cells is mechanosensitive and requires Piezo2 to convert force into serotonin release. PNAS 115:E7632–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Najjar SA, Davis BM, Albers KM. 2020. Epithelial-neuronal communication in the colon: implications for visceral pain. Trends Neurosci. 43:170–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bohórquez DV, Shahid RA, Erdmann A, Kreger AM, Wang Y, et al. 2015. Neuroepithelial circuit formed by innervation of sensory enteroendocrine cells. J. Clin. Investig. 125:782–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bellono NW, Bayrer JR, Leitch DB, Castro J, Zhang C, et al. 2017. Enterochromaffin cells are gut chemosensors that couple to sensory neural pathways. Cell 170:185–98.e16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kaelberer MM, Buchanan KL, Klein ME, Barth BB, Montoya MM, et al. 2018. A gut-brain neural circuit for nutrient sensory transduction. Science 361:eaat5236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.McMahon SB, Koltzenburg M, Tracey I, Turk D. 2013. Wall & Melzack’s Textbook of Pain. Philadelphia: Elsevier/Saunders. 6th ed. [Google Scholar]
- 140.Prato V, Taberner FJ, Hockley JR, Callejo G, Arcourt A, et al. 2017. Functional and molecular characterization of mechanoinsensitive “silent” nociceptors. Cell Rep. 21:3102–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Marshall KL, Saade D, Ghitani N, Coombs AM, Szczot M, et al. 2020. PIEZO2 in sensory neurons and urothelial cells coordinates urination. Nature 588:290–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kumada M, Terui N, Kuwaki T. 1990. Arterial baroreceptor reflex: its central and peripheral neural mechanisms. Progress Neurobiol. 35:331–61 [DOI] [PubMed] [Google Scholar]
- 143.Miglis MG, Muppidi S. 2019. Ion channels PIEZOs identified as the long-sought baroreceptor mechanosensors for blood pressure control, and other updates on autonomic research. Clin. Auton. Res. 29:9–11 [DOI] [PubMed] [Google Scholar]
- 144.Won J, Vang H, Lee P, Kim Y, Kim H, et al. 2017. Piezo2 expression in mechanosensitive dental primary afferent neurons. J. Dent. Res. 96:931–37 [DOI] [PubMed] [Google Scholar]
- 145.Emrick J, von Buchholtz L, Ryba N. 2020. Transcriptomic classification of neurons innervating teeth. J. Dent. Res. 99:1478–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Moayedi Y, Duenas-Bianchi LF, Lumpkin EA. 2018. Somatosensory innervation of the oral mucosa of adult and aging mice. Sci. Rep. 8:9975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Du G, Li L, Zhang X, Liu J, Hao J, et al. 2020. Roles of TRPV4 and piezo channels in stretch-evoked Ca2+ response in chondrocytes. Exp. Biol. Med. 245:180–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lee W, Leddy HA, Chen Y, Lee SH, Zelenski NA, et al. 2014. Synergy between Piezo1 and Piezo2 channels confers high-strain mechanosensitivity to articular cartilage. PNAS 111:E5114–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lee W, Guilak F, Liedtke W. 2017. Role of Piezo channels in joint health and injury. Curr. Top. Membr. 79:263–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Assaraf E, Blecher R, Heinemann-Yerushalmi L, Krief S, Vinestock RC, et al. 2020. Piezo2 expressed in proprioceptive neurons is essential for skeletal integrity. Nat. Commun. 11:3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wu Z, Grillet N, Zhao B, Cunningham C, Harkins-Perry S, et al. 2017. Mechanosensory hair cells express two molecularly distinct mechanotransduction channels. Nat. Neurosci. 20:24–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Beurg M, Fettiplace R. 2017. PIEZO2 as the anomalous mechanotransducer channel in auditory hair cells. J. Physiol. 595:7039–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chambers MC, Maclean B, Burke R, Amodei D, Ruderman DL, et al. 2012. A cross-platform toolkit for mass spectrometry and proteomics. Nat. Biotechnol. 30:918–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Southam L, Gilly A, Süveges D, Farmaki A-E, Schwartzentruber J, et al. 2017. Whole genome sequencing and imputation in isolated populations identify genetic associations with medically-relevant complex traits. Nat. Commun. 8:15606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Eberhardt E, Havlicek S, Schmidt D, Link AS, Neacsu C, et al. 2015. Pattern of functional TTX-resistant sodium channels reveals a developmental stage of human iPSC- and ESC-derived nociceptors. Stem Cell Rep. 5:305–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Jones I, Yelhekar TD, Wiberg R, Kingham PJ, Johansson S, et al. 2018. Development and validation of an in vitro model system to study peripheral sensory neuron development and injury. Sci. Rep. 8:15961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Alshawaf AJ, Viventi S, Qiu W, D’Abaco G, Nayagam B, et al. 2018. Phenotypic and functional characterization of peripheral sensory neurons derived from human embryonic stem cells. Sci. Rep. 8:603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.McDermott LA, Weir GA, Themistocleous AC, Segerdahl AR, Blesneac I, et al. 2019. Defining the functional role of Nav1.7 in human nociception. Neuron 101:905–19.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Schrenk-Siemens K, Pohle J, Rostock C, El Hay MA, Lam RM, et al. 2019. HESC-derived sensory neurons reveal an unexpected role for PIEZO2 in nociceptor mechanotransduction. bioRxiv 741660. 10.1101/741660 [DOI] [Google Scholar]
- 160.Kupari J, Usoskin D, Parisien M, Lou D, Hu Y, et al. 2020. Single cell transcriptomics of primate sensory neurons identifies cell types associated with human chronic pain. Nat. Commun. 12:1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Syeda R, Xu J, Dubin AE, Coste B, Mathur J, et al. 2015. Chemical activation of the mechanotransduction channel Piezo1. eLife 4:e07369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wang Y, Chi S, Guo H, Li G, Wang L, et al. 2018. A lever-like transduction pathway for long-distance chemical- and mechano-gating of the mechanosensitive Piezo1 channel. Nat. Commun. 9:1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Feng J, Luo J, Yang P, Du J, Kim BS, Hu H. 2018. Piezo2 channel-Merkel cell signaling modulates the conversion of touch to itch. Science 360:530–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gnanasambandam R, Ghatak C, Yasmann A, Nishizawa K, Sachs F, et al. 2017. GsMTx4: mechanism of inhibiting mechanosensitive ion channels. Biophys. J. 112:31–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lewis AH, Cui AF, McDonald MF, Grandl J. 2017. Transduction of repetitive mechanical stimuli by Piezo1 and Piezo2 ion channels. Cell Rep. 19:2572–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wu J, Goyal R, Grandl J. 2016. Localized force application reveals mechanically sensitive domains of Piezo1. Nat. Commun. 7:12939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Liu C, Li T, Chen J. 2019. Role of high-throughput electrophysiology in drug discovery. Curr. Protoc. Pharmacol. 87:e69. [DOI] [PubMed] [Google Scholar]
- 168.Mohanraj B, Hou C, Meloni GR, Cosgrove BD, Dodge GR, Mauck RL. 2014. A high throughput mechanical screening device for cartilage tissue engineering. J. Biomech. 47:2130–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gregurec D, Senko AW, Chuvilin A, Reddy PD, Sankararaman A, et al. 2020. Magnetic vortex nanodiscs enable remote magnetomechanical neural stimulation. ACS Nano 14:8036–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Montel L, Sotiropoulos A, Hénon S. 2019. The nature and intensity of mechanical stimulation drive different dynamics of MRTF-A nuclear redistribution after actin remodeling in myoblasts. PLOS ONE 14:e0214385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Matsui TS, Wu H, Deguchi S. 2018. Deformable 96-well cell culture plate compatible with high-throughput screening platforms. PLOS ONE 13:e0203448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Xu J, Mathur J, Vessières E, Hammack S, Nonomura K, et al. 2018. GPR68 senses flow and is essential for vascular physiology. Cell 173:762–75.e16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Orefice LL, Zimmerman AL, Chirila AM, Sleboda SJ, Head JP, Ginty DD. 2016. Peripheral mechanosensory neuron dysfunction underlies tactile and behavioral deficits in mouse models of ASDs. Cell 166:299–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Neubarth NL, Emanuel AJ, Liu Y, Springel MW, Handler A, et al. 2020. Meissner corpuscles and their spatially intermingled afferents underlie gentle touch perception. Science 368:eabb2751. [DOI] [PMC free article] [PubMed] [Google Scholar]