Abstract
Most lung development occurs in the context of cyclic stretch. Alteration of the mechanical microenvironment is a common feature of many pulmonary diseases, with congenital diaphragmatic hernia (CDH) and fetal tracheal occlusion (FETO, a therapy for CDH) being extreme examples with changes in lung structure, cell differentiation, and function. To address limitations in cell culture and in vivo mechanotransductive models, we developed two mouse lung organoid (mLO) mechanotransductive models using postnatal day 5 (PND5) mouse lung CD326-positive cells and fibroblasts subjected to increased, decreased, and cyclic strain. In the first model, mLOs were exposed to forskolin (FSK) and/or disrupted (DIS) and evaluated at 20 h. mLO cross-sectional area changed by +59%, +24%, and −68% in FSK, control, and DIS mLOs, respectively. FSK-treated organoids had twice as many proliferating cells as other organoids. In the second model, 20 h of 10.25% biaxial cyclic strain increased the mRNAs of lung mesenchymal cell lineages compared with static stretch and no stretch. Cyclic stretch increased TGF-β and integrin-mediated signaling, with upstream analysis indicating roles for histone deacetylases, microRNAs, and long noncoding RNAs. Cyclic stretch mLOs increased αSMA-positive and αSMA-PDGFRα-double-positive cells compared with no stretch and static stretch mLOs. In this PND5 mLO mechanotransductive model, cell proliferation is increased by static stretch, and cyclic stretch induces mesenchymal gene expression changes important in postnatal lung development.
Keywords: lung development, lung mesenchyme, mechanotransduction
INTRODUCTION
From the 12th wk of gestation onward, the lungs develop and function in the context of cyclic stretch first from fetal breathing movements and then from the respiratory cycle. Most pulmonary disorders are associated with decreased [e.g., pneumonia, idiopathic pulmonary fibrosis, congenital diaphragmatic hernia (CDH)] or increased (e.g., emphysema, asthma) strain in some region of the lung. Fetal tracheal occlusion (FETO) is an emerging treatment for CDH, where an endoscopically placed tracheal balloon prevents egress of tracheal fluid from ∼26 to ∼34 wk of gestation, causing lung distention and compensatory growth and improving outcomes in fetuses with severe CDH (1). We use CDH and FETO as a case study in the importance of lung mechanics because the CDH, tracheal occlusion (TO), and normal states represent three different mechanodevelopmental environments: reduced stretch, increased stretch, and cyclic stretch, respectively. None of these three states is accounted for in cell culture or organoid models of lung development.
Emerging evidence indicates that although CDH severely limits lung growth, lung epithelial cell development is only modestly impacted. FETO, in contrast, positively impacts lung growth and increases airspace size but negatively impacts lung cell differentiation (2–4). FETO increased lung cellularity in rat and mouse models (5, 6), retarded lung maturation in a rabbit model (7), and caused proximalization of the distal lung in a sheep model (8). Although these animal models support the importance of normal lung mechanics, the elucidation of specific genes and pathways important in driving cell-specific changes is difficult in such models.
In vitro mechanical models have largely focused on cells cultured on two-dimensional (2-D) surfaces subjected to uniaxial or biaxial strain. These reductionist models provide important insights into the mechanoresponsiveness of specific cell types. For example, cyclic stretch increases serotonin release from cultured neuroendocrine cells (9), increases surfactant release from cultured alveolar type 2 (AT2) cells (10), and promotes AT2 cell maturation (4). In cultured lung fibroblasts, transient receptor potential cation channel subfamily V member 4 (TRVP4) is a key mechanotransductive regulator (2), and Piezo1 is important in regulating lung epithelial cell division in response to tension (11). However, understanding the relative importance of these findings in vivo and assessing cell-cell or cell-matrix interactions are difficult.
In vivo models using animal lungs overcome some of these difficulties, but mechanical inputs cannot be easily manipulated, and human cells cannot be easily studied. To overcome these limitations, we developed two lung organoid models of mechanotransduction. The first is a simple model of organoid disruption or forskolin (FSK)-induced organoid swelling to study the effects of increased and decreased static strain. FSK increases intracellular cAMP levels (12) and has been shown to cause organoid swelling of human nasal epithelial organoids (13). The second model applies cyclic stretch to the organoid. We found that a constant reduction in strain downregulates many lung developmental pathways, whereas increased strain activates the cell cycle but has no effect on lung developmental pathways. Cyclic stretch promotes the development of several different lung mesenchymal cell lineages, activates transforming growth factor-beta (TGF-β) and epigenetic and posttranscriptional regulatory mechanisms, and increases the abundance of α-smooth muscle actin (αSMA)- and platelet-derived growth factor receptor-α (PDGFRα)-positive cells.
METHODS
Animals
Animal use and care was approved by the Cincinnati Children’s Hospital Institutional Animal Care and Use Committee. Mice (C57BL/6) were housed in a pathogen-free facility with easy access to food and water ad libitum with 12:12-h light/dark cycles. Postnatal day 5 (PND5) mice of mixed sex were used for single-cell isolation. Pups were anesthetized with triple sedative (ketamine-xylazine-acepromazine; 70-10-2 mg/kg, respectively) intraperitoneally and euthanized by exsanguination. After chests were opened, the lungs were flushed with PBS via the right atrium and harvested.
Cell Isolation
Harvested lung lobes were digested with Dispase (Sigma Cat. No. 354335) in DNase (25 U/mL) and then transferred to gentle MACS C tubes (Miltenyi Biotec Cat. No. 130-093-237) using gentle MACS Octo Dissociator (Cat. No. 130-095-937) for single-cell suspensions. The cell suspension was passed through a 40-µm nylon mesh (Fisher Scientific Cat. No. 22363547) into 50-mL blue-cap tubes (Cat. No. 339653) and centrifuged at 10,000 rpm for 5 min. After discarding the supernatant, 1 mL of 1× RBC lysis buffer (eBioscience Cat. No. 004300-54) was added to the cell pellet, mixed well by pipetting, and incubated on ice for 10 min. PBS (5 mL) was added and the cells centrifuged for 5 min at 10,000 rpm. The supernatant was discarded, the cells were washed once with PBS, and then the cells were resuspended in DMEM with 10% FBS with penicillin-streptomycin and cultured in a 10-cm2 dish for 1 h in a 37°C tissue culture incubator. Nonadherent cells were collected in a 50-mL blue-cap tube, and adherent cells (previously shown to be lung fibroblasts) (14) were collected after treatment with 0.25% of trypsin. Cells were counted using a hemocytometer and kept on ice. Epithelial cells were isolated from the nonadherent fraction using a MACS manual separator after combining 90 µL (107 cells) in separator buffer (Cat. No. 130-091-376) and 10 µL of CD326 (EpCAM) mouse microbeads (Cat. No. 130-105-958) with incubation at 4°C. The suspension was incubated for 15 min. A MACS multistand separator with MACS LS Columns wings (Cat. No. 130-042-401) was rinsed twice with 5 mL of MACS rinsing buffer (Cat. No. 130-091-222). After 15 min, the labeled cells and 5 mL of cell separator buffer were passed through the column. The unlabeled cells were discarded, and the labeled cells were washed twice with separator buffer. The captured cells and column were taken off the magnetic separator and the labeled cells extracted into a new 50-mL tube with 5 mL of cell separator buffer and the supplied plunger. The removal step was performed two times more and then centrifuged for 5 min at 10,000 rpm. The supernatant was discarded, cells were resuspended in DMEM with FBS, and then the cells were counted.
Organoid Culture
Based on a protocol from Green et al. (15), lung fibroblasts were cocultured with CD326 (EpCAM)-positive cells in a ratio of 10:1. A volume (100 µL) of mixed cells was combined with Matrigel (BD Bioscience Cat. No. 354234) in 1:1 ratio and loaded to the Transwell insert (BD, Cat. No. 353095) in a 24-well plate and incubated at an air-liquid interface in MTEC/Plus media [DMEM-Ham’s F-12 (Life Technologies Cat. No. 11330-032), 1% penicillin-streptomycin, fungizone (250 ng/mL, Fisher Scientific 401100501), insulin (10 µg/mL, Sigma I-6634), apotransferrin (5 µg/mL, Sigma, T-1147), cholera toxin (0.1 µg/mL, Sigma, C-8052), epidermal growth factor (25 ng/mL, Sigma E-4127), bovine pituitary extract (30 µg/mL, Life Technologies 13028-014), and retinoic acid (Sigma,12.5 ng/mL) with 5% FBS. Media were changed 3 times/wk, and organoids became easily visible to the unaided eye at 3 wk, which is when they were used.
Forskolin-Induced Swelling of mLOs
On the day of experimentation, 10 µM Forskolin (Sigma, MO; Cat. No. 344270) was added to the MTEC/Plus with 5% FBS culture media, and this addition was defined as time zero. Phase contrast microscopy was performed at time 0, 4 h, and 20 h, and organoids were collected at 20 h for additional experiments.
Disruption of mLOs
mLOs were imaged by phase contrast microscopy, and with the lid of the 24-well plate open, a sterile 27-gauge needle (BD Biosciences Cat. No. 305109) was used to pierce one wall of the organoid by hand without removal or injection of any material. The plate lid was replaced and the organoid was reimaged ∼60 s after disruption and then at 4 h and 20 h, at which time, mLOs were collected for additional experiments. A video of this procedure is provided in Supplemental Material (see Supplemental Video S1 at https://doi.org/10.6084/m9.figshare.17313998.v1).
Static and Cyclic Stretch Model
Silicone inserts were created using custom three-dimensional (3-D)-printed molds. A cross-shaped bottom mold was fitted with four cut nylon sponges to yield a space in the middle. About 10 mL of high-quality silicone (Smooth-On Ecoflex Cat. No. 00-30) was added, and a 3-D-printed lid with eight spokes and a post to create a well in the middle was placed on top and the insert allowed to cure overnight. The following morning, any excess silicone was removed, and the insert was sterilized with 70% ethanol. On a sterile piece of parafilm, 50–100 µL of Matrigel was pipetted into the well and allowed to cure. A single intact organoid was liberated from Matrigel using cold PBS and pipetted into the center of the insert, and an additional 50–100 µL of Matrigel added alternately to the spokes of the insert to embed the organoid in the well of the insert. The insert and organoid were seated into a CellScale MCB1 device with addition of 100 mL of DMEM with 10% FBS and 0.1% penicillin-streptomycin. For 20 h, the device applied 5% cyclic stretch (10.25% change in area) with a 50% duty cycle at 0.1 Hz, and control organoids were identically mounted and cultured in 25-cm2 tissue culture dishes with the same media. A similar model using fibroblasts in collagen gels was previously published (16).
RNA Extraction
Organoids were collected in 1.5-mL microfuge tubes with 300 µL of Dispase and incubated at 37°C for 30 min. One milliliter of 4°C PBS was added and pipetted several times and centrifuged at 10,000 rpm at 4°C for 5 min to pellet the cells. RNA was extracted using QIAshredder (Cat. No. 79654) and RNeasy Kit (Cat. No. 74106) as per manufacturer instructions. The RNA quality and quantity were assessed by Agilent 2100.
RNA Amplification
Since the RNA concentration of cyclic stretch and the associated control organoids was too low for conventional mRNA sequencing, the RNA was amplified and barcoded cDNA libraries were created using the NEBNext Single Cell/Low Input RNA Library Prep Kit for Illumina (New England BioLabs Cat. No. E6420) with NEBNext Multiplex Oligos Dual Index Primers Set No. 1 (Cat. No. E7600S) as per manufacturer instructions.
RNA Sequencing, Alignment, and Analysis
RNA from both experiments was sequenced at a depth of ∼10 million reads per sample using paired-end sequencing with 150-base pair-long sequences using a HiSeq2500 (Illumina). Sequences were aligned to GRCh38 using STAR (17) with creation of a count file for each sample. In the R3.5.3 (18) environment, each experiment was analyzed using DESeq2 (19) separately, as library creation techniques were different. Genes with less than five reads in all specimens were excluded from analysis. DESeq2 principal component data were plotted in ggplot2 (20). For gene set enrichment analysis (GSEA), deferentially expressed genes (DEGs) with adjusted P values of <0.1 and at least twofold changed expression were analyzed by ToppGene (21) and ingenuity pathway analysis (IPA) (22). Terms were filtered for Bonferroni-adjusted P values of ≤ 0.1 and heatmaps generated (23) for presentation using adjusted P values for color scales with exclusion of similar terms for clarity [all terms are provided in Supplemental Fig. S2 (https://doi.org/10.6084/m9.figshare.12594656.v2) and Supplemental Fig. S3 (https://doi.org/10.6084/m9.figshare.12594653.v2)]. For IPA data activation, scores of ≤3 or >3 were filtered, and these scores were used for color scales. Venn diagrams were created using VennDiagram (24).
Confocal Microscopy
Organoids were fixed overnight in 4% paraformaldehyde in PBS and then immunostained for α-smooth muscle actin (Sigma A2547-.2ML, 1:400 dilution), proliferating cell nuclear antigen (PCNA) (BD Biosciences, 555566, 1:200), nerve growth factor receptor (AbCam ab8874, 1:200), Forkhead box J1 (FoxJ1) (eBioscience Cat. No. 1409965-82, 1:200), and platelet-derived growth factor receptor-α (Santa Cruz Biotechnology, sc-398206, 1:200) with appropriate secondary antibodies. For whole organoid immunofluorescent imaging, organoids were stained with MemGlow-488 (Cytoskeleton, Cat. No. PHDH1, 1:200). Organoids were imaged with Nikon A1 single-photon confocal, Nikon MP1 multiphoton confocal, and Nikon NiE widefield microscopes at ×10 and ×20 magnification.
Flow Cytometry
Single-cell suspensions from mLOs were stained fixed and permeabilized with BD Bioscience Fixation/Permeabilization kit (554714) and stained for α-smooth muscle actin (Sigma A2547-.2ML 1:200 dilution), PCNA (BD Biosciences, 555566, 1:200), and platelet-derived growth factor receptor-α (Santa Cruz Biotechnology, sc-398206, 1:200) using appropriate secondary antibodies at 1:2,000 dilution using Zombie Red (BioLegend Cat. No. 423109) as a dead cell exclusion dye. Cell suspensions were analyzed using BD Biosciences FACS Canto device and FlowJo (v. 10.7.1, BD Biosciences).
Statistical Analysis
Comparisons of fractional change were made by Kruskal–Wallis test in R with post hoc comparisons by Dunn’s test (25). Median and 25th and 75th percentile values are plotted. P values of <0.05 were considered significant.
RESULTS
Forskolin-Mediated Swelling and Organoid Disruption Models
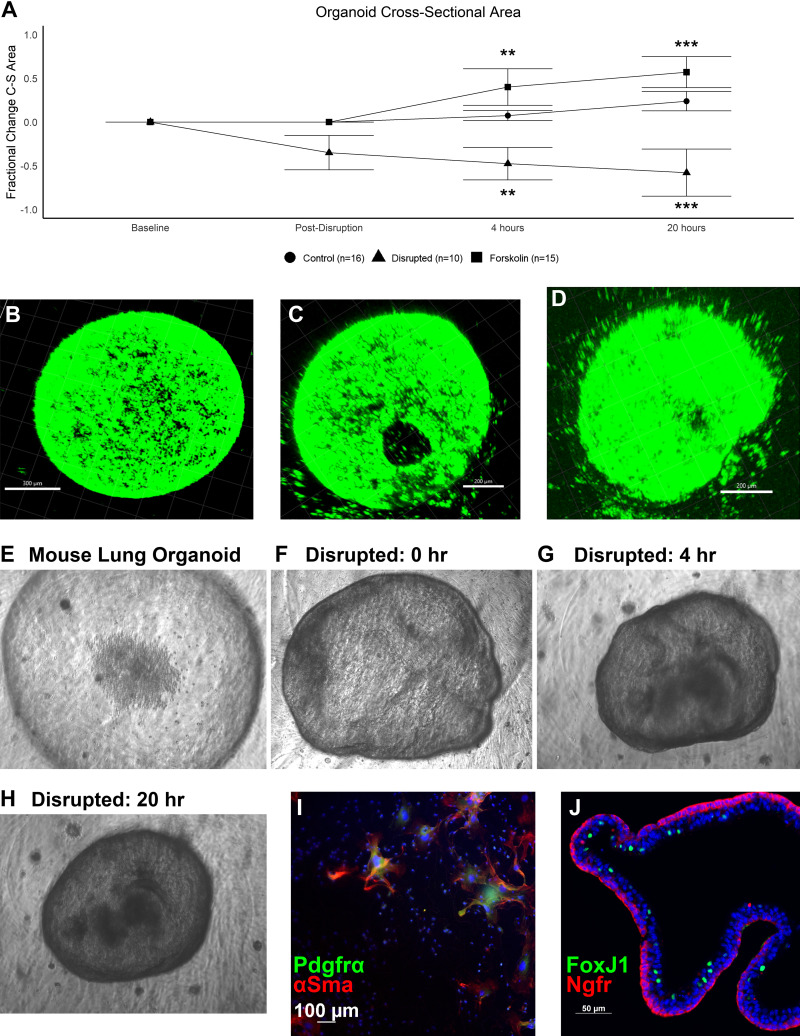
To model the impact of tracheal occlusion and congenital diaphragmatic hernia in an organoid model, we performed a 2 × 2 experiment with disruption and forskolin-mediated swelling of mouse lung organoids (mLOs). Forskolin induced a 37% and 59% increase in mLO cross-sectional area at 4 h and 20 h, whereas disruption caused a 48% and 68% decrease, respectively. Control mLO cross-sectional area increased by 7% and 24% over this same time, consistent with normal organoid growth (Fig. 1A). Two-photon confocal microscopy images of mLOs with membrane staining of live cells before (Fig. 1B), immediately after (Fig. 1C), and 20 h after (Fig. 1D) disruption revealed a sizable disruption in the organoid wall and reduction in organoid size. Phase contrast images of this process showed that the disruption process resulted in the organoid pulling away from the Matrigel with some wrinkling of the organoid wall in addition to being reduced in size (Fig. 1, E–H). A video of the disruption procedure is presented in Supplemental Material (see Supplemental Video S1) and measurement of the volume changes in this model is shown in Supplemental Fig. S1 (see https://doi.org/10.6084/m9.figshare.14192111.v5). Culture of the fibroblasts used in mLO creation showed α-smooth muscle actin (αSMA) and platelet-derived growth factor receptor-α (PDGFRα)-high cells, which formed syncytia after 1 wk of culture on coverslips (Fig. 1I). mLOs were composed of elements of the conducting airway epithelium with basal cell and ciliated cell markers (Fig. 1J).
Figure 1.
Mouse lung organoid (mLO) forskolin and disruption models. A: fractional change of mLO cross-sectional area at 0, 4, and 20 h after exposure to forskolin (n = 15) or after disruption (n = 10) compared with control (n = 16). Center bar represents median and whiskers 25th and 75th percentile. Kruskal–Wallis test at 4 and 20 h; P < 0.001. Statistical comparisons by Dunn’s post hoc test vs. control are shown with **P < 0.01 and ***P < 0.001. Membrane-stained mLO before (B), immediately after (C), and 20 h after disruption (D). Scale bars = 200 µm. E: representative phase contrast image of an mLO. Representative image of the same mLO immediately after disruption (F), 4 h after disruption (G), and 20 h after disruption (H). I: adherent cells were cultured for 1 wk for characterization showing both α-smooth muscle actin (αSMA) and platelet-derived growth factor receptor-α (PDGFR-α)-positive cells which formed syncytia over time. Negative and low-expressing cells were also observed. J: mLOs expressed proximal airway markers such as the basal cell marker nerve growth factor receptor (NGFR, red) and scattered ciliated cells marked by nuclear FoxJ1 (green). Scale bar = 50 µm. C-S area, cross-sectional area.
Identification of Mechanosensitive Genes in the FSK and DIS Mouse Lung Organoids
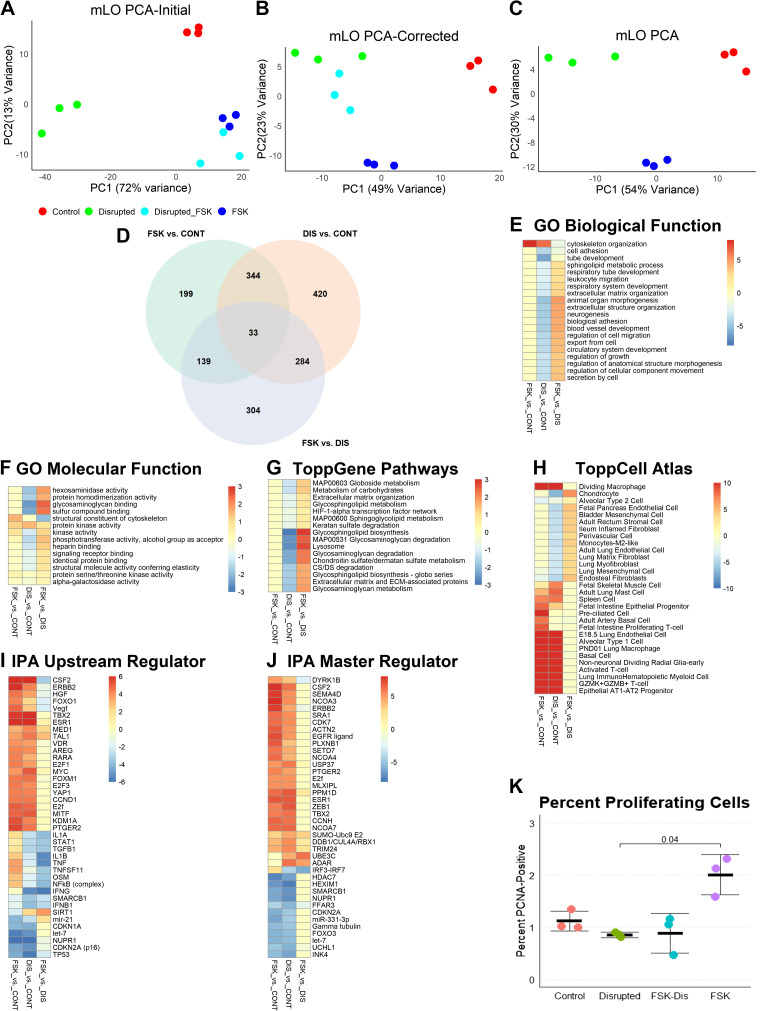
A challenge with this experimental approach was to differentiate those genes that were FSK-sensitive from those that were mechanosensitive, understanding that some genes may be both. RNA was extracted from three wells each of a 2 × 2 experiment of FSK and DIS, and sequences were mapped, genes filtered, and count files analyzed (14,353 genes). We first identified DEGs that were twofold increased or decreased in DIS versus FSK/DIS (2,538 genes) and removed these from further analysis. Among these removed genes, the top downregulated gene ontogeny (GO) molecular function was “inorganic molecular entity transmembrane transporter activity” and the top function among upregulated genes was “extracellular matrix structural constituent.” In all, 57 of the 58 genes in this upregulated GO term of 178 genes had adjusted P values of <0.1. Thus, FSK induced almost one-third of extracellular matrix genes, and this experimental model was biased against showing mechanotransductive gene induction. Similarities and differences in gene expression profiles were visualized by principal component analysis (PCA). Before exclusion of FSK-sensitive genes, FSK/DIS and FSK clustered closely together (Fig. 2A), but after removal of these genes, FSK/DIS clustered more closely with DIS (Fig. 2B). Only DIS, FSK, and control groups were used for gene set enrichment analysis (GSEA, Fig. 2C). After excluding these transcripts, 11,805 genes remained and were analyzed. Restricting transcripts to those with adjusted P values <0.1 and with twofold changed expression yielded 715 transcripts in FSK versus control (CONT), 1,081 in DIS versus CONT, and 760 in FSK versus DIS (Fig. 2D).
Figure 2.
Analysis of mLOs in forskolin and disruption model. A: both forskolin (FSK) and disrupted (DIS) mLOs (n = 3 per group; each specimen represents one well of treated organoids) exposed to FSK (Disrupted_FSK) had similarities in gene expression that were due to FSK and not mechanosensing. This similarity is visualized by the close clustering of these samples by principal component analysis (PCA). PC1, Principal Component 1; PC2, Principal Component 2. For each group, n = 3. B: to remove this effect, genes with a twofold change in expression between DIS and DIS/FSK were removed from analysis. After removal, FSK/DIS mLO gene expression profile was more like DIS than FSK or control. C: gene set enrichment analysis (GSEA) was performed on the 3 control, 3 FSK, and 3 DIS organoid mRNA-seq data sets using ToppGene. D: Venn diagram with number of differentially expressed genes for each comparison. E: comparison of gene ontogeny (GO) biological functions revealed greater upregulation of cytoskeleton-related functions in FSK compared with DIS and downregulation of many developmental processes in DIS, such as tube development and cell adhesion. Color scale represents −log10 Bonferroni-corrected P values for upregulated processes and log10 values for downregulated ones. A threshold of corrected P value of 0.1 was applied (i.e., values in this range are yellow). F: analysis of GO molecular functions showed upregulation of cytoskeleton-related functions in FSK and downregulation of processes related to extracellular matrix binding in DIS. G: pathway analysis showed no significant changes in FSK compared with control and downregulation of many processes related to cell-matrix interaction and lipid metabolism. H: to determine if any cell-specific gene sets were enriched, the 3 gene sets were compared with the ToppCell Atlas that computes a Bonferroni-corrected P value for each set compared with published single-cell mRNA-seq data sets. Both FSK and DIS had increased expression of genes found in inflammatory, basal, and AT2-AT1 progenitor cells. DIS had reduced abundances of mRNAs found in chondrocytes, and various lung lineage mesenchymal cells. I: ingenuity pathway analysis (IPA) found stronger activation of many upstream regulators in FSK compared with DIS with most of these regulators being involved in inflammation and cell cycle. Differences in FSK and DIS were noted in NFκB-related inflammation and TGF-β. Upregulation of the histone deacetylase sirtuin 1 (SIRT1) in DIS with downregulation in FSK suggests that changes in chromatin state may account for some of the differences between FSK and DIS. Color scale represents the IPA activation score with positive being activated and negative being inhibited. A threshold of 1 is applied. J: regulatory element depth analysis in IPA identified some of the same regulators as upstream analysis with additional identification of posttranslational modifications such as ubiquitination and sumoylation as being different between FSK and DIS and interferon response factors as accounting for the difference in inflammatory signaling. K: by flow cytometry, FSK organoids had significantly more proliferating cells than control, DIS, or FSK/DIS organoids. n = 3 per group, Kruskal–Wallis P = 0.01, Dunn’s post hoc test P value is shown. CONT, control; mLO, mouse lung organoid; PCNA, proliferating cell nuclear antigen; TGF-β, transforming growth factor-β.
Pathways and Processes Impacted by Forskolin-Induced Organoid Swelling and Organoid Disruption
Surprisingly, there were 344 shared DEGs between the FSK versus CONT and DIS versus CONT data sets. Genes that were activated in both FSK versus CONT and DIS versus CONT were largely involved in cell cycle and innate immune-related processes. A notable difference in the molecular functions between FSK and DIS were terms related to binding to extracellular matrix, such as “glycoaminoglycan binding” (Fig. 2E), despite the exclusion of many extracellular matrix genes as noted earlier. Although both FSK and DIS had changes in cytoskeletal organization, only DIS had downregulation of processes related to tube development, respiratory system development, and cell adhesion (Fig. 2F). In ToppGene GSEA, FSK had no significant differences compared with the control, but DIS had downregulation of many pathways related to glycosaminoglycan and glycosphingolipid metabolism (Fig. 2G). The ToppCell Atlas allows comparison of DEGs with published single-cell data sets. Both DIS and FSK had increased representation of genes related to immune response, although no immune cells were present in the system (Fig. 2H). Ingenuity pathway analysis (IPA) identified many common regulators of cell proliferation such as Yes-associated protein (YAP) and FOXM1 and reduced predicted regulatory effects of interferons and transforming growth factor-β (TGF-β) in DIS compared with FSK (Fig. 2I). Regulatory network analysis identified many of these same regulatory elements, with interferon-response factors being reduced in DIS and ubiquitin and sumoylation regulatory factors increased in DIS. Although regulators such as colony-stimulating factor-2 (CSF2), Erb-b2 receptor tyrosine kinase 2 (ERBB2), and epidermal growth factor receptor (EGFR) were increased in both DIS and FSK, they were generally higher in FSK, suggesting that the cell proliferation of FSK is more strongly influenced by soluble factors (Fig. 2J). A list of all GSEA terms and regulators can be found in Supplemental Fig. S2 (see https://doi.org/10.6084/m9.figshare.12594656.v2). To validate these findings, we performed flow cytometry for PCNA-positive cells. FSK mLOs showed evidence of increased cell proliferation compared with other groups. In summary, although this mLO FSK and DIS experimental design had decreased power to identify changes in FSK-sensitive genes, the model demonstrated that increased mechanical strain activates the cell cycle and that although FSK does not promote the development of mesenchymal cell lineage genes and associated regulatory pathways, DIS does inhibit them.
Cyclic Stretch Model
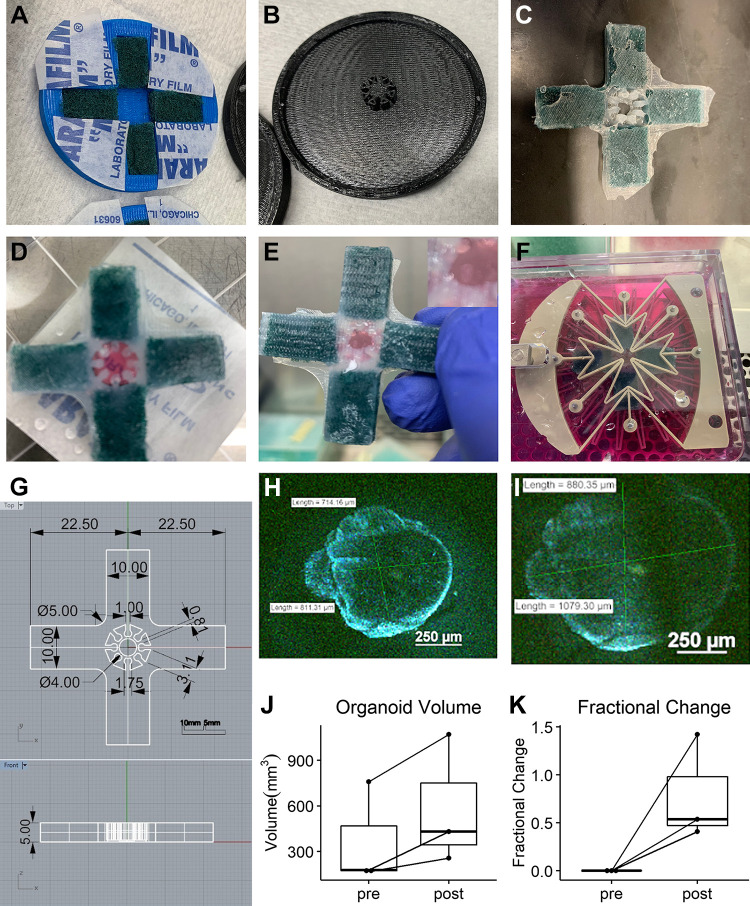
To characterize the differences in mLO gene expression in response to cyclic stretch, we developed a silicone insert to use with a CellScale MCB1 device that could apply biaxial cyclic stretch to an embedded lung organoid in tissue culture (Fig. 3A). This device consisted of two-piece 3-D printed mold in the shape of a cross with a lid with a spoke-and-arm pattern. A nylon fiber sponge was cut to occupy the majority of each arm and placed in the mold (Fig. 3, B and C). High-quality silicone was then added to the mold to minimize air bubbles. The top half of the mold was then applied, and the silicone was allowed to cure overnight. The following morning, the thin silicone membrane remaining in the center of the mold was removed (Fig. 3D). To mount the organoids, the silicone insert and CellScale A2 device were sterilized in 70% ethanol and the insert placed on a sterilized piece of Parafilm. Then, 50–100 µL of Matrigel was placed in the mounting chamber of the insert and a single mouse lung organoid pipetted into the center (Fig. 3E). An additional 50–100 µL of Matrigel was pipetted into the spokes of the insert until the organoid was covered. After curing for 10 min at 37°C, the insert was placed in the CellScale A2 device and cell culture media added (Fig. 3F). Dimensions are provided in Fig. 3G. For control mLOs, the insert and the mLO were cultured in a 25-cm2 dish. An appreciable increase in volume could be observed between unstretched (Fig. 3H) and stretched (Fig. 3I) states. Both the height of the Matrigel within the silicone insert and the volume of three mLOs at the baseline and after application of stretch were measured using a two-photon confocal microscope. Measurement of Matrigel height revealed a 7.6% decrease, but measurement of organoid volume revealed an ∼50% increase in volume (Fig. 3J), with smaller mLOs having a greater percentage volume increase (Fig. 3K). These data show that the application of cyclic biaxial strain to mLOs using this silicone insert and CellScale A2 device results in cyclic changes to organoid volume.
Figure 3.
Organoid cyclic stretch model. A: a 3-D-printed base has four cut nylon sponges placed in the four arms of the insert cross. B: a 3-D-printed lid has 8 radial spokes. C: after filling the mold with high-grade silicone and curing overnight, the insert is ready to be sterilized in 70% ethanol for use. D: after placing on a sterilized piece of parafilm, 50–100 µL of Matrigel is added. E: using a cut pipette tip, a single mouse lung organoid is placed in the well of the insert with minimal PBS and then covered with an additional 50–100 µL of Matrigel from the spaces between the spokes to keep the organoid in the well of the insert. F: the insert is placed into the biaxial stretching mount of a CellScale A2. G: schematic showing the dimensions of the silicone mold. H: 3-D-image of a membrane-stained mLO before stretch. Scale bar = 250 µm. Organoid dimensions are shown. (I) Image of the same organoid with static hold of 10.25% biaxial stretch. Scale bar = 250 µm. Quantification of the absolute (J) and relative change (K) in mLO volume with biaxial stretch. mLO, mouse lung organoid; 3-D, three-dimensional.
Impact of Cyclic Mechanical Stretch on mLO Gene Expression
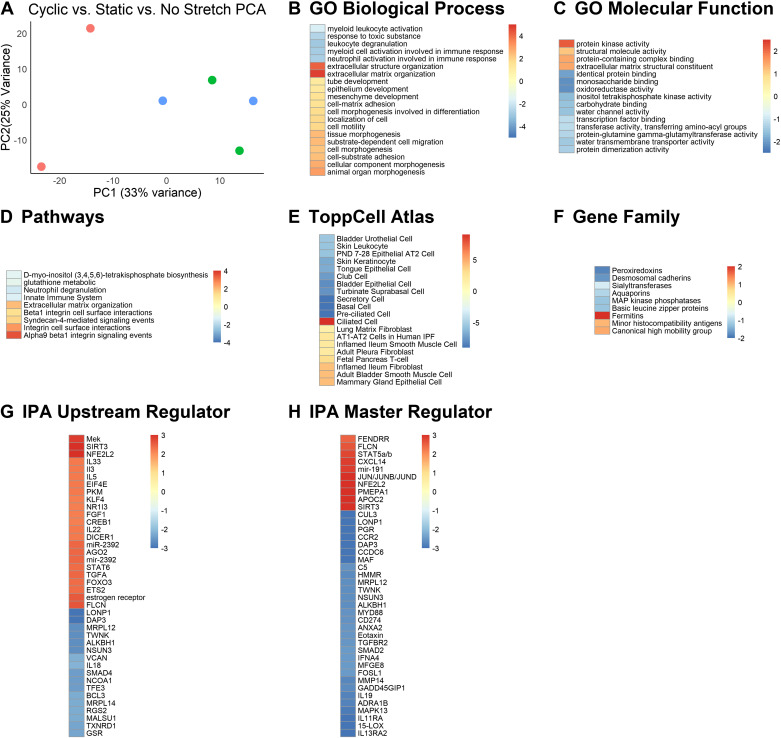
We subjected organoids to 20 h of cyclic stretch and extracted RNA from these mLOs, mLOs that were identically mounted and subjected to the same amount of static stretch, and mLOs mounted but not stretched. PCA revealed similarity between static and no stretch mLOs and good separation between these two groups and cyclic stretch (Fig. 4A). There were 281 genes with corrected P values of <0.1 that were twofold increased or decreased. Since static and no stretch mLOs were similar, we compared them with control cyclic stretch mLOs to determine whether cyclic stretch activated GO biological processes related to extracellular matrix structure and organization, cell-matrix adhesion, and mesenchyme development. Downregulated processes related to innate immunity (Fig. 4B). The statistical strength of GO molecular function terms were relatively weak with increased “protein kinase activity” and reduced “monosaccharide binding” and “oxioreductase activity” being most notable (Fig. 4C). ToppGene pathway analysis identified cell-matrix interactions via integrins as most significantly upregulated by cyclic stretch (Fig. 4D). Compared against static and no stretch, cyclic stretch reduced the abundance of genes associated with a host of lung epithelial cells and increased the number of genes associated with smooth muscle cells and fibroblasts (Fig. 4E). Similar to GO molecular function, gene family analysis was limited by statistical power, with fermitins being upregulated and peroxiredoxins being decreased. Fermitins regulate integrin-matrix interactions. Both IPA upstream regulatory (Fig. 4G) and master regulator (Fig. 4H) analysis were somewhat limited by weak signal strength, but results indicated that 1) cyclic stretch changes histone acetylation via SIRT3; 2) STAT signaling, MAP kinase activation, and transforming growth factor-α are upregulated; and 3) TGF-β and MyD88 signaling are reduced, and changes in microRNAs and long noncoding RNAs likely mediate many of the observed changes in gene expression. A list of all GSEA terms for this experiment are provided in Supplemental Fig. S3 (see https://doi.org/10.6084/m9.figshare.12594653.v2). Taken together, these data indicate that cyclic stretch promotes a shift away from the epithelial cell function and toward the maturation and organization of the extracellular matrix and interstitial cells.
Figure 4.
Analysis of mLOs in the cyclic stretch model. A: principal component analysis (PCA) of mLOs exposed to static stretch, cyclic stretch, or no stretch. PC1, Principal Component 1; PC2, Principal Component 2. B: gene ontogeny (GO) biological processes that were upregulated by cyclic stretch compared with no stretch were largely related to organization of the extracellular matrix and cell-matrix interaction. Color scale represents −log10 Bonferroni-corrected P value for upregulated processes and log10 for downregulated ones. A threshold of corrected P value of 0.1 was applied. C: GO molecular function analysis indicated activation of protein kinases and suppression of monosaccharide and reductase pathways related to carbohydrate metabolism. D: pathway analysis showed that cyclic stretch activated both extracellular matrix organization-related pathways and integrin signaling. E: the mRNAs of many epithelial cells were downregulated and the mRNAs of many fibroblasts and smooth muscle cells were upregulated by cyclic stretch. F: gene families that were downregulated included desmosomal cadherins likely related to suppression of epithelial cell-related processes and the antioxidant peroxiredoxins. Upregulated families included fermitins which regulate integrin-matrix interactions. G: ingenuity pathway analysis (IPA) for upstream regulators suggested that the MAP kinase Mek and the histone deacetylase SIRT3 were activated as were several microRNAs and the microRNA splicing gene DICER. Regulation by the TGF-β signaling molecule SMAD4 and the mitochondrial genes DAP3 and LONP1 was downregulated by cyclic stretch. Color scale represents the IPA activation score with positive being activated and negative being inhibited. H: regulatory network analysis identified the MAPK effector JUN, SIRT3, and the long-noncoding RNA FENDRR as positive regulators, and MYD88, SMAD2, and TGFBR2 as negative regulators. MAP, mitogen-activated protein; MAPK, mitogen-activated protein kinase; mLO, mouse lung organoid; TGF-β, transforming growth factor-β.
Changes in Mesenchymal Cells in Response to Cyclic Stretch
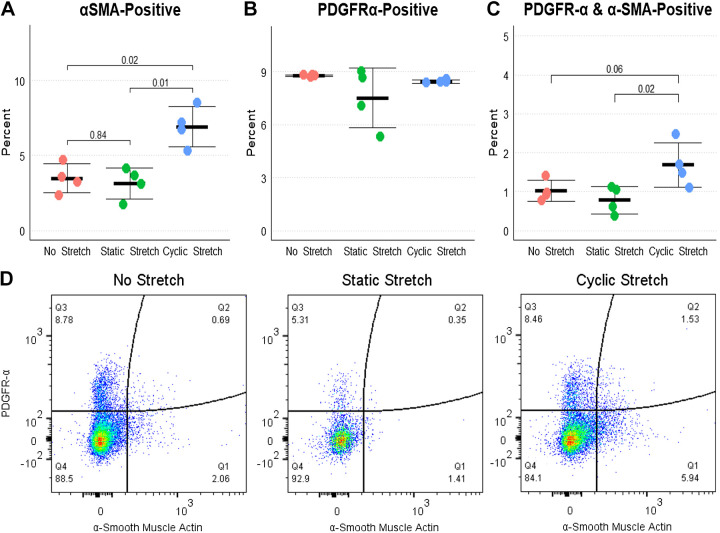
Since many of the pathways altered by cyclic stretch are known to be important in mesenchymal cell development, we quantified the percentages of αSMA-positive and PDGFRα-positive cells after 20 h of organoid embedding with no stretch, static stretch, or cyclic stretch. Cyclic stretch resulted in a near doubling of αSMA-positive and αSMA-PDGFRα-double-positive cells but no increase in the overall abundance of PDGFRα-positive cells (consistent with this receptor being expressed in several cell types; Fig. 5, A–D). These data support a role for cyclic stretch in maturation of lung mesenchymal cells.
Figure 5.
Changes in pulmonary fibroblasts with cyclic stretch. A: by flow cytometry, organoids subjected to 20 h of cyclic stretch had approximately double the number of α-smooth muscle actin (αSMA)-positive cells as unstretched and static stretch organoids. n = 4 per group. Kruskal–Wallis P < 0.01 and Dunn’s post hoc test results are shown. There was no overall difference in the total number of PDGFRα-positive cells (B), but the number of αSMA-PDGFRα-double-positive cells (C) was increased in cyclic stretch compared with static or no stretch. D: representative flow cytometry plots of the experiment. PDGFRα, platelet-derived growth factor receptor-α.
DISCUSSION
To the best of our knowledge, this is the first paper describing the impact of disruption, distention, and application of cyclic stretch on lung organoid gene expression. Since 1) the lung develops in the context of cyclic stretch after the 12th gestational week, 2) in humans many lung diseases such as chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis are marked by alterations in lung tissue dynamics, and 3) mechanical lung therapies such as FETO for CDH are emerging, these models and our findings are relevant to understanding normal and pathological lung mechanobiology.
Organoid disruption suppressed normal lung developmental processes. This observation likely reflects the loss of cell-matrix, cell-cell, and mechanotransductive cues that maintain cell homeostasis. Although hypercellularity is a characteristic of CDH, lung cell proliferation is actually decreased in animal CDH models (7, 8). However, in a mouse model of fetal tracheal occlusion (TO), lung DNA is increased and protein is decreased with hypercellularity (5). Thus, although the mLO disruption may not be a faithful model of CDH, it lends insight into how epithelial cells respond to the loss of matrix and mechanotransductive cues. These effects include lung cell differentiation. In a rabbit model of CDH, gene expression of CDH lung was less mature than the control, but it was actually more mature than that of TO or CDH/TO lung (7), and in a sheep model of TO, proximalization of the lung was observed (8). In DIS and FSK lung organoids, we observed increases in basal cell and AT1/AT2 progenitor cell-specific genes and a reduction in fibroblast and stromal cell genes.
A key element of this study was the inclusion of mesenchymal cells in mouse lung organoid models, which have been previously shown to support growth of larger mouse and human lung organoids (15). Many of the differences in gene expression in mLOs exposed to cyclic stretch involved pathways and processes associated with lung fibroblasts such as extracellular matrix organization and with cell-matrix interaction such as integrin-meditated signaling pathways. Comparison of genes increased by cyclic stretch with published single-cell data sets showed that cyclic stretch increased the abundance of many lung fibroblast-specific gene sets (26), consistent with the diversification of this lineage around the time that fetal breathing movements begin with continued differentiation during the alveolar stage of lung development. As studying subsets of lung mesenchymal cells presents a particular challenge because of limited, nonspecific, or timing-dependent genetic tools, this ex vivo cyclic stretch model represents a new tool by which these interactions and the importance of mechanical inputs in mesenchymal-epithelial interactions can be studied.
It is interesting to consider the pathways that were and were not identified in our study. Given the importance of PDGFRα and FGF signaling in alveolarization (15, 27), we expected to see differences in these pathways, but we did not. However, there were more PDGFRα-positive myofibroblasts in cyclic stretch organoids. Similarly, we did not identify difference in WNT (28), Sonic hedgehog (29, 30), or Notch (31) signaling as might be expected. YAP (32, 33) was upregulated in FSK but not identified in the cyclic stretch data set. FOXF1 adjacent noncoding developmental regulatory RNA (FENDRR) is a long noncoding RNA adjacent to the FoxJ1 locus important in suppressing fibrosis and cell proliferation (34). This suggests a potential link between the loss of normal lung mechanics and fibrotic progression. Many of the pathways altered in DIS, FSK, and cyclic stretch have also been shown to be changed in CDH and FETO. TGF-β (3, 35) and EGFR/TGF-α (4) have both been identified as dysregulated in rabbit CDH/FETO model, and changes in lung fibroblasts have been reported in mouse FETO with upregulation of myofibroblast and a downregulation of lipofibroblast mRNAs (26). These and other data sets (36) indicate the importance of extracellular matrix synthesis and organization in CDH and TO. We observed differences in extracellular matrix organization genes but were limited in our ability to detect differences in synthesis ones since forskolin directly induced many of these. Our data suggest that mitogen-activated protein (MAP) kinases are involved in mediating mechanotransductive signals, which is consistent with differences in MAP kinase activity in different regions of the TO lung (37) and a role in suppressing matrix genes (38). Finally, microRNA profile is predictive of positive response to FETO in human CDH (39). Our observation that cyclic stretch impacts several epigenetic programs and that αSMA increases with cyclic suggests a potential role for mechanical inputs epigenetically influencing mesenchymal cell fate. The microRNA splicer DICER was identified both in an ovine model of CDH/TO (8) and in our organoid data sets.
These models and our study have several limitations. We used postnatal day 5 epithelial cells and fibroblasts for organoid creation, and cell lineages at this age would be expected to be well established. The reason for using PND5 mice related to cell yield, but the use of cells from younger mice might have shown more significant differences in lung cell differentiation. We did not use any sorting technique to enhance lung epithelial cell homogeneity (40). At day 21, all mLOs were spheroid, and among DEGs, the only surfactant protein gene identified was surfactant protein D, which is known to be expressed in proximal airway epithelial cells. We thus believe that all or nearly all of the mLOs studied were proximal lung organoids (i.e., tracheospheres). These experiments cannot differentiate changes mediated by cell-cell signaling, cell-matrix interaction, or altered diffusion of soluble factors. Matrix and cell manipulations could help answer some of these important questions. Finally, we did not measure the direct mechanical forces imposed upon mLOs. For FSK and DIS experiments, RNA was collected from all of the organoids in a Transwell, and organoids of different sizes may have had different levels of increased or decreased strain or deformation with differences in gene expression that were lost, as the signal was averaged over all the cells in the well. For cyclic stretch experiments, we assume that the 5% stretch (10.25% change in area) was uniformly transmitted through the insert and Matrigel to the organoid, but we had no direct way of measuring this, as the organoid in the CellScale MCB1 device was outside the working distance of available microscopes.
Conclusion
In this mLO model, both increased and decreased static stretch induced cell proliferation, but decreased strain was associated with downregulation of important lung epithelial cell development programs. Cyclic stretch is important for lung mesenchymal cell-related processes.
SUPPLEMENTAL DATA
Supplemental Video S1: https://doi.org/10.6084/m9.figshare.17313998.v1.
Supplemental Fig. S1: https://doi.org/10.6084/m9.figshare.14192111.v5.
Supplemental Fig. S2: https://doi.org/10.6084/m9.figshare.12594656.v2
Supplemental Fig. S3: https://doi.org/10.6084/m9.figshare.12594653.v2.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant Nos. K08HL131261 and R01HL141229 (to B.M.V.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.J., M.R.B., and B.M.V. conceived and designed research; R.J., M.R.B., Q.F., and B.M.V. performed experiments; R.J., M.R.B., and B.M.V. analyzed data; R.J., M.R.B., and B.M.V. interpreted results of experiments; R.J. and B.M.V. prepared figures; R.J., M.R.B., and B.M.V. drafted manuscript; R.J., M.R.B., Q.F., and B.M.V. edited and revised manuscript; R.J., M.R.B., Q.F., and B.M.V. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Jenna Green in Dr. Anne Perl’s laboratory for assisting in developing the mLO model and Dr. John Brewington for helping develop the forskolin model. We thank Dr. John Matthew Kofron and Evan Meyer of CCHMC Confocal Imaging Core for assisting in organoid imaging. We thank the CCHMC Research Flow Cytometry Core for assistance.
REFERENCES
- 1.Al-Maary J, Eastwood MP, Russo FM, Deprest JA, Keijzer R. Fetal tracheal occlusion for severe pulmonary hypoplasia in isolated congenital diaphragmatic hernia: a systematic review and meta-analysis of survival. Ann Surg 264: 929–933, 2016. doi: 10.1097/SLA.0000000000001675. [DOI] [PubMed] [Google Scholar]
- 2.Rahman M, Sun R, Mukherjee S, Nilius B, Janssen LJ. TRPV4 stimulation releases ATP via pannexin channels in human pulmonary fibroblasts. Am J Respir Cell Mol Biol 59: 87–95, 2018. doi: 10.1165/rcmb.2017-0413OC. [DOI] [PubMed] [Google Scholar]
- 3.Vuckovic A, Herber-Jonat S, Flemmer AW, Ruehl IM, Votino C, Segers V, Benachi A, Martinovic J, Nowakowska D, Dzieniecka M, Jani JC. Increased TGF-β: a drawback of tracheal occlusion in human and experimental congenital diaphragmatic hernia? Am J Physiol Lung Cell Mol Physiol 310: L311–L327, 2016. doi: 10.1152/ajplung.00122.2015. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Huang Z, Nayak PS, Matthews BD, Warburton D, Shi W, Sanchez-Esteban J. Strain-induced differentiation of fetal type II epithelial cells is mediated via the integrin α6β1-ADAM17/tumor necrosis factor-α-converting enzyme (TACE) signaling pathway. J Biol Chem 288: 25646–25657, 2013. doi: 10.1074/jbc.M113.473777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aydin E, Joshi R, Oria M, Varisco BM, Lim FY, Peiro JL. Fetal tracheal occlusion in mice: a novel transuterine method. J Surg Res 229: 311–315, 2018. doi: 10.1016/j.jss.2018.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kitano Y, Yang EY, von Allmen D, Quinn TM, Adzick NS, Flake AW. Tracheal occlusion in the fetal rat: a new experimental model for the study of accelerated lung growth. J Pediatr Surg 33: 1741–1744, 1998. doi: 10.1016/s0022-3468(98)90275-5. [DOI] [PubMed] [Google Scholar]
- 7.Varisco BM, Sbragia L, Chen J, Scorletti F, Joshi R, Wong HR, Lopes-Figueira R, Oria M, Peiro J. Excessive reversal of epidermal growth factor receptor and ephrin signaling following tracheal occlusion in rabbit model of congenital diaphragmatic hernia. Mol Med 22: 398–411, 2016. doi: 10.2119/molmed.2016.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peiro JL, Oria M, Aydin E, Joshi R, Cabanas N, Schmidt R, Schroeder C, Marotta M, Varisco BM. Proteomic profiling of tracheal fluid in an ovine model of congenital diaphragmatic hernia and fetal tracheal occlusion. Am J Physiol Lung Cell Mol Physiol 315: L1028–L1041, 2018. doi: 10.1152/ajplung.00148.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pan J, Copland I, Post M, Yeger H, Cutz E. Mechanical stretch-induced serotonin release from pulmonary neuroendocrine cells: implications for lung development. Am J Physiol Lung Cell Mol Physiol 290: L185–L193, 2006. doi: 10.1152/ajplung.00167.2005. [DOI] [PubMed] [Google Scholar]
- 10.Pantazi D, Kitsiouli E, Karkabounas A, Trangas T, Nakos G, Lekka ME. Dipalmitoyl-phosphatidylcholine biosynthesis is induced by non-injurious mechanical stretch in a model of alveolar type II cells. Lipids 48: 827–838, 2013. doi: 10.1007/s11745-013-3800-8. [DOI] [PubMed] [Google Scholar]
- 11.Gudipaty SA, Lindblom J, Loftus PD, Redd MJ, Edes K, Davey CF, Krishnegowda V, Rosenblatt J. Mechanical stretch triggers rapid epithelial cell division through Piezo1. Nature 543: 118–121, 2017. doi: 10.1038/nature21407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barovsky K, Pedone C, Brooker G. Distinct mechanisms of forskolin-stimulated cyclic AMP accumulation and forskolin-potentiated hormone responses in C6-2B cells. Mol Pharmacol 25: 256–260, 1984. [PubMed] [Google Scholar]
- 13.Brewington JJ, Filbrandt ET, LaRosa FJ, Ostmann AJ, Strecker LM, Szczesniak RD, Clancy JP. Detection of CFTR function and modulation in primary human nasal cell spheroids. J Cyst Fibros 17: 26–33, 2018. doi: 10.1016/j.jcf.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Varisco BM, Ambalavanan N, Whitsett JA, Hagood JS. Thy-1 signals through PPARγ to promote lipofibroblast differentiation in the developing lung. Am J Respir Cell Mol Biol 46: 765–772, 2012. doi: 10.1165/rcmb.2011-0316OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Green J, Endale M, Auer H, Perl A-KT. Diversity of interstitial lung fibroblasts is regulated by platelet-derived growth factor receptor α kinase activity. Am J Respir Cell Mol Biol 54: 532–545, 2016. doi: 10.1165/rcmb.2015-0095OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen K, Vigliotti A, Bacca M, McMeeking RM, Deshpande VS, Holmes JW. Role of boundary conditions in determining cell alignment in response to stretch. Proc Natl Acad Sci USA 115: 986–991, 2018. doi: 10.1073/pnas.1715059115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29: 15–21, 2013. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.R Core Team. R: A Language and Environment for Statistical Computing (Online). Vienna, Austria: R Foundation for Statistical Computing, 2016. https://www.R-project.org/. [Google Scholar]
- 19.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15: 550, 2014. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wickham H. ggplot2: Elegant Graphics for Data Analysis. New York, NY: Springer-Verlag New York, Inc., 2019. https://ggplot2.tidyverse.org. [Google Scholar]
- 21.Chen J, Bardes EE, Aronow BJ, Jegga AG. ToppGene Suite for gene list enrichment analysis and candidate gene prioritization. Nucleic acids Res 37: W305–W311, 2009. doi: 10.1093/nar/gkp427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krämer A, Green J, Pollard J, Tugendreich S. Causal analysis approaches in ingenuity pathway analysis. Bioinformatics 30: 523–530, 2014. doi: 10.1093/bioinformatics/btt703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kolde R. pheatmap: Pretty Heatmaps (Online). https://CRAN.R-project.org/package=pheatmap [2020 Apr 28].
- 24.Chen H. VennDiagram: Generate High-Resolution Venn and Euler Plots (Online). https://CRAN.R-project.org/package=VennDiagram [2020 Jun 18].
- 25.Dinno A. dunn.test: Dunn’s Test of Multiple Comparisons Using Rank Sums (Online). https://CRAN.R-project.org/package=dunn.test [2020 May 28].
- 26.Seaborn T, St-Amand J, Cloutier M, Tremblay MG, Maltais F, Dinel S, Moulin V, Khan PA, Piedboeuf B. Identification of cellular processes that are rapidly modulated in response to tracheal occlusion within mice lungs. Pediatr Res 63: 124–130, 2008. doi: 10.1203/PDR.0b013e31815eba47. [DOI] [PubMed] [Google Scholar]
- 27.Chen L, Acciani T, Le Cras T, Lutzko C, Perl AK. Dynamic regulation of platelet-derived growth factor receptor α expression in alveolar fibroblasts during realveolarization. Am J Respir Cell Mol Biol 47: 517–527, 2012. doi: 10.1165/rcmb.2012-0030OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Langhe SP, Reynolds SD. Wnt signaling in lung organogenesis. Organogenesis 4: 100–108, 2008. doi: 10.4161/org.4.2.5856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Degirmenci B, Valenta T, Dimitrieva S, Hausmann G, Basler K. GLI1-expressing mesenchymal cells form the essential Wnt-secreting niche for colon stem cells. Nature 558: 449–453, 2018. doi: 10.1038/s41586-018-0190-3. [DOI] [PubMed] [Google Scholar]
- 30.Kugler MC, Loomis CA, Zhao Z, Cushman JC, Liu L, Munger JS. Sonic hedgehog signaling regulates myofibroblast function during alveolar septum formation in murine postnatal lung. Am J Respir Cell Mol Biol 57: 280–293, 2017. doi: 10.1165/rcmb.2016-0268OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guha A, Vasconcelos M, Zhao R, Gower AC, Rajagopal J, Cardoso WV. Analysis of Notch signaling-dependent gene expression in developing airways reveals diversity of Clara cells. PLoS One 9: e88848, 2014. doi: 10.1371/journal.pone.0088848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soldt Bj van, Qian J, Li J, Tang N, Lu J, Cardoso WV. Yap and its subcellular localization have distinct compartment-specific roles in the developing lung. Development 146: dev175810, 2019. doi: 10.1242/dev.175810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao R, Fallon TR, Saladi SV, Pardo-Saganta A, Villoria J, Mou H, Vinarsky V, Gonzalez-Celeiro M, Nunna N, Hariri LP, Camargo F, Ellisen LW, Rajagopal J. Yap tunes airway epithelial size and architecture by regulating the identity, maintenance, and self-renewal of stem cells. Dev Cell 30: 151–165, 2014. doi: 10.1016/j.devcel.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang C, Liang Y, Zeng X, Yang X, Xu D, Gou X, Sathiaseelan R, Senavirathna LK, Wang P, Liu L. Long noncoding RNA FENDRR exhibits antifibrotic activity in pulmonary fibrosis. Am J Respir Cell Mol Biol 62: 440–453, 2020. doi: 10.1165/rcmb.2018-0293OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Engels AC, Brady PD, Kammoun M, Finalet Ferreiro J, DeKoninck P, Endo M, Toelen J, Vermeesch JR, Deprest J. Pulmonary transcriptome analysis in the surgically induced rabbit model of diaphragmatic hernia treated with fetal tracheal occlusion. Dis Model Mech 9: 221–228, 2016. doi: 10.1242/dmm.021626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vuckovic A, Herber-Jonat S, Flemmer AW, Roubliova XI, Jani JC. Alveolarization genes modulated by fetal tracheal occlusion in the rabbit model for congenital diaphragmatic hernia: a randomized study. PloS One 8: e69210, 2013. doi: 10.1371/journal.pone.0069210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marwan AI, Shabeka U, Dobrinskikh E. Suggested mechanisms of tracheal occlusion mediated accelerated fetal lung growth: a case for heterogeneous topological zones. Front Pediatr 5: 295, 2017. doi: 10.3389/fped.2017.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu S, Parameswaran H, Young SM, Varisco BM. JNK suppresses pulmonary fibroblast elastogenesis during alveolar development. Respir Res 15: 34, 2014. doi: 10.1186/1465-9921-15-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pereira-Terra P, Deprest JA, Kholdebarin R, Khoshgoo N, DeKoninck P, Munck AA, Wang J, Zhu F, Rottier RJ, Iwasiow BM, Correia-Pinto J, Tibboel D, Post M, Keijzer R. Unique tracheal fluid microRNA signature predicts response to FETO in patients with congenital diaphragmatic hernia. Ann Surg 262: 1130–1140, 2015. doi: 10.1097/sla.0000000000001054. [DOI] [PubMed] [Google Scholar]
- 40.Tan Q, Ma XY, Liu W, Meridew JA, Jones DL, Haak AJ, Sicard D, Ligresti G, Tschumperlin DJ. Nascent lung organoids reveal epithelium- and bone morphogenetic protein–mediated suppression of fibroblast activation. Am J Respir Cell Mol Biol 61: 607–619, 2019. doi: 10.1165/rcmb.2018-0390OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Video S1: https://doi.org/10.6084/m9.figshare.17313998.v1.
Supplemental Fig. S1: https://doi.org/10.6084/m9.figshare.14192111.v5.
Supplemental Fig. S2: https://doi.org/10.6084/m9.figshare.12594656.v2
Supplemental Fig. S3: https://doi.org/10.6084/m9.figshare.12594653.v2.