Abstract
Disruption of the lung endothelial barrier is a hallmark of acute respiratory distress syndrome (ARDS), for which no effective pharmacologic treatments exist. Prior work has demonstrated that FTY720 S-phosphonate (Tys), an analog of sphingosine-1-phosphate (S1P) and FTY720, exhibits potent endothelial cell (EC) barrier protective properties. In this study, we investigated the in vitro and in vivo efficacy of Tys against methicillin-resistant Staphylococcus aureus (MRSA), a frequent bacterial cause of ARDS. Tys-protected human lung EC from barrier disruption induced by heat-killed MRSA (HK-MRSA) or staphylococcal α-toxin and attenuated MRSA-induced cytoskeletal changes associated with barrier disruption, including actin stress fiber formation and loss of peripheral VE-cadherin and cortactin. Tys-inhibited Rho and myosin light chain (MLC) activation after MRSA and blocked MRSA-induced NF-κB activation and release of the proinflammatory cytokines, IL-6 and IL-8. In vivo, intratracheal administration of live MRSA in mice caused significant vascular leakage and leukocyte infiltration into the alveolar space. Pre- or posttreatment with Tys attenuated MRSA-induced lung permeability and levels of alveolar neutrophils. Posttreatment with Tys significantly reduced levels of bronchoalveolar lavage (BAL) VCAM-1 and plasma IL-6 and KC induced by MRSA. Dynamic intravital imaging of mouse lungs demonstrated Tys attenuation of HK-MRSA-induced interstitial edema and neutrophil infiltration into lung tissue. Tys did not directly inhibit MRSA growth or viability in vitro. In conclusion, Tys inhibits lung EC barrier disruption and proinflammatory signaling induced by MRSA in vitro and attenuates acute lung injury induced by MRSA in vivo. These results support the potential utility of Tys as a novel ARDS therapeutic strategy.
Keywords: endothelium, inflammation, sepsis, S1P, Staphylococcus aureus
INTRODUCTION
Sepsis, a life-threatening syndrome caused by an overwhelming immune response to infection, is a leading cause of death among critically ill patients (1). A major complication of sepsis is the development of the acute respiratory distress syndrome (ARDS), a condition characterized by severely impaired gas exchange and high mortality (>30%), for which no effective pharmacologic treatments exist (2, 3). In patients with sepsis, the most common source of infection is the lungs (64%), and gram-positive bacteria are responsible for almost 50% of these cases (4). Staphylococcus aureus represents a frequently identified organism in gram-positive sepsis (4–6), and highly virulent, antibiotic-resistant strains such as methicillin-resistant S. aureus (MRSA) are particularly challenging to treat. In S. aureus-induced pneumonia, the intense inflammatory response leads to severe lung injury and ARDS (7), which is characterized by lung endothelial cell (EC) barrier disruption, increased permeability, extravasation of protein-rich fluid into alveoli, and severe pulmonary edema. Previous preclinical studies have demonstrated that EC injury is a major hallmark in MRSA-induced acute lung injury (ALI; 8, 9). Thus, therapeutic strategies are needed to protect EC barrier integrity, stabilize gas exchange, and improve MRSA-induced ARDS outcomes.
Sphingosine-1-phosphate (S1P) is an important regulator of vascular EC permeability in vitro and in vivo (10). It is homeostatically present in plasma and tissues and appears critical for the maintenance and repair of the endothelial barrier (10). A recent study demonstrated that serum levels of S1P are decreased significantly in sepsis-induced ARDS patients, with low S1P levels being associated with worse clinical outcomes (11), providing evidence for an important role in modulating ARDS. Mechanistically, S1P exerts its barrier-protective effects through ligation of the S1P receptor 1 (S1PR1), activating a series of downstream signaling events that include rapid and dramatic cortical actin ring formation at the EC periphery, Rac1 activation, peripheral cortactin recruitment, and myosin light chain phosphorylation, c-Abl activation, and junctional complex and focal adhesion rearrangement (10, 12). In vivo, S1P and its analog FTY720 (2-amino-2-[2-(4-octylphenyl)ethyl]propane-1,3-diol) significantly reduce pulmonary vascular leakage in some animal models of ALI, including LPS- and ventilator-induced ALI (13, 14). However, S1P and FTY720 carry potential risk as both may cause barrier disruption at higher concentrations (>10 μM), S1PR1 downregulation, and potential side effects that can be detrimental for patients with ARDS, such as bradycardia, increased airway hyperresponsiveness, and immunosuppression (15, 16).
Because of these limitations, there is significant interest in characterizing S1P and FTY720 analogs to identify those with superior therapeutic properties. Our prior work identified the FTY720 analog, (S)-FTY720-phosphonate (Tysiponate or Tys), as a promising barrier-protective candidate (15, 17, 18). Tys enhances EC barrier function in vitro and protects against multiple types of ALI in vivo, but unlike other similar agonists, it has the important property of preserving expression of the barrier promoting receptor S1PR1 (15, 17, 18). The mechanisms underlying the protective effects of Tys remain insufficiently characterized, and no prior reports have explored Tys-mediated protection in the clinically relevant MRSA model of ALI. Here, we characterize the effectiveness of Tys in attenuating MRSA-induced EC permeability, inflammation, and ALI in mice.
Some of these results previously have been reported in abstract form.
MATERIALS AND METHODS
Reagents
Unless otherwise specified, reagents were obtained from Millipore Sigma (St. Louis, MO). (S)-FTY720-phosphonate [(3S)-3-(amino)-3-(hydroxymethyl)-5-(4′-octylphenyl)-pentylphosphonic acid, Tys] was synthesized as previously described (19) and dissolved in ethanol, which also served as vehicle control. Anti-VE-cadherin (F-8) and VCAM-1 were purchased from Santa Cruz Biotechnology (Santa Cruz Biotechnology, CA). Alexa Fluor 568 Phalloidin was purchased from Thermo Fisher Scientific (Grand Island, NY). Anti-phospho-MLC, anti-p-NF-κB p65, pan-NF-κB, and anti-RhoA were purchased from Cell Signaling Technology (Danvers, MA). ROCK inhibitor Y-27632 was purchased from Selleckchem (Houston, TX). Human IL-6 and IL-8 ELISA kits and Alexa Fluor 488 anti-mouse Ly-6G/Ly-6C (Gr-1) were purchased from BioLegend (San Diego, CA). Mouse IL-6 ELISA kit was from Biolegend, and mouse KC kit from R&D Systems (Minneapolis, MN). Heat-killed Staphylococcus aureus (HK-SA) was from InvivoGen (San Diego, CA), and active recombinant Staphylococcus α-hemolysin protein (or α-toxin) from Abcam (Waltham, MA).
Cell Culture
Human pulmonary artery endothelial cells (HPAECs) and human lung microvascular endothelial cells (HLMVECs; Lonza, Walkersville, MD) were cultured in EGM-2 complete medium (Lonza) with 10% FBS. Passages 6–9 of EC were used for experimentation. ECs were maintained in EGM-2 with 2% FBS overnight before the indicated treatments.
Methicillin-Resistant Staphylococcus Aureus Preparation
USA300 CA-MRSA wild-type LAC strain [gift from Drs. Michael Otto (National Institute of Allergy and Infectious Diseases) and Juliane B. Wardenburg (Washington University)] was used in this study. To prepare an inoculum for animals, a frozen stock of S. aureus was incubated on a tryptic soy agar plate at 37°C overnight. A single colony was placed into tryptic soy broth (3 mL, TSB) and incubated overnight in a shaker set at 250 rpm and 37°C. One millilitre of overnight culture was then grown in 100 mL of TSB solution until OD650 was ∼0.5. Fifty millilitres of bacteria were then centrifuged at 6,000 g for 15 min at 4°C, washed in sterilized phosphate-buffered solution (PBS), and resuspended in PBS. For this ALI model, the volume of PBS for bacterial resuspension was 1.5 mL, resulting in an estimated concentration of 1.0 × 108 colony-forming units (CFUs) per 30 µL volume. After bacterial resuspension, the solution was immediately used for animal inoculation. All inocula were then quantified by plating serial dilutions in PBS on tryptic soy agar and counting colonies after overnight incubation at 37°C. Heat-killed MRSA (HK-MRSA) stocks were prepared by heating a suspension of MRSA at 60°C for 1 h.
Transendothelial Electrical Resistance Measurements
HPAECs or HLMVECs were seeded in EGM-2 (2% FBS) in polycarbonate wells containing evaporated gold microelectrodes. Transendothelial electrical resistance (TER) measurements were performed using an electric cell-substrate impedance sensing (ECIS) system (Applied Biophysics, Troy, NY), as we have previously described (15). TER values from each microelectrode were pooled at discrete time points and plotted versus time as the means ± SE. For statistical comparisons, the area under the curve (AUC) was calculated for each condition.
Immunofluorescence
Confluent HPAECs grown on 35-mm glass-bottom dishes (MatTek, MA) were treated with HK-MRSA and/or 1 μM Tys as indicated. Cells were then fixed in 4% paraformaldehyde for 20 min and permeabilized with 0.2% Triton X-100 for 5 min. After blocking with 2% BSA in PBS for 1 h, cells were incubated with primary antibodies overnight at 4°C, followed by staining with secondary antibodies conjugated to Alexa-488 or Alexa-568 for 1 h at room temperature. Actin was stained with phalloidin for 30 min at room temperature. Control and treatments groups were processed at the same time under the same conditions. Cells were analyzed using a Nikon Eclipse TE 300 microscope and imaged using a Sony Digital Photo camera DKC 5000.
Cytokine Measurements
IL-6 and IL-8 levels were determined using ELISA, according to the manufacturer’s instructions.
Immunoprecipitation for Assessment of Rho Activation
Seven hundred micrograms of cell lysates (in 500 μL of lysis buffer) were immunoprecipitated with 30 μg rhotekin RBD agarose by gentle agitation for 45 min at 4°C. The beads were washed with lysis buffer three times. The proteins were obtained by boiling beads in SDS-sample buffer at 95°C for 5 min and separated by SDS-electrophoresis.
Western Blotting
Proteins were separated in 4–20% SDS-PAGE gels (Bio-Rad, Hercules, CA) and transferred onto nitrocellulose membranes. The blots were incubated with appropriate primary antibodies overnight at 4°C or 1 h at room temperature, followed by incubation with horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature. Protein bands were detected with enhanced chemiluminescence (Pierce, Rockford, IL). Densitometric analysis was performed using ImageJ (NIH, Bethesda, MD).
Murine Model of MRSA-Induced ALI
Experimental conditions and animal care procedures were approved by the University of Illinois at Chicago Animal Care and Use Committee.
Pretreatment study.
Anesthetized (ketamine/xylazine) wild-type (WT) C57BL/6 female mice (age 8–12 wk) received FTY720 (S)-phosphonate (Tys) 0.5 mg/kg ip or vehicle 1 h before challenge with live MRSA (USA300 CA-MRSA wild-type LAC strain; 20) intratracheally (it) 1.25 × 108 CFU/mouse, or an equal volume of PBS (vehicle).
Posttreatment study.
Anesthetized WT male mice (8–12 wk) received live MRSA (it, 0.75 × 108 CFU/mouse) and then 1 h later were injected with Tys (0.5 mg/kg ip). In both studies, lung injury indices were measured 18 h after MRSA. Bronchoalveolar lavage (BAL) fluid and lung tissue were harvested and processed, as we have described (21). Briefly, BAL is centrifuged at 500 g for 20 min to pellet the BAL cells. Red blood cells were lysed using red blood cell lysis buffer (Qiagen, Valencia, CA). Total BAL cell counts were measured using the Bio-Rad TC10 automated cell counter device. For differential counting of BAL cells, cytospins were prepared and stained with Kwik Diff Stain (Thermo Fisher Scientific). BAL protein was measured with the BCA protein assay kit (Thermo Fisher Scientific) in the cell-free BAL fluid. For lung histology, lungs were fixed in paraformaldehyde and processed for hematoxylin and eosin (H&E) staining, as we have described before (21). Lung histology was performed at the Research Histology and Tissue Imaging Core of UIC. Lung tissues were scanned using a Leica Aperio AT2 at ×40. Images (.svs files) were analyzed using Aperio ImageScope (Leica).
Two-Photon Intravital Microscopy
Anesthetized (ketamine-xylazine) wild-type (WT) C57BL/6 mice (age 8–12 wk) received Tys 0.5 mg/kg ip or vehicle control 1 h before challenge with HK-MRSA intratracheally (it) 2 × 108 CFU/mouse. Control mice received an equal volume of PBS (vehicle). Eighteen hours following treatment, mice were again anesthetized, intubated, and placed on mechanical ventilation (Harvard Apparatus, Inspira Ventilator) at a tidal volume of 10 mL/kg, a respiratory rate of 100 breaths/min, and positive end-expiratory pressure (PEEP) = 0 cmH2O. To label blood plasma and neutrophils, tetramethylrhodamine (TMR)-conjugated Dextran (70 kDa, 20 mL/kg) and Alexa488-conjugated Gr1 antibody (0.1 mg/kg) were diluted in sterile saline and administered via intravenous injection. Subsequently, a left thoracotomy was performed with exposure of the pleural space, and a custom-made “lung window” equipped with low vacuum pressure was positioned over the exposed lung, similar to previously described (22). Gentle suction pressure was applied until the lung tissue was captured against a 12-mm glass coverslip.
Image acquisition.
An Ultima In vivo Microscopy System (Prairie Technologies) using a high-speed resonant scanner was used to obtain images of the live lung. The excitation wavelength was set to 810 nm and emission filters were set to 560–610 nm and 490–540 nm for the red and green channels, respectively. A ×60 Nikon water immersion objective was used for imaging. Time series between 30 s and 5 min in 2–5 different fields were obtained for each subject.
Image processing and data analysis.
To obtain stable and consistent images without respiratory motion artifact, each series of TIF files were processed through ImageJ software and a custom macro, which enabled isolation of images obtained during similar points in the respiratory cycle for further analysis. The number of neutrophils per field was counted for each time series and averaged per subject. The interstitium was defined as the TMR-stained area between individual alveoli, and interstitial thickness was quantified at the narrowest point between two alveoli at end inspiration (i.e., largest alveolar size). Lung fields, in which alveolar edema or hemorrhage was present, demonstrated uniform TMR staining and were not quantified. The interstitial thickness was calculated by measuring the rhodamine-labeled area between individual alveoli at 10–15 different locations in a given field and averaged for each subject. A typical analyzed image contained 5–6 alveoli for quantification.
Statistical Analysis
Results are expressed as means ± SD. For ECIS graphs and quantification, data are presented as means ± SE. Student’s t test or analysis of variance (ANOVA) was used to compare differences between two or more groups, respectively. Statistical analysis was performed using the GraphPad Prism 8 software. P values <0.05 were considered significant.
RESULTS
Tys Attenuates MRSA-Induced Endothelial Cell Barrier Disruption
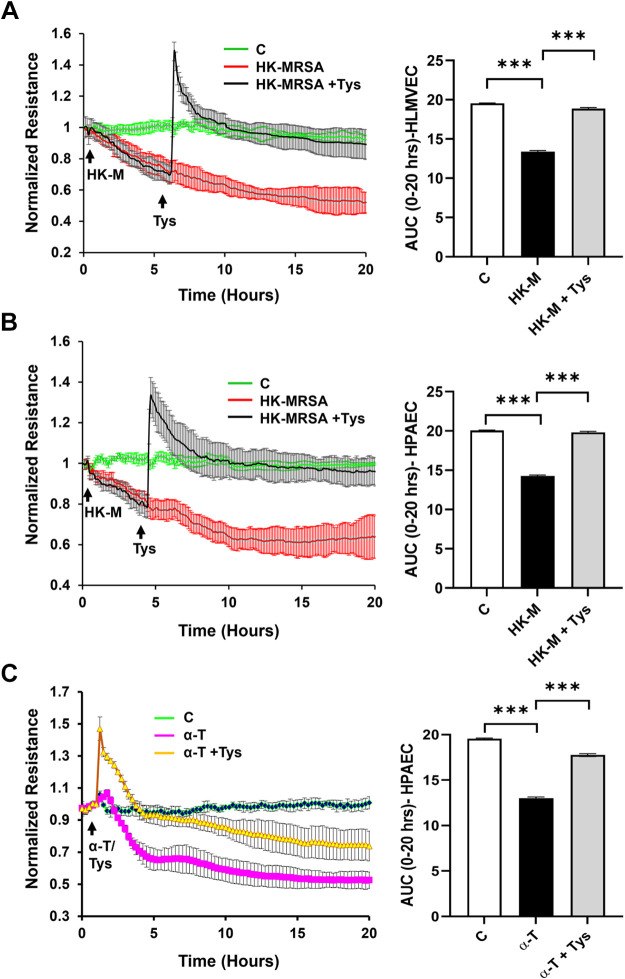
We first evaluated the impact of HK-MRSA on human lung endothelial permeability, using both HLMVECs and HPAECs. As depicted in Fig. 1, HK-MRSA exposure induced a consistent and sustained decrease in TER over time in both microvascular HLMVECs (Fig. 1A) and macrovascular HPAECs (Fig. 1B), indicative of EC barrier disruption. Area under the TER curves were quantified and pooled for multiple independent experiments to demonstrate statistical effects (bar graphs in Fig. 1). HK-MRSA-induced permeability was completely reversed in cells treated with Tys either 6 or 4 h after the onset of MRSA injury, respectively. Tys also was effective in inhibiting MRSA-induced EC permeability, when given either 1 h before or simultaneously with MRSA (Supplemental Fig. S1, A and B; all Supplemental Material is available at https://doi.org/10.6084/m9.figshare.16934899). Live S. aureus produces α-hemolysin (or α-toxin) as a potent pathogenic mediator (23, 24). Since this toxin is inactivated after heat-killing of the bacteria, we also investigated the effect of Tys on EC barrier function induced by α-toxin alone. As demonstrated in Fig. 1C, α-toxin caused a significant decrease in lung EC TER (i.e., increased permeability), which was ameliorated in cells treated with Tys.
Figure 1.
Tys protects against HK-MRSA- or α-toxin induced lung endothelial barrier disruption. Endothelial permeability was monitored using the electric cell-substrate impedance sensing (ECIS) system. HLMVEC (A) or HPAEC (B) on ECIS plates were treated with HK-MRSA (2 × 108/mL) for 6 h or 4 h, respectively. C: HPAECs were treated with α-toxin (150 ng/mL). Tys (1 μM) was added, as indicated by the arrow, and transendothelial resistance values (TERs) were recorded for up to 20 h. Resistance was normalized at the time point before HK-MRSA/α-toxin addition. Depicted are pooled TER tracings and corresponding area under the curve (AUC) quantifications for each condition. n = 3 or 4 independent experiments. One-way ANOVA, ***P < 0.001. α-T, α-toxin; C, control; HK-M, HK-MRSA, heat-killed MRSA; HLMVEC, human lung microvascular endothelial cell; HPAEC, human pulmonary artery endothelial cell; MRSA, methicillin-resistant Staphylococcus aureus.
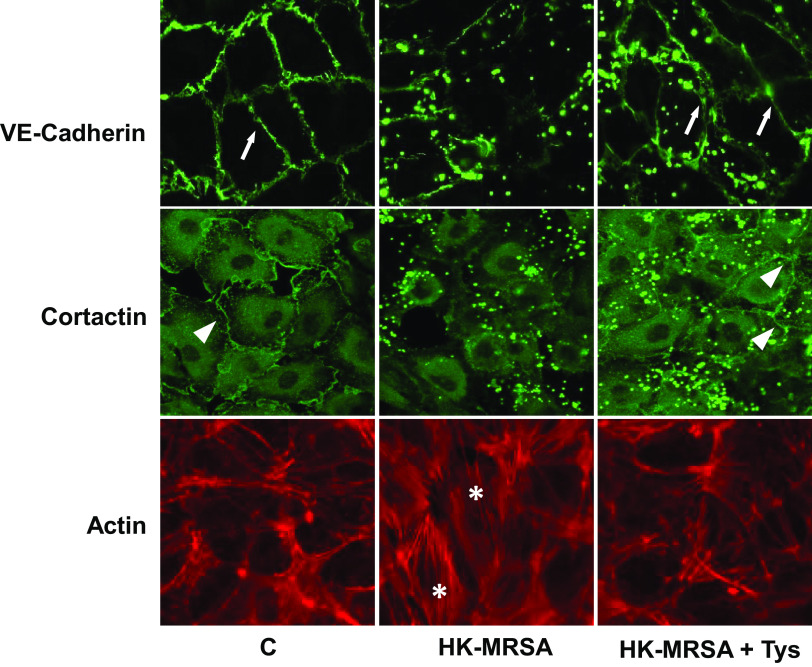
Tys Reverses MRSA-Induced Cytoskeletal Rearrangement and VE-Cadherin Junctional Disruption
Next, we explored the mechanisms by which Tys exerts its barrier protective properties against MRSA. The contraction of actin stress fibers leads to the formation of intercellular gaps between EC, whereas VE-cadherin is a junctional transmembrane protein that plays a key role in the maintenance and regulation of endothelial barrier integrity (12). In addition, cortactin is an actin-binding protein that participates in dynamic peripheral actin rearrangements that promote EC barrier function (12, 25, 26). Therefore, we determined via immunofluorescence the effects of HK-MRSA on the distributions of VE-cadherin and cortactin, and on the actin stress fiber formation in human lung EC. HK-MRSA stimulation for 6 h substantially reduced peripheral localization of VE-cadherin and cortactin and increased formation of actin stress fibers in HPAECs (Fig. 2). In contrast, Tys attenuated loss of VE-cadherin and cortactin from the cell-cell junctions and reduced actin stress fiber formation induced by HK-MRSA. Artifactual dot-shaped fluorescence also is observed in Fig. 2 images, when using the USA 300 MRSA strain. We performed additional immunofluorescence experiments using an alternative commercially available strain of methicillin-sensitive S. aureus that greatly reduced the background artifactual staining (Supplemental Fig. S2). In these supplemental experiments, HK-methicillin sensitive SA (HK-SA) caused intercellular gap formation and disruption of peripheral cortactin, and these effects were again ameliorated by Tys.
Figure 2.
Effect of Tys on cytoskeletal remodeling and junctional disruption induced by HK-MRSA. HPAECs were treated with HK-MRSA (2 × 108/mL) for 6 h and then treated with Tys (1 μΜ) for 15 min. Cells were fixed and analyzed by immunofluorescence after staining with VE-cadherin Ab, cortactin Ab, or phalloidin (actin staining). Each staining was performed in a different set of cells. Depicted are representative images from three or more independent experiments. White arrows and triangles indicate peripheral VE-cadherin and cortactin staining, respectively. *Stress fiber formation. Pictures were taken at ×40 magnification. HK-MRSA, heat-killed MRSA; HPAEC, human pulmonary artery endothelial cell; MRSA, methicillin-resistant Staphylococcus aureus.
Tys Inhibits MRSA-Induced MLC Phosphorylation and RhoA Activation
Rho family GTPases play a critical role in the regulation of endothelial barrier function (27, 28). Activation of Rho increases MLC phosphorylation and leads to the formation of actin stress fibers, cellular contraction, and barrier disruption. To investigate the effects of HK-MRSA on MLC phosphorylation and RhoA activation, HPAECs were exposed to HK-MRSA and Tys or vehicle for 0.5–1 h. HK-MRSA significantly increased MLC phosphorylation and Rho activation, although these effects were inhibited by Tys (Fig. 3, A–D). To determine the role of Rho activation in HK-MRSA-induced barrier disruption, HPAECs were pretreated with the Rho kinase inhibitor Y-27632 for 1 h and then exposed to HK-MRSA. ROCK inhibition decreased baseline EC resistance but also modestly attenuated HK-MRSA-induced EC barrier disruption (Fig. 3E), suggesting that Tys-mediated protection (Fig. 1) may be mediated in part via this pathway.
Figure 3.
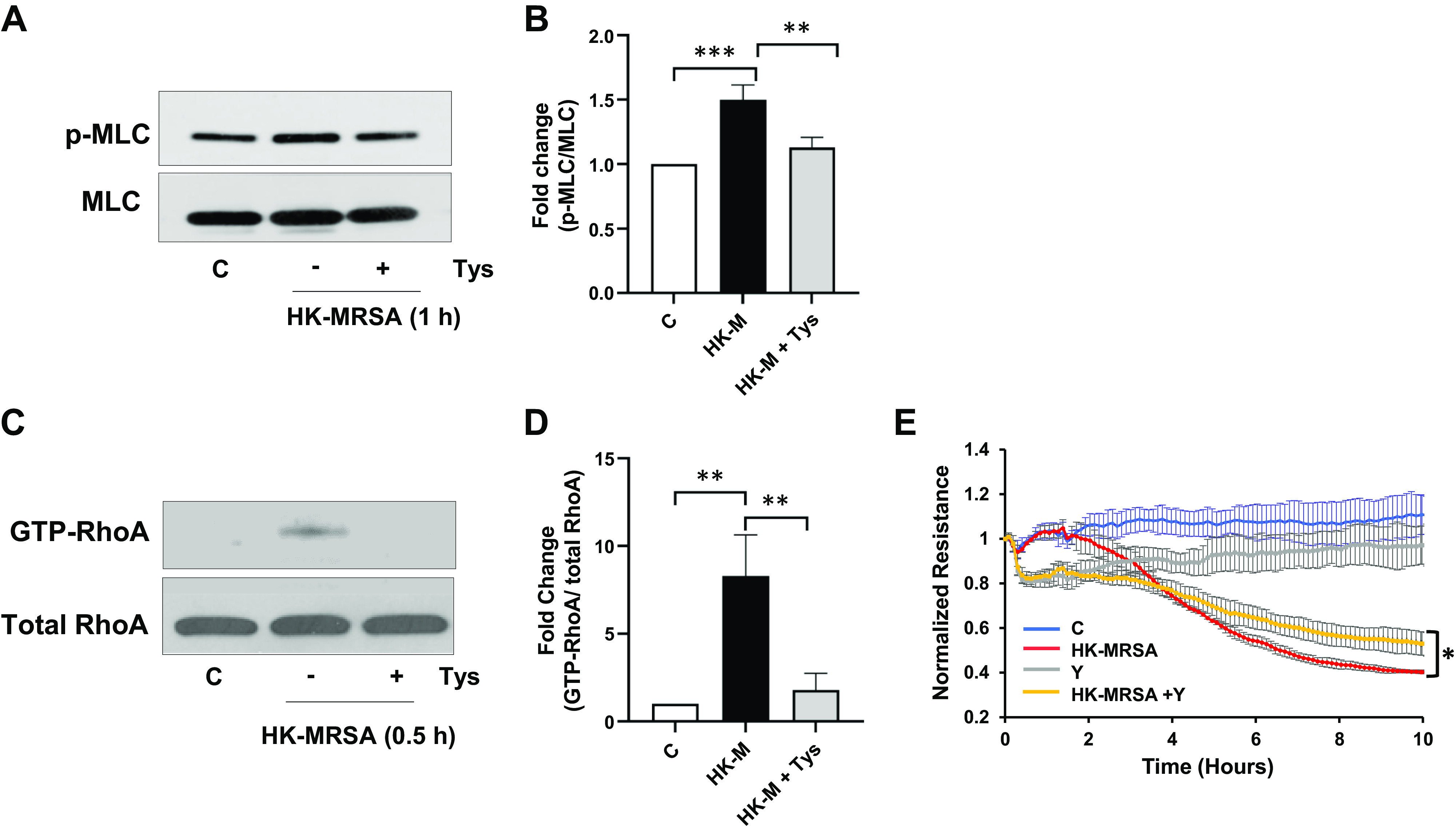
Tys inhibits MLC phosphorylation and RhoA activation induced by HK-MRSA. A and B: HPAECs were treated with HK-MRSA (2 × 108/mL) and Tys (1 μΜ) for 1 h. A: representative Western blots of pMLC and total MLC. B: densitometric analysis of Western blot data. The graph depicts the fold expression of pMLC/MLC. n = 3. C and D: HPAECs were treated with HK-MRSA (2 × 108/mL) and Tys (1 μΜ) for 0.5 h. RhoA activation was evaluated by Rho Activation Assay Biochem Kit. Depicted are representative blots of GTP-RhoA and total RhoA (C) and corresponding densitometric analysis (D). n = 3. E: HPAECs on ECIS plates were pretreated with 10 μM ROCK Inhibitor (Y-27632) for 1 h, then treated with 2 × 108/mL HK-MRSA. TER was monitored over a 10-h period using ECIS. Depicted are pooled TER tracings from three independent experiments. One-way ANOVA (B and D), t test (E). *P < 0.05, **P < 0.01, ***P < 0.001. C, control; ECIS, electric cell-substrate impedance sensing; HK-M, HK-MRSA, heat-killed MRSA; HPAEC, human pulmonary artery endothelial cell; MRSA, methicillin-resistant Staphylococcus aureus; TER, transendothelial electrical resistance; Y, Y-27632.
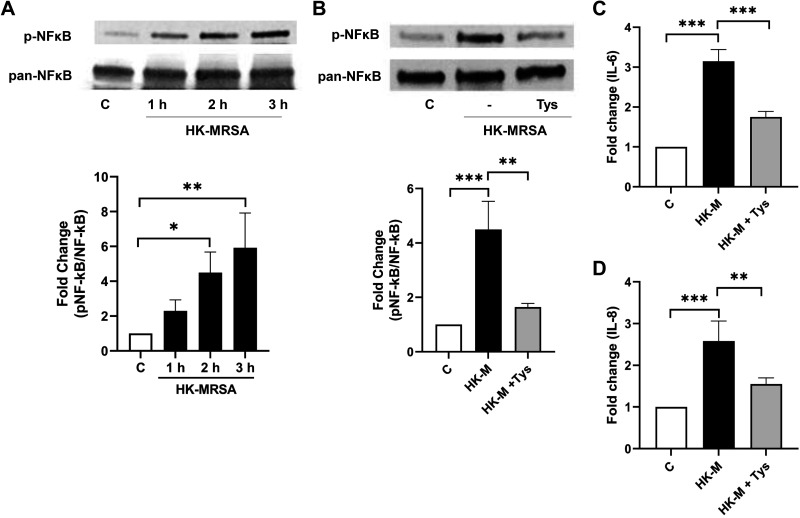
Tys Inhibits MRSA-Induced Proinflammatory Signaling
NF-κB pathways participate in MRSA-induced inflammatory responses (8, 29, 30). In HPAEC, HK-MRSA significantly increases phosphorylation of NF-κB in a time-dependent manner (Fig. 4A), indicating activation of the NF-κB pathway. Tys significantly attenuates this NF-κB phosphorylation (Fig. 4B) as well as MRSA-induced IL-6 (Fig. 4C) and IL-8 release (Fig. 4D) from HPAEC. Thus, treatment with Tys reduces several aspects of inflammatory signaling that occur in lung EC after exposure to MRSA.
Figure 4.
Tys inhibits inflammatory signaling induced by HK-MRSA. HPAECs were treated with HK-MRSA (2 × 108/mL) for 1, 2, or 3 h (A); or with or without Tys (1 µM) for 3 h (B). Cell lysates were analyzed by Western blotting. Depicted are representative Western blots of phospho-NF-κB (p-NF-κB) and total NF-κB (pan-NF-κB) and corresponding densitometric analyses. n = 3. C and D: HPAECs were treated with HK-MRSA (2 × 108/mL) with or without Tys (1 µM) for 6 h, and IL-6 (C), or IL-8 (D) levels were detected by ELISA in the cell supernatant. n = 4 independent experiments. One-way ANOVA, *P < 0.05, **P < 0.01, ***P < 0.001. C, control; HK-M, HK-MRSA, heat-killed MRSA; HPAEC, human pulmonary artery endothelial cell; MRSA, methicillin-resistant Staphylococcus aureus.
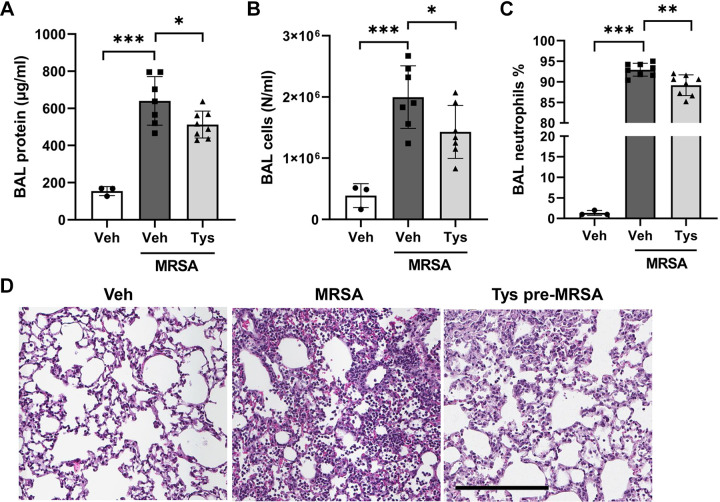
Tys Attenuates MRSA-Induced Lung Injury in Mice
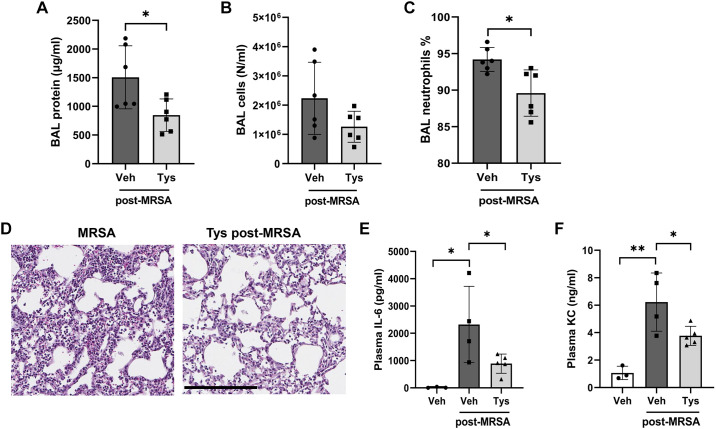
The protective properties of Tys were further examined in a murine model of MRSA-induced ALI. Intratracheal (it) administration of live MRSA caused significant increases in BAL protein levels, total cell counts, and neutrophils (Fig. 5, A–C), with neutrophils comprising over 90% of the total BAL cell count after MRSA (Fig. 5C). Histological analysis of lung tissue demonstrated dense inflammatory cell infiltrate and interstitial thickening in the lungs of MRSA-treated mice, findings indicative of acute lung injury (Fig. 5D). Pretreatment with Tys before MRSA administration significantly reduced BAL protein levels, total cell counts, and neutrophil percentage (Fig. 5, A–C). In addition, histological analysis of lung tissues demonstrated that Tys reduced the inflammatory cell infiltrate and interstitial thickening in mice receiving MRSA (Fig. 5D).
Figure 5.
Effects of Tys pretreatment on MRSA-induced acute lung injury. Wild-type mice were treated with Tys (0.5 mg/kg, ip) and then challenged with live bacteria-MRSA (1.25 × 108 CFU/mouse, intratracheally, 18 h). Bronchoalveolar lavage (BAL) protein (A), total cell counts (B), and neutrophil percentages were determined (C). D: H&E lung staining. Lung tissues were scanned with a digital slide scanner at ×40 and representative pictures were taken at ×20. Scale bar: 200 μm. Representative images are shown. n = 3–8 mice per condition. One-way ANOVA, *P < 0.05, **P < 0.01, ***P < 0.001. CFU, colony-forming unit; H&E, hematoxylin and eosin; MRSA, methicillin-resistant Staphylococcus aureus.
To assess the potential therapeutic effectiveness of Tys when administered postinfection, a separate group of mice received Tys 1 h after intratracheal administration of live MRSA. In this postinjury study, Tys significantly decreased BAL protein levels (Fig. 6A), trended toward reduced total cell counts by 44% (Fig. 6B), and significantly reduced the BAL neutrophil percentage induced by MRSA (Fig. 6C). When given post-MRSA infection, Tys also attenuated the neutrophil infiltration and other histologic changes in the lung tissues (Fig. 6D). Tys also significantly reduced the levels of VCAM-1 in the BAL of MRSA-injured mice (Supplemental Fig. S3, A and B), which links the effects of Tys to EC protection in vivo, since alveolar VCAM-1 is a putative biomarker of endothelial injury in ARDS (31). Moreover, Tys-mediated protection in our model of intratracheal MRSA was not limited to lung injury only. MRSA also caused systemic inflammation as demonstrated by significant increases in plasma levels of the inflammatory cytokines IL-6 and KC, which were greatly attenuated by Tys (Fig. 6, E and F). Taken together, these data demonstrate that Tys is protective when administered either pre- or post-MRSA infection in vivo.
Figure 6.
Effects of posttreatment with Tys on MRSA-induced acute lung injury. Wild-type mice were treated with live bacteria-MRSA (0.75 × 108 CFU/mouse, it) or PBS (control), and 1 h later received Tys (0.5 mg/kg, ip). BAL protein (A), total cell counts (B), and neutrophil percentages were determined (C). D: H&E lung staining. Lung tissues were scanned with a digital slide scanner at ×40 and representative pictures were taken at ×20. Scale bar: 200 μm. Representative images are shown. IL-6 (E) and KC (F) levels in plasma. n = 3–6 mice per condition. Unpaired t test (A–C), one-way ANOVA (E and F), *P < 0.05, **P < 0.01. BAL, bronchoalveolar lavage; CFU, colony-forming unit; H&E, hematoxylin and eosin; MRSA, methicillin-resistant Staphylococcus aureus; PBS, phosphate-buffered solution.
A recent report demonstrated that high concentrations of FTY720, a pharmaceutical compound related to Tys, displayed some antibacterial activity against S. aureus in vitro (32). To determine whether Tys has similar antibacterial properties against live MRSA at concentrations used in the current study, we performed assays to assess both bacterial growth and potential bactericidal effects of Tys. As seen in Supplemental Fig. S4A, neither Tys (1 μM) nor FTY720 affected MRSA growth in a plate assay, in contrast to the inhibitory effects of the antibiotic vancomycin (positive control). Similarly, neither Tys nor FTY720 affected MRSA viability as assessed by OD reading or colony counts (CFU—see Supplemental Methods), whereas vancomycin exhibited potent activity against live MRSA (Supplemental Fig. S4, B and C). These data argue against a direct antibacterial effect contributing to the protection mediated by Tys in the current study.
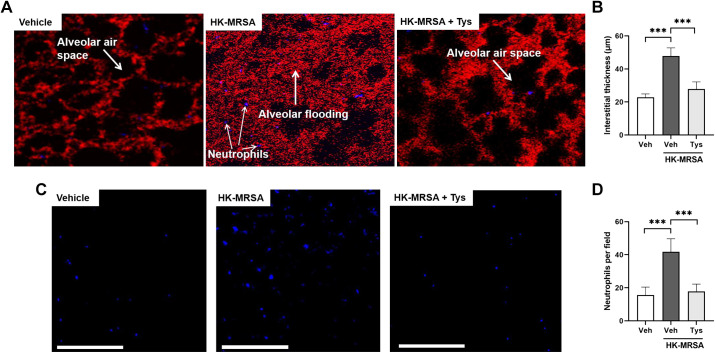
Intravital microscopy is a valuable tool for imaging biological processes in living animals in real time, including the lung tissue (22, 33–36). We used this imaging modality to dynamically assess the effects of Tys on lung neutrophil recruitment and interstitial edema induced by intratracheal administration of HK-MRSA. Live MRSA was not used in these experiments due to potential biohazard exposure concerns during the imaging process. After accessing the lung via thoracotomy, two-photon microscopy was performed to visualize labeled neutrophils and dextran within individual alveolar-capillary units. HK-MRSA resulted in substantial increases in neutrophil recruitment and interstitial thickening between alveolar airspaces (Fig. 7, A–D). However, pretreatment with Tys significantly attenuated these indices of ALI. These intravital data demonstrate the protective effects of Tys in live lung tissue and provide further evidence in support of its therapeutic potential.
Figure 7.
Intravital microscopy demonstrates attenuation of MRSA-induced neutrophil recruitment and interstitial edema after treatment with Tys. Wild-type mice were treated with Tys (0.5 mg/kg, ip) or vehicle 1 h before administration of HK-MRSA (2 × 108 CFU/mouse, it) or PBS (control). Live animal imaging by two-photon microscopy was performed 18 h following treatment. A: representative images showing the interstitial space visualized by TMR-labeled dextran (red) between alveolar airspaces (black) in each condition. Individual neutrophils labeled with fluorescent Gr1 antibody appear purple. B: quantification of interstitial thickness in µm. Additional representative images showing only fluorescent-labeled neutrophils (purple dots; C), and quantification of neutrophils per field (D). White scale bar = 200 μm, n = 5 mice per group. One-way ANOVA, ***P < 0.001. CFU, colony-forming unit; HK-MRSA, heat-killed MRSA; MRSA, methicillin-resistant Staphylococcus aureus; PBS, phosphate-buffered solution; TMR, tetramethylrhodamine.
DISCUSSION
This study demonstrates that (S)-FTY720-phosphonate (Tys), an analog of the endogenous sphingolipid S1P and the pharmaceutical agent FTY-720 (37), attenuates methicillin-resistant S. aureus (MRSA)-induced lung EC permeability and inflammation in vitro, and protects against acute lung injury in vivo. S. aureus is one of the most prominent bacterial pathogens in hospitalized patients, with total annual deaths comparable to acquired immunodeficiency syndrome (AIDS), tuberculosis, and viral hepatitis combined (38, 39). MRSA causes an intense inflammatory response that can lead to the development of pneumonia, severe respiratory failure, and ARDS (40–42). Because these severe clinical consequences of MRSA persist despite current treatment approaches, improved understanding of the pathophysiology underlying S. aureus infections is needed to facilitate the development of novel therapies.
Some prior studies have explored the effects of S. aureus on lung endothelial function in vitro and in vivo (8, 9, 30). Recently, it was demonstrated that heat-killed S. aureus (HK-SA) causes significant pulmonary EC barrier disruption in vitro and lung injury in vivo (8, 9, 30). The effects of MRSA have been less well-studied in these preclinical studies compared with antibiotic-sensitive S. aureus. In this study, we substantially advance prior work by investigating the protective effects of Tys on MRSA-induced injury in cultured human pulmonary endothelial cells, and in a clinically relevant mouse model of ALI caused by live bacteria. This study also builds upon our prior reports demonstrating that Tys has protective effects in vivo and in vitro against multiple sterile inflammatory stimuli, such as LPS, radiation, and bleomycin (15, 17, 18), and our recent report that live MRSA induces substantial ALI in mice (43).
Lung endothelial barrier disruption and inflammation are key features of ALI, and therefore compounds that protect EC barrier integrity and exert anti-inflammatory properties have significant therapeutic potential. In the current study, we demonstrate that Tys significantly reverses MRSA-induced barrier disruption in both HPAEC and HLMVEC (macro and microvascular lung EC; Fig. 1, A and B). Moreover, Tys protects the EC barrier when given prior or simultaneously with HK-MRSA (Supplemental Fig. S1, A and B). Endothelial barrier integrity is regulated by intracellular contractile forces, balanced by cell-cell and cell-matrix adhesive forces (12). Our previous work has revealed that Tys rapidly increases the EC barrier through a series of events that involve S1PR1 signaling, Rac1 activation, enhanced cortical actin, peripheral cortactin translocation, and junctional protein rearrangement (15, 17, 44). We hypothesize that Tys-mediated protection against HK-MRSA-induced EC permeability involves similar mechanisms. Here, we observed a profound loss of VE-cadherin from cell-cell junctions after HK-MRSA treatment (Fig. 2), consistent with prior studies using HK-SA (9). VE-cadherin is a major structural protein of cell-cell adherens junctions (AJs) that are critical for maintaining EC barrier integrity (45). Disruption of VE-cadherin complexes significantly increases vascular permeability (46). Tys-induced barrier protection is characterized by partial restoration of VE-cadherin at the cellular junctions (Fig. 2). Although we and others have previously demonstrated that Tys, and its endogenous analog S1P, enhance the presence of VE-cadherin in these junctions (44, 47), this is the first report to suggest that Tys increases VE-cadherin at cell-cell contact regions to protect against bacterial injury. Additional studies are needed to investigate the role of other AJ components such as β-catenin and P120, and tight junction complexes in mediating the protective effects of Tys after MRSA-induced barrier disruption.
Our studies also provide additional mechanistic insights underlying MRSA-induced lung EC barrier disruption and its amelioration by Tys. Similar to other barrier disrupting agents such as thrombin and LPS (48, 49), HK-MRSA causes actin stress fiber formation, Rho activation, MLC phosphorylation, and loss of peripheral cortactin (Figs. 2 and 3). These effects are consistent with those recently described in lung EC treated with HK-SA or the SA components peptidoglycans and lipoteichoic acid (8, 9, 30). Actin stress fibers confer contractile force that results in intercellular gap formation during EC barrier disruption (50). MLC phosphorylation is necessary for the formation and maintenance of stress fibers (25, 52), a process regulated by Rho signaling pathway (51). In the current study, we demonstrate that blocking Rho activation with the Rho kinase inhibitor Y-27632 attenuates MRSA-induced barrier disruption (Fig. 3E), providing further evidence of the involvement of Rho in MRSA-induced EC barrier disruption. Importantly, our data indicate that Tys provides EC barrier protection by attenuating these critical responses.
Severe S. aureus infection in the lung causes an intense inflammatory response characterized by dysregulated immune responses, influx of neutrophils, and increased cytokine levels in the alveolar space (53–55). Excessive proinflammatory cytokine production contributes to the development of severe respiratory failure and ARDS and is associated with poor outcomes in MRSA-related infections (56, 57). Previous studies have demonstrated that S. aureus activates the nuclear factor-κB (NF-κB) pathway in EC and induces release of multiple cytokines, including monocyte chemoattractant protein-1 (MCP-1), interleukin-8 (IL-8), IL-6, macrophage inflammatory protein-1, and tumor necrosis factor-α (TNF-α; 8, 9, 58). In agreement with these studies, we found that HK-MRSA significantly increases NF-κB activation, and IL-6 and IL-8 release from human lung EC, inflammatory effects each reduced by Tys (Fig. 4). Although this anti-inflammatory effect against MRSA by an S1P analog has not previously been described, other studies have reported that S1P receptor agonists may suppress NF-κB signaling and cytokine release from activated EC in the lung and other organs (59–61). More specifically, overexpression of S1PR1 in lung EC decreases NF-κB p65 phosphorylation and translocation into the nucleus (62). These studies highlight that S1PR1 receptor activation can play a protective role against endothelial proinflammatory signaling, possibly via direct effects on the NF-κB pathway. Our prior work demonstrated that Tys enhances EC barrier function by activating S1PR1 (15), but it remains to be investigated whether Tys suppresses NF-κB signaling and cytokine release upon MRSA infection by specifically targeting S1PR1 to affect the NF-κB pathway.
Our previous studies have demonstrated that Tys has superior protective properties compared with its analogs S1P or FTY720 in vitro and in vivo against LPS, radiation, and bleomycin (15, 17, 18). These models have provided important insights into the mechanisms underlying ALI and the protective properties of Tys; however, they lack the direct host-pathogen interaction that underlies bacterial lung injury and sepsis, which are the most common causes of ARDS. Therefore, in this study, we determined the efficacy of Tys against live MRSA infection, a more clinically relevant ALI model. MRSA causes a dramatic increase in BAL protein and neutrophil infiltration indicative of lung vascular leakage and inflammation, observations confirmed by lung histology (Figs. 5 and 6). Other groups have reported similar effects using either live MRSA, SA, or HK-SA (7–9, 63, 65). More importantly, Tys protects against MRSA-induced lung vascular leakage and neutrophil cell infiltration, not only when given pre-MRSA exposure, but also post-MRSA inoculation (Figs. 5 and 6). The protective effects of Tys include significantly attenuating MRSA-induced levels of VCAM-1 in BAL (Supplemental Fig. S3). VCAM-1 is an adhesion protein expressed primarily in vascular endothelium that is upregulated in lung EC during inflammation (64). It serves as a biomarker of endothelial injury and is increased in the alveolar space during ARDS (31). In the current study, BAL VCAM-1 levels were significantly increased in mice injured by MRSA but reduced by Tys, demonstrating that intratracheal MRSA in this preclinical model causes endothelial injury that can be ameliorated by Tys.
These in vivo observations are supported by the in vitro data, which indicate that Tys can completely restore EC barrier integrity compromised by MRSA (Fig. 1). This protection of the EC barrier by Tys in combination with the suppression of cytokine release in vitro (Fig. 4) represent mechanisms that together may reduce neutrophil infiltration into the lungs during MRSA-induced injury. Similar to this, other reports have suggested that S1P receptor agonists can ameliorate neutrophil infiltration by blocking EC dysfunction (60, 66). In addition, in our study, intratracheal MRSA causes not only local lung injury but also a systemic inflammation as assessed by the increased levels of circulating IL-6 and KC in the plasma (Fig. 6, E and F). Posttreatment with Tys ameliorated the levels of these inflammatory cytokines, providing in vivo evidence that Tys can reduce systemic inflammation induced by MRSA in this model. Although our in vitro data provide solid evidence that Tys can protect endothelial cells against MRSA, it is also possible that Tys may exert some protective effects via other key cell types involved in S. aureus-induced ALI (20, 63, 67, 68), such as immune cells or lung epithelium, as well as regulation of leukotrienes and other inflammatory signaling (69). It is also conceivable that Tys could exert some protective effects through direct action on bacteria. A recent study reported that the related compound FTY720 possesses antibacterial properties against several bacteria including S. aureus (32). However, that study utilized FTY720 concentrations more than 10 times greater than the Tys used in our study. In our experiments, neither Tys nor FTY720 displayed antibacterial activity against MRSA growth or viability at concentrations relevant to our in vitro assays and in vivo models (Supplemental Fig. S4). Thus, it seems unlikely that the protective effects of Tys observed in this study are caused by direct antibacterial effects on live MRSA.
Intravital microscopy techniques provide additional insights by illustrating that Tys attenuates MRSA-induced neutrophil recruitment and interstitial edema in lung tissue (Fig. 7). Live animal imaging of the intact lung enhances our understanding of the physiologic consequences of inflammatory lung injury and has the potential to demonstrate the detailed time course of pathologic events. Other groups previously used two-photon microscopy to examine neutrophil (36) and platelet (35) lung infiltration following inflammatory stimuli. Similar intravital techniques were used to characterize pulmonary edema in rat lungs following injury with hyperoxia or bleomycin (34), and we recently used two-photon microscopy to characterize the effects of group V phospholipase A2 in a murine MRSA pneumonia model (43). Our current work demonstrates a further role for intravital microscopy in understanding the immunologic and pulmonary vascular effects of MRSA infection, as well as the protective effects of potential therapeutic agents.
Our study has several important limitations. First, we primarily used HPAECs, which are macrovascular EC, to investigate the underlying mechanisms of Tys on MRSA-induced EC dysfunction. Although HPAECs are widely used in the field as a model for lung EC in ARDS (8, 70), vascular leakage occurs primarily in the microvasculature. Therefore, we first demonstrated that Tys has potent protective properties against MRSA-induced EC permeability in both HLMVEC and HPAEC (Fig. 1), suggesting it has similar effects in both microvascular and macrovascular lung EC. Second, there are potential mechanistic discrepancies between our in vitro experiments that used heat-killed bacteria and the in vivo mouse studies in which live MRSA were primarily used. There are multiple mechanisms by which live bacteria are likely to be more injurious than HK-bacteria, including the secretion of toxins that are inactivated by heat treatment. For example, live S. aureus produces α-hemolysin (or α-toxin), which induces potent proinflammatory signaling and can form pores in the host cell membranes leading to loss of membrane integrity and cell lysis (23, 24). As a preliminary assessment of its effects against α-toxin, we demonstrated that Tys significantly protects against EC permeability induced by the toxin (Fig. 1C). In vivo, HK-MRSA was used only for the intravital imaging studies due to biosafety concerns and required a higher concentration of bacteria than live MRSA to induce similar severity of lung injury (interstitial edema and neutrophil infiltration in Fig. 7). However, Tys was protective in vivo against both live and HK-MRSA, suggesting that it has potent protective properties against multiple aspects of MRSA-induced injury. Finally, we have focused our in vitro studies on the potent protective effects of Tys on lung EC, but Tys likely exerts its effects in vivo on multiple cellular targets, including nonendothelial cell types, that we have not yet characterized.
In conclusion, MRSA induces significant barrier disruption and activates proinflammatory signaling in lung endothelial cells, processes that can be suppressed by the S1P analog Tys. In vivo, Tys exerts protective effects in a clinically relevant mouse model of MRSA-induced ALI when administrated both before and after MRSA challenge. These results provide new insights into MRSA pathophysiology and suggest potential future utility of Tys in ARDS.
SUPPLEMENTAL DATA
Supplemental Figs. S1–S4: https://doi.org/10.6084/m9.figshare.16934899.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants P01 HL126609 (to J.G.N.G.), R01 HL133059 (to S.M.D.), P01 HL98050 (to S.M.D.), and K08 HL135318 (to P.B.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.G.N.G. and S.M.D conceived and designed research; L.W., E.L., H.W., P.B., L.N.M., M.E.B., and M.B. performed experiments; L.W., E.L., P.B., and M.E.B. analyzed data; L.W., E.L., and P.B. interpreted results of experiments; L.W., E.L., and M.E.B. prepared figures; L.W. drafted manuscript; E.L., P.B., J.C., J.G.N.G., and S.M.D. edited and revised manuscript; L.W., E.L., H.W., P.B., L.N.M., M.E.B., J.C., J.G.N.G., and S.M.D. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Benjamin Gantner for assistance with the Image J custom macro.
REFERENCES
- 1.Hershey TB, Kahn JM. State sepsis mandates—A new era for regulation of hospital quality. N Engl J Med 376: 2311–2313, 2017. doi: 10.1056/NEJMp1611928. [DOI] [PubMed] [Google Scholar]
- 2.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med 353: 1685–1693, 2005. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 3.Matthay MA, Zemans RL, Zimmerman GA, Arabi YM, Beitler JR, Mercat A, Herridge M, Randolph AG, Calfee CS. Acute respiratory distress syndrome. Nat Rev Dis Primers 5: 18, 2019. doi: 10.1038/s41572-019-0069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Font MD, Thyagarajan B, Khanna AK. Sepsis and septic shock—Basics of diagnosis, pathophysiology and clinical decision making. Med Clin North Am 104: 573–585, 2020. doi: 10.1016/j.mcna.2020.02.011. [DOI] [PubMed] [Google Scholar]
- 5.Schreiber MP, Chan CM, Shorr AF. Bacteremia in Staphylococcus aureus pneumonia: outcomes and epidemiology. J Crit Care 26: 395–401, 2011. doi: 10.1016/j.jcrc.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Dolin HH, Papadimos TJ, Chen X, Pan ZK. Characterization of pathogenic sepsis etiologies and patient profiles: a novel approach to triage and treatment. Microbiol Insights 12: 1178636118825081, 2019. doi: 10.1177/1178636118825081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayashida A, Bartlett AH, Foster TJ, Park PW. Staphylococcus aureus beta-toxin induces lung injury through syndecan-1. Am J Pathol 174: 509–518, 2009. doi: 10.2353/ajpath.2009.080394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karki P, Ke Y, Tian Y, Ohmura T, Sitikov A, Sarich N, Montgomery CP, Birukova AA. Staphylococcus aureus-induced endothelial permeability and inflammation are mediated by microtubule destabilization. J Biol Chem 294: 3369–3384, 2019. doi: 10.1074/jbc.RA118.004030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meliton AY, Meng F, Tian Y, Sarich N, Mutlu GM, Birukova AA, Birukov KG. Oxidized phospholipids protect against lung injury and endothelial barrier dysfunction caused by heat-inactivated Staphylococcus aureus. Am J Physiol Lung Cell Mol Physiol 308: L550–L562, 2015. doi: 10.1152/ajplung.00248.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia JG, Liu F, Verin AD, Birukova A, Dechert MA, Gerthoffer WT, Bamberg JR, English D. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J Clin Invest 108: 689–701, 2001. doi: 10.1172/JCI12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao J, Tan Y, Wang L, Su X, Shi Y. Serum sphingosine-1-phosphate levels and sphingosine-1-phosphate gene polymorphisms in acute respiratory distress syndrome: a multicenter prospective study. J Transl Med 18: 156, 2020. doi: 10.1186/s12967-020-02322-y. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Wang L, Dudek SM. Regulation of vascular permeability by sphingosine 1-phosphate. Microvasc Res 77: 39–45, 2009. doi: 10.1016/j.mvr.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peng X, Hassoun PM, Sammani S, McVerry BJ, Burne MJ, Rabb H, Pearse D, Tuder RM, Garcia JG. Protective effects of sphingosine 1-phosphate in murine endotoxin-induced inflammatory lung injury. Am J Respir Crit Care Med 169: 1245–1251, 2004. doi: 10.1164/rccm.200309-1258OC. [DOI] [PubMed] [Google Scholar]
- 14.McVerry BJ, Peng X, Hassoun PM, Sammani S, Simon BA, Garcia JG. Sphingosine 1-phosphate reduces vascular leak in murine and canine models of acute lung injury. Am J Respir Crit Care Med 170: 987–993, 2004. doi: 10.1164/rccm.200405-684OC. [DOI] [PubMed] [Google Scholar]
- 15.Wang L, Sammani S, Moreno-Vinasco L, Letsiou E, Wang T, Camp SM, Bittman R, Garcia JG, Dudek SM. FTY720 (s)-phosphonate preserves sphingosine 1-phosphate receptor 1 expression and exhibits superior barrier protection to FTY720 in acute lung injury. Crit Care Med 42: e189–e199, 2014. doi: 10.1097/CCM.0000000000000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roviezzo F, Di Lorenzo A, Bucci M, Brancaleone V, Vellecco V, De Nardo M, Orlotti D, De Palma R, Rossi F, D'Agostino B, Cirino G. Sphingosine-1-phosphate/sphingosine kinase pathway is involved in mouse airway hyperresponsiveness. Am J Respir Cell Mol Biol 36: 757–762, 2007. doi: 10.1165/rcmb.2006-0383OC. [DOI] [PubMed] [Google Scholar]
- 17.Camp SM, Bittman R, Chiang ET, Moreno-Vinasco L, Mirzapoiazova T, Sammani S, Lu X, Sun C, Harbeck M, Roe M, Natarajan V, Garcia JG, Dudek SM. Synthetic analogs of FTY720 [2-amino-2-(2-[4-octylphenyl]ethyl)-1,3-propanediol] differentially regulate pulmonary vascular permeability in vivo and in vitro. J Pharmacol Exp Ther 331: 54–64, 2009. doi: 10.1124/jpet.109.153544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mathew B, Jacobson JR, Berdyshev E, Huang Y, Sun X, Zhao Y, Gerhold LM, Siegler J, Evenoski C, Wang T, Zhou T, Zaidi R, Moreno-Vinasco L, Bittman R, Chen CT, LaRiviere PJ, Sammani S, Lussier YA, Dudek SM, Natarajan V, Weichselbaum RR, Garcia JG. Role of sphingolipids in murine radiation-induced lung injury: protection by sphingosine 1-phosphate analogs. FASEB J 25: 3388–3400, 2011. doi: 10.1096/fj.11-183970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu X, Sun C, Valentine WJ, Shuyu E, Liu J, Tigyi G, Bittman R. Chiral vinylphosphonate and phosphonate analogues of the immunosuppressive agent FTY720. J Org Chem 74: 3192–3195, 2009. doi: 10.1021/jo900023u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen J, Feng G, Guo Q, Wardenburg JB, Lin S, Inoshima I, Deaton R, Yuan JX, Garcia JG, Machado RF, Otto M, Wunderink RG. Transcriptional events during the recovery from MRSA lung infection: a mouse pneumonia model. PLoS One 8: e70176, 2013. doi: 10.1371/journal.pone.0070176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Letsiou E, Rizzo AN, Sammani S, Naureckas P, Jacobson JR, Garcia JG, Dudek SM. Differential and opposing effects of imatinib on LPS- and ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol 308: L259–L269, 2015. doi: 10.1152/ajplung.00323.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Looney MR, Bhattacharya J. Live imaging of the lung. Annu Rev Physiol 76: 431–445, 2014. doi: 10.1146/annurev-physiol-021113-170331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foletti D, Strop P, Shaughnessy L, Hasa-Moreno A, Casas MG, Russell M, Bee C, Wu S, Pham A, Zeng Z, Pons J, Rajpal A, Shelton D. Mechanism of action and in vivo efficacy of a human-derived antibody against Staphylococcus aureus α-hemolysin. J Mol Biol 425: 1641–1654, 2013. doi: 10.1016/j.jmb.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 24.Wilke GA, Bubeck Wardenburg J. Role of a disintegrin and metalloprotease 10 in Staphylococcus aureus alpha-hemolysin-mediated cellular injury. Proc Natl Acad Sci USA 107: 13473–13478, 2010. doi: 10.1073/pnas.1001815107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dudek SM, Jacobson JR, Chiang ET, Birukov KG, Wang P, Zhan X, Garcia JG. Pulmonary endothelial cell barrier enhancement by sphingosine 1-phosphate: roles for cortactin and myosin light chain kinase. J Biol Chem 279: 24692–24700, 2004. doi: 10.1074/jbc.M313969200. [DOI] [PubMed] [Google Scholar]
- 26.Schnoor M, Stradal TE, Rottner K. Cortactin: cell functions of a multifaceted actin-binding protein. Trends Cell Biol 28: 79–98, 2018. doi: 10.1016/j.tcb.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 27.Vouret-Craviari V, Bourcier C, Boulter E, van Obberghen-Schilling E. Distinct signals via Rho GTPases and Src drive shape changes by thrombin and sphingosine-1-phosphate in endothelial cells. J Cell Sci 115: 2475–2484, 2002. doi: 10.1242/jcs.115.12.2475. [DOI] [PubMed] [Google Scholar]
- 28.Garcia JG, Verin AD, Schaphorst K, Siddiqui R, Patterson CE, Csortos C, Natarajan V. Regulation of endothelial cell myosin light chain kinase by Rho, cortactin, and p60(src). Am J Physiol Lung Cell Mol Physiol 276: L989–L998, 1999. doi: 10.1152/ajplung.1999.276.6.L989. [DOI] [PubMed] [Google Scholar]
- 29.Maiti A, Jiranek WA. Inhibition of Methicillin-resistant Staphylococcus aureus-induced cytokines mRNA production in human bone marrow derived mesenchymal stem cells by 1,25-dihydroxyvitamin D3. BMC Cell Biol 15: 11, 2014. doi: 10.1186/1471-2121-15-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu T, Xing J, Birukova AA. Cell-type-specific crosstalk between p38 MAPK and Rho signaling in lung micro- and macrovascular barrier dysfunction induced by Staphylococcus aureus-derived pathogens. Transl Res 162: 45–55, 2013. doi: 10.1016/j.trsl.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Attia EF, Jolley SE, Crothers K, Schnapp LM, Liles WC. Soluble vascular cell adhesion molecule-1 (sVCAM-1) is elevated in bronchoalveolar lavage fluid of patients with acute respiratory distress syndrome. PLoS One 11: e0149687, 2016. doi: 10.1371/journal.pone.0149687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gilbert-Girard S, Savijoki K, Yli-Kauhaluoma J, Fallarero A. Screening of FDA-approved drugs using a 384-well plate-based biofilm platform: the case of fingolimod. Microorganisms 8: 1834, 2020. doi: 10.3390/microorganisms8111834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Z, Li J, Cho J, Malik AB. Prevention of vascular inflammation by nanoparticle targeting of adherent neutrophils. Nat Nanotechnol 9: 204–210, 2014. doi: 10.1038/nnano.2014.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chagnon F, Fournier C, Charette PG, Moleski L, Payet MD, Dobbs LG, Lesur O. In vivo intravital endoscopic confocal fluorescence microscopy of normal and acutely injured rat lungs. Lab Invest 90: 824–834, 2010. doi: 10.1038/labinvest.2010.76. [DOI] [PubMed] [Google Scholar]
- 35.Cleary SJ, Hobbs C, Amison RT, Arnold S, O'Shaughnessy BG, Lefrançais E, Mallavia B, Looney MR, Page CP, Pitchford SC. LPS-induced lung platelet recruitment occurs independently from neutrophils, PSGL-1, and P-selectin. Am J Respir Cell Mol Biol 61: 232–243, 2019. doi: 10.1165/rcmb.2018-0182OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kreisel D, Nava RG, Li W, Zinselmeyer BH, Wang B, Lai J, Pless R, Gelman AE, Krupnick AS, Miller MJ. In vivo two-photon imaging reveals monocyte-dependent neutrophil extravasation during pulmonary inflammation. Proc Natl Acad Sci USA 107: 18073–18078, 2010. doi: 10.1073/pnas.1008737107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Natarajan V, Dudek SM, Jacobson JR, Moreno-Vinasco L, Huang LS, Abassi T, Mathew B, Zhao Y, Wang L, Bittman R, Weichselbaum R, Berdyshev E, Garcia JG. Sphingosine-1-phosphate, FTY720, and sphingosine-1-phosphate receptors in the pathobiology of acute lung injury. Am J Respir Cell Mol Biol 49: 6–17, 2013. doi: 10.1165/rcmb.2012-0411TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moellering RC Jr. The growing menace of community-acquired methicillin-resistant Staphylococcus aureus. Ann Intern Med 144: 368–370, 2006. doi: 10.7326/0003-4819-144-5-200603070-00014. [DOI] [PubMed] [Google Scholar]
- 39.van Hal SJ, Jensen SO, Vaska VL, Espedido BA, Paterson DL, Gosbell IB. Predictors of mortality in Staphylococcus aureus Bacteremia. Clin Microbiol Rev 25: 362–386, 2012. doi: 10.1128/CMR.05022-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karchmer AW, Bayer AS. Methicillin-resistant Staphylococcus aureus: an evolving clinical challenge. Clin Infect Dis 46, Suppl 5: S342–S343, 2008. doi: 10.1086/533589. [DOI] [PubMed] [Google Scholar]
- 41.Defres S, Marwick C, Nathwani D. MRSA as a cause of lung infection including airway infection, community-acquired pneumonia and hospital-acquired pneumonia. Eur Respir J 34: 1470–1476, 2009. doi: 10.1183/09031936.00122309. [DOI] [PubMed] [Google Scholar]
- 42.Napolitano LM, Brunsvold ME, Reddy RC, Hyzy RC. Community-acquired methicillin-resistant Staphylococcus aureus pneumonia and ARDS: 1-year follow-up. Chest 136: 1407–1412, 2009. doi: 10.1378/chest.07-1511. [DOI] [PubMed] [Google Scholar]
- 43.Htwe YM, Wang H, Belvitch P, Meliton L, Bandela M, Letsiou E, Dudek SM. Group V phospholipase A2 mediates endothelial dysfunction and acute lung injury caused by methicillin-resistant Staphylococcus aureus. Cells 10: 1731, 2021. doi: 10.3390/cells10071731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang L, Bittman R, Garcia JG, Dudek SM. Junctional complex and focal adhesion rearrangement mediates pulmonary endothelial barrier enhancement by FTY720 S-phosphonate. Microvasc Res 99: 102–109, 2015. doi: 10.1016/j.mvr.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dejana E, Orsenigo F, Lampugnani MG. The role of adherens junctions and VE-cadherin in the control of vascular permeability. J Cell Sci 121: 2115–2122, 2008. doi: 10.1242/jcs.017897. [DOI] [PubMed] [Google Scholar]
- 46.Corada M, Liao F, Lindgren M, Lampugnani MG, Breviario F, Frank R, Muller WA, Hicklin DJ, Bohlen P, Dejana E. Monoclonal antibodies directed to different regions of vascular endothelial cadherin extracellular domain affect adhesion and clustering of the protein and modulate endothelial permeability. Blood 97: 1679–1684, 2001. doi: 10.1182/blood.v97.6.1679. [DOI] [PubMed] [Google Scholar]
- 47.Lee MJ, Thangada S, Claffey KP, Ancellin N, Liu CH, Kluk M, Volpi M, Sha'afi RI, Hla T. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell 99: 301–312, 1999. doi: 10.1016/S0092-8674(00)81661-X. [DOI] [PubMed] [Google Scholar]
- 48.Morel NM, Petruzzo PP, Hechtman HB, Shepro D. Inflammatory agonists that increase microvascular permeability in vivo stimulate cultured pulmonary microvessel endothelial cell contraction. Inflammation 14: 571–583, 1990. doi: 10.1007/BF00914277. [DOI] [PubMed] [Google Scholar]
- 49.Sukriti S, Tauseef M, Yazbeck P, Mehta D. Mechanisms regulating endothelial permeability. Pulm Circ 4: 535–551, 2014. doi: 10.1086/677356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Amano M, Chihara K, Kimura K, Fukata Y, Nakamura N, Matsuura Y, Kaibuchi K. Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science 275: 1308–1311, 1997. doi: 10.1126/science.275.5304.1308. [DOI] [PubMed] [Google Scholar]
- 51.Amano M, Nakayama M, Kaibuchi K. Rho-kinase/ROCK: a key regulator of the cytoskeleton and cell polarity. Cytoskeleton (Hoboken) 67: 545–554, 2010. doi: 10.1002/cm.20472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dudek SM, Birukov KG, Zhan X, Garcia JG. Novel interaction of cortactin with endothelial cell myosin light chain kinase. Biochem Biophys Res Commun 298: 511–519, 2002. doi: 10.1016/S0006-291X(02)02492-0. [DOI] [PubMed] [Google Scholar]
- 53.Fournier B, Philpott DJ. Recognition of Staphylococcus aureus by the innate immune system. Clin Microbiol Rev 18: 521–540, 2005. doi: 10.1128/CMR.18.3.521-540.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McGuinness WA, Kobayashi SD, DeLeo FR. Evasion of neutrophil killing by Staphylococcus aureus. Pathogens 5: 32, 2016. doi: 10.3390/pathogens5010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ventura CL, Higdon R, Hohmann L, Martin D, Kolker E, Liggitt HD, Skerrett SJ, Rubens CE. Staphylococcus aureus elicits marked alterations in the airway proteome during early pneumonia. Infect Immun 76: 5862–5872, 2008. doi: 10.1128/IAI.00865-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tisoncik JR, Korth MJ, Simmons CP, Farrar J, Martin TR, Katze MG. Into the eye of the cytokine storm. Microbiol Mol Biol Rev 76: 16–32, 2012. doi: 10.1128/MMBR.05015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McNicholas S, Talento AF, O'Gorman J, Hannan MM, Lynch M, Greene CM, Humphreys H, Fitzgerald-Hughes D. Cytokine responses to Staphylococcus aureus bloodstream infection differ between patient cohorts that have different clinical courses of infection. BMC Infect Dis 14: 580, 2014. doi: 10.1186/s12879-014-0580-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McLoughlin A, Rochfort KD, McDonnell CJ, Kerrigan SW, Cummins PM. Staphylococcus aureus-mediated blood-brain barrier injury: an in vitro human brain microvascular endothelial cell model. Cell Microbiol 19: e12664, 2017. doi: 10.1111/cmi.12664. [DOI] [PubMed] [Google Scholar]
- 59.Zhao Y, Shi D, Cao K, Wu F, Zhu X, Wen S, You Q, Zhang K, Liu L, Zhou H. Fingolimod targets cerebral endothelial activation to block leukocyte recruitment in the central nervous system. J Leukoc Biol 103: 107–118, 2018. doi: 10.1002/JLB.3A0717-287R. [DOI] [PubMed] [Google Scholar]
- 60.Teijaro JR, Walsh KB, Cahalan S, Fremgen DM, Roberts E, Scott F, Martinborough E, Peach R, Oldstone MB, Rosen H. Endothelial cells are central orchestrators of cytokine amplification during influenza virus infection. Cell 146: 980–991, 2011. doi: 10.1016/j.cell.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jiang H, Shen SM, Yin J, Zhang PP, Shi Y. Sphingosine 1-phosphate receptor 1 (S1PR1) agonist CYM5442 inhibits expression of intracellular adhesion molecule 1 (ICAM1) in endothelial cells infected with influenza A viruses. PLoS One 12: e0175188, 2017. doi: 10.1371/journal.pone.0175188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang L, Jiang H, Shen SM, Wen CX, Xing Z, Shi Y. Inhibition of autophagy and chemokine induction by sphingosine 1-phosphate receptor 1 through NF-κB signaling in human pulmonary endothelial cells infected with influenza A viruses. PLoS One 13: e0205344, 2018. doi: 10.1371/journal.pone.0205344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Parker D, Ryan CL, Alonzo F 3rd, Torres VJ, Planet PJ, Prince AS. CD4+ T cells promote the pathogenesis of Staphylococcus aureus pneumonia. J Infect Dis 211: 835–845, 2015. doi: 10.1093/infdis/jiu525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cook-Mills JM, Marchese ME, Abdala-Valencia H. Vascular cell adhesion molecule-1 expression and signaling during disease: regulation by reactive oxygen species and antioxidants. Antioxid Redox Signal 15: 1607–1638, 2011. doi: 10.1089/ars.2010.3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu F, Diao R, Liu J, Kang Y, Wang X, Shi L. Curcumin attenuates Staphylococcus aureus-induced acute lung injury. Clin Respir J 9: 87–97, 2015. [Erratum in Clin Respir J 13: 338, 2019]. doi: 10.1111/crj.12113. [DOI] [PubMed] [Google Scholar]
- 66.Burg N, Swendeman S, Worgall S, Hla T, Salmon JE. Sphingosine 1-phosphate receptor 1 signaling maintains endothelial cell barrier function and protects against immune complex-induced vascular injury. Arthritis Rheumatol 70: 1879–1889, 2018. doi: 10.1002/art.40558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Suliman HB, Kraft B, Bartz R, Chen L, Welty-Wolf KE, Piantadosi CA. Mitochondrial quality control in alveolar epithelial cells damaged by S. aureus pneumonia in mice. Am J Physiol Lung Cell Mol Physiol 313: L699–L709, 2017. doi: 10.1152/ajplung.00197.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cohen TS, Jones-Nelson O, Hotz M, Cheng L, Miller LS, Suzich J, Stover CK, Sellman BR. S. aureus blocks efferocytosis of neutrophils by macrophages through the activity of its virulence factor alpha toxin. Sci Rep 6: 35466, 2016. doi: 10.1038/srep35466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fettel J, Kühn B, Guillen NA, Sürün D, Peters M, Bauer R, Angioni C, Geisslinger G, Schnütgen F, Meyer Zu Heringdorf D, Werz O, Meybohm P, Zacharowski K, Steinhilber D, Roos J, Maier TJ. Sphingosine-1-phosphate (S1P) induces potent anti-inflammatory effects in vitro and in vivo by S1P receptor 4-mediated suppression of 5-lipoxygenase activity. FASEB J 33: 1711–1726, 2019. doi: 10.1096/fj.201800221R. [DOI] [PubMed] [Google Scholar]
- 70.Kim J, Nguyen TTT, Li Y, Zhang CO, Cha B, Ke Y, Mazzeffi MA, Tanaka KA, Birukova AA, Birukov KG. Contrasting effects of stored allogeneic red blood cells and their supernatants on permeability and inflammatory responses in human pulmonary endothelial cells. Am J Physiol Lung Cell Mol Physiol 318: L533–L548, 2020. doi: 10.1152/ajplung.00025.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figs. S1–S4: https://doi.org/10.6084/m9.figshare.16934899.