Abstract
Tracheobronchomalacia and complete tracheal rings are congenital malformations of the trachea associated with morbidity and mortality for which the etiology remains poorly understood. Epithelial expression of Wls (a cargo receptor mediating Wnt ligand secretion) by tracheal cells is essential for patterning the embryonic mouse trachea’s cartilage and muscle. RNA sequencing indicated that Wls differentially modulated the expression of BMP signaling molecules. We tested whether BMP signaling, induced by epithelial Wnt ligands, mediates cartilage formation. Deletion of Bmp4 from respiratory tract mesenchyme impaired tracheal cartilage formation that was replaced by ectopic smooth muscle, recapitulating the phenotype observed after epithelial deletion of Wls in the embryonic trachea. Ectopic muscle was caused in part by anomalous differentiation and proliferation of smooth muscle progenitors rather than tracheal cartilage progenitors. Mesenchymal deletion of Bmp4 impaired expression of Wnt/β-catenin target genes, including targets of WNT signaling: Notum and Axin2. In vitro, recombinant (r)BMP4 rescued the expression of Notum in Bmp4-deficient tracheal mesenchymal cells and induced Notum promoter activity via SMAD1/5. RNA sequencing of Bmp4-deficient tracheas identified genes essential for chondrogenesis and muscle development coregulated by BMP and WNT signaling. During tracheal morphogenesis, WNT signaling induces Bmp4 in mesenchymal progenitors to promote cartilage differentiation and restrict trachealis muscle. In turn, Bmp4 differentially regulates the expression of Wnt/β-catenin targets to attenuate mesenchymal WNT signaling and to further support chondrogenesis.
Keywords: Bmp4, cartilage, trachea, trachealis muscle, Wnt
INTRODUCTION
The large airways of the respiratory tract are supported by cartilage located in the ventrolateral mesenchyme and smooth muscle occupying the dorsal mesenchyme. Defects in the supporting structure of the large airways of the respiratory tract are common congenital malformations affecting cartilage and muscle of the trachea and bronchi, causing airway obstruction. Resultant malformations include tracheobronchomalacia (TBM), a disorder characterized by absent or weakened cartilaginous rings, and complete tracheal rings (CTR), a condition involving cartilage rings that encompass all, or nearly all, of the tracheal circumference (1). While mild cases of TBM may resolve during childhood, more severe malformations such as CTR require surgical intervention. An emotional and economic burden is placed upon patients and families when tracheostomy and surgery are required (2–6). While the etiologies of TBM and CTR are poorly understood, recent studies support a role for signaling pathways such as HH, WNT, and FGF as underlying causes of the disorders in humans. The aforementioned signaling pathways are essential for patterning of the conducting airways in the mouse (1, 7, 8).
Wnt signaling is critical for specification of the respiratory tract, (9, 10) and tracheal formation. We previously demonstrated the importance of Wnt signaling in the differentiation of tracheal mesenchyme into cartilage and trachealis muscle in the mouse (7). Epithelial Wnt ligands promote ventral cartilage upstream of Sox9 (a master regulator of cartilage formation), likely by activation of mesenchymal β-catenin (11, 12). Epithelial ligands induce genes mediating cartilage formation, including Notum, a gene that plays a pivotal role in facilitating mesenchymal cell condensation and attenuating Wnt/β-catenin canonical signaling (8). While the molecular mechanism remains unknown, epithelial Wnt ligands are necessary to restrict trachealis muscle to the dorsal aspect of the tracheal mesenchyme. Preventing the secretion of Wnt ligands from the tracheal epithelium via deletion of the cargo receptor Wls inhibited cartilage formation, which was replaced by ectopic muscle in the ventrolateral aspect of the trachea of the ShhCre;Wlsf/f mice. After epithelial deletion of Wls, expressions of Bmp4 and its targets Msx1 and Msx2 were diminished in tracheal mesenchyme (7, 8). Ex vivo studies demonstrated that treatment with recombinant (r)BMP2 rescued expression of Sox9 in ShhCre;Wlsf/f tracheal tissue (7).
Bmp ligands are highly conserved signaling molecules expressed dynamically during development (13). Bmp2 and Bmp4 are derived from an ancestral common gene (14) and preferentially signal through a specific set of type I receptors, BMPR1A (Alk3) and BMPR1B (Alk6), that activate transcription through phosphorylation of SMADs 1, 5, and 8 (15). Bmp4 and Bmp2 genetically interact to mediate morphogenetic processes leading to cardiac, eye, and limb development (16). Bmp2 is required for cardiac and chondrocyte maturation during endochondral bone formation (17, 18). Whether Bmp2 is required for respiratory tract formation is presently unknown.
On the other hand, Bmp4 plays a critical role in morphogenesis of the foregut and respiratory tract formation. Bmp4 expression is detected at embryonic day (E)8.5 in ventral foregut mesenchyme, a pattern that becomes more evident by E10.5 and is maintained after separation of the trachea and esophagus by E11.5 (19). Deletion of Noggin, a gene encoding a secreted protein that binds and inactivates Bmp4, prevents separation of embryonic trachea and esophagus (20). Bmp4 promotes differentiation of the lung epithelial cells toward a distal identity (21). In vitro studies show that Bmp4 modulates proliferation of proximal airway cells (22). Bmp4 is also required for tracheal development. Deletion of Bmp4 in foregut mesenchyme or deletion of genes encoding BMP type I receptors Bmpr1a and Bmpr1b in developing foregut endoderm causes tracheal agenesis (19, 23). Together these studies highlight the importance of the BMP pathway in foregut and respiratory tract development.
The role of Bmp4 in promoting cartilage and bone in the appendicular skeleton, cranial base, and vertebrae of the embryo has been established (24–27). In vitro studies in mouse models of tracheomalacia and in mouse and human induced pluripotent stem cells (iPSCs) predict a role for Bmp4 in tracheal cartilage development (7, 28, 29); however, it is unclear how BMP and other signaling pathways cross talk during the earlier stages of tracheal formation to promote cartilage formation in ventral tracheal mesenchyme. Based on the finding that Bmp4 is downregulated after epithelial deletion of Wls, we sought to test whether Bmp4 acts downstream of WNT signaling to promote the ventral cartilage identity in tracheal mesenchyme. Using a conditional allele targeting exon 4 of Bmp4 (30), we deleted Bmp4 from the foregut mesenchyme, resulting in abnormal trachea and lung development. Strikingly, in the Bmp4-mutant trachea, ectopic muscle replaced the cartilage, recapitulating the phenotype caused by epithelial deletion of Wls. Furthermore, targets and modulators of Wnt/β-catenin signaling Notum (required for patterning of tracheal cartilage) and Axin2 are regulated by Bmp4, demonstrating interplay between BMP and Wnt/β-catenin signaling during patterning of tracheal mesenchyme in the developing mouse embryo.
MATERIALS AND METHODS
Mouse Breeding and Genotyping
Animals were housed in a pathogen-free environment and handled according to protocols approved by the Cincinnati Children’s Hospital Medical Center (CCHMC) Institutional Animal Care and Use Committee. Generation of the Wntless (Wls) conditional knockout (CKO) mouse has been described previously (31). ShhCre/wt;Wlsf/f embryos were obtained by breeding Wlsf/f mice with ShhCre/wt mice and rebreeding the resulting mice with Wlsf/f mice (32). Foxg1Cre/wt;Bmp4f/f embryos were generated by breeding Bmp4f/f with Foxg1Cre/wt mice and mating the resulting mice to Bmp4f/f (Bmp4tm1Jfm/J mouse strain was obtained from Jackson Laboratories, Stock No. 016878). This allele of Bmp4 contains LoxP sites surrounding exon 4, encoding the mature Bmp4 peptide essential for its function. Foxg1Cre/wt;Bmp4f/f;γSMA eGFP embryos were generated by breeding Bmp4f/f with γSMA eGFP mice (33) and mating the resulting mice to Foxg1Cre/wt;Bmp4f/f. Sox9f/f mice (34) were mated with FoxG1Cre/wt and subsequently rebred to generate Foxg1Cre/wt; Sox9f/f embryos. Genotypes of transgenic mice were determined by PCR using genomic DNA isolated from mouse tails or embryonic tissue. Primers used for genotyping are provided in Supplemental Table S1 (all Supplemental Material is available at https://figshare.com/s/5eef680cb43d87548f20).
DNA Constructs
Luciferase pLightSwitch_Prom Axin2 and Notum vectors containing 1-kb regions of both AXIN2 and NOTUM human promoters upstream of RenSP luciferase gene were obtained from Active Motif (S711025 and S711417, respectively). A vector containing a random sequence upstream of the luciferase gene was used as control (S790001).
Transcriptomic Analyses
RNA sequencing (RNA-seq) of ShhCre;Wlsf/f tissue was performed on pooled (n = 8), isolated tracheas from control and ShhCre;Wlsf/f E13.5 embryos. RNA was isolated with TRIzol according to manufacturer’s instructions. RNA sequencing was performed by Cincinnati Children’s Hospital Medical Center’s Gene Expression Core with the Ovation RNA-Seq System V2 (NuGEN) and Nextera XT DNA Sample Preparation kits (Illumina Technologies). RNA-seq Fastq files generated from E13.5 Wlsf/f (n = 2) and ShhCre;Wlsf/f (n = 1) tracheas were combined with our previously published data (8). Previously published BAM files were converted to Fastq files with Picard and then realigned, along with the newly generated Fastq files, to mm10 with Bowtie2 (35, 36). Sorted Bam files were used to generate raw counts with Bioconductor’s GenomicAlignments. Differentially expressed genes were identified with DESeq (n = 5 controls, n = 4 mutants) (37, 38). Fragments per kilobase (FPKM) values were calculated with Cufflinks (39). Differentially expressed genes had a P value < 0.05, fold change (FC) > 1.5, and FPKM > 1 in over half of the replicates in at least one condition. RNA-seq related to Foxg1Cre;Bmp4f/f was performed on chondrocytes from Foxg1Cre;γSMAeGFP;Bmp4f/f tracheas by excluding dead cells based on Sytox staining and live epithelial and smooth muscle cells based on APC-epithelial cellular adhesion molecule (EpCAM) staining and enhanced green fluorescent protein (eGFP) fluorescence, respectively. Ultra-low Input RNA-Seq was performed by GENEWIZ. Resulting Fastq files were trimmed by TrimGalore and then followed the same analysis pipeline as previously outlined (n = 3 control, n = 2 Foxg1Cre;γSMAeGFP;Bmp4f/f). Differentially expressed genes had a P value < 0.05, FC > 2, and FPKM > 1 in over half of the replicates in at least one condition. Heat maps were generated with normalized counts generated by DESeq and pheatmap or from RNA-seq fold changes. Functional enrichment was performed with ToppFun, and hits relevant to this project were visualized in a −log10(P value) bar graph. System models were created with IPA’s Path Designer (40). Sequencing data have been deposited in the GEO repository under GSE158452.
Histology and Immunofluorescence Staining
Embryonic tissue was fixed in 4% paraformaldehyde (PFA) overnight and embedded in paraffin or OCT to generate 7-μm sections. For general immunofluorescence staining, antigen retrieval was performed with 10 mM citrate buffer, pH 6. Slides were blocked for 2 h in Tris-buffered saline (TBS) with 10% normal donkey serum and 1% BSA, followed by overnight incubation at 4°C in the primary antibody, diluted accordingly in blocking solution. Slides were washed in 1× TBS-Tween 20, incubated in secondary antibody at 1:200, diluted accordingly in blocking solution, at room temperature (RT) for 1 h, washed, and coverslipped with Vectashield mounting medium with or without DAPI. For phospho-SMAD1/5/8 staining, frozen sections were fixed in 4% PFA for 10 min, washed with PBS and permeabilized in PBS-0.1% Triton X-100 for 10 min, washed, and blocked in Image-iT FX signal enhancer (Invitrogen R10477) for 1 h at RT. Slides were then blocked for 1 h in PBS-0.1% Triton X-100 with 10% normal donkey serum and 3% BSA, followed by overnight incubation at 4°C in the primary antibody at a dilution of 1:100 in blocking solution. After washes, slides were incubated in a 1:200 dilution of secondary antibody in PBS-0.1% Tween 20 for 1 h at RT, washed, and coverslipped with or without DAPI. Fluorescent staining was visualized and photographed with automated fluorescence microscopes (Zeiss and Nikon). Source and dilution of primary and secondary antibodies used for this study are provided in Supplemental Table S2.
Whole Mount Staining
Tracheal lung tissue isolated at E11.5, E13.5, and E14.5 was subject to whole mount immunofluorescence as previously described (1). Embryonic tissue was fixed in 4% PFA overnight and then stored in 100% MeOH at −20°C. For staining, whole mounts were permeabilized in Dent’s bleach (4:1:1 MeOH-DMSO-30% H2O2) for 2 h and then taken from 100% MeOH to 100% PBS through a series of washes. After washes, whole mounts were blocked in a 5% (wt/vol) blocking solution for 2 h and then incubated overnight at 4°C in primary antibody diluted accordingly in the blocking solution. After five 1-h washes in PBS, whole mounts were incubated with a secondary antibody dilution of 1:500 overnight at 4°C. Samples were then washed three times in PBS and cleared in Murray’s clear. Images of whole mounts were obtained by confocal microscopy (Nikon A1R). Imaris imaging software was used to convert z-stack image slices obtained by confocal microscopy to three-dimensional renderings of whole mount samples.
Lectin Staining
Samples were fixed overnight in 4% PFA and rinsed multiple times in PBS. Afterward, samples were incubated for 1 h at room temperature in blocking buffer comprised of 2% goat serum, 1% BSA, 0.3% Triton X, and PBS. After blocking buffer was removed, peanut agglutinin (PNA) lectin solution (in 10% normal goat serum and PBS) was added to the samples at a final concentration of 10 µg/mL. Samples were covered in aluminum foil and incubated overnight at 4°C, rinsed with PBS multiple times, and photographed with a Leica fluorescence dissecting microscope.
Cell Proliferation
E10.5 pregnant mice were injected with BrdU at a concentration of 100 μg/g body wt. Embryos were isolated at E12.5, coronal sections were stained with anti-BrdU, α-smooth muscle actin (αSMA), Nkx2.1, and Sox9, and cell proliferation was analyzed in tracheal epithelium and mesenchyme. With the ImageJ multipoint tool, αSMA, Nkx2.1, and Sox9 were each labeled as an individual “counter” to enable the counting of positively stained cells. Labeled cells and total cells were counted per each field photograph at ×20, and ratios of proliferating cells to total cells, defined as number of Sox9- and αSΜΑ-stained cells, were calculated. Average mitotic index was determined for three different samples, each one of them photographed five times to capture different fields.
Tracheal Mesenchymal Cell Isolation and Culture
Primary cells were isolated as previously described (8). Briefly, E13.5 tracheas of at least five embryos of the same genotype were isolated, washed in PBS, minced in trypsin, and incubated for 10 min at 37°C. After incubation, tissue was pipetted until cell suspension formed. Cells were seeded in flasks containing mouse embryonic fibroblast (MEF) tissue culture medium composed of DMEM, 1% penicillin-streptomycin, 2% antibiotic-antimycotic, and 20% non-heat-inactivated FBS. Only mesenchymal cells were attached, as we confirmed expression of Sox9, Col2a1, and Myh11 but no expression of Nkx2.1 was detected (data not shown). For studies involving monolayers, 1 × 105 cells were seeded in six-well plates. For micromass experiments, 5 × 104 cells in 8-µL drops were seeded in six-well plates (5 micromasses per well) for qPCR analysis and one micromass per well in an eight-well slide for Alcian blue staining. After 4 h, micromasses were flooded with culture medium. All cells were treated on the consecutive day and harvested after a week, with treatments changed every 48 h. Cells were treated with 500–750 ng/mL recombinant (r)BMP2 (355-BM-CF, R&D; 10426-HNAE, Sino Biological), rBMP4 (314-BP-050, R&D), 1:1 (vol/vol) Wnt3a conditioned medium from L cells (CRL-2647, ATCC), and 5 µM DMH1 (D8946, Sigma).
Chemotaxis Assay
Procedure was performed according to manufacturer’s instructions with chemotaxis assay chambers (ibiTreat µSlide 80326, Ibidi). A suspension of 2 × 106 trachea MEF cells/mL was used. After 24 h of seeding cells, serum-free DMEM medium containing the live cell nuclear dye (NucBlue ReadyProbe R10477, Invitrogen) was added to the cells 4 h before initiation of the assay. The following conditions were tested on the chemoattractant reservoir: FBS 5% (S11150, Atlanta), rBMP2 (355-BM-CF, R&D), and rBMP4 (314-BP-050, R&D). The chemoattractant-free reservoir was filled with chemoattractant-free medium (high-glucose DMEM; 30-2002, ATCC). FBS 5% and rBMP2 or rBMP4 were also tested on both reservoirs at the same time. Time-lapse images were taken for a total of 72 h on Evos M7000 (Thermo Fisher) or Ti-E SpectraX inverted wide-field (Nikon) microscopes with a 20-min time interval between images for bright-field, GFP, and DAPI channels. Time-lapse images were analyzed with FastTrack AI software to obtain forward migration index (FMI) and center of mass (CoM) displacement for each condition. FMI indicates which axes have the highest percentage of the total CoM displacement. FMIs in y- and x-axes are represented as parallel and perpendicular FMI, respectively.
Embryonic Whole Tracheal-Lung Culture
Embryonic tracheas were harvested at E11.5 or E12.5 and cultured at air-liquid interphase as described previously (41). rBMP4 (500 ng/mL) or DMH1 (5 µM) was added to the medium 1 h after initiation of culture. Explants were photographed every 24 h and harvested after 120 h. Culture media and treatments were replaced every 48 h.
In Situ Hybridization
Procedure was performed according to a protocol developed by Advanced Cell Diagnostics (ACD) (42). In situ probes were designed by ACD. Slides were baked and deparaffinized. In situ probes were added to the slides, and hybridization was performed for 2 h at 40°C, followed by several rounds of amplification steps. For chromogenic assays, signal was detected with 3,3′-diaminobenzidine (DAB) or Fast Red or Green, and slides were counterstained with hematoxylin. For fluorescence detection, Opal dyes were used to detect the localization of the transcripts. After mounting with permanent mounting medium, slides were photographed with a wide-field Nikon i90 or Nikon confocal microscope.
RNA Isolation and RT-PCR
Gene expression was determined by quantitative RT-PCR. RNA was isolated from E13.5 tracheas and embryonic tracheal MEF cells (monolayers and micromasses) with a commercially available kit (Zymo Research Quick-RNA Micro Kit, S1050). Reverse transcription was performed according to manufacturer’s instructions (High-Capacity cDNA Reverse Transcription kit, Applied Biosystems), and TaqMan probes were used to detect differential expression with StepOne Plus and the QuantStudio3 RT-PCR system. Gene expression was normalized to Gapdh.
Motif Enrichment Analyses
Promoter regions 2 kb upstream of the predicted transcriptional start site of select genes and luciferase constructs were analyzed with MEME Suite’s FIMO coupled with Meme Suite’s Motif database (43). Promoter sequences were downloaded with the UCSC Table Browser. Selective motif locations in DNA sequences of interest were identified with MEME Suite’s FIMO package. IGV was used to visualize motif and luciferase construct locations within promoters.
Transfections and Luciferase Assay
NIH3T3 cells were cultured in 24-well plates at 37°C and 5% CO2. Cells (60–70% confluent) were transfected with a total of 250 ng of plasmidic DNA with FuGENE (E231A, Promega) according to manufacturer’s instructions. After 24 h, cells were incubated in DMEM-2.5% FBS and treated with 1:1 Wnt3a conditioned media from L cells, 500–750 ng/mL rBMP4, and a combination of these two. Cells were harvested 48 h after transfection with a passive lysis buffer reagent. Luciferase activity was determined with LightSwitch Luciferase Assay Reagent (LS010, Active Motif) over 2-s integration time with a GloMax 96 microplate luminometer (Promega).
Dissociation and FACS of Embryonic Trachea
Cell dissociation was performed following a previously published protocol (44) with modifications as follows. E13.5 Bmp4f/f;γSMAeGFP and Foxg1Cre;Bmp4f/f;γSMAeGFP tracheas were dissected in cold PBS and dissociated to single cells with TrypLE Express (phenol red free; Thermo, 12604013) at 37°C for 10 min, followed by trituration for 30 s at RT. Cells were washed twice with fluorescence-activated cell sorting (FACS) buffer containing 1 mM EDTA, 2% FBS, and 25 mM HEPES in phenol red-free HBSS. To identify epithelial cells, cells were stained with APC anti-mouse CD326/EpCAM (Invitrogen, Ref. 17-5791-82; used at 1:50) at 4°C for 30 min, followed by two washes with FACS buffer. Cells were resuspended in FACS buffer and passed through a 35-µm cell strainer. To stain dead cells, Sytox Blue nucleic acid stain (Thermo, S11348; used at 1 µM) was added to the cell suspension. Cells were sorted with BD FACSAria I and II. Single live “chondroblast” cells were collected after size selection and gating for Sytox-negative, EpCAM-negative, and eGFP-negative cells. Cells were sorted directly into RNA lysis buffer (Zymo Research Quick RNA Micro Kit, S1050).
Alcian Blue Staining
Tracheal MEF cells were cultured into micromasses and subsequently fixed in 4% PFA for 20 min at room temperature. After fixation, the micromasses underwent a series of PBS washes and were stained in Alcian blue solution for 2 h, followed by one rinse with 3% glacial acetic acid. Afterward, micromasses underwent a series of PBS washes and were ultimately mounted with Permount and a coverslip for imaging. Photographs of micromasses were obtained with a Nikon confocal microscope. For whole embryonic trachea staining we followed a previously published procedure (45).
Human Tissue
Sections of tracheal human tissue were provided by the Discover Together Biobank at CCHMC, which obtained consent from families to use the tissue for research purposes. Samples were deidentified; only age, sex, and disease status were provided to the researchers.
Statistics
Quantitative data are presented as means ± SE. For animal experiments, a minimum of three different litters for genotype were studied. Experiments were repeated at least twice with a minimum of three biological replicates for each group. Statistical analysis was performed with Graph Pad Prism versions 8.2.0 and 9.0.0 for MacOS. Statistically significant differences were determined by paired t test or one-way or two-way ANOVA followed by post hoc pairwise multiple comparison procedures (Dunnett or Holm–Sidak test). Significance was set at P < 0.05.
RESULTS
BMP Signaling Is a Target of Wls-Mediated Wnt Signaling in Developing Trachea
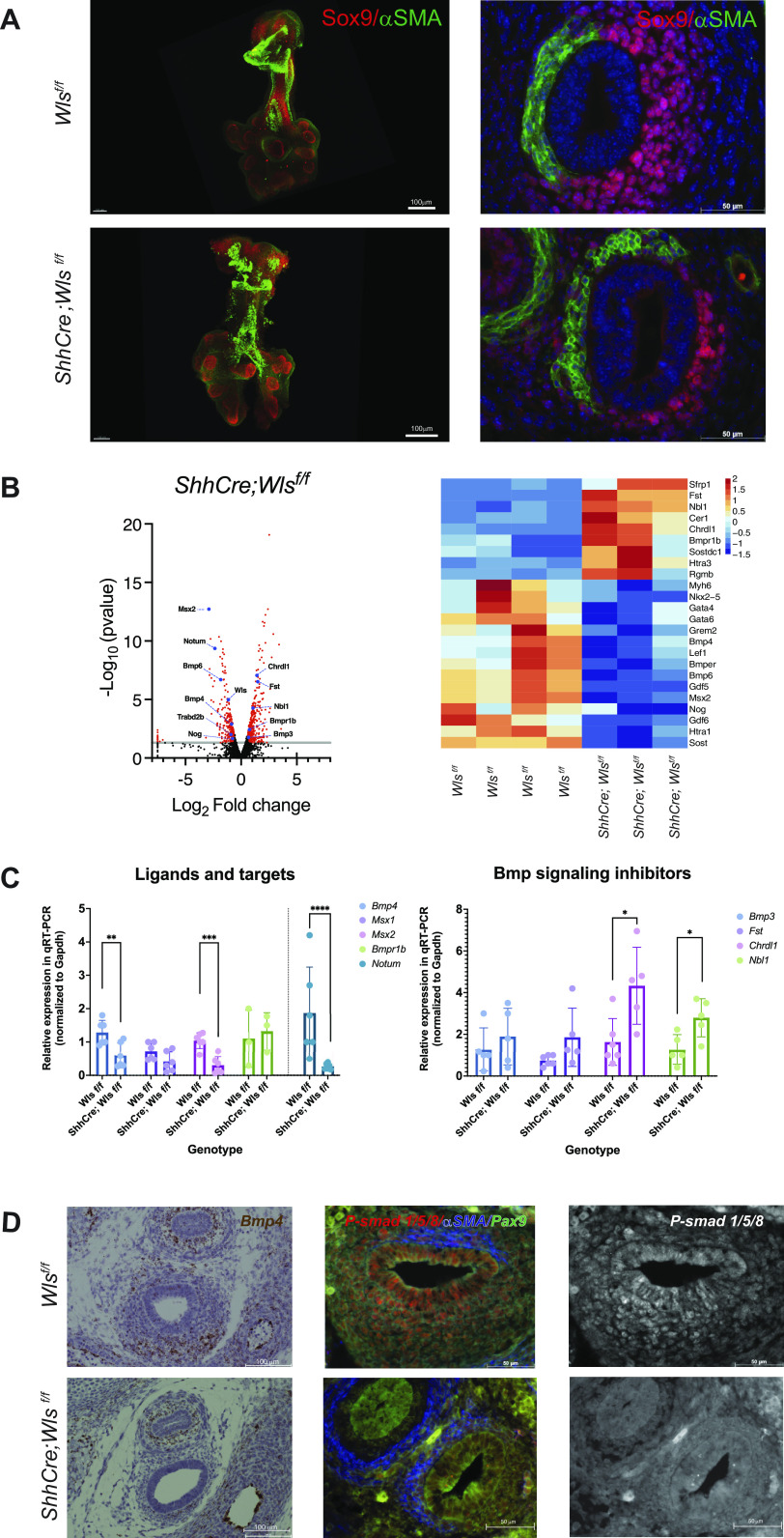
Deletion of Wls from tracheal epithelium disrupted patterning of the tracheal mesenchyme, causing loss of cartilage and anomalous expansion of the muscle layer into the ventral aspect of the trachea (Fig. 1A and Supplemental Fig. S1A). An unbiased analysis of RNA sequencing data from E13.5 ShhCre;Wlsf/f tracheas identified differential regulation of the BMP signaling pathway. Expressions of genes encoding ligands and targets of Bmp signaling including Bmp4 (Bone Morphogenetic Protein 4), Msx2 (Msh homeobox 2), Gdf5 (Growth Differentiation Factor 5), and Bmp6 were decreased, while expression of negative regulators of BMP signaling, including Bmp3, Nbl1 (Neuroblastoma 1, Dan family BMP antagonist), Chrdl1 (Chordin like1), and Fst (Folistatin), was increased. Bmpr1b, encoding a receptor required for BMP4 signaling, was increased, and transcripts encoding extracellular negative regulators of BMP signaling, Bmper (BMP binding endothelial regulator) and Noggin, were decreased, indicating that epithelial secretion of Wnt ligands influences BMP signaling activity (Fig. 1B). Genes encoding modulators of Wnt signaling Notum and Trabd2b (TraB Domain Containing 2B) as well as the Wnt cargo receptor Wls were downregulated (Fig. 1B). To validate the RNA sequencing data, RNA was isolated from whole E13.5 control and ShhCre;Wlsf/f tracheas and expression of selected Bmp signaling components analyzed. Bmp4 and Msx2 were downregulated while Chrld1 and Nbl1 were upregulated after epithelial deletion of Wls. Notum, a target of Wnt/β-catenin, was downregulated in ShhCre;Wlsf/f tracheas, thus validating the RNA-seq data (Fig. 1C). To further investigate whether lack of secretion of Wnt ligands from the tracheal epithelium affected BMP signaling, we analyzed phospho-SMAD1/5/8 levels in E13.5 ShhCre;Wlsf/f tracheas. At this early stage of chondrogenesis, phospho-SMAD1/5/8 staining was present in the epithelium and mesenchyme of the developing trachea. Consistent with decreased number of Bmp4 transcripts, phospho-SMAD1/5/8 staining was decreased in Wls-deficient trachea (Fig. 1D). Taken together, the data support the concept that Wnt ligands produced by the embryonic tracheal epithelium modulate BMP signaling in tracheal mesenchyme and epithelium.
Figure 1.
BMP signaling is modulated by Wnt ligands produced by the epithelium. A: as observed in whole mount and cross-sectional immunofluorescence staining of embryonic day (E)13.5 ShhCre;Wlsf/f tracheas, deletion of Wls from the epithelium resulted in ectopic presence of muscle [α-smooth muscle actin (αSMA), green] and decreased Sox9 (red) expression on the ventral side compared with the control tracheas. B: RNA sequencing (RNA-seq) analyses of ShhCre;Wlsfl/fl tracheas indicated changes in known tracheal patterning genes (volcano plot) and Bmp4 signaling components or regulators (heat map). C: gene expression analysis by RT-PCR in control and ShhCre;Wlsf/f E13.5 tracheas demonstrates robustness of RNA-seq data. As predicted by the unbiased analysis, Bmp4 and Msx2 were downregulated whereas Chrdl1 and Nbl1 were upregulated. Notum, a Wnt/β-catenin target, was downregulated. n = 5. Student’s t test; *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. D: E13.5 RNA in situ hybridization revealed that deletion of Wls from the epithelium results in reduced expression of Bmp4 (brown) in the tracheal mesenchyme and epithelium, further validated by decreased phospho-SMAD1/5/8 signal (red), in the Wls-deficient trachea compared with control, as detected by immunofluorescence. The same image is shown in grayscale.
Deletion of Bmp4 from Developing Foregut Mesenchyme Impairs Trachea and Lung Morphogenesis
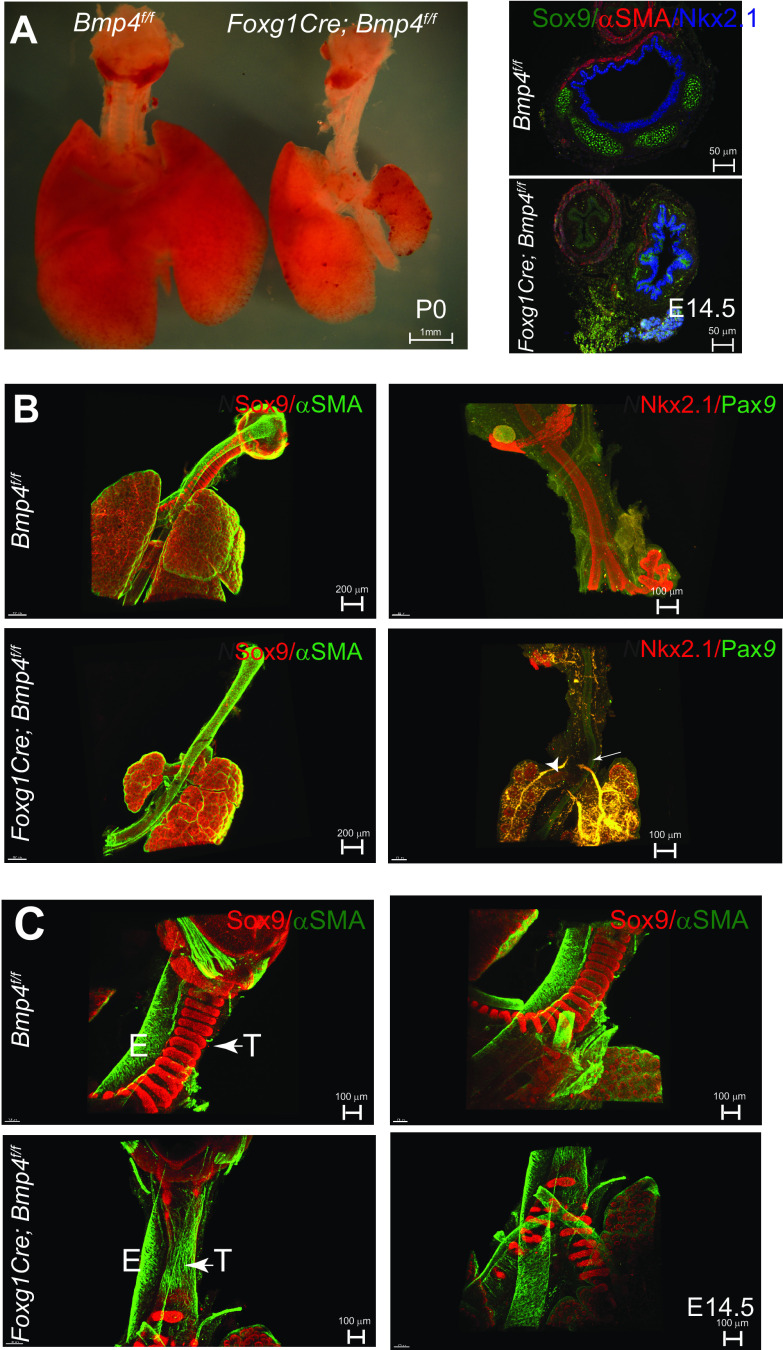
In vitro studies support a role for Bmp4 in promoting tracheal cartilage differentiation (28). However, in vivo testing during tracheal chondrogenesis has been hampered by the lack of suitable models. We generated Bmp4-deficient mutant mice in which Bmp4 (30) was deleted from tracheal mesenchyme using Dermo1Cre and Foxg1Cre or from developing chondrocytes using Col2a1Cre mice (46–48). Using Dermo1Cre, which mediates robust Cre recombinase in foregut mesenchyme at E10.5 (49, 50), or Col2a1Cre to delete Bmp4 did not alter tracheal morphogenesis or levels of Bmp4 transcripts, suggesting defective recombination (Supplemental Fig. S1, B and C). Conditional deletion of Bmp4 using Foxg1Cre caused lethality at birth due to abnormal tracheal and lung development (Fig. 2A) as well as craniofacial defects and microphthalmia as previously described (51–53). Foxg1 is first detected in foregut endoderm at E8.5, and its expression becomes uniform and robust at E9.5 in foregut mesenchyme and endoderm (19). With Rosa26mT;mG reporter mice, Cre-mediated recombinase activity was detected as early as E9.5 in mesenchyme surrounding the foregut epithelium of the Foxg1Cre;Rosa26mT/mG mice (50) (Supplemental Fig. S2). While Cre-mediated recombination occurred in mesenchymal cells of Foxg1Cre developing trachea, a small number of cells in the epithelium of the trachea were also targeted by the driver at E11.5 (Supplemental Fig. S2) (54, 55). We assessed Foxg1Cre;Bmp4f/f embryos and detected a number of phenotypes ranging from absent or extremely short and truncated tracheas, as previously described by Li and colleagues (19), to a full-length trachea lacking cartilage (Fig. 2, B and C, and Table 1). Heterozygous embryos were indistinguishable from control embryos. Male and female fetuses were similarly affected by the loss of Bmp4 (Supplemental Table S3). In embryos in which trachea was “truncated,” the larynx was present and the esophagus and the shortened trachea were distinct and separated; however, tracheal lumen was collapsed, reflecting the lack of cartilage (Fig. 2, A and B). Bronchi were observed emerging from the carina positioned adjacent to the esophagus but with no connection to the esophagus, as determined by Pax9 staining (Fig. 2B, Fig. 3A). Cartilage in the carina and bronchi was present, although mispatterned or reduced. These findings are consistent with tracheal atresia in Floyd type II malformations, in which the anterior portion of the trachea ends in a blind tube and the carina and bronchi are in normal position adjacent to the esophagus (56). In embryos with full-length trachea, SOX9 staining was detected, although reduced and mesenchymal condensations were lacking (Fig. 2C and Supplemental Fig. S3B), indicating lack of cartilage resulting in tracheomalacia. Ectopic expression of αSMA was detected in the ventral trachea mesenchyme, in contrast to the dorsal expression of αSMA in control trachea (Fig. 2C). In addition to the defects observed in tracheal tissue, mesenchymal deletion of Bmp4 severely impaired lung morphogenesis, causing hypoplastic and in some cases absent left lungs (Fig. 2A). Lung malformations were present regardless of the severity of the tracheal malformations. Taken together, mesenchymal Bmp4 is required for formation of tracheal cartilage, trachealis muscle patterning, and normal growth of the lung.
Figure 2.
Mesenchymal deletion of Bmp4 impairs respiratory tract development and patterning of the tracheal mesenchyme. A: postnatal day (P)0 image of Foxg1Cre; Bmp4f/f respiratory tract depicts the poorly developed trachea and lungs compared with the control. Cross section staining of embryonic day (E)14.5 trachea shows that deletion of Bmp4 from the mesenchyme renders poor development with nonexistent cartilage on the ventral side, resulting in a flaccid trachea. Respiratory identity was confirmed by NKX2.1 (blue) staining. B: whole mount immunofluorescence of E14.5 Foxg1Cre;Bmp4f/f truncated tracheas stained for smooth muscle [α-smooth muscle actin (αSMA), green] and cartilage (SOX9, red) reveal the ectopic presence of muscle on the ventral side and mispatterned or reduced cartilage near the carina and bronchi. Trachea and esophagus were stained with NKX2.1 (red) and PAX9 (green), respectively. In Foxg1Cre;Bmp4f/f, the carina and bronchi (arrowheads) are adjacent to the esophagus (arrow) and seem to emerge from a blind tube as opposed to the continuous structure in the control. C: whole mount immunofluorescence of E14.5 Foxg1Cre;Bmp4f/f “complete” tracheas revealed nearly nonexistent cartilage (SOX9, red) in the trachea with poor mesenchymal condensations in carina and bronchi and anomalous presence of smooth muscle (αSMA, green) in the ventral aspect of the tracheal mesenchyme. E, esophagus; T, trachea.
Table 1.
Incidence of tracheal phenotypes observed after mesenchymal deletion of Bmp4
| Phenotype | Genotype | No. of Embryos (in 44 Litters) | Percentage |
|---|---|---|---|
| Normal trachea | Bmp4f/f | 79 | 23.37% |
| Normal trachea | Bmp4f/− and Foxg1Cre; Bmp4f/− | 177 | 52.37% |
| Absent or truncated trachea | Foxg1Cre; Bmp4f/f | 31 | 9.17% |
| Full trachea with absent or mispatterned cartilage | Foxg1Cre; Bmp4f/f | 51 | 15.09% |
Genetically engineered mice were used to conditionally delete Bmp4 from the developing tracheal mesenchyme. Embryos from 44 litters were obtained to analyze genotype and phenotype correlations. While Bmp4f/f and Foxg1Cre;Bmp4f/− embryos displayed a normal trachea, analysis of Foxg1Cre;Bmp4f/f embryos depicted phenotypes ranging from an absent or extremely short, truncated trachea to a full trachea with lack of patterning. We observed that 9.17% of the total samples were of the Foxg1Cre;Bmp4f/f genotype and displayed a truncated trachea, while 15.09% of the samples with the same genotype revealed a full trachea characterized by mispatterned cartilage or without cartilage.
Respiratory Identity Is Preserved in Epithelium of Bmp4-Deficient Tracheas
To determine whether the lack of cartilage and ectopic muscle resulted from the abnormal specification of the mesenchymal lineages or altered dorsal-ventral patterning of the foregut epithelium, we analyzed earlier stages of tracheal development. At E11.5, we detected similar levels of Nkx2.1 and Wnt7b transcripts, two markers that define the respiratory identity of the endoderm (57) (Supplemental Fig. S3C). Similarly, NKX2.1 was detected in both controls and Bmp4-deficient tracheas as determined by whole mount immunofluorescence (Fig. 3A). The specific epithelial expression pattern of NKX2.1 and PAX9 in the E12.5 trachea and esophagus, respectively, further supports that proper foregut endodermal patterning took place in mutants with an entire trachea (Fig. 3B). Furthermore, Wnt7b transcripts were also detected at similar levels in the control and Bmp4-deficient tracheas, confirming the respiratory identity of the tracheal epithelium (Fig. 3C). We also analyzed Sox2, which is dynamically expressed in the developing proximal respiratory tract and plays a critical role in establishing the dorsal-ventral patterning of the foregut endoderm, antagonizing Nkx2.1 (58, 59). At E11.5 no significant differences were detected in Sox2 transcript levels (Supplemental Fig. S3). Sox2 was detected in the epithelium of the esophagus and the dorsal aspect of the E12.5 control trachea. In Bmp4 mutants, a similar expression pattern of Sox2 was observed, although in some cases expanding more ventrally than in control tracheal epithelium (Fig. 3C). However, Nkx2.1 expression was observed in the entire epithelium of the Bmp4-deficient trachea (Fig. 3A and Supplemental Fig. S3, C and D). At E12.5, ectopic muscle is observed expanding ventrally into the mesenchymal layer, whereas Col2a1 transcripts were reduced in the ventral tracheal mesenchyme (Fig. 3, C and D). The increased muscle was evident in whole mounts of E13.5 Foxg1Cre;Bmp4f/f;γSMAeGFP tracheas, in which ectopic muscle was present in ventral mesenchyme (Fig. 3D). Thus, Bmp4 is necessary for mesenchymal cell differentiation of the tracheal muscle and cartilage but is not required for the epithelial expression of Nkx2.1 and Wnt7b.
Figure 3.
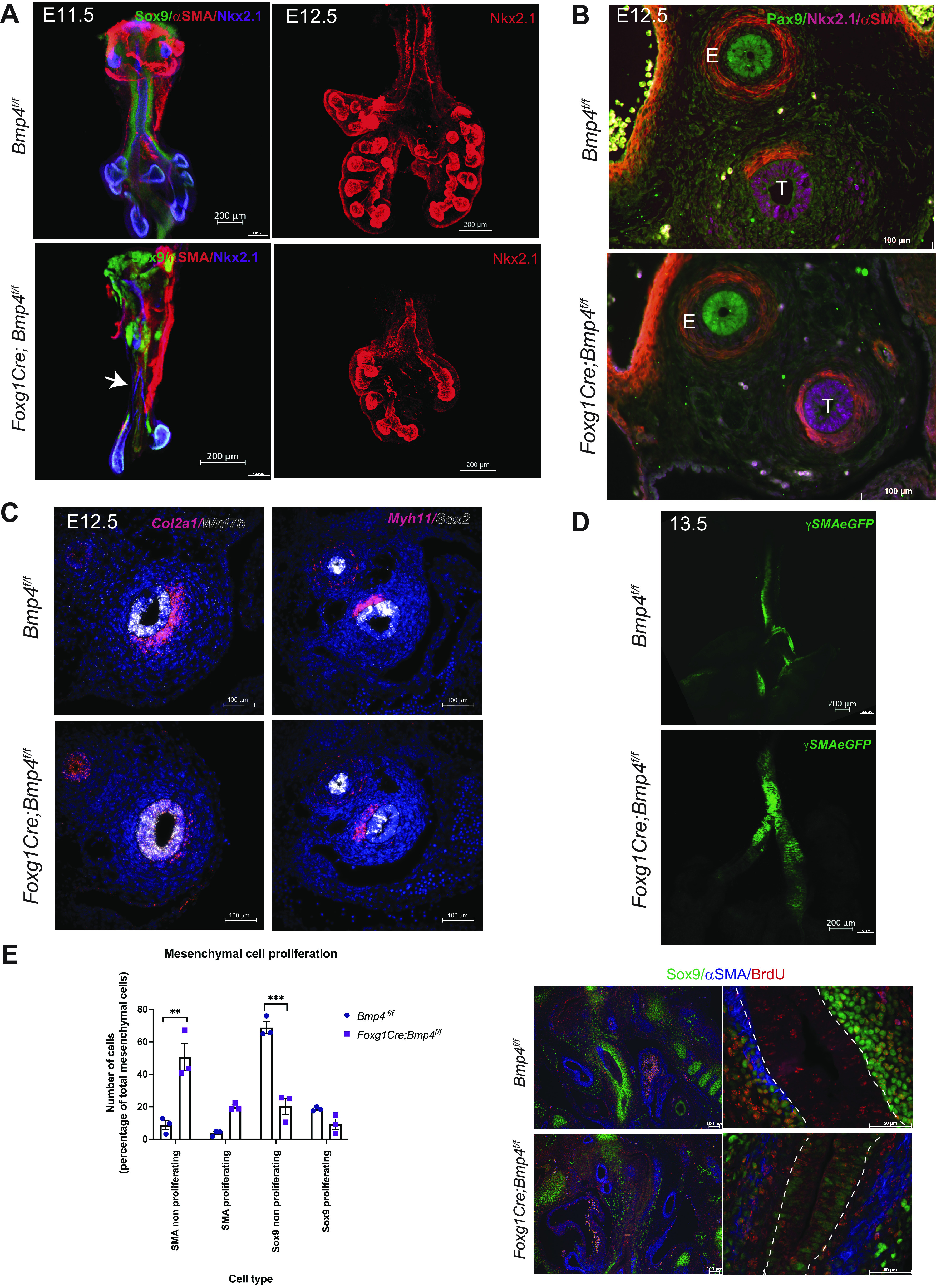
Bmp4 affects cell differentiation proliferation of tracheal mesenchymal cells. A, left: whole mount immunofluorescence of embryonic day (E)11.5 Foxg1Cre;Bmp4f/f respiratory tract tissue shows decreased chondroblasts (Sox9, green) in the trachea and close to the carina (arrow) compared with controls. Muscle cells [α-smooth muscle actin (αSMA), red] were present to some extent in both controls and mutants. Respiratory epithelial marker Nkx2.1 shows hypoplastic lungs with reduced branching compared with control lungs. Right: whole mount immunofluorescence of E12.5 confirms the respiratory identity of the Foxg1Cre;Bmp4f/f trachea and the hypoplasia of the lungs by Nkx2.1 staining (red). B: cross sections of E12.5 tracheas were stained with NKX2.1 (magenta) and PAX9 (green) to confirm the respiratory vs. esophageal identity. Ectopic smooth muscle (αSMA, red) was detected in the subepithelial mesenchymal layer of Foxg1Cre;Bmp4f/f tracheas. E, esophagus; T, trachea. C: E12.5 RNA in situ hybridization in transverse sections shows canonical Wnt ligand Wnt7b (white) transcripts localized in the epithelium of Foxg1Cre;Bmp4f/f tracheas similarly as observed in control tracheas. Sox2 transcripts (white) were detected in esophagus and in the dorsal aspect of the E12.5 control and Foxg1Cre;Bmp4f/f tracheas. Chondroblasts (Col2a1) and muscle (Myh11) restricted to the ventral and dorsal sides of the control trachea, respectively. Foxg1Cre;Bmp4f/f transverse sections depict muscle cells expanding laterally and reduced Col2a1 transcripts on the ventral side of the trachea. D: E13.5 whole mount explant of Foxg1Cre;Bmp4f/f;γSMAeGFP shows the presence of ectopic muscle in ventral trachea compared with control. E: E12.5 Foxg1Cre;Bmp4f/f sections show a decrease in the total mesenchymal population (αSMA+ and Sox9+), with significant increase in the percentage of nonproliferating muscle cells (αSMA, blue), likely at the expense of chondroblasts (Sox9, green), which are significantly reduced, as seen in the mesenchymal cell proliferation graph. We observed an increase in proliferating muscle cells (αSMA/BrdU+) in Foxg1Cre;Bmp4f/f. Dotted line represents epithelium edges. Two-way ANOVA; **P < 0.01, ***P < 0.001. n = 3.
Bmp4 Influences Mesenchymal Cell Proliferation and Differentiation
Tracheal morphogenesis depends on processes mediating cell proliferation, differentiation, and migration, which regulate formation of tracheal cartilage and muscle. To determine whether cell proliferation contributes to the differences observed in cartilage and muscle formation caused by mesenchymal deletion of Bmp4, we performed a BrdU incorporation assay. Pregnant mice were injected with BrdU at E10.5, when lung buds emerge and when precursors of the trachealis muscle and cartilage are first detected. At E12.5, mesenchymal cells, comprised of αSMA- and Sox9-positive cells, were diminished in Foxg1Cre;Bmp4f/f tracheas, with smooth muscle cells (αSMA positive) being the more abundant type of cells present in the tracheal mesenchyme and a reduced number of cartilage precursors (Sox9-positive cells). BrdU labeling of tracheal mesenchymal cells indicated increased proliferation of smooth muscle cells in mutant trachea, perhaps consistent with decreased numbers of Sox9-positive cells. On the contrary, Sox9-positive cells were reduced in mesenchyme of the trachea and trended to proliferate less in Foxg1Cre;Bmp4f/f tracheas, although the difference in proliferation did not reach statistical significance (Fig. 3E). We also observed Sox9-positive cells in the epithelium of the Bmp4-deficient trachea; however, the intensity of the signal was weak compared with the Sox9-positive cells present in the tracheal mesenchyme. BrdU incorporation in Nkx2.1-positive cells was not changed in mutant tracheas compared with control (Supplemental Fig. S3D). Thus, differential proliferation of smooth muscle progenitor is likely to contribute, at least in part, to the abnormal expansion of smooth muscle after mesenchymal deletion of Bmp4. To test whether directional migration of smooth muscle cells from the dorsal aspect of the trachea may contribute to the ectopic muscle observed in Foxg1Cre;Bmp4f/f, we performed chemotaxis assays on cells isolated from E13.5 control and Foxg1Cre;Bmp4f/f;γSMAeGFP tracheas (60). Despite cells moving, neither rBMP2 nor rBMP4 induced directional migration (chemotaxis) of the mesenchymal cells, as there were no significant differences in forward migration index (see supplemental data and methods) among treatments with recombinant proteins and media with serum (Supplemental Fig. S4A). Furthermore, treatment with rBMP4 did not act as a repellent for smooth muscle cells, as they move similarly to muscle cells cultured in the presence of serum (Supplemental Fig. S4B). To test whether inhibition of Bmp signaling affects trachealis muscle differentiation and growth, E11.5 and E12.5 γSMA:eGFP tracheal lung tissue was incubated in the presence of the small molecule DMH1 [an inhibitor of Bmp signaling (61)]. Lung branching morphogenesis was disrupted; however, formation of tracheal muscle proceeded normally (Supplemental Fig. S5A). In contrast, treatment with rBMP4 appears to impair the muscle development, as determined by a decrease in fluorescence of tracheal muscle cells in the ex vivo assay with disorganized muscle tissue (Supplemental Fig. S5B). Taken together, Bmp4 influences mesenchymal tracheal lineages by modulating cell proliferation and cell differentiation at early stages of tracheal mesenchyme differentiation.
Deletion of Bmp4 Partially Recapitulates the ShhCre;Wlsf/f Phenotype
The altered patterning of the Foxg1Cre;Bmp4f/f tracheal mesenchyme is similar to ShhCre;Wlsf/f tracheas. At E12.5, ectopic muscle cells in ventral mesenchyme were detected in Foxg1Cre;Bmp4f/f and ShhCre;Wlsf/f tracheas, a phenotype that was accentuated at E13.5 (Fig. 4A). Since deletion of Bmp4 affects Sox9 and αSMA patterning, we tested whether aberrant presence of muscle in ventral mesenchyme is associated with reduced Sox9 expression. RNA in situ hybridization demonstrated that deletion of Sox9 from foregut mesenchyme, using Foxg1cre, decreased expression of Col2a1; however, Myh11, a muscle marker, was not affected (Supplemental Fig. S6). In contrast, in Foxg1Cre;Bmp4f/f tracheas decreased levels of Col2a1 transcripts were accompanied by ectopic expression of αSMA (Fig. 3, B and C, and Fig. 4A). Based on the phenotypes observed, Bmp4 acts downstream of Wls-induced Wnt signaling and upstream of Sox9 and Col2a1 to promote cartilage development and to prevent ectopic muscle in developing trachea.
Figure 4.
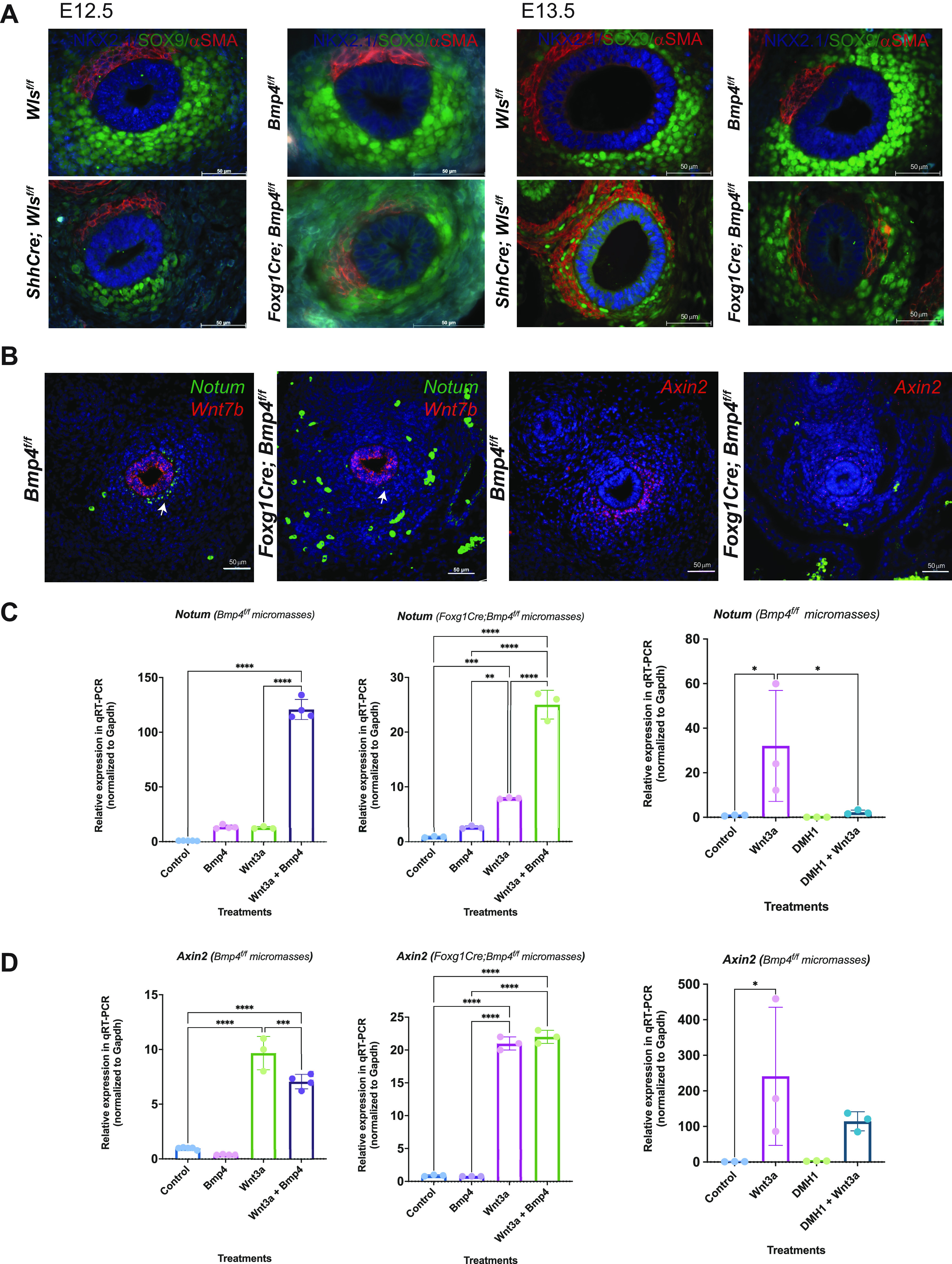
BMP signaling modulates expression of Wnt signaling targets. A: At embryonic day (E)12.5, in both ShhCre;Wlsf/f and Foxg1Cre;Bmp4f/f trachea muscle cells (red) are observed ventrolaterally, a pattern that at E13.5 was accentuated with reduced chondrocytes (Sox9, green) on the ventral side. B: at E12.5, Notum and Axin2 transcripts were reduced in the ventral tracheal mesenchyme. Axin2 transcripts were also observed ectopically in the dorsal mesenchyme of Foxg1Cre;Bmp4f/f tracheas. No changes were observed in the transcript levels of Wnt7b in Foxg1Cre;Bmp4f/f tracheas. C: micromasses of tracheal mesenchymal cells were treated with recombinant (r)BMP4 (500 ng/mL) and Wnt3a conditioned medium (a ligand typically inducing Wnt/β-catenin signaling) or DMH1 (20 µM). In micromasses of Foxg1Cre;Bmp4f/f cells, Wnt3a-mediated induction of Notum was significantly increased by cotreatment with rBMP4. Consistently, cotreatment with Wnt3a and DMH1 reduces Wnt3a-mediated Notum induction. D: Wnt3a-induced expression of Axin2 was attenuated by the cotreatment with rBMP4 and Wnt3a in control micromasses. Induction of Axin2 expression by Wnt3a in mutant micromasses was not affected by the cotreatment with Wnt3a and rBMP4; however, treatment with DMH1 reduces Wnt3a-mediated Axin2 expression. n = 3. One-way ANOVA; *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Bmp4 Modulates Expression of Wnt/β-Catenin Targets in Vivo and in Vitro
Since Wls-induced Wnt signaling promoted Bmp4 expression, and the phenotypes observed after epithelial deletion of Wls and mesenchymal deletion of Bmp4 were similar, we analyzed expression of Wnt signaling activity in Bmp4-deficient tracheas. At E12.5, transcripts of two direct Wnt/β-catenin targets, Notum and Axin2, were decreased in the ventral subepithelial mesenchyme (Fig. 4B). Notum and Axin2 transcripts were also diminished at E14.5, the stage when chondrogenesis starts in control tracheas (not shown). Conversely, Wnt7b, a ligand promoting Wnt/β-catenin signaling and required for development of the respiratory tract, was not affected by the mesenchymal deletion of Bmp4 (Fig. 4B). To further investigate how Bmp4 modulates expression of Wnt targets, we performed in vitro studies using mesenchymal cells isolated from E13.5 Bmp4f/f and Foxg1Cre;Bmp4f/f trachea. In Foxg1Cre;Bmp4f/f mesenchymal cells, expression levels of Bmp4 and its target Msx2 were diminished, further validating the efficiency of the mesenchymal deletion (Supplemental Fig. S7A). Next, tracheal mesenchymal cells were cultured in monolayers, which are representative of the stage before mesenchymal condensations, or cultured in micromasses, a model representing a stage when cells are beginning to aggregate in prechondrogenic nodules. To validate the in vitro system, we treated monolayers and micromasses with rBMP4, which induced the expression of Col2a1 in both monolayers and micromasses of Bmp4f/f and Foxg1Cre;Bmp4f/f cells (Supplemental Fig. S7B). Treatment with rBMP4 induced the deposition of cartilaginous extracellular matrix as determined by Alcian blue staining in micromasses of Bmp4f/f cells and to some extent Foxg1Cre;Bmp4f/f cells (Supplemental Fig. S7C). Next, we analyzed the effects of rBMP4 on expression of Wnt signaling targets Notum and Axin2. Notum expression was induced in monolayers and micromasses of control and Bmp4-deficient cells by Wnt3a conditioned media and by treatments with rBMP4 (micromasses in Fig. 4C and monolayers in Supplemental Fig. S7D). The inductive effects of rBMP4 on Notum expression were increased by cotreatment with Wnt3a in monolayers and micromasses of Bmp4-deficient cells. In contrast, inhibition of BMP signaling with DMH1 significantly reduced the Wnt3a-mediated Notum expression. While Axin2 was downregulated in vivo after mesenchymal deletion of Bmp4 (Fig. 4B), treatment with rBMP4 did not induce Axin2 and, to a certain extent, rBMP4 impaired Wnt3a-induced expression of Axin2 in monolayers of Bmp4f/f cells (Fig. 4D). Notwithstanding, cotreatment of micromasses with Wnt3a and DMH1 impaired the Wnt3a-mediated Axin2 expression. Taken together, Bmp4 is necessary to express Wnt signaling targets Notum and Axin2 before and after mesenchymal condensation occurs. In vitro, Bmp4 cooperates with Wnt/β-catenin signaling to promote the Wnt signaling modulator Notum, a gene that influences tracheal cartilage patterning and whose deletion causes tracheal stenosis and abnormal cartilage (8).
BMP Signaling Induces Notum Promoter Activity
To further investigate whether Bmp4 influences gene expression of Wnt targets via binding of SMAD1/5/8 to regulatory regions, we analyzed the 2-kb human and mouse promoter regions and 5′-untranslated regions (5′-UTRs) of Notum and Axin2. In silico analysis predicted both TCF (transcription factor mediating Wnt/β-catenin signaling) and SMAD1/5/8 binding sites in the fragments analyzed (human regulatory regions in Fig. 5, A and B, and mouse promoters in Supplemental Fig. S8). We performed luciferase assays and demonstrated that whereas Wnt3a induced luciferase activity in both human NOTUM and AXIN2 reporters, rBMP4 treatment promoted transcriptional activity in human NOTUM promoter alone, concordant with the presence of putative Smad1/5 binding sites (Fig. 5, C and D). Cotreatment with rBMP4 and Wnt3a further increased the activity of the NOTUM promoter construct (Fig. 5C). While treatment with rBMP4 did not induce luciferase activity of the AXIN2 reporter, cotreatment with Wnt3a and rBMP4 increased the activity of the reporter compared with Wnt3a treatment alone (Fig. 5D). Negative control and random sequence constructs did not respond to treatments (not shown). We searched publicly available ChIP-Seq databases and found that in human pulmonary artery endothelial cells (HPAECs) SMAD1/5 binds to NOTUM’s first exon and intron (62), with one site partially overlapping with the reporter sequence used in this study (Fig. 5E). Thus, the studies support a role for Bmp4 in regulating expression of Wnt/β-catenin modulator Notum at the transcriptional level via SMAD1/5.
Figure 5.
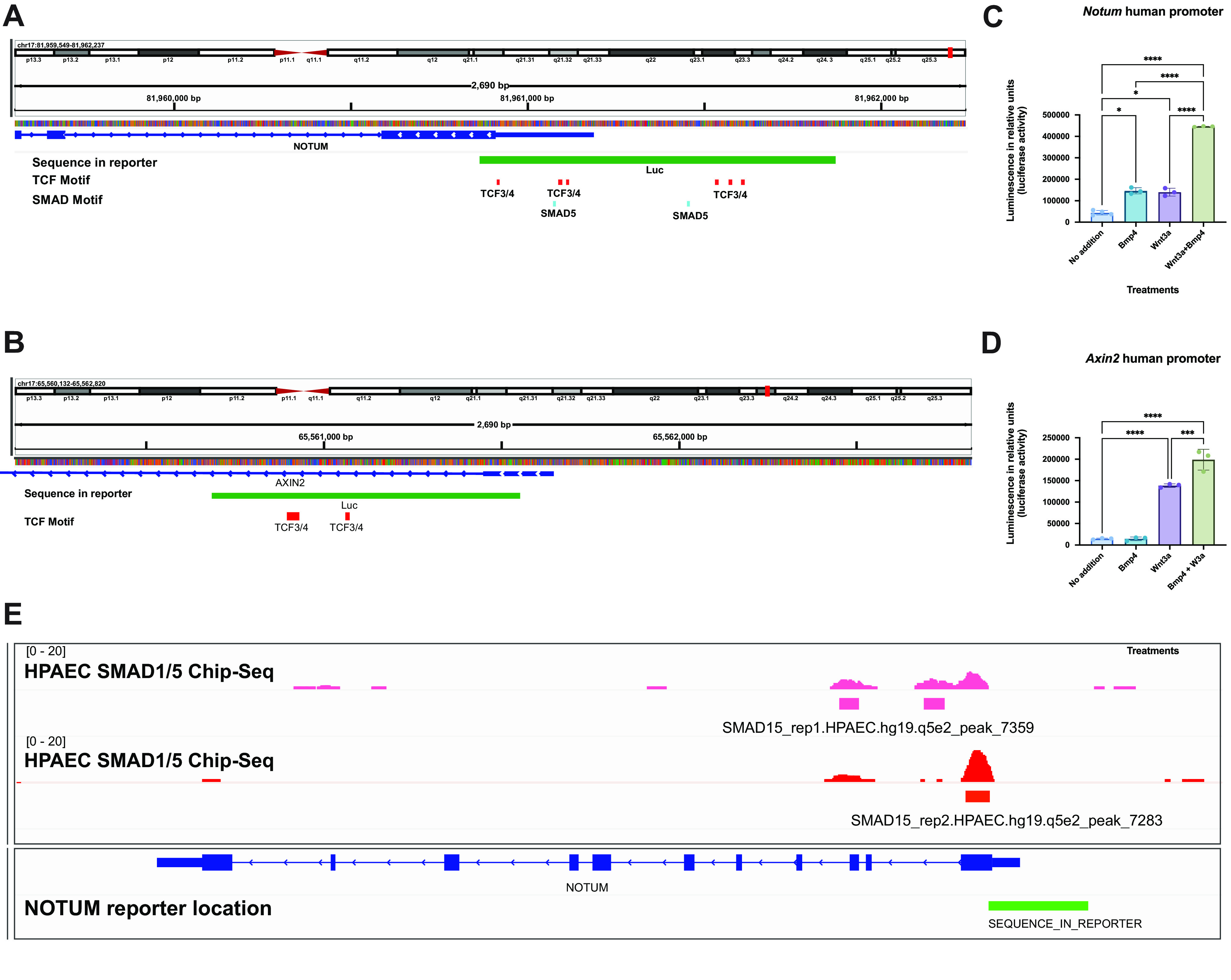
Bmp4 synergizes with Wnt/β-catenin to promote expression of NOTUM at the promoter level. A and B: promoter regions for human NOTUM (A) and AXIN2 (B) reveal putative binding sites for TCF and SMAD1/5 transcription factors. FIMO; P value < 0.0001 for TCF motifs and P value < 0.001 for SMAD motifs. C: addition of Wnt3a conditioned medium alone induced the relative luciferase activity of human NOTUM and AXIN2 promoters. Cotreatment of Wnt3a and recombinant (r)BMP4 induced a higher transcriptional activation of NOTUM promoter compared with individual treatments. D: as opposed to NOTUM promoter, no significant induction was observed in the luciferase activity of AXIN2 promoter when cells were treated with rBMP4 compared with control. Cotreatment of Wnt3a and rBMP4 increased luciferase activity compared with treatment with Wnt3a. One-way ANOVA, n = 3; *P < 0.05, ***P < 0.001, ****P < 0.0001. E: analysis of publicly available ChIP-seq studies performed on human pulmonary artery endothelial cells (HPAECs) identified SMAD1/5 binding sites in the first exon and intron of NOTUM, with 1 binding site overlapping partially with the reporter sequence used in this study.
Bmp and Wnt Signaling Coordinately Modulate a Common Subset of Genes during Tracheal Chondrogenesis
To study whether Bmp signaling has a broader effect on expression of Wnt signaling targets during early stages of chondrogenesis, we analyzed gene expression in E13.5 tracheas. To isolate a population of cells enriched in chondroblasts, we performed fluorescence-activated cell sorting (FACS), using cells isolated from Foxg1Cre;γSMAeGFP;Bmp4f/f tracheas. After dead cells were excluded based on Sytox staining, live epithelial and smooth muscle cells were isolated based on APC-EpCAM staining and eGFP fluorescence, respectively. APC-EpCAM staining allowed us to isolate epithelial cells, as confirmed by Nkx2.1 expression, whereas eGFP staining allowed us to isolate the smooth muscle as determined by Acta2 and Myocd expression (Supplemental Fig. S9B). Live cells without APC or eGFP fluorescence (apc−;gfp−) were considered a chondroblast-enriched population, based on Col2a1 expression that was concordantly not detected in APC+ or eGFP+ cells (Supplemental Fig. S9B). Unbiased RNA-seq gene expression analysis of Bmp4-deficient “chondroblasts” (apc−;gfp−cells) was performed (Fig. 6A). Mesenchymal deletion of Bmp4 caused a decrease in expression of genes mediating chondrogenesis, including Col2a1, Tbx5, Sox5, and Sox6. On the other hand, genes encoding extracellular matrix components were enriched in Bmp4-deficient chondroblasts (Fig. 6A). Functional enrichment analysis indicated that molecular processes related to muscle and cartilage development and signaling pathways such as WNT and BMP were differentially affected by the mesenchymal deletion of Bmp4 (Fig. 6B). Furthermore, we detected diminished expression of Wnt signaling target genes, including Wls, Lef1, and Myc. Notum levels were also diminished, trending toward significance, possibly because of the sorting strategy used, which did not allow recovery of a consistent ventrolateral subepithelial population of Foxg1Cre;Bmp4f/f cells (Supplemental Fig. S9A). We compared the gene changes detected in the Bmp4-deficient “chondroblast-enriched population” to the ShhCre;Wlsf/f tracheal RNA-seq and identified 132 common target genes coordinately regulated after mesenchymal deletion of Bmp4 and epithelial deletion of Wls (Fig. 6C). Supporting the RNA-seq data, analysis of the gene expression in Bmp4- and Wls-deficient tracheas confirmed diminished expression of Msx2 and Lef1, as assessed by in situ hybridization (Fig. 6D). Furthermore, using monolayers of control and Bmp4-deficient cells, we observed that both rBMP4 and Wnt3a can synergize to promote expression of Wnt and Bmp signaling targets (Fig. 6E and Supplemental Fig. 9C). Taken together, Bmp and Wnt signaling are necessary to promote gene expression mediating tracheal mesenchymal differentiation, and both signaling pathways can cooperate to regulate gene expression during tracheal chondrogenesis.
Figure 6.
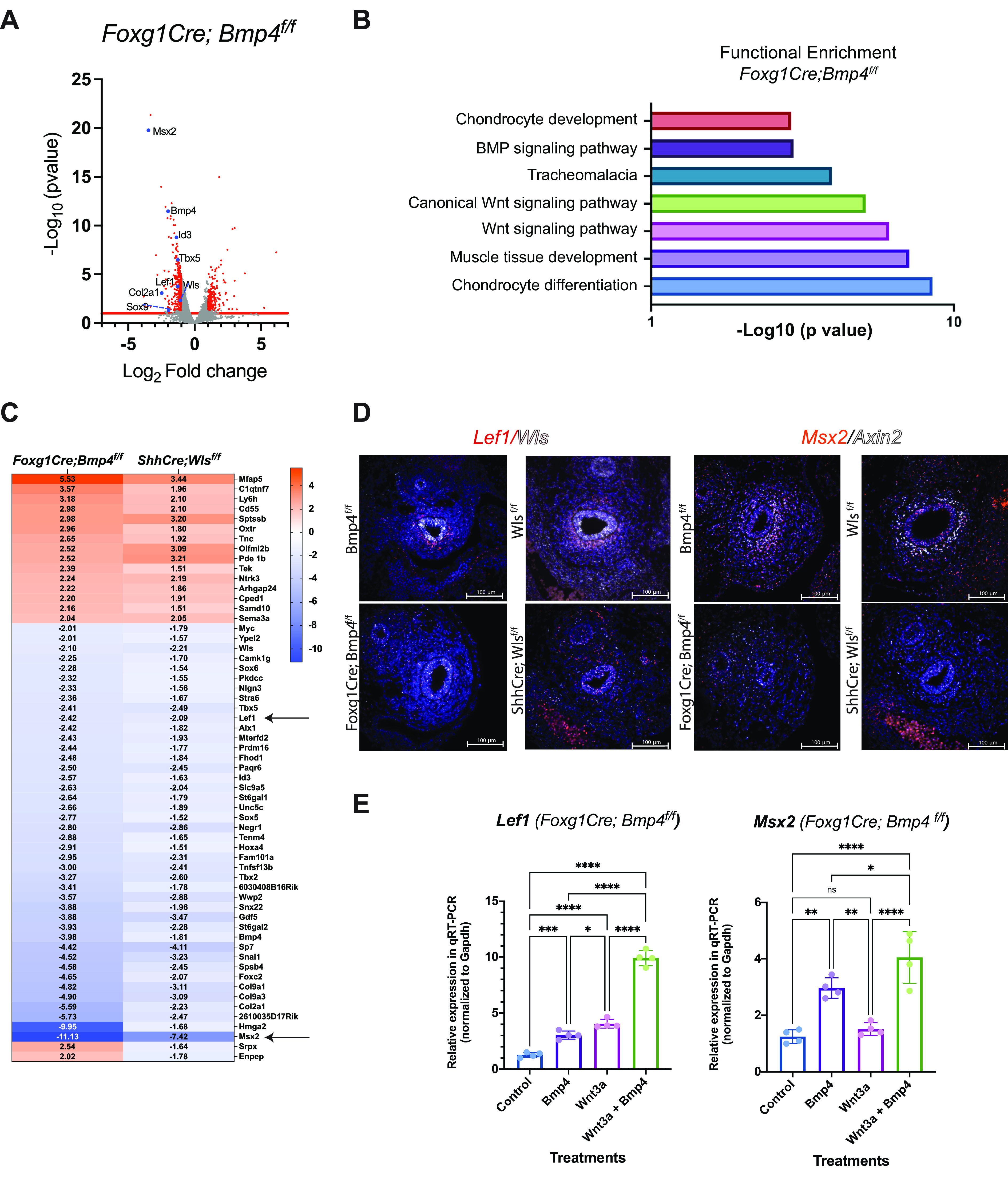
Wnt and BMP signaling modulate a common set of genes in ventral mesenchyme of developing trachea. A: volcano plot depicts differential regulation of Wnt signaling targets and genes mediating chondrogenesis in embryonic day (E)13.5 ventral tracheal mesenchymal cells after mesenchymal deletion of Bmp4. B: functional enrichment analysis indicates that in ventral tracheal mesenchyme Bmp4 modulates processes mediating chondrogenesis, muscle development, and the activity of Wnt signaling. C: heat map of common genes altered in ventral mesenchymal cells of Foxg1Cre;Bmp4f/f and ShhCre;Wlsf/f whole trachea reveals a set of common target genes regulated by Wnt and Bmp signaling during tracheal chondrogenesis. Lef1, a Wnt target, and Msx2, a Bmp4 target (arrows), were diminished after epithelial deletion of Wls and mesenchymal deletion of Bmp4. D: in situ hybridization detected decreased number of Lef1, Wls, Msx2, and Axin2 transcripts in ventral tracheal mesenchyme of E13.5 Foxg1Cre;Bmp4f/f and ShhCre;Wlsf/f tracheas. E: monolayers of Foxg1Cre;Bmp4f/f cells were treated with recombinant (r)BMP4 (500 ng/mL) and Wnt3a conditioned media. Lef1 expression was induced by treatment with rBMP4 or Wnt3a alone, whereas cotreatment with rBMP4 and Wnt3a further increased expression of Lef1. rBMP4 induced expression of Msx2. Cotreatment with rBMP4 and Wnt3a increased levels of Msx2 transcripts. n = 3. One-way ANOVA; *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. ns, Not significant.
Bmp4 Expression in Human Tracheal Tissue
We assessed expression of genes critical for cartilage and muscle formation in tracheal samples from children. Sox9 staining was observed circumferentially in mesenchyme of complete tracheal rings (CTR) tissue, whereas in tracheobronchomalacia (TBM), Sox9 was decreased, consistent with reduced cartilage. In CTR pathology trachealis muscle was absent; however, in TBM samples, ectopic muscle cells expressing αSMA were observed ectopically where cartilage is normally present (Supplemental Fig. S10A). BMP4 was detected at low levels in the tracheal epithelium and in submucosal glands as well as the muscle and the perichondrium of nondisease and TBM samples. In CTR samples, BMP4 transcripts were only detected in epithelium and to some extent in cells of submucosal glands (Supplemental Fig. S10B). Expression of BMP4 in large airways of children resembles the expression pattern of Bmp4 observed in large airways of E14.5, E16.5, and postnatal day (P)0 mice in which cartilage is already formed (Supplemental Fig. S10C). Taken together, expression pattern of genes promoting cartilage and muscle are altered in TBM and CTR pathology, whereas BMP4 is present in epithelium, muscle, and perichondrium of nondisease and TBM samples.
DISCUSSION
The present study identified an interplay between WNT and BMP signaling, facilitating the tracheal mesenchymal cell differentiation, and defines a role for Bmp4 in tracheal cartilage development. During the early stages of tracheal formation, WNT signaling from the epithelium induces Bmp4 in tracheal mesenchyme to promote cartilage differentiation and prevent ectopic trachealis muscle formation. Bmp4 cross talks with WNT signaling by regulating the expression of Wnt/β-catenin attenuators Notum and Axin2 (Fig. 7). WNT and BMP signaling regulate a common subset of genes expressed in ventral tracheal mesenchyme promoting chondrogenesis, including Col2a1, Msx2, Tbx5, Sox5, and Sox6. Thus, a coordinated activity of WNT and BMP signaling in developing mouse trachea is necessary for mesenchymal cell differentiation and tracheal cartilage and smooth muscle patterning (Fig. 7).
Figure 7.
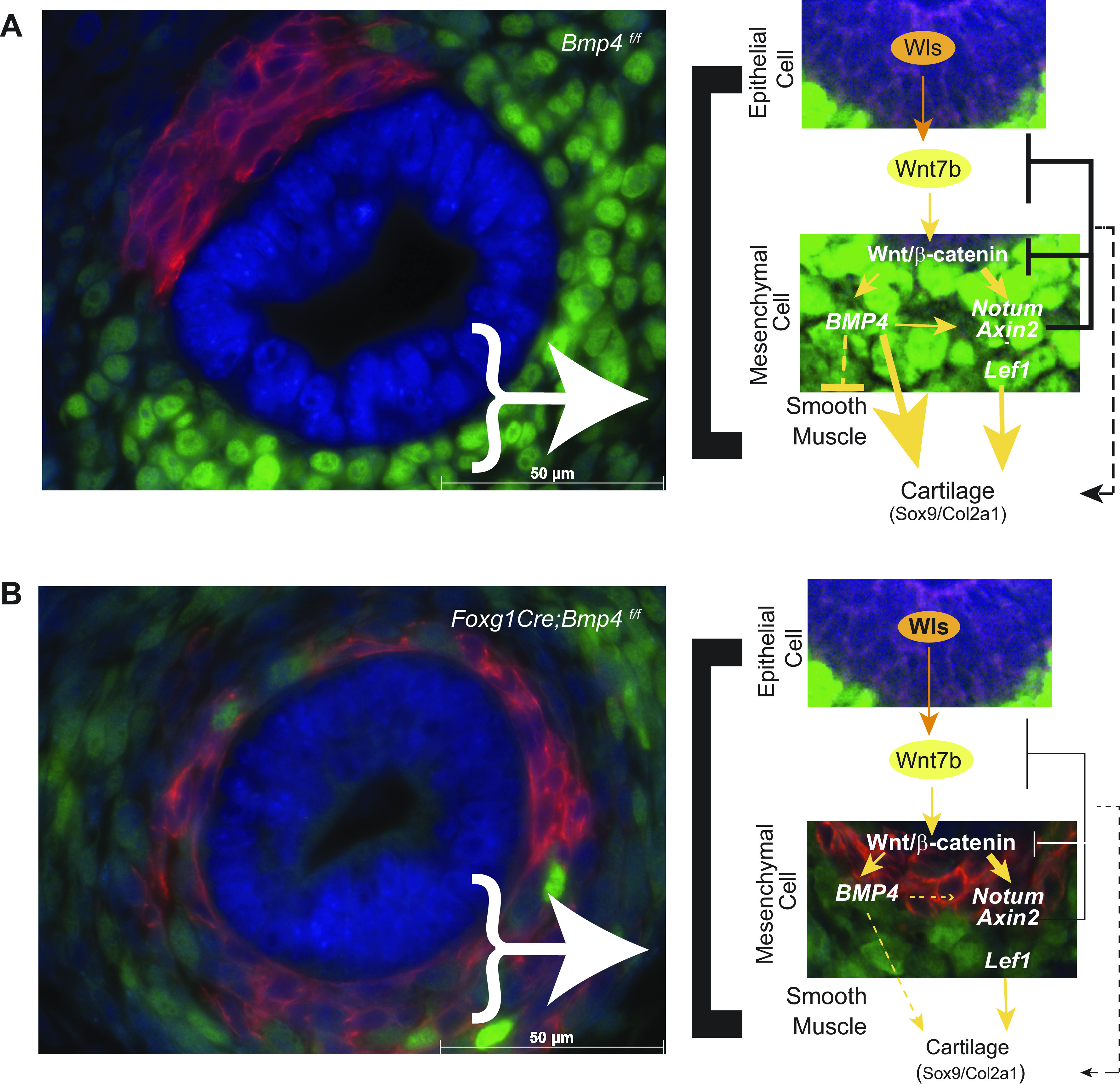
Putative network among BMP and Wnt molecules functionally interacting to promote tracheal cartilage and repress ectopic muscle. A: epithelial Wnt ligands induce Wnt signaling in developing mesenchyme of the trachea, which in turn promotes the expression of Bmp4, Lef1, Axin2, and Notum. Notum and Axin2 attenuate the strength of Wnt/β-catenin signaling to allow differentiation of tracheal mesenchyme into cartilage, a process also facilitated by Bmp4, by promoting cartilage development via Sox9 and Col2a1. Bmp4 promotes expression of Notum and Axin2, likely to contribute to attenuation of Wnt/β-catenin signaling. Bmp4 also promotes Lef1 expression, which in turn promotes expression of Wnt/β-catenin signaling targets including Bmp4, Sox9, and Col2a1 necessary for chondrogenesis. Simultaneously, Bmp4, via a mechanism yet to be determined, prevents ectopic smooth muscle in the ventral aspect of the trachea. B: mesenchymal deletion of Bmp4 causes lack of cartilage and ectopic muscle formation and a decrease in Wnt/β-catenin target gene expression, further contributing to impaired ventral tracheal mesenchyme differentiation.
Bmp4 Is Required for Tracheal Cartilage Development and Trachealis Muscle Patterning
In the present study, we formally demonstrated that Bmp4 promotes tracheal cartilage in vivo. In our model, after the mesenchymal deletion of Bmp4 we detected two main phenotypes. The first phenotype was the absence of or a poorly developed and very short, “truncated” trachea, resembling previously described phenotypes resulting from endodermal deletion of Bmpr1a and Bmpr1b or deletion of Bmp4 in lateral plate mesoderm (19, 23). The second phenotype observed was a complete trachea lacking cartilage. The phenotype may result from partial Cre-mediated recombination allowing some Bmp4 expression, enough for tracheal tube formation with endodermal cells expressing respiratory tract markers Nkx2.1 and Wnt7b (57). In our model, the residual levels of Bmp4 may also suffice for repression of Sox2 in the ventral foregut endoderm to promote respiratory identity and tracheal formation (20, 23, 58). While the respiratory identity of the epithelial cells was maintained, the mesenchyme was poorly developed, lacking cartilage replaced by ectopic muscle. Moreover, in Bmp4-deficient trachea, mesenchymal cells were diminished and proliferation was abnormal, a finding in agreement with studies by Li and colleagues (19) in which the deletion of Bmp4 in foregut caused reduction of mesenchymal proliferation at the time when tracheal primordium is specified. The lack of phenotype observed with Dermo1Cre and Col2a1Cre could be associated to different efficiencies of the Cre-mediated deletion. The timing of recombination may also account for the lack of phenotype, as Dermo1Cre and Col2a1Cre induce Cre recombination at later stages of development than Foxg1Cre, in which robust recombination is detected in foregut at E9.5 (50). Thus, is possible that Bmp4 is required early in tracheal development to influence mesenchymal cell lineages.
Our studies demonstrated that Bmp4 promotes tracheal chondrogenesis by inducing gene expression and influencing cell processes required for tracheal cartilage formation. Sox9 and Col2a1 are essential genes mediating tracheal cartilage development (11, 54). In Bmp4-deficient tracheas, SOX9 expression was reduced. While it is unclear whether Sox9 is a direct target of Bmp4, studies have shown that Bmp2 can activate the Sox9 promoter in committed chondrocytes during postnatal development and in progenitor cells in vitro (63). Deletion of Bmp4 abrogated Col2a1 expression, a direct target of Sox9, in tracheal mesenchyme. Bmp4 induces Col2a1 likely by promoting Sox9 expression and cooperating with Sox9 to induce Col2a1 expression (64, 65). Bmp4 may influence cartilage formation by promoting cell rearrangements required for chondroblast aggregation in mesenchymal condensations (66, 67). Our data support this concept, as lack of mesenchymal Bmp4 suppressed mesenchymal condensations and in vitro rBMP2 and rBMP4 favor cell chemokinesis (Supplemental Figs. S3B and S4). Thus, Bmp4 is essential for tracheal chondrogenesis by promoting gene expression and influencing cellular processes required for chondroblast cell condensation.
Bmp4 plays a role in patterning the trachealis muscle. Deletion of Bmp4 caused ectopic smooth muscle, a phenotype also observed after epithelial deletion of Wls (7, 45). While Bmp4 likely acts as a repressor of muscle development in ventral trachea mesenchyme, the mechanism remains unknown. It is possible that Bmp4 represses myogenic genes such as Myh11, Acta2, or Actg2 in partnership with gene(s) only expressed in ventral trachea mesenchyme or by inducing a muscle repressor in tracheal ventral mesenchyme. In vascular smooth muscle cells, Bmp-induced expression of Msx1 and Msx2 represses smooth muscle gene expression during onset and progression of atherosclerosis (68). After deletion of Myocd from respiratory tract, phospho-SMAD1/5/8 staining in mesenchyme of peripheral lung was increased where muscle was lacking (66). On the other hand, enhanced activity of BMP repressors increased myogenic differentiation and improved the dystrophic phenotype characteristic of Duchenne muscular dystrophy (69). Conditional deletion of Fstl1, a secreted BMP antagonist, causes reduction of trachealis smooth muscle (70), supporting a role for BMP4 in repressing muscle. The concept is partially supported by the ex vivo experiments in which treatment of explants with rBMP4 causes a decrease in smooth muscle cells. It is noteworthy that in ShhCre;Wlsf/f tracheas, in which Bmp4 is reduced, several Bmp4 antagonists are increased. Thus, increased activity of BMP antagonists may contribute to the ectopic presence of muscle after mesenchymal deletion of Bmp4. In Bmp4-deficient tracheas cartilage is lacking; however, absence of cartilage cannot account for ectopic muscle in the trachea, as the ablation of Sox9 from tracheal mesenchyme does not cause ectopic muscle ventrally (Supplemental Fig. S6) (7, 12, 54). Finally, ventral expression of Bmp4 in tracheal mesenchyme may act as a chemorepellent for migration of dorsally located myoblasts; however, the cell migration assays in the presence of rBMP4 or rBMP2 do not support the concept (Supplemental Fig. S4). Thus, Bmp4 prevents ectopic muscle in ventral mesenchyme either directly or indirectly, likely by a mechanism that involves differentiation of ventral mesenchymal cells.
WNT Regulates BMP Signaling to Influence WNT Activity in Developing Trachea
Interactions between Wnt and BMP pathways have been described in different systems including the mammalian limb (71) and in colon cancer (72), where Wnt/β-catenin induced the expression of BMP ligands. During neural development or in colon carcinoma cells Wnt/β-catenin also regulated the expression of BMP signaling modulators, including Xiro, Bambi, and PRDC (73–75). During tracheal chondrogenesis, Wnt ligands produced by the tracheal epithelium are critical for BMP signaling via SMAD1/5/8 and for expression of BMP ligands and Bmp4 antagonists such as Nbl1 and Chrdl1 (Fig. 1, B–D). Thus, epithelial-induced Wnt signaling can modulate the strength of BMP activity in the developing trachea mesenchyme (Fig. 1).
Deletion of Bmp4 impaired expression of direct targets of Wnt/β-catenin, including Axin2 and Notum (Fig. 4B). Published studies have shown that BMP and WNT synergize at the transcriptional level to induce the expression of Msx2 via the formation of a complex including β-catenin, TCF/LEF1, and SMAD4 on the promoter of Msx2 (76). We demonstrated that Bmp4 and Wnt/β-catenin signaling synergize to promote Notum expression. Notum, an extracellular deacylase, is expressed in developing trachea and attenuates the strength of Wnt/β-catenin signaling in the ventral mesenchyme. Notum mediates the timely formation of mesenchymal condensations, a prerequisite for tracheal cartilage formation and patterning. Ablation of Notum caused abnormal cartilage and tracheal stenosis (8). In silico and in vitro analysis of the 2-kb human and mouse Notum promoters predicted the presence of SMAD1/5 and TCF binding sites, indicating direct regulation of Notum expression via BMP4-SMAD1/5 (Fig. 5 and Supplemental Fig. S8). The findings are supported by ChIP-seq studies in HPAECs, in which SMAD1/5 binds to NOTUM (62). Whereas WNT and BMP cooperate at the transcriptional level to induce Notum, Axin2, whose expression was reduced in Bmp4-deficient tracheas, was not induced by rBMP4 in in vitro assays, likely reflecting limitations in the in vitro system. We have shown that increased levels of Wnt/β-catenin activity abolish differentiation of tracheal cartilage (8). Therefore, by promoting Notum and Axin2, Bmp4 modulates Wnt/β-catenin activity necessary for cartilage differentiation.
Our studies identified common target genes regulated by WNT and BMP signaling in tracheal ventral mesenchyme. The unbiased analysis of gene expression identified Wnt signature genes such as Lef1, Wls, and Myc decreased after mesenchymal deletion of Bmp4. Conversely, Msx2, Gdf5, and Bmp4 were downregulated after epithelial deletion of Wls (Fig. 6). Wnt/β-catenin and Bmp4 can coregulate gene expression via Smad1 and β-catenin co-occupying cis-regulatory DNA elements to mediate patterning of Xenopus’ digestive tract (77). While it is unclear whether TCF and SMADs physically interact to bind promoters of common WNT and BMP target genes in developing trachea, inducing both pathways coordinately affected gene expression as demonstrated by promoter analysis and in vitro analysis (Fig. 5, Fig. 6E). A recent study focusing on HH signaling identified differential gene expression in E11.5 tracheas, in which imbalance of Gli2/3 activator/repressor leads to lack of specification of chondrocyte progenitors and tracheomalacia (50). Among genes differentially regulated identified by Nasr and colleagues (50) were Sox9, Notum, and Msx2. In our studies, the same genes were downregulated in E13.5 Bmp4- and Wls-deficient tracheas. The findings support the presence of a core gene network that is active at different stages of tracheal development regulated by HH, WNT, and BMP. Whereas genes mediating chondrogenesis, such as Tbx5, Col2a1, and Sox5 and Sox6, were downregulated in Bmp4 and Wls-deficient tracheas, genes encoding extracellular matrix components Mfap5, Tnc, and Olfml2b were upregulated. Extracellular matrix influences development of the pulmonary and trachealis smooth muscle (78, 79); however, whether the observed changes in gene expression account for the ectopic muscle observed in both Wls- and Bmp4-deficient trachea requires further analysis. Thus, the presence of common targets in ventral tracheal mesenchyme further supports the cross talk between Wnt and BMP during tracheal cartilage formation.
BMP Signaling in TBM and CTR
Whole exome sequencing studies have shown the significance of HH and WNT signaling in the pathology of CTR (1); however, it is unknown whether disrupted BMP signaling could contribute to the pathogenesis of TBM or CTR. Clinical reports on patients diagnosed with orofacial cleft 11 (caused by heterozygous mutation in BMP4) identified VACTERL associations, although it was not reported whether or how trachea was affected (80). A recent study focusing on transcriptional and histochemical profile of tracheoesophageal fistulas reported decreased Bmp signaling and lack of cartilage in the abnormal tissue. The study could not detect BMP4 protein in the diseased or the nondiseased infant trachea or esophagus; however, the investigators detected BMPR1A, BMPR1B, and BMPR2 (81). In tracheal tissue obtained from infants diagnosed with TBM, cartilage was decreased and muscle was increased, partially recapitulating the phenotypes observed in mouse after deletion of Bmp4. Despite similarities in phenotype between mouse model and human disease, BMP4 expression patterns were similar among TBM, CTR, and control/nondisease airway (Supplemental Fig. S10, A and B). While no differences were observed postnatally, significant differences in BMP4 expression and pattern may exist at the early stages of tracheal development, contributing to abnormal chondrogenesis. A caveat associated with interpreting studies in children is the complex etiology of TBM and CTR, which is likely to involve several signaling pathways. Injury to the airways caused by surgical and medical treatment may also influence the findings. In humans, tracheal morphogenesis occurs around 4 wk of pregnancy (82), a time when the requirements of BMP4 are likely critical for cartilage formation. A role for Bmp4 during early foregut mesenchyme patterning is supported by recent studies in organoid cultures from human and mouse iPSCs (29, 83), further underpinning the role of BMP4 in tracheal development and chondrogenesis.
In summary, the present work highlights how the cross talk of two essential signaling pathways, WNT and BMP, promotes trachea morphogenesis, thus informing the etiology of human tracheal pathologies necessary to develop novel treatments.
SUPPLEMENTAL DATA
Supplemental Tables S1-S3 and Supplemental Figs. S1-S10: https://doi.org/10.6084/m9.figshare.16934125.
GRANTS
This work has been partially supported by National Institutes of Health National Heart, Lung, and Blood Institute Grant R01 144774 and Just in Time-Center for Clinical and Translational Science (JIT-CCTST Award Program Grant 5UL1TR001425-04) to D.S.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
N.B.-A. and D.S. conceived and designed research; N.B.-A., L.L., K.B., R.S., M.M., and D.S. performed experiments; N.B.-A., L.L., K.B., J.S., R.S., M.M., Y.X., and D.S. analyzed data; N.B.-A., L.L., K.B., J.S., R.S., Y.X., and D.S. interpreted results of experiments; N.B.-A., L.L., K.B., J.S., R.S., and D.S. prepared figures; D.S. drafted manuscript; N.B.-A., L.L., K.B., J.S., R.S., M.M., Y.X., and D.S. edited and revised manuscript; N.B.-A., L.L., K.B., J.S., R.S., M.M., Y.X., and D.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Gail Macke for assistance with cryosections, Chuck Crimmel for support with graphic design, and Alison Kissling for assistance with reference formatting. Evan Meyer provided support with confocal microscopy. Jamie Russ at CCHMC Pathology assisted in identifying and locating relevant tissue from CCHMC Biorepository. We appreciate the comments on the manuscript by Dan Swarr and Aaron Zorn and the critical input on the project by Jeff Whitsett. We acknowledge Celine Silva-Lages and the Research Flow Cytometry Core personnel for assistance. All flow cytometric data were acquired with equipment maintained by the Research Flow Cytometry Core in the Division of Rheumatology at Cincinnati Children’s Hospital Medical Center.
Present address of L. Leesman: Heritage College of Osteopathic Medicine, Ohio University, Athens, OH.
REFERENCES
- 1.Sinner DI, Carey B, Zgherea D, Kaufman KM, Leesman L, Wood RE, Rutter MJ, de Alarcon A, Elluru RG, Harley JB, Whitsett JA, Trapnell BC. Complete tracheal ring deformity. a translational genomics approach to pathogenesis. Am J Respir Crit Care Med 200: 1267–1281, 2019. doi: 10.1164/rccm.201809-1626OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kamran A, Jennings RW. Tracheomalacia and tracheobronchomalacia in pediatrics: an overview of evaluation. Medical management, and surgical treatment. Front Pediatr 7: 512, 2019. doi: 10.3389/fped.2019.00512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ridge CA, O’donnell CR, Lee EY, Majid A, Boiselle PM. Tracheobronchomalacia: current concepts and controversies. J Thorac Imaging 26: 278–289, 2011. doi: 10.1097/RTI.0b013e3182203342. [DOI] [PubMed] [Google Scholar]
- 4.Rutter MJ, Cotton RT, Azizkhan RG, Manning PB. Slide tracheoplasty for the management of complete tracheal rings. J Pediatr Surg 38: 928–934, 2003. doi: 10.1016/S0022-3468(03)00126-X. [DOI] [PubMed] [Google Scholar]
- 5.Wilcox LJ, Hart CK, de Alarcon A, Schweiger C, Peddireddy NS, Tabangin M, Rutter MJ. Unrepaired complete tracheal rings: natural history and management considerations. Otolaryngol Head Neck Surg 158: 729–735, 2018. doi: 10.1177/0194599817751889. [DOI] [PubMed] [Google Scholar]
- 6.Wilcox LJ, Schweiger C, Hart CK, de Alarcon A, Peddireddy NS, Rutter MJ. Growth and management of repaired complete tracheal rings after slide tracheoplasty. Otolaryngol Head Neck Surg 161: 164–170, 2019. doi: 10.1177/0194599819841893. [DOI] [PubMed] [Google Scholar]
- 7.Snowball J, Ambalavanan M, Whitsett J, Sinner D. Endodermal Wnt signaling is required for tracheal cartilage formation. Dev Biol 405: 56–70, 2015. doi: 10.1016/j.ydbio.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerhardt B, Leesman L, Burra K, Snowball J, Rosenzweig R, Guzman N, Ambalavanan M, Sinner D. Notum attenuates Wnt/betacatenin signaling to promote tracheal cartilage patterning. Dev Biol 436: 14–27, 2018. doi: 10.1016/j.ydbio.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goss AM, Tian Y, Tsukiyama T, Cohen ED, Zhou D, Lu MM, Yamaguchi TP, Morrisey EE. Wnt2/2b and beta-catenin signaling are necessary and sufficient to specify lung progenitors in the foregut. Dev Cell 17: 290–298, 2009. doi: 10.1016/j.devcel.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goss AM, Morrisey EE. Wnt signaling and specification of the respiratory endoderm. Cell Cycle 9: 10–11, 2010. doi: 10.4161/cc.9.1.10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elluru RG, Whitsett JA. Potential role of Sox9 in patterning tracheal cartilage ring formation in an embryonic mouse model. Arch Otolaryngol Head Neck Surg 130: 732–736, 2004. doi: 10.1001/archotol.130.6.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turcatel G, Rubin N, Menke DB, Martin G, Shi W, Warburton D. Lung mesenchymal expression of Sox9 plays a critical role in tracheal development. BMC Biol 11: 117, 2013. doi: 10.1186/1741-7007-11-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Massagué J. How cells read TGF-beta signals. Nat Rev Mol Cell Biol 1: 169–178, 2000. doi: 10.1038/35043051. [DOI] [PubMed] [Google Scholar]
- 14.McCauley DW, Bronner-Fraser M. Conservation and divergence of BMP2/4 genes in the lamprey: expression and phylogenetic analysis suggest a single ancestral vertebrate gene. Evol Dev 6: 411–422, 2004. doi: 10.1111/j.1525-142X.2004.04054.x. [DOI] [PubMed] [Google Scholar]
- 15.Gipson GR, Goebel EJ, Hart KN, Kappes EC, Kattamuri C, McCoy JC, Thompson TB. Structural perspective of BMP ligands and signaling. Bone 140: 115549, 2020. doi: 10.1016/j.bone.2020.115549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldman DC, Donley N, Christian JL. Genetic interaction between Bmp2 and Bmp4 reveals shared functions during multiple aspects of mouse organogenesis. Mech Dev 126: 117–127, 2009. doi: 10.1016/j.mod.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma L, Lu MF, Schwartz RJ, Martin JF. Bmp2 is essential for cardiac cushion epithelial-mesenchymal transition and myocardial patterning. Development 132: 5601–5611, 2005. doi: 10.1242/dev.02156. [DOI] [PubMed] [Google Scholar]
- 18.Shu B, Zhang M, Xie R, Wang M, Jin H, Hou W, Tang D, Harris SE, Mishina Y, O’Keefe RJ, Hilton MJ, Wang Y, Chen D. BMP2, but not BMP4, is crucial for chondrocyte proliferation and maturation during endochondral bone development. J Cell Sci 124: 3428–3440, 2011. doi: 10.1242/jcs.083659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y, Gordon J, Manley NR, Litingtung Y, Chiang C. Bmp4 is required for tracheal formation: a novel mouse model for tracheal agenesis. Dev Biol 322: 145–155, 2008. doi: 10.1016/j.ydbio.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Que J, Choi M, Ziel JW, Klingensmith J, Hogan BL. Morphogenesis of the trachea and esophagus: current players and new roles for noggin and Bmps. Differentiation 74: 422–437, 2006. doi: 10.1111/j.1432-0436.2006.00096.x. [DOI] [PubMed] [Google Scholar]
- 21.Weaver M, Dunn NR, Hogan BL. Bmp4 and Fgf10 play opposing roles during lung bud morphogenesis. Development 127: 2695–2704, 2000. doi: 10.1242/dev.127.12.2695. [DOI] [PubMed] [Google Scholar]
- 22.Hyatt BA, Shangguan X, Shannon JM. BMP4 modulates fibroblast growth factor-mediated induction of proximal and distal lung differentiation in mouse embryonic tracheal epithelium in mesenchyme-free culture. Dev Dyn 225: 153–165, 2002. doi: 10.1002/dvdy.10145. [DOI] [PubMed] [Google Scholar]
- 23.Domyan ET, Ferretti E, Throckmorton K, Mishina Y, Nicolis SK, Sun X. Signaling through BMP receptors promotes respiratory identity in the foregut via repression of Sox2. Development 138: 971–981, 2011. doi: 10.1242/dev.053694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duprez D, Bell EJ, Richardson MK, Archer CW, Wolpert L, Brickell PM, Francis-West PH. Overexpression of BMP-2 and BMP-4 alters the size and shape of developing skeletal elements in the chick limb. Mech Dev 57: 145–157, 1996. doi: 10.1016/0925-4773(96)00540-0. [DOI] [PubMed] [Google Scholar]
- 25.Pizette S, Niswander L. BMPs are required at two steps of limb chondrogenesis: formation of prechondrogenic condensations and their differentiation into chondrocytes. Dev Biol 219: 237–249, 2000. doi: 10.1006/dbio.2000.9610. [DOI] [PubMed] [Google Scholar]
- 26.Shum L, Wang X, Kane AA, Nuckolls GH. BMP4 promotes chondrocyte proliferation and hypertrophy in the endochondral cranial base. Int J Dev Biol 47: 423–431, 2003. [PubMed] [Google Scholar]
- 27.Hatakeyama Y, Tuan RS, Shum L. Distinct functions of BMP4 and GDF5 in the regulation of chondrogenesis. J Cell Biochem 91: 1204–1217, 2004. doi: 10.1002/jcb.20019. [DOI] [PubMed] [Google Scholar]
- 28.Park J, Zhang JJ, Moro A, Kushida M, Wegner M, Kim PC. Regulation of Sox9 by Sonic Hedgehog (Shh) is essential for patterning and formation of tracheal cartilage. Dev Dyn 239: 514–526, 2010. doi: 10.1002/dvdy.22192. [DOI] [PubMed] [Google Scholar]
- 29.Kishimoto K, Furukawa KT, Luz-Madrigal A, Yamaoka A, Matsuoka C, Habu M, Alev C, Zorn AM, Morimoto M. Bidirectional Wnt signaling between endoderm and mesoderm confers tracheal identity in mouse and human cells. Nat Commun 11: 4159, 2020. doi: 10.1038/s41467-020-17969-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu W, Selever J, Wang D, Lu MF, Moses KA, Schwartz RJ, Martin JF. Bmp4 signaling is required for outflow-tract septation and branchial-arch artery remodeling. Proc Natl Acad Sci USA 101: 4489–4494, 2004. doi: 10.1073/pnas.0308466101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carpenter AC, Rao S, Wells JM, Campbell K, Lang RA. Generation of mice with a conditional null allele for Wntless. Genesis 48: 554–558, 2010. doi: 10.1002/dvg.20651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harfe BD, Scherz PJ, Nissim S, Tian H, McMahon AP, Tabin CJ. Evidence for an expansion-based temporal Shh gradient in specifying vertebrate digit identities. Cell 118: 517–528, 2004. doi: 10.1016/j.cell.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 33.Szucsik JC, Lewis AG, Marmer DJ, Lessard JL. Urogenital tract expression of enhanced green fluorescent protein in transgenic mice driven by a smooth muscle gamma-actin promoter. J Urol 171: 944–949, 2004. doi: 10.1097/01.ju.0000099168.25976.9a. [DOI] [PubMed] [Google Scholar]
- 34.Kist R, Schrewe H, Balling R, Scherer G. Conditional inactivation of Sox9: a mouse model for campomelic dysplasia. Genesis 32: 121–123, 2002. doi: 10.1002/gene.10050. [DOI] [PubMed] [Google Scholar]
- 35.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods 9: 357–359, 2012. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Broad Institute. Picard. https://broadinstitute.github.io/picard/.
- 37.Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol 11: R106, 2010. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lawrence M, Huber W, Pagès H, Aboyoun P, Carlson M, Gentleman R, Morgan MT, Carey VJ. Software for computing and annotating genomic ranges. PLoS Comput Biol 9: e1003118, 2013. doi: 10.1371/journal.pcbi.1003118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, Salzberg SL, Wold BJ, Pachter L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol 28: 511–515, 2010. doi: 10.1038/nbt.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qiagen. IPA. www.qiagen.com/ingenuity.
- 41.Hyatt BA, Shangguan X, Shannon JM. FGF-10 induces SP-C and Bmp4 and regulates proximal-distal patterning in embryonic tracheal epithelium. Am J Physiol Lung Cell Mol Physiol 287: L1116–L1126, 2004. doi: 10.1152/ajplung.00033.2004. [DOI] [PubMed] [Google Scholar]
- 42.Wang F, Flanagan J, Su N, Wang LC, Bui S, Nielson A, Wu X, Vo HT, Ma XJ, Luo Y. RNAscope: a novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J Mol Diagn 14: 22–29, 2012. doi: 10.1016/j.jmoldx.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grant CE, Bailey TL, Noble WS. FIMO: scanning for occurrences of a given motif. Bioinformatics 27: 1017–1018, 2011. doi: 10.1093/bioinformatics/btr064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuwahara A, Lewis AE, Coombes C, Leung FS, Percharde M, Bush JO. Delineating the early transcriptional specification of the mammalian trachea and esophagus. Elife 9: e55526, 2020. doi: 10.7554/eLife.55526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Snowball J, Ambalavanan M, Sinner D. Studying Wnt signaling during patterning of conducting airways. J Vis Exp 116: 53910, 2016. doi: 10.3791/53910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hébert JM, McConnell SK. Targeting of cre to the Foxg1 (BF-1) locus mediates loxP recombination in the telencephalon and other developing head structures. Dev Biol 222: 296–306, 2000. doi: 10.1006/dbio.2000.9732. [DOI] [PubMed] [Google Scholar]
- 47.Šošić D, Richardson JA, Yu K, Ornitz DM, Olson EN. Twist regulates cytokine gene expression through a negative feedback loop that represses NF-kappaB activity. Cell 112: 169–180, 2003. doi: 10.1016/s0092-8674(03)00002-3. [DOI] [PubMed] [Google Scholar]
- 48.Ovchinnikov DA, Deng JM, Ogunrinu G, Behringer RR. Col2a1-directed expression of Cre recombinase in differentiating chondrocytes in transgenic mice. Genesis 26: 145–146, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- 49.Cornett B, Snowball J, Varisco BM, Lang R, Whitsett J, Sinner D. Wntless is required for peripheral lung differentiation and pulmonary vascular development. Dev Biol 379: 38–52, 2013. doi: 10.1016/j.ydbio.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nasr T, Holderbaum AM, Chaturvedi P, Agarwal K, Kinney JL, Daniels K, Trisno SL, Ustiyan V, Shannon JM, Wells JM, Sinner D, Kalinichenko VV, Zorn AM. Disruption of a hedgehog-foxf1-rspo2 signaling axis leads to tracheomalacia and a loss of sox9+ tracheal chondrocytes. Dis Model Mech 14: dmm046573, 2020. doi: 10.1242/dmm.046573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu W, Sun X, Braut A, Mishina Y, Behringer RR, Mina M, Martin JF. Distinct functions for Bmp signaling in lip and palate fusion in mice. Development 132: 1453–1461, 2005. doi: 10.1242/dev.01676. [DOI] [PubMed] [Google Scholar]
- 52.Liu W, Selever J, Murali D, Sun X, Brugger SM, Ma L, Schwartz RJ, Maxson R, Furuta Y, Martin JF. Threshold-specific requirements for Bmp4 in mandibular development. Dev Biol 283: 282–293, 2005. doi: 10.1016/j.ydbio.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 53.Goldman DC, Hackenmiller R, Nakayama T, Sopory S, Wong C, Kulessa H, Christian JL. Mutation of an upstream cleavage site in the BMP4 prodomain leads to tissue-specific loss of activity. Development 133: 1933–1942, 2006. doi: 10.1242/dev.02368. [DOI] [PubMed] [Google Scholar]
- 54.Hines EA, Jones MK, Verheyden JM, Harvey JF, Sun X. Establishment of smooth muscle and cartilage juxtaposition in the developing mouse upper airways. Proc Natl Acad Sci USA 110: 19444–19449, 2013. doi: 10.1073/pnas.1313223110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kishimoto K, Tamura M, Nishita M, Minami Y, Yamaoka A, Abe T, Shigeta M, Morimoto M. Synchronized mesenchymal cell polarization and differentiation shape the formation of the murine trachea and esophagus. Nat Commun 9: 2816, 2018. doi: 10.1038/s41467-018-05189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Floyd J, Campbell DC, Dominy DE. Agenesis of the trachea. Am Rev Respir Dis 86: 557–560, 1962. doi: 10.1164/arrd.1962.86.4.557. [DOI] [PubMed] [Google Scholar]
- 57.Miller MF, Cohen ED, Baggs JE, Lu MM, Hogenesch JB, Morrisey EE. Wnt ligands signal in a cooperative manner to promote foregut organogenesis. Proc Natl Acad Sci USA 109: 15348–15353, 2012. doi: 10.1073/pnas.1201583109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Que J, Okubo T, Goldenring JR, Nam KT, Kurotani R, Morrisey EE, Taranova O, Pevny LH, Hogan BL. Multiple dose-dependent roles for Sox2 in the patterning and differentiation of anterior foregut endoderm. Development 134: 2521–2531, 2007. doi: 10.1242/dev.003855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gontan C, de Munck A, Vermeij M, Grosveld F, Tibboel D, Rottier R. Sox2 is important for two crucial processes in lung development: branching morphogenesis and epithelial cell differentiation. Dev Biol 317: 296–309, 2008. doi: 10.1016/j.ydbio.2008.02.035. [DOI] [PubMed] [Google Scholar]
- 60.Biswenger V, Baumann N, Jürschick J, Häckl M, Battle C, Schwarz J, Horn E, Zantl R. Characterization of EGF-guided MDA-MB-231 cell chemotaxis in vitro using a physiological and highly sensitive assay system. PLoS One 13: e0203040, 2018. doi: 10.1371/journal.pone.0203040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hao J, Ho JN, Lewis JA, Karim KA, Daniels RN, Gentry PR, Hopkins CR, Lindsley CW, Hong CC. In vivo structure-activity relationship study of dorsomorphin analogues identifies selective VEGF and BMP inhibitors. ACS Chem Biol 5: 245–253, 2010. doi: 10.1021/cb9002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Morikawa M, Mitani Y, Holmborn K, Kato T, Koinuma D, Maruyama J, Vasilaki E, Sawada H, Kobayashi M, Ozawa T, Morishita Y, Bessho Y, Maeda S, Ledin J, Aburatani H, Kageyama R, Maruyama K, Heldin CH, Miyazono K. The ALK-1/SMAD/ATOH8 axis attenuates hypoxic responses and protects against the development of pulmonary arterial hypertension. Sci Signal 12: eaay4430, 2019. doi: 10.1126/scisignal.aay4430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Park Y, Sugimoto M, Watrin A, Chiquet M, Hunziker EB. BMP-2 induces the expression of chondrocyte-specific genes in bovine synovium-derived progenitor cells cultured in three-dimensional alginate hydrogel. Osteoarthritis Cartilage 13: 527–536, 2005. doi: 10.1016/j.joca.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 64.Bridgewater LC, Lefebvre V, de Crombrugghe B. Chondrocyte-specific enhancer elements in the Col11a2 gene resemble the Col2a1 tissue-specific enhancer. J Biol Chem 273: 14998–15006, 1998. doi: 10.1074/jbc.273.24.14998. [DOI] [PubMed] [Google Scholar]
- 65.Oh CD, Lu Y, Liang S, Mori-Akiyama Y, Chen D, de Crombrugghe B, Yasuda H. SOX9 regulates multiple genes in chondrocytes, including genes encoding ECM proteins, ECM modification enzymes, receptors, and transporters. PLoS One 9: e107577, 2014. [Erratum in PLoS One 10: e0143156, 2015]. doi: 10.1371/journal.pone.0107577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Young RE, Jones MK, Hines EA, Li R, Luo Y, Shi W, Verheyden JM, Sun X. Smooth muscle differentiation is essential for airway size, tracheal cartilage segmentation, but dispensable for epithelial branching. Dev Cell 53: 73–85.e5, 2020. doi: 10.1016/j.devcel.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yoshida T, Matsuda M, Hirashima T. Incoherent feedforward regulation via Sox9 and ERK underpins mouse tracheal cartilage development. Front Cell Dev Biol 8: 585640, 2020. doi: 10.3389/fcell.2020.585640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hayashi K, Nakamura S, Nishida W, Sobue K. Bone morphogenetic protein-induced MSX1 and MSX2 inhibit myocardin-dependent smooth muscle gene transcription. Mol Cell Biol 26: 9456–9470, 2006. doi: 10.1128/MCB.00759-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shi S, Hoogaars WM, de Gorter DJ, van Heiningen SH, Lin HY, Hong CC, Kemaladewi DU, Aartsma-Rus A, ten Dijke P, ’t Hoen PA. BMP antagonists enhance myogenic differentiation and ameliorate the dystrophic phenotype in a DMD mouse model. Neurobiol Dis 41: 353–360, 2011. doi: 10.1016/j.nbd.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu X, Liu Y, Li X, Zhao J, Geng Y, Ning W. Follistatin like-1 (Fstl1) is required for the normal formation of lung airway and vascular smooth muscle at birth. PLoS One 12: e0177899, 2017. doi: 10.1371/journal.pone.0177899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hill TP, Taketo MM, Birchmeier W, Hartmann C. Multiple roles of mesenchymal beta-catenin during murine limb patterning. Development 133: 1219–1229, 2006. doi: 10.1242/dev.02298. [DOI] [PubMed] [Google Scholar]
- 72.Kim JS, Crooks H, Dracheva T, Nishanian TG, Singh B, Jen J, Waldman T. Oncogenic beta-catenin is required for bone morphogenetic protein 4 expression in human cancer cells. Cancer Res 62: 2744–2748, 2002. [PubMed] [Google Scholar]
- 73.Gómez-Skarmeta J, de La Calle-Mustienes E, Modolell J. The Wnt-activated Xiro1 gene encodes a repressor that is essential for neural development and downregulates Bmp4. Development 128: 551–560, 2001. doi: 10.1242/dev.128.4.551. [DOI] [PubMed] [Google Scholar]
- 74.Sekiya T, Adachi S, Kohu K, Yamada T, Higuchi O, Furukawa Y, Nakamura Y, Nakamura T, Tashiro K, Kuhara S, Ohwada S, Akiyama T. Identification of BMP and activin membrane-bound inhibitor (BAMBI), an inhibitor of transforming growth factor-beta signaling, as a target of the beta-catenin pathway in colorectal tumor cells. J Biol Chem 279: 6840–6846, 2004. doi: 10.1074/jbc.M310876200. [DOI] [PubMed] [Google Scholar]
- 75.Im J, Kim H, Kim S, Jho EH. Wnt/beta-catenin signaling regulates expression of PRDC, an antagonist of the BMP-4 signaling pathway. Biochem Biophys Res Commun 354: 296–301, 2007. doi: 10.1016/j.bbrc.2006.12.205. [DOI] [PubMed] [Google Scholar]
- 76.Hussein SM, Duff EK, Sirard C. Smad4 and beta-catenin co-activators functionally interact with lymphoid-enhancing factor to regulate graded expression of Msx2. J Biol Chem 278: 48805–48814, 2003. doi: 10.1074/jbc.M305472200. [DOI] [PubMed] [Google Scholar]
- 77.Stevens ML, Chaturvedi P, Rankin SA, Macdonald M, Jagannathan S, Yukawa M, Barski A, Zorn AM. Genomic integration of Wnt/β-catenin and BMP/Smad1 signaling coordinates foregut and hindgut transcriptional programs. Development 144: 1283–1295, 2017. doi: 10.1242/dev.145789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cohen ED, Ihida-Stansbury K, Lu MM, Panettieri RA, Jones PL, Morrisey EE. Wnt signaling regulates smooth muscle precursor development in the mouse lung via a tenascin C/PDGFR pathway. J Clin Invest 119: 2538–2549, 2009. doi: 10.1172/JCI38079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yin W, Kim HT, Wang S, Gunawan F, Li R, Buettner C, Grohmann B, Sengle G, Sinner D, Offermanns S, Stainier DY. Fibrillin-2 is a key mediator of smooth muscle extracellular matrix homeostasis during mouse tracheal tubulogenesis. Eur Respir J 53: 1800840, 2019. doi: 10.1183/13993003.00840-2018. [DOI] [PubMed] [Google Scholar]
- 80.Castilla EE, Martínez-Frías ML. Congenital healed cleft lip. Am J Med Genet 58: 106–112, 1995. doi: 10.1002/ajmg.1320580203. [DOI] [PubMed] [Google Scholar]
- 81.Brosens E, Felix JF, Boerema-de Munck A, de Jong EM, Lodder EM, Swagemakers S, Buscop-van Kempen M, de Krijger RR, Wijnen RM, van IJcken WF, van der Spek P, de Klein A, Tibboel D, Rottier RJ. Histological, immunohistochemical and transcriptomic characterization of human tracheoesophageal fistulas. PLoS One 15: e0242167, 2020. doi: 10.1371/journal.pone.0242167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fockens MM, de Bakker BS, Oostra RJ, Dikkers FG. Development pattern of tracheal cartilage in human embryos. Clin Anat 34: 668–672, 2021. doi: 10.1002/ca.23688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kishimoto K, Morimoto M. Mammalian tracheal development and reconstruction: insights from in vivo and in vitro studies. Development 148: dev198192, 2021. doi: 10.1242/dev.198192. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Tables S1-S3 and Supplemental Figs. S1-S10: https://doi.org/10.6084/m9.figshare.16934125.