Abstract
Over one million people in the United States suffer from seizures that are not controlled with anti-seizure medications. Targeted interventions like surgery and deep brain stimulation can confer seizure reduction or even freedom in many of these drug resistant epilepsy patients, but success critically depends on identification epileptogenic zones through magnetic resonance imaging. Ultrahigh field imaging facilitates improved sensitivity and resolution across many imaging modalities and may facilitate better detection of epileptic markers than is achieved at lower field strengths. Though challenges remain, in particular with field homogeneity, the increasing availability and clinical adoption of ultrahigh field scanners may play an important role in characterizing drug-resistant epilepsy and planning for its treatment.
Keywords: 7T, Epilepsy, Ultrahigh, MRI
1.0. Introduction
Magnetic resonance imaging (MRI) at ultrahigh field strengths, often defined as 7 Tesla or greater, has gained increasing interest in clinical imaging, particularly following the first 510(k) approval of a 7T MRI scanner by the Food and Drug Administration in 2017 (Terra 7T, Siemens Healthineers, Erlangen, Germany). As of June 2020, at least 88 in vivo human MRI scanners of 7T or greater have been installed worldwide, including both research and clinical units. Higher field strengths facilitate proportional gains in signal-to-noise ratio over lower field strengths (SNR) [1, 2], which in turn may be leveraged for higher imaging resolution, greater tissue contrast or faster scan times, or some combination thereof. These gains may confer improved sensitivity in various advanced imaging applications to improve detection or characterization of small or poorly-differentiated imaging features [3, 4].
Among the most promising clinical applications of ultrahigh field imaging is the characterization and treatment planning of epilepsy. Affecting up to 1% of the total population, epilepsy is a heterogeneous group of neurological disorders associated with recurrent seizures [5]. There is also mounting evidence that epilepsy is a complex network disease with long term frequent seizures causing changes in the brain beyond the primary epileptic regions into other areas affected by seizure activity [6–9]. Factors such as seizure frequency, severity and duration of disease and seizure severity all contribute to the breadth of abnormalities that underlie these seizure networks [8, 10–17].
In the one-third of patients in whom anti-epilepsy drugs are ineffective, ablative or resective surgery may be considered. Minimally-invasive alternatives to surgery have also emerged, including responsive neuromodulation (RNS) and deep brain stimulation. The success of these targeted interventions is critically dependent on the localization of seizure onset zones (SOZs), typically through neuroimaging. While a majority of epilepsy cases exhibit focal lesions at conventional field strengths, in about one-third of refractory epilepsy patients lesions are not identified on routine clinical imaging [18]. Identifying the SOZs in these patients may require more advanced techniques to detect smaller or more subtle imaging features. These subtle imaging findings may include cortical abnormalities (including migrational disorders), atrophy or asymmetry in subcortical structures [19]. Though not suspected to cause seizure activity, abnormally large or numerous perivascular spaces (PVSs) may also be a biomarker for epilepsy indicative of regional volume loss [20] or inflammatory activity [21].
Advanced MRI techniques such as susceptibility weighted imaging (SWI), diffusion-weighted (DWI) or diffusion tensor imaging (DTI), and functional MRI (fMRI) each benefit from the SNR advantages at ultrahigh field [22]. SWI also receives the further benefit of higher susceptibility at ultrahigh field to improve lesional conspicuity. DTI, DWI and fMRI heavily depend on gradient performance and newer ultrahigh field scanners typically employ more powerful gradients for the benefit of these techniques. These advanced imaging techniques at ultrahigh field may facilitate improved subtyping of epilepsy through the detection vascular abnormalities or irregularities in structural or functional connectivity [23, 24].
2.0. Imaging Modalities
High-resolution structural and functional sequences are required to analyze sub-regions of the hippocampus, amygdala and thalamus which may act as sensitive markers for seizure pathology and more accurate targets for DBS and RNS therapies [25–30]. The addition of high-resolution functional and structural connectivity analysis with fMRI and DWI/DTI, respectively, has been shown to clarify regions of the brain involved in abnormal seizure activity when analyzed alongside structural images [7, 23, 31–36]. Magnetic resonance spectroscopic imaging (MRSI) captures signal from metabolites such as N-acetyl aspartate (NAA) and creatine (Cr) to provide information about neuronal integrity and total energy metabolism. MRSI studies have shown that NAA levels may be used to lateralize and localize the brain regions where the seizures originate in temporal lobe epilepsy (TLE) [37–39]. Ultrahigh field MRI significantly increases the sensitivity of all of these neuroimaging modalities and enables image acquisitions at appreciably finer resolution [22]. Table 1 summarizes the value of each of these methods at 7T for epilepsy. Multimodal pre-operative MRI at 7T should also result in improved placement of intracranial EEG (iEEG). Stereo-EEG (SEEG) is a technique allows for collection of iEEG data from virtually any location in the brain with great precision and could benefit significantly from high resolution multimodal imaging. Improved diagnostic accuracy of such an implant may lead to greater success in the following resective surgery, ablative surgery, or neuromodulation treatment of the epileptic network. This results in decreased infection rates, increased monitoring accuracy, reducing the duration of hospital stays [40–42].
Table 1:
MRI imaging modality, advantage at 7T and detected epileptogenic abnormalities
| Technique | Advantage at 7T | Abnormalities detected and imaging markers |
|---|---|---|
| T1 | Increased SNR enables higher isotropic resolution | Cortical, migrational and hippocampal abnormalities. Hippocampal subfield and thalamic subnuclei volumetrics |
| T2 | Increased SNR and enhanced contrast results in increased conspicuity of lesions | Cortical abnormalities (dysplasias, malformations, heterotopias), hippocampal sclerosis, lesions. Hippocampal subfield and thalamic subnuclei volumetrics |
| SWI | Increased sensitivity to susceptibility effects and venous anomalies | Vascular lesions, hypervascularity, and cavernomas, associated with or acting as seizure source |
| MRSI | Increased SNR and spectral separation. | Neuronal loss detected through NAA/Cr levels. |
| dMRI | Increased SNR. Hippocampal subfield- specific connectivity may be performed | Abnormal structural connectivity underlying seizure networks. Hippocampal subfield-specific tract density. |
| fMRI | Increased SNR and BOLD contrast | Abnormal functional connectivity in seizure networks. |
2.1. Structural Imaging
The most basic applications of MRI utilizes inherent differences in tissue T1 and T2 relaxation properties to generate image contrast. Examples include magnetization-prepared rapid gradient echo (MPRAGE) based on T1-weighting, and fast spin-echo (FSE) or turbo spin-echo (TSE) based on T2-weighting. A variant of T2-weighting called fluid-attenuated inversion recovery (FLAIR) uses an inversion pulse to lower the signal of normal cerebrospinal fluid (CSF) to increase conspicuity of edema especially near bulk fluid. Reviews of published ultrahigh field research generally reveal improved resolution compared to lower field strengths, with T1-weighted imaging yielding sub-millimeter isotropic resolution and two-dimensional T2-weighted imaging also producing in-plane resolution well under 1 mm. Clinically, ultrahigh field imaging has demonstrated improved visualization of subtle features like malformations of cortical development [43] compared to lower field strengths. Still other novel structural imaging contrasts have been developed including fast gray matter acquisition T1 inversion recovery (FGATIR), which improves gray matter-white matter contrast through selective suppression of white matter signal with inversion recovery pulses [44].
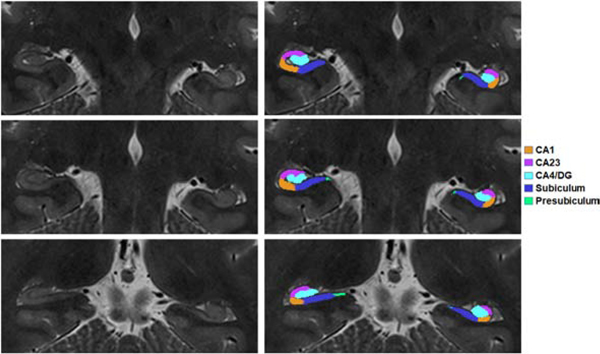
Although T2 TSE and T2 FLAIR can potentially offer higher SNR than lower field strengths, techniques must also recognize and accommodate the associated increase specific absorption rate (SAR) that high field imaging imparts (see section 4.2). SAR limitations can, therefore, temper some of the putative ultra-high field gains. Figure 1 shows hippocampal subfields of a 27 year-old female MTLE patient segmented with the benefit of higher resolution at 7T. The patient was both qualitatively observed to have asymmetric hippocampus on clinical assessment and showed increased asymmetry on quantitative anatomical segmentation.
Figure 1:
Hippocampal subfield segmentation of a MTLE patient overlayed on coronal-oblique T2-weighted TSE image acquired at 7T. At left are three unmarked slices of TSE images are shown at right are segmented cornu ammonis 1 (CA1), cornu ammonis 2+3 (CA2/3), cornu ammonis 4 + dentate gyrus (CA4/DG), subiculum and presubiculum.
2.2. Susceptibility Weighted Imaging
Susceptibility-weighted imaging (SWI) and the related quantitative susceptibility mapping (QSM) take advantage of the susceptibility differences caused by the paramagnetic, diamagnetic and ferromagnetic properties of local tissues. As magnetic susceptibility increases proportionately with field strength, SWI benefits greatly from ultrahigh fields. SWI utilizes the paramagnetic properties of deoxygenated blood to visualize tissue vasculature, reducing signal in post-capillary vessels so they appear darker than surrounding tissues. SWI is also useful for detection of micro-hemorrhages and of vascular, iron-rich or calcified lesions like cavernous malformations [45]. One study exploiting SWI in drug-resistant lesional epilepsy patients [46] demonstrated the ability of SWI to identify focal thickening and abnormal vasculature such as tortuous veins, which may be implicated in the pathogenesis of epilepsy. A second study identified abnormal blood vessel morphology and reduced gray-white contrast and presence of thin vessels in the regions of tuberous sclerosis [47].
2.3. Diffusion-weighted Imaging and Diffusion Tensor Imaging
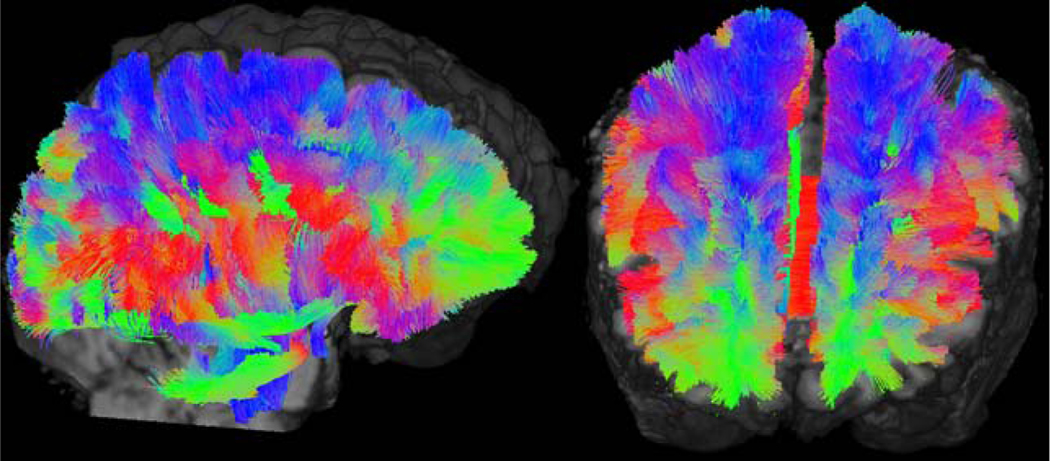
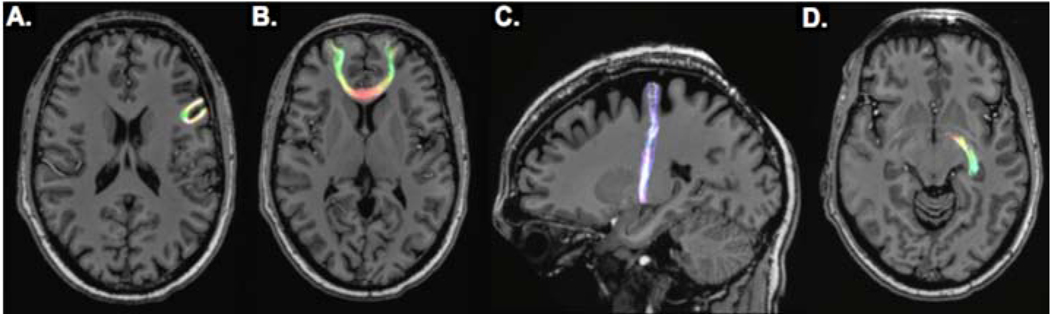
Diffusion based imaging techniques, including diffusion weighted (DWI) and diffusion tensor imaging (DTI), probe the structural connectivity of brain tissue by characterizing diffusion restriction of water molecules in the presence of an applied directional magnetic gradient. DWI and DTI receive a nominal SNR benefit of ultrahigh field, so these gradient-dependent techniques separately benefit from the better gradient performance typical in these newer MRI systems. Because of its greater directional specificity, DTI can be used for fiber tractography through sophisticated reconstruction algorithms, such as those offered by MRtrix3 (Brain Research Institute, Melbourne, VIC, Australia). These techniques can leverage constrained spherical deconvolution to calculate fiber orientation distributions (FOD) and resolve crossing fibers. From pre-selected volumetric seeds, for example within individual hippocampal subfields [25], tractography can elucidate the structural connectivity between the hippocampal subfields and other parts of the brain. With adequate resolution afforded by the high performance magnetic gradients a strong main field at ultra-high field strengths, DTI tractography can potentially identify epileptogenic foci in patients whose seizure onset zones are otherwise poorly visualized through conventional imaging [48, 49]. Figure 2 shows whole brain-tractography performed using TractSeg (German Cancer Research Center, Heidelberg, Germany) and displayed using MrTrix3 with 7T DTI data acquired from a TLE patient. Red, green and blue coloring is incorporated reflecting predominantly left-right, anterior-posterior and superior-inferior fiber directionality, respectively. Figures 3 and 4 show reconstructed seeded tractography performed by MRTrix3 superimposed against a co-registered structural T1-weighted MRI. Figure 3 shows cortical, limbic and cortico-limbic tractography, while Figure 4 shows tractography specific to segmented hippocampal subfields. Fiber coloring follows a typical MR convention, where fiber orientation is encoded as red, green and blue to reflect left-right, anterior-posterior and superior-inferior fibers, respectively.
Figure 2:
Sagittal (left) and coronal-oblique (right) images showing whole-brain tractography performed using TractSeg and displayed with MRTrix3 using 7T DTI data from an epilepsy patient. Red, green and blue coloring represents fibers whose dominant orientations are left-right, anterior-posterior and superior-inferior, respectively.
Figure 3:
Seed-based tractography images from DTI scan of epilepsy patient at 7T. Shown are U-fiber bundle between adjacent cortical nodes (A), long-range fibers connecting distant cortical nodes (B), cortico-limbic tract (C) and inter-limbic tract (D).
Figure 4:
Hippocampal subfield-specific tractography overlayed on T1-weighted MPRAGE imaging and generated using 7T DTI data acquired from epilepsy patient. Shown are coronal slice with CA1 overlay in yellow (A) and Subiculum overlay (C). Seeded tractography from CA1 and Subiculum are shown in B and D, respectively.
2.4. Functional Magnetic Resonance Imaging
Functional magnetic resonance imaging (fMRI) uses a time designated series of images to measure the blood oxygenation level dependent (BOLD) response. Task-dependent BOLD imaging characterizes changes in blood flow and oxygenation elucidated by alternating task and rest periods to determine changes in relative vascular oxygenation, with vascular susceptibility varying between paramagnetic deoxygenated blood and diamagnetic oxygenated blood. Of increasing interest is resting state fMRI, in which low-frequency cycling can be leveraged to characterize functional connectivity among brain regions [50, 51]. Ultra-high field imaging may improve upon lower field techniques since fMRI enjoys better than linear gains in susceptibility in proportion to field strength. One comparison study between 3T and 7T demonstrated up to three times improvements in SNR and resting-state functional connectivity coefficients at the higher field [52]. Another study of mesial temporal lobe epilepsy (TLE) patients using BOLD-fMRI at 7T [53] demonstrated functional network asymmetry among TLE patient in the region around the mesial temporal lobe.
2.5. Magnetic Resonance Spectroscopic Imaging
Magnetic resonance spectroscopic imaging (MRSI) assays the metabolic composition of tissue by detecting subtle differences in resonance frequency according to the molecular environment around atomic nuclei. These differences, called chemical shifts, stem from partial shielding or de-shielding of the observed magnetic field due to the electrons in the surrounding molecular structure. MRSI receives a double benefit from ultrahigh field, with both proportional gains in SNR and in spectral separation, facilitating better differentiation and quantification of metabolite signals [54–56]. Because spatial localization of a volume of interest (VOI) is typically performed using 180 degree refocusing RF pulses in MRSI, the technique is highly sensitive to B1 inhomogeneity, leading to the implementation of B1-insensitive semi-adiabatic techniques [57] to compensate.
Studies employing MRSI at 7T in patients with epilepsy have demonstrated that metabolic abnormalities may be present in the epileptogenic region [58–61]. For example, one study demonstrated improved surgical outcomes in patients where metabolic abnormality was detected in the region of surgical resection [59] and a second study demonstrated abnormalities in the ratio between n-acetyl aspartate (NAA) and creatine (Cr) among mesial temporal lobe epilepsy (MTLE) patients [58].
3.0. Imaging Features
3.1. Focal Cortical Dysplasia
Focal cortical dysplasia (FCD) is a congenital abnormality that may be manifest clinically as drug-resistant epilepsy, and a common target for resective surgery in these patients. A 7T study of patients suspected of FCD [62] demonstrated the superiority of ultrahigh field imaging in detecting these lesions. Structural 7T MRI identified all the lesions previously seen at 1.5T and 3T, yet also detected additional lesions undetected at those lower fields. That study suggested T2 FLAIR showed the best visualization of FCD lesions among the commonly-applied structural MRI contrasts. Figure 5 coronal-oblique T2-weighted TSE and axial SWI images showing focal cortical defect in 19 year-old male epilepsy patient.
Figure 5:
Focal cortical defect in a 19yo male with history of generalized tonic-clonic convulsions (GTCC). Coronal T2 TSE weighted image demonstrates a 3mm hypervascular cortical malformation in the left frontal operculum, manifest as an area of diminished signal (arrows in A and in the magnified inset A1, whose location is indicated by dashed box in A). This area blooms to a larger size and conspicuity on a minimum intensity projection of susceptibility weighted imaging (arrows in B and in the magnified inset B1, using the same localizing conventions).
3.2. Hippocampal Sclerosis
Hippocampal sclerosis is the prototypical pathology in mesial temporal lobe epilepsy, in reports from surgery and autopsy. Hippocampal sclerosis appears on structural imaging as diminished volume (atrophy) within the whole hippocampus or its component subfields, as well as a disruption in the laminar architecture with associated signal changes. Automated segmentation algorithms, like FreeSurfer [63] and ASHS (Automated Segmentation of Hippocampal Subfields) [64], have been developed in recent years to quantify the volume of the hippocampi and their subfields, using a probabilistic approach based on voxel position and contrast. A tissue-contrast based segmentation approach would likely benefit from the improved contrast offered by ultrahigh field imaging, and multiple studies have consequently reported reliable segmentation of hippocampal subfields at 7T [25, 61, 65, 66].
Studies of hippocampal sclerosis at 7T [67–70] demonstrated correlation between mesial temporal lobe epilepsy and ipsilateral sclerosis of the hippocampus with manual segmentation [69]. These studies also revealed some limitations of ultrahigh field scanners in imaging the hippocampus including increased field B1 field inhomogeneity in the region and limitations on minimum repetition time for high radiofrequency (RF) power sequences due to the specific absorption rate (SAR) limits. Figure 6 shows hippocampal asymmetry detected in a coronal-oblique T1-weighted MP2RAGE image acquired at 7T.
Figure 6:
Coronal-oblique T1-weighted MP2RAGE image (top) acquired at 7T with 0.7 mm3 isotropic resolution and T2-weighted TSE (bottom) with 0.4 mm in-plane resolution showing hippocampal asymmetry in 64-year old patient with intractable temporal lobe epilepsy. Both images show reduced left hippocampal volume (yellow arrows) compared with the normal configuration on the right (blue arrows). Larger than normal left temporal horn indicative of volume loss is marked by red asterisk.
3.3. Polymicrogyria
Polymicrogyria (PMG) is a cortical malformation characterized by abnormally prominent infolding or nodularity of one or more cortical locations, giving rise to paradoxical thickening of the cortical band on imaging with fusion of the molecular layer across sulci. Though its pathogenesis is not well-understood, causal factors include congenital infections, in utero ischemia and genetic mutations. PMG may be unilateral or bilateral; focal or diffuse; and symmetric or asymmetric, and may be associated with epilepsy. Zones of PMG also serve as potential targets for surgical treatment of epilepsy. A study of ten patients with polymicrogyria already diagnosed at 3T demonstrated improved visualization at 7T [71], with SWI angiography revealing dilated superficial veins in associated with the polymicrogyria. Figure 7 shows images of polymicrogyria acquired with multiple imaging modalities at 7T.
Figure 7:
Images of a temporal lobe epilepsy patient showing polymicrogyria at 7T. The large image at left is an axial T2-weighted TSE image with 0.4 mm in-plane resolution and 2 mm slice thickness. The four images at right are T1-weighted MP2RAGE and MPRAGE (coronal-oblique, both with 0.7 mm isotropic resolution) along with axial T2-weighted FLAIR (0.4 mm in-plane resolution) and SWI (0.2 mm in-plane resolution).
3.4. Perivascular Spaces
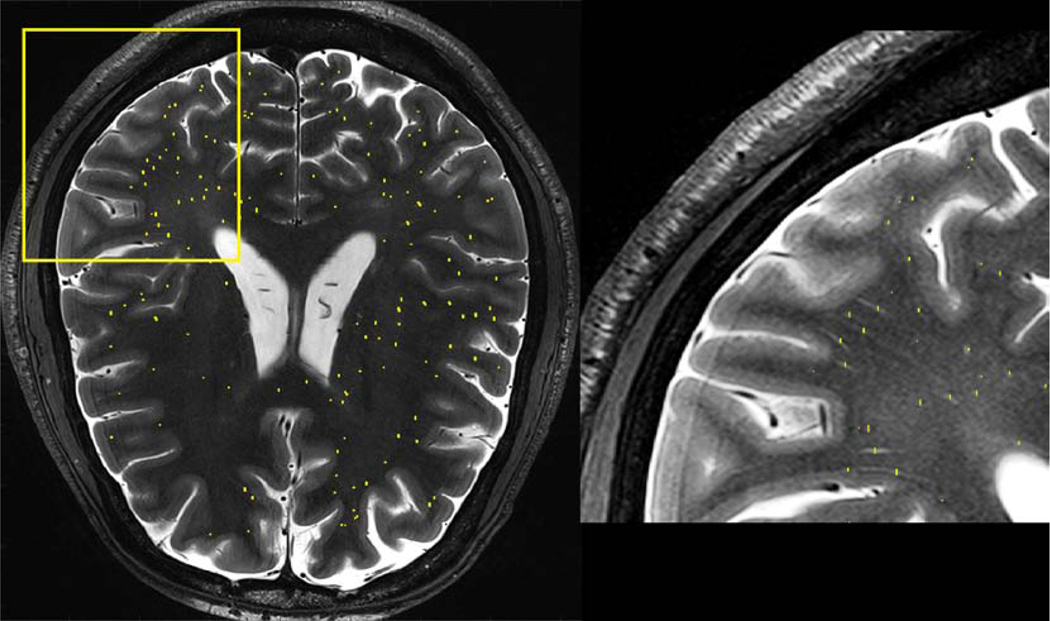
Perivascular spaces (PVSs) are small fluid-filled spaces within white matter which appear iso-intense to static CSF-filled regions on structural imaging. These spaces coincide with arterial trajectories, and excess size, number or asymmetry may be a marker for neurological disorders such as epilepsy. This prominence is attributed to regional volume loss. Because many are small or at least start small (perhaps a half-millimeter in transverse diameter or less) these spaces are more readily detected using high-resolution ultrahigh field imaging. In particular, two-dimensional (2D) T2 TSE, which shows high contrast between the CSF-filled spaces and the dark surrounding white matter, has been very effective in demonstrating regional variability in PVSs. Manual segmentation of PVSs revealed significant differences between the asymmetry index of PVSs compared to healthy controls [20]. Because a single subject may exhibit hundreds or thousands of MR-visible PVSs, particularly at ultrahigh field, segmentation of these spaces is a laborious process when performed manually and may benefit from automation based on imaging contrast. Figure 8 shows a zoomed in section of an axial T2-weighted TSE image showing the presence of multiple perivascular spaces, detected and marked by a manual reader.
Figure 8:
Whole-brain axial T2-weighted TSE image (acquired at 7T with 0.4 mm in-plane resolution, 2.0 mm slice thickness) and zoomed-in section indicated by yellow square. Manually demarcated cross sections of detected perivascular spaces are marked by yellow lines.
4.0. Technical Limitations
4.1. B1 Field Inhomogeneity
Ultrahigh field imaging suffers from poorer homogeneity of the applied B1 RF field compared to lower field strengths. Image homogeneity results from the interaction between the radiofrequency pulses and the dielectric properties of the scanned tissue, including conductivity and permittivity. The shorter wavelengths at ultrahigh field can result in standing wave patterns of high and low interference within the scanned field of view, producing characteristic bright and dark regions in the image. The resultant inhomogeneity can be particularly problematic in the visualization of deep brain structures and temporal lobe, complicating ultrahigh field imaging based assessment of epilepsy. Multiple techniques have emerged for mitigating the effects of this inhomogeneity including B1 insensitive adiabatic pulses [72–74] and parallel transmit (PTx) coils, which employ multiple transmission elements working independently and pre-determined B1 field maps to compensate for inhomogeneity with hardware [75]. The use of high permittivity dielectric pads, such as a deuterium-based suspension of barium titanate [76, 77], have demonstrated improved coverage in regions with poor B1-homogeneity at ultrahigh field strengths.
4.2. Specific Absorption Rate
RF power deposition scales with the square of the main field strength, making sequences employing multiple 180-degree RF pulses such as T2 TSE and T2 FLAIR particularly SAR-intensive at ultrahigh fields. For these sequences, modification of technique to comply with FDA-designated guidelines for SAR (3.2 W/kg over 10 minutes for brain imaging) can impose restrictions on potential signal gains, which can then adversely affect resolution and scan time. SAR limitations further complicate strategies for mitigating B1 inhomogeneity, since adiabatic pulses tend to be SAR-intensive and PTx coils [78] require complex calculations – and often restrictive approximations – to calculate the maximum local SAR. Though issues related to SAR are a major consideration across most applications of ultrahigh field imaging, they may be particularly limiting in epilepsy imaging due to the need to visualize deep brain structures, which typically require high transmission power to compensate for the poorer penetration of short-wavelength RF in the brain at ultrahigh field.
4.3. Other Limitations
To date, no commercial human 7T MRI system includes a body transmit RF coil, necessitating the integration of RF transmit elements into detection coils. These coils may thus exhibit RF localization effects with reduced sensitivity away from the coil elements – for example, in the skull base or brain stem region on birdcage head coils.
Anatomical imaging contrast is dependent on the T1 and T2 properties of the local tissue, which in turn are dependent on the B0 field strength, and may not perfectly match the contrast of 1.5T and 3T clinical imaging systems. Adoption of ultrahigh field imaging for routine clinical imaging will require familiarization of clinicians to those contrasts, and automatic segmentation algorithms dependent on these contrasts may require specific calibration for ultrahigh field.
5.0. Technical Advances
5.1. Deep Brain Stimulation
In recent years, DBS has received increasing clinical interest in the characterization and treatment of movement disorders and depression, and has also demonstrated efficacy in reducing or eliminating seizures in refractory temporal lobe epilepsy without the need for surgical resection [79–81]. DBS treatment of epilepsy typically targets the thalamus, and in particular the anterior or centromedian subthalamic nuclei. Resolving these subthalamic structures is complicated by poor anatomical contrast on conventional imaging, yet may benefit from both the improved contrast at ultrahigh field and the implementation of high-contrast FGATIR [44] to improve visualization of these deep gray matter structures. The thalamus has also shown organization by fiber connectivity, and it may therefore be amenable to segmentation through clustering of DTI data including fiber direction or long distance connectivity [82–86].
5.2. Advanced post-processing and Automated Segmentation
Volumetric atrophy and asymmetry have been implicated in a number of neurological disorders including epilepsy [53]. This has led to increasing interest in development of automatic segmentation algorithms which can provide volumetric statistics of whole brain, cortical and subcortical regions, and even substructures of the cortical regions including hippocampal subfields, amygdala nuclei and thalamic nuclei. Algorithms such as FreeSurfer [63] utilize spatial positioning and imaging contrast to develop probabilistic models for brain regions which can then be quantified for volume and contrast, or used in conjunction with seed-based tractography to study structural connectivity between brain regions. Because of the dependence of these automatic segmentation algorithms on resolution and image contrast, they are likely to benefit from SNR and resolution gains of ultrahigh field imaging.
6.0. Future Directions and Conclusions
The greater sensitivity and resolution afforded by ultrahigh field imaging have translated into promising increases in clinical benefit. Ultrahigh field imaging still faces challenges, particularly those related to reliable image quality such as B1 field inhomogeneity. As strategies such as adiabatic pulses and parallel transmission emerge to address these challenges, the case for clinical adoption of this more sensitive technology becomes more straightforward.
Epilepsy, in particular, stands to benefit from these sensitivity benefits in the detection of subtle imaging features which may not be apparent at clinically-standard field strengths. These features may provide biomarkers to improve the characterization of this highly heterogeneous disorder, and facilitate techniques including deep brain stimulation and resective surgery to provide treatment in cases where non-invasive therapy proves ineffective.
Key Points:
Magnetic resonance imaging (MRI) at ultrahigh field strengths, often defined as 7 Tesla or greater, has gained increasing interest in clinical imaging
Among the most promising clinical applications of ultrahigh field imaging is the characterization and treatment planning of epilepsy.
Minimally-invasive alternatives to surgery have also emerged, including responsive neuromodulation (RNS) and deep brain stimulation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
7.0 References
- 1.Pohmann R, Speck O, and Scheffler K, Signal-to-noise ratio and MR tissue parameters in human brain imaging at 3, 7, and 9.4 tesla using current receive coil arrays. Magnetic resonance in medicine, 2016. 75(2): p. 801–809. [DOI] [PubMed] [Google Scholar]
- 2.Pfrommer A. and Henning A. On the superlinear increase of the ultimate intrinsic signal-to-noise ratio with regard to main magnetic field strength in a spherical sample. in 2017 International Conference on Electromagnetics in Advanced Applications (ICEAA). 2017. IEEE. [Google Scholar]
- 3.Van Der Kolk A, et al. , Imaging the intracranial atherosclerotic vessel wall using 7T MRI: initial comparison with histopathology. American Journal of Neuroradiology, 2015. 36(4): p. 694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Middlebrooks EH, Ver Hoef L, and Szaflarski JP, Neuroimaging in epilepsy. Current neurology and neuroscience reports, 2017. 17(4): p. 32. [DOI] [PubMed] [Google Scholar]
- 5.Bell GS, Neligan A, and Sander JW, An unknown quantity—the worldwide prevalence of epilepsy. Epilepsia, 2014. 55(7): p. 958–962. [DOI] [PubMed] [Google Scholar]
- 6.Spencer SS, Neural networks in human epilepsy: evidence of and implications for treatment. Epilepsia, 2002. 43(3): p. 219–227. [DOI] [PubMed] [Google Scholar]
- 7.Stefan H. and Lopes Da Silva FH, Epileptic neuronal networks: methods of identification and clinical relevance. Frontiers in neurology, 2013. 4: p. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laxer KD, et al. , The consequences of refractory epilepsy and its treatment. Epilepsy & behavior, 2014. 37: p. 59–70. [DOI] [PubMed] [Google Scholar]
- 9.Fang M, et al. , A new hypothesis of drug refractory epilepsy: neural network hypothesis. Medical Hypotheses, 2011. 76(6): p. 871–876. [DOI] [PubMed] [Google Scholar]
- 10.Bernasconi N, Natsume J, and Bernasconi A, Progression in temporal lobe epilepsy: differential atrophy in mesial temporal structures. Neurology, 2005. 65(2): p. 223–228. [DOI] [PubMed] [Google Scholar]
- 11.Tosun D, et al. , Deformation-based morphometry of prospective neurodevelopmental changes in new onset paediatric epilepsy. Brain, 2011. 134(4): p. 1003–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coan A, et al. , Seizure frequency and lateralization affect progression of atrophy in temporal lobe epilepsy. Neurology, 2009. 73(11): p. 834–842. [DOI] [PubMed] [Google Scholar]
- 13.Bonilha L, et al. , Gray matter atrophy associated with duration of temporal lobe epilepsy. Neuroimage, 2006. 32(3): p. 1070–1079. [DOI] [PubMed] [Google Scholar]
- 14.Fuerst D, et al. , Volumetric MRI, pathological, and neuropsychological progression in hippocampal sclerosis. Neurology, 2001. 57(2): p. 184–188. [DOI] [PubMed] [Google Scholar]
- 15.Bernhardt BC, et al. , Longitudinal and cross-sectional analysis of atrophy in pharmacoresistant temporal lobe epilepsy. Neurology, 2009. 72(20): p. 1747–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Briellmann RS, et al. , Seizure-associated hippocampal volume loss: a longitudinal magnetic resonance study of temporal lobe epilepsy. Annals of neurology, 2002. 51(5): p. 641–644. [DOI] [PubMed] [Google Scholar]
- 17.Cendes F, Progressive hippocampal and extrahippocampal atrophy in drug resistant epilepsy. Current opinion in neurology, 2005. 18(2): p. 173–177. [DOI] [PubMed] [Google Scholar]
- 18.Sidhu MK, Duncan JS, and Sander JW, Neuroimaging in epilepsy. Current opinion in neurology, 2018. 31(4): p. 371–378. [DOI] [PubMed] [Google Scholar]
- 19.McDonald CR, et al. , Subcortical and cerebellar atrophy in mesial temporal lobe epilepsy revealed by automatic segmentation. Epilepsy research, 2008. 79(2–3): p. 130–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feldman RE, et al. , Quantification of perivascular spaces at 7 T: A potential MRI biomarker for epilepsy. Seizure, 2018. 54: p. 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wuerfel J, et al. , Perivascular spaces—MRI marker of inflammatory activity in the brain? Brain, 2008. 131(9): p. 2332–2340. [DOI] [PubMed] [Google Scholar]
- 22.Balchandani P. and Naidich T, Ultra-high-field MR neuroimaging. American Journal of Neuroradiology, 2015. 36(7): p. 1204–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liao W, et al. , Altered functional connectivity and small-world in mesial temporal lobe epilepsy. PloS one, 2010. 5(1): p. e8525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waites AB, et al. , Functional connectivity networks are disrupted in left temporal lobe epilepsy. Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society, 2006. 59(2): p. 335–343. [DOI] [PubMed] [Google Scholar]
- 25.Rutland JW, et al. , Subfield-specific tractography of the hippocampus in epilepsy patients at 7 Tesla. Seizure, 2018. 62: p. 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cukiert A, et al. , Intraoperative neurophysiological responses in epileptic patients submitted to hippocampal and thalamic deep brain stimulation. Seizure, 2011. 20(10): p. 748–753. [DOI] [PubMed] [Google Scholar]
- 27.Brown SS, et al. , Ultra-High-Resolution Imaging of Amygdala Subnuclei Structural Connectivity in Major Depressive Disorder. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 2020. 5(2): p. 184–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown S, et al. , Structural MRI at 7T reveals amygdala nuclei and hippocampal subfield volumetric association with major depressive disorder symptom severity. Scientific reports, 2019. 9(1): p. 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elder C, et al. , Responsive neurostimulation targeting the anterior nucleus of the thalamus in 3 patients with treatment-resistant multifocal epilepsy. Epilepsia Open, 2019. 4(1): p. 187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abosch A, et al. , An assessment of current brain targets for deep brain stimulation surgery with susceptibility-weighted imaging at 7 tesla. Neurosurgery, 2010. 67(6): p. 1745–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duncan JS, Imaging in the surgical treatment of epilepsy. Nature Reviews Neurology, 2010. 6(10): p. 537. [DOI] [PubMed] [Google Scholar]
- 32.Tracy JI and Doucet GE, Resting-state functional connectivity in epilepsy: growing relevance for clinical decision making. Current opinion in neurology, 2015. 28(2): p. 158–165. [DOI] [PubMed] [Google Scholar]
- 33.He X, et al. , Reduced thalamocortical functional connectivity in temporal lobe epilepsy. Epilepsia, 2015. 56(10): p. 1571–1579. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Z, et al. , Altered functional–structural coupling of large-scale brain networks in idiopathic generalized epilepsy. Brain, 2011. 134(10): p. 2912–2928. [DOI] [PubMed] [Google Scholar]
- 35.Liao W, et al. , Default mode network abnormalities in mesial temporal lobe epilepsy: a study combining fMRI and DTI. Human brain mapping, 2011. 32(6): p. 883–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duncan JS, et al. , Brain imaging in the assessment for epilepsy surgery. The Lancet Neurology, 2016. 15(4): p. 420–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuzniecky R, Clinical applications of MR spectroscopy in epilepsy. Neuroimaging Clinics of North America, 2004. 14(3): p. 507–516. [DOI] [PubMed] [Google Scholar]
- 38.Woermann FG, et al. , Short echo time single-voxel 1H magnetic resonance spectroscopy in magnetic resonance imaging–negative temporal lobe epilepsy: Different biochemical profile compared with hippocampal sclerosis. Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society, 1999. 45(3): p. 369–376. [DOI] [PubMed] [Google Scholar]
- 39.Pan JW, Lo KM, and Hetherington HP, Role of very high order and degree B0 shimming for spectroscopic imaging of the human brain at 7 tesla. Magnetic resonance in medicine, 2012. 68(4): p. 1007–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O’Muircheartaigh J, et al. , Clustering probabilistic tractograms using independent component analysis applied to the thalamus. Neuroimage, 2011. 54(3): p. 2020–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vadera S, et al. , Stereoelectroencephalography following subdural grid placement for difficult to localize epilepsy. Neurosurgery, 2013. 72(5): p. 723–729. [DOI] [PubMed] [Google Scholar]
- 42.Jayakar P, et al. , Diagnostic utility of invasive EEG for epilepsy surgery: indications, modalities, and techniques. Epilepsia, 2016. 57(11): p. 1735–1747. [DOI] [PubMed] [Google Scholar]
- 43.Guye M, Bartolomei F, and Ranjeva J-P, Malformations of cortical development: The role of 7-Tesla magnetic resonance imaging in diagnosis. Revue neurologique, 2019. 175(3): p. 157–162. [DOI] [PubMed] [Google Scholar]
- 44.Sudhyadhom A, et al. , A high resolution and high contrast MRI for differentiation of subcortical structures for DBS targeting: the Fast Gray Matter Acquisition T1 Inversion Recovery (FGATIR). Neuroimage, 2009. 47: p. T44–T52. [DOI] [PubMed] [Google Scholar]
- 45.Morrison L. and Akers A, Cerebral cavernous malformation, familial, in GeneReviews®[Internet]. 2016, University of Washington, Seattle. [Google Scholar]
- 46.Pittau F, et al. , MP2RAGE and susceptibility-weighted imaging in lesional epilepsy at 7T. Journal of Neuroimaging, 2018. 28(4): p. 365–369. [DOI] [PubMed] [Google Scholar]
- 47.Sun K, et al. , Magnetic resonance imaging of tuberous sclerosis complex with or without epilepsy at 7 T.Neuroradiology, 2018. 60(8): p. 785–794. [DOI] [PubMed] [Google Scholar]
- 48.Feldman RE, et al. , 7T MRI in epilepsy patients with previously normal clinical MRI exams compared against healthy controls. Plos one, 2019. 14(3): p. e0213642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O’Halloran R, et al. , A Method for U-Fiber Quantification from 7T Diffusion-Weighted MRI Data Tested in Subjects with Non-Lesional Focal Epilepsy. Neuroreport, 2017. 28(8): p. 457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen W. and Ogawa S, 10 Principles of BOLD Functional MRI. Red, 1999. 10: p. 1. [Google Scholar]
- 51.Strangman G, et al. , A quantitative comparison of simultaneous BOLD fMRI and NIRS recordings during functional brain activation. Neuroimage, 2002. 17(2): p. 719–731. [PubMed] [Google Scholar]
- 52.Morris LS, et al. , Ultra-high field MRI reveals mood-related circuit disturbances in depression: A comparison between 3-Tesla and 7-Tesla. Translational Psychiatry, 2019. 9(1): p. 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shah P, et al. , Structural and functional asymmetry of medial temporal subregions in unilateral temporal lobe epilepsy: A 7T MRI study. Human brain mapping, 2019. 40(8): p. 2390–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stephenson MC, et al. , Applications of multi-nuclear magnetic resonance spectroscopy at 7T. World journal of radiology, 2011. 3(4): p. 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Verma G, et al. , Implementation of two-dimensional L-COSY at 7 tesla: An investigation of reproducibility in human brain. Journal of Magnetic Resonance Imaging, 2013. [DOI] [PubMed] [Google Scholar]
- 56.Terpstra M, et al. , Test-retest reproducibility of neurochemical profiles with short-echo, single-voxel MR spectroscopy at 3T and 7T. Magnetic resonance in medicine, 2016. 76(4): p. 1083–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Feldman RE and Balchandani P, A semiadiabatic spectral-spatial spectroscopic imaging (SASSI) sequence for improved high-field MR spectroscopic imaging. Magnetic resonance in medicine, 2016. 76(4): p. 1071–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pan JW and Kuzniecky RI, Utility of magnetic resonance spectroscopic imaging for human epilepsy. Quantitative imaging in medicine and surgery, 2015. 5(2): p. 313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pan JW, et al. , 7 T MR spectroscopic imaging in the localization of surgical epilepsy. Epilepsia, 2013. 54(9): p. 1668–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Avdievich N, et al. , Short echo spectroscopic imaging of the human brain at 7T using transceiver arrays. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine, 2009. 62(1): p. 17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Voets NL, et al. , Hippocampal MRS and subfield volumetry at 7T detects dysfunction not specific to seizure focus. Scientific reports, 2017. 7(1): p. 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Colon A, et al. , Detection superiority of 7 T MRI protocol in patients with epilepsy and suspected focal cortical dysplasia. Acta Neurologica Belgica, 2016. 116(3): p. 259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fischl B, FreeSurfer. Neuroimage, 2012. 62(2): p. 774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yushkevich PA, et al. , Nearly automatic segmentation of hippocampal subfields in in vivo focal T2-weighted MRI. Neuroimage, 2010. 53(4): p. 1208–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wisse LE, et al. , Automated hippocampal subfield segmentation at 7T MRI. American Journal of Neuroradiology, 2016. 37(6): p. 1050–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schoene-Bake JC, et al. , In vivo mapping of hippocampal subfields in mesial temporal lobe epilepsy: relation to histopathology. Human brain mapping, 2014. 35(9): p. 4718–4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Santyr BG, et al. , Investigation of hippocampal substructures in focal temporal lobe epilepsy with and without hippocampal sclerosis at 7T. Journal of Magnetic Resonance Imaging, 2017. 45(5): p. 1359–1370. [DOI] [PubMed] [Google Scholar]
- 68.Coras R, et al. , 7 T MRI features in control human hippocampus and hippocampal sclerosis: An ex vivo study with histologic correlations. Epilepsia, 2014. 55(12): p. 2003–2016. [DOI] [PubMed] [Google Scholar]
- 69.Henry TR, et al. , Hippocampal sclerosis in temporal lobe epilepsy: findings at 7 T. Radiology, 2011. 261(1): p. 199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Breyer T, et al. , Imaging of patients with hippocampal sclerosis at 7 Tesla: initial results. Academic radiology, 2010. 17(4): p. 421–426. [DOI] [PubMed] [Google Scholar]
- 71.De Ciantis A, et al. , Ultra-high-field MR imaging in polymicrogyria and epilepsy. American Journal of Neuroradiology, 2015. 36(2): p. 309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wrede KH, et al. , Caudal image contrast inversion in MPRAGE at 7 Tesla: problem and solution. Academic radiology, 2012. 19(2): p. 172–178. [DOI] [PubMed] [Google Scholar]
- 73.Balchandani P, Pauly J, and Spielman D, Designing adiabatic radio frequency pulses using the Shinnar–Le Roux algorithm. Magnetic resonance in medicine, 2010. 64(3): p. 843–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Balchandani P. and Qiu D, Semi-adiabatic Shinnar–Le Roux pulses and their application to diffusion tensor imaging of humans at 7T. Magnetic resonance imaging, 2014. 32(7): p. 804–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gumbrecht R, et al. E, A. Fast high-flip pTx pulse design to mitigate B1+ inhomogeneity using composite pulses at 7T. in Proc Intl Soc Mag Reson Med. 2010. [Google Scholar]
- 76.O’Brien KR, et al. , Dielectric pads and low-adiabatic pulses: Complementary techniques to optimize structural T1w whole-brain MP2RAGE scans at 7 tesla. Journal of Magnetic Resonance Imaging, 2014. 40(4): p. 804–812. [DOI] [PubMed] [Google Scholar]
- 77.!!! INVALID CITATION !!!
- 78.Zelinski AC, et al. , Specific absorption rate studies of the parallel transmission of inner-volume excitations at 7T. Journal of Magnetic Resonance Imaging: An Official Journal of the International Society for Magnetic Resonance in Medicine, 2008. 28(4): p. 1005–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Boon P, et al. , Deep brain stimulation in patients with refractory temporal lobe epilepsy. Epilepsia, 2007. 48(8): p. 1551–1560. [DOI] [PubMed] [Google Scholar]
- 80.Lega BC, et al. , Deep brain stimulation in the treatment of refractory epilepsy: update on current data and future directions. Neurobiology of disease, 2010. 38(3): p. 354–360. [DOI] [PubMed] [Google Scholar]
- 81.Charbades S, et al. , Deep brain stimulation in epilepsy with particular reference to the subthalamic nucleus. Epileptic disorders, 2002. 4: p. S83–S93. [PubMed] [Google Scholar]
- 82.Akram H, et al. , Connectivity derived thalamic segmentation in deep brain stimulation for tremor. NeuroImage: Clinical, 2018. 18: p. 130–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pouratian N, et al. , Multi-institutional evaluation of deep brain stimulation targeting using probabilistic connectivity-based thalamic segmentation. Journal of neurosurgery, 2011. 115(5): p. 995–1004. [DOI] [PubMed] [Google Scholar]
- 84.Traynor C, et al. , Reproducibility of thalamic segmentation based on probabilistic tractography. Neuroimage, 2010. 52(1): p. 69–85. [DOI] [PubMed] [Google Scholar]
- 85.Ziyan U, Tuch D, and Westin C-F. Segmentation of thalamic nuclei from DTI using spectral clustering. in International Conference on Medical Image Computing and Computer-Assisted Intervention. 2006. Springer. [DOI] [PubMed] [Google Scholar]
- 86.Wiegell MR, et al. , Automatic segmentation of thalamic nuclei from diffusion tensor magnetic resonance imaging. NeuroImage, 2003. 19(2): p. 391–401. [DOI] [PubMed] [Google Scholar]