Abstract
Background:
In light of the increasing trend in the global number of individuals affected by dementia and the lack of any available disease-modifying therapies, it is necessary to fully understand and quantify the global burden of dementia. This work aimed to estimate the proportion of dementia due to Down syndrome, Parkinson’s disease, clinical stroke, and traumatic brain injury (TBI), globally and by world region, in order to better understand the contribution of clinical diseases to dementia prevalence.
Methods:
Through literature review, we obtained data on the relative risk of dementia with each condition and estimated relative risks by age using a Bayesian meta-regression tool. We then calculated population attributable fractions (PAFs), or the proportion of dementia attributable to each condition, using the estimates of relative risk and prevalence estimates for each condition from the Global Burden of Disease Study 2019. Finally, we multiplied these estimates by dementia prevalence to calculate the number of dementia cases attributable to each condition.
Findings:
For each clinical condition, the relative risk of dementia decreased with age. Relative risks were highest for Down syndrome, followed by Parkinson’s disease, stroke, and TBI. However, due to the high prevalence of stroke, the PAF for dementia due to stroke was highest. Together, Down syndrome, Parkinson’s disease, stroke, and TBI explained 10.0% (95% UI: 6.0–16.5) of the global prevalence of dementia.
Interpretation:
Ten percent of dementia prevalence globally could be explained by Down syndrome, Parkinson’s disease, stroke, and TBI. The quantification of the proportion of dementia attributable to these 4 conditions constitutes a small contribution to our overall understanding of what causes dementia. However, epidemiological research into modifiable risk factors as well as basic science research focused on elucidating intervention approaches to prevent or delay the neuropathological changes that commonly characterize dementia will be critically important in future efforts to prevent and treat disease.
Keywords: Dementia, Global health, Burden of disease, Meta-analysis, Public health
Introduction
Over the past few decades, there have been large increases in the numbers of people affected by dementia due to population aging and population growth [1–3]. These rising trends in the global burden of dementia are only expected to continue into the future [4–6]. Given these increases and the lack of any significant progress toward disease-modifying therapies, increasing attention has been directed toward dementia prevention and the development of a better understanding of disease etiology [7].
Recently, modifiable risk factors for dementia have received increasing attention, with the 2020 update to the Lancet Commission report concluding that 39.7% of dementia can be attributed to 12 major modifiable risk factors encompassing a range of modifiable lifestyle factors and clinical conditions [8]. Additionally, evidence based on autopsy studies has pointed to several neuropathological features, including neuritic and diffuse plaques, neurofibrillary tangles, and small vessel vascular disease, as the key drivers of dementia at the biological level [9–11]. However, these analyses do not directly address the fraction of dementia that can be etiologically caused by other clinical diseases, such as clinical stroke (stroke) or Parkinson’s disease. Interactions with the health system for the diagnosis or treatment of these other clinical conditions may provide a critical delivery point for interventions designed to prevent or delay the development of dementia in individuals with these conditions.
This paper will specifically focus on dementia due to stroke, Parkinson’s disease, Down syndrome, and traumatic brain injury (TBI). These diverse conditions were selected as the set of clinical conditions currently quantified within the Global Burden of Diseases, Injuries, and Risk Factors (GBD) Study 2019 and for which there is evidence supporting a quantifiable and potentially causal association with dementia [12–16]. Evidence suggesting a link between these conditions and dementia may indicate that preventing or treating these conditions could delay the onset of dementia. Risk factors for some of these conditions may also overlap with known modifiable risk factors for dementia, strengthening the evidence for previous identified associations.
Previous efforts to quantify the relationships between dementia and clinical conditions such as stroke and Parkinson’s disease have remained isolated, and results have not been summarized across disease topics [12, 16–19] Additionally, while recent work has shown that the proportion of dementia attributable to lifestyle factors may vary substantially by geography, the impact of differences in the prevalence of clinical disease by geography on the proportion of dementia prevalence that can be attributed to these conditions has not previously been explored [20]. This paper aims to synthesize the evidence on the association between dementia and each of these disease categories and to calculate the proportion of dementia attributable to each disease by age and sex, both globally and by world region.
Materials and Methods
The entity “dementia” as referenced in this paper is equivalent to the disease category of “Alzheimer’s disease and other dementias” within the Global Burden of Disease (GBD) study. The case definition for dementia is a clinical or adjudicated diagnosis of dementia using Diagnostic Statistical Manual of Mental Disorders (DSM) (III, III-R, IV, or 5) or International Classification of Diseases (ICD) definitions from within population-representative studies [21, 22]. Briefly, dementia prevalence in the GBD study is estimated by collating all information on prevalence and incidence globally through systematic review, adjusting the data to account for nonstandard case definitions or ascertainment methods, and using Bayesian meta-regression methods to estimate prevalence [23]. Other general GBD methods can be found in previous publications on dementia and the GBD overview papers [3, 23, 24].
Literature Reviews
We conducted literature reviews on the association between dementia and Parkinson’s disease, Down syndrome, stroke, and TBI. We searched all articles in PubMed and did not restrict articles based on publication date. The PubMed search for Down syndrome resulted in 355 hits, and 25 were ultimately extracted. For Parkinson’s disease, the PubMed search terms yielded 1,475 hits, of which 53 were accepted and extracted. A recent systematic review (2018) on the relationship between stroke and dementia yielded 31 sources [17]. This review was updated with a PubMed search on articles published after the most recent source identified in the systematic review. Of 504 hits, 2 were accepted and added. Three recent meta-analyses (2016, 2016, and 2019) were identified on the relationship between TBI and dementia, and we cross-checked all articles cited by these reviews to identify 47 unique sources for inclusion [19, 25, 26]. As the most recent systematic review was published within a year of this analysis, we did not conduct an additional literature review. Additional details on literature reviews including search strings are available in online suppl. Table 1 (for all online suppl. material, see www.karger.com/doi/10.1159/000515393).
All of the literature reviews targeted papers on population-representative samples and excluded studies on patients in nursing homes. However, clinical samples (studies that recruited patients from a health-care setting) were accepted and were adjusted for in the meta-regression. We accepted case-control, cross-sectional, and longitudinal studies that reported on relative risks, hazard ratios, and odds ratios for dementia or the proportion of exposed individuals with dementia. We required that each source reported on the mean age of the study population or presented results by age. For stroke, we excluded studies on the risk of dementia with transient ischemic attacks or other markers of subclinical vascular disease, as this analysis was limited to overt clinical stroke, a subset of all vascular dementia.
The available data on stroke and TBI primarily described the relationship between these causes and dementia using relative risks or odds ratios, and these data were modeled together. However, the literature on dementia and Parkinson’s disease or Down syndrome more commonly reported the proportion of patients who had dementia. Using these data, we approximated relative risks by dividing each extracted proportion by the age-sex-location-year-specific dementia prevalence estimate from GBD.
Relative Risk Models
We modeled the relative risk of dementia, given the exposure of each disease included, using a Bayesian meta-regression modeling framework. This model includes trimming, a form of outlier detection within the likelihood function. Using this framework, a data point with very low variance, and which is a moderate distance from the mean at a given set of age and covariate values, is more likely to be identified as an outlier than a data point with high variance farther from the mean (additional details are given in the online suppl. material).
Each model included dummy variables on the following study characteristics: study population (clinical vs. population-based samples), diagnosis (Alzheimer’s disease vs. all dementia), and factors controlled for in each component study, such as education, smoking, or vascular risks (full list of study characteristics is given in the online suppl. material). We also specified cubic splines on age with 3 knots equally spaced across the density of the input data and priors of zero slope on the terminal segments to prevent the extrapolation of trends based on small amounts of data at the youngest and oldest ages. Relative risks were estimated by predicting out 1,000 draws from each model for the set of gold standard covariates in each analysis (e.g., exposure definition of DSM dementia rather than Alzheimer’s disease). Negative draws were set to zero as these exposures were a priori believed to be harmful, not protective.
Calculation of Population Attributable Fractions and Attributable Prevalence
Population attributable fractions (PAFs) were estimated using relative risk estimates from the meta-regression models and the GBD 2019 age-sex-location-year-specific estimates of the prevalence of each clinical condition included. They were calculated using the formula below [27]:
PAFs were then multiplied by the corresponding age-sex-location-year-specific estimates of dementia prevalence from GBD to estimate the amount of dementia prevalence attributable to each disease.
Compilation of Results
Age-standardized estimates were calculated using weights derived from the global age distribution of dementia cases in 2019 [23]. All calculations were done with 1,000 draws to incorporate uncertainty from each step of the modeling process. Uncertainty intervals were defined as the 25th and 975th values of the ordered draws. Differences were defined as statistically significant if the uncertainty intervals did not intersect the null value.
Results
The literature reviews for the 4 conditions modeled in this analysis yielded 158 sources on the association between dementia and Down syndrome (n = 25), Parkinson’s disease (n = 53), stroke (n = 33), and TBI (n = 47). The majority of the data were from North America and Western Europe (shown in Table 1 and online suppl. Appendix 1).
Table 1.
Distribution of data sources by world region and disease exposure, N (%)
| Down syndrome, n (%) | Parkinson’s disease, n (%) | Stroke, n (%) | TBI, n (%) | |
|---|---|---|---|---|
| Sources, N | 25 | 54 | 36 | 49 |
| Global | 0 (0) | 0 (0) | 0 (0) | 3 (6.1) |
| Andean Latin America | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Australasia | 1 (4) | 4 (7.4) | 3 (8.3) | 2 (4.1) |
| Caribbean | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Central Asia | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Central Europe | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Central Latin America | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Central sub-Saharan Africa | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| East Asia | 0 (0) | 3 (5.6) | 3 (8.3) | 4 (8.2) |
| Eastern Europe | 0 (0) | 1 (1.9) | 0 (0) | 1 (2) |
| Eastern sub-Saharan Africa | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| High-income Asia Pacific | 1 (4) | 4 (7.4) | 2 (5.6) | 1 (2) |
| High-income North America | 5 (20) | 11 (20.4) | 15 (41.7) | 25 (51) |
| North Africa and Middle East | 0 (0) | 1 (1.9) | 0 (0) | 0 (0) |
| Oceania | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| South Asia | 0 (0) | 1 (1.9) | 0 (0) | 0 (0) |
| Southeast Asia | 0 (0) | 1 (1.9) | 0 (0) | 0 (0) |
| Southern Latin America | 0 (0) | 2 (3.7) | 0 (0) | 0 (0) |
| Southern sub-Saharan Africa | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Tropical Latin America | 1 (4) | 2 (3.7) | 0 (0) | 0 (0) |
| Western Europe | 17 (68) | 24 (44.4) | 13 (36.1) | 12 (24.5) |
| Western sub-Saharan Africa | 0 (0) | 0 (0) | 0 (0) | 1 (2) |
Sources labeled “Global” used individual level data in >1 region and therefore are not tagged to 1 specific location. TBI, traumatic brain injury.
For all 4 diseases included in the analysis, although the absolute risk of dementia increased with age, the relative risk of dementia decreased with age due to the increasing prevalence of dementia in the oldest age groups of the unexposed (individuals without each clinical condition) populations. This decrease in relative risk was most extreme for Down syndrome. The relationship between stroke and dementia was approximately logarithmic over age, and for Parkinson’s disease, the relative risk of dementia was high in the youngest ages but decreased fairly quickly from age 70 onward. When compared to the other diseases evaluated, the relative risks for dementia in those with TBI were the lowest in the youngest age group and decreased further across the age range (shown in Fig. 1).
Fig. 1.
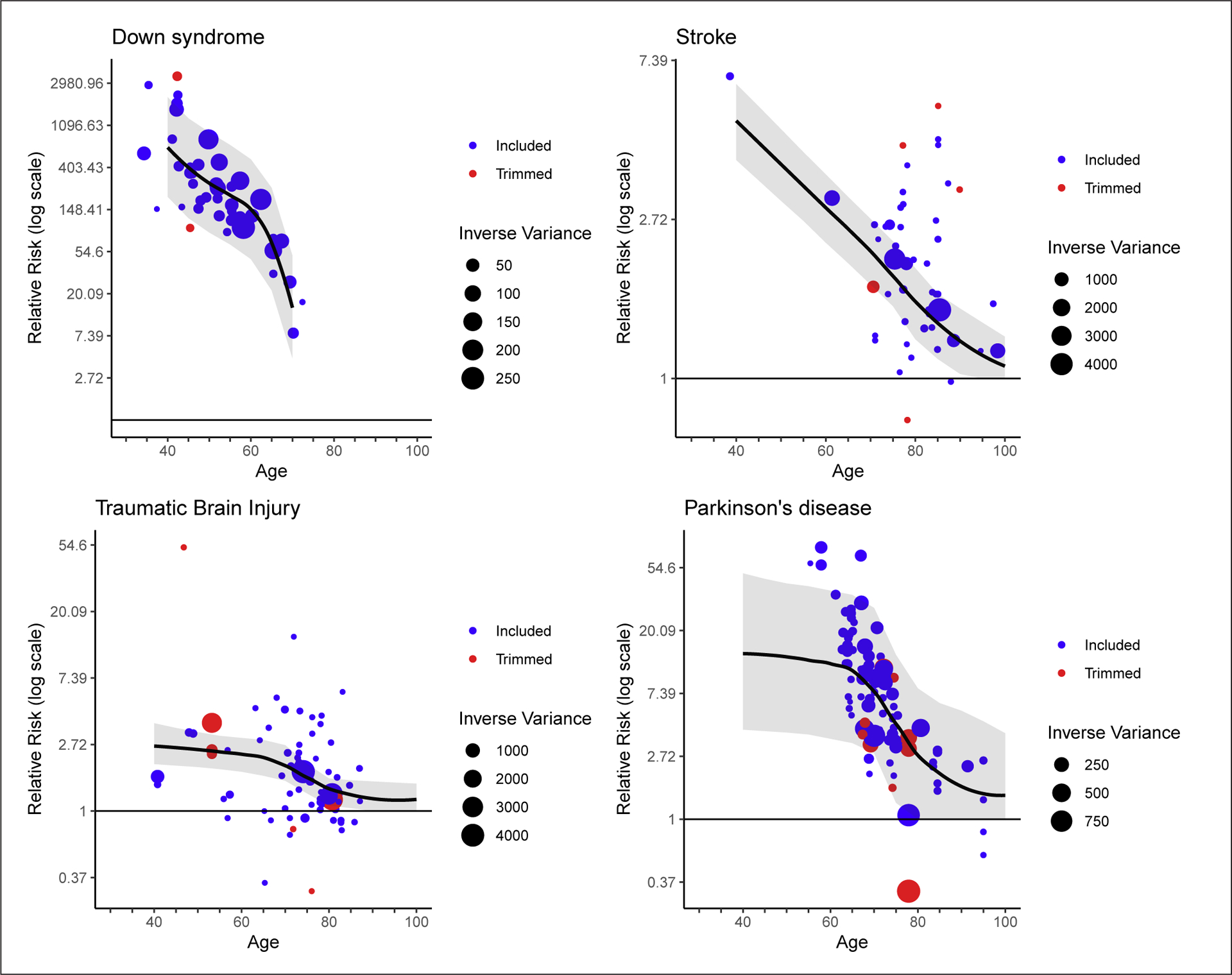
Meta-regression of relative risk of dementia by age for Down syndrome, stroke, TBI, and Parkinson’s disease. Trimmed data refer to data points that were identified as outliers in the modeling framework. The inverse variance scale sizes the data points according to the certainty of the data such that larger points have less uncertainty and more weight in the model. TBI, traumatic brain injury.
The proportion of dementia cases due to Down syndrome decreased with age, while for the other diseases, the proportion of dementia cases attributable displayed an inverted U-shaped curve over age, increasing until approximately age 70 years before decreasing. For TBI, Parkinson’s disease, and stroke (in younger ages), the proportions of dementia attributable to each condition were higher in men than in women, owing to the higher prevalence of these conditions in men (shown in Fig. 2). However, these sex differences were not statistically significant.
Fig. 2.
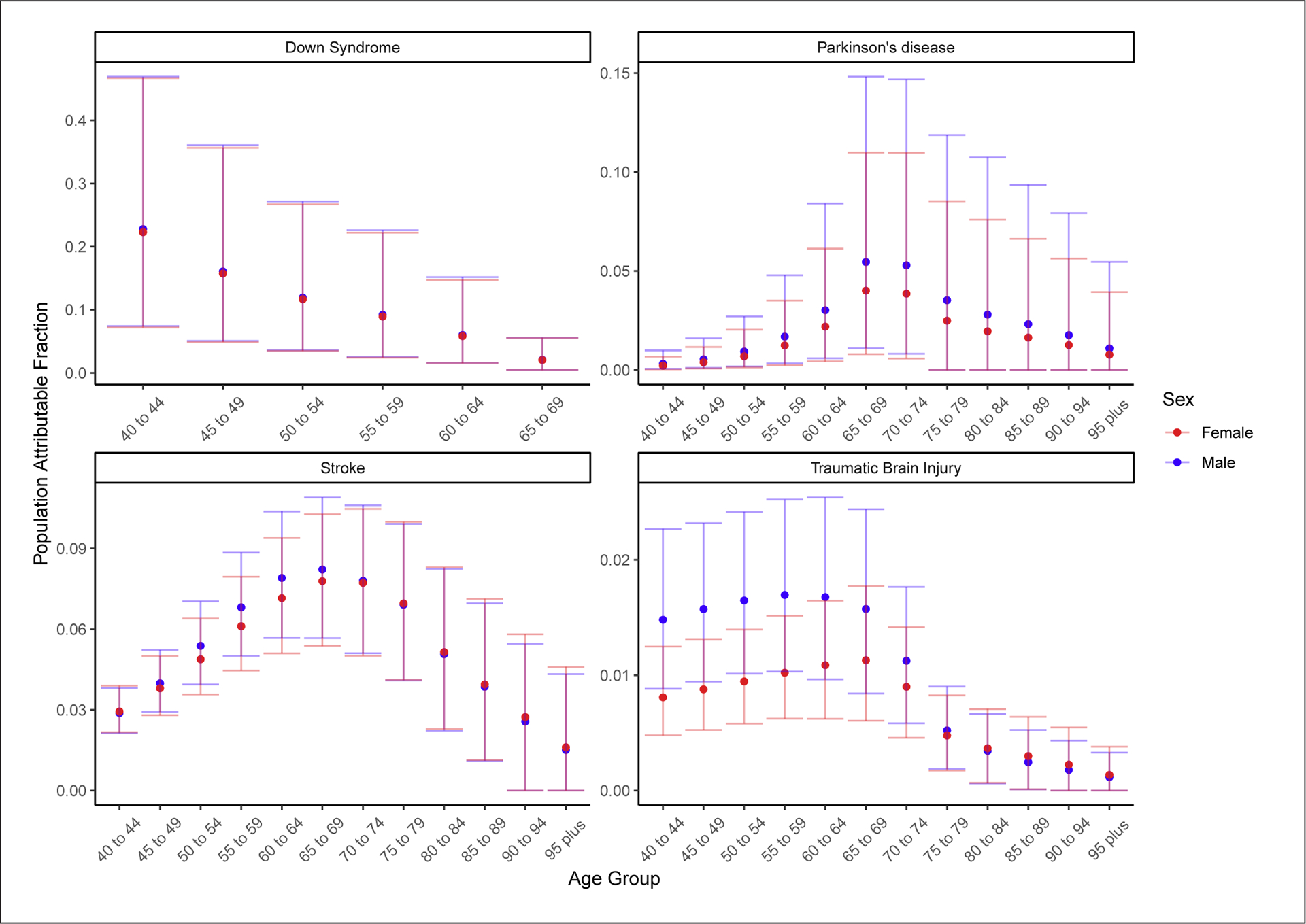
Global PAFs of dementia for Down syndrome, stroke, TBI, and Parkinson’s disease, by age and sex in 2019. PAF, population attributable fraction; TBI, traumatic brain injury.
Down syndrome, Parkinson’s disease, stroke, and TBI together accounted for 10.0% (95% UI 6.0–16.5) of the all-age global prevalence of dementia. There was a strong gradient with age, with only 2.6% (0.1–6.7) of the global prevalence of dementia in 2019 explained by these conditions in the 95 years and older age group, whereas 26.8% (11.6–50.8) of dementia prevalence was explained by the 4 diseases in the 40–44 years age group (shown in Fig. 3).
Fig. 3.
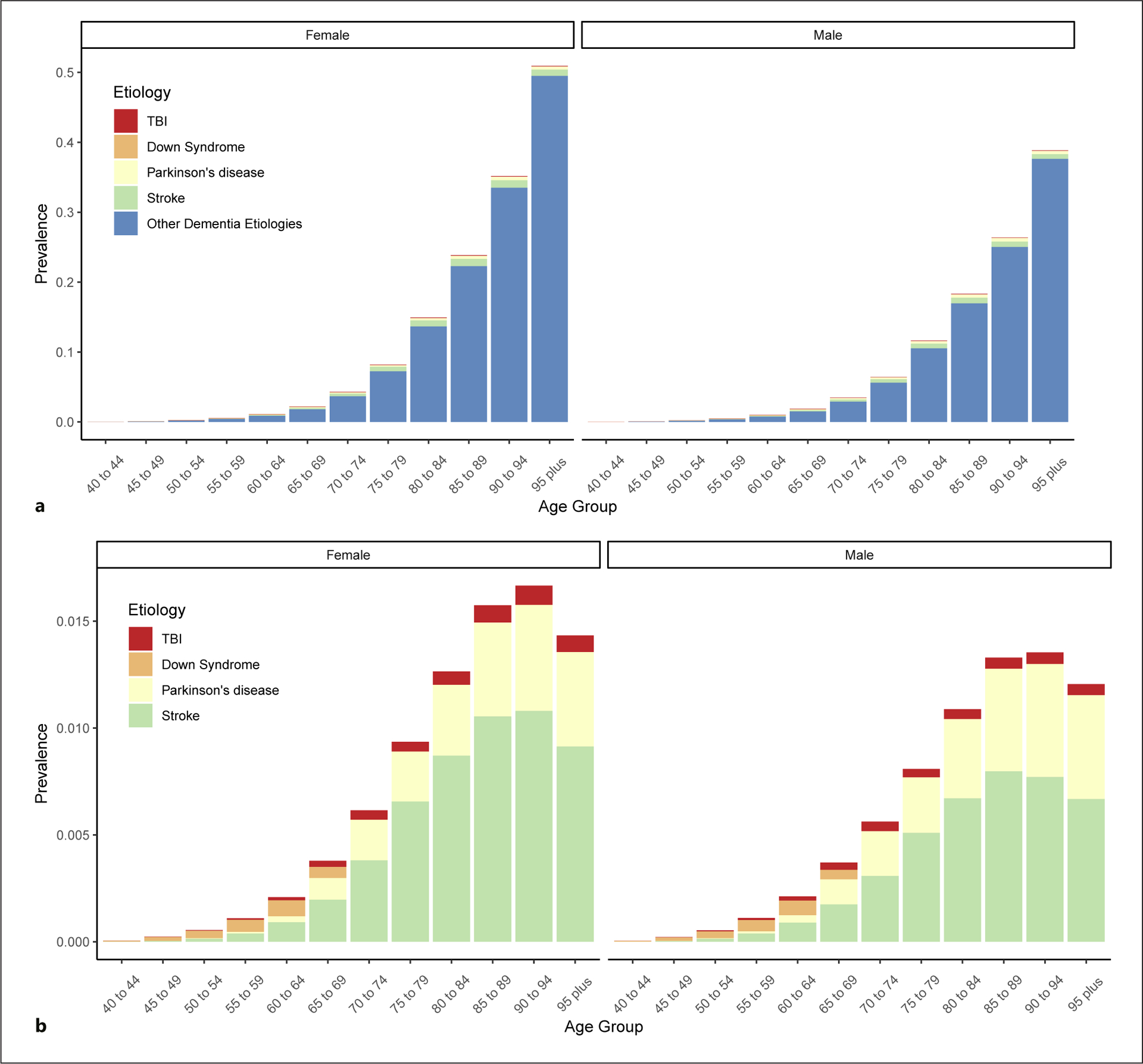
Global dementia prevalence due to Down syndrome, stroke, TBI, and Parkinson’s disease, and the prevalence of dementia unaccounted for by these 4 clinical conditions in 2019. a All categories including the residual category of dementia prevalence not attributable to the clinical conditions examined in this paper. b A zoomed-in view of the prevalence attributable to each clinical condition. TBI, traumatic brain injury.
For every region, stroke accounted for the largest number of dementia cases, totaling 3.70 million (95% UI 2.00–5.68) cases globally. Parkinson’s disease accounted for the second largest number of global cases (1.71 million cases; 0.14–5.79), followed by Down syndrome (0.80 million cases; 0.23–2.03) and then TBI (0.42 million cases; 0.20–0.69).
Eastern Europe was the region with the highest age-standardized proportion of dementia attributable to stroke (8.5%; 95% UI 4.6–12.5). East Asia had the highest age-standardized PAFs of dementia attributable to Parkinson’s disease (3.0%; 0.3–9.8). Australasia had the highest age-standardized PAF of dementia attributable to Down syndrome (2.3%; 0.7–5.3), and both central Europe and Australasia had the highest age-standardized prevalence of dementia attributable to TBI (central Europe: 1.9% [0.9–3.1]; Australasia: 1.9% [0.8–3.1]) (shown in Table 2).
Table 2.
Global and regional numbers of attributable cases of dementia and age-standardized PAFs for dementia cases attributable to Down’s syndrome, Parkinson’s disease, stroke and TBI in 2019
| Location | Down’s syndrome | Parkinson’s disease | Stroke | TBI | ||||
|---|---|---|---|---|---|---|---|---|
| cases, n (in 1,000s) (95% UI) | age-standardized PAF (95% UI) | cases, n (in 1,000s) (95% UI) | age-standardized PAF (95% UI) | cases, n (in 1,000s) (95% UI) | age-standardized PAF (95% UI) | cases, n (in 1,000s) (95% UI) | age-standardized PAF (95% UI) | |
| Global | 802.2 (226.3–2,030.7) | 1.2 (0.3–2.9) | 1,705.2 (141.8–5,787.1) | 2.6 (0.2–8.6) | 3,689.2 (2,003.6–5,677.7) | 5.6 (3.0–8.5) | 415.9 (197.0–694.5) | 0.6 (0.3–1.0) |
| Central Asia | 10.0 (2.8–25.2) | 1.5 (0.4–3.6) | 11.6 (1.0–37.6) | 2.6 (0.2–8.6) | 28.3 (16.3–42.7) | 5.9 (3.3–8.8) | 4.4 (2.3–7.1) | 0.8 (0.4–1.3) |
| Central Europe | 28.1 (8.0–69.8) | 1.9 (0.5–4.5) | 61.0 (3.9–207.1) | 2.7 (0.2–9.1) | 139.1 (70.3–218.2) | 6.2 (3.3–9.4) | 38.7 (17.3–64.6) | 1.9 (0.9–3.1) |
| Eastern Europe | 56.3 (16.3–136.5) | 2.2 (0.6–5.1) | 79.9 (5.6–276.8) | 2.5 (0.2–8.2) | 272.4 (143.8–420.3) | 8.5 (4.6–12.5) | 39.6 (18.9–66.7) | 1.3 (0.7–2.1) |
| Australasia | 7.1 (2.1–16.9) | 2.3 (0.7–5.3) | 11.4 (0.8–38.6) | 2.6 (0.2–8.5) | 13.3 (6.2–22.0) | 3.0 (1.5–4.6) | 8.0 (3.1–14.3) | 1.9 (0.8–3.1) |
| High-income Asia Pacific | 47.2 (13.6–116.9) | 1.9 (0.5–4.5) | 95.9 (4.9–346.5) | 1.9 (0.2–6.4) | 248.9 (114.6–411.8) | 5.1 (2.7–7.6) | 21.8 (8.9–38.3) | 0.5 (0.3–0.8) |
| High-income North America | 84.8 (25.0–206.2) | 1.8 (0.5–4.4) | 177.7 (11.6–602.4) | 2.8 (0.2–9.2) | 344.7 (163.0–557.7) | 5.4 (2.7–8.2) | 26.3 (12.2–44.0) | 0.5 (0.2–0.7) |
| Southern Latin America | 11.2 (3.3–26.7) | 2.0 (0.6–4.6) | 19.6 (1.2–66.3) | 2.8 (0.2–9.3) | 26.1 (13.1–41.7) | 3.8 (2.0–5.7) | 5.4 (2.5–9.2) | 0.8 (0.4–1.4) |
| Western Europe | 82.8 (24.0–202.9) | 1.7 (0.5–4.2) | 221.8 (11.2–775.0) | 2.7 (0.2–9.0) | 296.9 (134.9–489.8) | 3.6 (1.9–5.5) | 71.9 (27.7–128.9) | 1.0 (0.5–1.6) |
| Andean Latin America | 4.8 (1.3–12.5) | 1.1 (0.3–2.7) | 8.4 (0.7–28.6) | 2.4 (0.2–8.0) | 12.1 (6.6–18.7) | 3.3 (1.8–5.0) | 1.8 (0.8–3.0) | 0.5 (0.2–0.8) |
| Caribbean | 7.3 (2.2–17.5) | 1.9 (0.5–4.4) | 7.0 (0.5–23.8) | 2.2 (0.2–7.3) | 14.1 (7.7–21.8) | 4.3 (2.4–6.5) | 1.6 (0.7–2.7) | 0.5 (0.2–0.8) |
| Central Latin America | 35.5 (10.5–87.0) | 1.8 (0.5–4.3) | 31.3 (2.4–106.3) | 2.2 (0.2–7.4) | 46.9 (25.1–73.5) | 3.1 (1.7–4.8) | 5.1 (2.4–8.7) | 0.3 (0.2–0.6) |
| Tropical Latin America | 32.1 (8.8–82.5) | 1.2 (0.3–3.0) | 46.2 (4.2–157.3) | 2.1 (0.2–7.0) | 96.8 (54.2–148.0) | 4.3 (2.3–6.5) | 4.5 (2.2–7.3) | 0.2 (0.1–0.3) |
| North Africa and Middle East | 47.0 (12.9–120.6) | 1.1 (0.3–2.7) | 77.1 (6.9–255.9) | 2.3 (0.2–8.0) | 229.4 (128.7–344.9) | 6.8 (3.8–10.0) | 20.6 (10.5–33.6) | 0.6 (0.3–0.9) |
| South Asia | 58.7 (15.7–157.0) | 0.7 (0.2–1.8) | 143.3 (14.0–470.1) | 2.3 (0.2–7.7) | 241.7 (142.3–362.0) | 3.6 (2.0–5.4) | 30.3 (15.5–48.6) | 0.4 (0.2–0.7) |
| East Asia | 230.4 (62.4–599.3) | 1.1 (0.3–2.7) | 579.8 (57.0–1,857.4) | 3.0 (0.3–9.8) | 1,390.7 (784.1–2,114.7) | 7.3 (3.9–11.0) | 107.9 (56.3–174.9) | 0.5 (0.3–0.8) |
| Oceania | 0.5 (0.1–1.4) | 0.9 (0.2–2.3) | 0.9 (0.1–2.9) | 2.9 (0.3–9.5) | 2.0 (1.2–3.0) | 6.1 (3.3–9.1) | 0.1 (0.1–0.2) | 0.3 (0.2–0.5) |
| Southeast Asia | 34.2 (9.1–91.6) | 0.6 (0.1–1.5) | 91.5 (9.6–291.6) | 2.5 (0.2–8.2) | 190.7 (111.8–285.7) | 5.0 (2.7–7.5) | 15.8 (8.4–25.3) | 0.4 (0.2–0.6) |
| Central sub-Saharan Africa | 3.5 (1.0–9.3) | 0.8 (0.2–2.0) | 5.4 (0.5–17.6) | 1.8 (0.1–6.3) | 12.5 (7.4–18.7) | 4.0 (2.2–6.0) | 1.5 (0.8–2.4) | 0.4 (0.2–0.7) |
| Eastern sub-Saharan Africa | 6.4 (1.7–17.5) | 0.5 (0.1–1.3) | 13.3 (1.2–43.9) | 1.7 (0.1–5.6) | 31.3 (17.9–47.4) | 3.7 (2.0–5.6) | 4.6 (2.4–7.5) | 0.5 (0.2–0.8) |
| Southern sub-Saharan Africa | 4.0 (1.1–10.5) | 1.0 (0.3–2.4) | 5.7 (0.4–19.7) | 1.7 (0.1–6.0) | 15.6 (8.6–24.1) | 4.6 (2.4–7.1) | 1.8 (0.9–3.0) | 0.5 (0.2–0.8) |
| Western sub-Saharan Africa | 10.1 (2.7–26.6) | 0.7 (0.2–1.8) | 16.4 (1.4–54.2) | 2.2 (0.1–7.5) | 35.5 (20.4–53.6) | 4.3 (2.3–6.4) | 4.0 (2.1–6.5) | 0.4 (0.2–0.7) |
PAF, population attributable fraction; TBI, traumatic brain injury.
Before age 60 years, Down syndrome was responsible for the largest number of dementia cases of the diseases evaluated. However, as age increases, the fraction of dementia due to both stroke and Parkinson’s disease increases. The absolute numbers of dementia cases attributable to Down syndrome, Parkinson’s disease, TBI, and stroke are fairly similar between men and women, despite men having a lower prevalence of dementia in each age group.
Discussion
Globally, the proportion of dementia prevalence attributable to Down syndrome, Parkinson’s disease, stroke, and TBI was 10.0% (95% UI 6.0–16.5). Of these diseases, stroke accounted for the largest total number of dementia cases. However, at the youngest ages (40–50 years), Down syndrome and TBI accounted for larger proportions of dementia prevalence.
These results demonstrate the relative impact of a diverse set of clinical conditions (Down syndrome, Parkinson’s disease, stroke, and TBI) on dementia prevalence. Relative risks were highest in Down syndrome, particularly at the youngest ages, which is hypothesized to be due to the overexpression of genes involved in the processing of amyloid precursor protein and Alzheimer’s neuropathic changes caused by trisomy 21 in combination with the low background risk of dementia in the youngest ages [28]. Despite the fact the development of dementia is likely an eventuality among those with Down syndrome, the quantification of dementia in this population is still of interest, as risk factor-based interventions or amyloid-targeting therapies may still be able to delay the onset of dementia in this population. The estimated relative risks were also high for Parkinson’s disease, and these relative risks remained higher in older ages, where individuals may be affected by the combined burden of Lewy body pathology due to Parkinson’s disease as well as other neuropathologies, which often jointly lead to the expression of clinical dementia [29].
Despite the higher relative risks in Down syndrome and Parkinson’s disease, stroke was responsible for the largest number of global dementia cases due to the larger prevalence of stroke. Given that an estimated 88.8% (95% UI 86.5–90.9) of the global burden of stroke can be attributed to modifiable risk factors, this presents opportunities for interventions that could impact not only stroke outcomes but dementia outcomes as well [30]. As cardiovascular disease risk factors have also been shown to be associated with dementia independently of clinical stroke, addressing these risk factors may have an impact above and beyond affecting dementia through clinical stroke [31]. Potential gains may also be larger in low-income countries as compared to high-income countries, where there is currently inadequate identification and treatment of many of these risks [32].
The largest portion of dementia cases (90.0%; 95% UI 83.5–94.0) remained unexplained by the clinical conditions examined in this paper. Many of these cases are likely attributable to pathologies such as amyloid beta, tau, α-synuclein, or a mix of these pathologies along with subclinical vascular disease, TDP-43, and other pathological changes [33–37]. Prior work on autopsy samples reports that 25% of dementia risk is attributable to Alzheimer’s disease pathologies (including amyloid plaques, neurofibrillary tangles, and cerebral amyloid angiopathy) [9]. However, additional large population-based autopsy studies are needed to better characterize the distribution of these and other etiologies.
Furthermore, the quantification of the residual dementia category within this study, that is, dementia not due to other clinical conditions, is important within the context of the GBD project, as it ensures that GBD results are mutually exclusive and collectively exhaustive. This principle of GBD facilitates valid comparisons between diseases by avoiding double counting any health loss across disease categories [38].
A number of limitations require consideration. First, we only examined 4 clinical conditions, rather than all possible causes of dementia. We chose to focus on these clinical conditions because the ascertainment of these conditions is more widely standardized as compared to other causes of dementia, including Lewy body dementia and frontotemporal dementia. Additionally, while the quantification of dementia due to clinical conditions has received less attention compared to research on modifiable risk factors or neuropathological etiologies, focusing on clinical conditions that require interaction with health-care systems ensures that there are points of contact to enable the rollout of preventative interventions or potential future amyloid-targeting treatments. Stroke, Parkinson’s disease, Down syndrome, and TBI were specifically chosen for inclusion in this analysis because they are currently quantified within the GBD framework, allowing for global estimation. Although HIV fulfilled our criteria for inclusion in this study, we were unable to include HIV due to the use of a different definition of dementia (HIV-associated dementia) in the HIV literature, which relies principally on neuropsychological testing cutoffs rather than the more comprehensive DSM-based diagnostic criteria for dementia [39]. Second, there was a large amount of heterogeneity in the literature on the relative risk of dementia due to Down syndrome, Parkinson’s disease, stroke, and TBI. We attempted to control for some of these differences through the use of covariates, but some bias due to these study attributes may remain due to measurement error in the discretization and categorization of study traits. Third, the majority of the sparse data available on relative risks came from high-income settings in North America and Western Europe, and we have assumed that these data apply globally. To the extent that the increased risk due to each clinical condition is attributable to the biological link between each exposure and dementia, this may be a reasonable assumption, but the addition of new data sources would greatly strengthen the analysis. Fourth, when individual draws from our models of relative risk estimated protective effects (relative risks <1), we set these draws to 1 to conceptually align with our belief that these clinical conditions should lead to increased risk rather than acting as protective factors. However, this adjustment biases the mean of the draws upward. For example, in Parkinson’s disease, which had the largest number of draws under 1 at the oldest ages, the relative risk in the 95 years and older age group increased from 1.50 to 1.62 after adjustment. Fifth, the estimation of attributable burden is dependent on the estimation of dementia prevalence, which is subject to limitations related to data sparsity and heterogeneity.
Further inclusion of other clinical conditions such as diabetes, alcohol use disorders, and encephalitis should be considered as well, contingent on the evaluation of the strength of the evidence suggesting an association with dementia. Additionally, to refine our current estimates, future work should seek to distinguish between ischemic and hemorrhagic stroke, as recent research indicates that the association with dementia differs by stroke subtype [40].
Despite the limitations of the data and methods, this study synthesized available data to compare the relative risk and attributable burden of clinical diseases associated with dementia. This analysis complements prior work on modifiable risk factors and neuropathological etiologies by more fully elucidating the contribution of clinical conditions to dementia prevalence. The ability to compare both across clinical conditions and across regions supports more informed decision-making related to distributing research funds across the clinical conditions examined, as well as planning interventions to target modifiable risks for conditions such as TBI or stroke.
Supplementary Material
Acknowledgements
R.O.A. is supported by Grant U01HG010273 from the National Institutes of Health (NIH) as part of the H3Africa Consortium and by the FLAIR fellowship funded by the UK Royal Society and the African Academy of Sciences. F.C. and E.F. acknowledge UID/MULTI/04378/2019 and UID/QUI/50006/2019 support with funding from FCT/MCTES through national funds. L.F.S.C.A. is supported by Medical Research Council (London) Grant No. MR/T03355X/1. A.G. was supported by Fondazione Umberto Veronesi. M.R.H. is supported by Ohio University Research Council (OURC) Spring 2020 funding. Y.J.K. was supported by Research Management Centre, Xiamen University Malaysia (No.: XMUMRF/2020-C6/ITCM/0004). W.A.K. is part of the Alzheimer Advisory Group to IHME sponsored by Gates Ventures and is principally supported at UW by NIH Grant U01 AG016976. M.K. would like to acknowledge FIC/NIMH K43 TW010716-03. I.L. is a member of the Sistema Nacional de Investigación (SNI), which is supported by the Secretaría Nacional de Ciencia, Tecnología e Innovación (SENACYT), Panama. S.L. acknowledges institutional support from the Competence Cluster for Nutrition and Cardiovascular Health (nutriCARD) Halle-Jena-Leipzig (Germany; German Federal Ministry of Education and Research; Grant Agreement no. 01EA1808A). N.M. acknowledges support from the National Institute of Mental Health and Neurosciences, Bengaluru, India. S.M. is supported by Grant GR-2013-02354960 from the Italian Ministry of Health. A.R., D.S., and S.S. acknowledge support by a grant from the Italian Ministry of Health (Ricerca Corrente, Fondazione Istituto Neurologico C. Besta, Linea 4 – Outcome Research: dagli Indicatori alle Raccomandazioni Cliniche). P.S.S. acknowledges funding support from the NHMRC of Australia (GNT1093083) and the NIH (USA) (1RF1AG057531-01). J.P.S. acknowledges support by Grant No. UIDB/04378/2020 from the Applied Molecular Biosciences Unit (UCIBIO), supported through Portuguese national funds via FCT/MCTES. C.E.I. acknowledges support by the National Health and Medical Research Council. C.W. acknowledges support from the Ministry of Science and Technology in China (2020YFC2005600) and the Suzhou Science and Technology Bureau SS2019069 and partial support by the Kunshan Municipal Government research funding.
Conflict of Interest Statement
G.J.H. reports personal honoraria from AC Immune for serving as Chair, Data Safety Monitoring Committee, of ACI-24-701 and AC-35-1201 trials of an immune therapy for Alzheimer’s disease. J.J.J. reports personal fees from Amgen, ALAB Laboratories, Teva, Synexus, Boehringer Ingelheim, and Zentiva, outside the submitted work. W.A.K. reports NIH research Grant U01 AG016976. S.L. reports personal fees from Akcea Therapeutics, Amedes, Amgen, Berlin-Chemie, Boehringer Ingelheim Pharma, Daiichi Sankyo, Lilly, Merck Sharp & Dohme, Novo Nordisk, Sanofi-Aventis, SYNLAB, Unilever, and Upfield, and nonfinancial support from Preventicus, all outside the submitted work. P.S.S. reports personal fees from Biogen Australia Advisory Committee, outside the submitted work. M.S. reports being an employee of Bayer. J.A.S. reports personal fees from Crealta/Horizon, Medisys, Fidia, UBM LLC, Trio health, Medscape, WebMD, Clinical Care options, ClearView Healthcare Partners, Putnam Associates, Focus Forward, Navigant Consulting, Spherix, Practice Point Communications, the National Institutes of Health, the American College of Rheumatology, and Simply Speaking; owning stock options in Amarin, Viking, Moderna and Vaxart Pharmaceuticals, and Charlotte’s Web Holdings; membership in the FDA Arthritis Advisory Committee, Steering Committee of OMERACT (an international organization that develops measures for clinical trials and receives arm’s length funding from 12 pharmaceutical companies), Veterans Affairs Rheumatology Field Advisory Committee, and the UAB Cochrane Musculoskeletal Group Satellite Center on Network Meta-Analysis, all outside the submitted work. C.E.I.S. has provided clinical consultancy and has been on scientific advisory committees for the Australian Commonwealth Scientific and Industrial Research Organisation and the Federal Department of Health; her research program has received support from the National Health and Medical Research Council (Grants 547500, 1032350, and 1062133), National Institute on Aging (Grant 320312, Alzheimer’s Association), and the Royal Australian College of Physicians; she may accrue revenues from a patent in pharmacogenomics prediction of seizure recurrence. C.W. reports grants from the Ministry of Science and Technology in China, personal fees from HealthKeepers, and grants from Suzhou Municipal Science and Technology Bureau and the Kunshan Government, outside the submitted work.
Funding Sources
This work was funded by the Bill & Melinda Gates foundation and Gates Ventures. The funder of the study had no role in study design, data collection, data analysis, data interpretation, or the writing of the report.
Online Supplementary Appendix 1
Emma Nichols1, Foad Abd-Allah2, Amir Abdoli3, Eman Abu-Gharbieh4, Jaimie D Adelson1, Rufus Olusola Akinyemi5 6, Fahad Mashhour Alanezi7, Vahid Alipour8 9, Jalal Arabloo8, Getinet Ayano10, Atif Amin Baig11, Maciej Banach12 13, Miguel A Barboza14 15, Suzanne Lyn Barker-Collo16, Bernhard T Baune17 18, Akshaya Srikanth Bhagavathula19 20, Krittika Bhattacharyya21 22, Ali Bijani23, Antonio Biondi24, Atanu Biswas25, Srinivasa Rao Bolla26, Archith Boloor27, Carol Brayne28, Hermann Brenner29, Katrin Burkart1 30, Richard A Burns31, Sharath Burugina Nagaraja32, Luis Alberto Cámera33 34, Felix Carvalho35, Luis F S Castro-de-Araujo36, Ester Cerin37 38, Achille Cernigliaro39, Nicolas Cherbuin31, Jee-Young Jasmine Choi40, Dinh-Toi Chu41, Rosa A S Couto42, Baye Dagnew43, Anh Kim Dang44, Daniel Diaz45 46, Zahra Sadat Dibaji Forooshani47, Shirin Djalalinia48, Paul Narh Doku49, Shaimaa I El-Jaafary2, Khalil Eskandari50 51, Sharareh Eskandarieh52, Farshad Farzadfar53, Valery L Feigin54 1 55, Seyed-Mohammad Fereshtehnejad56 57, Eduarda Fernandes58, Pietro Ferrara59, Irina Filip60 61, Florian Fischer62, Shilpa Gaidhane63, Ahmad Ghashghaee8 64, Alessandro Gialluisi65, Elena V Gnedovskaya66, Mahaveer Golechha67, Mohammad Rifat Haider68, Samer Hamidi69, Graeme J Hankey70 71, Amr Hassan2, Simon I Hay1 30, Mohamed I Hegazy2, Golnaz Heidari72, Reza Heidari-Soureshjani73, Mowafa Househ74, Bing-Fang Hwang75, Licia Iacoviello65 76, Olayinka Stephen Ilesanmi77 78, Irena M Ilic79, Milena D Ilic80, Seyed Sina Naghibi Irvani81, M Mofizul Islam82, Hiroyasu Iso83, Masao Iwagami84 85, Ravi Prakash Jha86 87, Jost B Jonas88 89, Jacek Jerzy Jozwiak90, Rizwan Kalani91, André Karch92, Ayele Semachew Kasa93, Mahalaqua Nazli Khatib94, Yun Jin Kim95, Adnan Kisa97 98, Sezer Kisa96, Ai Koyanagi99 100, Walter A Kukull101, Manasi Kumar102 103, Iván Landires104 105, Kate E LeGrand1, Matilde Leonardi106, Bingyu Li107, Xuefeng Liu108, Giancarlo Logroscino109 110, Stefan Lorkowski111 112, Hawraz Ibrahim M. Amin113 114, Shilpashree Madhava Kunjathur115, Narayana Manjunatha116, Birhanu Geta Meharie117, Man Mohan Mehndiratta118 119, Workua Mekonnen Metekiya120, Norlinah Mohamed Ibrahim121, Yousef Mohammad122, Salahuddin Mohammed123 124, Archisman Mohapatra125, Ali H Mokdad1 30, Stefania Mondello126, Ghobad Moradi127 128, Tilahun Belete Mossie129, Gabriele Nagel130, Mukhammad David Naimzada131 132, Dr Muhammad Naveed133, Vinod C Nayak134, Cuong Tat Nguyen44, Huong Lan Thi Nguyen44, Son Hoang Nguyen135, Virginia Nunez-Samudio136 137, Andrew T Olagunju138 139, Sergej M Ostojic140, Samuel M Ostroff1 141, Nikita Otstavnov131, Stanislav S Otstavnov131 142, Mayowa O Owolabi143 144, Songhomitra Panda-Jonas88, Urvish K Patel145, Mona Pathak146, Hamidreza Pazoki Toroudi147 148, Carrie B Peterson149, Hai Quang Pham44, Marina Pinheiro150, Michael A Piradov55, Faheem Hyder Pottoo151, Akram Pourshams152, Sergio I Prada153 154, Dimas Ria Angga Pribadi155, Amir Radfar156, Alberto Raggi106, Fakher Rahim157 158, Pradhum Ram159, Juwel Rana160 161, Vahid Rashedi162, David Laith Rawaf165 166, Salman Rawaf163 164, Nickolas Reinig1, Nima Rezaei167 168, Aziz Rezapour8, Stephen R Robinson169, Michele Romoli170 171, Gholamreza Roshandel172, Perminder S Sachdev173 174, Amirhossein Sahebkar175 176, Mohammad Ali Sahraian52, Davide Sattin106, Monika Sawhney177, Mete Saylan178, Silvia Schiavolin106, Feng Sha179, Izza Shahid180, Masood Ali Shaikh181, David H Shaw1, Mika Shigematsu182, Jae Il Shin183, Rahman Shiri184, Tariq Jamal Siddiqi185, João Pedro Silva35, Jasvinder A Singh186 187, Deepika Singhal188 189, Amin Soheili190, Cassandra E I Szoeke191 192, Rafael Tabarés-Seisdedos193 194, Biruk Wogayehu Taddele195, Bhaskar Thakur196 197, Marcos Roberto Tovani-Palone198 199, Bach Xuan Tran200, Ravensara S Travillian1, Gebiyaw Wudie Tsegaye201, Muhammad Shariq Usman202, Marco Vacante24, Pascual R Valdez203 204, Diana Zuleika Velazquez46, Narayanaswamy Venketasubramanian205 206, Vasily Vlassov207, Giang Thu Vu135, Yuan-Pang Wang208, Abrha Hailay Weldemariam209, Ronny Westerman210, Chenkai Wu211 212, Ali Yadollahpour213, Kazumasa Yamagishi214 215, Yuichiro Yano216, Yordanos Gizachew Yeshitila217, Naohiro Yonemoto218 219, Chuanhua Yu220 221, Zhi-Jiang Zhang222, Christopher J L Murray1 30, and Theo Vos1 30.
1 Institute for Health Metrics and Evaluation, University of Washington, Seattle, WA, USA.
2 Department of Neurology, Cairo University, Cairo, Egypt.
3 Department of Parasitology and Mycology, Jahrom University of Medical Sciences, Jahrom, Iran.
4 Department of Clinical Sciences, University of Sharjah, Sharjah, United Arab Emirates.
5 Institute for Advanced Medical Research and Training, University of Ibadan, Ibadan, Nigeria.
6 Institute of Neuroscience, Newcastle University, Newcastle upon Tyne, UK.
7 Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia.
8 Health Management and Economics Research Center, Iran University of Medical Sciences, Tehran, Iran.
9 Health Economics Department, Iran University of Medical Sciences, Tehran, Iran.
10 School of Public Health, Curtin University, Perth, WA, Australia.
11 Unit of Biochemistry, Sultan Zainal Abidin University (Universiti Sultan Zainal Abidin), Kuala Terengganu, Malaysia.
12 Department of Hypertension, Medical University of Lodz, Lodz, Poland.
13 Polish Mothers’ Memorial Hospital Research Institute, Lodz, Poland.
14 Department of Neurosciences, Costa Rican Department of Social Security, San Jose, Costa Rica.
15 School of Medicine, University of Costa Rica, San Pedro, Costa Rica.
16 School of Psychology, University of Auckland, Auckland, New Zealand.
17 Department of Psychiatry, University of Münster, Münster, Germany.
18 Department of Psychiatry, Melbourne Medical School, Melbourne, VIC, Australia.
19 Department of Social and Clinical Pharmacy, Charles University, Hradec Kralova, Czech Republic.
20 Institute of Public Health, United Arab Emirates University, Al Ain, United Arab Emirates.
21 Department of Statistical and Computational Genomics, National Institute of Biomedical Genomics, Kalyani, India.
22 Department of Statistics, University of Calcutta, Kolkata, India.
23 Social Determinants of Health Research Center, Babol University of Medical Sciences, Babol, Iran.
24 Department of General Surgery and Medical-Surgical Specialties, University of Catania, Catania, Italy.
25 Department of Neurology, Institute of Post-Graduate Medical Education and Research and Seth Sukhlal Karnani Memorial Hospital, Kolkata, India.
26 Department of Biomedical Sciences, Nazarbayev University, Nur-Sultan City, Kazakhstan.
27 Department of Internal Medicine, Manipal Academy of Higher Education, Mangalore, India.
28 Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK.
29 Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany.
30 Department of Health Metrics Sciences, School of Medicine, University of Washington, Seattle, WA, USA.
31 Research School of Population Health, Australian National University, Canberra, ACT, Australia.
32 Department of Community Medicine, Employee State Insurance Post Graduate Institute of Medical Sciences and Research, Bangalore, India.
33 Internal Medicine Department, Hospital Italiano de Buenos Aires, Buenos Aires, Argentina.
34 Board of Directors, Argentine Society of Medicine, Buenos Aires, Argentina.
35 Research Unit on Applied Molecular Biosciences (UCIBIO), University of Porto, Porto, Portugal.
36 Department of Psychiatry, University of Melbourne, Melbourne, VIC, Australia.
37 Mary MacKillop Institute for Health Research, Australian Catholic University, Melbourne, VIC, Australia.
38 School of Public Health, University of Hong Kong, Hong Kong, China.
39 Regional Epidemiological Observatory Department, Sicilian Regional Health Authority, Palermo, Italy.
40 Biomedical Informatics, Seoul National University Hospital, Seoul, South Korea.
41 Faculty of Biology, Hanoi National University of Education, Hanoi, Vietnam.
42 Department of Chemical Sciences, University of Porto, Porto, Portugal.
43 Department of Human Physiology, University of Gondar, Gondar, Ethiopia.
44 Institute for Global Health Innovations, Duy Tan University, Hanoi, Vietnam.
45 Center of Complexity Sciences, National Autonomous University of Mexico, Mexico City, Mexico.
46 Faculty of Veterinary Medicine and Zootechnics, Autonomous University of Sinaloa, Culiacán Rosales, Mexico.
47 Tehran University of Medical Sciences, Tehran, Iran.
48 Development of Research and Technology Center, Ministry of Health and Medical Education, Tehran, Iran.
49 School of Nursing and Midwifery, University of Cape Coast, Cape Coast, Ghana.
50 Department of Medicinal Chemistry, Kerman University of Medical Sciences, Kerman, Iran.
51 Pharmaceutics Research Center, Kerman University of Medical Sciences, Kerman, Iran.
52 Multiple Sclerosis Research Center, Tehran University of Medical Sciences, Tehran, Iran.
53 Non-communicable Diseases Research Center, Tehran University of Medical Sciences, Tehran, Iran.
54 National Institute for Stroke and Applied Neurosciences, Auckland University of Technology, Auckland, New Zealand.
55 Research Center of Neurology, Moscow, Russia.
56 Department of Neurobiology, Karolinska Institute, Stockholm, Sweden.
57 Division of Neurology, University of Ottawa, Ottawa, ON, Canada.
58 Associated Laboratory for Green Chemistry (LAQV), University of Porto, Porto, Portugal.
59 Research Center on Public Health, University of Milan Bicocca, Monza, Italy.
60 Psychiatry Department, Kaiser Permanente, Fontana, CA, USA.
61 School of Health Sciences, A.T. Still University, Mesa, AZ, USA.
62 Institute of Gerontological Health Services and Nursing Research, Ravensburg-Weingarten University of Applied Sciences, Weingarten, Germany.
63 Department of Medicine, Datta Meghe Institute of Medical Science, Wardha, India.
64 Student Research Committee, Iran University of Medical Sciences, Tehran, Iran.
65 Department of Epidemiology and Prevention, IRCCS Neuromed, Pozzilli, Italy.
66 Third Department of Neurology, Research Center of Neurology, Moscow, Russia.
67 Health Systems and Policy Research, Indian Institute of Public Health Gandhinagar, Gandhinagar, India.
68 Department of Social and Public Health, Ohio University, Athens, OH, USA.
69 School of Health and Environmental Studies, Hamdan Bin Mohammed Smart University, Dubai, United Arab Emirates.
70 Medical School, University of Western Australia, Perth, WA, Australia.
71 Department of Neurology, Sir Charles Gairdner Hospital, Perth, WA, Australia.
72 Independent Consultant, Santa Clara, CA, USA.
73 School of Nursing and Midwifery, Tehran University of Medical Sciences, Tehran, Iran.
74 College of Science and Engineering, Hamad Bin Khalifa University, Doha, Qatar.
75 Department of Occupational Safety and Health, China Medical University, Taichung, Taiwan.
76 Research Center in Epidemiology and Preventive Medicine (EPIMED), Department of Medicine and Surgery, University of Insubria, Varese, Italy.
77 Department of Community Medicine, University of Ibadan, Ibadan, Nigeria.
78 Department of Community Medicine, University College Hospital, Ibadan, Ibadan, Nigeria.
79 Faculty of Medicine, University of Belgrade, Belgrade, Serbia.
80 Department of Epidemiology, University of Kragujevac, Kragujevac, Serbia.
81 Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
82 School of Psychology and Public Health, La Trobe University, Melbourne, VIC, Australia.
83 Public Health Department of Social Medicine, Osaka University, Suita, Japan.
84 Department of Health Services Research, University of Tsukuba, Tsukuba, Japan.
85 Department of Non-Communicable Disease Epidemiology, London School of Hygiene & Tropical Medicine, London, UK.
86 Department of Community Medicine, Baba Saheb Ambedkar Medical College & Hospital, Delhi, India.
87 Department of Community Medicine, Banaras Hindu University, Varanasi, India.
88 Department of Ophthalmology, Heidelberg University, Heidelberg, Germany.
89 Beijing Institute of Ophthalmology, Beijing Tongren Hospital, Beijing, China.
90 Department of Family Medicine and Public Health, University of Opole, Opole, Poland.
91 Department of Neurology, University of Washington, Seattle, WA, USA.
92 Institute for Epidemiology and Social Medicine, University of Münster, Münster, Germany.
93 Department of Adult Health Nursing, Bahir Dar University, Bahir Dar, Ethiopia.
94 Global Evidence Synthesis Initiative, Datta Meghe Institute of Medical Sciences, Wardha, India.
95 School of Traditional Chinese Medicine, Xiamen University Malaysia, Sepang, Malaysia.
96 Department of Nursing and Health Promotion, Oslo Metropolitan University, Oslo, Norway.
97 School of Health Sciences, Kristiania University College, Oslo, Norway.
98 Global Community Health and Behavioral Sciences, Tulane University, New Orleans, LA, USA.
99 CIBERSAM, San Juan de Dios Sanitary Park, Sant Boi de Llobregat, Spain.
100 Catalan Institution for Research and Advanced Studies (ICREA), Barcelona, Spain.
101 Department of Epidemiology, University of Washington, Seattle, WA, USA.
102 Department of Psychiatry, University of Nairobi, Nairobi, Kenya.
103 Division of Psychology and Language Sciences, University College London, London, UK.
104 Unit of Genetics and Public Health, Institute of Medical Sciences, Las Tablas, Panama.
105 Ministry of Health, Herrera, Panama.
106 Neurology, Public Health and Disability Unit, Carlo Besta Neurological Institute IRCCS (Fondazione IRCCS Istituto Neurologico Carlo Besta), Milan, Italy.
107 Department of Sociology, Shenzhen University, Shenzhen, China.
108 Department of Systems, Populations, and Leadership, University of Michigan, Ann Arbor, MI, USA.
109 Department of Basic Medical Sciences, Neuroscience and Sense Organs, University of Bari Aldo Moro, Bari, Italy.
110 Department of Clinical Research in Neurology, Fondazione Cardinale Giovanni Panico Hospital, Tricase, Italy.
111 Institute of Nutritional Sciences, Friedrich Schiller University Jena, Jena, Germany.
112 Competence Cluster for Nutrition and Cardiovascular Health (nutriCARD), Jena, Germany.
113 Department of Pharmaceutical Science, University of Eastern Piedmont, Novara, Italy.
114 Chemistry Department, Salahaddin University-Erbil, Erbil, Iraq.
115 Department of Biochemistry, BGS Global Institute of Medical Sciences, Bengaluru, India.
116 Department of Psychiatry, National Institute of Mental Health and Neurosciences, Bengalore, India.
117 Department of Pharmacy, Wollo University, Dessie, Ethiopia.
118 Neurology Department, Janakpuri Super Specialty Hospital Society, New Delhi, India.
119 Department of Neurology, Govind Ballabh Institute of Medical Education and Research, New Delhi, India.
120 Department of Psychiatry, Mekelle University, Mekelle, Ethiopia.
121 Department of Medicine, National University of Malaysia Medical Center, Bandar Tun Razak, Malaysia.
122 Internal Medicine Department, King Saud University, Riyadh, Saudi Arabia.
123 Department of Biomolecular Sciences, University of Missippi, Oxford, MS, USA.
124 Department of Pharmacy, Mizan-Tepi University, Mizan, Ethiopia.
125 Epidemiology Department, GRID Council, Bhubaneswar, India.
126 Department of Biomedical and Dental Sciences and Morphofunctional Imaging, Messina University, Messina, Italy.
127 Social Determinants of Health Research Center, Kurdistan University of Medical Sciences, Sanandaj, Iran.
128 Department of Epidemiology and Biostatistics, Kurdistan University of Medical Sciences, Sanandaj, Iran.
129 Department of Psychiatry, Bahir Dar University, Bahir Dar, Ethiopia.
130 Institute of Epidemiology and Medical Biometry, Ulm University, Ulm, Germany.
131 Laboratory of Public Health Indicators Analysis and Health Digitalization, Moscow Institute of Physics and Technology, Dolgoprudny, Russia.
132 Experimental Surgery and Oncology Laboratory, Kursk State Medical University, Kursk, Russia.
133 Department of Biotechnology, University of Central Punjab, Lahore, Pakistan.
134 Department of Forensic Medicine and Toxicology, Manipal Academy of Higher Education, Manipal, India.
135 Center of Excellence in Behavioral Medicine, Nguyen Tat Thanh University, Ho Chi Minh City, Vietnam.
136 Unit of Microbiology and Public Health, Institute of Medical Sciences, Las Tablas, Panama.
137 Department of Public Health, Ministry of Health, Herrera, Panama.
138 Department of Psychiatry and Behavioural Neurosciences, McMaster University, Hamilton, ON, Canada.
139 Department of Psychiatry, University of Lagos, Lagos, Nigeria.
140 Department of Biomedical Sciences, University of Novi Sad, Novi Sad, Serbia.
141 Henry M Jackson School of International Studies, University of Washington, Seattle, WA, USA.
142 Department of Project Management, National Research University Higher School of Economics, Moscow, Russia.
143 Department of Medicine, University of Ibadan, Ibadan, Nigeria.
144 Department of Medicine, University College Hospital Ibadan, Ibadan, Nigeria.
145 Department of Neurology and Public Health, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
146 Research & Development Department, Kalinga Institute of Medical Sciences, Bhubaneswar, India.
147 Department of Physiology, Iran University of Medical Sciences, Tehran, Iran.
148 Physiology Research Center, Iran University of Medical Sciences, Tehran, Iran.
149 Gerontology, Dementia and Digital Health, Independent Consultant, Copenhagen, Denmark.
150 Department of Chemistry, University of Porto, Porto, Portugal.
151 Department of Pharmacology, Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia.
152 Digestive Diseases Research Institute, Tehran University of Medical Sciences, Tehran, Iran.
153 Clinical Research Center, Valle del Lili Foundation (Centro de Investigaciones Clinicas, Fundación Valle del Lili), Cali, Colombia.
154 PROESA, ICESI University (Centro PROESA, Universidad ICESI), Cali, Colombia.
155 Health Sciences Department, Muhammadiyah University of Surakarta, Sukoharjo, Indonesia.
156 College of Medicine, University of Central Florida, Orlando, FL, USA.
157 Thalassemia and Hemoglobinopathy Research Center, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
158 Metabolomics and Genomics Research Center, Tehran University of Medical Sciences, Tehran, Iran.
159 Department of Cardiology, Emory University, Atlanta, GA, USA.
160 Department of Public Health, North South University, Dhaka, Bangladesh.
161 Department of Biostatistics and Epidemiology, University of Massachusetts Amherst, Amherst, MA, USA.
162 Tehran Institute of Psychiatry, Iran University of Medical Sciences, Tehran, Iran.
163 Department of Primary Care and Public Health, Imperial College London, London, UK.
164 Academic Public Health England, Public Health England, London, UK.
165 WHO Collaborating Centre for Public Health Education and Training, Imperial College London, London, UK.
166 University College London Hospitals, London, UK.
167 Research Center for Immunodeficiencies, Tehran University of Medical Sciences, Tehran, Iran.
168 Network of Immunity in Infection, Malignancy and Autoimmunity (NIIMA), Universal Scientific Education and Research Network (USERN), Tehran, Iran.
169 Department of Psychology, Royal Melbourne Institute of Technology University, Bundoora, VIC, Australia.
170 Department of Neuroscience, University of Perugia, Perugia, Italy.
171 Department of Neurology, Rimini ‘Infermi’ Hospital - AUSL Romagna, Rimini, Italy.
172 Golestan Research Center of Gastroenterology and Hepatology (GRCGH), Golestan University of Medical Sciences, Gorgan, Iran.
173 School of Psychiatry, University of New South Wales, Kensington, NSW, Australia.
174 Neuropsychiatric Institute, Prince of Wales Hospital, Randwick, NSW, Australia.
175 Halal Research Center of IRI, Food and Drug Administration of the Islamic Republic of Iran, Tehran, Iran.
176 Neurogenic Inflammation Research Center, Mashhad University of Medical Sciences, Mashhad, Iran.
177 Department of Public Health Sciences, University of North Carolina at Charlotte, Charlotte, NC, USA.
178 Market Access, Bayer, Istanbul, Turkey.
179 Center for Biomedical Information Technology, Shenzhen Institutes of Advanced Technology, Shenzhen, China.
180 Department of Internal Medicine, Ziauddin University, Karachi, Pakistan.
181 Independent Consultant, Karachi, Pakistan.
182 National Institute of Infectious Diseases, Tokyo, Japan.
183 College of Medicine, Yonsei University, Seoul, South Korea.
184 Finnish Institute of Occupational Health, Helsinki, Finland.
185 Department of Medicine, Dow University of Health Sciences, Karachi, Pakistan.
186 School of Medicine, University of Alabama at Birmingham, Birmingham, AL, USA.
187 Medicine Service, US Department of Veterans Affairs (VA), Birmingham, AL, USA.
188 Department of Ophthalmology, Gmers Medical College and Civil Hospital, Ahmedabad, India.
189 Department of Opthalmology, Datta Meghe Institute of Medical Sciences, Wardha, India.
190 Nursing Care Research Center, Semnan University of Medical Sciences, Semnan, Iran.
191 Faculty of Medicine, Dentistry and Health Sciences, University of Melbourne, Melbourne, VIC, Australia.
192 The Brain Institute, Australian Healthy Ageing Organisation, Melbourne, VIC, Australia.
193 Department of Medicine, University of Valencia, Valencia, Spain.
194 Carlos III Health Institute, Biomedical Research Networking Center for Mental Health Network (CiberSAM), Madrid, Spain.
195 Department of Pharmacy, Arbaminch College of Health Sciences, Arba Minch, Ethiopia.
196 Division of Biostatistics and Epidemiology, Texas Tech University, El Paso, TX, USA.
197 Massachusetts Veterans Epidemiology Research and Information Center (MAVERIC) & CSP Coordinating Center, Harvard University, Boston, MA, USA.
198 Department of Pathology and Legal Medicine, University of São Paulo, Ribeirão Preto, Brazil.
199 Modestum LTD, London, UK.
200 Department of Health Economics, Hanoi Medical University, Hanoi, Vietnam.
201 College of Medicine and Health Sciences, Bahir Dar University, Bahir Dar, Ethiopia.
202 Department of Internal Medicine, Dow University of Health Sciences, Karachi, Pakistan.
203 Argentine Society of Medicine, Buenos Aires, Argentina.
204 Velez Sarsfield Hospital, Buenos Aires, Argentina.
205 Raffles Neuroscience Centre, Raffles Hospital, Singapore, Singapore.
206 Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore.
207 Department of Health Care Administration and Economics, National Research University Higher School of Economics, Moscow, Russia.
208 Department of Psychiatry, University of São Paulo, São Paulo, Brazil.
209 Department of Adult Health Nursing, Aksum University, Aksum, Ethiopia.
210 Competence Center of Mortality-Follow-Up of the German National Cohort, Federal Institute for Population Research, Wiesbaden, Germany.
211 Global Health Research Center, Duke Kunshan University, Kunshan, China.
212 Duke Global Health Institute, Duke University, Durham, NC, USA.
213 Psychology Department, University of Sheffield, Sheffield, UK.
214 Research and Development Center for Health Services, University of Tsukuba, Tsukuba, Japan.
215 Graduate School of Medicine, Osaka University, Suita, Japan.
216 Department of Family Medicine and Community Health, Duke University, Durham, IL, USA.
217 Department of Nursing, Arba Minch University, Arba Minch, Ethiopia.
218 Department of Neuropsychopharmacology, National Center of Neurology and Psychiatry, Kodaira, Japan.
219 Department of Public Health, Juntendo University, Tokyo, Japan.
220 Department of Epidemiology and Biostatistics, Wuhan University, Wuhan, China.
221 Global Health Institute, Wuhan University, Wuhan, China.
222 School of Medicine, Wuhan University, Wuhan, China.
Footnotes
The ‘GBD 2019 Dementia Collaborators’ are listed in the online supplementary material www.karger.com/doi/10.1159/000515393.
Statement of Ethics
This GBD study used de-identified data, and the waiver of informed consent was reviewed and approved by the University of Washington Institutional Review Board (Study 9060).
References
- 1.Prince MJ. World Alzheimer report 2015: the global impact of dementia [Internet]. 2015. Aug [cited 2018 Apr 8]. Available from: https://www.alz.co.uk/research/world-report-2015.
- 2.Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement. 2013. Jan;9(9):63–e2. [DOI] [PubMed] [Google Scholar]
- 3.Nichols E, Szoeke CEI, Vollset SE, Abbasi N, Abd-Allah F, Abdela J, et al. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019. Jan;18(1): 88–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer’s disease. Alzheimers Dement. 2007. Jul;3(3):186–91. [DOI] [PubMed] [Google Scholar]
- 5.Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology. 2013. May;80(19):1778–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matthews KA, Xu W, Gaglioti AH, Holt JB, Croft JB, Mack D, et al. Racial and ethnic estimates of Alzheimer’s disease and related dementias in the United States (2015–2060) in adults aged ≥65 years. Alzheimers Dementia. 2019. Jan;15(1):17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waite LM. Treatment for Alzheimer’s disease: has anything changed? Aust Prescr. 2015. Apr;38(2):60–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020. Aug; 396(10248):413–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matthews FE, Brayne C, Lowe J, McKeith I, Wharton SB, Ince P. Epidemiological pathology of dementia: attributable-risks at death in the medical research council cognitive function and ageing study. PLoS Med. 2009. Nov; 6(6):e1000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brayne C, Richardson K, Matthews FE, Fleming J, Hunter S, Xuereb JH, et al. Neuropathological correlates of dementia in over-80-year-old brain donors from the population-based Cambridge city over-75s Cohort (CC75C) Study. J Alzheimers Dis. 2009. Jan; 18(3):645–58. [DOI] [PubMed] [Google Scholar]
- 11.Savva GM, Wharton SB, Ince PG, Forster G, Matthews FE, Brayne C. Age, neuropathology, and dementia. N Engl J Med. 2009. May; 360(22):2302–9. [DOI] [PubMed] [Google Scholar]
- 12.Barba R, Martínez-Espinosa S, Rodríguez-García E, Pondal M, Vivancos J, Del Ser T. Poststroke dementia: clinical features and risk factors. Stroke. 2000. Jul;31(7):1494–501. [DOI] [PubMed] [Google Scholar]
- 13.Nath A, Schiess N, Venkatesan A, Rumbaugh J, Sacktor N, Mcarthur J. Evolution of HIV dementia with HIV infection. Int Rev Psychiatry. 2008. Jan;20(20):25–31. [DOI] [PubMed] [Google Scholar]
- 14.Oliver C, Holland AJ. Down’s syndrome and Alzheimer’s disease: a review. Psychol Med. 1986. May;16(2):307–22. [DOI] [PubMed] [Google Scholar]
- 15.Lye TC, Shores EA. Traumatic brain injury as a risk factor for Alzheimer’s disease: a review. Neuropsychol Rev. 2000. Jun;10(2):115–29. [DOI] [PubMed] [Google Scholar]
- 16.Aarsland D, Zaccai J, Brayne C. A systematic review of prevalence studies of dementia in Parkinson’s disease. Mov Disord. 2005. Oct; 20(10):1255–63. [DOI] [PubMed] [Google Scholar]
- 17.Kuźma E, Lourida I, Moore SF, Levine DA, Ukoumunne OC, Llewellyn DJ. Stroke and dementia risk: a systematic review and metaanalysis. Alzheimers Dement. 2018. Nov; 14(14):1416–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wisniewski KE, Wisniewski HM, Wen GY. Occurrence of neuropathological changes and dementia of Alzheimer’s disease in down’s syndrome. Ann Neurol. 1985;17(3): 278–82. [DOI] [PubMed] [Google Scholar]
- 19.Hicks AJ, James AC, Spitz G, Ponsford JL. Traumatic brain injury as a risk factor for dementia and Alzheimer disease: critical review of study methodologies. J Neurotrauma. 2019. Aug;36(23):3191–219. [DOI] [PubMed] [Google Scholar]
- 20.Mukadam N, Sommerlad A, Huntley J, Livingston G. Population attributable fractions for risk factors for dementia in low-income and middle-income countries: an analysis using cross-sectional survey data. Lancet Glob Health. 2019. May;7(7):e596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization. The ICD-10 classification of mental and behavioural disorders: clinical descriptions and diagnostic guidlines. Geneva: World Health Organization; 1992. [Google Scholar]
- 22.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington D.C.; 2000. [Google Scholar]
- 23.Vos T, Lim SS, Abbafati C, Abbas KM, Abbasi M, Abbasifard M, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020. Oct;396(10258): 1204–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang H, Abbas KM, Abbasifard M, Abbasi-Kangevari M, Abbastabar H, Abd-Allah F, et al. Global, age-sex-specific fertility, mortality, healthy life expectancy (HALE) and population estimates in 204 countries and territories, 1950–2019: a comprehensive demographic analysis for the Global Burden of Disease Study 2019. Lancet. 2020. Oct;396(10258): 1160–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dams-O’Connor K, Guetta G, Hahn-Ketter AE, Fedor A. Traumatic brain injury as a risk factor for Alzheimer’s disease: current knowledge and future directions. Neurodegener Dis Manag. 2016;6(5):417–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perry DC, Sturm VE, Peterson MJ, Pieper CF, Bullock T, Boeve BF, et al. Association of traumatic brain injury with subsequent neurological and psychiatric disease: a metaanalysis. J Neurosurg. 2016. Feb;124(124): 511–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miettinen OS. Proportion of disease caused or prevented by a given exposure, trait or intervention. Am J Epidemiol. 1974. May;99(5): 325–32. [DOI] [PubMed] [Google Scholar]
- 28.Ballard C, Mobley W, Hardy J, Williams G, Corbett A. Dementia in Down’s syndrome. Lancet Neurol. 2016. May;15(15):622–36. [DOI] [PubMed] [Google Scholar]
- 29.Irwin DJ, White MT, Toledo JB, Xie SX, Robinson JL, Deerlin VV, et al. Neuropathologic substrates of Parkinson disease dementia. Ann Neurol. 2012;72(4):587–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson CO, Nguyen M, Roth GA, Nichols E, Alam T, Abate D, et al. Global, regional, and national burden of stroke, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019. May; 18(5):439–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kivipelto M, Ngandu T, Fratiglioni L, Viitanen M, Kåreholt I, Winblad B, et al. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol. 2005. Oct;62(10):1556–60. [DOI] [PubMed] [Google Scholar]
- 32.Gaziano TA. Reducing the growing burden of cardiovascular disease in the developing world. Health Aff. 2007. Jan–Feb;26(1):13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med. 2016;8(6):595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mandelkow EM, Mandelkow E. Tau in Alzheimer’s disease. Trends Cell Biol. 1998. Nov; 8(11):425–7. [DOI] [PubMed] [Google Scholar]
- 35.Spillantini MG, Schmidt ML, Lee VM-Y, Trojanowski JQ, Jakes R, Goedert M. Alphasynuclein in Lewy bodies. Nature. 1997. Aug; 388(6645):839–40. [DOI] [PubMed] [Google Scholar]
- 36.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007. Dec;69(69): 2197–204. [DOI] [PubMed] [Google Scholar]
- 37.James BD, Wilson RS, Boyle PA, Trojanowski JQ, Bennett DA, Schneider JA. TDP-43 stage, mixed pathologies, and clinical Alzheimer’s-type dementia. Brain. 2016. Nov;139(11): 2983–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murray CJL, Lopez AD. Measuring the global burden of disease. N Engl J Med. 2013. Aug; 369(5):448–57. [DOI] [PubMed] [Google Scholar]
- 39.Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007. Oct;69(18):1789–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Corraini P, Henderson VW, Ording AG, Pedersen L, Horváth-Puhó E, Sørensen HT. Long-term risk of dementia among survivors of ischemic or hemorrhagic stroke. Stroke. 2017;48(48):180–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


