Abstract
This systematic review aims to summarize cognitive reserve (CR) evaluation approaches and to examine the role of seven selected modifiable lifestyle factors (diet, smoking, alcohol consumption, physical activity, cognitive leisure activity, sleep, and meditation) in mitigating the impacts of age- or disease-related brain changes on cognition. Eighteen population-based English empirical studies were included. We summarize the study designs and identify three CR models that were broadly used in these studies, including a residual model assessing lifestyle factors in relation to unexplained variance in cognition after accounting for brain markers, a moderation model testing whether lifestyle factors moderate the relationship between brain status and cognition, and a controlling model examining the associations between lifestyle factors and cognition when controlling for brain measures. We also present the findings for the impact of each lifestyle factor. No studies examined diet, sleep, or meditation, and only two studies focused on smoking and alcohol consumption each. Overall, the studies suggest lifestyle activity factors (physical and cognitive leisure activities) may contribute to CR and attenuate the damaging impact of brain changes on cognition. Standardized measurements of lifestyle factors and CR are needed, and mechanisms underlying CR need to be further addressed as well.
Keywords: Cognitive reserve, modifiable lifestyle factors, brain markers, cognition
1. Introduction
Numerous previous systematic reviews and/or meta-analysis have found that various modifiable lifestyle factors, including diet (Gu and Scarmeas, 2011), smoking (Peters et al., 2008b), alcohol consumption (Peters et al., 2008a), physical activity (Carvalho et al., 2014), cognitive leisure activity (Fallahpour et al., 2016; Sajeev et al., 2016), sleep (Bubu et al., 2017) and meditation (Gard et al., 2014), were beneficial to the cognitive function in older adults (Baumgart et al., 2015; Law et al., 2014), although the underlying mechanisms remain unclear. One potential pathway may be that lifestyle factors supply cognitive reserve (CR) that mitigates the relationship between age- or disease-related brain changes and cognition (Clarke et al., 2012; Dik et al., 2007; Murphy and O’Leary, 2010; Scarmeas, 2007; Scarmeas and Stern, 2003; Scarmeas and Stern, 2004; Stern, 2012).
CR is the adaptability of cognitive process, referring to the brain’s ability to cope with changes due to age, pathology, or insult without developing cognitive impairment (Cheng, 2014; Stern et al., 2020; Tucker and Stern, 2011). Building up CR may help attenuate cognition decline and delay the onset of dementia and thus have important implications for dementia prevention (Cheng, 2014). Traditionally, studies have used certain proxies to represent CR, with the most common proxies including educational attainment and quality (Bennett et al., 2003; Fyffe et al., 2011; Meng and D’Arcy, 2012), occupational complexity, and general intelligence (Habeck et al., 2019; Scarmeas, 2007; Stern et al., 2020). The proxy role of these factors have been established through numerous studies that consistently show that they can moderate or attenuate the association between brain pathology and the clinical outcomes (Bennett et al., 2003; Perneczky et al., 2009); more pathology is required to manifest a clinically cognitive impairment for individuals with higher educational attainment, intelligence, or occupational complexity (Ewers et al., 2013; Hanyu et al., 2008; Morbelli et al., 2013); faster cognitive decline, probably due to a more advanced pathology, can be seen after diagnosis of Alzheimer’s disease (AD) in those with higher education compared with lower education (Andel et al., 2006; Conde-Sala et al., 2013; Scarmeas et al., 2006). Reed et al. proposed a more direct measure of CR as the unexplained variance (residuals) in cognition after taking demographic and brain markers into account, usually through regression models (Reed et al., 2010; Stern et al., 2020). A common estimation of residual is to compare the difference between the expected cognition given someone’s state of brain health and the observed cognition (Anaturk et al., 2021; Yao et al., 2020). Higher IQ and education have been shown to be related to such residuals (Habeck et al., 2017), thus adding another line of evidence supporting the measure of CR. To evaluate whether modifiable lifestyle factors can provide CR when coping with brain pathologies to slow the cognition decline, strong evidence similar to that found for the common proxies (education, premorbid intelligence) needs to be established.
As CR is rarely assessed directly, current studies used various approaches to measure CR and to evaluate the role of modifiable lifestyle factors in CR, which may result in some confusions about the reasonable CR measurements and how to apply them to research (Stern et al., 2020). Categorizing and summarizing the appropriate CR models for various lifestyle factors will assist in providing evidence to confirm whether a lifestyle factor promotes CR.
Thus, we undertook a review aiming to identify and summarize CR evaluation approaches and to examine the role of selected modifiable lifestyle factors in the associations between brain markers and cognition.
2. Methods
Our review was conducted based on the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) (Moher et al., 2015).
We systematically searched PubMed, Web of Science, Embase, PsycINFO and CINAHL databases on April 20, 2021 to identify the empirical studies assessing the role of lifestyle factors in the associations between brain and cognition. Based on the previous systematic reviews (Baumgart et al., 2015; Law et al., 2014), seven modifiable lifestyle factors which were testified to be associated with cognition were selected and analyzed in this study, including (1) diet (Gu and Scarmeas, 2011), (2) smoking (Peters et al., 2008b), (3) alcohol consumption (Peters et al., 2008a), (4) physical activity (Carvalho et al., 2014), (5) cognitive leisure activity (Fallahpour et al., 2016; Sajeev et al., 2016), (6) sleep (Bubu et al., 2017) and (7) meditation (Gard et al., 2014), among which the searching terms included “lifestyle*”, “life style*”, “diet*”, “nutrition*”, “nutrient*”, “vitamin*”, “fish*”, “meat*”, “vegetable*”, “food*”, “fatty acid*”, “smok*”, “tobacco*”, “drink*”, “alcohol*”, “beer*”, “liquor*”, “wine*”, “exercise*”, “physical activit*”, “physical inactivit*”, “sedentary”, “leisure activit*”, “cognitive activit*”, “sleep*” and “meditation*”. Besides these lifestyle factors, searching terms also included “cognitive reserve*”, “brain reserve*”, “cognitive resilience” and “brain resilience”. Free text words were applied to the searching strategy in every database; Medical Subject Headings (MESH) and Embase Subject Headings (Emtree) terms were also used when searching from PubMed and Embase, respectively. More detailed information of searching terms in each database is provided in Supplementary Table 1. To identify potential studies not captured by our searching strategy, we also searched studies listed in the references of relevant articles.
The search was limited to human studies published in English as well as empirical studies. Reviews and non-articles (e.g., books, book sections, generic, thesis, case reports, commentaries, editorials, perspectives, letters, conference abstracts) were excluded. We included studies that (1) included lifestyle factors of interest, brain markers and cognition; (2) examined the role of lifestyle factors in the relationship between brain markers and cognition (Figure 1).
Figure 1.
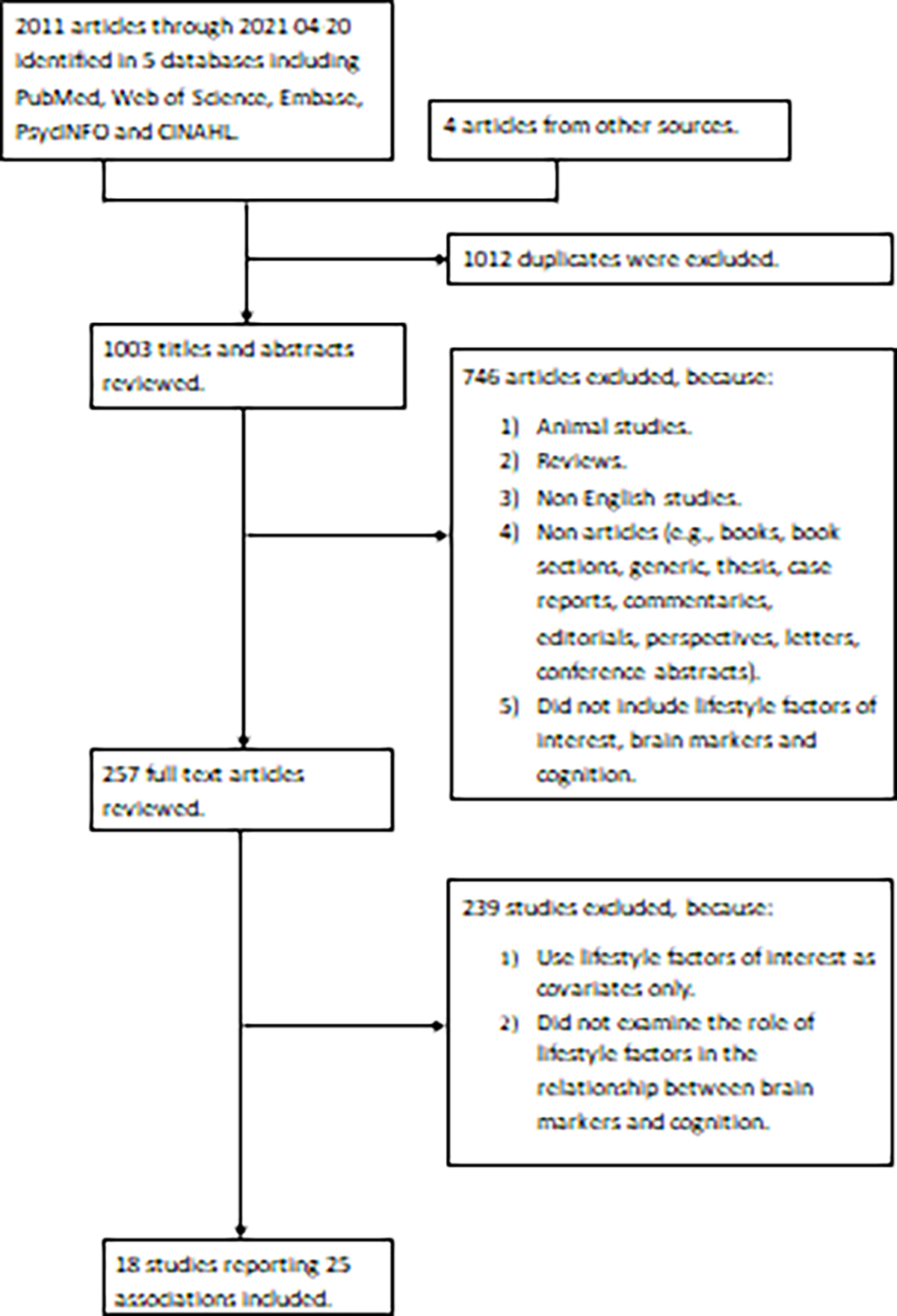
PRISMA diagram.
Duplicate citations were removed prior to screening. The study selection process was conducted in two steps. First, the first reviewer (SS) and the second reviewer (YG) reviewed the titles and abstracts of all retrieved citations independently, and assessed identified studies for inclusion, facilitated by grading each eligibility criterion as eligible/not eligible/might be eligible. Second, all citations deemed to be eligible/might be eligible by either reviewer at the title and abstract stage were pulled for full-text review. The two reviewers (SS and YG) independently reviewed all citations pulled for full-text review and disagreements were reconciled.
We developed a standardized data extraction form specifying key data elements to be extracted. Data items from articles that met inclusion and/or exclusion criteria were independently extracted by the first reviewer (SS), and the second reviewer (YG) checked the data for consistency, clarity, and accuracy independently. Data elements extracted included descriptive information about each study (study design, study objectives, country, characteristics and eligibility criteria of the subjects, sample size, datasets, statistical methods, CR model, outcome variables, cognition measurements, brain markers and their measurements, lifestyle factors and their measurements, covariates, comparators, key findings, study time, future directions), the basic information of the articles (ID, first author, publish year, title, abstract, keywords, journal) and decisions by reviewers. Records were managed through Endnote X8 and extracted through Excel.
The risk of bias and study quality were assessed from six bias domains, including study participation, attrition, measurements of prognostic factors (lifestyle factors, brain markers, and CR), measurement of outcome (cognition), confounding and statistical analysis and reporting, according to Quality in Prognosis Studies (QUIPS) tool, which is used for assessing the risk of bias in studies of prognostic factors (Daskalopoulou et al., 2017; Hayden et al., 2013; Kuiper et al., 2015).
3. Results
The search in five databases yielded a total of 2011 citations as of April 20, 2021 (Figure 1). An additional four studies were identified from the references of relevant articles. After eliminating the 1012 duplicates, 1003 articles remained for the title and abstract screening. Of these, 746 studies were excluded since they were animal studies, reviews, not written in English, not empirical studies or not including brain markers, cognition or lifestyle factors of interest. The 257 remaining articles were retrieved in full-text to be examined in more detail. Of these, 239 were ultimately excluded for not meeting other inclusion criteria. Eighteen studies met the inclusion criteria and were included in the review. Among them, four out of 18 studies included more than one lifestyle factor, resulting in a total of 25 associations of lifestyle factors in summarized CR models.
3.1. Cognitive reserve models
This study summarizes three CR models (Figure 2) that have been used most frequently in the previous studies, and which appropriately take into account the three necessary components: lifestyle factors, brain measures, and cognition (Reserve and Resilience Workshop). The first model, residual model, examines the associations between lifestyle factors and CR residuals (Anaturk et al., 2021; Negash et al., 2013; Reed et al., 2011; Yao et al., 2020). The second model, moderation model, tests whether lifestyle factors moderate the association between brain status and cognition with the expectation that the negative effect of the brain pathology or insult on cognitive impairment is smaller among those with healthier lifestyle behaviors under examination compare to those with unhealthier lifestyle behaviors (Amato et al., 2013; Bartres-Faz et al., 2009; Buchman et al., 2019; Casaletto et al., 2020a; Casaletto et al., 2020b; Chan et al., 2018; Chirles et al., 2017; Nunnari et al., 2016; Rouillard et al., 2017; Snitz et al., 2020; Sumowski et al., 2013). The last model, controlling model, examines the association between lifestyle factors and cognition while holding brain status constant (Borroni et al., 2009; Harris et al., 2015; Scarmeas et al., 2003; Sumowski et al., 2010; Xu et al., 2020; Xu et al., 2019), with the expectation of healthy lifestyle factors being associated with better cognition when there is a similar level of brain pathology or insult, thus directly fitting the concept of CR theory. A slight variation of this model is to examine the association between lifestyle factors with brain measures among individuals with the same cognitive abilities, with the expectation of individuals with healthier lifestyle factors can harbor worse brain status (more brain pathologies) when controlling for the cognition level. For example, previous evidence reported the more engagement in cognitive leisure activities is associated with more prominent cerebral blood flow deficits, accounting for the severity of AD among AD patients (Scarmeas et al., 2003).
Figure 2. Cognitive reserve mechanisms for the role of lifestyle factors in the associations between brain markers and cognition.
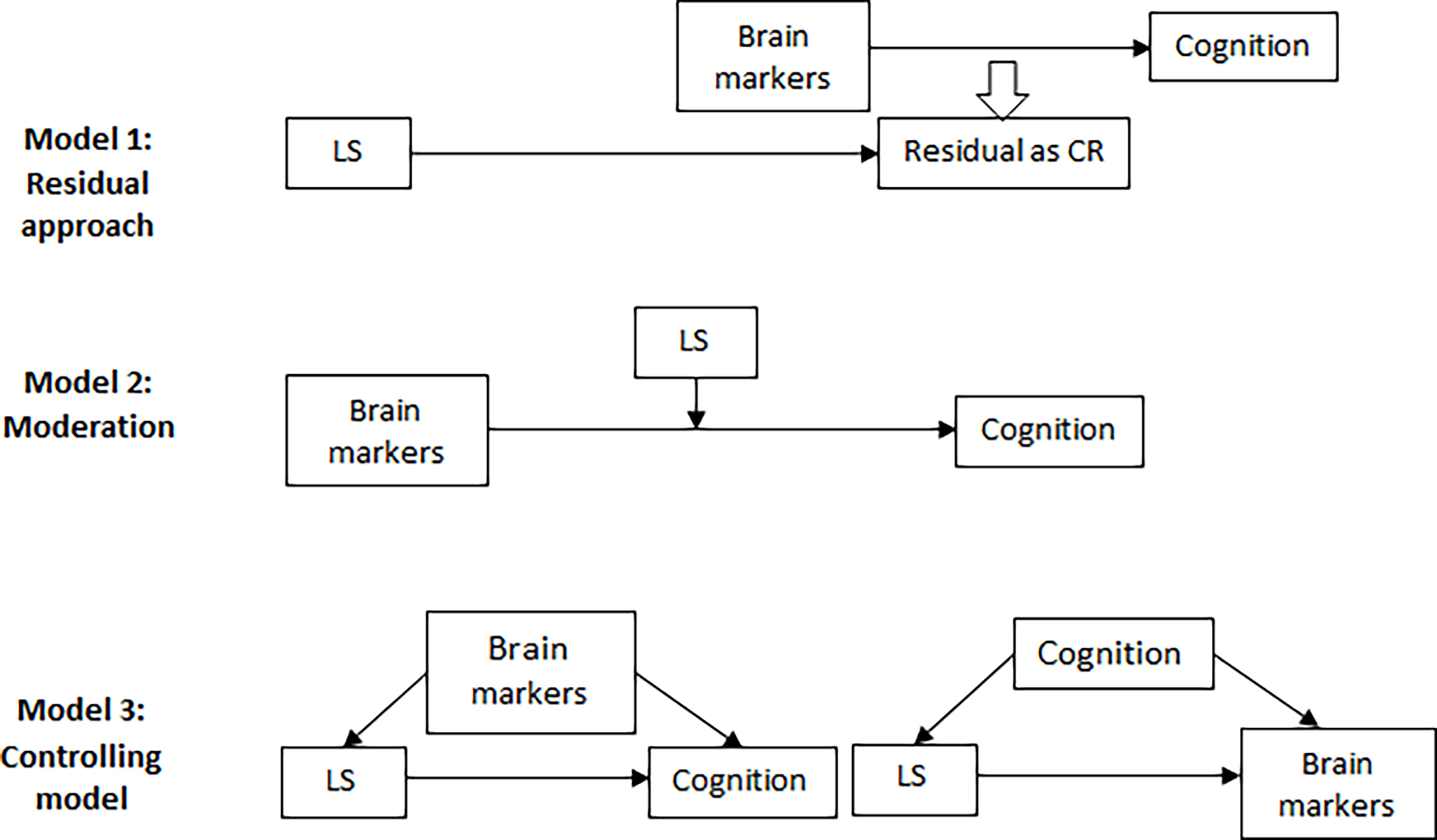
Abbreviations: LS: lifestyle factors; CR: cognitive reserve.
Note:
Model 1-Residual: analysing the associations between lifestyle factors and CR residuals, which are quantified as the unexplained variance in cognition after taking demographic and brain markers of cognition into account.
Model 2-Moderation: testing whether lifestyle factors moderate the association between brain status and cognition by examining the interaction between lifestyle factors and brain markers on cognition.
Model 3-Controlling model: conducting multivariable regressions with lifestyle factors as the predictors and cognition as the outcomes while accounting for brain markers, or regressing lifestyle factors with brain markers, controlling for cognition.
Besides the three models, some other approaches have also been used to examine whether a specific lifestyle factor could supply CR. But those approaches need to be interpreted with caution. For example, some studies estimated the association between lifestyle factors and a CR proxy (education or IQ) as a way to evaluate whether the lifestyle factor has CR capacities (Deary et al., 2006; Morales Ortiz and Fernandez, 2020), and some other studies directly explored a lifestyle factor’s association with cognition and interpret the lifestyle factors as a CR proxy (Power et al., 2018). However, such interpretation about CR may not be completely justifiable without in vivo biomarker imaging or postmortem data confirming neuropathology (Stern et al., 2020). In some other studies, CR proxies (e.g., education, IQ, occupational complexity) were combined together into a composite CR score and its association with cognition was then evaluated (Amato et al., 2013; Christensen et al., 2007; Clare et al., 2017; Consonni et al., 2020; Forcada et al., 2015; Harris et al., 2015; Nunnari et al., 2016; Xu et al., 2020; Xu et al., 2019). However, it is not able to distinguish the unique contributions of the individual component, even though it is still useful in some research or situations (Richards and Sacker, 2003; Stern et al., 2020). In another scenario, a composite CR score was used to test whether it mediated the association between lifestyle factors and cognition, which is not strictly aligned with the CR concept (Clare et al., 2017). Finally, the CR analysis was conducted among lifestyle factors, time to dementia diagnosis instead of brain measures, and cognitive outcomes (Hall et al., 2009; Helzner et al., 2007). Finally, the concept of CR has also been expanded to measure resilience in face of various chronic diseases or conditions such as obesity (Ihle et al., 2016), hearing loss (Chen and Lu, 2020), among others (Ihle et al., 2018; Ihle et al., 2017). In all these scenarios, an important limitation is the missing of the brain measure, which is one of the three necessary components for CR evaluation (Stern et al., 2020).
3.2. Overview of studies
Table 1 shows the summary of lifestyle factors and CR models. Among the seven selected lifestyle factors, the included studies most frequently focused on cognitive leisure activity (n=14), and physical activity (n=5), accounting for 76% (19/25) associations of lifestyle factors in the CR models, followed by multidomain lifestyle factors (n=2), smoking (n=2), and alcohol consumption (n=2). No studies on diet, sleep, or meditation were found after the systematically searching and the independent review. In terms of the three CR models, 10, nine and six out of the 25 associations were assessed by moderation analysis, residual approaches, and controlling models, respectively. The methodological features and major results by lifestyle factors are summarized in Table 2.
Table 1.
Summary of three models used for testing the role of lifestyle factors in the associations between brain markers and cognition.
| Lifestyle factors | Residual | Moderation | Controlling model | Total |
|---|---|---|---|---|
|
| ||||
| Diet | 0 | 0 | 0 | 0 |
| Smoking | 2 | 0 | 0 | 2 |
| Alcohol consumption | 2 | 0 | 0 | 2 |
| Physical activity | 2 | 3 | 0 | 5 |
| Cognitive leisure activity | 2 | 6 | 6 | 14 |
| Sleeping | 0 | 0 | 0 | 0 |
| Meditation | 0 | 0 | 0 | 0 |
| Multidomain lifestyle factors | 1 | 1 | 0 | 2 |
| Total | 9 | 10 | 6 | 25 |
Note:
Model 1-Residual: analysing the associations between lifestyle factors and CR residuals, which are quantified as the unexplained variance in cognition after taking demographic and brain markers of cognition into account.
Model 2-Moderation: testing whether lifestyle factors moderate the association between brain status and cognition by examining the interaction between lifestyle factors and brain markers on cognition.
Model 3-Controlling model: conducting multivariable regressions with lifestyle factors as the predictors and cognition as the outcomes while accounting for brain markers, or regressing lifestyle factors with brain markers, controlling for cognition.
Table 2.
The methodological features and major results by lifestyle factors and cognitive reserve models.
| Author (year) | Country | Study design | Sample size | Population | Dataset | Measurement of lifestyle factors | Brain markers | Cognition | CR model | CR hypothesis |
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Smoking | ||||||||||
| Anatürk et al. (2021) | UK, London | Cohort | 537 | Aging | Whitehall II imaging substudy | Never having smoked and former/current smokers | Gray matter and white matter features | Cognitive battery | Residual | Smoking was not significantly associated with cognitive age gap (CAG) (machine learning metric of how much each individual’s cognition deviates from healthy aging patterns). |
| Yao et al. (2020) | US | Cohort | 980 | Deceased AD patients | Rush Memory and Aging Project (MAP) and Religious Orders Study (ROS) | Never having smoked and former/current smokers | AD pathology | Global cognition estimated from a cognitive battery | Residual | Smoking was not significantly associated with AD-CR score (the CR residual). |
| Alcohol consumption | ||||||||||
| Anatürk et al. (2021) | UK, London | Cohort | 537 | Aging | Whitehall II imaging substudy | Total units of alcohol consumed on a weekly basis (over the last year) and the frequency. | Gray matter and white matter features | Cognitive battery | Residual | Alcohol consumption was not significantly associated with cognitive age gap (machine learning metric of how much each individual’s cognition deviates from healthy aging patterns). |
| Yao et al. (2020) | US | Cohort | 980 | Deceased AD patients | Rush Memory and Aging Project (MAP) and Religious Orders Study (ROS) | The average grams or number of alcoholic drinks consumed per day. | AD pathology | Global cognition estimated from a cognitive battery | Residual | Alcohol consumption was not significantly associated with AD-CR score (the CR residual). |
| Physical activity (PA) | ||||||||||
| Anatürk et al. (2021) | UK, London | Cohort | 537 | Aging | Whitehall II imaging substudy | Binary variable of whether the participants reported at least 2.5 h of moderate to vigorous intensity activities per week | Gray matter and white matter features | Cognitive battery | Residual | PA was not significantly associated with cognitive age gap (machine learning metric of how much each individual’s cognition deviates from healthy aging patterns). |
| Yao et al. (2020) | US | Cohort | 980 | Deceased AD patients | Rush Memory and Aging Project (MAP) and Religious Orders Study (ROS) | Physical activity scale | AD pathology | Global cognition estimated from a cognitive battery | Residual | PA was not significantly associated with AD-CR score (the CR residual). |
| Buchman et al. (2019) | US | Cross-sectional | 454 | Deceased aging | Rush Memory and Aging Project (MAP) | Continuously 24 h per day of the daily physical activities for up to 10 days | AD pathology, Lewy bodies, nigral neuronal loss, TAR DNA-binding protein 43, hippocampal sclerosis, macroinfarcts, microinfarcts, atherosclerosis, arteriolosclerosis, and cerebral amyloid angiopathy | Global cognition | Moderation | Moderation: with the exception of atherosclerosis, the association of the other 9 indices of pathology with global cognition did not vary with the levels of physical activity. Without interactions, the higher level of physical activity was significantly associated with higher levels of cognition, after controlling for the 10 brain pathologies. |
| Casaletto et al. (2020a) | US, Bay area | Cross-sectional | UCSF 344, UCD 485 | Aging | Two independent study cohorts from University of California, San Francisco (UCSF) and Davis (UCD) | Physical activity scale | Global fractional anisotropy, white matter hyperintensities (WMH) | Global cognition | Moderation | Within the UCSF sample, PA was not significantly moderated the relationship between global fractional anisotropy and cognitive performance; while in the UCD sample, PA attenuated the association between white matter hyperintensities and cognition. |
| Casaletto et al. (2020b) | US | Cohort | 174 | Frontotemporal lobar degeneration (FTLD) patients | Longitudinal Evaluation of Familial Frontotemporal Dementia Subjects (LEFFTDS) or Advancing Research and Treatment in Frontotemporal Lobar Degeneration (ARTFL) studies | Physical activity scale | Frontotemporal volume | Global cognition, processing speed, episodic memory and executive functioning | Moderation | Baseline: PA significantly moderated the relationship between frontotemporal volume and global cognition among mutation carriers with FTLD, indicating MCs with higher reported PA showed a significantly attenuated relationship between frontotemporal volumes and clinical functioning compared to their less active peers; not significantly moderated volume and processing speed, episodic memory and executive functioning. Longitudinal: PA significantly moderated frontotemporal atrophy on changes in global cognition and processing speed; not significantly moderated volume and episodic memory and executive functioning. |
| Cognitive leisure activity (CLA) | ||||||||||
| Negash et al. (2013) | US | Cohort | 966 | Deceased aging | Rush Memory and Aging Project (MAP) and Religious Orders Study (ROS) | Cognitive leisure activity scale | Global pathology (neuritic plaques, diffuse plaques, and neurofibrillary tangles) | Global cognition | Residual | Individuals with more frequent participation in cognitive leisure activities showed higher resilience (residual of global cognition variable on global pathology). |
| Reed et al. (2011) | US | Cross-sectional | 652 | Deceased aging | Rush Memory and Aging Project (MAP) and Religious Orders Study (ROS) | Cognitive leisure activity scale | Neuritic plaques, diffuse plaques, neocortical neurofibrillary tangles, medial temporal neurofibrillary tangles, and brain weight as well as single summary measures of Lewy bodies, chronic microscopic infarctions, and macroinfarcts. | Global cognition | Residual | CA at 40 years and CA at baseline had the strongest effects on Reserve (global cognitive residual) with statistical significance. |
| Amato et al. (2013) | Italy | Cohort | 52 | Multiple sclerosis patients | First handed | Cognitive Reserve Index | Normalized cortical volume (NCV) | Cognitive battery | Moderation | Baseline: an interaction between CRI and NCV predicted better verbal memory and attention/information processing speed significantly. Longitudinal: the progression of cortical atrophy (change in NCV) remained the only predictor of deteriorating cognitive performance. |
| Casaletto et al. (2020a) | US, Bay area | Cross-sectional | UCSF 344, UCD 485 | Aging | Two independent study cohorts from University of California, San Francisco (UCSF) and Davis (UCD) | Cognitive leisure activity scale | Global fractional anisotropy, white matter hyperintensities (WMH) | Cognitive performance | Moderation | Within the UCSF sample, CA was significantly moderated the relationship between global fractional anisotropy and cognitive performance, such that adults with greater CA demonstrated disproportionately better global cognition given their white matter integrity compared to less active peers; while in the UCD sample, CA was not significantly moderated the relationship between white matter hyperintensities and global cognitive performances. |
| Casaletto et al. (2020b) | US | Cohort | 174 | Frontotemporal lobar degeneration (FTLD) patients | Longitudinal Evaluation of Familial Frontotemporal Dementia Subjects (LEFFTDS) or Advancing Research and Treatment in Frontotemporal Lobar Degeneration (ARTFL) studies | Cognitive leisure activity scale | Frontotemporal volume | Global cognition, processing speed, episodic memory and executive functioning | Moderation | Baseline: CA significantly moderated the relationship between frontotemporal volume and global cognition, episodic memory and executive functioning among mutation carriers with FTLD, indicating MCs with higher reported CA showed a significantly attenuated relationship between frontotemporal volumes and clinical functioning compared to their less active peers; not significantly moderated volume and processing speed. Longitudinal: CA significantly moderated frontotemporal atrophy on changes in episodic memory; not significantly moderated volume and other clinical outcomes. |
| Nunnari et al. (2016) | Italy | Cross-sectional | 66 | Multiple sclerosis patients | First handed | Cognitive Reserve Index | Normalized cortical volume (NCV) | Cognitive battery | Moderation | The interaction between NCV and CRI did not account for significant variance. |
| Rouillard et al. (2017) | UK | Cross-sectional | 96 | Aging | First handed | Cognitive leisure activity scale | Level of brain atrophy | Global cognition, visuospatial perception, verbal episodic memory, processing and perceptual speed, and executive function | Moderation | In healthy participants: Concerning cognitive leisure activities at various levels of brain atrophy, the only effect that remains significant from the 75th percentile is on attention/perceptual speed, and no more effect was observed for executive processes. In the Parkinson’s disease group, no significant associations. |
| Sumowski et al. (2013) | US | Cross-sectional | 62 | Multiple sclerosis patients | First handed | Cognitive leisure activity scale | T2 lesion load | Global cognition | Moderation | The interaction between T2 lesion load and cognitive leisure activities was significant, with greater cognitive leisure activities moderating/attenuating the negative impact of T2 lesion load on cognitive status. |
| Borroni et al. (2009) | Italy | Cross-sectional | 54 | Frontotemporal dementia (FTD) patients | First handed | Cognitive leisure activity scale | Regional cerebral blood flow (rCBF) | FTD-modified Clinical Dementia Rating (FTD-modified CDR) | Controlling model | LA was not correlated with rCBF, controlling for age and gender and for FTD-modified CDR. |
| Harris et al. (2015) | Argentina | Cross-sectional | 33 | Aging | Argentina Alzheimer’s Disease Neuroimaging Initiative (ADNI) database | Cognitive Reserve Index | Cerebrospinal fluid levels, levels of Aβ1–42. | Cognitive battery | Controlling model | Higher levels of CSF Aβ31–42 were associated with higher CRQ scores, controlling for cognitive status among MCI patients. |
| Scarmeas et al. (2003) | US | Cross-sectional | 25 | Aging | First handed | Cognitive Reserve Index | Cerebral blood flow (CBF) | Cognitive battery | Controlling model | LA was inversely correlated with CBF when controlling for certain socio-demographic variables and mMMS score among AD patients. Significant associations were localized mainly to the temporal lobe but also in temporal-parietal-occipital association areas. With the exception of 2 voxels (1 in the cerebellum, and 1 in the lentiform nucleus), no significant associations were detected for the controls. |
| Sumowski et al. (2010) | US | Cross-sectional | 36 | Multiple sclerosis patients | First handed | Cognitive leisure activity scale | Third ventricle width (TVW) | Cognitive battery | Controlling model | There was a positive partial correlation between premorbid cognitive leisure and current cognitive status controlling for brain atrophy, estimated by TVW. |
| Xu et al. (2020) | US | Cohort | 1602 | Aging | Rush Memory and Aging Project (MAP) | Cognitive Reserve Index | Global Alzheimer’s disease (AD) pathology burden, including amyloid beta protein and neurofibrillary tangles; chronic infarcts, including gross infarcts and microinfarcts; cerebral vascular disease pathology, including atherosclerosis, arteriolosclerosis, and cerebral amyloid angiopathy; Lewy bodies; and typical hippocampal sclerosis were assessed as present or absent. | Dementia, AD-related dementia, and mild cognitive impairment | Controlling model | Compared to the lowest CR indicator, the association of highest CR indicator with MCI and dementia risk remained significant after further adjustment for gross infarcts and global AD pathology. |
| Xu et al. (2019) | US | Cohort | 1602 | Aging | Rush Memory and Aging Project (MAP) | Cognitive Reserve Index | Global Alzheimer’s disease (AD) pathology burden, including amyloid beta protein and neurofibrillary tangles; chronic infarcts, including gross infarcts and microinfarcts; cerebral vascular disease pathology, including atherosclerosis, arteriolosclerosis, and cerebral amyloid angiopathy; Lewy bodies; and typical hippocampal sclerosis were assessed as present or absent. | Dementia, AD-related dementia, and mild cognitive impairment | Controlling model | The highest CR score tertile was significantly associated with a reduction in risk of dementia and AD-related dementia compared with the lowest CR score tertile controlling for global AD pathology and other brain pathologies. |
| Multidomain lifestyle factors | ||||||||||
| Anatürk et al. (2021) | UK, London | Cohort | 537 | Aging | Whitehall II imaging substudy | Lifestyle index | Gray matter and white matter features | Cognitive battery | Residual | Cumulative lifestyle index was not significantly associated with cognitive age gap (CAG) (machine learning metric of how much each individual’s cognition deviates from healthy aging patterns). |
| Chan et al. (2018) | UK | Cross-sectional | 205, 196 with MRI | Aging | Cambridge Centre for Ageing and Neuroscience cohort | Lifestyle index | Total gray matter volume (TGM) | Cognitive ability | Moderation | The moderating term of an interaction between TGM and MAs was negative, meaning that the relationship between cognitive ability and brain health diminished with higher MA. |
3.2.1. Smoking
Two cohort studies focused on the impact of smoking on the relationship between brain markers and cognition using the residual model (Anaturk et al., 2021; Yao et al., 2020). One study includes cognitively intact participants in Whitehall II imaging substudy (Anaturk et al., 2021) and the other study includes deceased AD patients in Rush Memory and Aging Project and Religious Orders Study (Yao et al., 2020). The measures of smoking were consistent across the two studies, which was a self-reported smoking status, including never having smoked, former or current smokers. One study estimated the residual between a cognitive score from a neuropsychological battery and MRI-measured grey/white matter structural features, and defined CR as the difference between the cognitive age and chronological age (Anaturk et al., 2021), while the other study estimated the residual between pre-mortem global cognition and AD pathology burden (neuritic plaques, diffuse plaques, and neurofibrillary tangles) among deceased AD patients, and defined CR as the difference between observed cognition and expected cognition (Yao et al., 2020).
Both studies found that the associations between smoking status and CR residuals were not statistically significant (Anaturk et al., 2021; Yao et al., 2020). However, despite the different construction of CR residuals, both studies found that the current smokers tended to be more likely than former smokers or non-smokers to have a worse-observed cognition than expected cognition (Anaturk et al., 2021; Yao et al., 2020).
3.2.2. Alcohol consumption
The above two studies also examined alcohol consumption (Anaturk et al., 2021; Yao et al., 2020). Self-reported frequency of alcohol consumption and the average grams or number of alcoholic drinks consumed were used to measure alcohol consumption.
Both studies reported no significant associations between alcohol consumption and CR residual scores (Anaturk et al., 2021; Yao et al., 2020). However, both studies reported that people with more alcohol consumption tended to have a better-observed cognition than expected cognition (Anaturk et al., 2021; Yao et al., 2020).
3.2.3. Physical activity
Of the five studies, four in the US (Buchman et al., 2019; Casaletto et al., 2020a; Casaletto et al., 2020b; Yao et al., 2020) and one in the UK (Anaturk et al., 2021), examined physical activity (Anaturk et al., 2021; Buchman et al., 2019; Casaletto et al., 2020a; Casaletto et al., 2020b; Yao et al., 2020). Among them, two are embedded in cross-sectional studies (Buchman et al., 2019; Casaletto et al., 2020a) and three are longitudinal studies (Anaturk et al., 2021; Casaletto et al., 2020b; Yao et al., 2020). The sample size of the five studies ranged from 174 to 980. Special populations were included in one study—subjects aged about 50 years with frontotemporal lobar degeneration (FTLD) (Casaletto et al., 2020b). Among the other four studies, two included older adults who were dementia-free (Anaturk et al., 2021; Casaletto et al., 2020a) and two studies used postmortem data (Buchman et al., 2019; Yao et al., 2020).
The subjective measure was used in four studies to estimate a total physical activity score, which was summed based on the self-reported engagement in activities and the length or frequency of each activity (Anaturk et al., 2021; Casaletto et al., 2020a; Casaletto et al., 2020b; Yao et al., 2020). In one study, daily physical activities were objectively measured using an omnidirectional accelerometer (Buchman et al., 2019).
The findings of the role of physical activity varied by CR models. Two studies with residual approaches did not find any significant associations (Anaturk et al., 2021; Yao et al., 2020); three studies with moderation model reported both significant and nonsignificant results (Buchman et al., 2019; Casaletto et al., 2020a; Casaletto et al., 2020b). Specifically, it was found that individuals with greater physical activity demonstrated an attenuated relationship between various brain pathological markers, including frontotemporal volume (Casaletto et al., 2020b), white matter hyperintensities (Casaletto et al., 2020a), and atherosclerosis (but not with other brain pathologies) (Buchman et al., 2019), and cognition, compared to their low activity peers. However, the moderating impact of physical activity on the association between other brain pathologies (e.g., AD pathology, Lewy bodies, Nigral neuronal loss) and other cognition-related outcomes (e.g., processing speed, episodic memory) among cognitively intact older adults did not reach statistical significance (Buchman et al., 2019; Casaletto et al., 2020a; Casaletto et al., 2020b).
One study using post-mortem data (Buchman et al., 2019) found physical activity was positively associated with global cognition after controlling for AD pathology and other common brain pathologies, suggesting that physical activity may provide CR to maintain cognitive function among people with similar burdens of brain pathologies (Buchman et al., 2019).
3.2.4. Cognitive leisure activity
Five longitudinal studies (Amato et al., 2013; Casaletto et al., 2020b; Negash et al., 2013; Xu et al., 2020; Xu et al., 2019) and nine cross-sectional studies (Borroni et al., 2009; Casaletto et al., 2020a; Harris et al., 2015; Nunnari et al., 2016; Reed et al., 2011; Rouillard et al., 2017; Scarmeas et al., 2003; Sumowski et al., 2013; Sumowski et al., 2010) on cognitive leisure activities were conducted in the US (n=9) (Casaletto et al., 2020a; Casaletto et al., 2020b; Negash et al., 2013; Reed et al., 2011; Scarmeas et al., 2003; Sumowski et al., 2013; Sumowski et al., 2010; Xu et al., 2020; Xu et al., 2019), Italy (n=3) (Amato et al., 2013; Borroni et al., 2009; Nunnari et al., 2016), Argentina (n=1) (Harris et al., 2015) and the UK (n=1) (Rouillard et al., 2017) with a sample size ranging from 25 to 1602. Special populations were targeted in six studies: patients with multiple sclerosis (MS) (n=4/5) (Amato et al., 2013; Nunnari et al., 2016; Sumowski et al., 2013; Sumowski et al., 2010) and frontotemporal dementia (FTD) (n=2/5) (Borroni et al., 2009; Casaletto et al., 2020b); six studies focused on cognitively intact older adults (Casaletto et al., 2020a; Harris et al., 2015; Rouillard et al., 2017; Scarmeas et al., 2003; Xu et al., 2020; Xu et al., 2019); the other two studies with the residual model were post-mortem studies (Negash et al., 2013; Reed et al., 2011).
Cognitive leisure activities include social, intellectual, and cultural activities, such as reading books, magazines or newspapers, producing art, producing nonartistic writing, playing a musical instrument, playing structured games, participating in hobbies, watching television or listening to the radio, going to movies or restaurants or sporting events, complex cooking, walking for pleasure or excursion, physical conditioning, visiting/being visited by friends or relatives, doing unpaid community volunteer work, going to a club, library, museum or center, going to church or synagogue or temple, learning new skills and using the computer (Borroni et al., 2009; Casaletto et al., 2020a; Casaletto et al., 2020b; Negash et al., 2013; Reed et al., 2011; Rouillard et al., 2017; Sumowski et al., 2013; Sumowski et al., 2010). Eight studies measured cognitive leisure activity with questionnaires including 7–25 items, asking whether the participants participated in cognitive leisure activities throughout their life and/or the frequency of the participation (Borroni et al., 2009; Casaletto et al., 2020a; Casaletto et al., 2020b; Negash et al., 2013; Reed et al., 2011; Rouillard et al., 2017; Sumowski et al., 2013; Sumowski et al., 2010). For the remaining six studies, a composite CR score was calculated based on several CR proxies, including cognitive leisure activity, education, IQ, working activity, multilingualism, social activity and social network in late life (Amato et al., 2013; Harris et al., 2015; Nunnari et al., 2016; Scarmeas et al., 2003; Xu et al., 2020; Xu et al., 2019).
All three CR models were used to examine the CR potential of cognitive leisure activity. Two residual studies reported significant positive associations between past cognitive leisure activity and global cognitive residual over and beyond what can be explained by neuropathology (e.g., neuritic plaques, diffuse plaques, and neurofibrillary tangles) (Negash et al., 2013; Reed et al., 2011).
Moderation analysis was used in six studies each to examine the role of cognitive leisure activity, reporting mixed findings. Four out of six moderation studies reported that cognitive leisure activity significantly moderated the relationships between global brain atrophy and attention/perceptual speed among cognitively intact participants who obtained the top 25% scores in the Symbol Digit Modality Test, a test for attention/perceptual speed (Rouillard et al., 2017), between disease burden (T2 lesion load) and cognitive status (cognitive efficiency and memory composites) among MS patients (Sumowski et al., 2013), between white matter tract integrity and global cognitive performance among dementia-free older adults (Casaletto et al., 2020a), and between frontotemporal volume and global cognition, episodic memory and executive functioning among mutation carriers with FTLD (Casaletto et al., 2020b), indicating that adults with greater cognitive leisure activity significantly attenuated the relationship between brain markers and cognition compared to those with less cognitive leisure activity. However, cognitive leisure activity did not significantly moderate the relationship between white matter hyperintensities and global cognition (Casaletto et al., 2020a) or between frontotemporal volume and processing speed, between brain atrophy and any other neuropsychological clinical outcomes (i.e., global cognition, episodic memory, executive functioning and processing speed) among mutation carriers with FTLD (Casaletto et al., 2020b). It is worth noting that the remaining two moderation studies among Italian MS patients reported inconsistent findings—one found participants with greater composite CR scores significantly attenuated the relationship between normalized cortical volume (NCV) and verbal memory and attention/information processing speed, compared to those with lower composite CR scores (Amato et al., 2013), while the other study reported no impact of the composite CR score on the relationship between NCV and cognitive status (Nunnari et al., 2016), despite that they measured cognitive leisure activity, cognitive function and NCV in a similar way and both of them were analyzed among Italian MS patients. Both studies summarized the CR index including cognitive leisure activity and education, but one also included working activities (Nunnari et al., 2016) and the other one included premorbid IQ (Amato et al., 2013). Thus, the authors interpreted that the inconsistent findings could be due to the different calculations of the CR index (Nunnari et al., 2016).
Among the six controlling models, one reported a significant positive association between cognitive leisure activity and cognition controlling for brain atrophy (third ventricle width) among MS patients (Sumowski et al., 2010); two reported that a higher level of the composite CR score including cognitive leisure activities was associated with reduced risk of MCI after controlling for global AD pathology and gross infarcts (Xu et al., 2020), and with reduced risks of dementia or AD-related dementia after controlling for global AD pathology and other brain pathologies (e.g., gross infarcts, and microscopic infarcts) (Xu et al., 2019). Two studies among older adults reported significant correlations between composite CR scores and brain markers—one reported a higher level of the composite CR scores was associated with a higher level of Amyloid β1–42 controlling for cognitive status among mild cognitive impairment (MCI) patients (Harris et al., 2015), and the other one reported that a higher composite CR score was associated with more prominent cerebral blood flow deficits (localized mainly to the temporal lobe and temporal-parietal-occipital areas) controlling for modified Mini-Mental State examination among AD patients (Scarmeas et al., 2003). However, one study among FTD patients reported no correlation between cognitive leisure activities and regional cerebral blood flow, controlling for cognitive performance (Borroni et al., 2009).
3.2.5. Multidomain lifestyle factors
One cohort study (Anaturk et al., 2021) and one cross-sectional study (Chan et al., 2018) of multidomain lifestyle factors were conducted in the UK (Anaturk et al., 2021; Chan et al., 2018). The sample size of those studies ranged from 205 (Chan et al., 2018) to 537 (Anaturk et al., 2021). The two studies estimated a lifestyle index including multiple lifestyle factors of interest—one study included smoking, alcohol consumption and physical activity (Anaturk et al., 2021); the other one focused on lifestyle activities including social, intellectual and physical activities measured by Lifetime of Experiences Questionnaire (Chan et al., 2018).
One study using the residual approach observed no relationship of the multidomain lifestyle with cognitive residuals over and beyond grey/white matter features (Anaturk et al., 2021). However, the other study showed that multidomain lifestyle factors attenuated the association between grey matter volumes and cognitive ability (Chan et al., 2018).
3.2.6. Summary of the results
Table 3 summarizes the current evidence concerning the role of each lifestyle factor on CR. Studies focused on cognitive leisure activity in residual model provided strong evidence, with consistent findings from well-designed studies, that cognitive leisure activity had the capacity to supply CR, and studies of cognitive leisure activity in moderation and controlling models provided emerging evidence that cognitive leisure activity was associated with cognition accounting for certain brain markers. Emerging evidence supports that physical activity and multidomain lifestyle factors may also help maintain specific cognitions in face of certain brain markers. With limited evidence, smoking and alcohol consumption may not influence the CR residuals. We did not find any studies examining diet, sleep or meditation.
Table 3.
Current evidence concerning the role of lifestyle factors in the associations between brain markers and cognition.
| Lifestyle factors | Residual | Moderation | Controlling model | Total |
|---|---|---|---|---|
|
| ||||
| Diet | d | d | d | d |
| Smoking | c | d | d | c |
| Alcohol consumption | c | d | d | c |
| Physical activity | c | b | d | b |
| Cognitive leisure activity | a | b | b | b |
| Sleeping | d | d | d | d |
| Meditation | d | d | d | d |
| Multidomain lifestyle factors | c | b | d | b |
Note:
Model 1-Residual: analysing the associations between lifestyle factors and CR residuals, which are quantified as the unexplained variance in cognition after taking demographic and brain markers of cognition into account.
Model 2-Moderation: testing whether lifestyle factors moderate the association between brain status and cognition by examining the interaction between lifestyle factors and brain markers on cognition.
Model 3-Controlling model: conducting multivariable regressions with lifestyle factors as the predictors and cognition as the outcomes while accounting for brain markers, or regressing lifestyle factors with brain markers, controlling for cognition.
Strong evidence (i.e., evidence from well-designed studies with consistent findings).
Emerging evidence (i.e., less consistent findings).
Limited evidence with no significance (i.e., limited number of studies reporting no significant associations).
No evidence (i.e., no studies).
3.3. Bias and Quality of Studies
Based on the six domains included in the QUIPS tool, five out of 18 studies reviewed here were assessed as having moderate risk of bias and 13 as low, indicating that the quality of the included studies was high (Table 4). Study participation and attrition domains were reported to have the most prompting items with moderate and/or high risk of bias. Ten out of 18 studies were evaluated as having moderate risk of bias in the participation domain, since eight studies included small sample size, with less than 100 subjects, and 10 studies didn’t report the period and place of recruitment of participants, such analysis and findings may not be stable, reliable and comparable. In the attrition domain, seven out of 18 studies were cohort studies, with three and four studies reporting moderate and high risk of bias, respectively, regarding the lack of information on the participants lost to follow-up, which may be associated with the effect of lifestyle factors on cognition in face of the brain markers. Among the seven cohort studies, two included only a few follow-ups within two years (Amato et al., 2013; Casaletto et al., 2020b). The short follow-up timeframe of these two studies may limit the ability to investigate the role of lifestyle factors in the relationship between brain markers and cognition, so those studies may not provide informative evidence (Fratiglioni et al., 2004). The remaining 11 studies were cross-sectional studies, which cannot supply causal inferences, but rather can help identify potential associations to be further explored in future longitudinal studies. In terms of the prognostic factor measurement domain, with the exception of one study focusing on physical activity with an objective measurement (Buchman et al., 2019), all the other 17 studies measured lifestyle factors with self-reported data, which could result in recall and response biases, especially for some questions inquiring information at a younger age or some questions answered by informants on behalf of the study participants (Reed et al., 2011; Rouillard et al., 2017; Xu et al., 2020; Xu et al., 2019). The measures for each lifestyle factor and CR varied across studies, which may contribute to the inconsistent findings (Nunnari et al., 2016). Controlling for relevant variables is also a crucial issue, as many factors (e.g., age, sex, occupation) may be related to lifestyle behaviors and such factors may also be associated with brain markers and cognition. In the analysis, all studies controlled for demographics and/or education; some of the studies controlled for occupation attainment and medical comorbidities or health status; two studies didn’t control for any of these potential confounders (Harris et al., 2015; Rouillard et al., 2017). Outcome measurement and statistical analysis domains were assessed as having low risk of bias among all the included studies.
Table 4.
Methodological quality assessment per quality item and per study, according to the Quality of Prognosis Studies in Systematic Reviews (QUIPS).
| Bias domains and Prompting items | Amato et al. (2013) | Anatürk et al. (2021) | Borroni et al. (2009) | Buchman et al. (2019) | Casaletto et al. (2020a) | Casaletto et al. (2020b) | Chan et al. (2018) | Harris et al. (2015) | Negash et al. (2013) | Nunnari et al. (2016) | Reed et al. (2011) | Rouillard et al.(2017) | Scarmeas et al. (2003) | Sumowski et al. (2010) | Sumowski et al. (2013) | Xu et al. (2019) | Xu et al. (2020) | Yao et al. (2020) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||||
| 1. Study Participation | ||||||||||||||||||
| a. Adequate participation in the study by eligible persons | P | Y | P | Y | Y | Y | Y | N | Y | P | Y | P | N | N | P | Y | Y | Y |
| b. Description of the source population or population of interest | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| c. Description of the baseline study sample | Y | Y | Y | Y | Y | Y | P | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| d. Adequate description of the sampling frame and recruitment | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| e. Adequate description of the period and place of recruitment | N | Y | Y | P | Y | N | N | Y | Y | Y | N | N | N | N | N | Y | Y | N |
| f. Adequate description of inclusion and exclusion criteria | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Overall rating of study participation domain | M | L | L | L | L | M | M | M | L | L | M | M | M | M | M | L | L | M |
| 2. Study Attrition | ||||||||||||||||||
| a. Adequate response rate for study participants | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| b. Description of attempts to collect information on participants who dropped out | N | N | NA | NA | NA | N | NA | NA | N | NA | NA | NA | NA | NA | NA | N | N | N |
| c. Reasons for loss to follow-up are provided | N | Y | NA | NA | NA | P | NA | NA | N | NA | NA | NA | NA | NA | NA | Y | Y | N |
| d. Adequate description of participants lost to follow-up | N | P | NA | NA | NA | Y | NA | NA | Y | NA | NA | NA | NA | NA | NA | Y | Y | N |
| e. There are no important differences between participants who completed the study and those who did not | N | N | NA | NA | NA | Y | NA | NA | P | NA | NA | NA | NA | NA | NA | N | N | N |
| Overall rating of study attrition domain | H | H | L | L | L | M | L | L | H | L | L | L | L | L | L | M | M | H |
| 3. Prognostic Factor Measurement | ||||||||||||||||||
| a. A clear definition or description of the PF is provided | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| b. Method of PF measurement is adequately valid and reliable | P | Y | Y | Y | Y | Y | Y | P | Y | P | Y | Y | P | Y | Y | P | P | Y |
| c. Continuous variables are reported or appropriate cut points are used | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| d. The method and setting of measurement of PF is the same for all study participants | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| e. Adequate proportion of the study sample has complete data for the PF | P | Y | P | Y | Y | Y | Y | N | Y | P | Y | P | N | N | P | Y | Y | Y |
| f. Appropriate methods of imputation are used for missing PF data | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Overall rating of prognostic factor measurement domain | L | L | L | L | L | L | L | M | L | L | L | L | M | M | L | L | L | L |
| 4. Outcome Measurement | ||||||||||||||||||
| a. A clear definition of the outcome is provided | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| b. Method of outcome measurement used is adequately valid and reliable | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| c. The method and setting of outcome measurement is the same for all study participants | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Overall rating of outcome measurement domain | L | L | L | L | L | L | L | L | L | L | L | L | L | L | L | L | L | L |
| 5. Study Confounding | ||||||||||||||||||
| a. All important confounders are measured | Y | Y | Y | Y | Y | Y | P | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| b. Clear definitions of the important confounders measured are provided | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| c. Measurement of all important confounders is adequately valid and reliable | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| d. The method and setting of confounding measurement are the same for all study participants | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| e. Appropriate methods are used if imputation is used for missing confounder data | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| f. Important potential confounders are accounted for in the study design | Y | Y | Y | Y | Y | Y | P | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| g. Important potential confounders are accounted for in the analysis | Y | Y | Y | P | Y | Y | P | N | Y | P | Y | N | Y | Y | Y | Y | Y | P |
| Overall rating of study confounding domain | L | L | L | L | L | L | M | M | L | L | L | M | L | L | L | L | L | L |
| 6. Statistical Analysis and Reporting | ||||||||||||||||||
| a. Sufficient presentation of data to assess the adequacy of the analytic strategy | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| b. Strategy for model building is appropriate and is based on a conceptual framework or model | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| c. The selected statistical model is adequate for the design of the study | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| d. There is no selective reporting of results | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Overall rating of statistical analysis and reporting domain | L | L | L | L | L | L | L | L | L | L | L | L | L | L | L | L | L | L |
| Overall risk of bias | M | M | L | L | L | L | L | M | M | L | L | L | L | L | L | L | L | M |
Abbreviation: QUIPS = Quality of Prognosis Studies in Systematic Reviews; Y = Yes; P = Partly; N = No; L = Low risk of bias; M = Moderate risk of bias; H = High risk of bias; NA = Not applicable; PF = Prognostic factor.
Note: Yes: referring to low risk of bias, the prompting item was met; No: referring to high risk of bias, the prompting item was not met; Partly: referring to moderate risk of bias, the prompting item was partly met. Low risk of bias: ≤ two “Partly” prompting items (moderate bias domains); Moderate risk of bias: ≥ three “Partly” prompting items (moderate bias domains) without “No” prompting item (high bias domain), ≤ two “No” prompting items (high bias domains), and one “No” prompting item (high bias domain) and one “Partly” prompting item (moderate bias domain); High risk of bias: ≥ three “No” prompting items (high bias domains), two “No” prompting items (high bias domains) and ≥ one “Partly” prompting item (moderate bias domain).
In summary, although risk of bias, poor quality of study design, and sample size were commonly stated in the limitation section of those studies, the summary of their main findings is broadly applicable to conclude that certain lifestyle activity factors, including physical activity and cognitive leisure activity, seem to have a beneficial effect on the association between brain markers and cognitive function.
4. Discussion
This review summarized three CR models (residual, moderation, and controlling models) that were commonly used in previous studies, and examined the role of seven selected modifiable lifestyle factors in the association between brain markers and cognition. This review included 18 studies with 25 associations among four lifestyle factors, confirming lifestyle activity factors (physical activity and cognitive leisure activity) may provide CR and have a protective impact on the associations between brain markers and cognition. The residual model revealed less relationship between modifiable lifestyle factors and CR residuals, compared to the moderation and controlling models. This may be because these residuals cannot fully explain the majority of the variance in cognition due to the limited number of brain markers and other unmeasurable factors (Habeck et al., 2017; Zahodne et al., 2015; Zahodne et al., 2013). Moderation model examines the attenuated effects of lifestyle activity factors on the relationship between specific brain markers and cognition, which indicates that the lifestyle activity factors may exert moderation impacts according to the type of brain characteristics or pathologies and cognition (Bennett et al., 2003; Xu et al., 2015).
This review yielded results similar to previous reviews, indicating that lifestyle factors might impart cognitive reserve (Amanollahi et al., 2021; Fratiglioni et al., 2004; Scarmeas and Stern, 2003). However, previous reviews were not designed to include studies analyzing all three essential components of CR (lifestyle factors, brain markers and cognition) (Amanollahi et al., 2021; Fratiglioni et al., 2004). Based on the CR concept, it may not be appropriate to test CR capacity without accounting for brain markers (Stern et al., 2020; Reserve and Resilience Workshop). Furthermore, we have summarized the various CR models used to examine the role of each lifestyle factor in the relationship between brain markers and cognition. Clarifying these analytical models can help guide future studies on this research topic and avoid study design pitfalls. Finally, previous reviews only included a few different lifestyle activity factors, like social networks (Fratiglioni et al., 2004), social engagement (Amanollahi et al., 2021) and bilingualism (Amanollahi et al., 2021). As per recent reports (Livingston et al., 2020; World Health Organization, 2019) suggest the risk of dementia and cognitive decline can be reduced through modifying risk factors. It is therefore important to comprehensively evaluate and elucidate the mechanisms for a wider range of modifiable lifestyle factors. This may encourage at-risk populations to adopt healthy behaviors in order to attenuate cognitive decline, and may help decision-makers tailor the policies or recommendations based on the role of each lifestyle factor.
While the exact biological mechanisms remain unclear and different lifestyle factors might have different underlying mechanisms, many studies are ongoing to investigate the neural basis for CR. A few potential mechanisms have been proposed, including compensation and efficiency mechanisms (Barulli and Stern, 2013). CR may exert a compensatory effect before the damaging effect of neuropathology is manifested. For example, there was a weaker association between brain markers (e.g., Amyloid β) and cognitive performance among people with higher CR (Barulli and Stern, 2013; Yaffe et al., 2011). The biological mechanism for compensation lies in that actively engaged in lifestyle activities may increase synaptogenesis of the unaffected neurons, which may compensate for the brain damage to help the brain tolerate more loss before developing cognitive impairment (Churchill et al., 2002; Fratiglioni et al., 2004; Milgram et al., 2006; Oh et al., 2018; Scarmeas, 2007; Scarmeas and Stern, 2003). Lifestyle activity factors may also enhance the efficiency or capacity of brain networks when performing the cognitive functions, and the flexibility of the brain networks when shifting operations to alternate circuits, or non-neuronal components of the brain (Churchill et al., 2002; Scarmeas, 2007; Scarmeas and Stern, 2003). More studies are needed to confirm those mechanisms and explore other possible biological mechanisms.
Overall, some research gaps that are worthy of further exploration. To begin with, more studies are needed for some lifestyle factors, such as diet, sleep, meditation, smoking, and alcohol assumption, when exploring the mechanisms underlying the association between those lifestyle factors and cognition. Finding more factors that influence CR may tailor multidomain interventions to reduce the risk of cognitive diseases and delay the onset of these diseases. In addition, the measurements used in the previous studies varied and might hinder the consistent findings. Thus, it may be helpful to develop a standardized measurement of lifestyle factors (Casaletto et al., 2020b; Chan et al., 2018; Ikanga et al., 2017; Levi et al., 2013). Moreover, future studies may want to compare the quantity and reliability of each CR model and to identify the appropriate CR models in the study. Furthermore, better and higher-quality cohort or experimental designs with larger sample sizes are warranted to help validate and distinguish the metrics and effect sizes with different sample characteristics (Anaturk et al., 2021; Bartres-Faz et al., 2009; Casaletto et al., 2020b; Chirles et al., 2017; Clare et al., 2017; Harris et al., 2015; Morales Ortiz and Fernandez, 2020; Rouillard et al., 2017; Sobral et al., 2014; Sobral et al., 2015; Sumowski et al., 2013; Xu et al., 2020; Xu et al., 2019; Yao et al., 2020). As a long time window and a long duration of healthy behaviors may be needed to observe the protective impact of lifestyle factors on cognition among the population with similar brain status, it will be helpful to expand the follow-up timeframe and assess the health behaviors in early-life or mid-life (Amato et al., 2013; Buchman et al., 2019; Helzner et al., 2007; Sumowski et al., 2010). Finally, biology mechanisms, particularly through experimental studies with animals (Corpas et al., 2019; Frolinger et al., 2019; Rungratanawanich et al., 2019; Zhang et al., 2018) or cells (Mota and Kelly, 2020), need to be further elucidated to ensure the causal effects (Buchman et al., 2019; Casaletto et al., 2020b; Chan et al., 2018; Fratiglioni et al., 2004; Snitz et al., 2020; Xu et al., 2019). Data-driven approaches (e.g., functional MRI) may also help clarify the mechanisms underlying CR in the setting of brain pathology (Anaturk et al., 2021; Casaletto et al., 2020a; Chirles et al., 2017; Negash et al., 2013; Snitz et al., 2020; Sumowski et al., 2013).
This study has certain limitations. First, as we focused on more general modifiable lifestyle factors in this review, some other lifestyle factors potentially relevant to CR but less studied were excluded from the literature search, e.g., multilingualism, the single activity like visiting the museum, volunteering, musical practice, training, social network, genetic variability and personality, etc. (Anaturk et al., 2021; Craik et al., 2010; Crane et al., 2010; Fancourt et al., 2018; Fratiglioni et al., 2004; Hall et al., 2009; Jha et al., 2017; Kail and Carr, 2020; Lombardi et al., 2018; Negash et al., 2013; Perquin et al., 2013; Reed et al., 2011; Seinfeld et al., 2013; Strong and Mast, 2019; Sumowski et al., 2010; Vernooij-Dassen et al., 2021; Yao et al., 2020; Zheng et al., 2018). In addition, there are some intrinsic limitations of the three CR models we reviewed here. For the moderation model, unless there is a way to consolidate all brain measures, multiple models will have to be tested for each brain measure one by one. Residual models or controlling models can overcome this problem by including all available brain measures in the same model, however, it may still not be able to capture a true “reserve of cognition”, as some unmeasured factors may still explain the remaining cognitive variations (Habeck et al., 2017; Zahodne et al., 2015; Zahodne et al., 2013).
Our study has some novelties and advantages. We summarized the models currently used in the literature in CR analysis. As can be seen from Table 1, for each lifestyle factor, multiple models have been used which may help explain the inconsistency of the results. By summarizing the models and identifying certain pitfalls in previous studies, our review also provides a solid foundation in terms of study design and model selection for future studies aiming to examine the CR potential of lifestyles factors. The current review focused on multiple modifiable lifestyle factors together and identified factors that share the same CR mechanism for cognitive benefits, which may be helpful for future multidomain intervention, since combinations of these health factors and behaviors seem to be more effective at reducing the risk of cognitive diseases than individual factors (Guo et al., 2021).
5. Conclusion
This review provides evidence to support the hypothesis that lifestyle activity factors may mitigate the associations between brain markers and cognition, suggesting that tailored health policies on lifestyle activities may have far-reaching consequences to attenuate cognition decline and delay the onset of dementia. Significance of the role of each lifestyle activity factor varied by CR models. In general, it is less likely to find significant impacts of lifestyle factors using the residual models compared to the moderation or controlling models. Lifestyle factors only moderated specific, but not all, brain markers on cognition. Standardized measurements of lifestyle factors and CR and underlying mechanisms need to be further addressed.
Supplementary Material
Highlights.
Three CR models have been used commonly in the literature.
Lifestyle activity factors (physical/cognitive leisure activities) may supply CR.
Lifestyle activity factors moderate effect of specific brain markers on cognition.
Data on other lifestyle factors (diet, sleep, and meditation, etc.) are scarce.
Standardized measurements of lifestyle factors and CR are needed.
Funding
This work was supported by grants AG061008, AG061421, AG026158 funded by the National Institute on Aging (NIA). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Declaration of Competing Interest
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference:
- Amanollahi M, Amanollahi S, Anjomshoa A, et al. , 2021. Mitigating the negative impacts of aging on cognitive function; modifiable factors associated with increasing cognitive reserve. Eur J Neurosci. 53(9), 3109–3124. 10.1111/ejn.15183. [DOI] [PubMed] [Google Scholar]
- Amato MP, Razzolini L, Goretti B, et al. , 2013. Cognitive reserve and cortical atrophy in multiple sclerosis A longitudinal study. Neurology. 80(19), 1728–1733. 10.1212/WNL.0b013e3182918c6f. [DOI] [PubMed] [Google Scholar]
- Anaturk M, Kaufmann T, Cole JH, et al. , 2021. Prediction of brain age and cognitive age: Quantifying brain and cognitive maintenance in aging. Hum Brain Mapp. 42(6), 1626–1640. 10.1002/hbm.25316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andel R, Vigen C, Mack WJ, et al. , 2006. The effect of education and occupational complexity on rate of cognitive decline in Alzheimer’s patients. J Int Neuropsychol Soc. 12(1), 147–152. 10.1017/s1355617706060206. [DOI] [PubMed] [Google Scholar]
- Bartres-Faz D, Sole-Padulles C, Junque C, et al. , 2009. Interactions of cognitive reserve with regional brain anatomy and brain function during a working memory task in healthy elders. Biol Psychol. 80(2), 256–259. 10.1016/j.biopsycho.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Barulli D, Stern Y, 2013. Efficiency, capacity, compensation, maintenance, plasticity: emerging concepts in cognitive reserve. Trends Cogn Sci. 17(10), 502–509. 10.1016/j.tics.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgart M, Snyder HM, Carrillo MC, et al. , 2015. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: A population-based perspective. Alzheimers Dement. 11(6), 718–726. 10.1016/j.jalz.2015.05.016. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Wilson RS, Schneider JA, et al. , 2003. Education modifies the relation of AD pathology to level of cognitive function in older persons. Neurology. 60(12), 1909–1915. 10.1212/01.wnl.0000069923.64550.9f. [DOI] [PubMed] [Google Scholar]
- Borroni B, Premi E, Agosti C, et al. , 2009. Revisiting Brain Reserve Hypothesis in Frontotemporal Dementia: Evidence from a Brain Perfusion Study. Dement Geriatr Cogn Disord. 28(2), 130–135. 10.1159/000235575. [DOI] [PubMed] [Google Scholar]
- Bubu OM, Brannick M, Mortimer J, et al. , 2017. Sleep, Cognitive impairment, and Alzheimer’s disease: A Systematic Review and Meta-Analysis. Sleep. 40(1). 10.1093/sleep/zsw032. [DOI] [PubMed] [Google Scholar]
- Buchman AS, Yu L, Wilson RS, et al. , 2019. Physical activity, common brain pathologies, and cognition in community-dwelling older adults. Neurology. 92(8), E811–E822. 10.1212/WNL.0000000000006954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho A, Rea IM, Parimon T, et al. , 2014. Physical activity and cognitive function in individuals over 60 years of age: a systematic review. Clin Interv Aging. 9, 661–682. 10.2147/cia.s55520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casaletto KB, Renteria MA, Pa J, et al. , 2020a. Late-Life Physical and Cognitive Activities Independently Contribute to Brain and Cognitive Resilience. J Alzheimers Dis. 74(1), 363–376. 10.3233/JAD-191114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casaletto KB, Staffaroni AM, Wolf A, et al. , 2020b. Active lifestyles moderate clinical outcomes in autosomal dominant frontotemporal degeneration. Alzheimers Dement. 16(1), 91–105. 10.1002/alz.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan D, Shafto M, Kievit R, et al. , 2018. Lifestyle activities in mid-life contribute to cognitive reserve in late-life, independent of education, occupation, and late-life activities. Neurobiol Aging. 70, 180–183. 10.1016/j.neurobiolaging.2018.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Lu B, 2020. Cognitive reserve regulates the association between hearing difficulties and incident cognitive impairment evidence from a longitudinal study in China. Int Psychogeriatr. 32(5), 635–643. 10.1017/S1041610219001662. [DOI] [PubMed] [Google Scholar]
- Cheng S-T, 2014. Double Compression: A Vision for Compressing Morbidity and Caregiving in Dementia. Gerontologist. 54(6), 901–908. 10.1093/geront/gnu015. [DOI] [PubMed] [Google Scholar]
- Chirles TJ, Reiter K, Weiss LR, et al. , 2017. Exercise Training and Functional Connectivity Changes in Mild Cognitive Impairment and Healthy Elders. J Alzheimers Dis. 57(3), 845–856. 10.3233/JAD-161151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen H, Anstey KJ, Parslow RA, et al. , 2007. The brain reserve hypothesis, brain atrophy and aging. Gerontology. 53(2), 82–95. 10.1159/000096482. [DOI] [PubMed] [Google Scholar]
- Churchill JD, Galvez R, Colcombe S, et al. , 2002. Exercise, experience and the aging brain. Neurobiol Aging. 23(5), 941–955. 10.1016/s0197-4580(02)00028-3. [DOI] [PubMed] [Google Scholar]
- Clare L, Wu Y-T, Teale JC, et al. , 2017. Potentially modifiable lifestyle factors, cognitive reserve, and cognitive function in later life: A cross-sectional study. PLoS Med. 14(3). 10.1371/journal.pmed.1002259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke PJ, Ailshire JA, House JS, et al. , 2012. Cognitive function in the community setting: the neighbourhood as a source of ‘cognitive reserve’? J Epidemiol Community Health. 66(8), 730–736. 10.1136/jech.2010.128116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde-Sala JL, Garre-Olmo J, Vilalta-Franch J, et al. , 2013. Cognitive decline in Alzheimer’s disease. A follow three or more years of a sample of patients. Rev Neurol. 56(12), 593–600. [PubMed] [Google Scholar]
- Consonni M, Dalla Bella E, Bersano E, et al. , 2020. Cognitive reserve is associated with altered clinical expression in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 10.1080/21678421.2020.1849306. [DOI] [PubMed] [Google Scholar]
- Corpas R, Grinan-Ferre C, Rodriguez-Farre E, et al. , 2019. Resveratrol Induces Brain Resilience Against Alzheimer Neurodegeneration Through Proteostasis Enhancement. Mol Neurobiol. 56(2), 1502–1516. 10.1007/s12035-018-1157-y. [DOI] [PubMed] [Google Scholar]
- Craik FIM, Bialystok E, Freedman M, 2010. Delaying the onset of Alzheimer disease Bilingualism as a form of cognitive reserve. Neurology. 75(19), 1726–1729. 10.1212/WNL.0b013e3181fc2a1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane PK, Gruhl JC, Erosheva EA, et al. , 2010. Use of Spoken and Written Japanese Did Not Protect Japanese-American Men From Cognitive Decline in Late Life. J Gerontol B Psychol Sci Soc Sci. 65(6), 654–666. 10.1093/geronb/gbq046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskalopoulou C, Stubbs B, Kralj C, et al. , 2017. Physical activity and healthy ageing: A systematic review and meta-analysis of longitudinal cohort studies. Ageing Res Rev. 38, 6–17. 10.1016/j.arr.2017.06.003. [DOI] [PubMed] [Google Scholar]
- Deary IJ, Whalley LJ, Batty GD, et al. , 2006. Physical fitness and lifetime cognitive change. Neurology. 67(7), 1195–1200. 10.1212/01.wnl.0000238520.06958.6a. [DOI] [PubMed] [Google Scholar]
- Dik MG, Deeg DJH, Visser M, et al. , 2007. Association between early life physical activity and late-life cognition: Evidence for cognitive reserve, in: Stern Y (Ed.), Cognitive reserve: Theory and applications. Taylor & Francis, Philadelphia, PA, pp. 143–157. [Google Scholar]
- Ewers M, Insel PS, Stern Y, et al. , 2013. Cognitive reserve associated with FDG-PET in preclinical Alzheimer disease. Neurology. 80(13), 1194–1201. 10.1212/WNL.0b013e31828970c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallahpour M, Borell L, Luborsky M, et al. , 2016. Leisure-activity participation to prevent later-life cognitive decline: a systematic review. Scand J Occup Ther. 23(3), 162–197. 10.3109/11038128.2015.1102320. [DOI] [PubMed] [Google Scholar]
- Fancourt D, Steptoe A, Cadar D, 2018. Cultural engagement and cognitive reserve: museum attendance and dementia incidence over a 10-year period. Br J Psychiatry. 213(5), 661–663. 10.1192/bjp.2018.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forcada I, Mur M, Mora E, et al. , 2015. The influence of cognitive reserve on psychosocial and neuropsychological functioning in bipolar disorder. Eur Neuropsychopharmacol. 25(2), 214–222. 10.1016/j.euroneuro.2014.07.018. [DOI] [PubMed] [Google Scholar]
- Fratiglioni L, Paillard-Borg S, Winblad B, 2004. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurol. 3(6), 343–353. 10.1016/S1474-4422(04)00767-7. [DOI] [PubMed] [Google Scholar]
- Frolinger T, Sims S, Smith C, et al. , 2019. The gut microbiota composition affects dietary polyphenols-mediated cognitive resilience in mice by modulating the bioavailability of phenolic acids. Sci Rep. 9. 10.1038/s41598-019-39994-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyffe DC, Mukherjee S, Barnes LL, et al. , 2011. Explaining differences in episodic memory performance among older African Americans and Whites: the roles of factors related to cognitive reserve and test bias. J Int Neuropsychol Soc. 17(4), 625–638. 10.1017/s1355617711000476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard T, Hölzel BK, Lazar SW, 2014. The potential effects of meditation on age-related cognitive decline: a systematic review. Ann N Y Acad Sci. 1307, 89–103. 10.1111/nyas.12348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Scarmeas N, 2011. Dietary patterns in Alzheimer’s disease and cognitive aging. Curr Alzheimer Res. 8(5), 510–519. 10.2174/156720511796391836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Brickman AM, Manly JJ, et al. , 2021. Association of Life’s Simple 7 with incident dementia and its modification by the apolipoprotein E genotype. Alzheimers Dement. 10.1002/alz.12359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habeck C, Eich TS, Gu Y, et al. , 2019. Occupational Patterns of Structural Brain Health: Independent Contributions Beyond Age, Gender, Intelligence, and Age. Front Hum Neurosci. 13. 10.3389/fnhum.2019.00449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habeck C, Razlighi Q, Gazes Y, et al. , 2017. Cognitive Reserve and Brain Maintenance: Orthogonal Concepts in Theory and Practice. Cereb Cortex. 27(8), 3962–3969. 10.1093/cercor/bhw208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall CB, Lipton RB, Sliwinski M, et al. , 2009. Cognitive activities delay onset of memory decline in persons who develop dementia. Neurology. 73(5), 356–361. 10.1212/WNL.0b013e3181b04ae3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanyu H, Sato T, Shimizu S, et al. , 2008. The effect of education on rCBF changes in Alzheimer’s disease: a longitudinal SPECT study. Eur J Nucl Med Mol Imaging. 35(12), 2182–2190. 10.1007/s00259-008-0848-4. [DOI] [PubMed] [Google Scholar]
- Harris P, Suarez MF, Surace EI, et al. , 2015. Cognitive reserve and AβI-42 in mild cognitive impairment (Argentina-Alzheimer’s disease neuroimaging initiative). Neuropsychiatr Dis Treat. 11, 2599–2604. 10.2147/NDT.S84292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden JA, van der Windt DA, Cartwright JL, et al. , 2013. Assessing bias in studies of prognostic factors. Ann Intern Med. 158(4), 280–286. 10.7326/0003-4819-158-4-201302190-00009. [DOI] [PubMed] [Google Scholar]
- Helzner EP, Scarmeas N, Cosentino S, et al. , 2007. Leisure activity and cognitive decline in incident Alzheimer disease. Arch Neurol. 64(12), 1749–1754. 10.1001/archneur.64.12.1749. [DOI] [PubMed] [Google Scholar]
- Ihle A, Gouveia ER, Gouveia BR, et al. , 2018. The Relation of Hypertension to Performance in Immediate and Delayed Cued Recall and Working Memory in Old Age: The Role of Cognitive Reserve. J Aging Health. 30(8), 1171–1187. 10.1177/0898264317708883. [DOI] [PubMed] [Google Scholar]
- Ihle A, Gouveia ER, Gouveia BR, et al. , 2017. High-Density Lipoprotein Cholesterol Level Relates to Working Memory, Immediate and Delayed Cued Recall in Brazilian Older Adults: The Role of Cognitive Reserve. Dement Geriatr Cogn Disord. 44(1–2), 84–91. 10.1159/000477846. [DOI] [PubMed] [Google Scholar]
- Ihle A, Mons U, Perna L, et al. , 2016. The Relation of Obesity to Performance in Verbal Abilities, Processing Speed, and Cognitive Flexibility in Old Age: The Role of Cognitive Reserve. Dement Geriatr Cogn Disord. 42(1–2), 117–126. 10.1159/000448916. [DOI] [PubMed] [Google Scholar]
- Ikanga J, Hill EM, MacDonald DA, 2017. The conceptualization and measurement of cognitive reserve using common proxy indicators: Testing some tenable reflective and formative models. J Clin Exp Neuropsychol. 39(1), 72–83. 10.1080/13803395.2016.1201462. [DOI] [PubMed] [Google Scholar]
- Jha AP, Morrison AB, Parker SC, et al. , 2017. Practice Is Protective: Mindfulness Training Promotes Cognitive Resilience in High-Stress Cohorts. Mindfulness. 8(1), 46–58. 10.1007/s12671-015-0465-9. [DOI] [Google Scholar]
- Kail BL, Carr DC, 2020. More Than Selection Effects: Volunteering Is Associated With Benefits in Cognitive Functioning. J Gerontol B Psychol Sci Soc Sci. 75(8), 1741–1746. 10.1093/geronb/gbaa101. [DOI] [PubMed] [Google Scholar]
- Kuiper JS, Zuidersma M, Oude Voshaar RC, et al. , 2015. Social relationships and risk of dementia: A systematic review and meta-analysis of longitudinal cohort studies. Ageing Res Rev. 22, 39–57. 10.1016/j.arr.2015.04.006. [DOI] [PubMed] [Google Scholar]
- Law LL, Barnett F, Yau MK, et al. , 2014. Effects of combined cognitive and exercise interventions on cognition in older adults with and without cognitive impairment: a systematic review. Ageing Res Rev. 15, 61–75. 10.1016/j.arr.2014.02.008. [DOI] [PubMed] [Google Scholar]
- Levi Y, Rassovsky Y, Agranov E, et al. , 2013. Cognitive Reserve Components as Expressed in Traumatic Brain Injury. J Int Neuropsychol Soc. 19(6), 664–671. 10.1017/S1355617713000192. [DOI] [PubMed] [Google Scholar]
- Livingston G, Huntley J, Sommerlad A, et al. , 2020. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 396(10248), 413–446. 10.1016/s0140-6736(20)30367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi G, Polito C, Berti V, et al. , 2018. Contribution of Bilingualism to Cognitive Reserve of an Italian Literature Professor at High Risk for Alzheimer’s Disease. J Alzheimers Dis. 66(4), 1389–1395. 10.3233/JAD-180736. [DOI] [PubMed] [Google Scholar]
- Meng X, D’Arcy C, 2012. Education and dementia in the context of the cognitive reserve hypothesis: a systematic review with meta-analyses and qualitative analyses. PLoS One. 7(6), e38268. 10.1371/journal.pone.0038268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milgram NW, Siwak-Tapp CT, Araujo J, et al. , 2006. Neuroprotective effects of cognitive enrichment. Ageing Res Rev. 5(3), 354–369. 10.1016/j.arr.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Moher D, Shamseer L, Clarke M, et al. , 2015. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 4(1), 1. 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales Ortiz M, Fernandez A, 2020. Assessment of Cognitively Stimulating Activity in a Spanish Population. Assessment. 27(6), 1310–1319. 10.1177/1073191118774620. [DOI] [PubMed] [Google Scholar]
- Morbelli S, Perneczky R, Drzezga A, et al. , 2013. Metabolic networks underlying cognitive reserve in prodromal Alzheimer disease: a European Alzheimer disease consortium project. J Nucl Med. 54(6), 894–902. 10.2967/jnumed.112.113928. [DOI] [PubMed] [Google Scholar]
- Mota BC, Kelly AM, 2020. Exercise alters LPS-induced glial activation in the mouse brain. Neuronal signal. 4(4), NS20200003–NS20200003. 10.1042/NS20200003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M, O’Leary E, 2010. Depression, cognitive reserve and memory performance in older adults. Int J Geriatr Psychiatry. 25(7), 665–671. 10.1002/gps.2404. [DOI] [PubMed] [Google Scholar]
- Negash S, Wilson RS, Leurgans SE, et al. , 2013. Resilient brain aging: characterization of discordance between Alzheimer’s disease pathology and cognition. Curr Alzheimer Res. 10(8), 844–851. 10.2174/15672050113109990157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunnari D, De Cola MC, Costa A, et al. , 2016. Exploring cognitive reserve in multiple sclerosis: New findings from a cross-sectional study. J Clin Exp Neuropsychol. 38(10), 1158–1167. 10.1080/13803395.2016.1200538. [DOI] [PubMed] [Google Scholar]
- Oh H, Razlighi QR, Stern Y, 2018. Multiple pathways of reserve simultaneously present in cognitively normal older adults. Neurology. 90(3), e197–e205. 10.1212/wnl.0000000000004829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perneczky R, Wagenpfeil S, Lunetta KL, et al. , 2009. Education attenuates the effect of medial temporal lobe atrophy on cognitive function in Alzheimer’s disease: the MIRAGE study. J Alzheimers Dis. 17(4), 855–862. 10.3233/jad-2009-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perquin M, Vaillant M, Schuller A-M, et al. , 2013. Lifelong Exposure to Multilingualism: New Evidence to Support Cognitive Reserve Hypothesis. PLoS One. 8(4). 10.1371/journal.pone.0062030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters R, Peters J, Warner J, et al. , 2008a. Alcohol, dementia and cognitive decline in the elderly: a systematic review. Age Ageing. 37(5), 505–512. 10.1093/ageing/afn095. [DOI] [PubMed] [Google Scholar]
- Peters R, Poulter R, Warner J, et al. , 2008b. Smoking, dementia and cognitive decline in the elderly, a systematic review. BMC Geriatr. 8, 36. 10.1186/1471-2318-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JM, Tang J, Lawlor B, et al. , 2018. Mediators of the relationship between social activities and cognitive function among older Irish adults: results from the Irish longitudinal study on ageing. Aging Ment Health. 22(1), 129–134. 10.1080/13607863.2016.1233935. [DOI] [PubMed] [Google Scholar]
- Reed BR, Dowling M, Farias ST, et al. , 2011. Cognitive Activities During Adulthood Are More Important than Education in Building Reserve. J Int Neuropsychol Soc. 17(4), 615–624. 10.1017/S1355617711000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed BR, Mungas D, Farias ST, et al. , 2010. Measuring cognitive reserve based on the decomposition of episodic memory variance. Brain. 133(Pt 8), 2196–2209. 10.1093/brain/awq154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reserve and Resilience Workshop. Framework for Terms Used in Research of Reserve and Resilience. https://reserveandresilience.com/framework/. Accessed June 22, 2021.
- Richards M, Sacker A, 2003. Lifetime antecedents of cognitive reserve. J Clin Exp Neuropsychol. 25(5), 614–624. 10.1076/jcen.25.5.614.14581. [DOI] [PubMed] [Google Scholar]
- Rouillard M, Audiffren M, Albinet C, et al. , 2017. Contribution of four lifelong factors of cognitive reserve on late cognition in normal aging and Parkinson’s disease. J Clin Exp Neuropsychol. 39(2), 142–162. 10.1080/13803395.2016.1207755. [DOI] [PubMed] [Google Scholar]
- Rungratanawanich W, Cenini G, Mastinu A, et al. , 2019. -Oryzanol Improves Cognitive Function and Modulates Hippocampal Proteome in Mice. Nutrients. 11(4). 10.3390/nu11040753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajeev G, Weuve J, Jackson JW, et al. , 2016. Late-life Cognitive Activity and Dementia: A Systematic Review and Bias Analysis. Epidemiology. 27(5), 732–742. 10.1097/ede.0000000000000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarmeas N, 2007. Lifestyle patterns and cognitive reserve, in: Stern Y (Ed.), Cognitive reserve: Theory and applications. Taylor & Francis, Philadelphia, PA, pp. 187–206. [Google Scholar]
- Scarmeas N, Albert SM, Manly JJ, et al. , 2006. Education and rates of cognitive decline in incident Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 77(3), 308–316. 10.1136/jnnp.2005.072306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarmeas N, Stern Y, 2003. Cognitive reserve and lifestyle. J Clin Exp Neuropsychol. 25(5), 625–633. 10.1076/jcen.25.5.625.14576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarmeas N, Stern Y, 2004. Cognitive reserve: implications for diagnosis and prevention of Alzheimer’s disease. Curr Neurol Neurosci Rep. 4(5), 374–380. 10.1007/s11910-004-0084-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarmeas N, Zarahn E, Anderson KE, et al. , 2003. Association of life activities with cerebral blood flow in Alzheimer disease - Implications for the cognitive reserve hypothesis. Arch Neurol. 60(3), 359–365. 10.1001/archneur.60.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seinfeld S, Figueroa H, Ortiz-Gil J, et al. , 2013. Effects of music learning and piano practice on cognitive function, mood and quality of life in older adults. Front Physiol. 4. 10.3389/fpsyg.2013.00810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snitz BE, Chang Y, Tudorascu DL, et al. , 2020. Predicting resistance to amyloid-beta deposition and cognitive resilience in the oldest-old. Neurology. 95(8), E984–E994. 10.1212/WNL.0000000000010239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobral M, Pestana MH, Paul C, 2014. Measures of cognitive reserve in Alzheimer’s disease. Trends Psychiatry Psychother. 36(3), 160–168. 10.1590/2237-6089-2014-0012. [DOI] [PubMed] [Google Scholar]
- Sobral M, Pestana MH, Paúl C, 2015. The impact of cognitive reserve on neuropsychological and functional abilities in Alzheimer’s disease patients. Psychol Neurosci. 8(1), 39–55. 10.1037/h0101022. [DOI] [Google Scholar]
- Stern Y, 2012. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 11(11), 1006–1012. 10.1016/S1474-4422(12)70191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y, Arenaza-Urquijo EM, Bartrés-Faz D, et al. , 2020. Whitepaper: Defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimers Dement. 16(9), 1305–1311. 10.1016/j.jalz.2018.07.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong JV, Mast BT, 2019. The cognitive functioning of older adult instrumental musicians and non-musicians. Aging Neuropsychol Cog. 26(3), 367–386. 10.1080/13825585.2018.1448356. [DOI] [PubMed] [Google Scholar]
- Sumowski JF, Rocca MA, Leavitt VM, et al. , 2013. Brain reserve and cognitive reserve in multiple sclerosis What you’ve got and how you use it. Neurology. 80(24), 2186–2193. 10.1212/WNL.0b013e318296e98b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumowski JF, Wylie GR, Gonnella A, et al. , 2010. Premorbid cognitive leisure independently contributes to cognitive reserve in multiple sclerosis. Neurology. 75(16), 1428–1431. 10.1212/WNL.0b013e3181f881a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker AM, Stern Y, 2011. Cognitive Reserve in Aging. Curr Alzheimer Res. 8(4), 354–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernooij-Dassen M, Moniz-Cook E, Verhey F, et al. , 2021. Bridging the divide between biomedical and psychosocial approaches in dementia research: the 2019 INTERDEM manifesto. Aging Ment Health. 25(2), 206–212. 10.1080/13607863.2019.1693968. [DOI] [PubMed] [Google Scholar]
- World Health Organization, 2019. Risk reduction of cognitive decline and dementia: WHO guidelines. https://www.who.int/publications/i/item/risk-reduction-of-cognitive-decline-and-dementia Accessed June 22, 2021. [PubMed]
- Xu H, Yang R, Dintica C, et al. , 2020. Association of lifespan cognitive reserve indicator with the risk of mild cognitive impairment and its progression to dementia. Alzheimers Dement. 16(6), 873–882. 10.1002/alz.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Yang R, Qi X, et al. , 2019. Association of Lifespan Cognitive Reserve Indicator With Dementia Risk in the Presence of Brain Pathologies. JAMA Neurol. 76(10), 1184–1191. 10.1001/jamaneurol.2019.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Yu J-T, Tan M-S, et al. , 2015. Cognitive Reserve and Alzheimer’s Disease. Mol Neurobiol. 51(1), 187–208. 10.1007/s12035-014-8720-y. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Weston A, Graff-Radford NR, et al. , 2011. Association of plasma β-amyloid level and cognitive reserve with subsequent cognitive decline. JAMA. 305(3), 261–266. 10.1001/jama.2010.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao T, Sweeney E, Nagorski J, et al. , 2020. Quantifying cognitive resilience in Alzheimer’s Disease: The Alzheimer’s Disease Cognitive Resilience Score. PLoS One. 15(11):e0241707. 15(11 November). 10.1371/journal.pone.0241707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahodne LB, Manly JJ, Brickman AM, et al. , 2015. Is residual memory variance a valid method for quantifying cognitive reserve? A longitudinal application. Neuropsychologia. 77, 260–266. 10.1016/j.neuropsychologia.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahodne LB, Manly JJ, Brickman AM, et al. , 2013. Quantifying cognitive reserve in older adults by decomposing episodic memory variance: replication and extension. J Int Neuropsychol Soc. 19(8), 854–862. 10.1017/s1355617713000738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Zhai Y, Sun Y, et al. , 2018. Swimming improves cognitive reserve in ovariectomized rats and enhances neuroprotection after global cerebral ischemia. Brain Res. 1692, 110–117. 10.1016/j.brainres.2018.05.020. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Wu Q, Su F, et al. , 2018. The Protective Effect of Cantonese/Mandarin Bilingualism on the Onset o Alzheimer Disease. Dement Geriatr Cogn Disord. 45(3–4), 210–219. 10.1159/000488485. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


