Abstract
Neuroinflammation is a central factor in neuropathic pain (NP). Ginger is a promising bioactive compound in NP management due to its anti-inflammatory property. Emerging evidence suggests that gut microbiome and gut-derived metabolites play a key role in NP. We evaluated the effects of two ginger root extracts rich in gingerols (GEG) and shogaols (SEG) on pain sensitivity, anxiety-like behaviors, circulating cell-free mitochondrial DNA (ccf-mtDNA), gut microbiome composition, and fecal metabolites in rats with NP. Sixteen male rats were divided into four groups: sham, spinal nerve ligation (SNL), SNL+0.75%GEG in diet, and SNL+0.75%SEG in diet groups for 30 days. Compared to SNL group, both SNL+GEG and SNL+SEG groups showed a significant reduction in pain- and anxiety-like behaviors, and ccf-mtDNA level. Relative to the SNL group, both SNL+GEG and SNL+SEG groups increased the relative abundance of Lactococcus, Sellimonas, Blautia, Erysipelatoclostridiaceae, and Anaerovoracaceae, but decreased that of Prevotellaceae UCG-001, Rikenellaceae RC9 gut group, Mucispirillum and Desulfovibrio, Desulfovibrio, Anaerofilum, Eubacterium siraeum group, RF39, UCG-005, Lachnospiraceae NK4A136 group, Acetatifactor, Eubacterium ruminantium group, Clostridia UCG-014, and an uncultured Anaerovoracaceae. GEG and SEG had differential effects on gut-derived metabolites. Compared to SNL group, SNL+GEG group had higher level of 1'-acetoxychavicol acetate, (4E)-1,7-Bis(4-hydroxyphenyl)-4-hepten-3-one, NP-000629, 7,8-Dimethoxy-3-(2-methyl-3-buten-2-yl)-2H-chromen-2-one, 3-{[4-(2-Pyrimidinyl)piperazino]carbonyl}-2-pyrazinecarboxylic acid, 920863, and (1R,3R,7R,13S)-13-Methyl-6-methylene-4,14,16-trioxatetracyclo[11.2.1.0~1,10~.0~3,7~]hexadec-9-en-5-one, while SNL+SEG group had higher level for (+/−)-5-[(tert-Butylamino)-2'-hydroxypropoxy]-1_2_3_4-tetrahydro-1-naphthol and dehydroepiandrosteronesulfate. In conclusion, ginger is a promising functional food in the management of NP, and further investigations are necessary to assess the role of ginger on gut-brain axis in pain management.
Keywords: bioactive compound, neuropathic pain, gut microbiome, fecal metabolites, anxiety, animals
Introduction
Neuropathic pain (NP) arises from damage to the peripheral or central nervous system [1]. The burden of chronic NP is related to the complexity of NP symptoms (e.g., anxiety and depression), poor outcomes, and limited available treatment options. Opioid analgesics are the most common forms of treatment for NP; unfortunately, they have severe side effects and can result in opioid use disorder [2]. Nerve injury in NP leads to neuroinflammation and neuroplastic changes in peripheral and central neurons associated with sensitization and hyperexcitability [3]. Previous studies have shown that NP could be a consequence of an imbalance between reactive oxygen species (ROS) and endogenous antioxidants after nerve injury, leading to neuroinflammation [4]. Therefore, the development of new effective and safe analgesic and anti-inflammatory alternatives is urgently needed.
Newly developed evidence suggests that the gut microbiome may serve as an important element between the neuroimmune-endocrine and gut-brain axes, forming a sophisticated network that directly and indirectly affects critical factors in the manifestations of NP [5]. For instance, spinal cord injury (SCI)-induced NP increases intestinal permeability and bacterial translocation from the gut, resulting in gut dysbiosis [6, 7]. Gut dysbiosis is associated with profound changes in gut-associated lymphoid tissues immune cell activation, and dysbiosis also exacerbates spinal inflammation/lesion, leading to impaired recovery of neurological function [6, 8]. Moreover, gut dysbiosis has been implicated in the onset or progression of pain-associated behavior, such as pain sensitivity and depression [9-12].
Convincing evidence suggests that gut microbiome-derived metabolites (mediators) may play a key role in the direct or indirect regulation of peripheral and central sensitization mechanisms in the development of NP [9, 13, 14]. In the peripheral nervous system, gut microbiome-derived mediators (e.g., pathogen-associated molecular patterns) can also indirectly increase the excitability of DRG neurons by inducing pro-inflammatory factors (e.g., tumor necrosis factor-α, interluekin-1 β, and interluekin-6) and chemokine (e.g., monocyte chemoattractant protein-1) release from immune cells to enhance pain, whereas other mediators (e.g., short-chain fatty acids (SCFA) and bile acids) can indirectly decrease the excitability of dorsal root ganglion (DRG) neurons by releasing anti-inflammatory cytokines (i.e., interluekin-4, IL-4) or neuropeptides (e.g., opioids) from immune cells to inhibit pain [14]. Several cell types in the central nervous system (CNS), including microglia, astrocytes, and immune cells, can receive information from the periphery (e.g., gastrointestinal tract) [15-17]. In the CNS, gut microbiome-derived mediators may regulate energy metabolism and neuroinflammation, which involves the activation of cells in the blood-brain barrier, microglia, and infiltrating immune cells via their receptors, transporters, and histone deacetylase [18] to modulate induction and maintenance of central sensitization [9, 13, 14] and depression [18]. The activation of glial cells (e.g., microglia, astrocytes) can produce pro-inflammatory cytokines and chemokines, which have been shown to alter glutamatergic synaptic neurotransmission and GABAergic neurotransmission [19-22], contributing to central sensitization and leading to pain hypersensitivity [14]. Since the gut microbiome and metabolites may be linked to neuroinflammation, neuronal sensitization, and hyperexcitability in the development of NP, targeting gut microbiome and metabolites using bioactive compounds (in food) represents a new therapeutic strategy to manage NP.
Ginger (Zingiber officinale Roscoe) consists of a complex combination of biologically active constituents, among which the compounds gingerols (6-, 8-, and 10-gingerol) and shogaols (6-, 8-, and 10-shogaol) reportedly account for the majority of ginger’s anti-inflammatory properties [23]. Various ginger compounds and extracts have been tested as anti-inflammatory agents, where the length of side chains determines the level of effectiveness [24]. Among all the gingerols, 6-gingerol is the most abundant and potent anti-oxidant that yields the greatest anti-inflammatory effects [24]. A combination of gingerols and shogaols is more effective in decreasing inflammatory mediators than the individual compounds [25]. Furthermore, ginger and its bioactive components have been shown to penetrate the blood-brain barrier via passive diffusion, suggesting the positive effects of ginger in CNS [26].
Due to ginger’s anti-inflammatory and antioxidant properties, ginger has been used for inflammation-associated knee osteoarthritis in vitro [27], ex vivo [28], and in humans [29]. Ginger elicits antinociceptive properties and intensifies morphine-induced analgesia during the rat radiant heat tail-flick test [30]. Recently Borgonetti et al. reported oral administration of CO2-extracted ginger attenuated spared nerve injury (SNI)-induced NP symptoms by reducing spinal neuroinflammation [31]. Administration of ginger root extract or its individual bioactive compound (e.g., 6-gingerol, 6-shogaol, 8-shogaol) has also shown to mitigate pain in animals with SNI-induced NP [31-38]. Mata-Bermudez et al. reported that in a spinal nerve ligation (SNL)-induced rat model, the antiallodynic effect induced by 6-gingerol is mediated by the serotoninergic system involving the activation of 5-HT1A/1B/1D/5A receptors and the NO-cyclic guanosine monophosphate-ATP-sensitive K+ channel pathway, but not by the opioidergic system [39]. On the other hand, ginger supplementation has shown to enhance the abundance of SCFA-producing favorable microbiota species in obese mice [40]. However, no study has evaluated the effects of two ginger root extracts, namely, gingerols-enriched ginger (GEG) and shogaols-enriched ginger (SEG), respectively, on pain sensitivity and anxiety-like behaviors and their potential impacts on gut microbiome composition and gut microbiome-derived metabolites in animals with spinal nerve ligation (SNL)-induced NP.
We designed this study to assess the effects of GEG and SEG on (1) pain- and anxiety-like behaviors, (2) the plasma circulating cell-free mitochondrial DNA (ccf-mtDNA) damage, a biomarker of excessive mitochondria-derived ROS linked to inflammation, (3) the composition of gut microbiota, and (4) gut microbiota-derived metabolites in the feces of SNL-treated rats. We hypothesized that dietary supplementation with GEG and SEG would reduce SNL-induced pain-associated sensory and affective behaviors compared to SNL control animals. Such changes in pain-associated behaviors may be mediated by reducing mitochondrial DNA damage and modulating gut microbiome composition and fecal metabolites. In this study, we combined microbiome and metabolome approaches to explore the effects of ginger bioactive compounds on metabolic pathways relevant to NP in the development of personalized nutrition therapy for NP management.
Materials and Methods
Animals
16 male Sprague-Dawley rats (4-5 week-old, 150-180 g, Harlan Laboratories, Indianapolis, IN) were housed under a 12-hour light-dark cycle with food and water ad libitum. All experimental procedures were approved by the Institutional Animal Care and Use Committee at Texas Tech University Health Sciences Center. Body weights, food intake, and water consumption were recorded weekly.
Introduction of neuropathic pain
The SNL model is widely used for the preclinical study of NP mechanisms and the development of new analgesic drugs/compounds. The nerve injury (SNL) results in acute hypersensitivity within 1 week that persists for weeks [41]. In this well-established SNL-induced NP model, prolonged changes in inflammatory and pronociceptive mediators, neurotransmitters, and receptor expression were observed, producing peripheral and central sensitization [42]. Spontaneous pain behaviors, increased emotional responses, anxiety-like and depression-like behaviors are also observed and have been studied by our group [3, 43, 44].
After 5-day acclimatization, we randomly assigned 4 animals as sham-controls receiving sham surgery, while the remaining 12 animals underwent SNL surgery. The SNL model of NP was used to induce peripheral neuropathy in the left hind paw, as described in our previous work [3, 43]. In brief, isoflurane was used for induction (3%) and maintenance (2%) of anesthesia throughout the procedure. After removing the L5/L6 level paraspinal muscles and underlying L6 transverse process, the L5 spinal nerve was removed from adjacent structures and tightly ligated with 6-0 silk thread. The paraspinal muscles were sutured closed, and the skin clipped together. Sham-operated animals served as controls for the NP model, receiving the same surgical procedure without the L5 spinal nerve ligation. After surgery, all animals were given antibiotic treatment (1 dose of gentamycin, 8 mg/kg, subcutaneously, s.c.; VetOne, Boise, ID) and were monitored for any signs of infection or distress. Throughout the study period, the animals were monitored to reduce unnecessary stress or pain following ethical guidelines of the International Association for the Study of Pain [45].
Dietary treatments
We randomly divided the 16 animals into four dietary treatment groups: sham group, SNL group, SNL+GEG group, and SNL+SEG group. All animals were given AIN-93G diet (catalog number # D10012G, Research Diet, Inc., New Brunswick, NJ, USA). For the SNL+GEG group and SNL+SEG group, after the SNL procedure, the animals were given GEG at 0.75% (wt/wt diet) and SEG at 0.75% (wt/wt diet) into AIN-93G diet for 30 days, respectively. GEG administration to rats (range between 100 mg and 400 mg/kg of body weight) has been shown to reduce inflammation in various inflammation models [46, 47]. In this study, the dose of 0.75% (wt/wt diet) corresponds 400 mg/kg body weight/day via oral gavage for rat, showing a reduction in mechanical hyperalgesia in streptozotocin-induced NP by ginger root extract [35].
Based on the results of gas chromatography-mass spectrometry, GEG consists of 18.7% 6-gingerol, 1.81% 8-gingerol, 2.86% 10-gingerol, 3.09% 6-shogoal, 0.39% 8-shogaol, and 0.41% 10-shogaol and SEG consists of 3.29% 6-gingerol, 0.06% 8-gingerol, 0.43% 10-gingerol, 17.4% 6-shogaol, 1.94% 8-shogaol, and 1.49% 10-shogaol [48]. Both GEG and SEG were obtained from Sabinsa, Inc., Piscataway, NJ.
Sample collection
At 1 day before- and 10, 20, and 30 days post-sham or SNL operation, (i) paw withdrawal mechanical thresholds were measured by von Frey test (VFT) using von Frey filaments for pain sensory assessment and (ii) center frequency and center duration in the open field test (OFT) were measured to assess anxiety-like behavior. After behavioral tests, the fecal samples of the animals were collected via individual metabolic cage and stored at −80°C for later microbiomes and metabolites analyses. On collection day, the animals were anesthetized, euthanized, and blood was drawn for plasma collection. Plasma samples were stored at −80°C until ccf-mtDNA concentration measurement.
Assessment of pain-related behaviors
The von Frey test was used to measure mechanosensitivity as described in our previous studies [3]. Mechanical withdrawal thresholds of spinal reflexes were measured using Electronic von Frey Aesthesiometer (IITC Life Science, Woodland Hills, CA). The tip made contact with the base of the third or fourth toe perpendicularly, with increasing force until a flexion reflex was provoked. The physical reaction was automatically recorded as the paw withdrawal threshold (in grams). The average of triplicate measurements at least 30s apart was utilized.
Open field test (OFT) was used to measure exploratory behavior of the animal in an arena (70 cm × 70 cm) with acrylic walls (height, 45 cm) for 15 min, using a computerized video tracking and analysis system (EthoVisionXT 11 software, Noldus Information Technology). Duration and entries in the center area (35 cm × 35 cm) were calculated for the first 5 min [3, 43]. Avoidance of the center area, in terms of duration and frequency, in the OFT suggests anxiety-like behavior.
Assessment of circulating cell-free mitochondrial DNA (ccf-mtDNA)
Quantitative PCR (qPCR) of COX2 and GAPDH genes was used to measure the copy numbers of mitochondrial DNA (mtDNA) and nuclear DNA (nDNA), respectively. Plasma DNA (mtDNA and nDNA) were isolated via the QIAamp DNA Blood Mini Kit (Qiagen Inc., Valencia, CA) according to the manufacturer’s protocol. Standard curves were generated by dilution series of 20, 10, 5, 2, 1, and 0.1 ng of total rat liver DNA per reaction. We obtained the average threshold cycle (Ct) values for mtDNA and nDNA to determine the quantities of mtDNA and nucDNA present in the experimental samples. The cycle number (Ct) at which the fluorescent signal of a given reaction crosses the threshold value was used to quantify mtDNA and nucDNA copy numbers. We used the following primers to amplify the COX2 gene from mtDNA: forward – 5’CAC ACA AGC ACA ATA GAC GC-3’; reverse – 5’TAG GGA GGG AAG GGC AAT TA −3’. For amplification of the GAPDH gene from nDNA, the following primers were used: forward – 5’TGG CCT CCA AGG AGT AAG AAA C-3’; reverse – 5’-GGC CTC TCT CTT GCT CTC AGT ATC-3’. Primers were chosen using BLAST database (http://blast.ncbi.nlm.nih.gov/Blast.cgi) so that the amplicon from mtDNA had no significant homology with the nuclear genome. Primers for qPCR were synthesized by SYBRGreenER Mix (Invitrogen, Paisley, Scotland), and qPCRs were performed in a 20-μl volume containing 10 μl of TaqMan Universal PCR Master Mix 2× Buffer (Applied Biosystems, Foster City, CA), 2 μl of the DNA solution, and 250 nM of each primer. The PCR cycles were as follows: 5 min at 95 °C followed by 40 amplification cycles (95 °C for 30 s, annealing, and elongation at 60 °C for 1 min). PCRs were performed in a 20-μl volume containing 10 μl of TaqMan Universal PCR Master Mix 2× Buffer, 2 μl of the DNA solution, and 250 nM of each primer. The gene relative expression level was analyzed by 2−ΔΔCt method.
Gut microbiota profiling via 16S rRNA gene amplification and sequencing
Microbiota DNA was isolated from mouse feces using PowerFecal DNA isolation kit (Qiagen Inc., Germantown, MD, USA) following the manufacturer’s instructions. Amplicon sequencing of V4 region of the16S rRNA gene was performed by MR DNA (Molecular Research LP, Shallowater, TX, USA.). Briefly, V4 variable region was amplified using PCR primers 515F/806R. Samples were multiplexed and pooled together in equal proportions based on their molecular weight and DNA concentrations. Pooled samples were purified using calibrated Ampure XP beads, then used in Illumina DNA library preparation. Sequencing was performed at MR DNA (www.mrdnalab.com, Shallowater, TX, USA) on a MiSeq following the manufacturer’s guidelines. Raw sequencing data have been deposited under BioProject accession numbers PRJNA715490 in the National Center for Biotechnology Information (NCBI) BioProject database.
Fecal metabolites profiling
Untargeted metabolomics profile in feces was determined using LC-MS/MS analysis [49]. In brief, 50 mg of fecal samples were homogenized using bead beater in PBS buffer followed by centrifugation. 100 μL of homogenized sample (supernatant) was transferred to a glass tube, and metabolites were extracted with dichloromethane/methanol/water (1:2:1 v/v) mixture and vortexed. After centrifugation, the aqueous phase was collected for LC-MS/MS analysis. The metabolite analysis was done by Q-Exactive HF mass spectrometer in both positive and negative ion mode using a Vanquish LC-system at a flow rate of 450 μL/min. An Acquity UPLC column (HSS T3, 1.7μm, 2.1 x 100mm) was used in a 15 min gradient for each run, and the temperature was kept at 50°C during the run.
Statistical analysis
Data analysis for body weight, food intake, water consumption, and pain parameters
The data of final body weight, food intake, water consumption, and pain-associated parameters were analyzed by one-way analysis of variance (ANOVA) test, followed by post hoc Fisher’s Least Significant Difference (LSD) test using GraphPad Prism software version 6.0. A significance level of P-value < 0.05 applies to all statistical tests.
Data Analysis for gut microbiome
16S rRNA gene sequencing data was analyzed using QIIME 2 [50]. In brief, reads were filtered, denoised, and merged. DADA2 was used to identify exact amplicon sequence variants (ASVs). For taxonomy assignment, Silva database version 132 database was used. To compare the relative abundance of taxa between groups, we used the non-parametric Kruskal–Wallis test followed by Dunn's test for multiple comparisons. Results were regarded as significant when P-value < 0.05.
Data Analysis for fecal metabolites
Principle Component Analysis (PCA) was performed to assess the different profiling of the metabolites detected and quantified among 4 groups. For data analysis, the following parameters were applied: mass tolerance for the precursor ion= 5 ppm, Intensity tolerance = 30%, minimum peak intensity= 1×106, mass tolerance for alignment= 5 ppm, maximum shift for peak alignment= 2min and mass tolerance of fragment ion= 10 ppm. Data (peak areas of metabolites) were analyzed using Compound Discoverer software (3.1) to identify and quantify metabolites. Compound Discoverer 3.1 detected compounds with “Predicted Formula”, followed by automatic online library search against mzCloud and ChemSpider database. The compounds identified from the mzCloud library can be checked using the mirror plot of MS/MS spectra of identified compounds with library standards. The database search results yield spectra fit and matching score based on mass accuracy and isotope pattern and fragment ions obtained by MS/MS. For quantitative analysis, the area under the peak of each identified compound was calculated with proper peak alignment parameters described below. The t-test was performed to determine the number of statistically different metabolites in the SNL group compared to the other groups. Metabolites were filtered based on their statistical significance (P < 0.01), and results with >10 fold-change were presented.
Results
Final body weight, food intake, and water consumption
Throughout the study, there was no statistical difference in body weights among all groups, and the average final body weights of animals were 316.0±11.1 g, 312.8±11.6 g, 309.2±15.0 g, and 307.8±10.4 g for the sham, SNL, SNL+GEG, and SNL+SEG groups, respectively (P > 0.05). In addition, there was no significant difference in the food intake and water consumption of animals among all 4 groups (P > 0.05, data not shown).
Pain-like behaviors
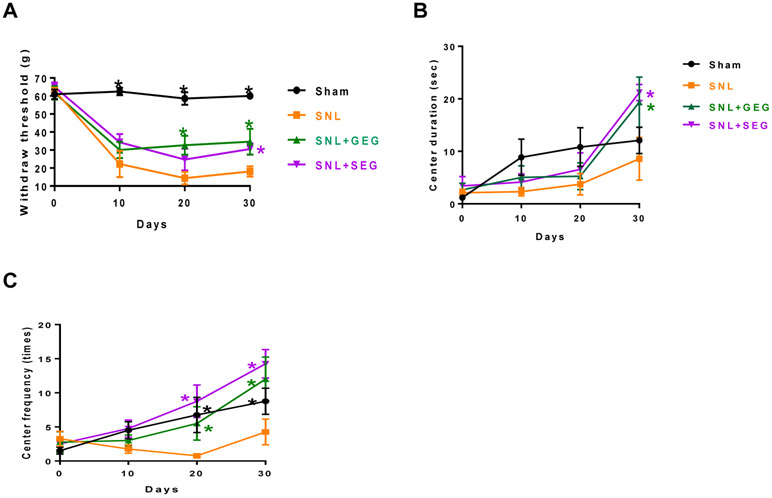
The effects of GEG and SEG supplementation on NP-associated behaviors were assessed using von Frey test (Figure 1). Compared to the sham group, the SNL group had significantly greater sensitivity to mechanical stimuli starting at 10 days post-operation and sustained throughout the observation period (until 30 days after SNL induction). Compared to the SNL group, (i) both SNL+GEG and SNL+SEG groups showed significantly reduced pain sensitivity as early as 10 days post-operation and sustained through 20 and 30 days, as shown by increased mechanical thresholds; and (ii) there was no difference in pain sensitivity between the SNL+GEG group and the SNL+SEG group (Figure 1A). At the end of the study (30 days after supplements started), the order of pain sensitivity was SNL group > SNL+GEG group = SNL+SEG group > sham group.
Figure 1.
Effects of GEG and SEG on NP-associated behaviors. Pain sensitivity was assessed in the von Frey test (Figure 1A). Anxiety-like behaviors were assessed in the open field test as duration in the central area (Figure 1B) and frequency of entering the central area (Figure 1C) in animals with NP.
In terms of anxiety-like behaviors, relative to the sham group the SNL group had anxiety-like behaviors as shown by decreased duration in the center of an open field area (Figure 1B) and frequency of entering the central area (Figure 1C). Compared to the animals in the SNL group without supplements, the addition of GEG and SEG to the diet significantly improved NP-associated anxiety-like behaviors, as indicated by prolonged center duration at 30 days (Figure 1B) and increased center frequency at 20 and 30 days (Figure 1C).
Plasma ccf-mtDNA
We used ccf-mtDNA as a circulating biomarker indicative of oxidative stress from SNL and assessed the effect of GEG and SEG on reducing ccf-mtDNA. The SNL group had significantly higher plasma ccf-mtDNA levels relative to the sham group (Figure 2). When SNL-operated animals were fed a diet supplemented with either GEG or SEG, both SNL+GEG and SNL+SEG groups showed significant reduction in mitochondrial oxidative stress, as reflected in the decreased plasma ccf-mtDNA levels, relative to those in the SNL-only group (Figure 2).
Figure 2.
Effects of GEG and SEG on ccf-mtDNA concentration in animals with NP.
Gut microbiome composition and function
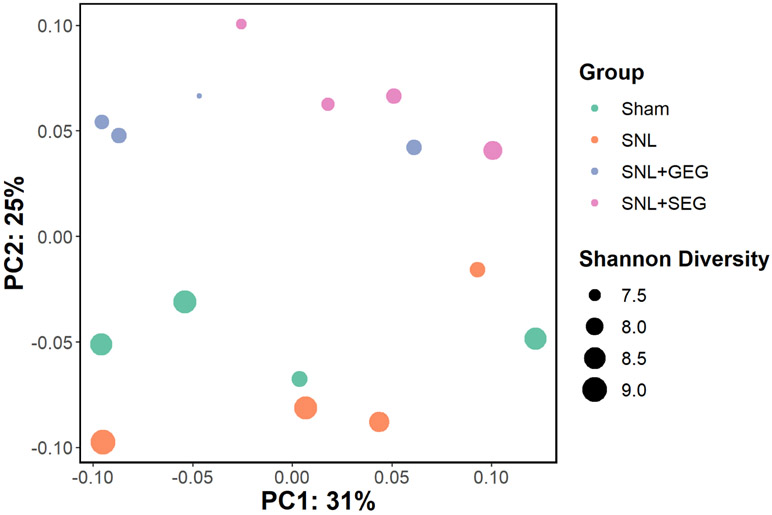
After data processing using QIIME 2, the number of unique amplicon sequence variants (ASVs) across all samples was 7,100, and the median number of ASVs per sample was 53,729 (range: 27,594–64,395). Beta-diversity analysis using weighted UniFrac distance metrics showed a slight separation between the different groups along PC2 (i.e., the sham group and the SNL group on one side and the SNL+GEG group and the SNL+SEG group on the other side) (Figure 3). This group separation was statistically significant using PERMANOVA (P = 0.003, pseudo-F = 2.34, and number of permutations = 999). Further, pairwise PERMANOVA was used to examine the separation of each group based on the distance between each pair. Only the distance between either sham group or SNL groups vs. SNL+SEG group was statistically significant (P = 0.03). In contrast, the distance between all other groups was not significantly different (P > 0.05). This indicates that while SNL didn't significantly impact the microbiome profile, the microbiome profile was strongly altered after SEG treatment.
Figure 3.
Alpha-diversity analysis of the gut microbiome. Principal component analysis (PCA) based on weighted UniFrac distance metric. Samples are colored based on the respective groups, while the size corresponds to the Shannon diversity index.
The sham and the SNL groups showed slightly higher alpha-diversity than other groups (Figure 3), calculated using the Shannon diversity index. The SNL+SEG microbiome showed lower diversity than either the Sham or the SNL groups (P = 0.043). In contrast, the SNL+GEG microbiome showed lower diversity than the sham group (P = 0.021) but not the SNL group (P = 0.083). We did not find a significant difference between the alpha-diversity of Sham and SNL groups (P = 0.772).
Then we identified taxa with the relative abundance that was altered with GEG or SEG supplementation. To achieve this, we used the Kruskal–Wallis test, which was followed by the post-hoc Dunn's multiple comparison test. We considered P < 0.05 as statistically significant (Figure 4). Overall, the SNL group has a small effect on the relative abundance of taxa in comparison with the sham group. The SNL group increased the abundance of Prevotellaceae UCG-001, and two Clostridia members, Roseburia and Eubacterium ruminantium group (Figure 4). In contrast, the SNL group showed a decrease in the abundance of Morganellaceae (Figure 4).
Figure 4.
A heatmap showing significant alterations in the relative abundance of the gut microbiome ASVs. The color scale represents the log10 relative abundance of each ASV as the mean per group. The taxon at the genus level is shown on the left side, while the right side shows the respective phylum. Statistical test used was Kruskal–Wallis test followed by post-hoc Dunn's multiple comparison test (* and ** denote P < 0.05 and P < 0.01, respectively). Asterisks in SNL column represents significance in comparison to Sham, while asterisks in the SNL+GEG or SNL+SEG columns represents significance in comparison to SNL.
A common signature was observed with the GEG or SEG supplementation in the microbiome compared to the SNL group (Figure 4). Compared to the SNL group, both the SNL+GEG and the SNL+SEG groups had increased abundance of Lactococcus, Sellimonas, Blautia, Erysipelatoclostridiaceae (a family belonging to Firmicutes phylum), and Anaerovoracaceae (a family in the Clostridia class), but a decrease of Prevotellaceae UCG-001, Rikenellaceae RC9 gut group, Mucispirillum and Desulfovibrio (a member of Deferribacterota phylum), Desulfovibrio (a member of Desulfobacterota phylum), Anaerofilum, Eubacterium siraeum group (a member of Ruminococcaceae family, Firmicutes phylum), RF39 (belongs to Bacilli class, Firmicutes phylum), UCG-005 (a member of Oscillospiraceae family, Firmicutes phylum), Lachnospiraceae NK4A136 group, Acetatifactor (a member of Lachnospiraceae family, Firmicutes phylum), Eubacterium ruminantium group, Clostridia UCG-014, and an uncultured Anaerovoracaceae (Figure 4). We found that the abundance of Rikenellaceae and Prevotellaceae in the SNL+GEG and SNL+SEG groups was below or similar to the those in the Sham group (Figure 4).
The SNL+GEG group and the SNL+SEG group had a distinct effect on the gut microbiome as well (Figure 4). Relative to the SNL group, the SNL+GEG group showed an increase in the relative abundance of Parvibacter, Faecalitalea, Dubosiella, and Erysipelotrichaceae, and a decrease in the relative abundance of Muribaculaceae, Bacteroidales, and Ruminococcaceae. Furthermore, relative to the SNL group, the SNL+SEG group showed an increase in the relative abundance of Butryicimonas, Staphylococcus, Jeotgalicoccus, Erysipelatoclostridiaceae, Aerococcus, and Bacillales, and a decrease in relative abundance of Ruminococcaceae UCG-010, Ruminococcus, and Oscillospiraceae NK4A214 group.
Fecal metabolites
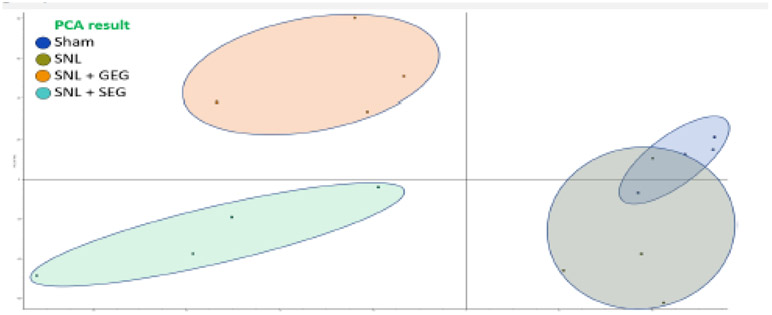
Overall, GEG and SEG supplementation had an impact on the fecal metabolite profiles (Figure 5). PCA of the experimental groups demonstrates less variance and good reproducibility among the 4 groups and significant difference between treated groups (i.e., the SNL+GEG group and the SNL+SEG group) and the sham/SNL groups (Figure 5). The level of 637 and 379 metabolites was significantly altered with GEG and SEG supplementation, respectively; fold-change ≥ 2 and adjusted P < 0.05.
Figure 5.
Effects of GEG and SEG on fecal metabolites of animals with NP, as shown in PCA
Due to the high number of metabolites, we focused on the top metabolites using these two filtering methods. First, we considered the top metabolites with more stringent statistical significance, i.e., adjusted P < 0.001 and fold-change ≥ 10 (Table 1). As a result of this filtering, compared to the SNL group the SNL+GEG group had a higher level of ‘seven’ metabolites including 1'-acetoxychavicol acetate, (4E)-1,7-Bis(4-hydroxyphenyl)-4-hepten-3-one, NP-000629, 7,8-Dimethoxy-3-(2-methyl-3-buten-2-yl)-2H-chromen-2-one, 3-{[4-(2-Pyrimidinyl)piperazino]carbonyl}-2-pyrazinecarboxylic acid, 920863, and (1R,3R,7R,13S)-13-Methyl-6-methylene-4,14,16-trioxatetracyclo[11.2.1.0~1,10~.0~3,7~]hexadec-9-en-5-one (Table 1). Relative to the SNL group, the SNL+SEG group had higher levels for two metabolites, including (+/−)-5-[(tert-Butylamino)-2'-hydroxypropoxy]-1_2_3_4-tetrahydro-1-naphthol and dehydroepiandrosteronesulfate (DHEAS) (Table 1).
Table 1.
Top enriched metabolites in either SNL+GEG or SNL+SEG vs SNL group
| SNL+GEG group vs SNL group* | ||||
|---|---|---|---|---|
| Metabolite Name | Formula | Log2 Fold Change (SNL+GEG/SNL) |
P-value | Adj. P-value |
| 1'-Acetoxyeugenol acetate | C14 H16 O5 | 13.10 | 5.52E-05 | 0.0009558 |
| (4E)-1,7-Bis(4-hydroxyphenyl)-4-hepten-3-one | C19 H20 O3 | 11.20 | 2.30E-07 | 5.86E-05 |
| NP-000629 | C14 H16 O4 | 10.86 | 1.34E-05 | 0.00045946 |
| 7,8-Dimethoxy-3-(2-methyl-3-buten-2-yl)-2H-chromen-2-one | C16 H18 O4 | 10.32 | 1.57E-06 | 0.00015679 |
| 3-{[4-(2-Pyrimidinyl)piperazino]carbonyl}-2-pyrazinecarboxylic acid | C14 H14 N6 O3 | 10.30 | 1.60E-07 | 5.46E-05 |
| 920863 | C16 H20 O5 | 10.27 | 3.01E-07 | 6.50E-05 |
| (1R,3R,7R,13S)-13-Methyl-6-methylene-4,14,16-trioxatetracyclo[11.2.1.0~1,10~.0~3,7~]hexad ec-9-en-5-one | C15 H18 O4 | 10.11 | 5.83E-06 | 0.00029074 |
| SNL+SEG group vs SNL group* | ||||
| Metabolite Name | Formula | Log2 Fold Change (SNL+SEG/SNL) |
P-value | Adj. P-value |
| (+/−)-5-[(tert-Butylamino)-2'-hydroxypropoxy]-1_2_3_4-tetrahydro-1-naphthol | C17 H27 N O3 | 11.57 | 1.93E-08 | 7.44E-05 |
| Dehydroepiandrosterone sulfate (DHEAS) | C19 H28 O5 S | 10.16 | 8.99E-07 | 0.00043157 |
Top metabolites were included if fold change ≥ 10 and adjusted P-value ≤ 0.001
Second, since our physiological results show that either GEG or SEG supplementation have a similar beneficial effect on NP-associated behaviors, we reported common metabolites that occurred in both the SNL+GEG and SNL+SEG groups. We filtered the metabolites for those with similar trends in both SNL+GEG and SNL+SEG groups and selected top shared metabolites (Table 2). Twenty-eight fecal metabolites were enriched with either GEG or SEG supplementation of SNL-treated animals with NP (Table 2).
Table 2.
Top enriched and shared metabolites in both SNL+GEG and SNL+SEG groups vs SNL group
| Metabolite Name* | Log2 Fold Change |
Log2 Fold Change |
|---|---|---|
| (SNL+GEG/SNL) | (SNL+SEG/SNL) | |
| (+)-(1R_2R)-1_2-Diphenylethane-1_2-diol | 7.19 | 6.19 |
| [ST(3:0)]estra-1_3_5(10)-triene-2_4_17beta-triol | 8.15 | 6.95 |
| 1-(4-Hydroxy-3-methoxyphenyl)-3-oxo-5-decanesulfonic acid | 6.7 | 8.61 |
| 1-[2-(3-Hydroxy-1-propen-2-yl)-2,3-dihydro-1-benzofuran-5-yl]ethanone | 9.79 | 10.6 |
| 16a-Hydroxyestrone | 6.24 | 6.18 |
| 1'-Acetoxychavicol acetate | 5.35 | 6.55 |
| 1-Hydroxy-4-isopropyl-7-methoxy-1,6-dimethyl-2(1H)-naphthalenone | 7.6 | 7.12 |
| 3,7,15-Trihydroxy-12,13-epoxytrichothec-9-en-8-one | 6.81 | 7.67 |
| 3-{[4-(2-Pyrimidinyl)piperazino]carbonyl}-2-pyrazinecarboxylic acid | 10.3 | 8.15 |
| 4,4'-(3,4-Dimethyltetrahydrofuran-2,5-diyl)bis(2-methoxyphenol) | 6.35 | 6.59 |
| 5-[(1E)-3-hydroxy-3-methylbut-1-en-1-yl]-2-methylcyclohex-5-ene-1,2,4-triol | 8.26 | 5.99 |
| 6-{4-Oxo-5-[(2E)-2-penten-1-yl]-2-cyclopenten-1-yl}hexanoic acid | 5.16 | 6.23 |
| 7,8-Dihydroxy-3,7-dimethyl-6-oxo-7,8-dihydro-6H-isochromene-5-carbaldehyde | 7 | 6.64 |
| 8'-Hydroxyabscisate | 6.45 | 8.38 |
| alpha-Santonin | 6.87 | 5.19 |
| Benzylideneacetone | 6.56 | 6.21 |
| Dihydrokavain | 6.03 | 7.52 |
| Di-n-Amyl phthalate | 5.78 | 6.7 |
| Ethyl vanillin isobutyrate | 5.91 | 11.34 |
| Mazindol-d4 | 9.04 | 5.68 |
| Methyl (2E,4E)-5-(3,8-dihydroxy-1,5-dimethyl-6-oxabicyclo[3.2.1]oct-8-yl)-3-methyl-2,4-pentadienoate | 6.91 | 5.35 |
| methyl 2-[4-ethenyl-2,6-dihydroxy-3-(3-hydroxyprop-1-en-2-yl)-4-methylcyclohexyl]prop-2-enoate | 5.49 | 5.17 |
| Methyl N-formylnorleucylleucylphenylalaninate | 8.3 | 8.7 |
| Resorcinol diglycidyl ether | 6.35 | 8.32 |
| Sakuranetin | 8.1 | 5.48 |
| Trepibutone | 8.39 | 6.9 |
| Ubiquinone-1(CoQ1) | 5.31 | 8.36 |
| zinniol | 6.04 | 5.94 |
Shared metabolites were included if fold change for both SNL+GEG/SNL and SNL+SEG/SNL ≥ 5 and adjusted P-value ≤ 0.05
DISCUSSION
In the present investigation, the SNL model of NP was successfully employed to investigate the impact of two ginger extracts, GEG and SEG, supplemented into the diet for 30 days in NP-associated behaviors. Compared to Sham rats, SNL rats showed pain-like behaviors (mechanical hypersensitivity) and anxiety-like behavior consistent with previous studies [3, 43]. Ginger and its bioactive components have been shown to reduce pain in a variety of NP models [31, 32, 39, 51]. The present study demonstrated potent effects of dietary GEG and SEG supplementation in mitigating mechanical hypersensitivity in the SNL-operated male rats during chronic pain. The capacity of 30-day GEG and SEG supplementation post SNL to decrease indices of mechanical allodynia in SNL-treated male rats corroborates the findings of Mata-Bermudez et al [39] and Fajirin et al. [35], respectively.
The current findings that both GEG and SEG supplementation decrease anxiety-like behaviors in the SNL model of NP further corroborates ginger’s anxiolytic-like role in vivo through serotonin [5-HydroxyTryptamine (5-HT)] and its receptors (5-HT1, 5-HT1A, 5-HT2, 5-HT3, 5-HT7 receptors) [52-57]. Ginger and its constituents 6-shogaol, 1-dehydro-6-gingerdione, 8- and 10-gingerol have shown to attenuate serotonin-induced hypothermia in mice [25]. Nievergelt et al. demonstrated 10-shogaol and 1-dehydro-6- gingerdione partially activated the serotonin 5-HT1A receptor [53]. Gingerols or the diterpenoid galanolactone are potent antagonists at the 5-HT3 receptor [56, 57].
Although we did not measure pro-inflammatory cytokines in the present study, previous studies have shown the inhibitory impacts of GEG or SEG on mechanical hypersensitivity may be mediated, in part, through inhibition of neuroinflammation and oxidative stress in vitro studies [31, 58-60]. Significant anti-neuroinflammatory effects of GEG and SEG have been reported in animals with NP. For instance, Mata-Bermudez et al. demonstrated that the antiallodynic effect induced by 6-gingerol in SNL-treated rats is mediated by activating the serotoninergic system and NO-cyclic guanosine monophosphate-ATP-sensitive K+ channel pathway [39]. Fajrin et al. reported 6-shogaol oral feeding reduced mechanical hyperalgesia in streptozotocin-induced NP mice via reduction in axon myelination damage from the sciatic nerve [35] and mRNA expression of transient receptor potential vanilloid-1 (TRPV-1) and N-methyl-D-aspartate receptor subunit 2B (NMDAR2B) in the spinal cord [51].
Excessive oxidative stress has been associated with the progression of chronic NP. NP is initiated by increases in ROS, which leads to increased mitochondrial damage and increased plasma-free mitochondrial DNA (mtDNA) [41, 61, 62]. The present study is the first study to show that both GEG and SEG significantly reduced ccf-mtDNA levels in the SNL-treated animals with NP, further corroborating the previous studies, showing ginger root extract improved mitochondrial function through enhancing antioxidant capacity and decreasing ROS production [18, 63].
Microbiome analysis conducted in the present study showed a shift in the composition of the gut microbiome in the SNL-operated rats with mechanical hypersensitivity and anxiety-like behaviors compared to sham-operated rats, suggesting a link between the gut microbiome and behavioral abnormalities in these SNL rats. From our collective knowledge, this is the first study establishing that mechanical hypersensitivity and anxiety-like behaviors in SNL-operated rats were mitigated by GEG and SEG supplementation, by means of an alteration of the gut microbiome, namely a decrease in the abundance of Eubacterium ruminantium, Clostridia UCG-014, and especially Prevotellaceae UCG-001 [11, 64]. Yang et al. reported that compared to the sham group, SNI-treated rats with depression-like/anhedonia-like phenotypes had increased levels of Prevotellaceae UCG-001, and fecal transplantation from SNI rats with or without anhedonia can exaggerate or mitigate pain and depression-like/anhedonia-like phenotypes in the pseudo-germ-free mice, respectively [64]. The current observation that GEG or SEG caused a decrease in Prevotellaceae UCG-001 in SNL rats with less anxiety-like phenotypes agrees with Yang’s study [64].
Ginger has been widely used for centuries to treat intestinal inflammation, both in vitro and in vivo. For instance, 6-gingerol has been shown to maintain barrier function in a cellular model of gut inflammation [65]. In animals, 6-gingerol abates colonic injury [66] and restores colonic permeability [67], in part mediated through downregulation of oxidative stress and inflammation in the inflamed colon [67-69]. Zingerone (chemical components of ginger) significantly reduced colonic transit and attenuated anxiety-like behavior in rats with irritable bowel disorder [70]. 6-shogoal prevents TNF-α-induced barrier loss, as shown by downregulation of claudin-2 via inhibition of PI3K/Akt and NF-κB signaling [71]. 6-gingerol prevents chronic ulcerative colitis via anti-inflammatory and anti-oxidative mechanisms and preservation of Wnt/β-catenin signaling pathway [72]. In the present study, the findings that the increased intestinal “potential pro-inflammatory” taxa (Prevotellaceae UCG-001 [73], Eubacterium ruminantium group, and Mucispirillum [74] in the SNL-operated rats were decreased when treated with GEG or SEG supplementation, further corroborating the anti-inflammatory role of GEG and SEG on colon health via modification of gut microbiome composition. Furthermore, Sun et al. recently reported that an increase in the abundance of Rikenellaceae RC9 gut group has been associated with increased intestinal permeability and oxidative stress, subsequently impairing the intestinal barrier and stimulating gut inflammation in coronary heart disease [75]. In the present study, we found that both GEG and SEG supplementation reduced the abundance of Rikenellaceae RC9 gut group which was even below the sham level along with a reduction of ccf-mtDNA in SNL-treated rats, supporting the protective role of GEG and SEG in gut health, presumably through decreased intestinal permeability and oxidative stress.
In the present study, GEG and SEG had differential effects on the gut microbiome composition. In general, the present study shows the gut micro-ecosystem of GEG and SEG-fed rats was shifted at the phylum level by favoring Firmicutes at expense of Bacteroidetes. Numerous studies in mice and humans indicated that the higher ratio of Firmicutes to Bacteroidetes might play an important role in energy uptake and nutrient utilization [76, 77]. The findings that an increase in abundance of SCFA-associated beneficial gut microbiome in the present study suggests that GEG and SEG supplementation can contribute to the improvement of gut function [78].
Wang et al. recently reported that ginger supplementation modulated the gut microbiota composition by increased amounts of species belonging to the Bifidobacterium genus as well as SCFA-producing bacteria (Alloprevotella and Allobaculum), along with increases in fecal SCFA (e.g., butyrate) concentrations in obese mice [40]. Feng et al. also showed that 6-gingerol increased the relative abundance of beneficial Bacteroidetes while decreasing Firmicutes on the phylum level in rats treated with cisplatin [79]. The present study supports the findings that GEG and SEG supplementation restores gut dysbiosis by elevating SCFA-producing beneficial bacteria, e.g., Lactococcus [80], Sellimonas [81], Blautia [82], and Erysipelatoclostridiaceae [83], while decreasing potential pro-inflammatory bacteria, such as Mucispirillum [84], Desulfovibrio [84], Anaerofilum [85], Eubacterium siraeum group, Eubacterium ruminantium group [85], Lachnospiraceae NK4A136 group [86], and Clostridia UCG-014 [87].
Ginger has been shown to benefit glucose homeostasis in diabetic animals [88, 89]. Gut microbiome is considered to be closely related to the occurrence and development of Type 1 diabetes mellitus in recent years [90, 91]. Specifically, Ma et al. reported an imbalance of the gut microbiota in individuals with Type 1 Diabetes presented by higher pathogenic bacteria (e.g., Ruminococcaceae, Shigella, Enterococcus, Streptococcus, Rothia, and Alistipes) and decreased beneficial bacteria (e.g., Lactobacillus, Faecalitalea, Butyricicoccus, Allobaculum, and Parvibacter) that are associated with infection and inflammation in diabetic subjects [90]. Although our SNL model is not a diabetic model, the findings that GEG supplementation increased the abundance of Faecalitalea and Parvibacter in animals further supports the beneficial role of GEG in gut health of SNL-treated animals. Moreover, recent studies have shown that the decreased abundance of Butryicimonas [92], Staphylococcus [91], Jeotgalicoccus [91], Aerococcus [93], and Bacillales [94] is associated with depression-like behaviors in animals. Intriguingly, in addition to an increased abundance of Prevotellaceae UCG-001, we have also found increased abundance of Butryicimonas, Staphylococcus, Jeotgalicoccus, Aerococcus, and Bacillales, while improving NP-associated negative anxiety-like behaviors. Together, these collective findings corroborate the SEG’s potential in ameliorating depression-like behaviors in SNL-treated rats.
The present study is the first study to demonstrate that compared to the SNL group, the SNL+GEG and SNL+SEG groups had relative higher intensity of fecal 1'-acetoxychavicol acetate that may be involved in inhibition of NF-κB [95, 96] and ERK/MAPK signaling [97, 98, 99]. Our findings of decreased neuroinflammation in SNL-treated animals due to GEG and SEG can be partially explained by the increased 1'-acetoxychavicol acetate levels in both SNL+GEG and SNL+SEG groups.
In the present study, we also demonstrated that the anti-neuroinflammatory effects of GEG and SEG are not only due to their anti-inflammatory property, as shown by increased levels in alpha-santonin [100], dihydrokavain [101], trepibutone [93], and sakuranetin [102], but also due to their anti-oxidant property, as shown by increased levels of sakuranetin [102, 103] and ubiquinone-1 (also called CoQ1) [104]. alpha-Santonin has exhibited diverse bioactivities including antioxidant [105, 106], anti-inflammation [107], and analgesic [108]. Dihydrokavain has exhibited a greater analgesic effect than aspirin in morphine-induced pain [109] due to its anti-inflammatory activity [110]. Dihydrokavain was reported to contribute significantly to the stress-induced anxiolytic effects in chicks under social separation stress [111]. In the present study, the observation that both GEG and SEG supplementation into the diet significantly reduces pain and anxiety-associated behaviors in SNL-treated animals may be, in part, due to elevation in the dihydrokavin levels. Sakuranetin, a group of methoxylated flavanones, was reported to have potent anti-inflammatory, antioxidant, antimicrobial, antiparasitic, antimutagenic, and antiallergic properties [102]. Ubiquinone-1 (also called CoQ1), a member of polyprenylbenzoquinones, acts as an antioxidant with the ability to transfer electrons in the electron transport chain and to stabilize mitochondrial complex in the status of inflammation [104]. Our observation that the elevation of fecal anti-inflammatory, anti-oxidant, and anxiolytic effects of GEG and SEG mitigated the SNL-induced NP parameters are corroborated with properties of metabolites as discussed above [102, 104, 109-111].
Based on our best knowledge, the present research is the first study to show that both GEG and SEG have antinociceptive effects in SNL-treated animals through a modulation of fecal metabolites, as shown in the increased levels of mazindol-d4 [112] and trepibutone [93, 113]. Mazindol is a non-selective catecholamine reuptake inhibitor, which blocks dopamine reuptake and thus could be used as an enhancer of dopamine release by neuropsychological tasks [114]. Rothman et al. reported that mazindol attenuates cocaine craving and euphoria by the inhibition of dopamine reuptake [115]. Robledo-González et al. showed that repeated mazindol administration significantly decreased spontaneous pain-like behaviors in arthritic joints animals through activation of dopaminergic and opioid receptors [112]. In clinic, trepibutone is widely used to mitigate cholestatic disease-associated pain [116]. In the mice with cholestatic disease, the G-protein–coupled BA receptor 1 (TGR5) is expressed by primary sensory neurons, and TGR5’s activation by bile acid induces neuronal hyperexcitability and scratching [113], resulting in the spinal cord releasing neuropeptides [117]. Trepibutone promotes secretion of bile and pancreatic juice, accelerating flaccidity of the smooth muscle in the gastrointestinal tract to decrease internal pressure of the gallbladder and bile duct, resulting in pain reduction in cholestatic disease [116]. The fact that elevated levels of mazindol and trepibutone in SNL-treated animals due to GEG and SEG further elucidate their roles in SNL-induced pain reduction.
In this study, we observed there were differential effects of GEG and SEG on fecal metabolites in SNL-treated rats. In general, these fecal metabolites possessed antioxidant and anti-inflammatory activities in the SNL+GEG group. In the SNL+GEG group, the elevated levels of 1’-acetoxyeugenol acetate [118, 119], (4E)-1,7-bis(4-hydroxyphenyl)-4-hepten-3-one [120], and 3-{[4-(2-pyrimidinyl)piperazino]carbonyl}-2-pyrazinecarboxylic acid [121] possessed antioxidant and anti-inflammatory activities. On the other hand, in the SNL+SEG group, the increased fecal level of (+/−)-5-[(tert- Butylamino)-2'-hydroxypropoxy]-1_2_3_4-tetrahydro-1-naphthol was involved in conversion of NADP+ to NADPH, suggesting SEG’s role in energy metabolism [122]. Dehydroepiandrosterone sulfate (DHEAS), an endogenous androstane steroid that is produced by the adrenal cortex, is considered a neuroactive neurosteroid [123]. DHEAS can modulate inhibitory gamma-aminobutyric acid (GABA) and excitatory N-methyl-D-aspartate (NMDA) receptors, producing complex neuronal effects. Naylor et al. reported that serum DHEAS levels were inversely correlated to the level of chronic low back pain in female Veterans, indicating a role for DHEAS in pain analgesia [124].
CONCLUSION
Both GEG and SEG supplementation into the diet decreased mechanical hypersensitivity and improved anxiety-like behavior mediated in part by suppressing oxidative stress (ccf-mtDNA). GEG and SEG exhibited differential effects on the microbiome composition and fecal metabolites, suggesting a prebiotic potential for dietary ginger root intake in the management of NP.
Highlights.
Beneficial effects of two ginger root extracts rich in gingerols and shogaols on pain sensitivity and anxiety-like behaviors, and circulating cell-free mitochondria DNA in the development of neuropathic pain
Dietary gingerols and shogaols favored microbiome composition in rats with neuropathic pain
Differential impacts of gingerols and shogaols on fecal metabolites in rats with neuropathic pain
Acknowledgment
This study was supported by the United States Department of Agriculture-NIFA, GRANT2021-67017-34026) (Shen and Neugebauer), NIH grants R01NS038261 (Neugebauer), and Texas Tech University Health Sciences Center (Shen and Neugebauer). Authors thank Jacob Lovett for editorial work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Colloca L, et al. Neuropathic pain. Nat Rev Dis Primers, 2017. 3: p. 17002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finnerup NB, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol, 2015. 14(2): p. 162–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ji G, et al. Fear extinction learning ability predicts neuropathic pain behaviors and amygdala activity in male rats. Mol Pain, 2018. 14: p. 1744806918804441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teixeira-Santos L, Albino-Teixeira A, and Pinho D, Neuroinflammation, oxidative stress and their interplay in neuropathic pain: Focus on specialized pro-resolving mediators and NADPH oxidase inhibitors as potential therapeutic strategies. Pharmacol Res, 2020. 162: p. 105280. [DOI] [PubMed] [Google Scholar]
- 5.Zhong S, et al. Targeting strategies for chemotherapy-induced peripheral neuropathy: does gut microbiota play a role? Crit Rev Microbiol, 2019. 45(4): p. 369–393. [DOI] [PubMed] [Google Scholar]
- 6.Kigerl KA, et al. Gut dysbiosis impairs recovery after spinal cord injury. J Exp Med, 2016. 213(12): p. 2603–2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Du J, et al. Spinal Cord Injury Changes the Structure and Functional Potential of Gut Bacterial and Viral Communities. mSystems, 2021. 6(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kigerl KA, Mostacada K, and Popovich PG, Gut Microbiota Are Disease-Modifying Factors After Traumatic Spinal Cord Injury. Neurotherapeutics, 2018. 15(1): p. 60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Defaye M, et al. Microbiota: a novel regulator of pain. J Neural Transm (Vienna), 2020. 127(4): p. 445–465. [DOI] [PubMed] [Google Scholar]
- 10.Ait-Belgnaoui A, et al. Lactobacillus farciminis treatment suppresses stress induced visceral hypersensitivity: a possible action through interaction with epithelial cell cytoskeleton contraction. Gut, 2006. 55(8): p. 1090–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin B, et al. Gut microbiota regulates neuropathic pain: potential mechanisms and therapeutic strategy. J Headache Pain, 2020. 21(1): p. 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rousseaux C, et al. Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nat Med, 2007. 13(1): p. 35–7. [DOI] [PubMed] [Google Scholar]
- 13.Abdel-Haq R, et al. Microbiome-microglia connections via the gut-brain axis. J Exp Med, 2019. 216(1): p. 41–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo R, et al. Pain regulation by gut microbiota: molecular mechanisms and therapeutic potential. Br J Anaesth, 2019. 123(5): p. 637–654. [DOI] [PubMed] [Google Scholar]
- 15.Duan L, et al. PDGFRbeta Cells Rapidly Relay Inflammatory Signal from the Circulatory System to Neurons via Chemokine CCL2. Neuron, 2018. 100(1): p. 183–200 e8. [DOI] [PubMed] [Google Scholar]
- 16.Rafalski VA, Merlini M, and Akassoglou K, Pericytes: The Brain's Very First Responders? Neuron, 2018. 100(1): p. 11–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang AT, et al. Endothelial TLR4 and the microbiome drive cerebral cavernous malformations. Nature, 2017. 545(7654): p. 305–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li J, et al. 6-gingerol ameliorates age-related hepatic steatosis: Association with regulating lipogenesis, fatty acid oxidation, oxidative stress and mitochondrial dysfunction. Toxicol Appl Pharmacol, 2019. 362: p. 125–135. [DOI] [PubMed] [Google Scholar]
- 19.Chen G, et al. Microglia in Pain: Detrimental and Protective Roles in Pathogenesis and Resolution of Pain. Neuron, 2018. 100(6): p. 1292–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao YJ and Ji RR, Targeting astrocyte signaling for chronic pain. Neurotherapeutics, 2010. 7(4): p. 482–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao YJ and Ji RR, Chemokines, neuronal-glial interactions, and central processing of neuropathic pain. Pharmacol Ther, 2010. 126(1): p. 56–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsuda M, Huh Y, and Ji RR, Roles of inflammation, neurogenic inflammation, and neuroinflammation in pain. J Anesth, 2019. 33(1): p. 131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tjendraputra E, et al. Effect of ginger constituents and synthetic analogues on cyclooxygenase-2 enzyme in intact cells. Bioorg Chem, 2001. 29(3): p. 156–63. [DOI] [PubMed] [Google Scholar]
- 24.Dugasani S, et al. Comparative antioxidant and anti-inflammatory effects of [6]-gingerol, [8]-gingerol, [10]-gingerol and [6]-shogaol. J Ethnopharmacol, 2010. 127(2): p. 515–20. [DOI] [PubMed] [Google Scholar]
- 25.Lantz RC, et al. The effect of extracts from ginger rhizome on inflammatory mediator production. Phytomedicine, 2007. 14(2-3): p. 123–8. [DOI] [PubMed] [Google Scholar]
- 26.Simon A, et al. Blood-brain barrier permeability study of ginger constituents. J Pharm Biomed Anal, 2020. 177: p. 112820. [DOI] [PubMed] [Google Scholar]
- 27.Shen CL, Hong KJ, and Kim SW, Comparative effects of ginger root (Zingiber officinale Rosc.) on the production of inflammatory mediators in normal and osteoarthrotic sow chondrocytes. J Med Food, 2005. 8(2): p. 149–53. [DOI] [PubMed] [Google Scholar]
- 28.Shen CL, Hong KJ, and Kim SW, Effects of ginger (Zingiber officinale Rosc.) on decreasing the production of inflammatory mediators in sow osteoarthrotic cartilage explants. J Med Food, 2003. 6(4): p. 323–8. [DOI] [PubMed] [Google Scholar]
- 29.Bartels EM, et al. Efficacy and safety of ginger in osteoarthritis patients: a meta-analysis of randomized placebo-controlled trials. Osteoarthritis Cartilage, 2015. 23(1): p. 13–21. [DOI] [PubMed] [Google Scholar]
- 30.Sepahvand R, et al. Ginger (Zingiber officinale Roscoe) elicits antinociceptive properties and potentiates morphine-induced analgesia in the rat radiant heat tail-flick test. J Med Food, 2010. 13(6): p. 1397–401. [DOI] [PubMed] [Google Scholar]
- 31.Borgonetti V, et al. Zingiber officinale Roscoe rhizome extract alleviates neuropathic pain by inhibiting neuroinflammation in mice. Phytomedicine, 2020. 78: p. 153307. [DOI] [PubMed] [Google Scholar]
- 32.Gauthier ML, Beaudry F, and Vachon P, Intrathecal [6]-gingerol administration alleviates peripherally induced neuropathic pain in male Sprague-Dawley rats. Phytother Res, 2013. 27(8): p. 1251–4. [DOI] [PubMed] [Google Scholar]
- 33.Chia JSM, et al. Zerumbone alleviates chronic constriction injury-induced allodynia and hyperalgesia through serotonin 5-HT receptors. Biomed Pharmacother, 2016. 83: p. 1303–1310. [DOI] [PubMed] [Google Scholar]
- 34.Zulazmi NA, et al. Zerumbone Alleviates Neuropathic Pain through the Involvement of l-Arginine-Nitric Oxide-cGMP-K(+) ATP Channel Pathways in Chronic Constriction Injury in Mice Model. Molecules, 2017. 22(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fajrin F, et al. The improvement of pain behavior and sciatic nerves morphology in mice model of painful diabetic neuropathy upon administration of ginger (Zingiber officinale Roscoe.) extract and its pungent compound, 6-shogaol. Journal of Natural Science, Biology and Medicine, 2019. 10(2): p. 149–156. [Google Scholar]
- 36.Fajrin FA, et al. The activity of red ginger oil in antioxidant study in vitro and antihyperalgesia effect in alloxan-induced painful diabetic neuropathy in mice. The Thai Journal of Pharmaceutical Sciences, 2019. 43(2): p. 69–75. [Google Scholar]
- 37.Fajrin FA, et al. Antihyperalgesia potency of Zingiber officinale var. Rubrum in inflammatory and neuropathy-induced chronic pain condition in mice. Pak J Pharm Sci, 2019. 32(4): p. 1663–1669. [PubMed] [Google Scholar]
- 38.Chia JSM, et al. Zerumbone Modulates alpha2A-Adrenergic, TRPV1, and NMDA NR2B Receptors Plasticity in CCI-Induced Neuropathic Pain In Vivo and LPS-Induced SH-SY5Y Neuroblastoma In Vitro Models. Front Pharmacol, 2020. 11: p. 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mata-Bermudez A, et al. Antiallodynic effect induced by [6]-gingerol in neuropathic rats is mediated by activation of the serotoninergic system and the nitric oxide-cyclic guanosine monophosphate-adenosine triphosphate-sensitive K(+) channel pathway. Phytother Res, 2018. 32(12): p. 2520–2530. [DOI] [PubMed] [Google Scholar]
- 40.Wang J, et al. Beneficial effects of ginger on prevention of obesity through modulation of gut microbiota in mice. Eur J Nutr, 2020. 59(2): p. 699–718. [DOI] [PubMed] [Google Scholar]
- 41.Chung JM, Kim HK, and Chung K, Segmental spinal nerve ligation model of neuropathic pain. Methods Mol Med, 2004. 99: p. 35–45. [DOI] [PubMed] [Google Scholar]
- 42.Bennett GJ, et al. Models of neuropathic pain in the rat. Curr Protoc Pharmacol, 2003. Chapter 5: p. Unit5 32. [DOI] [PubMed] [Google Scholar]
- 43.Ji G, et al. 5-HT2C Receptor Knockdown in the Amygdala Inhibits Neuropathic-Pain-Related Plasticity and Behaviors. J Neurosci, 2017. 37(6): p. 1378–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Navratilova E, et al. Kappa opioid signaling in the central nucleus of the amygdala promotes disinhibition and aversiveness of chronic neuropathic pain. Pain, 2019. 160(4): p. 824–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zimmermann M, Ethical guidelines for investigations of experimental pain in conscious animals. Pain, 1983. 16(2): p. 109–110. [DOI] [PubMed] [Google Scholar]
- 46.Li XH, et al. Attenuation of liver pro-inflammatory responses by Zingiber officinale via inhibition of NF-kappa B activation in high-fat diet-fed rats. Basic Clin Pharmacol Toxicol, 2012. 110(3): p. 238–44. [DOI] [PubMed] [Google Scholar]
- 47.Mansour DF, et al. The Carcinogenic Agent Diethylnitrosamine Induces Early Oxidative Stress, Inflammation and Proliferation in Rat Liver, Stomach and Colon: Protective Effect of Ginger Extract. Asian Pac J Cancer Prev, 2019. 20(8): p. 2551–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shao X, et al. Quantitative analysis of ginger components in commercial products using liquid chromatography with electrochemical array detection. J Agric Food Chem, 2010. 58(24): p. 12608–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zierer J, et al. The fecal metabolome as a functional readout of the gut microbiome. Nat Genet, 2018. 50(6): p. 790–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bolyen E, et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol, 2019. 37(8): p. 852–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fajrin FA, et al. Ginger extract and its compound, 6-shogaol, attenuates painful diabetic neuropathy in mice via reducing TRPV1 and NMDAR2B expressions in the spinal cord. J Ethnopharmacol, 2020. 249: p. 112396. [DOI] [PubMed] [Google Scholar]
- 52.Cortes-Altamirano JL, et al. Review: 5-HT1, 5-HT2, 5-HT3 and 5-HT7 Receptors and their Role in the Modulation of Pain Response in the Central Nervous System. Curr Neuropharmacol, 2018. 16(2): p. 210–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nievergelt A, et al. Identification of serotonin 5-HT1A receptor partial agonists in ginger. Bioorg Med Chem, 2010. 18(9): p. 3345–51. [DOI] [PubMed] [Google Scholar]
- 54.Kilpatrick GJ, Jones BJ, and Tyers MB, Identification and distribution of 5-HT3 receptors in rat brain using radioligand binding. Nature, 1987. 330(6150): p. 746–8. [DOI] [PubMed] [Google Scholar]
- 55.Costall B, et al. The effect of the 5-HT3 receptor antagonist, RS-42358-197, in animal models of anxiety. Eur J Pharmacol, 1993. 234(1): p. 91–9. [DOI] [PubMed] [Google Scholar]
- 56.Huang QR, et al. Anti-5-hydroxytryptamine3 effect of galanolactone, diterpenoid isolated from ginger. Chem Pharm Bull (Tokyo), 1991. 39(2): p. 397–9. [DOI] [PubMed] [Google Scholar]
- 57.Yamahara J, et al. Active components of ginger exhibiting anti-serotonergic action. Phytotherapy Research, 1989. 3(2): p. 70–71. [Google Scholar]
- 58.Jung HW, et al. Hexane fraction of Zingiberis Rhizoma Crudus extract inhibits the production of nitric oxide and proinflammatory cytokines in LPS-stimulated BV2 microglial cells via the NF-kappaB pathway. Food Chem Toxicol, 2009. 47(6): p. 1190–7. [DOI] [PubMed] [Google Scholar]
- 59.Ho SC, Chang KS, and Lin CC, Anti-neuroinflammatory capacity of fresh ginger is attributed mainly to 10-gingerol. Food Chem, 2013. 141(3): p. 3183–91. [DOI] [PubMed] [Google Scholar]
- 60.Ha SK, et al. 6-Shogaol, a ginger product, modulates neuroinflammation: a new approach to neuroprotection. Neuropharmacology, 2012. 63(2): p. 211–23. [DOI] [PubMed] [Google Scholar]
- 61.West AP, Shadel GS, and Ghosh S, Mitochondria in innate immune responses. Nat Rev Immunol, 2011. 11(6): p. 389–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ermakov AV, et al. Oxidized extracellular DNA as a stress signal in human cells. Oxid Med Cell Longev, 2013. 2013: p. 649747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hosseinzadeh A, et al. Protective Effect of Ginger (Zingiber officinale Roscoe) Extract against Oxidative Stress and Mitochondrial Apoptosis Induced by Interleukin-1beta in Cultured Chondrocytes. Cells Tissues Organs, 2017. 204(5-6): p. 241–250. [DOI] [PubMed] [Google Scholar]
- 64.Yang C, et al. Key role of gut microbiota in anhedonia-like phenotype in rodents with neuropathic pain. Transl Psychiatry, 2019. 9(1): p. 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim Y, Kim DM, and Kim JY, Ginger Extract Suppresses Inflammatory Response and Maintains Barrier Function in Human Colonic Epithelial Caco-2 Cells Exposed to Inflammatory Mediators. J Food Sci, 2017. 82(5): p. 1264–1270. [DOI] [PubMed] [Google Scholar]
- 66.Ajayi BO, Adedara IA, and Farombi EO, 6-Gingerol abates benzo[a]pyrene-induced colonic injury via suppression of oxido-inflammatory stress responses in BALB/c mice. Chem Biol Interact, 2019. 307: p. 1–7. [DOI] [PubMed] [Google Scholar]
- 67.Deol PK, et al. Managing colonic inflammation associated gut derangements by systematically optimised and targeted ginger extract-Lactobacillus acidophilus loaded pharmacobiotic alginate beads. Int J Biol Macromol, 2017. 105(Pt 1): p. 81–91. [DOI] [PubMed] [Google Scholar]
- 68.Deol PK, et al. Coadministration of ginger extract-Lactobacillus acidophilus (cobiotic) reduces gut inflammation and oxidative stress via downregulation of COX-2, i-NOS, and c-Myc. Phytother Res, 2018. 32(10): p. 1950–1956. [DOI] [PubMed] [Google Scholar]
- 69.Guo S, et al. Ginger Alleviates DSS-Induced Ulcerative Colitis Severity by Improving the Diversity and Function of Gut Microbiota. Front Pharmacol, 2021. 12: p. 632569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Banji D, et al. Zingerone regulates intestinal transit, attenuates behavioral and oxidative perturbations in irritable bowel disorder in rats. Phytomedicine, 2014. 21(4): p. 423–9. [DOI] [PubMed] [Google Scholar]
- 71.Luettig J, et al. The ginger component 6-shogaol prevents TNF-alpha-induced barrier loss via inhibition of PI3K/Akt and NF-kappaB signaling. Mol Nutr Food Res, 2016. 60(12): p. 2576–2586. [DOI] [PubMed] [Google Scholar]
- 72.Ajayi BO, Adedara IA, and Farombi EO, Protective mechanisms of 6-gingerol in dextran sulfate sodium-induced chronic ulcerative colitis in mice. Hum Exp Toxicol, 2018. 37(10): p. 1054–1068. [DOI] [PubMed] [Google Scholar]
- 73.Ibrahim A, et al. Colitis-induced colorectal cancer and intestinal epithelial estrogen receptor beta impact gut microbiota diversity. Int J Cancer, 2019. 144(12): p. 3086–3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Caruso R, et al. A specific gene-microbe interaction drives the development of Crohn's disease-like colitis in mice. Sci Immunol, 2019. 4(34). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sun L, et al. Cecal Gut Microbiota and Metabolites Might Contribute to the Severity of Acute Myocardial Ischemia by Impacting the Intestinal Permeability, Oxidative Stress, and Energy Metabolism. Front Microbiol, 2019. 10: p. 1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bervoets L, et al. Differences in gut microbiota composition between obese and lean children: a cross-sectional study. Gut Pathog, 2013. 5(1): p. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ley RE, et al. Microbial ecology: human gut microbes associated with obesity. Nature, 2006. 444(7122): p. 1022–3. [DOI] [PubMed] [Google Scholar]
- 78.Zhou J, et al. Dietary supplemental xylooligosaccharide modulates nutrient digestibility, intestinal morphology, and gut microbiota in laying hens. Anim Nutr, 2021. 7(1): p. 152–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Feng X, et al. Effects of ondansetron and [6]-gingerol on pica and gut microbiota in rats treated with cisplatin. Drug Des Devel Ther, 2019. 13: p. 2633–2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Markowiak-Kopec P and Slizewska K, The Effect of Probiotics on the Production of Short-Chain Fatty Acids by Human Intestinal Microbiome. Nutrients, 2020. 12(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu YS, et al. Microbiota populations and short-chain fatty acids production in cecum of immunosuppressed broilers consuming diets containing gamma-irradiated Astragalus polysaccharides. Poult Sci, 2021. 100(1): p. 273–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang C, et al. Algal Oil Rich in Docosahexaenoic Acid Alleviates Intestinal Inflammation Induced by Antibiotics Associated with the Modulation of the Gut Microbiome and Metabolome. J Agric Food Chem, 2021. [DOI] [PubMed] [Google Scholar]
- 83.Bojovic K, et al. Gut Microbiota Dysbiosis Associated With Altered Production of Short Chain Fatty Acids in Children With Neurodevelopmental Disorders. Front Cell Infect Microbiol, 2020. 10: p. 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li Y, et al. The Anti-Inflammatory Effect and Mucosal Barrier Protection of Clostridium butyricum RH2 in Ceftriaxone-Induced Intestinal Dysbacteriosis. Front Cell Infect Microbiol, 2021. 11: p. 647048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nagao-Kitamoto H and Kamada N, Host-microbial Cross-talk in Inflammatory Bowel Disease. Immune Netw, 2017. 17(1): p. 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xia T, et al. Polyphenol-rich vinegar extract regulates intestinal microbiota and immunity and prevents alcohol-induced inflammation in mice. Food Res Int, 2021. 140: p. 110064. [DOI] [PubMed] [Google Scholar]
- 87.Emami NK, et al. Effect of Probiotics and Multi-Component Feed Additives on Microbiota, Gut Barrier and Immune Responses in Broiler Chickens During Subclinical Necrotic Enteritis. Front Vet Sci, 2020. 7: p. 572142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Akhani SP, Vishwakarma SL, and Goyal RK, Anti-diabetic activity of Zingiber officinale in streptozotocin-induced type I diabetic rats. J Pharm Pharmacol, 2004. 56(1): p. 101–5. [DOI] [PubMed] [Google Scholar]
- 89.Abdulrazaq NB, et al. Beneficial effects of ginger (Zingiber officinale) on carbohydrate metabolism in streptozotocin-induced diabetic rats. Br J Nutr, 2012. 108(7): p. 1194–201. [DOI] [PubMed] [Google Scholar]
- 90.Ma Q, et al. Investigation of gut microbiome changes in type 1 diabetic mellitus rats based on high-throughput sequencing. Biomed Pharmacother, 2020. 124: p. 109873. [DOI] [PubMed] [Google Scholar]
- 91.Feng Z, et al. Wenyang Jieyu Decoction Alleviates Depressive Behavior in the Rat Model of Depression via Regulation of the Intestinal Microbiota. Evid Based Complement Alternat Med, 2020. 2020: p. 3290450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang C, et al. Possible role of the gut microbiota-brain axis in the antidepressant effects of (R)-ketamine in a social defeat stress model. Transl Psychiatry, 2017. 7(12): p. 1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sun L, et al. Fluoxetine ameliorates dysbiosis in a depression model induced by chronic unpredicted mild stress in mice. Int J Med Sci, 2019. 16(9): p. 1260–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Song X, et al. Puerarin ameliorates depression-like behaviors of with chronic unpredictable mild stress mice by remodeling their gut microbiota. J Affect Disord, 2021. 290: p. 353–363. [DOI] [PubMed] [Google Scholar]
- 95.Ito K, et al. 1'-acetoxychavicol acetate is a novel nuclear factor kappaB inhibitor with significant activity against multiple myeloma in vitro and in vivo. Cancer Res, 2005. 65(10): p. 4417–24. [DOI] [PubMed] [Google Scholar]
- 96.Satoh R, et al. Identification of ACA-28, a 1'-acetoxychavicol acetate analogue compound, as a novel modulator of ERK MAPK signaling, which preferentially kills human melanoma cells. Genes Cells, 2017. 22(7): p. 608–618. [DOI] [PubMed] [Google Scholar]
- 97.Shih RH, Wang CY, and Yang CM, NF-kappaB Signaling Pathways in Neurological Inflammation: A Mini Review. Front Mol Neurosci, 2015. 8: p. 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cao Y, et al. Central sensitization and MAPKs are involved in occlusal interference-induced facial pain in rats. J Pain, 2013. 14(8): p. 793–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu MG, et al. Differential roles of ERK, JNK and p38 MAPK in pain-related spatial and temporal enhancement of synaptic responses in the hippocampal formation of rats: multi-electrode array recordings. Brain Res, 2011. 1382: p. 57–69. [DOI] [PubMed] [Google Scholar]
- 100.Perri F, et al. Naturally occurring sesquiterpene lactones and their semi-synthetic derivatives modulate PGE2 levels by decreasing COX2 activity and expression. Heliyon, 2019. 5(3): p. e01366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xuan TD and Teschke R, Dihydro-5,6-dehydrokavain (DDK) from Alpinia zerumbet: Its Isolation, Synthesis, and Characterization. Molecules, 2015. 20(9): p. 16306–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Stompor M, A Review on Sources and Pharmacological Aspects of Sakuranetin. Nutrients, 2020. 12(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shanmugavasan A and Ramachandran T, Investigation of the extraction process and phytochemical composition of preparations of Dodonaea viscosa (L.) Jacq. J Ethnopharmacol, 2011. 137(3): p. 1172–6. [DOI] [PubMed] [Google Scholar]
- 104.Bongard RD, Townsley MI, and Merker MP, The effects of mitochondrial complex I blockade on ATP and permeability in rat pulmonary microvascular endothelial cells in culture (PMVEC) are overcome by coenzyme Q1 (CoQ1). Free Radic Biol Med, 2015. 79: p. 69–77. [DOI] [PubMed] [Google Scholar]
- 105.Khan H, et al. Antioxidant profile of constituents isolated from Polygonatum verticillatum rhizomes. Toxicol Ind Health, 2016. 32(1): p. 138–42. [DOI] [PubMed] [Google Scholar]
- 106.Seitembetova AZ, Preparation of alpha-santonin and estafiatin derivatives and their effect on biochemiluminescence kinetics. Chemistry of Natural Compounds, 1999. 35(6): p. 631–634. [Google Scholar]
- 107.Kittayaruksakul S, et al. Identification of three novel natural product compounds that activate PXR and CAR and inhibit inflammation. Pharm Res, 2013. 30(9): p. 2199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.al-Harbi MM, et al. Studies on the antiinflammatory, antipyretic and analgesic activities of santonin. Jpn J Pharmacol, 1994. 64(3): p. 135–9. [DOI] [PubMed] [Google Scholar]
- 109.Bruggemann F and Meyer HJ, [The analgesic effect of kava constituents dihydrokavain and dihydromethysticine]. Arzneimittelforschung, 1963. 13: p. 407–9. [PubMed] [Google Scholar]
- 110.Pollastri MP, et al. Identification and characterization of kava-derived compounds mediating TNF-alpha suppression. Chem Biol Drug Des, 2009. 74(2): p. 121–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Feltenstein MW, et al. Anxiolytic properties of Piper methysticum extract samples and fractions in the chick social-separation-stress procedure. Phytother Res, 2003. 17(3): p. 210–6. [DOI] [PubMed] [Google Scholar]
- 112.Robledo-Gonzalez LE, et al. Repeated administration of mazindol reduces spontaneous pain-related behaviors without modifying bone density and microarchitecture in a mouse model of complete Freund's adjuvant-induced knee arthritis. J Pain Res, 2017. 10: p. 1777–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Alemi F, et al. The TGR5 receptor mediates bile acid-induced itch and analgesia. J Clin Invest, 2013. 123(4): p. 1513–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kimura Y, et al. Measurement of psychological state changes at low dopamine transporter occupancy following a clinical dose of mazindol. Psychopharmacology (Berl), 2017. 234(3): p. 323–328. [DOI] [PubMed] [Google Scholar]
- 115.Rothman RB, High affinity dopamine reuptake inhibitors as potential cocaine antagonists: a strategy for drug development. Life Sci, 1990. 46(20): p. PL17–21. [DOI] [PubMed] [Google Scholar]
- 116.Sun Z, et al. Pharmacokinetics and Metabolite Profiling of Trepibutone in Rats Using Ultra-High Performance Liquid Chromatography Combined With Hybrid Quadrupole-Orbitrap and Triple Quadrupole Mass Spectrometers. Front Pharmacol, 2019. 10: p. 1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dawson PA and Karpen SJ, Bile acids reach out to the spinal cord: new insights to the pathogenesis of itch and analgesia in cholestatic liver disease. Hepatology, 2014. 59(4): p. 1638–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Matsuda H, et al. Gastroprotective effects of phenylpropanoids from the rhizomes of Alpinia galanga in rats: structural requirements and mode of action. Eur J Pharmacol, 2003. 471(1): p. 59–67. [DOI] [PubMed] [Google Scholar]
- 119.Jayasingh Chellammal HS, et al. Neuroprotective effects of 1’delta-1’-acetoxyeugenol acetate on Abeta(25-35) induced cognitive dysfunction in mice. Biomed Pharmacother, 2019. 109: p. 1454–1461. [DOI] [PubMed] [Google Scholar]
- 120.Sun Y, et al. Bioactivity and Synthesis of Diarylheptanoids From Alpinia officinarum. Studies in Natural Products Chemistry, 2016. 49: p. 157–187. [Google Scholar]
- 121.Tinschert A, et al. Novel regioselective hydroxylations of pyridine carboxylic acids at position C2 and pyrazine carboxylic acids at position C3. Appl Microbiol Biotechnol, 2000. 53(2): p. 185–95. [DOI] [PubMed] [Google Scholar]
- 122.Leinweber FJ, et al. Bunolol metabolism by cell-free preparations of human liver: biosynthesis of dihydrobunolol. Xenobiotica, 1972. 2(2): p. 191–202. [DOI] [PubMed] [Google Scholar]
- 123.Baulieu EE and Robel P, Dehydroepiandrosterone (DHEA) and dehydroepiandrosterone sulfate (DHEAS) as neuroactive neurosteroids. Proc Natl Acad Sci U S A, 1998. 95(8): p. 4089–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Naylor JC, et al. An exploratory pilot investigation of neurosteroids and self-reported pain in female Iraq/Afghanistan-era Veterans. J Rehabil Res Dev, 2016. 53(4): p. 499–510. [DOI] [PubMed] [Google Scholar]