ABSTRACT
Background: After Neisseria gonorrhoeae FC428 was first found in Japan, ceftriaxone-resistant strains disseminated globally, and the gonococcal resistance rate increased remarkably. Epidemiological investigations are greatly significant for the analysis of antimicrobial resistance (AMR) trends, molecular features and evolution. Objectives: To clarify the AMR trend from 2016–2019 and reveal the molecular characteristics and evolution of ceftriaxone-resistant penA 60.001 isolates. Methods: The minimum inhibitory concentrations (MICs) of antibiotics against 4113 isolates were detected by the agar dilution method. N. gonorrhoeae multiantigen sequence typing (NG-MAST), multilocus sequence typing (MLST) and N.gonorrhoeae sequence typing for antimicrobial resistance (NG-STAR) were used to identify the sequence types. Genome analysis was conducted to analyze resistance genes, virulence factors, and evolutionary sources. Results: Isolates with decreased ceftriaxone susceptibility have increased from 2.05% (2016) to 16.18% (2019). Six ceftriaxone-resistant isolates possessing penA 60.001 appeared in Guangdong Province, and were resistant to ceftriaxone, penicillin, tetracycline, ciprofloxacin and cefixime, but susceptible to azithromycin and spectinomycin. Single-nucleotide polymorphisms (SNPs) in the porB gene were the major cause of different NG-MAST types. ST1903 was the main NG-STAR genotype and only strain-ZH545 was ST7365, with molecular features consistent with the MICs. Furthermore, different MLSTs suggested diverse evolutionary sources. Genome analysis revealed a set of virulence factors along with the resistance genes “penA” and “blaTEM-1B”. Half of penA 60.001 strains were fully mixed with global FC428-related strains. Conclusions: Global FC428-related clones have disseminated across Guangdong, possibly causing decreased ceftriaxone susceptibility. Enhanced gonococcal surveillance will help elucidate the trajectory of transmission and curb further dissemination.
KEYWORDS: N. gonorrhoeae, ceftriaxone-resistant, penA 60.001, epidemiological investigation
Introduction
Gonorrhea is a sexually transmitted disease caused by Neisseria gonorrhoeae, and more than 87 million cases are currently infected globally [1]. Currently, the World Health Organization (WHO) has included N. gonorrhoeae as a high priority pathogen because of therapy failures and dramatic case number increases [2]. The pathogen has developed antimicrobial resistance (AMR) to every antibiotic currently approved for treatment [3], and the effectiveness of the last remaining option for first-line antimicrobial monotherapy, ceftriaxone, has come into question due to the evolving resistance and, especially, spread of the FC428 clone [4].
The presence of FC428, which is a globally disseminated ceftriaxone-resistant clone characterized by mosaic penA 60.001, has been widely documented, including in Japan, Denmark, South Korea, Ireland, Canada, Australia, Singapore, France and the UK, since 2015 [4], raising concerns about the long-term effectiveness of ceftriaxone therapy. In a study analyzing FC428 isolates from 2016 to 2019, the first case was identified in northern China (July 2016, Beijing) [5] and then FC428 disseminated to the central (2017 June to September, Hunan) [6], western (2018, Sichuan) [7] and eastern (May to October 2019, Zhejiang) [8] region, showing that highly resistant to ceftriaxone FC428 related clones had already appeared in China. The wide spread of FC428 might have a considerable impact on the molecular characteristics and composition of local strains. The further dissemination of resistant/virulence factors, without new antibiotics/vaccines for gonorrhea will be a major challenge in the future.
In China, the epidemic of gonococcal infections and AMR trends has been the most severe in Guangdong for nearly ten years. As an important part of the WHO Western Pacific Regional (WPR) Resistance Surveillance Program, southern China (Guangdong Province) reported a dramatic increase in N. gonorrhoeae in 2017, including 29,945 new isolates (accounting for 21.6% of notifiable cases in China), which is consistent with the situation in other countries, such as the United States, Japan and South Africa [9]. However, no research has been conducted to explore whether this phenomenon is related to the spread of FC428. Since this is a region undergoing rapid economic development with high personnel flow and close interactions with other countries, gonococcal AMR surveillance must be conducted to identify emerging AMR, monitor AMR trends and provide evidence for the revision of global, regional and national gonorrhea management guidelines and public health strategies and policies. To date, research on penA 60.001 strains in this area is lacking, and detailed resistance and virulence features have rarely been presented.
In our study, we performed ceftriaxone susceptibility testing of 4113 N. gonorrhoeae isolates from 2016 to 2019 and subsequently found that the rate of decreased ceftriaxone susceptibility (minimum inhibitory concentration (MIC) ≥0.125 mg/L) increased sharply from 2.05% in 2016–16.18% in 2019. Through penA gene sequence typing, six resistant strains with MIC=0.25∼0.5 mg/L harbouring penA 60.001 were found. The emergence of such highly ceftriaxone resistant isolates raised grave concerns because no FC428 clone or high-level ceftriaxone-resistant penA 60.001 strain had previously been identified in Guangdong Province. Further genome analysis revealed the sequence types (STs), resistance genes and virulence factors of penA 60.001 strain. Finally, evolutionary analysis showed that half of isolates were closely related to the FC428-related clones detected overseas, and remaining penA 60.001 strains have a similar evolutionary origin to the strain 18DG342. Overall, FC428-related clones have disseminated across Guangdong, and the need to strengthen antibiotic and molecular resistance monitoring should be emphasized.
Materials and methods
Strain collection, cultivation, preservation
A total of 4113 strains were collected from 2016 to 2019 in Guangdong, China (including Guangzhou, Shenzhen, Jiangmen, Dongguan, Zhuhai, Shantou, Maoming, Shaoguan, Zhongshan, Zhanjiang and Foshan cities). Specifically, we collected 634, 758, 1633 and 1088 strains in 2016, 2017, 2018, and 2019 respectively. Then, the isolates were identified by gram staining and oxidase, catalase and sugar fermentation tests recommended by the WHO. Furthermore, the strains were cultured in Thayer-Martin (TM) medium and gonococcal agar supplemented with 10% defibrinated sheep blood for 18 h in a 37 incubator at 5% CO2. All strains were stored in fetal bovine serum containing 10% DMSO at −80°C.
Antimicrobial susceptibility testing
The MICs of ceftriaxone, penicillin, ciprofloxacin, tetracycline, spectinomycin, azithromycin and cefixime were determined by the agar dilution method according to the WHO Western Pacific N. gonorrhoeae Monitoring Program. WHO reference strains D, G, J, L, K and P were used for quality control.
Whole genome sequencing
The genomic DNA of ceftriaxone-resistant strains harbouring penA 60.001 was extracted for sequencing, assembly and analysis by Wentao Chen.
Genotyping
N. gonorrhoeae sequence typing for antimicrobial resistance (NG-STAR), N. gonorrhoeae multiantigen sequence typing (NG-MAST), and multilocus sequence typing (MLST) were performed to define the molecular epidemiological characteristics (https://pubmlst.org/).
Resistance, virulence and phylogenetic analysis
Raw reads were downloaded from the Sequence Read Archive (SRA) database, except for a subset of strains for which complete genomes were unavailable. Sequencing reads were preprocessed with fastp (v0.20.1) to remove adaptors and low-quality reads. The assembled contigs were generated using SPAdes (v3.13) and then utilized to analyze the acquired resistance genes and virulence factors using ABRicate (v1.0.0). Phylogenetic analysis was conducted according to the pipeline described previously [10]. Variant calling and full-length genome alignment were performed using Snippy (v4.6) (https://github.com/tseemann/snippy) with FC428 (GenBank accession NZ_AP018377 set as the reference). The full-length alignment was used as an input into Gubbins (v2.3.1) with default parameters to predict and filter regions of homologous recombination, which was followed by re-construction of the filtered alignment [11]. The following default parameters of Gubbins were used: min SNPs to identify a recombination block (default: 3); minimum window size (default: 100); and maximum window size (default: 10000). SNP-sites (v2.5.1) was used to reduce the filtered alignment to the core polymorphic sites [12]. The core alignment containing 254 core polymorphic sites was used to create the maximum likelihood tree in RAxML-NG (v1.0.3) with 1,000 bootstrap replicates under a best-fit model (TPMu1f) obtained from Modeltest-NG [13,14]. The tree was visualized and mid-rooted using iTOL (v6.1.1). The tree scale bar indicates the average number of SNPs per site.
Results and discussion
Antimicrobial susceptibility
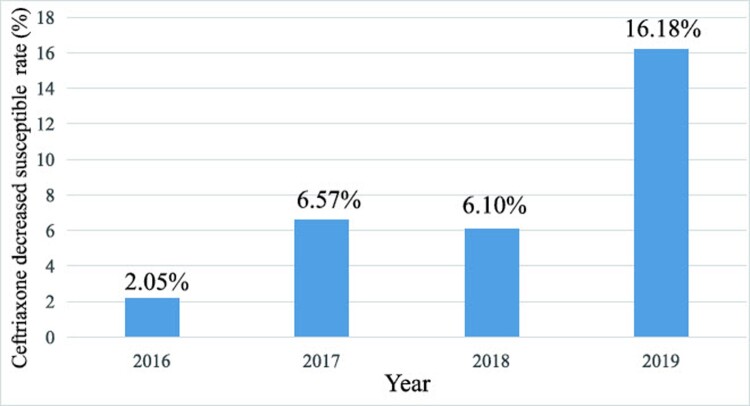
Between 2016 and 2019, a total of 4113 isolates were collected for ceftriaxone MIC testing, and the results showed that the prevalence rate of decreased susceptibility to ceftriaxone (ceftriaxone DS, MIC≥0.125 mg/L) increased from 2.05% (13 of 634) in 2016–16.18% (176 of 1088) in 2019 (Figure 1). As reported previously, the FC428 epidemic is one of the reasons for the increase in the rate of ceftriaxone resistance [12]. Thus, to evaluate the penA allele of ceftriaxone-resistant strains (MIC≥0.25 mg/L) and determine the prevalence of dominant ceftriaxone-resistant strains, we collected a total of 50 ceftriaxone-resistant N. gonorrhoeae samples from 2016 to 2019 for penA allele identification. Then, six clones were first identified as penA 60.001 strains. Furthermore, the antimicrobial susceptibility of such penA 60.001 clones to seven drugs was similar to that of the original FC428 strain. As shown, six isolates all displayed resistance to ceftriaxone (MIC=0.25-0.5 mg/L), cefixime (MIC≥1 mg/L), ciprofloxacin (MIC≥8 mg/L), tetracycline (MIC=2-4 mg/L) and penicillin (MIC≥2 mg/L), but were susceptible to spectinomycin (MIC=8-16 mg/L) and azithromycin (MIC=0.06-0.5 mg/L) (Table 1A).
Figure 1.
Ceftriaxone decreased susceptibility testing of N. gonorrhoeae in Guangdong, China from 2016 to 2019.
Table 1.
Characteristics of penA 60.001 isolates.
| (A) Minimum inhibitory concentrations of antibiotics against penA 60.001 isolates. | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MIC | (mg/L) | |||||||||
| Number | Strain | City | Year | CRO | CFM | PEN | TET | CIP | SPT | AZM |
| 1 | MM08 | Maoming | 2017 | 0.25 | ≥1 | 8 | 2 | 16 | 16 | 0.25 |
| 2 | DG18193 | Dongguang | 2018 | 0.5 | ≥1 | 2 | 2 | 8 | 16 | 0.5 |
| 3 | MM14 | Maoming | 2018 | 0.5 | ≥1 | 2 | 4 | ≥32 | 16 | 0.25 |
| 4 | ZH545 | Zhuhai | 2018 | 0.5 | ≥1 | 2 | 4 | 16 | 8 | 0.25 |
| 5 | DG19112 | Dongguang | 2019 | 0.5 | ≥1 | 2 | 2 | ≥32 | 8 | 0.25 |
| 6 | SS74 | Guangzhou | 2019 | 0.5 | ≥1 | ≥32 | 2 | 16 | 16 | 0.06 |
| CRO: Ceftriaxone; CFM: Cefixime; PEN: Penicillin; TET: Tetracycline; CIP: Ciprofloxacin; SPT: Spectinomycin; AZM: Azithromycin | ||||||||||
| (B) Sequence typing of penA 60.001 strains | |||
|---|---|---|---|
| Stain name | porB | tbpB | NG-MAST ST |
| MM14 | 581 | 21 | 1086 |
| MM08 | 581 | 21 | 1086 |
| DG19112 | 2035 | 21 | 20408 |
| ZH545 | 1132 | 21 | 13272 |
| SS74 | 3723 | 21 | New |
| DG18193 | 2682 | 21 | New |
| Stain name | penA | 23srRNA | mtrR | ponA | parC | gyr | porB | NG-STAR ST |
|---|---|---|---|---|---|---|---|---|
| MM14 | 60.001 | 100 | 1 | 1 | 3 | 7 | 8 | 233 |
| MM08 | 60.001 | 100 | 1 | 1 | 3 | 7 | 8 | 233 |
| DG19112 | 60.001 | 100 | 1 | 1 | 3 | 7 | 8 | 233 |
| ZH545 | 60.001 | 100 | 1 | 1 | 3 | 7 | 4 | 2239 |
| DG18193 | 60.001 | 100 | 1 | 1 | 3 | 7 | 13 | NEW |
| SS74 | 60.001 | 100 | 1 | 1 | 3 | 21 | 8 | NEW |
| Stain name | abcZ | adk | aroE | fumC | gdh | pdhC | pgm | MLST ST |
|---|---|---|---|---|---|---|---|---|
| SS74 | 126 | 39 | 67 | 157 | 148 | 153 | 65 | 1903 |
| MM14 | 126 | 39 | 67 | 157 | 148 | 153 | 65 | 1903 |
| DG18193 | 126 | 39 | 67 | 157 | 148 | 153 | 65 | 1903 |
| DG19112 | 126 | 39 | 170 | 157 | 148 | 153 | 65 | 13943 |
| MM08 | 126 | 39 | 67 | 157 | 148 | 153 | 65 | 1903 |
| ZH545 | 126 | 39 | 67 | 111 | 148 | 153 | 65 | 7365 |
Molecular surveillance of AMR in penA 60.001 N. gonorrhoeae
To clarify the molecular characteristics of penA 60.001 isolates, NG-MAST, NG-STAR and MLST were performed for genotyping. As shown in Table 1B, NG-MAST divided the six isolates into five STs, and differences were mainly identified in the SNPs in the porB gene, which were clearly different from those in the original FC428 (porB-1053, tbpB-21, ST3435) [13]. ST233, which is identical to FC428, was the main genotype determined by NG-STAR. Strains ZH545, DG18193 and SS74 were characterized as having different STs because of their differences in the porB and gyr genes. In conclusion, all molecular features were consistent with the antibiotic phenotype. For MLST, ST1903 was the most prevalent sequence type (66.7%, n=6), which is also a typical feature of FC428 found in Japan. However, ZH545 strain possessing ST7365 and ST13943 from strain DG19112 suggested that the origin of the six penA 60.001 isolates might have included different evolutionary trajectories.
To optimize the analysis of possible resistance genes, we used ABRicate for further analysis. As shown in Table 2A, the resistance gene “penA” was indeed the most important factor affecting cephalosporin efficacy, and one of the isolates also carried “blaTEM-1B”, which was consistent with the antibiotic phenotype. In addition, the genomes of these six penA 60.001 strains were fully sequenced and analyzed to obtain a complete picture of virulence gene repertoires. The data revealed that all examined strains harboured the antigen 85 proteins (fbpB and fbpC), high-affinity ABC importer pathway for Mn(II) (mntB and mntC), mtrC-mtrD-mtrE efflux pump, Pil (msrA/B (pilB), pilF, pilG, pilH, pilK, pilM, pilN, pilO, pilP, pilT, pilT2, pilU, pilV, pilW, pilI and pilJ), porin porB, ABC transporter farB, DNA repair gene (recN), lactoferrin import receptor (lbpA), hemoglobin receptor gene (hmbR) and surface protein A (nspA). The coverage of the virulence factors pilD, pilX, pilZ, lbpB, fbpA, dLdH catalase (katA), transferrin receptor (tbpA) and outer membrane proteins (hpuA and hpuB) in the genome reached more than 80%, which might also play a key role in gonococcal infection and AMR. Only the mntA virulence factor was not detected in some strains (DG19112, SS74 and ZH545); thus, the importance of this virulence factor remains to be discussed at a later stage (Table 2B).
Table 2.
Resistance genes and virulence factor analysis.
| (A) Resistance genes identified among penA 60.001 isolates | ||||||
|---|---|---|---|---|---|---|
| DG18193 | DG19112 | MM08 | MM14 | SS74 | ZH545 | |
| penA | 100 | 100 | 100 | 100 | 100 | 100 |
| blaTEM-1B | 0 | 0 | 0 | 0 | 100 | 0 |
| (B) Virulence factors analysis of penA 60.001 isolates | ||||||
|---|---|---|---|---|---|---|
| DG18193 | DG19112 | MM08 | MM14 | SS74 | ZH545 | |
| farB | 100 | 100 | 100 | 100 | 100 | 100 |
| fbpB | 100 | 100 | 100 | 100 | 100 | 100 |
| fbpC | 100 | 100 | 100 | 100 | 100 | 100 |
| hmbR | 100 | 100 | 100 | 100 | 100 | 100 |
| mntB | 100 | 100 | 100 | 100 | 100 | 100 |
| mntC | 100 | 100 | 100 | 100 | 100 | 100 |
| msrA/B(pilB) | 100 | 100 | 100 | 100 | 100 | 100 |
| mtrC | 100 | 100 | 100 | 100 | 100 | 100 |
| lbpA | 100 | 100 | 100 | 100 | 100 | 100 |
| mtrD | 100 | 100 | 100 | 100 | 100 | 100 |
| mtrE | 100 | 100 | 100 | 100 | 100 | 100 |
| nspA | 100 | 100 | 100 | 100 | 100 | 100 |
| pilF | 100 | 100 | 100 | 100 | 100 | 100 |
| pilG | 100 | 100 | 100 | 100 | 100 | 100 |
| pilH | 100 | 100 | 100 | 100 | 100 | 100 |
| pilK | 100 | 100 | 100 | 100 | 100 | 100 |
| pilM | 100 | 100 | 100 | 100 | 100 | 100 |
| pilN | 100 | 100 | 100 | 100 | 100 | 100 |
| pilO | 100 | 100 | 100 | 100 | 100 | 100 |
| pilP | 100 | 100 | 100 | 100 | 100 | 100 |
| pilT | 100 | 100 | 100 | 100 | 100 | 100 |
| pilT2 | 100 | 100 | 100 | 100 | 100 | 100 |
| pilU | 100 | 100 | 100 | 100 | 100 | 100 |
| pilV | 100 | 100 | 100 | 100 | 100 | 100 |
| pilW | 100 | 100 | 100 | 100 | 100 | 100 |
| pilZ | 100 | 100 | 100 | 100 | 100 | 100 |
| porB | 100 | 100 | 100 | 100 | 100 | 100 |
| recN | 100 | 100 | 100 | 100 | 100 | 100 |
| lbpB | 100 | 81.43 | 100 | 100 | 81.43 | 81.43 |
| katA | 99.88 | 99.88 | 99.88 | 99.88 | 99.88 | 99.88 |
| pilX | 99.8 | 99.8 | 99.8 | 99.8 | 99.8 | 99.8 |
| fbpA | 99.8 | 99.8 | 99.8 | 99.8 | 99.8 | 99.8 |
| hpuA | 99.83 | 99.83 | 99.83 | 99.83 | 99.83 | 99.83 |
| pilJ | 99.68 | 99.68 | 99.68 | 99.68 | 99.68 | 99.68 |
| tbpA | 99.67 | 99.67 | 99.67 | 99.67 | 99.67 | 99.67 |
| pilI | 99.51 | 99.51 | 99.51 | 99.51 | 99.51 | 99.51 |
| hpuB | 98.32 | 98.32 | 98.32 | 98.32 | 98.32 | 98.32 |
| pilD | 99.3 | 99.3 | 99.3 | 99.3 | 99.3 | 99.3 |
| mntA | 97.43 | 0 | 97.43 | 97.43 | 0 | 0 |
An absent gene is denoted “0” and a present gene is represented by its “% COVERAGE”.
Phylogenomic analysis
Finally, phylogenomic analysis was performed to track evolution. The results in Figure 2 show that the six strains were scattered in different evolutionary branches. ZH545 was the closest strain to the classic FC428 clone found in Japan in 2015, SS74 alone constituted an evolutionary branch near A7536 (2017, Australia), and MM14, MM08 and DG18193 were classified into another subclade close to 18DG342 from Singapore in 2018. DG19112 was not mixed with the globally abundant FC428-related strains. In addition, the purple-labelled strains in the figure are all FC428-related clones identified in China, but they were alienated from the six isolates in Guangdong. Moreover, it was reported that FC428-related clones found in Australia have a history of dissemination via sexual intercourse in China, and strains of similar evolutionary origin “SS74” were locally detected among FC428-related strains, indicating that foreign strains have already affected the composition of local N. gonorrhoeae strains.
Figure 2.
Maximum likelihood phylogeny of 35 globally disseminated N.gonorrhoeae strains of penA 60.001 clones. The ML phylogeny was constructed based on 254 SNVs in non-recombination regions along the whole genomes. The tree was rooted by mid-rooting method. The six isolates from the current study are coloured red, purple represents known FC428-related clones found in other regions of China, and the rest are foreign penA 60.001 strains. Bootstrap value was labelled as green gradient dot on the branch. The tree scale bar indicates the average number of SNPs per site.
Discussion
N. gonorrhoeae is a major public health threat worldwide, and is the second most common cause of bacterial STI after Chlamydia trachomatis [15]. Penicillin, tetracycline, ciprofloxacin, and cefixime were all first-line drugs used to treat gonorrhea until the resistance rate exceeded the WHO threshold of 5% [15]. Currently, the state of gonorrhea treatment is extremely serious because only ceftriaxone remains as a last-line effective monotherapy [16]. As reported, the prevalence of decreased ceftriaxone susceptibility in many countries, including China, has reached 10% [17]. According to clinical guidelines in the United States [18], UK [19] and China [17], one gram of ceftriaxone is recommended as the typical does. However, given the irregular use of antibiotics and spread of FC428, ceftriaxone resistance has become more severe. Overall, antibiotic resistance in gonorrhea is extremely severe, and the clinical use of ceftriaxone might need to be re-standardized.
Currently, whole-genome sequencing is an emerging drug resistance monitoring method, and the MICs of antibiotics can be reflected by gene features to an extent. As reported, 23S rRNA-100 both possessed wild-type genes, which was consistent with the azithromycin-sensitive phenotype. PonA-1, which include the AMR marker L421P, can significantly reduce the rate of penicillin acylation, leading to treatment failure [20]. ParC-3, containing S87R, was first found in Canada and is correlated with ciprofloxacin resistance [20]. In addition, mutations in gyrA and porB reduce drug intake and determine resistance [21,22]. PenA 60.001, which a characteristic of FC428 and possesses A311V and T483S, was confirmed to be related to cephalosporin and penicillin resistance [23]. Moreover, the widespread distribution of FC428 has become a major threat to ceftriaxone-based therapy [24]; thus, it is essential to enhance penA sequencing to identify FC428 clone. Obviously, molecular features were consistent with the antibiotic phenotype. In summary, molecular epidemiological investigations are of great significance for gonorrhea prevention and control.
Since 1996, the Guangdong Gonococcal Antibiotic Resistance Monitoring Network has been part of the Gonococcal Antibiotic Susceptibility Programme (GASP). By 2020, we established a monitoring network covering 44 medical institutions across Guangdong Province and integrated antibiotic susceptibility testing and sequence genotyping, including NG-MAST, NG-STAR and MLST, into sentinel hospitals. The strategy of enhanced AMR surveillance in China was addressed at the 2017 International Forum on Gonococcal Infections and Resistance [25]. Expanding this network and collecting more isolates are essential to improving representative surveillance.
Along with the previous study in supplemental Table 1, our study is the first report of penA 60.001 under large-sample screening in Guangdong Province. The data illustrated that ceftriaxone susceptibility has decreased, and penA 60.001 strains are mostly scattered in common global FC428-related clones. It is necessary to monitor the genetic characteristics of this strain and its subsequent dissemination. In addition to the major resistance gene penA, we revealed for the first time that penA 60.001 strains harboured a set of virulence factors related to the Mn(II) uptake system, ferric ion (Fe(III))-binding protein transport, drug efflux, adhesion, and detoxification, which are related to infection and AMR [26–34].
In conclusion, the ancestral FC428 lineage has disseminated into Guangdong, China. Enhanced epidemiological surveillance will help shed light on the molecular properties, transmission trajectory and antibiotic resistance rate, which will curb the dissemination of gonorrhea.
Supplementary Material
Funding Statement
This study was supported by grants from the Medical Science and Technolog y Foundation of Guangdong Province (No. B2020149), Guangdong Traditional Chinese Medicine Research Project (No. 202105291302207230), and “Overseas Famous Teacher” Project of Guangdong Provincial Science and Technology Department (No. 2020A1414010136).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Author contributions
Xiaomian Lin designed the study. Xiaomian Lin, Wentao Chen, Qinghui Xie, Yuqi Yu, Yiwen Liao, Xiaolin Qin, Zhanjin Feng, Xingzhong Wu, Sanmei Tang, and Heping Zheng conducted the experiments, analyzed the results and wrote the manuscript. All authors reviewed the final version of the manuscript.
Others
Raw reads of high-level ceftriaxone-resistant penA 60.001 Neisseria gonorrhoeae strains could be downloaded from the Sequence Read Archive (SRA) database (PRJNA778600).
References
- 1.Rowley J, Vander Hoorn S, Korenromp E, et al. . Chlamydia, gonorrhoea, trichomoniasis and syphilis: global prevalence and incidence estimates, 2016. Bull World Health Organ. 2019;97(8):548–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tacconelli E, Carrara E, Savoldi A, et al. . Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018;18(3):318–327. [DOI] [PubMed] [Google Scholar]
- 3.Ohnishi M, Golparian D, Shimuta K, et al. . Is Neisseria gonorrhoeae initiating a future era of untreatable gonorrhea?: detailed characterization of the first strain with high-level resistance to ceftriaxone. Antimicrob Agents Chemother. 2011;55(7):3538–3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin EY, Adamson PC, Klausner JD, et al. . And Vaccine development for antimicrobial-resistant Neisseria gonorrhoeae: Current strategies and future directions. Drugs. 2021;81(10):1153–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen SC, Han Y, Yuan LF, et al. . Identification of internationally disseminated ceftriaxone-resistant Neisseria gonorrhoeae strain FC428, China. Emerg Infect Dis. 2019;25(7):1427–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiu L, Yuan Q, Li Y, et al. . Emergence of ceftriaxone-resistant Neisseria gonorrhoeae strains harbouring a novel mosaic penA gene in China. J Antimicrob Chemother. 2020;75(4):907–910. [DOI] [PubMed] [Google Scholar]
- 7.Wang H, Wang Y, Yong G, et al. . Emergence and genomic characterization of the ceftriaxone-resistant Neisseria gonorrhoeae FC428 clone in chengdu, China. J Antimicrob Chemother. 2020;75(9):2495–2498. [DOI] [PubMed] [Google Scholar]
- 8.Yan J, Chen Y, Yang F, et al. . High percentage of the ceftriaxone-resistant Neisseria gonorrhoeae FC428 clone among isolates from a single hospital in hangzhou, China. J Antimicrob Chemother. 2021;76(4):936–939. [DOI] [PubMed] [Google Scholar]
- 9.Qin X, Zhao Y, Chen W, et al. . Changing antimicrobial susceptibility and molecular characterisation of Neisseria gonorrhoeae isolates in guangdong, China: in a background of rapidly rising epidemic. Int J Antimicrob Agents. 2019;54(6):757–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas JC, Joseph SJ, Cartee JC, et al. . Phylogenomic analysis reveals persistence of gonococcal strains with reduced-susceptibility to extended-spectrum cephalosporins and mosaic penA-34. Nat Commun. 2021;12(1):3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Croucher NJ, Page AJ, Connor TR, et al. . Rapid phylogenetic analysis of large samples of recombinant bacterial whole genome sequences using gubbins. Nucleic Acids Res. 2015;43(3):e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Page AJ, Taylor B, Delaney AJ, et al. . SNP-sites: rapid efficient extraction of SNPs from multi-FASTA alignments. Microb Genom. 2016;2(4):e000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kozlov AM, Darriba D, Flouri T, et al. . RAxML-NG: a fast, scalable and user-friendly tool for maximum likelihood phylogenetic inference. Bioinformatics. 2019;35(21):4453–4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Darriba D, Posada D, Kozlov AM, et al. . ModelTest-NG: A New and scalable tool for the selection of DNA and protein evolutionary models. Mol Biol Evol. 2020;37(1):291–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Unemo M, Seifert HS, Hook EW, et al. . Gonorrhoea. Nat Rev Dis Primers. 2019;5(1):79. [DOI] [PubMed] [Google Scholar]
- 16.Sánchez-Busó L, Yeats CA, Taylor B, et al. . A community-driven resource for genomic epidemiology and antimicrobial resistance prediction of Neisseria gonorrhoeae at pathogenwatch. Genome Med. 2021;13(1):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yin YP, Han Y, Dai XQ, et al. . Susceptibility of Neisseria gonorrhoeae to azithromycin and ceftriaxone in China: A retrospective study of national surveillance data from 2013 to 2016. PLoS Med. 2018;15(2):e1002499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.St Cyr S, Barbee L, Workowski KA, et al. . Update to CDC's treatment guidelines for gonococcal infection, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(50):1911–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Unemo M, Ross J, Serwin AB, et al. . European guideline for the diagnosis and treatment of gonorrhoea in adults. Int J STD AIDS. 2020;2020:956462420949126. [DOI] [PubMed] [Google Scholar]
- 20.Ropp PA, Hu M, Olesky M, et al. . Mutations in ponA, the gene encoding penicillin-binding protein 1, and a novel locus, penC, are required for high-level chromosomally mediated penicillin resistance in Neisseria gonorrhoeae. Antimicrob Agents Chemother. 2002;46(3):769–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaskolskiy B, Dementieva E, Kandinov I, et al. . Resistance of Neisseria gonorrhoeae isolates to beta-lactam antibiotics (benzylpenicillin and ceftriaxone) in russia, 2015-2017. PLoS One. 2019;14(7):e0220339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drlica K. Mechanism of fluoroquinolone action. Curr Opin Microbiol. 1999;2(5):504–508. [DOI] [PubMed] [Google Scholar]
- 23.Singh A, Tomberg J, Nicholas RA, et al. . Recognition of the β-lactam carboxylate triggers acylation of Neisseria gonorrhoeae penicillin-binding protein 2. J Biol Chem. 2019;294(38):14020–14032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen SC, Yuan LF, Zhu XY, et al. . Sustained transmission of the ceftriaxone-resistant Neisseria gonorrhoeae FC428 clone in China. J Antimicrob Chemother. 2020;75(9):2499–2502. [DOI] [PubMed] [Google Scholar]
- 25.Chen XS. Proceedings of the 2017 International Forum on Gonococcal Infections and Resistance in shenzhen, China. Sex Transm Dis. 2018;45(10):e75–e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu HJ, Seib KL, Srikhanta YN, et al. . Perr controls Mn-dependent resistance to oxidative stress in Neisseria gonorrhoeae. Mol Microbiol. 2006;60(2):401–416. [DOI] [PubMed] [Google Scholar]
- 27.Ohneck EA, Zalucki YM, Johnson PJ, et al. . A novel mechanism of high-level, broad-spectrum antibiotic resistance caused by a single base pair change in Neisseria gonorrhoeae. mBio. 2011;2(5):e00187–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rudel T, Facius D, Barten R, et al. . Role of pili and the phase-variable PilC protein in natural competence for transformation of Neisseria gonorrhoeae. Proc Natl Acad Sci U S A. 1995;92(17):7986–7990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Russell MA, Darzins A.. The pilE gene product of pseudomonas aeruginosa, required for pilus biogenesis, shares amino acid sequence identity with the N-termini of type 4 prepilin proteins. Mol Microbiol. 1994;13(6):973–985. [DOI] [PubMed] [Google Scholar]
- 30.Gong Z, Lai W, Liu M, et al. . Novel genes related to ceftriaxone resistance found among ceftriaxone-resistant Neisseria gonorrhoeae strains selected In vitro. Antimicrob Agents Chemother. 2016;60(4):2043–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Criss AK, Katz BZ, Seifert HS.. Resistance of Neisseria gonorrhoeae to non-oxidative killing by adherent human polymorphonuclear leucocytes. Cell Microbiol. 2009;11(7):1074–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baarda BI, Zielke RA, Holm AK, et al. . Comprehensive bioinformatic assessments of the variability of Neisseria gonorrhoeae Vaccine candidates. mSphere. 2021;6(1):e00977–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noinaj N, Buchanan SK, Cornelissen CN.. The transferrin-iron import system from pathogenic Neisseria species. Mol Microbiol. 2012;86(2):246–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jean S, Juneau RA, Criss AK, et al. . Neisseria gonorrhoeae evades calprotectin-mediated nutritional immunity and survives neutrophil extracellular traps by production of TdfH. Infect Immun. 2016;84(10):2982–2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.