Abstract
Background
Valproic acid (VPA) has shown beneficial effects in vitro against SARS-CoV-2 infection, but no study has analyzed its efficacy in the clinical setting.
Methods
This multicenter, retrospective study included 165 adult patients receiving VPA at the time of admission to hospital, and 330 controls matched for sex, age and date of admission. A number of clinical, outcome and laboratory parameters were recorded to evaluate differences between the two groups. Four major clinical endpoints were considered: development of lung infiltrates, in-hospital respiratory worsening, ICU admissions and death.
Results
VPA-treated patients had higher lymphocyte (P<0.0001) and monocyte (P = 0.0002) counts, and lower levels of diverse inflammatory parameters, including a composite biochemical severity score (P = 0.016). VPA patients had shorter duration of symptoms (P<0.0001), were more commonly asymptomatic (P = 0.016), and developed less commonly lung infiltrates (65.8%/88.2%, P<0.0001), respiratory worsening (20.6%/30.6%, P = 0.019) and ICU admissions (6.1%/13.0%, P = 0.018). There was no difference in survival (84.8%/88.8%, P = 0.2), although death was more commonly related to non-COVID-19 causes in the VPA group (36.0%/10.8%, P = 0.017). The cumulative hazard for developing adverse clinical endpoints was higher in controls than in the VPA group for infiltrates (P<0.0001), respiratory worsening (P<0.0001), and ICU admissions (P = 0.001), but not for death (0.6). Multivariate analysis revealed that VPA treatment was independently protective for the development of the first three clinical endpoints (P = 0.0002, P = 0.03, and P = 0.025, respectively), but not for death (P = 0.2).
Conclusions
VPA-treated patients seem to develop less serious COVID-19 than control patients, according to diverse clinical endpoints and laboratory markers.
Introduction
In December 2019 a cluster of severe pulmonary infections requiring hospital admission and, in many cases, mechanical ventilation in workers of the wet market of Wuhan, Hubei province, China, was reported [1]. These pulmonary infections were due to a new beta-coronavirus, named SARS-CoV-2, responsible for the so called COVID-19. Since then this infection became pandemic. As for November 7, 2021, 248 million of patients with confirmed COVID-19 had been reported worldwide, including 5 million of deaths [2]. The COVID-19 pandemic has collapsed the health services of most of the countries throughout the world, even of the wealthiest, and ruined the world economy. In spite of intensive efforts by the pharmaceutical companies to obtain an effective antiviral drug against COVID-19, most of the drugs tested so far proved ineffective or even harmful such as hydroxychloroquine, azithromycin or lopinavir/ritonavir [3–5]. Remdesivir, an antiviral previously designed to treat Ebola virus, is the only licensed drug with some antiviral effect on COVID-19. Remdesivir treatment seems to reduce the hospital admission days, but neither affects mortality nor decreases the viral load from nasopharyngeal or pulmonary exudates [6, 7]. So far, only dexamethasone has proved clinical benefit in reducing deaths in certain subgroups of patients [8] and fluvoxamine, a serotonin reuptake inhibitor, in reducing hospitalization among high-risk outpatients with early infection [9]. Molnupiravir might become the first oral antiviral COVID treatment in the coming months. This antiviral, a prodrug of the synthetic nucleoside derivative N4-hydroxycytidine (also called EIDD-1931), forces the SARS-CoV-2 to mutate itself to death by introducing copying errors during viral RNA replication [10].
Valproic acid (VPA) is a branched, short-chain fatty acid, and the 2-n-propyl derivative of valeric acid. VPA has been used for more than 60 years to treat epilepsy and other neurologic and psychiatric diseases including bipolar disorders, neuropathic pain and migraine. In the last years an antiviral effect of VPA on herpes viruses, including herpes simplex virus 1 and 2, varicella zoster virus, Epstein-Barr virus and cytomegalovirus, has been reported [11–16]. Very recently an antiviral effect of VPA on dengue and SARS-CoV-2 has also been suggested in a virtual simulation study [17]. Interestingly, VPA administration decreased the LPs-induced lung inflammation in murine models, suggesting a protective effect of VPA on acute lung injury [18, 19], and suppressed TNF-alpha and IL-6 production via inhibition of NF-kappaB activation, pointing towards a modulating role of VPA on immune responses [20].
VPA also inhibits histone deacetylase 2 (HDAC2), responsible for the deacetylation of lysine residues on the N-terminal region of the core histones, and HDAC2 inhibition modifies gene transcription. Thus VPA inhibits the expression of angiotensin-converting enzyme (ACE-2) receptors that are the entry door for SARS-CoV-2 into the cells [21]. Likewise, VPA decreases the expression of IL-6 in endothelial cells, a key element of the cytokines storm, the inflammatory event leading to lung infiltrates and pulmonary insufficiency in severe COVID-19, and also decreases the expression of the intercellular adhesion molecule 1(ICAM-1), a cell surface glycoprotein that enhances viral adhesion to the capillary endothelial cells, and facilitates intravascular thrombosis, a severe complication of COVID-19 [21].
A recent study using diverse cell lines found that VPA blocks three important processes involved in the severity of COVID-19 infection: the drug downregulates the expression of ACE2 and neuropili-1 (NRP1) decreasing viral infectivity, it reduces viral yields, probably because of alterations of the virus budding or virions stability, and it diminishes the inflammatory response to the viral infection [22].
Thus VPA is in an excellent position to be tested in patients with COVID-19 due to its antiviral and inflammatory properties. However, no study to date has evaluated the possible usefulness of VPA in the clinical setting.
Because many individuals are exposed for years to VPA to treat their neuropsychiatric disorders, the easiest way to test VPA efficacy on COVID-19 is to analyze the presentation, evolution and outcome of COVID-19 in patients who are taking VPA at the time of admission to the hospital. In this line of thought, we have carried out a retrospective, observational, multicenter study to compare the clinical and lab characteristics, course, treatments and outcomes of patients exposed and not exposed to VPA, matched by age, sex, and COVID-19 pandemic wave.
Patients and methods
This retrospective, case control study was developed in the following 14 Spanish hospitals, totalizing 10,917. hospital beds and providing care for a population of 4,612,712 inhabitants, about one-tenth of the total population of our country: Hospital de la Santa Creu i Sant Pau (Barcelona), Hospital General Universitario Gregorio Marañón (Madrid), Hospital Universitario Fundación Jiménez Díaz (Madrid), Hospital Universitario Central de Asturias (Oviedo), Hospital Universitari Son Espases (Palma de Mallorca), Hospital Universitario 12 de Octubre (Madrid), Hospital Universitario La Princesa (Madrid), Hospital Universitario y Politécnico La Fe (Valencia), Hospital Son Llàtzer (Palma de Mallorca), Hospital Regional Universitario (Málaga), Hospital Clínico Universitario (Valladolid), Hospital General Universitario (Alicante), Hospital Universitario Dr Peset (Valencia), and Hospital POVISA (Vigo).
Patients were considered for inclusion if admitted to the participating hospitals during a period of one year, from 1 March 2020 to 28 February 2021, covering the three major epidemic waves suffered in Spain from 2020 to 2021 (spring, autumn and winter). Cases were identified through electronic records. The first step was the detection of all in-hospital VPA prescriptions by means of the Pharmacy departments of the respective hospitals. The patients thus identified were scrutinized for simultaneous SARS-CoV-2 infection by means of microbiology records, PCR, antigen and serology results, codified diagnoses and/or discharge reports.
Adult patients fulfilling the criteria of being treated with VPA at the time of arrival, SARS-CoV-2 infection and hospital admission were included in the study as cases. Two controls were selected for each case matched by age, sex and date of admission, as each of these three features might have influence on the outcome. Particular attention was devoted to the choice of the controls to prevent selection bias. Thus, control patients were selected at each participating hospital from electronic, non-clinical records, in which only the date of birth, gender and date of admission were available. Therefore, no clinical, laboratory or outcome data were known at the time of selection of the controls. The two closest patients to the index VPA case according to these three matching factors were selected as controls.
A number of demographic, comorbid, microbiological, clinical, diagnostic, laboratory, imaging, hospitalization, prognostic, and therapeutic data were collected from medical records and compared in cases and controls. As many patients lacked diverse biochemical determinations, a composite biochemical severity score was constructed to evaluate the impact of the infection from a biochemical point of view in those parameters commonly affected by COVID-19 (C-reactive protein, alanine aminotransferase, lactic dehydrogenase, ferritin, procalcitonin, interleukin-6 (IL-6), D-dimer, troponin and N-terminal pro B-type natriuretic peptide (NT-proBNP)). This biochemical score was calculated for each patient by dividing the available determinations into their respective upper normal ranges, adding the resulting values, and dividing this result into the number of parameters measured.
Patients were also classified into two categories according to their respiratory evolution during the in-hospital stay. Worsening respiratory status was considered if the patients required additional oxygen supplementation or mechanical ventilation in the clinical ward or Intensive Care Unit (ICU) relative to their requirements at the time of arrival at the Emergency Department, whereas no worsening was considered if the need for oxygen supplementation did not increase during hospitalization. Four major clinical endpoints were considered for analysis: development of lung infiltrates, in-hospital respiratory worsening, ICU admission, and death.
This was a retrospective, observational study using anonymized data from electronic records, based on patients who had undergone routine clinical care for COVID-19. Therefore, according to the Spanish law, no formal written informed consent was obtained from the patients, who had been already discharged from the hospital by then and some of whom even had died. The study was approved by the Research Ethics Committee of the Principality of Asturias, Spain, which also granted a formal waiver of requiring the consent from the patients.
Statistical analysis
As the distribution of continuous variables was non-Gaussian, according to the Kolmogorov-Smirnov test, original values underwent natural logarithmic transformation for analysis. The reported values are the result of back-transformation into the original units, and are expressed as geometric mean and 95% CI. Variables not suitable for logarithmic transformation, such as those containing 0 or negative values, are reported as median, IQ range. The t-test and the Mann-Whitney U test were used for the comparison of continuous variables, according to the nature of the variable. Proportions were compared with the chi-square test or Fisher’s exact test, as appropriate. Correlations among doses and duration of VPA treatment with other parameters were assessed with the Pearson’s correlation coefficient. Receiver operating characteristic (ROC) curves and the area under the ROC curve (AUROC) were calculated to identify the laboratory parameters more discriminant of clinical endpoints, whereas the differential association between cases and controls with these endpoints was analyzed with the Kaplan-Meier hazard function, using the log-rank Mantel-Cox test to assess their statistical significance. Logistic regression analysis models were constructed to identify the factors independently associated with clinical outcomes. SPSS v. 25 software (IBM Corp., Armonk, NY, USA) was used for statistical calculations. A P value <0.05 for a two-tailed test was considered statistically significant.
Results
A total of 165 cases and 330 controls were included in the study. The mean age was 60.1 years (95% CI 58.3–61.9) and the male to female ratio was 1.3/1. Regarding the epidemic waves, 159 patients (32.1%) were admitted during the first, 213 (43.0%) during the second and 123 (24.9%) during the third wave. We also calculated the incidences of hospital admissions by dividing the corresponding cases (165 for VPA patients and 43,637 for overall COVID-19 patients) into the total population covered by the 14 participating hospitals (4,612,712). Thus, the estimated incidence of hospital admissions because of COVID-19 of the VPA-treated patients during the year of study was 3.58/100,000 individuals, whereas the overall incidence of COVID-19 cases admitted to the participant hospitals during the same period was 946/100,000 individuals. Therefore, admissions of VPA-treated patients represented 0.38% of the total admissions for COVID-19.
Table 1 shows the demographic features, habits, comorbidities and treatments at the hospital arrival. There were no significant differences between the two groups in any of the parameters evaluated, with the only exception of a somewhat higher frequency of antihypertensive treatment with angiotensin receptor blockers in the control group, which was only marginally significant. However, this treatment was not significantly associated with the development of lung infiltrates (P = 0.3), respiratory worsening (P = 0.2), ICU admission (P = 0.2) or death (P = 0.9).
Table 1. Demography, habits, comorbidities and treatment at the time of hospital arrival.
| All | VPA cases | Controls | P value | ||
|---|---|---|---|---|---|
| (n = 495) | (n = 165) | (n = 330) | |||
| Demography & anthropometry | |||||
| Gender | Male | 282 (57.0%) | 94 (57.0%) | 188 (57.0%) | 1 |
| Female | 213 (43.0%) | 71 (43.0%) | 142 (43.0%) | ||
| Age | years (n = 495) | 60.1 (58.3–61.9) | 59.9 (56.5–62.8) | 60.3 (58.2–62.6) | 0.8 |
| Weight | kg (n = 279) | 77.3 (75.4–79.2 | 77.2 (73.6–80.9) | 77.3 (75.1–79.6) | 0.9 |
| Body mass index | (n = 232) | 28.34 (27.62–29.07) | 27.96 (26.59–29.41) | 28.52 (27.70–29.37) | 0.5 |
| Comorbidities / habits | |||||
| Diabetes | Yes | 95 (19.2%) | 30 (18.2%) | 65 (19.7.0%) | 0.7 |
| No | 400 (80.8%) | 135 (81.8%) | 265 (80.3%) | ||
| Hypertension | Yes | 231 (46.7%) | 68 (41.2%) | 163 (49.4%) | 0.09 |
| No | 264 (53.3%) | 97 (58.8%) | 167 (50.6%) | ||
| Immunosuppression | Yes | 49 (9.9%) | 19 (11.5%) | 30 (9.1%) | 0.4 |
| No | 446 (90.1%) | 146 (88.5%) | 300 (90.9%) | ||
| Immunosuppression, causes* | Cancer | 28 (57.1%) | 8 (42.1%) | 20 (66.7%) | 0.15 |
| HIV | 6 (12.2%) | 4 (21.1%) | 2 (6.7%) | ||
| Immunosuppressors | 9 (18.4% | 3 (15.8%) | 6 (20.0%) | ||
| Other conditions | 6 (12.2%) | 4 (21.1%) | 2 (6.7%) | ||
| Smoking | Yes | 32 (6.5%) | 15 (9.1%) | 17 (5.2%) | 0.09 |
| No | 461 (93.5%) | 149 (90.0%) | 310 (94.8%) | ||
| Alcohol (>50 gr/d for ≥5 years) | Yes | 20 (4.1%) | 7 (4.3%) | 13 (4.0%) | 0.9 |
| No | 472 (95.9%) | 156 (95.7%) | 316 (96.0%) | ||
| Antiepileptic/psychoactive therapy* | |||||
| VPA doses | mg/day (n = 165) | - | 1098 (1001–1196) | - | - |
| VPA therapy duration | years (n = 142) | - | 6.77 (5.75–7.79) | - | - |
| Reason for VPA therapy | Epilepsy | - | 84 (50.9%) | - | - |
| Mental disorder | - | 75 (45.5%) | - | ||
| Other | 6 (3.6%) | ||||
| Concomitant use of other psychoactive drugs | Yes | - | 68 (41.2%) | - | - |
| No | - | 97 (58.8%) | - | ||
| Antihypertensive therapy* | |||||
| Angiotensin-converting enzyme inhibitors | Yes | 75 (33.8%) | 16 (23.9%) | 59 (38.1%) | 0.04 |
| No | 147 (66.2%) | 51 (76.1%) | 96 (61.9%) | ||
| Angiotensin receptor blockers | Yes | 84 (37.8%) | 21 (31.3%) | 63 (40.6%) | 0.19 |
| No | 138 (62.2%) | 46 (68.7%) | 92 (59.4%) | ||
| Other antihypertensive drugs | Yes | 140 (63.1%) | 43 (64.2%) | 97 (62.6%) | 0.8 |
| No | 82 (36.9%) | 24 (35.8%) | 58 (37.4%) | ||
VPA denotes Valproic acid.
Values are expressed as mean (95% CI) or %.
* Only in patients who fulfilled the condition.
Table 2 depicts the clinical features, radiological findings, laboratory determinations at admission and diagnostic procedures for SARS-CoV-2 infection. As compared to VPA cases, controls were more likely to be symptomatic and to have pulmonary infiltrates. Controls had also longer duration of symptoms and time to infiltrates detection and higher values of CRP, transaminases, ferritin and the biochemical severity score than the VPA group. Regarding hematological parameters, controls had significantly lower lymphocyte, monocyte and basophil counts than VPA-treated patients.
Table 2. Clinical, radiologic, laboratory and diagnostic procedures at admission.
| All | VPA cases | Controls | P value | ||
|---|---|---|---|---|---|
| (n = 495) | (n = 165) | (n = 330) | |||
| Clinical features | |||||
| Duration of symptoms* | days (n = 472) | 5.0 (2.0–9.0) | 3.0 (1.0–7.0) | 7.0 (3.0–10.0) | <0.0001 |
| Asymptomatic | Yes | 23 (4.6%) | 13 (7.9%) | 10 (3.0%) | 0.016 |
| No | 472 (95.4%) | 152 (92.1%) | 320 (97.0%) | ||
| Temperature | °C (n = 488) | 37.03 (36.90–37.16) | 37.03 (36.88–37.19) | 37.03 (36.84–37.21) | 1 |
| Respiratory rate | per minute (n = 391) | 19.80 (19.308–20.31) | 20.24 (19.32–21.22) | 19.59 (18.99–20.31) | 0.2 |
| Heart rate | per minute (n = 486) | 87.65 (86.11–89.22) | 86.74 (83.99–89.57) | 88.10 (86.24–90.00) | 0.4 |
| Oxygen saturation | % (n = 491) | 93.5 (93.0–94.0) | 93.2 (92.4–94.1) | 93.6 (93.1–94.2) | 0.4 |
| pO2 | mm Hg (n = 243) | 70.2 (67.4–73.1) | 69.2 (64.1–74.8) | 70.6 (67.4–74.1) | 0.6 |
| Need for supplementary oxygen | Yes | 282 (57.0%) | 106 (64.2%) | 176 (53.3%) | 0.02 |
| No | 213 (43.0%) | 59 (35.8%) | 154 (46.7%) | ||
| Pulmonary infiltrates | Yes | 390 (80.7%) | 106 (65.8%) | 284 (88.2%) | <0.0001 |
| No | 93 (19.3%) | 55 (34.2%) | 38 (11.8%) | ||
| Bilateral pulmonary infiltrates* | Yes | 328 (84.1%) | 87 (82.1%) | 241 (84.9%) | 0.5 |
| No | 62 (15.9%) | 19 (17.9%) | 43 (15.1%) | ||
| Time to infiltrates detection since the onset of symptoms* § | days (n = 384) | 6.0 (3.0–9.0) | 5.0 (2.0–8.0) | 7.0 (4.0–10.0) | 0.002 |
| Laboratory blood determinations | |||||
| Total leukocyte count | cells/μL (n = 485) | 6411.3 (6163.1–6669.6) | 6439.2 (5995.1–6916.2) | 6397.6 (6100.7–6700.9) | 0.9 |
| Neutrophils | cells/μL (n = 485) | 4521.4 (4303.0–4750.8) | 4249.4 (3888.9–4643.3) | 4662.9 (4393.0–4949.4) | 0.08 |
| Lymphocytes | cells/μL (n = 485) | 1033.3 (985.2–1083.7) | 1193.7 (1089.6–1307.7) | 961.8 (911.4–1014.8) | <0.0001 |
| Monocytes | cells/μL (n = 485) | 459.8 (434.4–486.8) | 535.8 (483.9–593.1) | 426.2 (398.5–455.9) | 0.0002 |
| Eosinophils | cells/μL (n = 485) | 7.73 (6.58–9.09) | 9.73 (7.19–13.18) | 6.90 (5.70–8.34) | 0.049 |
| Basophils | cells/μL (n = 485) | 8.89 (7.83–10.08) | 13.42 (10.79–16.68) | 7.24 (6.23–8.41) | <0.0001 |
| C-reactive protein | mg/L (n = 474) | 46.26 (41.28–51.85) | 35.93 (29.09–44.39) | 52.56 (46.02–60.02) | 0.002 |
| Procalcitonin | ng/mL (n = 255) | 0.10 (0.09–0.11) | 0.10 (0.08–0.13) | 0.10 (0.09–0.12) | 0.9 |
| Aspartate aminotransferase | U/L (n = 357) | 32.66 (30.49–34.99) | 29.43 (26.07–33.21) | 34.39 (31.64–37.38) | 0.036 |
| Alanine aminotransferase | U/L (n = 453) | 26.21 (24.49–28.06) | 21.63 (19.09–24.51) | 28.82 (26.63–31.20) | 0.0001 |
| Creatine kinase | U/L (n = 298) | 102.6 (91.4–115.1) | 115.0 (90.9–145.5) | 96.5 (85.2–109.3) | 0.15 |
| Lactate dehydrogenase | U/L (n = 398) | 293.6 (282.6–304.6) | 280.4 (261.0–301.2) | 299.4 (286.6–312.8) | 0.11 |
| Ferritin | ng/mL (n = 295) | 460.4 (409.2–518.0) | 352.5 (286.8–433.1) | 524.7 (455.6–604.4) | 0.002 |
| IL-6 | pg/mL (n = 210) | 18.75 (15.16–23.18) | 18.85 (12.72–27.94) | 18.70 (14.50–24.11) | 0.9 |
| D-dimer | ng/mL (n = 392) | 585.3 (524.5–648.8) | 593.4 (489.2–719.8) | 578.6 (508.99–657.7) | 0.8 |
| Troponin | ng/L (n = 190) | 9.27 (8.05–10.67) | 9.60 (7.35–12.53) | 9.13 (7.72–10.79) | 0.7 |
| NT-proBNP | pg/mL (n = 132) | 262.0 (199.3–344.3 | 278.5 (179.6–431.9) | 254.1 (178.7–361.3) | 0.8 |
| Biochemical severity score | (n = 484) | 3.19 (2.94–3.45) | 2.77 (2.37–3.24) | 3.42 (3.12–3.74) | 0.016 |
| Diagnostic procedures for SARS-CoV-2 infection | |||||
| Nasopharyngeal PCR | Yes | 451 (94.0%) | 154 (93.9%) | 297 (94.0%) | 1 |
| No | 29 (6.0%) | 10 (6.1%) | 19 (6.0%) | ||
| Nasopharyngeal viral load* | log copies/1000 cells (n = 45) | 6.437 (6.010–6.864) | 6.541 (5.877–7.204) | 6.380 (5.798–6.961) | 0.7 |
| Other samples PCR | Yes | 4 (2.2%) | 0 (0.0%) | 4 (3.4%) | 0.14 |
| No | 174 (97.8%) | 61 (100.0%) | 113 (96.6%) | ||
| Nasopharyngeal antigen | Yes | 67 (63.8%) | 19 (59.4%) | 48 (65.8%) | 0.5 |
| No | 38 (36.2%) | 13 (40.6%) | 25 (34.2%) | ||
| Positive IgM serology | Yes | 37 (39.4%) | 12 (30.8%) | 25 (45.5%) | 0.15 |
| No | 57 (60.6%) | 27 (69.2%) | 30 (54.5%) | ||
| Positive IgG serology | Yes | 45 (37.5%) | 17 (34.7%) | 28 (39.4%) | 0.6 |
| No | 75 (62.5%) | 32 (65.3%) | 43 (60.6%) | ||
| Positive IgM and/or IgG | Yes | 51 (42.9%) | 18 (36.7%) | 33 (47.1%) | 0.3 |
| No | 68 (57.1%) | 31 (63.3%) | 37 (52.9%) | ||
VPA denotes Valproic acid.
Values are expressed as mean (95% CI), §median (IQ range) or %.
* Only in patients who fulfilled the condition.
The severity biochemical score was also significantly increased in patients with lung infiltrates (mean 3.63, 95% CI 3.35–3.94, vs 1.92 95% CI 1.56–2.36, P<0.0001), respiratory worsening (mean 3.87, 95% CI 3.37–4.46 vs 2.96, 95% CI 2.68–3.25, P = 0.003), ICU admission (mean 5.49, 95% CI 4.66–6.47 vs 2.98, 95% CI 2.73–3.25, P<0.0001) and in those who died (mean 5.82, 95% CI 4.69–7.24 vs 2.93, 95% CI 2.69–3.18, P<0.0001). The AUROCs for these four outcomes were 0.687 (95% CI 0.622–0.752) P<0.0001; 0.588 (0.533–0.642) P = 0.003; 0.700 (0.638–0.761) P<0.0001; and 0.719 (0.646–0.793) P<0.0001, respectively. In multivariate analyses this biochemical severity score was independently predictive of the development of infiltrates (OR 1.641, 95% CI 1.161–2.319, P = 0.005), ICU admission (OR 1.939, 1.030–3.649, P = 0.04) and death (OR 2.361, 1.331–4.190, P = 0.003), but not of respiratory worsening (P = 0.2).
VPA daily dosage only correlated with higher lymphocyte (n = 161, r = 0.20, P = 0.01) and mononuclear cell counts (n = 161, r = 0.19, P = 0.017), whereas duration of VPA treatment correlated with higher procalcitonin levels (n = 69, r = 0.30, P = 0.01), lower nasopharyngeal viral load (n = 7, r = -0.80, P = 0.029), and lower aspartate aminotransferase levels (n = 96, r = -0.24, P = 0.02). Dosage of VPA was not significantly associated with the development of lung infiltrates (P = 0.3), respiratory worsening (P = 0.9), ICU admission (P = 0.2) or death (P = 0.6).
Table 3 shows the management and treatments during the hospital stay. There were no significant differences in the management of the two groups, with the exception of a significantly higher rate of dexamethasone treatment, as well as a trend towards a higher rate of tocilizumab therapy, in controls as compared to VPA patients.
Table 3. In-hospital management and treatment.
| All | VPA cases | Controls | P value | ||
|---|---|---|---|---|---|
| (n = 495) | (n = 165) | (n = 330) | |||
| Supplementary oxygen in clinical ward/ICU | Yes | 352 (71.1%) | 126 (76.4%) | 226 (68.5%) | 0.07 |
| No | 143 (28.9%) | 39 (23.6%) | 104 (31.5%) | ||
| Treatment | |||||
| Dexamethasone | Yes | 192 (38.9%) | 53 (32.3%) | 139 (42.1%) | 0.03 |
| No | 302 (61.1%) | 111 (67.7%) | 191 (57.9%) | ||
| Dexamethasone duration* | days (n = 192) | 7.45 (6.78–8.18) | 7.06 (5.70–8.75) | 7.60 (6.85–8.42) | 0.5 |
| Methylprednisolone | Yes | 141 (28.5%) | 40 (24.2%) | 101 (30.7%) | 0.13 |
| No | 353 (71.5%) | 125 (75.8%) | 228 (69.3%) | ||
| Methylprednisolone duration* | days (n = 139) | 3.92 (3.44–4.47) | 4.67 (3.59–6.08) | 3.66 (3.15–4.26) | 0.1 |
| Tocilizumab | Yes | 47 (9.5%) | 10 (6.1%) | 37 (11.2%) | 0.07 |
| No | 448 (90.5%) | 155 (93.9%) | 293 (88.8%) | ||
| Tocilizumab duration* | days (n = 46) | 1.16 (1.04–1.30) | 1.41 (0.87–2.29) | 1.10 (1.01–1.20) | 0.07 |
| Remdesivir | Yes | 29 (5.9%) | 8 (4.8%) | 21 (6.4%) | 0.5 |
| No | 466 (94.1%) | 157 (95.2%) | 309 (93.6%) | ||
| Remdesivir duration* | days (n = 28) | 5.04 (4.56–5.58 | 4.46 (3.40–5.84) | 5.30 (4.77–5.88) | 0.12 |
| Low-molecular weight heparin (≥0,5 mg/Kg/d) | Yes | 420 (84.8%) | 140 (84.8%) | 280 (84.8%) | 1 |
| No | 75 (15.2%) | 25 (15.2%) | 50 (15.2%) | ||
| Heparin duration* | days (n = 418) | 9.52 (8.81–10.29) | 9.80 (8.51–11.28) | 9.39 (8.55–10.30) | 0.6 |
| Other commonly used drugs | |||||
| Hydroxychloroquine | Yes | 112 (22.6%) | 39 (23.6%) | 73 (22.1%) | 0.7 |
| No | 383 (77.4%) | 126 (76.4%) | 257 (77.9%) | ||
| Azithromycin | Yes | 103 (20.8%) | 29 (17.6%) | 74 (22.4%) | 0.2 |
| No | 392 (79.2%) | 136 (82.4%) | 256 (77.6%) | ||
| Lopinavir/ritonavir | Yes | 53 (10.7%) | 13 (7.9%) | 40 (12.1%) | 0.15 |
| No | 442 (89.3%) | 152 (92.1%) | 290 (87.9%) | ||
| Ceftriaxone | Yes | 100 (20.2%) | 38 (23.0%) | 62 (18.8%) | 0.3 |
| No | 395 (79.8%) | 127 (77.0%) | 268 (81.2%) |
VPA denotes Valproic acid.
Values are expressed as median (IQ range) or %.
* Only in patients who fulfilled the condition.
Table 4 describes the in-hospital course and outcomes. Controls experienced significantly higher rates of ICU admissions and respiratory worsening during the hospital stay than VPA-treated patients. On the contrary, no differences in the overall death rate were observed between cases and controls, although deaths specifically related to COVID-19 causes were significantly less common in the VPA than in the control groups in those patients who died.
Table 4. In-hospital course and outcomes.
| All | VPA cases | Controls | P value | ||
|---|---|---|---|---|---|
| (n = 495) | (n = 165) | (n = 330) | |||
| Nosocomial acquisition | Yes | 8 (1.6%) | 6 (3.6%) | 2 (0.6%) | 0.01 |
| No | 487 (98.4%) | 159 (96.4%) | 328 (99.4%) | ||
| Duration of in-hospital stay | days (n = 495) | 9.07 (8.41–9.77) | 9.76 (8.49–11.21) | 8.74 (8.00–9.55) | 0.17 |
| Time to discharge since the onset of symptoms* § | days (n = 472) | 16.0 (11.0–22.0) | 15.0 (10.0–21.75) | 16.0 (12.0–22.0) | 0.07 |
| Time to negative PCR since the onset of symptoms* § | days (n = 159) | 20.0 (14.0–32.0) | 18.0 (13–29.5) | 21.0 (14.0–33.0) | 0.3 |
| Respiratory worsening | Yes | 135 (27.3%) | 34 (20.6%) | 101 (30.6%) | 0.019 |
| No | 360 (72.7%) | 131 (79.4%) | 229 (69.4%) | ||
| Intensive Care Unit admission | Yes | 53 (10.7%) | 10 (6.1%) | 43 (13.0%) | 0.018 |
| No | 442 (89.3%) | 155 (93.9%) | 287 (87.0%) | ||
| Duration of ICU stay* | days (n = 53) | 14.06 (10.94–18.07) | 17.45 (9.21–33.04) | 13.35 (10.06–17.72) | 0.4 |
| Need for mechanical ventilation | Yes | 58 (11.7%) | 14 (8.5%) | 44 (13.3%) | 0.11 |
| No | 437 (88.3%) | 151 (91.5%) | 286 (86.7%) | ||
| Complications | Yes | 122 (24.6%) | 43 (26.1%) | 79 (23.9%) | 0.6 |
| No | 373 (75.4%) | 122 (73.9%) | 251 (76.1%) | ||
| Sequelae at discharge | Yes | 39 (8.9%) | 16 (11.4%) | 23 (7.7%) | 0.2 |
| No | 399 (91.1%) | 124 (88.6%) | 275 (92.3%) | ||
| Positive IgM serology at discharge | Yes | 66 (57.9%) | 19 (47.5%) | 47 (63.5%) | 0.098 |
| No | 48 (42.1%) | 21 (52.5%) | 27 (36.5%) | ||
| Positive IgG serology at discharge | Yes | 129 (75.4%) | 46 (74.2%) | 83 (76.1%) | 0.8 |
| No | 42 (24.6%) | 16 (25.8%) | 26 (23.9%) | ||
| Positive IgM and/or IgG at discharge | Yes | 129 (75.9%) | 47 (75.8%) | 82 (75.9%) | 1 |
| No | 41 (24.1%) | 15 (24.2%) | 26 (24.1%) | ||
| Outcome | Survival | 433 (87.5%) | 140 (84.8%) | 293 (88.8%) | 0.2 |
| Death | 62 (12.5%) | 25 (15.2%) | 37 (11.2%) | ||
| Death caused by COVID-19* | Yes | 49 (79.0%) | 16 (64.0%) | 33 (89.2%) | 0.017 |
| No | 13 (21.0%) | 9 (36.0%) | 4 (10.8%) |
VPA denotes Valproic acid.
Values are expressed as mean (95% CI), §median (IQ range) or %.
* Only in patients who fulfilled the condition.
These favorable effects on certain clinical endpoints were not due to the higher rate of asymptomatic patients at admission in the VPA treated group, because significant differences were also observed when the asymptomatic patients were excluded from the analysis for lung infiltrates (P<0.0001), respiratory worsening (P = 0.026), and ICU admissions (P = 0.015).
ROC curves revealed that lymphocyte and monocyte counts had similar predictive values and were the most discriminant hematological cell types for the four clinical endpoints, as well as for VPA treatment. The combination of these two mononuclear cells was moderately but significantly discriminatory for the development of lung infiltrates (AUROC 0.661, 95% CI 0.598–0.725, P<0.0001), respiratory worsening (AUROC 0.584, 0.528–0.640, P = 0.004), ICU admissions (AUROC 0.677, 0.602–0.753, P<0.0001), and death (AUROC 0.604, 0.522–0.687, P = 0.009), as well as to identify the VPA group (AUROC 0.642, 0.589–0.696, P<0.0001).
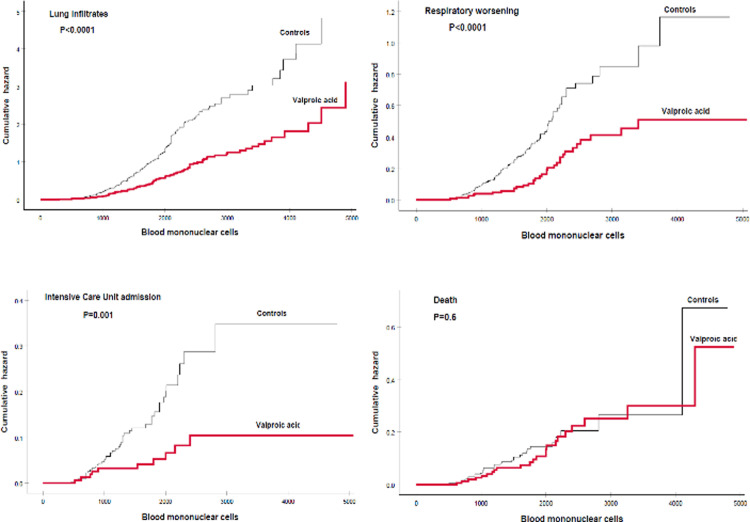
Taking into account the somewhat better predictive value of the combination of these two mononuclear cell types, hazard functions were calculated for the development of the four clinical outcomes evaluated in case and control patients (Fig 1).
Fig 1. Hazard functions for the development of diverse clinical outcomes according to the blood mononuclear cell count (cells/μ).
As seen in the Figure, control patients had significantly higher cumulative hazards for these adverse outcomes than VPA-treated individuals across the mononuclear cell range for the development of lung infiltrates, respiratory worsening and ICU admissions, but not for death. Regarding biochemical parameters, control patients also had higher cumulative hazards than VPA cases according to the biochemical severity index, although the association was somewhat less marked: lung infiltrates, P = 0.03; respiratory worsening, P = 0.06; ICU admissions, P = 0.027; death, P = 0.4.
To identify the factors independently predictive of each of the four clinical endpoints evaluated, backward stepwise logistic regression analyses were carried out, including as explanatory variables those parameters that were significantly associated with the respective clinical endpoint in the univariate analyses, although VPA treatment was entered into the analysis for death despite the lack of univariate association. In these models, which adequately fitted the data according to the Hosmer–Lemeshow goodness-of-fit statistic, VPA treatment was independently protective for the development of lung infiltrates (OR 0.309, 95% CI 0.168–0.568, P = 0.0002), in-hospital respiratory worsening (OR 0.508, 95% CI 0.273–0.945, P = 0.03), and ICU admissions (OR 0.251, 95% CI 0.075–0.843, P = 0.025), whereas it was not predictive of death (P = 0.2).
Discussion
To our knowledge, this is the first clinical study that evaluates the effect of VPA therapy on patients with COVID-19. We found that VPA-treated patients, as compared to control patients matched for age, sex and admission date, developed less commonly and had lower hazards for the development of pulmonary infiltrates, in-hospital respiratory worsening and ICU admissions, as well as had higher lymphocyte and monocyte counts and lower levels of diverse biochemical inflammatory markers. Beneficial effects that were confirmed by multivariate analyses after adjusting for covariates.
Rates of ICU admissions should be interpreted cautiously, as diverse factors such as medical judgment, hospital policies and bed availability, among others, may influence this decision. However, our findings of a substantial reduction in ICU admissions in the VPA group seem consistent. Firstly, because these confounding factors would expectedly affect to both groups. Secondly, because of the degree of improvement, as control patients more than doubled the rate of ICU admission as compared to the VPA group. Finally, improvements in other clinical endpoints, such as development of lung infiltrates and respiratory worsening, as well as substantial reductions in diverse hematological and biochemical parameters known to be associated with severe COVID-19 were also objective indicators or a more benign disease in the VPA-treated patients.
Despite these improvements in clinical endpoints and laboratory parameters, no improvement was observed in survival. However, it should be considered that all VPA-treated patients had additional neuropsychiatric comorbidities, some of them serious, which may account for additional deaths, both related and unrelated to COVID-19. In fact, we found that deaths not strictly related to-COVID-19 were significantly more common in the VPA than in the control group. In addition, control patients received more commonly than VPA patients some treatments aimed to improve COVID-19 outcomes, such as tocilizumab, methylprednisolone and, particularly, dexamethasone, a drug that has proved to be efficacious in reducing mortality rates [8], and that was administered significantly more frequently to control patients.
Epilepsy was the most common cause of VPA treatment in our study, involving 51% of the patients. If patients with epilepsy suffer from more severe COVID-19 is controversial, as exemplified by two large studies, one of which found that the infection was more prevalent, required more hospital and ICU admissions and was more lethal in epileptic patients than in the general population [23], whereas the other did not find differences between these two groups [24].
Low lymphocyte and monocyte counts have been associated with serious COVID-19 [25–30]. In this regard, we found significantly higher lymphocyte and monocyte and lower neutrophil counts in VPA-treated than in control patients, and there was also a positive correlation between VPA doses and lymphocyte counts, supporting the beneficial effects of such a therapy. A non-COVID-19 study also found higher lymphocyte and lower neutrophil counts in patients treated with VPA than in those treated with other antiepileptic drugs, although in this study there was a similar negative correlation between VPA plasma levels and both neutrophil and lymphocyte counts [31]. The preservation of the blood mononuclear cells in VPA treated patients may have contributed to the clinical benefits observed in our study. Benefits of VPA treatment that are also supported by experimental studies [18, 19, 22] and by our findings of lower levels of some inflammatory biochemical parameters, including the biochemical severity score, as well as the lower hazards for developing certain adverse clinical endpoints according to the blood mononuclear counts. Therefore, the immunomodulatory and anti-inflammatory effects of VPA were presumably responsible for the improvements in these clinical endpoints.
From our study we cannot derive which would be the optimal VPA dosage for obtaining the best results. Although there was a significant positive correlation between daily dosage and lymphocyte counts, indicating that higher doses were associated with higher improvements in lymphocyte counts, such association was only moderate, there is little correlation between the dose administered and serum levels due to its high plasma protein binding [32], and no significant relationship was found between dosage and clinical endpoints, suggesting that low VPA doses may be similarly efficacious as larger ones.
Likewise, our study was based on VPA pre-treated patients and, therefore, the inferences about the effect that new treatments (e.g. at or before the onset of symptoms) could have on the course of the infection should be cautious. However, VPA is fully and rapidly absorbed, reaching peak plasma concentrations 1–4 hours after oral administration, and the steady state is reached 2–4 days after the onset of oral treatment, minutes in the case of intravenous administration [32, 33]. Therefore, its effects would expectedly be rapid, as also indicated by in vitro studies [18–22], and, consequently, its early administration might not only have a beneficial effect in hospitalized patients, but also a potential role in the prevention of hospital admissions.
Dexamethasone is nowadays the most effective therapy for serious COVID-19 due to its well-known anti-inflammatory properties. Treatment with this drug resulted in lower 28-day mortality in patients receiving either invasive mechanical ventilation or oxygen alone, but not in those who did not receive respiratory support [8]. On the other hand, the effect of the anti-IL-6 monoclonal antibody tocilizumab on patients hospitalized with COVID-19 is more controversial, although tocilizumab use may be associated with a short-term mortality and a reduction in the need for mechanical ventilation [34, 35]. Interestingly VPA-treated patients needed less dexamethasone and tocilizumab therapy, as well as methylprednisolone and remdesivir, compared to controls in our study, and had lower rates, not only of pulmonary infiltrates or respiratory worsening, but also ICU admission, an aspect that none of these drugs could demonstrate.
The main limitations of this study are those related to retrospective studies, including the lack of certain laboratory data in many patients and the inferences regarding causality, as well as the limited number of VPA-treated patients and the small number of deaths, which precluded us to perform a more precise mortality analysis. Also, we cannot entirely dismiss the existence of any occult VPA-unrelated factor that could favor the outcomes of the VPA-treated group, although this possibility is very unlikely and, in fact, these patients had additional comorbidities such as epilepsy, dementia and mental disorders. The main strengths of the study are its novelty and the enrollment of hospitalized patients throughout our country, covering about one-tenth of the Spanish population and the three COVID-19 major waves that ours and other Western European countries have suffered so far.
Conclusions
We conclude that exposure to VPA seems to protect against the development of severe COVID-19, reducing the development of lung infiltrates, respiratory worsening and ICU admissions. This treatment was also associated with lower serum levels of diverse biochemical inflammatory markers, and higher peripheral blood lymphocyte and monocyte counts, objective laboratory parameters that support the clinical observations. However, large, prospective studies are needed to further clarify the role of VPA on COVID-19.
Acknowledgments
This work was done by the Valproic Acid in COVID-19 Study Group whose leading member is Dr Julio Collazos, email: med0053033@gmail.com
Other members of the Valproic Acid in COVID-19 Study Group are:
Virginia Pomar (Hospital de la Santa Creu i Sant Pau, Barcelona); Silvia Manrique-Rodríguez and Irene Taladriz-Sender (Hospital General Universitario Gregorio Marañón, Madrid); Raquel Bravo-Ruiz, Paula Asensio-Matthews, Barbara Soler-Bonafont, and Marina Bernal-Palacios (Fundación Jiménez Díaz, Madrid); Sara Rodríguez-Suárez, Laura Pérez-Is, Carlos López-Larrea, Miguel Alaguero, and Alberto García (Hospital Universitario Central de Asturias, Oviedo); Adrián Ferre-Beltrán (Hospital Universitari Son Espases, Palma de Mallorca); Elisa Cerezo-Benichou (Hospital 12 de Octubre, Madrid); Aresio Sancha-Lloret and Jesús Sanz-Sanz (Hospital Universitario La Princesa, Madrid); Marino Blanes (Hospital Universitario y Politécnico La Fe, Valencia); Lidia Cobos-Palacios, Almudena López-Sampalo, Rosa M. Bernal-López and Ricardo Gómez-Huelgas (Hospital Regional Universitario, Málaga); Ana Martín-Pastor and Rosario Sánchez-Martínez (Hospital General Universitario, Alicante); Sara Gutiérrez-González, Laura Rodríguez-Fernández and Genoveva Zapico-Aldea (Hospital Clínico Universitario, Valladolid); Javier de la Fuente (Hospital POVISA, Pontevedra).
Data Availability
The data for this study were obtained from the 14 participating hospitals, which belong to the Spanish National Health System, a third-party organization. Data are available on request to interested researchers by writing to: Dr Gonzalo Solis Presidente del Comite Ético de la Investigacion del Principado de Asturias Avenida de Roma s/n 33011 Oviedo,Spain Email: ceim.asturias@asturias.org. The authors had no special access privileges others would not have.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med 2020;382:1199–07. doi: 10.1056/NEJMoa2001316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO Coronavirus (COVID-19) Dash Board. https://covid19.who.int/
- 3.Rentsch TC, DeVito NJ, Mackenna B, Morton CE, Bhaskaran K, Brown JP,et al. Effect of pre-exposure use of hydroxychloroquine on COVID-19 mortality: a population-based cohort study in patients with rheumatoid arthritis or systemic lupus erythematosus using the OpenSAFELY platform. Lancet Rheumatol 2021;3:e19–e27. doi: 10.1016/S2665-9913(20)30378-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Furtado RHM, Berwanger O, Fonseca HA, D´Correa T; Ferraz LR, Lapa MG, et al. Azithromycin in addition to standard of care versus standard of care alone in the treatment of patients admitted to the hospital with severe COVID-19 in Brazil (COALITION II): a randomised clinical trial. Lancet 2020;396:959–67. doi: 10.1016/S0140-6736(20)31862-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med 2020;382:1787–99. doi: 10.1056/NEJMoa2001282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beigel JH, Tomashek KH, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the treatment of COVID-19—Final report. N Engl J Med 2020;383;1813–26. doi: 10.1056/NEJMoa2007764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldman JD, Lye DCB, Hui DS, Marks KM, Bruno R, Montejano R, et al. Remdesivir for 5 or 10 days in patients with severe Covid-19. N Engl J Med 2020;383;1827–37. doi: 10.1056/NEJMoa2015301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med 2021;384:693–704. doi: 10.1056/NEJMoa2021436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reis G, Moreira-Silva EAS, Medeiros Silva DC, Thabane L, Milagres AC, Ferreira TS, et al. Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial. Lancet Glob Health, published Online October 27, 2021, doi: 10.1016/S2214-109X(21)00448-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fischer W, Eron JJ, Holman W, Cohen MS, Fang L, Szzewczyk, et al. Molnupiravir, an oral antiviral treatment for COVID-19. medRxiv [Preprint] 2021. jun 17: 2021. 06. 17. 21258639. doi: 10.1101/2021.06.17.21258639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vázquez-Calvo A, Saiz JC, Sobrino F,. Martín-Acebes MA. Inhibition of enveloped virus infection of cultured cells by valproic acid. J Virol 2011;85:1267–74; doi: 10.1128/JVI.01717-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujii K, Suzuki N, Yamamoto T, Suzuki D, Iwatsuki K. Valproic acid inhibits proliferation of EB virus-infected natural killer cells. Hematology 2012; 17: 163–9. doi: 10.1179/102453312X13376952196494 [DOI] [PubMed] [Google Scholar]
- 13.Ornaghi S, Davis JN, Gorres KL, Miller G, Paidas MJ, van den Pol AN. Mood stabilizers inhibit cytomegalovirus infection Virology 2016; 499:121–35. doi: 10.1016/j.virol.2016.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andreu S, Ripa I, Bello-Morales R, López-Guerrero JA. Valproic acid and its amidic derivatives as new antivirals against alphaherpesviruses. Viruses 2020; 12: 1356. doi: 10.3390/v12121356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gil M. González-González R, Vázquez-Calvo A, Álvarez Gutiérrez A, Martín-Acebes MA, Praena B, et al. Clinical infections by herpesviruses in patients treated with valproic acid: a nested case-control study in the Spanish Primary Care Database, BIFAP. J Clin Med 2019; 8.1442 doi: 10.3390/jcm8091442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao H, Liu R, Wu N, Mo XD, Han W, Huang X, et al. Valproic acid enhances pamidronate-sensitized cytotoxicity of Vδ2+ T cells against EBV-related lymphoproliferative cells. Int Immunopharmacol 2020; 88:106890. doi: 10.1016/j.intimp.2020.106890 [DOI] [PubMed] [Google Scholar]
- 17.Patra A, Bhavesh NS. Virtual screening and molecular dynamics simulation suggest Valproic acid-Co-A could bind to SARS-CoV2 RNA depended RNA polymerase. www.Preprints.org, posted on 26 march 2020. doi: 10.20944/preprints2020203.0393.v1 [DOI]
- 18.Bhargava P, Panda P, Ostwal V, Ramaswamy A. Repurposing valproate to prevent acute respiratory distress syndrome/acute lung injury in COVID-19: A review of immunomodulatory action. Cancer Res Stat Treat 2020;3 Suppl S1:65–70. doi: 10.4103/CRST.CRST_156_20 [DOI] [Google Scholar]
- 19.Unal G, Turan B, Balcioglu YH. Immunopharmacological management of COVID-19. Potential therapeutic role of valproic acid. Med Hypotheses 2020,143: 109891. doi: 10.1016/j.mehy.2020.109891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ichiyama T, Okada K, Lipton JM, Matsubara T, Hayashi T, Furukawa S. Sodium valproate inhibits production of TNF-alpha and IL-6 and activation of NF-kappaB. Brain Res 2000;857:246–51. doi: 10.1016/s0006-8993(99)02439-7 [DOI] [PubMed] [Google Scholar]
- 21.Singh S, Singh KK. Valproic acid in prevention and treatment of COVID-19. Int J Respir Pulm Med 2020; 7:138. doi: 10.23937/2378-3516/1410138 [DOI] [Google Scholar]
- 22.Saiz ML, De Diego ML, López-García D, Corte-Iglesias V, Raneros AB, AstolaI, et al. Epigenetic targeting of the ACE2 and NRP1 viral receptors limits SARS-CoV-2 infectivity. Clin Epigenet 2021;13:187. doi: 10.1186/s13148-021-01168-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanchez-Larsen A, Conde-Blanco E, Viloria-Alebesque A, Sánchez-Vizcaíno Buendía C, Espinosa Oltra T, Alvarez-Noval A, et al. COVID-19 prevalence and mortality in people with epilepsy: A nation-wide multicenter study. Epilepsy Behav. 2021;125:108379. doi: 10.1016/j.yebeh.2021.108379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Asadi-Pooya AA, Emami A, Akbari A, Javanmardi F. COVID-19 presentations and outcome in patients with epilepsy. Acta Neurol Scand 2021;143:624–628. doi: 10.1111/ane.13404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 2020;180:1–11. doi: 10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang F, Nie J, Wang H, Zhao Q, Xiong Y, Deng L. Characteristics of peripheral lymphocyte subset alteration in COVID-19 pneumonia. J Infect Dis 2020;221:1762–9. doi: 10.1093/infdis/jiaa150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Agbuduwe C, Basu S. Haematological manifestations of COVID-19: From cytopenia to coagulopathy. Eur J Haematol 2020;105:540–6. doi: 10.1111/ejh.13491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruan Q,Yang K,Wang W,Jiang L,Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China Intensive Care Med. 2020. May;46(5):846–848. doi: 10.1007/s00134-020-05991-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anurag A, Jha PK, Kumar A. Differential white blood cell count in the COVID-19: A cross-sectional study of 148 patients Diabetes Metab Syndr 2020;14:2099–2102. doi: 10.1016/j.dsx.2020.10.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun S, Cai X, Wang H, He G, Lin Y, Lu B, et al., Abnormalities of peripheral blood system in patients with COVID-19 in Wenzhou, China. Clin Chim Acta 2020;507:174–80. doi: 10.1016/j.cca.2020.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bartels M, van Solinge WW, den Breeijen HJ, Bierings MB, Coffer PJ, Egberts TCG. Valproic acid treatment is associated with altered leukocyte subset development. J Clin Psychopharmacol 2012;32:832–4. doi: 10.1097/JCP.0b013e318270e5e2 [DOI] [PubMed] [Google Scholar]
- 32.Aldaz A, Ferriols R, Aumente D, Calvo MV, Farre MR, García B, et al. Pharmacokinetic monitoring of antiepileptic drugs. Farm Hosp 2011;35:326–39. doi: 10.1016/j.farma.2010.10.005 [DOI] [PubMed] [Google Scholar]
- 33.Patsalos PN, Spencer EP, Berry DJ. Therapeutic drug monitoring of antiepileptic drugs in epilepsy: A 2018 Update. Ther Drug Monit 2018;40:526–48. doi: 10.1097/FTD.0000000000000546 [DOI] [PubMed] [Google Scholar]
- 34.Stone JH, Frigault MJ, Serling-Boyd NJ, Fernandes AD, Harvey L, Foulkes As, et al. Efficacy of tocilizumab in patients hospitalized with Covid-19. N Engl J Med 2020;383:2333–44. doi: 10.1056/NEJMoa2028836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Snow TAC, Saleem N, Ambler G, Nastouli E, Singer M, Arulkumaran N. Tocilizumab in COVID-19: a meta-analysis, trial sequential analysis, and meta-regression of randomized-controlled trials. Intensive Care Med 2021; May 21. doi: 10.1007/s00134-021-06416-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data for this study were obtained from the 14 participating hospitals, which belong to the Spanish National Health System, a third-party organization. Data are available on request to interested researchers by writing to: Dr Gonzalo Solis Presidente del Comite Ético de la Investigacion del Principado de Asturias Avenida de Roma s/n 33011 Oviedo,Spain Email: ceim.asturias@asturias.org. The authors had no special access privileges others would not have.