Abstract
Background
Opioid-induced respiratory depression driven by ligand binding to mu-opioid receptors is a leading cause of opioid-related fatalities. Buprenorphine, a partial agonist, binds with high affinity to mu-opioid receptors but displays partial respiratory depression effects. The authors examined whether sustained buprenorphine plasma concentrations similar to those achieved with some extended-release injections used to treat opioid use disorder could reduce the frequency and magnitude of fentanyl-induced respiratory depression.
Methods
In this two-period crossover, single-centre study, 14 healthy volunteers (single-blind, randomized) and eight opioid-tolerant patients taking daily opioid doses ≥90 mg oral morphine equivalents (open-label) received continuous intravenous buprenorphine or placebo for 360 minutes, targeting buprenorphine plasma concentrations of 0.2 or 0.5 ng/mL in healthy volunteers and 1.0, 2.0 or 5.0 ng/mL in opioid-tolerant patients. Upon reaching target concentrations, participants received up to four escalating intravenous doses of fentanyl. The primary endpoint was change in isohypercapnic minute ventilation (VE). Additionally, occurrence of apnea was recorded.
Results
Fentanyl-induced changes in VE were smaller at higher buprenorphine plasma concentrations. In healthy volunteers, at target buprenorphine concentration of 0.5 ng/mL, the first and second fentanyl boluses reduced VE by [LSmean (95% CI)] 26% (13–40%) and 47% (37–59%) compared to 51% (38–64%) and 79% (69–89%) during placebo infusion (p = 0.001 and < .001, respectively). Discontinuations for apnea limited treatment comparisons beyond the second fentanyl injection. In opioid-tolerant patients, fentanyl reduced VE up to 49% (21–76%) during buprenorphine infusion (all concentration groups combined) versus up to 100% (68–132%) during placebo infusion (p = 0.006). In opioid-tolerant patients, the risk of experiencing apnea requiring verbal stimulation following fentanyl boluses was lower with buprenorphine than with placebo (odds ratio: 0.07; 95% CI: 0.0 to 0.3; p = 0.001).
Interpretation
Results from this proof-of-principle study provide the first clinical evidence that high sustained plasma concentrations of buprenorphine may protect against respiratory depression induced by potent opioids like fentanyl.
Introduction
Opioid use disorder (OUD) is a major source of morbidity and mortality [1]. The opioid epidemic has been fuelled in recent years by increasingly widespread prescription and illicit opioid consumption for many indications [2–5], including the treatment of non-cancer pain [6]. Fatalities attributable to opioid misuse and overdose in the USA increased six-fold between 1999 and 2017 to an estimated 47,600 [7]. The alarming increase in mortality has been observed in other countries and is largely driven by the increasing use of fentanyl and fentanyl analogues, often surreptitiously mixed with heroin [8–10].
Potentially fatal respiratory depression is the main hazard associated with opioid use and abuse [11]. Opioid-induced respiratory depression (OIRD) is driven by ligand binding to mu-opioid receptors (MORs) expressed on neurons in brainstem respiratory centres [12]. Binding to MORs induces complex changes in respiratory regulation that result in increased arterial carbon dioxide concentrations and reduced tidal volume and minute ventilation [13]. Breathing slows and becomes irregular, potentially culminating in fatal apnea, the major cause of death in opioid overdose [14]. As an additional complication, development of tolerance to opioid analgesic/euphoric effects often precedes the development of tolerance to OIRD, which may lead to dangerous self-regulated dose escalation [15].
Buprenorphine has been proven as an effective medication for the treatment of OUD [16]. Buprenorphine is a semi-synthetic MOR partial agonist that binds to MORs with high affinity and slowly dissociates from the receptors, enabling it to displace MOR full agonists such as fentanyl and mitigate their physiological effects [17, 18]. Buprenorphine itself is associated with OIRD, but a study in healthy volunteers at intravenous bolus doses ranging from 0.05 to 0.60 mg/70 kg demonstrated an apparent maximum, or ceiling, effect on respiratory depression [19, 20]. Based on a pharmacokinetic-pharmacodynamic model of OIRD reversal, the authors previously proposed that at maximum buprenorphine MOR occupancy (S1 Fig), the effect of fentanyl on respiration would be limited, even at high fentanyl doses [12, 21]. The present study aimed to provide proof of principle for this hypothesis. The results of this study confirm that high sustained buprenorphine plasma concentrations can reduce the respiratory depression caused by injection of a potent, short-acting MOR full agonist such as fentanyl.
Methods
Trial design
This was a two-part, placebo-controlled crossover study. Both Parts A and B included two study periods, during which participants received continuous intravenous infusion of buprenorphine or placebo co-administered with up to four escalating fentanyl doses. For healthy volunteers in Part A, treatment sequence was randomly assigned so participants received placebo or buprenorphine infusion during Period 1 and the alternate infusion during Period 2. Because tolerance to opioid effects is poorly characterized in patients receiving long-term opioids, opioid-tolerant patients in Part B had a fixed treatment sequence, receiving placebo infusion plus fentanyl challenges in Period 1 to optimize the fentanyl dose escalation before buprenorphine and fentanyl were co-administered in Period 2.
There were no major changes to trial design after commencement of each study part, other than an amendment of the eligibility criteria for Part B to enable recruitment of a broader group of patients. Changes regarded concurrent use of CNS depressants (e.g. benzodiazepines), inclusion of smokers (measurements not affected), and exclusion of patients with clinically significant risks of Torsades de Pointes instead of a history of risk factors. There were no changes to trial endpoints after the trial commenced.
Participants
The study enrolled healthy volunteers (Part A) and opioid-tolerant patients (Part B). All participants provided written informed consent prior to any study-related procedure and screening was completed within 30 days of the first study drug administration. In Part A, male and female healthy volunteers, aged 18 to 45 years with a body mass index of 18 to 30 kg/m2, were eligible. Exclusion criteria included history of any clinically relevant medical, psychiatric, or neurologic condition; positive pregnancy test; history of current substance use disorder according to the criteria of the Diagnostic and Statistical Manual of Mental Disorders, 5th edition; [22] smoking or having smoked in the last 6 months; alcohol consumption >20 units/week (men) or >13 units/week (women); use of any medication within 14 days or 5 half-lives before dosing; opioid use (including opioid antagonists) within 30 days before dosing; use of medication that induces/inhibits relevant cytochrome P450 enzymes; history of suicidal ideation within 30 days or suicide attempt within 6 months prior to informed consent; or any other condition that, in the opinion of the investigators, could interfere with the ability to participate in the study.
For Part B, male and female opioid-tolerant patients, aged 18 to 55 years, with a body mass index of 18 to 32 kg/m2 using daily doses of opioids ≥ 90 mg oral morphine equivalents [23], and who were in stable condition based on their medical evaluation were eligible. All exclusion criteria were similar to Part A, except for modified alcohol consumption limits to >27 units/week (men) or >20 units/week (women); broadened nicotine permissions to no smoking on dosing days; and specifically no use of buprenorphine within 10 days of the first study drug administration. Opioid-tolerant patients were recruited through national advertisements, out-patient clinics with expertise in the treatment of pain, and in collaboration with specialized opioid-abuse treatment clinics.
All eligibility criteria are provided in the study protocol, which is available as S1 File.
Setting and location of data collection
This study was conducted in Leiden, The Netherlands. Dosing day procedures were performed at the department of anaesthesiology of the Leiden University Medical Centre (LUMC) and all other activities regarding trial execution were performed at the Centre for Human Drug Research (CHDR). The study was conducted in accordance with the principles of the Declaration of Helsinki, the International Conference on Harmonisation Good Clinical Practice (ICH GCP), and ethical principles as referenced in EU Directive 2001/20/EC. The protocol (EudraCT 2017‐004858‐42) was approved by the Medical Review and Ethics Committee of the BEBO foundation (Assen, The Netherlands).
Interventions
Healthy volunteers in Part A were admitted the day prior to the experiment for each study period, with a washout of two weeks between periods. Opioid-tolerant patients in Part B were admitted to the clinic 2–5 days before the first study period and remained in the clinic until completion of the both study periods. To ensure washout of each patient’s usual opioids, tailored substitution schedules with oxycodone began a minimum of 48 hours before Period 1, and the last dose of oxycodone was administered at least 15 hours before study drug administration. Due to the short half-life of fentanyl, Period 2 was separated from Period 1 by 40 hours. During this washout period, patients again received oxycodone for opioid substitution.
On the morning of each study period, an intravenous line was placed for administration of study medication and an arterial line was placed for blood sampling in the opposite arm. Isohypercapnic ventilation was measured during buprenorphine/placebo infusion for approximately 6 hours using the dynamic end-tidal forcing technique, as described elsewhere [20, 21], allowing the investigator to direct ventilation towards pre-defined end-tidal PCO2 (7 kPa) and PO2 (14.5 kPa) values. A combination of oxygen, carbon dioxide, and nitrogen was delivered to the participants through a face mask and inspired minute ventilation was measured by pneumotachography. A finger probe with pulse oximeter was used for continuous surveillance of arterial oxygen saturation (SpO2). These ventilation parameters were captured as one-minute breath-to-breath averages.
Intravenous infusion with buprenorphine (Indivior UK Ltd., UK) or placebo started once baseline minute ventilation (VE) had stabilized at 20 ± 2 L/min (about 4-fold above normal resting VE). In healthy volunteers, an infusion rate of 0.02 or 0.05 mg/70 kg/h buprenorphine was selected to target plasma concentrations of 0.2 or 0.5 ng/mL, respectively. In opioid-tolerant patients, higher buprenorphine infusion rates were administered: 0.1, 0.2 or 0.5 mg/70 kg/h targeting plasma concentrations of 1.0, 2.0 or 5.0 ng/mL, respectively. In both healthy volunteers and opioid-tolerant patients, a 10-fold higher infusion rate was used over the first 15 minutes to speed attainment of steady-state buprenorphine concentrations at the site of action. In order to manage possible gastrointestinal side effects, all participants received 4 mg of ondansetron prior to infusion.
At 120, 180, 240, and 300 minutes after the start of the buprenorphine or placebo infusion, escalating intravenous fentanyl doses (Hameln Pharmaceuticals Ltd., UK) were administered over 90 seconds. The planned fentanyl doses in healthy volunteers were 0.075, 0.15, 0.25 and 0.35 mg/70 kg. In opioid-tolerant patients, the planned fentanyl doses were 0.25, 0.35, 0.50 and 0.70 mg/70 kg.
Arterial blood samples for analysis of buprenorphine and fentanyl plasma concentrations were collected at multiple timepoints over 540 minutes after the start of buprenorphine or placebo infusion. Buprenorphine and fentanyl plasma concentrations were assessed using liquid chromatography with tandem mass spectrometry (LC-MS/MS) methods validated over a range of 0.02 to 10.0 ng/mL for buprenorphine and 0.1 to 50.0 ng/mL for fentanyl.
Pharmacodynamic and pharmacokinetic outcomes
The primary study endpoint was maximum decrease in minute ventilation, defined as the minimum value of isohypercapnic VE observed during each fentanyl dosing period compared to pre-fentanyl baseline. The pre-fentanyl baseline value was defined as the average of the last 5 minutes prior to the first fentanyl dose. Secondary endpoints included the number and percentage of participants who experienced apnea (defined as ≥20 s loss of respiratory activity) and required verbal stimulation to breath after a fentanyl dose. Any subject who desaturated below 92% without spontaneous recovery within seconds after, was verbally stimulated to breathe, regardless of intervention.
Buprenorphine average plasma concentration (Cavg) at steady-state was calculated as the area under the plasma concentration-time curve between 120 and 360 minutes after the start of buprenorphine infusion divided by the time interval. Treatment-emergent adverse events (TEAEs) were recorded from time of first screening visit through the end of the last visit. Fentanyl dose escalation was halted if a participant did not breathe for a prolonged period or SpO2 dropped below 85% despite active verbal stimulation by the investigator, or if the investigators deemed necessary (i.e. other TEAEs). Drug plasma concentrations and safety measures (SpO2, TEAEs) were exploratory endpoints.
Sample size
In the absence of informed priors for the interaction between fentanyl and buprenorphine, no a priori sample size calculation was performed, and statistical testing was descriptive. A post-hoc power analysis, calculated by paired sample t-test for the primary endpoint, showed that a sample size of 8 yields >96% power, when the treatment difference is 50.8%, standard deviation is 32.7% and alpha is set to 0.05 two-sided.
Randomisation
Sequence generation
Healthy volunteers in Part A were randomly assigned to one of two treatment sequences (buprenorphine-placebo or placebo-buprenorphine). A blocked randomisation schedule was generated by an independent statistician using SAS version 9.4. A block size of 2 was chosen to ensure the best possible balancing if the study would be prematurely halted.
Part B was an open-label, single-sequence crossover study where participants received placebo treatment and then buprenorphine treatment.
Allocation concealment and implementation
For Part A, participants were single-blinded. The independent statistician who generated the random allocation sequence was not involved in recruiting nor randomising participants. To prevent selection bias, CHDR staff not involved in generating the random allocation sequence assigned the randomisation numbers to participants sequentially, in the order of completed medical screenings. An independent LUMC study pharmacist prepared masked infusion syringes for administration by LUMC staff.
Treatment sequence for Part B was not randomised. Dose group allocation in Part B was performed by the investigators within dose ranges specified per protocol.
Statistical methods
To reduce the impact of technical artifacts that could introduce measurement noise on VE measures, analysis of VE endpoints was conducted after post-hoc adjustment of data sets. The adjusted data sets reflect imputations based on clinical notes to account for the impact of concurrent clinical events such as facemask removal, urinating with facemask on and severe itching. Stimulated, nonspontaneous breathing data were set at zero (apnea) for analyses on ventilation data.
Maximum percent decreases in VE relative to baseline were compared between treatment groups using a mixed effects model with treatment as a fixed effect. For Part B, all buprenorphine concentration groups were combined to perform the treatment comparison. Maximum percent decreases in VE were assessed within the first 10 minutes after each fentanyl bolus to accurately characterise the peak pharmacodynamic effect of fentanyl by minimizing the impact of random variation evident over the full 60-minute intervals. Secondary endpoints were compared between treatment groups by exact conditional logistic regression and Fisher’s exact test. The exploratory safety endpoint (SpO2) was analysed in a similar manner to changes in VE.
The primary and secondary endpoint analyses were performed on participants who received at least 1 dose of fentanyl and had at least 1 post-dose assessment, excluding one participant who received the wrong buprenorphine infusion rate. TEAEs were summarized for participants who received at least one dose of study medication. The buprenorphine and fentanyl plasma concentrations were summarized for all participants who received at least 1 dose of the medication and had an adequate number of pharmacokinetic samples collected.
Statistical analyses were performed using SAS version 9.4.
Results
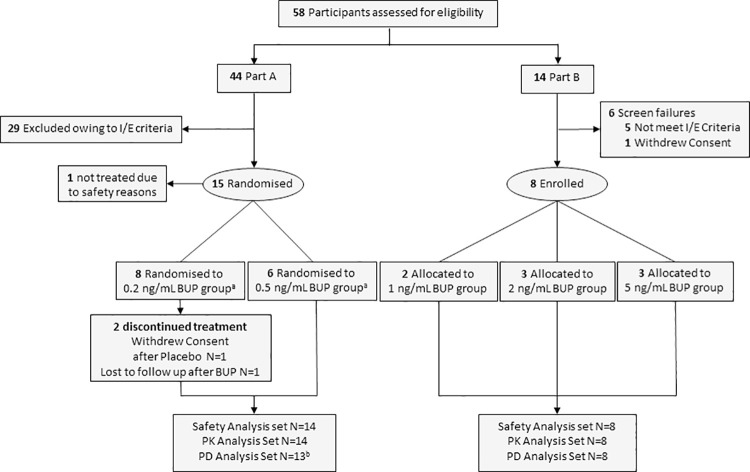
In total, 58 participants were screened for the study, which commenced on 22 March 2018 and completed on 04 January 2019, enrolling a total of 22 participants. Fourteen healthy volunteers and eight opioid-tolerant patients who used high-dose opioids for at least three months (range 0.25–29 years; see Table 1 for baseline characteristics) were included in the study. The CONSORT diagram summarizes participant disposition (Fig 1).
Table 1. Participant demographic and clinical characteristics.
| Part A: Healthy Volunteers | Part B: Opioid-tolerant Patients | |||||
|---|---|---|---|---|---|---|
| Buprenorphine concentration | 0.2 ng/mL | 0.5 ng/mL | 1 ng/mL | 2 ng/mL | 5 ng/mL | Grouped |
| (n = 8) | (n = 6) | (n = 2) | (n = 3) | (n = 3) | (n = 8) | |
| Sex, N (%) | ||||||
| Male | 4 (50) | 3 (50) | 1 (50) | 1 (33) | 1 (33) | 3 (38) |
| Female | 4 (50) | 3 (50) | 1 (50) | 2 (67) | 2 (67) | 5 (63) |
| Age, mean (SD) or range, y | 23.8 ± 4.6 | 24.5 ± 2.4 | 44–46 | 31–43 | 34–52 | 42 ± 8 |
| Ethnicity, N (%) | ||||||
| White | 8 (100) | 5 (83) | 2 (100) | 3 (100) | 3 (100) | 8 (100) |
| Native Hawaiian | 1 (17) | |||||
| Weight, mean (SD) or range, kg | 74.2 ± 6.9 | 67.9 ± 6.6 | 70–93 | 70–87 | 65–89 | 78 ± 10 |
| BMI, mean (SD) or range, kg/m2 | 23.5 ± 2.2 | 22.4 ± 1.6 | 23.6–29.6 | 22.0–30.8 | 21.0–31.5 | 25.9 ± 4.2 |
| Daily MME, mean (SD) or range, mg | NA | NA | 90–150 | 90–480 | 90–270 | 203 ± 135 |
| Drug Usage per Participanta | NA | NA | • Oxycodone 60 mg/d | • Fentanyl patch 75 mcg/h; oxycodone 90 mg/d; tapentadol 50 mg/d | • Heroin 250 mg/d (smoke); cocaine; marijuana | |
| • Fentanyl patch 25 mcg/h; oxycodone 60 mg/d; marijuana | • Buprenorphine 16 mg/d; cocaine; marijuana | • Fentanyl patch 50 mcg/h | ||||
| • Oxycodone 60 mg/d; marijuana | • Fentanyl patch 75 mcg/h; oxycodone 60 mg/d; marijuana | |||||
BMI, body mass index; MME, Morphine Milligram Equivalents; N, sample size; NA, not applicable; SD: standard deviation.
aTailored substitution schedules with oxycodone began a minimum of 48 hours before the first experiment to ensure washout of each patient’s usual opioids at baseline.
Fig 1. CONSORT flow diagram.
BUP, buprenorphine; PD, pharmacodynamic; PK, pharmacokinetic. aRandomised sequences for Part A were Placebo:BUP N = 5, BUP:Placebo N = 3 for the 0.2 ng/mL group and Placebo:BUP N = 2, BUP:Placebo N = 4 for the 0.5 ng/mL group. bOne volunteer in the lower dose group received the incorrect buprenorphine dose and was excluded from the PD analyses. Data were available for six healthy volunteers in each treatment (placebo and buprenorphine) for the PD analyses in the lower dose group due to two volunteers in the lower dose group who completed only one study period.
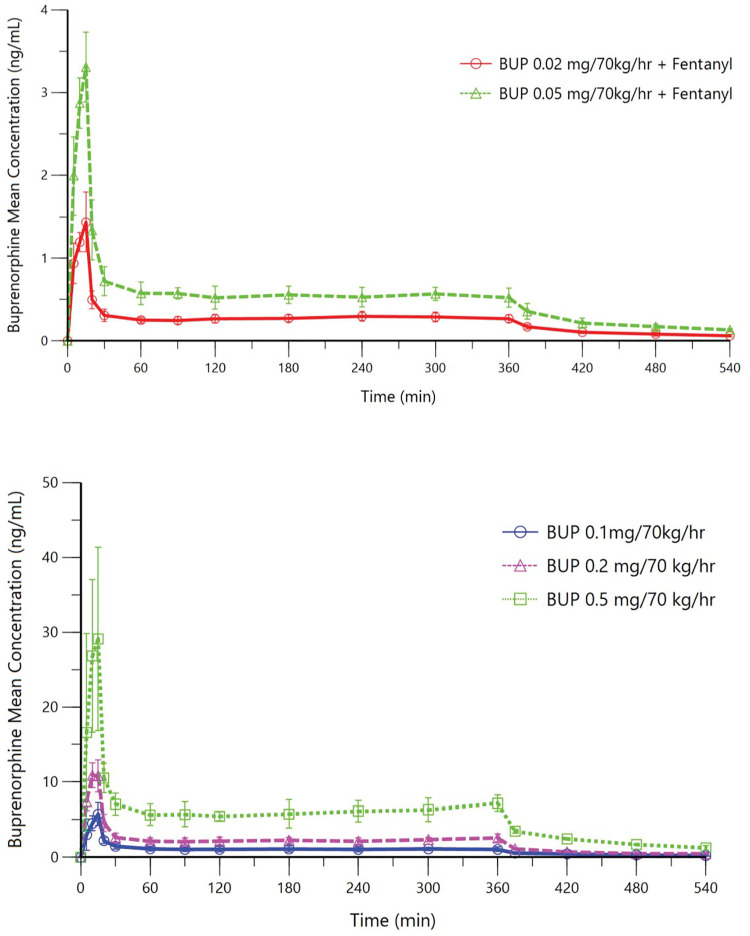
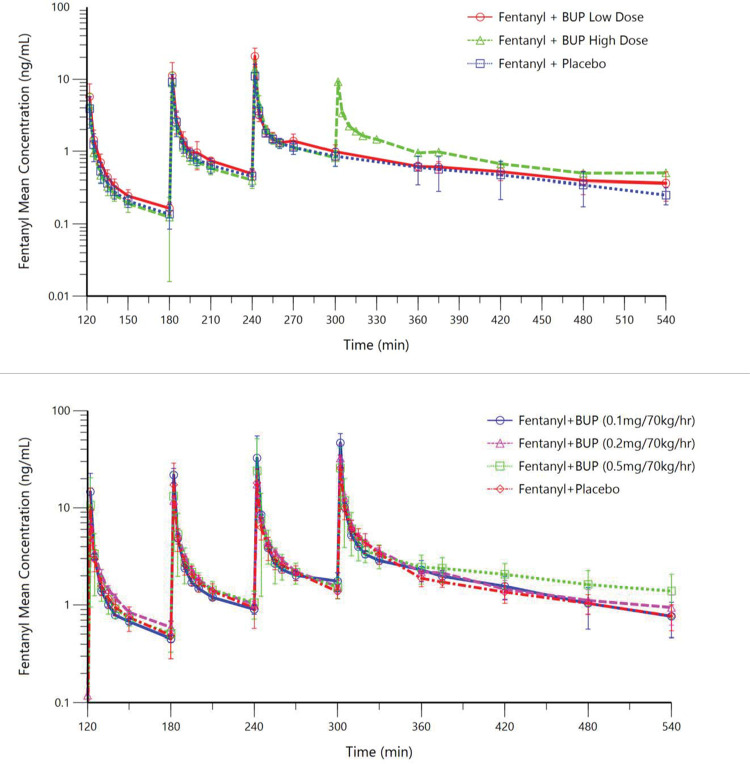
In healthy volunteers, steady-state buprenorphine plasma concentrations (mean ± SD) were 0.28 ± 0.05 and 0.54 ± 0.08 ng/mL, respectively (Fig 2), consistent with the target concentrations of 0.2 and 0.5 ng/mL. In opioid-tolerant patients, steady-state buprenorphine plasma concentrations were 1.08 ± 0.33, 2.28 ± 0.40, and 6.12 ± 1.26 ng/mL, respectively (Fig 2), all consistent with the targeted concentrations. Mean fentanyl plasma concentrations are shown for both participant populations in Fig 3. Table 2 lists fentanyl doses administered to healthy volunteers and opioid-tolerant patients and results for the number of participants who experienced persistent apnea that required verbal stimulation.
Fig 2. Mean buprenorphine plasma concentration-time curves.
Upper panel: Part A, healthy volunteers; Lower panel: Part B, opioid-tolerant patients. In both healthy volunteers and opioid-tolerant patients, a 10-fold higher infusion rate was used over the first 15 minutes to speed attainment of steady-state buprenorphine concentrations at the site of action. Infusions were stopped at 360 min. Steady-state buprenorphine infusion rates are labelled in the graphs.
Fig 3. Mean fentanyl plasma concentration-time curves.
Upper panel: Part A, healthy volunteers; Lower panel: Part B, opioid-tolerant patients. At 120, 180, 240, and 300 minutes after the start of the buprenorphine or placebo infusion, escalating intravenous fentanyl doses were administered over 90 seconds. Planned fentanyl bolus doses are labelled in the graphs. Higher doses were not administered to participants if they did not tolerate lower fentanyl doses.
Table 2. Number and percentage of participants who experienced apnea that required stimulation (i.e. persistent apnea).
| Part A: Healthy Volunteers | Part B: Opioid-tolerant Patients | |||||||
|---|---|---|---|---|---|---|---|---|
| Fentanyl Dose | Fentanyl Dose Number | Placebo for 0.2 ng/mL (N = 6) | Buprenorphine 0.2 ng/mL (N = 6) | Placebo for 0.5 ng/mL (N = 6) | Buprenorphine 0.5 ng/mL (N = 6) | Fentanyl Dose Number | Placebo (N = 8) | Buprenorphinea (N = 8) |
| 0.075 mg/70 kg | 1 | 0/6 (0) | 0/6 (0) | 0/6 (0) | 0/6 (0) | |||
| 0.15 mg/70 kg | 2 | 1/6 (17) | 0/4 (0) b | 0/6 (0) | 0/6 (0) | |||
| 0.25 mg/70 kg | 3 | 2/2 (100)b | 2/4 (50) | 3/4 (75)b | 1/6 (17) | 1 | 0/8 (0) | 0/8 (0) |
| 0.35 mg/70 kg | 4 | 0/0 | 0/0b | 0/0b | 0/1 (0)b | 2 | 2/8 (25) | 0/8 (0) |
| 0.50 mg/70 kg | 3 | 1/6 (17) | 0/8 (0) | |||||
| 0.70 mg/70 kg | 4 | 3/4 (75)b | 0/8 (0) | |||||
aThe three buprenorphine dose groups in opioid-tolerant patients (target plasma concentrations of 1, 2 and 5 ng/mL) were grouped for this analysis.
bSome participants did not receive some fentanyl doses due to adverse events, apnea events that did not require stimulation or abnormalities in other ventilatory parameters (i.e. unstable breathing, drop in ventilation or saturation and high end-tidal CO2).
During the placebo study periods, five of the six healthy volunteers (83%) who progressed to the third fentanyl dose had persistent apnea versus only three out of ten (30%) during the buprenorphine study period. Four opioid-tolerant patients progressed to the fourth fentanyl bolus during the placebo period, three of which (75%) experienced persistent apnea. In contrast, all eight opioid-tolerant patients progressed to the fourth bolus during the buprenorphine study period, and none of them (0%) experienced persistent apnea. In opioid-tolerant patients, the risk of experiencing apnea requiring verbal stimulation following fentanyl boluses was significantly lower when receiving buprenorphine than when receiving placebo, with an odds ratio of 0.07 (95% CI, 0.0 to 0.3; p = 0.001).
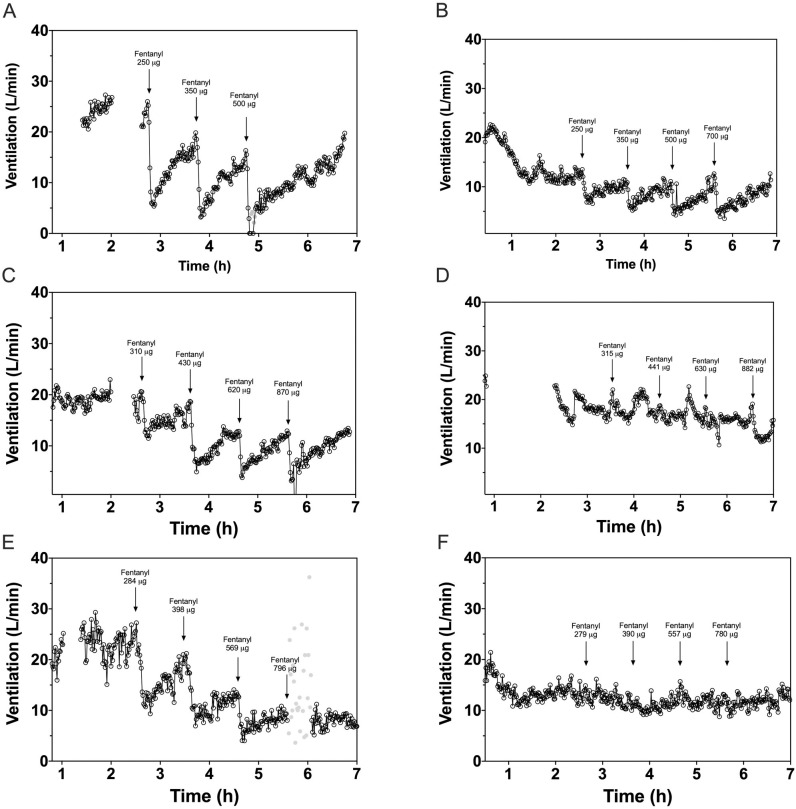
In opioid-tolerant patients, fentanyl reduced VE up to 49% (21–76%) during buprenorphine infusion (all concentration groups combined) versus up to 100% (68–132%) during placebo infusion (p = 0.006). Example tracings for representative opioid-tolerant patients in the 1, 2 and 5 ng/mL concentration groups, show VE during the placebo and buprenorphine infusion study periods (Fig 4) and graphs depicting individual VE per concentration level are provided as S2 Fig. The tracings indicate that buprenorphine itself decreased VE compared to placebo; in healthy volunteers, the decrease in ventilation caused by buprenorphine was more pronounced. After fentanyl injections, significant treatment differences for healthy volunteers in the 0.5 ng/mL buprenorphine versus placebo groups were observed, with lower decreases in VE [least squares mean difference (95% CI), p-value] following the first [25.1% (13.4–36.8%), 0.001] and second [31.6%, (19.3–43.8%), < .001] fentanyl bolus compared to pre-fentanyl baseline (Table 3). For the combined group of opioid-tolerant patients, significantly smaller reductions in VE after fentanyl bolus 1 [29.9% (19.6–40.3%), < .001], 2 [42.8%, (23.8–61.8%), 0.001], 3 [39.4%, (15.7–63.1%), 0.008], and 4 [50.8%, (27.7–73.9%), 0.006] were measured when patients received buprenorphine infusion compared to placebo (Table 3). When the three buprenorphine concentration groups were compared in opioid-tolerant patients, fentanyl effects on VE appeared greater for the 1 ng/mL group than for the 2 and 5 ng/mL groups.
Fig 4. Example graphs showing the effect of fentanyl on minute ventilation in three opioid-tolerant patients during placebo infusion and buprenorphine infusion.
(1) Placebo infusion (A, C and E) and buprenorphine infusion (B, D, F) at target plasma concentrations of 1 ng/mL (top row), 2 ng/mL (middle row) and 5 ng/mL (lower row). (2) Open spaces in the beginning of graphs A, C, D and E relate to concurrent clinical events such as temporary removal of the facemask. (3) Grey dots are stimulated breaths in case of an apnea episode. (4) The time on the x-axis in the graphs is related to the start time of the ventilation experiment, not the timing of the buprenorphine/placebo infusion and fentanyl injections.
Table 3. Maximum decreases in minute ventilation after fentanyl bolus administration (%).
| Healthy volunteers | Opioid-tolerant patients | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.2 ng/mL group | 0.5 ng/mL group | 1 ng/mL group | 2 ng/mL group | 5 ng/mL group | ||||||||||
| Placebo | BUP | Treatment Difference | Placebo | BUP | Treatment Difference | Placebo | BUP | Placebo | BUP | Placebo | BUP | Treatment Differenceb | ||
| Fentanyl Dose 1 a | N | 6 | 6 | 6 | 6 | 2 | 2 | 3 | 3 | 3 | 3 | |||
| LSM (95% CI) | -60.9 (-73.5, -48.2) | -53.1 (-65.8, -40.5) | 7.7 (-3.8, 19.3) | -51.3 (-64.3, -38.3) | -26.2 (-40.0, -12.5) | 25.1 (13.4, 36.8) | -82.3 (-105.2, -59.3) | -49.2 (-72.1, -26.3) | -44.3 (-63.0, -25.6) | -22.2 (-40.9, -3.5) | -62.5 (-81.2, -43.8) | -26.8 (-45.5, -8.1) | 29.9 (19.6, 40.3) | |
| p-value | 0.1590 | 0.001 | < .001 | |||||||||||
| Fentanyl Dose 2 a | N | 6 | 4 | 6 | 6 | 2 | 2 | 3 | 3 | 3 | 3 | |||
| LSM (95% CI) | -82.4 (-92.7, -72.0) | -70.3 (-82.8, -57.8) | 12.1 (-2.5, 26.7) | -79.0 (-89.4, -68.6) | -47.4 (-57.8, -37.0) | 31.6 (19.3, 43.8) | -93.5 (-122.3, -64.6) | -57.3 (-86.1, -28.4) | -68.4 (-91.9, -44.8) | -35.9 (-59.5, -12.4) | -87.5 (-111.0, -63.9) | -30.0 (-53.5, -6.5) | 42.8 (23.8, 61.8) | |
| p-value | 0.0916 | < .001 | 0.001 | |||||||||||
| Fentanyl Dose 3 a | N | 2 | 4 | 4 | 6 | 1 | 2 | 3 | 3 | 2 | 3 | |||
| LSM (95% CI) | -100.0 (-142.3, -57.7) | -83.2 (-113.2, -53.3) | 16.8 (-35.1, 68.7) | -93.6 (-123.6, -63.7) | -71.9 (-96.4, -47.5) | 21.7 (-16.9, 60.4) | -100.0 (-145.3, -54.7) | -71.8 (-103.8, -39.8) | -79.3 (-105.5, -53.2) | -46.1 (72.3, -20.0) | -88.1 (-120.1, -56.1) | -30.7 (-56.9, -4.6) | 39.4 (15.7, 63.1) | |
| p-value | 0.3788 | 0.1716 | 0.008 | |||||||||||
| Fentanyl Dose 4 a | N | 0 | 0 | 0 | 1 | 0 | 2 | 3 | 3 | 1 | 3 | |||
| LSM (95% CI) | NA | NA | NA | NA | NA | NA | NA | -68.7 (-140.2, 2.8) | -82.3 (-140.7, -23.9) | -33.7 (-92.1, 24.7) | -116.3 (-189.3, -42.2) | -50.5 (-108.9, 7.8) | 50.8 (27.7, 73.9) | |
| p-value | NA | NA | 0.006 | |||||||||||
BUP, buprenorphine; CI, confidence interval; LSM, least square mean; NA, not applicable. p<0.05 are presented in bold. Differences are LSM estimated treatment differences between buprenorphine and placebo.
aMaximum changes (%) in minute ventilation during first 10 minutes after each fentanyl administration compared to pre-fentanyl baseline.
b The three buprenorphine concentration level groups in patients were grouped for this analysis.
All participants in each treatment period reported at least one TEAE and at least one TEAE-related to buprenorphine/placebo treatment. Overall, most events were mild or moderate in severity. In healthy volunteers, the most frequent TEAEs were nausea, apnea, and somnolence in both periods. The most frequent TEAEs in opioid-tolerant patients were apnea, dizziness, and somnolence. In the placebo period, 88% of opioid-tolerant patients experienced apnea compared to 13% during the buprenorphine period. Apneas reported as TEAEs did not necessarily require verbal stimulation. All the TEAEs were expected for administration of opioid agonists, including a high incidence of nausea among healthy volunteers who were opioid-naive.
In opioid-tolerant patients, SpO2 levels were significantly decreased after placebo treatment relative to buprenorphine after the first, third and fourth fentanyl boluses (Table 4). No other consistent differences in safety parameters were observed between treatment groups.
Table 4. Maximum change from pre-fentanyl baseline in oxygen saturation (%) in opioid-tolerant patients.
| Placebo | Buprenorphine | Treatment Difference (BUP-Placebo) | |
|---|---|---|---|
| Fentanyl Dose 1 | |||
| N | 8 | 8 | |
| LS mean | -2.9 | -1.5 | 1.4 |
| 95% CI | -3.8, -1.9 | -2.4, -0.6 | 0.1, 2.7 |
| P-value | 0.041 | ||
| Fentanyl Dose 2 | |||
| N | 8 | 8 | |
| LS mean | -5.2 | -3.5 | 1.8 |
| 95% CI | -8.2, -2.3 | -6.4, -0.5 | -2.4, 5.9 |
| P-value | 0.353 | ||
| Fentanyl Dose 3 | |||
| N | 6 | 8 | |
| LS mean | -8.1 | -2.7 | 5.4 |
| 95% CI | -12.1, -4.2 | -6.2, -0.7 | 0.8, 10.0 |
| P-value | 0.030 | ||
| Fentanyl Dose 4 | |||
| N | 4 | 8 | |
| LS mean | -11.2 | -2.6 | 8.6 |
| 95% CI | -14.7, -7.6 | -5.0, -0.1 | 4.3, 12.9 |
| P-value | 0.008 |
CI, confidence interval; LS, least squares. (p<0.05) are presented in bold.
Discussion
The present study is, to the best of our knowledge, the first to provide clinical evidence for the protective effects of buprenorphine in limiting fentanyl-induced respiratory depression. Previous studies in animal models and in healthy volunteers have shown that respiratory depression induced by buprenorphine is characterized by a ceiling effect at higher concentrations [19–21]. It was demonstrated that, unlike some other opioids, respiratory depression associated with buprenorphine is relatively resistant to naloxone reversal, likely because of high receptor affinity and slow dissociation from the receptor [17, 21]. The authors hypothesized that because of its special properties, high concentrations of buprenorphine that sustain maximum MOR occupancy could limit the extent of OIRD induced by fentanyl, a potent MOR full agonist.
The results demonstrate that in patients with higher tolerance to the effects of opioids, sustained high plasma concentrations of buprenorphine significantly reduced the magnitude of fentanyl-induced respiratory depression relative to placebo. This effect was observed with escalating fentanyl doses up to 0.70 mg/70 kg (total administered dose 1.8 mg/70kg over 180 minutes). Each fentanyl bolus was infused over 90 seconds, resulting in an immediate ventilatory response. This pharmacodynamic effect was well defined within the first 10 minutes of each fentanyl bolus; the ventilatory response slowly decreased thereafter (i.e. breathing recovered) and became more susceptible to random variation the longer after a bolus was administered. Apneic periods directly following a drug injection can be fatal in real-life situations. Therefore, it was regarded justified to only include the ventilatory response during the first 10 minutes after each bolus in the analysis. Buprenorphine administration was itself associated with a decrease in VE, but at the highest dose there was little or no additional decrease after subsequent fentanyl administration. The numbers in each buprenorphine dose group of opioid-tolerant patients were small, but there was a trend consistent with a buprenorphine concentration-response with highest levels of buprenorphine achieving greater suppression of VE as evidenced by the tracings in Fig 4 (suggesting greater MOR occupancy). The impact of the fourth fentanyl bolus on VE appears to be greater in the highest buprenorphine dose group than in the middle dose group of opioid-tolerant patients. This is due to a few isolated low values directly following fentanyl administration in two thirds of patients in the high-dose group. These data points were not excluded from the analysis but might be considered outliers. Because dose level groups were small, the statistical analysis was performed by grouping data across the buprenorphine dose levels.
Apnea events were less frequent and less severe following fentanyl administration during buprenorphine infusion than during placebo infusion. Opioid-tolerant patients treated with the highest dose of buprenorphine had no meaningful apnea events or changes in SpO2 after fentanyl boluses. At lower buprenorphine doses, fentanyl had an appreciable effect on ventilation. These results are consistent with the expected greater MOR occupancy at higher buprenorphine plasma levels [24]. Inhibition of fentanyl-induced respiratory depression by buprenorphine in healthy volunteers was observed only to a limited extent in this study. Although fentanyl did cause respiratory depression during buprenorphine infusion in healthy volunteers, especially at high-dose buprenorphine infusion, the decrease in VE was significantly lower compared to the fentanyl effect during placebo infusion. Comparisons were difficult for the third and fourth fentanyl boluses, as only six of 12 healthy volunteers progressed to the third bolus during the placebo period compared to ten during the buprenorphine treatment. The only healthy volunteer who tolerated all four fentanyl boluses received buprenorphine at the highest dose. Collectively, the results suggest that buprenorphine at high concentrations reduces respiratory depression induced by fentanyl administration and suggest that sustained high concentrations of buprenorphine, such as those achieved with some extended-release injections used to treat OUD [25], may protect against inadvertent fentanyl overdose.
A possible limitation of this study is the relatively small number of participants with limited racial diversity. Moreover, the opioid-tolerant patient group is somewhat heterogeneous, including six patients chronically using opioids for pain, and two chronic drug abusers, and might not fully represent the real-world population of patients with OUD. However, ventilatory responses to buprenorphine and fentanyl were consistent between all opioid-tolerant patients with relatively low inter-subject variability. In addition, the observed effects of buprenorphine on fentanyl-induced respiratory depression were substantial and significant, so the authors regard these results as clinically relevant despite the small sample size, and valid from the perspective of a single-centre trial.
In conclusion, data from this study provide clinical evidence that buprenorphine reduces the harmful effects of fentanyl on ventilation and protects against fentanyl-induced respiratory depression in a concentration-dependent manner. Future research, including studies with larger sample sizes and combining other populations in clinical practice, designed to confirm the potential protective effect of buprenorphine against this fatal consequence of opioid misuse, is warranted.
Supporting information
(PDF)
(TIF)
(PDF)
(PDF)
Acknowledgments
Editorial support was provided by Peloton Advantage, LLC, an OPEN Health company, Parsippany, NJ.
The authors thank Dr. Karen Broekhuizen who provided medical writing support on behalf of the Centre for Human Drug Research, Leiden, The Netherlands.
S1 Fig was created with BioRender.com.
Registration
This study is registered with trialregister.nl, number NL7028 (https://www.trialregister.nl/trial/7028). Due to administrative reasons, trial registry was completed in May 2018, after 5 participants of the 22 in total completed their treatment visits, and prior to any analyses performed with study data. The authors confirm that all ongoing and related trials for the study drug are registered.
Data Availability
All relevant data are located at Dryad: doi:10.5061/dryad.j3tx95xdb.
Funding Statement
This study was supported by Indivior Inc., North Chesterfield, VA. The funder provided support in the form of salaries for authors [RD, FG, SS, AH, CL]. The specific roles of these authors are articulated in the ‘author contributions’ section. The funder of the study and affiliated authors participated in designing the study, data analysis, and interpretation of the data.
References
- 1.United Nations Office on Drugs and Crime. World Drug Report 2020, (United Nations publication, Sales No. E.20.XI.6).
- 2.van Amsterdam J, Philips L, Henderson G, Bell J, Bowden-Jones O, Hammersley R, et al. Ranking the harm of non-medically used prescription opioids in the UK. Regul Toxicol Pharmacol 2015; 73, 999–1004. doi: 10.1016/j.yrtph.2015.09.014 [DOI] [PubMed] [Google Scholar]
- 3.Stokes A, Berry KM, Hempstead K, Lundberg DJ, Neogi T. Trends in prescription analgesic use among adults with musculoskeletal conditions in the United States, 1999–2016. JAMA Netw Open 2019; 2: e1917228. doi: 10.1001/jamanetworkopen.2019.17228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dahan A, van der Schrier R, Smith T, Aarts L, van Velzen M, Niesters M. Averting opioid-induced respiratory depression without affecting analgesia. Anesthesiology 2018; 128: 1027–37. doi: 10.1097/ALN.0000000000002184 [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 2019 Annual Surveillance Report of Drug-Related Risks and Outcomes—United States Surveillance Special Report. Centers for Disease Control and Prevention, U.S. Department of Health and Human Services. Published November 1, 2019. https://www.cdc.gov/drugoverdose/pdf/pubs/2019-cdc-drug-surveillance-report.pdf. Accessed August 25, 2020.
- 6.Atluri S, Akbik H, Sudarshan G. Prevention of opioid abuse in chronic non-cancer pain: an algorithmic, evidence based approach. Pain Physician 2012; 15: ES177–89. [PubMed] [Google Scholar]
- 7.Wilson N, Kariisa M, Seth P, Smith H IV, Davis NL. Drug and Opioid-Involved Overdose Deaths—United States, 2017–2018. MMWR Morb Mortal Wkly Rep 2020;69: 290–7. doi: 10.15585/mmwr.mm6911a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G. Drug and Opioid-Involved Overdose Deaths–United States, 2013–2017. MMWR Morb Mortal Wkly Rep 2019; 67: 1419–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Donnell JK, Halpin J, Mattson CL, Goldberger BA, Gladden RM. Deaths involving fentanyl, fentanyl analogs, and U-47700–10 states, July–December 2016. MMWR Morb Mortal Wkly Rep 2017; 66: 1197–202. doi: 10.15585/mmwr.mm6643e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ochalek TA, Parker MA, Higgins ST, Sigmon SC. Fentanyl exposure among patients seeking opioid treatment. J Subst Abuse Treat 2019; 96: 23–5. doi: 10.1016/j.jsat.2018.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dahan A, Aarts L, Smith TW. Incidence, reversal, and prevention of opioid-induced respiratory depression. Anesthesiology 2010; 112: 226–38. doi: 10.1097/ALN.0b013e3181c38c25 [DOI] [PubMed] [Google Scholar]
- 12.Algera MH, Kamp J, van der Schier R, vanVelzen M, Niesters M, Aarts L, et al. Opioid-induced respiratory depression in humans: a review of pharmacokinetic-pharmacodynamic modelling of reversal. Br J Anaesth 2019; 122: e168–e79. doi: 10.1016/j.bja.2018.12.023 [DOI] [PubMed] [Google Scholar]
- 13.Boom M, Niesters M, Sarton E, Aarts L, Smith TW, Dahan A. Non-analgesic effects of opioids: opioid-induced respiratory depression. Curr Pharm Des 2012; 18: 5994–6004. doi: 10.2174/138161212803582469 [DOI] [PubMed] [Google Scholar]
- 14.White JM, Irvine RJ. Mechanisms of fatal opioid overdose. Addiction 1999; 94: 961–72. [PubMed] [Google Scholar]
- 15.Volkow ND, McLellan AT. Opioid abuse in chronic pain—misconceptions and mitigation strategies. N Engl J Med 2016; 374: 1253–63. doi: 10.1056/NEJMra1507771 [DOI] [PubMed] [Google Scholar]
- 16.Schuckit MA. Treatment of Opioid-Use Disorders. N Engl J Med 2016; 375: 357–68. doi: 10.1056/NEJMra1604339 [DOI] [PubMed] [Google Scholar]
- 17.Megarbane B, Hreiche R, Pirnay S, Marie N, Baud FJ. Does high-dose buprenorphine cause respiratory depression?: possible mechanisms and therapeutic consequences. Toxicol Rev 2006; 25: 79–85. doi: 10.2165/00139709-200625020-00002 [DOI] [PubMed] [Google Scholar]
- 18.Khanna IK, Pillarisetti S. Buprenorphine—an attractive opioid with underutilized potential in treatment of chronic pain. J Pain Res 2015; 8: 859–70. doi: 10.2147/JPR.S85951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dahan A, Yassen A, Romberg R, Sarton E, Teppema L, Olofsen E, et al. Buprenorphine induces ceiling in respiratory depression but not in analgesia. Br J Anaesth 2006; 96: 627–32. doi: 10.1093/bja/ael051 [DOI] [PubMed] [Google Scholar]
- 20.Dahan A, Yassen A, Bijl H, Romberg R, Sarton E, Teppema L, et al. Comparison of the respiratory effects of intravenous buprenorphine and fentanyl in humans and rats. Br J Anaesth 2005; 94: 825–834. doi: 10.1093/bja/aei145 [DOI] [PubMed] [Google Scholar]
- 21.Yassen A, Olofsen E, Romberg R, Sarton E, Teppema L, Danhof M, et al. Mechanism-based PK/PD modeling of the respiratory depressant effect of buprenorphine and fentanyl in healthy volunteers. Clin Pharmacol Ther 2007; 81: 50–58. doi: 10.1038/sj.clpt.6100025 [DOI] [PubMed] [Google Scholar]
- 22.American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 5th edn. Arlington: American Psychiatric Association, 2013. [Google Scholar]
- 23.National Center for Injury Prevention and Control. CDC compilation of benzodiazepines, muscle relaxants, stimulants, zolpidem, and opioid analgesics with oral morphine milligram equivalent conversion factors, 2018 version. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Department of Health and Human Services. 2018; https://www.cdc.gov/drugoverdose/resources/data.html. Accessed June 8, 2020. [Google Scholar]
- 24.Greenwald M, Johanson CE, Bueller J, Chang Y, Moody DE, Kilbourn M, et al. Buprenorphine duration of action: mu-opioid receptor availability and pharmacokinetic and behavioral indices. Biol Psychiatry 2007; 61: 101–10. doi: 10.1016/j.biopsych.2006.04.043 [DOI] [PubMed] [Google Scholar]
- 25.Haight BR, Learned SM, Laffont CM, Fudala PJ, Zhao Y, Garofalo AS, et al. Efficacy and safety of a monthly buprenorphine depot injection for opioid use disorder: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2019; 393: 778–790. doi: 10.1016/S0140-6736(18)32259-1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(TIF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are located at Dryad: doi:10.5061/dryad.j3tx95xdb.