Abstract
Acute damage to the heart, as in the case of myocardial infarction (MI), triggers a robust inflammatory response to the sterile injury that is part of a complex and highly organized wound-healing process. Cortical bone stem cell (CBSC) therapy after MI has been shown to reduce adverse structural and functional remodeling of the heart after MI in both mouse and swine models. The basis for these CBSC treatment effects on wound healing are unknown. The present experiments show that CBSCs secrete paracrine factors known to have immunomodulatory properties, most notably macrophage colony-stimulating factor (M-CSF) and transforming growth factor-β, but not IL-4. CBSC therapy increased the number of galectin-3+ macrophages, CD4+ T cells, and fibroblasts in the heart while decreasing apoptosis in an in vivo swine model of MI. Macrophages treated with CBSC medium in vitro polarized to a proreparative phenotype are characterized by increased CD206 expression, increased efferocytic ability, increased IL-10, TGF-β, and IL-1RA secretion, and increased mitochondrial respiration. Next generation sequencing revealed a transcriptome significantly different from M2a or M2c macrophage phenotypes. Paracrine factors from CBSC-treated macrophages increased proliferation, decreased α-smooth muscle actin expression, and decreased contraction by fibroblasts in vitro. These data support the idea that CBSCs are modulating the immune response to MI to favor cardiac repair through a unique macrophage polarization that ultimately reduces cell death and alters fibroblast populations that may result in smaller scar size and preserved cardiac geometry and function.
NEW & NOTEWORTHY Cortical bone stem cell (CBSC) therapy after myocardial infarction alters the inflammatory response to cardiac injury. We found that cortical bone stem cell therapy induces a unique macrophage phenotype in vitro and can modulate macrophage/fibroblast cross talk.
Listen to this article’s corresponding podcast at https://ajpheart.podbean.com/e/cortical-bone-stem-cells-effects-on-cardiac-wound-healing/.
Keywords: immune modulation, inflammation, macrophage, myocardial infarction, stem cell therapy
INTRODUCTION
Cardiovascular disease is the leading cause of death for both men and women in the United States (1). Coronary heart disease (CHD) accounts for a plurality of 42.6% of all cardiovascular diseases and contributes to up to 68% of all cases of heart failure (1, 2). International efforts to promote the current standard therapy of reperfusing the ischemic heart tissue have decreased the 30-day MI mortality rate from 18% to 16% (3). However, the 5-yr mortality rate of patients diagnosed with heart failure (HF) after MI remains above 50% (4). Despite the massive need for novel therapeutics aimed toward preventing myocardial damage, adverse remodeling, and the progression to HF after MI, no such therapies have been approved.
Stem cell therapy has been shown to be safe in humans and has been used in clinical trials with modest beneficial effects (5). We have previously shown that stem cells isolated from the outer cortex of bone, termed cortical bone stem cells (CBSCs), have unique proliferation, survival, and immunomodulatory properties compared with other stem cell types (6). In addition, CBSCs decreased mortality, increased cardiac function, and prevented adverse cardiac remodeling in a mouse model of MI (7). More recently, we demonstrated that CBSCs reduced scar size, improved ejection fraction, and prevented left ventricle (LV) dilation after 3 mo compared with vehicle controls in a clinically translational swine model of ischemia-reperfusion (IR)-induced MI (8). This large animal model is uniquely well-suited for translational studies due to the similarity in cardiac physiology between swine and human hearts. Furthermore, in the same swine model, CBSCs reduced myocyte apoptosis 3 days after MI and may be altering macrophage and T cell populations in vivo (9). The mechanisms through which CBSCs improve cardiac structure and function after MI and alter the immune response and wound healing after MI are currently unknown. Understanding how these cells function in the healing heart could lead to the development of new therapies to treat MI patients and reduce their risk of developing HF.
Given our previous work indicating that CBSCs may be interacting with immune cells in the infarcted heart, we hypothesized that CBSCs modulate the wound healing response to cardiac IR injury by inducing an acute proreparative immune milieu in the infarct that alters fibroblast phenotype, ultimately leading to the reduction in scar size and preservation of LV geometry and function observed previously in vivo (8).
Both the initial inflammatory and subsequent reparative phases of the immune response to cardiac damage are necessary for proper healing of the infarct (10). Macrophages play critical roles in all phases of cardiac wound healing and, through the process of efferocytosis of apoptotic neutrophils and myocytes, may act as the fulcrum on which the infarct tips from inflammation to repair by the secretion of anti-inflammatory cytokines like interleukin-10 (IL-10) and IL-1 receptor antagonist (IL-1RA) (10, 11). Macrophages are also crucial for fibroblast proliferation and myofibroblast activation through the secretion of transforming growth factor-β (TGF-β) and IL-10 (12). Initial experiments sought to determine if CBSCs were changing myocardial immune cell populations and cytokines in the swine model of MI. Next, we determined if CBSCs were changing the wound healing and scar formation process by altering fibroblast populations in the same swine model. In addition, we examined the ability of CBSCs to inhibit cell death in the infarct. Given these data, we then pursued the characterization of macrophages treated with CBSC-secreted paracrine factors in vitro. We found that CBSCs increase macrophage and CD4+ T cells, decrease CD8+ T cells, and increase anti-inflammatory cytokines in vivo. We also find that CBSC treatment increases fibroblasts in the infarct and decreases apoptosis in macrophages in vivo. In vitro, CBSC paracrine factors polarize macrophages to a phenotype that shares characteristics of both M2a and M2c macrophage phenotypes, inhibit inflammatory activation of macrophages by LPS, and induce fibroblast proliferation while inhibiting fibroblast activation. Next generation sequencing and gene ontology enrichment analysis of macrophage RNA revealed that CBSC-treated macrophages have a unique transcriptome in vitro that mirrors both our in vivo and in vitro data. Together these data indicate that CBSCs can have far-reaching effects on the innate and adaptive immune system in vivo and have a potent anti-inflammatory, proreparative effect on macrophages and fibroblasts in vitro. These data help to explain the structural and functional improvement of swine hearts treated with CBSCs 3 mo after MI and provide insight into the potential function of stem-cell therapy in post-MI wound healing.
METHODS
Study Design
The aim of this study was to assess the immunomodulatory capabilities of CBSCs and their link to the potential benefits of CBSC therapy in a model of myocardial ischemia/reperfusion. To study changes to heart function, immune cell phenotype, and fibroblast composition in vivo, we employed a large animal swine model of MI. All procedures performed on animals were approved by the Temple University Institutional Animal Care and Use Committee. After blockage of the lateral anterior descending (LAD) coronary artery, female Gottingen miniswine were randomly assigned to either the CBSC or vehicle (saline only) treatment groups. All researchers involved in the surgery, data collection, and data analysis were blinded to the treatments until analysis was complete. Transthoracic echocardiography was used to assess heart function. 2,3,5-Triphenyltetrazolium chloride (TTC) staining was used to determine infarct size as a percentage of the total LV area. Fifteen animals underwent MI surgery. One animal developed unresponsive ventricular arrhythmias and died during surgery, one animal sustained a major injury to the hoof before surgery and was excluded from the study, and three animals did not meet the injection site retention cutoff of 70% and were excluded from analysis. Isolated nonmyocytes from tissue-punch sections of each heart were stained and assess by flow cytometry to measure changes in immune cell populations. Tissue was also fixed and stained using immunofluorescence techniques and confocal microscopy to assess immune cell and fibroblast populations. For in vitro experiments, all samples were run in duplicate at minimum. In addition, all in vitro tests were confirmed in at least three independent experiments. Data presented in the figures of this paper are representative of these independent experiments. No in vitro data were excluded from analysis. All primary animal and in vitro data are reported in Supplemental Data File S1; all Supplemental material is available at https://doi.org/10.6084/m9.figshare.14710635.
Statistical Analysis
All data are presented as means ± SD. For all collection and analysis of in vivo data pertaining to the swine studies, including but not limited to echocardiography analysis, flow cytometric analysis of immune cells, protein quantification, and gross histology, the researchers performing the collection and analysis of the data were blinded to the treatment of each animal until analysis was complete. For all comparisons between two distinct groups, an unpaired, two tailed t test was used to determine significantly distinct groups. For all grouped analyses, a two-way analysis of variance (ANOVA) was performed with post hoc Tukey’s correction for multiple comparisons. For all data, a P value of less than 0.05 was considered significant. All graphs were visualized and analyzed using GraphPad Prism software (GraphPad Holdings, LLC, CA). RNA sequencing and gene ontology data were analyzed using R programming language, and the statistical analysis of these data is outlined in detail in RNA Sequencing and Analysis of these methods.
Induction of MI and Functional Measurements In Vivo
Gottingen miniswine aged 9–12 mo and weighing 25–35 kg at the time of surgery were sedated with 6.0 mg/kg toletamine-zolazapam (Telazol; Fort Dodge Animal Health, Fort Dodge, IA). Animals were then intubated with a 5.5-mm-internal diameter endotracheal tube and general anesthesia was maintained with 1.5%–2% isoflurane (IsoFlo; Zoetis Inc., Kalamazoo, MI). Myocardial infarction was induced by percutaneous transluminal coronary angioplasty as previously described (8, 13). An angioplasty balloon was guided through the femoral artery to the mid-LAD past the first diagonal branch. The balloon was inflated in the LAD for 90 min. Location and ischemia were confirmed by perfusion of the coronary arteries with the radio-opaque contrast lopamidol (ISOVUE; Bracco Diagnostic, Inc., Milan, Italy) and fluoroscopy. After 90 min, the angioplasty balloon was deflated and removed, reperfusing the ischemic tissue. The animal was left to recover for 30 min, after which blood was drawn to assess plasma levels of cardiac troponin I. After 30 min, the inside of the LV was mapped using a Noga mapping catheter (Biosense Webster, Irvine, CA). Infarcted and stunned tissue were visualized by a reduction in conductivity and wall movement as previously described (8). After mapping, 10 injections of 2 million CBSCs each or sterile saline (vehicle) were performed using a Noga injection catheter targeting the infarct and border zone of the infarcted tissue. All injections contained fluorescent microspheres (FluoSpheres, Invitrogen, Carlsbad, CA) for localization after excision of the heart. Transthoracic echocardiography was performed pre-MI and on days 1, 3, and 7 post-MI using a Vivid q Vet Premium BT’12 (General Electric, Boston, MA) and analyzed with EchoPAC SW v201 (General Electric, Boston, MA). Infarct size was determined using 2,3,5- triphenyltetrazolium chloride (TTC, Sigma Aldrich, St. Louis, MO). Plasma levels of cardiac troponin I was analyzed using an enzyme-linked immunosorbent assay (ELISA).
Immunofluorescence
Cardiac tissue was fixed in 10% formalin (Fisher Chemical, Walther, MA) before paraffin embedding with Histoplast LP paraffin wax (ThermoScientific, Waltham, MA) using a Leica ASP300S (Leica Microsystems Inc., Bannockburn, IL). Tissue was sliced in 5-µm sections and mounted by AML Laboratories (Jacksonville, FL). Immunofluorescent staining was performed with the TSA Plus Fluorescence kit (PerkinElmer, Inc., Waltham, MA) as per the manufacturer’s protocol. TUNEL staining was performed using the DeadEnd Fluorometric TUNEL System kit (Promega, Madison, WI) as per the manufacturer’s protocol. A full list of antibodies and their concentrations can be found in Supplemental Table S1. All images were captured using a Nikon Eclispse Ti confocal microscope (Nikon, Tokyo, Japan) with a ×20 objective. Representative images were taken with the same ×20 objective with ×2 optical zoom to enhance detail. Images were analyzed using the NIS Elements imaging software for object counting.
Tissue Protein Quantification
Cardiac tissue isolated for protein quantification was snap-frozen in liquid nitrogen within 5 min of excision. All proteomic quantification was done by Ray Biotech (Peach Tree Corners, GA) using their 50-marker porcine protein screen.
Flow Cytometry
All flow cytometric analysis was performed on a BD LSRII flow cytometer. For in vivo analysis, swine cardiac tissue was digested, and myocytes removed by filtration. Nonmyocytes were then stained for viability with Zombie Aqua fixable viability dye in protein-free PBS to exclude any dead cells during analysis. The cells were then blocked with FcR Blocking Reagent (Miltenyi Biotec, Bergisch Gladbach, Germany) diluted 1:200 in FACS staining buffer (5% bovine serum albumin, 0.5% sodium azide in PBS) for 30 min. Samples were split, and antibodies were added in two separate panels for analysis of myeloid and nonmyeloid cell populations (Supplemental Table S2). All antibodies were titrated for minimal spectral overlap and tested for specificity. After 30 min, the cells were washed and resuspended in FACS buffer for analysis.
For in vitro experiments, macrophage supernatant was removed and saved for analysis by ELISA and replaced by ice-cold PBS. Macrophages were scraped and moved to a 96-well plate for staining. Macrophages were then blocked as previously described for 30 min. Antibodies were added for different experiments as detailed in Supplemental Table S3.
Bone Marrow-Derived Macrophage Generation
All macrophages were derived from C57BL/6 female mice aged 4–6 wk of age at the time of isolation. After anesthesia and cervical dislocation, the legs were removed at the hip, leaving the femur and tibia intact. The bones were separated, muscle tissue was removed, and the bone marrow flushed using a 27-gauge tuberculin needle. Red blood cells were lysed, and macrophages were plated in complete RPMI medium with 50 ng/mL macrophage-colony stimulating factor (M-CSF) to encourage macrophage differentiation. Cells were plated in tissue culture coated 24-well plates at 1 × 106 cells/well. Medium was changed 72 h after plating. Treatments were performed on the 6th day after plating for 24 h and analysis performed on day 7.
Fibroblast Cell Culture and Compound Treatment
Normal human cardiac (ventricular) fibroblasts (NHCFs) were purchased from Lonza (CC-2904) and expanded using their recommended growth medium [FGM-3 Cardiac Fibroblast Growth Medium-3 Bullet Kit (CC-4526; Lonza)]. Cells were harvested at passage 4 and stored in liquid nitrogen. For each experiment, NHCFs were used at passage 5 after thawing and recovery in the above indicated growth medium. Cell counting and viability were assessed using a LUNA automated cell counter (L10001; Logos Biosystems). Cells were plated at 2,000 cells/well into 96-well clear-bottom plates (Cat. No. 655090; Greiner) in 100 µL of the same medium and were grown overnight at 37°C, 5% CO2, and 100% humidity.
The following day, cells were washed and maintained with 100 μL/well of serum-free medium (FBM, Cat. No. CC-3131; Lonza) supplemented with 1% penicillin-streptomycin using a BioTek ELx406 washer dispenser. Conditioned media samples were added 80 μL/well in duplicate and 80 μL/well of serum starvation medium was added to the control wells. The known TGF-β1 receptor inhibitor SB525334 (Cat. No. S1476; SelleckChem) was used to generate an 8-point concentration response curve in duplicate using threefold dilutions with a maximal final concentration of 10 μM in the assay and with a final DMSO concentration of 0.1%. Cells were incubated for 1 h after dosing with SB525334 and TGFβ1 (10 ng/mL, 100-21 C; Preprotech) was added to the SB525334-treated cells. A single column of negative control (DMSO, 8 wells) and a single column of positive control (TGFβ1 10 ng/mL, 8 wells) were included on all plates. The cells were incubated at 37°C, 5% CO2, and 100% humidity for 72 h before fixation.
Fibroblast Staining and Imaging
Cells were washed two times with Dulbecco’s phosphate buffered saline with Ca and Mg (DPBS, Cat. No. 14040216; ThermoFisher Scientific) and fixed for 20 min with 100% methanol (Cat. No. A412-4; Fisher Scientific) at −20°C. Fixed cells were washed three times with DPBS without calcium and magnesium containing 0.02% NaN3 (Cat. No. 14200166; Gibco) and blocked with 1% BSA in Tris-buffered saline (TBS, Cat. No. BP24711; Fisher BioReagents) for 1 h. The primary antibody for anti-α-SMA (1:2,000 anti-α-SMA, Cat. No. ab7817; Abcam) in blocking buffer and incubated overnight at 4°C. Cells were washed with DPBS + 0.02% NaN3 and incubated with a cocktail of secondary antibody and nuclear stain (1:400 goat anti-mouse Alexa488, Thermo; Hoechst 33342, 1:2,000) in blocking buffer and incubated for 1 h in the dark. Cells were washed and stored on sealed plates in DPBS until imaged. All imaging was performed on a CellInsight CX7 High-Content Platform (Cat. No. CX7A1110; Thermo Scientific). Two fluorescence wavelengths (386 and 488 nm) were measured for each field using a ×10 objective. Nine fields were imaged in each well.
High Content Image Analysis
A multistep screening protocol was developed to quantitatively analyze fibroblast morphology and biomarker expression using the HCS Studio software that is part of the CellInsight CX7. The primary mask was generated from the nuclear stain (386 nm) to identify and segment cells. Two masks were used to quantify α-SMA expression. The first mask (RingAvgInten) forms a doughnut shape to measure average perinuclear α-SMA fluorescence intensity (488 nm), which excludes the nuclear fluorescence. The second mask (CircAvgInten) quantifies average α-SMA fluorescence intensity (488 nm) including the entire cytoplasmic and nuclear regions.
Fibroblast Contraction Assay
Compressible collagen matrices were prepared in 24-well plates using PureCol EZ Gel solution (Cat. No. 5074, Advanced BioMatrix) by incubating at 37°C for 1 h. NHCFs suspended in serum-supplemented growth medium were seeded (50,000 cells/gel) on the collagen gels for 24 h before equilibration by serum deprivation (0.1% FBS) overnight. At the initiation of contraction, gels were released from the wells and cells were treated with DMSO (vehicle; 0.05% final concentration), TGF-β1 (10 ng/mL) for up to 72 h. Well images were captured every 24 h; gel area for each well was determined using ImageJ software and data are reported as percent contraction.
RNA Sequencing and Analysis
Stranded mRNA-seq library: 100–1,000 ng of total RNAs from each sample were used to make library according to the product guide of stranded mRNA library kit. In short, mRNAs were enriched twice via poly-T-based RNA purification beads and subjected to fragmentation at 94° for 15 min via divalent cation method. The first strand cDNA was synthesized by reverse transcriptase and random primers at 42°C for 15 min, followed by second strand synthesis at 16°C for 1 h. During second strand synthesis, the dUTP was used to replace dTTP, thereby the second strand was quenched during amplification. A single “A” nucleotide is added to the 3′-ends of the blunt fragments at 37°C for 30 min. Adapters with illuminaP5, P7 sequences as well as indices were ligated to the cDNA fragment at 30°C for 10 min. After SPRIselect beads (Cat. No. B23318; Beckman Coulter) purification, a 15-cycle PCR reaction was used to enrich the fragments. PCR was set at 98°C for 10 s, 60°C for 30 s, and extended at 72°C for 30 s. Libraries were again purified using SPRIselect beads, with a quality check on Agilent 2100 bioanalyzer (Serial No. DE34903146) using Agilent high-sensitive DNA kit (Cat. No. 5067-4626) and quantified with Qubit 3.0 fluorometer (Cat. No. Q33216; ThermoFisher Scientific) using Qubit 1x dsDNA HS assay kit (Cat. No. Q33230). Sample libraries were subsequently pooled and loaded to the Hiseq 2500 (Serial No. SN930; Illumina). Paired end reads at 75 bp were generated by using Nextseq 500 high-output reagent kit v2.5 (Cat. No. 20024907; Illumina). Fastq files were obtained at Illumina base space (https://basespace.illumina.com). For each sample, ∼40 million paired end reads were obtained. Raw fastq files were indexed to mouse genome mm10 and quantified for downstream analysis by Salmon. Quantified transcripts were imported as a matrix of average transcript length using package tximport to package DESeq2 for downstream differential expression analysis in R. Genes were identified and those with less than 5 reads per sample were removed. Wald test was used to statistically identify differential expressed genes and P values adjusted by Benjamin–Hochberg method (Padjust) < 0.05 and presented. With the regularized logarithm (rlog) function, count data were transformed for visualization on a log2 scale. The volcano plots created had thresholds of log fold change set at ±1.5 and false discovery rate value set at <0.05. Differentially expressed genes (DEGs) were used for functional analysis. Gene ontology (GO) enrichment analysis was performed using package goseq to classify genes in terms within biological process. Threshold of Padjust < 0.05 was implemented and the top overrepresented GO terms were analyzed.
RESULTS
CBSCs Alter Immune Cell Populations and Cytokines In Vivo
We have previously shown CBSCs to be effective at reducing scar size and preserving cardiac function 3 mo after MI in a swine model of lateral anterior descending (LAD) coronary artery occlusion with reperfusion (8). However, CBSCs had no acute cardioprotective effect nor did they reduce infarct size 3 days after MI (8). Evidence from animal models as well as clinical studies shows that the resolution of inflammation and the transition to fibroblast proliferation and scar formation culminate ∼7 days after MI (10, 14–17). Therefore, given the potential for immunomodulation and alteration of wound healing by CBSCs, we sought to determine if CBSCs were changing the healing infarct at a cellular level 7 days after MI in ways that could explain the long-term benefits of CBSC therapy.
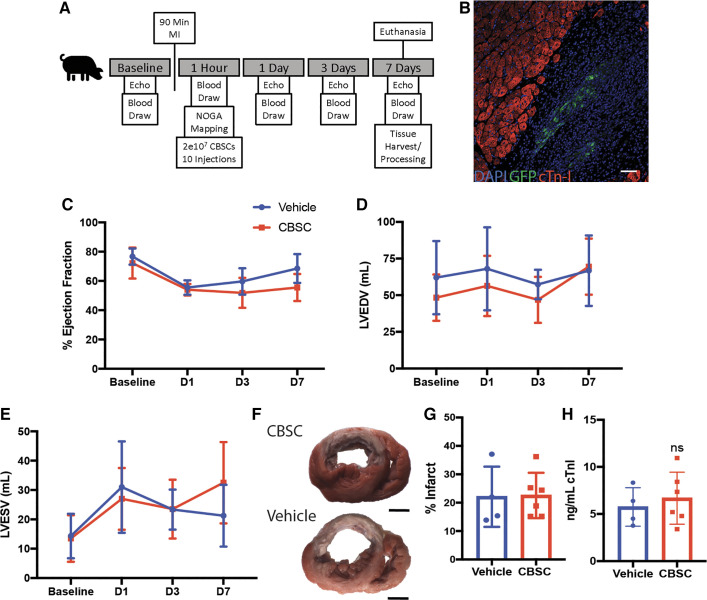
We designed a 7-day experiment with serial echocardiography and blood analyses to first assess any functional changes taking place over the first week post-MI (Fig. 1A). CBSCs injected into swine expressed lentiviral-transfected green fluorescent protein (GFP) to aid in their identification. Additionally, all injections contained fluorescent microbeads detectable with ultraviolet light so that injection sites could be identified on cardiac excision. CBSCs were detected at injection sites by immunofluorescent staining for GFP in all CBSC-treated hearts 7 days after MI (representative image Fig. 1B). Consistent with previous work, CBSCs did not have a functional cardioprotective effect measured by changes in ejection fraction, end-diastolic, or end-systolic volumes in the first 7 days after MI (Fig. 1, C–E). On the 7th day after MI, swine hearts were excised, and the tissue processed for analysis. 2,3,5-Triphenyltetrazolium chloride (TTC) staining revealed no difference in infarct size as a percentage of total LV area between CBSC and vehicle-treated animals 7 days after MI (Fig. 1, F and G). There was no difference in plasma levels of cardiac troponin I 60 min after reperfusion between CBSC- or vehicle-treated swine, indicating cardiac damage was similar between treatment groups (Fig. 1H). These data are consistent with previous data in this model that showed no acute cardioprotective effect or reduction in infarct size 3 days after MI (8).
Figure 1.
CBSCs do not have an acute cardioprotective effect. A: outline of in vivo study design. B: representative image of GFP + CBSCs in the border zone of CBSC-treated female swine myocardium. Serial transthoracic echocardiographic measurement of ejection fraction (C), left ventricle end-diastolic volume (LVEDV; D), and left ventricle end-systolic volume (LVESV; E) of swine on day 1 (D1), 3 (D3), and 7 (D7) after MI. Representative images of TTC-stained heart slices from vehicle- and CBSC-treated swine hearts (F) and quantification of infarct size (G). H: quantification of cardiac troponin I 1 h after MI by ELISA. n = 4 swine for vehicle and n = 6 swine for CBSC for all figures. Scale bar in B = 100 µm. CBSCs, cortical bone stem cells; GFP, green fluorescent protein; MI, myocardial infarction; TTC, 2,3,5-triphenyltetrazolium chloride.
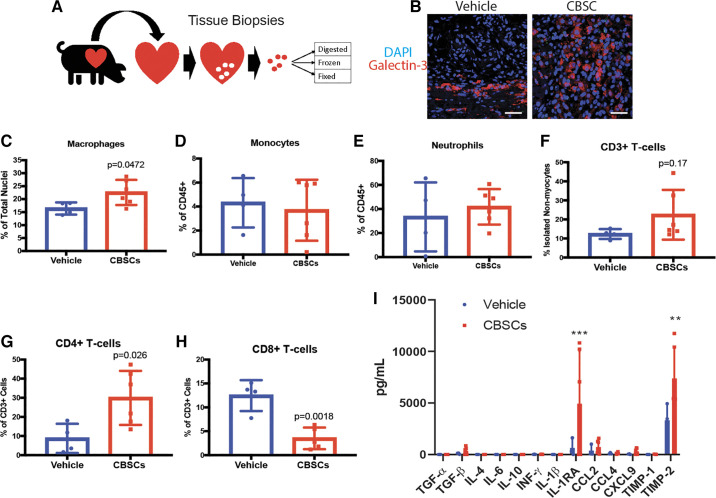
After cardiac excision on day 7, heart tissue was processed for various applications (Fig. 2A). Immunofluorescent staining of fixed tissue for galectin-3 was used to quantify the number of macrophages present in the infarct 7 days after MI (Fig. 2B). Galectin-3 is highly expressed in macrophages and is involved in the uptake of dead cells and macrophages deficient in galectin-3 display reduced phagocytosis of apoptotic cells in vitro and in vivo (18–20). CBSC-treated animals had significantly more galectin-3+ macrophages in the border zone of the infarct 7 days after MI as measured by immunofluorescent staining (Fig. 2C, CBSCs = 22.58 ± 4.84, vehicle = 16.4 ± 2.34, P = 0.047, n = 6 for CBSCs, n = 4 for vehicle).
Figure 2.
A: CBSC treatment alters the inflammatory response to MI in vivo. Summary of tissue harvest for analysis 7 days after MI. Representative images (B) and quantification of the number of macrophages by immunofluorescent staining for Galectin-3 in vehicle (saline) or CBSC-treated female swine hearts (C). C–H: quantification of immune cell popualtions by flow cytometry using the gating strategies outlined in Fig. S1. I: quantification of protein levels of various cytokines, chemokines, and growth factors in CBSC or vehicle (saline)-treated swine hearts. n = 3 for vehicle and n = 6 for CBSC. ***P = 0.0006 vs. vehicle. **P = 0.0014 vs. vehicle. n = 4 for vehicle, n = 6 for CBSC for all figures except I. Scale bar in B = 25 µm. CBSCs, cortical bone stem cells; IL-1RA, IL-1 receptor antagonist; MI, myocardial infarction; TGF-β, transforming growth factor-β.
Multicolor flow cytometry of isolated nonmyocytes was used to examine the levels of neutrophils, monocytes, and T cells from the swine heart 7 days after MI (Supplemental Fig. S1). No difference in monocytes or neutrophils was observed 7 days after MI (Fig. 2, D and E). However, there was a trend toward increased CD3+ T cells in the tissue of CBSC-treated animals (Fig. 2F, vehicle = 12.34 ± 2.6%, CBSC = 22.5 ± 13.1%, P = 0.17, n = 4 for vehicle, n = 6 for CBSC). Within this CD3+ T-cell population, there was a significantly higher proportion of CD4+ T-helper cells (Fig. 2G, vehicle = 8.75 ± 7.7%, CBSC = 29.97 ± 14.2%, P = 0.026, n = 4 for vehicle, n = 6 for CBSC) and a lower proportion of CD8+ cytotoxic T cells (Fig. 2H, vehicle = 12.46 ± 3.2%, CBSC = 3.51 ± 2.3%, P = 0.0018, n = 4 for vehicle, n = 5 for CBSC). These data indicate that CBSCs do not affect neutrophil or monocyte levels 7 days after MI. CBSCs do, however, affect cells in the infarct, such as macrophages and CD4+ T cells, involved in processes taking place during the resolution of inflammation and the transition to scar formation 7 days post-MI. Interestingly, CBSCs, either directly or indirectly, appear to change the numbers of cells involved in independent arms of the immune system (innate vs. adaptive). These data speak to the broad-reaching changes possible with intramuscular injection of CBSCs.
To further assess changes to the healing infarct brought about by these changes in cell populations, we quantified protein levels of immune-relevant cytokines, chemokines, and growth factors. Of the factors analyzed, IL-1RA (Fig. 2I, vehicle = 649.1 ± 865.39 pg/mL , CBSC = 4,935.3 ± 5,053.9 pg/mL, P = 0.0006, n = 3 for vehicle, n = 6 for CBSC, two-way ANOVA with Bonferroni’s correction for multiple comparisons) and tissue inhibitor of metalloproteinase-2 (TIMP-2, Fig. 2J, vehicle = 3,315.2 ± 1,700.6 pg/mL, CBSC = 7,384.9 ± 3,009.9 pg/mL, P = 0.0014, n = 3 for vehicle, n = 6 for CBSC, two-way ANOVA with Bonferroni’s correction for multiple comparisons) were increased in CBSC-treated animals 7 days after MI. An increase in IL-1RA is consistent with an anti-inflammatory infarct environment and IL-1RA administered after MI leads to decreased apoptosis and LV remodeling in animal models of MI (21). TIMP-2 can act as both an inhibitor and activator of MMP-2, regulating extracellular matrix (ECM) processing and production (22). Understanding the role of its upregulation in the infarct of CBSC-treated animals requires further study, but efficient ECM regulation is necessary for proper scar development and maturation (23). These data together with the evidence for altered immune cell populations in the infarct of CBSC-treated hearts suggest that CBSCs are altering the inflammatory state of the healing cardiac wound in a large animal model of MI.
CBSC Treatment Increases Fibroblast Populations In Vivo
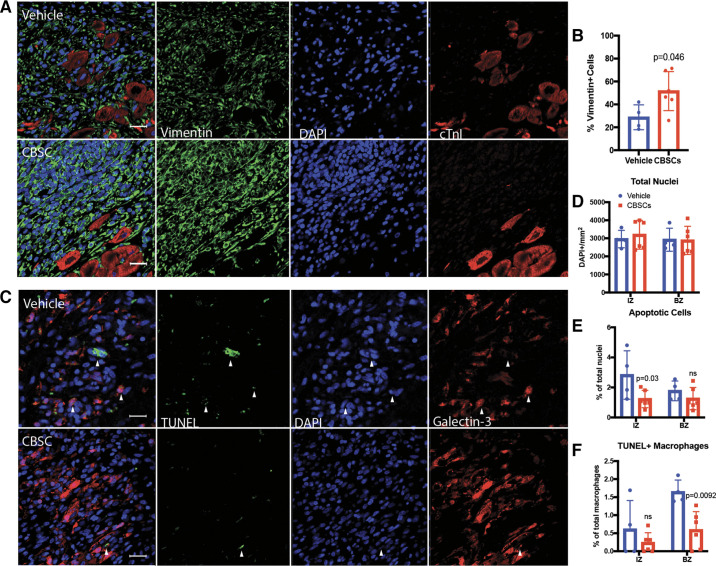
As the inflammatory response to MI resolves, wound healing transitions to a proliferative phase where fibroblasts increase in number and begin producing collagen I and III, the primary scaffolding proteins of the post-MI scar (24). The peak of fibroblast proliferation occurs 3–7 days after MI in animal models, and disruption of fibroblast proliferation and activation leads to impaired wound healing and impaired cardiac function (25, 26). Due to the link between inflammation resolution and fibroblast proliferation, we quantified the number of fibroblasts in the infarct of CBSC- or vehicle-treated swine hearts 7 days after MI by immunofluorescent staining for Vimentin (Fig. 3A). CBSC-treated animals had significantly more Vimentin+ cells in the border zone of the infarct 7 days after MI (Fig. 3B, vehicle = 28.7 ± 10.9%, CBSC = 51.6 ± 17.1%, P = 0.046, n = 4 for vehicle, n = 6 for CBSC). These data suggest that CBSCs, either directly or indirectly through their modification of the immune response, may stimulate fibroblast proliferation or migration during the resolution of inflammation beyond the normal amounts observed in this phase of the wound healing process. These results support the idea that the resolution of inflammation occurs earlier after MI due to an anti-inflammatory effect of CBSCs, and CBSC-treated animals have entered the proliferative fibroblast phase sooner, allowing for the accumulation of fibroblasts earlier than their vehicle-treated counterparts. Further studies are needed to examine the effects of CBSC treatment on fibroblast populations in vivo.
Figure 3.
CBSCs increase fibroblasts and decrease apoptosis in vivo 7 days after MI. Representative confocal images (A) and quantification (B) of immunofluorescent staining for Vimentin, DAPI, and cardiac troponin I in swine myocardium 7 days after MI. Representative images of terminal deoxynucleotidyl transferase dUTP nuck end labeling (TUNEL), DAPI, and Galectin-3 staining in the infarct zone of swine myocardium 7 days after MI (C) and their quantification (D–F). n = 4 for vehicle, n = 6 for CBSC for all figures. Scale bar = 25 µm. White arrows indicate TUNEL+/DAPI+ cells. Staining for Galectin-3 and TUNEL was performed and analyzed concurrently from the same images. CBSCs, cortical bone stem cells; MI, myocardial infarction.
CBSC Treatment Decreases Apoptosis 7 Days after MI In Vivo
Scar formation is a necessary mechanism to preserve cardiac structure and function in response to the death of cardiac tissue. At the most basic level, less dead cardiac tissue should require smaller scar, and scar size is a primary indicator of clinical outcomes for MI patients (25, 27). Necrosis of myocardial cells in the infarct core occurs rapidly after the onset of ischemia and is the primary method of cell death in the first hours of myocardial infarction (28). Apoptosis, however, occurs more slowly in humans and continues days and weeks past the initial ischemic insult (29). Additionally, inflammation can be a major source of myocardial apoptosis on reperfusion of the ischemic tissue (30). We have previously shown that CBSCs reduce myocyte apoptosis 3 days after MI (9). In the current study, we determined if CBSC treatment had antiapoptotic effects in nonmyocytes. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining along with antibody-based immunofluorescent staining was performed on paraffin-embedded swine myocardium and visualized with confocal microscopy (Fig. 3C). The total number of cells in the infarct and border zones was not different between groups (Fig. 3D). The number of TUNEL+ apoptotic nuclei was lower in the infarct zone of CBSC-treated animals (Fig. 4E, vehicle = 2.83 ± 1.6%, CBSC = 1.23 ± 0.58%, P = 0.03, n = 4 for vehicle, n = 6 for CBSC). In addition to lowering apoptosis overall, there were fewer TUNEL+ Galectin-3+ macrophages in the border zone of CBSC-treated animals (Fig. 4F, vehicle = 1.64 ± 0.3%, CBSC = 0.59 ± 0.5%, P = 0.0092, n = 4 for vehicle, n = 6 for CBSC). These data, together with our previously published myocyte apoptosis data (9), indicate that CBSCs are reducing cell death by apoptosis throughout the wound healing process. Although the infarct size of CBSC-treated animals may not be reduced at these acute time points, the inhibition of apoptosis throughout the first 7 days after MI may indicate a continued protective effect that could account for some of the reduction in scar size seen in CBSC-treated swine 3 mo after MI (8).
Figure 4.
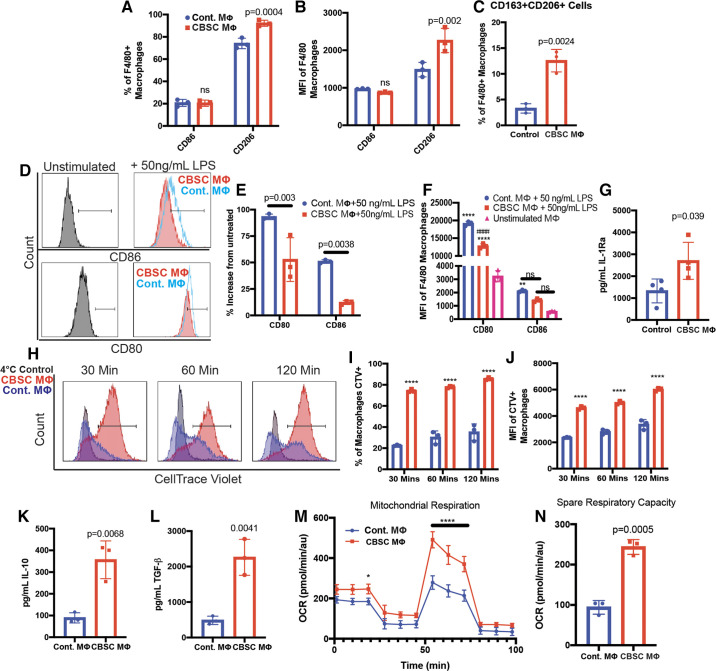
CBSCs induce a unique macrophage phenotype in vitro. A–C: flow cytometric analysis of macrophage surface markers after stimulation with CBSC-conditioned medium (CBSC) or normal complete RPMI (control). D: representative flow cytometric analysis of inflammatory macrophage markers after treatment with 50 ng/mL LPS in CBSC or control medium for 24 h. E–F: quantification of flow cytometry analysis, ****P < 0.0001 compared with untreated, #### P < 0.0001 compared with Cont. + 50 ng/mL LPS, **P = 0.0031. G: quantification of IL-1RA in macrophage supernatant by ELISA after stimulation with CBSC-conditioned RPMI for 24 h. H: flow cytometric analysis of macrophage efferocytosis of dead cardiac myocytes after 24-h stimulation with CBSC or control medium. I and J: quantification of flow cytometry analysis; ****P < 0.0001 compared with Cont; CTV, CellTrace Violet. K and L: quantification of IL-10 and TGF-β in the supernatant of macrophages that had undergone efferocytosis of dead myocytes after stimulation with CBSC or control medium by ELISA. M and N: quantification of mitochondrial metabolism and spare respiratory capacity by Seahorse Assay, ****P < 0.0001 compared with Cont., *P = 0.042. n = 3. CBSCs, cortical bone stem cells; IL-1RA, IL-1 receptor antagonist; TGF-β, transforming growth factor-β; OCR, oxygen consumption rate.
CBSC-Secreted Factors Induce a Novel M2-like Macrophage Phenotype
Macrophages are crucial for cardiac wound healing and are mediators of cardiac remodeling in mice, swine, and humans (31–35). CBSCs secrete paracrine factors known to stimulate macrophage survival and differentiation (Table 1). Furthermore, CBSCs increased macrophage numbers in vivo. To investigate the effects of CBSC-secreted factors on macrophages, we exposed bone marrow-derived macrophages (BMDMs) from female mice aged 4–6 wk to complete RPMI cell culture medium in which CBSCs had been grown (CBSC MΦ) or normal complete RPMI medium in which no CBSCs had been grown (control MΦ). Flow cytometric analysis of the mannose receptor CD206 and the T-cell costimulatory protein CD86 was used to get a baseline assessment of the polarization of macrophages to M2 or M1 phenotypes, respectively (40–42). CBSC MΦ had significantly higher CD206 surface expression than control MΦ (Fig. 4A, control MΦ = 74.08 ± 4.7%, CBSC MΦ = 92.3 ± 2.7%, P = 0.0004, n = 3). CD86 expression was not different between groups (Fig. 4A, control MΦ = 20.72 ± 3.1%, CBSC MΦ = 20.42 ± 2.8%, P = 0.99, n = 3). This pattern remained consistent when measured by the median fluorescence intensity (MFI) of each marker (Fig. 4B).
Table 1.
CBSCs secrete immunomodulatory factors
| Secreted Factor | Concentration, pg/mL | Function |
|---|---|---|
| Macrophage-colony stimulating factor | 7.8 ± 2.0 | Growth factor critical for the survival and differentiation of macrophages (36) |
| Transforming growth factor-β | 2,385.8 ± 647.3 | Pleiotropic growth factor involved in inflammation, fibroblast activation (37) |
| Tissue inhibitor of metalloproteinase-1 | 30,421.6 ± 2,840.7 | Inhibitor of metalloproteinases with antiapoptotic properties (38) |
| Stromal cell-derived factor-1 | 1,096.3 ± 258.4 | Strong lymphocyte chemoattractant, proangiogenic effects, procardiomyocyte survival, upregulated in response to inflammation (39) |
Concentration of factors secreted by mouse cortical bone stem cells (CBSCs) in vitro. Factors were chosen based on unbiased dot-blot screen and the positive results quantified by ELISA. CBSCs, cortical bone stem cells.
M2 macrophage subtypes differ in their surface molecule profiles. Both M2a and M2c macrophages express CD206 whereas only M2c macrophages coexpress CD206 and CD163 (43). To assess whether CBSCs were inducing coexpression of CD206 and CD163 in CBSC MΦs, we quantified the number of cells expressing both markers after stimulation with either CBSC or control medium by flow cytometry. CBSC MΦ had significantly increased coexpression of CD206 and CD163 compared with control MΦ (Fig. 4C, control = 3.3 ± 0.9%, CBSC = 12.6 ± 2.2%, P = 0.0024, n = 3). However, the majority of CBSC MΦ expressed only CD206 in the absence of further stimulation, indicating that the BMDMs may be heterogeneous in culture and do not represent a uniform M2c population.
The ability of CBSCs to suppress the expression of inflammatory macrophage markers was examined by incubating BMDMs with either the CBSC or control medium containing 50 ng/mL lipopolysaccharide (LPS). LPS is a potent inflammatory signal and a canonical inducer of M1 proinflammatory macrophages (44). Both CD80 and CD86 are T-cell costimulatory proteins involved in the priming and activation of T cells and are upregulated in response to LPS (45). Multicolor flow cytometry was used to compare surface expression of CD80 and CD86 in both treatment groups to unstimulated macrophages [unstimulated MΦ (Fig. 4D)]. After stimulation with LPS in CBSC medium for 24 h, the number of cells expressing either CD86 (Fig. 4E, control MΦ = 51.0 ± 1.7%, CBSC MΦ = 12.2 ± 1.9%, P = 0.0038, n = 3) or CD80 (Fig. 4E, control MΦ = 93.2 ± 2.6%, CBSC MΦ = 52.8 ± 20.6%, P = 0.003, n = 3) was significantly reduced compared with macrophages exposed to control medium with 50 ng/mL LPS. Again, this trend was continued when measuring the MFI of the total F4/80+ macrophage population (Fig. 4F). These data suggest CBSC-secreted paracrine factors inhibit the expression of proteins involved in proinflammatory activation between the innate and adaptive immune system in the presence of inflammatory stimuli.
IL1-RA is an anti-inflammatory cytokine and a hallmark of M2a macrophages (46, 47). To further characterize the phenotype of CBSC-treated macrophages, we measured IL-1RA production by macrophages exposed to CBSC or control medium by enzyme-linked immunosorbent assay (ELISA). CBSC-treated MΦ produced significantly more IL-1RA after 24 h than control MΦ (Fig. 4G, control MΦ = 1,326 ± 544.6 pg/mL, CBSC MΦ = 2,700 ± 843.0 pg/mL, P = 0.039, n = 4). Importantly, IL-1RA was not present in the CBSC medium alone, indicating that the IL-1RA was produced by the macrophages specifically. The production of IL-1RA in response to CBSC-conditioned medium supports the notion that CBSC-secreted factors induce an anti-inflammatory phenotype in macrophages and supports the increase of IL-1RA in CBSC-treated myocardium in vivo.
Clearance of dead cells and cellular debris by efferocytosis is a primary function of macrophages in the infarcted heart and inhibition of this process leads to decreased wound debridement and reduced fibroblast activation (12, 48). The enhanced ability to phagocytose dead cells is associated with M2c macrophages (43). We measured the efferocytic ability of CBSC MΦ by flow cytometric analysis. Myocytes from C57BL/6 mouse hearts were isolated and left in low-calcium isolation buffer at room temperature overnight to induce cell death. The myocytes were then stained with the protein-adherent dye CellTrace Violet and added to macrophage cultures previously incubated with either CBSC or control medium for 24 h at a ratio of 1:3 myocytes to BMDMs. Macrophages were analyzed by flow cytometry and gated for F4/80 expression followed by CellTrace Violet intensity (Fig. 4H, full gating strategy, Supplemental Fig. S2). To ensure the uptake of CellTrace Violet was efferocytosis-dependent, we included a control in which macrophages were exposed to dead myocytes at 4°C (4°C control). At this temperature, no efferocytosis should take place and any dye present in the macrophages would be transferred through nonspecific dye uptake. Uptake of dye in this way was negligible and positive gates for analyzing dye positivity of the experimental groups was set at the upper limit of this 4°C control group to remove any potential confounding effects of nonspecific dye uptake. A higher percentage CBSC MΦ efferocytosed myocyte debris after all timepoints indicated by the percentage of macrophages positive for the dye (Fig. 4I, 30 min: control MΦ = 14.83 ± 0.45%, CBSC MΦ = 66.13 ± 1.7%, P < 0.0001, n = 3) and appear to have efferocytosed more myocyte debris after all timepoints indicated by the MFI of the dye within the dye + macrophage population only for both groups (Fig. 4J, 30 min: control MΦ = 1,823.67 ± 23 au , CBSC MΦ = 4,140.67 ± 140.32 au, P < 0.0001). These data indicate that CBSC-conditioned medium increases the ability of BMDMs to take up cellular debris. In addition, it increased the rate at which the debris was taken up by BMDMs. Increasing efferocytic capabilities could improve wound debridement and alter the activation of fibroblasts, improving the wound healing environment of the infarct.
Efferocytosis can induce the production and release of IL-10 and TGF-β from macrophages (49). IL-10 was not detectable after 30 or 60 min of exposure to dead myocytes whereas TGF-β was detected at all timepoints. Supernatant from CBSC MΦ exposed to dead myocytes contained more IL-10 (Fig. 4K, control MΦ = 89.28 ± 23.4 pg/mL, CBSC MΦ = 356.4 ± 86.9 pg/mL, P = 0.0068, n = 3) and TGF-β (Fig. 4L, control MΦ = 539.3 ± 72.9 pg/mL, CBSC MΦ = 2262.1 ± 413.4 pg/mL, P = 0.0021, n = 3) than RPMI controls after 120 min. IL-10 was not detected in CBSC-conditioned medium alone and so must have originated from the macrophages in culture. However, TGF-β is present in CBSC-conditioned medium (Table 1). To determine if the macrophages were producing TGF-β apart from the TGF-β already present in the conditioned medium, we examined the percent increase in concentration of TGF-β over time. From 30 to 120 min, the average concentration of TGF-β in the supernatant of control MΦ increased by 13.9%, whereas the concentration of TGF-β in the supernatant of CBSC MΦ increased by 43.8% (Supplemental Fig. S3A). These data indicate that both groups of macrophages produced TGF-β on exposure to dead myocytes. The production of IL-10 and TGF-β after exposure to cellular debris has been indicated as the possible point from which inflammation in the infarcted heart begins to resolve. Increasing the efferocytic ability of macrophages and subsequently the production of IL-10 and TGF-β may resolve inflammation sooner and alter the wound healing process by differential activation of fibroblasts. Indeed, disrupting the clearance of apoptotic cells in the infarct increases infarct size and decreases cardiac function in mice, whereas the adoptive transfer of efferocytosis-competent phagocytes restores function and reduces infarct size (50). In addition, efficient clearance of apoptotic cells in humans requires the polarization of macrophages to an M2c phenotype (51). These changes in infarct healing may be linked to the resolution of inflammation that results from efficient efferocytosis.
Phenotypic changes to macrophages are accompanied by corresponding metabolic shifts (52). M2 macrophages rely more heavily on oxidative phosphorylation and exhibit larger spare respiratory capacities (52–54). A Seahorse Extracellular Flux analyzer was used to measure the oxygen consumption rate of CBSC MΦ and control MΦ (Fig. 4M). The spare respiratory capacity of CBSC MΦ was significantly greater than in control MΦ (Fig. 4N, control MΦ = 94.02 ± 16.9 pmol/min/au, CBSC MΦ = 243.7 ± 18.31 pmol/min/au, P = 0.0005, n = 3). There was also a trend toward a higher basal metabolic respiration, ATP production, and nonmitochondrial respiration in CBSC MΦ (Supplemental Fig. S3, n = 3). This metabolic profile matches that of alternatively activated macrophages corroborates the phenotypic changes of CBSC MΦ.
Taken together, these data indicate that CBSC MΦ are polarized to an M2-like phenotype. This phenotype spans surface marker expression, cytokine secretion, and functional ability profiles of multiple M2 subtypes (Table 2). Secreted paracrine factors of CBSCs that induce MΦ to an M2-like phenotype may be a novel combination of stimuli that ultimately results in anti-inflammatory, highly phagocytic macrophages capable of modulating the wound healing response to MI in unique ways. Finally, these data suggest that intercellular signaling may induce a vast array of macrophage phenotypes beyond the commonly studied phenotypes induced by single cytokines in vitro like M(IL-4)/M2a and M(IL-10)/M2c.
Table 2.
Summary of CBSC-induced changes to macrophages
| Factor/Function | M2a (43, 47) | M2c (43, 47) | CBSC-Mac |
|---|---|---|---|
| CD206+ | + | + | + |
| CD163+ | − | + | +/− |
| IL-1RA | + | − | + |
| IL-10 | +/− | + | + |
| TGF-β | +/− | + | + |
| TNF-α | + | − | − |
| Enhanced efferocytosis | − | + | + |
| Stimulated by IL-4/IL-13 | + | − | − |
| Stimulated by TGF-β/IL-10 | − | + | + |
Functions and secreted factors of M2a and M2c macrophages are listed. +consistent high expression/secretion, +/− inconsistent or low expression, and − no expression. CBSC, cortical bone stem cell.
CBSC-Treated Macrophages Have a Unique RNA Transcriptome Profile
We performed next-generation RNA sequencing (RNAseq) on CBSC-treated macrophages, M2a macrophages (40 ng/mL IL-4), M2c macrophages (40 ng/mL IL-10 and TGF-β), M1 macrophages (50 ng/mL LPS), M0 macrophages (unstimulated control), and compared transcriptomes to gain a deeper understanding of the differences and similarities between groups. Principal component analysis (PCA) revealed CBSC macrophages separated from M2a, M2c, and M0 control macrophages along the second principal component, whereas, unsurprisingly, M1 macrophages were separated from all other groups along the first principal component (Fig. 5A). Together, principal components 1 and 2 accounted for 64% of variance. In a similar way, hierarchical clustering heat mapping showed high correlation within groups and lower correlation between groups, with the biggest differences occurring between CBSC-treated macrophages and M1 (Fig. 5B). The mean Pearson’s correlation coefficient (r) remained above 0.95, likely due to the fact that all groups were murine macrophages.
Figure 5.
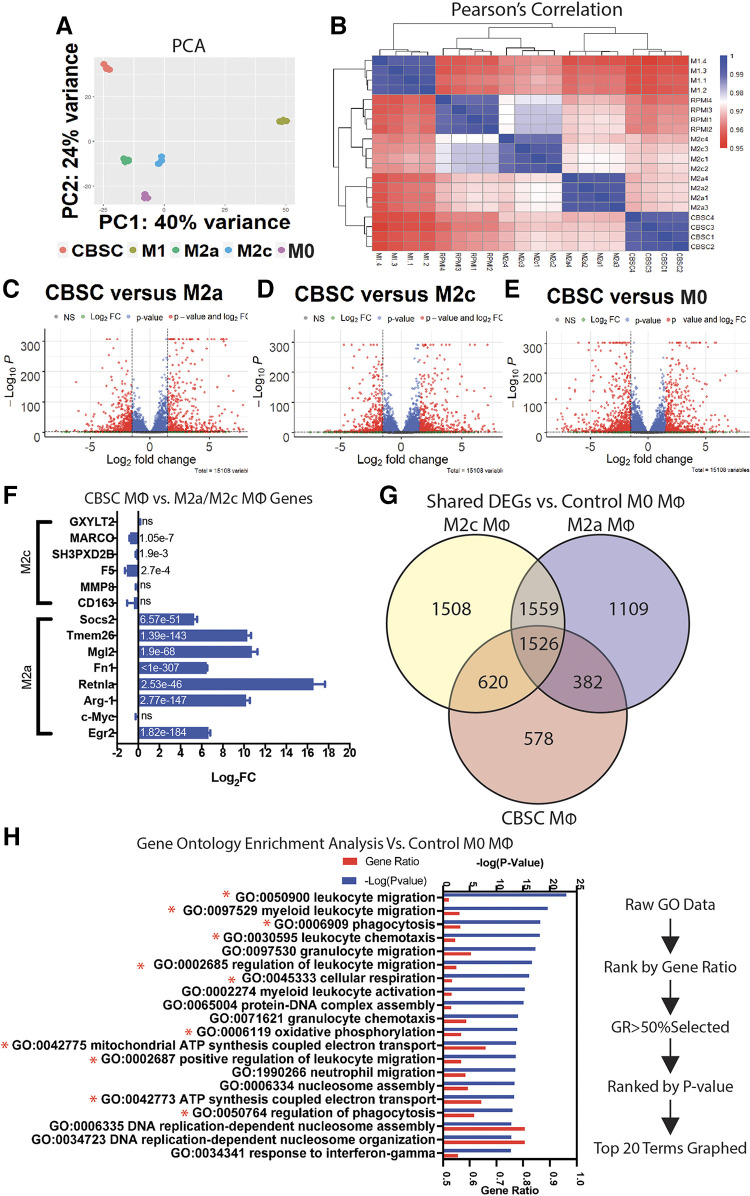
A: CBSC-treated macrophages display a unique transcriptome. Principal component analysis of CBSC, M1 (50 ng/mL LPS), M2a (40 ng/mL IL-4), M2c (40 ng/mL IL-10), and M0 (no treatment) macrophages; principal components 1 and 2 represent 64% of total variance. B: heatmap of Pearson’s correlation coefficients between all groups. C–E: volcano plots of fold change vs. −log(P value) representing differentially expressed genes (DEGs) in CBSCs vs. M2a, M2c, and M0 macrophages. F: log2(fold change) of specific M2a- and M2c-related genes with adjusted P value in bar; values derived from CBSC vs. M2c for M2c genes, CBSC vs. M2a for M2a genes. G: Venn diagram showing DEGs and common genes between M2c, M2a, and CBSCs vs. M0 control macrophages. H: gene ontology enrichment analysis and ranking system based on DEGs of CBSC vs. M0 control. n = 4 for all groups. CBSCs, cortical bone stem cells.
Analysis of differentially expressed genes (DEGs) between macrophage groups revealed that CBSC macrophages differentially express a large number of genes compared with M2a, M2c, and M0 control macrophages (Fig. 5, C–E). Of these DEGs, we analyzed 14 genes specifically upregulated in M2a and M2c transcriptomes (55, 56). Interestingly, all but one of the genes associated with M2a macrophages were significantly upregulated in CBSC-treated macrophages, including Arg-1, Retnla, Socs2, and Egr2, but not C-Myc (Fig. 5F). Conversely, the genes MARCO, SH3PXD2B, and F5 (associated with M2c macrophages) were significantly downregulated in CBSC-treated macrophages whereas the remaining half of M2c-associated genes were not significantly different (Fig. 5F). However, looking at the total set of DEGs, CBSCs-treated macrophages differentially express 578 unique genes compared with both M2a and M2c macrophages and share 1.6 times more DEGs with M2c macrophages than M2a (620 M2c vs. 382 M2a; Fig. 5G). These data suggest that CBSC MΦ are differentially expressing M2a-like genes at levels higher than in M2a macrophages themselves while secreting cytokines, sharing a larger number of genes, and performing functionally akin to M2c macrophages, adding to the complexity of the CBSC macrophage phenotype.
Gene ontology (GO) enrichment analysis was performed comparing CBSC MΦ with M0 control macrophages. Raw GO data were sorted in descending order based on gene ratio (the ratio of differentially expressed genes to total genes in a GO set). GO sets containing a majority of DEGs (gene ratio >50%) were then sorted again in ascending order according to their adjusted P value. The top 20 most significant terms were then extracted from the list and graphed to show gene ratio and −log(P value) (Fig. 5H). Interestingly, 11 of the top 20 terms obtained from this unbiased approach directly pertain to observations made about CBSC-treated macrophages in Figs. 2 and 4, including positive regulation of leukocyte and myeloid cell migration, oxidative phosphorylation and mitochondrial ATP synthesis, and phagocytosis. GO terms for the positive regulation of phagocytosis, macrophage migration, and aerobic respiration were also present within the top-50 most enriched GO terms (Supplemental Data File S1). Identical sorting and comparisons of M2a versus M0 and M2c versus M0 GO terms yielded lists with entirely different enriched term sets (Supplemental Figs. S4 and S5).
Together these data show that CBSC-treated macrophages display a unique transcriptome compared with M2a and M2c macrophages, implying that CBSC-treated macrophages may represent a unique macrophage polarization state exhibiting qualities from a unique combination of previously described phenotypes. The GO enrichment analysis also reaffirms some of the functional and phenotypic data observed previously, strengthening the concept of CBSC-treated macrophages as functionally unique.
CBSC-Treated Macrophages Induce Fibroblast Proliferation While Inhibiting Activation
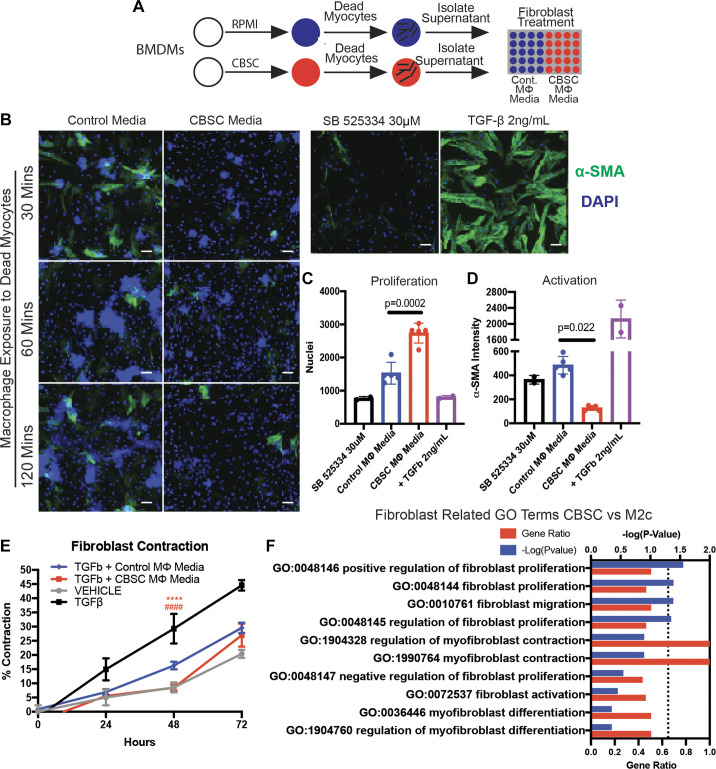
The resolution of the inflammatory phase of wound healing and the transition to a healing phase dominated by fibroblast proliferation and the production of scar is a major focus of this work. The communication and coordination between fibroblasts and macrophages in the healing infarct are well documented, although the effects of modulating this aspect of wound healing remain largely unknown (24). Due to the unique polarization of CBSC MΦ, we sought to determine the effects of secreted factors from CBSC-treated macrophages exposed to dead myocytes (CBSC MΦ media) or equivalent control macrophages exposed to dead myocytes (control MΦ media) on fibroblast proliferation and activation (Fig. 6A). Supernatant from these groups was isolated and any remaining cells removed. Human cardiac fibroblasts were then exposed to the different conditioned media for 72 h and proliferation and activation were measured by high content quantitative fluorescent imaging (Fig. 6B). CBSC MΦ media significantly increased the number of DAPI+ fibroblast nuclei (Fig. 6C, control MΦ media = 1,528 ± 329 DAPI+ cells, CBSC MΦ media = 2,738 ± 300 DAPI+ cells, P = 0.0002, n = 5). Inversely, the same medium inhibited α-smooth muscle actin (α-SMA) expression in the fibroblasts (Fig. 6D, control MΦ media = 484.1 ± 73.2 au, CBSC MΦ media = 126.7 ± 19.6 au, P = 0.022, n = 5). α-SMA is upregulated in myofibroblasts and its expression indicates the transition from fibroblast to myofibroblast.
Figure 6.
CBSC-treated macrophages induce proliferation and inhibit activation and contraction of human fibroblasts. A: schematic of media generation for treatment of fibroblasts. B: representative images of human fibroblasts in culture treated with CBSC or control medium outlined in A; SB525334 was used as a TGF-β inhibitor and TGF-β was used as a positive control for fibroblast activation and α-SMA. Scale bar = 50 µm. Quantification of the number of nuclei (C) and the intensity of α-SMA (D) of the 60-min treatment group. E: quantification of the percent contraction from baseline of human fibroblasts cultured on collagen gels and treated with TGF-β; vehicle group received no TGF-β. ****P < 0.0001 CBSC vs. TGF-β, ####P < 0.0001 CBSC vs. TGF-β + control media. n = 5 for all groups. F: gene ontology enrichment analysis of differentially expressed genes (DEGs) of CBSC vs. M2c of terms including search term “fibroblast,” plotted with gene ratio and −log(adjusted P value); dotted line = −log(0.05) = 1.3 indicating cutoff for significance. n = 4 for gene ontology analysis. α-SMA, α-smooth muscle actin; CBSC, cortical bone stem cell; TGF-β, transforming growth factor-β.
Another hallmark of fibroblast activation and transition to myofibroblasts is their contraction. To measure fibroblast contraction in the presence of CBSC MΦ or control MΦ medium, fibroblasts were seeded and cultured on collagen gels, grown, and stimulated with TGF-β. As the fibroblasts contracted over 72 h, the area of the gel was measured and compared with the baseline area to get the percent contraction. By 24 h, fibroblasts exposed to vehicle (no TGF-β), control MΦ, or CBSC MΦ medium in the presence of added TGF-β had contracted significantly less than fibroblasts exposed to TGF-β alone (Fig. 6E). Furthermore, fibroblasts exposed to CBSC MΦ medium contracted 51% less than those exposed to the equivalent control MΦ media at 48 h (Fig. 6E, TGF-β + control MΦ media = 16.3 ± 1.3% TGF-β + CBSC MΦ media = 8.4 ± 1.1%, P < 0.0001, n = 5) and was approximately equivalent to the vehicle control. By 72 h, the CBSC MΦ, control MΦ media, and vehicle effects were no longer significantly different. All three groups remained significantly below the TGF-β group contraction for the entirety of the study.
M2c macrophages are known to secrete IL-10 and TGF-β, both of which have potent inhibitory and stimulatory effects on fibroblasts depending on how and when they are engaged in the wound healing process (37, 43, 47, 57, 58). Due to the affect CBSC MΦ medium had on fibroblast proliferation and activation, we sought to compare the CBSC MΦ transcriptome to the M2c MΦ transcriptome. Using the same GO data presented in Fig. 5, we performed a biased term-based search for fibroblast-related terms in both significantly and nonsignificantly enriched term lists comparing CBSC MΦ versus M2c MΦ. Using the search term “fibroblast,” 10 relevant terms were categorized and graphed according to ascending adjusted P value (Fig. 6F). The four significantly enriched terms [P < 0.05, or −log(P value) > 1.3] pertained to positive regulation of fibroblast proliferation and the regulation of fibroblast migration. The remaining nonsignificant six terms pertained to myofibroblast contraction, differentiation, and the negative regulation of fibroblast proliferation, indicating that these biological systems were not enriched in CBSC MΦ compared with M2c MΦ.
These data show that CBSCs’ influence on macrophage phenotype results in conditions under which fibroblasts proliferate rapidly without undergoing myofibroblast activation. This is supported by the fact that fibroblasts exposed to CBSC MΦ -treated macrophage supernatant contract less than controls. Finally, GO analysis supports the conclusion that CBSC-treated macrophages are enriched for gene sets responsible for the proliferation and migration of fibroblasts. These changes in fibroblast phenotype may also provide insight into the extent to which macrophage phenotype can determine fibroblast phenotype and function. Overall, these data suggest CBSCs possess the ability to modify the scar formation and the wound healing process by altering macrophage-fibroblast cross talk.
DISCUSSION
Our group has previously shown the unique qualities and beneficial results of CBSC therapy in both small and large animal models of MI (6–8). In this study, we examined the cellular changes induced by CBSC treatment that may lead to these effects. Our findings can be separated into two categories of effects: immunomodulation within the infarct and modulation of the fibroblast response to injury. These two functions are indelibly linked as major arms of the wound healing process.
We found in a large animal swine model of MI that injection of CBSCs into the MI border zone alters the infiltrating immune cell populations and cytokine milieu present 7 days after MI. This in vivo model of MI is unique in its translational nature in that the swine heart bears physiological and functional similarities to the human heart that rodent models do not possess. In this way, we can more accurately predict changes that could occur should CBSCs or other immunomodulatory cells be used clinically. Injected CBSCs increased macrophages and CD4+ T cells within the healing infarct border zone while simultaneously increasing anti-inflammatory IL-1RA and TIMP-2 in the infarct compared with vehicle controls. IL-1RA is produced by M2a-polarized macrophages and plays a number of roles in inflammation resolution and healing in the infarct (59–62). TIMP-2, on the other hand, uniquely regulates the production and breakdown of ECM proteins and deficiency of TIMP-2 accelerates adverse remodeling after MI (63). The role of increased levels of TIMP-2 is not clear and uncontrolled TIMP expression has been linked with fibrosis in humans (64). However, we have shown that CBSC-treated swine hearts have less scar. The role of CBSC-induced TIMP-2 requires further study.
Identifying the exact subtype of these cells in the swine model with the specificity of in vitro characterization of murine macrophages and T cells is not currently feasible due to a lack of readily available and reliable swine-specific reagents and antibodies. Nonetheless, these data indicate significant changes to broad categories of immune cells after CBSC treatment using company-validated antibodies directed toward swine antigens. These changes in immune cells also occurred in parallel with a reduction in cell (myocyte and nonmyocyte) death in the infarct. Although this correlation in no way identifies a causative link between CBSC-induced immunomodulation and reduced cell death, it does provide evidence for a CBSC-induced cytoprotective environment within the infarct. Although the survival of cardiomyocytes in the infarct would almost certainly be beneficial, the death of immune cells during the healing phase of the cardiac wound is an important factor in reducing inflammation and healing the infarcted tissue and it is unclear what role the reduction of macrophage apoptosis plays in CBSC therapy. Alternatively, given the increased phagocytic ability of CBSC-treated macrophages in vitro, fewer apoptotic cells in the infarct could be a result of increased cell clearance. One implication of these data paired with our previously published data showing reduced numbers of apoptotic myocytes with CBSC treatment is that CBSCs may be altering cell clearance and the wound healing response in ways that protect damaged but not yet dead myocardium after MI. Although these changes may not be robust enough to alter infarct size at the level of gross histology at 7 days post-MI, if sustained they could slow infarct expansion so that scar size is reduced in CBSC-treated hearts 3 mo after MI, as seen in our previously published results in this model (8, 9).
To better characterize how CBSC-secreted factors influence macrophage phenotype, we turned to reliable and well-studied in vitro methods of macrophage generation and analysis. Through the stimulation of BMDMs with CBSC-conditioned medium, we found that the CBSC secretome induces a macrophage phenotype that does not neatly fit into predefined macrophage phenotypes like M2a and M2c despite the fact that the CBSC secretome contained well-defined and well-studied elements of macrophage polarization, such as TGF-β and M-CSF. Although it is possible that the novel nature of these macrophages could indicate a new state of macrophage polarization in vitro, it is likely that the stimulation of macrophages with CBSC-conditioned medium induces an anti-inflammatory, prophagocytic macrophage phenotype reminiscent of the more nuanced macrophage phenotypes found in vivo. The in vitro characterization and standardization of macrophages based on individual stimuli like IL-4, IL-10, and TGF-β may not represent the full array of possible macrophage phenotypes likely to be seen in complex in vivo conditions. Macrophages in the infarcted heart exist on a spectrum of activation in response to a vast array of stimuli. In support of cell-based macrophage polarization, the commonly studied mesenchymal stem cells (MSCs) have documented immunomodulatory properties and their effects on macrophages are documented both in vitro and in vivo (65–67). Although the necessity of identifying macrophage phenotypes based on specific and clear stimuli is readily apparent, we argue that room be made for M(CBSC) and M(MSC) alongside M(IL-4) and M(IL-10). Our future work will focus on the secreted factors responsible for controlling the functional outputs of CBSC treatment, like phagocytosis, aerobic respiration, and fibroblast modulation.
Independent from but related to the immunomodulatory properties of CBSCs are the apparent affects they have on fibroblasts. We found that CBSC injections in vivo increased the number of Vimentin+ cells in the infarct. Although it is possible that other mesenchymal-derived cells may be included within this Vimentin+ population, it is certain that this population contains the fibroblasts of the heart. Furthermore, Vimentin plays key roles in fibroblast proliferation and activation, making it a relevant marker to assess in the healing infarct (68). When we examined the cross talk of macrophages exposed to CBSC medium and human fibroblasts in vitro, we found that the CBSC-macrophage-conditioned medium greatly increased fibroblast proliferation without simulating the expression of the myofibroblast marker α-SMA. In addition, CBSC-macrophage-conditioned medium inhibited the contraction of human fibroblasts with TGF-β stimulation. Although the exact signals responsible for the effects seen on fibroblasts have yet to be determined, IL-10 has been previously identified as an inhibitor of fibroblast activation and fibrosis in various models of scarring including myocardial infarction (69). In addition, the activation and inhibition of fibroblasts by TGF-b and IL-10, respectively, appear to be reversible and time-dependent in a 3 D collagen matrix model of tissue (58). Lastly, IL-10 has been shown to decrease levels of collagens I and III and alpha-smooth muscle actin (70). Indeed, IL-10 is increased in the media of CBSC-treated macrophages by ∼3.5-fold (Fig. 4K). The presence of IL-10 in CBSC-macrophage-conditioned media makes it a strong candidate for future studies aimed at understand the molecular mechanisms behind the CBSC-macrophage-fibroblast interactions.
The stiffening of cardiac scar during the maturation phase of the infarct plays a significant role in infarct thinning and LV chamber dilation that are hallmarks of HF (25). These data are particularly relevant in light of our previously published data showing a lack of infarct thinning and a preservation of LV chamber dimensions and cardiac function 3 mo after MI (8). It is possible that the interaction between CBSCs, macrophages, and fibroblasts changes the maturation of the scar, preventing the dilation and thinning seen in vehicle-treated swine hearts. Future studies will focus on the characterization of CBSC-treated fibroblasts by single-cell RNA sequencing and ECM by mass spectrometry to assess the fibroblast phenotype and protein make-up of CBSC-treated scar. These data also raise the question of the direct versus indirect action of CBSCs: Are macrophages necessary for the same effect of CBSCs? Future work is needed to determine the role macrophages play in mediating fibroblast-CBSC interactions.
The limitations of this study lie primarily in the correlative relationships between our in vitro and in vivo findings. For example, the in vitro characterization of macrophages in mouse BMDMs cannot be directly applied to the increase in macrophage number in swine. In vitro experiments naturally cannot replicate the complex and highly organized environment of the living infarct and must be viewed as such. In addition, the swine model of MI is best suited to functional measurements of the heart. The reagents currently available to study the phenotype of immune cells and fibroblasts by immunofluorescence and flow cytometry are limited. However, the swine model provides us with heart physiology and anatomy closer to that of humans than rodent models and is a powerful tool to examine the possible effects of stem cell treatment in the clinic. Our examination of day 7 post-MI captures only a narrow slice of the entire wound healing process, and effects of CBSC therapy on cell types like neutrophils and monocytes, which are critical to the immune response to cardiac wound healing, may not be accurately represented looking at this time point. Although the analysis of swine cardiac immune cells by flow cytometry may not yet be commonplace in basic cardiovascular research, all immune cell markers used in this study were taken directly from previously established cell-membrane markers of porcine immune cell populations (71–74). Lastly, the pig heart is a vast organ compared with a mouse or rat, and although we have taken targeted approaches in our analysis of the infarct, it is not feasible to analyze the pig heart as thoroughly as a mouse or rat heart.
The original intent of cell therapy was to induce the differentiation of highly plastic stem cells to contractile, organized, myocytes that contribute to the pump function of the heart. Instead, studies have shown that stem cells have little ability to become cardiac tissue, yet possess unique immunomodulatory capabilities through their secretomes and these abilities are beneficial to healing and preventing disease (65, 66, 75, 76). Understanding the mechanisms by which stem cells sense, interact, and affect their environment is crucial to the successful implementation of more effective post-MI wound healing therapies in patients. Large animal models, although limited in their own way, provide the best model in which to study these effects.
We conclude from this study that CBSCs are altering the immune cell and fibroblast populations of the infarcted heart in unique ways, and these modifications of the healing process may rescue scar size and result in improvements in the way the heart functions after MI.
SUPPLEMENTAL DATA
Supplemental Data File S1, Supplemental Figs. S1–S3, and Supplemental Tables S1–S3: https://doi.org/10.6084/m9.figshare.14710635.
GRANTS
This work was supported by American Heart Association Predoctoral Grant 18PRE33960122 (to A.R.H.H.), Postdoctoral Grant 20POST35210627 (to M.R.), and Grant 16SFRN31400013 (to T.A.M.); and National Institutes of Health Grants 5R01HL139960-03 and 5P01HL134608-04 (to S.R.H.), HL116848, DK119594, HL127240, and HL150225 (to T.A.M.), and HL147558 (to T.A.M. and S.R.H.).
DISCLOSURES
S. Houser is a named inventor on intellectual property filings that are related to the subject of this paper. In addition, S. Houser is a cofounder, scientific advisor, and holds equity in MyocardTherapeutics, LLC, a biotech startup. MyocardTherapeutics, LLC, has not funded any aspect of this research. Patent information is available upon request. None of the other authors has any conflicts of interest, financial or otherwise, to disclose. T.A.M. is on the SAB of Artemes Bio, received funding from Italfarmaco for an unrelated project, and has a subcontract from Eikonizo Therapeutics for an SBIR grant from the National Institutes of Health (HL154959).
AUTHOR CONTRIBUTIONS
A.R.H.H., R.M.B., S.M., S.G., T.A.M., and S.R.H. conceived and designed research; A.R.H.H., R.M.B., D.M.E., H.K., E.F., Y.Y., A.L.H., K.A.K., M.R., and Y.T. performed experiments; A.R.H.H., A.L.H., K.A.K., M.R., J.K., M.K., Y.T., and Y.Y. analyzed data; A.R.H.H., Y.Y., K.A.K.., J.K., M.K., S.M., S.G., T.A.M., and S.R.H. interpreted results of experiments; A.R.H.H. prepared figures; A.R.H.H. drafted manuscript; A.R.H.H., S.M., S.G., T.A.M., and S.R.H. edited and revised manuscript; A.R.H.H., R.M.B., D.M.E., H.K., Y.Y., A.L.H., K.A.K., M.R., J.K., Y.T., S.M., S.G., T.A.M., and S.R.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank all the members of the Houser and McKinsey laboratories who aided in producing this manuscript and the members of Temple University’s Cardiovascular Research Center for making this research possible.
Present address of A. R. H. Hobby: Div. of Cardiology, Dept. of Medicine, Univ. of Colorado Anschutz Medical Campus, Aurora, CO.
REFERENCES
- 1.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart Disease and Stroke Statistics-2019 Update: a report from the American Heart Association. Circulation 139: e56–e528, 2019. [Erratum in Circulation 141: e33, 2020]doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 2.Gheorghiade M, Bonow RO. Chronic heart failure in the United States: a manifestation of coronary artery disease. Circulation 97: 282–289, 1998. doi: 10.1161/01.cir.97.3.282. [DOI] [PubMed] [Google Scholar]
- 3.Krumholz HM, Wang Y, Chen J, Drye EE, Spertus JA, Ross JS, Curtis JP, Nallamothu BK, Lichtman JH, Havranek EP, Masoudi FA, Radford MJ, Han LF, Rapp MT, Straube BM, Normand SL. Reduction in acute myocardial infarction mortality in the United States: risk-standardized mortality rates from 1995-2006. JAMA 302: 767–773, 2009. doi: 10.1001/jama.2009.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levy D, Kenchaiah S, Larson MG, Benjamin EJ, Kupka MJ, Ho KK, Murabito JM, Vasan RS. Long-term trends in the incidence of and survival with heart failure. N Engl J Med 347: 1397–1402, 2002. doi: 10.1056/NEJMoa020265. [DOI] [PubMed] [Google Scholar]
- 5.Madigan M, Atoui R. Therapeutic use of stem cells for myocardial infarction. Bioengineering (Basel) 5: 28, 2018. doi: 10.3390/bioengineering5020028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohsin S, Troupes CD, Starosta T, Sharp TE, Agra EJ, Smith S, Duran JM, Zalavadia N, Zhou Y, Kubo H, Berretta RM, Houser SR. Unique features of cortical bone stem cells associated with repair of the injured heart. Circ Res 117: 1024–1033, 2015. [Erratum in Circ Res 127: e271, 2020]. doi: 10.1161/CIRCRESAHA.115.307362. [DOI] [PubMed] [Google Scholar]
- 7.Duran JM, Makarewich CA, Sharp TE, Starosta T, Zhu F, Hoffman NE, Chiba Y, Madesh M, Berretta RM, Kubo H, Houser SR. Bone-derived stem cells repair the heart after myocardial infarction through transdifferentiation and paracrine signaling mechanisms. Circ Res 113: 539–552, 2013. doi: 10.1161/CIRCRESAHA.113.301202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharp TE, Schena GJ, Hobby AR, Starosta T, Berretta RM, Wallner M 3rd, Borghetti G, Gross P, Yu D, Johnson J, Feldsott E, Trappanese DM, Toib A, Rabinowitz JE, George JC, Kubo H, Mohsin S, Houser SR. Cortical bone stem cell therapy preserves cardiac structure and function after myocardial infarction. Circ Res 121: 1263–1278, 2017. doi: 10.1161/CIRCRESAHA.117.311174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hobby ARH, Sharp TE 3rd, Berretta RM, Borghetti G, Feldsott E, Mohsin S, Houser SR. Cortical bone-derived stem cell therapy reduces apoptosis after myocardial infarction. Am J Physiol Heart Circ Physiol 317: H820–H829, 2019.doi: 10.1152/ajpheart.00144.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frangogiannis NG. The immune system and cardiac repair. Pharmacol Res 58: 88–111, 2008. doi: 10.1016/j.phrs.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frangogiannis NGRegulation of the inflammatory response in cardiac repair. Circ Res 110: 159–173, 2012. doi: 10.1161/CIRCRESAHA.111.243162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lambert JM, Lopez EF, Lindsey ML. Macrophage roles following myocardial infarction. Int J Cardiol 130: 147–158, 2008. doi: 10.1016/j.ijcard.2008.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valina C, Pinkernell K, Song Y-H, Bai X, Sadat S, Campeau RJ, Le Jemtel TH, Alt E. Intracoronary administration of autologous adipose tissue-derived stem cells improves left ventricular function, perfusion, and remodelling after acute myocardial infarction. Eur Heart J 28: 2667–2677, 2007. doi: 10.1093/eurheartj/ehm426. [DOI] [PubMed] [Google Scholar]
- 14.Liu J, Wang H, Li J. Inflammation and inflammatory cells in myocardial infarction and reperfusion injury: a double-edged sword. Clin Med Insights Cardiol 10: 79–84, 2016. doi: 10.4137/CMC.S33164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saparov A, Ogay V, Nurgozhin T, Chen WCW, Mansurov N, Issabekova A, Zhakupova J. Role of the immune system in cardiac tissue damage and repair following myocardial infarction. Inflamm Res 66: 739–751, 2017. doi: 10.1007/s00011-017-1060-4. [DOI] [PubMed] [Google Scholar]
- 16.Timmers L, Pasterkamp G, de Hoog VC, Arslan F, Appelman Y, de Kleijn DP. The innate immune response in reperfused myocardium. Cardiovasc Res 94: 276–283, 2012. doi: 10.1093/cvr/cvs018. [DOI] [PubMed] [Google Scholar]
- 17.Yan X, Anzai A, Katsumata Y, Matsuhashi T, Ito K, Endo J, Yamamoto T, Takeshima A, Shinmura K, Shen W, Fukuda K, Sano M. Temporal dynamics of cardiac immune cell accumulation following acute myocardial infarction. J Mol Cell Cardiol 62: 24–35, 2013.doi: 10.1016/j.yjmcc.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 18.Sano H, Hsu DK, Apgar JR, Yu L, Sharma BB, Kuwabara I, Izui S, Liu FT. Critical role of galectin-3 in phagocytosis by macrophages. J Clin Invest 112: 389–397, 2003.doi: 10.1172/JCI17592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shirakawa K, Endo J, Kataoka M, Katsumata Y, Yoshida N, Yamamoto T, Isobe S, Moriyama H, Goto S, Kitakata H, Hiraide T, Fukuda K, Sano M. IL (interleukin)-10–STAT3–galectin-3 axis is essential for osteopontin-producing reparative macrophage polarization after myocardial infarction. Circulation 138: 2021–2035, 2018. doi: 10.1161/CIRCULATIONAHA.118.035047. [DOI] [PubMed] [Google Scholar]
- 20.MacKinnon AC, Farnworth SL, Hodkinson PS, Henderson NC, Atkinson KM, Leffler H, Nilsson UJ, Haslett C, Forbes SJ, Sethi T. Regulation of alternative macrophage activation by galectin-3. J Immunol 180: 2650–2658, 2008. doi: 10.4049/jimmunol.180.4.2650. [DOI] [PubMed] [Google Scholar]
- 21.Abbate A, Salloum FN, Vecile E, Das A, Hoke NN, Straino S, Biondi-Zoccai GG, Houser JE, Qureshi IZ, Ownby ED, Gustini E, Biasucci LM, Severino A, Capogrossi MC, Vetrovec GW, Crea F, Baldi A, Kukreja RC, Dobrina A. Anakinra, a recombinant human interleukin-1 receptor antagonist, inhibits apoptosis in experimental acute myocardial infarction. Circulation 117: 2670–2683, 2008.doi: 10.1161/CIRCULATIONAHA.107.740233. [DOI] [PubMed] [Google Scholar]
- 22.Bourboulia D, Stetler-Stevenson WG. Matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs): positive and negative regulators in tumor cell adhesion. Semin Cancer Biol 20: 161–168, 2010. doi: 10.1016/j.semcancer.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun Y, Weber KT. Infarct scar: a dynamic tissue. Cardiovasc Res 46: 250–256, 2000. doi: 10.1016/S0008-6363(00)00032-8. [DOI] [PubMed] [Google Scholar]
- 24.Deb A, Ubil E. Cardiac fibroblast in development and wound healing. J Mol Cell Cardiol 70: 47–55, 2014. doi: 10.1016/j.yjmcc.2014.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holmes JW, Borg TK, Covell JW. Structure and mechanics of healing myocardial infarcts. Annu Rev Biomed Eng 7: 223–253, 2005. doi: 10.1146/annurev.bioeng.7.060804.100453. [DOI] [PubMed] [Google Scholar]
- 26.Duan J, Gherghe C, Liu D, Hamlett E, Srikantha L, Rodgers L, Regan JN, Rojas M, Willis M, Leask A, Majesky M, Deb A. Wnt1/βcatenin injury response activates the epicardium and cardiac fibroblasts to promote cardiac repair. EMBO J 31: 429–442, 2012. doi: 10.1038/emboj.2011.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richardson WJ, Clarke SA, Quinn TA, Holmes JW. Physiological implications of myocardial scar structure. Compr Physiol 5: 1877–1909, 2015. doi: 10.1002/cphy.c140067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pasotti M, Prati F, Arbustini E. The pathology of myocardial infarction in the pre- and post-interventional era. Heart 92: 1552–1556, 2006. doi: 10.1136/hrt.2005.086934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saraste A, Pulkki K, Kallajoki M, Henriksen K, Parvinen M, Voipio-Pulkki LM. Apoptosis in human acute myocardial infarction. Circulation 95: 320–323, 1997. doi: 10.1161/01.cir.95.2.320. [DOI] [PubMed] [Google Scholar]
- 30.Toldo S, Mauro AG, Cutter Z, Abbate A. Inflammasome, pyroptosis, and cytokines in myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 315: H1553–H1568, 2018. p doi: 10.1152/ajpheart.00158.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weinberger T, Schulz C. Myocardial infarction: a critical role of macrophages in cardiac remodeling. Front Physiol 6: 107, 2015. doi: 10.3389/fphys.2015.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Amerongen MJ, Harmsen MC, van Rooijen N, Petersen AH, van Luyn MJA. Macrophage depletion impairs wound healing and increases left ventricular remodeling after myocardial injury in mice. Am J Pathol 170: 818–829, 2007. doi: 10.2353/ajpath.2007.060547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leblond A-L, Klinkert K, Martin K, Turner EC, Kumar AH, Browne T, Caplice NM. Systemic and cardiac depletion of M2 macrophage through CSF-1R signaling inhibition alters cardiac function post myocardial infarction. PLoS One 10: e0137515, 2015. doi: 10.1371/journal.pone.0137515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vilahur G, Juan-Babot O, Peña E, Oñate B, Casaní L, Badimon L. Molecular and cellular mechanisms involved in cardiac remodeling after acute myocardial infarction. J Mol Cell Cardiol 50: 522–533, 2011. doi: 10.1016/j.yjmcc.2010.12.021. [DOI] [PubMed] [Google Scholar]
- 35.Ma Y, Mouton AJ, Lindsey ML. Cardiac macrophage biology in the steady-state heart, the aging heart, and following myocardial infarction. Transl Res 191: 15–28, 2018. doi: 10.1016/j.trsl.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ushach I, Zlotnik A. Biological role of granulocyte macrophage colony-stimulating factor (GM-CSF) and macrophage colony-stimulating factor (M-CSF) on cells of the myeloid lineage. J Leukoc Biol 100: 481–489, 2016. doi: 10.1189/jlb.3RU0316-144R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bujak M, Frangogiannis NG. The role of TGF-beta signaling in myocardial infarction and cardiac remodeling. Cardiovasc Res 74: 184–195, 2007. doi: 10.1016/j.cardiores.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li G, Fridman R, Kim H-RC. Tissue inhibitor of metalloproteinase-1 inhibits apoptosis of human breast epithelial cells. Cancer Res 59: 6267–6275, 1999. [PubMed] [Google Scholar]
- 39.Bleul CC, Fuhlbrigge RC, Casasnovas JM, Aiuti A, Springer TA. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1). J Exp Med 184: 1101–1109, 1996. doi: 10.1084/jem.184.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol 177: 7303–7311, 2006. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- 41.Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci 29: 13435–13444, 2009. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stein M, Keshav S, Harris N, Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med 176: 287–292, 1992. doi: 10.1084/jem.176.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yao Y, Xu XH, Jin L. Macrophage polarization in physiological and pathological pregnancy. Front Immunol 10: 792, 2019. doi: 10.3389/fimmu.2019.00792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Orecchioni M, Ghosheh Y, Pramod AB, Ley K. Macrophage polarization: different gene signatures in M1(LPS+) vs. classically and M2(LPS–) vs. alternatively activated macrophages. Front Immunol 10: 1084, 2019. doi: 10.3389/fimmu.2019.01084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wong CK, Lit LCW, Tam LS, Li EK, Lam CWK. Aberrant production of soluble costimulatory molecules CTLA-4, CD28, CD80 and CD86 in patients with systemic lupus erythematosus. Rheumatology 44: 989–994, 2005. doi: 10.1093/rheumatology/keh663. [DOI] [PubMed] [Google Scholar]
- 46.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 25: 677–686, 2004. p doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 47.Rőszer T. Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediators Inflamm 2015: 816460, 2015. . doi: 10.1155/2015/816460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Duffield JS, Tipping PG, Kipari T, Cailhier J-F, Clay S, Lang R, Bonventre JV, Hughes J. Conditional ablation of macrophages halts progression of crescentic glomerulonephritis. Am J Pathol 167: 1207–1219, 2005. doi: 10.1016/S0002-9440(10)61209-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chung EY, Kim SJ, Ma XJ. Regulation of cytokine production during phagocytosis of apoptotic cells. Cell Res 16: 154–161, 2006. doi: 10.1038/sj.cr.7310021. [DOI] [PubMed] [Google Scholar]
- 50.Wan E, Yeap XY, Dehn S, Terry R, Novak M, Zhang S, Iwata S, Han X, Homma S, Drosatos K, Lomasney J, Engman DM, Miller SD, Vaughan DE, Morrow JP, Kishore R, Thorp EB. Enhanced efferocytosis of apoptotic cardiomyocytes through myeloid-epithelial-reproductive tyrosine kinase links acute inflammation resolution to cardiac repair after infarction. Circ Res 113: 1004–1012, 2013. doi: 10.1161/CIRCRESAHA.113.301198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zizzo G, Hilliard BA, Monestier M, Cohen PL. Efficient clearance of early apoptotic cells by human macrophages requires M2c polarization and MerTK induction. J Immunol 189: 3508–3520, 2012. doi: 10.4049/jimmunol.1200662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O'Neill LA, Pearce EJ. Immunometabolism governs dendritic cell and macrophage function. J Exp Med 213: 15–23, 2016. doi: 10.1084/jem.20151570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Caputa G, Flachsmann LJ, Cameron AM. Macrophage metabolism: a wound-healing perspective. Immunol Cell Biol 97: 268–278, 2019. doi: 10.1111/imcb.12237. [DOI] [PubMed] [Google Scholar]
- 54.Huang SC-C, Smith AM, Everts B, Colonna M, Pearce EL, Schilling JD, Pearce EJ. Metabolic reprogramming mediated by the mTORC2-IRF4 signaling axis is essential for macrophage alternative activation. Immunity 45: 817–830, 2016. doi: 10.1016/j.immuni.2016.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lurier EB, Dalton D, Dampier W, Raman P, Nassiri S, Ferraro NM, Rajagopalan R, Sarmady M, Spiller KL. Transcriptome analysis of IL-10-stimulated (M2c) macrophages by next-generation sequencing. Immunobiology 222: 847–856, 2017. doi: 10.1016/j.imbio.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gerrick KY, Gerrick ER, Gupta A, Wheelan SJ, Yegnasubramanian S, Jaffee EM. Transcriptional profiling identifies novel regulators of macrophage polarization. PloS One 13: e0208602, 2018. doi: 10.1371/journal.pone.0208602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jung M, Ma Y, Iyer RP, DeLeon-Pennell KY, Yabluchanskiy A, Garrett MR, Lindsey ML. IL-10 improves cardiac remodeling after myocardial infarction by stimulating M2 macrophage polarization and fibroblast activation. Basic Res Cardiol 112: 33, 2017. doi: 10.1007/s00395-017-0622-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sapudom J, Wu X, Chkolnikov M, Ansorge M, Anderegg U, Pompe T. Fibroblast fate regulation by time dependent TGF-β1 and IL-10 stimulation in biomimetic 3D matrices. Biomater Sci 5: 1858–1867, 2017. doi: 10.1039/c7bm00286f. [DOI] [PubMed] [Google Scholar]
- 59.Brønnum H, Eskildsen T, Andersen DC, Schneider M, Sheikh SP. IL-1β suppresses TGF-β-mediated myofibroblast differentiation in cardiac fibroblasts. Growth Factors 31: 81–89, 2013. doi: 10.3109/08977194.2013.787994. [DOI] [PubMed] [Google Scholar]
- 60.Madej MP, Töpfer E, Boraschi D, Italiani P. Different regulation of interleukin-1 production and activity in monocytes and macrophages: innate memory as an endogenous mechanism of IL-1 inhibition. Front Pharmacol 8: 335, 2017. doi: 10.3389/fphar.2017.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saxena A, Chen W, Su Y, Rai V, Uche OU, Li N, Frangogiannis NG. IL-1 induces proinflammatory leukocyte infiltration and regulates fibroblast phenotype in the infarcted myocardium. J Immunol 191: 4838–4848, 2013. doi: 10.4049/jimmunol.1300725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shen Y, Qin J, Bu P. Pathways involved in interleukin-1β-mediated murine cardiomyocyte apoptosis. Tex Heart Inst J 42: 109–116, 2015. doi: 10.14503/THIJ-14-4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kandalam V, Basu R, Abraham T, Wang X, Soloway PD, Jaworski DM, Oudit GY, Kassiri Z. TIMP2 deficiency accelerates adverse post-myocardial infarction remodeling because of enhanced MT1-MMP activity despite lack of MMP2 activation. Circ Res 106: 796–808, 2010. doi: 10.1161/CIRCRESAHA.109.209189. [DOI] [PubMed] [Google Scholar]
- 64.Heymans S, Schroen B, Vermeersch P, Milting H, Gao F, Kassner A, Gillijns H, Herijgers P, Flameng W, Carmeliet P, Van de Werf F, Pinto YM, Janssens S. Increased cardiac expression of tissue inhibitor of metalloproteinase-1 and tissue inhibitor of metalloproteinase-2 is related to cardiac fibrosis and dysfunction in the chronic pressure-overloaded human heart. Circulation 112: 1136–1144, 2005. doi: 10.1161/CIRCULATIONAHA.104.516963. [DOI] [PubMed] [Google Scholar]
- 65.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105: 1815–1822, 2005. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 66.Kyurkchiev D, Bochev I, Ivanova-Todorova E, Mourdjeva M, Oreshkova T, Belemezova K, Kyurkchiev S. Secretion of immunoregulatory cytokines by mesenchymal stem cells. World J Stem Cells 6: 552–570, 2014. doi: 10.4252/wjsc.v6.i5.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vasandan AB, Jahnavi S, Shashank C, Prasad P, Kumar A, Prasanna SJ. Human mesenchymal stem cells program macrophage plasticity by altering their metabolic status via a PGE2-dependent mechanism. Sci Rep 6: 38308, 2016. doi: 10.1038/srep38308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cheng F, Shen Y, Mohanasundaram P, Lindström M, Ivaska J, Ny T, Eriksson JE. Vimentin coordinates fibroblast proliferation and keratinocyte differentiation in wound healing via TGF-β–Slug signaling. Proc Natl Acad Sci USA 113: E4320–E4327, 2016. doi: 10.1073/pnas.1519197113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Singampalli KL, Balaji S, Wang X, Parikh UM, Kaul A, Gilley J, Birla RK, Bollyky PL, Keswani SG. The role of an IL-10/hyaluronan axis in dermal wound healing. Front Cell Dev Biol 8: 636, 2020. doi: 10.3389/fcell.2020.00636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shi J, Li J, Guan H, Cai W, Bai X, Fang X, Hu X, Wang Y, Wang H, Zheng Z, Su L, Hu D, Zhu X. Anti-fibrotic actions of interleukin-10 against hypertrophic scarring by activation of PI3K/AKT and STAT3 signaling pathways in scar-forming fibroblasts. PLoS One 9: e98228, 2014. doi: 10.1371/journal.pone.0098228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ezquerra A, Revilla C, Alvarez B, Pérez C, Alonso F, Domínguez J. Porcine myelomonocytic markers and cell populations. Dev Comp Immunol 33: 284–298, 2009. doi: 10.1016/j.dci.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 72.Pelosi G. Phenotype changes of circulating monocytes in a hypercholesterolemic swine model of coronary artery disease. J Cytol Histol 05, 2014. doi: 10.4172/2157-7099.1000270. [DOI] [Google Scholar]
- 73.Piriou-Guzylack L, Salmon H. Membrane markers of the immune cells in swine: an update. Vet Res 39: 54, 2008. doi: 10.1051/vetres:2008030. [DOI] [PubMed] [Google Scholar]
- 74.Zuckermann FA, Husmann RJ. Functional and phenotypic analysis of porcine peripheral blood CD4/CD8 double-positive T cells. Immunology 87: 500–512, 1996. [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang M, Mal N, Kiedrowski M, Chacko M, Askari AT, Popovic ZB, Koc ON, Penn MS. SDF-1 expression by mesenchymal stem cells results in trophic support of cardiac myocytes after myocardial infarction. FASEB J 21: 3197–3207, 2007. doi: 10.1096/fj.06-6558com. [DOI] [PubMed] [Google Scholar]
- 76.Yan W, Abu-El-Rub E, Saravanan S, Kirshenbaum LA, Arora RC, Dhingra S. Inflammation in myocardial injury: mesenchymal stem cells as potential immunomodulators. Am J Physiol Heart Circ Physiol 317: H213–H225, 2019. doi: 10.1152/ajpheart.00065.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Data File S1, Supplemental Figs. S1–S3, and Supplemental Tables S1–S3: https://doi.org/10.6084/m9.figshare.14710635.