Abstract
Posttraumatic stress disorder (PTSD) and insomnia are characterized by sleep disturbances and daytime functional impairments. Actigraphy metrics can quantify diurnal rhythms via interdaily stability, intradaily variability, relative amplitude, and sleep regularity. Here, we (a) compared diurnal rhythms in PTSD, insomnia, and healthy control samples using linear mixed modeling; (b) compared inter-individual variability of diurnal rhythms between groups using variance ratio tests; and (c) examined correlations between diurnal rhythms and sleep measures within the clinical samples. Participants (N = 98) wore wrist-activity monitors for one week and completed the Insomnia Severity Index and Pittsburgh Sleep Quality Index. Both clinical samples displayed significantly lower interdaily stability, relative amplitude, and sleep regularity compared with controls. Individuals with PTSD and insomnia did not differ on mean diurnal rhythm metrics. Both clinical samples showed more inter-individual variability in relative amplitude compared with controls, and the individuals with PTSD were distinguished from those with insomnia by greater inter-individual variability in interdaily stability and relative amplitude. Relative amplitude in the clinical samples was positively correlated with objective sleep efficiency and total sleep time. This is the first study to compare individuals with PTSD and insomnia on measures of diurnal rhythms, revealing those with PTSD and insomnia to have less robust and more variable diurnal rhythms compared with controls. Individuals with PTSD differed from those with insomnia in inter-individual variability of diurnal rest-activity stability and amplitude, highlighting this population as particularly heterogenous. Diurnal rhythm robustness might be considered an intervention target in insomnia and PTSD populations.
Keywords: insomnia, posttraumatic stress disorder, diurnal, actigraphy, amplitude, stability, variability, regularity, sleep
Posttraumatic stress disorder (PTSD) can occur in response to life-threatening events, with a lifetime prevalence of 3.9% in adults worldwide (Koenen et al., 2017). One of the hallmark features of PTSD is sleep disturbance, which is reported by approximately 70% of those with PTSD (Babson and Feldner, 2010; Germain, 2013). While having insomnia increases the risk of developing PTSD after trauma exposure, once PTSD develops, insomnia and PTSD appear to have a bidirectional relationship (Germain, 2013; Spoormaker and Montgomery, 2008). For example, PTSD treatment can lead to small improvements in sleep outcomes, and cognitive-behavioral therapy for insomnia (CBT-I) can improve daytime PTSD symptoms (Talbot et al., 2014; Walters et al., 2020). Despite the overlap in nighttime symptoms between insomnia and PTSD, the sleep disturbance seen in PTSD is worse in certain ways, relative to non-comorbid insomnia. Specifically, Straus et al. (2015) found that while mean actigraphy-based sleep measures were very similar between the two groups, individuals with PTSD were distinguished from those with non-comorbid insomnia by greater night-to-night intraindividual variability (for sleep efficiency [SE]) and greater inter-individual variability (for total sleep time [TST] and SE). While the source of increased variability in these sleep measures is not known, such differences in variability, especially night-to-night variability, suggest that robustness and/or regularity of diurnal rhythms in PTSD might play a role. In addition, other clinical features of PTSD, such as nightmares and increased hyperarousal at night (Pigeon et al., 2013), suggest diurnal rhythms may be affected in this population more so than in insomnia. It is important to better understand diurnal rhythms in PTSD, as disturbed rhythms have been shown to affect sleep (Kim et al., 2020), mood (Luik et al., 2013), academic performance (Phillips et al., 2017), and quality of life (Carvalho-Bos et al., 2007) in other populations. In addition, it is essential to explore inter-individual variability of diurnal rhythms in PTSD because (a) prior literature has suggested variability is a key feature of sleep in PTSD (Straus et al., 2015); (b) sleep variability is associated with clinical outcomes such as depression (Suh et al., 2012); and (c) demonstration of high inter-individual variability in a clinical group would be helpful for informing clinical targets (e.g., highly heterogenous symptom presentation suggests individualized treatment plans would be most effective).
There are multiple ways to assess diurnal rhythms. Van Someren et al. (1999) described three nonparametric measures derived from diurnal rest-activity rhythms. Interdaily stability estimates the variability in rest-activity patterns across all days, intradaily variability quantifies fragmentation and magnitude of rest-activity transitions within each day, and relative amplitude measures the robustness of the 24-h rest-activity rhythm. These nonparametric measures are well suited to activity data, which do not typically follow a sinusoidal waveform (Gonçalves et al., 2015). In contrast to rest-activity rhythms, the Sleep Regularity Index (SRI; Phillips et al., 2017) focuses more specifically on sleep-wake patterns. The SRI measures the likelihood of the same sleep-wake state occurring in epochs that are 24 h apart, thereby measuring similarity of sleep-wake patterns between consecutive days. The SRI incorporates the entire 24-h cycle, thus extending upon more traditional measures of sleep-timing variability that focus on main sleep episodes only (Bei et al., 2016; Sánchez-Ortuño and Edinger, 2012).
To our knowledge, only one study has examined diurnal rest-activity rhythms in PTSD, and found only intradaily variability to be significantly different from a healthy control sample (Tsanas et al., 2020). A comparison of PTSD with sleep-disturbed populations on measures of diurnal rest-activity (i.e., interdaily stability, intradaily variability, and relative amplitude) and diurnal sleep regularity (SRI) has never been reported. The current study aimed to compare the diurnal rhythms of PTSD, insomnia, and healthy control groups on diurnal rhythms. We hypothesized that (a) individuals with PTSD would have lower interdaily stability, higher intradaily variability, lower relative amplitude, and lower SRI than both the insomnia sample and healthy controls; (b) the PTSD sample would show the greatest inter-individual variability in these measures compared with the other two groups; and (c) within the clinical groups, less impaired diurnal rhythms would be associated with greater objective TST and SE, and lower severity on self-report measures of insomnia and sleep quality.
Methods
Participants
This study combined data from a PTSD sample collected as part of a larger study, Project NITES (described by Straus et al., 2015), with data from individuals with insomnia and healthy controls recruited from a number of studies running concurrently in the same lab at the time. All studies were approved by the University of California San Diego and/or Veteran Affairs San Diego Healthcare System (VASDHS) institutional review boards. Project NITES was also approved by the Naval Medical Centre San Diego (Protocol Approvals: 1150445, 120841, 1155063). For the current data analysis project combining the results of multiple studies, the research was conducted according to the World Medical Association Declaration of Helsinki.
A total of 98 participants were eligible for analysis. Details on participant age, gender, education, race, and ethnicity are reported in Table 1. The PTSD sample consisted of U.S. military veterans (n = 44) seeking care at the VASDHS and/or recruited from the Naval Medical Centre, San Diego (84.1% veterans; 4.5% reserves; 11.4% active duty). The insomnia (n = 21) and healthy control (n = 33) samples were recruited from the community.
Table 1.
Participant demographics split by patient group.
| Sample | Healthy Controls (n = 33) | Insomnia (n = 21) | PTSD (n = 44) | p |
|---|---|---|---|---|
| Age (years) (M [SD]) | 30.88 (5.95) | 31.76 (6.66) | 35.53 (9.17) | .047* |
| Range | 23–49 | 23–48 | 23–58 | |
| Gender (n [%]) | .04* | |||
| Male | 21 (63.6%) | 12 (57.1%) | 37 (84.1%) | |
| Female | 12 (36.4%) | 9 (42.9%) | 7 (15.9%) | |
| Education (n [%]) | <.001** | |||
| College Graduate | 26 (78.8%) | 12 (57.1%) | 9 (20.5%) | |
| Less than College | 7 (21.2%) | 9 (42.9%) | 35 (79.5%) | |
| Race (n [%]) | .36 | |||
| Caucasian | 20 (60.6%) | 17 (81%) | 28 (63.6%) | |
| African American | 1 (3%) | 3 (14.3%) | 10 (22.7%) | |
| Pacific Islander/Hawaiian Native | 1 (3%) | 0 | 3 (6.8%) | |
| Asian | 5 (15.2%) | 0 | 1 (2.3%) | |
| Mixed Race | 1 (3%) | 1 (4.8%) | 2 (4.5%) | |
| [Not Reported] | 5 (15.2%) | — | — | |
| Ethnicity (n [%]) | .10 | |||
| Latino/Hispanic | 8 (24.2%) | 1 (4.8%) | 11 (25%) | |
| Not Latino/Hispanic | 20 (60.6%) | 20 (95.2%) | 33 (75%) | |
| [Not Reported] | 5 (15.2%) | — | — |
Abbreviation: PTSD = posttraumatic stress disorder. Age differences tested by one-way ANOVA. Gender, education, and ethnicity differences tested by chi-square test. Differences in race tested by chi-square test after collapsing groups to Yes/No Caucasian due to low cell counts.
p < .05, two-tailed.
p < .001, two-tailed.
Eligibility criteria for the PTSD sample included (a) at least 18 years of age; (b) deployed at least once as part of Operation Enduring Freedom/Operation Iraqi Freedom/Operation New Dawn (OEF/OIF/OND); (c) diagnosed PTSD following military exposure; (d) meets criteria for comorbid insomnia; (e) no unmanaged psychosis or mania; (f) no current or prior substance or alcohol abuse in the past 6 months; (g) no untreated sleep disorders other than insomnia or nightmares; (h) no current use of hypnotics or Prazosin; (i) no history of severe traumatic brain injury (TBI); and (j) no concurrent psychotherapy for PTSD.
The insomnia patients and healthy controls shared eligibility criteria included (a) stable preferred sleep timing between 2200 and 0800 h, (b) no current Axis 1 disorder (except for insomnia disorder for the insomnia sample) and no personal or family history of Axis 1 disorders, (c) no sleep disorder (other than insomnia disorder for the insomnia sample), (d) no current use of hypnotics or psychotropic medication, and (e) no history of TBI. In addition, the insomnia patients received a sleep disorder interview and also completed a sleep-diary prior to data collection to confirm insomnia, and subsequent additional eligibility criteria included (a) sleep difficulties at least 3 nights a week for at least 3 months, (b) average 45-min sleep latency and/or wake after sleep onset, and (c) self-reported less than 6 h sleep or less than 80% SE. In addition to the shared criteria, all healthy controls were required to self-report 7 to 9 h average TST per night.
As Project NITES had the most stringent inclusion and exclusion criteria, the insomnia sample and the healthy control participants were age-matched to this sample. However, after removing participants with insufficient or faulty actigraphy data, the PTSD sample was slightly, but significantly, older than the other groups (Table 1). There was also a significant difference between groups in gender and whether a college level education was achieved. There were no significant differences in race or ethnicity. In addition, given we followed recommendations not to use depression as an exclusion criterion for the PTSD group (Miller and Chapman, 2001), that group reported moderate levels of depression, on average, based on the Patient Health Questionnaire–9 (PHQ-9; Kroenke et al., 2001) (M = 14.70, SD = 5.61; 80% scoring 10 or above indicating risk of moderate depression), and several were taking antidepressants (18 of 44 individuals) or other psychotropics (11 of 44; 5 of which in conjunction with an antidepressant). Given current psychiatric diagnoses were an exclusion for the healthy controls and individuals with insomnia, these groups, by definition, showed very low levels of depression symptoms and none were taking psychotropic medications.
Procedure
All studies had a screening and written informed consent phase where a psychiatric nurse or trained researcher interviewed participants for demographic information and to screen for eligibility criteria. The Structured Clinical Interview for DSM-IV (SCID) (First et al., 2012) was administered to screen for Axis 1 disorders. The Duke Structured Interview Schedule (Edinger et al., 2006) was administered to screen for sleep disorders. The Brief Screening for Traumatic Brain Injury as per VASDHS protocol was used to screen for TBI, whereby respondents who screened positive on this questionnaire were followed up with an interview. For the PTSD sample, the interview confirmed deployment as part of OEF/OIF/OND and screened for medication use or concurrent psychotherapy. For the insomnia and healthy control samples, a sleep-diary was completed for a week to confirm eligibility criteria. Items included self-reported time in to bed, sleep onset time, duration of wake time since sleep onset, time of final wakening, and time out of bed, and subsequent calculated variables of TST and SE (a percentage of sleep time over time in bed). In all studies, participants completed measures of insomnia severity and sleep quality. Participants then were given a wrist-worn actigraphy device and sleep diaries to complete a week of at-home rest-activity monitoring, following their habitual sleep schedule.
Measures and Materials
Actigraphy
The Respironics Actiwatch 2 (Respironics) was used to collect actigraphy data. Participants wore the actigraph on their non-dominant wrist to collect rest-activity data via an accelerometer (piezoelectric accelerometer; sensitivity = 0.025 G-force; sampling rate = 32 Hz). Data were collected in 60-s epochs. Although participants were asked to wear the actigraph for a week at a time, there was some variation in recording length due to faulty data or differences in the date of actigraph administration and collection. Actigraphy data were excluded from analysis when the number of days collected was less than 5 to ensure an accurate reflection of a participant’s activity patterns (Aili et al., 2017; Van Someren, 2007). At analysis (N = 98), the range of days of data collected was 5 to 14 (M = 7.88, SD = 2.14) after excluding participants with faulty data and/or insufficient days.
The Actiwatch 2 has been validated against the gold-standard PSG, demonstrating high sensitivity (to detect sleep; 0.97), slightly poorer specificity (to detect wake; 0.77), and overall high accuracy (0.90) (Meltzer et al., 2012), and this is consistent with other actigraph models and scoring algorithms used across healthy adults (Aili et al., 2017; Sadeh, 2011). Although the validity and reliability of actigraphy somewhat decreases when studying sleep-disordered populations such as insomnia, the use of actigraphy is still recommended by the American Academy of Sleep Medicine for the assessment of sleep disturbance, when PSG is not available (Morgenthaler et al., 2007). Actiware Version 6.0.9 (Philips Respironics, 2017) was used to analyze sleep data. An automatic algorithm determined sleep and wake states, and thus calculated objective TST and SE. Rest periods were defined as the sleep-diary reported time in bed, and the data were cleaned manually by a single scorer, not blinded by group status, to reflect this. To account for obvious sleep outside the reported lights-out/lights-on times, rest intervals could be extended up to 60 min either side. This process is similar to methods used in other actigraphy studies (Goldman et al., 2007; Straus et al., 2015; Walters et al., 2020).
Diurnal Rest-activity Rhythms
Diurnal rest-activity rhythms were quantified using interdaily stability, intradaily variability, and relative amplitude (Van Someren et al., 1999). The accelerometry data from the actigraphy were exported using Actiware and analyzed in MATLAB R2019a (The MathWorks Inc, 2019) to calculate the diurnal rest-activity rhythm variables for each participant.
Interdaily stability calculates the similarity of each day’s activity patterns to the average activity across days. Values range from 0 to 1, with higher values indicating higher stability between days. Intradaily variability estimates the fragmentation of rest-activity behavior relative to a 24-h profile. Values range from 0 to 2, with values closer to 0 indicating lower rest-activity variability, and values closer to 2 indicating higher frequency of transitions between rest and activity (e.g., a greater number of naps and/or nighttime awakenings). Relative amplitude estimates the robustness of the 24-h rest-activity rhythm, by calculating the normalized mean difference in activity between the most active 10-h (M10) and least active 5-h (L5) periods. Values range from 0 to 1, with higher values indicating lower activity during the night and/or higher activity when awake. Formulas for these measures are described by Van Someren et al. (1999).
Diurnal Sleep Regularity
The SRI (Phillips et al., 2017) measures the similarity of sleep-wake patterns from one day to the next. The SRI is based on the probability that an individual will be in the same sleep-wake state in epochs that are 24 h apart, with values ranging 0 to 100. A higher score indicates higher sleep regularity. This provides complementary data to interdaily stability in using sleep-wake rather than rest-activity patterns. At least 5 days of overlap (i.e., non-missing epochs 24 h apart) was required for the SRI to be calculated; 11 healthy controls, 4 insomnia patients, and 8 individuals with PTSD had missing SRI data.
Insomnia Severity Index (ISI)
The ISI (Morin, 1993) was used to assess self-reported insomnia symptom severity from the past week. There are seven items, and participants are required to respond on a 5-point Likert-type scale ranging from 0 to 4. A higher overall score represents worse symptom severity. The ISI demonstrates high internal consistency (α = .91), and a high sensitivity of 99.4% (using a cut-off of 8 or more) in an insomnia population, and 95.8% in a community sample, to detect subthreshold insomnia (Morin et al., 2011).
Pittsburgh Sleep Quality Index (PSQI)
The PSQI (Buysse et al., 1989) was used to assess self-reported sleep quality from the past month. There are 19 items, the first 5 are open-ended sleep-timing questions, and 14 items are on a Likert-type scale from 0 to 3. A higher overall score represents worse sleep quality, with scores equal to or more than 5 indicating a clinically impaired sleep disturbance. The PSQI demonstrates high internal consistency (α = .83) and high sensitivity (89.6%) to detect good or poor sleepers at the cut-off of 5 (Buysse et al., 1989).
Statistical Analysis
All analyses were conducted in IBM SPSS Statistics Package for the Social Sciences for Windows (Version 25) or Stata 14 (StataCorp, 2015). An alpha value of less than .05, two-tailed, was used as significance for all tests.
Basic descriptive statistics for each diurnal rhythm measure within each group were reported using means and standard deviations. Since we were interested in testing differences in both means and inter-individual variability by diagnostic category, we then used a linear mixed-effects model for each diurnal rhythm measure to test for group differences and model the three group variances separately (Brown and Prescott, 2014). Due to significant group differences in age, gender, and education, these covariates were included in each omnibus test. If the omnibus test showed significant group differences among the three groups for a given diurnal rhythm measure, we followed up with pairwise post hoc tests.
To investigate differences between groups in inter-individual variability, we used our linear mixed-effects model for each diurnal rhythm measure to model the three group variances separately, controlling for covariates as noted above. We followed up each omnibus test by performing pairwise variance ratio tests using standard deviations of the residuals for each group.
To investigate the relationship between diurnal rhythm measures and sleep-related outcomes, bivariate correlations were conducted. The correlations were run in the clinical populations only, as inclusion criteria ensured the healthy controls had no sleep complaints. The decision to combine the PTSD and insomnia sample was made to strengthen any possible findings such that they could be more widely generalized, and is justified by the outcome of the linear mixed-effects model post hoc pairwise comparisons between the two clinical groups for each diurnal rhythm measure (Table 3). The correlations were between the diurnal rhythm measures (interdaily stability, intradaily variability, relative amplitude, and SRI), and actigraphy-derived TST and SE, ISI, and PSQI scores. Pearson’s correlations were calculated when both variables met the normality assumption. Spearman’s rho correlation was calculated when at least one of the variables was non-normally distributed. The alpha threshold for significance was not corrected for multiple comparisons. Partial correlations, controlling for age, gender, and education, were conducted in all instances due to significant group differences in these demographics.
Table 3.
Linear mixed-effect models omnibus tests and follow-up pairwise comparisons.
| ANOVA | HC vs. INS | HC vs. PTSD | INS vs. PTSD | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| F | p | t | p | t | p | t | p | ||||
| IS | 8.55 | <.001** | −4.27 | <.001** | −4.06 | .001 * | −0.49 | .84 | |||
| IV | 0.63 | 0.68 | n/a | n/a | n/a | ||||||
| RA | 10.76 | <.001** | −4.45 | <.001** | −5.21 | <.001** | −1.77 | .08 | |||
| L5 | 8.03 | <.001** | 4.27 | <.001** | 4.59 | <.001** | 1.63 | .11 | |||
| M10 | 0.88 | 0.50 | n/a | n/a | n/a | ||||||
| SRI | 14.25 | <.001** | −3.44 | .001 * | −4.27 | <.001** | −0.75 | .46 | |||
Abbreviations: HC = healthy controls; INS = insomnia; PTSD = posttraumatic stress disorder; IS = interdaily stability; IV = intradaily variability; RA = relative amplitude; SRI = Sleep Regularity Index. Age, gender, and education included as covariates in all models. Negative t values represent the second group listed has the lower value.
p < .05, two-tailed.
p < .001, two-tailed.
Results
Diurnal Rhythms Between Groups
There was a main effect of group for interdaily stability, relative amplitude, L5, and SRI (Tables 2 and 3; Figure 2). Both clinical samples (PTSD and insomnia) had significantly lower interdaily stability, relative amplitude, and SRI, and greater L5 compared with healthy controls. There were no significant differences between groups in intradaily variability and M10. The PTSD and insomnia samples did not show a significant difference on any diurnal rhythm measure. Figure 1 depicts the diurnal rhythms of an insomnia and PTSD patient.
Table 2.
Descriptive statistics for diurnal rhythm measures by patient group.
| Controls (n = 33) | Insomnia (n = 21) | PTSD (n = 44) | |
|---|---|---|---|
| IS | 0.56 (0.10) | 0.44 (0.10) | 0.43 (0.13) |
| IV | 0.77 (0.18) | 0.72 (0.20) | 0.77 (0.21) |
| RA | 0.93 (0.03) | 0.86 (0.06) | 0.82 (0.12) |
| L5 | 5.80 (2.99) | 13.41 (7.58) | 18.03 (17.31) |
| M10 | 158.74 (44.67) | 175.02 (41.52) | 166.12 (58.31) |
| SRIa | 88.60 (6.42) | 80.43 (6.80) | 75.54 (8.15) |
Abbreviations: PTSD = posttraumatic stress disorder; IS = interdaily stability; V = intradaily variability; RA = relative amplitude; SRI = Sleep Regularity Index. Presented are M (SD) for each measure.
Smaller n due to missing data.
Figure 2.
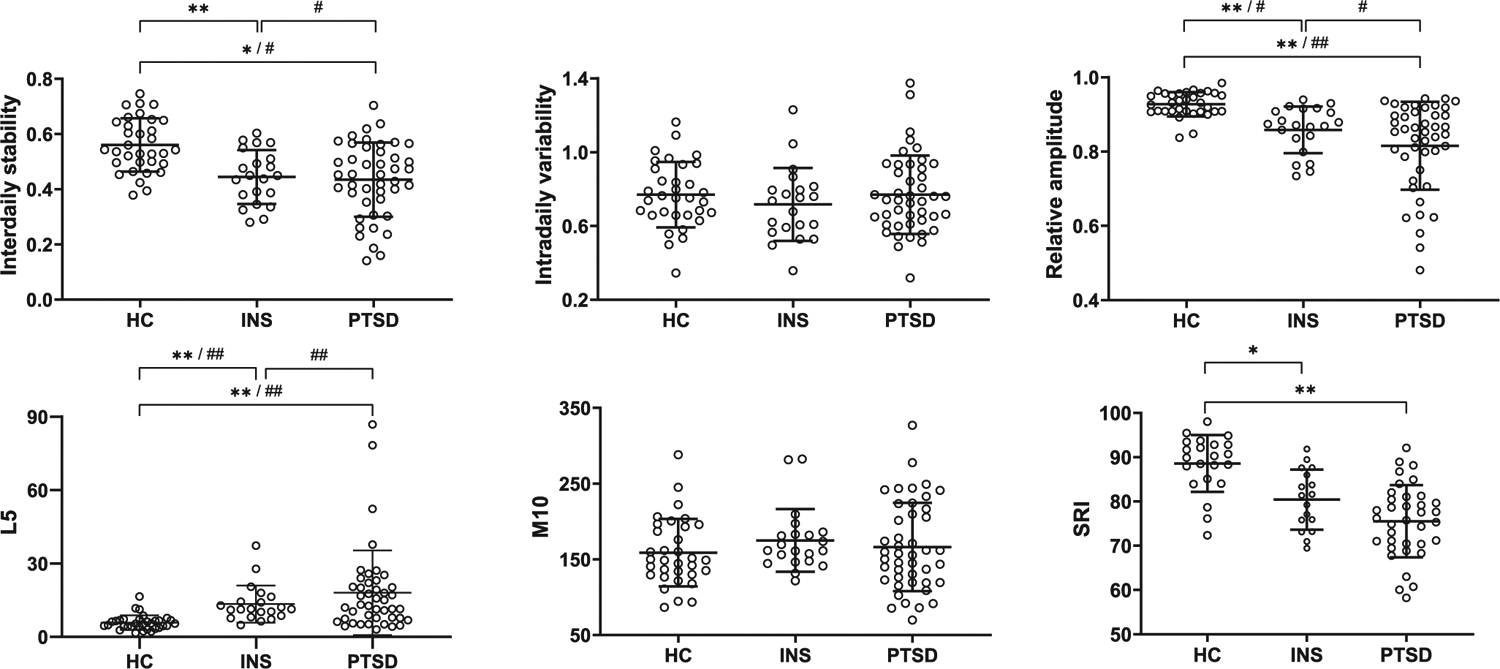
Inter-individual variability of diurnal rhythm measures by diagnostic group. Means and standard deviations are indicated by the lines. Significant between group mean values, after controlling for demographics is indicated by: * p value < 0.05, two-tailed. ** p value < 0.001, two-tailed. Significant differences on inter-individual variability between groups, after controlling for demographics, is indicated by: # p value < 0.05, two-tailed. ## p value < 0.001, two-tailed.
Figure 1.
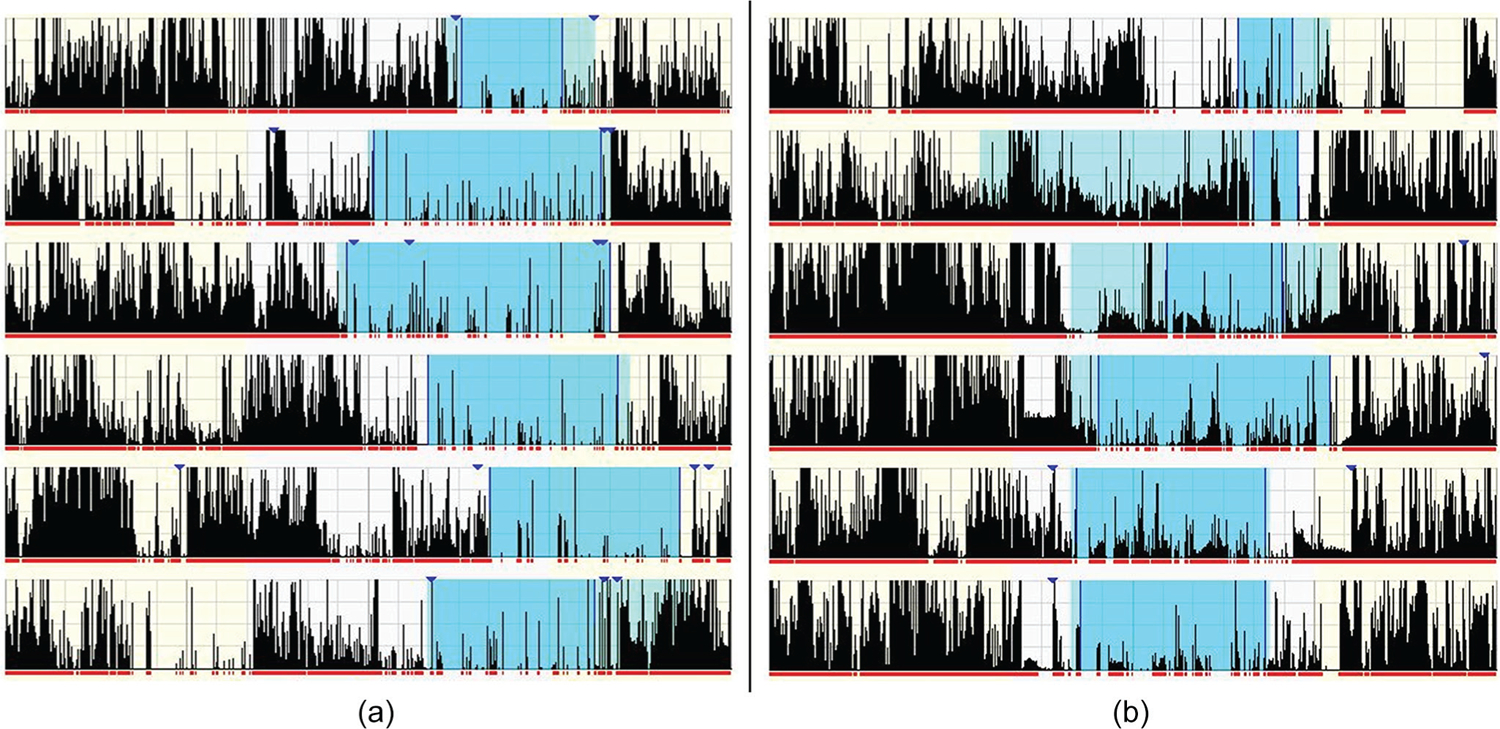
Representative actograms of diurnal rhythms. Panel A represents an individual with insomnia (IS = .38, IV = 0.79, RA = .84, SRI = 75.82). Panel B represents an individual with PTSD (IS = .35, IV = 0.91, RA = .62, SRI = 77.65). Each row indicates 24-hours from mid-day to mid-day. Diary-reported rest intervals are the shaded sections, and activity counts per 60-second epoch are indicated by the height of each vertical line.
Inter-individual Variability in Diurnal Rhythms
Both the PTSD and Insomnia groups had greater inter-individual (i.e., within-group) variability in relative amplitude and L5 compared with the healthy control group. The PTSD group also showed greater inter-individual variability in interdaily stability compared with controls. Finally, the PTSD group had greater inter-individual variability than the Insomnia group in interdaily stability, relative amplitude, and L5 (Table 4; Figure 2). No significant differences in inter-individual variability were found in intradaily variability, M10, or SRI across any of the pairwise comparisons.
Table 4.
Pairwise variability comparisons between groups.
| HC | INS | PTSD | HC vs. INS | HC vs. PTSD | INS vs. PTSD | |
|---|---|---|---|---|---|---|
| SD | p | |||||
| IS | 0.09 | 0.09 | 0.13 | .62 | .046 * | .03 * |
| IV | 0.18 | 0.20 | 0.22 | .55 | .23 | .67 |
| RA | 0.03 | 0.06 | 0.12 | .002 * | <.001** | .002 * |
| L5 | 2.94 | 7.72 | 17.46 | <.001** | <.001** | <.001** |
| M10 | 42.94 | 42.87 | 59.62 | .98 | .06 | .11 |
| SRI | 5.72 | 7.19 | 7.42 | .32 | .21 | .93 |
Abbreviations: HC = healthy controls; INS = insomnia; PTSD = posttraumatic stress disorder; IS = interdaily stability; IV = intradaily variability; RA = relative amplitude; SRI = Sleep Regularity Index. Variance ratio tests from linear mixed-effects models. Same covariates applied.
p < .05, two-tailed.
p < .001, two-tailed.
Correlations With Sleep Outcomes
Relative amplitude had a large (Cohen, 1988) positive correlation with TST, and a medium positive correlation with SE. L5 showed a large negative correlation with both TST and SE. There was also a small negative correlation between M10 and TST (see Table 5).
Table 5.
Correlations between diurnal rhythm measures and sleep outcomes in the clinical samples after controlling for demographics.
| TST | SEa | PSQI | ISI | |
|---|---|---|---|---|
| IS | .24 | .04 | .11 | −.08 |
| IV | −.07 | .05 | .01 | .06 |
| RAa | .55 ** | .44 ** | −.12 | −.18 |
| L5a | -.56 ** | -.53 ** | .13 | .05 |
| M10 | −.34 * | −.24 | −.03 | −.23 |
| SRI | −.07 | .12 | .13 | −.02 |
Abbreviations: TST = total sleep time; SE = sleep efficiency; PSQI = Pittsburgh Sleep Quality Index; ISI = Insomnia Severity Index; IS = interdaily stability; IV = intradaily variability; RA = relative amplitude; SRI = Sleep Regularity Index.
Spearman’s rho used for correlations with these variables.
p < .05, two-tailed.
p < .001, two-tailed.
Discussion
Both PTSD and insomnia are associated with subjective sleep disturbances and daytime functional impairment. While sleep has been extensively studied in both populations, little work has examined the 24-h profile of the disorders via diurnal rhythms. Therefore, the goal of this study was to quantify differences in objective 24-h rest-activity and sleep-wake patterns over several days and examine any possible associations with more commonly assessed sleep measures. Both clinical samples showed more impaired diurnal rhythms compared with healthy controls on average interdaily stability, relative amplitude, L5, and SRI, as well as greater inter-individual variability of relative amplitude and L5. PTSD also showed greater inter-individual variability in interdaily stability and relative amplitude compared with insomnia, and greater inter-individual variability in interdaily stability compared with controls. Finally, within the clinical samples, TST and SE had positive correlations with relative amplitude and negative correlations with L5.
Interdaily stability compares the similarity of each day’s activity patterns with the average across days, and this measure separated the clinical samples from healthy controls. This finding complements and extends upon the nighttime variability findings by Straus et al. (2015), by highlighting instability in activity throughout the 24-h period. Relative amplitude is the normalized difference of the most active (during the daytime) and least active (during the nighttime) periods. The group differences in diurnal rhythm robustness in the clinical samples compared with healthy controls appear driven by a higher L5 (a less restful rest period) rather than a lower M10 (being less active during the most active periods). A higher L5 would also be expected from objectively disturbed sleep, thus, it is perhaps not surprising L5 is driving the differences in relative amplitude. SRI compares sleep/wake patterns from one day with the next (i.e., on a circadian timescale). By considering previously known significant differences in the patient samples from healthy controls in night-to-night variability and mean sleep parameters (Straus et al., 2015), and the difference in interdaily stability revealed in our study, the SRI differences here are not surprising.
It should be noted that while no study has compared these groups on SRI, two studies (Kim et al., 2020; Natale et al., 2014) have compared insomnia disorder with healthy controls on the other measures (except for L5) and found no differences. Kim et al. (2020) utilized samples much older than ours, and so the difference between the studies may relate to changes in diurnal rest-activity rhythms observed during later adulthood (Huang et al., 2002). Natale et al. (2014) did not require a sleep-diary to corroborate interview-assessed insomnia, and so it is possible our sample had stricter exclusion criteria, thus revealing greater differences from healthy controls. To our knowledge, just one study has reported diurnal rest-activity in PTSD compared with healthy controls (Tsanas et al., 2020), finding differences in intradaily variability but not on interdaily stability and relative amplitude, although they used a less well-validated rest-activity monitoring device.
No mean diurnal rhythm measure could separate individuals with PTSD from individuals with insomnia, after adjusting for other covariates. This suggests that, contrary to our hypothesis and to night-to-night variability findings by Straus et al. (2015), diurnal impairments in rest-activity rhythms and sleep-wake regularity are similar between these sleep-disturbed populations. It is possible that a lack of difference in daytime activity (e.g., as shown with M10) might balance the differences in nighttime activity; however, future research is required to clarify the source of rest-activity similarities between the clinical groups.
The two clinical groups showed greater inter-individual variability in diurnal rest-activity rhythms than did the healthy controls (Figure 2). Both insomnia and PTSD groups showed greater inter-individual variability in relative amplitude and L5 than controls. This suggests the overall robustness of the rest-activity rhythm is not only affected by the presence of insomnia or PTSD (reflected in mean group differences) but also by individual-level factors (reflecting the inter-individual variability findings) within these populations. Although relative amplitude in community settings has been found to be associated with age, sex, race, and education (Mitchell et al., 2017), our sample did not have significant differences in race, and age, gender, and education were controlled for in our analyses. Thus, we demonstrate relative amplitude might be particularly heterogenous in PTSD and insomnia, even after accounting for the effects of demographics. Furthermore, inter-individual variability in relative amplitude appeared to be driven by inter-individual variability in overnight activity (i.e., the variable L5). This is not surprising given both PTSD and insomnia are characterized by an increased number of awakenings and increased minutes Wake After Sleep Onset, relative to healthy controls.
Perhaps even more interesting than differences between the clinical groups and controls are the findings the PTSD sample had greater inter-individual variability in interdaily stability, relative amplitude, and L5 compared with the insomnia sample, underscoring the heterogeneity of PTSD symptoms and extending this to include measures of rest-activity rhythms too. The reasons for PTSD showing greater variability than insomnia are unclear. One possible explanation is the presence of nightmares in the PTSD group. In the current PTSD sample, individuals reported experiencing one or more nightmares on an average of 5.0 ± 2.6 days (0–14), for a total of 7.9 ± 6.2 (range 0–34) nightmares per participant for the recording period. Nightmare awakenings are often associated with significant hyperarousal and the time to fall back asleep can be highly variable. Thus, inter-individual differences in nightmare frequency and/or intensity could certainly contribute to greater levels of inter-individual variability in both overnight activity (i.e., L5 and, thus, relative amplitude) and consistency of rhythms from day to day (i.e., interdaily stability). In addition, the fact many in our PTSD sample, but no one in the insomnia sample, experienced comorbid psychiatric symptoms, especially depression, and/or were taking psychotropics, may explain some of the group differences. Future studies should assess the unique contributions of these factors, relative to the contribution of PTSD, itself.
We observed a large positive correlation between TST and relative amplitude, which replicates findings by Cespedes Feliciano et al. (2017) in a community sample. This is also consistent with Kim et al. (2020) who suggested relative amplitude has a positive impact on SE, regardless of insomnia disorder or healthy sleepers. Here, we extend these previous findings by, perhaps intuitively, observing it is those who remain more inactive during the sleep period (lower L5) who also show the greatest TST and SE.
The study has a few limitations that must be recognized when interpreting results. Although we controlled for age, gender, and education as demographics significantly differed between our patient groups (Table 1) and are factors shown to affect rest-activity rhythms (Mitchell et al., 2017), the analyses could not account for other lifestyle factors such as family or occupation, which may limit interpretation of findings. In addition, the larger study from which we recruited the PTSD sample was a clinical trial investigating both trauma and sleep problems in PTSD, which meant comorbid insomnia was an inclusion criterion, and all participants were treatment seeking. The comorbid insomnia criterion aligns with the high rates of comorbid insomnia in PTSD (Pigeon et al., 2013). However, recruiting treatment seekers for this study may limit generalizability, considering up to 50% of war veterans do not seek treatment (Kulesza et al., 2015). In addition, inclusion criteria for the PTSD sample allowed comorbid psychiatric symptoms, as is recommended for research in PTSD (Miller and Chapman, 2001), while psychiatric diagnoses were exclusions for the insomnia and healthy controls samples. This difference could have influenced group differences, especially differences between PTSD and insomnia.
Despite these limitations, our study highlights several novel findings with important implications. We found individuals with insomnia and individuals with PTSD appear to exhibit similar diurnal rest-activity rhythms. Thus, interventions aimed to increase rest-activity stability and amplitude and decrease fragmentation may have a similar effect across both insomnia and PTSD. Notably, brief behavioral treatment for insomnia (BBTI) has been found to consolidate sleep overnight and improve rest-activity stability in veterans with insomnia (Kanady et al., 2020). Presumably, full CBT for insomnia would accomplish the same goal, given the emphasis on maintaining regular sleep-wake schedules and the documented benefit of consolidating overnight sleep (Talbot et al., 2014; Taylor and Pruiksma, 2014; Walters et al., 2020). Bright light therapy would also be expected to perhaps even more directly address the impaired diurnal rhythms in these populations (Winkler et al., 2005).
At the same time, the PTSD sample showed greater inter-individual variability in interdaily stability and relative amplitude than did the insomnia sample. This suggests the similarity in rest-activity between days and overall robustness of diurnal rest-activity rhythms is more variable within a PTSD population, compared with non-comorbid insomnia. While we were not able to examine the source of this difference here, one possible cause could be nightmares experienced in PTSD. To the extent the nightmares occur randomly in their frequency, intensity, and timing from night to night and between individuals, this would be expected to influence PTSD patients more than sleep-disturbed or rest-activity-impaired populations without nightmares as a primary symptom. If confirmed, such knowledge would help inform modifications to current sleep or nightmare interventions in PTSD, with the knowledge that clinicians should expect heterogenous presentations of rest-activity stability and robustness. Finally, we found few significant associations between diurnal rest-activity and sleep estimates, which emphasizes diurnal rhythms and nightly mean sleep estimates are distinct constructs that may be useful in parallel.
Acknowledgements:
We would like to thank the research staff at the Veteran Affairs San Diego Health Care System for their time and effort in the projects, as well as the veterans for their time and service. The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institute of Nursing Research 1-RC1-NR011728-01. J. W. Clark receives financial support from the Australian Government through a Research Training Program (RTP) Scholarship, and L. Mascaro receives financial support from the Australian Government through a strategically allocated RTP scholarship aligned with the Elite Sports Strategy. Funders played no role in the design or implementation of the project, nor analysis or interpretation of the data.
Footnotes
Trial Registry Project NITES; www.clinicaltrials.gov; Registration number: NCT01009112
Conflict of Interest Statement: The author(s) have no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Aili K, Astrom-Paulsson S, Stoetzer U, Svartengren M, and Hillert L (2017) Reliability of Actigraphy and Subjective Sleep Measurements in Adults: The Design of Sleep Assessments. J Clin Sleep Med 13:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babson KA, and Feldner MT (2010) Temporal relations between sleep problems and both traumatic event exposure and PTSD: a critical review of the empirical literature. J Anxiety Disord 24:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bei B, Wiley JF, Trinder J, and Manber R (2016) Beyond the mean: a systematic review on the correlates of daily intraindividual variability of sleep/wake patterns. Sleep Med Rev 28:108–124. [DOI] [PubMed] [Google Scholar]
- Brown H, and Prescott R (2014) Applied mixed models in medicine, third edition. John Wiley & Sons, West Sussex, United Kingdom. [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, and Kupfer DJ (1989) The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res 28:193–213. [DOI] [PubMed] [Google Scholar]
- Carvalho-Bos SS, Riemersma-Van Der Lek RF, Waterhouse J, Reilly T, and Van Someren EJW (2007) Strong Association of the Rest-Activity Rhythm With Well-Being in Demented Elderly Women. The American Journal of Geriatric Psychiatry 15:92–100. [DOI] [PubMed] [Google Scholar]
- Cespedes Feliciano EM, Quante M, Weng J, Mitchell JA, James P, Marinac CR, Mariani S, Redline S, Kerr J, Godbole S, Manteiga A, Wang D, and Hipp JA (2017) Actigraphy-Derived Daily Rest-Activity Patterns and Body Mass Index in Community-Dwelling Adults. Sleep 40:zsx168:161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J (1988) Statistical power analysis for the behavioral sciences. Routledge, New York, NY. [Google Scholar]
- Edinger J, Kirby AC, Lineberger MD, Loiselle MM, Wohlgemuth WK, and Means MK (2006) Duke Structured Interview Schedule for DSM-IV-TR and International Classification of Sleep Disorders, Second Edition, Sleep Disorders Diagnoses. In, Veterans Affairs and Duke University Medical Centers, Durham, NC. [Google Scholar]
- First M, Spitzer R, Gibbon M, and Williams J (2012) Structured Clinical Interview for DSM-IV Axis I Disorders, Administration Booklet. In, American Psychiatric Publishing, Arlington, VA. [Google Scholar]
- Germain A (2013) Sleep disturbances as the hallmark of PTSD: where are we now? Am J Psychiatry 170:372–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman SE, Stone KL, Ancoli-Israel S, Blackwell T, Ewing SK, Boudreau R, Cauley JA, Hall M, Matthews KA, and Newman AB (2007) Poor sleep is associated with poorer physical performance and greater functional limitations in older women. Sleep 30:1317–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves BSB, Adamowicz T, Louzada FM, Moreno CR, and Araujo JF (2015) A fresh look at the use of nonparametric analysis in actimetry. Sleep Med Rev 20:84–91. [DOI] [PubMed] [Google Scholar]
- Huang YL, Liu RY, Wang QS, Van Someren EJ, Xu H, and Zhou JN (2002) Age-associated difference in circadian sleep–wake and rest–activity rhythms. Physiol Behav 76:597–603. [DOI] [PubMed] [Google Scholar]
- Kanady JC, Straus LD, Gloria R, Neylan TC, and Maguen S (2020) Reductions in Sleep and Daily Rhythm Variability Following Brief Behavioral Treatment for Insomnia (BBTI). In Associated Professional Sleep Societies (SLEEP), Virtual Meeting. [Google Scholar]
- Kim SJ, Lim YC, Kwon HJ, and Lee JH (2019) Association of rest–activity and light exposure rhythms with sleep quality in insomnia patients. Chronobiol Int:1–11. [DOI] [PubMed] [Google Scholar]
- Koenen KC, Ratanatharathorn A, Ng L, McLaughlin KA, Bromet EJ, Stein DJ, Karam EG, Meron Ruscio A, Benjet C, Scott K, Atwoli L, Petukhova M, Lim CCW, Aguilar-Gaxiola S, Al-Hamzawi A, Alonso J, Bunting B, Ciutan M, de Girolamo G, Degenhardt L, Gureje O, Haro JM, Huang Y, Kawakami N, Lee S, Navarro-Mateu F, Pennell BE, Piazza M, Sampson N, ten Have M, Torres Y, Viana MC, Williams D, Xavier M, and Kessler RC (2017) Posttraumatic stress disorder in the World Mental Health Surveys. Psychol Med 47:2260–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, and Williams JBW (2001) The PHQ-9. J Gen Intern Med 16:606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulesza M, Pedersen ER, Corrigan PW, and Marshall GN (2015) Help-Seeking Stigma and Mental Health Treatment Seeking Among Young Adult Veterans. Military Behavioral Health 3:230–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luik AI, Zuurbier LA, Hofman A, Van Someren EJ, and Tiemeier H (2013) Stability and fragmentation of the activity rhythm across the sleep-wake cycle: the importance of age, lifestyle, and mental health. Chronobiol Int 30:1223–1230. [DOI] [PubMed] [Google Scholar]
- Meltzer LJ, Walsh CM, Traylor J, and Westin AML (2012) Direct Comparison of Two New Actigraphs and Polysomnography in Children and Adolescents. Sleep 35:159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GA, and Chapman JP (2001) Misunderstanding analysis of covariance. J Abnorm Psychol 110:40–48. [DOI] [PubMed] [Google Scholar]
- Mitchell JA, Quante M, Godbole S, James P, Hipp JA, Marinac CR, Mariani S, Cespedes Feliciano EM, Glanz K, Laden F, Wang R, Weng J, Redline S, and Kerr J (2017) Variation in actigraphy-estimated rest-activity patterns by demographic factors. Chronobiol Int 34:1042–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenthaler T, Alessi C, Friedman L, Owens J, Kapur V, Boehlecke B, Brown T, Chesson A Jr., Coleman J, Lee-Chiong T, Pancer J, and Swick TJ (2007) Practice Parameters for the Use of Actigraphy in the Assessment of Sleep and Sleep Disorders: An Update for 2007. Sleep 30:519–529. [DOI] [PubMed] [Google Scholar]
- Morin CM (1993) Insomnia: Psychological assessment and management. Guilford Press, New York. [Google Scholar]
- Morin CM, Belleville G, Bélanger L, and Ivers H (2011) The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep 34:601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natale V, Leger D, Martoni M, Bayon V, and Erbacci A (2014) The role of actigraphy in the assessment of primary insomnia: a retrospective study. Sleep Med 15:111–115. [DOI] [PubMed] [Google Scholar]
- Respironics Philips (2017) Actiware. In, Royal Philips Electronics, Bend, OR. [Google Scholar]
- Phillips AJK, Clerx WM, O’Brien CS, Sano A, Barger LK, Picard RW, Lockley SW, Klerman EB, and Czeisler CA (2017) Irregular sleep/wake patterns are associated with poorer academic performance and delayed circadian and sleep/wake timing. Sci Rep 7:3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigeon WR, Campbell CE, Possemato K, and Ouimette P (2013) Longitudinal relationships of insomnia, nightmares, and PTSD severity in recent combat veterans. J Psychosom Res 75:546–550. [DOI] [PubMed] [Google Scholar]
- Sadeh A (2011) The role and validity of actigraphy in sleep medicine: an update. Sleep Med Rev 15:259–267. [DOI] [PubMed] [Google Scholar]
- Sánchez-Ortuño MM, and Edinger JD (2012) Internight sleep variability: its clinical significance and responsiveness to treatment in primary and comorbid insomnia. J Sleep Res 21:527–534. [DOI] [PubMed] [Google Scholar]
- Spoormaker VI, and Montgomery P (2008) Disturbed sleep in post-traumatic stress disorder: secondary symptom or core feature? Sleep Med Rev 12:169–184. [DOI] [PubMed] [Google Scholar]
- StataCorp (2015) Stata Statistical Software: Release 14. In, StataCorp LLC, College Station, TX. [Google Scholar]
- Straus LD, Drummond SP, Nappi CM, Jenkins MM, and Norman SB (2015) Sleep variability in military-related PTSD: a comparison to primary insomnia and healthy controls. J Trauma Stress 28:8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh S, Nowakowski S, Bernert RA, Ong JC, Siebern AT, Dowdle CL, and Manber R (2012) Clinical significance of night-to-night sleep variability in insomnia. Sleep Med 13:469–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot LS, Maguen S, Metzler TJ, Schmitz M, McCaslin SE, Richards A, Perlis ML, Posner DA, Weiss B, Ruoff L, Varbel J, and Neylan TC (2014a) Cognitive Behavioral Therapy for Insomnia in Posttraumatic Stress Disorder: A Randomized Controlled Trial. Sleep 37:327–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot LS, Maguen S, Metzler TJ, Schmitz M, McCaslin SE, Richards A, Perlis ML, Posner DA, Weiss B, Ruoff L, Varbel J, and Neylan TC (2014b) Cognitive behavioral therapy for insomnia in posttraumatic stress disorder: a randomized controlled trial. Sleep 37:327–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DJ, and Pruiksma KE (2014) Cognitive and behavioural therapy for insomnia (CBT-I) in psychiatric populations: A systematic review. Int Rev Psychiatry 26:205–213. [DOI] [PubMed] [Google Scholar]
- The MathWorks Inc (2019) MATLAB. In, Natick, MA. [Google Scholar]
- Tsanas A, Woodward E, and Ehlers A (2020) Objective Characterization of Activity, Sleep, and Circadian Rhythm Patterns Using a Wrist-Worn Actigraphy Sensor: Insights Into Posttraumatic Stress Disorder. JMIR mHealth uHealth 8:e14306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Someren EJ (2007) Improving actigraphic sleep estimates in insomnia and dementia: how many nights? J Sleep Res 16:269–275. [DOI] [PubMed] [Google Scholar]
- Van Someren EJ, Swaab DF, Colenda CC, Cohen W, McCall WV, and Rosenquist PB (1999) Bright Light Therapy: Improved Sensitivity to Its Effects on Rest-Activity Rhythms in Alzheimer Patients by Application of Nonparametric Methods. Chronobiol Int 16:505–518. [DOI] [PubMed] [Google Scholar]
- Walters EM, Jenkins MM, Nappi CM, Clark J, Lies J, Norman SB, and Drummond SPA (2019) The impact of prolonged exposure on sleep and enhancing treatment outcomes with evidence-based sleep interventions: A pilot study. Psychological Trauma: Theory, Research, Practice, and Policy:Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler D, Pjrek E, Praschak-Rieder N, Willeit M, Pezawas L, Konstantinidis A, Stastny J, and Kasper S (2005) Actigraphy in Patients with Seasonal Affective Disorder and Healthy Control Subjects Treated with Light Therapy. Biol Psychiatry 58:331–336. [DOI] [PubMed] [Google Scholar]


