Abstract
In this review, we discuss a logical approach to diagnosis of histiocytic and dendritic cell lesions of the mediastinum. We break down the differential diagnosis between true neoplasms of histiocytic and dendritic cells [Rosai-Dorfman disease (RDD), Langerhans cell histiocytosis (LCH), and follicular dendritic cell sarcoma (FDCS)] versus selected neoplasms of other lineages which frequently attract non-neoplastic histiocytes or resemble them morphologically (carcinoma, melanoma, sarcoma, germinoma, mesothelioma, and lymphoma). As neoplasms in the latter category are more common, they should be stringently excluded before diagnosing a lesion in the first group, particularly given enormous differences in clinical management. We also consider histiocytic sarcoma (HS), an extremely rare lesion which, in some cases is likely of intrinsic histiocytic differentiation, whereas in others represents clonal evolution from an underlying non-histiocytic neoplasm.
Keywords: Histiocytic, dendritic cell, sarcoma, Rosai-Dorfman disease (RDD), Langerhans cell histiocytosis (LCH)
Histiocytic and dendritic cell lesions of the mediastinum can pose significant diagnostic challenges, because often these lesions consist of a complex admixture of cell types, only a subset of which are truly neoplastic. Particularly in small biopsies, the true nature of the lesion can be difficult to discern, given that the neoplastic cells can be few and far between. To break down this difficult group of lesions, we conceptually divide them into two broad categories. First, one can consider those entities in which histiocytic or dendritic cells are neoplastic, and themselves contain clonal genetic abnormalities leading to their autonomous proliferation. Due to the inherently social nature of histiocytic and dendritic cells, it is not surprising that neoplasms composed of them often attract brisk non-neoplastic inflammatory infiltrates; this phenomenon leads to distinctive morphologic patterns that can be readily recognized and used to narrow the differential diagnosis. This group of tumors includes Langerhans cell histiocytosis (LCH), Rosai-Dorfman disease (RDD), and follicular dendritic cell sarcoma (FDCS). While each has consistent morphologic features, a targeted immunohistochemical panel can also reliably separate these entities and confirm the diagnosis.
The second group encompasses lesions that are quantitatively rich in histiocytic or dendritic cells, but these cells are non-neoplastic bystanders; these neoplasms are driven by clonal cells of non-histiocytic or dendritic cell lineage, which attract large numbers of benign histiocytic and/or dendritic cells. This second group includes B and T cell lymphoma, carcinoma, sarcoma, mesothelioma, and germ cell tumors, and constitutes a critical checklist of potentially deceptive entities which must be systemically considered and excluded before diagnosing a lesion in the first category. The morphologic overlap between these two groups of lesions can be extensive, and this difficulty is further compounded by profound differences in prognosis and therapy between many of these lesions. Lastly, histiocytic sarcoma (HS) is perhaps the most challenging diagnosis of all and straddles these two categories; some cases appear to arise de novo, whereas others seem to evolve or transdifferentiate from an underlying non-histiocytic neoplasm, and thus only acquire histiocytic differentiation secondarily.
RDD
Prototypical examples of the intrinsic histiocytic neoplasms each exhibit quite characteristic morphology. Due to the inherent biologic properties of the antigen presenting cells they phenotypically resemble, these neoplasms have a striking ability to attract brisk, non-neoplastic inflammatory infiltrates, which can be exuberant enough to obscure or overshadow the neoplastic cells. However, in general, these inflammatory ‘smokescreens’ can actually be quite helpful morphologically, as they may be independently suggestive of the diagnosis even if the neoplastic cells themselves are not immediately obvious. Therefore, the first category of tumors can be subdivided by two essential parameters: not only by the morphology and immunophenotype of the neoplastic cells, but also by the lineage and quality of their associated non-neoplastic inflammatory infiltrates. RDD forms one end of the spectrum with the lesion quantitatively consisting predominantly of a non-neoplastic infiltrate of hematolymphoid cells, which is intimately admixed with the neoplastic cells. This infiltrate is typically strikingly rich in lymphocytes and especially plasma cells (Figure 1). These brisk plasma-cell rich infiltrates can easily distract from the often subtle and singly distributed neoplastic cells, leading one to entertain a diagnosis of an inflammatory or lymphoproliferative disorder, or even IgG4 related disease. Bona fide examples of RDD can easily satisfy the quantitative criteria for IgG4 related disease if one focuses solely on the highest density of IgG4 expressing plasma cells. In fact, due to its unique ability to attract and concentrate plasma cells, the presence of an increase in IgG4+ plasma cells should actually independently raise the differential diagnosis of RDD, and it is prudent to perform an S100 stain before suggesting the diagnosis of IgG4 related disease. Similar issues apply to inflammatory myofibroblastic tumor (IMT) as well as the inflammatory variant of well-differentiated liposarcoma, which also certainly enter into the differential diagnosis of RDD, and the appropriate studies should be performed in the appropriate morphologic context; overexpression or amplification of MDM2 would be in keeping with well-differentiated liposarcoma, whereas confirmation of a myofibroblastic phenotype with evidence of fusions involving tyrosine kinases such as ALK would be indicative of IMT. Finally, it is certainly reasonable to consider and exclude lymphoma or a plasma cell neoplasm in these situations, particularly given that focal areas morphologically identical to RDD have been observed in association with true lymphoproliferative disorders (1). Thus, in the biopsy setting, no matter how characteristic the morphology, one should maintain a high threshold for making a definite diagnosis of RDD, particularly if there is clinical suspicion for a lymphoproliferative disorder. Although others have been reported, it is useful to note that the most common lesions reported to harbor RDD-like proliferations in the aforementioned study were nodular lymphocyte predominant Hodgkin lymphoma (NLPHL) and classical Hodgkin lymphoma; thus particular care should be taken to consider and exclude these in the setting of morphology suggestive of RDD.
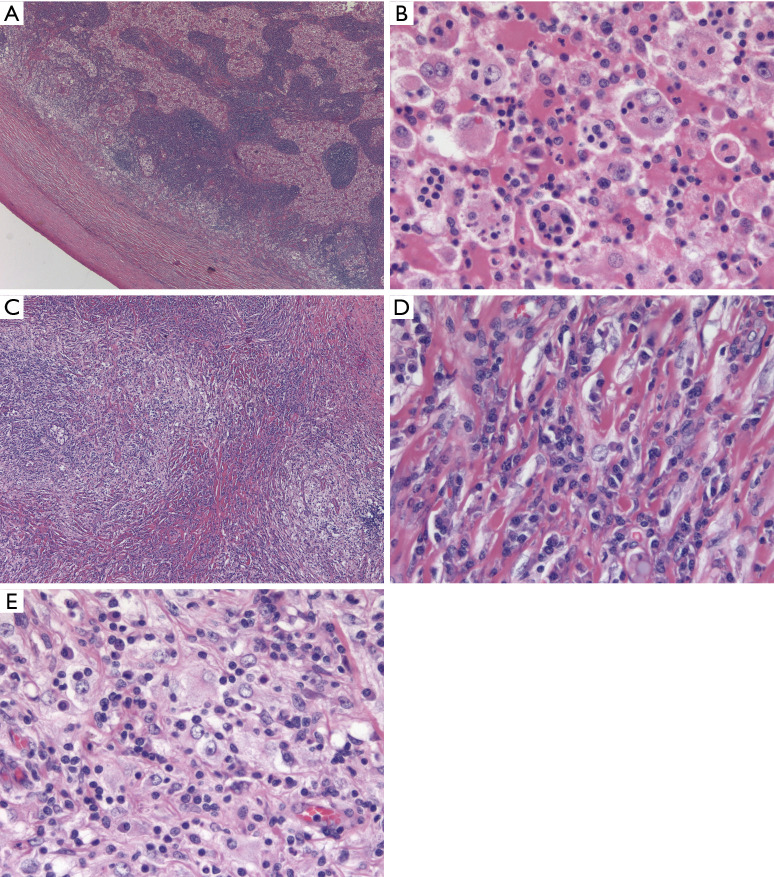
Figure 1.
RDD. (A) Classic low power appearance of nodal RDD with a thickened capsule and a serpiginous anastomosing network of expanded sinuses distended by large histiocytes with abundant eosinophilic cytoplasm (H&E stain, 25× magnification). (B) Higher magnification (400×) shows prototypical emperipolesis, with small inflammatory cells appearing superimposed upon the cytoplasm of voluminous histiocytes with large vesicular nuclei and prominent central nucleoli. (C) Same case with transition to distinct morphology of extranodal RDD with areas of marked sclerosis alternating with more cellular, paler areas (H&E stain, 100× magnification). (D) Typical sclerotic areas of extranodal RDD consist of large numbers of plasma cells with rare interspersed RDD histiocytes, which could easily be overlooked on a small biopsy (H&E stain, 400× magnification). (E) Looser areas of extranodal RDD with dense lymphoplasmacytic infiltrate with interspersed RDD histiocytes (H&E stain, 400× magnification). RDD, Rosai-Dorfman disease.
Therefore, from a practical standpoint, one could consider a definitive diagnosis of RDD on a needle biopsy just as potentially treacherous as rendering a diagnosis of classical Hodgkin lymphoma; the list of mimickers is long, and the implications of a misdiagnosis are potentially high. Therefore, a low threshold for performing the relevant studies to pursue these differential diagnoses, and/or requesting additional tissue if needed is of the essence. From an immunohistochemical standpoint, S100 positivity is a hallmark of RDD, and is essentially diagnostic when coupled with the classic cytomorphology of large histiocytoid cells with voluminous cytoplasm, nearly perfectly round nuclei with vesicular chromatin, and prominent centrally placed nucleoli. Emperipolesis, an intimate embrace between the intact cytoplasmic compartments of the lesional histiocytes and admixed inflammatory cells, is also a classic finding, which is often highlighted by the S100 stain as a negative image within the positive cytoplasm of the lesional cells.
The etiology of RDD was a matter of long-standing debate until a groundbreaking study that identified mutually exclusive mutations in the mitogen-activated protein kinase (MAPK) pathway; this work established the neoplastic nature of RDD and paved the way for targeted therapies via selective inhibition of this ubiquitously dysregulated kinase cascade (2). Subsequent work has shown that RDD cells express nuclear cyclinD1, which appears to correlate with their underlying genetically deranged MAPK pathway (3). CyclinD1 expression is certainly not specific to RDD, but like S100, is not usually brightly expressed by benign histiocytes, and, particularly in small biopsies, can highlight subtle emperipolesis nicely. Importantly, some pathologists may harbor a specific picture of the morphology of RDD similar to that of the original classic description of the entity, which clinically manifested as massive cervical adenopathy in young African men (4,5). Classic cases of nodal RDD feature a characteristic architecture, with patent sinuses distended to such an extent that a serpiginous, anastomosing network of pale areas, together with lamellated thickening of the nodal capsule, are readily recognizable at low magnification. However, the clinical and morphological spectrum of RDD have expanded significantly since the original description, with nodal and extranodal cases being reported at essentially any anatomic site. Further, the morphology of extranodal RDD is somewhat distinct from the classic nodal sinus pattern, with collagenous fibrosis, exuberant lymphoplasmacytic infiltrates, and spindling of the neoplastic cells representing a significant departure compared to nodal cases. It is these cases where a low threshold for S100 and/or cyclin D1 staining, and even molecular analysis in search of MAPK pathway related mutations can be critical or even necessary for the diagnosis. Remembering that RDD cells are typically SOX10 negative despite strong S100 expression is also useful given that melanoma should always be considered in the differential diagnosis of a histiocytoid neoplasm with a rich lymphoplasmacytic infiltrate (6). In contrast to RDD, melanoma typically exhibits both SOX10 and S100 expression, with the former often stronger and more diffuse. Expression of HMB45, Melan-A and other more specific melanocytic markers is not seen in RDD. We also note that both RDD and melanoma may certainly express both S100 and CyclinD1, which is not surprising given that they share hyperactivation of the MAPK pathway at the genetic level. Although KRAS and MAP2K1 mutations are the most common alterations seen in RDD, the canonical BRAF V600E mutation has also been reported, and thus there is substantial genetic overlap between these two neoplasms as well (7).
LCH
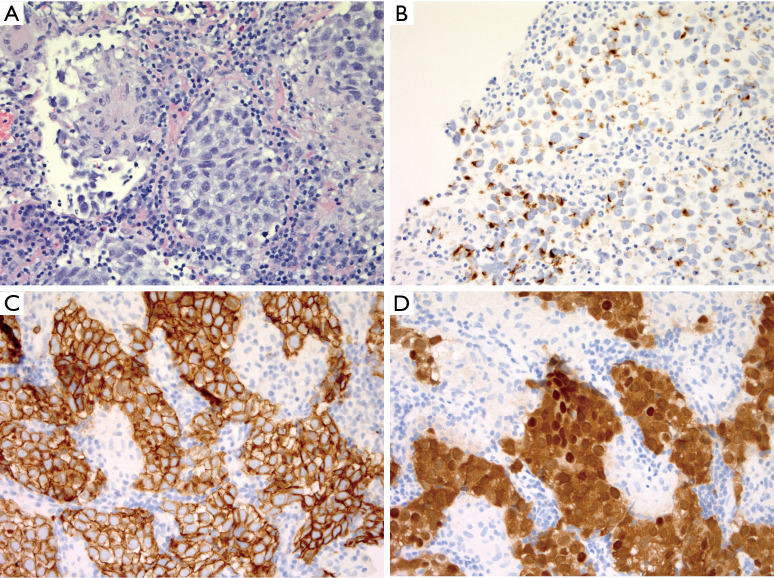
Like RDD, LCH is also a histiocytic neoplasm that characteristically attracts a brisk inflammatory infiltrate, but in LCH the inflammatory background is typically of the myeloid lineage, and contains abundant eosinophils. In fact, if the telltale eosinophil rich infiltrate is not prominent, LCH can be much more difficult to recognize. Like RDD, LCH consistently expresses S100 and cyclinD1, and harbors genetic mutations leading to hyperactivation of the MAPK pathway (8,9). Fortunately, these similarities are mainly immunohistochemical and genetic—morphologically these entities have very little in common. Furthermore, the combination of CD1a and Langerin expression is highly specific for LCH and not seen in RDD. The cytology of the tumor cells in LCH is quite distinctive—the nuclei are characteristically not circular, rather they are angulated and elliptical with a nuclear silhouette reminiscent of a boomerang. This nuclear morphology and the reliable absence of prominent nucleoli, can thus be extremely helpful when eosinophils are not present in abundance. Nuclear grooves are also typical, and thus the overall nuclear cytology of LCH cells is quite reminiscent of that seen in adult granulosa cell tumor of the ovary. In contrast to RDD, the diagnosis of LCH is most challenging within the lymph node where exuberant reactive, physiologic proliferations of Langerhans cells are frequently seen in the setting of nodular paracortical hyperplasia, which in the appropriate clinical setting can also be referred to as dermatopathic lymphadenitis. This benign nodal proliferation beautifully encapsulates the normal immunologic function of cutaneous Langerhans cells, which phagocytose antigens locally and subsequently migrate to draining lymph nodes and present their findings to paracortical CD4+ T cells. Chronic cutaneous inflammatory processes of any etiology can accelerate this process leading to clinically detectable and potentially concerning lymphadenopathy. An impressive population of S100+CD1a+ and Langerin+ Langerhans cells can be found in the paracortices of such lymph nodes (Figure 2). Importantly, the lack of admixed eosinophils, subcapsular sinus involvement, BRAF V600E or other MAPK pathway mutations, and the lack of cyclinD1 expression all support a reactive Langerhans cell proliferation. Just as with RDD, the concept of focal LCH-like proliferations adjacent to lymphoma has been established, with Hodgkin lymphoma again representing a commonly associated lesion (10). Thus a “second diagnosis” should always be sought in the setting of LCH, particularly in a small biopsy. In the physiologic setting, after migrating to the lymph node paracortex, Langerhans cells downregulate CD1a and Langerin, while retaining S100 expression, and are thus designated interdigitating dendritic cells. Thus, the neoplasm corresponding to this developmental stage of the Langerhans cell lineage, the interdigitating dendritic cell sarcoma, is always in the differential diagnosis of an intranodal S100+ neoplasm.
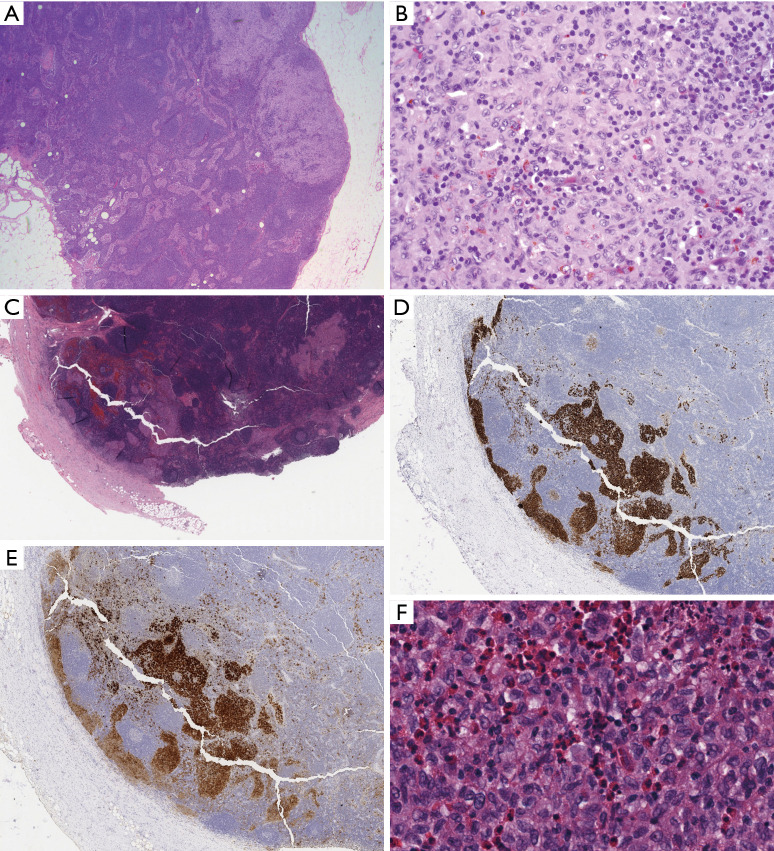
Figure 2.
Nodular paracortical hyperplasia versus nodal involvement by LCH. (A) Typical low power appearance of nodular paracortical hyperplasia, showing pale nodular aggregates within lymph node parenchyma (H&E stain, 25× magnification); (B) higher power of paracortex shows numerous Langerhans cells with abundant eosinophilic cytoplasm, and angulated, vesicular nuclei admixed with numerous small lymphocytes (H&E stain, 400× magnification); (C) in contrast, at low power, nodal involvement by LCH replaces the subcapsular sinus (H&E stain, 50× magnification). The sinus distribution is highlighted by immunohistochemistry for CD1a (D, 50× magnification) and S100 (E, 50× magnification), which also highlights scattered and singly distributed interdigitating dendritic cells. (F) High magnification demonstrates similar cytologic features including nuclear angulation and grooves, but numerous admixed eosinophils are indicative of LCH (H&E stain, 400× magnification). LCH, Langerhans cell histiocytosis.
Follicular dendritic cell sarcoma (FDCS)
Like RDD and LCH, FDCS may arise within or outside lymph nodes, and often contains exuberant infiltrates of admixed benign inflammatory cells. However, as the normal function of follicular dendritic cells is to organize B-cells into follicular structures and to present antigens to the B-cell receptor to facilitate affinity maturation, FDCS at least theoretically will harbor an infiltrate relatively rich in B lymphocytes. In fact, in some cases these lesions can concentrate B-cells so intensely that B-cell lymphoma will enter the differential diagnosis (11). However, compared to LCH and RDD, the quality of the non-neoplastic infiltrate is less consistent and unfortunately less specific diagnostically. The morphology of FDCS can vary widely, and thus it is reasonable to apply the relevant markers CD21, CD23, CD35, D240, and/or SSTR2a liberally when confronted with an epithelioid, syncytial, and/or spindle cell lesion with abundant admixed inflammatory cells (12). However, there are some important morphologic and immunophenotypic clues that can be helpful. At low magnification, FDCS often demonstrates a whirling architecture reminiscent of an aerial view of a hurricane. This appearance presumably results from the slender and serpiginous cytoplasmic processes of the neoplastic cells, which squeeze and distort the surrounding cells, a phenomenon which is confirmed with immunohistochemistry. Cytologically, the neoplastic cells typically harbor eosinophilic nucleoli, just as benign follicular dendritic cells do (Figure 3). Perhaps the most useful feature in the mediastinum is identifying adjacent or associated areas of lymph node tissue showing features of hyaline vascular Castleman’s disease, which is postulated to represent the precursor lesion to a subset of FDCS, and itself typically harbors atretic germinal centers containing variable expansions of follicular dendritic cells (13-16).
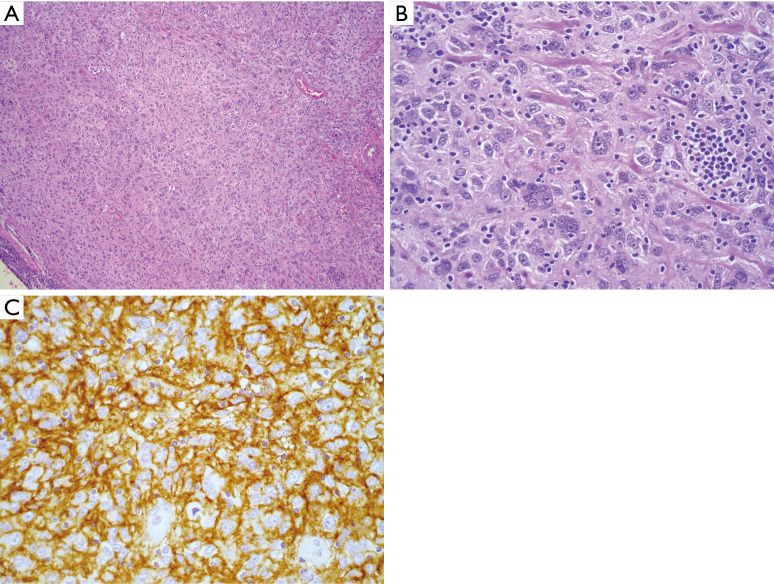
Figure 3.
FDCS. (A) H&E stained section (100×) showing a histiocytoid cell proliferation with a loose whirling architecture; (B) higher power (400×) demonstrates tumor cells, many with eosinophilic nucleoli, and prominent admixed small, mature lymphoid cells; (C) strong CD35 expression confirming follicular dendritic cell lineage (immunohistochemistry, 400× magnification). FDCS, follicular dendritic cell sarcoma.
Histiocytic sarcoma
The last tumor in the intrinsic histiocytic and dendritic cell category we will consider is HS. By definition this lesion expresses multiple histiocytic markers and consists of cytologically malignant cells (Figure 4), while requiring exclusion of other more specific diagnostic entities, a task which is quite challenging pathologically and important clinically, given the extensive list of diverse lesions we have already discussed (17). Although an extremely rare diagnosis, HS is still a heterogeneous category, and likely encompasses distinct tumor types with overlapping morphology and immunophenotype. Conceptually, one can divide HS into cases in which no underlying lower grade lesion can be identified, versus cases which appear to have transformed into HS via a non-histiocytic neoplasm. Therefore, after excluding the extensive list of competing differential diagnoses, perhaps the most important task when encountering HS is attempting to identify morphologic, immunohistochemical, or clinical evidence of an underlying leukemia or lymphoma from which the lesion arose. For example, cases of HS have been shown to harbor the same t(14;18) or t(11;14) as an underlying follicular lymphoma or mantle cell lymphoma and thus appear to represent an unusual trans-differentiation phenomenon (18-21). Further, it has been suggested that weak expression of B-cell markers such as PAX-5 in HS can be a subtle clue to an underlying B-cell lymphoma (22). A truly unusual case was recently reported wherein HS appeared to originate from an underlying germ cell tumor, as demonstrated by shared clonal genetic abnormalities (23). Thus, whenever HS is encountered, underlying neoplasms should be sought and if possible, genetic comparisons between the lesions should be performed as appropriate (24). Interestingly, there is some evidence that HS arising from an underlying non-histiocytic neoplasm may be related to acquisition of additional MAPK related mutations superimposed on the genome of the originating neoplasm (25). Cases in which no pre-existing neoplasm can be identified may be considered de novo. Perhaps unsurprisingly, these lesions frequently harbor mutations in MAPK related genes similar to those seen in the aforementioned, low-grade intrinsic histiocytic neoplasms (26-28). In short, both primary and secondary HS cases appear to frequently harbor mutations leading to MAPK pathway activation; these genetic findings, as in other diverse tumor types, may ultimately pave the way for targeted therapy for patients with HS (29,30).
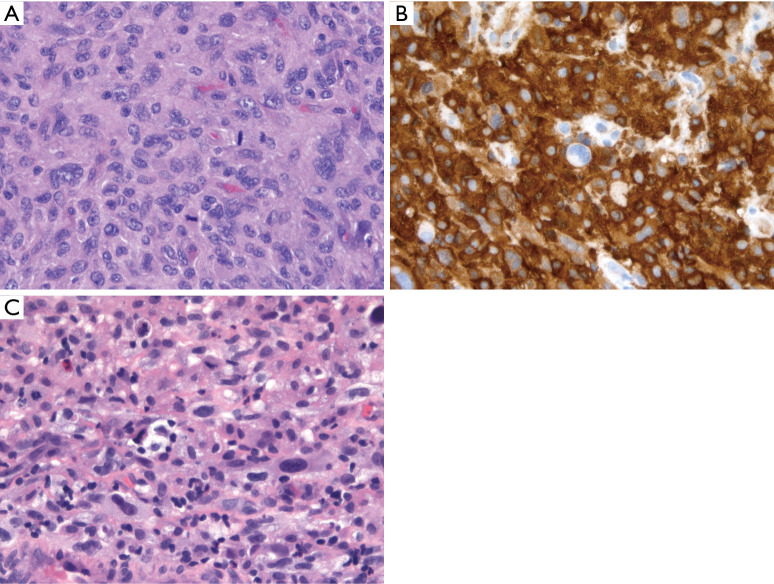
Figure 4.
HS. (A) Syncytial sheets of markedly atypical cells with eosinophilic cytoplasm, prominent nucleoli, and mitotic activity (H&E stain, 400× magnification); (B) diffuse CD163 reactivity (immunohistochemistry, 400× magnification); (C) separate case showing sheets of tumor cells with marked nuclear pleomorphism, foamy cytoplasm, and scattered admixed non-neoplastic inflammatory cells (H&E stain, 400× magnification). The morphology of histiocytic sarcoma is not specific and would be compatible with other pleomorphic tumors given a different immunoprofile. Thus, diagnosis of HS requires a broad panel of immunohistochemical stains to exclude other tumors. HS, histiocytic sarcoma.
Non-histiocytic & dendritic cell lesions
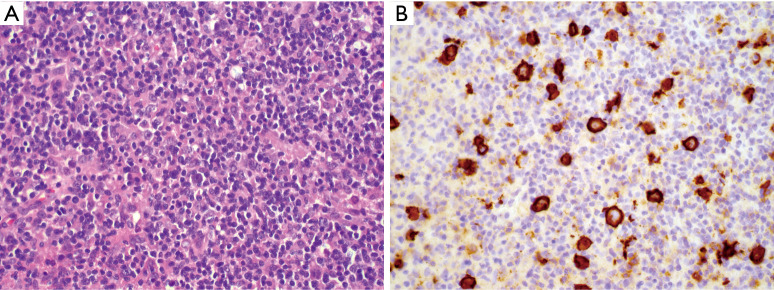
It is important to recognize that primary, intrinsic histiocytic and dendritic cell lesions are relatively rare compared to malignancies that may morphologically mimic them. Thus, from a probabilistic standpoint, one must always consider an unusual morphologic variant of a more common lesion before diagnosing a true histiocytic or dendritic cell neoplasm. At least three lymphoid lesions merit specific consideration because of their infamous ability to hide behind innocuous appearing infiltrates of histiocytes. First, T-cell histiocyte rich large B-cell lymphoma (TCHRLBCL) as the name suggests consists of a vast infiltrate of unremarkable mature T-cells and histiocytes. Careful examination will reveal large highly atypical lymphoid cells, interspersed singly within the lesion, which immunohistochemistry will reveal to be of B lineage (Figure 5). This lesion therefore represents an important diagnostic pitfall of any histiocyte rich lesion and demands careful attention to morphology and immunophenotype. Importantly, small biopsies of TCHRLBCL can overlap extensively with NLPHL, and this distinction may be impossible without a larger biopsy, as the phenotype of the large atypical cells is essentially identical; the key to separating these lesions lies in the nodular nature and specific constituents of the background non-neoplastic infiltrate, which as mentioned can be impossible to appreciate in a core biopsy (31).
Figure 5.
TCHRLBCL. (A) H&E stained section (100×) showing sheets of mature small lymphocytes and histiocytes, with occasional large atypical lymphoid cells; (B) CD20 stain highlights many larger atypical B-cells than were apparent by H&E (immunohistochemistry, 400× magnification). TCHRLBCL, T-cell histiocyte rich large B-cell lymphoma.
The lymphohistiocytic variant of peripheral T-cell lymphoma, or so-called Lennert lymphoma, poses similar challenges, and can be just as morphologically subtle given the ostensibly banal infiltrate of benign histiocytes and lymphocytes (32-34). Finally, the lymphohistiocytic variant of anaplastic large cell lymphoma (ALCL) can readily overlap morphologically with the above entities (35). CD30 and ALK immunohistochemistry make this diagnosis possible even on a small biopsy, provided one can exclude other ALK+ entities including IMT, diffuse large B-cell lymphoma, and lung adenocarcinoma.
The lymphohistiocytoid variant of mesothelioma is yet another tumor that reinforces the importance of excluding non-histiocytic lesions despite histiocytoid morphology (36). Rather than attracting a distracting density of benign histiocytes, in this entity, the tumor cells themselves have abundant eosinophilic cytoplasm and deceptively bland nuclear features.
As with any situation where mesothelioma is a consideration, immunohistochemistry is mandatory, and expression of multiple mesothelial markers such as calretinin, WT1, D240, and CK5/6, with negativity for multiple carcinomas associated antigens such as MOC31, BerEP4, and carcinoembryonic antigen (CEA) should be established (37). If a proliferation is confirmed to be mesothelial in nature, but invasion cannot be documented, BAP1 loss can be a useful marker of malignancy, and this finding has been demonstrated in the rare lymphohistiocytoid variant (38). Bi-allelic CDKN2A deletion and lack of expression of MTAP, its genetic neighbor which serves as an immunohistochemical surrogate, are also recently established tools which support the diagnosis of malignant mesothelioma (39,40).
Germ cell tumors, particularly seminomas [referred to as germinomas in the central nervous system (CNS) or mediastinum and dysgerminoma in the ovary], are notorious for attracting robust histiocytic and often granulomatous infiltrates that can obscure the malignant cells. In the mediastinum, several clinical clues are extremely helpful in navigating this diagnostic category. First, epidemiological data is instructive, as mediastinal germinomas overwhelmingly occur in post-pubertal males, and are essentially unheard of in female patients. Although mediastinal germ cell tumors do occur in females (with approximately ten times less frequency compared to males), teratomas constitute the vast majority in females and thus germinomas and their associated histiocytic infiltrates are essentially not a diagnostic consideration in female patients (41). Therefore, in an adult male patient, one simply must entertain the possibility of a germinoma when encountering a histiocyte rich lesion of the mediastinum, because these tumors are exquisitely sensitive to radiation and/or chemotherapy (42). Once suspected, immunohistochemistry makes confirmation straightforward, with the large atypical cells with clear cytoplasm and prominent nucleoli showing strong nuclear expression of OCT3/4 and SALL4 (Figure 6) (43). The possibility of a malignant mixed germ cell tumor is always an important consideration, and correlation with tumor markers is essential, as elevations of alpha fetoprotein (AFP) or B-HCG can alert the pathologist to the possibility of unsampled yolk sac tumor or choriocarcinoma respectively. Embryonal carcinoma does not secrete any of the conventionally measured tumor markers and poses two significant pathologic pitfalls; diffuse cytokeratin expression can readily mislead one toward a diagnosis of carcinoma or mesothelioma, while CD30 expression can also of course be seen in lymphoid lesions such as primary mediastinal large B-cell lymphoma, ALCL, and classic Hodgkin lymphoma. Finally, establishing the diagnosis of a primary mediastinal germ cell tumor requires correlation with clinical and radiologic findings to exclude a gonadal primary.
Figure 6.
Mediastinal germinoma with histiocytic infiltrate. (A) Core biopsy showing high magnification comparison between histiocytic and giant cell reaction (left) versus sheets of large epithelioid germinoma cells with vesicular nuclei (H&E, 400× magnification). (B) Importantly, focal keratin expression can be seen in germinoma (immunohistochemistry, 400× magnification), and if considered alongside diffuse CD117 expression (C, 400× magnification), this lesion could easily be mistaken for thymic carcinoma. The nested architecture and sclerosis would certainly also raise the differential diagnosis of primary mediastinal large B-cell lymphoma. (D) Nuclear OCT3/4 and SALL4 expression (not pictured) are confirmatory (immunohistochemistry, 400× magnification).
Acknowledgments
We are grateful to Professor Dale Frank for graciously providing case material for Figures 2, 3, and 5.
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Provenance and Peer Review: This article was commissioned by the Guest Editors (Saul Suster and David Suster) for the series “Mediastinal Sarcomas” published in Mediastinum. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/med-20-31). The series “Mediastinal Sarcomas” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
References
- 1.Garces S, Yin CC, Patel KP, et al. Focal Rosai-Dorfman disease coexisting with lymphoma in the same anatomic site: a localized histiocytic proliferation associated with MAPK/ERK pathway activation. Mod Pathol 2019;32:16-26. 10.1038/s41379-018-0152-1 [DOI] [PubMed] [Google Scholar]
- 2.Garces S, Medeiros LJ, Patel KP, et al. Mutually exclusive recurrent KRAS and MAP2K1 mutations in Rosai-Dorfman disease. Mod Pathol 2017;30:1367-77. 10.1038/modpathol.2017.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baraban E, Sadigh S, Rosenbaum J, et al. Cyclin D1 expression and novel mutational findings in Rosai-Dorfman disease. Br J Haematol 2019;186:837-44. 10.1111/bjh.16006 [DOI] [PubMed] [Google Scholar]
- 4.Rosai J, Dorfman RF. Sinus histiocytosis with massive lymphadenopathy: a pseudolymphomatous benign disorder. Analysis of 34 cases. Cancer 1972;30:1174-88. [DOI] [PubMed] [Google Scholar]
- 5.Rosai J, Dorfman RF. Sinus histiocytosis with massive lymphadenopathy. A newly recognized benign clinicopathological entity. Arch Pathol 1969;87:63-70. [PubMed] [Google Scholar]
- 6.Xie DY, Costello CM, Liang HJ, et al. Melanoma mimicking Rosai-Dorfman disease. J Cutan Pathol 2018;45:355-9. 10.1111/cup.13121 [DOI] [PubMed] [Google Scholar]
- 7.Fatobene G, Haroche J, Hélias-Rodzwicz Z, et al. BRAF V600E mutation detected in a case of Rosai-Dorfman disease. Haematologica 2018;103:e377-9. 10.3324/haematol.2018.190934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shanmugam V, Craig JW, Hornick JL, et al. Cyclin D1 Is Expressed in Neoplastic Cells of Langerhans Cell Histiocytosis but Not Reactive Langerhans Cell Proliferations. Am J Surg Pathol 2017;41:1390-6. 10.1097/PAS.0000000000000897 [DOI] [PubMed] [Google Scholar]
- 9.Badalian-Very G, Vergilio JA, Degar BA, et al. Recurrent BRAF mutations in Langerhans cell histiocytosis. Blood 2010;116:1919-23. 10.1182/blood-2010-04-279083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pina-Oviedo S, Medeiros LJ, Li S, et al. Langerhans cell histiocytosis associated with lymphoma: an incidental finding that is not associated with BRAF or MAP2K1 mutations. Mod Pathol 2017;30:734-44. 10.1038/modpathol.2016.235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lorenzi L, Lonardi S, Petrilli G, et al. Folliculocentric B-cell-rich follicular dendritic cells sarcoma: a hitherto unreported morphological variant mimicking lymphoproliferative disorders. Hum Pathol 2012;43:209-15. 10.1016/j.humpath.2011.02.029 [DOI] [PubMed] [Google Scholar]
- 12.Tao LL, Huang YH, Chen YL, et al. SSTR2a Is a Useful Diagnostic Marker for Follicular Dendritic Cells and Their Related Tumors. Am J Surg Pathol 2019;43:374-81. 10.1097/PAS.0000000000001205 [DOI] [PubMed] [Google Scholar]
- 13.Chan JK, Fletcher CD, Nayler SJ, et al. Follicular dendritic cell sarcoma. Clinicopathologic analysis of 17 cases suggesting a malignant potential higher than currently recognized. Cancer 1997;79:294-313. [DOI] [PubMed] [Google Scholar]
- 14.Pauwels P, Dal Cin P, Vlasveld LT, et al. A chromosomal abnormality in hyaline vascular Castleman's disease: evidence for clonal proliferation of dysplastic stromal cells. Am J Surg Pathol 2000;24:882-8. 10.1097/00000478-200006000-00016 [DOI] [PubMed] [Google Scholar]
- 15.Chan AC, Chan KW, Chan JK, et al. Development of follicular dendritic cell sarcoma in hyaline-vascular Castleman's disease of the nasopharynx: tracing its evolution by sequential biopsies. Histopathology 2001;38:510-8. 10.1046/j.1365-2559.2001.01134.x [DOI] [PubMed] [Google Scholar]
- 16.Jain P, Prieto VG, Manning JT, et al. Synchronous presentation of intra-nodal follicular dendritic cell sarcoma and Castleman disease. Am J Hematol 2017;92:478-9. 10.1002/ajh.24603 [DOI] [PubMed] [Google Scholar]
- 17.Hung YP, Qian X. Histiocytic Sarcoma. Arch Pathol Lab Med 2020;144:650-4. 10.5858/arpa.2018-0349-RS [DOI] [PubMed] [Google Scholar]
- 18.Wang E, Hutchinson CB, Huang Q, et al. Histiocytic sarcoma arising in indolent small B-cell lymphoma: report of two cases with molecular/genetic evidence suggestive of a 'transdifferentiation' during the clonal evolution. Leuk Lymphoma 2010;51:802-12. 10.3109/10428191003699845 [DOI] [PubMed] [Google Scholar]
- 19.Feldman AL, Arber DA, Pittaluga S, et al. Clonally related follicular lymphomas and histiocytic/dendritic cell sarcomas: evidence for transdifferentiation of the follicular lymphoma clone. Blood 2008;111:5433-9. 10.1182/blood-2007-11-124792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Péricart S, Waysse C, Siegfried A, et al. Subsequent development of histiocytic sarcoma and follicular lymphoma: cytogenetics and next-generation sequencing analyses provide evidence for transdifferentiation of early common lymphoid precursor-a case report and review of literature. Virchows Arch 2020;476:609-14. 10.1007/s00428-019-02691-w [DOI] [PubMed] [Google Scholar]
- 21.Hure MC, Elco CP, Ward D, et al. Histiocytic sarcoma arising from clonally related mantle cell lymphoma. J Clin Oncol 2012;30:e49-53. 10.1200/JCO.2011.38.8553 [DOI] [PubMed] [Google Scholar]
- 22.Skala SL, Lucas DR, Dewar R. Histiocytic Sarcoma: Review, Discussion of Transformation From B-Cell Lymphoma, and Differential Diagnosis. Arch Pathol Lab Med 2018;142:1322-9. 10.5858/arpa.2018-0220-RA [DOI] [PubMed] [Google Scholar]
- 23.Tashkandi H, Dogan A. Histiocytic sarcoma arising in patient with history of clonally-related germ cell tumour and myelodysplastic syndrome. Br J Haematol 2020;188:482. 10.1111/bjh.16372 [DOI] [PubMed] [Google Scholar]
- 24.Tzankov A, Kremer M, Leguit R, et al. Histiocytic cell neoplasms involving the bone marrow: summary of the workshop cases submitted to the 18th Meeting of the European Association for Haematopathology (EAHP) organized by the European Bone Marrow Working Group, Basel 2016. Ann Hematol 2018;97:2117-28. 10.1007/s00277-018-3436-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rassidakis GZ, Stromberg O, Xagoraris I, et al. Trametinib and Dabrafenib in histiocytic sarcoma transdifferentiated from chronic lymphocytic leukemia with a K-RAS and a unique BRAF mutation. Ann Hematol 2020;99:649-51. 10.1007/s00277-020-03941-7 [DOI] [PubMed] [Google Scholar]
- 26.Go H, Jeon YK, Huh J, et al. Frequent detection of BRAF(V600E) mutations in histiocytic and dendritic cell neoplasms. Histopathology 2014;65:261-72. 10.1111/his.12416 [DOI] [PubMed] [Google Scholar]
- 27.Said J. Genomic profiling of histiocytic sarcoma: new insights into pathogenesis and subclassification. Haematologica 2020;105:854-5. 10.3324/haematol.2019.246314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shanmugam V, Griffin GK, Jacobsen ED, et al. Identification of diverse activating mutations of the RAS-MAPK pathway in histiocytic sarcoma. Mod Pathol 2019;32:830-43. 10.1038/s41379-018-0200-x [DOI] [PubMed] [Google Scholar]
- 29.Kumamoto T, Aoki Y, Sonoda T, et al. A case of recurrent histiocytic sarcoma with MAP2K1 pathogenic variant treated with the MEK inhibitor trametinib. Int J Hematol 2019;109:228-32. 10.1007/s12185-018-2553-9 [DOI] [PubMed] [Google Scholar]
- 30.Takada M, Hix JML, Corner S, et al. Targeting MEK in a Translational Model of Histiocytic Sarcoma. Mol Cancer Ther 2018;17:2439-50. 10.1158/1535-7163.MCT-17-1273 [DOI] [PubMed] [Google Scholar]
- 31.Hartmann S, Eichenauer DA. Nodular lymphocyte predominant Hodgkin lymphoma: pathology, clinical course and relation to T-cell/histiocyte rich large B-cell lymphoma. Pathology 2020;52:142-53. 10.1016/j.pathol.2019.10.003 [DOI] [PubMed] [Google Scholar]
- 32.Kurita D, Miyoshi H, Yoshida N, et al. A Clinicopathologic Study of Lennert Lymphoma and Possible Prognostic Factors: The Importance of Follicular Helper T-cell Markers and the Association With Angioimmunoblastic T-cell Lymphoma. Am J Surg Pathol 2016;40:1249-60. 10.1097/PAS.0000000000000694 [DOI] [PubMed] [Google Scholar]
- 33.Etebari M, Navari M, Agostinelli C, et al. Transcriptional Analysis of Lennert Lymphoma Reveals a Unique Profile and Identifies Novel Therapeutic Targets. Front Genet 2019;10:780. 10.3389/fgene.2019.00780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Summers TA, Jr, Rush W, Aguilera N, et al. Cutaneous involvement in the lymphoepithelioid variant of peripheral T-cell lymphoma, unspecified (Lennert lymphoma). Report of a case and review of the literature. J Cutan Pathol 2009;36 Suppl 1:25-30. 10.1111/j.1600-0560.2008.01203.x [DOI] [PubMed] [Google Scholar]
- 35.Kosari F, Saffar H, Izadi B. Hypocellular/lymphohistiocytic variant of Anaplastic Large Cell Lymphoma of lymph node, mimicking granulation tissue. Arch Iran Med 2013;16:424-7. [PubMed] [Google Scholar]
- 36.Khalidi HS, Medeiros LJ, Battifora H. Lymphohistiocytoid mesothelioma. An often misdiagnosed variant of sarcomatoid malignant mesothelioma. Am J Clin Pathol 2000;113:649-54. 10.1309/7ECY-KN61-PP1M-52EB [DOI] [PubMed] [Google Scholar]
- 37.Chapel DB, Schulte JJ, Husain AN, et al. Application of immunohistochemistry in diagnosis and management of malignant mesothelioma. Transl Lung Cancer Res 2020;9:S3-27. 10.21037/tlcr.2019.11.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matsubara T, Toyokawa G, Yamada Y, et al. A Case of the Resected Lymphohistiocytoid Mesothelioma: BAP1 Is a Key of Accurate Diagnosis. Anticancer Res 2017;37:6937-41. [DOI] [PubMed] [Google Scholar]
- 39.Chapel DB, Schulte JJ, Berg K, et al. MTAP immunohistochemistry is an accurate and reproducible surrogate for CDKN2A fluorescence in situ hybridization in diagnosis of malignant pleural mesothelioma. Mod Pathol 2020;33:245-54. 10.1038/s41379-019-0310-0 [DOI] [PubMed] [Google Scholar]
- 40.Althakfi W, Gazzo S, Blanchet M, et al. The value of BRCA-1-associated protein 1 expression and cyclin-dependent kinase inhibitor 2A deletion to distinguish peritoneal malignant mesothelioma from peritoneal location of carcinoma in effusion cytology specimens. Cytopathology 2020;31:5-11. 10.1111/cyt.12788 [DOI] [PubMed] [Google Scholar]
- 41.Kalhor N, Moran CA. Primary germ cell tumors of the mediastinum: a review. Mediastinum 2018;2:4. 10.21037/med.2017.12.01 [DOI] [Google Scholar]
- 42.Bokemeyer C, Droz JP, Horwich A, et al. Extragonadal seminoma: an international multicenter analysis of prognostic factors and long term treatment outcome. Cancer 2001;91:1394-401. [DOI] [PubMed] [Google Scholar]
- 43.Baraban EG, Cooper K. Pathogenesis of Testicular Germ Cell Neoplasia: A Conceptual Approach. Adv Anat Pathol 2019;26:241-5. 10.1097/PAP.0000000000000233 [DOI] [PubMed] [Google Scholar]