Abstract
The recurrent excitatory circuits in dlPFC underlying working memory are known to require activation of glutamatergic NMDA receptors (NMDAR). The neurons in these circuits also rely on acetylcholine to maintain persistent activity, with evidence for actions at both nicotinic α7 receptors and muscarinic M1 receptors (M1R). It is known that nicotinic α7 receptors interact with NMDAR in these circuits, but the interactions between M1R and NMDAR on dlPFC neuronal activity are unknown. Here, we investigated whether M1Rs contribute to the permissive effects of ACh in dlPFC circuitry underlying working memory via interactions with NMDA receptors. We tested interactions between M1Rs and NMDARs in vivo on single neuron activity in rhesus macaques performing a working memory task, as well as on working memory behavior in rodents following infusion of M1R and NMDAR compounds into mPFC. We report that M1R antagonists block the enhancing effects of NMDA application, consistent with M1R permissive actions. Conversely, M1R positive allosteric modulators prevented the detrimental effects of NMDAR blockade in single neurons in dlPFC and on working memory performance in rodents. These data support an interaction between M1R and NMDARs in working memory circuitry in both primates and rats, and suggest M1Rs contribute to the permissive actions of ACh in primate dlPFC. These results are consistent with recent data suggesting that M1R agonists may be helpful in the treatment of schizophrenia, a cognitive disorder associated with NMDAR dysfunction.
Keywords: NMDA receptor, Acetylcholine, Muscarinic m1 receptor, Working memory, Prefrontal cortex, dlPFC
Graphical abstract

Highlights
-
•
Working memory-related persistent firing in primate prefrontal cortex relies on NMDAR.
-
•
Unlike classic circuits, NMDAR transmission requires permissive acetylcholine actions.
-
•
Muscarinic M1R blockade prevents the excitatory effects of NMDA on neuronal firing.
-
•
M1R stimulation averts the harmful effects of NMDAR blockade on cell firing and memory.
1. Introduction
Cognitive deficits arising from prefrontal cortical dysfunction are a central symptom in many psychiatric and age-related disorders, with no current available treatment options for these symptoms. In particular, cognitive disorders often involve deficits in working memory – the ability to hold information “in mind”. Studies in nonhuman primates have shown that working memory relies on recurrent excitatory circuits in the dorsolateral prefrontal cortex (dlPFC).
Much work has been done to understand the cellular basis of spatial working memory in dlPFC of rhesus monkeys, where neurons are able to maintain elevated, persistent firing for several seconds in the absence of continued sensory stimulation. These pyramidal neurons, termed Delay cells, reside in deep layer III of dlPFC, and also show extensive horizontal projections (Funahashi et al., 1989; Goldman-Rakic, 1995; Gonzalez-Burgos et al., 2000), allowing for neurons with similar spatial tuning to recurrently excite one another across delays (Goldman-Rakic, 1995). These recurrent excitatory connections have unique neurotransmission: they rely on NMDA receptors (NMDARs), including those with NR2B subunits, which are expressed exclusively within the postsynaptic density (PSD) (Wang et al., 2013). Importantly, Delay cells have only subtle reliance on AMPA receptors (AMPAR), and the permissive role normally played by AMPAR is instead provided by acetylcholine (ACh) actions at nicotinic α7 receptors which are co-localized in the PSD and are needed for Delay cell firing (Yang et al., 2013). Thus, local iontophoresis of a nicotinic α7 receptor antagonist onto Delay cells markedly reduced delay related firing, and prevented the enhancing effects of direct NMDAR application, while nicotinic α7 receptor stimulation reduced the deleterious effects of NMDAR blockade (Yang et al., 2013). These physiological data are consistent with behavioral studies showing a critical role for ACh in dlPFC for working memory, where depletion of ACh from dlPFC causes working memory deficits in monkeys equitable to total tissue ablation (Croxson et al., 2011).
There is also longstanding evidence of a role for ACh actions at muscarinic receptors in cognitive function, as systemic administration of muscarinic antagonists impairs cognitive performance in non-human primates (Bartus and Dean, 1988) and humans (Green et al., 2005). Systemic delivery of anti-muscarinic agents also significantly worsen symptoms in patients with schizophrenia (Veselinović et al., 2015), further implicating ACh actions at muscarinic receptors for optimal cognitive functioning. Muscarinic M1 receptors (M1Rs) are also known to be expressed postsynaptically within or proximal to the PSD of glutamatergic spines on layer III in primate dlPFC (Galvin et al., 2020; Mrzljak et al., 1993). Recent work has shed light on the role of M1Rs in the dlPFC circuits underlying working memory, revealing an important role for M1R for delay firing. In both adult and aged primates, a nonselective muscarinic antagonist (Major et al., 2015), as well as selective M1R antagonists (Galvin et al., 2020; Vijayraghavan et al., 2018) significantly impaired persistent firing of dlPFC neurons, while selective agonists and positive allosteric modulators have mixed effects in adult primates but consistently enhance firing in aged monkeys with naturally-occurring cholinergic depletion (Galvin et al., 2020; Vijayraghavan et al., 2018). Thus, aged monkeys can be particularly helpful in examining the beneficial effects of cholinergic actions in dlPFC which are saturated in the healthy young monkey.
An important remaining question is whether M1Rs contribute to ACh's permissive role for NMDAR actions in dlPFC, similar to nicotinic α7 receptors. Furthermore, as both the PFC and the cholinergic system vary significantly between rodents and primates (Coppola and Disney, 2018; Mesulam et al., 1983a; Rye et al., 1984; Amatrudo et al., 2012; Gilman et al., 2017), it is not known whether M1R contribute to the working memory functions in the rodent medial PFC (mPFC). The current study investigated M1R interactions with NMDAR using both in vivo iontophoretic recordings from aged monkey dlPFC, and intra-mPFC infusions of M1R and NMDAR compounds in rat medial PFC, the subregion linked to working memory performance in a T maze (Larsen and Div, 1978). We tested whether stimulation of M1R could rescue impairments induced by NMDAR blockade (monkey physiology, rat behavior), and conversely, whether blockade of M1R would prevent the excitatory effects of NMDA application onto monkey dlPFC neurons. The results are consistent with cholinergic M1R actions contributing to NMDAR actions in PFC.
2. Material and methods
2.1. Electrophysiology and iontophoresis
2.1.1. Subjects
Two aged rhesus monkeys (Macaca mulatta; Monkey C, 20 years old, male; Monkey T, 21 years old, female) were used in the current study, and were cared for under the guidelines of the National Institutes of Health (NIH) and the Yale Institutional Animal Care and Use Committee (IACUC).
2.2. Oculomotor delayed response (ODR) task
Two rhesus macaques were trained to perform the ODR task (Fig. 1A) to test visuospatial working memory. A central light is illuminated on an LED display monitor, which the subject must fixate for 0.5s to initiate a trial (fixation period). Following the 0.5s fixation, a cue light is illuminated for 0.5s (cue period) at 1 of 8 peripheral targets located at an eccentricity of 13° with respect to the fixation point. After the 0.5s cue presentation, the cue spot is extinguished, and a 2.5s delay period follows (delay period). The subject must maintain fixation on the central point through the cue presentation and the subsequent delay period. At the end of the delay period, the fixation point is extinguished, instructing the animal to make a memory-guided eye saccade to the previously cued location. A trial is successful if the animal makes a saccade to within 2 degrees of the location of the prior cue point within 0.5s after the offset of the fixation point. Correctly completed trials are rewarded with juice immediately after the successful response. The inter-trial interval is 3s. The animal's eye position was monitored with ISCAN Eye Movement Monitoring System, and the ODR task was generated by PictoBox System (developed by Dr. Daeyeol Lee and colleagues, Yale University) or Monkey Logic (developed by Dr. Wael Asaad, Brown University).
Fig. 1.
Electrophysiological methods to probe M1R interactions with NMDAR in primate dlPFC. (A) The oculomotor delayed response (ODR) task where animals must remember one of eight potential spatial locations over a multi-second delay. (B) Diagram of the seven barrel glass electrodes used for combined neuronal recording and iontophoresis. (C) The recording location in dlPFC, at the posterior end of the principal sulcus. (D) An example Delay cell showing elevated, persistent activity across the delay for the preferred direction. (E) Top. Schematic of spine dynamics in dlPFC Delay cell excitatory synaptic connections. NMDARs are expressed within the PSD, and M1R and KCNQ channels are both found proximal to the PSD or extrasynaptically. Bottom. Example immunoelectron microscopy localizing M1R on dendritic spines in layer III dlPFC within the PSD and extrasynaptically. Synapses are between arrows. Color-coded arrowheads (green) point to M1R immunoreactivity. Profiles are pseudocolored for clarity. Ax, axon; Sp, dendritic spine. Scale bar, 200 nm. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
2.3. Location of recording site
Animals underwent a magnetic resonance imaging (MRI) brain scan prior to placement of implants to obtain the exact anatomical coordinates of the desired recording site over the caudal principal sulcus of cortical area 46 (shown in Fig. 1C). These coordinates were then used to guide placement of the chronic recording chamber and electrophysiological recordings.
2.4. Recording and iontophoresis
Electrodes for dual recording and iontophoresis (Fig. 1B) were constructed with a 20um-pitch carbon fiber inserted into the central barrel of a 7-barrel nonfilamented capillary glass (Friedrich and Dimmock). The assembled carbon fiber and glass were then pulled using a custom electrode puller (PMP-107, Microdata Instrument Inc.) and the tip was hand beveled to reach an impedance of 0.3–1.0 MΩ with tip sizes of 30–40μm. After pulling and beveling, the outer barrels of the electrode were filled with up to 3 different drug solutions (2 consecutive barrels per drug), which were pushed through the tip of the electrode using air. A Neurophore BH2 iontophoretic system (Medical Systems Corp.) was used to deliver the drugs at ejection currents that varied from 5 nA–25 nA. Retaining currents of 5 nA at the opposite polarity were used in a cycled manner (1s ON, 1s OFF) when drugs were not being actively applied. Drug ejection did not create noise in the recording, and there was no iontophoresis-related change in spike waveforms at any ejection current.
The compounds used in the current study are the M1R antagonist telenzepine, M1R PAM VU0357017, NMDAR antagonist MK801, NMDAR NR2B antagonist TCN237, and NMDAR agonist NMDA. Each compound was from Tocris Bioscience and dissolved at 0.01 M concentration in sterile water. This study used iontophoresis to apply each compound near dlPFC neurons.
2.5. Electrophysiology data analyses
Each trial in the ODR task was divided into four epochs – initial Fixation, Cue, Delay and Response (Saccade). The initial Fixation epoch lasted for 0.5 s. The Cue epoch lasted for 0.5 s and corresponds to the stimulus presentation phase of the task. The Delay lasted for 2.5 s and reflects the mnemonic component of the task. The Response phase started immediately after the Delay epoch and lasted ~1.5 s. Data analysis was performed in MATLAB, SPSS and GraphPad Prism 7.01. This study focused on Delay cells (Fig. 1D) that represent working memory. The delay cells were recorded within or on the dorsal bank of the principal sulcus. Delay cells exhibited elevated persistent firing during the delay epoch (delay cells) at one or more than one directions. The direction with highest delay firing was defined as the cell's preferred direction, and its opposite direction was defined as the nonpreferred direction. Two-way analysis of variance (ANOVA) was used to examine the spatial tuned delay-related activity with regard to: (1) different periods of the task (delay vs. fixation) and (2) different cue locations (preferred direction vs. nonpreferred direction). In our studies, there were typically three distinct subtypes of delay cells. One prominent subset of delay cells, termed “memory delay cells,” fired throughout the delay period. The second subset of delay cells, so-called preparatory set delay cells or ramp-up delay cells, began firing in the early or middle of the delay period and increased their firing in anticipation of the motor act. Finally, there were delay cells with a combined phenotype that fired throughout the delay period but ramped down during later delay period, so-called ramp-down delay cells. The combined firing of these neurons provides the temporal integration needed to guide response in the absence of sensory stimulation. All three subtypes of delay cells were influenced by drug in a similar fashion, and thus were combined for these analyses. Many Delay cells fire during the cue and/or response epochs as well as the delay epoch; given their variable responses to the cue and response epochs, data analyses focused on the delay epoch. Unpaired t-test with Welch's correction and one-way ANOVA were employed to assess the effects of drug application on task-related activity or each single Delay cell. Two-tailed paired t-test t or repeated measures one-way ANOVA with Tukey's multiple comparisons or repeated measures two-way ANOVA with Sidak's multiple comparisons were employed to assess the effects of drug application on task-related activity for the population analysis. In the interest of brevity, figures often show the neurons' preferred direction in comparison to just one non-preferred direction, the “anti-preferred” direction directly opposed to the neurons' preferred direction. For Delay cells, the spatial tuning was assessed by comparing firing levels for the neuron's preferred direction vs. its non-preferred directions. Quantification of spatial tuning was performed by calculating a measure of d’ using the formula:
2.6. Rodent intra-mPFC infusions and delayed alternation task
2.6.1. Subjects
Male Sprague Dawley rats aged 5–18 months were independently housed under standard laboratory conditions and kept on a 12 h light/dark cycle. Behavioral experiments were conducted during the light phase. Highly palatable rewards (mini chocolate chips) were used during experiments to minimize the need for food restriction. Animals were fed 12–16 g of rat chow immediately following testing, and weighed weekly to maintain weight at 400–450 g. Animals were habituated to all procedures and tested by a single experimenter who was blind to drug treatment conditions. Rats were cared for under the guidelines of the National Institutes of Health (NIH) and the Yale IACUC.
2.6.2. Delayed alternation T-maze
Animals were trained in a delayed alternation T-maze, a test of working memory. The animals started each trial at the bottom of the ‘T’ in the Start Box. For the first trial, the animal was rewarded for entering either arm. For each subsequent trial, they were only rewarded for choosing the arm that they had not visited in the previous trial. Between trials, the animal was picked up and returned to the start box for the duration of the delay period, requiring the animal to update and maintain spatial information over the delay period for each trial. Delay lengths were adjusted for each rat to maintain a stable baseline of 60–80% before an infusion treatment. The choice point, where the animal must decide which arm to choose, was wiped with 80% ethanol in between trials to erase scent cues.
2.6.3. Intra-mPFC infusion
The effects of the M1R PAM VU0453595 on working memory performance in rodents was tested independently, and in combination with the NMDA receptor antagonist MK801, through direct infusions intothe medial PFC (mPFC). Adult male Sprague-Dawley rats were implanted with chronic infusion cannulae directly above the prelimbic PFC (anterior-posterior +3.2 mm; medial-lateral ± 0.75 mm; dorsal-ventral −4.2 mm). Prior to a drug infusion, animals had to test within 60–80% correct for three consecutive test days. After two days of stable 60–80% correct testing, the cannulae cap and stylet were removed and reinserted prior to testing on the third day. Only if the animal successfully scored within 60–80% after the cap and stylet removal was an infusion done the following test day. A minimum one week washout period was maintained between infusions. Experimenters conducting the behavioral testing were blind to drug condition, and the order of doses and vehicle infusions was randomized for each animal.
2.6.4. Behavioral analysis
Data were analyzed using SPSS Statistics v26 (IBM). Drug effects were tested with 1-way ANOVA-R with paired comparisons, and 2-tailed paired-samples t-test. P < 0.05 was predetermined as the threshold for statistical significance.
3. Results
Our previous study showed that generating persistent delay activity is highly dependent on NMDAR, but not dependent on AMPAR, instead relying on ACh actions at both nicotinic α7 receptors and muscarinic M1Rs (Wang et al., 2013; Yang et al., 2013; Galvin et al., 2020). As stimulation of M1R enhanced delay activity, at least partly through KCNQ channel closure, and M1Rs have been localized within or proximal to the PSD (Fig. 1E), here we tested the hypothesis that M1R interact with NMDAR and contribute to the permissive effects of ACh for working memory in aged primates. In order to best view interactions without the confound of additive effects of combined drug treatment, we used very low doses of cholinergic agents with minimal effects when administered alone. A total of 21 delay cells from a total of 15 recording sessions were successfully tested with all drug treatments. Of these, 6 were from Monkey C (20 years old, male) and 15 from Monkey T (21 years old, female). The delay cells included in the study were tested with the following conditions: Study 1: M1R PAM alone, M1R PAM + NMDA antagonist, and NMDA antagonist alone (n = 10 neurons). Study 2: M1R antagonist alone, M1R antagonist + NMDAR agonist, NMDAR agonist alone, and recovery (n = 11 neurons). The number of neurons is relatively low compared to a typical paper in this field because 1): Each experiment used multiple drug application conditions and thus required the aged monkey to perform at least 300 trials for each recording. Aged monkeys perform a limited number of trials compared to young adults, and often stop working in the middle of a recording session. Thus, we could only use the sessions/neurons where the aged monkeys kept working and finished all drug conditions. 2): The studies of NMDAR (Wang et al., 2013) and M1R (Galvin et al., 2020) alone have already been published, thus, the current work focused on the interaction between NMDAR and M1R.
3.1. M1R stimulation prevents the reduction effects of NMDAR blockade on delay firing of dlPFC delay cells in primates
We first examined whether co-application of an M1R agonist or PAM could prevent the reduction in delay cell firing caused by NMDAR blockade. In this experiment, we first treated Delay cells with low dose of the M1R PAM, VU0357017 (VU), then co-applied VU with the NMDAR NR2B antagonist, TCN237 (TCN), or the general NMDAR antagonist, MK801 (MK). Last, we ceased VU application and applied MK or TCN on their own. We found that MK or TCN reduced delay firing significantly when applied alone, consistent with our previous findings, but that co-application of VU with the NMDAR antagonist prevented the reduction in firing caused by either MK or TCN. An example delay cell is shown in Fig. 2A–B, where VU was able to prevent the loss of firing caused by the general NMDA antagonist, MK. This neuron showed weak but significant delay related firing for its preferred direction, but not non-preferred direction in the control condition (two-way ANOVA, Fdirectionxepoch(1,28) = 5.464, p = 0.0268; Sidak's multiple comparisons: delay vs fixation: preferred direction, p = 0.0003; non-preferred direction, p = 0.4814; preferred vs non-preferred direction: delay, p = 0.0077; fixation, p = 0.9846), and the subsequent application of a low doses of VU tended to enhance delay firing (Fig. 2A–B; control vs. VU condition: two-way ANOVA, Fdirectionxdrug(1,28) = 0.8891, p = 0.3538; Fdrug(1,28) = 4.131, p = 0.0517; Fdirection (1,28) = 13.91, p = 0.0009; Sidak's multiple comparisons: preferred direction, p = 0.087 and non-preferred direction, p = 0.6947). Importantly, co-application of VU and MK maintained delay activity at the same level as in the VU alone condition (Fig. 2A–B; VU vs. VU + MK condition: two-way ANOVA, Fdirectionxdrug(1,28) = 0.3057, p = 0.5847; Fdrug(1,28) = 0.8493, p = 0.3646; Fdirection (1,28) = 16.11, p = 0.0004; Sidak's multiple comparisons: preferred direction, p = 0.5184 and non-preferred direction, p = 0.9585), while after removing VU, MK significantly reduced Delay cell firing (Fig. 2A–B; VU + MK vs. MK condition: two-way ANOVA, Fdirectionxdrug(1,28) = 4.223, p = 0.0493; Fdrug(1,28) = 15.99, p = 0.0004; Fdirection (1,28) = 11.08, p = 0.0024; Sidak's multiple comparisons: preferred direction, p = 0.0004 and non-preferred direction, p = 0.328). Thus, even a low dose of VU was able to prevent the loss of firing caused by NMDAR blockade.
Fig. 2.
M1R stimulation prevents the reducing effects of NMDAR blockade on delay firing of dlPFC delay cells. (A) A single Delay cell example of M1R PAM, VU0357017 (VU) preventing the reducing effects of NMDAR blockade by MK801 (MK). From top panel to bottom panel, rasters and histograms for preferred direction and non-preferred direction during the control condition, VU application condition, VU + MK co-application condition and MK application condition are shown. (B) The average delay firing rate for the example neuron in (A). Low dose application of VU non-significantly increase delay-related firing, and blocked the detrimental effects of MK when co-applied, as MK applied alone after the removal of VU significantly eroded delay firing. (C) A single cell example mirroring the conditions in (A), but with the NR2B selective antagonist TCN237 (TCN). (D) The average delay firing rate of the above delay cell for the preferred and non-preferred directions during control, application of VU alone, application of VU + TCN, and application of TCN alone, where TCN alone significantly reduced firing compared to control.
Fig. 2C–D shows another example where VU prevented the marked reduction in firing caused by the selective NMDAR-NR2B antagonist, TCN. This delay cell showed cue and ramping up delay firing for its preferred direction, but not non-preferred direction in the control condition (two-way ANOVA, Fdirectionxepoch(1,46) = 5.216, p = 0.027; Sidak's multiple comparisons: delay vs fixation: preferred direction, p < 0.0001; non-preferred direction, p = 0.0918; preferred vs non-preferred direction: delay, p = 0.0123; fixation, p = 0.8523). Application of a low dose of VU significantly enhanced delay firing (Fig. 2C–D; control vs. VU condition: two-way ANOVA, Fdirectionxdrug(1,41) = 4.186, p = 0.0472; Fdrug(1,41) = 9.272, p = 0.0041; Fdirection (1,41) = 26.44, p < 0.0001; Sidak's multiple comparisons: preferred direction, p = 0.0015 and non-preferred direction, p = 0.7393). In the following condition, co-application of VU and TCN kept delay activity at the same level as the VU only condition (Fig. 2C–D; VU vs. VU + TCN condition: two-way ANOVA, Fdirectionxdrug(1,44) = 0.3119, p = 0.5793; Fdrug(1,44) = 0.1306, p = 0.7195; Fdirection (1,44) = 42.45, p < 0.0001; Sidak's multiple comparisons: preferred direction, p = 0.7684 and non-preferred direction, p = 0.9879), however, when VU was no longer applied, TCN significantly reduced delay firing (Fig. 2C–D; VU + TCN vs. TCN condition: two-way ANOVA, Fdirectionxdrug(1,40) = 18.92, p < 0.0001; Fdrug(1,40) = 26.82, p < 0.0001; Fdirection (1,40) = 11.21, p = 0.0018; Sidak's multiple comparisons: preferred direction, p < 0.0001 and non-preferred direction, p = 0.8071).
We replicated these results in 10 Delay cells (4 tested with MK, 6 tested with TCN), showing the consistency of this effect. As illustrated in Fig. 3A, low doses of VU slightly increased the firing rate for the neurons' preferred directions, and kept the delay firing above the control level when subsequently co-applied with either NMDAR antagonist (Fig. 3A; preferred direction: Repeated one-way ANOVA, F(1.573,14.15) = 2.995, p = 0.0913; Tukey's multiple comparisons: control vs. VU, p = 0.4742; VU vs. VU + NMDAR antagonist, p = 0.4975). Conversely, both NMDAR antagonists significantly reduced delay firing when VU was no longer applied (Fig. 3A; preferred direction: VU + NMDAR antagonist vs. NMDAR antagonist: two-tailed paired t-test, t = 5.872, df = 9, p = 0.0002). These were not simply additive effects of the two treatments, as the reduction in firing with the NMDAR antagonists by themselves was significantly greater than the improvement caused by VU treatment alone (Fig. 3B; t-test to compare the effects of MK/TCN and VU relative to control, p = 0.03). There data are consistent with an interaction between M1R and NMDAR.
Fig. 3.
M1R stimulation consistently prevents the effects of NMDAR antagonists across a population of Delay cells. (A) As both MK and TCN consistently and similarly reduced Delay cell firing, and both were equally prevented by preapplication of VU, we combined the population of neurons tested with either NMDAR antagonist (n = 10). Compared to control activity, VU modestly increases delay firing and blocks any effect of NMDAR blockade, while delay firing is significantly reduced once VU is no longer co-applied. (B) Comparison of the effects of MK/TCN and VU alone as a percent change in firing from control levels, showing MK produced a stronger reduction.
3.2. M1R blockade prevents the enhancing effect of NMDA on delay firing of dlPFC delay cells in primates
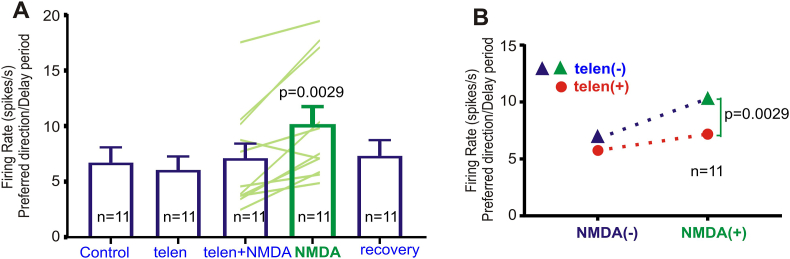
To further test whether there is an interaction between M1R and NMDAR, we conducted another set of experiments to examine whether NMDA is ineffective in exciting a neuron under conditions of M1R blockade. In this experiment, we first treated Delay cells with a very low dose of the M1R antagonist, telenzepine by itself, and then co-applied telenzepine with the NMDAR agonist, NMDA; we then ceased telenzepine application and applied only the NMDA. As shown in a single delay cell example in Fig. 4 (control condition: two-way ANOVA, Fdirectionxepoch(1,26) = 5.362, p = 0.0287; Sidak's multiple comparisons: delay vs fixation: preferred direction, p = 0.0166; non-preferred direction, p = 0.8961; preferred vs non-preferred direction: delay, p = 0.0225; fixation, p = 0.452), the application of a low dose (20 nA) of telenzepine had non-significant decreasing effects on delay firing for the preferred direction (Fig. 4 A-B; control vs telenzepine condition: two-way ANOVA, Fdirectionxdrug(1,23) = 2.596, p = 0.120.8; Fdrug(1,23) = 3.205, p = 0.0866; Fdirection (1,23) = 1.154, p = 0.2939; Sidak's multiple comparisons: preferred direction, p = 0.1452 and non-preferred direction, p = 0.9111). When NMDA was then co-applied with the telenzepine, there were no significant changes to neuronal firing (Fig. 4 A-B; telenzepine vs telenzepine + NMDA condition: two-way ANOVA, Fdirectionxdrug(1,24) = 1.07, p = 0.3112; Fdrug(1,24) = 0.7071, p = 0.4087; Fdirection (1,24) = 0.3733, p = 0.5469; Sidak's multiple comparisons: preferred direction, p = 0.9463 and non-preferred direction, p = 0.443). However, when telenzepine was no longer co-applied, NMDA alone significantly increased delay firing for the preferred direction (Fig. 4 A-B; telenzepine + NMDA vs NMDA condition: two-way ANOVA, Fdirectionxdrug(1,27) = 16.05, p = 0.0004; Fdrug(1,27) = 1.732, p = 0.1992; Fdirection (1,27) = 2.364, p = 0.1358; Sidak's multiple comparisons: preferred direction, p = 0.0009 and non-preferred direction, p = 0.1855). The firing rate returned to control levels during the subsequent recovery condition (Fig. 4 A-B; NMDA vs recovery condition: two-way ANOVA, Fdirectionxdrug(1,30) = 5.255, p = 0.0291; Fdrug(1,30) = 1.075, p = 0.3081; Fdirection (1,30) = 0.4011, p = 0.5313; Sidak's multiple comparisons: preferred direction, p = 0.0923 and non-preferred direction, p = 0.4375). This was a consistent pattern across all 11 Delay cells tested, as shown in Fig. 5. There was no difference in delay firing between the control condition and the telenzepine or telen + NMDA conditions, while NMDA alone significantly increased the delay firing compared to control (preferred direction: Repeated one-way ANOVA, F(1.779, 17.79) = 16.21, p = 0.0001; Tukey's multiple comparisons: control vs. telen, p = 0.6575; control vs. telen + NMDA, p = 0.8341; control vs. NMDA, p = 0.0023; telen vs. NMDA, p = 0.0015; telen + NMDA vs. NMDA, p = 0.0242). Therefore, NMDA showed different effects on delay firing with and without the presence of telenzepine (Fig. 5B, two-tailed paired t-test: telen + NMDA vs. NMDA, t = 3.916, df = 10, p = 0.0029), confirming there is an interaction between M1R and NMDAR. These results are consistent with a permissive effect of M1R stimulation for NMDAR excitatory actions.
Fig. 4.
M1R blockade prevents the enhancing effect of NMDA on delay firing. A single cell example of rasters and histograms for the preferred direction are shown with each condition from left to right in an arch. The average delay firing rate of the same delay cell for the preferred and non-preferred directions during control, application of telen alone, application of telen + NMDA, and application of NMDA alone are shown in the center of the graph. Both a low dose of telen application and telen + NMDA co-application had no significant effect on delay firing. After stopping telen application, NMDA significantly enhances Delay cell firing for both the preferred and non-preferred directions, and cell firing returns to control rates following the removal of NMDA.
Fig. 5.
M1R blockade consistently prevents delay firing enhancement with NMDA across a population of Delay cells. (A) Average delay firing rate for the preferred directions for each condition across the population. No difference is observed compared to controls for telenzepine alone, or telenzepine + NMDA. However, when telenzepine is no longer applied, NMDA alone, at the same dose, significantly enhanced delay-related firing. The firing returned to control levels after NMDA was no longer applied. (B) NMDA had the different effects on delay firing with (red circle) and without (green triangle) the presence of telenzepine. NMDA is unable to excite a neuron under the condition of telenzepine application, confirming there is an interaction between M1R and NMDAR. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.3. M1R stimulation with M1R PAM prevents working memory deficits following NMDAR blockade in rodents
To assess the impact of these same experiments on behavior, we tested the effect of M1R blockade or activation and interactions with NMDAR on working memory performance in rats, as assessed by delayed alternation performance in a T maze (Fig. 6A). Animals were implanted with cannulae targeting prelimbic cortex for direct medial PFC (mPFC) infusions of telenzepine, VU0453595 (VU045) and MK (example in Fig. 6B; see methods). Post-mortem analyses showed that all cannula were properly placed in the prelimbic PFC.
Fig. 6.
Infusion of M1R antagonist telenzepine into rat mPFC impairs working memory performance. (A) The delayed alternation T-maze used to test working memory. The animal starts each trial in the Start Box. For the first trial, the animal is rewarded for choosing either arm at the Choice Point. For each subsequent trial, the animal is only rewarded if it correctly chooses the opposite arm to the one previously visited on the last trial for a total of 10 trials. (B) The location in mPFC where chronic cannulae where implanted and drug infusions occurred. Asterisk and the arrowhead represent the location of the tip of the cannulae. (C) The effect of infusion of three different doses of telenzepine on T-maze performance in rats (n = 10 for Veh, 0.001 and 0.01, and n = 9 for dose 0.1 due to one animal dying before receiving final dose).
We first tested a variety of doses of telenzepine on rat working memory performance (0.001, 0.01, 0.1 μg/0.5 μL), the results of which are shown in Fig. 6C. Intra-PFC infusion of telenzepine 15 min prior to testing significantly impaired animal performance at two of the three doses tested (R-one-way ANOVA, F(1,9) = 3.517, p = 0.0285; Sidak's multiple comparisons: Veh vs. 0.001, p = 0.0275 (n = 10); Veh vs. 0.01, p = 0.1283 (n = 10); Veh vs 0.1, p = 0.02 (n = 9)).
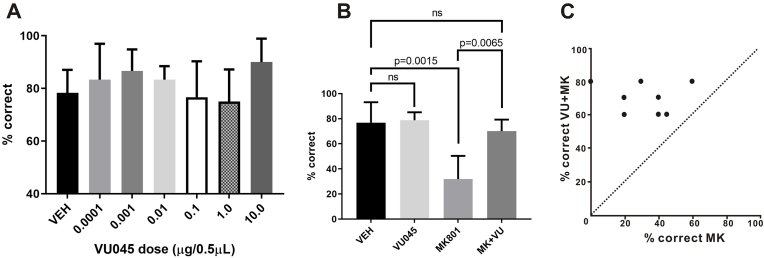
As the M1R PAM VU045 is a new compound, we tested a wide range of doses for intra-PFC infusions in rats (0.0001, 0.001, 0.01, 0.1, 1.0, 10.0 μg/0.5 μL). After correcting for multiple comparisons, there was no significant improvement in working memory performance for any dose tested (Fig. 7A, p > 0.1). This may be due to substantial variability we observed between effective doses from animal to animal, our small group size (n = 6), or possible ceiling effects where M1R actions in rat PFC were already optimally engaged, so no improvement was observable as we tested these doses on adult rats aged 5–18 months (similar to the mixed results observed by Vijayraghavan and colleagues testing M1R PAM on neuronal activity in young adult monkeys (Vijayraghavan et al., 2018)). Though we saw no significant improvement with VU045 infusions, this allowed us to use the individualized rat dose response data to select a low dose that had no impact on behavior when infused alone, to use to investigate interactions with NMDAR behaviorally. Of the animals used for the wider VU045 dose response, three of these six were additionally used to test interactions with NMDAR blockade.
Fig. 7.
Infusion of an M1R PAM prevents working memory deficits from NMDAR blockade. (A) The full dose response for effects of VU045 infusion into mPFC on working memory performance. No dose significantly improved performance across the population, and a low dose that did not improve performance was chosen for each animal (n = 6 for all doses). (B). Infusion of VU045 had no effect on performance when infused alone compared to an infusion of vehicle, while infusion of MK alone significantly impaired performance. This impairment was blocked when VU045 was co-infused with MK (n = 8). (C) The behavioral performance following the infusion of VU045 + MK801 was significantly different from the infusion of MK801 alone.
To assess interactions between NMDAR with M1R, we conducted a small pilot to find the lowest dose of MK801 producing impairment in each animal (5.0, 10.0 or 15.0 μg/0.5 μL, n = 8). We then co-infused this impairing dose of MK801 with a dose of VU045 selected for each animal where no improvement was found compared to vehicle (Sidak's multiple comparisons test: vehicle vs. VU045, p = 0.4430). Initial VU045 doses to test with MK were doses within the middle of the tested range for VU045 (0.01 and 0.1 μg/0.5 μL) which produced the lowest score variability and were lower than the higher doses that improved performance in some animals. These two doses were then tested on the additional five animals used for the MK-VU045 interaction experiments. For one animal, an additional dose of VU045 (1.0 μg/0.5 μL) was used in combination with MK after both initial doses failed to block the impairment with MK. For each animal, the VU045 dose did not improve performance above the score following vehicle infusion. This low dose of VU045 completely blocked the behavioral impairment seen with MK801 infusion alone, where behavioral performance following the combined infusion was not significantly different from a vehicle infusion (Fig. 7B, Sidak's multiple comparisons test: vehicle vs. MK + VU, p = 0.1304). The behavioral performance following the combined infusion was significantly different from the infusion of MK801 alone (Fig. 7B–C, Sidak's multiple comparisons test: MK801 vs. MK + VU, p = 0.0065). These results support a key role for M1R in ACh gating of NMDAR in working memory circuitry in rodent dlPFC.
4. Discussion
Here we report M1Rs interact with NMDARs in dlPFC circuitry underlying working memory, contributing to the permissive actions of ACh on Delay cell firing. First, we show that a low dose of an M1R PAM, VU0357017, that has no effect on delay firing on its own, prevented the detrimental effects of blocking NMDARs on Delay cell activity. Second, a low dose of the M1R antagonist, telenzepine, that does not alter delay firing alone, blocked the excitatory effect of NMDA application when co-applied to the same neuron. Lastly, we tested whether this interaction existed in rat mPFC while animals performed a working memory task. We found results similar to the neuronal recordings in monkeys, where intra-mPFC infusion of a low dose of an M1R PAM, that did not alter behavioral performance alone, blocked the detrimental effects of infusing the NMDAR antagonist, MK801. The reliance of both primate and rodent NMDAR actions on M1R stimulation points to the important role played by the arousal systems in allowing PFC circuits to come “online” during waking when ACh is released in cortex.
4.1. Potential mechanisms for M1R interaction with NMDAR
M1R are metabotropic receptors linked to Gq signaling. Thus, it is not immediately apparent how they might depolarize the PSD to relieve the Mg2+ block of the NMDAR pore and permit NMDAR transmission. There are two likely possibilities. One mechanism may involve M1R increasing internal calcium release near the PSD via Gq-IP3 signaling, as IP3R are seen on the endoplasmic reticulum near the PSD in dlPFC spines (Paspalas and Goldman-Rakic, 2004). A second possibility is the closure of KCNQ “m” channels, which like M1R, are localized within and near the PSD in dlPFC spines (Galvin et al., 2020). Physiological data support this possibility, as the beneficial effects of M1R stimulation were reversed by increasing the open state of KCNQ channels, while the loss of firing with the M1R antagonist telenzepine was reversed by the KCNQ channel blocker, XE991. Studies of M1R activation closing KCNQ channels in vitro have shown complete reduction of the KCNQ current within 5–16s of M1R stimulation (Suh and Hille, 2002), with closure being controlled by rapid hydrolysis of the second messenger PIP2, which keeps KCNQ channels open. The localization of both KCNQ and M1R within and near the PSD in layer III dlPFC spines suggest that this mechanism may contribute to the M1R interactions with NMDAR transmission reported here.
4.2. Species differences
There are multiple differences between rodents and primates in PFC structure and physiology, and in cholinergic mechanisms. The dlPFC does not exist in rodents, and even the mPFC in rodents differs in its organization from the mPFC in primates (Wallis et al., 1073). Furthermore, the primate dlPFC contains circuits with extensive local recurrent excitation to generate persistent firing over many seconds, which depend on NMDAR with slowly closing NR2B subunits that flux high levels of calcium (Wang et al., 2013). In contrast, rodent mPFC neurons have very limited persistent firing (~1sec), and limited recurrent circuits within the mPFC, and are more dependent on mPFC interactions with the hippocampus to sustain working memory (Spellman et al., 1038). However, we did find working memory performance was impaired by infusion of the NMDAR antagonist, MK801 in mPFC in rats. As rodents are widely used as models for neuropsychiatric disorders that involve alterations in NMDAR signaling, understanding these species differences is critical to effective translation. Several studies have investigated the contribution of NMDAR subtype composition for working memory in rodents, with mixed results. There are reports of NR2B-containing NMDARs underlying connections between layer V pyramidal neurons in rat PFC (Wang et al., 2008), the layer much more expansive in rodent PFC. Additionally, overexpression of NR2B in PFC enhances working memory in mice (Cui et al., 2011). However, other studies report that only NMDAR with NR2A subunits are required, and blockade of NR2B-containing NMDAR had no effect on behavioral performance for working memory (McQuail et al., 2016). Further investigation is needed to determine the precise role of NMDAR composition in rodent PFC circuits.
The cholinergic system also exhibits significant differences across species. This is evident in the proportion of ACh-producing neurons in the basal forebrain between rodents and primates, the specificity and density of projection patterns (Mesulam et al., 1983b, 1986; Zaborszky et al., 2015), and receptor expression patterns (Disney et al., 2006; Disney and Reynolds, 2014) (species differences reviewed by Coppola and Disney, (2018)). Relevant to this study are the differences in ACh projections to PFC and the distribution of M1Rs. Studies in rats have reported high M1R expression on pyramidal neurons and astrocytes, as well as very high expression in GABAergic interneurons, with 90% of PV+ neurons expressing M1R (Oda et al., 2018). Studies in primate show some similarities, though fewer anatomical studies have been conducted. The data from primate PFC have localized M1R expression predominantly postsynaptically on spines in layer III of dlPFC, with some expression on dendritic shafts and many positive astrocyte processes surrounding synapses (Galvin et al., 2020; Mrzljak et al., 1993). While the circuitry in PFC and the specific role of NMDARs may differ from rodents to primates, our results presented here support an interaction between M1R and NMDAR in both species, where M1R contribute to the permissive effects of ACh on dlPFC circuitry underlying working memory, and activation of M1R can block the detrimental behavioral effects of NMDAR antagonists. Whether this interaction is governed by the same underlying mechanisms remains to be studied.
4.3. Relevance to schizophrenia
Alterations in glutamatergic signaling and NMDAR function are thought to contribute to cognitive deficits and PFC dysfunction in schizophrenia, as the NMDAR antagonist ketamine both worsens symptoms in schizophrenic patients (Malhotra et al., 1997) and recapitulates symptoms in healthy volunteers (Corlett et al., 2006). Interestingly, muscarinic antagonists have also been reported to produce these effects, both worsening patient symptoms (Veselinović et al., 2015) and producing positive and cognitive symptoms in healthy volunteers (Green et al., 2005; Ellis et al., 2006). Genetic studies have found insults to genes causing weakened NMDAR signaling (Banerjee et al., 2010; Javitt, 2010), and Weickert and colleagues (2012) (Weickert et al., 2012) reported a link between allelic alterations in NR2B and impaired reasoning abilities in schizophrenic patients.
Prior theories of glutamate dysfunction in schizophrenia have proposed a hyperglutamate problem (reviewed in Kantrowitz and Javitt (2012) (Kantrowitz and Javitt, 1097)) following studies of NMDAR actions in rodent PFC showing NMDAR antagonists increase neuronal firing and glutamate release (Jackson et al., 2004). Reports in schizophrenic patients, however, have reported reduced fMRI BOLD response in dlPFC while performing a working memory task, and the degree of reduced activation correlated with severity of thought disorder in these subjects (Perlstein et al., 2001). Additionally, reduced NMDAR glutamate signaling has been directly associated with impaired cognitive abilities in patients with schizophrenia (Bustillo et al., 2011), further supporting a hypoglutamate problem in the dlPFC circuits underlying working memory and high order cognition.
Despite potential differences in working memory circuitry and effects of NMDAR antagonists, our data presented here from both primate and rat support the pursuit of M1R agonists or PAMs for clinical development to treat dlPFC hypofunction in schizophrenia. Indeed, the M1R agonist xanomeline is currently being tested in Phase 3 trials by Karuna Pharmaceuticals after producing an 11.6 point improvement on total PANSS scores in patients with schizophrenia in a phase 2 trial (https://www.businesswire.com/news/home/20191118005243/en/Karuna-Therapeutics-Announces-KarXT-Met-Primary-Endpoint).
4.4. Relevance to aging and Alzheimer's disease
The cholinergic neurons in the basal forebrain degenerate in AD (Whitehouse et al., 1982). The association cortical regions most afflicted in AD receive strong basal forebrain cholinergic innervation, and depend on ACh for optimal circuit function. AD patients show reduced cholinergic innervation of cortical and paralimbic areas, and the extent of BF neuronal loss correlates with cognitive dysfunction (Mesulam et al., 1986; Geula and Mesulam, 1989; Gibson et al., 1981; Gibson and Peterson, 1981). This degeneration of the cholinergic system inspired the earliest medication options to improve function in patients, providing acetylcholinesterase inhibitors to prolong ACh actions in the synapse (Rogers et al., 1998). Although these compounds are in widespread use, they provide only temporary relief and do not halt the underlying degeneration of association cortex. The critical role for ACh in recurrent dlPFC circuits, and the sensitivity of these circuits to insult due to a high level of recurrent excitatory connections, may thus be one mechanism behind the sensitivity of this region to impairment in AD. The enhancing effects of M1R stimulation in aged monkeys in the current study, and their important consequences to NMDAR transmission, highlight the importance of maintaining cholinergic stimulation for strong working memory function.
5. Conclusions
Our study shows that a low dose of M1R stimulation prevented the reducing effect of NMDAR blockade, while M1R blockade blocked the excitatory effect of NMDA application. At the behavior level, we found that M1R stimulation blocked the working memory deficits by NMDAR blockade. Our results confirm that there is an interaction between M1R and NMDAR, and that M1R may play a permissive role for NMDAR actions in dlPFC.
Funding
This work was supported by National Institutes of Health R01 MH093354 to M.W., 1RL1AA017536 within U54RR024350 to A.A., and MH113257 to A.D.
CRediT authorship contribution statement
Veronica C. Galvin: Methodology, Investigation, Writing – original draft, Writing – review & editing. Shengtao Yang: Methodology, Investigation, Writing – review & editing. Adam S. Lowet: Methodology, Investigation, Writing – review & editing. Dibyadeep Datta: Methodology, Investigation, Writing – review & editing. Alvaro Duque: Funding acquisition, Methodology, Investigation. Amy FT. Arnsten: Funding acquisition, Writing – original draft, Writing – review & editing. Min Wang: Funding acquisition, Methodology, Investigation, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank Dr. Jeffery Conn for providing the novel M1R positive allosteric modulator VU0453595 used for systemic behavioral testing. We thank MacBrainResource (MBR, https://medicine.yale.edu/neuroscience/macbrain/) for allowing use of its equipment. MBR is supported by MH113257 to Dr. Alvaro Duque. We also thank L Ciavarella, S Johnson, T Sadlon, and M Wilson for their invaluable technical support.
Footnotes
A Peer Review Overview and (sometimes) Supplementary data associated with this article: https://doi.org/10.1016/j.crneur.2021.100016.
Appendix A. Peer Review Overview and Supplementary data
A Peer Review Overview and (sometimes) Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.crneur.2021.100016
References
- Amatrudo J.M., Weaver C.M., Crimins J.L., Hof P.R., Rosene D.L., Luebke J.I. Influence of highly distinctive structural properties on the excitability of pyramidal neurons in monkey visual and prefrontal cortices. J. Neurosci. 2012;32:13644–13660. doi: 10.1523/JNEUROSCI.2581-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A., Macdonald M.L., Borgmann-Winter K.E., Hahn C.-G. Neuregulin 1-erbB4 pathway in schizophrenia: from genes to an interactome. Brain Res. Bull. 2010;83:132–139. doi: 10.1016/j.brainresbull.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartus R.T., Dean R.L. Tetrahydroaminoacridine, 3,4 diaminopyridine and physostigmine: direct comparison of effects on memory in aged primates. Neurobiol. Aging. 1988;9:351–356. doi: 10.1016/s0197-4580(88)80080-0. [DOI] [PubMed] [Google Scholar]
- Bustillo J.R., Chen H., Gasparovic C., Mullins P., Caprihan A., Qualls C., Apfeldorf W., Lauriello J., Posse S. Glutamate as a marker of cognitive function in schizophrenia: a proton spectroscopic imaging study at 4 tesla. BPS. 2011;69:19–27. doi: 10.1016/j.biopsych.2010.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppola J.J., Disney A.A. Is there a canonical cortical circuit for the cholinergic system? Anatomical differences across common model systems. Front. Neural Circ. 2018;12:1–13. doi: 10.3389/fncir.2018.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corlett P.R., Honey G.D., Aitken M.R.F., Dickinson A., Shanks D.R., Absalom A.R., Lee M., Pomarol-Clotet E., Murray G.K., McKenna P.J., et al. Frontal responses during learning predict vulnerability to the psychotogenic effects of ketamine: linking cognition, brain activity, and psychosis. Arch. Gen. Psychiatr. 2006;63:611–621. doi: 10.1001/archpsyc.63.6.611. [DOI] [PubMed] [Google Scholar]
- Croxson P.L., Kyriazis D.A., Baxter M.G. Cholinergic modulation of a specific memory function of prefrontal cortex. Nat. Neurosci. 2011;14:1510–1512. doi: 10.1038/nn.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y., Jin J., Zhang X., Xu H., Yang L., Du D., Zeng Q., Tsien J.Z., Yu H., Cao X. Forebrain NR2B overexpression facilitating the prefrontal cortex long-term potentiation and enhancing working memory function in mice. PLoS One. 2011;6 doi: 10.1371/journal.pone.0020312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disney A.A., Reynolds J.H. Expression of m1-type muscarinic acetylcholine receptors by parvalbumin-immunoreactive neurons in the primary visual cortex: a comparative study of rat, Guinea pig, ferret, macaque, and human. J. Comp. Neurol. 2014;522:986–1003. doi: 10.1002/cne.23456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disney A.A., Domakonba K.V., Aoki C. Differential expression of muscarinic acetylcholine receptors across excitatory and inhibitory cells in visual cortical areas V1 and V2 of the macaque monkey. J. Comp. Neurol. 2006;499:49–63. doi: 10.1002/cne.21096. [DOI] [PubMed] [Google Scholar]
- Ellis J.R., Ellis K.A., Bartholomeusz C.F., Harrison B.J., Wesnes K.A., Erskine F.F., Vitetta L., Nathan P.J. Muscarinic and nicotinic receptors synergistically modulate working memory and attention in humans. Int. J. Neuropsychopharmacol. 2006;9:175–189. doi: 10.1017/S1461145705005407. [DOI] [PubMed] [Google Scholar]
- Funahashi S., Bruce C.J., Goldman-Rakic P.S. Mnemonic coding of visual space in the monkey's dorsolateral prefrontal cortex. J. Neurophysiol. 1989;61:331–349. doi: 10.1152/jn.1989.61.2.331. [DOI] [PubMed] [Google Scholar]
- Galvin V.C., Yang S., Paspalas C.D., Yang Y., Jin L.E., Datta D., Morozov Y.M., Lightbourne T.C., Lowet A.S., Rakic P., et al. Muscarinic M1 receptors modulate working memory performance and activity via KCNQ potassium channels in primate prefrontal cortex. Neuron. 2020 doi: 10.2139/ssrn.3492110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geula C., Mesulam M.M. Cortical cholinergic fibers in aging and Alzheimer's disease: a morphometric study. Neuroscience. 1989;33 doi: 10.1016/0306-4522(89)90399-0. [DOI] [PubMed] [Google Scholar]
- Gibson G.E., Peterson C. Aging decreases oxidative metabolism and the release and synthesis of acetylcholine. J. Neurochem. 1981;37:978–984. doi: 10.1111/j.1471-4159.1981.tb04484.x. [DOI] [PubMed] [Google Scholar]
- Gibson G.E., Peterson C., Jenden D.J. Brain acetylcholine synthesis declines with senescence. Science (80 ) 1981;213:674–676. doi: 10.1126/science.7256270. [DOI] [PubMed] [Google Scholar]
- Gilman J.P., Medalla M., Luebke J.I. Area-specific features of pyramidal neurons-a comparative study in mouse and rhesus monkey. Cerebr. Cortex. 2017;27:2078–2094. doi: 10.1093/cercor/bhw062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic P.S. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos G., Barrionuevo G., Lewis D.A. Horizontal synaptic connections in monkey prefrontal cortex: an in vitro electrophysiological study. Cerebr. Cortex. 2000;10:82–92. doi: 10.1093/cercor/10.1.82. [DOI] [PubMed] [Google Scholar]
- Green A., Ellis K.A., Ellis J., Bartholomeusz C.F., Ilic S., Croft R.J., Phan K.L., Nathan P.J. Muscarinic and nicotinic receptor modulation of object and spatial n-back working memory in humans. Pharmacol. Biochem. Behav. 2005;81:575–584. doi: 10.1016/j.pbb.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Jackson M.E., Homayoun H., Moghaddam B. 2004. NMDA Receptor Hypofunction Produces Concomitant Firing Rate Potentiation and Burst Activity Reduction in the Prefrontal Cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt D.C. 2010. Glutamatergic Theories of Schizophrenia. [PubMed] [Google Scholar]
- Kantrowitz J, Javitt DC: Glutamatergic Transmission in Schizophrenia: from Basic Research to Clinical Practice. [date unknown], doi:10.1097/YCO.0b013e32835035b2. [DOI] [PMC free article] [PubMed]
- Larsen J.K., Div Ac I. 1978. Selective Ablations within the Prefrontal Cortex of the Rat and Performance of Delayed Alternation. [Google Scholar]
- Major A.J., Vijayraghavan S., Everling S. Muscarinic attenuation of mnemonic rule representation in macaque dorsolateral prefrontal cortex during a pro-and anti-saccade task. J. Neurosci. 2015;35:16064–16076. doi: 10.1523/JNEUROSCI.2454-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra A.K., Pinals D.A., Adler C.M., Elman I., Clifton A., Pickar D., Breirer A. vol. 17. 1997. pp. 141–150. (Ketamine-Induced Exacerbation of Psychotic Symptoms and Cognitive Impairment in Neuroleptic-free Schizophrenics). [DOI] [PubMed] [Google Scholar]
- McQuail J.A., Beas B.S., Kelly K.B., Simpson K.L., Frazier C.J., Setlow B., Bizon J.L. NR2A-containing NMDARs in the prefrontal cortex are required for working memory and associated with age-related cognitive decline. J. Neurosci. 2016;36:12537–12548. doi: 10.1523/JNEUROSCI.2332-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M -Marsel, Mufson E.J., Levey A.I., Wainer B.H. Cholinergic innervation of cortex by the basal forebrain: cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (Substantia innominata), and hypothalamus in the rhesus monkey. J. Comp. Neurol. 1983;214:170–197. doi: 10.1002/cne.902140206. [DOI] [PubMed] [Google Scholar]
- Mesulam M.M., Mufson E.J., Wainer B.H., Levey A.I. Central cholinergic pathways in the rat: an overview based on an alternative nomenclature (Ch1-Ch6) Neuroscience. 1983;10:1185–1201. doi: 10.1016/0306-4522(83)90108-2. [DOI] [PubMed] [Google Scholar]
- Mesulam M Marsel, Volicer L., Marquis J.K., Mufson E.J., Green R.C. Systematic regional differences in the cholinergic innervation of the primate cerebral cortex: distribution of enzyme activities and some behavioral implications. Ann. Neurol. 1986;19:144–151. doi: 10.1002/ana.410190206. [DOI] [PubMed] [Google Scholar]
- Mrzljak L., Levey A.I., Goldman-Rakic P.S. Proceedings of the National Academy of Sciences of the United States of America. 1993. Association of m1 and m2 muscarinic receptor proteins with asymmetric synapses in the primate cerebral cortex: morphological evidence for cholinergic modulation of excitatory neurotransmission; pp. 5194–5198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda S., Tsuneoka Y., Yoshida S., Adachi-Akahane S., Ito M., Kuroda M., Funato H. Immunolocalization of muscarinic M1 receptor in the rat medial prefrontal cortex. J. Comp. Neurol. 2018;526:1329–1350. doi: 10.1002/cne.24409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paspalas C.D., Goldman-Rakic P.S. 2004. Cellular/Molecular Microdomains for Dopamine Volume Neurotransmission in Primate Prefrontal Cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlstein W.M., Carter C.S., Noll D.C., Cohen J.D. Relation of prefrontal cortex dysfunction to working memory and symptoms in schizophrenia. Am. J. Psychiatr. 2001;158:1105–1113. doi: 10.1176/appi.ajp.158.7.1105. [DOI] [PubMed] [Google Scholar]
- Rogers S.L., Doody R.S., Mohs R.C., Friedhoff L.T. Donepezil improves cognition and global function in Alzheimer disease: a 15-week, double-blind, placebo-controlled study. Arch. Intern. Med. 1998;158:1021–1031. doi: 10.1001/archinte.158.9.1021. [DOI] [PubMed] [Google Scholar]
- Rye D.B., Wainer B.H., Mesulam M.M., Mufson E.J., Saper C.B. Cortical projections arising from the basal forebrain: a study of cholinergic and noncholinergic components employing combined retrograde tracing and immunohistochemical localization of choline acetyltransferase. Neuroscience. 1984;13:627–643. doi: 10.1016/0306-4522(84)90083-6. [DOI] [PubMed] [Google Scholar]
- Spellman T, Rigotti M, Ahmari SE, Fusi S, Gogos JA, Gordon JA: Hippocampal-prefrontal Input Supports Spatial Encoding in Working Memory. [date unknown], doi:10.1038/nature14445. [DOI] [PMC free article] [PubMed]
- Suh B.C., Hille B. Recovery from muscarinic modulation of M current channels requires phosphatidylinositol 4,5-bisphosphate synthesis. Neuron. 2002;35:507–520. doi: 10.1016/s0896-6273(02)00790-0. [DOI] [PubMed] [Google Scholar]
- Veselinović T., Vernaleken I., Janouschek H., Kellermann T., Paulzen M., Cumming P., Gründer G. Effects of anticholinergic challenge on psychopathology and cognition in drug-free patients with schizophrenia and healthy volunteers. Psychopharmacology (Berl) 2015;232:1607–1617. doi: 10.1007/s00213-014-3794-9. [DOI] [PubMed] [Google Scholar]
- Vijayraghavan S., Major A.J., Everling S. Muscarinic M1 receptor overstimulation disrupts working memory activity for rules in primate prefrontal cortex. Neuron. 2018;98:1256–1268.e4. doi: 10.1016/j.neuron.2018.05.027. [DOI] [PubMed] [Google Scholar]
- Wallis CU, Cardinal RN, Alexander L, Roberts AC, Clarke HF: Opposing Roles of Primate Areas 25 and 32 and Their Putative Rodent Homologs in the Regulation of Negative Emotion. [date unknown], doi:10.1073/pnas.1620115114. [DOI] [PMC free article] [PubMed]
- Wang H., Stradtman G.G., Wang X.J., Gao W.J. A specialized NMDA receptor function in layer 5 recurrent microcircuitry of the adult rat prefrontal cortex. Proc. Natl. Acad. Sci. U. S. A. 2008;105:16791–16796. doi: 10.1073/pnas.0804318105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Yang Y., Wang C.J., Gamo N.J., Jin L.E., Mazer J.A., Morrison J.H., Wang X.J., Arnsten A.F.T. NMDA receptors subserve persistent neuronal firing during working memory in dorsolateral prefrontal cortex. Neuron. 2013;77:736–749. doi: 10.1016/j.neuron.2012.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weickert C.S., Fung S.J., Catts V.S., Schofield P.R., Allen K.M., Moore L.T., Newell K.A., Pellen D., Huang X.-F., Catts S.V., et al. Molecular evidence of N-methyl-D-aspartate receptor hypofunction in schizophrenia. Mol. Psychiatr. 2012;18:1185–1192. doi: 10.1038/mp.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehouse P.J., Price D.L., Struble R.G., Clark A.W., Coyle J.T., DeLong M.R. Alzheimer's disease and senile dementia: loss of neurons in the basal forebrain. Science (80 ) 1982;215:1237–1239. doi: 10.1126/science.7058341. [DOI] [PubMed] [Google Scholar]
- Yang Y., Paspalas C.D., Jin L.E., Picciotto M.R., Arnsten A.F.T., Wang M. Nicotinic α7 receptors enhance NMDA cognitive circuits in dorsolateral prefrontal cortex. Proc. Natl. Acad. Sci. U. S. A. 2013;110 doi: 10.1073/pnas.1307849110. 12078–12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaborszky L., Csordas A., Mosca K., Kim J., Gielow M.R., Vadasz C., Nadasdy Z. Neurons in the basal forebrain project to the cortex in a complex topographic organization that reflects corticocortical connectivity patterns: an experimental study based on retrograde tracing and 3D reconstruction. Cerebr. Cortex. 2015;25:118–137. doi: 10.1093/cercor/bht210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.